Abstract
Expression of functional transforming growth factor β (TGF-β) receptors (TβR) is required for the invasion of mammalian cells by the protozoan parasite Trypanosoma cruzi. However, the precise role of this host cell signaling complex in T. cruzi infection is unknown. To investigate the role of the TGF-β signaling pathway, infection levels were studied in the mink lung epithelial cell lines JD1, JM2, and JM3. These cells express inducible mutant TβR1 proteins that cannot induce growth arrest in response to TGF-β but still transmit the signal for TGF-β-dependent gene expression. In the absence of mutant receptor expression, trypomastigotes invaded the cells at a low level. Induction of the mutant receptors caused an increase in infection in all three cell lines, showing that the requirement for TGF-β signaling at invasion can be divorced from TGF-β-induced growth arrest. TGF-β pretreatment of mink lung cells expressing wild-type TβR1 caused a marked enhancement of infection, but no enhancement was seen in JD1, JM2, and JM3 cells, showing that the ability of TGF-β to stimulate infection is associated with growth arrest. Likewise, expression of SMAD7 or SMAD2SA, inhibitors of TGF-β signaling, did not block infection by T. cruzi but did block the enhancement of infection by TGF-β. Taken together, these results show that there is a dual role for TGF-β signaling in T. cruzi infection. The initial invasion of the host cell is independent of both TGF-β-dependent gene expression and growth arrest, but TGF-β stimulation of infection requires a fully functional TGF-β signaling pathway.
Trypanosoma cruzi is the agent responsible for Chagas' disease, a chronic infection widespread in Central and South America (4). T. cruzi is an intracellular parasite in the mammalian host. Infectious metacyclic trypomastigotes introduced in the feces of the hemophagic reduviid bug invade host cells. Initially the parasite is located in a lysosomal compartment, but it rapidly escapes into the cytoplasm. Amastigotes, the intracellular form of the parasite, divide in the host cell cytoplasm and then differentiate back into trypomastigotes, which are released, destroying the cell and allowing continuation of infection in new cells.
The process of mammalian cell invasion by T. cruzi and the mechanisms of parasite survival within the host cell are poorly understood. Entry is accompanied by stimulation of several host cell signaling pathways by trypomastigotes. Calcium transients (16), tyrosine phosphorylation (20), MAP kinase activation (20), and transforming growth factor β (TGF-β) receptor (TβR) activation (10) are all important in invasion. The role of TGF-β signaling in T. cruzi infection is demonstrated by the fact that infection is very low in the absence of TβR1 or -2 and is restored by expression of the deficient receptor (10). Preincubation of host cells with TGF-β causes a marked enhancement of infection (10), but the mechanism by which TGF-β promotes T. cruzi infection is not known.
TGF-β is a pleiotropic factor that regulates a number of cellular functions and can stimulate expression of extracellular matrix proteins, cell cycle arrest, cell growth, and apoptosis in different cell types (15). The TβR complex is made up of two transmembrane serine/threonine kinases, TβR1 and TβR2 (21). TβR2 kinase is constitutively active but cannot induce signaling until TGF-β binding induces association with TβR1 (22). Phosphorylation of TβR1 by TβR2 activates kinase activity and stimulates association with SMAD2 and SMAD3 (6, 9, 12). In unstimulated cells, these proteins are present in an inactive form in the cytoplasm. Phosphorylation by TβR1 activates SMAD2 and SMAD3, causing association with the related protein SMAD4 and translocation to the nucleus, where the SMAD complex binds to DNA and triggers gene expression (1, 8, 9, 19). SMAD7, an inhibitory member of the SMAD family, is induced by TGF-β signaling (2, 13). This protein binds to TβR1 and prevents association with the signaling SMAD proteins, thereby blocking TGF-β signaling.
TGF-β signaling has been extensively studied by overexpression of mutant receptors and SMAD proteins to dissect the function of different components of the pathway. The mutant TβR1 receptors, JD1, JM2, and JM3, all contain mutations in the juxtamembrane region of the receptor (17). JM2 and JM3 are mutated at specific phosphorylation sites, Ser172 and Thr176, respectively, while JD1 lacks the entire juxtamembrane region from amino acid 150 to 181. All three mutants are active in kinase activity and can trigger changes in expression of the matrix proteins fibronectin and plasminogen activator inhibitor, but they cannot induce growth arrest. Overexpression of SMAD7 or of SMAD2SA, a mutant of SMAD2 in which the phosphorylation sites (Ser465 and Ser247) are mutated to alanine residues, both block TGF-β signaling completely (1, 13, 19). Expression of SMAD2SD, which contains aspartate residues in place of Ser465 and Ser467, leads to constitutive activation of these pathways (19).
