Abstract
Circadian rhythms are generated by a series of genes, collectively named clock genes, which act as a self-sustained internal 24 h timing system in the body. Many physiological processes, including metabolism and the endocrine system, are regulated by clock genes in coordination with environmental cues. Loss of the circadian rhythms has been reported to contribute to widespread obesity, particularly in the pediatric population, which is increasingly exposed to chronodisruptors in industrialized society. The aim of the present study was to evaluate the DNA methylation status of seven clock genes, namely clock, arntl, per1-3 and cry1-2, in a cohort of chronobiologically characterized obese adolescents (n: 45: F/M: 28/17; age ± SD: 15.8 ± 1.4 yrs; BMI SDS: 2.94 [2.76; 3.12]) hospitalized for a 3-week multidisciplinary body weight reduction program (BWRP), as well as a series of cardiometabolic outcomes and markers of hypothalamo–pituitary–adrenal (HPA) function. At the end of the intervention, an improvement in body composition was observed (decreases in BMI SDS and fat mass), as well as glucometabolic homeostasis (decreases in glucose, insulin, HOMA-IR and Hb1Ac), lipid profiling (decreases in total cholesterol, LDL-C, triglycerides and NEFA) and cardiovascular function (decreases in systolic and diastolic blood pressures and heart rate). Moreover, the BWRP reduced systemic inflammatory status (i.e., decrease in C-reactive protein) and HPA activity (i.e., decreases in plasma ACTH/cortisol and 24 h urinary-free cortisol excretion). Post-BWRP changes in the methylation levels of clock, cry2 and per2 genes occurred in the entire population, together with hypermethylation of clock and per3 genes in males and in subjects with metabolic syndrome. In contrast to the pre-BWRP data, at the end of the intervention, cardiometabolic parameters, such as fat mass, systolic and diastolic blood pressures, triglycerides and HDL-C, were associated with the methylation status of some clock genes. Finally, BWRP induced changes in clock genes that were associated with markers of HPA function. In conclusion, when administered to a chronodisrupted pediatric obese population, a short-term BWRP is capable of producing beneficial cardiometabolic effects, as well as an epigenetic remodeling of specific clock genes, suggesting the occurrence of a post-BWRP metabolic and endocrine chronoresynchronization, which might represent a “biomolecular” predictor of successful antiobesity intervention.
Keywords: obesity, clock genes, DNA methylation, chronobiology, body weight reduction program, childhood, cardiometabolic outcomes, HPA function
1. Introduction
Owing to the changeable nature of environmental conditions in living beings, including the daily or seasonal availability of food, a complex system of chronobiological regulation has been evolved. This system imparts circadian rhythmicity to a variety of biological phenomena, including hormonal secretion, consummatory behaviors and metabolism [1].
The suprachiasmatic nucleus (SCN), located at the hypothalamic level, is monosinaptically connected to photosensitive ganglion neurons that, within the retina, detect the light/dark cycle from the external environment, with ensuing internal chronosynchronization [2]. Therefore, SCN has been acknowledged to act as master pacemaker, upon which the circadian rhythmicity of many peripheral organs depends, including pituitary and pineal gland, liver and adipose tissue, which, when disconnected from hierarchical SCN control, maintain a proper chronobiological autonomy [3].
Specific genes collectively known as clock genes have been identified for a long time. In particular, they encode nuclear transcriptional factors or coactivator/repressors that modulate the expression of a multitude of genes (about 20% of all genes present in the nucleus of a eukaryotic cell) [1].
In particular, CLOCK (circadian locomotor output cycles kaput) and ARNTL (aryl hydrocarbon receptor nuclear translocator-like, which is alternatively named BMAL1 (brain- and muscle-ANRT-like protein) are (transcriptionally) positive nuclear factors taking part in a chronobiological feedback loop in which NPAS2 (name derived from PERARNT-SIM protein-2) is also engaged. The CLOCK-ARNTL heterodimer binds to a specific DNA sequence named E-box, which is present within the promoter region of some target genes, which, in turn, regulate the transcription of other clock genes, such as the PER (period genes) family and the CRY (cryptochrome genes) family. The protein products of per and cry genes dimerize and are translocated into the nucleus. The transcription of genes encoding the repressors is blocked when the levels of PER-CRY are sufficient to antagonize the positive effect of CLOCK-ARNTL, with subsequent inhibition of the CLOCK-ARNTL-dependent transcription.
Other nuclear receptors and coactivators/repressors, such as peroxisome proliferator-activated receptors (PPARs) and PPAR-γ coactivator 1α (PGC 1α), have been recognized as modulators of arntl/ARNTL and clock/CLOCK [4]. Taking into account the pre-eminent role exerted by PPARs in the regulation of glucose and lipid metabolism in the liver, muscles and adipose tissue, as well as in the proliferation/differentiation of adipocyte progenitors [5,6], the strong interrelationships among chronobiology, obesity and metabolic syndrome are not surprising [7].
In this context, clock mutant mice, as other genetically modified (knockout and transgene) animal models in clock genes, are obese due to hypertrophied visceral adipose tissue, together with liver steatosis, hyperglycemia, hyperinsulinemia, hypertriglyceridemia and hyperleptinemia, which, overall, resemble (human) metabolic syndrome [8,9].
In humans, a considerable number of epidemiological studies have demonstrated the existence of a strong association of obesity with known chronodisruptors, including working night shifts, long-lasting exposure to artificial light, sleep deprivation, nocturnal snacking, irregular daily eating times, etc. [10]. Most chronodisruptors are typically present in the so-called nocturnal chronotype, which is becoming increasingly prevalent in the modern civilized population living in the urban centers [11].
Many endocrine axes, such as the hypothalamo–pituitary–adrenal (HPA) axis, show a typical circadian secretory pattern [12]. In particular, secretion of corticotropin (ACTH) and cortisol is generally higher in the morning and falls throughout the day [13]. There is strong evidence that in obese subjects, accumulation of visceral adipose tissue and dysmetabolism are associated with a chronodisruption of HPA function [14,15]. Furthermore, even in normal-weight subjects, many chronodisruptors negatively affect HPA function (e.g., psychological stress, jet lag or working nocturnal shifts) [16]. As clock gene expression and diet rhythmicity have been demonstrated to be regulated by glucocorticoid receptors [17,18], the existence of a link among clock genes, the HPA axis and obesity cannot be ruled out [19].
Obesity is a multifactorial disease derived from a combination of environmental factors (e.g., hypercaloric diet and sedentary lifestyle) with a polygenetic predisposition [20]. In recent years, an epigenetic dysregulation has been documented in obesity and its cardiometabolic comorbidities, particularly alterations in DNA methylation status, such as hypo/hypermethylation of clock genes [21,22]. DNA methylation is an epigenetic mechanism involving the transfer of a methyl group to the C5 position of the cytosine to form 5-methylcytosine [23]. DNA methylation occurring in gene promoters is mostly associated with the silencing of gene expression through the recruitment of proteins involved in gene repression or the inhibition of transcription factor binding to DNA [24]. The pattern of DNA methylation is continuously modified by external stimuli, in a dynamic process involving both de novo DNA methylation and demethylation [25]. This plasticity also involves clock genes [26].
In this regard, diet patterns have been reported to change the methylation status of CpG sequences within clock genes [27,28]. Moreover, some protein products of clock genes have been reported to be directly involved in the biochemical mechanisms underlying epigenetics (e.g., CLOCK as histone-acetyltransferase) [29]. Accordingly, in a study carried out in obese adults, DNA methylation of clock, bmal1 (or arntl) and per2 genes was shown to be associated with anthropometric and biochemical parameters that are related to obesity, metabolic syndrome and weight loss after a program of metabolic rehabilitation [30].
Based on previous considerations, with the spread of obesity and the nocturnal chronotype in the pediatric population [31], the aim of the present clinical study was to evaluate, in a context of chronobiological parameters, a cohort of obese adolescents hospitalized for a short-term (3-week) body weight reduction program (BWRP). In particular, we determined the DNA methylation of a series of clock genes (i.e., clock, arntl, cry1-2 and per1-3), which were associated with clinical, anthropometric and biochemical parameters in peripheral leukocytes collected before and after the 3-week BWRP, stratifying the study population by sex and metabolic syndrome. Furthermore, chronotype and HPA function were evaluated.
Whereas BWRP-induced cardiometabolic benefits are well-documented in the medical literature [32,33,34], our hypothesis was that a short-term BWRP is capable of modifying the DNA methylation pattern of clock genes, providing novel insight into the relationships between epigenetics and chrononutrition, with the possibility of therapeutically resynchronizing chronodisrupted obese subjects through easy lifestyle modifications, including diet and exercise [27].
2. Materials and Methods
2.1. Subjects
A set of adolescents was selected from a patient population admitted to the Division of Auxology of the Istituto Auxologico Italiano, Piancavallo (VB), Italy, for a 3-week in-hospital multidisciplinary BWRP.
The inclusion criteria were individuals of both sexes aged ≤ 18 yrs with a body mass index (BMI) (or BMI deviation standard score, BMI-SDS) > 97th percentile according with age- and sex-specific Italian growth charts [35], with or without metabolic syndrome (see below for its definition). The exclusion criteria were: (1) secondary causes of obesity (e.g., Prader–Willi syndrome, steroid–induced or medication-induced obesity); (2) individuals with systolic blood pressure (SBP) ≥ 180 mmHg and diastolic blood pressure (DBP) ≥ 110 mmHg; (3) cardiovascular disease clinically evident in the previous 6 months; (4) psychiatric, neurological, osteomuscular or rheumatologic diseases that limit the ability to undertake a (standard) 3-week in-hospital period of metabolic rehabilitation, including physical activity (see below for details); and (5) individuals (and/or their parents) who refused to sign the consent form.
The study protocol was approved by the Ethical Committee of the Istituto Auxologico Italiano, IRCCS, Milan, Italy (research project code: 01C922; acronym: GENICLOCK); the protocol was explained to the patients and/or their parents, who gave their written informed consent.
2.2. Body Weight Reduction Program (BWRP)
The BWRP consisted of a 3-week multidisciplinary in-hospital metabolic rehabilitation, entailing an energy-restricted diet, physical rehabilitation (moderate aerobic activity), psychological counseling and nutritional education. The amount of energy to be provided by diet was calculated by subtracting approximately 500 kcal from the measurement of resting energy expenditure (REE), which was determined after an overnight fast by means of open-circuit, indirect computerized calorimetry (Vmax 29, Sensor Medics, Yorba Linda, CA, USA) with a rigid, transparent, ventilated canopy. In terms of macronutrients, the diet contained approximately 21% proteins, 53% carbohydrates and 26% lipids; the daily estimated water content was 1000 mL, whereas the estimated salt content was 1560 mg Na+, 3600 mg K+ and 900 mg Ca2+. Extra water intake of at least 2000 mL/day was encouraged.
