Abstract
The receptor for urokinase-type plasminogen activator (uPAR) (CD87) plays an important role in leukocyte adhesion and migration. To assess the effect of endotoxin on cellular uPAR, uPAR expression was determined on leukocytes by fluorescence-activated cell sorter analysis in seven healthy subjects following intravenous injection of endotoxin (lot G; 4 ng/kg). Endotoxin induced a transient increase in uPAR expression on monocytes, reaching a 92% ± 46% increase over baseline expression after 6 h (P < 0.05). Endotoxin did not influence uPAR expression on granulocytes, while uPAR remained undetectable on lymphocytes. Endotoxin also increased soluble uPAR levels in plasma (P < 0.05). Stimulation of human whole blood with endotoxin or gram-positive stimuli in vitro also resulted in an upregulation of monocyte uPAR expression. Although tumor necrosis factor alpha (TNF) upregulated monocyte uPAR expression, anti-TNF did not influence the endotoxin-induced increase in monocyte uPAR expression. These data suggest that infectious stimuli may influence monocyte function in vivo by enhancing the expression of uPAR.
Transendothelial migration of leukocytes can be seen as a three-step process that includes rolling of cells along the luminal side of the vascular endothelium (a process mediated by selectins), firm adherence to the endothelium (mediated by β2-integrins), and subsequent transmigration across the endothelial monolayer, presumably over a chemotactic gradient generated by chemokines (15). The urokinase plasminogen activator receptor (uPAR) (CD87), which is widely expressed on many different cell types including hematopoietic cells, has been implicated as an important factor in the regulation of leukocyte trafficking (9). Although uPAR is a glycosylphosphatidylinositol (GPI)-linked membrane protein and therefore lacks the transmembrane and cytoplasmatic sequences to induce signal transduction, it has an obligate function in chemotaxis of monocytes and neutrophils. The capacity of uPAR to act as an adhesion receptor depends on a functional and physical association with integrins. Indeed, uPAR can form complexes with complement receptor type 3 (CR3) (CD11b/CD18), an integrin adhesion protein, on the surface of monocytes and neutrophils, thereby facilitating the adhesive capacity of the latter (3, 11, 17, 18, 27, 32, 34). Furthermore, studies with uPAR-deficient mice have demonstrated that β-integrin-mediated leukocyte recruitment to inflamed areas in vivo requires the presence of uPAR (29).
Relatively little is known about the regulation of uPAR expression. Lipopolysaccharide (LPS), the toxic moiety of the gram-negative bacterial cell membrane, can enhance uPAR expression on monocytes (5). Although tumor necrosis factor alpha (TNF) is capable of increasing uPAR mRNA levels in monocytic cells and colon cancer cell lines (21, 35), reports on the ability of this proinflammatory cytokine to induce uPAR expression on the surface of monocytic cells are conflicting (31, 35). After LPS administration to mice, increased uPAR mRNA levels were detected in most tissues examined (4).
Intravenous administration of low-dose LPS to healthy subjects is a widely adopted human model of acute inflammation (40). Experimental human endotoxemia is characterized by systemic release of cytokines, endothelial cell activation, and activation of leukocytes with sequestration of granulocytes in the lungs (14, 20, 41). Furthermore, LPS administration to humans is associated with enhanced expression of CD11b on circulating granulocytes (41) and with a transient monocytopenia, presumably related to enhanced adhesion of these cells to the vascular wall (14, 20, 40, 41). In the present study, we used this model to determine the in vivo effect of LPS on the expression of uPAR on different leukocyte subsets in the circulation.
MATERIALS AND METHODS
Human endotoxemia model.
