Abstract
Background: Gynecological cancers, including cervical cancer, ovarian cancer and endometrial cancer are leading causes of cancer-related death in women worldwide. Diet plays an important role in cancer development, which is widely accepted. However, the associations between dietary intakes and gynecological cancers remain unclear. Methods: A total of 12,437 women aged over 20 years from the National Health and Nutrition Examination Survey (NHANES), conducted from 2007–2016, were included in this study. The relationships between 30 dietary factors (4 macronutrients, 15 vitamins, 9 minerals, caffeine and alcohol) and gynecological cancers were assessed. Results: We observed negative correlations of intakes of phosphorus (odds ratio (OR), 95% confidence interval (CI); 0.998 (0.996, 0.999), p = 0.002) with cervical cancer, and intakes of vitamin B12 (0.812 (0.714, 0.925), p = 0.002), phosphorus (0.997 (0.996, 0.999), p < 0.001) and alcohol (0.971 (0.950, 0.992), p = 0.009) with endometrial cancer. The data showed positive associations of intake of caffeine (1.002 (1.001, 1.003), p = 0.003) with cervical cancer, and intake of copper (2.754 (1.313, 5.778), p = 0.009) with endometrial cancer. In addition, we found potential negative correlations between intake of vitamin B1 (p = 0.025) and cervical cancer; zinc (p = 0.048) and ovarian cancer; and potassium (p = 0.032) and endometrial cancer. Potential positive associations were found between intake of calcium and cervical cancer (p = 0.026) and endometrial cancer (p = 0.034), and between sodium (p = 0.042) and endometrial cancer. Intakes of protein, total sugars, total fat, cholesterol, vitamin A, alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, vitamin B2, niacin, vitamin B6, food folate, vitamin C, vitamin D, vitamin E, vitamin K, magnesium, iron and selenium showed no relationship with gynecological cancers (p > 0.05). Conclusions: Specific dietary factors were associated with gynecological cancers. More epidemiological studies are needed to validate our results.
Keywords: gynecological cancers, cervical cancer, ovarian cancer, endometrial cancer, dietary factors, nutrients, NHANES
1. Introduction
The incidence of gynecological cancers is growing worldwide and poses a serious public health problem [1]. Gynecological cancers, including cervical cancer, ovarian cancer and endometrial cancer, are leading causes of cancer-related death in women globally [2]. Ovarian cancer ranks fifth and endometrial cancer ranks sixth in cancer-related mortality in the United States (U.S.), separately [3]. Although significant progress has been made toward early detection and treatment, cervical cancer, ovarian cancer and endometrial cancer are usually detected at a late stage and have a poor prognosis [4,5]. Thirty to sixty percent of cancers are closely related to dietary factors in the developed countries [6]. The associations between dietary intakes and cancers, such as colorectal cancer [7], breast cancer [8], prostate cancer [9] and lung cancer [10], have been studied extensively so far. Lifestyle changes such as dietary transformation are typically proposed to prevent the development of gynecological cancers, and to mitigate severity once the disease has developed [11].
There has been renewed interest in relationships between dietary factors and gynecological cancer risk [12,13,14]. Antioxidants, such as some vitamins, carotenoids, vegetables and fruits, exhibit different effects on the natural history of gynecological cancers [15,16,17]. However, results on the relationships between dietary intakes and gynecological cancers remain limited and inconclusive [18,19,20]. For instance, recent studies showed that alcohol intake has variably shown positive (odds ratio (OR), 95% confidence interval (CI), 1.6 (1.2, 2.2)) [21], inverse (risk ratio (RR), 95%CI, 0.81 (0.68, 0.96)) [22] or null association (RR, 95%CI, 1.04 (0.88, 1.22)) [23] with endometrial cancer. Moreover, previous studies only focused on a single dietary factor, ignoring the complexity and multitude of dietary variables, so that reported results on the associations between dietary intakes and gynecological cancers have been one-sided [14,24,25,26,27]. Modifications in one dietary characteristic usually led to compensatory changes in other dietary characteristics. Instead, focusing on food groups or dietary patterns could avoid the collinearity of dietary variables, and avoid finding chance associations due to analyses with multiple individual nutrients as the exposure [28,29]. Therefore, by considering multiple dietary factors, a comprehensive perspective of associations of diet with disease process can be provided [30].
The National Health and Nutrition Examination Survey (NHANES), as a consecutive design, was committed to measuring the health and nutrients status of non-institutionalized U.S. civilians [31]. This is the first study, as far as we know, to comprehensively investigate the relationships between dietary intakes and gynecological cancers using NHANES data. This study aimed to examine the associations between dietary intakes and gynecological cancers with a representative sample of Americans. In-depth understanding of the associations between dietary intakes and gynecological cancers provides a reference for preventing the occurrence and development of gynecological cancers.
2. Methods
2.1. Study Design and Population
Samples for this study were extracted from the NHANES, a multistage stratified composite design survey of a representative selection of the noninstitutionalized U.S. population. Participants were interviewed at home, followed by various clinical and laboratory examinations performed in a mobile examination center (MEC). We combined 5 consecutive NHANES surveys, 2007–2008, 2009–2010, 2011–2012, 2013–2014 and 2015–2016, into a single analytic sample. A total of 12,437 women aged over 20 years who were interviewed regarding their dietary intakes and medical conditions were included.
2.2. Outcomes
The primary outcomes were the diagnoses of gynecological cancers. Cancer types were defined using items on the Medical Status Questionnaire: “Have you ever been told by a doctor or other health professional that you had cancer or malignancy?” and “What kind of cancer was it?”. Answerers which indicated only cervical cancer, ovarian cancer and endometrial cancer were classified as outcome variables.
2.3. Dietary Intakes
Trained interviewers conducted two consecutive 24-h dietary recalls to assess total dietary intakes by comprehensive reference in the NHANES. The first was conducted face-to-face at the MEC examination and the second was collected by telephone after 3–10 days. Dietary intakes were calculated from the average of data from two dietary recalls (if available); otherwise, single dietary recall data were used.
We included 30 dietary factors from the dietary questionnaire of the NHANES database. These factors encompassed 4 macronutrients, 15 vitamins and 9 minerals—including protein (g), total sugars (g), total fat (g), cholesterol (mg), vitamin A (vitamin A, RAE) (μg), alpha-carotene (μg), beta-carotene (μg), beta-cryptoxanthin (μg), lycopene (μg), vitamin B1 (thiamin) (mg), vitamin B2 (riboflavin) (mg), niacin (mg), vitamin B6 (mg), food folate (μg), vitamin B12 (μg), vitamin C (μg), vitamin D (D2 + D3) (μg), vitamin E (vitamin E as alpha-tocopherol) (mg), vitamin K (μg), calcium (mg), phosphorus (mg), magnesium (mg), iron (mg), zinc (mg), copper (mg), sodium (mg), potassium (mg) and selenium (mcg)—as well as caffeine (mg) and alcohol (g).
2.4. Covariates
Covariates were previously identified as potential prognostic factors in recent literature [32,33,34]. We assessed demographic covariates including age, body mass index (BMI), poverty-to-income ratio (PIR), energy intake (expressed in kcal), education level, race, work activity and recreational activity [11,34,35,36].
2.5. Statistical Analysis
Descriptive analysis was used for exploring demographic characteristics of included participants. Considering that all continuous variables did not obey normal distribution, the median with interquartile range (IQR) was used to describe continuous variables. Significance difference between the two groups was appraised by Wilcoxon rank-sum test. Frequency and percent were used to describe categorical variables. The distribution of categorical variables was appropriately compared by chi-squared test.
