Abstract
The degree of protection against Plasmodium yoelii asexual blood stages induced by immunization of mice with the 19-kDa region of merozoite surface protein 1 (MSP119) is H-2 dependent. As a strategy to improve the protection, mouse strains with disparate H-2 haplotypes were immunized with glutathione S-transferase (GST)–MSP119 proteins including either a universal T-cell epitope from tetanus toxin (P2) or an I-Ak-restricted T-cell epitope (P8) from Plasmodium falciparum Pf332. In H-2k mice which are poorly protected following immunization with GST-MSP119, GST-P2-MSP119 significantly improved the protection. In mice partially (H-2k/b) or well protected by GST-MSP119 (H-2d and H-2b), P2 did not further increase the protection. However, the protection of H-2k/b mice and to some extent H-2k mice was improved by immunization with GST-P8-MSP119. The magnitudes of immunoglobulin G1 (IgG1) and IgG2a responses in mice immunized with the GST-MSP119 variants correlated with low peak parasitemia, indicating a protective capacity of these IgG subclasses. In H-2k mice immunized with GST-P2-MSP119, both IgG1 and IgG2a responses were significantly enhanced. The epitope P2 appeared to have a general ability to modulate the IgG subclass response since all four mouse strains displayed elevated IgG2a and/or IgG2b levels after immunization with GST-P2-MSP119. In contrast, GST-P8-MSP119 induced a slight enhancement of IgG responses in H-2k/b and H-2k mice without any major shift in IgG subclass patterns. The ability to improve the protective immunity elicited by P. yoelii MSP119 may have implications for improvement of human vaccines based on P. falciparum MSP119.
A number of malaria proteins have been proposed as candidates for inclusion in subunit vaccines against asexual blood stages of Plasmodium falciparum (30). One of the most promising antigens in this respect is the 19-kDa C-terminal region of merozoite surface protein 1 (MSP1). P. falciparum MSP1 is expressed on the surfaces of merozoites and is proteolytically processed in two steps to generate first a complex of polypeptides including a 42-kDa fragment (MSP142) that is subsequently cleaved to a 19-kDa fragment (MSP119) and a 33-kDa fragment (MSP133). The complex containing MSP133 is shed (7), and MSP119 is the only part of MSP1 that remains on the merozoite surface during parasite invasion of red blood cells (RBC) (6). The MSP119 region consists of two epidermal growth factor-like domains (8). A limited number of allelic variants of P. falciparum MSP119 occur, with minor amino acid differences between them, making MSP119 one of the most conserved regions of P. falciparum MSP1 (48, 57). Importantly, MSP1 is expressed in other Plasmodium species and the processing that generates MSP119 appears to be similar (5, 17, 29, 43), enabling the use of various malaria parasites and animal models for the study of MSP119-induced protection.
Both antibodies and effector T cells appear to play important roles in the protection of mice against Plasmodium yoelii (4, 23, 28). However, the protective immunity induced by vaccination with recombinant P. yoelii MSP119 is mediated largely by antibodies (14, 28, 36). Passive transfer of MSP119-specific antiserum or monoclonal antibodies (MAb) into naive mice suppressed an otherwise lethal P. yoelii infection (14, 39, 55), whereas vaccination with MSP119 failed to protect mice deficient in immunoglobulin μ-chains (28). In contrast, immunization with defined MSP119-derived T-cell epitopes does not induce protective immunity and transfer of T-cell lines from MSP119-immunized mice does not confer protection to naive recipients (58). Nevertheless, a functional CD4+ T-cell response is a prerequisite for the generation of potent humoral responses by vaccination with protein antigens.
Several parameters can be modified in order to enhance the protection induced by vaccination with pathogen-derived proteins or protein subunits. Optimized immunization protocols as well as the use of different adjuvants have been shown to have an impact on protective immunity elicited by P. yoelii MSP119 (15, 28, 38). Another strategy would be to link additional T-cell epitopes to MSP119 to overcome limited recognition of T-helper-cell epitopes. This procedure may be suitable for improving the H-2-dependent protection against lethal P. yoelii infection induced by glutathione S-transferase (GST)–MSP119 fusion proteins (59). In inbred mouse strains, GST-MSP119 elicited a higher degree of protection in C57BL/10 (H-2b) mice than in B10.A(4R) mice (H-2k/b), and B10.BR mice (H-2k) were poorly protected (59). Partial protection against P. falciparum and Plasmodium vivax has been obtained in monkeys vaccinated with the respective MSP119 proteins linked to T-cell epitopes from tetanus toxoid (TT) (34, 61). However, the impact of the T-cell epitopes on protective immune responses is uncertain, as comparisons with MSP119 alone were not made. Moreover, expression of P. falciparum MSP119 in the yeast Saccharomyces cerevisiae resulted, to a large extent, in proteolytic cleavage of the linked T-cell epitopes (34).
To evaluate a strategy to improve the protection induced by P. yoelii MSP119, GST fusion proteins containing MSP119 were expressed in bacteria with or without additional T-cell epitopes inserted between the GST and MSP119. The epitopes selected were the universal TT-derived epitope P2 (44) and an I-Ak-restricted epitope (P8) from the P. falciparum antigen Pf332 (3). The proteins were used for immunization of four H-2 congenic mouse strains, of haplotypes H-2b, H-2d, H-2k, and H-2k/b (I-Ak/I-Eb). Immune responses after vaccination and protection against parasite challenge were assessed. The results demonstrate that insertion of T-cell epitopes into MSP119-based immunogens can enhance the immunoglobulin G (IgG) responses to MSP119 and can improve the protection against malaria parasite challenge. Additionally, it is shown that the P2 epitope, but not the P8 epitope, modulates the IgG subclass response to MSP119.
MATERIALS AND METHODS
Construction of plasmids encoding variants of recombinant GST-MSP119.
A plasmid encoding GST-MSP119 (36), residues 1649 to 1754, of P. yoelii YM MSP1 (35) was modified to encode proteins with T-cell epitopes inserted between GST and MSP119. A BamHI site in the plasmid, between the GST and the MSP119 genes, was digested with BamHI (Promega, Madison, Wis.), and the plasmid was dephosphorylated using alkaline phosphatase (Boehringer GmbH, Mannheim, Germany). Complementary oligonucleotides (VH BIO, Newcastle, United Kingdom) encoding the epitope P2 (IQYIKANSKFIGITEL) or P8 (EEGPVDEEIVQEEGTV) were annealed by heating to 95°C followed by slow cooling to 20°C. The duplex oligonucleotide was phosphorylated using T4 polynucleotide kinase (Promega) and ligated into the BamHI site of the plasmid using T4 DNA ligase (Promega). Ligation reactions with a fivefold molar excess of oligonucleotide were used to transform Escherichia coli strain DH5α. The oligonucleotides used were 5′-GAT CCA GTA CAT CAA AGC TAA CTC CAA ATT CAT CGG TAT CAC CGA ACT GGG TCA GGT-3′ (P2 sense), 5′-GAT CAC CTG ACC CAG TTC GGT GAT ACC GAT GAA TTT GGA GTT AGC TTT GAT GTA CTG-3′ (P2 antisense), 5′-GAT CCT GGA AGA AGG TCC GGT TGA CGA AGA AAT CGT TCA GGA AGA AGG TAC CGT-3′ (P8 sense), and 5′-GAT CAC GGT ACC TTC TTC CTG AAC GAT TTC TTC GTC AAC CGG ACC TTC TTC CAG-3′ (P8 antisense) (nucleotides in boldface ensured re-creation of the BamHI site in the 5′ ends of correct inserts and in the 3′ ends of reversed inserts; underlined nucleotides encode a stop codon in reversed inserts). To identify plasmids with inserts, proteins expressed by transformed bacterial clones were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11). Plasmids with correct inserts resulted in the expression of proteins larger than GST-MSP119, and those with reversed inserts resulted in the expression of GST only. The number of inserts was determined by BamHI and EcoRI (Promega) restriction of plasmids. The EcoRI site is located immediately after the MSP119 sequence, and excised DNA fragments with correct inserts included the MSP119 sequence plus 50 to 60 bp for each insert, unlike the DNA fragments from plasmids with reversed inserts or the parental plasmid. Plasmids encoding GST-P2-MSP119 including two copies of P2 and GST-P8-MSP119 including one copy of P8 were identified, and the inserts were confirmed by DNA sequencing.