Seven men (mean age, 22 years; range, 20 to 25 years) were admitted to the Clinical Research Unit of the Academic Medical Center, where they received an intravenous injection with Escherichia coli LPS (lot G; U.S. Pharmacopeial Convention, Rockville, Md.) over 1 min in an antecubital vein at a dose of 4 ng/kg of body weight. All subjects were in good health, as documented by history, physical examination, and hematological and biochemical screening. Blood for soluble uPAR measurement was obtained directly before LPS administration (t = 0 h) and at 1, 2, 4, 6, 8, 12 and 24 h thereafter. Blood was drawn in K3-EDTA-containing tubes and centrifuged at 2,000 × g for 20 min at 4°C, after which plasma was stored at −20°C until the assay was performed. Soluble uPAR was measured by an enzyme-linked immunosorbent assay (detection limit, 0.25 ng/ml), as specified by the manufacturer (American Diagnostica, Inc., Greenwich, Conn.). The assay detects the domain 2/3 fragment of uPAR and measures total uPAR, i.e., free uPAR, uPAR-uPA, and uPAR–uPA–PAI-1 complexes (information provided by American Diagnostica, Inc.).
Whole-blood stimulation.
Whole blood was stimulated as described previously (2, 13). Blood was collected aseptically from healthy subjects using a sterile collecting system consisting of a butterfly needle connected to a syringe (Becton Dickinson & Co., Rutherford, N.J.). For anticoagulation, sterile heparin (LEO Pharmaceutical, Weesp, The Netherlands) (final concentration, 10 U/ml of blood) was used. Whole blood, diluted 1:1 in sterile RPMI 1640 (Gibco BRL, Life Technologies, Inc., Grand Island, N.Y.), was stimulated for 4, 8, or 24 h at 37°C with different stimuli in sterile polypropylene tubes (Becton Dickinson & Co.). For these experiments, polypropylene tubes were prefilled with 1 ml of RPMI 1640 containing the appropriate concentrations of the stimuli, after which 1 ml of heparinized blood was added. The contents of the tubes were gently mixed, and the tubes were placed in the incubator. Each test was performed at least four times with blood from different healthy donors. The stimuli used were LPS (from Escherichia coli serotype O111:B4 (1 to 1,000 ng/ml) (Sigma, St. Louis, Mo.), staphylococcal enterotoxin B (SEB) (1 and 10 μg/ml) (Sigma), heat-killed Staphylococcus aureus (106 and 107 CFU/ml), and recombinant human TNF (1 to 100 ng/ml). In some experiments, a neutralizing anti-TNF monoclonal antibody (MAb) (MAK 195F) (10 μg/ml) was used (10). Recombinant TNF and anti-TNF were kindly provided by Knoll, Ludwigshafen, Germany. After incubation, the blood samples were immediately placed on ice and processed for fluorescence-activated cell sorter (FACS) analysis as described below.
Measurement of cell-associated uPAR.
Blood for FACS analysis was obtained directly before LPS administration (t = 0 h) and at 1, 2, 4, 6, and 24 h thereafter. These blood samples were drawn in heparin-containing Vacutainer tubes and immediately placed on ice. For FACS analysis, erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA [pH 7.4]) for 10 min. The cells were centrifuged at 600 × g for 5 min at 4°C. The remaining cells were brought to a concentration of 4 × 106 cells/ml in FACS buffer (phosphate-buffered saline supplemented with 0.5% bovine serum albumin [BSA], 0.01% NaN3, and 100 mM EDTA). Expression of cell-bound uPAR was determined using a mouse anti-human uPAR MAb (clone VIM-5; Instruchemie, Hilversum, The Netherlands) (28) at the concentrations recommended by the manufacturer. To correct for nonspecific staining, an appropriate control antibody (murine immunoglobulin G1; Becton Dickinson & Co.) was used. For each test, 105 cells and at least 103 (in vivo studies) or 104 (in vitro studies) monocytes were counted. The mean cell fluorescence (MCF) at >570 nm of forward- and side-angle scatter-gated granulocytes, monocytes, and lymphocytes was assessed. Data are presented as the difference between MCF intensities of specifically and nonspecifically stained cells.
Stimulation of isolated leukocyte fractions.
Heparinized blood was placed on an equal volume of Polymorphrep (Nycomed Pharma AS, Oslo, Norway) and centrifuged at 500 × g for 30 min at 20°C. The different cell subsets were diluted 1:2 in 0.5 N RPMI 1640. Erythrocytes were lysed with ice-cold isotonic NH4Cl solution for 10 min and centrifuged at 400 × g for 10 min at 4°C. The remaining cells were resuspended in equal volumes of RPMI 1640 (106 cells/ml) in combination with 5% heat-inactivated human serum (BioWhittaker) plus the different stimuli and kept at 37°C for 8 h. After the samples were centrifuged for 10 min at 2,000 × g, the cell pellet was resuspended in 1 ml of RPMI 1640 and immediately stored at −80°C until needed for further analysis.