Outcome variables were imbalanced due to the relatively low incidence of gynecological cancers. Thus the “ROSE” package in R software was used to correct the imbalanced data [37]. Furthermore, we considered the stratified, multi-stage probabilistic sampling approach of NHANES, using the “survey” package to adjust complex sampling weights for dietary analysis. Five cycles of continuous NHANES data from 2007–2016 were included. Two-year cycle weights were divided by 5 to reflect 10 survey years. Estimates and standard errors were analyzed using sampling weights, stratification and clustering provided in the NHANES dataset.
Logistic regression was performed using the “glmnet” package in R software. For the logistic regression, self-reported gynecological cancers were included as the dependent variables. All statistical analyses were conducted using IBM SPSS (version 24.0.) and R (version 4.1.2.). The p-value was adjusted for multiple comparisons using the Bonferroni correction (Bonferroni: 0.05/3 = 0.0167). A value of p < 0.0167 was considered statistically significant and 0.0167 < p < 0.05 indicated potential association.
3. Results
3.1. Characteristics of Included Participants
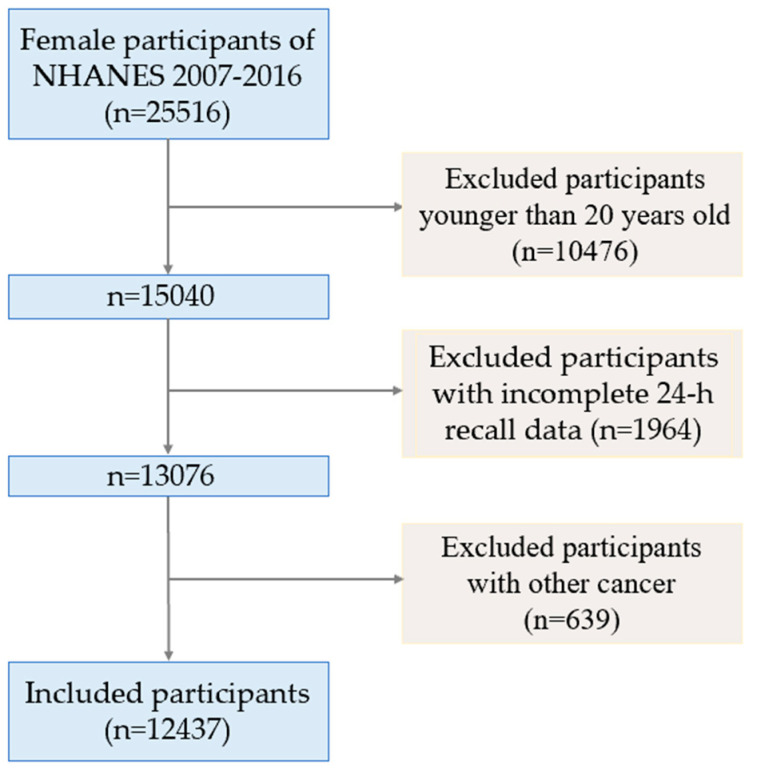
The flow chart of participants is shown in Figure 1. Compared to the participants without cancers, those with cervical cancer had a lower PIR (p < 0.001), represented lower educational level (less than 9th grade, p = 0.009), tended to be non-Hispanic white (p < 0.001) and represented more work activity (p = 0.005); those with ovarian cancer were more likely to be older (p < 0.001), represented lower educational level (p = 0.010) and lower recreational activity (p = 0.025); and those with endometrial cancer were more likely to be older (p < 0.001), had a potentially lower PIR (p = 0.047), had higher BMI (p < 0.001), represented lower educational level (p < 0.001), tended to be non-Hispanic white (p = 0.012) and had lower recreational activity (p = 0.004). Characteristics of the included participants are summarized in Table 1.
Figure 1.
Flowchart of study participants.
Table 1.
Characteristics of participants with and without self-reported cervical cancer, ovarian cancer and endometrial cancer, separately.
| Variables | Women with Cervical Cancer (n = 162) |
Women without Cervical Cancer (n = 12,275) |
p-Value | Women with Ovarian Cancer (n = 66) |
Women without Ovarian Cancer (n = 12,371) |
p-Value | Women with Endometrial Cancer (n = 104) | Women without Endometrial Cancer (n = 12,333) |
p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Age (years), median (IQR) a | 46.50 (35.00, 58.25) | 48.00 (34.00, 63.00) | 0.401 | 62.50 (48.00, 71.00) | 48.00 (34.00, 63.00) | <0.001 *** | 60.00 (48.25, 69.00) | 48.00 (34.00, 63.00) | <0.001 *** |
| Family Poverty-Income-Ratio, median (IQR) a | 1.63 (0.77, 2.69) | 2.19 (1.08, 3.57) | <0.001 *** | 1.73 (1.09, 3.33) | 2.19 (1.08, 3.55) | 0.584 | 2.05 (0.79, 2.57) | 2.19 (1.08, 3.57) | 0.047 * |
| BMI (kg/m3), median (IQR) a | 29.26 (24.20, 34.15) | 28.60 (24.19, 33.70) | 0.521 | 29.39 (26.50, 35.06) | 28.60 (24.17, 33.70) | 0.079 | 31.24 (26.08, 37.18) | 28.60 (24.16, 33.70) | <0.001 *** |
| Energy (kcal), median (IQR) a | 1722.00 (1278.50, 2136.50) | 1681.00 (1263.00, 2169.00) | 0.658 | 1549.50 (1041.25, 2292.75) | 1682.00 (1264.00, 2167.00) | 0.325 | 1631.50 (1144.75, 1999.50) | 1683.00 (1264.00, 2169.00) | 0.153 |
| Education, n (%) b | - | - | 0.009 ** | - | - | 0.010 * | - | - | <0.001 *** |
| Less than 9th grade | 12 (7.4%) | 1306 (10.6%) | - | 14 (21.2%) | 1304 (10.5%) | - | 24 (23.1%) | 1294 (10.5%) | - |
| 9th−11th grade | 36 (22.2%) | 1731 (14.1%) | - | 5 (7.6%) | 1762 (14.2%) | - | 19 (18.3%) | 1748 (14.2%) | - |
| More than 12th grade | 114 (70.4%) | 9238 (75.3%) | - | 47 (71.2%) | 9305 (75.2%) | - | 61 (58.7%) | 9291 (75.3%) | - |
| Race, n (%) b | - | - | <0.001*** | - | - | 0.791 | - | - | 0.012 * |
| Mexican American | 15 (9.3%) | 1960 (16.0%) | - | 14 (21.2%) | 1961 (15.9%) | - | 16 (15.4%) | 1959 (15.9%) | - |
| Other Hispanic | 13 (8.0%) | 1450 (11.8%) | - | 7 (10.6%) | 1456 (11.8%) | - | 19 (18.3%) | 1444 (11.7%) | - |
| Non-Hispanic White | 114 (70.4%) | 4909 (40.0%) | - | 26 (39.4%) | 4997 (40.4%) | - | 51 (49.0%) | 4972 (40.3%) | - |
| Non-Hispanic Black | 12 (7.4%) | 2713 (22.1%) | - | 14 (21.2%) | 2711 (21.9%) | - | 12 (11.5%) | 2713 (22.0%) | - |
| Other Race—including Multi-Racial | 8 (4.9%) | 1243 (10.1%) | - | 5 (7.6%) | 1246 (10.1%) | - | 6 (5.8%) | 1245 (10.1%) | - |
| Work activity, n (%) b | - | - | 0.005 ** | - | - | 0.950 | - | - | 0.145 |
| Vigorous | 30 (18.5%) | 1347 (11.0%) | - | 8 (12.1%) | 1369 (11.1%) | - | 7 (6.7%) | 1370 (11.1%) | - |
| Moderate | 39 (24.1%) | 2736 (22.3%) | - | 14 (21.2%) | 2761 (22.3%) | - | 30 (28.8%) | 2745 (22.3%) | - |
| Other | 93 (57.4%) | 8192 (66.7%) | - | 44 (66.7%) | 8241 (66.6%) | - | 67 (64.4%) | 8218 (66.6%) | - |
| Recreational activity, n (%) b | - | - | 0.076 | - | - | 0.025 * | - | - | 0.004 ** |
| Vigorous | 18 (11.1%) | 2092 (17.0%) | - | 5 (7.6%) | 2105 (17.0%) | - | 6 (5.8%) | 2104 (17.1%) | - |
| Moderate | 42 (25.9%) | 3390 (27.6%) | - | 14 (21.2%) | 3418 (27.6%) | - | 27 (26.0%) | 3405 (27.6%) | - |
| Other | 102 (63.0%) | 6793 (55.3%) | - | 47 (71.2%) | 6848 (55.4%) | - | 71 (68.3%) | 6824 (55.3%) | - |
ap value was tested by Wilcoxon rank-sum test; b p value was tested by Pearson chi-square test. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Dietary Intakes and Gynecological Cancer Risk
Table 2 presents the dietary intakes of the included participants. Women suffering from cervical cancer took less alpha-carotene (p < 0.001), beta-carotene (p < 0.001), beta-cryptoxanthin (p = 0.001), vitamin B1 (p = 0.003), niacin (p = 0.009), vitamin B6 (p = 0.001), food folate (p = 0.007), vitamin C (p < 0.001), vitamin E (p = 0.015), vitamin K (p = 0.003) and iron (p = 0.003). In addition, women suffering from cervical cancer potentially took less protein (p = 0.048), vitamin A (p = 0.018) and copper (p = 0.048). Women suffering from ovarian cancer potentially took less zinc (p = 0.030) and selenium (p = 0.038).
