Abstract
Simple Summary
The integument of leopard coral grouper (Plectropomus leopardus) becomes black, brown, and red under intensive culture. Fish skin color is one of the most important commercial traits in aquaculture and is strongly affected by the background color. The tank colors used to rear P. leopardus are generally gray or blue; however, the effect of tank color on fish physiological status is poorly understood. No studies related to background color on skin color have been conducted in P. leopardus. To further understand the molecular mechanisms of skin pigmentation in P. leopardus, fifteen experimental aquaria with circulating water were prepared, and 12 aquaria were pasted with the labels Blue, Red, Black, or White on opaque polypropylene plastic board. Three aquaria were Transparent. The results showed that lighter colors inhibited the formation of melanocytes and had a significant effect on carotenoid and lutein contents. Pigment-related genes were involved in the regulation of fish skin color and were affected by background color in P. leopardus. These results indicate that a white background is more conducive to maintaining red skin color in juvenile P. leopardus. Our findings provide a new idea on the culture of P. leopardus.
Abstract
Fish skin color is usually strongly affected by the background color of their environment. The study investigated the effects of five different background colors on the skin color of leopard coral groupers (Plectropomus leopardus). More than 450 juveniles were reared in Blue, Red, Black, White, and Transparent background tanks for 56 days. The paraffin section showed that the skin melanin zone of fish in the White group was smaller, whereas the Black and Red groups (especially Black) were nearly the largest. The apparent skin color of P. leopardus was red on the white background, which darkened in response to the other color backgrounds. The Black group revealed the blackest skin color, followed by the transparent group. Moreover, the White group had the highest L*, a*, and b* values. The melanin content and tyrosinase activity in the dorsal and ventral skin of the Black group were significantly higher than those in the other groups (p < 0.05), and the serum α-MSH level was higher in the Black group as well. The carotenoid and lutein contents showed completely different trends among the experimental groups, as carotenoid content was higher in the Red and White groups, while lutein content was higher in the Transparent group. The expression level of scarb1 was highest in the Blue and White groups, followed by the Transparent group, and lowest in the Black group (p < 0.05). The expression trend of scarb1 was similar to the skin color in different backgrounds, indicating that the background color regulated scarb1 expression level through visual center, then influenced the uptake and transport of carotenoids, then influenced the skin color formation of P. leopardus. Moreover, lighter colors inhibited the formation of melanocytes and had a significant effect on carotenoid and lutein contents. Pigment-related genes were involved in the regulation of fish skin color, and they were affected by background color in P. leopardus. These results indicate that a white background is more conducive to maintaining red skin color in juvenile P. leopardus.
Keywords: background color, MSH content, Plectropomus leopardus, pigments, skin color, tyrosinase activity
1. Introduction
Fish skin color is one of the most important commercial traits in aquaculture. Skin color has been classified into morphological and physiological types, both of which are affected by the interaction between environmental factors and genetics [1,2,3,4,5]. Teleosts can change their skin color or hue quickly by translocating melanosomes within the skin chromatophores, depending on differences in the light intensity and background color [5,6]. The color change is closely associated with neuroendocrine and endocrine systems in the hypothalamus–pituitary axis [7,8].
Five different pigment cell types have been identified in teleosts, including melanocytes, xantho-/erythrophores, iridophores, white leucophores, and blue cyanophores, and the color of the fish body is determined by the changes in pigment cells [9]. In many teleosts, adaptation to the background occurs as a physiological response, including aggregation and dispersion of pigments triggered by neural stimuli [8,10]. The apparent body color of Malaysian red tilapia is paler on a white background and darker on a black background, and the body color varies in response to transfer to the opposite background color [11]. Bright light causes aggregation of melanosomes, leading to a pale skin color, and dim light disperses the melanosomes [12]. In Oryzias latipes, the density of chromatic nerve fibers changes along with changes in the number of melanophores during prolonged background adaptation; melanophore size decreases first, followed by a decrease in the density of melanophores caused by gradual cell death on a white background [13,14]. Oreochromis niloticus exhibits a high cortisol level when maintained on blue and brown backgrounds [15], and fish reared on a black background are distinctively darker than those reared on a white, blue, or clear background [16,17,18]. Furthermore, the background color could influence the capture of food and consumption, and consequently, the growth of the fish [19,20,21].
Melanin-concentrating hormone (mch) and α-melanophore-stimulating hormone (α-MSH) are two peptide hormones controlling body color with opposite functions in the chromatophores of fish [7,8,22]. Body color in Oncorhynchus mykiss is affected by tank brightness; fish in white tanks have the brightest body color and the highest mch expression levels, and proopiomelanocortins (pomc-a and pomc-b) are more highly expressed in black tanks [23]. The body color of Carassius auratus exposed to fluorescent light on a white background is paler than that of fish held on a black background [23]. The aggregation of pigment induced by adapting to a white background has been associated with increased mch expression [24]. Recent research has shown that the expression levels of prepro-melanin-concentrating hormone 1 (pmch1) and proopiomelanocortin (pomc) are affected by the background color in C. auratus [25]. Moreover, carotenoids are also one of the main pigments that affect the color characteristics of fish. In Dawkinsia filamentosa, the black background tank helped body pigmentation and promoted carotenoid accumulation [26]. In addition, the carotenoid content was regulated by scarb1 (Scavenger receptor class B type I) [27,28]. The scarb1 gene is involved in the selective absorption and transport of carotenoids, and this function is conservative among different species [29].
The leopard coral grouper (Plectropomus leopardus) is a valuable marine fish in the family Epinephelidae. Moreover, this species is an important resource for intensive industrial farming in recirculating aquaculture systems due to their high nutritional value, tender flesh, beautiful skin color, and high breeding density. P. leopardus has high commercial value and broad market prospects. The integument of this fish becomes black, brown, and red under intensive culture [30], which is an important factor when determining fish quality, as Chinese markets prefer fish with a bright red skin color. Research has shown that different proportions of carotenoids change the skin color in this species [30]. The tank colors used to rear P. leopardus are generally gray or blue; however, the effect of tank color on fish physiological status is poorly understood. No studies related to the effect of background color on body color have been conducted in P. leopardus. To further understand the molecular mechanisms of skin pigmentation in P. leopardus, this study investigated the effects of background color on skin color and the endocrine system concerning body color changes and metabolism. This is the first study to examine the skin changes associated with the background color of P. leopardus and provide important guide for breeding of red skin color of P. leopardus, which will help to establish a new method of skin color regulation.
