Abstract
To identify potential immunodominant and/or adhesin binding domains of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae (NTHI), three sets of synthetic peptides were synthesized and assayed in an adherence inhibition assay, by Western blotting, and in a biomolecular interaction analysis (BIA) system. The first series of 34 8- to 10-mer peptides represented the entire mature protein sequentially. The second set of four peptides (each 19 to 28 residues) represented the four predicted major surface-exposed regions (or loops) of this adhesin. The third series of seven peptides (each 27 to 34 residues) were specifically designed to map the third surface-exposed region. Data obtained by BIA indicated limited reactivity of a panel of high-titered immune chinchilla sera to the 8- to 10-mer peptides representing the mature protein, likely because these linear peptides did not represent continuous epitopes. However, several of these short peptides did inhibit adherence of multiple NTHI strains to a human respiratory epithelial cell. Overall, greatest relative reactivity in both BIA and adherence inhibition assays was demonstrated against, or shown by, peptides mapping to the third and fourth predicted surface-exposed regions of this adhesin, thereby indicating the presence of immunodominant and adhesin binding domains at these sites. Middle ear fluids sequentially recovered from a chinchilla with an ongoing NTHI-induced otitis media (OM) as well as sera from children with OM due to NTHI also reacted exclusively with peptides representing the third and fourth surface-exposed regions of the P5-fimbrin adhesin, indicating a similarity in immune recognition of this bacterial protein by these two hosts. Collectively, these data together with the previously demonstrated protective efficacy of immunogens derived from this adhesin in chinchilla models support the continued development of P5-fimbrin based vaccine components.
Nontypeable Haemophilus influenzae (NTHI) is an important causative agent of bacterial otitis media (OM). Of interest are several conserved surface proteins of this heterogeneous group of organisms that could potentially serve as components in a vaccine. We have focused in recent years on a structure observed on 100% of middle ear and nasopharyngeal NTHI isolates recovered from children with chronic OM (6). These appendages, referred to as fimbriae due to their filamentous appearance, are composed of a 36.4-kDa protein subunit (fimbrin) that is highly homologous to outer membrane protein (OMP) P5 of H. influenzae type b (39, 40). The P5-homologous fimbrin protein (or P5-fimbrin) is an adhesin to human oropharyngeal (OP) cells (51) and mucin (44, 45), chinchilla eustachian tube mucus (37), and respiratory syncytial virus-infected A549 cells (24).
We have been conducting studies to determine whether or not P5-fimbrin can serve as a protective immunogen and have demonstrated that, like whole OMP preparations, immunization with P5-fimbrin does provide significant protection against homologous NTHI challenge (3, 4, 51). However, as with other isolated OMPs, induction of protective activity against heterologous strains was less effective (4). To overcome this obstacle, we chose a strategy in which we attempted to identify a minimal region of this antigen that could elicit a protective response (13). Thus, we elected to focus on a specific predicted surface-exposed region of P5-fimbrin for further development as an epitope-targeted peptide based vaccine rather than attempt to develop a component derived from the whole native protein (4).
Multiple algorithmic analyses of the deduced amino acid sequence of this protein (4) indicate that there are four predicted surface-exposed regions located in the N-terminal half, the general location of which are consistently reported among laboratories (15, 55, 55a). Duim et al. (15) recently reported that all four of these regions, or loops, were hypervariable in five NTHI isolates and their variants recovered from chronic bronchitic patients. They attributed this variability to nonsynonymous point mutations induced by selective immunological pressures exerted during chronic disease. Webb and Cripps (55) also fully sequenced the gene that encodes this surface protein from 13 Australian isolates recovered from diverse anatomical sites, as well as the portion of the gene corresponding to the region of the first loop from an additional 10 isolates. These investigators noted sequence diversity in the predicted surface-exposed regions as well. However, in the Australian isolates, which were all recovered from instances of acute rather than chronic disease, the greatest diversity was found in the first loop (or region). More limited diversity was found in loops 2 and 3; loop 4 was found to be highly conserved. We recently reported the sequence of the third surface-exposed region of P5-fimbrin from 99 European and U.S. NTHI isolates (3). Based on the observed sequence diversity in region 3, we assigned NTHI strains to three major subclasses or groupings (with a subgroup for a minority of group 2 strains). The majority of all isolates sequenced (76%) belong to group 1.
Due to interest in this and similar proteins as a protective antigen in animal models, these surface-exposed regions, and particularly the more conserved among them, have been the target of peptide vaccine design strategies (3, 4, 23, 55a). Prior to having a complete understanding of the diversity in these regions, but due to its predicted antigenicity, we have to date focused our efforts on a region 3-based immunogen. Thus, a 19-mer peptide representing the majority of the third surface-exposed region of this adhesin, expressed by NTHI strain 1128 (4, 51), was selected and subsequently incorporated into both a synthetic chimeric peptide immunogen known as LB1; variants of it are encoded in a recombinant fusion peptide vaccinogen called LPD-LB1(f)2,1,3. Coincidentally, this 19-mer region shares limited or no homology with equivalent regions of other OmpA family members (51).
We have recently demonstrated that induction of antibodies against the two immunogens derived from this focused region (3, 4, 30) provide significant protection against homologous NTHI challenge in both an active immunization regimen and one of passive transfer in a chinchilla model of viral-bacterial superinfection. In vitro assays have shown that antibodies directed against these immunogens do have bactericidal activity (9, 30); however, they also strongly inhibit adherence of NTHI to human oropharyngeal cells (3; Jurcisek and Bakaletz, Abstr. 7th Int. Symp. Recent Adv. Otitis Media, 1999). This latter finding indicates that one primary functional activity of antibodies directed against these immunogens may be to block NTHI colonization or augment bacterial clearance. In fact, animals immunized with these derivatives of P5-fimbrin demonstrate significantly earlier clearance of NTHI from the nasopharynx and markedly lower incidence of eustachian tube ascension and OM induction than do sham-immunized cohorts (3, 4).
As we have been continuing to refine these immunogens and attempting to better understand the mechanism(s) behind the significant protection observed in chinchilla models, we have been interested in further defining those portions of this adhesin that are immunodominant and/or represent epithelial cell binding domains for both the chinchilla and human hosts. In addition, we wanted to better understand the significance of the demonstrated sequence diversity in the 19-mer region of the adhesin protein (3, 15, 55), called LB1(f), that is targeted in our P5-fimbrin based vaccinogen design. Toward this goal, we created three sets of synthetic peptides, derived from the deduced amino acid sequence of the P5-fimbrin adhesin of NTHI strain 1128, a pediatric middle ear isolate. Using these peptides and isolated P5-fimbrin from several NTHI strains plus a panel of polyclonal antisera and a murine monoclonal antibody, four assays were performed: an enzyme-linked immunosorbent assay (ELISA) to determine serum titer, Western blotting to define antibody specificity, a bacterial adherence inhibition assay to identify adhesin domains, and a biomolecular interaction analysis (BIA) (26–28, 32, 34, 49) to examine the relative binding affinity between these peptides or isolated P5-fimbrin and antibodies present in both chinchilla and human sera or in middle ear effusions.
These data were collectively used to determine which portions of this surface protein were immunodominant not only to the chinchilla host, which serves as a model of human disease, but also to children, and thus identify epitopes and perhaps adhesin binding domains.
(This work was presented in part at the Seventh International Symposium on Recent Advances in Otitis Media, Fort Lauderdale, Fla., June 1 to 5, 1999.)
MATERIALS AND METHODS
Synthesis of peptides.
Synthesis, purification, and sequence confirmation of all synthetic peptides were performed essentially as described by Kaumaya et al. (29) with established techniques of the Peptide and Protein Engineering Laboratory at The Ohio State University. Briefly, synthetic peptides were assembled stepwise by 9-fluorenylmethoxycarbonyl–t-butyl strategy with a benzotriazolyloxy-tris-(dimethylamino)phosphonium hexafluorophosphate–N-hydroxybenzotriazole protocol on a Milligen/Biosearch 9600 synthesizer. Peptides were purified by high-performance liquid chromatography on a Vydac C4 (10 mm by 25 μm) column using CH3CN containing 0.1% trifluoroacetic acid. Amino acid analysis and mass spectral analysis (not shown) confirmed the composition and amino acid sequence of each peptide.
