Abstract
The role of the interactions between endophytes and host plants is unclear in invasive plants from different geographical latitudes. In this study, we aimed to explore the relationship between endophytic microbes and the functional traits of the invasive plant Wedelia trilobata. We explored the relationship between endophytes and the clonal growth traits of the invasive clonal plant Wedelia trilobata from different geographical latitudes using high-throughput sequencing technology and a common garden-planting experiment. We found that: (1) Different W. trilobata populations had similar endophytic fungi but different endophytic bacteria. However, no latitudinal variation pattern of the overall microbial community was found; (2) plant clonal growth performance (i.e., spacer length) was significantly correlated with endophytic bacterial diversity but not fungal diversity; and (3) the latitudinal variation pattern of the plant clonal growth performance of W. trilobata populations was found in pre-cultivated (i.e., wild) individuals but disappeared in post-cultivated W. trilobata. Our results suggest both environmental adaptability and the endophytic bacterial community are linked to the functional traits of the invasive clonal plant W. trilobata, and these functional traits tend to increase its invasiveness, which may enhance its invasion success.
Keywords: environmental adaptation, endophytic diversity, functional traits, invasive plant, Wedelia trilobata
1. Introduction
Microorganisms and plants in the natural environment are connected in various ways, resulting in a variety of plant–microbe symbiotic interactions. Plant endophytes are generally a group of microorganisms that exist as symbionts in plants, mainly including endophytic bacteria and endophytic fungi, and these microorganisms provide profound benefits for plants through their interactions with the plants [1,2,3]. For example, endophytic bacteria promote plant growth through conducting nitrogen fixation [4], regulating the synthesis of phytohormones [5], and improving the host plant’s resistance to abiotic (e.g., cold, drought, saline-alkali) [6,7] and biotic (e.g., plant diseases) [8] environmental stresses. Invasion of alien plant species, as a consequence of global changes, usually causes significant losses of biodiversity and economy in the invaded regions [9,10,11,12]. Several studies in recent years have connected endophyte traits to invasive plant behavior. Studies [13,14] have proved that endophytes can indeed enhance the ability of invasive plants directly or indirectly. For example, Spartina alterniflora not only directly competes with native plants, it also indirectly causes pathogen infection on native plants via endophytic fungi [15]. In addition, endophytic bacteria can promote the production of phytohormones to help plant growth and indirectly influence the functional features of the plant host [16]. The functional traits of invasive clonal plants play an important role in their invasion process, which often makes them more competitive than native plants [17]. Thus, interactions between invasive plants and endophytes have an impact on the ecology, distribution, and variety of flora and wildlife. Over the past ten years, interest has grown in how important these interactions are to ecosystem function by encouraging nutrient intake and changing the defense mechanisms of plants [18].
Despite the fact that diverse geographical regions are connected with endophytes of host plants [18,19,20], the role of endophytes on plant invasion is still unclear, as cultivable endophytes or endophytic communities of invasive plants were explored separately in previous studies. For instance, Fang et al. [21] showed that the community of cultivable endophytic fungi based on tissues within the invasive plant Ageratina adenophora changed across geographic areas. Cheng et al. [22] discovered numerous invasive plant populations. A basic group of endophytic bacteria was present in Senecio vulgaris. These studies concentrated on endophyte variations without demonstrating the impact of functional variations on plant invasion success. Therefore, it is crucial to understand the link between invasive plants and endophytes because doing so could lead to the development of novel biological methods for controlling invasive plants.
The invasive clonal plant Wedelia trilobata (L.) Hitchc., one of the 100 worst invasive alien species in the world [23], is a creeping herb native to tropical Central America and has invaded many areas of the tropics and subtropics. Clonal propagation, with extremely strong phenotypic plasticity and adaptability, is the main mode of reproduction of W. trilobata [24]. At present, W. trilobata has been further expanding from South China to Central China. We also found that W. trilobata successfully expanded northward to the central and northern parts of East China, such as Wenzhou and Taizhou Cities, Zhejiang Province. Current research on W. trilobata focuses on resource utilization [25,26,27], allelopathy [28,29,30], clonal propagation strategy analysis [24,31], and environmental factor stress response [32,33,34]. Considerable beneficial effects of tissue-cultivable endophytic bacteria on the clonal growth of W. trilobata have been confirmed [2], yet few studies investigate the community composition of the endophytic microorganism community of W. trilobata from different populations and its role on the growth performance of this invasive plant. Therefore, exploring the endophytic microbial composition and its association with the growth phenotypes of different populations of W. trilobata in the invasion areas is of great ecological significance. The results will provide better understanding in prediction of further invasion and expansion of alien plants.
We investigated the functional characteristics of the corresponding population of W. trilobata under a homogeneous growth environment, as well as the endophytic microbial community of four distinct populations of W. trilobata from four different provinces in China. We tested the following hypotheses: (1) a latitudinal gradient of the four populations of W. trilobata is associated with the pattern of or the difference in endophytic microbial diversity and communities, and (2) the functional traits of W. trilobata are related to the geographic pattern of endophytic microbial diversity and communities.
2. Results
2.1. Endophytic Microbial Diversity and Composition
The rarefaction curve (Figure S1) indicated sufficient sequencing depth. Sequencing of microbial communities generated a total of 0.93 million bacterial sequences (average of 77,590 sequences per sample) and 1.38 million fungal sequences (average of 114,902 sequences per sample). More detailed results are shown in the Supplementary Materials Table S1. These sequences were clustered into 436 and 1987 distinct operational taxonomic units (OTUs) for bacteria and fungi, respectively. In addition, the number of core flora of endophytic bacteria and fungi differed significantly (Figure S3).