Statistical analysis.
Data are presented as means ± standard errors (SE) or as individual values. Changes over time were analyzed by one-way analysis of variance. A P value of <0.05 was considered significant.
RESULTS
Endotoxemia in healthy subjects.
Injection of LPS induced a decrease in peripheral blood monocyte counts (Table 1). Granulocyte numbers showed an initial decrease followed by an increase from 2 h onward (data not shown).
TABLE 1.
Effect of LPS administration on monocyte counta
| Time (h) after LPS administration |
Monocyte counts (109/liter) |
|---|---|
| 0 | 0.55 ± 0.4 |
| 1 | 0.05 ± 0.01 |
| 2 | 0.04 ± 0.01 |
| 4 | 0.06 ± 0.01 |
| 6 | 0.29 ± 0.05 |
| 24 | 0.79 ± 0.07 |
Values are means ± SE for seven healthy subjects who received an intravenous injection of LPS (4 ng/kg) at t = 0 h. LPS induced a significant decrease in monocyte counts (P < 0.05 by one-way analysis of variance).
At baseline, uPAR was detectable at the surface of peripheral blood monocytes and granulocytes but not of lymphocytes. LPS administration was associated with a rise in the surface expression of uPAR on monocytes, reaching a 92% ± 46% increase over baseline expression after 6 h (P < 0.05) (Fig. 1, top panel). In contrast, granulocyte uPAR expression did not change after LPS injection, while uPAR remained undetectable on lymphocytes throughout (data not shown).
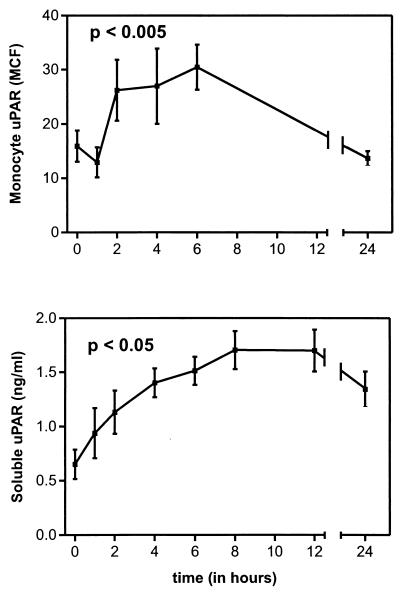
FIG. 1.
Monocyte uPAR (top) and plasma-soluble uPAR (bottom) after intravenous injection of LPS (lot G; 4 ng/kg) into seven healthy subjects (P < 0.005). uPAR expression on monocytes was determined by FACS analysis as described in Materials and Methods. Results are expressed as the difference between specific MCF and nonspecific MCF (means and SE). P < 0.05 for changes over time by one-way analysis of variance.
Soluble uPAR was detectable in the plasma of all seven volunteers at baseline (0.65 ± 0.14 ng/ml). LPS induced a sustained increase in soluble uPAR concentrations, reaching a plateau between 8 and 12 h (8 h, 1.71 ± 0.18 [Fig. 1, bottom panel, P < 0.005]). At 24 h after LPS administration, the soluble uPAR levels were still elevated.
Effect of different bacterial stimuli on uPAR expression on monocytes in whole blood in vitro.
Next we determined whether the effect of LPS on monocyte uPAR expression could be reproduced in whole blood in vitro. LPS was found to enhance uPAR expression on monocytes, with maximum effects detected after 4 or 8 h of incubation (Fig. 2). After stimulation with LPS for 24 h, uPAR expression on monocytes had returned to control levels in three of the four subjects tested. In the LPS concentration range from 1 to 1,000 ng/ml, no clear dose dependency was found. LPS did not have a consistent effect on uPAR expression on granulocytes (data not shown). LPS stimulation of whole blood, isolated neutrophils, or peripheral blood mononuclear cells did not influence soluble uPAR levels (data not shown).
