Abstract
Campylobacter fetus is a cause of enteritis and invasive extraintestinal disease in humans. In order to develop an animal model of C. fetus infection, outbred ICR SCID mice were orally challenged with a clinical isolate of C. fetus. The stomachs of SCID mice were heavily colonized with C. fetus, and colonization was associated with the development of chronic atrophic gastritis. This lesion was characterized by an inflammatory infiltrate of granulocytes and macrophages that over time resulted in a loss of specialized parietal and chief cells in the corpus and the appearance of a metaplastic mucous epithelium. This lesion bears similarity to that encountered during experimental murine infection with Helicobacter pylori or Helicobacter felis. Despite colonization of the cecum and colon tissues by C. fetus in SCID mice, no lesions were noted in these tissues. A follow-up study confirmed these findings for SCID mice and also demonstrated that C. fetus could also infect the gastric mucosa of wild-type, outbred ICR mice. However, in ICR mice, the anatomic extent of colonization was more limited and the severity of inflammation and epithelial alterations was significantly less than that observed in infected SCID mice. The stomach may represent an unrecognized environmental niche for Campylobacter species.
Campylobacter species are important causes of enteritis and invasive disease in humans. In the United States, C. jejuni and C. coli are the most common causes of bacterial enteritis (3). C. fetus subsp. fetus (referred to as C. fetus) is also a cause of enteritis but is generally isolated as a cause of bacteremia and invasive extraintestinal infections in patients with underlying disease. It has been associated with septic arthritis, mycotic aneurysms, endocarditis, septic thrombophlebitis, salpingitis, spontaneous bacterial peritonitis, meningitis, and cholecystitis (8). The prevalence of C. fetus enteritis is probably underestimated because many strains do not grow well under the selective culture conditions developed specifically for the isolation of C. jejuni and C. coli from stool (2, 8).
Experimental infection of ferrets and nonhuman primates by C. jejuni and C. coli can result in acute enteritis (15, 27). In addition, a number of mouse models of infection with Campylobacter species have been described. A major shortcoming of many of the murine models is the failure to reproduce the most common symptom encountered in human infections, namely, enteritis. A number of models that result in stable colonization of mice with C. jejuni, without the development of diarrhea, have been introduced. They have been used to demonstrate the importance of several colonization factors in establishment of mucosal colonization (10, 25). In addition, colonization models have been used to aid in the development of potential vaccines against C. jejuni (1, 4, 6). In other models, pretreatment of mice with iron gives lethality as a measurable end point following C. fetus infection (24). Intranasal challenge with C. jejuni also results in measurable lethality, but this is not the usual route of infection among mammals (5).
Immunocompromised mice have been challenged with Campylobacter species. Athymic germfree mice are consistently colonized with C. fetus, without observable mortality or morbidity (33, 34). At necropsy, the gastrointestinal tracts of infected mice are grossly normal and no specific histopathologic lesions are observed. Conversely, athymic germfree mice challenged with C. jejuni develop clinical enteritis and inflammation of the lower gastrointestinal tract (33, 34).
The previous studies used germfree mice, which are known to have altered expression of mucosal antigens that may result in a different environmental niche being presented to the challenge microorganisms as well as the absence of competition from resident microbiota (13). In fact, when a normal fecal microbiota was introduced to ex-germfree mice monoassociated with Campylobacter, the Campylobacter species could no longer be cultured from the feces (33). This is in contrast to the majority of studies, in which mice with a normal fecal microbiota are persistently colonized with C. jejuni (1, 7, 25). In the present study we extended the previous studies of Campylobacter infection in immunocompromised mice by challenging outbred SCID mice colonized with a normal fecal microbiota with fresh clinical isolates of Campylobacter species.
MATERIALS AND METHODS
Animals and housing.
Four-week old Tac:Icr:Ha(ICR) and Tac:Icr:Ha(ICR) scid/scid (severe combined immunodeficient [SCID]) mice, free of murine pathogens including all Helicobacter species, were obtained from Taconic Farms (Germantown, N.Y.). A pilot experiment was performed with male and female SCID mice. As similar results were obtained for both sexes, male animals were used in the follow-up experiment. Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility in groups of five animals, separated by sex, in sterile polycarbonate microisolator cages. For SCID animals, all food, water, and bedding were autoclaved. All experiments were approved by the MIT Animal Care and Use Committee.
Bacterial strains and culture conditions.
Campylobacter species used in this study were isolated during a clinical study designed to characterize microaerobic spiral bacteria isolated from clinical stool samples (Young, Ferraro, Kachoris, Murtagh, Dewhirst, and Schauer, submitted for publication). C. fetus strain MGH 97-3574 was isolated from a human immunodeficiency virus (HIV)-infected patient who presented with an acute episode of enteritis manifested as 2 weeks of diarrhea and fever. C. fetus strain MGH 97-2126 was isolated from a patient with acute diarrhea and no underlying disease. C. hyointestinalis strain MGH 97-2652 was isolated from an HIV-infected patient who presented with an acute diarrheal illness. Species level identification of these strains was based on routine biochemical characterization (including catalase, oxidase, and urease activity, hippurate and indoxyl acetate hydrolysis, and sensitivity to cephalothin and nalidixic acid) and was confirmed by determination of the complete 16S rRNA gene sequence.
After minimal passage (less than five passages) on tryptic soy agar (TSA) supplemented with 5% sheep blood, bacteria were stored at −70°C in tryptic soy broth with 40% glycerol. Campylobacter species were grown at 37°C in a microaerobic environment which was maintained in vented GasPak jars without a catalyst by evacuation to −20 mm Hg and then repressurization with a gas mixture consisting of 80% N2, 10% H2, and 10% CO2 to yield a final O2 concentration of 5% (17).
