Abstract
Cystic fibrosis (CF) is characterized by dysfunction of the digestive and respiratory tracts resulting in generalized malnutrition and chronic respiratory infections. Chronic lung infections with Pseudomonas aeruginosa, intense neutrophil-dominated airway inflammation, and progressive lung disease are the major cause of high morbidity and mortality in CF. Here we investigated the effects of malnutrition in CF on innate lung defenses, susceptibility to P. aeruginosa colonization, and associated inflammation, using aerosol models of acute and chronic infections in normal, malnourished, and transgenic mice. CFTRm1Unc−/− knockout mice displayed body weight variations and showed variable pulmonary clearance of P. aeruginosa. This variability was not detected in bitransgenic CFTRm1Unc−/−(FABP-hCFTR) mice in which the intestinal defect had been corrected. Diet-induced protein calorie malnutrition in C57BL/6J mice resulted in impaired pulmonary clearance of P. aeruginosa. Tumor necrosis factor alpha (TNF-α) and nitrite levels detected upon exposure to P. aeruginosa aerosols were lower in the lungs of the malnourished C57BL/6J mice relative than in lungs of mice fed a normal diet. The role of TNF-α and reactive nitrogen intermediates in P. aeruginosa clearance was tested in TNF-α and inducible nitric oxide synthase (iNOS) knockout mice. P. aeruginosa clearance was diminished in transgenic TNF-α- and iNOS-deficient mice. In contrast to the effects of TNF-α and iNOS, gamma interferon knockout mice retained a full capacity to eliminate P. aeruginosa from the lung. Malnutrition also contributed to excessive inflammation in C57BL/6J mice upon chronic challenge with P. aeruginosa. The repeatedly infected malnourished host did not produce interleukin-10, a major anti-inflammatory cytokine absent or diminished in the bronchoalveolar fluids of CF patients. These results are consistent with a model in which defective CFTR in the intestinal tract leads to nutritional deficiency which in turn contributes to compromised innate lung defenses, bacterial colonization, and excessive inflammation in the CF respiratory tract.
Cystic fibrosis (CF), the most common inheritable lethal disease among Caucasians, is caused by mutations in the CFTR gene encoding a chloride channel (CF transmembrane conductance regulator [CFTR]) (10). CF is characterized by chronic obstructive pulmonary disease, intestinal problems, and generalized malnutrition (30). As the disease progresses, the lungs of CF patients become infected and colonized with a variety of pathogens. Among these, Pseudomonas aeruginosa represents the predominant CF pathogen (15). Persistent pulmonary infections with P. aeruginosa, intense neutrophil-dominated airway inflammation, and progressive lung disease are presently the major cause of high morbidity and mortality in CF (23).
Several concurrent proposals have been put forward to explain the relationship between the defect in CFTR and predilection for P. aeruginosa infections (4, 5, 12, 22, 31, 36, 40). The altered electrolyte composition of CF epithelial secretions has been linked to reduced bactericidal properties of defensins (12, 36). CFTR has also been implicated in P. aeruginosa uptake by respiratory epithelial cells (31). Reduced sialylation of glycoconjugates on the surface of epithelial cells in CF has been associated with increased P. aeruginosa adhesion (40). Other studies focusing on cytokine profiles in bronchoalveolar lavage fluids of CF patients have suggested that the excessive inflammation in the CF lung may be attributed to endogenously increased levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin (IL-8) (5, 22). Considering the multitude of sometimes conflicting models, factors rendering the CF lung prone to infections are still not conclusively defined.
Chronic malnutrition with progressive weight loss has been recognized as a problem in CF (30). Almost 50% of newly diagnosed CF infants suffer from various degrees of malnutrition (25), maldigestion, and malabsorption (21, 28). About 85% of CF patients show pancreatic insufficiency and severe steatorrhoea due to the problems with their digestive systems (30). Approximately 16% of CF newborns and adults also present with meconium ileus or meconium ileus equivalent, one of the most common causes of intestinal obstruction in CF (7, 11). CF patients have increased energy expenditure and energy deficit and are underweight. The combined effects of malabsorption and anorexia in CF result in inefficient utilization of proteins, lipids, vitamins, and trace elements. Significant growth retardation with weight loss has been seen in all age groups of CF patients (30). The issues related to protein energy malnutrition (PEM) in CF are of particular significance due to their import on long-term prognosis in this disease (24).