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
All experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals in China. All experimental procedures and sample collection were approved by the Institutional Animal Care and Use Committee of the College of Ocean of Hainan University, Hainan, China (protocol code HNUAUCC-2021-00007, 26 February 2021).
2.2. Fish
The experimental fish were obtained from the Dongfang Star Technology Co., Ltd. (Ledong, Hainan Province, China). Juvenile fish were about 10 g at 4 months post-hatch. They were maintained in experimental culture facilities during the acclimation and experimental periods under a 12 h natural photoperiods light/dark cycle at 28 ± 1 °C, pH range at 7.5~8.0, NH4—N < 0.5 mg/L, NO2—N < 0.1 mg/L, NO3—N < 12 mg/L. Aeration was supplied to each tank for 24 h and about a quarter of water was exchanged per day, and fish fed three times daily with a compound feed (Guangdong Yuequn Biotechnology Co., Ltd., Jieyang, China).
2.3. Experimental Tanks
Fifteen experimental aquaria with circulating water (length × width × depth = 60 × 40 × 40 cm) were prepared, and 12 aquaria were pasted with the labels Blue (Blue-1, Blue-2, Blue-3), Red (Red-1, Red-2, Red-3), Black (Black-1, Black-2, Black-3), or White (White-1, White-2, White-3) on opaque polypropylene plastic board. Three aquaria were Transparent (Transparent-1, Transparent-2, Transparent-3) (Figure S1). Fishing nets were placed over the aquaria to prevent the fish from escaping.
2.4. Experiment
These juveniles P. leopardus were randomly divided into five groups of Blue, Red, Black, White, or Transparent, with three replicates in each group. At the beginning of the experiment, we randomly chose 10 fish to measure the L* (lightness), a* (redness), and b* (yellowness) values of the dorsal, ventral, head, and caudal peduncle skin. Photographs were taken of the three fish. Then, healthy acclimated fish (average initial body weight 10.5 g) were randomly stocked into the 15 experimental aquaria with 30 fish per aquarium on 23 November 2020. The fish were reared in the indoor aquaria (a room with transparent roof) for 56 days from 23 November 2020 to 17 January 2021 and fed a commercially prepared diet (Guangdong Yuequn Biotechnology Co., Ltd., Jieyang, China) to satiation three times daily at about 08:00, 12:00, and 17:00.
2.5. Fish Sampling and Tissue Preparation
At the end of the experiment, photographs were taken of three fish per group. We randomly chose 10 fish from each group to measure the L*, a*, and b* values of the dorsal, ventral, head, and caudal peduncle skin with the ColorQuest XE (HunterLab, Reston, VA, USA). Nine fish were randomly selected from each group with three fish in each replicate. Then, blood samples were taken from the caudal vein with syringes, and serum samples were obtained for tyrosinase activity and α-MSH analyses after centrifugation (3000 g for 15 min) at 4 °C. Tissue samples, including dorsal and ventral skin of the fish from each group, were collected, snap-frozen in liquid nitrogen, and stored at −80 °C until processed.
2.6. Determination of Pigments, MSH Content, and Tyrosinase Activity
Nine fish per group were examined for the contents of skin melanin, carotenoids, lutein, and α-MSH, and serum α-MSH and tyrosinase activity were measured by ELISA kit (Zhenke Industrial International, Zhongshan, China). The skin was washed with precooled PBS (0.01 M, pH = 7.4), weighed, homogenized with cold PBS, and centrifuged (2000 g for 20 min) to obtain the supernatant for determination. The standard wells followed the test sample wells and the blank wells on the antibody-coated plate. Various 50 μL aliquots of different concentrations of the standard solution were added to each well. A 40 μL aliquot of the Sample Diluent and 10 μL of sample were added to each sample well. A 100 μL aliquot of HRP Conjugate was added to all wells except the blank well. The plate was covered and incubated at 37 °C for 60 min. The liquid in the wells was discarded and washing buffer was added to each well and allowed to stand for 30 s. Then, the liquid was discarded. These steps were repeated five times. A 50 μL aliquot of Chromogen Solution A and 50 μL of Chromogen Solution B were added to each well, mixed, and held at 37 °C for 15 min in the dark. A 50 μL aliquot of Stop solution was added to each well to stop the chromogenic reaction. The solution in the wells changed from blue to yellow. The absorbance of each well was read at 450 nm within 15 min after stopping the reaction. The zero was set with the blank well.
2.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from dorsal and ventral skin using TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, and nine fish per group were used. Genomic DNA was removed from the RNA samples using DNase I (New England Biolabs, Ipswich, MA, USA). The concentration of total RNA was measured with a UV spectrophotometer (NanoDrop 2000, Thermo Scientific, Waltham, MA, USA) and quality and integrity were checked at an OD of 260/280 by 1% agarose gel electrophoresis. First-strand cDNA was synthesized using the PrimeScript RT Master Mix (Takara, Shiga, Japan), and qPCR was performed on the ABI PRISM 7500 Real-time PCR System (ABI, Foster City, CA, USA). The amplification reactions were performed in a total volume of 25 μL, including 12.5 μL of 2× SYBR Green MasterMix reagent, 1 μL of cDNA, 1 μL of each primer (10 μM), and 9.5 μL of PCR-grade water. The thermal cycling profile consisted of initial denaturation at 95 °C for 5 min followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension for 45 s at 60 °C. The details are shown in Song et al. [31]. All primers were designed using Primer Premier 5 (Table 1).
Table 1.
