Abstract
Group B streptococci (GBS) adhere to surface receptors present on epithelial cells; these receptors include fibronectin and laminin. To identify other possible receptors, plasma membranes from A549 cells, a respiratory tract epithelial cell line, were prepared. These plasma membranes were tested in a protein blot analysis using radiolabeled GBS as a probe. GBS adhered to two species, with molecular masses of 50 kDa (p50) and 57 kDa (p57). We concluded that p50 and p57 correspond to two forms of cytokeratin 8 (CK8) on the basis of the following results: (i) protein blot results demonstrated that p50 and p57 exactly comigrated with two forms of CK8 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE); (ii) p50 and p57 exactly comigrated with CK8 after separation by two-dimensional PAGE; (iii) CK8 in solution bound to GBS, as demonstrated by immunoblot analysis of proteins from A549 lysates that bound to GBS in a liquid-phase assay; and (iv) radiolabeled GBS bound to A549 lysate-derived CK8 that had been captured in anti-CK8-coated microtiter wells. CK8 bound to COH1-13, an acapsular mutant of COH1, demonstrating that adherence is not mediated by capsular polysaccharide. Trypsin-treated GBS did not bind to CK8, indicating that adherence is mediated via a protein on the surface of GBS. Soluble CK8 bound to six of six GBS strains tested. Soluble CK8 also bound to Staphylococcus aureus, Lactococcus lactis, Enterococcus faecalis, and Streptococcus pyogenes. We hypothesize that adherence of GBS to cytokeratin may be important for maintenance of colonization at sites of keratinized epithelium, such as the vagina, or for adherence of these bacteria to damaged epithelial cells at other sites.
Group B streptococci (GBS) are a leading cause of sepsis and meningitis in the United States and Western Europe, as well as a leading cause of infectious morbidity and mortality in neonates (1). GBS infection generally occurs by passage of bacteria from colonized mothers to their newborns. Mothers are colonized in the rectum and/or vagina. Following maternal colonization, infection of the infant occurs either through ascent of bacteria to the amniotic sac or following aspiration of vaginal contents during parturition. Pneumonia is the primary presentation of early-onset GBS disease, implicating the neonatal lung as the site of initial infection.
The ability to adhere to epithelial surfaces has been demonstrated to be an important virulence factor for many bacterial pathogens (12, 16). Adherence of GBS to epithelial cells in the lung, the urogenital tract, and the gastrointestinal tract may play an important role in preventing these bacteria from being cleared by bulk-flow host defense mechanisms. Studies of GBS adherence to epithelial cells have implicated a number of bacterial factors, including surface proteins and lipoteichoic acid (19). However, relatively little is known about the adhesin receptors that are present on host epithelial cells to which GBS adhere. Previous studies have demonstrated that GBS bind to fibronectin (18) and laminin, and the laminin receptor has recently been cloned (15). However, a role for these interactions in either adherence to epithelial cells or pathogenesis of infection of the neonate has yet to be demonstrated.
Many bacterial pathogens express multiple adhesins that adhere to numerous adhesin receptors present on epithelial surfaces. We have focused on the adhesin receptors which might be present in the lung of the neonate and which might allow bacteria to avoid clearance by pulmonary defense mechanisms, such as the mucociliary ladder. In previous studies, we have studied adherence with the A549 cell line, a well-differentiated pulmonary epithelial cell line with many of the characteristics of type I alveolar pneumocytes (19). We hypothesized that adhesin receptors which mediate attachment of GBS to pulmonary epithelial cells would be present in plasma membrane preparations from A549 cells and that identification of these adhesin receptors could be accomplished by allowing GBS to adhere to individual proteins from these plasma membrane preparations. In this study, we demonstrated that GBS adhere to two proteins, with molecular masses of approximately 50 and 57 kDa, which are present in plasma membrane preparations of A549 epithelial cells, and that these proteins correspond to two forms of cytokeratin 8 (CK8).
MATERIALS AND METHODS
Bacterial strains, epithelial cell lines, and growth conditions.
All GBS strains were obtained from Craig Rubens, who maintains a bank of GBS isolates as part of the National Institutes of Health Initiative on the Prevention of Group B Streptococcal Infections in Neonatal and Infant Populations (RFP-NIH-NIAID-DMID-92-13). COH1 is a virulent type III GBS isolate from a septic neonate, and COH1-13 is an acapsular mutant of COH1 (14). Strains CS101 (Streptococcus pyogenes), RH110 (Enterococcus faecalis), LM0230 (Lactococcus lactis), and SA25923 (Staphylococcus aureus) were obtained from the American Type Culture Collection (Manassas, Va.). All bacterial strains were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) at 37°C in a shaking incubator except for CS101, which was grown in stationary tubes. A549, an epithelial cell line from a human lung carcinoma, which has many characteristics of type I alveolar pneumocytes, was obtained from the American Type Culture Collection and was grown as described previously (19).