The physical activity program consisted of 5 days per week training, including (i) 1 h dynamic aerobic standing and floor exercise with arms and legs at moderate intensity and under the guidance of a therapist and (ii) either 20–30 min cycloergometer exercise at 60 W or 3–4 km outdoor walking on flat terrain according to individual capabilities and clinical status.
The subjects also underwent a psychological counseling program consisting of two or three sessions per week of individual and/or group psychotherapy performed by clinical psychologists. Furthermore, lectures, demonstrations and group discussions with or without a supervisor took place daily.
2.3. Anthropometric Measurements
A scale with a stadiometer was used to determine height and weight (Wunder Sa.Bi., WU150, Trezzo sull’Adda, Italy). Waist circumference (WC) was measured with a flexible tape in standing position, halfway between the inferior margin of the ribs and the superior border of the crista, whereas hip circumference (HC) was measured at the largest parts around the buttocks. Body composition was measured by bioimpedance analysis (Human-IM Scan, DS-Medigroup, Milan, Italy) after 20 min of supine resting and in accordance with the international guidelines [36]. BMI, fat mass (FM) and fat-free mass (FFM) were determined in all subjects.
2.4. Metabolic, Biochemical and Hormonal Evaluation
Blood samples (about 10 mL) were collected at around 8:00 AM after an overnight fast at the beginning of the BWRP at T0 and at the end (i.e., 21st day, T1). A further blood sample was drawn at 15:00 (only for evaluation of HPA axis). Total cholesterol (T-C), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TGs), glucose, insulin, C-reactive protein (CRP), cortisol (at 08:00 AM and 03:00 PM, i.e., cortisol-8AM and cortisol-3PM, respectively) and ACTH (at 08:00 AM and 03:00 PM, i.e., ACTH-8AM and ACTH-3PM, respectively) were measured. The 24 h urine was collected (at T0 and T1) for the determination of free cortisol excretion.
Colorimetric enzymatic assays (Roche Diagnostics, Monza, Italy) were used to determine serum T-C, LDL-C, HDL-C and TG levels. The sensitivities of the assays were 3.86 mg/dL [1 mg/dL = 0.03 mmol/L], 3.87 mg/dL [1 mg/dL = 0.03 mmol/L], 3.09 mg/dL [1 mg/dL = 0.03 mmol/L] and 8.85 mg/dL [1 mg/dL = 0.01 mmol/L], respectively.
The serum glucose level was measured by the glucose oxidase enzymatic method (Roche Diagnostics, Monza, Italy). The sensitivity of the method was 2 mg/dL [1 mg/dL = 0.06 mmol/L].
The serum insulin concentration was determined by a chemiluminescent immuno-metric assay using a commercial kit (Elecsys Insulin, Roche Diagnostics, Monza, Italy). The sensitivity of the method was 0.2 µIU/mL [1 µU/mL = 7.18 pmol/L].
The intra- and interassay coefficients of variation (CVs) were 1.1% and 1.6% for T-C, 1.2% and 2.5% for LDL-C, 1.8% and 2.2% for HDL-C, 1.1% and 2.0% for TG, 1.0% and 1.3% for glucose, and 1.5% and 4.9% for insulin.
CRP was measured using an immunoturbidimetric assay (CRP RX, Roche Diagnostics GmbH, Mannheim, Germany). The sensitivity of the method was 0.03 mg/dL.
Cortisol (in plasma and urine) was detected using a commercial ELISA kit (IBL-Hamburg Gmbh, Hamburg, Germany). Intra- and interassay CVs for this assay were <8.0% and <15%, respectively. The sensitivity of the method was 1.5 μg/L.
ACTH was determined by chemiluminescent enzyme immunoassay “ECLIA” Elecsys ACTH (Cobas, Roche diagnostics Gmbh, Mannheim, Germany). Intra- and interassay CVs for this analytical method were <3.2% and <5.4%, respectively. The sensitivity of the method was 1.00 ng/L.
For each patient, we also calculated the homeostatic model assessment of insulin resistance (HOMA-IR) according to the following formula: (insulin [μIU/mL] × glucose [mmol/L])/22.5.
2.5. Evaluation of Blood Pressure
Blood pressure was measured on the right arm using a sphygmomanometer with an appropriate pediatric cuff size, with the subject in a seated position and relaxed condition. The procedure was repeated three times at 10 min intervals; the means of the three values for SBP and DBP were recorded.
2.6. Definition of Metabolic Syndrome
According to the IDF (International Diabetes Federation) criteria for diagnosis of metabolic syndrome in children and adolescents [37], our patients were considered positive for the presence of metabolic syndrome if they had abdominal obesity (WC ≥ 90th percentile [38] for ages <16 years and ≥94 cm for males and ≥80 cm for female for ages >16 years) plus two or more of the following factors: (i) increased TG level ≥ 150 mg/dL (1.7 mmol/L) for ages < 16 years and the same cutoff or specific treatment for this lipid abnormality for ages > 16 years, (ii) reduced HDL-C < 40 mg/dL (1.03 mmol/L) for males and females for ages < 16 years and <40 mg/dL for males and <50 mg/dL (1.29 mmol/L) for females or specific treatment for this lipid abnormality for ages > 16 years, (iii) increased BP: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg for ages < 16 years and the same cutoff or treatment of previously diagnosed hypertension for ages > 16 years and (iv) increased fasting glucose concentration ≥ 100 mg/dL (5.6 mmol/L) or previously diagnosed type 2 diabetes mellitus for all ages.
2.7. Evaluation of Chronodisruptors
Before BWRP (basal condition, T0), each subject was chronobiologically characterized by the following tests.
2.7.1. Sleep Duration
Habitual sleep time was estimated by a questionnaire containing the following questions:
-
(1).
During week days: how many hours (and minutes) do you usually sleep?
-
(2).
During weekend days: how many hours (and minutes) do you usually sleep?
A total weekly sleep score was calculated as [(min weekdays × 5) + (min weekend days × 2)]/7 [39].
2.7.2. Eveningness
Eveningness was assessed by the Horne and Ostberg [40] questionnaire to assess morningness–eveningness (MEQSA, morningness–eveningness questionnaire self-assessment).
This questionnaire establishes not only a (quantitative) total score, but also five behavioral categories, which, in the present study, were reduced to three ones: definitively morning types (score = 70–86), intermedial types (score = 31–69), and definitively evening types (score = 16–30).
2.7.3. Sleepiness
The Cleveland Adolescent Sleepiness Questionnaire (CASQ), a brief, self-completed instrument to measure excessive daytime sleepiness specifically developed for adolescents was administered [41].
The total CASQ score, as well as sleepiness and alertness scores, was used for statistical analysis.
2.8. DNA Extraction and Bisulfite Treatment
Another 7 mL of whole blood was collected into EDTA tubes from each participant (at T0 and T1). After centrifuging the blood tubes at 1200× g for 15 min to separate plasma, buffy coat and erythrocytes, genomic DNA was extracted from the buffy-coat fraction using a Wizard Genomic DNA Purification Kit (Promega; Madison, WI, USA) according to the manufacturer’s instructions. The concentration of the purified DNA was measured using a NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific; Waltham, MA, USA). The DNA samples were plated at a concentration of 25 ng/µL in 96-well plates and treated with sodium bisulfite using an EZ-96 DNA Methylation-Gold™ kit (Zymo Research; Irvine, CA, USA) following the manufacturer’s instructions. After elution, each DNA sample was divided into 10 µL aliquots using a Microlab STAR Automated Liquid Handling Workstation (Hamilton Company; Reno, NV, USA), and the plates were stored at −80 °C until use.
2.9. DNA Amplification and Pyrosequencing
DNA methylation was analyzed via previously published methods with minor changes [42,43]. Briefly, 10 µL of bisulfite-treated template DNA was added to 25 µL of GoTaq Hot Start Green Master Mix (Promega), 1 µL of forward primer (10 µM) and 1 µL of 5-t-end-biotinylated reverse primer (10 µM) to set up a 50 µL PCR reaction [44]. PCR cycling conditions and primer sequences are reported in Supplementary Table S1.
The biotin molecule at the 5 t extremity of reverse primers was exploited to isolate a single DNA filament, which was subsequently used as a template for pyrosequencing. The whole procedure was performed using a Pyromark® Gold Q96 kit (QIAGEN GmbH, Hilden, Germany). Briefly, after incubating 15 µL of PCR product with streptavidin–Sepharose HP beads (Amersham BioSciences Ltd., Little Chalfont, UK), the biotin-labeled single-stranded DNA was purified, washed, denatured with 0.2 M NaOH and washed again using a Pyrosequencing Vacuum Prep Tool (QIAGEN). After elution, the purified DNA filament was briefly incubated in an annealing mix containing the sequencing primer (0.3 µM), and the plates were then heated up to 85 °C. Pyrosequencing was performed with a PyroMark MD system (QIAGEN). CpG sites were queried within the promoter regions of the following genes: arntl, clock, cry1, cry2, per1, per2 and per3.
Quantitative analysis of the methylation level at individual CpG positions within each gene’s promoter region was carried out using Pyro Q-CpG software (Biotage, Uppsala, Sweden), which indicates the percentage of methylated cytosines among the total number of cytosines (5-methyl-cytosine + unmethylated cytosines) at each CpG site of interest. Measures of individual CpGs were averaged and used in the statistical analysis. Every sample was tested twice for each gene to guarantee the reproducibility of the experimental setting.
2.10. Statistical Analysis
Pre- and post-BWRP demographic, lifestyle, biochemical and clinical characteristics were compared as continuous variables using linear regression models for paired data. Categorical data were compared with a McNemar test for paired data.
Linear mixed regression models for paired data were used to evaluate associations between time (pre/post BWRP) and clock gene methylation.
We applied linear mixed regression models for paired data to evaluate the modifier effect of gender and metabolic syndrome (yes/no) on associations between time (pre/post BWRP) and biochemical/clinical characteristics or methylation of clock genes, classified as continuous variables; for categorical variables, we applied the McNemar test for paired data.
Linear regression models were applied to evaluate the associations between methylation of clock genes and the lifestyle, biochemical and clinical characteristics measured pre or post BWRP. Models were adjusted for sex, smoking habits and BMI SDS.