In order to study the role of TGF-β signaling in T. cruzi infection, we utilized an inducible expression system in mink lung epithelial (Mv1Lu) cells to determine the effect of expression of mutant TβR1 proteins and SMAD family proteins on infection.
MATERIALS AND METHODS
Parasites and cells.
T. cruzi Silvio strain trypomastigotes were maintained in Vero cells in RPMI medium supplemented with 10% fetal bovine serum, 12.5 mM HEPES, 2 g of sodium bicarbonate per liter, penicillin, and streptomycin (Gibco, Rockville, Md.). Released trypomastigotes were harvested by centrifugation at 500 × g for 5 min to remove host cells and debris and at 1,200 × g for 10 min to recover parasites. All experiments were carried out using wild-type Mv1Lu or R4.2 cells, which are mutant Mv1Lu cells lacking functional TβR1. Stable transfectants of Mv1Lu and R4.2 cells were generated with pMEP4-derived plasmids—pMEP4 expresses genes under the control of the metallothionein promoter, which is activated by Zn2+ (17, 21). Construction of the plasmids and development of stable transfectants containing mutant TβR1, SMAD2SA, and SMAD7 proteins are described in references 17, 19, and 7, respectively. Functional studies revealed that the TβR1 transfectants were resistant to growth arrest by 2 ng of TGF-β1 per ml, as described in references 7, 17, and 19. Mv1Lu cells were maintained in minimal essential medium (MEM) supplemented with nonessential amino acids, 10% fetal bovine serum, 12.5 mM HEPES, 2 g of sodium bicarbonate per liter, penicillin, and streptomycin (Gibco).
Infection assays.
Mv1Lu cells were plated into 96-well plates at 2 × 103/well and incubated overnight. Expression of receptors or mutant SMAD proteins was induced by replacement of medium with MEM containing 0.2% serum and 100 μM zinc chloride and incubation for 4 to 6 h (17). For TGF-β pretreatment, cells were incubated for 24 h in the presence of various concentrations of recombinant human TGF-β1 (R&D Systems, Minneapolis, Minn.). For infection, trypomastigotes were added at a concentration of 106/ml in RPMI medium–1% bovine serum albumin for 2 h. The plates were washed with RPMI medium and then incubated in MEM containing 2.5% Nu serum at 37°C for 48 h. Plates were washed with phosphate-buffered saline and stained with DiffQuik as described in reference 14. The percent infection and number of parasites were determined for at least 300 cells per well. Significance was determined using Student's t test.
RESULTS AND DISCUSSION
In order to determine the role of the growth arrest pathway in infection with T. cruzi, R4.2 cells, whose TβR1 is nonfunctional, were induced to express wild-type TβR1 and mutant TβR1 proteins JD1, JM2, and JM3. The various transfectants were then infected with T. cruzi trypomastigotes, and after 2 days, the infection level in the cells was determined. Addition of 100 μM zinc chloride had no significant effect on infection of wild-type Mv1Lu (Fig. 1). R4.2 cells transfected with pMEP4 alone show significantly lower levels of infection than wild-type cells (P < 0.02). This is in accordance with the observations made previously with the mutant Mv1Lu line R1B, which also bears nonfunctional TβR1 (10). The results with both R1B and R4.2 cells show that T. cruzi requires functional TβR1 expression for proper invasion, at least of epithelial cells.
FIG. 1.
Expression of wild-type and mutant TβR1 increases the infection level in Mv1Lu cells. Wild-type (WT) Mv1Lu cells and R4.2 clone cells (bearing a mutated TβR1 gene) were transfected with pMEP4 carrying wild-type TβR1 or mutant receptors JD1, JM2, and JM3 and incubated in the presence or absence of 100 μM ZnCl2 for 4 h prior to infection. Infection with Silvio strain trypomastigotes was carried out as described in Materials and Methods. Cells containing at least two amastigotes were scored as infected. At least 300 cells were counted per well. Each bar represents the mean of triplicate wells + the standard error of the mean. This graph is representative of eight experiments with similar results.