Generation of antiserum and middle ear fluids.
Chinchilla naive and immune sera and middle ear fluids were retrieved from archived samples. Sera obtained from chinchillas immunized with NTHI strain 1128 whole OMP preparation, isolated P5-fimbrin, or with other immunogens derived from this strain were selected for use. Pooled immune chinchilla serum samples were collected from animals immunized as follows: whole OMP delivered in complete Freund adjuvant (CFA) (51), isolated P5-fimbrin delivered in CFA (3), isolated P5-fimbrin delivered in A1PO4 (3), LB1 delivered in CFA (3), and LPD-LB1(f)2,1,3 delivered in A1PO4 plus monophosphoryl lipid A (MPL) (3). LB1 is a 40-mer synthetic chimeric peptide composed of a putative B-cell epitope of P5-fimbrin of NTHI strain 1128 [called LB1(f)] that was colinearly synthesized with a T-cell promiscuous epitope of measles virus fusion protein (4). LPD-LB1(f)2,1,3 is a recombinant fusion peptide composed of a lipoprotein D (LPD) moiety followed by three sequential unique LB1(f) P5-fimbrin epitopes (3, 30).
Chinchilla middle ear fluids were retrieved every 3 to 7 days over a 5-week period by epitympanic tap from an immunologically naive animal with an ongoing NTHI-induced OM resulting from transbullar challenge with 2,500 CFU of NTHI strain 86-028NP (Bakaletz et al., Abstr. 7th Int. Symp. Recent Adv. Otitis Media, 1999). Human sera from children undergoing tympanocentesis for acute OM were collected from children age 3 months to 11 years with a confirmed culture-positive NTHI or Streptococcus pneumoniae OM from which the bacteria were isolated in pure culture. A murine monoclonal antibody, 2C7, produced against H. influenzae biogroup aegyptius (strain F3031) that recognizes P5 (40), was generously provided by Alan Lesse, State University of New York at Buffalo, Buffalo, N.Y.
Bacterial strains.
All NTHI strains used were minimally passaged clinical isolates obtained from the nasopharynges (86-028NP) or middle ears (1128, 86-028L, 1885MEE, and 1728MEE) of children undergoing tympanostomy and tube insertion for chronic OM with effusion at Columbus Children's Hospital. Isolates were maintained frozen in a skim milk plus 20% (vol/vol) glycerol solution until used. NTHI strains 1128 and 86-028NP (and strain 86-028L from the left ear of the same child) are group 1 isolates based on our classification of region 3 sequences (3); strain 1885MEE is a group 2a isolate, and strain 1728MEE is a group 3 isolate.
OMP and P5-fimbrin isolation.
Whole OMP preparations or isolated P5-fimbrin were recovered from NTHI strains 1128, 86-028NP, 1885MEE, and 1728MEE as previously described (51).
ELISA and Western blotting assays.
ELISA assays were performed with dilutions of pooled chinchilla sera that were assayed against whole NTHI OMP preparations (0.5 μg/well), isolated P5-fimbrin (0.2 μg/well), or synthetic peptides (0.2 μg/well) in 96-well microtiter plates as described previously (2, 4). The titer of a serum pool was defined as the reciprocal of the dilution that consistently yielded an optical density at 490 nm showing a twofold increase over that of wells containing all components except immune serum. Assays were conducted a minimum of three times, and median reciprocal titers are reported.
Western blotting was performed after separation of proteins by electrophoresis in 7.5% sodium dodecyl sulfate-polyacrylamide gels as described previously (5). Human sera or pooled chinchilla sera diluted 1:100 served as the primary antibody, and horseradish peroxidase-conjugated protein A (diluted 1:200; Zymed) served as the secondary antibody. We used a miniblot apparatus (Mini-Protean II multiscreening apparatus; Bio-Rad) to conserve sera and reagents. Color was developed with 4-chloro-1-naphthol (Sigma).
Adherence inhibition ELISA.
An adherence inhibition ELISA was developed for use with synthetic peptides as the blocking agent and using human OP cells as the epithelial target cell. Human OP cells were collected from 12 healthy volunteers, washed twice in 10 mM phosphate-buffered saline (PBS)–0.05% bovine serum albumin (PBS-BSA) and fixed to the bottoms of wells in a 96-well microtiter plate as previously described (3). All incubations were at 37°C in a humidified chamber, and all washes were performed with an Ultrawash Plus microtiter plate washer (Dynatech) set at 5 cycles of 300 μl of PBS-BSA per cycle. Wells were blocked with 300 μl of 1.0% skim milk (Upstate Biotechnology) in 10 mM PBS. Peptides (0.2 μg/ml of 10 mM PBS) were then added to wells and incubated with OP cells for 1 h. The plate was washed, and biotinylated NTHI strain 86-028L, 1885MEE, or 1728MEE (51) was added at a bacterium-to-target cell ratio of 500:1. NTHI strain 86-028L was used in adherence assays instead of strain 86-028NP because it is slightly less prone to autoagglutinate, resulting in more consistent interassay results. Positive control wells contained all components except the individual synthetic peptides. After a 1-h incubation with the bacterial cells, plates were washed again and adherent bacteria were detected by incubations with ExtrAvidin-horseradish peroxidase (Sigma) diluted 1:100 followed by 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (Zymed) diluted 1:100. Percent inhibition of bacterial adherence for each peptide was calculated by comparing the average reading of the test wells to the average reading of the positive control wells at an optical density of 405 nm. Each of the 10-mer peptides and region 1 to 4 peptides was assayed against each of the NTHI strains a total of three times with the mean value ± standard deviation reported.
Sensor chip preparation and BIA.
Analysis of interaction between the synthetic peptides and serum or middle ear fluids was performed with a BIAcore 2000 instrument (Biacore AB, Uppsala, Sweden). This system detects the specific interactions between a ligand and an analyte in real time, without labeling any component (34). The BIAcore instrument utilizes the optical phenomenon of surface plasmon resonance, a quantum mechanical phenomenon, to detect changes in the refractive index at the surface of a sensor chip (26, 28). Briefly, an analyte free in solution flows across the surface of a sensor chip to which a ligand is bound (32). As the specific interaction between the analyte and ligand occurs, the increase in mass is monitored near the sensor chip surface and the change is expressed as relative resonance units (RU), which are plotted against time (27). The sensor chip may be used for multiple analyses following a regeneration step to remove the bound analyte.
All reagents were obtained from Biacore AB. A reagent-grade CM5 sensor chip was activated by injection of 35 μl of a 400 mM N-ethyl-N′-(3-diethylaminopropyl)carbodiimide–100 mM N-hydroxysuccinimide solution (25). Synthetic peptides, suspended in 10 mM acetate buffer (pH 4.5), were manually injected over one flow cell of an activated chip to bind approximately 0.1 to 0.5 ng of peptide per mm2 of chip surface. Excess ester groups were then deactivated by injection of 35 μl of 1.0 M ethanolamine hydrochloride-NaOH. To optimize experimental conditions, a flow rate test was performed whereby sample injection flow rates of 5, 10, 15 and 20 μl/min were assayed to detect mass transport limitation. For interaction analysis, 10 μl of each serum sample, diluted 1:5 with HBS-EP buffer (0.01 M HEPES [pH 7.4], 0.15 M NaCl, 3 mM EDTA, 0.005% [vol/vol] Surfactant P20), was exposed to the immobilized peptides. The sensor chip surface was then regenerated with a 5- or 10-μl injection of 10 mM glycine-HCl (pH 1.5). HBS-EP served as the continuous running buffer. The relative amount of antibody bound to each peptide was determined by comparing the change in RU between sample injection cycles.
All chinchilla sera were assayed against each of the 34 10-mer peptides twice and against all remaining region and partial sequence peptides a minimum of three times, with the mean RU ± standard deviation reported. Due to the very limited volume of chinchilla middle ear fluids and human sera, these fluids were assayed against the region 1 to 4 peptides only once by BIA.