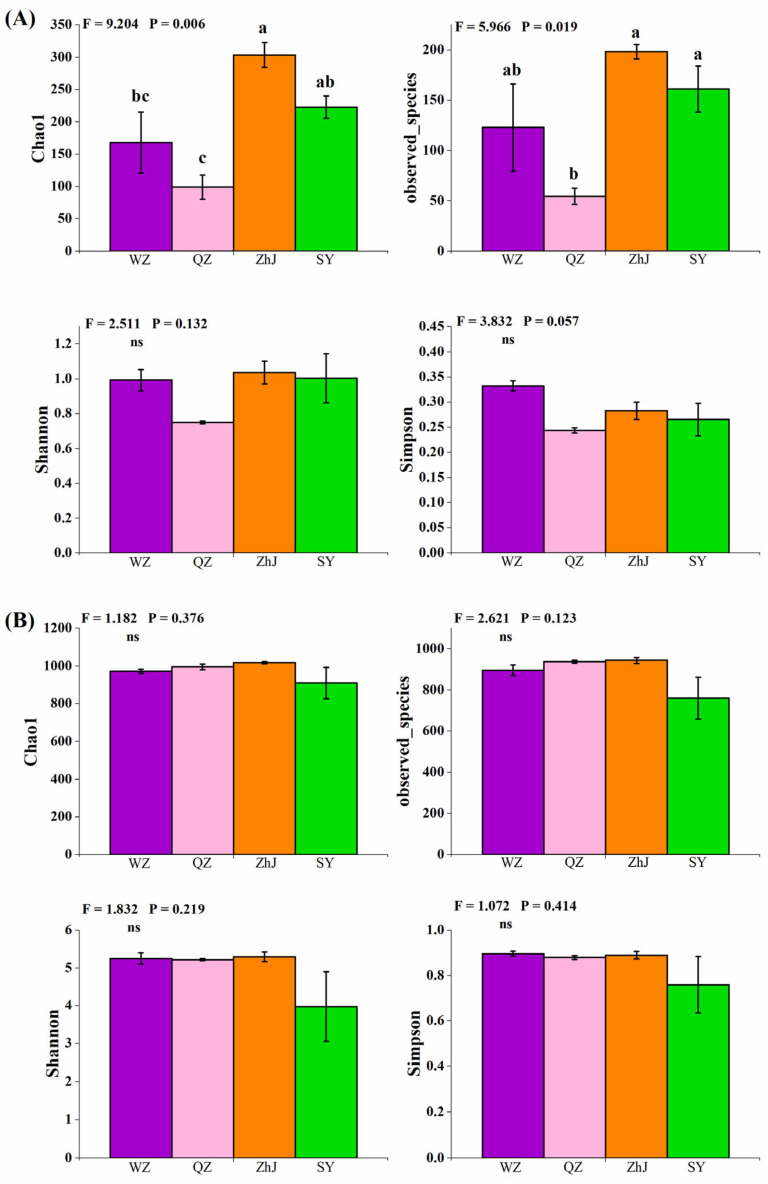
We found that the alpha diversities of endophytic bacterial communities of W. trilobata from different populations were significantly different (Figure 1A). The Chao1 index and the observed species of W. trilobata from the QZ population were significantly lower than those of W. trilobata from other places, while the alpha diversities of W. trilobata from ZhJ and SY were similar (Figure 1A). However, the alpha diversities of soil fungal communities did not change significantly among W. trilobata from different geographic regions (Figure 1B).
Figure 1.
Differences in the α diversities of endophytic bacterial (A) and fungal (B) communities of W. trilobata from different populations (WZ, Wenzhou City; QZ, Quanzhou City; ZhJ, Zhanjiang City; SY, Sanya City). Bars (mean with standard error, n = 7) with different lowercase letters represent statistically significant differences (p < 0.05). “ns” means no statistically significant difference (p > 0.05).
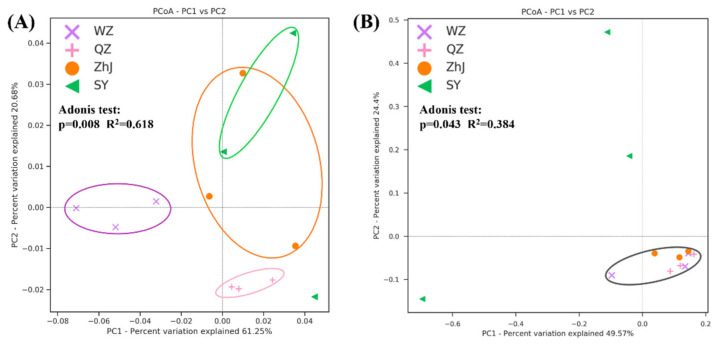
Beta-diversity analysis showed that the bacterial community compositions of W. trilobata from different populations were significantly different (Figure 2A). However, there was little difference in the composition of the fungal community except W. trilobata from the SY population (Figure 2B). The community compositions of W. trilobata in endophytic bacterial communities from QZ and WZ differed distinctly from the W. trilobata from ZhJ and SY, according to the Bray–Curtis distances (Figure 2A). W. trilobata from the ZhJ and SY populations had similar community structures for bacteria and fungi. In addition, the β diversity of W. trilobata in endophytic bacterial communities from QZ differed significantly from that of W. trilobata in endophytic bacterial communities from the most northern population of WZ (Figure 2A).
Figure 2.
PCoA plots of endophytic bacterial (A) and fungal (B) communities of W. trilobata from different places based on the Bray–Curtis distance (WZ, Wenzhou City; QZ, Quanzhou City; ZhJ, Zhanjiang City; SY, Sanya City).
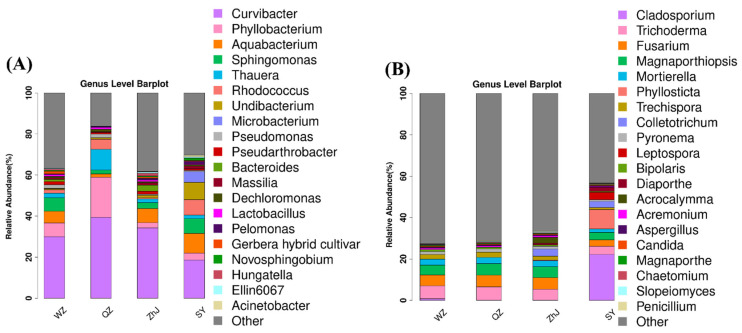
Although the compositions of core endophytic bacteria of W. trilobata were similar at the phylum level (Figure S4), the relative abundances of endophytic bacteria in the stems of W. trilobata varied greatly among different populations at the genus level. Plant samples from different populations were found to be dominated by bacteria from the genera Curvibacter, Phyllobacterium, Aquabacterium, and Sphingomonas (Figure 3A). Compared with W. trilobata from other populations, the relative abundances of Curvibacter, Phyllobacterium, and Thauera in the QZ population were significantly greater than that of other populations, while the relative abundances of Sphingomonas and Aquabacterium were significantly lower than that of other populations (Figure 3A). Similarly, Circos analysis at the phylum level showed differences in the endophytic core flora of W. trilobata (Figure S5).
Figure 3.
The relative abundances of endophytic bacterial (A) and fungal (B) communities at the genus level (WZ, Wenzhou City; QZ, Quanzhou City; ZhJ, Zhanjiang City; SY, Sanya City); ‘Other’ indicates OTUs that have not been annotated.
The relative abundance of endophytic fungi in the stems of W. trilobata from the southernmost population SY was significantly different from that of other populations. However, there were no significant differences in the relative abundances of fungi in other populations of W. trilobata (Figure 3B). Different from the observed bacterial community, there were no significant differences in the fungal community structure. The most abundant fungi found in W. trilobata were Trichoderma, Fusarium, Magnaporthiopsis, and Mortierella (Figure 3B).