No change in infection was seen when R4.2 cells were incubated with zinc prior to infection. By contrast, the percentage of cells infected was markedly increased in R4.2 cells transfected with pMEP4 carrying the wild-type TβR1 gene and exposed to zinc to induce receptor expression, which is under the control of the zinc-sensitive metallothionein promoter. Basal levels of infection in the transfectants (i.e., in the absence of zinc stimulation) were also higher in these cells, probably due to a low level of constitutive receptor expression. Expression of the mutant receptors JD1, JM2, and JM3 enhanced the infection level in each case. Consistent results were obtained in eight experiments. These results show that expression of TβR1 is sufficient to enhance infection level, irrespective of the ability of the receptor to induce growth arrest. Thus, although the receptor is required for invasion, induction of growth arrest by the parasite is unlikely to be the basis of that requirement.
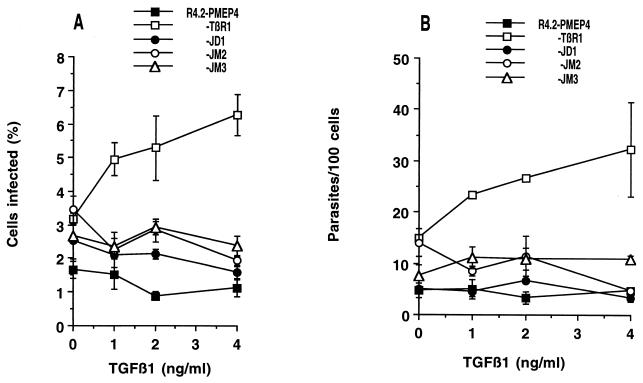
Exposure of Mv1Lu cells to TGF-β enhances infection dramatically (10). To determine whether the growth arrest pathway might be involved in this enhancement, R4.2 cells expressing wild-type or mutant receptors were incubated with various concentrations of TGF-β 24 h before infection. In the absence of zinc, infection levels were uniformly low in the presence or absence of TGF-β (data not shown). TGF-β did not have any effect on infection of R4.2 cells transfected with an empty plasmid (Fig. 2). Cells expressing wild-type TβR1 showed a dose-dependent enhancement of infection with increasing concentrations of TGF-β1. However, in JD1, JM2, and JM3, the lines expressing mutant TβR1, TGF-β had very little effect on the infection level, either in terms of the percentage of cells infected (Fig. 2A) or in terms of parasite replication (Fig. 2B). The ability of TGF-β to enhance infection is therefore closely associated with the stimulation of growth arrest.
FIG. 2.
Dose response to TGFβ in Mv1Lu cells expressing wild-type and mutant TβR1. R4.2 cells (bearing mutated TβR1) were transfected with pMEP4 alone or pMEP4 carrying TβR1, JD1, JM2, or JM3 and incubated with 100 μM ZnCl2 for 4 h. Cells were incubated overnight in the presence of the indicated concentrations of TβR1 and infected with trypomastigotes as described in Materials and Methods. Cells containing at least two amastigotes were scored as infected, and the numbers of parasites in each infected cell were also counted. At least 300 cells were counted per well. Each point represents the mean of triplicate wells ± the standard error of the mean.
To determine the role of signaling events downstream of the TGF-β1 receptor in T. cruzi infection, we employed Mv1Lu cells expressing two different mutant SMAD2 proteins, SMAD2SA and SMAD2SD, and SMAD7. SMAD2SA and SMAD7 are dominant negative inhibitors of TGF-β signaling, while SMAD2SD is constitutively active (13, 19). Like wild-type cells, Mv1Lu cells transfected with the plasmid pMEP4 alone showed similar levels of infection in the presence and absence of zinc (Fig. 3). In cells expressing SMAD2D, however, infection levels were significantly enhanced (P < 0.01). The activation of signaling in this case mimicked the effect of addition of exogenous TGF-β1. Interestingly, expression of either SMAD2SA or SMAD7 did not alter infection. The two cell lines showed levels of infection similar to that of the control cells transfected with pMEP4 alone, and addition of zinc had no effect. Neither the number of cells infected nor the number of parasites per cell (i.e., parasite replication) was affected by the ectopic expression of SMAD2SA and SMAD7, suggesting that the SMAD pathway has no direct involvement in infection of host cells by T. cruzi.