Table 2.
Dietary intakes in women with and without self-reported cervical cancer, ovarian cancer and endometrial cancer, separately.
| Variables Median (IQR) |
Women with Cervical Cancer (n = 162) |
Women without Cervical Cancer (n = 12,275) |
p-Value | Women with Ovarian Cancer (n = 66) |
Women without Ovarian Cancer (n = 12,371) |
p-Value | Women with Endometrial Cancer (n = 104) | Women without Endometrial Cancer (n = 12,333) |
p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Protein (g) | 58.53 (39.76, 79.42) | 63.16 (45.76, 84.87) | 0.048 * | 56.77 (41.53, 79.73) | 63.12 (45.76, 84.88) | 0.086 | 62.50 (46.05, 82.96) | 63.10 (45.72, 84.87) | 0.708 |
| Total sugars (g) | 93.95 (52.88, 148.83) | 86.85 (56.02,127.72) | 0.166 | 77.21 (56.50, 143.16) | 86.94 (55.97, 127.87) | 0.807 | 84.36 (49.52, 129.05) | 86.92 (56.03, 127.96) | 0.601 |
| Total fat (g) | 62.00 (38.65, 87.61) | 61.37 (41.07, 87.05) | 0.888 | 56.65 (35.14, 84.04) | 61.41 (41.08, 87.06) | 0.166 | 56.41 (37.26, 82.12) | 61.41 (41.09, 87.10) | 0.144 |
| Cholesterol (mg) | 183.00 (97.00, 328.00) | 187.00 (109.00, 323.00) |
0.871 | 160.50 (100.75, 283.75) | 187.00 (109.00, 324.00) |
0.138 | 169.50 (93.25, 312.50) | 187.00 (109.00, 323.00) |
0.396 |
| Vitamin A (μg) | 362.00 (198.00, 612.25) | 435.00 (240.00, 717.00) | 0.018 * | 464.00 (190.75, 690.25) | 435.00 (239.00, 717.00) | 0.719 | 490.00 (259.75, 819.25) | 434.00 (239.00, 716.00) | 0.203 |
| Alpha-carotene (μg) | 20.50 (1.00, 166.25) | 48.00 (11.00, 270.00) | <0.001 *** | 43.00 (10.75, 319.75) | 47.00 (11.00, 269.00) | 0.877 | 68.00 (18.25, 538.50) | 47.00 (11.00, 267.00) | 0.101 |
| Beta-carotene (μg) | 448.00 (137.75, 1294.50) | 733.00 (267.00, 2376.00) |
<0.001 *** | 904.00 (148.00, 3051.00) | 727.00 (264.00, 2351.00) |
0.874 | 1061.50 (386.00, 2777.00) | 726.00 (263.00, 2349.00) |
0.089 |
| Beta-cryptoxanthin (μg) | 16.50 (3.75, 54.50) | 27.00 (7.00, 90.00) | 0.001 ** | 45.50 (15.50, 150.50) | 26.00 (7.00, 90.00) | 0.011 * | 26.00 (10.00, 108.75) | 26.00 (7.00, 90.00) | 0.440 |
| Lycopene (μg) | 921.00 (0.00, 4612.75) | 1422.00 (1.00, 4591.00) | 0.153 | 1482.00 (0.00, 6221.25) | 1417.00 (1.00, 4583.00) | 0.796 | 1317.50 (5.25, 3957.75) | 1418.00 (1.00, 4595.00) | 0.844 |
| Vitamin B1 (mg) | 1.13 (0.80, 1.44) | 1.24 (0.86, 1.68) | 0.003 ** | 1.19 (0.76, 1.61) | 1.24 (0.86, 1.68) | 0.153 | 1.25 (0.86, 1.54) | 1.24 (0.86, 1.68) | 0.751 |
| Vitamin B2 (mg) | 1.61 (1.12, 2.34) | 1.59 (1.12, 2.20) | 0.429 | 1.52 (1.06, 2.04) | 1.59 (1.12, 2.20) | 0.270 | 1.55 (1.08, 2.11) | 1.59 (1.12, 2.20) | 0.517 |
| Niacin (mg) | 16.95 (12.34, 22.38) | 18.70 (13.16, 25.71) | 0.009 ** | 17.14 (12.45, 26.28) | 18.67 (13.16, 25.66) | 0.326 | 16.55 (12.52, 23.64) | 18.68 (13.16, 25.69) | 0.119 |
| Vitamin B6 (mg) | 1.25 (0.79, 1.88) | 1.48 (1.00, 2.11) | 0.001 ** | 1.27 (0.97, 2.01) | 1.48 (1.00, 2.10) | 0.303 | 1.38 (0.94, 1.90) | 1.48 (1.00, 2.10) | 0.230 |
| Food folate (μg) | 154.00 (92.50, 213.25) | 165.00 (110.00, 240.00) | 0.007 ** | 172.00 (115.00, 233.25) | 165.00 (110.00, 240.00) | 0.981 | 151.50 (104.50, 244.75) | 165.00 (110.00, 240.00) | 0.801 |
| Vitamin B12 (μg) | 3.05 (1.63, 5.02) | 3.17 (1.78, 5.14) | 0.586 | 2.59 (1.76, 4.08) | 3.17 (1.78, 5.14) | 0.185 | 3.24 (1.76, 5.07) | 3.16 (1.78, 5.14) | 0.914 |
| Vitamin C (μg) | 24.70 (9.15, 83.58) | 51.30 (20.90, 107.50) | <0.001 *** | 64.40 (24.93, 122.58) | 51.00 (20.60, 107.30) | 0.220 | 59.65 (25.73, 101.45) | 50.90 (20.70, 107.40) | 0.493 |
| Vitamin D (μg) | 2.70 (0.80, 4.73) | 2.80 (1.10, 5.40) | 0.240 | 3.05 (1.55, 5.23) | 2.80 (1.10, 5.40) | 0.623 | 3.10 (1.53, 5.45) | 2.80 (1.10, 5.40) | 0.485 |
| Vitamin E (mg) | 5.21 (3.14, 8.31) | 5.96 (3.80, 9.00) | 0.015 * | 5.20 (3.20, 9.31) | 5.95 (3.80, 9.00) | 0.407 | 5.45 (3.70, 8.04) | 5.96 (3.79, 9.00) | 0.216 |
| Vitamin K (μg) | 51.50 (25.43, 88.73) | 58.60 (32.50, 112.80) |
0.003 ** | 60.35 (25.30, 115.55) | 58.60 (32.40, 112.30) |