Production, purification, and analysis of recombinant proteins.
Recombinant proteins were produced as previously described, with modifications (36, 54). Briefly, protein expression in transformed E. coli DH5α cells was induced with 1 mM isopropylthio-β-galactosidase (Sigma) for 3 h at 37°C. The cells were harvested and lysed in phosphate-buffered saline (PBS). After centrifugation, the supernatant was incubated with glutathione-agarose (Sigma). Fusion proteins were eluted with 10 mM reduced glutathione (Sigma) and dialyzed against PBS. Solubilization of GST-P2-MSP119 required sonication of bacteria in 2 M urea. The corresponding supernatant was diluted to 0.5 M urea in PBS to enable binding to glutathione-agarose beads. The beads were washed with decreasing concentrations of urea in PBS and eventually with pure PBS before elution and dialysis as described above. To obtain GST-free MSP119, GST-MSP119 was bound to glutathione-agarose and treated for 12 h at 22°C with factor Xa (Sigma), which cleaves a site right after the C terminus of GST. Factor Xa was removed by incubation with p-aminobenzamidine-agarose (Sigma). Simultaneous incubation with glutathione-agarose ensured that any contaminating GST was removed before dialysis against PBS. Protein concentrations were determined by multiplying the absorbance at 280 nm with a factor calculated for each protein according to protein size and content of aromatic amino acids (26). The GST-MSP119 variants were further analyzed by Western blotting (11), and GST-free MSP119 was subjected to a dot blot assay (2) using the MSP119-specific MAb B10 and F5 (55).
MAPs.
Multiple-antigen peptide (MAP) constructs (56) were made as control immunogens as well as for measurement of specific antibodies. Synthesis and analysis of MAP constructs were performed as described previously (1). The MAP constructs had four identical peptide chains synthesized in parallel on the tetrameric lysine core. Amino acid analysis confirmed the right amino acid composition of each MAP. The sequences of MAP-P2 and MAP-P8 corresponded to those of the P2 and P8 epitopes in the recombinant proteins, respectively.
P. yoelii parasites.
Lethal P. yoelii YM parasites (63) were kindly provided by D. Walliker, Edinburgh University, and were kept in liquid nitrogen and by passage in mice. All infected mice were given drinking water supplemented with 2.5 g of p-aminobenzoic acid/liter (47).
Immunization and parasite challenge of mice.
Female B10.A(4R), B10.BR, C57BL/6, and B10.D2 mice (Harlan, Oxford, United Kingdom) (Table 1) were used at 7 to 10 weeks of age. Groups of five mice were injected intraperitoneally (i.p.) with 40 μg of GST-MSP119, GST-P2-MSP119, or a mixture of GST and MAP-P2 emulsified in complete Freund's adjuvant (Sigma). B10.A(4R), B10.BR, and C57BL/6 mice were also immunized with GST-P8-MSP119, GST, or MAP-P8. Booster injections with 40 μg of immunogen in incomplete Freund's adjuvant (Sigma) were given at weeks 3 and 6, and blood to obtain serum was drawn from the tail at week 8. One week later, mice were inoculated intravenously with 104 P. yoelii-infected RBC. Giemsa-stained blood smears were made daily from day 3 of the infection. Parasitemia was assessed by counting the percentage of infected RBC using light microscopy. Two additional groups of B10.A(4R) mice were immunized subcutaneously (s.c.) in the base of the tail with either GST-MSP119 or GST-free MSP119. Otherwise, the immunizations were made as described above.
TABLE 1.
Titers of IgG to MSP119 in mice immunized with GST-MSP119 with or without additional T-cell epitopes, as measured by ELISA
| Mouse strain | H-2 haplotypea
|
Mean titer of IgG (103) to MSP119b ± SD for immunogen
|
||||||
|---|---|---|---|---|---|---|---|---|
| K (I) | I-A (II) | I-E (II) | S (III) | D (I) | GST-MSP119 | GST-P2-MSP119 | GST-P8-MSP119 | |
| B10.A(4R) | k | k | (b) | b | b | 150 ± 60 | 330 ± 110 | 190 ± 80 |
| B10.BR | k | k | k | k | k | 115 ± 40 | 280 ± 55 | 135 ± 95 |
| C57BL/6 | b | b | (b) | b | b | 270 ± 100 | 270 ± 95 | ndc |
| B10.D2 | d | d | d | d | d | 290 ± 115 | 220 ± 25 | nd |
Classes (I to III) are in parentheses. (b), the class II molecule I-Eb is not expressed.
Individual sera from five mice per group immunized i.p. were titrated for reactivity with MSP119. The reciprocal titers were estimated as the dilution corresponding to an absorbance value of 0.6. Mean reactivities of serum plus two standard deviations from all groups of mice immunized with GST/MAP-P2 were below 0.3 at dilutions of 1:1,000.
nd, not done.
Assessment of antibody responses.
For the enzyme-linked immunosorbent assay (ELISA), GST-free MSP119, GST, or MAP constructs were adsorbed at 2 μg/ml in PBS to Immulon 4 plates (Dynatech Laboratories, Inc., Billingshurst, United Kingdom) overnight at 8°C. The wells were blocked with 1% bovine serum albumin in PBS for 1 h at room temperature, and then duplicates of serum diluted in PBS with 0.1% bovine serum albumin and 0.05% Tween were added, and the wells were incubated for 1 h at 37°C. Between incubations, wells were washed with PBS containing 0.05% Tween. Specific antibodies were detected by incubation with rabbit anti-mouse IgG (Sigma), goat anti-mouse IgG1, IgG2b, or IgG3 (Southern Biotechnology, Birmingham, Ala.), or sheep anti-mouse IgG2a (The Binding Site, Birmingham, United Kingdom) conjugated to horseradish peroxidase. Absorbance was measured at 492 nm after adding o-phenylenediamine (Sigma) as the chromogen and H2O2 as the substrate and later 1 M sulfuric acid to stop the reaction. Analyses were standardized by including a pool of mouse antisera to GST-MSP119 in all assays.