Here we tested whether some aspects of P. aeruginosa infections in CF could be explained by effects of malnutrition on relevant innate defenses of the respiratory system. We used an aerosol infection model in combination with several strains of transgenic mice and normal mice subjected to PEM (which represents one aspect of nutritional problems in CF) to model the role of innate defenses in P. aeruginosa pulmonary clearance. We report the roles of malnutrition, relevant proinflammatory and anti-inflammatory cytokines, and other mediators of innate lung immunity in P. aeruginosa clearance from the respiratory tract.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
For nebulization, P. aeruginosa PAO1 was grown in 200 ml of Luria broth at 37°C for 12 h and harvested by centrifugation at 4,000 rpm for 15 min at 4°C. Pellets (1.2 g [wet cell mass]) were washed once in cold 1% Proteose Peptone (Difco) phosphate-buffered saline (pH 7.4) and resuspended in cold phosphate-buffered saline. The suspension was adjusted to 1011 CFU/ml, and 5 ml was used for nebulization as previously described (39).
Animals.
Mice were 8 weeks old at the inception of single- or repeated-aerosol-exposure experiments as previously described (39). All animals were housed under specific-pathogen-free conditions. C57BL/6J and transgenic mice (four per group) were from The Jackson Laboratory (Bar Harbor, Maine). Gamma interferon (IFN-γ) knockout mice were C57BL/6-Ifnγtm1Ts (JR2287); inducible nitric oxide synthase 2 (iNOS-2) knockout mice were C57BL/6-Nos2tmlLau (JR2609). CFTR transgenic animals were CFTRm1UNC−/− homozygotes (37) obtained by breeding heterozygous CFTRm1UNC+/− mice purchased from The Jackson Laboratory and genotyping mice following instructions from the supplier. CFTRm1Unc−/−(FABP-hCFTR) mice were purchased from The Jackson Laboratory and breeding colony maintained without further typing. CFTRm1Unc−/−(FABP-hCFTR) mice have their intestinal defect corrected by the presence of a functional human CFTR gene expressed from a rat intestinal fatty acid-binding protein gene promoter (41).
Infection model.
The aerosol infection model and equipment (Glas-Col, Terre Haute, Ind.) were as previously described (39). For single-exposure experiments, animals were sacrificed 18 h upon exposure to P. aeruginosa aerosols. For chronic infection model (39), animals were repeatedly subjected to P. aeruginosa aerosols every 72 h (eight times over a period of 22 days) and sacrificed 18 h following the last exposure. Bacteriological analysis and histopathology workup were as previously described (39). Relative bacterial survival represents the percent of CFU remaining in the lungs compared to the initially deposited CFU in the lungs determined by sacrificing a group of animals immediately following the aerosol exposure (39).
Generation of PEM in mice.
A standard protocol was followed (38). Low (2%)- and full (20%)-protein and protein-free diets were from BIOSERV (Frenchtown, N.J.). These diets were made isocaloric to the basic diet (casein-based mouse diet) by carbohydrate supplement. The protein-free diet consisted of 7% fat, 5% fiber, 3% ash, and 79% carbohydrate. Compositions of low- and normal-protein diets, respectively, were as follows: protein (2 and 20%), fat (7 and 7%), fiber (5 and 5%), ash (3 and 3%), and carbohydrate (77 and 57%). The protein component in these diets was hydrolyzed casein. The caloric content of the diet was 3.6 kcal/g. An adult mouse usually consumes about 3 g/day (10.8 kcal). Analysis of protein, fat, and carbohydrate content of diets was performed for quality control. After 3 weeks on protein-free diet, during which animals typically lose about 50% of their body weight (38), mice were placed on low-protein or normal-protein diet. The time of infection following the introduction of low (PEM)- or normal (control)-protein diets was 2 weeks or after switching from low- to normal-protein diet for additional 2 weeks (PEM-recovered [PEM-R] animals). In single-aerosol-exposure experiments, PEM animals had body weights of 10.7 ± 1.2 g (n = 4); control mice on the normal diet weighed 19.1 ± 1.3 g (n = 4), and PEM-R animals had body weights of 20.9 ± 2.1 g (n = 8). The animals subjected to repeated P. aeruginosa aerosol exposure (chronic infection/inflammation model [39]) weighed 13.5 ± 0.9 g (n = 5) in the PEM group, versus 19.6 ± 1.1 g (n = 5) in the control group fed normal diet. A sentinel group of five PEM mice were found free of viral and bacterial infections.
Cytokine levels, MPD activity, and nitrite concentration.