FIG. 2.
LPS upregulates uPAR expression on monocytes in whole blood in vitro. Whole blood was incubated for 4, 8, or 24 h in the presence or absence of increasing concentrations of LPS. Bars represent the difference between specific MCF and nonspecific MCF for four different donors.
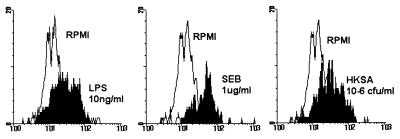
Besides LPS, gram-positive stimuli also increased uPAR expression on monocytes in whole blood in vitro. Indeed, both heat-killed S. aureus and the superantigen SEB enhanced uPAR expression on monocytes (Fig. 3).
FIG. 3.
Upregulation of uPAR expression on monocytes by LPS, heat-killed S. aureus (HKSA), or SEB. Whole blood was incubated for 8 h with or without LPS (1 ng/ml), SEB (1 μg/ml) or heat-killed S. aureus (106 CFU/ml). Data are from one representative experiment from a total of four experiments with four different donors.
Role of TNF in uPAR expression on monocytes.
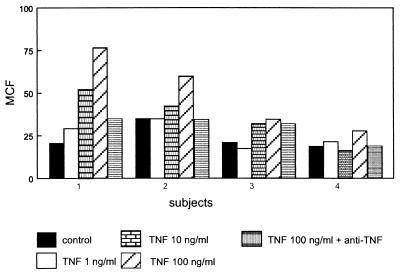
TNF has been implicated as an important mediator of LPS-induced effects in vitro and in vivo (20, 23, 26). Since TNF has been reported to enhance uPAR expression on colon cancer cell lines and monocytes (21, 35), we considered it of interest to determine the role of TNF in the LPS-induced increase in uPAR expression on monocytes. First, we confirmed that recombinant TNF is capable of increasing uPAR levels on monocytes in our whole-blood system, although this TNF effect clearly was less potent than that of LPS. The effect of TNF (100 ng/ml) was specific, since it could be prevented by a neutralizing anti-TNF MAb (10 μg/ml) (Fig. 4). Incubation with TNF did not alter soluble uPAR levels (data not shown).
FIG. 4.
TNF enhances uPAR expression on monocytes in whole blood. Whole blood was incubated for 4 h in the presence or absence of increasing concentrations of recombinant human TNF. Bars represent the difference between specific MCF and nonspecific MCF for four different donors.
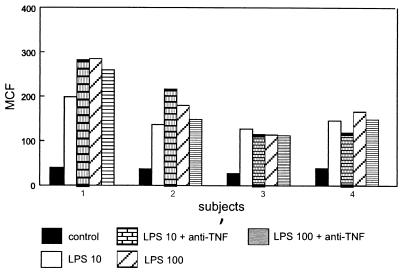
We next assessed the effect of anti-TNF (10 μg/ml) in whole-blood cultures stimulated with LPS (10 or 100 ng/ml). For these experiments, blood was cultured with LPS for 8 h, since (i) this was associated with consistent increases in uPAR expression on monocytes and (ii) this incubation period allows endogenous TNF to reach peak levels in whole blood (23, 30). It was found that anti-TNF did not influence the LPS-induced rise in uPAR levels on monocytes (Fig. 5).
FIG. 5.
Anti-TNF MAb does not influence LPS-induced upregulation of uPAR expression on monocytes in whole blood. Whole blood was incubated for 8 h in the presence or absence of LPS (10 or 100 ng/ml) with or without anti-TNF MAb (10 μg/ml). Bars represent the difference between specific MCF and nonspecific MCF for four different donors.