Statistical analysis.
Student's t test was used to determine differences between arithmetic means. A P value of ≤0.05 was accepted as significant.
RESULTS
Western blotting and ELISA.
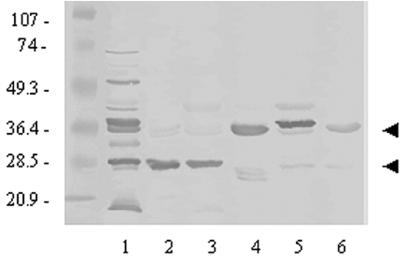
Chinchillas immunized with a whole NTHI OMP preparation recognized many OMPs as expected, as well as both species of P5-fimbrin (Fig. 1, lane 1) by Western blotting as we have reported previously (4, 5, 51). Chinchilla polyclonal antiserum pools, generated by immunization with isolated P5-fimbrin and delivered in either CFA or A1PO4, each predominantly recognized the partially denatured species of P5-fimbrin at ca. 25 kDa (Fig. 1, lanes 2 and 3). Chinchilla anti-LB1 predominantly recognized the fully denatured species of P5-fimbrin at approximately 37 kDa (lane 4). Anti-LPD-LB1(f)2,1,3 predominantly recognized a band at approximately 42 kDa which is presumed to be LPD (lane 5) and also both P5-fimbrin species. Monoclonal antibody 2C7 recognized both species of the P5-fimbrin adhesin; however, recognition of the 37-kDa species was slightly stronger (lane 6) as has been reported elsewhere (40).
FIG. 1.
Western blotting of chinchilla sera and a murine monoclonal antibody versus NTHI strain 1128 whole OMP preparation (in all lanes). Chinchilla serum pools: lane 1, anti-whole OMP (delivered in CFA); lane 2, anti-isolated P5-fimbrin (delivered in CFA); lane 3, anti-isolated P5-fimbrin (delivered in A1PO4); lane 4, anti-LB1 (delivered in CFA); lane 5, anti-LPD-LB1(f)2,1,3 (delivered in A1PO4 plus MPL); lane 6, monoclonal antibody 2C7. Arrows indicate approximate recognition of the fully and partially denatured species of P5-fimbrin at 37 and 25 kDa, respectively. The major reactivity observed in lane 5 is directed against LPD.
The relative median reciprocal titers for each serum pool characterized above, against the immunogen delivered, as determined by ELISA were 5 × 104 for anti-whole OMP/CFA, 5 × 104 for anti-isolated P5-fimbrin/CFA, 104 for anti-isolated P5-fimbrin/A1PO4, 2 × 105 for anti-LB1/CFA, and 5 × 104 for anti-LPD-LB1(f)2,1,3/A1PO4 plus MPL. These titers indicated the similarity between CFA and a mixture of A1PO4 plus MPL as adjuvants in this host, as well as the relatively weaker effectiveness of alum alone as an adjuvant as we (3) and others (22) have previously reported.
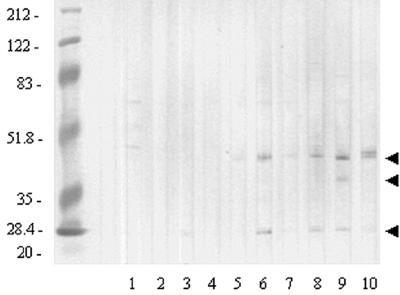
Two of the five human sera obtained from children with S. pneumoniae culture-positive OM were not reactive in Western blotting with proteins in a NTHI whole OMP preparation (Fig. 2, lanes 2 and 4). Faint bands at approximately 25 kDa (lane 3) and in the approximate range of 42 to 80 kDa (lanes 1 and 5) were detected with the three other sera collected from children with pneumococcal OM, indicating that these children may have had a prior episode of OM resulting from NTHI infection. Sera obtained from children with OM that was culture positive for NTHI predominantly recognized proteins in a whole OMP preparation at ca. 25 and 42 kDa (Fig. 2, lanes 6 to 10). The presumption that these bands represented recognition of the partially denatured fimbrin species at 25 kDa and OMP P2 at 42 kDa (rather than the similarly migrating LPD) were supported by repeating the blot using these sera and assaying them against isolated P5-fimbrin and recombinant LPD (not shown). An additional band in lane 9 at approximately 37 kDa and in lane 10 at approximately 45 kDa was observed as well, but the specific OMPs being recognized here have not been identified.
FIG. 2.
Western blotting of human sera versus NTHI strain 1128 whole OMP preparation (in all lanes). Lanes 1 to 5, sera from children with S. pneumoniae culture-positive OM; lanes 6 to 10, sera from children with NTHI culture-positive OM. Arrows indicate recognition (from top to bottom) at approximately 42, 37, and 25 kDa, respectively.
Peptide synthesis.
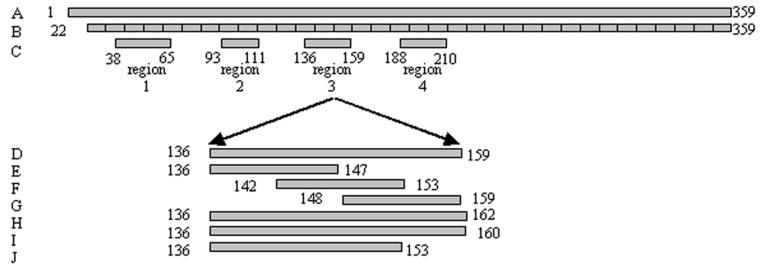
Three sets of peptides based on the deduced amino acid sequence of P5-fimbrin of NTHI strain 1128 (51) were synthesized to map epitopes of this adhesin. These peptides are depicted schematically in Fig. 3. The first series of 34 peptides represented mature P5-fimbrin sequentially in 10-residue segments, except the C-terminal peptide 34, which was 8 residues long. The second series of four region peptides represented each of the predicted major surface-exposed regions of this adhesin (Fig. 3 and Table 1) (15, 55). A final series of seven peptides was designed to specifically map the third predicted surface-exposed region (Fig. 3 and Table 2). Recently, the loop 3 region of the P5-fimbrin gene from 99 clinical NTHI isolates from the United States and Europe was PCR amplified and analyzed for sequence heterogeneity within the 19-mer region that has been the basis of our development of a vaccine component. Within this region, called LB1(f), three major groups and one subgroup were identified, and thus a peptide representing each of these groups was also created. These are included in the group peptides: LB1(1), LB1(2a), LB1(2b), and LB1(3). In addition, a set of three short, overlapping partial sequence peptides (LB1ps1, LB1ps2, and LB1ps3) were synthesized to more precisely map the consensus sequence of the group 1 NTHI strains.
FIG. 3.
Schematic diagram of synthetic peptides. (A) P5-fimbrin with leader peptide; (B) 8- to 10-mer peptides representing the mature protein; (C) predicted surface-exposed region peptides; (D) region 3 peptide LB1(1); E to G, partial sequence peptides LB1ps1 to 3; H to J, group peptides LB1(2a), LB1(2b), and LB1(3), respectively.
TABLE 1.
Amino acid sequences of peptides representing the four predicted surface-exposed regions of P5-fimbrin
| Peptide | Amino acid sequence |
|---|---|
| Region 1 | SFHDGINNNGAIKKGLSSSNYGYRRNTF |
| Region 2 | GRAKLREAGKPKAKHTNHG |
| Region 3a | LVRSDYKFYEDANGTRDHKKGRHT |
| Region 4 | TRVGKYRPQDKPNTAINYNPWIG |
Identical in sequence to LB1(1) peptide noted in text and Table 2.
TABLE 2.
Amino acid sequences of peptides designed to map LB1(f)
| Peptide | Amino acid sequencea |
|---|---|
| LB1(f) | RSDYKFYEDANGTRDHKKG |
| LB1(1)b | LVRSDYKFYEDANGTRDHKKGRHT |
| LB1ps1 | LVRSDYKFYEDA |
| LB1ps2 | KFYEDANGTRDH |
| LB1ps3 | NGTRDHKKGRHT |
| LB1(2a) | LVRSDYKLYNKNSSSNSTLKNLGEHHR |
| LB1(2b) | LVRSDYKLYNKNSS--NTLKDLGEHHR |
| LB1(3) | LVRSDYKFYDN------KRID---SHR |
Dashes were inserted to align sequences.