Owing to the large number of comparisons, we used a multiple comparison method based on Benjamini–Hochberg false-discovery rate (FDR) to calculate the FDR p-value.
The statistical analyses were performed using SAS software (version 9.4, SAS Institute, Milan, Italy). p-values below 0.05 were considered statistically significant.
3. Results
3.1. Subject Characteristics
Forty-five obese adolescents (F/M: 28/17; mean age ± SD: 15.8 ± 1.4 yrs; BMI SDS: 2.94 [2.76; 3.12]) were recruited and, having completed the 3 weeks of BWRP, included in the study. Table 1 summarizes the demographic, lifestyle, biochemical and clinical characteristics of the entire population in basal condition (at T0, i.e., before the BWRP) and at the end of the intervention (at T1, i.e., after the 3-week BWRP).
Table 1.
Demographic, lifestyle, biochemical and clinical characteristics of study participants: comparison pre vs. post BWRP (N = 45).
| Characteristics | Pre | Post | p-Value | FDR p-Value |
|---|---|---|---|---|
| Age, years | 15.8 ± 1.4 | - | - | |
| Gender | ||||
| Males | 17 (37.8%) | - | - | |
| Females | 28 (62.2%) | |||
| Smoking status | ||||
| Yes | 8 (17.8%) | - | - | |
| No | 37 (82.2%) | |||
| BMI, kg/m2 | 37.5 (35.7;39.3) | 36.0 (34.2;37.8) | <0.0001 | <0.0001 |
| BMI SDS | 2.94 (2.76;3.12) | 2.82 (2.64;3) | <0.0001 | <0.0001 |
| WC, cm | 114.8 (109.9;119.7) | 110.2 (105.4;115.1) | <0.0001 | <0.0001 |
| 50 Hz ohm | 549.3 (529.3;569.2) | 554.33 (534.4;574.3) | 0.0018 | 0.002 |
| FFM, kg | 57.1 (53.9;60.4) | 55.24 (52.04;58.45) | <0.0001 | <0.0001 |
| FFM, % | 55.0 (53.0;57.1) | 55.47 (53.43;57.52) | 0.0008 | 0.0009 |
| FM, kg | 47.9 (43.4;52.4) | 44.82 (40.29;49.35) | <0.0001 | <0.0001 |
| FM, % | 45.0 (43.1;46.8) | 43.7 (41.83;45.56) | <0.0001 | <0.0001 |
| REE, kcal | 1884 (1788;1981) | 1853 (1756;1950) | 0.0577 | 0.0598 |
| NEFA, mmol/L | 0.88 (0.78;0.99) | 0.7 (0.59;0.81) | <0.0001 | <0.0001 |
| Heart rate, bpm | 83.3 (79.8;86.8) | 77.69 (74.17;81.21) | <0.0001 | <0.0001 |
| SBP, mmHg | 128.6 (126.0;131.1) | 119.44 (116.88;122.01) | <0.0001 | <0.0001 |
| DBP, mmHg | 79.22 (77.84;80.61) | 74.44 (73.06;75.83) | <0.0001 | <0.0001 |
| MAP, mmHg | 95.67 (94.05;97.28) | 89.44 (87.83;91.06) | <0.0001 | <0.0001 |
| Glucose, mmol/L | 4.97 (4.74;5.2) | 4.63 (4.4;4.86) | <0.0001 | <0.0001 |
| Insulin, mU/L | 24.32 (21.58;27.06) | 17.14 (14.4;19.88) | <0.0001 | <0.0001 |
| HbA1c, mmol/L | 5.41 (5.19;5.63) | 5.2 (4.97;5.42) | <0.0001 | <0.0001 |
| HOMA-IR | 5.39 (4.61;6.17) | 3.58 (2.8;4.36) | <0.0001 | <0.0001 |
| Metabolic syndrome | ||||
| Yes | 17 (37.8%) | 12 (26.7%) | 0.2594 | 0.2597 |
| No | 28 (62.2%) | 33 (73.3%) | ||
| T-C, mg/dL | 157.04 (150.14;163.95) | 134.18 (127.27;141.09) | <0.0001 | <0.0001 |
| HDL-C, mg/dL | 43.96 (41.37;46.54) | 38.36 (35.77;40.94) | <0.0001 | <0.0001 |
| LDL-C, mg/dL | 100.38 (93.88;106.88) | 83.73 (77.23;90.24) | <0.0001 | <0.0001 |
| TG, mg/dL | 122.2 (107.32;137.08) | 101.11 (86.23;115.99) | <0.0001 | <0.0001 |
| CRP, mg/dL | 0.48 (0.37;0.59) | 0.32 (0.21;0.43) | <0.0001 | <0.0001 |
| Cortisol-8AM, μg/L | 16.06 (14.89;17.23) | 14.74 (13.57;15.91) | <0.0001 | <0.0001 |
| Cortisol-3PM, μg/L | 6.26 (5.41;7.1) | 6.88 (6.03;7.72) | <0.0001 | <0.0001 |
| Urinary cortisol (24 h), µg | 101.58 (86.78;116.37) | 75.16 (60.34;89.98) | <0.0001 | <0.0001 |
| ACTH-8AM, ng/L | 48.26 (41.76;54.76) | 43.57 (37.07;50.07) | 0.0001 | 0.0001 |
| ACTH-3PM, ng/L | 17.9 (16.09;19.71) | 17.43 (15.62;19.24) | 0.0383 | 0.0411 |
| ALT, U/L | 21 [13;32] | - | - | |
| Creatinine, mg/dL | 0.69 ± 0.11 | - | - | |
| CASQ score | 37.7 ± 5.6 | - | - | |
| CASQ alertness score | 16.4 ± 4.3 | - | - | |
| CASQ sleepiness score | 21.3 ± 5.8 | - | - | |
| Weekly sleep score, min | 435 ± 72 | - | - | |
| Sleep time during weekdays, min | 407 ± 78 | - | - | |
| Sleep time during weekend days, min | 504 ± 105 | |||
| MEQSA score | 46.1 ± 8.3 | - | - | |
| Chronotype MEQSA score | ||||
| Morning (16–41) | 2 (4.4%) | - | - | |
| Intermediate (42–58) | 30 (66.7%) | - | - | |
| Evening (59–86) | 13 (28.9%) | - | - | |
In descriptive statistics, data with normal distribution are expressed as mean ± standard deviation. When not normally distributed, values are expressed as median (Q1, Q3). Categorical data are reported as frequencies and percentage. We applied linear regression models for paired data to evaluate the associations between time (pre/post BWRP) and continuous variables; for categorical variables, we applied the McNemar test for paired data.
3.2. Effects of the BWRP in the Entire Population
At the end of the BWRP, among the entire population, BMI or BMI SDS significantly decreased (vs. BMI or BMI SDS at T0, p < 0.0001). Changes in body composition were produced by the BWRP (pre vs. post BWRP); in particular, the BWRP significantly reduced both FM % and FFM kg (p < 0.0001), with an unchanged post-BWRP value of REE. The BWRP also produced beneficial metabolic effects (pre-vs. post BWRP); in particular, there were significant decreases in glucose (p < 0.0001), insulin (p < 0.0001), HOMA-IR (p < 0.0001), HbA1c (p < 0.0001), T-C (p < 0.0001), LDL-C (p < 0.0001), HDL-C (p < 0.0001), NEFA (p < 0.0001) and TG (p < 0.0001). An improvement in cardiovascular function was also evident at the end of the BWRP (pre vs. post BWRP); significant reductions in SBP (p < 0.0001), DBP (p < 0.0001) and HR (p 0.0171) were observed. The BWRP significantly reduced some markers of systemic inflammation (pre vs. post BWRP), such as CRP (p < 0.0001). Finally, despite the post-BWRP cardiometabolic improvements, the prevalence of metabolic syndrome did not change.
Serum levels of ACTH-8AM (p 0.0001), cortisol-8AM (p < 0.0001) and ACTH-3PM (p 0.0383) and 24 h urinary free-cortisol excretion (p < 0.0001) were significantly reduced at the end of BWRP, with the exception of serum levels of cortisol-3PM, which significantly increased (p < 0.0001).
BWRP induced a significant hypermethylation of clock (p 0.0489) and cry2 (p 0.0102) genes, together with a significant hypomethylation of the per2 gene (p 0.0307), with unchanged post-BWRP methylation status of arntl, cry1, per1 and per3 genes (Table 2).
Table 2.
Gene-specific clock methylation means (pre and post 3-week BWRP).
| Gene methylation | TIME | Mean | (95% CI) | p-Value |
|---|---|---|---|---|
| arntl | pre | 0.9 | (0.77;1.03) | 0.0592 |
| post | 1.1 | (0.96;1.23) | ||
| clock | pre | 1.05 | (0.95;1.16) | 0.0489 |
| post | 1.21 | (1.09;1.32) | ||
| cry1 | pre | 1.49 | (1.31;1.67) | 0.5149 |
| post | 1.58 | (1.4;1.75) | ||
| cry2 | pre | 1.13 | (1.03;1.22) | 0.0102 |
| post | 1.31 | (1.21;1.41) | ||
| per1 | pre | 0.9 | (0.76;1.05) | 0.6478 |
| post | 0.96 | (0.82;1.11) | ||
| per2 | pre | 79.83 | (78.8;80.85) | 0.0307 |
| post | 79.33 | (78.3;80.36) | ||
| per3 | pre | 83.87 | (82.84;84.89) | 0.2493 |
| post | 84.62 | (83.58;85.67) |
We applied linear mixed regression models for paired data to evaluate associations between time (pre/post BWRP) and clock gene methylation. Mean and 95% CI are reported.