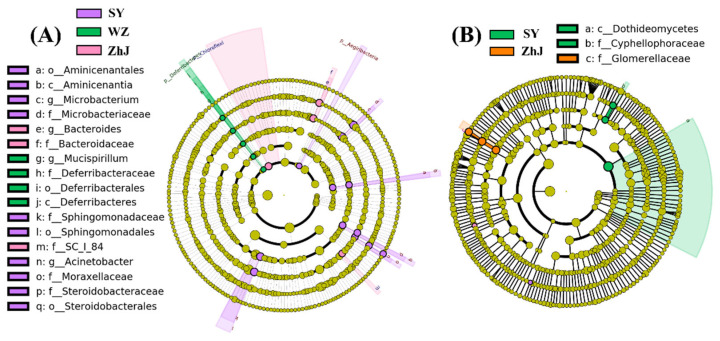
LEfSe analyses were implemented to assess whether any statistically significant differences occurred in the taxon abundance of endophytic microorganisms and to check the biological relevance of the species in different populations of W. trilobata (Figure 4). Specifically, Deferribacterales and Mucispirillum were the main biomarkers in endophytic bacteria of WZ; Bacteroides, Bacteroidaceae, Chloroflexi, and SC_I_84 were the main biomarkers in endophytic bacteria of ZhJ; and Microbacterium, Sphingomonadales, Aegiribacteria, Steroidobacteraceae, Moraxellaceae, Acinetobacterjohnsonii, Acinetobacter, and Aminicenantales were the main biomarkers in endophytic bacteria of SY (Figure 4A). In addition, Microascus and Agrocybe were the only biomarkers in WZ and QZ endophytic fungi, respectively; Glomerellaceae, Colletotrichum, and Colletotrichum_brevisporum were the main biomarkers in endophytic fungi of ZhJ; Dothideomycetes and Cyphellophoraceae were the main the biomarkers in endophytic fungi of SY (Figure 4B).
Figure 4.
The LEfSe analysis of endophytic bacteria (A) and fungi (B) in W. trilobata. (No biomarkers were found in the bacterial community of QZ.)
2.2. Plant Phenotypic Growth
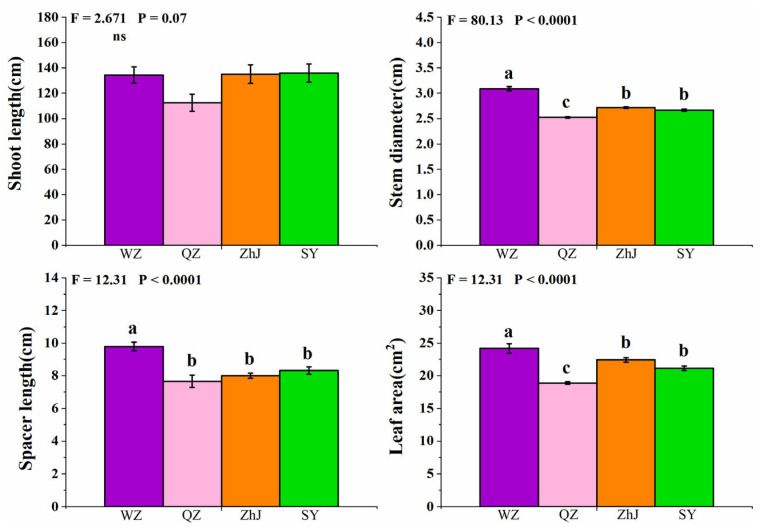
We counted the plant phenotypic growth in the common garden. Stem diameter, spacer length, and leaf area of the northernmost population WZ were significantly higher than those of other populations, and the stem diameter and leaf area index of the QZ population were significantly lower than other populations. However, there were no significant differences between the ZhJ and SY populations in any index (Figure 5).
Figure 5.
Shoot length, stem diameter, spacer length, and leaf area of different populations of W. trilobata (WZ, Wenzhou City; QZ, Quanzhou City; ZhJ, Zhanjiang City; SY, Sanya City). Bars (mean with standard error, n = 7) with different lower case letters represent statistically significant differences (p < 0.05).
2.3. Correlation Patterns between Microbial Diversity and Growth Indices of W. trilobata
Pearson correlation showed that the stem diameter and spacer length of W. trilobata in the pre- and post-cultivated environments were significantly positively correlated with the Simpson diversity index of endophytic bacteria (Table 1; p < 0.05). There was a correlation between the growth indicators of pre-cultured W. trilobata and its geographical location (Table 1; p < 0.05); however, the correlation was not found after cultivating it in the common environment.
Table 1.
Relationships among plant growth performances, geographical latitudes, and diversities of endophytic microbial communities. p-values equal to or lower than 0.05 are in bold print.