Identical in sequence to region 3 peptide noted in text and in Table 1.
NTHI adherence inhibition ELISA.
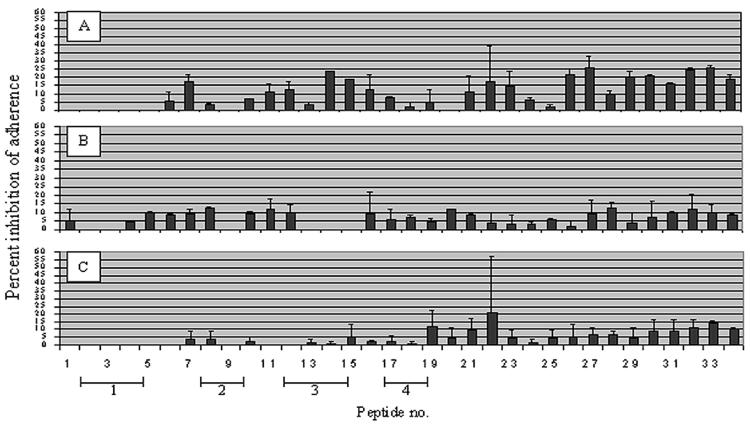
Inhibition of adherence of three NTHI strains representing groups 1, 2a, and 3 to human OP cells by the 10-mer linear peptides demonstrated a group-specific inhibitory effect (Fig. 4). Substantial inhibition of adherence against the group 1 isolate (86-028L) is demonstrated by peptides representing the N-terminal portion of this adhesin (peptides 1 to 19) (Fig. 4A). These peptides were based on the sequence from another group 1 NTHI strain. Less activity was shown against the group 2a strain, 1885MEE (Fig. 4B), and minimal activity was demonstrated against the group 3 strain, 1728MEE (Fig. 4C). For the group 1 strain 86-028L, of those peptides that approximated the four predicted surface-exposed regions, those that spanned region 3 (peptides 12 to 15) showed the greatest relative ability to inhibit adherence of this isolate to human OP cells. Significant adherence inhibition activity against all three isolates examined was demonstrated by peptides representing the C-terminal half of this protein (peptides 20 to 34).
FIG. 4.
Ability of 8- to 10-mer linear peptides representing mature P5-fimbrin of strain 1128 to block adherence of NTHI strains to human OP cells. Inhibition of binding of NTHI strain: (A) 86-028L (representative of group 1); (B) 1885MEE (representative of group 2a); (C) 1728MEE (representative of group 3). Brackets roughly approximate location of the four predicted surface-exposed regions of this OMP.
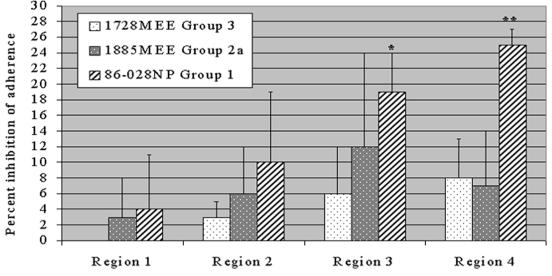
Longer peptides representing each of the four predicted surface exposed regions of P5-fimbrin confirmed the group-specific result obtained with the 10-mer peptides (Fig. 5). Peptides representing regions 1 and 2 did not inhibit adherence above a mean value of 10% for either the group 1, 2a, or 3 NTHI isolate. The region 3 peptide was significantly more inhibitory to adherence of a group 1 isolate (P ≤ 0.05) than to the group 3 isolate, with greatest interassay variability shown against the group 2a strain. These data further indicated a potential adhesin binding domain within the fourth predicted surface-exposed region that had not been detected with the 10-mer peptides. The region 4 peptide significantly inhibited the adherence of a group 1 but not a group 2a or 3 isolate to human OP cells (P ≤ 0.05).
FIG. 5.
Percent inhibition of adherence of NTHI strains to human OP cells by synthetic peptides representing the four predicted surface exposed regions of P5-fimbrin. ∗, significant difference from strain 1728MEE; ∗∗, significant difference from strains 1728MEE and 1885MEE (P ≤ 0.05).
BIA.
Before using BIA in epitope mapping studies, we examined the relationship between RU affinity values obtained by biosensor and reciprocal titer of chinchilla serum pools obtained by ELISA. Serial dilutions of polyclonal anti-LB1 serum were injected across a sensor chip to which the peptide immunogen LB1 was bound. A linear relationship was noted when reciprocal titer values between those obtained with undiluted serum and those serially diluted out to 1:1,000 were plotted against relative RU values. An affinity value of 100 RU was approximated to equal a reciprocal titer value of 1.3 × 103 (not shown). Naive or sham-immunized chinchilla sera did not yield an RU value of ≥100 when assayed against any of the 45 peptides described here.
Regardless of the relative titer or whether the antiserum pool was directed against a whole OMP preparation, isolated P5-fimbrin, LB1, or LPD-LB1(f)2,1,3, with one exception, the relative RU affinity values of pooled immune chinchilla serum to the 10-mer peptides did not exceed an arbitrarily selected baseline value of 100 RU for any serum tested (data not shown), again indicating the likelihood that these short, sequential linear peptides did not represent continuous epitopes. A single mean affinity peak greater than 100 RU shown by antiserum raised against whole OMP when reacted with peptide 17 was not likely significant, as this was not a consistent observation among the antisera tested. Further, depending on the model used, the majority or all of the residues incorporated into peptide 17 are not predicted to be surface accessible.
However, greater relative affinity values were obtained when some of these sera were reacted with longer peptides that represented the four surface-exposed regions of this adhesin, and specifically those mapping residues within the third predicted surface-exposed region (Table 3). Despite reactivity in Western blotting and by ELISA, antiserum raised against a whole OMP preparation was largely unreactive by BIA against the synthetic peptides designed to map the focused regions of P5-fimbrin, indicating perhaps a relatively low concentration or avidity of antibodies specific for these epitopes available in this serum pool. Similarly, sera generated in chinchillas against isolated P5-fimbrin by delivery in either a strong (CFA) or a weak (A1PO4) adjuvant were unreactive except for relatively modest recognition of the 40-mer synthetic chimeric peptide LB1, where RU values of 110 and 100, respectively, were obtained for these two serum pools (only anti-LB1/CFA values are shown in Table 3). As with anti-whole OMP serum, this may reflect the relative concentration or avidity of specific antibodies available in the serum pool. However, the additional lack of reactivity of either of these latter two sera by BIA with sensor chip-bound P5-fimbrin that had been isolated from any of three NTHI isolates (not shown) demonstrated that there was also likely a conformational aspect to both the immune recognition as well as perhaps to the assay system itself in which the orientation of bound peptides could not be controlled. Monoclonal antibody 2C7 did not yield an affinity value of greater than 5 RU against any peptide depicted in Table 3 despite reactivity in Western blotting, and thus is not shown, again indicating both a conformational aspect of recognition as well as the need to use multiple systems to fully evaluate antibodies or immune sera for reactivity and specificity. Conversely, chinchilla serum obtained by immunization with LB1 was strongly reactive against both the immunogen itself and against the region 3 peptide from which it was derived, as expected. The RU affinity value obtained against the bound LB1 40-mer immunogen was about 95% of that obtained against the LB1(1) region 3 peptide, indicating that antibodies generated upon immunization with the chimeric peptide LB1 were largely directed against the incorporated 19-mer P5-fimbrin B-cell epitope and not the promiscuous T-cell epitope from measles virus fusion protein, as anticipated. These RU values approximate a reciprocal titer of 1.9 × 104 in this serum, which is consistent with what we have reported previously as determined by ELISA (3). The anti-LB1 serum pool also recognized peptides representing the LB1(f) moiety from both a group 2b and a group 3 isolate but was not reactive with the group 2a peptide. While this finding may again indicate a limitation of the BIA system, it may also indicate that the amino acid sequence diversity demonstrated in the group 2 isolates that resulted in the establishment of a subgroup 2a and 2b (3) was perhaps immunologically significant as well.