DISCUSSION
The involvement of uPAR in leukocyte invasion through extracellular matrices is suggested by its expression on a variety of migratory cells and its polarization at the leading edge of migrating monocytes (3, 16, 32). In vitro studies have further indicated that uPAR is crucial for chemotaxis of neutrophils and monocytes, suggesting that uPAR plays an important role in the orchestration of inflammatory reactions (3, 9, 27, 32). However, knowledge of the regulation of uPAR expression during inflammation in vivo is highly limited. We demonstrate here that a single intravenous injection of low-dose LPS induced a transient upregulation of uPAR expression on circulating monocytes in healthy humans. This monocyte response to LPS and to other bacterial stimuli may be a good indication of adequate innate immunity. This LPS effect could be reproduced in whole blood in vitro. Gram-positive stimuli also enhanced uPAR expression on monocytes in whole-blood cultures. Although TNF was capable of upregulating uPAR expression at the surface of monocytes in whole blood, anti-TNF did not influence the LPS-induced increase in uPAR expression on monocytes. These data show for the first time that LPS and presumably other infectious stimuli may directly influence the migratory capacity of monocytes in vivo by exerting an effect on uPAR expression.
The LPS-induced upregulation of uPAR expression on monocytes in vivo coincided with a decrease in monocyte counts. We consider our measurements of uPAR levels on the surface of monocytes reliable, since at each time point at least 103 cells were analyzed. It should be noted that our FACS results applied only for cells that remained in the circulation after LPS injection. However, in our opinion, it is likely that monocytes adhering to endothelium (and thereby disappearing from the circulating pool) also display enhanced uPAR expression after LPS administration, since they presumably reflect cells that have become even more activated than monocytes that do not adhere.
In the present study, FACS analysis was performed on unseparated leukocytes obtained from lysed human whole blood. In accordance with earlier studies using isolated leukocyte subsets, uPAR was expressed on the surface of resting monocytes and granulocytes but not on resting lymphocytes (5, 6, 8, 12, 35). Previous evidence that LPS can upregulate uPAR expression on monocytes was derived from experiments in which isolated human monocytes and monocytic U937 cells were incubated with various concentrations of LPS for up to 24 h (5). In these studies, LPS concentrations in excess of 1 ng/ml stimulated maximal degrees of uPAR expression on cells stimulated for 20 to 24 h. Only a modest increase in uPAR expression was detected after incubations of 6 h (5). Although we also observed no clear dose dependency of the effect of LPS in whole blood in vitro at LPS concentrations exceeding 1 ng/ml, uPAR expression peaked after incubations of 4 to 8 h, presumably reflecting differences in culture conditions (whole blood versus isolated cells). The kinetics of LPS-stimulated uPAR expression on monocytes in whole blood in vitro closely mimicked the kinetics of upregulation of uPAR expression on monocytes after intravenous injection of LPS in humans in vivo, which reached maximal values 6 h postinjection.
To the best of our knowledge, only one earlier study evaluated LPS effects on uPAR expression in vivo (4). Intraperitoneal administration of LPS to mice was found to increase the steady-state levels of uPAR mRNA in most tissues examined, with the greatest induction being detected after 1 to 3 h. Although the uPAR protein levels were not measured, these data are in line with our findings in normal humans exposed to LPS. Remarkably, in mice the increase in uPAR mRNA expression was located primarily in tissue macrophages and lymphocytes (4). We were unable to detect uPAR protein at the surface of circulating lymphocytes at any time point during the study. One possible explanation for the apparent discrepancy with the mouse study is that the increased uPAR mRNA concentrations do not lead to enhanced expression of uPAR at the cell surface. In addition, differences in body compartments (tissue versus peripheral blood lymphocytes) and differences in the LPS dose used (relatively high in the mouse study) may play a role. In this respect, it should be noted that human lymphocytes are capable of upregulating uPAR expression upon activation in vitro (6, 8).
In granulocytes, uPAR is stored in secretory vesicles, distinct from primary and specific granules, and in specific granules (24). Stimulation of isolated granulocytes with phorbol myristate acetate, formyl methionyl leucyl phenylalanine, or TNF in vitro resulted in enhanced expression of uPAR (24). We did not find any effect of LPS on uPAR expression on granulocytes in vivo or in vitro. In addition, gram-positive stimuli had an inconsistent effect on uPAR expression on granulocytes in whole blood in vitro. Therefore, our data suggest that of all the leukocyte subsets in peripheral blood, monocytes are the most sensitive cell type in terms of uPAR upregulation in response to infectious stimuli. Further studies are needed to determine whether granulocytes and/or lymphocytes do respond to infectious stimuli with upregulation of uPAR expression in an inflammatory environment in tissues (which is not mimicked in our studies using blood cells).