0.518 | 54.80 (34.03, 106.63) | 58.60 (32.30, 112.40) |
0.563 |
| Calcium (mg) | 671.50 (412.75, 1111.25) | 732.00 (479.00, 1054.00) |
0.257 | 653.00 (459.75, 982.75) | 732.00 (477.00, 1055.00) |
0.332 | 742.50 (477.00, 1004.75) | 731.00 (477.00, 1055.00) | 0.946 |
| Phosphorus (mg) | 1022.00 (703.50, 1391.00) | 1075.00 (783.00, 1429.00) | 0.075 | 947.50 (660.50, 1313.25) | 1075.00 (782.00, 1429.00) |
0.140 | 968.50 (789.75, 1406.00) | 1075.00 (782.00, 1429.00) |
0.205 |
| Magnesium (mg) | 224.00 (145.75, 306.25) | 239.00 (175.00, 320.00) | 0.093 | 229.50 (162.75, 323.00) | 239.00 (175.00, 320.00) | 0.447 | 230.00 (189.25, 303.25) | 239.00 (175.00, 320.00) | 0.800 |
| Iron (mg) | 10.04 (7.12, 13.62) | 11.22 (7.83, 15.60) | 0.003 ** | 9.84 (6.77, 13.83) | 11.20 (7.83, 15.60) | 0.064 | 10.58 (7.77, 15.25) | 11.20 (7.82, 15.59) | 0.745 |
| Zinc (mg) | 7.70 (5.39, 11.27) | 8.29 (5.76, 11.63) | 0.221 | 7.43 (5.15, 9.38) | 8.29 (5.76, 11.63) | 0.030 * | 8.47 (5.64, 11.39) | 8.28 (5.76, 11.62) | 0.820 |
| Copper (mg) | 0.95 (0.60, 1.30) | 0.99 (0.71, 1.35) | 0.048 * | 0.90 (0.61, 1.33) | 0.99 (0.71, 1.35) | 0.153 | 1.03 (0.72, 1.39) | 0.99 (0.71, 1.35) | 0.638 |
| Sodium (mg) | 2579.00 (1807.50, 3561.75) | 2729.00 (1955.00, 3676.00) | 0.172 | 2359.00 (1701.50, 3648.25) | 2726.00 (1954.00, 3674.00) | 0.164 | 2735.50 (1836.75, 3607.75) | 2725.00 (1952.50, 3675.50) | 0.576 |
| Potassium (mg) | 2063.50 (1370.75, 2881.00) | 2151.00 (1567.00, 2851.00) | 0.125 | 2303.50 (1454.50, 2996.00) | 2150.00 (1565.00, 2850.00) | 0.773 | 2163.00 (1604.75, 2905.00) | 2150.00 (1564.50, 2851.00) | 0.866 |
| Selenium (μg) | 80.95 (51.45, 111.60) | 86.50 (60.80, 118.90) |
0.057 | 75.65 (51.35, 112.70) | 86.50 (60.80, 118.90) |
0.038 * | 80.30 (56.63, 120.68) | 86.50 (60.70, 118.80) |
0.341 |
| Caffeine (mg) | 185.00 (44.50, 329.75) | 83.00 (9.00, 180.00) | <0.001 *** | 105.50 (22.50, 201.00) | 83.00 (9.00, 183.00) | 0.229 | 76.00 (6.50, 169.25) | 84.00 (9.00, 183.00) | 0.458 |
| Alcohol (g) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.711 | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.894 | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.005 ** |
p value was tested by Wilcoxon rank-sum test, median (IQR). * p < 0.05, ** p < 0.01, *** p < 0.001.
Participants with cervical cancer tended to consume more caffeine (p < 0.001). Participants with ovarian cancer tended to consume more beta-cryptoxanthin (p = 0.011). Participants with endometrial cancer tended to consume more alcohol (p = 0.005). No statistical difference was observed in the other dietary factors with gynecological cancers (p > 0.05).
The correlations between dietary intakes and the gynecological cancers after adjustment for potential confounders are shown in Table 3. After adjusting for covariates, intake of phosphorus (OR, 95% CI; 0.998 (0.996, 0.999), p = 0.002) was negatively linked with cervical cancer; intakes of vitamin B12 (0.812 (0.714, 0.925), p = 0.002), phosphorus (0.997 (0.996, 0.999), p < 0.001) and alcohol (0.971 (0.950, 0.992), p = 0.009) were negatively linked with endometrial cancer. Intake of caffeine (1.002 (1.001, 1.003), p = 0.003) was positively associated with cervical cancer and intake of copper (2.754 (1.313, 5.778), p = 0.009) was positively associated with endometrial cancer. In addition, intake of vitamin B1 (p = 0.025) had a potential negative link with cervical cancer; intake of zinc (p = 0.048) had a potential negative link with ovarian cancer; and intake of potassium (p = 0.032) had a potential negative link with endometrial cancer. Intake of calcium (p = 0.026) had a potential positive association with cervical cancer and intakes of calcium (p = 0.034) and sodium (p = 0.042) had potential positive associations with endometrial cancer.
Table 3.
ORs with 95% CIs of the associations between dietary intakes, cervical cancer, ovarian cancer and endometrial cancer, separately.