TNF-α, macrophage inflammatory protein 2 (MIP-2), and chemokine-induced neutrophil chemoattractant KC (KC) were measured in lung tissue (left lung lobe homogenates), using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. Detection limits for murine TNF-α, MIP-2, and KC were 5.0, 1.5, and 2.0 pg/ml, respectively. Myeloperoxidase (MPD) in the lung tissues was measured as described by Hobden et al. (17) after dilution 1:1 with cetyltrimethylammonium bromide (0.75%, final concentration; United States Biochemical). Samples were freeze-thawed three times and sonicated once before centrifugation at 14,000 rpm for 10 min at 4°C. An aliquot of the supernatant was mixed with 2.9 ml of 50 mM potassium phosphate buffer (pH 6.0) containing o-dianisidine dihydrochloride (0.53 mM; Sigma) and H2O2 (0.15 mM). One unit of MPO activity is defined as the decomposition of 1 mmol of peroxide/min at 25°C, representing a change in A460 of 1.13 × 10−2/min. Lung homogenates were first centrifuged at 10,000 rpm for 20 min at 4°C and filtered (0.45 μm-pore-size filter) before nitrate determination. Total nitrite (nitrite plus nitrate reduced enzymatically to nitrite) in lung homogenates (expressed in nanomoles per gram of lung tissue) was determined using a kit from Cayman Chemical (Ann Arbor, Mich.). The levels of TNF-α and NO in uninfected animals were below detection limits (TNF-α, 34.0 pg/g of tissue; NO, 14 nmol of nitrite/g of tissue). The levels of MIP-2 and KC in uninfected animals were 195 ± 15 and 210 ± 14 pg/g of tissue, respectively.
Statistical analysis.
Analysis of variance (ANOVA), t test, and post hoc analyses were performed with SuperANOVA (version 1.11; Abacus Concepts) and StatView (version 4.5; Abacus Concepts).
RESULTS
Respiratory clearance in CFTR transgenic mice with and without correction of the intestinal defect: effects of malnutrition.
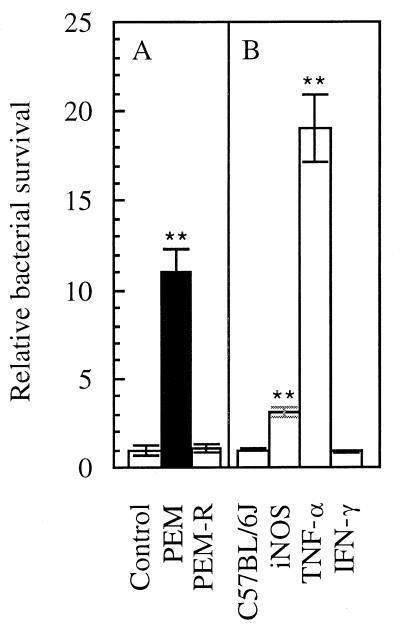
Using an aerosol infection model (39), we observed variability among CF knockout mice (37) in the ability to clear P. aeruginosa (Table 1). The CFTRm1UNC−/− mice presented with two extremes of either clearing or not clearing P. aeruginosa, a phenomenon not encountered with any other strains of age-matched mice in the mice used. This finding appeared to be associated with variations in body weight and nutritional status of the mice. In CFTRm1UNC−/− mice that had their intestinal defect corrected by the presence of a functional human CFTR gene expressed from a rat intestinal fatty acid-binding protein gene promoter CFTRm1Unc−/−(FABP-hCFTR mice) (41), P. aeruginosa was efficiently cleared from the lung and the variability was no longer observed (Table 1). One interpretation of these observations is that repairing the CFTR defect in the intestinal tract improved lung defenses against P. aeruginosa. Normal C57BL/6J mice in which PEM was induced showed an 11-fold-reduced capacity to clear P. aeruginosa (Fig. 1A, PEM). This defect was promptly restored by placing the malnourished mice on a normal-protein (20%) diet (Fig. 1A, PEM-R). These results indicate that malnutrition impairs pulmonary clearance of P. aeruginosa.
TABLE 1.
Pulmonary clearance of P. aeruginosa in CFTRm1Unc−/− mice, CFTR+/+ littermates, and CFTRm1Unc−/−(FABP-hCFTR) bitransgenic mice with corrected intestinal defecta
| Genotype | Body wt (g) | % Bacterial survival |
|---|---|---|
| CFTR+/+ | 21.0 ± 1.4 | 0.17 ± 0.03 |
| CFTRm1Unc−/− | 6.5–20.7 | 0.12–122 |
| CFTRm1Unc−/−(FABP-hCFTR) | 22.4 ± 2.5 | 0.10 ± 0.08 |
Pulmonary clearance (n = 18) was determined 18 h following exposure to P. aeruginosa aerosols. Bacterial survival at 18 h upon exposure to aerosols was expressed as percentage of the initial bacterial deposition (3 × 106 CFU per lung) determined in animals sacrificed immediately upon exposure.