| Plant Growth Performance | Geographical Latitude |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Cultivate | Post-Cultivate | |||||||||||
| Shoot Length |
Stem Diameter | Spacer Length | Stem Number | Shoot Length |
Stem Diameter | Spacer Length | Stem Number |
Leaf Area | ||||
| Bacterial | Chao1 index | r | −0.151 | 0.1418 | −0.195 | −0.0411 | 0.3882 | 0.1187 | 0.3078 | −0.012 | 0.372 | −0.512 |
| p | 0.6384 | 0.6602 | 0.5432 | 0.8990 | 0.2124 | 0.7133 | 0.3304 | 0.97 | 0.234 | 0.089 | ||
| Observed species | r | −0.048 | 0.2336 | −0.064 | −0.0417 | 0.3847 | 0.2136 | 0.4645 | −0.202 | 0.417 | −0.478 | |
| p | 0.8823 | 0.4649 | 0.8438 | 0.8975 | 0.2169 | 0.5051 | 0.1282 | 0.53 | 0.178 | 0.116 | ||
| Shannon diversity | r | 0.0521 | 0.4535 | 0.2475 | −0.1538 | 0.336 | 0.3786 | 0.4867 | −0.282 | 0.446 | −0.237 | |
| p | 0.8723 | 0.1387 | 0.4381 | 0.6331 | 0.2856 | 0.2249 | 0.1086 | 0.374 | 0.147 | 0.458 | ||
| Simpson diversity | r | −0.185 | 0.776 | 0.702 | −0.542 | 0.3396 | 0.713 | 0.596 | −0.407 | 0.636 | 0.406 | |
| p | 0.565 | 0.003 | 0.011 | 0.0687 | 0.2801 | 0.0093 | 0.041 | 0.19 | 0.026 | 0.191 | ||
| Fungal | Chao1 index | r | −0.304 | −0.0951 | −0.139 | −0.0851 | −0.053 | −0.0198 | 0.0321 | −0.081 | 0.0689 | 0.248 |
| p | 0.3371 | 0.7688 | 0.6664 | 0.7926 | 0.8704 | 0.9512 | 0.9211 | 0.803 | 0.8314 | 0.436 | ||
| Observed species | r | −0.495 | −0.0517 | −0.101 | −0.2352 | −0.186 | 0.0044 | 0.0069 | −0.143 | 0.0714 | 0.418 | |
| p | 0.1018 | 0.8732 | 0.7551 | 0.4618 | 0.5625 | 0.9891 | 0.983 | 0.657 | 0.8255 | 0.176 | ||
| Shannon diversity | r | −0.452 | 0.0807 | 0.0735 | −0.3209 | −0.026 | 0.1604 | 0.1581 | −0.187 | 0.1718 | 0.472 | |
| p | 0.1399 | 0.8031 | 0.8204 | 0.3091 | 0.9355 | 0.6186 | 0.6236 | 0.561 | 0.5934 | 0.121 | ||
| Simpson diversity | r | −0.369 | 0.0875 | 0.1297 | −0.3018 | 0.0319 | 0.173 | 0.2058 | −0.21 | 0.1606 | 0.420 | |
| p | 0.2385 | 0.7868 | 0.6879 | 0.3404 | 0.9215 | 0.5908 | 0.5211 | 0.511 | 0.6181 | 0.174 | ||
| Geographical Latitude | r | r | 0.536 | 0.64 | −0.737 | −0.203 | 0.523 | 0.328 | −0.51 | 0.285 | - | |
| p | p | 0.73 | 0.025 | 0.006 | 0.528 | 0.081 | 0.299 | 0.091 | 0.368 | - | ||
3. Discussion
The results of this study found that: (1) the endophytic bacterial biodiversity differed significantly among W. trilobata populations, but the fungal biodiversity did not. However, no latitudinal variation pattern of the overall microbial community was found. (2) Plant clonal growth performance was significantly correlated with endophytic bacterial diversity but not fungal diversity. (3) The latitudinal variation patterns of plant clonal growth performances of W. trilobata populations were found in pre-cultivated (i.e., wild) individuals but disappeared in post-cultivated W. trilobata. In summary, our results suggested that both environmental adaptability and the endophytic bacterial, but not fungal, community could affect functional traits in invasive clonal W. trilobata.
3.1. Geographical Changes of Endophytic Microbe and Clonal Growth Performances in Different Populations of W. trilobata
In general, the sorts of endophytic bacteria that a plant tissue hosts can be determined by the genotype of the host plant, by geographic location, and even by specific plant parts [35]. For example, different tissue parts and geographical locations of the invasive Ageratina adenophora affected the composition of the endophytic fungal community [21]. In addition, the endophytic bacteria in Senecio vulgaris among different geographical locations were also significantly different [22]. Similarly, we also found that there were significant differences in endophytic bacteria of W. trilobata among different geographical locations, although no significant differences were found in the compositions of endophytic fungi (Figure 1A,B). It can be said that the differences in the composition of endophytic bacteria caused by geographical differences among different populations of W. trilobata are obvious.
Geographical location has a far-reaching impact on the growth of plants, not only on the phenotypic growth of plants [36] but also on the composition of plant microbial communities [37]. Numerous studies have demonstrated that the diversity of endogenous microorganisms is influenced by the environmental variations brought on by geographic variances [38,39]. However, the results of this study’s correlation analysis revealed that there were no meaningful associations between variations in W. trilobata populations’ microbial diversities and latitudes (Table 1). In a prior investigation, we discovered that W. trilobata exhibited a high level of local adaptation to boost the success of its invasion [24]. In this study, the correlation between the growth indicators of pre-cultured, not post-cultured, W. trilobata and its geographical location (Table 1) may therefore represent adaptive differences among populations, which could contribute to the invasion success of W. trilobata.
3.2. The Endophytic Microbial Composition May Contribute to the Clonal Phenotypic Growth of Invasive W. trilobata
Generally, clonal characteristics can be divided into clonal integration and clonal growth. Clonal growth is an asexual reproduction method used by plants that produces genetically identical but possibly independent ramets. This method allows a species to procreate and avoid inbreeding depression, even in the presence of modest initial population levels [40]. It also offers competitive advantages, such as the ability to nurse new ramets, opportunities of scale and division of labor through resource sharing between ramets, and pre-emption of resources through spatial occupation, as well as avoidance of the costs and risks involved in sexual reproduction [41]. For instance, stolons and rhizome internodes of clonal plants can function as places to store resources, such as glucose, which represents a poor method to deal with stressful situations and boosts the survival and re-growth ability of clonal plants, especially following disturbances. Clonal plants are able to withstand harsh conditions and successfully occupy new habitats or endure disruptions thanks to the mobilization of carbohydrates stored in clonal organs such as stolons or rhizomes [42,43,44,45].
The higher performance of growth, competitiveness, and stress resistance is critical for alien plants’ invasion success in the early colonization stage [46]. The clonal growth traits can help plants adapt to new environments more quickly and enable alien clonal plants to colonize and compete successfully in a wide range of habitats [47] by mediating environmental stresses and sharing resources between ramets (i.e., stolon or rhizome structures) [48]. As a successful creeping clonal plant invader [26,34], W. trilobata may be subject to environmental stresses in the new areas during the colonization stage of its invasion. In the homogeneous garden environment, stem diameters, spacer lengths, and leaf areas of the WZ population were all at the highest level (Figure 5). Stem diameter can reflect the resource storage capacity of cloned plants [47], so thicker stems mean more resource storage in the plant stems; invasive plants with slender stems may have weak viability in the new environment. Spacer length of cloned plants can often reflect the space occupation ability of invasive plants in a new habitat [45] and predict the spatial structure pattern of their growth [49]. Several studies have shown that some plants have successfully invaded certain environments due to higher leaf area [50,51], which can often enable invasive species to obtain more ground resources and grow rapidly [31]. Considering that the climates of the three other populations outside Wenzhou are warm, and cold weather is the main limiting factor for the expansion of W. trilobata, higher levels of stem diameters, spacer lengths, and leaf areas of the WZ population area therefore more conducive to the expansion of invasive clonal W. trilobata in a new cold habitat. Our findings suggest that the clonal phenotypic growth difference in W. trilobata may be due to different climatic and environmental conditions. Specially, the colder climate of the WZ population may shape the different clonal phenotypic growth for W. trilobata.