| Variables | Cervical Cancer OR (CI) | p-Value | Ovarian Cancer OR (CI) | p-Value | Endometrial Cancer OR (CI) | p-Value |
|---|---|---|---|---|---|---|
| Protein (g) | 1.013 (0.990, 1.037) | 0.266 | 1.012 (0.986, 1.039) | 0.367 | 1.026 (0.998, 1.053) | 0.064 |
| Total sugars (g) | 1.003 (0.995, 1.010) | 0.468 | 1.005 (0.995, 1.015) | 0.332 | 1.005 (0.993, 1.017) | 0.386 |
| Total fat (g) | 1.010 (0.992, 1.028) | 0.293 | 1.009 (0.985, 1.033) | 0.469 | 0.987 (0.968, 1.006) | 0.160 |
| Cholesterol (mg) | 0.999 (0.998, 1.001) | 0.533 | 0.999 (0.997,1.001) | 0.313 | 1.001 (0.999, 1.002) | 0.477 |
| Vitamin A (μg) | 0.999 (0.998, 1.000) | 0.123 | 0.999 (0.998, 1.000) | 0.151 | 1.000 (0.999, 1.001) | 0.450 |
| Alpha-carotene (μg) | 1.000 (1.000, 1.000) | 0.981 | 1.000 (1.000, 1.000) | 0.214 | 1.000 (1.000, 1.000) | 0.253 |
| Beta-carotene (μg) | 1.000 (1.000, 1.000) | 0.213 | 1.000 (1.000, 1.000) | 0.072 | 1.000 (1.000, 1.000) | 0.460 |
| Beta-cryptoxanthin (μg) | 1.001 (0.999, 1.002) | 0.356 | 1.000 (0.999, 1.001) | 0.505 | 1.000 (0.999, 1.001) | 0.705 |
| Lycopene (μg) | 1.000 (1.000, 1.000) | 0.905 | 1.000 (1.000, 1.000) | 0.194 | 1.000 (1.000, 1.000) | 0.775 |
| Vitamin B1 (mg) | 0.518 (0.293, 0.916) | 0.025 * | 1.099 (0.678, 1.780) | 0.695 | 0.565 (0.276, 1.155) | 0.115 |
| Vitamin B2 (mg) | 1.362 (0.861, 2.154) | 0.182 | 0.775 (0.378, 1.586) | 0.476 | 1.465 (0.770, 2.785) | 0.237 |
| Niacin (mg) | 0.965 (0.921, 1.012) | 0.134 | 0.990 (0.930, 1.054) | 0.749 | 1.011 (0.950, 1.075) | 0.731 |
| Vitamin B6 (mg) | 1.408 (0.883, 2.246) | 0.146 | 1.170 (0.726, 1.885) | 0.510 | 1.232 (0.884, 1.717) | 0.212 |
| Food folate (μg) | 0.999 (0.996, 1.002) | 0.597 | 0.999 (0.996, 1.002) | 0.484 | 1.001 (0.998, 1.003) | 0.509 |
| Vitamin B12 (μg) | 1.067 (0.968, 1.177) | 0.186 | 1.031 (0.942,1.129) | 0.494 | 0.812 (0.714, 0.925) | 0.002 ** |
| Vitamin C (μg) | 0.999 (0.995, 1.003) | 0.639 | 1.001 (0.995, 1.006) | 0.837 | 1.000 (0.996, 1.004) | 0.993 |
| Vitamin D (μg) | 1.032 (0.966, 1.102) | 0.349 | 1.015 (0.959, 1.075) | 0.595 | 1.026 (0.949, 1.110) | 0.504 |
| Vitamin E (mg) | 0.979 (0.932, 1.029) | 0.392 | 0.979 (0.922, 1.039) | 0.471 | 0.990 (0.938, 1.045) | 0.715 |
| Vitamin K (μg) | 0.999 (0.997, 1.001) | 0.210 | 0.999 (0.997, 1.001) | 0.455 | 0.999 (0.997, 1.001) | 0.277 |
| Calcium (mg) | 1.001 (1.000, 1.002) | 0.026 * | 1.001 (1.000, 1.002) | 0.102 | 1.001 (1.000, 1.002) | 0.034 * |
| Phosphorus (mg) | 0.998 (0.996, 0.999) | 0.002 ** | 0.999 (0.997, 1.000) | 0.144 | 0.997 (0.996, 0.999) | <0.001 *** |
| Magnesium (mg) | 0.999 (0.995, 1.003) | 0.619 | 1.000 (0.996, 1.005) | 0.832 | 1.000 (0.995, 1.005) | 0.894 |
| Iron (mg) | 0.972 (0.913, 1.036) | 0.372 | 1.014 (0.947, 1.085) | 0.683 | 0.969 (0.893, 1.053) | 0.451 |
| Zinc (mg) | 1.015 (0.995, 1.079) | 0.624 | 0.886 (0.787, 0.999) | 0.048 * | 1.099 (0.990, 1.220) | 0.074 |
| Copper (mg) | 1.450 (0.796, 2.642) | 0.218 | 1.042 (0.404, 2.687) | 0.931 | 2.754 (1.313, 5.778) | 0.009 ** |
| Sodium (mg) | 1.000 (1.000, 1.000) | 0.194 | 1.000 (1.000, 1.001) | 0.487 | 1.000 (1.000, 1.001) | 0.042 * |
| Potassium (mg) | 1.000 (0.999, 1.000) | 0.607 | 1.000 (1.000, 1.001) | 0.516 | 0.999 (0.999, 1.000) | 0.032 * |
| Selenium (μg) | 1.002 (0.993, 1.012) | 0.625 | 0.997 (0.985, 1.010) | 0.674 | 1.000 (0.989, 1.011) | 0.969 |
| Caffeine (mg) | 1.002 (1.001, 1.003) | 0.003 ** | 1.000 (0.998, 1.002) | 0.861 | 0.999 (0.996, 1.001) | 0.297 |
| Alcohol (g) | 1.011 (0.996, 1.027) | 0.146 | 1.013 (0.994, 1.031) | 0.170 | 0.971 (0.950, 0.992) | 0.009 ** |
* p < 0.05, ** p < 0.01, *** p < 0.001.
Intakes of protein (p = 0.266; p = 0.367; p = 0.064), total sugars (p = 0.468; p = 0.332; p = 0.386), total fat (p = 0.293; p = 0.469; p = 0.160), cholesterol (p = 0.533; p = 0.313; p = 0.477), vitamin A (p = 0.123; p = 0.151; p = 0.450), alpha-carotene (p = 0.981; p = 0.214; p = 0.253), beta-carotene (p = 0.213; p = 0.072; p = 0.460), beta-cryptoxanthin (p = 0.356; p = 0.505; p = 0.705), lycopene (p = 0.905; p = 0.194; p = 0.775), vitamin B2 (p = 0.182; p = 0.476; p = 0.237), niacin (p = 0.134; p = 0.749; p = 0.731), vitamin B6 (p = 0.146; p = 0.510; p = 0.212), food folate (p = 0.597; p = 0.484; p = 0.509), vitamin C (p = 0.639; p = 0.837; p = 0.993), vitamin D (p = 0.349; p = 0.595; p = 0.504), vitamin E (p = 0.392; p = 0.471; p = 0.715), vitamin K (p = 0.210; p = 0.455; p = 0.277), magnesium (p = 0.619; p = 0.832; p = 0.894), iron (p = 0.372; p = 0.683; p = 0.451) and selenium (p = 0.625; p = 0.674; p = 0.969) showed no relationship with cervical cancer, ovarian cancer and endometrial cancer, separately.
4. Discussion
In this cross-sectional study, we revealed the relationships between dietary intakes and gynecological cancers. Intakes of certain vitamins, minerals, caffeine and alcohol were positively associated with gynecological cancers. In addition, statistical diversities were represented between dietary factors and various types of gynecological cancers. As far as we know, it is the first study to comprehensively examine the relationships between dietary factors and gynecological cancers among the U.S. population using the NHANES database.
The prospect that intakes of certain vitamins might confer protection against cancer has received much attention during recent years [38,39,40]. Our study found that intake of vitamin B12 was negatively associated with endometrial cancer. However, epidemiological evidence exploring the link between vitamin B12 intake and endometrial cancer is limited, and the results are not consistent [41,42]. A study reported that no relationship between vitamin B12 consumption and endometrial cancer was observed [41]. In a prospective study, it was found that an initial increase in consumption of vitamin B12 did not enhance endometrial cancer risk, but the women in the highest consumption quintile had a significantly increased endometrial cancer risk compared to women in the lowest quintile [42]. In fact, such association was only observed in women with BMI ≥ 25 kg/m2 [42]. Our findings suggested that intake of vitamin B12 might have a negative relationship with endometrial cancer. It might be attributed to the potential mechanism whereby vitamin B12 deficiency could result in altered expression of cancer-related genes by reducing DNA synthesis, leading to cancer pathogenesis [43].
Whether dietary phosphorus affects the development of gynecological cancers is unclear [44]. Our findings suggested that phosphorus was protective against cervical cancer and endometrial cancer. Similar to our findings, recent studies have indicated that dietary phosphorus had a protective role in cervical intraepithelial neoplasia (CIN) and colorectal adenoma [45,46,47]. Combining both low calcium and high phosphorus might affect cancer development [48]. A statistical relationship between dietary ratio of calcium-to-phosphorus and CIN risk was reported in a previous case–control study [49]. However, these studies only focused on the dietetical calcium-to-phosphorus ratio and phosphorus inorganic salt status, not dietary phosphorus intake. The phosphorus intake reflected normal daily intake, but it might not embody an accurate level in cells [47]. The associations between phosphorus intake and gynecological cancers require further epidemiological studies to verify. In addition, we found that intake of copper might be positively associated with endometrial cancer. Elevated copper concentrations have been reported in many types of cancers, including gynecological cancers [50], breast cancer [51], lung cancer [52] and gastrointestinal cancer [53]. Relationships between copper and cancers have been widely accepted, as there is a requirement for higher levels of copper for cancer cells compared with non-dividing cells [54]. Copper appears to drive the estrogen-dependent cell proliferation [55,56,57].