Purification of plasma membranes.
For a single membrane preparation, three 150-cm2 flasks of A549 cells were grown to confluence, and cells were loosened by incubation for 30 min at 25°C with 50 ml of phosphate-buffered saline (PBS) containing 10 mM EDTA. The cells were removed by vigorous pipetting, centrifuged at 800 × g for 10 min, and washed with 50-ml volumes of PBS by centrifugation three times. Cells were then resuspended in lysis buffer consisting of 5 mM HEPES (pH 7.4), 50 mM mannitol, and 40 μg of soybean trypsin inhibitor (Sigma, St. Louis, Mo.)/ml. Nuclei were then removed by low-speed centrifugation at 200 × g for 10 min. The pellet was resuspended in lysis buffer, and the centrifugation repeated. The supernatants, containing crude membranes, were pooled. Plasma membranes were then isolated by sucrose density gradient centrifugation. Sucrose (10.8 g) was added to pooled crude membranes along with lysis buffer for a final volume of 20 ml (54% sucrose). The membrane preparation was placed in a centrifuge tube for an SW28 swinging-bucket rotor (Beckman Instruments, Palo Alto, Calif.) and overlaid with 8 ml of 40% sucrose in lysis buffer, then 8 ml of 33% sucrose in lysis buffer, and then 3 ml of lysis buffer. The tube was centrifuged at 4°C and 100,000 × g for 18 h. Membranes at the 33%/40% sucrose interface were recovered, brought to 40 ml with lysis buffer, and recovered by centrifugation at 100,000 × g for 1 h. The final pellet was resuspended in 1 ml of lysis buffer without mannitol and stored at −20°C. Purification was monitored by determining alkaline phosphatase activity with an alkaline phosphatase kit from Sigma. Protein content was analyzed by using the Bio-Rad protein assay reagent. In all plasma membrane preparations, a 10- to 20-fold relative purification of plasma membrane proteins, as determined by assay of alkaline phosphatase activity, was achieved.
Protein blot analysis of adhesin receptors, using radiolabeled bacteria as a probe.
A 5- 20-μg quantity of plasma membrane proteins was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore, Burlington, Mass.), and probed with 35S-labeled COH1 as previously described (18). For two-dimensional analysis, proteins were separated as described previously (11) and then transferred and probed as described above.
Immunoblot analysis.
Immunoblot analysis was carried out as previously described (20). Briefly, blots were prepared as described above, blocked with 1% bovine serum albumin (BSA) in PBS for 2 h, and then probed with monoclonal antibody diluted 1:200 in immunoblot-blocking solution (PBS containing 5% nonfat dry milk and 0.1% Tween 20) overnight at 4°C. The blot was washed briefly and then incubated with an anti-mouse immunoglobulin–horseradish peroxidase conjugate diluted 1:1,000 in immunoblot-blocking solution for 1 h at room temperature. The blot was washed and then developed by the use of an enhanced chemiluminescence system (Pierce, Rockford, Ill.) per the manufacturer's protocol. Anti-CK8 (M20) and anti-CD14 (uchm-1) are monoclonal antibodies and were obtained from Sigma.
Adherence of CK8 from A549 epithelial cell lysates to GBS.
A549 cells from one 150-cm2 flask were removed and washed as described above. Cells were pelleted and then resuspended on 5 ml of phosphate lysis buffer (PLB; PBS with 1% Triton X-100 and 0.1% SDS). Cells were allowed to lyse for 30 min on a rocking platform, and nuclei and other insoluble material were pelleted for 30 min in a refrigerated microcentrifuge (model 5417R; Eppendorf, Hamburg, Germany). The supernatant was aliquoted and stored at −70°C for later use. Bacterial strains were grown overnight as described above, diluted 1:10 in fresh Todd-Hewitt broth, and grown to an optical density at 600 nm of 0.4 to 0.5. A 1-ml volume of bacterial culture was centrifuged, and the pelleted cells were washed twice with PBS, resuspended in 0.5 ml of PBS with 5% BSA (Sigma), and incubated for 2 h on ice to prevent nonspecific binding of proteins. BSA was added to A549 lysates to a concentration of 5% (wt/vol). Bacteria were pelleted, resuspended in 100 μl of A549 lysate, and incubated on a rocking platform for 2 to 4 h at 4°C. Bacteria were pelleted and then washed four times with 1-ml volumes of PLB, and bound proteins were eluted with 50 μl of 2× SDS-PAGE sample buffer at 100°C for 5 min. Eluted proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and blocked with immunoblot blocking solution. The blot was probed overnight at 4°C with 5 ml of M20 (anti-CK8; Sigma) ascites fluid diluted 1:500 in immunoblot-blocking solution. The blot was washed briefly and then incubated for 1 h at 4°C with a goat anti-mouse immunoglobulin–horseradish peroxidase conjugate (Sigma) diluted 1:1,000 in immunoblot-blocking solution.