Primer sequences.
| Primer | Sequences (5′–3′) | |
|---|---|---|
| tyr | F | GGTCGCATAGACAGTGCTTCC |
| R | GTCTTCAACATCCTCAGCGGT | |
| mch | F | TGCTCTGTCAGTGGCGATAC |
| R | GAGGGACAGTCCGTTGTGTT | |
| pomc | F | AGTCAGTGCTGGGAACATCC |
| R | GTCGAGATCTGACGGAGGAG | |
| scarb1 | F | CACCGTGTCCTACAGGGAGT |
| R | ACCAGTCCGCTGTCATAACC | |
| β-actin | F | CACCACAGCCGAGAGGGA |
| R | TCTGGGCAACGGAACCTCT | |
2.8. Statistical Analysis
All data are presented as mean ± standard error and were analyzed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). All data was analyzed by one-way ANOVA after a homogeneity of variance test. A p-value < 0.05 was considered significant. Duncan’s multiple range tests were used to identify differences between experimental groups when significant differences were found. Comparisons between the two groups were performed using Student’s t-test.
3. Results
3.1. Effects of Background Color on Skin Color
The skin color of P. leopardus reared in this study is shown in Figure 1. At the end of the experiment, the White group maintained a red skin color compared to the Initial group, followed by the Blue group. Nevertheless, the skin color of the fish in the Red, Black, and Transparent groups blackened, followed by that in the Transparent group.
Figure 1.
The apparent skin color of P. leopardus rearing in different background tanks.
3.2. Slice Microstructure Observations of the Skin
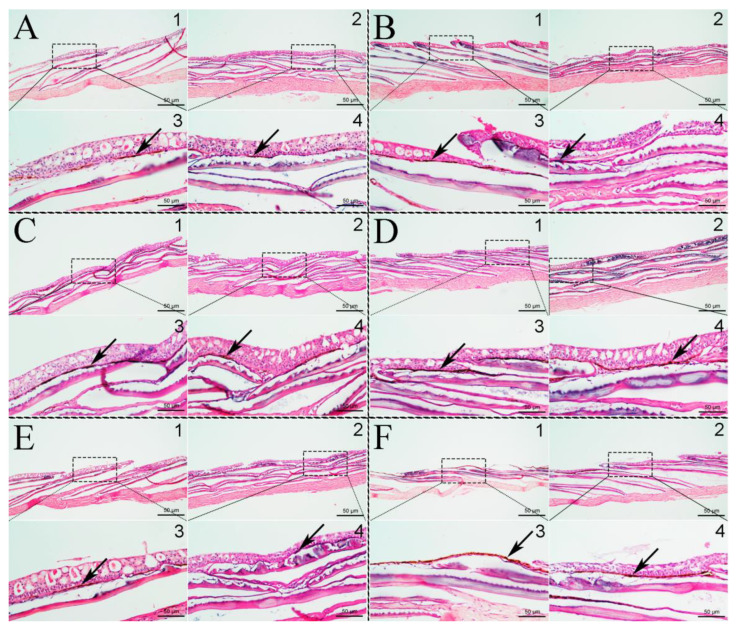
Paraffin sections of the dorsal and ventral skin were prepared to observe the effect of background color on skin pigment cells (Figure 2). In the Initial group (Figure 2A), the melanin zone in the dorsal and ventral skin was smaller than that in the other groups. The melanin zone of the fish skin in the White group was smaller, whereas that in the Black and Red groups was nearly the largest (Figure 2C,D). The melanin zone on the dorsal skin was larger than that of the ventral skin in all groups. Interestingly, the melanin granules on the dorsal skin were almost at the surface of the skin in the Transparent group (Figure 2F3).
Figure 2.
Slice microstructure observation of melanin zone in dorsal and ventral skin of P. leopardus. (A): Initial group; (B): Blue group; (C): Red group; (D): Black group; (E): White group; (F): Transparent group. Black arrows indicate melanin. In the subfigures, 1 is the dorsal skin and 2 is the ventral skin. 3 is the local amplification of 1, and 4 is the local amplification of 2.
3.3. Effects of Background Color on the L*, a*, and b* Values
The L*, a*, and b* values of the dorsal, ventral, head, and caudal peduncle skin of P. leopardus are shown in Table 2 and Table 3. As shown in Table 2, the average L* value of the ventral skin was higher than that of the dorsal skin (p < 0.05). The L* value of fish skin in the White group was the highest among all experimental groups (p < 0.05), whereas that in the Black group was the lowest (p < 0.05). Moreover, the trend in the a* value of the dorsal skin and the b* values of the dorsal and ventral skin were similar to the L* value. Nevertheless, the a* value of the ventral skin was highest in the White group among all experimental groups (p < 0.05), whereas the a* value of the Red group was the lowest (p < 0.05). In Table 3, no significant differences in the L* or a* values were observed between the head skin and caudal peduncle skin. The b* values of the head skin were higher than those of the caudal peduncle skin in the Initial and White groups (p < 0.05); however, no significant differences were detected among the other experimental groups.
Table 2.
Effects of background color on the L*, a*, and b* values of the dorsal and ventral skin.
| Dorsal Skin | Ventral Skin | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Initial | 37.95 ± 1.22 Ae | 5.34 ± 0.20 d | 6.20 ± 0.37 Ad | 52.73 ± 0.50 Bd | 6.10 ± 0.37 c | 9.40 ± 0.46 Bd |
| Blue | 29.10 ± 0.63 Ac | 1.92 ± 0.14 Ab | 3.57 ± 0.18 c | 42.93 ± 2.26 Bc | 3.85 ± 0.50 Bb | 3.78 ± 0.48 c |
| Red | 25.90 ± 0.84 Ab | 0.80 ± 0.09 a | 2.27 ± 0.20 Ab | 37.00 ± 1.66 Bb | 1.13 ± 0.15 a | 3.32 ± 0.30 Bb |
| Black | 22.62 ± 0.46 Aa | 0.70 ± 0.06 Aa | 1.32 ± 0.16 Aa | 31.62 ± 1.33 Ba | 1.63 ± 0.17 Ba | 2.67 ± 0.28 Ba |
| White | 33.45 ± 0.88 Ad | 2.57 ± 0.25 c | 4.23 ± 0.24 c | 51.33 ± 2.23 Bd | 3.72 ± 0.27 b | 4.45 ± 0.11 d |
| Transparent | 26.00 ± 0.85 Ab | 0.85 ± 0.08 Aa | 2.32 ± 0.18 b | 31.60 ± 1.24 Ba | 1.52 ± 0.16 Ba | 2.50 ± 0.23 a |
Note: Values are means ± standard error for ten replicates. Significant differences for means within experimental groups are indicated with different lowercase letter superscripts (p < 0.05, n = 10); Significant differences between means in the dorsal skin and ventral skin groups are indicated with different capital letter superscripts (p < 0.05, n = 10).