3.3. Effects of the BWRP in Females and Males
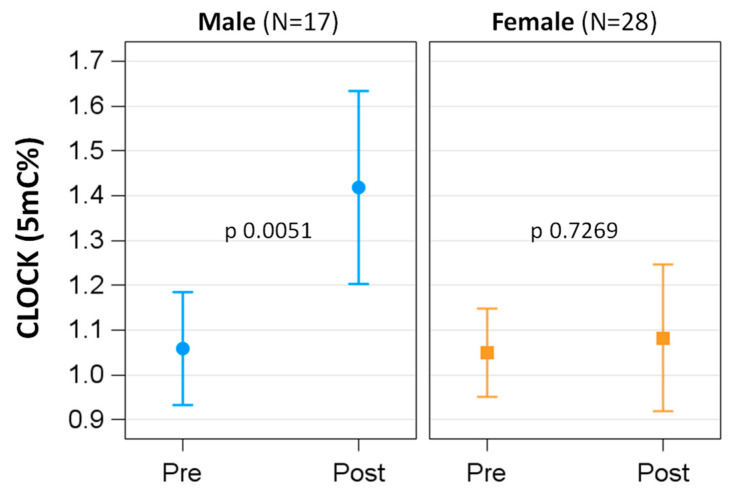
Gender modified the effect of BWRP on most demographic, lifestyle, biochemical and clinical parameters (Table 3). In particular, females showed a smaller change than males, except for HR, BP, glucose and CRP. When considering the methylation status of the single clock genes, at T0, the methylation level of per2 was significantly higher in females than in males (F vs. M: 80.47 ± 0.63 vs. 78.31 ± 0.82, p 0.0445), whereas the BWRP induced a significant hypermethylation only of the clock gene in the male group (p of interaction term time × sex 0.0386; in the male group, T0 vs. T1: 1.06 ± 0.06 vs. 1.42 ± 0.11, p 0.0051) (Figure 1). The methylation of the other genes was not associated with different behavior between males and females.
Table 3.
Demographic, lifestyle, biochemical and clinical characteristics of study participants: pre- vs. post-BWRP comparison in males and females.
| Male (n = 17) | Female (n = 28) | p-Value of Interaction | FDR p-Value of Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Pre | Post | Post-Pre | p-Value | Pre | Post | Post-Pre | p-Value | ||
| Mean (95% CI) | Mean (95% CI) | β ± SE | Mean (95% CI) | Mean (95% CI) | β ± SE | |||||
| BMI, kg/m2 | 38.02 (35.05;40.98) | 36.33 (33.36;39.29) | −1.69 ± 0.03 | <0.0001 | 37.17 (34.85;39.48) | 35.77 (33.46;38.08) | −1.39 ± 0.03 | <0.0001 | <0.0001 | 0.0003 |
| BMI SDS | 3.1 (2.81;3.4) | 2.88 (2.59;3.17) | −0.22 ± 0.01 | <0.0001 | 2.85 (2.62;3.08) | 2.78 (2.55;3.01) | −0.07 ± 0.01 | <0.0001 | <0.0001 | 0.0003 |
| WC, cm | 123.59 (116.16;131.01) | 117.18 (109.75;124.6) | −6.41 ± 0.48 | <0.0001 | 109.43 (103.64;115.21) | 106.04 (100.25;111.82) | −3.39 ± 0.37 | <0.0001 | <0.0001 | 0.0003 |
| 50 Hz ohm | 511.06 (481.74;540.38) | 516.64 (487.3;545.98) | 5.58 ± 2.52 | 0.0321 | 572.46 (549.62;595.31) | 577.21 (554.37;600.06) | 4.75 ± 1.9 | 0.0166 | 0.7934 | 0.7934 |
| FFM, kg | 66 (62.02;69.98) | 64.18 (60.19;68.16) | −1.82 ± 0.21 | <0.0001 | 51.78 (48.68;54.88) | 49.82 (46.72;52.92) | −1.95 ± 0.16 | <0.0001 | 0.6179 | 0.6654 |
| FFM, % | 58.78 (55.8;61.76) | 59.77 (56.79;62.75) | 0.99 ± 0.21 | <0.0001 | 52.74 (50.42;55.06) | 52.89 (50.56;55.21) | 0.15 ± 0.16 | 0.3536 | 0.0023 | 0.0047 |
| FM, kg | 48.12 (40.67;55.58) | 44.96 (37.5;52.42) | −3.16 ± 0.23 | <0.0001 | 47.79 (41.98;53.6) | 44.73 (38.92;50.54) | −3.06 ± 0.18 | <0.0001 | 0.7272 | 0.7541 |
| FM, % | 41.21 (38.48;43.94) | 40.22 (37.49;42.95) | −0.99 ± 0.24 | 0.0002 | 47.26 (45.14;49.39) | 45.81 (43.69;47.94) | −1.45 ± 0.18 | <0.0001 | 0.1355 | 0.1807 |
| REE, kcal | 2067 (1921;2213) | 2002 (1856;2148) | −65.06 ± 25.84 | 0.0159 | 1775 (1663;1888) | 1765 (1652;1877) | −10.56 ± 20.26 | 0.6051 | 0.1047 | 0.1494 |
| NEFA, mmol/L | 1.15 (0.97;1.32) | 0.64 (0.47;0.82) | −0.5 ± 0.04 | <0.0001 | 0.73 (0.59;0.86) | 0.72 (0.59;0.86) | 0.01 ± 0.03 | 0.9972 | <0.0001 | 0.0003 |
| Heart rate, bpm | 76.94 (71.48;82.4) | 74.12 (68.66;79.58) | −2.82 ± 0.93 | 0.0039 | 87.14 (82.89;91.4) | 79.86 (75.6;84.11) | −7.29 ± 0.72 | <0.0001 | 0.0005 | 0.0011 |
| SBP, mmHg | 127.06 (122.84;131.28) | 120.29 (116.07;124.52) | −6.76 ± 0.84 | <0.0001 | 129.46 (126.17;132.75) | 118.93 (115.64;122.22) | −10.54 ± 0.65 | <0.0001 | 0.0010 | 0.002 |
| DBP, mmHg | 79.71 (77.44;81.97) | 75.29 (73.03;77.56) | −4.41 ± 0.48 | <0.0001 | 78.93 (77.16;80.69) | 73.93 (72.16;75.69) | −5 ± 0.37 | <0.0001 | 0.3355 | 0.3915 |
| MAP, mmHg | 95.49 (92.84;98.14) | 90.29 (87.64;92.94) | −5.2 ± 0.5 | <0.0001 | 95.77 (93.71;97.84) | 88.93 (86.86;90.99) | −6.85 ± 0.39 | <0.0001 | 0.0121 | 0.0211 |
| Glucose, mmol/L | 4.94 (4.56;5.31) | 4.72 (4.35;5.1) | −0.22 ± 0.07 | 0.0022 | 4.99 (4.7;5.28) | 4.58 (4.29;4.87) | −0.42 ± 0.05 | <0.0001 | 0.0209 | 0.0344 |
| Insulin, mU/L | 27.44 (23.01;31.86) | 18.85 (14.44;23.25) | −8.59 ± 0.56 | <0.0001 | 22.59 (19.16;26.02) | 16.11 (12.68;19.54) | −6.48 ± 0.4 | <0.0001 | 0.0038 | 0.0071 |
| HbA1c, mmol/L | 5.35 (4.99;5.72) | 5.15 (4.78;5.51) | −0.21 ± 0.02 | <0.0001 | 5.44 (5.16;5.73) | 5.22 (4.94;5.51) | −0.22 ± 0.01 | <0.0001 | 0.5821 | 0.652 |
| HOMA-IR | 5.59 (4.32;6.87) | 4 (2.73;5.28) | −1.59 ± 0.18 | <0.0001 | 5.25 (4.26;6.25) | 3.32 (2.33;4.32) | −1.93 ± 0.14 | <0.0001 | 0.1481 | 0.1884 |
| Metabolic syndrome | ||||||||||
| Yes | 6 (35.3%) | 4 (23.5%) | - | 0.4516 | 11 (39.3%) | 8 (28.6%) | - | 0.3972 | - | - |
| No | 11 (64.7%) | 13 (76.5%) | 17 (60.7%) | 20 (71.4%) | ||||||
| T-C, mg/dL | 157.12 (145.79;168.44) | 128.65 (117.32;139.97) | −28.47 ± 1.16 | <0.0001 | 157 (148.18;165.82) | 137.54 (128.71;146.36) | −19.46 ± 0.91 | <0.0001 | <0.0001 | 0.0003 |
| HDL-C, mg/dL | 39.82 (36.01;43.64) | 32.88 (29.07;36.7) | −6.94 ± 0.35 | <0.0001 | 46.46 (43.49;49.44) | 41.68 (38.7;44.65) | −4.79 ± 0.27 | <0.0001 | <0.0001 | 0.0003 |
| LDL-C, mg/dL | 103.88 (93.19;114.57) | 82.94 (72.25;93.63) | −20.94 ± 1.14 | <0.0001 | 98.25 (89.92;106.58) | 84.21 (75.88;92.55) | −14.04 ± 0.89 | <0.0001 | <0.0001 | 0.0003 |
| TG, mg/dL | 131.18 (106.74;155.61) | 101.76 (77.33;126.2) | −29.41 ± 2.07 | <0.0001 | 116.75 (97.71;135.79) | 100.71 (81.68;119.75) | −16.04 ± 1.61 | <0.0001 | <0.0001 | 0.0003 |
| CRP, mg/dL | 0.34 (0.16;0.51) | 0.34 (0.17;0.52) | 0.01 ± 0.02 | 0.7772 | 0.57 (0.43;0.71) | 0.3 (0.17;0.44) | −0.27 ± 0.02 | <0.0001 | <0.0001 | 0.0003 |
| Cortisol-8AM, μg/L | 15.55 (13.74;17.35) | 11.86 (10.06;13.67) | −3.68 ± 0.36 | <0.0001 | 16.37 (14.96;17.77) | 16.49 (15.08;17.9) | 0.12 ± 0.28 | 0.6709 | <0.0001 | 0.0003 |
| Cortisol-3PM μg/L | 4.66 (3.43;5.9) | 5.12 (3.89;6.35) | 0.46 ± 0.19 | 0.0174 | 7.23 (6.27;8.18) | 7.94 (6.98;8.9) | 0.71 ± 0.14 | <0.0001 | 0.2835 | 0.3452 |
| Urinary cortisol (24 h), µg | 102.22 (78.21;126.23) | 59.17 (35.06;83.28) | −43.05 ± 4.58 | <0.0001 | 100.85 (82.09;119.6) | 84.64 (65.88;103.4) | −16.21 ± 3.59 | 0.0001 | <0.0001 | 0.0003 |
| ACTH-8AM, ng/L | 53.84 (43.28;64.41) | 46.8 (36.23;57.37) | −7.04 ± 1.81 | 0.0003 | 44.87 (36.64;53.1) | 41.61 (33.38;49.84) | −3.26 ± 1.41 | 0.0256 | 0.1067 | 0.1494 |
| ACTH-3PM, ng/L | 20.14 (17.33;22.95) | 20.29 (17.48;23.1) | 0.15 ± 0.36 | 0.6728 | 16.55 (14.36;18.74) | 15.69 (13.5;17.88) | −0.85 ± 0.28 | 0.004 | 0.0327 | 0.0508 |
In descriptive statistics, normally distributed data are expressed as mean ± standard deviation. When not normally distributed, values are expressed as median (Q1, Q3). Categorical data are reported as frequencies and percentage. We applied linear mixed regression models for paired data to evaluate the modifier effect of sex on associations between time (pre/post BWRP) and continuous variables; for categorical variables, we applied the McNemar test for paired data.