Adherence of GBS to CK8 captured via a monoclonal antibody.
U-bottom microtiter plates (Becton Dickinson, Franklin Lakes, N.J.) were coated with 50 μl of a goat anti-mouse immunoglobulin solution (100 μg/ml; Sigma) overnight at 4°C. This solution was aspirated and saved for reuse in subsequent experiments. The plate was then washed three times with PBS. A 50-μl volume of monoclonal antibody (ascites fluid diluted 1:100 in PBS) was then added, and the plate was incubated overnight at 4°C. It was then washed three times with PBS containing 5% nonfat dry milk and then three times with PBS. The plate was blocked with 90 μl of 5% BSA in PBS for 4 h at 4°C. A549 lysate (50 μl) prepared as described above was then added to each well and allowed to bind overnight at 4°C. The plate was then washed three times with PLB and three times with PBS containing 5% nonfat dry milk. The plate was again blocked with 5% BSA in PBS for 1 h at 4°C, and adherence of radiolabeled GBS was determined as previously described (18).
Determination of candidates for use in identification of p50 and p57.
A list of potential candidates for use in identifying p50 and p57 was obtained by using GuessProt. This site has now been renamed TagIdent (http://www.expasy.ch/tools/tagident.html). A pI range of from 4.6 to 6.1 and a molecular weight range of from 43,000 to 63,000 were used. The search was limited to proteins from humans.
RESULTS
GBS adhere to a 57-kDa and a 50-kDa protein present in A549 epithelial cell plasma membranes.
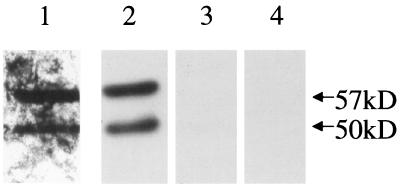
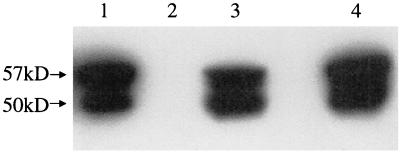
To identify additional receptors by which GBS might adhere to epithelial cells, we isolated plasma membrane proteins from A549 epithelial cells. We hypothesized that the plasma membrane would contain adhesin receptors. We further hypothesized that these adhesin receptors might retain their ability to bind to GBS after separation by SDS-PAGE and transfer to a PVDF membrane, thus allowing us to identify the molecular masses of proteins that bind to GBS. A549 epithelial cell plasma membranes were isolated as described in Materials and Methods, with sucrose density gradient centrifugation being used to separate plasma membranes from other cellular membranes. Alkaline phosphatase was used as a marker for the presence of plasma membranes. We achieved between 10- and 20-fold purification of plasma membrane proteins (data not shown). Potential adhesin receptors were then identified by protein blot analysis with radiolabeled GBS as a probe. A549 plasma membrane proteins were separated by SDS-PAGE and transferred to a PVDF membrane, which was then blocked and probed with 35S-labeled COH1. The results of this protein blot analysis are shown in Fig. 1 (lane 1). COH1 adhered to two proteins, with molecular masses of 50 kDa (p50) and 57kDa (p57). We concluded that p50 and p57 bound to immobilized GBS and were potential adhesin receptors.
FIG. 1.
Protein blot analysis of A549 membranes. A549 membrane proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and probed as follows: lane 1, 35S-labeled COH1; lane 2, M20 (anti-CK8); lane 3, uchm-1 (anti-CD14); and lane 4, no primary antibody. (Lanes 2 to 4 are immunoblots.)
p50 and p57 comigrate with two forms of CK8 on SDS-PAGE gels.
We then undertook identification of these proteins. By two-dimensional protein blot analysis (data not shown), we demonstrated that the pI of p50 and p57 was approximately 5.1. We then searched the Swiss-Prot database for candidates whose apparent molecular weights and pIs were close to those of p50 and p57, using GuessProt, as described in Materials and Methods. Several candidates were identified. We performed an immunoblot analysis (Fig. 1) of A549 plasma membrane proteins, using monoclonal antibodies to two of these candidate proteins, CK8 and CD14. M20 (anti-CK8) bound to two proteins that comigrated exactly with p50 and p57 (lane 2). The anti-CD14 antibody did not react with A549 plasma membrane proteins (lane 3). No signal was seen when primary antibody was not added (lane 4). These results demonstrate that a monoclonal antibody to CK8 recognized two distinct proteins from A549 cell membranes. The larger protein, p57, appears to be intact CK8, while the 50-kDa protein appears to be a degradation product of p57. Consistent with this interpretation, multiple degradation products of CK8 have been previously found in A549 cells (7) and colorectal carcinoma cells (9).