For indirect immunofluorescence antibody assay (IFAT), blood was drawn from P. yoelii YM-infected mice into heparinized tubes. The blood was washed and diluted 1:200 in phosphate-saline buffer with 0.2% glucose. Ten microliters of cell suspension was added to wells of 15-well multitest slides (ICN Biomedicals, Inc., Aurora, Ohio) that were air dried before storage at −20°C. Prior to the staining, the slides were fixed with acetone for 2 min and blocked in PBS with 1% bovine serum albumin for 30 min at room temperature. The wells were incubated for 1 h at 37°C with serum in consecutive twofold dilutions in PBS. Specific antibodies were detected by incubation for 1 h at 37°C with fluorescein isothiocyanate-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Southern Biotechnology). Between incubations the slides were washed in PBS. Titers were determined as the reciprocal of the last positive dilution when analyzed by UV microscopy.
Competition ELISA.
Wells were adsorbed with MSP119 or 1 μg of native MSP1/ml from P. yoelii YM as described above. Native MSP1 was purified as previously described (29). The wells were blocked and incubated with duplicates of serum dilutions, followed by incubation with biotinylated MAb at concentrations established by titration. MAb B10, F5, and D3 (55) were biotinylated by mixing the MAb in PBS with sulfosuccimidyl-6-(biotinamido)-hexanoate (Pierce, Rockford, Ill.) for 1 h at room temperature, followed by dialysis against PBS. Bound MAb was detected by incubation with 1 μg of avidin-peroxidase (Sigma)/ml and addition of the substrate as described above. The level of competition was estimated by comparison with wells incubated with biotinylated MAb only.
Statistical analyses.
Statistical analyses were made by linear regression or Student's t test (two-tailed, unpaired).
RESULTS
Production and analyses of recombinant proteins.
Recombinant GST-MSP119 with or without T-cell epitopes inserted between GST and MSP119 were made. GST-P2-MSP119 included two copies of the universal epitope P2, and GST-P8-MSP119 included one copy of the I-Ak-restricted epitope P8. According to SDS-PAGE, GST-MSP119 and variants thereof were predominantly full-length proteins and MSP119, from which GST had been removed, was free from contamination. Proteins including P2 or P8 displayed increased relative molecular masses compared to GST-MSP119 (42 kDa). Insertion of one P8 epitope resulted in an increase of 5 kDa, which is larger than expected (theoretically 1.5 kDa), most likely because of the highly negative charge of P8 (3). Insertion of two copies of P2 led to an expected increase of 3 kDa. MAb B10 and F5, which recognize parasite-derived MSP119 (55), bound all GST-MSP119 variants in Western blotting, provided the SDS-PAGE separation was performed under nonreducing conditions. MAb B10 and F5 also recognized GST-free MSP119 in a dot blot assay.
P. yoelii challenge in mice immunized with GST-MSP119 variants.
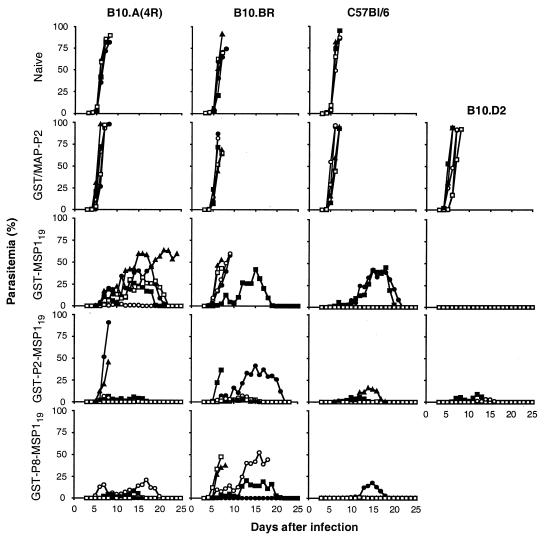
B10.A(4R) and B10.BR mice immunized with GST/MAP-P2, GST-MSP119, GST-P2-MSP119, and GST-P8-MSP119 were challenged with 104 P. yoelii YM-infected RBC (Fig. 1). Naive control mice and the mice immunized with GST/MAP-P2 rapidly developed high parasitemias and succumbed to the infection at days 7 to 9. Similarly, B10.A(4R) and B10.BR mice immunized with GST or MAP-P8 were not protected (data not shown). B10.A(4R) mice immunized with GST-MSP119 quickly developed high levels of parasites, but only one of the mice failed to clear the parasites and died at day 25. The protection of B10.BR mice immunized with GST-MSP119 was poor. Four mice died between days 8 and 10; one mouse survived but had a peak parasitemia of 42%. In B10.A(4R) mice, immunization with GST-P2-MSP119 resulted in good protection in three mice, which had parasitemias below 7%, but two mice developed high parasitemias and died at day 9. Immunization with GST-P8-MSP119 resulted in the survival of all five B10.A(4R) mice, with significantly lower peak parasitemias than were observed in the GST-MSP119 group (P = 0.027). In B10.BR mice, GST-P2-MSP119 induced better protection than GST-MSP119, in terms of increased survival as well as lower peak parasitemias (P = 0.0074). Four out of five mice survived, and three of these had parasitemias below 5%. In B10.BR mice, GST-P8-MSP119 did not significantly improve protection although two mice survived and the death of one mouse was delayed (day 19).
FIG. 1.
Parasite challenge of mice immunized with GST-MSP19 with or without additional T-cell epitopes. Two weeks after the third injection of immunogen, groups of five mice were inoculated intravenously with 104 P. yoelii YM-infected RBC. Parasitemia was monitored daily from day 3. B10.BR, B10.A(4R), and C57BL/6 mice were either naive or were immunized with GST-MSP119, GST-P2-MSP119, GST-P8-MSP119, or a mixture of GST and MAP-P2; B10.D2 mice were immunized with GST-MSP119, GST-P2-MSP119, or GST/MAP-P2. All groups were analyzed in parallel except for the C57BL/6 mice immunized with GST-P8-MSP119, which were analyzed in a separate experiment where C57BL/6 immunized with GST-MSP119 or GST-P8-MSP119 were found to be similarly protected (maximal parasitemias for the GST-MSP119 group were <0.001 [two mice], 0.22, 0.44, and 1.7% and for the GST-P8-MSP119 group were <0.001, 0.16, 0.18, 0.65, and 17%).
Levels of protection induced by GST-MSP119 and GST-P2-MSP119 in C57BL/6 and B10.D2 mice were also compared (Fig. 1). After immunization with GST-MSP119, C57BL/6 mice were reasonably well protected, with peak parasitemias below 0.05% in three mice, although two mice slowly developed high parasitemias, which were cleared by day 23. B10.D2 mice were well protected, and only two mice had detectable parasitemias. There was no significant change in C57BL/6 and B10.D2 mice immunized with GST-P2-MSP119. Immunization of C57BL/6 mice with GST-P8-MSP119 also did not change the level of protection.
IgG responses to MSP119 following immunization with GST-MSP119 constructs.