TNF did not play a major role in the LPS effect on uPAR expression on monocytes in whole blood in vitro, as indicated by the finding that saturating concentrations of a neutralizing anti-TNF MAb did not alter the upregulation of uPAR expression on monocytes after stimulation with LPS. Conceivably, LPS is able to directly stimulate uPAR expression. It should be noted that although anti-TNF is strongly protective in models of endotoxemia and gram-negative bacterial sepsis (7, 33), TNF is not required for a number of LPS-induced inflammatory responses in healthy humans, including activation of the coagulation system and downmodulation of TNF and interleukin-1 IL-1 receptor expression in monocytes and granulocytes (2, 20).
uPAR belongs to the family of GPI-anchored proteins, of which several members exist in both a membrane-associated form and a soluble form. We have previously found that intravenous LPS induces an increase in expression of soluble CD16, a protein known to be linked to cell membranes by a GPI anchor (36). We demonstrate now that LPS administration also is associated with a rise in the concentrations of soluble uPAR in plasma, which is in line with the finding of elevated levels of soluble uPAR in plasma in patients with sepsis syndrome (25). Other investigators also have reported elevated soluble uPAR concentrations in plasma in patients with paroxysmal nocturnal hemoglobinuria or advanced malignancies (1, 22, 38, 39). The source of soluble uPAR in these patients and in subjects exposed to LPS is unknown, although besides leukocytes, endothelial cells are likely candidates (19). LPS stimulation of whole blood, isolated neutrophils, or peripheral blood mononuclear cells did not induce a detectable release of soluble uPAR. In a previous in vitro study, human umbilical vein endothelial cells released considerable amounts of soluble uPAR after stimulation with phorbol myristate acetate while the monocytic cell lines U937 and HL-60 secreted modest quantities of this soluble receptor (19). The exact function of soluble uPAR remains to be established. It may facilitate the β2-integrin-dependent adhesion of cells and/or the vitronectin-mediated binding of uPA, especially when cells lack membrane-bound uPAR (19, 29). An “enhancing” role for soluble uPAR is not undisputed in the literature, however (11). Indeed, soluble uPAR inhibits uPAR binding to uPAR-expressing cells, conceivably by competing with cell-associated uPAR for the binding of free uPA (19, 37). uPAR plays an important role in leukocyte biology by influencing cell adhesion, chemotaxis, receptor clustering, and changes in cell shape (9). We found that intravenous administration of low-dose LPS induces a transient increase in uPAR expression at the surface of monocytes in peripheral blood. The capacity of LPS to enhance uPAR expression on monocytes was shared with gram-positive bacterial stimuli in whole blood in vitro. These data suggest that bacteria and bacterial products can influence monocyte function in vivo by exerting a stimulating effect on uPAR expression.
REFERENCES
- 1.Abe T, Hasegawa Y, Nagasawa T, Ninomiya H. Excess soluble urokinase-type plasminogen activator receptor in the plasma of patients with paroxysmal nocturnal hemoglobinuria inhibits cell-associated fibrinolytic activity. Int J Hematol. 1997;65:285–291. doi: 10.1016/s0925-5710(96)00559-2. [DOI] [PubMed] [Google Scholar]
- 2.Agosti J, Barbosa K, Coyle S M, Kumar A, Lowry S F, van der Poll T. Down-regulation of surface receptors for TNF and IL-1 on circulating monocytes and granulocytes during human endotoxemia. Effect of neutralization of endotoxin-induced TNF activity by infusion of a recombinant dimeric TNF receptor. J Immunol. 1997;158:1490–1497. [PubMed] [Google Scholar]