For infection studies, bacteria were harvested after 48 h of growth and resuspended in a small volume of tryptic soy broth. The optical density at 600 nm (OD600) of the inoculum was measured. Tenfold serial dilutions of the inoculum were plated to quantify the CFU used for infection.
Experimental infection.
In a pilot infection study, five male and five female SCID mice were challenged with C. fetus isolates MGH 97-3574 and MGH 97-2126 and C. hyointestinalis isolate MGH 97-2652, which were administered as a mixed inoculum. Prior to inoculation, it was determined that the three different bacterial strains could be distinguished on the basis of randomly amplified polymorphic DNA (RAPD)-PCR. As controls, five male and five female SCID mice were inoculated with sterile broth only. In a follow-up experiment, 20 immunocompetent ICR mice and 20 immunocompromised SCID mice were challenged orally with C. fetus strain MGH 97-3574.
Mice were inoculated with a suspension of bacteria with an OD600 of 1.0 (∼108 CFU) in a volume of 0.2 to 0.3 ml. For the pilot study involving mixed infection with C. fetus strains MGH 97-3574 and MGH 97-2126 and C. hyointestinalis strain MGH 97-2652, approximately equal numbers of each strain were combined into a single inoculum with an OD600 of 1.0. Bacteria were introduced directly into the stomach with a 24-gauge ball-tipped gavage needle. Mice were challenged with a total of three equal doses of bacteria on three alternating days. An equal number of control mice were inoculated with sterile tryptic soy broth.
Necropsy and histopathology.
At 1 week, 3 weeks, and 3 months in the initial experiment and at 1, 3, and 6 months in the follow-up study, mice were euthanized by CO2 asphyxiation. The stomach, the liver, and small and large bowel were fixed in 10% neutral buffered formalin and processed for histopathologic evaluation.
After 24 h of fixation in formalin, tissue samples were processed and embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin and by the Warthin-Starry method. Sections of the gastrointestinal mucosa were examined histologically for inflammatory and epithelial changes and for the presence of argyrophilic spiral organisms. Epithelial hyperplasia, atrophy (loss of normal corpus gland morphology), and inflammation were graded semiquantitatively as 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), or 4 (marked) based on the extent and intensity of the alteration.
Microbiological culture.
A single, fresh fecal pellet was collected from each mouse for culture. Feces were homogenized in 0.5 ml of phosphate-buffered saline, and 50 μl was plated on TSA supplemented with 5% sheep blood and 20 μg of cefoperazone, 10 μg of vancomycin, and 2 μg of amphotericin B/ml (TSA-CVA).
Portions of the antrum of the stomach, the distal ileum, the cecum, and the mid-colon were collected for culture at necropsy. The tissue was rinsed in sterile phosphate-buffered saline, and the mucosal surface was applied to a TSA-CVA plate. After application of the mucosal surface to the surface of the agar, the tissue was discarded, and a sterile, disposable inoculating loop was used to spread the sample over the surface of the plate.
Detection of spiral organisms in feces by multiplex PCR.
DNA was extracted from feces and subjected to analysis using a multiplex PCR assay as described previously (Young et al., submitted). This assay can detect and discriminate between bacteria belonging to the genera Arcobacter, Campylobacter, and Helicobacter.
RAPD-PCR.
RAPD-PCR was performed using the primer 5′-CAATCGCCGT-3′ as previously described (19).
Statistics.
Histologic scores for the different experimental groups were compared by nonparametric methods (Mann-Whitney U test) using StatView, version 5.0, for Macintosh (SAS Institute Inc., Cary, N.C.).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA genes of the clinical isolates described in this study have been entered in the GenBank database under accession numbers AF219233 (MGH 97-2126), AF219234 (MGH 97-3574), and AF219245 (MGH 97-2652).
RESULTS
Campylobacter infection of SCID mice results in persistent colonization and the development of gastritis.
To establish an SCID mouse model of infection with Campylobacter species, a pilot study was undertaken with low-passage clinical isolates. Animals were challenged with a mixed inoculum of two C. fetus isolates and one C. hyointestinalis isolate.
All mice challenged with the mixed inoculum of Campylobacter species shed microaerobic spiral bacteria in their feces throughout the 3 months of the experiment. Three independent bacterial isolates from each mouse were analyzed by RAPD-PCR. All isolated organisms were identified as C. fetus strain MGH 97-3574. C. fetus strain MGH 97-2126 and C. hyointestinalis strain MGH 97-2652 were never isolated from any of the mice after challenge with the mixed inoculum. Multiplex PCR of DNA extracted from the feces of infected mice detected Campylobacter at all time points (data not shown). No Helicobacter or Arcobacter was detected in these animals. PCR and culture for microaerobic spiral organisms were negative in all control animals at all time points.
Throughout the experiment, none of the animals displayed any clinical signs of disease. No diarrhea was noted, and there was no mortality among the infected or control animals.
At all time points microaerobic spiral organisms could be cultured from the stomach, distal ileum, cecum and colon tissues of infected animals at necropsy. PCR of these tissues was positive for Campylobacter, and RAPD-PCR of organisms obtained by culture demonstrated that these organisms were C. fetus MGH 97-3574.