FIG. 1.
(A) Pseudomonas clearance from the lungs of control C57BL/6J mice (normal-protein diet), PEM mice, and PEM-R mice (as defined in Materials and Methods). P. aeruginosa was delivered as aerosol (initial deposition was 2 × 106 to 8 × 106 CFU/g of lung tissue). Bacterial survival was expressed as the fraction of the initially deposited CFU (determined by sacrificing a group of animals immediately following exposure) remaining in the lung 18 h following infection (relative bacterial survival). (B) Pulmonary clearance in transgenic iNOS, TNF-α, and IFN-γ knockout mice. ∗∗, P < 0.01 (ANOVA post hoc t test; relative to control in panel A or relative to C57BL/6J in panel B).
Clearance of P. aeruginosa in IFN-γ, iNOS, and TNF-α transgenic mice.
As mutations in CFTR have severe effects on the digestive system, the resulting nutritional defect may impair the innate defense systems in the lung. PEM can selectively compromise the immune system in the lung, with IFN-γ, TNF-α, and iNOS being the major effector mechanisms affected (9). To test whether and which of these factors play a role in P. aeruginosa clearance from the lung, we investigated the roles of IFN-γ, iNOS, and TNF-α in corresponding transgenic mice. Age-matched IFN-γ, iNOS, and TNF-α transgenic knockout and C57BL/6J mice were exposed to P. aeruginosa aerosols. The strongest effect on P. aeruginosa was observed in TNF-α transgenic mice, which showed a 19-fold decrease in the efficiency of P. aeruginosa clearance relative to C57BL/6J mice (P < 0.01). The iNOS knockout animals also showed a threefold-reduced clearance (P < 0.01), while IFN-γ animals displayed no defect in removal of P. aeruginosa from the lungs (P = 0.786). These results suggest that iNOS and TNF-α play important roles in the innate resistance to P. aeruginosa and its clearance from the respiratory tract.
Malnutrition affects iNOS and TNF-α output in response to respiratory infection with P. aeruginosa.
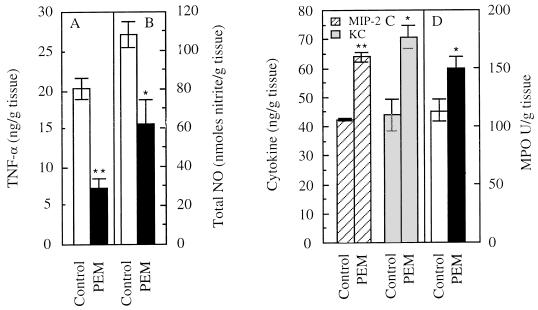
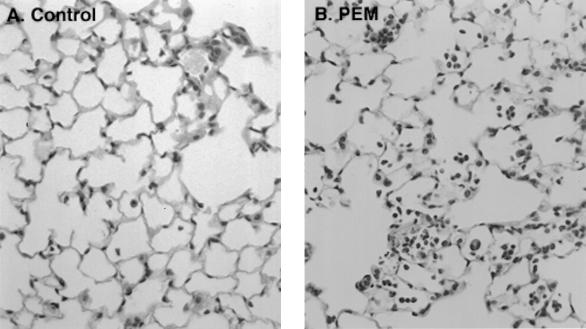
Considering the significant role of iNOS and TNF-α, we next tested whether NO and TNF-α elicited by respiratory infection with P. aeruginosa were affected by malnutrition. TNF-α was reduced 2.8-fold (P < 0.01) (Fig. 2A) and NO production (determined by measuring levels of its metabolite nitrite) was reduced by 58% (P < 0.01) (Fig. 2B) in C57BL/6J PEM mice relative to controls fed a normal-protein diet. Reduced iNOS levels in CF epithelial cells have been independently noted (19). Histopathological examination indicated an increase in the neutrophil and other inflammatory cell infiltration in the lungs of mice under PEM (Fig. 3). The neutrophil-recruiting chemokines MIP-2 and KC, which represent functional equivalents of human IL-8 in the mouse (16, 34, 35), were increased (50% for MIP-2 [P < 0.01] and 60% for KC [P < 0.05]) in C57BL/6J PEM animals relative to mice fed normal diet (Fig. 2C). There was also a 30% increase in MPO activity in the PEM mice (P < 0.05) (Fig. 2D).
FIG. 2.