- 3.Albrecht E, Brock T G, Gyetko M R, Shollenberger S B, Sitrin R G, Todd III R F, Petty H R. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Investig. 1996;97:1942–1951. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almus-Jacobs F, Loskutoff D J, Sawday M S, Varki N. Endotoxin stimulates expression of the murine urokinase receptor gene in vivo. Am J Pathol. 1995;147:688–698. [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez P A, Brott D A, Liu D Y, Todd R F., III Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J Immunol. 1985;135:3869–3877. [PubMed] [Google Scholar]
- 6.Andreasen P A, Christensen T, Gliemann J, Moller B, Nykjaer A, Petersen C M, Todd R F., III Urokinase receptor. An activation antigen in human T lymphocytes. J Immunol. 1994;152:505–516. [PubMed] [Google Scholar]
- 7.Beutler B, Cerami A, Milsark I W. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi E, Fazioli F, Ferrero E. Integrin-dependent induction of functional urokinase receptor in primary T lymphocytes. J Clin Investig. 1996;98:1133–1141. doi: 10.1172/JCI118896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasi F. uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol Today. 1997;18:415–417. doi: 10.1016/s0167-5699(97)01121-3. [DOI] [PubMed] [Google Scholar]
- 10.Blohm D, Emling F, Möller A, Schlick E, Schollmeier K. Monoclonal antibodies to human tumor necrosis factor: in vitro and in vivo application. Cytokine. 1990;2:162–169. doi: 10.1016/1043-4666(90)90011-h. [DOI] [PubMed] [Google Scholar]
- 11.Bodary S C, Chapman H A, Doyle M V, Lukashev M, Rosenberg S, Simon D I, Wei Y. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 12.Burgal M, Inglés-Esteve J, Jardi M. Distinct patterns of urokinase receptor (uPAR) expression by leukemic cells and peripheral blood cells. Thromb Haemostasis. 1996;76:1009–1019. [PubMed] [Google Scholar]
- 13.Calvano S E, Kumar A, van der Poll T. Endotoxin induces downregulation of tumor necrosis factor receptors on circulating monocytes and granulocytes in humans. Blood. 1995;86:2754–2759. [PubMed] [Google Scholar]
- 14.Camoglio L, Tiel-van Buul M C M, Pajkrt D. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia; the effect of timing of rhIL-10 administration. J Immunol. 1997;158:3971–3977. [PubMed] [Google Scholar]
- 15.Carlos T M, Harlan J M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 16.Carpentier J L, Estreicher A, Mülhauser J, Orci L, Vassali J D. The receptor for urokinase plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990;111:783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman H A, Drummond R J, Rao N, Rosenberg S, Waltz D A, Wei Y. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380–32388. [PubMed] [Google Scholar]
- 18.Chapman H A, Kobzik L, Majdic O, Rao N K, Ronne E, Simon D I, Wei Y, Xu H. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- 19.Chavakis T, Kanse S M, Lijnen H R, Preissner K T, Yutzy B. Vitronectin concentrates proteolytic activity on the cell surface and extracellular matrix by trapping soluble urokinase receptor-urokinase complexes. Blood. 1998;91:2305–2312. [PubMed] [Google Scholar]
- 20.Coyle S M, Levi M, van der Poll T. Effect of a recombinant dimeric tumor necrosis factor receptor on inflammatory responses to intravenous endotoxin in normal humans. Blood. 1997;89:3727–3734. [PubMed] [Google Scholar]
- 21.Dang J, Doe W F, Jones C J, Liang X, Olsen J E, Wang Y. Human urokinase receptor protein expression is inhibited by amiloride and induced by tumor necrosis factor and phorbol ester in colon cancer cells. FEBS Lett. 1994;353:138–143. doi: 10.1016/0014-5793(94)01032-3. [DOI] [PubMed] [Google Scholar]
- 22.Dano K, Eriksen J, Hansen N E, Plesner T, Ploug M. A soluble form of the glycolipid-anchored receptor for urokinase-type plasminogen activator is secreted from peripheral blood leukocytes from patients with paroxysmal nocturnal hemoglobinuria. Eur J Biochem. 1992;208:397–404. doi: 10.1111/j.1432-1033.1992.tb17200.x. [DOI] [PubMed] [Google Scholar]
- 23.DeForge L E, Jones M L, Kenney J S, Remick D G, Warren J S. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992;148:2133–2141. [PubMed] [Google Scholar]