Endophytic bacteria have been shown to provide several beneficial effects on their plant host directly or indirectly. They can benefit plants directly to assist plants in getting nutrients and to improve plant growth by modulating growth-related hormones, which can help plants grow better under normal and stressed conditions [52]. By deterring phytopathogens through the synthesis of antibiotics and lytic enzymes, making nutrients unavailable to the pathogens and priming plant defense mechanisms, endophytic bacteria indirectly promote plant growth while defending the plants from future pathogen attacks [53]. In this study, the endophytic bacterial diversity of different populations of W. trilobata was significantly and positively correlated with the clonal phenotypic growth of W. trilobata (Table 1). The lack of endophytic bacterial diversity in plant tissues may lead to the weakening of phenotypic growth [16]. On the contrary, diverse endophytic bacteria enable W. trilobata to gain advantages in growth phenotypes, such as spacer length [2], which make its invasive expansion more efficient. Thus, we speculate that the low bacterial diversity in the tissue may lead to poor phenotypic growth of W. trilobata from the QZ population (Figure 1).
Endophytic bacteria can also enhance nutrient accumulation and metabolism of host plants by producing growth-regulating phytohormones [54,55]. In addition to the lack of diversity, the abundances of some important endophytic bacteria of W. trilobata from QZ, such as Sphingomonas and Pseudarthrobacter, was significantly lower than those of the WZ population, which was shown to play an active role in plant growth (Figure 3) [56,57]. In addition, we found that the abundances of most of the endophytic bacteria with a positive role in plant growth in the WZ population were at high levels, such as Sphingomonas, Pseudarthrobacter, and Novosphingobium [58]. Therefore, we believe that the difference in endophytic bacterial community abundance also had a potential impact on the phenotypic growth of W. trilobata, although the specific mechanism of beneficial endophytes remains unclear.
In short, in addition to environmental adaptability, it is endophytic bacteria instead of endophytic fungi that mainly also affect the phenotypic growth of W. trilobata among different populations. However, future studies need to be further improved as follows: (1) long-term monitoring experiment combining environmental data on the different geographic environments associated with plant growth; (2) pure endophytes should be isolated from different plant tissue parts to explore the bioactivity of endophytes on plant invasion.
4. Materials and Methods
4.1. Plant Materials and Pretreatment
To assess whether there is a geographic pattern in endophytic microbial diversity and communities and whether these differences are associated with the growths of different populations of W. trilobata, stems of W. trilobata from public wastelands of four cities from north to south in China were collected: (1) Wenzhou City (WZ: 27.919617N, 120.698494E), Zhejiang Province; (2) Quanzhou City (QZ: 24.901539N, 118.616325E), Fujian Province; (3) Zhanjiang City (ZhJ: 21.270611N, 110.351982E), Guangdong Province; and (4) Sanya City (SY: 18.28169N, 109.508509E), Hainan Province. We collected complete individual plants of W. trilobata from the above cities and recorded the initial growth indicators (i.e., shoot length, stem diameter, spacer length, and stem number; more details can be found in the Supplementary Materials Figure S2), and then sent them to the lab for further cultivation. Stems with similar lengths and thicknesses were selected for the following common garden experiment for phenotypic growth analysis [29] and surface disinfection [59] for endophytic microbial community analysis using high-throughput sequencing of microbial amplicons.
4.2. Common Garden Experiment
To assess whether the geographic difference in endophytic microbial diversity and communities contribute to the growth of different populations of W. trilobata, a common garden planting experiment was conducted in a greenhouse at Jiangsu University. In the experiment, stem segments from these four populations of W. trilobata with similar lengths and thicknesses were selected; each stem segment retained two stem nodes. The stem segments were placed in plastic flowerpots of 90 mm × 60 mm × 80 mm, with one stem segment per pot, seven independent pots for each population of W. trilobata, resulting in a total of 28 pots. A mixture of commercial nutrient soil (commercial nutrient soil consisted of different organic matter, with a pH of about 6.5) and vermiculite (5:1 in volume ratio) of 400 g was put in each flowerpot. All the flowerpots were placed in the greenhouse (25 °C/20 °C, day/night average) at Jiangsu University and watered with 100 mL of pure water every day at noon. After 12 weeks of cultivation, all the plants were harvested to measure plant growth indicators (i.e., shoot length, stem diameter, spacer length, and leaf area).
4.3. Microbial Community Sequencing and Analysis
First, three stem segments with similar lengths and thicknesses from each W. trilobata population as plant tissue samples were selected. Next, in order to retain the endophytes in the plant tissue, the stems of W. trilobata from the field were surface-disinfected with 5% sodium hypochlorite one time and rinsed with sterile distilled water three times. The plant tissue samples (three replicates per W. trilobata population) were stored on dry ice and then sent to Genepioneer Biotechnologies Co., Ltd. (Nanjing, China) for DNA quantification and qualification. Library construction of endophytic bacteria and fungi and amplicon sequencing were used on the Illumina Novaseq 6000 platform to obtain paired-end (PE) reads. Amplification of the V3–V4 region of the bacterial 16S rRNA genes was completed using the universal primers (341F: 5′-CCTACGGGNGGCWGCAG-3′; 806R: 5′-GGACTACHVGGGTWTCTAAT-3′). The ITS2 region of the fungal rRNA genes was amplified with ITS1 and ITS2 primer (ITS1F: 5′-CTTGGTCATTTAGAGGAAGTAA-3′; ITS2R: 5′-TCCTCCGCTTATTGATATGC-3′). After adding adapters, we used the Illumina Novaseq6000 platform and performed sequencing to obtain 2 × 250 bp paired-end data. Through splicing, a longer sequence was obtained for subsequent analysis.
To make the results of information analysis more accurate and reliable, it was necessary to eliminate the interfering data. First, the original data (deposited in CNGBdb) were spliced and filtered to obtain valid data. Then, OTUs (operational taxonomic units) clustering and species classification analysis were performed based on valid data. According to the OTUs clustering results, species annotations were made for each OTU sequence to obtain corresponding species information and species-based abundance distribution. By using OTU-based abundance and annotation information, we counted the proportion of the sequence number of each sample at each classification level (phylum, class, order, family, genus) to the total sequence number, which can effectively evaluate the species annotation of the sample resolution (the higher the proportion of annotated to genus, the better the OTU annotation effect of the sample), and the species complexity of the sample. (The lower the proportion annotated to the genus, the higher the species complexity of the sample.) Clean reads with the same sequence were first grouped into a single tag, and the abundance (i.e., the number of reads) corresponding to each tag was counted; then, the tags were sorted according to abundance, and the sequences of singletons (corresponding to only one read) were filtered out, which were removed because singletons may be caused by sequencing errors. The clustered sequences were filtered out using a search at 0.97 similarity, and the species were classified in the chimeric OTU; then, the singleton sequences were compared with the representative OTU sequences with a similarity of 0.97. The unaligned sequence would not enter the subsequent analysis, and the sequence on the alignment would be used as one of the OTUs read for follow-up analysis. At the same time, the abundance and alpha diversity calculations of OTUs were analyzed to obtain the species richness and uniformity information in the sample and the common and unique OTUs information among different samples or groups. On the other hand, multiple sequence alignments of OTUs were performed, and a phylogenetic tree was constructed. More details can be found in the Supplementary Materials (Table S1).