TNF-α (A) and total nitrite (B) in the lungs of control C57BL/6J and PEM mice 18 h following exposure to P. aeruginosa aerosols; levels of MIP-2 (C), KC (C), and MPO (D) in the lungs of control (fed normal-protein diet) and PEM mice 18 h following exposure to P. aeruginosa aerosols. ∗∗, P < 0.01; ∗, P < 0.05 (ANOVA post hoc t test).
FIG. 3.
Increased neutrophil and inflammatory cell infiltration in PEM mice exposed to P. aeruginosa. (A) Control C57BL/6J mice fed normal-protein diet; (B) malnourished mice (PEM) fed low-protein diet. Both groups of mice were exposed as in Fig. 1.
Reduced levels of IL-10 in a chronically infected malnourished host.
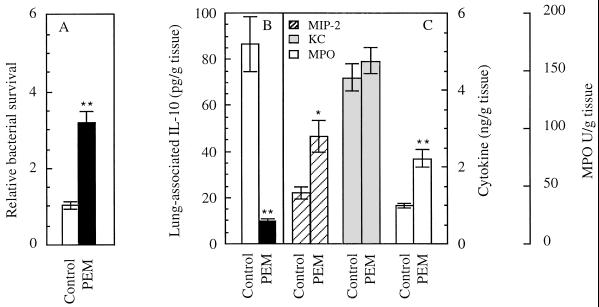
In addition to increased levels of proinflammatory cytokines and neutrophil infiltration, another hallmark of CF is a low level of the major anti-inflammatory cytokine IL-10 in the bronchoalveolar fluid (5). In a model of chronic infection with P. aeruginosa that has been previously used to test effects of IL-10 on inflammatory processes due to P. aeruginosa infection (39), the clearance of P. aeruginosa remained less efficient in malnourished animals (Fig. 4A). We also observed production of significant amounts of IL-10 in the well-nourished mice 22 days following the initiation of a regimen of repeated exposure to P. aeruginosa aerosols (Fig. 4B). In contrast, the malnourished animals had no detectable IL-10 (Fig. 4B). In normally fed mice (which prior to infection showed less than 9.0 pg of IL-10 per g), IL-10 was present at a level of 86.5 ± 11.9 pg/g of lung tissue. IL-10 levels were below the detection limit (9.0 pg/g) in the malnourished animals (Fig. 4B). In the chronic infection model, PEM also resulted in a 2.1-fold increase in MIP-2 relative to animals on the normal-protein diet (P < 0.05; Fig. 4C) although the levels of KC were similar in both groups of mice (P = 0.4679). MPO levels in chronically infected PEM mice were 2.3-fold higher than in the normally fed mice (P < 0.01; Fig. 4C).
FIG. 4.
(A) Pulmonary clearance in chronically infected PEM mice. Mice were repeatedly exposed (eight times, every 72 h) to P. aeruginosa as previously described (39). (B and C) IL-10 (B), MIP-2 (C), KC (C), and MPO (C) in chronically infected mice. ∗∗, P < 0.01; ∗, P < 0.05 (ANOVA post hoc t test).
DISCUSSION
In this work, we report reduced clearance of P. aeruginosa from the respiratory tract of transgenic mice lacking TNF-α and iNOS, suggesting that these mediators of innate immunity may be critical for lung defenses against Pseudomonas infection. In contrast to TNF-α and iNOS transgenic mice, IFN-γ knockout mice do not show reduced P. aeruginosa clearance, suggesting that this cytokine does not play a major role in the early stages of Pseudomonas colonization. Any iNOS role in the control of P. aeruginosa immediately upon aerosol delivery is most likely independent of the IFN-γ-mediated induction of iNOS in murine phagocytic cells. The production of iNOS has been reported to be constitutive in both human and murine airway epithelia (19), and these cells could be the source of the effects observed in our model system. In vitro killing of P. aeruginosa with sodium nitroprusside (19), NO-dependent killing of P. aeruginosa in excised murine lungs (19), and reduced control of P. aeruginosa in animals treated with the iNOS inhibitor aminoguanidine (13) have also been reported. These analyses along with our findings using iNOS knockout animals are consistent with the conclusion that NO plays a role in P. aeruginosa control in the respiratory tract. Since iNOS levels and NO output are reduced in CF epithelia (19, 27), this deficiency may contribute to the colonization with P. aeruginosa.