At all time points the liver, small intestine, cecum, and colon tissues of infected mice were histologically similar to those of uninfected controls. Marked lesions in the stomach tissue of infected mice were noted. One week postinfection (p.i.), spiral organisms in the pyloric region were noted, with accompanying hyperplasia and inflammation. Three weeks p.i., there was expansion of the area of colonization proximally to involve the antrum, again with hyperplasia and inflammation. By 3 months p.i. there was further expansion of colonization with organisms found in the corpus. Hyperplasia and inflammation now extended into the corpus as well as the antrum. Additionally, the corpus tissue of infected animals was notable for the development of an atrophic gastritis in which local inflammation was accompanied by loss of specialized parietal and chief cells and the appearance of mucous metaplasia (data not shown).
C. fetus strain MGH 97-3574 colonizes both ICR and SCID mice.
To confirm the finding of atrophic gastritis in mice challenged with a mixture of Campylobacter species where C. fetus strain MGH 97-3574 was the only apparent successful colonizer, a follow-up study using this C. fetus strain was performed. For this experiment, both wild-type ICR and SCID mice were infected to determine the influence of the adaptive immune system on the development of gastritis.
One week p.i., all mice (ICR and SCID) that had been challenged were culture positive for microaerobic spiral organisms. RAPD-PCR fingerprints of randomly selected isolated colonies matched the fingerprint of the input C. fetus strain (data not shown). All infected animals continued to shed C. fetus for the duration of the experiment. Multiplex PCR on feces from infected animals detected only Campylobacter species. No evidence of colonization with Helicobacter or Arcobacter was ever found (data not shown). Throughout the experiment, none of the animals displayed any clinical signs of disease. No diarrhea was noted, and there was no mortality among the infected or control animals. Control animals were negative for microaerobic spiral bacteria by culture and PCR throughout the study.
C. fetus strain MGH 97-3574 causes chronic atrophic gastritis in SCID mice.
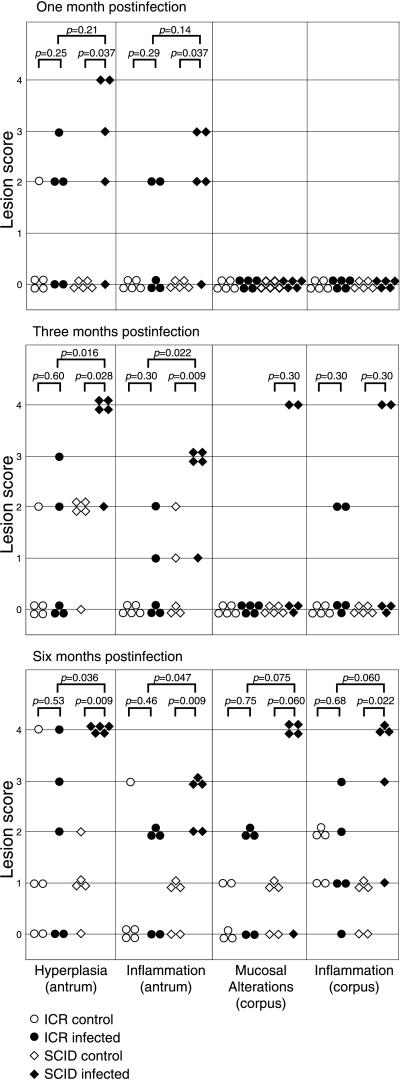
One month after infection, the stomachs of four of five SCID mice infected with C. fetus were notable for hyperplasia and inflammation involving the antrum. Warthin-Starry silver stain revealed the presence of large numbers of spiral bacteria in the mucosa of the pylorus and distal antrum of SCID mice with hyperplasia and inflammation (Fig. 1A and B). The inflammatory infiltrate consisted primarily of granulocytes accompanied by macrophages and involved both the mucosa and submucosa (Fig. 2). No inflammation or hyperplasia was seen in the corpus mucosa 1 month after infection (Fig. 3).
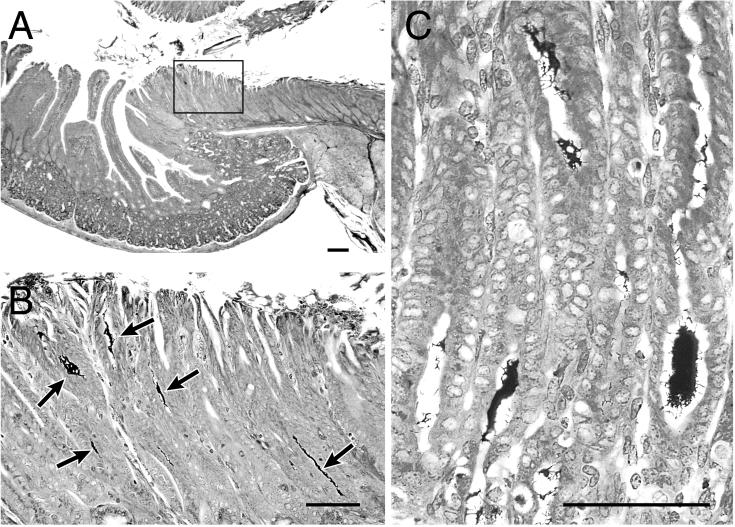
FIG. 1.
Colonization of the stomach tissue of ICR and SCID mice with C. fetus. Shown are low-power (A) and high-power (B) views of the pylorus of an ICR mouse 1 month after infection with C. fetus. Numerous argyrophilic spiral bacteria (arrows) are localized in the antral mucosa at the level of the pyloric sphincter. (C) High-power view of spiral organisms in the mucosa from the corpus of a SCID mouse 3 months after infection with C. fetus. The normal glandular zone has been replaced by a mucous epithelium. Warthin-Starry stain. Bar, 200 μm.