We found that intakes of caffeine and alcohol were associated with gynecological cancers. Previous studies have demonstrated that intake of caffeine promoted development of specific cancers, such as esophageal adenocarcinoma [58], vulvar cancer [59] and head and neck squamous cell carcinomas [60]. A positive association between intake of caffeine and cervical cancer was observed in this study. It might be in part explained by the fact that caffeine, acting as an adenosine receptor, has been shown to contribute to carcinogenesis indirectly [61]. Our results observed that alcohol consumption was inversely linked with endometrial cancer even after accounting for multiple dietary factors. It has been raised that alcohol consumption might enhance cancer risk [62]. However, a recent large prospective cohort study among 68,067 women reported that, compared with women who did not drink, women who drank < 5 g/day of ethanol had a 22% reduction in risk of endometrial cancer [22]. When stratifying by alcohol consumption and BMI, alcohol consumption was associated with high risk of endometrial cancer in thin women (BMI < 25 kg/m2), whereas heavier women had a decreased risk [63,64]. The association between alcohol consumption and endometrial cancer needs more epidemiological studies to validate.
We also found potential links between several dietary factors and gynecological cancers. Our study indicated that intakes of vitamin B1, zinc and potassium had potential negative associations with cervical cancer, ovarian cancer and endometrial cancer, separately. Zhou et al. [65] reported that moderate intake of vitamin B1 could prevent human papilloma virus (HPV) infection, suggesting a resistant effect of vitamin B1 in cervical cancer. Previous studies reported that intakes of zinc and potassium were negatively associated with several cancers, such as breast cancer [66,67], lung cancer [36,68] and colorectal cancer [69,70]. Additionally, in a mendelian randomization analysis, women with high circulating zinc concentration had a lower risk of ovarian cancer [71]. Our results suggested that calcium intake had a potential positive relationship with cervical cancer and endometrial cancer. An epidemiological study showed a positive relationship between calcium and prostate cancer, even adjusting for race, dietary phosphorus and BMI [72]. We found that dietary sodium might have a potential positive association with endometrial cancer. It has been reported that low sodium intake might have benefits for human cancers [73]. Although the role of sodium intake in gynecological cancers has not been fully elucidated, sodium intake has been shown to have a positive association with renal cell cancer [74], lung cancer [68] and gastric cancer [75].
Some limitations of the study need to be acknowledged. First, self-reported 24-h dietary recall information was subjected to measurement bias due to large day-to-day changes in dietary intakes. The analysis relied on a single measurement of diet at a dietary recall interview, whereas dietary behaviors might change over time. Second, there might have been misclassifications in cancer status due to the self-reported nature of the survey. Participants might not have reported accurate information about their cancer history. Third, there were likely complex added effects and biological interactions between multiple nutrients and non-nutrients in the usual diet [76]. Although we adjusted for known covariates, residual confounding secondary unknown or uncontrolled factors such as estrogen use might have interfered with our results. Lastly, in this study, the dietary assessment was conducted after cancer diagnosis, hence the diet could have been modified after cancer treatment. Due to the cross-sectional nature of this investigation, causality was hard to elucidate in this investigation.
5. Conclusions
In conclusion, we found that intakes of copper and caffeine were positively related to certain gynecological cancers, while intakes of vitamin B12, phosphorus and alcohol were negatively related to them. In addition, intakes of vitamin B1, zinc, calcium, sodium and potassium had potential associations with gynecological cancers. Nevertheless, no significant correlations were observed between other dietary factors and gynecological cancers. Further epidemiological studies are required to elucidate our findings.
Acknowledgments
The authors are grateful to all the NHANES study participants for their assistance.
Author Contributions
Study design: G.Z., Z.L. and L.S.; data extraction, quality assessment and data analysis: G.Z., Z.L. and Y.W.; manuscript writing and edition: G.Z., Z.L., L.T., Z.Z. and S.B.; technical support: Z.L., M.S. and Y.Z.; L.S. approved the final version of this manuscript and took responsibility for its content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the use of publicly available de-identified data, and it not being considered human subject research in the United States.
Informed Consent Statement
The National Center for Health Statistics obtained informed consent from all subjects.
Data Availability Statement
The data supporting the findings of this study are publicly available from the NHANES. (https://www.cdc.gov/nchs/nhanes/index.htm). Accessed on 4 January 2022.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
L.S. is supported by the Free Exploration Research Project of the Second Affiliated Hospital of Xi’an Jiaotong University (Grant number: 2020YJ(ZYTS)282).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician. 2016;93:937–944. [PubMed] [Google Scholar]
- 5.Wang Q., Peng H., Qi X., Wu M., Zhao X. Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Signal Transduct. Target. Ther. 2020;5:137. doi: 10.1038/s41392-020-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R. The lessons of life: Keynote address to the nutrition and cancer conference. Cancer Res. 1992;52:2024s–2029s. [PubMed] [Google Scholar]
- 7.Thanikachalam K., Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11:164. doi: 10.3390/nu11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicco P.D., Catani M.V., Gasperi V., Sibilano M., Quaglietta M., Savini I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients. 2019;11:1514. doi: 10.3390/nu11071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oczkowski M., Dziendzikowska K., Pasternak-Winiarska A., Włodarek D., Gromadzka-Ostrowska J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients. 2021;13:496. doi: 10.3390/nu13020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y., Li Z., Li J., Li Z., Han J. A Healthy Dietary Pattern Reduces Lung Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients. 2016;8:134. doi: 10.3390/nu8030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieck G., Fiander A. The effect of lifestyle factors on gynaecological cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2006;20:227–251. doi: 10.1016/j.bpobgyn.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Ciebiera M., Esfandyari S., Siblini H., Prince L., Elkafas H., Wojtyla C., Al-Hendy A., Ali M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients. 2021;13:1178. doi: 10.3390/nu13041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barchitta M., Maugeri A., Quattrocchi A., Agrifoglio O., Scalisi A., Agodi A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients. 2018;10:469. doi: 10.3390/nu10040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen C.W., Fontaine K.R., Arend R.C., Soleymani T., Gower B.A. Favorable Effects of a Ketogenic Diet on Physical Function, Perceived Energy, and Food Cravings in Women with Ovarian or Endometrial Cancer: A Randomized, Controlled Trial. Nutrients. 2018;10:1187. doi: 10.3390/nu10091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barchitta M., Maugeri A., La Mastra C., Rosa M.C., Favara G., Lio R.M.S., Agodi A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients. 