To investigate the relationship between prechallenge IgG levels and protection, antisera from mice immunized with GST-MSP119 variants were analyzed for IgG reactivity with MSP119 by ELISA (Table 1). Following immunization with GST-MSP119, C57BL/6 and B10.D2 mice had approximately two- to threefold-higher MSP119-specific IgG levels than B10.BR mice (P = 0.0061 and 0.0052, respectively) and B10.A(4R) mice (P = 0.032 and 0.032, respectively). The IgG titers in B10.A(4R) and B10.BR mice were increased two- to threefold after immunization with GST-P2-MSP119 compared to titers for mice immunized with GST-MSP119 (P = 0.006 and 0.001, respectively), whereas in C57BL/6 and B10.D2 mice, IgG titers were no different or lower in GST-P2-MSP119 antisera. B10.A(4R) and B10.BR antisera to GST-P8-MSP119 displayed only slightly elevated MSP119-specific IgG levels compared to sera from GST-MSP119-immunized mice.
In all mice taken together, there was a significant inverse correlation of peak parasitemias and IgG titers to MSP119 (r = 0.53; n = 50; P ≤ 0.0001).
IgG subclass responses in mice immunized with MSP119-containing immunogens.
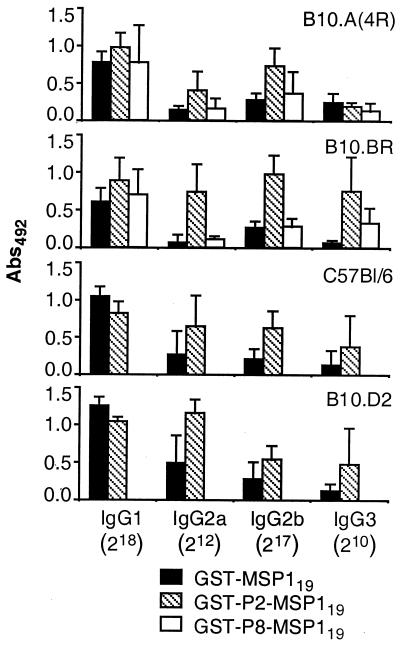
The IgG subclass distribution of antibodies to MSP119 in prechallenge antisera from mice immunized with GST-MSP119 was examined by ELISA and was found to differ between mouse strains although, in all mice, IgG1 was detected at higher dilutions than other subtypes (IgG2b > IgG2a > IgG3) (Fig. 2). In Fig. 2, the mean serum reactivities for each mouse strain are compared at a serum dilution appropriate for each IgG subclass (IgG1, 1:218; IgG2a, 1:212; IgG2b, 1:217; IgG3, 1:210). C57BL/6 and B10.D2 mice displayed significantly higher IgG1 reactivity than both B10.A(4R) (P = 0.015 and 0.0007, respectively) and B10.BR mice (P = 0.0038 and 0.0005, respectively). B10.D2 mice also had significantly higher levels of IgG2a than B10.A(4R) (P = 0.028) and B10.BR mice (P = 0.0009). No significant differences between IgG2b and IgG3 levels were observed.
FIG. 2.
MSP119-specific IgG subclass responses in mice immunized with GST-MSP119 with or without additional T-cell epitopes as measured by ELISA. Groups of five B10.A(4R), B10.BR, C57BL/6, and B10.D2 mice were immunized with GST-MSP119, GST-P2-MSP119, or GST-P8-MSP119 [B10.A(4R) and B10.BR mice only]. The bars display mean reactivities plus standard deviations of sera analyzed for reactivity with MSP119 at dilutions of 1:218 (IgG1), 1:212 (IgG2a), 1:217 (IgG2b), or 1:210 (IgG3). No significant reactivity was displayed by sera from GST-immunized or naive mice at these dilutions (not shown). Abs492, absorbance at 492 nm.
In mice immunized with GST-MSP119, there was an inverse correlation between peak parasitemia and the levels of IgG1 (r = 0.73; n = 20; P = 0.0003) and IgG2a (r = 0.50; n = 20; P = 0.026), but not levels of IgG2b or IgG3.
In mice vaccinated with GST-P2-MSP119, the IgG2a and IgG2b levels were elevated compared to levels in mice immunized with GST-MSP119 (Fig. 2). The increases of both IgG2a and IgG2b levels in B10.BR mice were significant (P = 0.0008 and P ≤ 0.0001, respectively), as was the increase of IgG2a in B10.D2 (P = 0.013) mice and the increases of IgG2b in B10.A(4R) (P = 0.0003) and C57BL/6 (P = 0.0073) mice. IgG1 levels were slightly increased in B10.(4R) and B10.BR mice and significantly decreased in C57BL/6 (P = 0.027) and B10.D2 mice (P = 0.0039). IgG3 was enhanced in all mice except B10.A(4R) mice after immunization with GST-P2-MSP119, but only significantly so in B10.BR mice (P = 0.0003). No significant change of any IgG subclass was observed in antisera to GST-P8-MSP119 (Fig. 4). An analysis of all groups of mice together found a significant association between low maximal parasitemia and high IgG1 (r = 0.43; n = 50; P = 0.0019) or IgG2a levels (r = 0.49; n = 50; P = 0.0003), but not IgG2b or IgG3 levels. Importantly, the levels of IgG1, IgG2a, and IgG2b detected by IFAT correlated with the reactivities of the corresponding IgG subclasses in the ELISA, confirming that the IgG levels determined by ELISA reflected reactivity with parasite-derived MSP119 (Table 2).
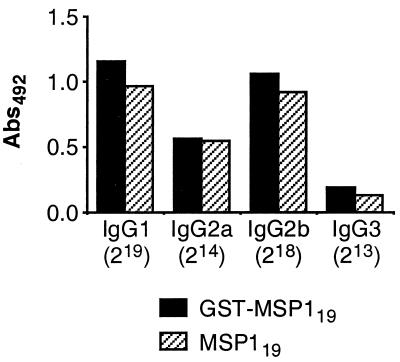
FIG. 4.
Comparison of MSP119-specific IgG subclass responses in B10.A(4R) mice immunized with GST-MSP119 or MSP119 as measured by ELISA. Groups of five mice were immunized s.c. with either GST-MSP119 or GST-free MSP119. The bars display mean reactivities of sera analyzed for reactivity with MSP119 at dilutions of 1:219 (IgG1), 1:214 (IgG2a), 1:218 (IgG2b), or 1:213 (IgG3). Abs492, absorbance at 492 nm.
TABLE 2.