Figure 1.
DNA methylation level of the clock gene in males and females: pre- vs. post-BWRP comparisons.
3.4. Effects of the BWRP in Subjects with and without Metabolic Syndrome
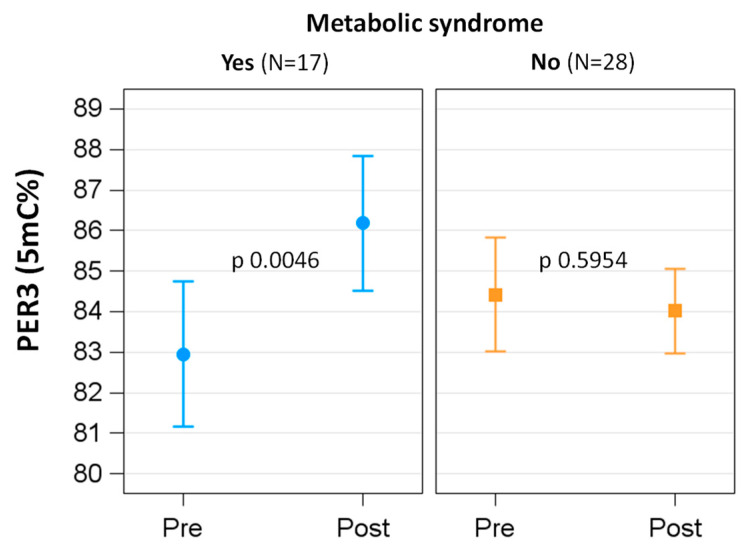
Metabolic syndrome modified the effect of BWRP on most demographic, lifestyle, biochemical and clinical parameters (Table 4). When considering the methylation status of the single clock genes, whereas at T0, there were no significant differences in methylation level of any clock genes, the BWRP induced a significant hypermethylation only of the per3 gene in the group with metabolic syndrome (p of interaction term time × metabolic syndrome 0.0095; in the group with metabolic syndrome, T0 vs. T1: 82.95 ± 0.89 vs. 86.19 ± 0.82, p 0.0046) (Figure 2).
Table 4.
Demographic, lifestyle, biochemical and clinical characteristics of study participants: pre- vs. post-BWRP comparison in subjects with (MetS+) or without (MetS-) metabolic syndrome.
| MetS+ (n = 17) | MetS− (n = 28) | p-Value of Interaction | FDR p-Value of Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Pre | Post | Post-Pre | p-Value | Pre | Post | Post-Pre | p-Value | ||
| Mean (95% CI) | Mean (95% CI) | β ± SE | Mean (95% CI) | Mean (95% CI) | β ± SE | |||||
| BMI, kg/m2 | 37.5 (35.7;39.3) | 35.8 (34.0;37.7) | −1.68 ± 0.04 | <0.0001 | 37.45 (35.65;39.26) | 36.03 (34.22;37.84) | −1.43 ± 0.03 | <0.0001 | <0.0001 | 0.0002 |
| BMI SDS | 2.83 (2.64;3.02) | 2.71 (2.52;2.91) | −0.12 ± 0.02 | <0.0001 | 3.01 (2.82;3.2) | 2.86 (2.67;3.04) | −0.16 ± 0.01 | <0.0001 | 0.0565 | 0.0659 |
| WC, cm | 113.6 (108.5;118.6) | 110.8 (105.7;115.8) | −2.79 ± 0.57 | <0.0001 | 115.52 (110.57;120.47) | 110.05 (105.12;114.99) | −5.46 ± 0.37 | <0.0001 | 0.0004 | 0.0008 |
| 50 Hz ohm | 555.05 (534.37;575.73) | 563.46 (542.3;584.63) | 8.42 ± 3.04 | 0.0084 | 545.76 (525.49;566.02) | 551.11 (530.94;571.28) | 5.35 ± 1.9 | 0.0074 | 0.4083 | 0.4234 |
| FFM, kg | 55.8 (52.53;59.07) | 54.43 (51.14;57.72) | −1.37 ± 0.25 | <0.0001 | 57.97 (54.72;61.22) | 55.55 (52.3;58.8) | −2.42 ± 0.15 | <0.0001 | 0.0010 | 0.0017 |
| FFM, % | 53.76 (51.72;55.8) | 54.8 (52.73;56.87) | 1.04 ± 0.25 | 0.0001 | 55.79 (53.78;57.8) | 55.74 (53.73;57.74) | −0.05 ± 0.15 | 0.7349 | 0.0007 | 0.0013 |
| FM, kg | 48.74 (44.25;53.23) | 45.66 (41.15;50.17) | −3.08 ± 0.28 | <0.0001 | 47.42 (42.94;51.89) | 44.51 (40.04;48.98) | −2.9 ± 0.18 | <0.0001 | 0.6145 | 0.6145 |
| FM, % | 45.58 (43.7;47.46) | 44.66 (42.73;46.59) | −0.92 ± 0.29 | 0.0029 | 44.61 (42.77;46.45) | 43.36 (41.53;45.19) | −1.25 ± 0.18 | <0.0001 | 0.3529 | 0.38 |
| REE, kcal | 1989 (1879;2100) | 1875 (1755;1995) | −114.06 ± 32.39 | 0.0011 | 1827.79 (1726.85;1928.73) | 1847.73 (1748.42;1947.03) | 19.93 ± 19.67 | 0.3169 | 0.0013 | 0.0022 |
| NEFA, mmol/L | 0.86 (0.71;1) | 0.77 (0.61;0.93) | −0.08 ± 0.06 | 0.1457 | 0.9 (0.78;1.02) | 0.67 (0.55;0.79) | −0.23 ± 0.04 | <0.0001 | 0.0231 | 0.0294 |
| Heart rate, bpm | 88.08 (84.17;91.99) | 74.48 (70.3;78.66) | −13.6 ± 1.05 | <0.0001 | 80.38 (76.74;84.02) | 78.86 (75.28;82.43) | −1.53 ± 0.69 | 0.0323 | <0.0001 | 0.0002 |
| SBP, mmHg | 138.02 (135.03;141.01) | 128.51 (125.27;131.76) | −9.51 ± 0.92 | <0.0001 | 122.81 (120.08;125.54) | 116.15 (113.48;118.81) | −6.66 ± 0.6 | <0.0001 | 0.0148 | 0.0208 |
| DBP, mmHg | 81.54 (79.93;83.15) | 74.98 (73.21;76.76) | −6.56 ± 0.56 | <0.0001 | 77.81 (76.38;79.25) | 74.25 (72.85;75.64) | −3.56 ± 0.36 | <0.0001 | 0.0001 | 0.0002 |
| MAP, mmHg | 100.34 (98.55;102.14) | 92.8 (90.85;94.75) | −7.54 ± 0.56 | <0.0001 | 92.83 (91.2;94.46) | 88.22 (86.63;89.81) | −4.6 ± 0.37 | <0.0001 | 0.0001 | 0.0002 |
| Glucose, mmol/L | 5.21 (4.95;5.48) | 4.38 (4.1;4.67) | −0.83 ± 0.08 | <0.0001 | 4.82 (4.58;5.06) | 4.72 (4.49;4.96) | −0.1 ± 0.05 | 0.0448 | <0.0001 | 0.0002 |
| Insulin, mU/L | 26.57 (23.69;29.46) | 18.25 (15.23;21.27) | −8.32 ± 0.63 | <0.0001 | 22.92 (20.15;25.69) | 16.74 (14.01;19.47) | −6.18 ± 0.41 | <0.0001 | 0.0084 | 0.0124 |
| HbA1c, mmol/L | 5.47 (5.24;5.69) | 5.12 (4.89;5.35) | −0.34 ± 0.02 | <0.0001 | 5.37 (5.15;5.6) | 5.22 (4.99;5.44) | −0.16 ± 0.01 | <0.0001 | <0.0001 | 0.0002 |
| HOMA-IR | 6.24 (5.4;7.09) | 3.49 (2.59;4.38) | −2.75 ± 0.21 | <0.0001 | 4.85 (4.05;5.65) | 3.61 (2.83;4.4) | −1.24 ± 0.14 | <0.0001 | <0.0001 | 0.0002 |
| T-C, mg/dL | 153.17 (145.23;161.11) | 125.92 (117.73;134.11) | −27.25 ± 1.4 | <0.0001 | 159.39 (151.7;167.09) | 137.18 (129.54;144.82) | −22.21 ± 0.91 | <0.0001 | 0.005 | 0.0078 |
| HDL-C, mg/dL | 42.33 (39.68;44.98) | 39.65 (36.94;42.36) | −2.68 ± 0.4 | <0.0001 | 44.94 (42.36;47.53) | 37.88 (35.31;40.46) | −7.06 ± 0.26 | <0.0001 | <0.0001 | 0.0002 |
| LDL-C, mg/dL | 97.33 (89.75;104.91) | 74.57 (66.75;82.39) | −22.76 ± 1.34 | <0.0001 | 102.23 (94.88;109.57) | 87.07 (79.78;94.36) | −15.16 ± 0.88 | <0.0001 | <0.0001 | 0.0002 |
| TG, mg/dL | 122.88 (106.26;139.49) | 80.83 (63.87;97.79) | −42.05 ± 2.35 | <0.0001 | 121.79 (105.5;138.07) | 108.49 (92.28;124.69) | −13.3 ± 1.53 | <0.0001 | <0.0001 | 0.0002 |
| CRP, mg/dL | 0.66 (0.54;0.77) | 0.4 (0.27;0.52) | −0.26 ± 0.02 | <0.0001 | 0.38 (0.26;0.49) | 0.29 (0.18;0.4) | −0.09 ± 0.02 | <0.0001 | <0.0001 | 0.0002 |
| Cortisol-8AM, μg/L | 16.22 (14.82;17.61) | 15.93 (14.41;17.45) | −0.29 ± 0.45 | 0.5259 | 15.96 (14.7;17.22) | 14.31 (13.08;15.54) | −1.65 ± 0.29 | <0.0001 | 0.0173 | 0.0231 |
| Cortisol-3PM, μg/L | 7.25 (6.28;8.21) | 7.15 (6.14;8.16) | −0.1 ± 0.22 | 0.65 | 5.66 (4.74;6.57) | 6.78 (5.87;7.68) | 1.12 ± 0.14 | <0.0001 | <0.0001 | 0.0002 |
| Urinary cortisol (24 h), µg | 108.94 (91.48;126.4) | 92.21 (72.69;111.74) | −16.73 ± 6.15 | 0.0096 | 96.8 (80.94;112.67) | 69.58 (54.09;85.07) | −27.23 ± 3.5 | <0.0001 | 0.1549 | 0.1735 |
| ACTH-8AM, ng/L | 43.06 (35.31;50.8) | 41.6 (33.3;49.9) | −1.46 ± 2.14 | 0.5005 | 51.42 (44.24;58.6) | 44.29 (37.24;51.33) | −7.13 ± 1.4 | <0.0001 | 0.0359 | 0.0437 |
| ACTH-3PM, ng/L | 16.35 (14.27;18.43) | 13.52 (11.36;15.68) | −2.83 ± 0.41 | <0.0001 | 18.85 (16.84;20.85) | 18.85 (16.87;20.83) | 0 ± 0.27 | 0.9859 | <0.0001 | 0.0002 |
In descriptive statistics, normally distributed are expressed as mean ± standard deviation. When not normally distributed, values are expressed as median (Q1, Q3). Categorical data are reported as frequencies and percentages. We applied linear mixed regression models for paired data to evaluate the modifier effect of metabolic syndrome on associations between time (pre/post-BWRP) and continuous variables; for categorical variables, we applied the McNemar test for paired data. MetS+ : presence of criteria for metabolic syndrome at baseline; MetS− : lack of criteria for metabolic syndrome at baseline.