2020;12:1384. doi: 10.3390/nu12051384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markowska A., Antoszczak M., Markowska J., Huczyński A. Role of Vitamin C in Selected Malignant Neoplasms in Women. Nutrients. 2022;14:882. doi: 10.3390/nu14040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricceri F., Giraudo M.T., Fasanelli F., Milanese D., Sciannameo V., Fiorini L., Sacerdote C. Diet and endometrial cancer: A focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer. 2017;17:757. doi: 10.1186/s12885-017-3754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H., Gong T.-T., Xia Y., Wen Z.-Y., Zhao L.-G., Zhao Y.-H., Wu Q.-J. Diet and ovarian cancer risk: An umbrella review of systematic reviews and meta-analyses of cohort studies. Clin. Nutr. 2021;40:1682–1690. doi: 10.1016/j.clnu.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh C., Baker J.A., Moysich K.B., Rivera R., Brasure J.R., McCann S.E. Dietary intakes of selected nutrients and food groups and risk of cervical cancer. Nutr. Cancer. 2008;60:331–341. doi: 10.1080/01635580701861769. [DOI] [PubMed] [Google Scholar]
- 20.Salazar-Martinez E., Lazcano-Ponce E., Sanchez-Zamorano L.M., Gonzalez-Lira G., Rios P.E.-D.L., Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int. J. Gynecol. Cancer. 2005;15:938–945. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 21.Parazzini F., Vecchia C.L., D’Avanzo B., Moroni S., Chatenoud L., Ricci E. Alcohol and endometrial cancer risk: Findings from an Italian case-control study. Nutr. Cancer. 1995;23:55–62. doi: 10.1080/01635589509514361. [DOI] [PubMed] [Google Scholar]
- 22.Je Y., Vivo I.D., Giovannucci E. Long-term alcohol intake and risk of endometrial cancer in the Nurses’ Health Study. Br. J. Cancer. 2014;111:186–194. doi: 10.1038/bjc.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q., Guo P., Li H., Chen X.-D. Does alcohol consumption modify the risk of endometrial cancer? A dose-response meta-analysis of prospective studies. Arch. Gynecol. Obstet. 2017;295:467–479. doi: 10.1007/s00404-016-4263-y. [DOI] [PubMed] [Google Scholar]
- 24.Sreeja S.R., Seo S.S., Kim M.K. Associations of Dietary Glycemic Index, Glycemic Load and Carbohydrate with the Risk of Cervical Intraepithelial Neoplasia and Cervical Cancer: A Case-Control Study. Nutrients. 2020;12:3742. doi: 10.3390/nu12123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman H.G., Kitahara C.M., Murray L.J., Dodd K.W., Black A., Stolzenberg-Solomon R.Z., Cantwell M.M. Dietary carbohydrate intake, glycemic index, and glycemic load and endometrial cancer risk: A prospective cohort study. Am. J. Epidemiol. 2014;179:75–84. doi: 10.1093/aje/kwt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou R., Wu Q.J., Gong T.T., Jiang L. Dietary fat and fatty acid intake and epithelial ovarian cancer risk: Evidence from epidemiological studies. Oncotarget. 2015;6:43099–43119. doi: 10.18632/oncotarget.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotsopoulos J., Hankinson S.E., Tworoger S.S. Dietary betaine and choline intake are not associated with risk of epithelial ovarian cancer. Eur. J. Clin. Nutr. 2010;64:111–114. doi: 10.1038/ejcn.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fung T.T., Brown L.S. Dietary Patterns and the Risk of Colorectal Cancer. Curr. Nutr. Rep. 2013;2:48–55. doi: 10.1007/s13668-012-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett W. Nutritional Epidemiology. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- 30.Hayden K.M., Beavers D.P., Steck S.E., Hebert J.R., Tabung F.K., Shivappa N., Casanova R., Manson J.E., Padula C.B., Salmoirago-Blotcher E., et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s Health Initiative Memory Study. Alzheimers Dement. 2017;13:1187–1196. doi: 10.1016/j.jalz.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. National Center for Health Statistics . National Health and Nutrition Examination Survey [Internet] Centers for Disease Control and Prevention; Atlanta, GA, USA: 2001. [Google Scholar]
- 32.Arli S.K., Bakan A.B., Aslan G. Distribution of cervical and breast cancer risk factors in women and their screening behaviours. Eur. J. Cancer Care. 2019;28:e12960. doi: 10.1111/ecc.12960. [DOI] [PubMed] [Google Scholar]
- 33.Fortner R.T., Poole E.M., Wentzensen N.A., Trabert B., White E., Arslan A.A., Patel A.V., Setiawan V.W., Visvanathan K., Weiderpass E., et al. Ovarian cancer risk factors by tumor aggressiveness: An analysis from the Ovarian Cancer Cohort Consortium. Int. J. Cancer. 2019;145:58–69. doi: 10.1002/ijc.32075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raglan O., Kalliala I., Markozannes G., Cividini S., Gunter M.J., Nautiyal J., Gabra H., Paraskevaidis E., Martin-Hirsch P., Tsilidis K.K., et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 35.Cramer D.W. The epidemiology of endometrial and ovarian cancer. Hematol. Oncol. Clin. N. Am. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charoenngam N., Ponvilawan B., Ungprasert P. Higher zinc intake is associated with decreased risk of lung cancer. J. Evid. Based Med. 2021;14:185–187. doi: 10.1111/jebm.12448. [DOI] [PubMed] [Google Scholar]
- 37.Lunardon N., Menardi G., Torelli N. ROSE: A Package for Binary Imbalanced Learning. R J. 2014;6:79–89. doi: 10.32614/RJ-2014-008. [DOI] [Google Scholar]
- 38.Muñoz A., Grant W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients. 2022;14:1448. doi: 10.3390/nu14071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venturelli S., Leischner C., Helling T., Burkard M., Marongiu L. Vitamins as Possible Cancer Biomarkers: Significance and Limitations. Nutrients. 2021;13:3914. doi: 10.3390/nu13113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markowska A., Antoszczak M., Markowska J., Huczyński A. Role of Vitamin K in Selected Malignant Neoplasms in Women. Nutrients. 2022;14:3401. doi: 10.3390/nu14163401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J.J., Hazra A., Giovannucci E., Hankinson S.E., Rosner B., Vivo I.D. One-carbon metabolism factors and endometrial cancer risk. Br. J. Cancer. 2013;108:183–187. doi: 10.1038/bjc.2012.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu J., Trabert B., Liao L.M., Pfeiffer R.M., Michels K.A. Dietary intake of nutrients involved in folate-mediated one-carbon metabolism and risk for endometrial cancer. Int. J. Epidemiol. 2019;48:474–488. doi: 10.1093/ije/dyy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi S.W., Mason J.B. Folate and carcinogenesis: An integrated scheme. J. Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 44.Spina A., Sapio L., Esposito A., Maiolo F.D., Sorvillo L., Naviglio S. Inorganic Phosphate as a Novel Signaling Molecule with Antiproliferative Action in MDA-MB-231 Breast Cancer Cells. Biores. Open Access. 2013;2:47–54. doi: 10.1089/biores.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kesse E., Boutron-Ruault M.-C., Norat T., Riboli E., Clavel-Chapelon F., Group E.N. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int. J. Cancer. 2005;117:137–144. doi: 10.1002/ijc.21148. [DOI] [PubMed] [Google Scholar]
- 46.Lee L.v., Heyworth J., McNaughton S., Iacopetta B., Clayforth C., Fritschi L. Selected dietary micronutrients and the risk of right- and left-sided colorectal cancers: A case-control study in Western Australia. Ann. Epidemiol. 2011;21:170–177. doi: 10.1016/j.annepidem.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z., Wang W., Yang A., Zhao W., Yang J., Wang Z., Wang W., Su X., Wang J., Song J., et al. Lower dietary mineral intake is significantly associated with cervical cancer risk in a population-based cross-sectional study. J. Cancer. 2021;12:111–123. doi: 10.7150/jca.39806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takata Y., Shu X.-O., Yang G., Li H., Dai Q., Gao J., Cai Q., Gao Y.-T., Zheng W. Calcium intake and lung cancer risk among female nonsmokers: A report from the Shanghai Women’s Health Study. Cancer Epidemiol. Biomark. Prev. 2013;22:50–57. doi: 10.1158/1055-9965.EPI-12-0915-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T., Soong S.J., Alvarez R.D., Jr C.E.B. A longitudinal analysis of human papillomavirus 16 infection, nutritional status, and cervical dysplasia progression. Cancer Epidemiol. Biomark. Prev. 1995;4:373–380. [PubMed] [Google Scholar]