We considered the possibility that one of the two peptides recognized by M20 was not CK8 but a peptide unrelated to CK8 that spuriously binds to M20. To eliminate this possibility, we performed immunoblot analysis with a second, independently derived monoclonal antibody, 35βH-11. 35βH-11 and M20 react with the same two peptides (data not shown), confirming that the two peptides seen in the immunoblot analysis correspond to two forms of CK8.
p50 and p57 comigrate with CK8 in two-dimensional electrophoresis.
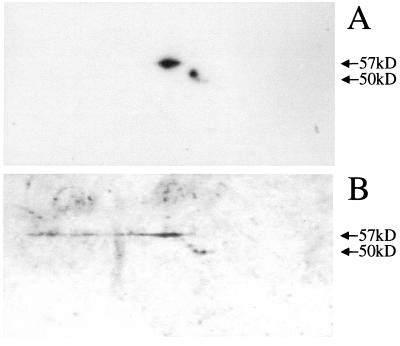
We considered the possibility that the comigration of p50 and p57 with CK8 seen on SDS-PAGE was coincidental and that p50 and p57 are unrelated to CK8. To test this possibility, we performed a two-dimensional protein blot analysis to test whether CK8 comigrates with p50 and p57 in both SDS-PAGE and isoelectric focusing. If p50 and p57 are unrelated to CK8, they should not comigrate with CK8 in isoelectric focusing. Alternatively, comigration of p50 and p57 with CK8 after two-dimensional electrophoresis would provide strong support for the hypothesis that p50 and p57 are CK8. Figure 2 shows the results of a two-dimensional protein blot analysis of A549 membranes probed with both M20 (anti-CK8) (Fig. 2A) and radiolabeled GBS (Fig. 2B). There are two isoelectric forms of the 57-kDa CK8 protein, and they exactly comigrate with the corresponding isoelectric forms of the p50 and p57 GBS adhesin receptors. There are three isoelectric forms of the 50-kDa CK8 protein, one major and two minor. Although the forms on the two blots differ in relative intensity, they do comigrate. We presume that the differences in intensity of the spots are due to differences in the affinities of the different degradation products of CK8 for the M20 antibody and for GBS. Thus, p50 and p57 comigrate with CK8 in both SDS-PAGE and isoelectric focusing, confirming that GBS adhere to CK8.
FIG. 2.
Two-dimensional protein blot analysis of A549 membranes. A549 membranes were separated by the method of O'Farrell (11), transferred to a PVDF membrane, and probed. (A) Immunoblot probed with M20 (anti-CK8). (B) Protein blot probed with 35S-labeled COH1.
GBS bind to p50 and p57 in solution.
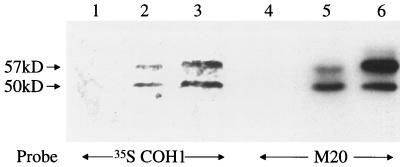
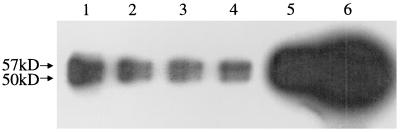
It remained formally possible that GBS were adhering to a molecule that comigrates with CK8 after both isoelectric focusing and SDS-PAGE. We hypothesized that if GBS bound to CK8 on a protein blot, they might also bind to CK8 in solution. Alternatively, if GBS bound to a molecule that comigrated with CK8, but not to CK8 itself, then we would not expect GBS to bind to CK8 in solution. To distinguish between these two possibilities, we tested the ability of GBS to bind CK8 in solution. However, we have previously demonstrated that GBS will only bind fibronectin when it is attached to a solid substrate, and we first sought to confirm that p50 and p57 would bind to GBS both when attached to a solid substrate and when in solution. We incubated COH1 with detergent-solubilized A549 proteins to allow p50 and p57 to bind to COH1, then washed away nonadherent proteins and eluted bound proteins with SDS-PAGE sample buffer. Proteins that eluted from COH1 were subjected to a protein blot analysis and probed with radiolabeled GBS. As shown in Fig. 3, two peptides that bound to 35S-labeled GBS were eluted from COH1. These peptides exactly comigrated with the p57 and p50 present in plasma membranes. These results demonstrate that GBS bind the p57 and p50 adhesin receptors in solution. Proteins eluted from COH1 were also analyzed by SDS-PAGE and stained with Coomassie blue; the only bands visualized corresponded to p50 and p57 (data not shown), suggesting that these are the major A549 cell proteins that bind to COH1. We attempted to confirm the identities of these peptides by performing amino acid sequence analysis, but these attempts were unsuccessful as the N termini appear to be blocked (data not shown).
FIG. 3.