- 24.Ellis V, Plesner T, Ploug M. The receptor for urokinase-type plasminogen activator and urokinase is translocated from two distinct intracellular compartments to the plasma membrane on stimulation of human neutrophils. Blood. 1994;83:808–815. [PubMed] [Google Scholar]
- 25.Faulkner N E, Gyetko M R, Mizukami I F, Sitrin R, Todd R F., III Enzyme-linked immunoabsorbent assay detection of a soluble form of urokinase plasminogen activator receptor in vivo. Blood. 1995;86:203–211. [PubMed] [Google Scholar]
- 26.Fong Y, Moldawer L L, Tracey K J. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1β and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170:1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller J A, Gyetko M R, Standiford T J, Sitrin R G, Todd III R F, Petty H. Function of the urokinase receptor (CD87) in neutrophil chemotaxis. J Leukoc Biol. 1995;58:533–538. doi: 10.1002/jlb.58.5.533. [DOI] [PubMed] [Google Scholar]
- 28.Gadd S J, Kasinrerk W, Majdic O. M5, a phosphoinositol-linked human myelomonocytic activation-associated antigen. Clin Exp Immunol. 1990;80:252–256. doi: 10.1111/j.1365-2249.1990.tb05243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisler R H, Imhof B A, Kanse S M, Lund L R, May A E, Preissner K T. Urokinase receptor (CD87) regulates leukocyte recruitment via β2 integrins in vivo. J Exp Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths J K, van der Meer J W M, Nerad J L. Interleukin-1β, IL-1 receptor antagonist, and TNFα production in whole blood. J Leukoc Biol. 1992;52:687–692. doi: 10.1002/jlb.52.6.687. [DOI] [PubMed] [Google Scholar]
- 31.Gross T J, Gyetko M R, Mizukami I F, Sitrin R G, Sollenberger S B, Todd R F., III Cytokine-specific regulation of urokinase receptor (CD87) expression by U937 mononuclear phagocytes. Blood. 1994;84:1268–1275. [PubMed] [Google Scholar]
- 32.Gyetko M R, Sitrin R C, Todd III R F, Wilkinson C C. The urokinase receptor is required for human monocyte chemotaxis in vitro. J Clin Investig. 1994;93:1380–1387. doi: 10.1172/JCI117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesse D G, Fong Y, Tracey K J. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 34.Kindzelskii H R, Petty A L, Todd III R F, Xue W. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152:4630–4640. [PubMed] [Google Scholar]
- 35.Kirchheimer J C, Nong Y H, Remold H G. IFN-γ, tumor necrosis factor-α and urokinase regulate the expression of urokinase receptors on human monocytes. J Immunol. 1988;141:4229–4234. [PubMed] [Google Scholar]
- 36.Minnema M, Pajkrt D, Stokkers P. Reduction of tumor necrosis factor release by an orally administered metalloproteinase inhibitor (BB 2516) during human endotoxemia. In: Pajkrt D, editor. Modulation of inflammatory responses during human endotoxemia. Academic thesis. Amsterdam, The Netherlands: University of Amsterdam; 1996. pp. 143–150. . Thesis Publishers, Amsterdam, The Netherlands. [Google Scholar]
- 37.Mizukami I F, Todd R F., III A soluble form of the urokinase plasminogen activator receptor (suPAR) can bind to hematopoietic cells. J Leukoc Biol. 1998;64:203–213. doi: 10.1002/jlb.64.2.203. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen H J, Pedersen A N, Stephens R W. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem. 1997;43:1868–1876. [PubMed] [Google Scholar]
- 39.Pedersen N, Ronne E, Schmitt M. A ligand-free, soluble urokinase receptor is present in the ascitic fluid from patients with ovarian cancer. J Clin Investig. 1993;92:2160–2167. doi: 10.1172/JCI116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Deventer S J H, Pajkrt D, van der Poll T. Inflammatory responses during human endotoxemia. In: Vincent J L, editor. Yearbook of intensive care and emergency medicine 1997. New York, N.Y: Springer-Verlag; 1997. pp. 14–30. [Google Scholar]
- 41.van Deventer S J H, ten Cate J W, Jansen J, Pajkrt D, Manten A, Tiel-van Buul M M C, van der Poll T. Modulation of cytokine release and neutrophil function by granulocyte colony-stimulating factor during endotoxemia in humans. Blood. 1997;90:1415–1424. [PubMed] [Google Scholar]