4.4. Statistical Analyses
The alpha diversity of the endophytic microorganism was weighed using the succeeding indexes, i.e., Chao1 index, observed species, Shannon diversity, and Simpson dominance. Further, the correlations among the beta diversity estimates of the endophytic microorganism were estimated using the Bray–Curtis algorithm via principal coordinates analysis (PCoA). Through PCoA, dimensionality reduction analysis, and display, the differences in the community structure between different samples or groups were explored. The significance of microbial community variation between treatments was assessed using the Adonis test at α level = 0.05.
Endophytic microbial features in four population were categorized using the linear discriminant analysis (LDA) effect size (LEfSe) method for biomarker discovery, which emphasized the statistical significance and biological relevance. With the normalized relative abundance matrix, the LEfSe method used the Kruskal–Wallis rank-sum test to unearth the features with significantly different abundances between the assigned taxa and performed LDA to estimate the effect size of each feature. A significance level of 0.05 and an effect size threshold of 3 were used for all the biomarkers evaluated in this study.
Differences in the phenotypic growth indices, as well as alpha diversity of endophytic microbial communities, were examined by ANOVA, followed by multiple comparisons via Duncan’s test. The correlation analysis (using the Pearson methods) was performed to analyze the relationships between each phenotypic growth index and alpha diversity of endophytic microbial communities. Statistical analysis processing was completed using IBM SPSS Statistics (version 26.0; IBM Corp., Armonk, NY, USA). The significance level was set at p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11233369/s1, Figure S1: Rarefaction curve of endophytic bacteria (A) and fungi (B) in four populations of W. trilobata; Figure S2: Phenotypic growth indexes of four populations of W. trilobata collected from different regions; Figure S3: Petal pattern analysis of endophytic bacteria (A) and fungi (B) in four populations of W. trilobata; Figure S4: The relative abundances of endophytic bacterial (A) and fungal (B) communities of four populations of W. trilobata at the phylum level; Figure S5: Circos analysis of endophytic bacteria (A) and fungi (B) in four populations of W. trilobata at phylum level; Table S1: Sequencing evaluation of bacterial and fungal communities in four populations of W. trilobata.
Author Contributions
Conceptualization, S.-S.Q. and Z.-C.D.; methodology, X.L. and J.-Y.Z.; formal analysis, F.-L.K.; data curation, Y.-H.M.; writing—original draft preparation, Y.-H.M.; writing—review and editing, B.Z., M.N., D.-L.D. and Z.-C.D.; visualization, Y.-H.M.; supervision, S.-S.Q. and Z.-C.D.; project administration, S.-S.Q. and Z.-C.D.; funding acquisition, S.-S.Q., Z.-C.D. and D.-L.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The microbial sequencing data that supported the findings of this study have been deposited into the CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb, https://www.cngb.org/, accessed on 5 September 2022) with accession number CNP0003006. The data presented in this study are available upon request from the corresponding author (e-mail: daizhicong@163.com).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This study was funded by the National Natural Science Foundation of China (32271587, 32171509, 32071521), the Natural Science Foundation of Jiangsu (BK20211321), the Carbon Peak and Carbon Neutrality Technology Innovation Foundation of Jiangsu Province (BK20220030), and the Jiangsu Funding Program for Excellent Postdoctoral Talent (2022ZB658). Part of the funding for this research was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Special Scientific Research Project of the School of Emergency Management, Jiangsu University.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hardoim P.R., van Overbeek L.S., van Elsas J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008;16:463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Dai Z.C., Fu W., Wan L.Y., Cai H.H., Wang N., Qi S.S., Du D.L. Different Growth Promoting Effects of En-dophytic Bacteria on Invasive and Native Clonal Plants. Front. Plant Sci. 2016;7:706. doi: 10.3389/fpls.2016.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian B., Zhang C., Ye Y., Wen J., Wu Y., Wang H., Li H., Cai S., Cai W., Cheng Z., et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017;247:149–156. doi: 10.1016/j.agee.2017.06.041. [DOI] [Google Scholar]
- 4.Verma S.C., Ladha J., Tripathi A.K. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 2001;91:127–141. doi: 10.1016/S0168-1656(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 5.Egamberdieva D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009;31:861–864. doi: 10.1007/s11738-009-0297-0. [DOI] [Google Scholar]
- 6.Ait Barka E., Nowak J., Clément C. Enhancement of Chilling Resistance of Inoculated Grapevine Plantlets with a Plant Growth-Promoting Rhizobacterium, Burkholderia phytofirmans Strain PsJN. Appl. Environ. Microbiol. 2006;72:7246–7252. doi: 10.1128/AEM.01047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding S., Huang C.-L., Sheng H.-M., Song C.-L., Li Y.-B., An L.-Z. Effect of inoculation with the endophyte Clavibacter sp. strain Enf12 on chilling tolerance in Chorispora bungeana. Physiol. Plant. 2011;141:141–151. doi: 10.1111/j.1399-3054.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 9.Pyšek P., Richardson D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010;35:25–55. doi: 10.1146/annurev-environ-033009-095548. [DOI] [Google Scholar]