Protein blot analysis of A549 proteins that bind to COH1 in solution. Lanes: 1 to 3, protein blots probed with 35S-labeled COH1; 4 to 6, immunoblots probed with M20 (anti-CK8). Lanes 1 and 4 contain proteins eluted from COH1 alone; lanes 2 and 5 contain proteins eluted from COH1 incubated with A549 lysates, and lanes 3 and 6 contain whole A549 membranes.
GBS bind CK8 in solution.
Since the p50 and p57 adhesins bind to COH1 in solution, we hypothesized that CK8 would also bind to COH1 in solution, thereby confirming that p50 and p57 are two forms of CK8. To test this hypothesis, the protein blot described above, containing A549 lysate proteins that bound to COH1, was probed with M20 (anti-CK8). Two M20-reactive peptides, with molecular masses of 57 and 50 kDa, were eluted from COH1 (Fig. 3). No such peptides were eluted from COH1 that had not been incubated with A549 lysates, demonstrating that the immunoreactive proteins originated in the A549 lysates and were not from COH1. These data demonstrate that CK8 binds to COH1 and confirm that the p50 and p57 adhesin receptors are both forms of CK8.
GBS bind to immunoaffinity-purified CK8.
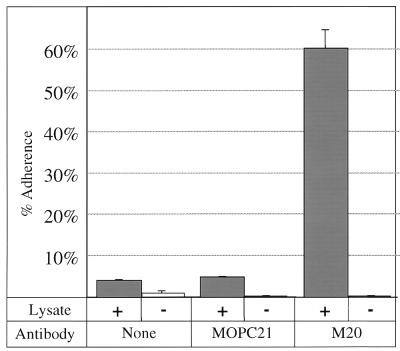
As further proof that GBS bind to CK8, we also tested the ability of GBS to bind to purified CK8. To perform this experiment, CK8 was immunoaffinity purified directly in microtiter wells (17). M20 was captured in microtiter wells coated with goat anti-mouse immunoglobulin G. CK8 from A549 lysates was then captured in the microtiter wells containing the bound M20 antibody. Radiolabeled COH1 was allowed to adhere to the CK8-coated microtiter wells, and adherent bacteria were assayed as described in Materials and Methods. As shown in Fig. 4, approximately 60% of the input COH1 bound to wells containing captured CK8. Adherence was dependent on addition of A549 lysate, demonstrating that the binding was not due to adherence of GBS to the M20 antibody itself. Minimal adherence was seen when no monoclonal antibody or MOPC21 (an isotype-matched control antibody) was used, demonstrating that binding was not due to Fc receptors contained in the A549 lysates but was dependent on the antigen-binding portion of M20. Together, these data demonstrate that COH1 binds to immunoaffinity-purified CK8.
FIG. 4.
COH1 adherence to CK8 captured by anti-CK8. 3H-labeled COH1 was allowed to bind to wells coated with anti-immunoglobulin; then no specific antibody (none), MOPC21 (an isotype control antibody), or M20 (anti-CK8) was added, followed by A549 lysate (+). Negative-control wells (−) did not have lysate added. Percent adherence was calculated as described in Materials and Methods.
Role of capsular polysaccharide and surface proteins in mediating adherence of GBS to CK8.
A number of components are present on the surface of GBS, including capsular polysaccharide, lipoteichoic acid, surface proteins, and group antigen. We tested the role of surface proteins and capsular polysaccharide in adherence of GBS to CK8. Previous studies had demonstrated that adherence of GBS to both epithelial cells and fibronectin is mediated by surface proteins (18, 19). We tested the role of surface proteins in adherence of GBS to CK8 by treating GBS with trypsin. As shown in Fig. 5, binding of CK8 to GBS (lane 1) was eliminated by trypsin treatment (lane 2). To ensure that this was not due to a contaminant in the trypsin, soybean trypsin inhibitor, a specific trypsin inhibitor, was added concomitantly with the trypsin, and adherence to CK8 was then tested. As shown in lane 3, soybean trypsin inhibitor restored adherence of GBS to CK8, demonstrating that the effect of trypsin was due to the proteolytic activity of trypsin and not to a contaminant in the trypsin preparation. Radiolabeled GBS also did not bind to p50 or p57 in protein blot assays when these bacteria were pretreated with trypsin (data not shown). These data demonstrate that adherence of GBS to CK8 is mediated by surface proteins.
FIG. 5.
CK8 binding to trypsin-treated COH1. COH1 was treated as indicated below and then incubated with A549 lysates. Bound proteins were eluted and analyzed by immunoblotting with M20 (anti-CK8). Lane 1, PBS-treated COH1; lane 2, trypsin-treated COH1; lane 3, trypsin- and soybean trypsin inhibitor-treated COH1; lane 4, A549 whole-cell lysate.