Table 3.
Effects of background on the L*, a*, and b* values of the head and caudal peduncle skin.
| Head Skin | Caudal Peduncle Skin | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Initial | 46.45 ± 0.44 d | 7.44 ± 0.29 c | 8.75 ± 0.26 Ac | 43.49 ± 0.59 d | 6.39 ± 0.18 c | 6.37 ± 0.29 Bc |
| Blue | 36.35 ± 1.13 b | 3.55 ± 0.42 b | 4.70 ± 0.38 b | 36.13 ± 0.56 c | 3.53 ± 0.37 b | 4.08 ± 0.21 b |
| Red | 31.57 ± 0.87 a | 1.27 ± 0.15 a | 2.40 ± 0.28 a | 32.02 ± 0.93 b | 1.55 ± 0.20 a | 2.73 ± 0.22 a |
| Black | 30.42 ± 0.83 a | 1.20 ± 0.13 a | 1.78 ± 0.07 a | 28.03 ± 0.99 a | 1.68 ± 0.19 a | 2.25 ± 0.18 a |
| White | 40.35 ± 1.14 c | 3.92 ± 0.42 b | 5.13 ± 0.50 Ab | 42.48 ± 1.81 d | 3.70 ± 0.29 b | 3.73 ± 0.50 Bb |
| Transparent | 30.88 ± 1.20 a | 1.23 ± 0.42 a | 1.94 ± 0.16 a | 27.58 ± 0.72 a | 1.62 ± 0.10 a | 2.30 ± 0.19 a |
Note: Values are means ± standard error for ten replicates. Significant differences for means within experimental groups are indicated with different lowercase letter superscripts (p < 0.05, n = 10); Significant differences for means between head skin and caudal peduncle skin groups are indicated with different capital letter superscripts (p < 0.05, n = 10).
3.4. Pigment Content and Tyrosinase Activity in Fish Skin
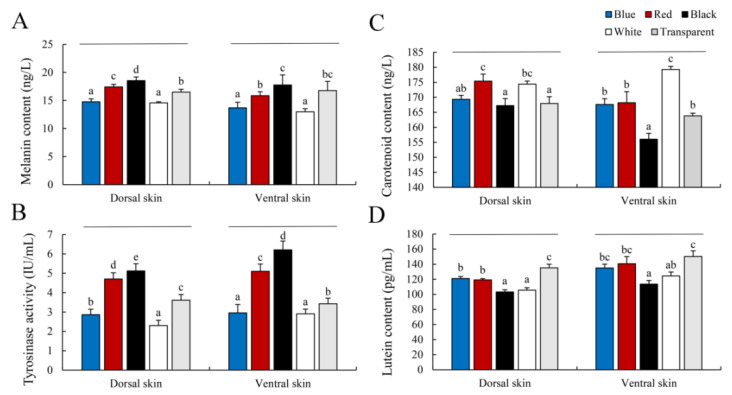
The pigment content and tyrosinase activity in fish skin from the experimental groups are shown in Figure 3. The melanin contents in the dorsal and ventral skin of the Black group were significantly higher than those in the other groups (p < 0.05), whereas the White and Blue group levels were the lowest (p < 0.05) (Figure 3A). In addition, the trends in tyrosinase activity were similar to the trends in melanin content among the groups (Figure 3B). Interestingly, carotenoid and lutein contents had completely different trends among the experimental groups. The carotenoid content was highest in the dorsal skin from the Red and White groups (p < 0.05) (Figure 3C). The ventral skin from the White group had the highest carotenoid content (p < 0.05), and the Black group had the lowest (p < 0.05) (Figure 3C). Lutein content was lower in the White and Black groups than the other groups (p < 0.05), and the Transparent group had the highest lutein content (p < 0.05) (Figure 3D).
Figure 3.
Pigment content and tyrosinase activity in skin. Values are means ± standard error for ten replicates. (A): Melanin content; (B): Tyrosinase activity; (C): Carotenoid content; (D): Lutein content. Significant differences for means within experimental groups are indicated with different lowercase letters (p < 0.05, n = 9).
3.5. Serum Tyrosinase Activity and α-MSH Level
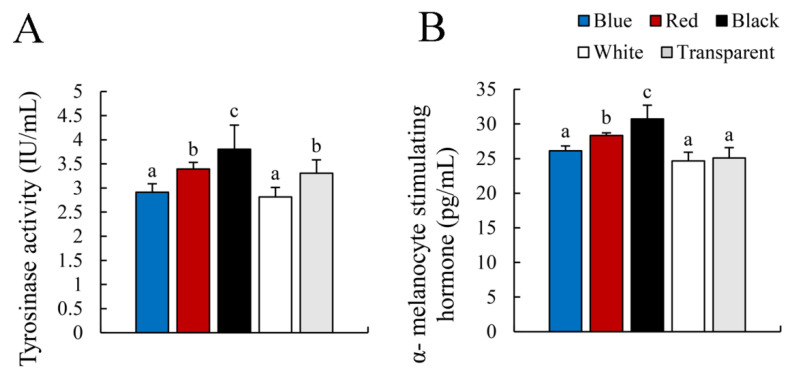
As shown in Figure 4, the Black group had the highest serum tyrosinase activity (p < 0.05), followed by the Red and Transparent groups. The serum tyrosinase activity of the Blue and White groups was the lowest (p < 0.05) (Figure 4A). The serum α-MSH levels were similar to those of tyrosinase activity except in the Transparent group (Figure 4B).