TABLE 3.
Affinities of pooled immune chinchilla sera to peptides derived from P5-fimbrin LB1(f) moiety
| Antiserum directed against (NTHI strain): | Adjuvant | Mean relative affinity (RU ± SD)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB1ps1 | LB1ps2 | LB1ps3 | LB1(2a) | LB1(2b) | LB1(3) | Region 1 | Region 2 | LB1(1) region 3 | Region 4 | LB1 (40-mer) | ||
| Whole OMP (1128) | CFA | 32 ± 3 | 72 ± 7 | 23 ± 8 | 38 ± 2 | 40 ± 5 | 35 ± 3 | 21 ± 9 | 25 ± 9 | 32 ± 10 | 33 ± 10 | 47 ± 4 |
| Isolated P5-fimbrin (1128) | CFA | 24 ± 9 | 32 ± 5 | 9 ± 3 | 38 ± 3 | 33 ± 10 | 56 ± 6 | 20 ± 4 | 22 ± 11 | 50 ± 5 | 41 ± 6 | 110 ± 15 |
| LB1 | CFA | 40 ± 7 | 49 ± 1 | 885 ± 67 | 38 ± 6 | 211 ± 27 | 564 ± 54 | 38 ± 12 | 21 ± 10 | 1,453 ± 70 | 45 ± 14 | 1,530 ± 63 |
| LPD-LB1(f)2,1,3 | A1PO4 MPL | 30 ± 7 | 25 ± 9 | 17 ± 1 | 29 ± 4 | 40 ± 6 | 63 ± 3 | 35 ± 11 | 19 ± 9 | 33 ± 10 | 43 ± 10 | 10 ± 5 |
Values of ≥100 RU are in boldface.
When we attempted to more precisely map the group 1 sequence, reactivity with anti-LB1 serum was greatest against the C-terminal portion of this 24-mer peptide, as was seen by comparison of RU values obtained against the LB1ps3 peptide relative to those obtained against peptides LB1ps1 and LB1ps2 (Table 3).
Serum antibodies available in the pool of serum obtained by immunization with LPD-LB1(f)2,1,3 were unreactive against the P5-fimbrin-derived panel of synthetic peptides in BIA despite demonstrated significant protective efficacy (3), a high reciprocal titer (5 × 104), and strong reactivity in Western blotting. Again, this may have been due to a conformational aspect of the BIA system; the lower concentration or avidity of LB1(f)-specific antibodies available in this serum pool or may more likely reflect the inability of antibodies raised against a large fusion protein to recognize linear peptides bound to a chip as in this assay system. Overall, BIA-obtained data clearly demonstrated the differences between an immune response raised to an intact protein versus one obtained by immunization with a peptide or recombinant immunogen.
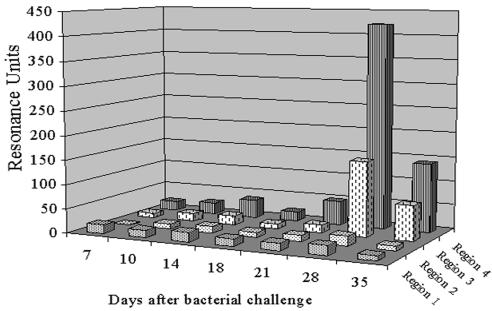
When middle ear fluids, obtained over a 5-week disease course from an immunologically naive chinchilla that had been transbullarly challenged with NTHI and was thus manifesting active OM, were assayed by BIA against the four region peptides, RU values above background were directed exclusively against those representing regions 3 and 4 (Fig. 6). This increase in affinity peaked on day 28 and represented estimated reciprocal titers of 2 × 103 against region 3 and 5.5 × 104 against region 4 in these middle ear fluids. By 5 weeks after challenge, RU affinity values had decreased by 53 and 67% against regions 3 and 4, respectively.
FIG. 6.
Relative affinity values of antibodies in sequentially collected chinchilla middle ear fluids to recognize bound peptides representing the four predicted surface-exposed regions of P5-fimbrin.
To determine if human serum antibodies similarly demonstrated preferential reactivity against any of the region 1 to 4 peptides as had chinchilla middle ear effusions, sera collected from 14 children with either pneumococcal OM or OM due to NTHI were assayed against these peptides (Table 4). RU values obtained with all seven sera collected from children with S. pneumoniae culture-positive OM were unremarkable against any of the peptides representing the four predicted surface-exposed regions; however, all seven sera collected from children with culture-positive NTHI OM were strongly reactive against selected peptides in this system. Whereas RU values against region 1 and region 2 peptides did not exceed a maximum value of 58, those obtained when assayed against the region 3 and region 4 peptides yielded mean values of 283 ± 130 and 487 ± 266 RU, respectively. Comparing mean RU values against region 3 and 4 peptides, greater overall reactivity was detected against the latter; however, this difference was not significant (P = 0.12). These mean RU values translate into approximate titers of 3.7 × 103 and 6.3 × 103 against regions 3 and 4, respectively, in these sera.
TABLE 4.
Relative affinities of human serum to peptides representing the four surface-exposed regions of P5-fimbrin
| Sera from children with: | RU against peptides representinga:
|
|||
|---|---|---|---|---|
| Region 1 | Region 2 | LB1(1) region 3 | Region 4 | |
| S. pneumoniae culture-positive OM | ||||
| 1 | 44 | 34 | 47 | 54 |
| 2 | 37 | 31 | 47 | 49 |
| 3 | 26 | 19 | 32 | 51 |
| 4 | 57 | 42 | 55 | 70 |
| 5 | 2 | 1 | 19 | 43 |
| 6 | 13 | 9 | 28 | 67 |
| 7 | 23 | 18 | 57 | 88 |
| NTHI culture-positive OM | ||||
| 1 | 58 | 43 | 109 | 118 |
| 2 | 16 | 21 | 260 | 501 |
| 3 | 27 | 27 | 312 | 478 |
| 4 | 7 | 14 | 321 | 609 |
| 5 | 39 | 51 | 453 | 1,005 |
| 6 | 0 | 7 | 287 | 480 |
| 7 | 5 | 3 | 151 | 215 |
Values of >100 RU are in boldface.
DISCUSSION
Despite their limitations, peptide-based vaccines have the potential to direct the immune response against prespecified immunodominant and protective epitopes of native proteins (14). Recent reports from several laboratories have shown that the inclusion of both B- and T-cell epitopes in synthetic peptide immunogens can induce antibodies of higher affinity for the incorporated B-cell epitopes (41, 50). The orientation, number, and secondary character of peptide sequences included in these immunogens have been found to influence antigen processing and presentation to T cells, thus affecting the specificity and affinity of the antibodies produced for native proteins (14, 41, 50). We have recently used this epitope-targeted peptide-based vaccine strategy to develop two immunogens [LB1 and LPD-LB1(f)2,1,3] based on the third surface-exposed region of the NTHI adhesin P5-fimbrin (24, 37, 44, 45, 51). While an earlier report from our lab, using the NEWCOILS algorithm of Lupas et al. (33), indicated a coiled-coil character for P5-fimbrin, Webb and Cripps (55) recently disputed this finding based on a similar analysis of the sequences of multiple NTHI isolates by the COILS algorithm. Recently, it was shown that NEWCOILS produced false positives by classifying noncoiled-coil alpha-helical regions incorrectly (8, 56). We thereby concur with conclusion of Webb and Cripps (55), that this surface protein does not have an unambiguous coiled-coil character overall; however, its role in pathogenesis and potential protection remains highly intriguing.