IgG subclass titers in mice immunized with GST-MSP119 variants as measured by IFAT
| Mouse strain | Immunogen | Mean IFAT titer (103)a ± SD for:
|
|||
|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | ||
| B10.A(4R) | GST-MSP119 | 4.9 ± 1.8 | —b | 0.4 ± 0.4 | — |
| GST-P2-MSP119 | 6.6 ± 2.2 | 0.4 ± 0.1 | 1.3 ± 0.7 | — | |
| GST-P8-MSP119 | 5.7 ± 2.2 | — | 0.4 ± 0.1 | — | |
| B10.BR | GST-MSP119 | 2.5 ± 0.9 | — | 0.2 ± 0.1 | — |
| GST-P2-MSP119 | 5.7 ± 2.2 | 0.4 ± 0.4 | 1.0 ± 0.6 | — | |
| GST-P8-MSP119 | 3.7 ± 2.9 | — | 0.3 ± 0.1 | — | |
| C57BL/6 | GST-MSP119 | 11.5 ± 4.5 | 0.2 ± 0.1 | 0.3 ± 0.1 | — |
| GST-P2-MSP119 | 7.4 ± 1.8 | 0.5 ± 0.4 | 1.8 ± 0.5 | — | |
| B10.D2 | GST-MSP119 | 19.7 ± 7.3 | 0.3 ± 0.2 | 0.5 ± 0.4 | — |
| GST-P2-MSP119 | 10.7 ± 5.5 | 0.7 ± 0.3 | 1.2 ± 0.8 | — | |
Sera from five mice per group were titrated for IgG subclass reactivity in IFAT. The titers were estimated as the reciprocal of the last positive dilution. Sera from mice immunized with GST/MAP-P2 were negative for all subclasses at the lowest dilution tested (1:64).
—, no reactivity at the lowest dilution tested.
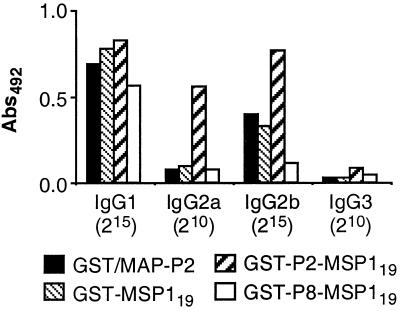
In order to determine if the insertion of P2 into GST-MSP119 also affected the IgG subclass response to the GST fusion partner, B10.BR antisera to GST fusion proteins were analyzed by ELISA (Fig. 3). The levels of IgG reactivity with GST in antisera to GST-MSP119 were lower than the levels of MSP119-reactive IgG but displayed a similar IgG subclass distribution. Interestingly, antiserum to GST-P2-MSP119 had increased levels of IgG2a and IgG2b also to GST, in comparison with antisera to GST-MSP119, GST-P8-MSP119, or GST/MAP-P2, suggesting that P2 influenced the IgG subclass response to GST as well as MSP119.
FIG. 3.
GST-specific IgG subclass responses in B10.BR mice immunized with GST fusion proteins, as determined by ELISA. Groups of five mice were immunized with GST/MAP-P2, GST-MSP119, GST-P2-MSP119, or GST-P8-MSP119. The bars display reactivities of pooled sera analyzed for reactivity with GST at dilutions of 1:215 (IgG1 and IgG2b) or 1:210 (IgG2a and IgG3). Sera from naive mice did not react significantly at these dilutions (not shown). Abs492, absorbance at 492 nm.
Moreover, B10.A(4R) antisera to GST-MSP119 and GST-free MSP119 were compared for reactivity with MSP119 (Fig. 4). Notably, these two groups of mice were immunized by s.c. injections, which resulted in slightly higher IgG responses to MSP119 than those in mice immunized i.p. (data not shown). Irrespective of that finding, the levels of IgG subclasses in the two groups immunized s.c. with GST-MSP119 and MSP119 were similar, indicating that the presence of GST did not influence the IgG subclass response to MSP119 (Fig. 4).
Analysis of the epitope specificity induced by the GST-MSP119 variants.
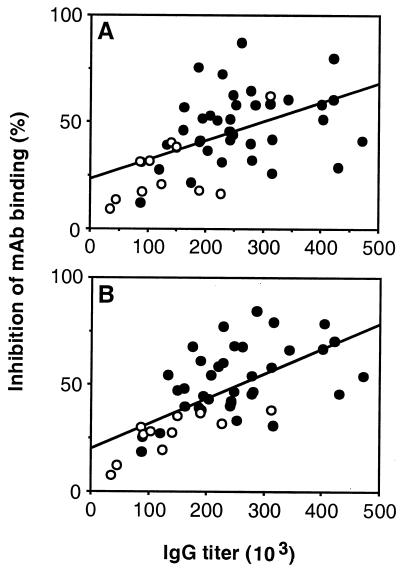
A competitive ELISA was used to analyze whether antisera from the different immunization groups differed not only in levels of MSP119-specific IgG but also in their specificities for known, protective epitopes. Sera from mice immunized with GST-MSP119 variants were tested for the capacity to inhibit the binding of MAb F5 and B10 to MSP119 or MAb D3 to purified native MSP1 protein. All three MAb confer partial protection when passively transferred to mice (55). MAb F5 recognizes an epitope in the first domain of MSP119; B10 requires both domains together for binding, and D3 binds P. yoelii MSP142 but not MSP119 or MSP133 separately (55). All sera inhibited, to a variable extent, the binding of MAb F5 or B10 to MSP119 (Fig. 5), whereas no inhibition of MAb D3 binding to MSP1 was observed. The inhibition of both MAb F5 and B10 was strongly associated with the levels of MSP119-reactive IgG in the sera (Fig. 5). Thus, although sera from well-protected mice displayed higher competition, the results do not suggest that the competitive capacity varied between groups due to major differences in epitope specificity. Competition with both MAb B10 and F5 was also inversely associated with peak parasitemia in the corresponding mice (r = 0.49, n = 50, P ≤ 0.0001, and r = 0.58, n = 50, P ≤ 0.0001, respectively).
FIG. 5.
Relationship between the MSP119-specific IgG titers of antisera to GST-MSP119 variants and the capacity to inhibit the binding of MAb F5 or MAb B10 to MSP119. The MSP119-specific titers of sera from B10.A(4R), B10.BR, C57BL/6, and B10.D2 mice immunized with GST-MSP119, GST-P2-MSP119, or GST-P8-MSP119 [B10.A(4R) and B10.BR mice]) were determined by ELISA. To establish the capacity of the sera to inhibit the binding of MAb to MSP119, ELISA plates were incubated first with serum diluted 1:600 and subsequently with biotinylated MAb F5 (A) or MAb B10 (B). The IgG titers of individual sera are plotted against the level of competition obtained with the corresponding sera. Open circles, mice that died following challenge infection. The lines display the association between IgG titer and inhibition of MAb F5 (r = 0.52; n = 50; P ≤ 0.0001) (A) and MAb B10 (r = 0.66; n = 50; P ≤ 0.0001) (B). The binding of biotinylated MAb could be inhibited by adding an excess of nonlabeled homologous but not heterologous MAb (not shown).
DISCUSSION
Numerous studies have indicated the protective potential of the malaria antigen MSP119. The levels of antibodies in malaria-exposed humans to P. falciparum MSP119, or epitopes within MSP119, are associated with clinical immunity (20, 51, 52). Moreover, immunization of monkeys with antigens that include either P. falciparum or Plasmodium cynomolgi MSP119 confers protection (12, 34, 46), although protection has not always been achieved or has been insufficient (10, 12, 34). Similarly, inbred mouse strains can be protected against P. yoelii by immunization with GST linked to P. yoelii MSP119 (16, 36) but are differently protected in an H-2-dependent manner; C57BL/10 (H-2b) mice are better protected than B10.A(4R) (H-2k/b) and B10.BR (H-2k) mice (59). The genetically restricted protection induced by GST-MSP119 was previously assessed using residues 1619 to 1754 of P. yoelii MSP1 (59). We found that using a shorter fragment of MSP1 (residues 1649 to 1754), still including both domains of MSP119 that are essential for induction of protection (37), did not change the relative protection observed in C57BL/6 (H-2b), B10.A(4R), and B10.BR mice. We also found that B10.D2 (H-2d) mice were similarly or slightly better protected than H-2b mice.