Figure 2.
DNA methylation level of the per3 gene in subjects with or without metabolic syndrome: pre- vs. post-BWRP comparisons.
3.5. Associations of Methylation Level of Clock Genes with Other Parameters
Neither the CASQ total score, MEQSA total score nor MEQSA chronotype (at T0) were associated with the methylation level of any clock genes. On the contrary, sleep time during a weekday (at T0) was significantly associated with the methylation levels of arntl (β = −0.0025, SE = 0.001 and p 0.0224) and per1 (β = −0.0028, SE = 0.001 and p 0.0137) genes. Finally, in contrast to the CASQ alertness score, the CASQ sleepiness score (at T0) was significantly associated with the methylation level of the cry1 gene (β = 0.0360, SE = 0.0169 and p 0.0397).
Before BWRP, cortisol-3PM was significantly associated with the methylation level of the clock gene (β = −0.0386, SE = 0.0159 and p 0.0198) and per3 (β = 0.5597, SE = 0.2303 and p 0.0197), whereas there was a significant association between ACTH-3PM and the methylation level of the per1 gene (β = 0.0386, SE = 0.0141 and p 0.0095). Finally, ACTH-8AM and the methylation level of the cry1 gene were significantly associated (β = 0.0081, SE = 0.0040 and p 0.0489). After BWRP, there were significant associations between ACTH-8AM and the methylation level of the per3 gene (β = 0.0464, SE = 0.0186 and p 0.0169) or the clock gene (β = 0.0054, SE = 0.0026 and p 0.0473).
Before BWRP, the methylation level of the arntl gene was significantly associated with CRP (β = -0.5526, SE = 0.2254 and p 0.00188), whereas that of the clock gene was associated with SBP (β = 0.0104, SE = 0.0030 and p 0.0015); that of the cry1 gene was associated with FM kg (β = 0.0231, SE = 0.0089 and p 0.0133) and REE (β = 0.0008, SE = 0.0003 and p 0.00135); that of the cry2 gene was associated with FFM kg (β = 0.0186, SE = 0.0090 and p 0.0433); that of the per1 gene was associated with CRP (β = -0.4876, SE = 0.2381 and p 0.047); that of the per2 gene was associated with T-C (β = -0.0440, SE = 0.0161 and p 0.0095) and LDL-C (β = −0.0466, SE = 0.0169 and p 0.0089); and that of the per3 gene was associated with HR (β = 0.0744, SE = 0.0344 and p 0.0366), FFM % (β = 0.2575, SE = 0.1141 and p 0.0295) and FM % (β = −0.2579, SE = 0.1141 and p 0.0293). After BWRP, the methylation level of the cry2 gene was significantly associated with FM % (β = −0.0202, SE = 0.0089 and p 0.0290), whereas that of the per1 gene was associated with FM kg (β = -0.0155, SE = 0.0070 and p 0.0330) and T-C (β = −0.0609, SE = 0.0275 and p 0.0327); finally, that of per2 was associated with DBP (β = -0.1919, SE = 0.0943 and p 0.0485).
For further details, the reader may consult the Supplemental Material (i.e., Tables S2 and S3).
4. Discussion
Chronobiological misalignment has been associated with obesity and its related comorbidities, including metabolic syndrome [45]. Massive exposure to chronodisruptors (e.g., sleep deprivation, living under constant artificial light, erratic and frequently changeable eating times, nocturnal snacking, etc.) has been invoked as a reason for the widespread prevalence of pediatric obesity, particularly in Western countries [46].
In the present study, in a cohort of obese adolescents, we investigated the effects of a short-term BWRP on the DNA methylation status of a series of clock genes, namely clock, arntl, cry1-2 and per1-3, which, at the molecular level, regulate the circadian rhythms in SCN neurons (master clock) and many peripheral organs (peripheral clocks), such as the liver, muscles, adipose tissue and even leucocytes, the latter being the source of the extracted DNA used in our epigenetic analyses [47]. Although this experimental condition (i.e., leucocyte-based source) may actually represent a limitation of the present study, hampering the derivation of definitive conclusions, the fact that leukocytes are recognized as a “peripheral biomarker” in many fields of clinical research, including obesity [48], as well as the simplicity of blood sampling against the invasiveness of tissue biopsy (not allowed by our Ethical Committee), provides support for the present choice.
Before admission to the 3-week BWRP, obese adolescents enrolled in this study were chronobiologically characterized using validated questionnaires to quantify chronodisruption, such CASQ, MESQA and total weekly sleep score [39,40,41]. Interestingly, the methylation level of some clock genes, particularly arntl, cry1 and per1, was associated with chronodisruption, i.e. hypermethylation of these clock genes with decreased sleep time and increased sleepiness, indicating that our epigenetic approach was (somewhat) methodologically adequate to evaluate the relationship between weight loss and chronodisruption.
At the end of the 3-week BWRP, a hypermethylation of clock and cry2 genes occurred, as well as a hypomethylation of the per2 gene. The epigenetic changes in the clock gene system were related to BWRP-induced favorable cardiometabolic outcomes, such as weight loss with WC and FM decreases, improved glucometabolic homeostasis, antidyslipidemic effects and cardiovascular benefits.
The design of our clinical study does not allow us to establish whether epigenetic changes in clock genes actually represent the cause or the effect of the global cardiometabolic improvement. In our opinion, the final effect of our BWRP, entailing hypocaloric diet, exercise and psychological support, appears to a sort of ”metabolic chrono-resynchronization” [49]. This is particularly evident when considering the increasing number of associations (statistically significant or close the statistical significance) of the methylation level of clock genes with cardiometabolic outcomes at the end of the BWRP, including FM (a surrogate of WC) (cry2 and per1), HDL-C (cry1), SBP (per2), DBP (per2) and TG (per2), which (non-surprisingly) represent the IDF criteria for metabolic syndrome [37] and (surprisingly) were not present before BWRP administration (i.e., T0).
In the present study, hypermethylation of the per3 gene was observed at the end of the BWRP only in the group with metabolic syndrome. Among the cardiometabolic outcomes that did not change in obese adolescents without metabolic syndrome, we might invoke REE, FFM and ACTH-3PM as potential causative factors of the missing post-BWRP hypermethylation of the per3 gene in this group. Being limited the number of the cardiometabolic outcomes that were evaluated in the present study, any attempt to explain the post-BWRP epigenetic differences among obese adolescents with or without metabolic syndrome might be too speculative. Further studies are thus needed to solve this issue, which might be of clinical interest due to the need of a “biomolecular marker” of BWRP effectiveness in metabolic syndrome, a condition that is more difficult to treat compared to essential obesity [50]. Importantly, the BWRP-induced hypermethylation of the per3 gene might be interpreted as a negative outcome due to the post-BWRP decrease in REE and FFM in the group with metabolic syndrome, which would clinically indicate energy storage and muscle protein waste [51].
A sexual dysmorphism in body clock has been reported in humans, explaining, at least in part, the differing chronobiology between female and male metabolism and behavior, including the well-known eveningness preferences of men relative to women [52]. In the present study, whereas we observed hypomethylation of the per2 gene in the male rather than female group before the BWRP, obese males showed a post-BWRP hypermethylation of the clock gene, an epigenetic change that did not occur in the female group.
Whereas mRNA levels of the clock gene were not evaluated in an in vitro study using subcutaneous and visceral adipose tissues from obese men and women, in whom a different gene expression of per2, cry1 and bmal1 (arntl) was instead found [53], in an animal model, the effects of calorie restriction on circadian rhythms in hepatic rev-erb-α, ror-γ (retinoic acid receptor-related orphan receptor-γ) and both cry1 and cry2 gene expression were demonstrated to be sex-dependent, with the exception of that of per1-3 genes [54]. Apart from these non-epigenetic studies, to the best of our knowledge, no study has evaluated BWRP-induced epigenetic changes in clock genes in obese adolescents with a sex-centered approach to date. Based on the results of the present study, we are unaware whether sex-related differences in epigenetic regulation of clock genes may explain the lower post-BWRP weight loss that is generally observed in obese females relative to males [55]. Because strong evidence supports the view that estrogens can modulate the expression of clock genes, an effect that is essential for orchestration of (even sex-related) circadian rhythms by SCN [56], a different sex-tailored BWRP might be customized, e.g., a more “chrono-resynchronizing” BWRP in females relative to males or, alternatively, a chrononutrition specific to women and men (see below).
Obesity, particularly that characterized by massive visceral adiposity, is reportedly associated with hypercortisolism, including increased 24 h urinary free cortisol excretion, cortisol secretion rate, plasma cortisol response to ACTH (or corticotropin-releasing hormone [CRH]) and salivary cortisol peak after mental stress [57,58,59,60]. Furthermore, plasma levels of cortisol-binding globulin (CBG) are lower in subjects with obesity and insulin resistance, explaining, at least in part, increased free cortisol under conditions of reduced negative feedback, such as stress [61]. Finally, adipose tissue may generate (active) cortisol from (inactive) cortisone via the 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) enzyme [62].