To determine whether the capsule mediates binding of GBS to CK8, we tested the ability of an acapsular mutant (COH1-13) to bind to CK8 in solution. As shown in Fig. 6, both COH1 (lane 6) and COH1-13 (lane 7) bound CK8. COH1-13 appeared to bind somewhat more CK8 than did COH1. These data indicate that adherence to CK8 is not mediated by capsular polysaccharide but that capsular polysaccharide may, in fact, slightly inhibit the binding of GBS to CK8.
FIG. 6.
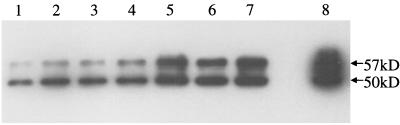
CK8 binding to different GBS strains. Various GBS strains were incubated with A549 lysate, and bound proteins were eluted and analyzed by immunoblotting with M20 (anti-CK8). Strains used were as follows: lane 1, A909 (type Ia); lane 2, DK14 (type Ib); lane 3, DK23 (type II); lane 4, NT6 (type VI); lane 5, NCTC 10/84 (type V); lane 6, COH1 (type III); and lane 7, COH1-13 (acapsular mutant of COH1). Lane 8 contained A549 whole-cell lysate.
CK8 binds to six of six GBS strains tested.
We also tested the possibility that GBS adherence to CK8 might be restricted to a small subset of GBS strains. Six GBS strains of different serotypes were tested for their ability to bind CK8 in solution (Fig. 6). GBS strains were incubated with A549 cell lysates, and after the bacteria were washed, adherent CK8 was eluted with SDS-PAGE sample buffer. Eluted CK8 was detected by immunoblot analysis. As shown in Fig. 6, all six strains tested bound CK8, although their levels of binding differed. NCTC 10/84 bound significantly more CK8 than did the other strains, while A909 bound significantly less than any of the other strains. These results demonstrate that adherence of GBS to CK8 is widespread among GBS strains of different serotypes and is not a peculiar property of COH1.
CK8 binds to four other species of mammalian-associated gram-positive cocci.
We also wished to know whether adherence to CK8 is unique to GBS or is a common property of gram-positive cocci which colonize mammalian hosts. We thus tested binding of four other gram-positive cocci to CK8 in solution. Three of these species (Streptococcus pyogenes, E. faecalis, and Staphylococcus aureus) are human pathogens. We also tested CK8 binding by L. lactis, which is considered a commensal of both humans and bovines. Binding of bacteria to CK8 present in A549 cell lysates was assayed as described above. As shown in Fig. 7, the four gram-positive species bound to CK8 to various degrees. Of note, Streptococcus pyogenes CS101 (lane 5) bound much higher levels of CK8 than did any of the other species tested, including GBS. These data indicate that binding to CK8 is not limited to GBS but may be a general property of gram-positive cocci whose ecological niches are associated with mammalian hosts.
FIG. 7.
CK8 binding to five species of gram-positive cocci. Various strains were incubated with A549 lysates, and bound proteins were eluted and analyzed by immunoblotting with M20 (anti-CK8). Lane 1, COH1 (Streptococcus agalactiae); lane 2, RH110 (E. faecalis); lane 3, LM0230 (L. lactis); lane 4, SA25923 (Staphylococcus aureus); lane 5, CS101 (Streptococcus pyogenes); lane 6, A549 whole-cell lysate.
DISCUSSION
Adherence of GBS to epithelial cells may play an important role in multiple stages in the pathogenesis of GBS disease, including colonization of rectal, vaginal, and pulmonary epithelial surfaces, and as a first step in the invasion of pulmonary epithelial cells. In our efforts to identify additional adhesin receptors on epithelial cell surfaces, we used respiratory epithelial cell plasma membranes to which GBS adhered in a protein blot analysis. Adhesin receptors on epithelial cells include fibronectin (18). Binding to fibronectin was not consistently observed because of the low levels of fibronectin in the membrane preparation (data not shown). Laminin may also be an adhesin receptor (15), although radiolabeled GBS do not appear to bind to purified laminin (18); we did not detect binding to laminin in our blots.
GBS did bind to two specific proteins present in A549 membranes. We have identified these proteins as two forms of CK8. Adherence is mediated by a surface protein; capsular polysaccharide does not appear to be involved. Adherence of GBS to CK8 appears to be a general phenomenon, since six of six GBS strains tested all bound CK8, to various degrees. Furthermore, all other gram-positive cocci tested (Staphylococcus aureus, Streptococcus pyogenes, E. faecalis, and L. lactis) all bound CK8, to various degrees. Streptococcus pyogenes exhibited the highest degree of binding among all strains tested.
