Abstract
T cell-based immunotherapy has demonstrated great therapeutic potential in recent decades, on the one hand, by using tumor-infiltrating lymphocytes (TILs) and, on the other hand, by engineering T cells to obtain anti-tumor specificities through the introduction of either engineered T cell receptors (TCRs) or chimeric antigen receptors (CARs). Given the distinct design of both receptors and the type of antigen that is encountered, the requirements for proper antigen engagement and downstream signal transduction by TCRs and CARs differ. Synapse formation and signal transduction of CAR T cells, despite further refinement of CAR T cell designs, still do not fully recapitulate that of TCR T cells and might limit CAR T cell persistence and functionality. Thus, deep knowledge about the molecular differences in CAR and TCR T cell signaling would greatly advance the further optimization of CAR designs and elucidate under which circumstances a combination of both receptors would improve the functionality of T cells for cancer treatment. Herein, we provide a comprehensive review about similarities and differences by directly comparing the architecture, synapse formation and signaling of TCRs and CARs, highlighting the knowns and unknowns. In the second part of the review, we discuss the current status of combining CAR and TCR technologies, encouraging a change in perspective from “TCR versus CAR” to “TCR and CAR”.
Keywords: immunotherapy, tumor immunology, adoptive T cell therapies, immune synapse, signaling, endosomal trafficking, T cell engineering, chimeric antigen receptor, T cell receptor
1. Introduction
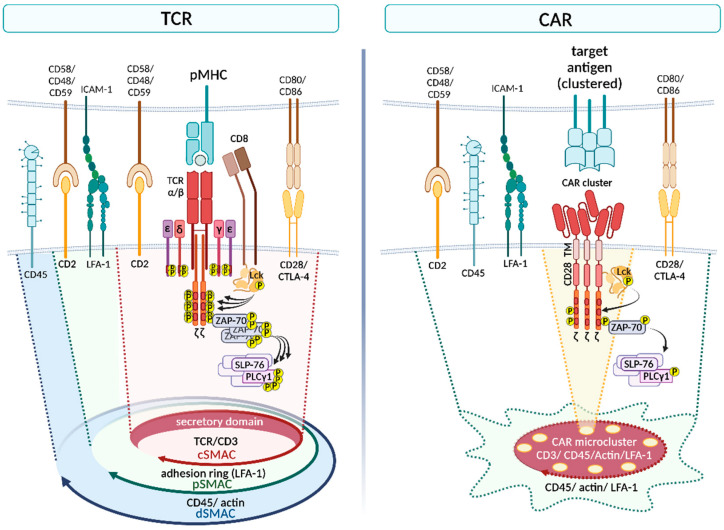
The beginning of adoptive T cell-based immunotherapies goes back to the 1980s when Rosenberg and colleagues successfully demonstrated the anti-cancer potential of tumor-infiltrating lymphocytes (TILs) [1]. Although TILs have shown encouraging results in several clinical trials [2,3,4,5], difficulties were reported in regard to the isolation and manufacturing of tumor-specific TILs [6]. This led to the approach of engineering T cells to express defined tumor-specific receptors, which are generally classified into T cell receptors (TCRs) and chimeric antigen receptors (CARs) [7]. CAR T cell therapy has led to great success in the treatment of hematological malignancies, resulting in the approval of several CAR products by the US Food and Drug Administration (FDA) [8]. However, antigen escape, therapy-associated toxicities and poor efficacy in solid tumors are some of the currently faced challenges in CAR T cell immunotherapy [9]. While transgenic TCRs have shown encouraging results in the treatment of solid tumors [10,11,12,13,14,15,16], the restriction to human leukocyte antigens (HLAs) constitutes a major constraint [17]. In this review, we outline the similarities and differences between TCRs and CARs in regard to architecture, immunological synapse formation and signaling, in order to establish a better understanding of the thereof resulting advantages and limitations. The main differences are summarized in Table 1 and illustrated in Figure 1.