Figure 4.
Tyrosinase activity and α-MSH levels in serum. (A): Tyrosinase activity; (B): α-melanocyte stimulating hormone. Values are means ± standard error for ten replicates. Significant differences for means within experimental groups are indicated with different lowercase letters (p < 0.05, n = 9).
3.6. Expression of Fish Skin Color-Related Genes
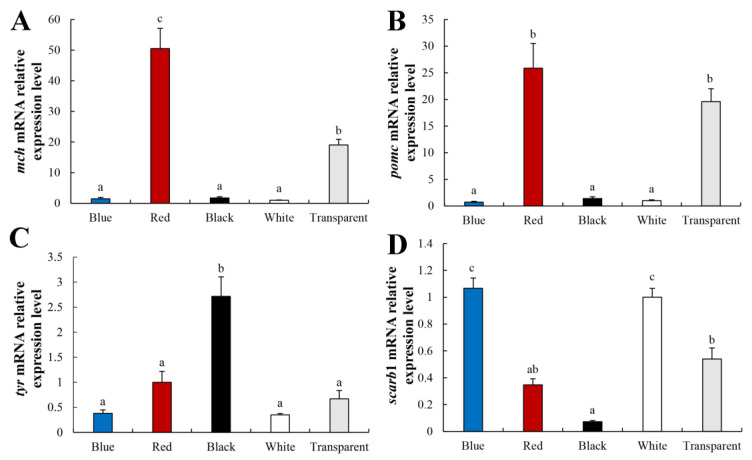
The results of fish skin melanin and carotenoid biosynthesis-related gene expression in the skin of P. leopardus are shown in Figure 5. The expression levels of mch and pomc in P. leopardus skin were significantly higher in the Red group (p < 0.05), followed by the Transparent group (p < 0.05). No significant differences in the mch or pomc expression levels were observed in the Blue, Black, or White groups (Figure 5A,B). The tyr expression level was highest in the Black group (p < 0.05), followed by the Red and Transparent groups (Figure 5C). The scarb1 expression level was highest in the Blue and White groups, followed by the Transparent group. scarb1 expression was lowest in the Black group (p < 0.05) (Figure 5D).
Figure 5.
The expression levels of mch (A), pomc (B), tyr (C), and scarb1 (D) in P. leopardus skin for different color. Values are means ± standard error for ten replicates. Significant differences for means within experimental groups are indicated with different lowercase letters (p < 0.05, n = 9).
4. Discussion
The skin color of P. leopardus is typically red; however, skin of most fish will turn black under artificial culture conditions, which affects their economic value [32]. Fish skin color is affected by the nervous and endocrine systems, as well as changes in nutrition and the environment. Here, we investigated whether the skin color of P. leopardus was affected by the background color of the tanks in which the fish were held. Studies have shown that background color can affect the skin color variation of aquatic animals [33].
Fish skin pigmentation or body color is one of the most important quality criteria affecting the economic value of fish for human consumption and ornamental use [34,35]. In this study, the apparent skin color of P. Leopardus was red on a white background, which darkened when the fish were held on the other color backgrounds. The Black group had the blackest skin color, followed by the Transparent group (Figure 1), which was consistent with the findings for C. auratus [12,24], red tilapia [11], and other fish [33]. Paraffin sections of the dorsal and ventral skin showed that darkening of the fish skin was caused by an increase in the number of melanocytes in the experimental groups (Figure 2). Paler colors indicated fewer melanocytes, and this was likely caused by the visual opsin perception of the tank colors [36].
The L*, a*, and b* values have been frequently used to quantify fish color [33,37,38]. The White group demonstrated the highest L*, a*, and b* values in the dorsal, ventral, head, and caudal peduncle skin of P. leopardus in this study (Table 2 and Table 3), which was consistent with the brighter, paler, more yellow and red skin color on the White background than that on the other color backgrounds. During our study, the fish subjected to the treatments with dark backgrounds had a deeper melanin pigmentation, which could have resulted from their camouflage mechanism and an attempt to simulate the environmental colors [39].
Fish body coloration is the result of diverse pigments synthesized by pigment cells or chromatophores [9]. Here, carotenoid and lutein contents showed completely different trends than melanin. Carotenoid content was higher in the Red and White groups, while lutein content was higher in the Transparent group (Figure 3). This is inconsistent with the results of C. auratus and D. filamentosa, which may be caused by different species [18,26]. Previous studies have shown that the coloring effect of carotenoids is affected by the distribution of melanocytes [29]. This result suggests that carotenoids and other pigments, such as lutein and melanin, regulate fish skin color. However, fish cannot synthesize carotenoids by themselves, as these come from algae and other foods [29]. Although carotenoid-based coloration is dietary-dependent, genetic factors also play an indispensable role in carotenoid pigmentation. scarb1 is a key gene directly related to carotenoid coloring in vertebrates that mediates carotenoid absorption and transportation [40]. Here, the scarb1 expression level was highest in the Blue and White groups, followed by the Transparent group, and lowest in the Black group (p < 0.05), showing the same trend as skin color (Figure 5D). Specific wavelengths could regulate the fish skin color through neuropeptide hormones and photoreceptors [41]. Our results suggest that the background color regulated scarb1 expression level through the visual center, then influenced the uptake and transport of carotenoids, then influenced the skin color formation of P. leopardus. These results indicate that the White background was more conducive for juvenile P. leopardus to maintain their red skin color.
Tyrosinase is a rate-limiting enzyme in the melanin synthetic pathway, and its activity plays an important role in regulating fish skin color. A correlation has been reported between tyrosinase activity and melanin content in fish, and it is affected by genetic, nutritional, and environmental conditions, as well as by the developmental stage of the fish [42,43,44,45,46]. The trend in tyrosinase activity and melanin content in skin of Cyprinus carpio is consistent with different skin colors [46]. In this study, the melanin content and tyrosinase activity in the dorsal and ventral skin of the Black group were significantly higher than those in the other groups (p < 0.05), and the highest tyr expression level was detected (Figure 3A,B). Therefore, it can be considered that when fish were reared in the dark background, the tyrosinase activity was increased, which stimulated the production of melanin pigment in the skin. In addition, the melanin synthesis affected the variations in fish skin color, and its content reflects differences in skin color.