Because of the promiscuous binding of CK8 to all bacteria tested, we also considered the possibility that CK8 was binding nonspecifically to COH1. This is unlikely for four reasons. First, GBS did not bind to any of the other proteins present in the plasma membrane preparation, demonstrating that the GBS were not interacting nonspecifically with all membrane proteins but were specifically reacting only with CK8. Second, the lack of CK8 binding to bacterial cells treated with trypsin demonstrates that GBS binding to CK8 is mediated by a protein or proteins that interact with CK8. Third, the lack of binding to trypsinized GBS also provides a negative control for CK8 binding to GBS, demonstrating that the apparent binding of GBS to CK8 in solution is not a result of carryover of CK8 due to inadequate washing during the assay procedure. Fourth, a large proportion of the CK8 present in A549 cell lysates (on the order of 1/10) bound to the bacteria, suggesting that this is a high-capacity, high-affinity interaction. Finally, in all experiments, GBS were pretreated with 5% BSA to block nonspecific interactions. We concluded that binding of GBS to CK8 was unlikely to be due to nonspecific interactions but appeared to be due to an interaction between CK8 and specific protein receptors on GBS. However, a definitive demonstration of this will require identification of GBS mutants that lack specific surface proteins which mediate the interaction of GBS with CK8.
The role, if any, of GBS adherence to CK8 in the pathogenesis of GBS disease is unclear. In general, adhesin receptors must be present on the surface of epithelial cells to interact with bacterial adhesins. CK8 is present, in general, on the cytoplasmic side of plasma membranes of simple epithelia, such as the respiratory epithelium, where it plays a role in anchoring the cytoskeleton to the plasma membrane (8). CK8 is generally not believed to be present on the surface of epithelial cells. There are several mechanisms by which adherence of GBS to CK8 may play a role in pathogenesis, including GBS binding to CK8 that may be present on simple epithelial surfaces, binding of GBS to other cytokeratins present on keratinized epithelial surfaces, binding of GBS to cytoplasmic CK8 after invasion of epithelial cells, or host recognition of CK8 receptors as part of host defense mechanisms. These possible mechanisms are described in further detail below.
First, CK8 may be present on the surface of some epithelial cells. Hembrough and colleagues found that CK8 is on the surface of hepatocytes (5, 6) and some malignant epithelial cells (4). The latter finding may be an artifact of the malignant transformation process, although nonmalignant epithelial cells have not, to our knowledge, been tested for surface CK8 expression. Thus, it is possible that GBS adhere to CK8 which may be present on the surface of some normal epithelial cells.
Second, GBS may adhere to CK8 present on the surface of damaged respiratory epithelial cells. GBS secrete a hemolysin/cytolysin which damages the surface of epithelial cells (10). Interactions between GBS and CK8 may take place after epithelial cells are damaged by hemolysin or other factors elicited by GBS. In preliminary experiments, we have demonstrated that damaged epithelial cells do have high levels of exposed CK8, as evidenced by the high-level binding of anti-CK8 to damaged A549 epithelial cells (data not shown). Alternatively, GBS may adhere preferentially to mucosal surfaces damaged by other mechanisms.
Third, GBS may adhere to damaged keratinized epithelia through CK8 receptors. Although CK8 is not felt to be present in the keratinized upper layers of the dermis (2), it does appear to be expressed in basal layers of the human skin. GBS could potentially interact with CK8 on squamous epithelial surfaces which have been damaged by trauma, exposing the CK8 that is present in the more basal elements of these surfaces.
Fourth, GBS may bind to keratinized epithelia through interactions with cytokeratins other than CK8. Many cytokeratins (not including CK8) are exposed on the surface of keratinized squamous epithelia such as the skin and vagina (8). These cytokeratins are exposed after the plasma membrane dissolves in the final stages of differentiation. In preliminary experiments, we found that GBS do appear to bind to at least two cytokeratins (with molecular masses of approximately 60 and 75 kDa) present in a cytokeratin preparation from human skin. We are presently performing experiments to identify these dermal cytokeratins, and we hypothesize that adherence of GBS to squamous epithelial cytokeratins plays an important role in colonization at these sites.
Fifth, GBS may also interact with cytoskeletal components during the invasion process. GBS are able to invade epithelial cells and enter the cytoplasm (13). We postulate that adherence of GBS to cytoplasmic CK8 plays a role in either the invasion process or transcytosis, the process by which GBS translocate across epithelial barriers. Cytoskeletal components are an important part of the cellular systems for cytoplasmic transport of vessicles and other organelles. GBS may take advantage of these systems by adhering to cytoskeletal components such as CK8.
Sixth, adherence of GBS to cytokeratins may not be a virulence factor but may in fact play a role in host defense. GBS may adhere to keratin exposed on squamous epithelial surfaces. When keratinized cells are exfoliated, GBS may then be eliminated. Studies of mastitis-producing GBS strains have demonstrated that keratin in the teat canal inhibits the ability of GBS to cause infection (3). These data are consistent with a role for adherence of GBS to teat canal keratin in host defense.