FIG. 3.
Infection in cells expressing mutant SMAD2 or SMAD7. Mv1Lu cells transfected with an empty vector (pMEP4) or a vector carrying SMAD2SD, SMAD2SA, or SMAD7 were incubated for 6 h in the presence or absence of 100 μM ZnCl2 and then infected with trypomastigotes. At least 400 cells were counted per well. Each bar represents the mean of triplicate wells + the standard error of the mean.
To determine the role of the SMAD pathway in the stimulation of infection by TGF-β, cells were induced to express SMAD2SA or SMAD7 and then incubated with 2 ng of TGF-β1 per ml prior to infection. In the absence of zinc, TGF-β1 pretreatment enhanced the percentage of cells infected more than threefold (Fig. 4A). The effect on parasite replication was even more dramatic (Fig. 4B). The levels of enhancement seen in cells transfected with pMEP4 containing the SMAD2SA or SMAD7 gene were similar to those in control cells transfected with pMEP4 alone. When zinc was added to induce expression of either SMAD2SA or SMAD7, the enhancement of infection by TGF-β was partially, but not completely, blocked (P < 0.01). This shows that the SMAD pathway is involved in the enhancement of infection by TGF-β, but it suggests that other signals may also play a role.
FIG. 4.
Expression of SMAD2SA and SMAD7 partially blocks the enhancement of infection by TGF-β1. Mv1Lu cells transfected with an empty vector (pMEP4) or a vector carrying SMAD2SA or SMAD7 were incubated for 4 h in the presence or absence of 100 μM ZnCl2, and then 2 ng of TGF-β1 per ml was added with or without ZnCl2. The cells were incubated overnight and then infected with trypomastigotes as described in Materials and Methods. At least 300 cells were counted per well. Each bar represents the mean of triplicate wells + the standard error of the mean.
The results presented here point to a dual role for TGF-β signaling in T. cruzi infection. The parasite requires receptor expression to enter host cells, but this requirement can be divorced from the downstream SMAD signaling pathway and from TGF-β-induced growth arrest. It has previously been shown that trypomastigotes can trigger expression of genes controlled by TGF-β and that kinase-inactive TβR1 does not support infection (10), but in apparent contradiction, the major downstream signals induced by TGF-β do not seem to be very important in invasion. However, TβR expression is required for trypomastigote entry into the host cell, rather than long-term establishment of infection (10). Invasion is a rapid process, taking only a few minutes. Changes in gene expression, while they may have an impact on parasite growth within the host cell, are unlikely to contribute to parasite entry. It is more likely that other yet-to-be-determined signals are involved as well in T. cruzi invasion. TGF-β has been shown to activate the GTPase Ras (25), which is required for control of transcription but not for growth arrest (24). TGF-β also activates the Rho family GTPases (3, 11, 24), which control cell shape and motility (5). Other pathways stimulated by TGF-β include activation of kinases such as TAK1 (23), SAPK (3), and Erk1 (11). These are early events in TGF-β signaling that may control infection. Other events, such as receptor clustering and association with cytoplasmic proteins, could also be important.
The role of TGF-β itself in infection by T. cruzi is quite distinct from the requirement for the TβR. The enhancement of infection is a long-term effect, closely associated with growth arrest and at least partially dependent on the SMAD signaling pathway. Previous work suggested that growth arrest per se is not sufficient to enhance infection (10). The effect of TGF-β may therefore depend on alterations in expression of specific genes during TGF-β-induced growth arrest, rather than the growth arrest itself. Although the role of exogenously added TGF-β is distinct from the role of parasite-induced receptor activation, the ability of TGF-β to enhance infection is highly relevant to parasite survival in vivo. TGF-β-producing cells are abundant in heart lesions of mice infected with T. cruzi (26). Infection of macrophages induces TGF-β expression, which in turn reduces the ability of gamma interferon to control intracellular infection (18). In addition, experimental T. cruzi infection is exacerbated by injection of TGF-β, showing that this cytokine is a key regulator of infection (18). The presence of TGF-β in infected tissues serves to enhance infection directly by increasing susceptibility to infection and indirectly by counteracting the effects of antiparasitic cytokines.