- 10.Bellard C., Cassey P., Blackburn T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016;12:20150623. doi: 10.1098/rsbl.2015.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paini D.R., Sheppard A.W., Cook D.C., De Barro P.J., Worner S.P., Thomas M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA. 2016;113:7575–7579. doi: 10.1073/pnas.1602205113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews J., van derVelde G., Collas F.P.L., de Hoop L., Koopman K.R., Hendriks A.J., Leuven R.S.E.W. Inconsistencies in the risk classification of alien species and implications for risk assessment in the European Union. Ecosphere. 2017;8:e01832. doi: 10.1002/ecs2.1832. [DOI] [Google Scholar]
- 13.Aschehoug E.T., Metlen K.L., Callaway R.M., Newcombe G. Fungal endophytes directly increase the competitive effects of an invasive forb. Ecology. 2012;93:3–8. doi: 10.1890/11-1347.1. [DOI] [PubMed] [Google Scholar]
- 14.Rout M.E., Chrzanowski T.H., Westlie T.K., DeLuca T.H., Callaway R.M., Holben W.E. Bacterial endophytes enhance competition by invasive plants. Am. J. Bot. 2013;100:1726–1737. doi: 10.3732/ajb.1200577. [DOI] [PubMed] [Google Scholar]
- 15.Li H., Zhang X., Zheng R., Li X., Elmer W.H., Wolfe L.M., Li B. Indirect effects of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native saltmarsh plants in China. J. Ecol. 2014;102:1112–1119. doi: 10.1111/1365-2745.12285. [DOI] [Google Scholar]
- 16.Afzal I., Shinwari Z.K., Sikandar S., Shahzad S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Liu J.H., Yong X.H., Han Q., Ali A., Wang Y.J. Response of plant functional traits to species origin and adaptive reproduction in weeds. Plant Biosyst. 2017;151:323–330. doi: 10.1080/11263504.2016.1174171. [DOI] [Google Scholar]
- 18.Naik B.S. Functional roles of fungal endophytes in host fitness during stress conditions. Symbiosis. 2019;79:99–115. doi: 10.1007/s13199-019-00635-1. [DOI] [Google Scholar]
- 19.Mei L., Zhu M., Zhang D.-Z., Wang Y.-Z., Guo J., Zhang H.-B. Geographical and Temporal Changes of Foliar Fungal Endophytes Associated with the Invasive Plant Ageratina adenophora. Microb. Ecol. 2014;67:402–409. doi: 10.1007/s00248-013-0319-8. [DOI] [PubMed] [Google Scholar]
- 20.Koyama A., Maherali H., Antunes P.M. Plant geographic origin and phylogeny as potential drivers of community structure in root-inhabiting fungi. J. Ecol. 2019;107:1720–1736. doi: 10.1111/1365-2745.13143. [DOI] [Google Scholar]
- 21.Fang K., Miao Y.-F., Chen L., Zhou J., Yang Z.-P., Dong X.-F., Zhang H.-B. Tissue-Specific and Geographical Variation in Endophytic Fungi of Ageratina adenophora and Fungal Associations with the Environment. Front. Microbiol. 2019;10:2919. doi: 10.3389/fmicb.2019.02919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng D.D., Tian Z.S., Feng L., Xu L., Wang H.M. Diversity analysis of the rhizospheric and endophytic bacterial communities of Senecio vulgaris L. (Asteraceae) in an invasive range. PeerJ. 2019;6:e6162. doi: 10.7717/peerj.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe S.J., Browne M., Boudjelas S. 100 of The World’s Worst Invasive Alien Species. A selection from the global invasive species database. Invasive Species Specialist Group; Auckland, New Zealand: 2000. [Google Scholar]
- 24.Si C.-C., Dai Z.-C., Lin Y., Qi S.-S., Huang P., Miao S.-L., Du D.-L. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island. Biol. Invasions. 2014;16:2323–2337. doi: 10.1007/s10530-014-0667-4. [DOI] [Google Scholar]
- 25.Chen S., Jin W., Liu A., Zhang S., Liu D., Wang F., Lin X., He C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013;160:222–229. doi: 10.1016/j.scienta.2013.05.039. [DOI] [Google Scholar]
- 26.Dai Z.-C., Fu W., Qi S.-S., Zhai D.-L., Chen S.-C., Wan L.-Y., Huang P., Du D.-L. Different Responses of an Invasive Clonal Plant Wedelia trilobata and its Native Congener to Gibberellin: Implications for Biological Invasion. J. Chem. Ecol. 2016;42:85–94. doi: 10.1007/s10886-016-0670-6. [DOI] [PubMed] [Google Scholar]
- 27.Sun J., Javed Q., Azeem A., Ullah I., Saifullah M., Kama R., Du D. Fluctuated water depth with high nutrient concentrations promote the invasiveness of Wedelia trilobata in Wetland. Ecol. Evol. 2020;10:832–842. doi: 10.1002/ece3.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z.H., Hu B.Q., Hu G. Assessment of allelopathic potential of Wedelia trilobata on the germination, seedling growth and chlorophyll content of rape. Adv. Mater. Res. 2013;807:719–722. [Google Scholar]
- 29.Dai Z.-C., Wang X.-Y., Qi S.-S., Cai H.-H., Sun J.-F., Huang P., Du D.-L. Effects of leaf litter on inter-specific competitive ability of the invasive plant Wedelia trilobata. Ecol. Res. 2016;31:367–374. doi: 10.1007/s11284-016-1344-0. [DOI] [Google Scholar]
- 30.Azizan K.A., Ibrahim S., Ghani N.H.A., Nawawi M.F. Metabolomics approach to investigate phytotoxic effects of Wedelia trilobata leaves, litter and soil. Plant Biosyst. 2019;153:691–699. doi: 10.1080/11263504.2018.1539042. [DOI] [Google Scholar]
- 31.Javed Q., Sun J.F., Azeem A., Jabran K., Du D.L. Competitive ability and plasticity of Wedelia trilobata (L.) under wetland hydrological variations. Sci. Rep. 2020;10:9431. doi: 10.1038/s41598-020-66385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song L., Chow W.S., Sun L., Li C., Peng C. Acclimation of photosystem II to high temperature in two Wedelia species from different geographical origins: Implications for biological invasions upon global warming. J. Exp. Bot. 2010;61:4087–4096. doi: 10.1093/jxb/erq220. [DOI] [PubMed] [Google Scholar]
- 33.Song L.-Y., Li C.-H., Peng S.-L. Elevated CO2 increases energy-use efficiency of invasive Wedelia trilobata over its indigenous congener. Biol. Invasions. 2010;12:1221–1230. doi: 10.1007/s10530-009-9541-1. [DOI] [Google Scholar]
- 34.Qi S.S., Dai Z.C., Miao S.L., Zhai D.L., Si C.C., Huang P., Wang R.P., Du D.L. Light limitation and litter of an invasive clonal plant, Wedelia trilobata, inhibit its seedling recruitment. Ann. Bot. 2014;114:425–433. doi: 10.1093/aob/mcu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallmann J., Berg G. Spectrum and Population Dynamics of Bacterial Root Endophytes. Microb. Root Endophytes. 2006;9:15–31. doi: 10.1007/3-540-33526-9_2. [DOI] [Google Scholar]
- 36.Pérez-Izquierdo L., Zabal-Aguirre M., González-Martínez S.C., Buée M., Verdú M., Rincón A., Goberna M. Plant intraspecific variation modulates nutrient cycling through its below ground rhizospheric microbiome. J. Ecol. 2019;107:1594–1605. doi: 10.1111/1365-2745.13202. [DOI] [Google Scholar]