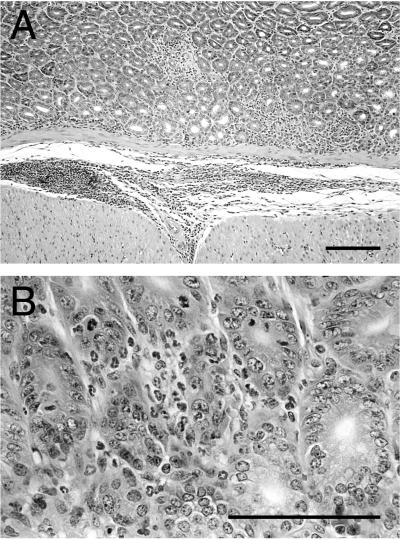
FIG. 2.
Antral inflammation in a SCID mouse 1 month after infection with C. fetus. (A) Low-power view of the antral mucosa demonstrating both mucosal and submucosal inflammation. (B) Higher-power view of the antral mucosa with an inflammatory infiltrate composed primarily of granulocytes. Hematoxylin and eoson stain. Bar, 200 μm.
FIG. 3.
Histopathologic scoring of stomach lesions in ICR and SCID mice infected with C. fetus. Five animals in each experimental group were necropsied 1, 3, and 6 months after infection. Each animal was graded for the degree of hyperplasia and inflammation in the antrum and for inflammation and mucosal alterations (loss of parietal and chief cells and mucous metaplasia) in the corpus. Experimental groups were compared by the Mann-Whitney U test.
Three months after infection, the gastritis in SCID mice had progressed. All infected SCID mice had marked antral hyperplasia and inflammation (Fig. 4), and the mucosa remained heavily colonized with argyrophilic spiral organisms. The region of colonization at 3 months expanded proximally from the pylorus to include the entire antrum and the antrum/corpus transitional zone. Additionally, in two of five infected SCID mice, colonization of the corpus was seen (Fig. 1C). In these two mice, there was loss of parietal and chief cells, with replacement by a hyperplastic and metaplastic mucous epithelium and a brisk submucosal and mucosal inflammatory infiltrate composed primarily of granulocytes with macrophages (Fig. 5). This was the same chronic atrophic gastritis lesion that was observed during the pilot study at 3 months p.i.
FIG. 4.
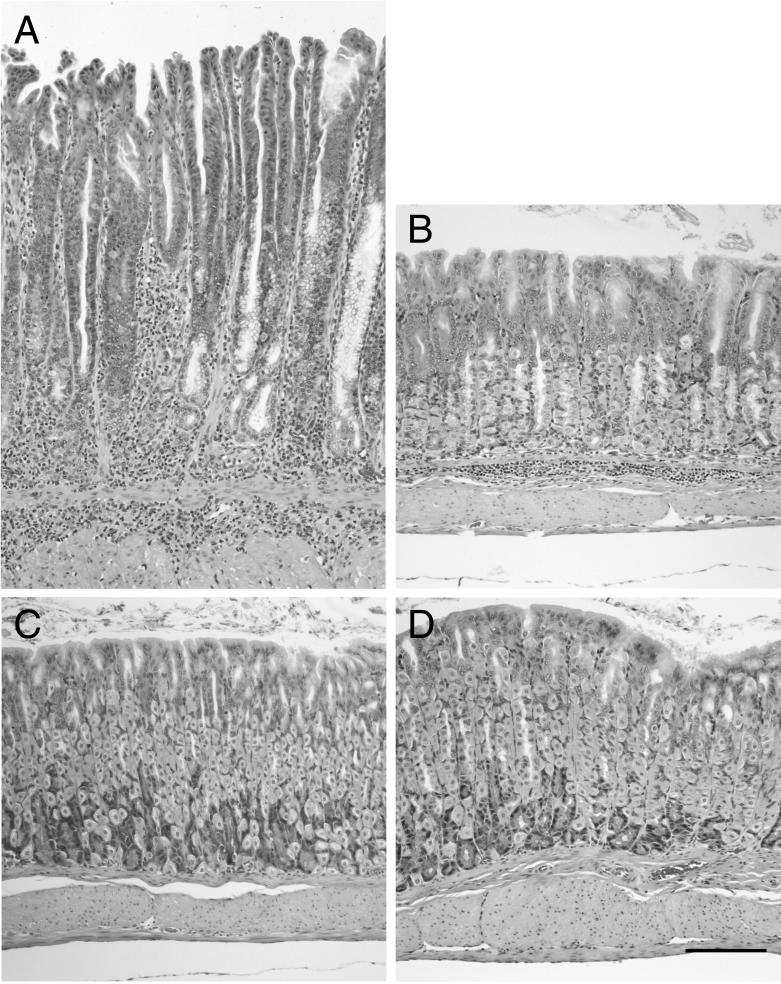
Antral inflammation and hyperplasia in ICR and SCID mice 3 months after infection with C. fetus. The antral mucosa of an infected SCID mouse (A) is markedly hyperplastic compared to that of an uninfected control (C). A predominantly granulocytic inflammatory infiltrate accompanied by macrophages is present. The antrum from an infected ICR mouse (B) has a lesser degree of inflammation and hyperplasia than that observed in infected SCID mice. The antrum from an uninfected control ICR mouse (D) is shown for comparison. Hematoxylin and eosin stain. Bar, 200 μm.
FIG. 5.