As to the role of P5-fimbrin as a protective immunogen, we have demonstrated the ability to significantly protect against homologous NTHI challenge by either active or passive immunization using the above-described region 3-based immunogens in a chinchilla model (3). To better understand the mechanisms behind this protection, and also perhaps better predict efficacy against future heterologous challenges relative to the specific region targeted, we have been characterizing both the functional activities of the protective sera (30) as well as the immunodominant and adhesin binding domains of this NTHI adhesin. Herein we report on the latter efforts. Toward this goal, we created a panel of 45 synthetic peptides representing mature P5-fimbrin as well as several areas within it that were of particular interest. The first series of 34 sequential 10-mer peptides were created to scan P5-fimbrin for sites throughout the mature protein that were potentially reactive with a panel of immune chinchilla sera of variable specificity and titer, including two serum pools that had proven to be highly protective in animal models. We also used this series of peptides to determine if any were able to directly inhibit the adherence of diverse NTHI strains to human respiratory epithelial cells, thus identifying the domains of the protein mediating binding to these cells.
We found that despite high titer and demonstrated reactivity by Western blotting, reactivity to the 10-mer peptides by all of the sera tested was fairly low overall by BIA, indicating that these short linear peptides did not likely represent complete epitopes. However, adherence inhibition assays, conducted with the same 10-mer peptides, suggested the presence of an adhesin binding domain in the area that maps to surface-exposed region 3 and also indicated a group-specific inhibitory effect relative to the reported sequence diversity in this area (3). Collectively, these data suggested the need to both focus further on the third surface-exposed region and better understand the noted sequence diversity herein (3).
The observation that the C-terminal 10-mer peptides inhibited adherence of particularly the group 1 isolate but also the group 2a and 3 isolates as well was unanticipated due to the prediction that this portion of the surface protein is largely periplasmically located and thus unavailable for cell-cell interactions. Thus, the significance of these observations is not known at this time. Several reports have challenged the periplasmic location of the C-terminal portion of similar members of the OmpA family of proteins (10, 20, 23), however, suggesting greater surface accessibility of portions of these proteins than predicted by the classic N-terminal eight-stranded β-barrel protein model as put forth by Morona et al. (38). While questions remain concerning the possible surface exposure of portions of the C terminus of P5-fimbrin and other members of the family of OmpA homologues (7, 11, 21, 23, 46, 52) as well as how they might be expressed on the bacterial surface, the role of P5-fimbrin as an adhesin for NTHI and for its homologs in other gram-negative mucosal pathogens (21, 37, 42–45, 51, 53–55) has gained acceptance. Likewise, the general location of the four surface-exposed regions of P5-fimbrin is consistently reported among labs (4, 15, 55, 55a).
We thereby synthesized a second set of peptides designed to map all four surface-exposed regions of this peptide as predicted by Duim et al. (15) and by Webb and Cripps (55). These regions are similar to those described for OmpA of Escherichia coli (31, 38) and the Opa proteins of Neisseria (35) and served as the original basis for the design of LB1 (4). These longer peptides were designed to more accurately mimic the surface-exposed regions in their entirety and thus help overcome the issue of noncontinuous epitopes presented by the shorter 10-mer peptides. It was also anticipated that the region 1 to 4 peptides would allow us to confirm the relative importance of the third surface-exposed region in NTHI adherence, as predicted by the 10-mer peptides, as well as perhaps identify others. These region peptides were also used to assay whether the highly protective anti-LB1 and anti-LPD-LB1(f)2,1,3 sera were indeed inducing antibody that was specific for the surface-accessible region(s) they were designed to target.
When used in the bacterial adherence inhibition assay, synthetic peptides representing regions 3 and 4 were significantly more inhibitory to adherence of a group 1 NTHI strain to human OP cells than to either a group 2a or group 3 isolate. These data thus substantiated the observation that surface-exposed region 3 was an adhesin binding domain and additionally identified a second domain in region 4 that was also group specific. When the two protective serum pools were reacted with the panel of peptides in the BIA, they displayed unique affinities. Whereas the anti-LPD-LB1(f)2,1,3 serum pool was unreactive, the anti-LB1 serum pool showed greatest relative affinity values against itself as well as the region 3 peptide as it had been designed to do. Thus, data obtained by BIA with anti-LB1 corroborated the likelihood that the 19-mer focused region of P5-fimbrin was a surface exposed B-cell epitope as had been predicted by algorithm (4, 15, 55).
To continue our attempt to determine if the recombinant fusion peptide immunogen LPD-LB1(f)2,1,3 did indeed induce antibodies that were more broadly reactive against NTHI strains heterogeneous in this 19-mer focused region than did the chimeric peptide LB1 (which was designed after majority group 1 only), we then assayed these two serum pools against the last set of seven synthetic peptides designed to specifically map this region and address the noted sequence diversity here (3). However, the anti-LPD-LB1(f)2,1,3 serum pool, while strongly reactive in BIA against itself and recombinant LPD, did not recognize the group or partial sequence peptide series. Anti-LB1 serum, on the other hand, demonstrated significantly greater affinity for the homologous group 1 sequence [LB1(1)] than for those representing groups 2a, 2b, or 3; however, there were interesting affinities noted in terms of heterologous recognition as well. This latter serum pool showed greatest relative affinity for the group 3 peptide and also reacted with a bound peptide representing group 2b but was unreactive with the peptide representing the group 2a sequence. Given the more extensive similarity in the consensus sequences of groups 2a and 2b to that of majority group 1, compared to the truncated group 3 consensus sequence (13 residues versus 19 to 22 for group 1, 2a, or 2b), this was an unexpected finding. These data again suggest the immunological significance of the sequence variability observed in the C-terminal portion of this focused region among NTHI strains.
When run against bound overlapping partial sequence peptides designed to map the majority group 1 sequence, anti-LB1 demonstrated a stronger affinity for the C-terminal half of this region, indicating perhaps greater accessibility of these residues. Based on the models put forth by Duim et al. (15) and as predicted by Webb and Cripps (55), the LB1ps3 sequence residues would be placed nearly centrally in this region or loop, placing them furthest away from any nonaccessible regions.
Overall, data obtained with the region 1 to 4 peptides suggested that regions 3 and 4 within the N-terminal half of P5-fimbrin were potentially significant in terms of both immune recognition and adherence activity. In addition, these findings suggested that for greatest future protective efficacy and to avoid a limited group-specific immunizing effect, the ideal immunogen for P5-fimbrin should incorporate sequences of all three NTHI groups relative to region 3. Thus, despite the unanticipated lack of reactivity shown by the anti-LPD-LB1(f)2,1,3 serum pool in the BIA, our data generally supported the rationale behind the creation of this recombinant fusion peptide immunogen. LPD-LB1(f)2,1,3 has indeed demonstrated significant protective activity in vivo against homologous challenge (3) and recently against heterologous challenge as well (30). Moreover, while some of the patterns of functional activity and immunological reactivity shown here could have been largely predicted for these sera, since they had been collected from animals actively and specifically immunized against the targeted epitopes, other patterns of group-specific functional activities and immunological cross-reactivity, or lack thereof, were heretofore unknown.
One other goal of our study was to determine if any of the four predicted surface-exposed regions of this adhesin might be immunodominant during induced and, perhaps more importantly, natural disease. To test this, we assayed middle ear fluids collected over a 5-week period from an immunologically naive chinchilla during an active middle ear infection due to direct challenge. When assayed against the four region peptides, the middle ear fluids specifically recognized and bound to the region 3 and 4 peptides, beginning approximately 4 weeks after challenge but already showing lessened reactivity 5 weeks after challenge. Since total antibody available in middle ear fluids represents both locally produced antibody as well as antibodies contributed by serum transuded into the middle ear space during inflammation (2, 16–19, 57), this delayed reactivity of the middle ear fluids to these peptides may have represented the prolonged period of time required for serum antibodies to transude into this highly inflamed space during an active disease of this type. However, these data may also be indicative of the time point at which region 3- and 4-specific antibodies were available for detection in this assay system despite their presence in these fluids at a much earlier point in time and for a more extended period of the disease course. At 28 days after transbullar challenge, the majority of naive animals inoculated in this manner with NTHI strain 86-028NP have begun to resolve their middle ear infections and most have cleared viable bacteria from the middle ear cleft (3); thus, less antibody may be bound by these microorganisms and more might be available for detection by BIA. While it would have been interesting to similarly assay serum samples collected from this animal on the same schedule as collection of middle ear fluids, these were unfortunately not available to us.