- 37.Rodríguez-Caballero G., Caravaca F., Díaz G., Torres P., Roldán A. The invader Carpobrotus edulis promotes a specific rhizosphere microbiome across globally distributed coastal ecosystems. Sci. Total Environ. 2020;719:137347. doi: 10.1016/j.scitotenv.2020.137347. [DOI] [PubMed] [Google Scholar]
- 38.Werner J.J., Zhou D., Caporaso J.G., Knight R., Angenent L.T. Comparison of Illumina paired-end and single-direction sequencing for microbial 16S rRNA gene amplicon surveys. ISME J. 2012;6:1273–1276. doi: 10.1038/ismej.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman-Derr D., Desgarennes D., Fonseca-Garcia C., Gross S., Clingenpeel S., Woyke T., North G., Visel A., Partida-Martinez L.P., Tringe S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016;209:798–811. doi: 10.1111/nph.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douhovnikoff V., Hazelton E.L.G. Clonal growth: Invasion or stability? A comparative study of clonal architecture and diversity in native and introduced lineages of Phragmites australis (Poaceae) Am. J. Bot. 2014;101:1577–1584. doi: 10.3732/ajb.1400177. [DOI] [PubMed] [Google Scholar]
- 41.Alpert P., Simms E.L. The relative advantages of plasticity and fixity in different environments: When is it good for a plant to adjust? Evol. Ecol. 2002;16:285–297. doi: 10.1023/A:1019684612767. [DOI] [Google Scholar]
- 42.Goulas E., Le Dily F., Teissedre L., Corbel G., Robin C., Ourry A. Vegetative storage proteins in white clover (Trifolium repens L.): Quantitative and qualitative features. Ann. Bot. 2001;88:789–795. [Google Scholar]
- 43.Stuefer J.F. The role of stolon internodes for ramet survival after clone fragmentation in Potentilla anserina. Ecol. Lett. 2010;2:135–139. doi: 10.1046/j.1461-0248.1999.00066.x. [DOI] [Google Scholar]
- 44.Suzuki J.-I., Stuefer J. On the ecological and evolutionary significance of storage in clonal plants. Plant Species Biol. 1999;14:11–17. doi: 10.1046/j.1442-1984.1999.00002.x. [DOI] [Google Scholar]
- 45.Bittebiere A.K., Benot M.L., Mony C. Clonality as a key but overlooked driver of biotic interactions in plants. Perspect. Plant Ecol. Evol. Syst. 2020;43:125510. doi: 10.1016/j.ppees.2020.125510. [DOI] [Google Scholar]
- 46.Dai Z.-C., Wan L.-Y., Qi S.-S., Rutherford S., Ren G.-Q., Wan J.S., Du D.-L. Synergy among hypotheses in the invasion process of alien plants: A road map within a timeline. Perspect. Plant Ecol. Evol. Syst. 2020;47:125575. doi: 10.1016/j.ppees.2020.125575. [DOI] [Google Scholar]
- 47.Roiloa S.R. Clonal traits and plant invasiveness: The case of Carpobrotus NE.Br. (Aizoaceae) Perspect. Plant Ecol. Evol. Syst. 2019;40:125479. doi: 10.1016/j.ppees.2019.125479. [DOI] [Google Scholar]
- 48.Dahal K., Kane K., Gadapati W., Webb E., Savitch L.V., Singh J., Sharma P., Sarhan F., Longstaffe F.J., Grodzinski B. The effects of phenotypic plasticity on photosynthetic performance in winter rye, winter wheat and Brassica napus. Physiol. Plant. 2012;144:169–188. doi: 10.1111/j.1399-3054.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- 49.Ott J.P., Klimešová J., Hartnett D.C. The ecology and significance of below-ground bud banks in plants. Ann. Bot. 2019;123:1099–1118. doi: 10.1093/aob/mcz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith M.D., Knapp A.K. Physiological and morphological traits of exotic, invasive exotic, and native plant species in tallgrass prairie. Int. J. Plant Sci. 2001;162:785–792. doi: 10.1086/320774. [DOI] [Google Scholar]
- 51.Van Kleunen M., Weber E., Fischer M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2009;13:235–245. doi: 10.1111/j.1461-0248.2009.01418.x. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y., Rajkumar M., Zhang C., Freitas H. Beneficial role of bacterial endophytes in heavy metal phytoremedia-tion. J. Environ. Manag. 2016;174:14–25. doi: 10.1016/j.jenvman.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 53.Miliute I., Buzaite O., Baniulis D., Stanys V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirb. Agric. 2015;102:465–478. doi: 10.13080/z-a.2015.102.060. [DOI] [Google Scholar]
- 54.Gravel V., Antoun H., Tweddell R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA) Soil Biol. Biochem. 2008;39:1968–1977. doi: 10.1016/j.soilbio.2007.02.015. [DOI] [Google Scholar]
- 55.Phetcharat P., Duangpaeng A. Screening of Endophytic Bacteria from Organic Rice Tissue for Indole Acetic Acid Production. Procedia Eng. 2012;32:177–183. doi: 10.1016/j.proeng.2012.01.1254. [DOI] [Google Scholar]
- 56.Ali A., Mohanta T.K., Asaf S., Rehman N., Al-Housni S., Al-Harrasi A., Khan A.L., Al-Rawahi A. Biotransformation of benzoin by Sphingomonas sp. LK11 and ameliorative effects on growth of Cucumis sativus. Arch. Microbiol. 2019;201:591–601. doi: 10.1007/s00203-019-01623-1. [DOI] [PubMed] [Google Scholar]
- 57.Ham S., Yoon H., Park J.-M., Park Y.G. Optimization of Fermentation Medium for Indole Acetic Acid Production by Pseudarthrobacter sp. NIBRBAC000502770. Appl. Biochem. Biotechnol. 2021;193:2567–2579. doi: 10.1007/s12010-021-03558-0. [DOI] [PubMed] [Google Scholar]
- 58.Vives-Peris V., Gómez-Cadenas A., Pérez-Clemente R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018;37:1557–1569. doi: 10.1007/s00299-018-2328-z. [DOI] [PubMed] [Google Scholar]
- 59.Pang F., Xia W.K., He M., Qi S.S., Dai Z.C., Du D.L. Nitrogen fixing bacteria alleviate the nutritional competition of arbuscular mycorrhizal fungi against Solidago canadensis in a nitrogen limited environment. Chin. J. Plant Ecol. 2020;44:782–790. doi: 10.17521/cjpe.2020.0114. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microbial sequencing data that supported the findings of this study have been deposited into the CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb, https://www.cngb.org/, accessed on 5 September 2022) with accession number CNP0003006. The data presented in this study are available upon request from the corresponding author (e-mail: daizhicong@163.com).