Corpus gastritis in ICR and SCID mice 3 months after infection with C. fetus. The mucosa from the corpus of an infected SCID mouse (A) is markedly hyperplastic compared to that of an uninfected control (C). The mucosa and submucosa contain a predominantly granulocytic inflammatory infiltrate accompanied by macrophages. There is marked loss of parietal cells and chief cells and replacement by a metaplastic mucous epithelium. An infected ICR mouse that had mild corpus colonization with spiral bacteria (B) has a mild, focal, submucosal inflammatory infiltrate. Widespread loss of parietal cells is not seen, although chief cells are not prominent. The overall mucosal height is similar to that of an uninfected control (D). Hematoxylin and eosin stain. Bar, 200 μm.
By 6 months after infection, four of five infected SCID mice had extensive corpus colonization with C. fetus and associated gastritis. There was further progression of the epithelial alterations originally observed 3 months p.i. Chronic atrophic gastritis persisted, but the mucosal hyperplasia that was predominant at 3 months was no longer apparent. Instead, there was a reduction in total mucosal height at 6 months p.i. (Fig. 6). Other mucosal lesions included vascular congestion in the superficial mucosa, focal erosions of the surface epithelium, and minute hemorrhages into the lamina propria. These changes coincided with the gross observation of dark, granular material consistent with partially digested blood in the gastric luminal contents apposed to the mucosa. No frank mucosal ulcerations were seen. All infected SCID mice, including the one animal without significant corpus gastritis, had marked hyperplasia and inflammation involving the antrum (Fig. 3). Only limited antral inflammation and hyperplasia were observed in uninfected control mice, significantly less than that observed in infected animals (Fig. 3). No corpus gastritis was observed in control mice, and organisms were not observed in gastric sections from control animals.
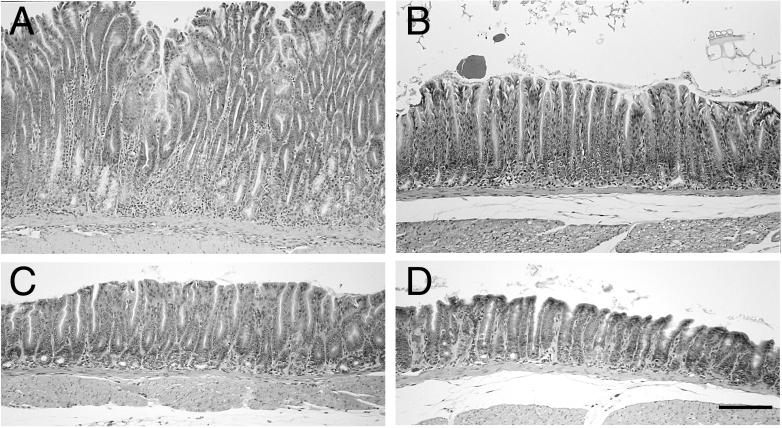
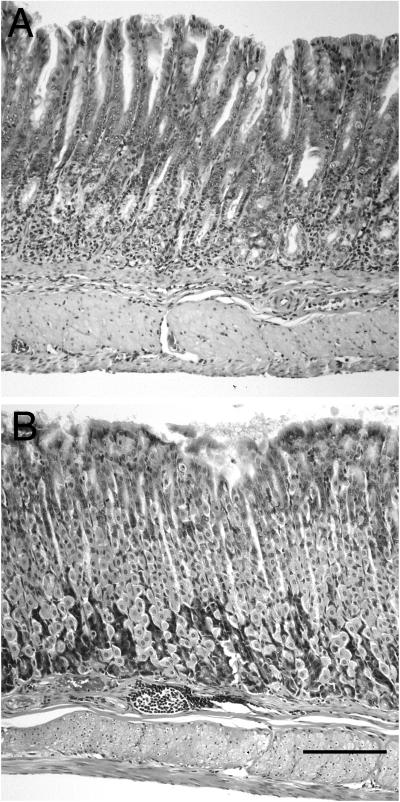
FIG. 6.
Corpus gastritis in ICR and SCID mice 6 months after infection with C. fetus. The mucosa from the corpus of an infected SCID mouse (A) shows continued inflammation and loss of parietal and chief cells. A metaplastic mucous epithelium remains, but the hypertrophy seen at 3 months is no longer seen, and instead there is a decrease in mucosal height. Infected ICR mice had focal areas of monocytic inflammatory infiltrate (B). However, similar lesions were also seen in uninfected control ICR mice. The severe mucosal alterations with loss of specialized cells and mucous metaplasia were not seen in infected ICR mice. Hematoxylin and eosin stain. Bar, 200 μm.
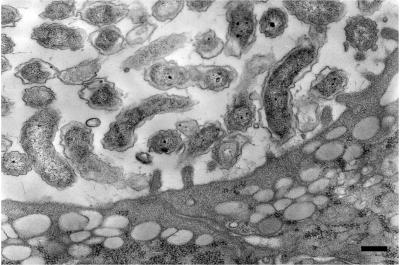
Warthin-Starry staining of gastric sections from infected SCID mice revealed the presence of large numbers of bacteria, packing many of the glands (Fig. 1C). Some of these organisms appeared to be in close apposition to cells. Electron microscopy confirmed the presence of large numbers of curved bacteria in the gastric glands (Fig. 7). Although bacteria were observed in close proximity to the apical surface of host cells, no intimate adherence to or alteration in the ultrastructure of host cells was seen.
FIG. 7.