To determine if children with OM due to NTHI similarly preferentially recognized any of the four surface-exposed region of this adhesin during natural disease, we assayed serum collected from 14 children aged 3 months to 11 years by Western blotting and against bound region peptides in the BIA system. All seven sera obtained from children with NTHI culture-positive OM recognized NTHI OMPs in Western blotting, including species of P5-fimbrin, and showed specific affinity for those peptides representing the third and fourth predicted surface-exposed regions of this adhesin as had the chinchilla middle ear fluids. Conversely, none of the seven serum samples collected from children with S. pneumoniae culture-positive OM strongly recognized NTHI OMPs by Western blotting, nor were they reactive with any of the four region peptides by BIA.
Thus, both immunodominant and adherence functional activities mapped to the third and fourth predicted surface-exposed regions of P5-fimbrin. Whereas region 3-based immunogens have shown efficacy (3, 4, 30), we have not been successful to date with an immunogen directed against region 4. A synthetic chimeric peptide immunogen designated LB2 that included a sequence representing this fourth region, N-terminal to the same T-cell promiscuous epitope as in LB1, induced high-titered antiserum that strongly recognized P5-fimbrin in both Western blotting and by ELISA. However, it did not inhibit NTHI adherence to chinchilla tracheal organ culture (4) or protect against homologous NTHI challenge in either a nasopharyngeal colonization model or one of OM in chinchillas (3). In fact, animals immunized with LB2 and later challenged with a homologous isolate demonstrated signs of severe immunopathology in sections of tympanic membrane and middle ear mucosa (3), similar to what we have observed in chinchillas immunized with OMP P2 that were also later homologously challenged (51). Webb and Cripps likewise synthesized an immunogen, in the reversed orientation, that contained an N-terminal T-cell promiscuous epitope from measles virus fusion protein and a C-terminal region 4 peptide (termed MVF/L4) and found a similar ineffectiveness of this construct as an immunogen in a rat model of lung clearance (55a). Thereby, despite predicted surface exposure of this fourth loop or region, and its potential role in adherence and immune recognition as demonstrated here, we have not further developed this moiety as a vaccine component against NTHI-induced OM.
In summary, peptides derived from a group 1 isolate representing the third surface-exposed region of P5-fimbrin significantly inhibited the adherence of NTHI strains to human OP cells in a group-specific manner, indicating the significance of the sequence diversity noted herein. By BIA, the immune reactivity of animals that had been significantly protected against nasopharyngeal colonization, ascension of the eustachian tube, and induction of OM mapped to the exact moiety or moieties of P5-fimbrin located within region 3 to which they had been specifically immunized. An animal with an active NTHI-induced OM preferentially recognized both regions 3 and 4 of P5-fimbrin, the latter of which has performed poorly as a protective immunogen to date in two animal models of NTHI colonization and/or disease, unlike region 3-based immunogens (3, 4). Region 3 was one of two areas of this adhesin that were recognized immunologically by children as young as 3 months of age and as old as 11 years with an NTHI-caused OM during natural infection.
If a human immune response could be directed toward this third region of P5-fimbrin, as our data indicate is feasible, based on the demonstrated immunoreactivity of pediatric sera during natural infection, our hypothesis is that higher-titered preexisting antibody present in the serum of immunized children could transude on to the mucosal surface upon upper respiratory tract virus infection that commonly precedes bacterial OM (1, 12, 36, 47, 48). In this scenario, these induced and transuded serum antibodies could inactivate NTHI colonizing the nasopharyngeal mucosa and thus prevent their ascension of the virus-compromised eustachian tube and development of bacterial OM, as we have been able to successfully demonstrate occurs in the chinchilla (3, 30).
ACKNOWLEDGMENTS
This research was funded by a grant from SmithKline Beecham Biologicals, Rixensart, Belgium, and by grant DC02830-03 from the NIDCD/NIH.
We thank Pravin T. P. Kaumaya and John Lowbridge (The Ohio State University Peptide and Protein Engineering Lab), Jon Nezezon (University of Rochester), and Joe Cohen and Yves Lobet (SmithKline Beecham Biologicals) for assistance with this project.
REFERENCES
- 1.Arola M, Ziegler T, Ruuskanen O. Respiratory virus infection as a cause of prolonged symptoms in acute otitis media. J Pediatr. 1990;116:697–701. doi: 10.1016/s0022-3476(05)82650-2. [DOI] [PubMed] [Google Scholar]
- 2.Bakaletz L O, Holmes K A. Evidence for transudation of specific antibody into the middle ears of parenterally immunized chinchillas after an upper respiratory tract infection with adenovirus. Clin Diagn Lab Immunol. 1997;4:223–225. doi: 10.1128/cdli.4.2.223-225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakaletz L O, Kennedy B J, Novotny L A, Dequesne G, Cohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun. 1999;67:2746–2762. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakaletz L O, Leake E R, Billy J M, Kaumaya P T. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine. 1997;15:955–961. doi: 10.1016/s0264-410x(96)00298-8. [DOI] [PubMed] [Google Scholar]
- 5.Bakaletz L O, Tallan B M, Andrzejewski W J, DeMaria T F, Lim D J. Immunological responsiveness of chinchillas to outer membrane and isolated fimbrial proteins of nontypeable Haemophilus influenzae. Infect Immun. 1989;57:3226–3229. doi: 10.1128/iai.57.10.3226-3229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakaletz L O, Tallan B M, Hoepf T, DeMaria T F, Birck H G, Lim D J. Frequency of fimbriation of nontypeable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun. 1988;56:331–335. doi: 10.1128/iai.56.2.331-335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck-Sickinger A G, Rotering H, Wiesmuller K H, Dorner F, Jung G. Mapping of antigenic and immunogenic sites of Haemophilus influenzae outer membrane protein P6 using synthetic lipopeptides. Biol Chem Hoppe Seyler. 1994;375:173–182. doi: 10.1515/bchm3.1994.375.3.173. [DOI] [PubMed] [Google Scholar]
- 8.Berger B. Algorithms for protein structural motif recognition. J Comput Biol. 1995;2:125–138. doi: 10.1089/cmb.1995.2.125. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein J M, Faden H S, Ogra P L. Nasopharyngeal colonization by nontypeable Hemophilus influenzae in children: the effect of serum bactericidal antibody. Otolaryngol Head Neck Surg. 1991;105:406–410. doi: 10.1177/019459989110500309. [DOI] [PubMed] [Google Scholar]
- 10.Blake M S, Wetzler L M, Gotschlich E C, Rice P A. Protein III: structure, function, and genetics. Clin Microbiol Rev. 1989;2 Suppl:S60–S63. doi: 10.1128/cmr.2.suppl.s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogdan J A, Jr, Apicella M A. Mapping of a surface-exposed, conformational epitope of the P6 protein of Haemophilus influenzae. Infect Immun. 1995;63:4395–4401. doi: 10.1128/iai.63.11.4395-4401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements D A, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995;149:1113–1117. doi: 10.1001/archpedi.1995.02170230067009. [DOI] [PubMed] [Google Scholar]
- 13.Cooper J A, Hayman W, Reed C, Kagawa H, Good M F, Saul A. Mapping of conformational B cell epitopes within alpha-helical coiled coil proteins. Mol Immunol. 1997;34:433–440. doi: 10.1016/s0161-5890(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 14.Craig L, Sanschagrin P C, Rozek A, Lackie S, Kuhn L A, Scott J K. The role of structure in antibody cross-reactivity between peptides and folded proteins. J Mol Biol. 1998;281:183–201. doi: 10.1006/jmbi.1998.1907. [DOI] [PubMed] [Google Scholar]
- 15.Duim B, Bowler L D, Eijk P P, Jansen H M, Dankert J, van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faden H, Bernstein J, Brodsky L, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children. I. The systemic immune response to nontypable Hemophilus influenzae. J Infect Dis. 1989;160:999–1004. doi: 10.1093/infdis/160.6.999. [DOI] [PubMed] [Google Scholar]