In the present study, the BWRP was able to tone down HPA function as documented by the decreased values of ACTH-8AM, cortisol-8AM, ACTH-3PM and 24 h urinary free-cortisol excretion at the end of our 3-week BWRP. Although conflicting results have been reported in calorically restricted obese subjects when evaluating their HPA function, a full discussion of this discrepancy is outside the scope of the present study [63].
On the contrary, this study highlights the BWRP-induced changes in the associations of some markers of HPA function with the methylation status of specific clock genes. For instance, pre-BWRP cortisol-3PM and ACTH-3PM were associated with the methylation status of clock and per1 genes, respectively. These were associations that, interestingly, were missing at the end of the intervention, when, instead, the association of ACTH-8AM with the methylation status of per3 was evident.
Whereas the pulsatile release of ACTH and cortisol is controlled by a negative feedback loop involving glucocorticoid receptor (GR) signaling in the hippocampus up to pituitary [64,65], circadian HPA rhythm is regulated by clock-gene-mediated mechanisms operating at different levels: (i) the endocrine HPA axis itself, (ii) SCN-controlled autonomic innervation and (iii) local adrenocortical circadian clocks [64,66].
Given that the DNA source of our epigenetic analyses was represented by peripheral leucocytes, which may not completely correspond to that from the HPA axis (e.g., pituitary or adrenals), the circadian rhythms of cortisol secretion and white blood cell count are, under physiological conditions, perfectly synchronized [67], implying common molecular mechanisms of circadian rhythmicity. Therefore, because clock and per1 are the clock genes with a methylation level that did change at the end of the intervention (in the entire subject population, male group or metabolic syndrome) and which are implicated in associations with HPA activity, our hypothesis is that the BWRP-induced “chrono-resynchronization” involved not only metabolism in se (see above) but also the endocrine system, namely the HPA axis. Further studies are needed to further explore the relationships between clock genes and effects of energy restriction on HPA activity in obesity, including human and animal models [63].
The present study is subject to some limitations.
First of all, evaluation of epigenetic remodeling (i.e., levels of DNA methylation in a specific gene) does not permit a complete definition of gene/protein expression. The clock gene system is regulated at different levels: genetic, epigenetic, translational and post-translational [47,68]. Therefore, some BWRP-induced effects on (final) gene/protein (e.g., clock/CLOCK) expression might have occurred but not been detected due to our methodological approach. These considerations may also explain the difficulty encountered in interpreting our results using genetically modified animal models (e.g., clock mutant mice), in which obesity and dysmetabolism represent a phenotype [9].
Second, given the clinical nature of this study, the BWRP-related molecular factor(s) that could have interfered with DNA methylation machinery (e.g., DNA methyltransferases/demethylases) are unknown. Some metabolites have been demonstrated to affect DNA methylation, such as some lipids, which, in the present study, decreased after the BWRP (e.g., T-C, TG or NEFA) [22]. Metabolomics studies might be useful to answer this question and permit the selection of so-called “bioactive” nutrients to be inserted in patient diets in order to achieve nutrition-based epigenetic remodeling [69]. Alternatively, we cannot rule out that the BWRP-induced metabolic effects are mediated by PPARs and PGC 1α, which, binding (even endogenous) lipidic ligands, have been recognized as modulators of arntl/ARNTL and clock/CLOCK [4].
Third, epigenetic remodeling of clock genes has been associated with obesity and other metabolic disorders [30], but changes in DNA methylation of clock genes might be a simple epiphenomenon with no causative implications. Therefore, other (unknown or yet to be investigated) molecular mechanisms might underlie the relationships from chronobiological misalignment and obesity to BWRP-induced benefits and weight loss.
5. Conclusions
A short-term (3-week) BWRP administered to a chronodisrupted pediatric obese population is capable of producing beneficial cardiometabolic effects, as well as an epigenetic remodeling of specific clock genes, suggesting the occurrence of a post-BWRP metabolic and endocrine “chrono-resynchronization”, which might represent a “biomolecular” predictor of successful antiobesity intervention.
Acknowledgments
The authors acknowledge the head nurse, Angela Seddone, and the nursing staff at the Division of Auxology, Istituto Auxologico Italiano, Piancavallo, VB, Italy. Our special thanks go to the subjects and their families for their willingness to participate in this research protocol. The authors thank S. Zajac for the careful English revision.
Abbreviations
11β-HSD1, 11β-hydroxysteroid dehydrogenase 1; ACTH, adrenocorticotropic hormone; ALT, alanine transaminase; ARNTL, aryl hydrocarbon receptor nuclear translocator like; ASP, aspartate transaminase; BMI, body mass index; CI, confidence interval; BMAL1, brain- and muscle-ANRT-like protein; bpm, beats per minute; CLOCK, circadian locomotor output cycles kaput; CRH, corticotropin-releasing hormone; CRP, C-reactive protein; CRY1-2, cryptochrome 1-2; DBP, diastolic blood pressure; DNAm, DNA methylation; ERB-α, reverse erythroblastosis virus-α; F, female; HDL-C, high-density lipoprotein cholesterol; gamma-GT, gamma-glutamyl transferase; HC, hip circumference; HOMA-IR, homeostatic model assessment for insulin resistance; HR, heart rate; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; M, male; MAP, mean arterial pressure; MetS, metabolic syndrome; NPAS2, name derived from PERARNT-SIM protein-2; OR, odds ratio; PER1-3, period 1-3; PGC 1α, PPAR-γ coactivator 1α; PPAR, peroxisome proliferator-activated receptor; ROR-α, retinoic acid receptor-related orphan receptor-α; SBP, systolic blood pressure; SD, standard deviation; SE, standard error; T-C, total cholesterol; TG, triglycerides; WC, waist circumference; yr, year.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192315492/s1, Table S1: Pyrosequencing assay information, Table S2: Association between chronodisruption parameters and clock gene DNA methylation, pre-BWRP; Table S3: Association between clinical and biochemical parameters and clock gene DNA methylation, pre-BWRP and post-BWRP.
Author Contributions
A.E.R., V.B. and A.S. designed the study. D.C. and A.D.C. recruited the subjects and collected all clinical data, elaborating the database, which was shared with and completed by the remaining authors. B.A. performed methylation analyses. C.F. statistically analyzed the data. A.E.R., together with V.B. and A.S., wrote the manuscript. S.G.C. contributed to data interpretation and writing of the Discussion section. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The present study was approved by the Ethical Committee of Istituto Auxologico Italiano (research project code: 01C922; acronym: GENICLOCK).
Informed Consent Statement
Written informed consent was obtained from the parents of the patients. See Methods for details.
Data Availability Statement
The datasets used and/or analyzed in the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported herein.
Funding Statement
This research was funded by the Italian Ministry of Health.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patke A., Young M.W., Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2. [DOI] [PubMed] [Google Scholar]
- 2.Hastings M.H., Maywood E.S., Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018;19:453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 3.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., Yang G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 2014;2014:653017. doi: 10.1155/2014/653017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthier A., Johanns M., Zummo F.P., Lefebvre P., Staels B. PPARs in liver physiology. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:166097. doi: 10.1016/j.bbadis.2021.166097. [DOI] [PubMed] [Google Scholar]
- 6.Sun C., Mao S., Chen S., Zhang W., Liu C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021;22:8974. doi: 10.3390/ijms22168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht U. The circadian clock, metabolism and obesity. Obes. Rev. 2017;18((Suppl. 1)):25–33. doi: 10.1111/obr.12502. [DOI] [PubMed] [Google Scholar]
- 8.Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D.R., et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan D., Lazar M.A. Interconnections between circadian clocks and metabolism. J. Clin. Invest. 2021;131:e148278. doi: 10.1172/JCI148278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covassin N., Singh P., Somers V.K. Keeping Up With the Clock: Circadian Disruption and Obesity Risk. Hypertension. 2016;68:1081–1090. doi: 10.1161/HYPERTENSIONAHA.116.06588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora T., Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int. J. Obes. 2015;39:39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuis J.D., Johnson M.L., Lizarralde G., Iranmanesh A. Rhythmic and nonrhythmic modes of anterior pituitary gland secretion. Chronobiol. Int. 1992;9:371–379. doi: 10.3109/07420529209064549. [DOI] [PubMed] [Google Scholar]
- 13.Oster H., Challet E., Ott V., Arvat E., de Kloet E.R., Dijk D.J., Lightman S., Vgontzas A., Van Cauter E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017;38:3–45. doi: 10.1210/er.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Safi Z.A., Polotsky A., Chosich J., Roth L., Allshouse A.A., Bradford A.P., Santoro N. Evidence for disruption of normal circadian cortisol rhythm in women with obesity. Gynecol. Endocrinol. 2018;34:336–340. doi: 10.1080/09513590.2017.1393511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicennati V., Pasquali R. Abnormalities of the hypothalamic-pituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: Evidence for a central and a peripheral alteration. J. Clin. Endocrinol. Metab. 2000;85:4093–4098. doi: 10.1210/jcem.85.11.6946. [DOI] [PubMed] [Google Scholar]
- 16.Liyanarachchi K., Ross R., Debono M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract. Res. Clin. Endocrinol. Metab. 2017;31:459–473. doi: 10.1016/j.beem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M., Schütz G., Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 18.Jaikumar G., Slabbekoorn H., Sireeni J., Schaaf M., Tudorache C. The role of the Glucocorticoid Receptor in the Regulation of Diel Rhythmicity. Physiol. Behav. 2020;223:112991. doi: 10.1016/j.physbeh.2020.112991. [DOI] [PubMed] [Google Scholar]
- 19.Kolbe I., Dumbell R., Oster H. Circadian Clocks and the Interaction between Stress Axis and Adipose Function. Int. J. Endocrinol. 2015;2015:693204. doi: 10.1155/2015/693204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sayed Moustafa J.S., Froguel P. From obesity genetics to the future of personalized obesity therapy. Nat. Rev. Endocrinol. 2013;9:402–413. doi: 10.1038/nrendo.2013.57. [DOI] [PubMed] [Google Scholar]