The transgenic animals lacking TNF-α showed the most striking defect in clearing P. aeruginosa in our infection model. A role for TNF-α in the innate resistance to P. aeruginosa infection has also been proposed by others based on a correlation between TNF-α levels in BALB/c and C57BL/6 strains of mice and their differential susceptibility to endobronchial instillation of P. aeruginosa embedded in agar beads (13). Furthermore, depletion of TNF-α increases bacterial loads in some strains of mice (13), in keeping with our observations with transgenic animals. Buret et al. (8) have noticed that intratracheal administration of TNF-α in rats improves P. aeruginosa clearance from the lungs. The function of TNF-α in resistance to P. aeruginosa colonization most likely involves a number of mechanisms, including recruitment of neutrophils and macrophages via effects on adhesion molecules or chemoattractants or by affecting bactericidal activities of phagocytic cells. Our unpublished results indicate that bone marrow-derived macrophages from TNF-α knockout mice are diminished in the ability to kill P. aeruginosa during early time points, suggesting that this defect could be one of the contributing factors to the observed reduced P. aeruginosa clearance in TNF-α transgenic mice. Paradoxically, TNF-α levels are elevated in bronchoalveolar lavage fluids in CF (5), and yet the patients cannot clear Pseudomonas from their lungs. This discrepancy can be best explained by the observation that the immunoreactive TNF-α in CF bronchoalveolar fluids is biologically inactive, as it is complexed with soluble TNF-α receptor which is also elevated in CF lung fluids (5). The TNF-α in CF is thus most likely not available to stimulate antipseudomonal activities in the respiratory tract.
In this report we also examined the effects of malnutrition on clearance and relevant cytokine profiles in the lung. A potential role for malnutrition in CF has been considered in a follow-up to the observation that variable body weight in CFTRm1Unc−/− mice with intestinal dysfunction was associated with variability in P. aeruginosa respiratory clearance. While we could not establish a statistically significant difference between CFTRm1Unc−/− and C57BL/6J mice, the variability in P. aeruginosa clearance was excessive in the CFTRm1Unc−/− group. Importantly, this variability was not observed in the bitransgenic mouse with the corrected CFTR defect in the intestinal tract. An observation suggesting increased susceptibility to P. aeruginosa infections of CFTR knockout mice relative to C57BL/6 mice has been reported in a model of intratracheal instillation of P. aeruginosa entrapped in agar beads with animal mortality as an outcome measure (14). Although in our studies we did not observe mortality in mice exposed to aerosolized P. aeruginosa, indicating obvious differences between the two models, the data shown in the study by Gosselin et al. (14) also point to a correlation between the reduced body weight in CFTR mice and increased bacterial burden relative to the control experimental group. In addition, other authors using strains of CFTR knockout mice prone to spontaneous early onset progressive lung disease have reported severe intestinal disease in such animals (20).
Nutritional problems in CF are complex and not limited to PEM, as they also include essential fatty acid and subclinical micronutrient deficiencies present even in well-nourished CF subjects (32, 33). Nevertheless, protein calorie malnutrition is a critical feature of CF and a significant determinant of clinical prognosis (24). It has been strongly associated with morbidity and mortality in young infants with CF, especially those fed soy formula and not receiving pancreatic enzyme supplements (1). Severe PEM is always accompanied by infection and decline in pulmonary function in CF (18). PEM status has significant impact on long-term prognosis in CF (24, 32). It has also been associated with essential fatty acid deficiencies often reported in CF patients (26), albeit some recent studies indicate that PEM and fatty acid abnormalities can be dissociated (33). Importantly, PEM in CF patents cannot be completely corrected despite regular treatments with pancreatic enzyme supplements (3) and further boosting of weight and linear growth in young patients with CF may require additional interventions, as recently illustrated in studies with growth hormone administration (2). PEM is further exacerbated during growth spurts, with increased demands for nutrient delivery often compounded by deterioration in dietary compliance during adolescence (6). It is worth noting that the incidence of P. aeruginosa infections in CF escalates with age, ranging from 1 to 10% in the age group from 0 to 4 years and rising to 80% in the age group from 10 to 14 and above (11, 29). The pattern of incidence of P. aeruginosa infections in CF, reaching a plateau between the age groups of 10 to 14 and 15 to 19 years, is often attributed to antibiotic treatment of infections caused by other pathogens (which conversely to P. aeruginosa decline in incidence with age), but additional or alternative explanations, potentially including effects of malnutrition, should not be excluded.
The results presented in this work indicate that malnutrition can compromise pulmonary defenses against P. aeruginosa colonization and is conducive to excessive inflammation in response to P. aeruginosa infection, resembling the situation in CF. PEM animals showed significantly reduced production of TNF-α and NO in response to P. aeruginosa challenge relative to the well-nourished experimental group. Importantly, neutrophil infiltration in the lungs of malnourished animals did not result in increased bacterial clearance from the lung and instead was a correlate of unproductive inflammatory response. PEM also prevented production of IL-10 during chronic infection with P. aeruginosa, the critical anti-inflammatory cytokine that is lacking in the bronchoalveolar fluids in CF (5) and is necessary to control excessive inflammation in the murine lung chronically infected with P. aeruginosa (39).