As a strategy to improve the protection induced by P. yoelii MSP119, we inserted additional T-cell epitopes into GST-MSP119 and compared the protective capacities of these immunogens with GST-MSP119. Insertion of the TT-derived T-cell epitope P2 in GST-MSP119 led to increased survival and protection against P. yoelii challenge infection in B10.BR mice, a mouse strain otherwise not protected by immunization with GST-MSP119. Inclusion of the I-Ak-restricted epitope P8 markedly improved the ability of B10.A(4R) mice to suppress parasitemia but had a lesser effect on B10.BR mice, suggesting that differences at the I-E locus or a minor histocompatibility locus may affect presentation of the P8 epitope. In mice well protected by GST-MSP119 (C57BL/6 and B10.D2 mice), insertion of P2 in the immunogen had no significant effect on protection. The course of infection in mice immunized with MAP constructs containing P2 or P8 was similar to that in GST-immunized or naive mice, demonstrating that immune responses to these epitopes were not protective.
The P2 epitope has been described as a universal T-cell epitope, and T cells from most TT-vaccinated humans recognize it (44). When tested with B10.A(4R), B10.BR, C57BL/6, and DBA/2 (H-2d) mice, incorporation of P2 in a peptide immunogen enhanced the total IgG responses to otherwise poorly immunogenic B-cell epitopes in all mouse strains (33). We have shown here that insertion of P2 into GST-MSP119 led to enhanced total IgG responses to MSP119 in B10.A(4R) and B10.BR mice but not in C57BL/6 and B10.D2 mice. However, in all mouse strains, GST-P2-MSP119 induced significantly increased levels of MSP119-reactive IgG2a and/or IgG2b. The ability to modulate the IgG subclass response may be a general feature of P2, as IgG2a and IgG2b responses to the GST moiety were also enhanced. In contrast, P8 did not significantly change the IgG subclass pattern induced.
In mice, IgG1 responses are usually associated with Th2 responses whereas high levels of IgG2a, sometimes associated with IgG2b and IgG3, are thought to reflect Th1 responses (18, 22, 25, 27, 42). Several parameters influence the IgG subclass responses to proteins, including the use of adjuvants or delivery systems as well as the intrinsic immunogenicity of the protein itself (13, 31, 53, 64). The response elicited by GST-MSP119, with high levels of multiple IgG subclasses, is thus likely to be a combined effect of several parameters. Nevertheless, Freund's adjuvant appears to be a strong regulator of immune responses and usually induces high IgG1 as well as considerable IgG2a and IgG2b levels, reflecting an adjuvant-dependent mixed Th1/Th2 response (13, 15, 31, 62). The modulation of IgG subclass responses induced by P2 suggests that T-cell responses to P2 can, to some extent, override other factors determining the IgG subclass response, possibly reflecting a polarization towards Th1, although studies on P2-induced cytokine responses are required to confirm this. The decreased IgG1 levels in H-2b and H-2d mice support this, but, in contrast, the IgG1 levels were slightly increased in H-2k and H-2k/b mice. The decrease of IgG1 in H-2b and H-2d mice may explain why no improvement of the protection was observed in these mice following immunization with GST-P2-MSP119. Moreover, the differential effect of P2 on the IgG response in mice with disparate H-2 haplotypes suggests that this epitope, despite being universally recognized, has a different impact on the IgG subclass response in a major histocompatibility complex-dependent manner.
The prechallenge levels of IgG to MSP119, as well as the levels of specific IgG1 and IgG2a, in mice immunized with GST-MSP119 variants were inversely correlated with peak parasitemia. This is in agreement with previous studies that have implicated antibodies to P. yoelii MSP119 as the main effector mechanism for protection (14, 28, 36). Specific IgG1 and IgG2a were found at higher levels in C57BL/6 and B10.D2 mice than in B10.BR or B10.A(4R) mice after immunization with GST-MSP119, providing a plausible explanation for the differential protection in these mouse strains. Moreover, the enhanced levels of these IgG subclasses may be responsible for the improved protection of B10.BR mice immunized with GST-P2-MSP119. Surprisingly, in the B10.A(4R) mice vaccinated with GST-P2-MSP119, a similar improvement of the protection was not observed despite increased IgG levels. However, three mice in this group had peak parasitemias comparable to that of the most protected B10.A(4R) mouse in the GST-MSP119 group.
Several pieces of evidence indicate an important role for IgG2a in protection against P. yoelii. Only the IgG2a fraction of isotype- and subclass-fractionated polyclonal antibodies to whole P. yoelii blood stage parasites was protective (60). Also, mice inoculated with recombinant Mycobacterium bovis BCG bacteria expressing MSP119 were partially protected against P. yoelii; this was accompanied by rapid production of MSP119-specific IgG2a (but not IgG1, IgG2b, or IgG3) during challenge infection (41). In contrast, in a study by Tian et al. (59), protection following immunization with recombinant GST-MSP119 was associated with high levels of MSP119-specific IgG1 and Hirunpetcharat et al. (28) found associations with IgG1 and IgG2b. The association between levels of both IgG1 and IgG2a to MSP119 and low peak parasitemia found in this study suggests that both of these IgG subclasses mediate protection against P. yoelii. Differences in immunization protocols and use of different mouse strains may to some extent underlie the different associations observed in these studies, but the choice of detection reagents can also affect measurement of IgG subclasses, especially IgG2a (40).
The protective capacity of the IgG subclasses could relate to their differential recognition by Fcγ receptors and thus their ability to trigger cell-mediated parasite clearance (19, 24, 49). However, a recent study showed that Fcγ-receptor γ-chain knockout mice were protected by passive transfer of antiserum to MSP119 (50). Since IgG1, IgG2a, and IgG2b cannot mediate antibody-dependent cell-mediated cytotoxicity or phagocytosis in such mice and since antisera with low levels of IgG3 were used, the observed protection suggests that IgG specific for MSP119 can exert a direct inhibitory effect on the parasite (50). However, it does not follow that the subclass of the IgG response to MSP119 is irrelevant to its protective capacity. IgG Fc regions vary in size, shape, and mobility and can thus affect the ability of Fab regions to gain access to, and bind to, their binding site. Also, complement-mediated mechanisms relying on specific IgG subclasses may also contribute to protective immunity.