The dual role for the TGF-β pathway in T. cruzi infection demonstrates the importance of host cell responses in parasite survival. Identification of the specific mechanisms underlying the two effects may reveal novel aspects of TGF-β signaling and lead to new targets for the therapy of Chagas' disease.
ACKNOWLEDGMENTS
We thank P. ten Dijke, M. Saitoh. H. Ichijo, S. Souchelnytsky, and S. Itoh for the stable transfectants of Mv1Lu containing TGF-β.
This work was supported by NIH grant AI18102.
REFERENCES
- 1.Abdollah S, Marcias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana J L. TβR1 phosphorylation of SMAD2 on Ser465 and Ser467 is required for SMAD2-SMAD4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- 2.Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin C H, Heldin N E, ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun. 1998;249:505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- 3.Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/cJun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- 4.Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- 5.Hall A. Rho-GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 6.Heldin C H, Miyazono K, ten Dijke P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 7.Itoh S, Landstrom M, Hermansson A, Itoh F, Heldin C H, Heldin N E, ten Dijke P. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J Biol Chem. 1998;273:29195–29201. doi: 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- 8.Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Partnership between DPC4 and SMAD proteins in TGF-β signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 9.Marcias-Silva M, Abdollah S, Hoodless P A, Pirone R, Attisano L, Wrana J L. MADR2 is a substrate for the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 10.Ming M, Ewen M E, Pereira M E A. Trypanosome invasion of mammalian cells requires activation of the TGFβ signalling pathway. Cell. 1995;82:287–296. doi: 10.1016/0092-8674(95)90316-x. [DOI] [PubMed] [Google Scholar]
- 11.Mucsi I, Skorecki K L, Goldberg H J. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-β1 on gene expression. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 12.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin C H, Miyazono K, ten Dijke P. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian J L, Heuchel R, Itoh S, Kawabata M, Heldin N E, Heldin C H, ten Dijke P. Identification of Smad7, a TGF-beta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 14.Ortega-Barria E, Pereira M E A. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 15.Roberts A, Sporn M. The transforming growth factor-betas. In: Sporn M B, Roberts A B, editors. Peptide growth factors and their receptors. Heidelberg, Germany: Springer-Verlag; 1990. pp. 419–472. [Google Scholar]
- 16.Rodriguez A, Rioult M G, Ora A, Andrews N W. A trypanosome-soluble factor induces IP3 formation, intracellular calcium mobilization and microfilament rearrangement in host cells. J Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh M, Nishitoh H, Amagase T, Miyazono K, Takagi M, Ichijo H. Identification of important regions in the cytoplasmic juxtamembrane domain of type I receptor that separate signaling pathways of transforming growth factor-β. J Biol Chem. 1996;271:2769–2775. doi: 10.1074/jbc.271.5.2769. [DOI] [PubMed] [Google Scholar]
- 18.Silva J S, Twardzic D R, Reed S G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-β) J Exp Med. 1991;174:539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souchelnytskyi S, Tamaki K, Engstrom U, Wernstedt C, ten Dijke P, Heldin C H. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-beta signaling. J Biol Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- 20.Villalta F, Zhang Y, Bibb K E, Burns J M, Lima M F. Signal transduction by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through MAP-kinase pathway. Biophys Biochem Res Commun. 1998;249:247–252. doi: 10.1006/bbrc.1998.9127. [DOI] [PubMed] [Google Scholar]
- 21.Wrana J L, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang X, Massague J. TGFβ signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 22.Wrana J L, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto H, Atsuchi N, Tanaka H, Ogawa W, Abe M, Takeshita A, Ueno H. Separate roles for H-Ras and Rac in signaling by transforming growth factor (TGF)-β. H-Ras is essential for activation of MAP kinase, partially required for transcriptional activation by TGFβ, but not required for signaling of growth suppression by TGFβ. Eur J Biochem. 1999;264:110–119. doi: 10.1046/j.1432-1327.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 25.Yan Z, Winawer S, Friedman E. Two different signal transduction pathways can be activated by transforming growth factor β1 in epithelial cells. J Biol Chem. 1994;269:13231–13237. [PubMed] [Google Scholar]
- 26.Zhang L, Tarleton R L. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]