In conclusion, we propose the following model: (i) defective CFTR in CF causes dysfunction of the digestive system, and (ii) the ensuing malnutrition adversely affects innate lung defenses contributing to bacterial colonization and associated inflammation in addition to other direct effects of CFTR in the lung (4, 5, 12, 22, 31, 36, 40). A prediction from the relationships uncovered in this work is that the function of CFTR in the intestinal tract may be as critical for lung defenses against P. aeruginosa, as its direct roles may be at the level of the respiratory epithelium (4, 5, 12, 22, 31, 36, 40). We propose that in order to improve the compromised innate immunity in the CF lung, future treatment efforts, including gene therapy and other means of correcting the CFTR defect, should consider potential benefits of similar interventions in the intestinal tract.
ACKNOWLEDGMENTS
This work was supported by grants AI31139 from National Institutes of Health and 96PO from Cystic Fibrosis Foundation and in part by a grant from the University of Michigan.
REFERENCES
- 1.Abman S H, Accurso F J, Bowman C M. Persistent morbidity and mortality of protein calorie malnutrition in young infants with cystic fibrosis. J Pediatr Gastroenterol Nutr. 1986;5:393–396. doi: 10.1097/00005176-198605000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Alemzadeh R, Upchurch L, McCarthy V. Anabolic effects of growth hormone treatment in young children with cystic fibrosis. J Am Coll Nutr. 1998;17:419–424. doi: 10.1080/07315724.1998.10718788. [DOI] [PubMed] [Google Scholar]
- 3.Benabdeslam H, Garcia I, Bellon G, Gilly R, Revol A. Biochemical assessment of the nutritional status of cystic fibrosis patients with pancreatic enzyme extracts. Am J Clin Nutr. 1998;67:817–818. doi: 10.1093/ajcn/67.5.912. [DOI] [PubMed] [Google Scholar]
- 4.Biwersi J, Verkman A S. Functions of CFTR other than as a plasma membrane chloride channel. In: Dodge J A, Brock D J H, Widdicombe J H, editors. Cystic fibrosis-current topics. Vol. 2. Chichester, England: John Wiley & Sons Ltd.; 1994. pp. 155–171. [Google Scholar]
- 5.Bonfield T L, Panuska J R, Konstan M W, Hillard K A, Hillard J B, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 6.Booth I W. The nutritional consequences of gastrointestinal disease in adolescence. Acta Paediatr Scand Suppl. 1991;373:91–102. doi: 10.1111/j.1651-2227.1991.tb18156.x. [DOI] [PubMed] [Google Scholar]
- 7.Borowitz D. Pathophysiology of gastrointestinal complications of cystic fibrosis. Semin Respir Crit Care Med. 1994;15:391–401. [Google Scholar]
- 8.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1 alpha. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Tian Y, Tanaka K E, Tsang M S, Yu K, Salgame P, Carroll D, Kress Y, Teitelbaum R, Bloom B R. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci USA. 1996;93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins F S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 11.FitzSimmons S C. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 13.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosselin D, Stevenson M M, Cowley E A, Griesenbach U, Eidelman D H, Boule M, Tam M F, Kent G, Skamene E, Tsui L C, Radzioch D. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157:1253–1262. doi: 10.1164/ajrccm.157.4.9702081. [DOI] [PubMed] [Google Scholar]
- 15.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicuddy D C, Standiford T J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumoniae. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 17.Hobden J A, Masinick S A, Barrett R P, Hazlett L D. Proinflammatory cytokine deficiency and pathogenesis of Pseudomonas aeruginosa keratitis in aged mice. Infect Immun. 1997;65:2754–2758. doi: 10.1128/iai.65.7.2754-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt T L, Ward L C, Francis P J, Isles A, Cooksley W G, Shepherd R W. Whole body protein turnover in malnourished cystic fibrosis patients and its relationship to pulmonary disease. Am J Clin Nutr. 1985;41:1061–1066. doi: 10.1093/ajcn/41.5.1061. [DOI] [PubMed] [Google Scholar]