In summary, we demonstrate here that GBS bind to CK8 both in solution and when attached to a solid phase. This binding is independent of serotype and is not restricted to GBS, since the four other gram-positive cocci tested also bind to CK8. Binding appears to be mediated by a protein and not by the capsule. This interaction may play an important role in the pathogenesis of GBS disease.
ACKNOWLEDGMENTS
This work was supported by PHS training grant AI01287 to G.S.T.
Craig Rubens, Amanda Jones, and Donald O. Chaffin provided technical advice for carrying out this project.
REFERENCES
- 1.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders; 1994. pp. 980–1054. [Google Scholar]
- 2.Bosch F X, Leube R E, Achtstatter T, Moll R, Franke W W. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol. 1988;106:1635–1648. doi: 10.1083/jcb.106.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capuco A V, Bright S A, Pankey J W, Wood D L, Miller R H, Bitman J. Increased susceptibility to intramammary infection following removal of teat canal keratin. J Dairy Sci. 1992;75:2126–2130. doi: 10.3168/jds.s0022-0302(92)77972-7. [DOI] [PubMed] [Google Scholar]
- 4.Hembrough T A, Kralovich K R, Li L, Gonias S L. Cytokeratin 8 released by breast carcinoma cells in vitro binds plasminogen and tissue-type plasminogen activator and promotes plasminogen activation. Biochem J. 1996;317:763–769. doi: 10.1042/bj3170763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hembrough T A, Li L, Gonias S L. Cell-surface cytokeratin 8 is the major plasminogen receptor on breast cancer cells and is required for the accelerated activation of cell-associated plasminogen by tissue-type plasminogen activator. J Biol Chem. 1996;271:25684–25691. doi: 10.1074/jbc.271.41.25684. [DOI] [PubMed] [Google Scholar]
- 6.Hembrough T A, Vasudevan J, Allietta M M, Glass II W F, Gonias S L. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci. 1995;108:1071–1082. doi: 10.1242/jcs.108.3.1071. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa A, Tachibana H, Kawamoto S, Kamei M, Honjoh T, Hashizume S, Shirahata S. Cytokeratin 8 and 19 as antigens recognized by adenocarcinoma-reactive human monoclonal antibody AE6F4. Hum Antib. 1997;8:195–202. [PubMed] [Google Scholar]
- 8.Moll R, Franke W W, Schiller D L, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 9.Nishibori H, Matsuno Y, Iwaya M, Osada T, Kubomura N, Iwamatsu A, Kohno H, Sato S, Kitajima M, Hirohashi S. Human colorectal carcinomas specifically accumulate Mr 42,000 ubiquitin-conjugated cytokeratin 8 fragments. Cancer Res. 1996;56:2752–2757. [PubMed] [Google Scholar]
- 10.Nizet V, Gibson R L, Chi E Y, Framson P E, Hulse M, Rubens C E. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 12.Reid G, Sobel J D. Bacterial adherence in the pathogenesis of urinary tract infection: a review. Rev Infect Dis. 1987;9:470–487. doi: 10.1093/clinids/9.3.470. [DOI] [PubMed] [Google Scholar]
- 13.Rubens C E, Smith S, Hulse M, Chi E Y, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubens C E, Heggen L M, Haft R F, Wessels M R. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol Microbiol. 1993;8:843–855. doi: 10.1111/j.1365-2958.1993.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 15.Spellerberg B, Rozdzinski E, Martin S, Weber-Heynemann J, Schnitzler N, Lütticken R, Podbielski A. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect Immun. 1999;67:871–878. doi: 10.1128/iai.67.2.871-878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svanborg C, Hedlund M, Connell H, Agace W, Duan R D, Nilsson A, Wullt B. Bacterial adherence and mucosal cytokine responses. Receptors and transmembrane signaling. Ann NY Acad Sci. 1996;797:177–190. doi: 10.1111/j.1749-6632.1996.tb52959.x. [DOI] [PubMed] [Google Scholar]
- 17.Tamura G S, Dailey M O, Gallatin W M, McGrath M S, Weissman I L, Pillemer E A. Isolation of molecules recognized by monoclonal antibodies and antisera: the solid phase immunoisolation technique. Anal Biochem. 1984;136:458–464. doi: 10.1016/0003-2697(84)90244-6. [DOI] [PubMed] [Google Scholar]
- 18.Tamura G S, Rubens C E. Group B streptococci adhere to a variant of fibronectin attached to a solid phase. Mol Microbiol. 1995;15:581–589. doi: 10.1111/j.1365-2958.1995.tb02271.x. [DOI] [PubMed] [Google Scholar]
- 19.Tamura G S, Kuypers J M, Smith S, Raff H, Rubens C E. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994;62:2450–2458. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]