In mice immunized with GST-P8-MSP119, protection was improved despite a relatively small increase of IgG levels compared to those in mice immunized with GST-MSP119. Similarly, improved protection was also observed in B10.A(4R) mice immunized with GST-free P8-MSP119 despite a modest increase of IgG levels in comparison with those in MSP119-immunized mice (data not shown). Although even a slight increase in IgG is likely to have a positive effect on protection, other explanations could be sought as well. For example, antibodies with different fine specificities for P. falciparum MSP119 exert variable effects on the processing of MSP142. Some antibodies inhibit the proteolytic processing, whereas others do not and may even block the effect of the inhibitory antibodies (9, 45). Whether or not IgG antibodies with such distinct activities are induced by P. yoelii MSP119 is not known. To examine if the groups of mice in the present study displayed different epitope specificities, antisera were analyzed for the capacity to inhibit the binding of MAb to P. yoelii MSP119. Antisera from well-protected mice generally displayed better inhibition, but the inhibition appeared to be related to the total level of MSP119-reactive IgG rather than reflecting drastically different epitope specificities between groups. A recent study indicated that the avidity of IgG elicited by GST-MSP119 is of importance for protection against P. yoelii. Prechallenge sera from protected mice vaccinated with GST-MSP119 protein not only had higher levels of multiple IgG subclasses but also displayed a higher avidity for MSP119 than sera from nonprotected mice immunized by DNA vaccination (32). It was also observed that B10.BR mice immunized with GST-MSP119 in Freund's adjuvant responded with lower avidity than C57BL/10 mice (32). Thus, since high-avidity responses depend on somatic mutation in B cells, which is a T-cell-dependent process, inclusion of additional T-cell epitopes in GST-MSP119 may improve the protection by enhancing IgG avidity as well as antibody titers.
In summary, the genetically restricted protection against P. yoelii induced by GST-MSP119 in mice can be overcome by inserting additional T-cell epitopes into the immunogen. Importantly, both T-cell epitopes used in the present study could enhance the IgG responses to MSP119 but one of them (P2) also modulated the IgG subclass response. The enhanced IgG responses induced by the modified GST-MSP119 variants are likely to explain the improved protection, as the prechallenge levels of both IgG1 and IgG2 were inversely correlated with peak parasitemia after infection. Whether or not inclusion of additional T-cell epitopes in a P. falciparum MSP119 vaccine will overcome problems of low immunogenicity and poor T-cell activation associated with immune responses to P. falciparum MSP119 in humans (21) remains to be investigated.
ACKNOWLEDGMENTS
We thank Wendy Howard for excellent technical assistance, David Walliker for providing parasites, Sola Ogun for purification of native MSP1, and Sue Fleck and Lilian Spencer Valero for monoclonal antibodies.
This work was supported by the Wenner-Gren Center Foundation and an EU Marie Curie grant, and partially by the Swedish Medical Research Council, The Swedish Institute, the Wellcome Trust, and the Wallenberg Foundation.
REFERENCES
- 1.Ahlborg N. Synthesis of a diepitope MAP containing two malaria antigen sequences using Fmoc chemistry. J Immunol Methods. 1995;179:269–275. doi: 10.1016/0022-1759(94)00328-t. [DOI] [PubMed] [Google Scholar]
- 2.Ahlborg N, Paulie S, Braesch-Andersen S. Generation of antibodies to human IL-12 and amphiregulin by immunization of Balb/c mice with diepitope multiple antigen peptides. J Immunol Methods. 1997;204:23–32. doi: 10.1016/s0022-1759(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 3.Ahlborg N, Sterky F, Haddad D, Perlmann P, Nygren P-Å, Andersson R, Berzins K. Predominance of H-2d and H-2k-restricted T-cell epitopes in the highly repetitive Plasmodium falciparum antigen Pf332. Mol Immunol. 1997;34:379–389. doi: 10.1016/s0161-5890(97)00046-1. [DOI] [PubMed] [Google Scholar]
- 4.Amante F, Good M. Prolonged Th1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol. 1997;19:111–126. doi: 10.1046/j.1365-3024.1997.d01-187.x. [DOI] [PubMed] [Google Scholar]
- 5.Blackman M, Dennis E, Hirst E, Kocken C, Scott-Finnigan T, Thomas A. Plasmodium knowlesi: secondary processing of the malaria merozoite surface protein-1. Exp Parasitol. 1996;83:229–239. doi: 10.1006/expr.1996.0069. [DOI] [PubMed] [Google Scholar]
- 6.Blackman M, Heidrich H, Donachie S, McBride J, Holder A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackman M, Holder A. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol. 1992;50:307–315. doi: 10.1016/0166-6851(92)90228-c. [DOI] [PubMed] [Google Scholar]
- 8.Blackman M, Ling I, Nicholls S, Holder A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–33. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 9.Blackman M, Scott-Finnigan T, Shai S, Holder A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burghaus P, Wellde B, Hall T, Richards R, Egan A, Riley E, Ballou W, Holder A. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect Immun. 1996;64:3614–3619. doi: 10.1128/iai.64.9.3614-3619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanagh D, McBride J. Antigenicity of recombinant proteins derived from Plasmodium falciparum merozoite surface protein 1. Mol Biochem Parasitol. 1997;85:197–211. doi: 10.1016/s0166-6851(96)02826-5. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Case S, Gosnell W, Hashimoto A, Kramer K, Tam L, Hshiro C, Nikaido C, Gibson H, Lee-Ng C, Barr P, Yokota B, Hui G. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comoy E, Capron A, Thyphronitis G. In vivo induction of type 1 and 2 immune responses against protein antigens. Int Immunol. 1997;9:523–531. doi: 10.1093/intimm/9.4.523. [DOI] [PubMed] [Google Scholar]
- 14.Daly T, Long C. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 15.Daly T, Long C. Influence of adjuvants on protection induced by a recombinant fusion protein against malarial infection. Infect Immun. 1996;64:2602–2608. doi: 10.1128/iai.64.7.2602-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David P, Hadley T, Aikawa M, Miller L. Processing of a major parasite surface glycoprotein during the ultimate stages of differentiation in Plasmodium knowlesi. Mol Biochem Parasitol. 1984;11:267–282. doi: 10.1016/0166-6851(84)90071-9. [DOI] [PubMed] [Google Scholar]
- 18.De Kruyff R, Rizzo L, Umetsu D. Induction of immunoglobulin synthesis by CD4+ T cell clones. Semin Immunol. 1993;5:421–430. doi: 10.1006/smim.1993.1048. [DOI] [PubMed] [Google Scholar]
- 19.Diamond B, Yelton D. A new Fc receptor on mouse macrophages binding IgG3. J Exp Med. 1981;153:514–519. doi: 10.1084/jem.153.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan A, Morris J, Barnish G, Allen S, Greenwood B, Kaslow D, Holder A, Riley E. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 21.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 23.Freeman R, Holder A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin Exp Immunol. 1983;54:609–616. [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin A, Barnes N, Dijstelbloem H, Hogarth P. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 25.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kolsch E, Podlaski F, Gately M, Rude E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 26.Gill S, von Hippel P. Calculation of protein extinction coefficients from amino acid sequence data Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. . (Erratum, 189:283, 1990.) [DOI] [PubMed] [Google Scholar]