- 21.van Dijk S.J., Molloy P.L., Varinli H., Morrison J.L., Muhlhausler B.S., Members of EpiSCOPE Epigenetics and human obesity. Int. J. Obes. 2015;39:85–97. doi: 10.1038/ijo.2014.34. [DOI] [PubMed] [Google Scholar]
- 22.Feng D., Lazar M.A. Clocks, metabolism, and the epigenome. Mol. Cell. 2012;47:158–167. doi: 10.1016/j.molcel.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kass S.U., Landsberger N., Wolffe A.P. DNA methylation directs a time-dependent repression of transcription initiation. Curr. Biol. 1997;7:157–165. doi: 10.1016/S0960-9822(97)70086-1. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari L., Carugno M., Bollati V. Particulate matter exposure shapes DNA methylation through the lifespan. Clin. Epigenetics. 2019;11:129. doi: 10.1186/s13148-019-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joska T.M., Zaman R., Belden W.J. Regulated DNA methylation and the circadian clock: Implications in cancer. Biology. 2014;3:560–577. doi: 10.3390/biology3030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oike H., Oishi K., Kobori M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014;3:204–212. doi: 10.1007/s13668-014-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen E.C., Dolinoy D., Peterson K.E., O’Brien L.M., Chervin R.D., Cantoral A., Tellez-Rojo M.M., Solano-Gonzalez M., Goodrich J. Adolescent sleep timing and dietary patterns in relation to DNA methylation of core circadian genes: A pilot study of Mexican youth. Epigenetics. 2021;16:894–907. doi: 10.1080/15592294.2020.1827719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi M., Hirayama J., Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Milagro F.I., Gómez-Abellán P., Campión J., Martínez J.A., Ordovás J.M., Garaulet M. CLOCK, PER2 and BMAL1 DNA methylation: Association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol. Int. 2012;29:1180–1194. doi: 10.3109/07420528.2012.719967. [DOI] [PubMed] [Google Scholar]
- 31.Malone S.K., Zemel B., Compher C., Souders M., Chittams J., Thompson A.L., Pack A., Lipman T.H. Social jet lag, chronotype and body mass index in 14–17-year-old adolescents. Chronobiol. Int. 2016;33:1255–1266. doi: 10.1080/07420528.2016.1196697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigamonti A.E., Cicolini S., Caroli D., De Col A., Scacchi M., Cella S.G., Sartorio A. Effects of a 3-Week In-Hospital Body Weight Reduction Program on Cardiovascular Risk Factors, Muscle Performance, and Fatigue: A Retrospective Study in a Population of Obese Adults with or without Metabolic Syndrome. Nutrients. 2020;12:1495. doi: 10.3390/nu12051495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigamonti A.E., Tringali G., Micheli R., De Col A., Tamini S., Saezza A., Cella S.G., Sartorio A. Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome. Nutrients. 2020;12:208. doi: 10.3390/nu12010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigamonti A.E., Caroli D., Grugni G., Cella S.G., Sartorio A. Frequent Medical Supervision Increases the Effectiveness of a Longitudinal Multidisciplinary Body Weight Reduction Program: A Real-World Experience in a Population of Children and Adolescents with Obesity. Nutrients. 2021;13:3362. doi: 10.3390/nu13103362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cacciari E., Milani S., Balsamo A., Spada E., Bona G., Cavallo L., Cerutti F., Gargantini L., Greggio N., Tonini G., et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) J. Endocrinol. Investig. 2006;29:581–593. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 36.Deurenberg P. International consensus conference on impedance in body composition. Age Nutr. 1994;5:142–145. [Google Scholar]
- 37.Zimmet P., Alberti K.G., Kaufman F., Tajima N., Silink M., Arslanian S., Wong G., Bennett P., Shaw J., Caprio S., et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.McCharty H.D., Jarret K.V., Crawley H.F. The development of waist circumference percentiles in British children aged 5.0_16.9 y. Eur. J. Clin. Nutr. 2001;55:902e7. doi: 10.1038/sj.ejcn.1601240. [DOI] [PubMed] [Google Scholar]
- 39.Garaulet M., Corbalán M.D., Madrid J.A., Morales E., Baraza J.C., Lee Y.C., Ordovas J.M. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int. J. Obes. 2010;34:516–523. doi: 10.1038/ijo.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horne J.A., Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 41.Spilsbury J.C., Drotar D., Rosen C.L., Redline S. The Cleveland adolescent sleepiness questionnaire: A new measure to assess excessive daytime sleepiness in adolescents. J. Clin. Sleep Med. 2007;3:603–612. doi: 10.5664/jcsm.26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantone L., Tobaldini E., Favero C., Albetti B., Sacco R.M., Torgano G., Ferrari L., Montano N., Bollati V. Particulate Air Pollution, Clock Gene Methylation, and Stroke: Effects on Stroke Severity and Disability. Int. J. Mol. Sci. 2020;21:3090. doi: 10.3390/ijms21093090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bollati V., Baccarelli A., Hou L., Bonzini M., Fustinoni S., Cavallo D., Byun H.M., Jiang J., Marinelli B., Pesatori A.C., et al. Changes in DNA Methylation Patterns in Subjects Exposed to Low-Dose Benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 44.Monti P., Iodice S., Tarantini L., Sacchi F., Ferrari L., Ruscica M., Buoli M., Vigna L., Pesatori A.C., Bollati V. Effects of PM Exposure on the Methylation of Clock Genes in a Population of Subjects with Overweight or Obesity. Int. J. Environ. Res. Public Health. 2021;18:1122. doi: 10.3390/ijerph18031122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmet P., Alberti K.G.M.M., Stern N., Bilu C., El-Osta A., Einat H., Kronfeld-Schor N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019;286:181–191. doi: 10.1111/joim.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter R.J., Tan D.X., Korkmaz A., Ma S. Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Ann. Med. 2012;44:564–577. doi: 10.3109/07853890.2011.586365. [DOI] [PubMed] [Google Scholar]
- 47.Partch C.L., Green C.B., Takahashi J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pecht T., Gutman-Tirosh A., Bashan N., Rudich A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes. Rev. 2014;15:322–337. doi: 10.1111/obr.12133. [DOI] [PubMed] [Google Scholar]
- 49.Johnston J.D., Ordovás J.M., Scheer F.A., Turek F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv. Nutr. 2016;7:399–406. doi: 10.3945/an.115.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srikanthan K., Feyh A., Visweshwar H., Shapiro J.I., Sodhi K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016;13:25–38. doi: 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiegler P., Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36:239–262. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- 52.Anderson S.T., FitzGerald G.A. Sexual dimorphism in body clocks. Science. 2020;369:1164–1165. doi: 10.1126/science.abd4964. [DOI] [PubMed] [Google Scholar]
- 53.Gómez-Abellán P., Madrid J.A., Luján J.A., Frutos M.D., González R., Martínez-Augustín O., de Medina F.S., Ordovás J.M., Garaulet M. Sexual dimorphism in clock genes expression in human adipose tissue. Obes. Surg. 2012;2:105–112. doi: 10.1007/s11695-011-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Astafev A.A., Patel S.A., Kondratov R.V. Calorie restriction effects on circadian rhythms in gene expression are sex dependent. Sci. Rep. 2017;7:9716. doi: 10.1038/s41598-017-09289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams R.L., Wood L.G., Collins C.E., Callister R. Effectiveness of weight loss interventions--is there a difference between men and women: A systematic review. Obes. Rev. 2015;16:171–186. doi: 10.1111/obr.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatcher K.M., Royston S.E., Mahoney M.M. Modulation of circadian rhythms through estrogen receptor signaling. Eur. J. Neurosci. 2020;51:217–228. doi: 10.1111/ejn.14184. [DOI] [PubMed] [Google Scholar]
- 57.Douyon L., Schteingart D.E. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol. Metab. Clin. North Am. 2002;31:173–189. doi: 10.1016/S0889-8529(01)00023-8. [DOI] [PubMed] [Google Scholar]
- 58.Marin P., Darin N., Amemiya T., Andersson B., Jern S., Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 59.Rosmond R., Dallman M.F., Bjorntorp P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998;83:1853–1859. doi: 10.1210/jc.83.6.1853. [DOI] [PubMed] [Google Scholar]
- 60.Pasquali R., Cantobelli S., Casimirri F., Capelli M., Bortoluzzi L., Flamia R., Labate A.M., Barbara L. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J. Clin. Endocrinol. Metab. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Real J.M., Pugeat M., Grasa M., Broch M., Vendrell J., Brun J., Ricartet W. Serum corticosteroid-binding globulin concentration and insulin resistance syndrome: A population study. J. Clin. Endocrinol. Metab. 2002;87:4686–4690. doi: 10.1210/jc.2001-011843. [DOI] [PubMed] [Google Scholar]
- 62.Tomlinson J.W., Moore J.S., Clark P.M., Holder G., Shakespeare L., Stewart P.M. Weight loss increases 11beta-hydroxysteroid dehydrogenase type 1 expression in human adipose tissue. J. Clin. Endocrinol. Metab. 2004;89:2711–2716. doi: 10.1210/jc.2003-031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seimon R.V., Hostland N., Silveira S.L., Gibson A.A., Sainsbury A. Effects of energy restriction on activity of the hypothalamo-pituitary-adrenal axis in obese humans and rodents: Implications for diet-induced changes in body composition. Horm. Mol. Biol. Clin. Investig. 2013;15:71–80. doi: 10.1515/hmbci-2013-0038. [DOI] [PubMed] [Google Scholar]
- 64.Leliavski A., Dumbell R., Ott V., Oster H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J. Biol. Rhythms. 2015;30:20–34. doi: 10.1177/0748730414553971. [DOI] [PubMed] [Google Scholar]
- 65.Walker J.J., Spiga F., Waite E., Zhao Z., Kershaw Y., Terry J.R., Lightman S.L. The origin of glucocorticoid hormone oscillations. PLoS Biol. 2012;10:e1001341. doi: 10.1371/journal.pbio.1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dickmeis T., Weger B.D., Weger M. The circadian clock and glucocorticoids— interactions across many time scales. Mol. Cell Endocrinol. 2013;380:2–15. doi: 10.1016/j.mce.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Thomson S.P., McMahon L.J., Nugent C.A. Endogenous cortisol: A regulator of the number of lymphocytes in peripheral blood. Clin. Immunol. Immunopathol. 1980;17:506–514. doi: 10.1016/0090-1229(80)90146-4. [DOI] [PubMed] [Google Scholar]
- 68.Papazyan R., Zhang Y., Lazar M.A. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat. Struct. Mol. Biol. 2016;23:1045–1052. doi: 10.1038/nsmb.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato T., Sassone-Corsi P. Nutrition, metabolism, and epigenetics: Pathways of circadian reprogramming. EMBO Rep. 2022;23:e52412. doi: 10.15252/embr.202152412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in the present study are available from the corresponding author upon reasonable request.