Transmission electron micrograph of infected gastric gland from a SCID mouse 6 months after infection with C. fetus. Large numbers of curved bacteria are seen in the lumen of the gland. The ultrastructure of the epithelial cells is unaltered. Although bacteria are in close proximity to apical surfaces of the epithelial cells, no intimate adherence is observed. Bar, 200 nm.
In contrast, 1 month after infection with C. fetus strain MGH 97-3574, three of five immunocompetent ICR mice exhibited colonization confined to the pyloric region that was visible by Warthin-Starry staining. Mild mucosal hyperplasia and inflammation were also present and tended to be less severe than that in infected SCID mice, although this difference did not reach statistical significance (Fig. 3). The inflammatory infiltrate in the ICR mice was composed of scattered foci of mononuclear cells without an active, granulocytic component.
Three months after infection, the ICR mice remained colonized in the distal antrum and pylorus of the stomach. The expansion of colonization to the proximal antrum that was observed in the SCID mice was not seen in ICR mice at this time point. Mild mucosal hyperplasia and inflammation persisted in the distal antrum but did not progress to the same degree as that observed in the SCID mice (Fig. 4). The degree of antral hyperplasia and inflammation in infected animals was not significantly greater than that in controls and was much less than that encountered in infected SCID animals at 3 months (Fig. 3). Two infected ICR mice did have foci of spiral bacteria in the mucosa of the corpus. These bacteria were located in the squamocolumnar transition zone (the “cardia equivalent” of the murine stomach). In these mice, there was moderate focal, submucosal inflammation, with a minor component of mucosal inflammation (Fig. 5). However, the widespread loss of parietal and chief cells that was observed in infected SCID mice was not seen in these ICR mice.
Six months p.i., all infected ICR mice remained colonized in the distal antrum and pylorus. In three animals, colonization extended proximally to involve that the mid-antrum. Minor inflammation and hyperplasia in the antrum persisted in infected ICR mice but remained significantly less than that observed in infected SCID mice (Fig. 3). Four of five infected ICR mice were noted to have scattered foci of lymphocytic submucosal inflammation involving the corpus (Fig. 6). The significance of these foci of inflammation is unclear, as similar lesions were found in control mice (Fig. 3). Small numbers of spiral bacteria were seen in the squamocolumnar transition zone of one infected ICR mouse. Minor epithelial alterations were seen in isolated regions of the corpus, manifested as focal loss of parietal cells and focal regions of mucous metaplasia. The widespread atrophic gastritis observed in the majority of infected SCID mice was not encountered in any ICR mice by 6 months after infection.
DISCUSSION
We describe a novel murine model of persistent infection with C. fetus that is characterized by gastric colonization and the development of chronic atrophic gastritis. To our knowledge, this is the first report of experimental Campylobacter-induced gastritis. Previous studies on germfree, athymic mice demonstrated that both C. jejuni and C. fetus are able to colonize the stomach, but no gastric lesions were noted (33, 34). In humans, a clear relationship between Helicobacter pylori infections and chronic gastritis, peptic ulcer disease, and gastric cancer has been established (9). Campylobacter species, however, are not generally thought to colonize the stomachs of humans. Campylobacter species do not possess urease activity, which has been shown to be required for gastric colonization by H. pylori in a mouse model (21, 30). C. jejuni has been cultured from a gastric biopsy of a patient with indomethacin-associated gastritis (28) who did not have evidence of H. pylori infection. It is not clear if this represents primary gastric colonization with C. jejuni and subsequent gastritis or secondary colonization of a preexisting gastric lesion.
Campylobacter species are generally thought to colonize the lower gastrointestinal tract in humans. Although we could isolate C. fetus from the ileum, cecum, and colon tissues of infected mice in the present study, no lesions were noted in these tissues. Lesions in both immunocompetent ICR mice and immunocompromised SCID mice were limited to the stomach. The gastric lesions seen in SCID mice after infection with C. fetus were particularly striking. These mice developed severe, chronic, active gastric inflammation that involved the entire antrum and by 6 months had progressed to involve the corpus of the majority of animals. Colonization and inflammation were associated with alterations in the cytology and architecture of the gastric mucosa. In the antrum this consisted of hyperplasia, and in the corpus this consisted initially of hyperplasia but progressed to atrophic gastritis, characterized by loss of parietal cells and chief cells and replacement by a metaplastic mucous epithelium. Over time, areas of overall mucosal atrophy developed in the corpus, with reduction of mucosal thickness and multifocal disruption of the surface epithelium. Frank mucosal ulceration was not seen however.
In both ICR and SCID mice, C. fetus initially colonized the antroduodenal transition zone (31). Over the 6-month time period of the study colonization expanded anatomically to a greater degree in SCID mice than in ICR mice. In animals that developed inflammation and mucosal changes in the corpus of the stomach, the gastric glands were colonized with bacteria. This expansion of colonization and inflammation mimics what has been described during long-term human infection with H. pylori (31). It is proposed that H. pylori initially colonizes the antrum-body transition zone, resulting in chronic inflammation and mucosal damage that eventually cause a decrease in local acid levels (31). This damage and changes in the local microenvironment at the proximal antrum-body transition zone allow the bacteria to colonize more-proximal portions of the stomach and thus induce a moving front of inflammation (31).