- 27.Hayglass K, Stefura W. Antigen-specific inhibition of ongoing murine IgE responses. II. Inhibition of IgE responses induced by treatment with glutaraldehyde-modified allergens is paralleled by reciprocal increases in IgG2a synthesis. J Immunol. 1991;147:2455–2460. [PubMed] [Google Scholar]
- 28.Hirunpetcharat C, Tian J, Kaslow D, van Rooijen N, Kumar S, Berzofsky J, Miller L, Good M. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 29.Holder A A, Freeman R R. Characterization of a high molecular weight protective antigen of Plasmodium yoelii. Parasitology. 1984;88:211–219. [PubMed] [Google Scholar]
- 30.Howard R, Pasloske B. Target antigens for asexual malaria vaccine development. Parasitol Today. 1993;9:369–372. doi: 10.1016/0169-4758(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 31.Hui G S, Hashimoto C N. Pathways for potentiation of immunogenicity during adjuvant-assisted immunizations with Plasmodium falciparum major merozoite surface protein 1. Infect Immun. 1998;66:5329–5336. doi: 10.1128/iai.66.11.5329-5336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang Y, Calvo P, Daly T, Long C. Comparison of humoral immune responses elicited by DNA and protein vaccines based on merozoite surface protein-1 from Plasmodium yoelii, a rodent malaria parasite. J Immunol. 1998;161:4211–4219. [PubMed] [Google Scholar]
- 33.Kumar A, Arora R, Kaur P, Chauhan V, Sharma P. “Universal” T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J Immunol. 1992;148:1499–1505. [PubMed] [Google Scholar]
- 34.Kumar S, Yadava A, Keister D, Tian J, Ohl M, Perdue-Greenfield K, Miller L, Kaslow D. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis A. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol Biochem Parasitol. 1989;36:271–282. doi: 10.1016/0166-6851(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 36.Ling I, Ogun S, Holder A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 37.Ling I, Ogun S, Holder A. The combined epidermal growth factor-like modules of Plasmodium yoelii merozoite surface protein-1 are required for a protective immune response to the parasite. Parasite Immunol. 1995;17:425–433. doi: 10.1111/j.1365-3024.1995.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 38.Ling I, Ogun S, Momin P, Richards R, Garcon N, Cohen J, Ballou W, Holder A. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15:1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 39.Majarian W, Daly T, Weidanz W, Long C. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984;132:3131–3137. [PubMed] [Google Scholar]
- 40.Martin R, Brady J, Lew A. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto S, Yukitake H, Kanbara H, Yamada T. Recombinant Mycobacterium bovis bacillus Calmette-Guérin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J Exp Med. 1998;188:845–854. doi: 10.1084/jem.188.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosmann T, Coffman R. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 43.O'Dea K P, McKean P G, Harris A, Brown K N. Processing of the Plasmodium chabaudi chabaudi AS merozoite surface protein 1 in vivo and in vitro. Mol Biochem Parasitol. 1995;72:111–119. doi: 10.1016/0166-6851(95)00090-n. [DOI] [PubMed] [Google Scholar]
- 44.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 45.Patino J, Holder A, McBride J, Blackman M. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J Exp Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perera K, Handunetti S, Holm I, Longacre S, Mendis K. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect Immun. 1998;66:1500–1506. doi: 10.1128/iai.66.4.1500-1506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters W. Chemotherapy of Plasmodium chabaudi infection in albino mice. Ann Trop Med Parasitol. 1967;61:52–56. doi: 10.1080/00034983.1967.11686457. [DOI] [PubMed] [Google Scholar]
- 48.Qari S, Shi Y-P, Goldman I, Nahlen B, Tibayrenc M, Lal A. Predicted and observed alleles of Plasmodium falciparum merozoite surface protein-1 (MSP1), a potential malaria vaccine antigen. Mol Biochem Parasitol. 1998;92:241–252. doi: 10.1016/s0166-6851(98)00010-3. [DOI] [PubMed] [Google Scholar]
- 49.Ravetch J, Kinet J-P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 50.Rotman H, Daly T, Clynes R, Long C. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J Immunol. 1998;161:1908–1912. [PubMed] [Google Scholar]
- 51.Shai S, Blackman M, Holder A. Epitopes in the 19kDa fragment of the Plasmodium falciparum major merozoite surface protein-1 (PfMSP-1(19)) recognized by human antibodies. Parasite Immunol. 1995;17:269–275. doi: 10.1111/j.1365-3024.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Sayed U, Qari S, Roberts J, Udhayakumar V, Oloo A, Hawley W, Kaslow D, Nahlen B, Lal A. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjölander A, Ahlborg N, Ståhl S, Andersson R. Characterization of immune responses to experimental polyvalent subunit vaccines assembled in iscoms. Mol Immunol. 1998;35:159–166. doi: 10.1016/s0161-5890(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 54.Smith D, Johnson K. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;6:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 55.Spencer Valero L M, Ogun S A, Fleck S L, Ling I T, Scott-Finnigan T J, Blackman M J, Holder A A. Passive immunization with antibodies against three distinct epitopes on Plasmodium yoelii merozoite surface protein 1 suppresses parasitemia. Infect Immun. 1998;66:3925–3930. doi: 10.1128/iai.66.8.3925-3930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tam J. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigen system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanabe K, Mackay M, Goman M, Scaife J. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 58.Tian J H, Good M F, Hirunpetcharat C, Kumar S, Ling I T, Jackson D, Cooper J, Lukszo J, Coligan J, Ahlers J, Saul A, Berzofsky J A, Holder A A, Miller L H, Kaslow D C. Definition of T cell epitopes within the 19 kDa carboxylterminal fragment of Plasmodium yoelii merozoite surface protein 1 (MSP1(19)) and their role in immunity to malaria. Parasite Immunol. 1998;20:263–278. doi: 10.1046/j.1365-3024.1998.00138.x. [DOI] [PubMed] [Google Scholar]
- 59.Tian J H, Miller L, Kaslow D, Ahlers J, Good M, Alling D, Berzofsky J, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]
- 60.White W, Evans C, Taylor D. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991;59:3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C, Collins W E, Sullivan J S, Kaslow D C, Xiao L, Lal A A. Partial protection against Plasmodium vivax blood-stage infection in Saimiri monkeys by immunization with a recombinant C-terminal fragment of merozoite surface protein 1 in block copolymer adjuvant. Infect Immun. 1999;67:342–349. doi: 10.1128/iai.67.1.342-349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang C, Collins W, Xiao L, Patterson P, Reed R, Hunter R, Kaslow D, Lal A. Influence of adjuvants on murine immune responses against the C-terminal 19 kDa fragment of Plasmodium vivax merozoite surface protein-1 (MSP-1) Parasite Immunol. 1996;18:182–185. doi: 10.1046/j.1365-3024.1996.d01-32.x. [DOI] [PubMed] [Google Scholar]
- 63.Yoeli M, Hargreaves B, Carter R, Walliker D. Sudden increase in virulence in a strain of Plasmodium berghei yoelii. Ann Trop Med Parasitol. 1975;69:173–178. doi: 10.1080/00034983.1975.11686998. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Mohaptra S S. Antigen- and isotype-specific immune responses to a recombinant antigen-allergen chimeric (RAAC) protein. J Immunol. 1993;151:791–799. [PubMed] [Google Scholar]