In teleosts, chronic treatment with α-MSH darkens body color [6,47]. Injecting red tilapia with α-MSH in the caudal vein results in significantly higher tyrosinase activity and melanin content in the dorsal and ventral skin [48]. In this study, the Black group had the highest serum α-MSH level, followed by the Red group. A similar trend was observed for serum tyrosinase activity (Figure 4). This is consistent with previous results, indicating that the α-MSH level can be affected by background color and that a dark color increases the α-MSH level. α-MSH is a peptide derived from pomc and mch that is competitively involved in body color regulation. α-MSH induces a dark body color, while Mch induces a pale body color [25]. Interestingly, the expression levels of pomc and mch in skin were highest in the Red group, followed by the Transparent group, while the Black group expressed the lowest levels of pomc and mch in this study (Figure 5). These results suggest that the red background had a stronger effect on pomc and mch gene expression than the other colors.
5. Conclusions
In this study, the apparent skin color of P. leopardus was red in a white background, which darkened in response to the other color backgrounds. The Black group exhibited the darkest skin color, followed by the Transparent group. The darkening of fish skin was caused by an increase in the number of melanocytes in the experimental groups. Moreover, the White group had the highest L*, a*, and b* values, which was consistent with the brighter, paler, more yellow and red skin color on the White background than that on the other color backgrounds. The melanin content, tyrosinase activity, and serum α-MSH level in the dorsal and ventral skin of the Black group were significantly higher than those in the other groups (p < 0.05). The carotenoid content was higher in the Red and White groups, while lutein content was higher in Transparent group. Moreover, the paler colors inhibited the formation of melanocytes and had a significant effect on carotenoid and lutein contents. Pigment-related genes are involved in the regulation of fish skin color, and they are affected by background color in P. leopardus. Therefore, a white background is more conducive for juvenile P. leopardus to maintain red skin color.
Acknowledgments
The authors were grateful to all of the laboratory members for technical advice and helpful discussions.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/ani12233349/s1, Figure S1: Aquaculture aquariums with different background colors (n = 3).
Author Contributions
F.S. and J.L. conceived and designed the experiments; F.Y., W.Z., Y.L. and M.Y. finished the rearing experiments and sample collection; L.S., Y.G., D.Z., L.W. and K.Z. performed the validation experiments and data analysis; J.S. assisted proofreading; F.S. wrote the paper; J.L. assisted with writing and proofreading. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals in China. The Institutional Animal Care and Use Committee of the College of Ocean of Hainan approved all experimental procedures and sample collection.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Hainan Provincial Natural Science Foundation of China (322QN236), the Key R&D Project in Hainan (ZDYF2020093), and the initial fund from Hainan University for R&D, KYQD (ZR)-2013.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnson S.L., Africa D., Walker C., Weston J.A. Genetic control of adult pigment stripe development in zebrafish. Dev. Biol. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard J.K., Uy J.A.C., Hauber M.E., Hoekstra H.E., Safran R.J. Vertebrate pigmentation: From underlying genes to adaptive function. Trends Genet. 2010;26:231–239. doi: 10.1016/j.tig.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Haque M.R., Islam M.A., Wahab M.A., Hoq M.E., Rahman M.M., Azim M.E. Evaluation of production performance and profitability of hybrid red tilapia and genetically improved farmed tilapia (GIFT) strains in the carbon/nitrogen controlled periphyton-based (C/N-CP) on-farm prawn culture system in Bangladesh. Aquac. Rep. 2016;4:101–111. doi: 10.1016/j.aqrep.2016.07.004. [DOI] [Google Scholar]
- 4.Nüsslein-Volhard C., Singh A.P. How fish color their skin: A paradigm for development and evolution of adult patterns: Multipotency, plasticity, and cell competition regulate proliferation and spreading of pigment cells in Zebrafish coloration. BioEssays. 2017;39:201600231. doi: 10.1002/bies.201600231. [DOI] [PubMed] [Google Scholar]
- 5.Cal L., Suarez-Bregua P., Cerdá-Reverter J.M., Braasch I., Rotllant J. Fish pigmentation and the melanocortin system. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017;211:26–33. doi: 10.1016/j.cbpa.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi A., Mizusawa K., Amano M. Multifunctional roles of melanocyte-stimulating hormone and melanin-concentrating hormone in fish: Evolution from classical body color change. Aqua-BioSci. Monogr. 2014;7:1–46. doi: 10.5047/absm.2014.00701.0001. [DOI] [Google Scholar]
- 7.Mizusawa K., Kobayashi Y., Yamanome T., Saito Y., Takahashi A. Interrelation between melanocyte-stimulating hormone and melanin-concentrating hormone in physiological body color change: Roles emerging from barfin flounder Verasper moseri. Gen. Comp. Endocrinol. 2013;181:229–234. doi: 10.1016/j.ygcen.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto M. Morphological color changes in fish: Regulation of pigment cell density and morphology. Microsc. Res. Tech. 2002;58:496–503. doi: 10.1002/jemt.10168. [DOI] [PubMed] [Google Scholar]