The above model can be used to explain the pathology seen in immunocompetent ICR mice and immunocompromised SCID mice in the present study. It is possible that ICR mice are able to mount an immune response in the setting of C. fetus infection, dependent on B and/or T cells, that is able to limit the extent of infection but that cannot eliminate the pathogen. There is little damage to the mucosa in infected ICR mice, and the bacteria remain localized to the initial area of colonization, which appears to be the antroduodenal transition zone. In addition, the transition zone between the glandular and squamous portions of the stomach (the cardia equivalent) may represent a second area permissive for colonization, similar to H. pylori colonization in humans (18). In SCID animals, colonization with C. fetus leads to the generation of a chronic, active inflammatory response composed chiefly of granulocytes and macrophages. We hypothesize that this immune response is also unable to eliminate the infection; however, it does lead to damage of the gastric mucosa including a loss of parietal cells. This mucosal damage leads to an expansion of the area of gastric mucosa that can support colonization by C. fetus, presumably via a decrease in local acid levels due to a loss of parietal cell mass. The end result is a progression of infection from the antroduodenal transition zone through the antrum to the corpus of the stomach.
Mice have been employed as model systems for infection by the gastric Helicobacter species H. pylori and H. felis. Different strains of mice exhibit varied histopathologic responses to infection with Helicobacter (14, 22, 23, 29, 32). Certain strains, exemplified by C57BL/6 mice, mount an intense inflammatory response to infection with H. pylori or H. felis (16, 22). These mice also develop marked histological changes in the corpus mucosa consisting of loss of specialized cells and the appearance of mucous metaplasia. Other mouse strains, exemplified by BALB/c mice, although chronically colonized by Helicobacter, develop minimal gastric inflammation, and marked alterations in the gastric mucosa are not encountered (29). Instead, after long-term (>22-month) infection, these mice reportedly develop low-grade gastric MALToma-like lesions (12).
The changes in epithelial cytology and architecture encountered in corpus tissue of SCID mice infected with C. fetus in the present study are similar to those noted in C57BL/6 mice infected with gastric Helicobacter. It is important to note that the mice used in the present study were regularly screened for Helicobacter species and that none were found prior to challenge or during the course of the study. In spite of their similarity to the lesions that develop in the corpus tissue of C57BL/6 mice infected with H. pylori or H. felis, the lesions that develop in SCID mice infected with C. fetus clearly occur in the setting of a fundamentally different immune response and are not dependent on an adaptive immune response. It has become apparent that the gastritis and epithelial changes encountered in C57BL/6 mice infected with gastric Helicobacter are dependent on cellular, probably T-cell-mediated, immune responses. Infection of C57BL/6 mice that were either RAG-1−/− or compound T-cell receptor βδ−/− with H. felis resulted in high levels of colonization but no detectable gastric pathology (26). The importance of T-cell-mediated immunity was underscored by the fact that infection of B-cell-deficient μMT mice with H. felis still resulted in gastritis and epithelial changes (26). In another study, SCID mice on a C57BL/6 background did not develop gastritis when colonized with H. pylori unless they received adoptively transferred splenocytes from C57BL/6 donor animals (11). Splenocytes from infected or uninfected donor mice induced severe gastritis and also suppressed bacterial colonization. In the present study, expansion of colonization and chronic active inflammation with corpus atrophy and mucous cell metaplasia occurred in the setting of infection with C. fetus without the presence of T or B cells. In fact, C. fetus infection of animals with an intact immune system resulted in more-limited colonization and minimal active inflammation.
The development of atrophic gastritis, characterized by loss of specialized parietal and chief cells with the appearance of mucous metaplasia, in SCID mice suggests that these changes are the end result of chronic gastric inflammation. It has been previously suggested that these epithelial lesions are dependent on the presence of inflammation and that, by themselves, even large numbers of bacteria in close contact with the gastric epithelium are unable to cause these lesions in the absence of a host response (11). Our results support this hypothesis and further suggest that these epithelial lesions can occur in the setting of non-antigen-specific (T-cell-independent) inflammatory responses. Additionally, our electron microscope results suggest that intimate adherence or contact between C. fetus and host cells may not be a prerequisite for inflammation and mucosal damage.
The finding of gastritis in ICR and SCID mice infected with C. fetus was unexpected; the current system was developed to model the lower intestinal tract disease that characterizes most Campylobacter infection in humans. During the course of the present study, it was reported that infection of C.B-17 scid beige mice with fresh clinical isolates of C. jejuni resulted in the development of diarrhea in about 10% of the infected mice (20). Histopathologic analysis of mice with diarrhea revealed the presence of marked colonic inflammation, and no gastric pathology was noted. It is likely that the different results seen in this study and in our present study are due to differences in the genetic background of the mice (inbred BALB/c versus outbred ICR) carrying the scid mutation. Alternately, there may be differences between the pathogenesis of C. jejuni infections and that of C. fetus infections. However, during the pilot infection study presented here, we also infected a group of SCID mice with a fresh clinical isolate of C. jejuni. Similar to what was found for mice infected with C. fetus, we noted gastric colonization with the development of antral gastritis. No lower-bowel lesions were seen in SCID mice infected with C. jejuni (data not shown).
Our findings suggest that the stomach may represent an unrecognized environmental niche within the gastrointestinal tract for Campylobacter species. Furthermore, this system may yield additional insight into the role chronic inflammation plays in the development of gastric pathology during infection with microaerobic spiral bacteria.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI01398 to V.B.Y. and by AI37750 and DK52413 from the National Institutes of Health.
We thank Floyd Dewhirst for performing 16S sequence analysis and Kimberly A. Knox for technical assistance.