- 17.Faden H, Brodsky L, Bernstein J, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children: local immune response to nontypeable Haemophilus influenzae. Infect Immun. 1989;57:3555–3559. doi: 10.1128/iai.57.11.3555-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 19.Faden H S. Immunology of the middle ear: role of local and systemic antibodies in clearance of viruses and bacteria. Ann N Y Acad Sci. 1997;830:49–60. doi: 10.1111/j.1749-6632.1997.tb51878.x. [DOI] [PubMed] [Google Scholar]
- 20.Finnen R L, Martin N L, Siehnel R J, Woodruff W A, Rosok M, Hancock R E. Analysis of the Pseudomonas aeruginosa major outer membrane protein OprF by use of truncated OprF derivatives and monoclonal antibodies. J Bacteriol. 1992;174:4977–4985. doi: 10.1128/jb.174.15.4977-4985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gousset N, Rosenau A, Sizaret P Y, Quentin R. Nucleotide sequences of genes coding for fimbrial proteins in a cryptic genospecies of Haemophilus spp. isolated from neonatal and genital tract infections. Infect Immun. 1999;67:8–15. doi: 10.1128/iai.67.1.8-15.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes E E, Gilleland L B, Gilleland H E., Jr Synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa that elicit antibodies reactive with whole cells of heterologous immunotype strains of P. aeruginosa. Infect Immun. 1992;60:3497–3503. doi: 10.1128/iai.60.9.3497-3503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Z, Nagata N, Molina E, Bakaletz L O, Hawkins H, Patel J A. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect Immun. 1999;67:187–192. doi: 10.1128/iai.67.1.187-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson U, Fagerstam L, Ivarsson B, Johnsson B, Karlsson R, Lundh K, Lofas S, Persson B, Roos H, Ronnberg I, et al. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. BioTechniques. 1991;11:620–627. [PubMed] [Google Scholar]
- 26.Karlsson R, Falt A. Experimental design for kinetic analysis of protein-protein interactions with surface plasmon resonance biosensors. J Immunol Methods. 1997;200:121–133. doi: 10.1016/s0022-1759(96)00195-0. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson R, Michaelsson A, Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991;145:229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson R, Stahlberg R. Surface plasmon resonance detection and multispot sensing for direct monitoring of interactions involving low-molecular-weight analytes and for determination of low affinities. Anal Biochem. 1995;228:274–280. doi: 10.1006/abio.1995.1350. [DOI] [PubMed] [Google Scholar]
- 29.Kaumaya P T, Berndt K D, Heidorn D B, Trewhella J, Kezdy F J, Goldberg E. Synthesis and biophysical characterization of engineered topographic immunogenic determinants with alpha alpha topology. Biochemistry. 1990;29:13–23. doi: 10.1021/bi00453a002. . (Erratum, 29:7780.) [DOI] [PubMed] [Google Scholar]
- 30.Kennedy, B., L. A. Novotny, J. A. Jurcisek, Y. Lobet, and L. O. Bakaletz. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 31.Koebnik R. Structural and functional roles of the surface-exposed loops of the beta-barrel membrane protein OmpA from Escherichia coli. J Bacteriol. 1999;181:3688–3694. doi: 10.1128/jb.181.12.3688-3694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lofas S, Johnsson B. A novel Hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J Chem Soc Chem Commun. 1990;21:1526–1528. [Google Scholar]
- 33.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 34.Malmqvist M. Epitope mapping by label-free biomolecular interaction analysis. Methods. 1996;9:525–532. doi: 10.1006/meth.1996.0060. [DOI] [PubMed] [Google Scholar]
- 35.Malorny B, Morelli G, Kusecek B, Kolberg J, Achtman M. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. J Bacteriol. 1998;180:1323–1330. doi: 10.1128/jb.180.5.1323-1330.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIntosh K, Halonen P, Ruuskanen O. Report of a workshop on respiratory viral infections: epidemiology, diagnosis, treatment, and prevention. Clin Infect Dis. 1993;16:151–164. doi: 10.1093/clinids/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto N, Bakaletz L O. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb Pathog. 1996;21:343–356. doi: 10.1006/mpat.1996.0067. [DOI] [PubMed] [Google Scholar]
- 38.Morona R, Klose M, Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984;159:570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munson R S, Jr, Granoff D M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985;49:544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munson R S, Jr, Grass S, West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993;61:4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Partidos C, Stanley C, Steward M. The influence of orientation and number of copies of T and B cell epitopes on the specificity and affinity of antibodies induced by chimeric peptides. Eur J Immunol. 1992;22:2675–2680. doi: 10.1002/eji.1830221030. [DOI] [PubMed] [Google Scholar]
- 42.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAc beta 1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy M S, Bernstein J M, Murphy T F, Faden H S. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect Immun. 1996;64:1477–1479. doi: 10.1128/iai.64.4.1477-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy M S, Murphy T F, Faden H S, Bernstein J M. Middle ear mucin glycoprotein: purification and interaction with nontypeable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg. 1997;116:175–180. doi: 10.1016/S0194-59989770321-8. [DOI] [PubMed] [Google Scholar]
- 46.Rosenqvist E, Musacchio A, Aase A, Hoiby E A, Namork E, Kolberg J, Wedege E, Delvig A, Dalseg R, Michaelsen T E, Tommassen J. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect Immun. 1999;67:1267–1276. doi: 10.1128/iai.67.3.1267-1276.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruuskanen O, Arola M, Putto-Laurila A, Mertsola J, Meurman O, Viljanen M K, Halonen P. Acute otitis media and respiratory virus infections. Pediatr Infect Dis J. 1989;8:94–99. [PubMed] [Google Scholar]
- 48.Ruuskanen O, Heikkinen T. Viral-bacterial interaction in acute otitis media. Pediatr Infect Dis J. 1994;13:1047–1049. doi: 10.1097/00006454-199411000-00034. [DOI] [PubMed] [Google Scholar]
- 49.Senpuku H, Miyauchi T, Hanada N, Nisizawa T. An antigenic peptide inducing cross-reacting antibodies inhibiting the interaction of Streptococcus mutans PAc with human salivary components. Infect Immun. 1995;63:4695–4703. doi: 10.1128/iai.63.12.4695-4703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw D M, Stanley C M, Partidos C D, Steward M W. Influence of the T-helper epitope on the titre and affinity of antibodies to B-cell epitopes after co-immunization. Mol Immunol. 1993;30:961–968. doi: 10.1016/0161-5890(93)90121-q. [DOI] [PubMed] [Google Scholar]
- 51.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugawara E, Steiert M, Rouhani S, Nikaido H. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J Bacteriol. 1996;178:6067–6069. doi: 10.1128/jb.178.20.6067-6069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virji M, Makepeace K, Ferguson D J, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 54.Virji M, Makepeace K, Ferguson D J, Achtman M, Sarkari J, Moxon E R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–2795. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 55.Webb D C, Cripps A W. Secondary structure and molecular analysis of interstrain variability in the P5 outer-membrane protein of non-typeable Haemophilus influenzae isolated from diverse anatomical sites. J Med Microbiol. 1998;47:1059–1067. doi: 10.1099/00222615-47-12-1059. [DOI] [PubMed] [Google Scholar]
- 55a.Webb D C, Cripps A W. A P5 peptide that is homologous to peptide 10 of OprF from Pseudomonas aeruginosa enhances clearance of nontypeable Haemophilus influenzae from acutely infected rat lung in the absence of detectable peptide-specific antibody. Infect Immun. 2000;68:377–381. doi: 10.1128/iai.68.1.377-381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf E, Kim P S, Berger B. MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 1997;6:1179–1189. doi: 10.1002/pro.5560060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamanaka N, Faden H. Local antibody response to P6 of nontypable Haemophilus influenzae in otitis-prone and normal children. Acta Otolaryngol (Stockholm) 1993;113:524–529. doi: 10.3109/00016489309135857. [DOI] [PubMed] [Google Scholar]