- 9.Braasch I., Schart M., Volff J.N. Evolution of pigment synthesis pathways by gene and genome duplication in fish. BMC Evol. Biol. 2007;7:74. doi: 10.1186/1471-2148-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii R. The regulation of motile activity of fish chromatophores. Pigment Cell Res. 2000;13:300–319. doi: 10.1034/j.1600-0749.2000.130502.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang L.M., Luo M.K., Yin H.R., Zhu W.B., Fu J.J., Dong Z.J. Effects of background adaptation on the skin color of Malaysian red tilapia. Aquaculture. 2020;521:735061. doi: 10.1016/j.aquaculture.2020.735061. [DOI] [Google Scholar]
- 12.Mizusawa K., Kasagi S., Takahashi K. Melanin-concentrating hormone is a major substance mediating light wavelength-dependent skin color change in larval zebrafish. Gen. Comp. Endocrinol. 2018;269:141–148. doi: 10.1016/j.ygcen.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M., Oshima N. Changes in adrenergic innervation to chromatophores during prolonged background adaptation in the medaka, Oryzias latipes. Pigment Cell Res. 1995;8:37–45. doi: 10.1111/j.1600-0749.1995.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto M., Uchida H., Hayayama M. Apoptosis in skin pigment cells of the medaka, Oryzias latipes (Teleostei), during long-term chromatic adaptation: The role of sympathetic innervation. Cell Tissue Res. 2000;301:205–216. doi: 10.1007/s004410000226. [DOI] [PubMed] [Google Scholar]
- 15.Merighe G.K.F., Pereira-Da-Silva E.M., Negrão J.A., Ribeiro S. Effect of background color on the social stress of nile tilapia (Oreochromis niloticus) Rev. Bras. Zootec. 2004;33:828–837. doi: 10.1590/S1516-35982004000400002. [DOI] [Google Scholar]
- 16.Opiyo M.A., Ngugi C.C., Rasowo J. Combined effects of stocking density and background colour on growth performance and survival of nile tilapia (Oreochromis niloticus, L.) fry reared in aquaria. J. Fish. Sci. 2014;8:228–237. doi: 10.3153/jfscom.201429. [DOI] [Google Scholar]
- 17.Boaventura T.P., Pedras P.P.C., Santos F.A.C., Ferreira A.L., Favero G.C., Palheta G.D.A., Melo N.F.A.C., Luz R.K. Cultivation of juvenile Colossoma macropomum in different colored tanks in recirculating aquaculture system (RAS): Effects on performance, metabolism and skin pigmentation. Aquaculture. 2021;532:736079. doi: 10.1016/j.aquaculture.2020.736079. [DOI] [Google Scholar]
- 18.Ninwichian P., Phuwan N., Limlek P. Effects of tank color on the growth, survival rate, stress response, and skin color of juvenile hybrid catfish (Clarias macrocephalus × Clarias gariepinus) Aquaculture. 2022;554:738129. doi: 10.1016/j.aquaculture.2022.738129. [DOI] [Google Scholar]
- 19.Banan A., Kalbassi M.R., Bahmani M., Sadati M.A.Y. Effects of colored light and tank color on growth indices and some physiological parameters of juvenile beluga (Huso huso). J. Appl. Ichthyol. 2011;27:565–570. doi: 10.1111/j.1439-0426.2011.01682.x. [DOI] [Google Scholar]
- 20.Eslamloo K., Akhavan S.R., Eslamifar A., Henry M.A. Effects of background colour on growth performance, skin pigmentation, physiological condition and innate immune responses of goldfish, Carassius auratus. Aquac. Res. 2013;46:202–215. doi: 10.1111/are.12177. [DOI] [Google Scholar]
- 21.Ninwichian P., Phuwan N., Jakpim K., Sae-Lim P. Effects of tank color on the growth, stress responses, and skin color of snakeskin gourami (Trichogaster pectoralis) Aquac. Int. 2018;26:659–672. doi: 10.1007/s10499-018-0242-6. [DOI] [Google Scholar]
- 22.Mizusawa K., Kobayashi Y., Sunuma T., Asahida T., Saito Y., Takahashi A. Inhibiting roles of melanin-concentrating hormone for skin pigment dispersion in barfin flounder, Verasper moseri. Gen. Comp. Endocrinol. 2011;171:75–81. doi: 10.1016/j.ygcen.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Kasagi S., Miura M., Okazaki T., Mizusawa K., Takahashi A. Effects of tank color brightness on the body color, somatic growth, and endocrine systems of rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2020;298:113581. doi: 10.1016/j.ygcen.2020.113581. [DOI] [PubMed] [Google Scholar]
- 24.Cerdá-Reverter J.M., Canosa L.F., Peter R.E. Regulation of the hypothalamic melanin-concentrating hormone neurons by sex steroids in the goldfish: Possible role in the modulation of luteinizing hormone secretion. Neuroendocrinology. 2006;84:364–377. doi: 10.1159/000098334. [DOI] [PubMed] [Google Scholar]
- 25.Yang T., Kasagi S., Takahashi A., Mizusawa K. Effects of background color and feeding status on the expression of genes associated with body color regulation in the goldfish Carassius auratus. Gen. Comp. Endocrinol. 2021;312:113860. doi: 10.1016/j.ygcen.2021.113860. [DOI] [PubMed] [Google Scholar]
- 26.Padhi N., Jena S.K., Ail S.K.S., Ferosekhan S., Sahoo S.N., Udit U.K., Bairwa M.K., Swain S.K. Does tank background colour influence the growth, survival, and carotenoid content in fishes? An illustration in filament barb, Dawkinsia filamentosa (Valenciennes, 1844) Aquaculture. 2022;560:738536. doi: 10.1016/j.aquaculture.2022.738536. [DOI] [Google Scholar]