REFERENCES
- 1.Abimiku A G, Dolby J M, Borriello S P. Comparison of different vaccines and induced immune response against Campylobacter jejuni colonization in the infant mouse. Epidemiol Infect. 1989;102:271–280. doi: 10.1017/s0950268800029940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allerberger F, Kasten M J, Anhalt J P. Campylobacter fetus subspecies fetus infection. Klin Wochenschr. 1991;69:813–816. doi: 10.1007/BF01744276. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Incidence of foodborne illnesses: preliminary data from the Foodborne Diseases Active Surveillance Network (FoodNet)—United States, 1998. Morbid Mortal Weekly Rep. 1999;48:189–194. [PubMed] [Google Scholar]
- 4.Baqar S, Applebee L A, Bourgeois A L. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect Immun. 1995;63:3731–3735. doi: 10.1128/iai.63.9.3731-3735.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baqar S, Bourgeois A L, Applebee L A, Mourad A S, Kleinosky M T, Mohran Z, Murphy J R. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect Immun. 1996;64:4933–4939. doi: 10.1128/iai.64.12.4933-4939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baqar S, Bourgeois A L, Schultheiss P J, Walker R I, Rollins D M, Haberberger R L, Pavlovskis O R. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13:22–28. doi: 10.1016/0264-410x(95)80006-y. [DOI] [PubMed] [Google Scholar]
- 7.Berndtson E, Danielsson-Tham M L, Engvall A. Experimental colonization of mice with Campylobacter jejuni. Vet Microbiol. 1994;41:183–188. doi: 10.1016/0378-1135(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 8.Blaser M J. Campylobacter and related bacteria. In: Bennett J E B, Mandell G L, Dolin R, editors. Principles and practice of infectious diseases. 3rd ed. Edinburgh, Scotland: Churchill Livingstone, Ltd.; 1995. pp. 1948–1956. [Google Scholar]
- 9.Blaser M J, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Investig. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diker K S, Hascelik G, Diker S. Colonization of infant mice with flagellar variants of Campylobacter jejuni. Acta Microbiol Hung. 1992;39:133–136. [PubMed] [Google Scholar]
- 11.Eaton K A, Ringler S R, Danon S J. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enno A, O'Rourke J L, Howlett C R, Jack A, Dixon M F, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995;147:217–222. [PMC free article] [PubMed] [Google Scholar]
- 13.Falk P G, Hooper L V, Midtvedt T, Gordon J I. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–1355. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox J G, Ackerman J I, Taylor N, Claps M, Murphy J C. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. Am J Vet Res. 1987;48:85–90. [PubMed] [Google Scholar]
- 16.Fox J G, Li X, Cahill R J, Andrutis K, Rustgi A K, Odze R, Wang T C. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 17.Fox J G, Maxwell K O, Taylor N S, Runsick C D, Edmonds P, Brenner D J. “Campylobacter upsaliensis” isolated from cats as identified by DNA relatedness and biochemical features. J Clin Microbiol. 1989;27:2376–2378. doi: 10.1128/jcm.27.10.2376-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genta R M, Huberman R M, Graham D Y. The gastric cardia in Helicobacter pylori infection. Hum Pathol. 1994;25:915–919. doi: 10.1016/0046-8177(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez J, Fayos A, Ferrus M A, Owen R J. Random amplified polymorphic DNA fingerprinting of Campylobacter jejuni and C. coli isolated from human faeces, seawater and poultry products. Res Microbiol. 1995;146:685–696. doi: 10.1016/0923-2508(96)81065-5. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson A E, McBride B W, Hudson M J, Hall G, Leach S A. Experimental campylobacter infection and diarrhoea in immunodeficient mice. J Med Microbiol. 1998;47:799–809. doi: 10.1099/00222615-47-9-799. [DOI] [PubMed] [Google Scholar]
- 21.Karita M, Tsuda M, Nakazawa T. Essential role of urease in vitro and in vivo Helicobacter pylori colonization study using a wild-type and isogenic urease mutant strain. J Clin Gastroenterol. 1995;21(Suppl. 1):S160–S163. [PubMed] [Google Scholar]
- 22.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei Z, Blaser M J. Pathogenesis of Campylobacter fetus infections. Role of surface array proteins in virulence in a mouse model. J Clin Investig. 1990;85:1036–1043. doi: 10.1172/JCI114533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei Z, Burucoa C, Grignon B, Baqar S, Huang X Z, Kopecko D J, Bourgeois A L, Fauchere J L, Blaser M J. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun. 1998;66:938–943. doi: 10.1128/iai.66.3.938-943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth K A, Kapadia S B, Martin S M, Lorenz R G. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 27.Russell R G, Sarmiento J I, Fox J, Panigrahi P. Evidence of reinfection with multiple strains of Campylobacter jejuni and Campylobacter coli in Macaca nemestrina housed under hyperendemic conditions. Infect Immun. 1990;58:2149–2155. doi: 10.1128/iai.58.7.2149-2155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahay P, West A P, Birkenhead D, Hawkey P M. Campylobacter jejuni in the stomach. J Med Microbiol. 1995;43:75–77. doi: 10.1099/00222615-43-1-75. [DOI] [PubMed] [Google Scholar]
- 29.Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Zanten S J, Dixon M F, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and Helicobacter ecology. Gastroenterology. 1999;116:1217–1229. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang T C, Goldenring J R, Dangler C, Ito S, Mueller A, Jeon W K, Koh T J, Fox J G. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 33.Yrios J W, Balish E. Colonization and infection of athymic and euthymic germfree mice by Campylobacter jejuni and Campylobacter fetus subsp. fetus. Infect Immun. 1986;53:378–383. doi: 10.1128/iai.53.2.378-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yrios J W, Balish E. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect Immun. 1986;53:384–392. doi: 10.1128/iai.53.2.384-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]