- 50.Margalioth E.J., Schenker J.G., Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::AID-CNCR2820520521>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 51.Adeoti M., Oguntola A., Akanni E., Agodirin O., Oyeyemi G. Trace elements; copper, zinc and selenium, in breast cancer afflicted female patients in LAUTECH Osogbo, Nigeria. Indian J. Cancer. 2015;52:106–109. doi: 10.4103/0019-509X.175573. [DOI] [PubMed] [Google Scholar]
- 52.Jin Y., Zhang C., Xu H., Xue S., Wang Y., Hou Y., Kong Y., Xu Y. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: A hospital based case-control study in China. Cancer Epidemiol. 2011;35:182–187. doi: 10.1016/j.canep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Sohrabi M., Gholami A., Azar M.H., Yaghoobi M., Shahi M.M., Shirmardi S., Nikkhah M., Kohi Z., Salehpour D., Khoonsari M.R., et al. Trace Element and Heavy Metal Levels in Colorectal Cancer: Comparison Between Cancerous and Non-cancerous Tissues. Biol. Trace Elem. Res. 2018;183:1–8. doi: 10.1007/s12011-017-1099-7. [DOI] [PubMed] [Google Scholar]
- 54.Lopez J., Ramchandani D., Vahdat L. Copper Depletion as a Therapeutic Strategy in Cancer. Met. Ions Life Sci. 2019;19:303–330. doi: 10.1515/9783110527872-018. [DOI] [PubMed] [Google Scholar]
- 55.Martin M.B., Reiter R., Pham T., Avellanet Y.R., Camara J., Lahm M., Pentecost E., Pratap K., Gilmore B.A., Divekar S., et al. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology. 2003;144:2425–2436. doi: 10.1210/en.2002-221054. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X., Wang Y., Zhao Y., Chen X. Experimental study on the estrogen-like effect of mercuric chloride. Biometals. 2008;21:143–150. doi: 10.1007/s10534-007-9102-y. [DOI] [PubMed] [Google Scholar]
- 57.Choe S.-Y., Kim S.-J., Kim H.-G., Lee J.H., Choi Y., Lee H., Kim Y. Evaluation of estrogenicity of major heavy metals. Sci. Total Environ. 2003;312:15–21. doi: 10.1016/S0048-9697(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 58.Kambhampati S., Tieu A.H., Luber B., Wang H., Meltzer S.J. Risk Factors for Progression of Barrett’s Esophagus to High Grade Dysplasia and Esophageal Adenocarcinoma. Sci. Rep. 2020;10:4899. doi: 10.1038/s41598-020-61874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturgeon S.R., Ziegler R.G., Brinton L.A., Nasca P.C., Mallin K., Gridley G. Diet and the risk of vulvar cancer. Ann. Epidemiol. 1991;1:427–437. doi: 10.1016/1047-2797(91)90012-2. [DOI] [PubMed] [Google Scholar]
- 60.Nour A., Joury E., Naja F., Hatahet W., Almanadili A. Diet and the risk of head and neck squamous cell carcinomas in a Syrian population: A case-control study. East Mediterr. Health J. 2015;21:629–634. doi: 10.26719/2015.21.9.629. [DOI] [PubMed] [Google Scholar]
- 61.VanderPloeg L.C., Wolfrom D.M., Welsch C.W. Influence of caffeine on development of benign and carcinomatous mammary gland tumors in female rats treated with the carcinogens 7,12-dimethylbenz(a)anthracene and N-methyl-N-nitrosourea. Cancer Res. 1991;51:3399–3404. [PubMed] [Google Scholar]
- 62.Rumgay H., Shield K., Charvat H., Ferrari P., Sornpaisarn B., Obot I., Islami F., Lemmens V.E.P.P., Rehm J., Soerjomataram I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021;22:1071–1080. doi: 10.1016/S1470-2045(21)00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Setiawan V.W., Monroe K.R., Goodman M.T., Kolonel L.N., Pike M.C., Henderson B.E. Alcohol consumption and endometrial cancer risk: The multiethnic cohort. Int. J. Cancer. 2008;122:634–638. doi: 10.1002/ijc.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster L.A., Weiss N.S. Alcoholic beverage consumption and the risk of endometrial cancer. Cancer and Steroid Hormone Study Group. Int. J. Epidemiol. 1989;18:786–791. doi: 10.1093/ije/18.4.786. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Y.-X., Zhu F.-F., Chen C., Zhang Y.-X., Lv X.-L., Li J.-W., Luo S.-P., Gao J. Association of Thiamine Intake with Human Papillomavirus (HPV) Infection in American Women: A Secondary Data Analysis Based on the National Health and Nutrition Examination Survey from 2003 to 2016. Med. Sci. Monit. 2020;26:e924932. doi: 10.12659/MSM.924932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez-Lazaro C.I., Martínez-González M.Á., Aguilera-Buenosvinos I., Gea A., Ruiz-Canela M., Romanos-Nanclares A., Toledo E. Dietary Antioxidant Vitamins and Minerals and Breast Cancer Risk: Prospective Results from the SUN Cohort. Antioxidants. 2021;10:340. doi: 10.3390/antiox10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levi F., Pasche C., Lucchini F., Vecchia C.L. Dietary intake of selected micronutrients and breast-cancer risk. Int. J. Cancer. 2001;91:260–263. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 68.You D., Zhang M., He W., Wang D., Yu Y., Yu Z., Lange T., Yang S., Wei Y., Ma H., et al. Association between dietary sodium, potassium intake and lung cancer risk: Evidence from the prostate, lung, colorectal and ovarian cancer screening trial and the Women’s Health Initiative. Transl. Lung Cancer Res. 2021;10:45–56. doi: 10.21037/tlcr-20-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papadimitriou N., Bouras E., Brandt P.A.v.d., Muller D.C., Papadopoulou A., Heath A.K., Critselis E., Gunter M.J., Vineis P., Ferrari P., et al. A Prospective Diet-Wide Association Study for Risk of Colorectal Cancer in EPIC. Clin. Gastroenterol. Hepatol. 2022;20:864–873. doi: 10.1016/j.cgh.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 70.Li P., Xu J., Shi Y., Ye Y., Chen K., Yang J., Wu Y. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin. Nutr. 2014;33:415–420. doi: 10.1016/j.clnu.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Lin S., Yang H. Ovarian cancer risk according to circulating zinc and copper concentrations: A meta-analysis and Mendelian randomization study. Clin. Nutr. 2021;40:2464–2468. doi: 10.1016/j.clnu.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Batai K., Murphy A.B., Ruden M., Newsome J., Shah E., Dixon M.A., Jacobs E.T., Hollowell C.M., Ahaghotu C., Kittles R.A. Race and BMI modify associations of calcium and vitamin D intake with prostate cancer. BMC Cancer. 2017;17:64. doi: 10.1186/s12885-017-3060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jansson B. Potassium, sodium, and cancer: A review. J. Environ. Pathol. Toxicol. Oncol. 1996;15:65–73. [PubMed] [Google Scholar]
- 74.Deckers I.A.G., Brandt P.A.v.d., Engeland M.V., Soetekouw P.M.M.B., Baldewijns M.M.L.L., Goldbohm R.A., Schouten L.J. Long-term dietary sodium, potassium and fluid intake; exploring potential novel risk factors for renal cell cancer in the Netherlands Cohort Study on diet and cancer. Br. J. Cancer. 2014;110:797–801. doi: 10.1038/bjc.2013.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pelucchi C., Tramacere I., Bertuccio P., Tavani A., Negri E., Vecchia C.L. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann. Oncol. 2009;20:160–165. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Michalak M., Agellon L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018;91:95–103. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are publicly available from the NHANES. (https://www.cdc.gov/nchs/nhanes/index.htm). Accessed on 4 January 2022.