- 27.Sundvold H., Helgeland H., Baranski M., Omholt S.W., Våge D.I. Characterisation of a novel paralog of scavenger receptor class B member I (SCARB1) in Atlantic salmon (Salmo salar) BMC Genet. 2011;12:52. doi: 10.1186/1471-2156-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H., Zheng H.P., Zhang H.K., Deng L.H., Liu W.H., Wang S.Q., Meng F., Wang Y.J., Guo Z.C., Li S.K., et al. A de novo transcriptome of the noble scallop, Chlamys nobilis, focusing on mining transcripts for carotenoid-based coloration. BMC Genom. 2015;16:44. doi: 10.1186/s12864-015-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T.L., Xu G.L., Xing W., Ma Z.H., Jiang N., Luo L. Mechanisms underlying carotenoid-based coloration in fishes. J. Shanghai Ocean. Univ. 2018;27:206–212. [Google Scholar]
- 30.Maoka T., Sato W., Nagai H., Takahashi T. Carotenoids of red, brown, and black specimens of Plectropomus leopardus, the coral trout (Suziara in Japanese) Oleo Sci. 2017;66:579–584. doi: 10.5650/jos.ess16179. [DOI] [PubMed] [Google Scholar]
- 31.Song F.B., Gu Y., Chen Y.M., Zhang K.X., Shi L.P., Sun J.L., Zhang Z.J., Luo J. Transcriptome analysis provides insights into differentially expressed long noncoding RNAs between the testis and ovary in golden pompano (Trachinotus blochii) Aquac. Rep. 2022;22:100971. doi: 10.1016/j.aqrep.2021.100971. [DOI] [Google Scholar]
- 32.Hao R.J., Zhu X.W., Tian C.X., Jiang M.Y., Huang Y., Zhu C.H. Integrated analysis of the role of miRNA-mRNA in determining different body colors of leopard coral grouper (Plectropomus leopardus) Aquaculture. 2022;548:737575. doi: 10.1016/j.aquaculture.2021.737575. [DOI] [Google Scholar]
- 33.McLean E. Fish tank color: An overview. Aquaculture. 2021;530:735750. doi: 10.1016/j.aquaculture.2020.735750. [DOI] [Google Scholar]
- 34.Bjerkeng B. Carotenoids in aquaculture: Fish and crustaceans. Carotenoids. 2008;4:237–254. [Google Scholar]
- 35.Vissio P.G., Darias M.J., Di Yorio M.P., Perez Sirkin D.I., Delgadin T.H. Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. Gen. Comp. Endocrinol. 2021;301:113662. doi: 10.1016/j.ygcen.2020.113662. [DOI] [PubMed] [Google Scholar]
- 36.Suliman T., Novales Flamarique I. Visual pigments and opsin expression in the juveniles of three species of fish (rainbow trout, zebrafish, and killifish) following prolonged exposure to thyroid hormone or retinoic acid: Opsin expression and nuclear receptor ligands. Comp. Neurol. 2014;522:98–117. doi: 10.1002/cne.23391. [DOI] [PubMed] [Google Scholar]
- 37.Dijkstra P.D., Maguire S.M., Harris R.M., Rodriguez A.A., DeAngelis R.S., Flores S.A., Hofmann H.A. The melanocortin system regulates body pigmentation and social behaviour in a colour polymorphic cichlid fish. Proc. Biol. Sci. 2017;284:2016–2838. doi: 10.1098/rspb.2016.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Border S.E., Piefke T., Fialkowski R., Tryc M., Funnell T., DeOliveira G., Dijkstra P.D. Color change and pigmentation in a color polymorphic cichlid fish. Hydrobiologia. 2019;832:175–191. doi: 10.1007/s10750-018-3755-0. [DOI] [Google Scholar]
- 39.Díaz-Jiménez L., Hernández-Vergara M.P., Pérez-Rostro C.I., Olvera-Novoa M.Á. The effect of two carotenoid sources, background colour and light spectrum on the body pigmentation of the clownfish Amphiprion ocellaris. Aquac. Res. 2021;52:3052–3061. doi: 10.1111/are.15149. [DOI] [Google Scholar]
- 40.Saunders L., Mishra A., Aman A.J., Lewis V.M., Toomey M.B., Packer J.S., Qiu X., Mcfalinefigueroa J.L., Corbo J.C., Trapnell C., et al. Thyroid hormone regulates distinct paths to maturation in pigment cell lineages. elife. 2019;8:e45181. doi: 10.7554/eLife.45181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin H.S., Choi C.Y. The stimulatory effect of LED light spectra on genes related to photoreceptors and skin pigmentation in goldfish (Carassius auratus) Fish Physiol. Biochem. 2014;40:1229–1238. doi: 10.1007/s10695-014-9918-7. [DOI] [PubMed] [Google Scholar]
- 42.Yin H.R., Luo M.K., Wang L.M., Dong Z.J., Zhu W.B., Fu J.J. Changes of pigment-related enzyme activity and gene expression at early developmental stage of koi carp. South China Fish. Sci. 2019;15:109–117. [Google Scholar]
- 43.Deng C., Chen S.L., Ye H.Z., Qi X.Z., Luo J. Analysis of pigment and enzyme levels of Plectropomus leopardus with body color difference. Life Sci. Res. 2020;24:15–20. [Google Scholar]
- 44.Cheng W., Xu G., Zhang L., Han M., Chen L., Wei Y. Effects of dietary inclusion of oxidized fish oil on melanin, melanin synthetic enzymes and hormones of Pelteobagrus fulvidraco. Acta Hydrobiol. Sin. 2017;41:1020–1026. [Google Scholar]
- 45.Chatzifotis S., Pavlidis M., Jimeno C.D., Vardanis G., Sterioti A., Divanach P. The effect of different carotenoid sources on skin coloration of cultured red porgy (Pagrus pagrus) Aquac. Res. 2005;36:1517–1525. doi: 10.1111/j.1365-2109.2005.01374.x. [DOI] [Google Scholar]
- 46.Fu J.J., Zhu W.B., Luo W.T., Wang L.M., Luo M.K., Dong Z.J. Comparison of growth, tyrosinase activity, melanin content, and gene expression between common carps with different pigmentations. J. Fish. Sci. China. 2021;28:939–947. [Google Scholar]
- 47.Yamanome T., Chiba H., Takahashi A. Melanocyte-stimulating hormone facilitates hypermelanosis on the non-eyed side of the barfin flounder, a pleuronectiform fish. Aquaculture. 2007;270:505–511. doi: 10.1016/j.aquaculture.2007.05.037. [DOI] [Google Scholar]
- 48.Wang L.M., Jiang B.J., Zhu W.B., Fu J.J., Luo M.K., Liu W., Dong Z.J. The role of melanocortin 1 receptor on melanogenesis pathway in skin color differentiation of red tilapia. Aquac. Rep. 2022;22:100946. doi: 10.1016/j.aqrep.2021.100946. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.