- 19.Kelley T J, Drumm M L. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Investig. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent G, Iles R, Bear C E, Huan L, Griesenbach U, McKerlie C, Frndova H, Ackerley C, Gosselin D, Razioch D, O'Brodovich H, Tsui L, Buchwald M, Tanswell A K. Lung disease in mice with cystic fibrosis. J Clin Investig. 1997;100:3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerem E, Corey B, Kerem B-S, et al. Clinical and genetic comparisons of patients with cystic fibrosis with or without meconium ileus. J Pediatr. 1989;114:767–773. doi: 10.1016/s0022-3476(89)80134-9. [DOI] [PubMed] [Google Scholar]
- 22.Khan T Z, Wagener J S, Bost T, Martinez J, Accurso F J, Riches D W H. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 23.Konstan M W, Berger M. Infection and inflammation of the lung in cystic fibrosis. In: Davis P B, editor. Cystic fibrosis. Vol. 64. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 219–276. [Google Scholar]
- 24.Kraemer R, Rudeberg A, Hadorn B, Rossi E. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr Scand. 1978;67:33–37. doi: 10.1111/j.1651-2227.1978.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 25.Lai H C, Kosorok M R, Sondel S A, Chen S T, FitzSimmons S C, Green C G, Shen G, Walker S, Farrell P M. Growth status in children with cystic fibrosis based on the national cystic fibrosis patient registry data: evaluation of various criteria used to identify malnutrition. J Pediatr. 1998;132:478–485. doi: 10.1016/s0022-3476(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 26.Landon C, Kerner J A, Castillo R, Adams L, Whalen R, Lewiston N J. Oral correction of essential fatty acid deficiency in cystic fibrosis. JPEN J Parenter Enteral Nutr. 1981;5:501–504. doi: 10.1177/0148607181005006501. [DOI] [PubMed] [Google Scholar]
- 27.Meng Q H, Springall D R, Bishop A E, Morgan K, Evans T J, Habib S, Gruenert D C, Gyi K M, Hodson M E, Yacoub M H, Polak J M. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol. 1998;184:323–331. doi: 10.1002/(SICI)1096-9896(199803)184:3<323::AID-PATH2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Mornet E, Serre J L, Farrall M E A. Genetic differences between cystic fibrosis with and without meconium ileus. Lancet. 1988;i:376–378. doi: 10.1016/s0140-6736(88)91180-4. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen S S, Jensen T, Pressler T, Hoiby N, Rosendal K. Does centralized treatment of cystic fibrosis increase the risk of Pseudomonas aeruginosa infection? Acta Paediatr Scand. 1986;75:840–845. doi: 10.1111/j.1651-2227.1986.tb10299.x. [DOI] [PubMed] [Google Scholar]
- 30.Pencharz P, Durie P. Nutritional management of cystic fibrosis. Annu Rev Nutr. 1993;13:111–136. doi: 10.1146/annurev.nu.13.070193.000551. [DOI] [PubMed] [Google Scholar]
- 31.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaska J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roulet M. Protein-energy malnutrition in cystic fibrosis patients. Acta Pediatr Suppl. 1994;395:43–48. doi: 10.1111/j.1651-2227.1994.tb13228.x. [DOI] [PubMed] [Google Scholar]
- 33.Roulet M, Frascarolo P, Rappaz I, Pilet M. Essential fatty acid deficiency in well nourished young cystic fibrosis patients. Eur J Pediatr. 1997;156:952–956. doi: 10.1007/s004310050750. [DOI] [PubMed] [Google Scholar]
- 34.Schall T J. The chemokines. In: Thomson A W, editor. The cytokine handbook. 2nd ed. San Diego, Calif: Academic Press; 1994. pp. 419–460. [Google Scholar]
- 35.Schmal H, Shanley T P, Jones M L, Friedl H P, Ward P A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996;156:1963–1972. [PubMed] [Google Scholar]
- 36.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:1–20. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 37.Snouwaert J N, Brigman K K, Latour A M, Malouf N N, Boucher R C, Smithies O, Koller B H. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 38.van Buren C T, Rudolph F B, Kulkarni A, Pizzini R, Fanslow W C, Kumar S. Reversal of immunosuppression induced by a protein-free diet: comparison of nucleotides, fish oil, and arginine. Crit Care Med. 1990;18:S114–S117. [PubMed] [Google Scholar]
- 39.Yu H, Hanes M, Chrisp C E, Boucher J C, Deretic V. Microbial pathogenesis in cystic fibrosis: pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated repiratory challenge. Infect Immun. 1998;66:280–288. doi: 10.1128/iai.66.1.280-288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zar H, Saiman L, Quittell L, Prince A. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995;126:230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Dey C R, Wert S E, DuVall M D, Frizzell R A, Whitsett J A. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]