Table 1.
Summary of similarities and differences between TCR and CAR.
| TCR | CAR | ||
|---|---|---|---|
| Structure | Receptor clustering |
One pMHC potentially enough [18,19] |
Clustering required [20,21,22] |
| ITAM number |
10 ITAMs provided by the CD3 complex [23] |
Up to 3 ITAMs per CAR [23] | |
| Affinity/ Sensitivity |
Lower affinity, higher sensitivity |
Higher affinity [24], lower sensitivity [25] |
|
| Phosph. of CD3 subunits |
Phosph. of CD3 ζ, γ, δ, ε [26] | Phosph. of only CD3 ζ [25] | |
| Signaling | Phosph. of signaling molecules |
Stronger phosph. of ZAP-70, ITAMs and PLCγ1 than in CAR [27,28] |
Stronger phosph. of Lck and ERK than in TCR [29] |
| Recruitment of signaling molecules |
More efficient recruitment of ZAP-70, CD2 and LFA-1 than in CAR [27,28] |
Less dependent on LFA-1:ICAM-1 interaction and LAT [21,25,29] |
|
| Upon increased antigen exposure |
Maintain an earlier differentiation phenotype upon strong stimulation [30] |
Higher levels of co-inhibitory molecules upon activation [30] | |
| IS structure | Classical “bull’s eye” structure [29] or multifocal structures formed by Th2 cells [31] or at the interface with DCs [32] | Non-classical, disorganized IS with multifocal pattern [29] | |
| Immunological Synapse | SMACs | Conventional IS consisting of cSMAC, pSMAC and dSMAC [33] |
Merged cSMAC and pSMAC, no adhesion molecule ring [21,29] |
| Lck | One central Lck cluster [29] | Disorganized Lck patches [29] | |
| Duration | Usually slower/weaker effector function [29,30]; longer IS duration, slower off-rate from target [29] |
Faster cytotoxic granule secretion and faster resolution of IS [29] |
|
| Resting state | Constitutive internalization of TCR complex through clathrin-dependent endocytosis (CDE) [34] |
Unknown | |
| Trafficking | Upon activation |
Engaged TCRs: Clathrin- independent endocytosis (CIE) for internalization, recycling or lysosomal degradation [34,35] Bystander TCRs: CDE for internalization and recycling [34,35] |
Engagement of antigens induced rapid lysosomal ubiquitination [36] High-affinity CAR T cells demonstrated enhanced trogocytosis [37] |
Figure 1.
Interface of TCR and CAR, illustrating differences in immunological synapse formation and essential signaling elements (created with BioRender.com).
2. Part I—TCR versus CAR
2.1. TCR versus CAR: Structure
T cells recognize intracellularly processed peptides [38,39,40] that are presented on major histocompatibility complexes (pMHCs) [41] through their endogenous T cell receptor (TCR) [42]. The majority of TCRs in humans are characterized by their heterodimeric architecture, assembled by an alpha and a beta chain (αβTCR), both consisting of a constant and a variable region. A minority of T cells express TCRs composed of a gamma and delta chain (γδTCR) which are not further addressed in this review. The variable region of an αβTCR (in the following only TCR) contains the three loop-forming complementary-determining regions CDR1, CDR2 and CDR3, being the main determinant for binding specificity [43]. Those structurally hypervariable CDRs are flanked by framework regions, which were reported to support CDR conformation. T cells are generally subdivided into CD8+ and CD4+ T cells and bind with their TCR to antigens displayed on class I and class II MHC molecules, respectively [41,44]. In humans, MHC class I and class II are referred to as HLA class I and HLA class II and are further subdivided into different HLA allele groups: HLA-A/-B/-C and HLA-DR/-DQ/-DM/-DP, respectively [45]. Hence, ligand recognition through the TCR is therefore restricted to both a specific peptide sequence and the peptide-presenting HLA molecule [46].
The extracellular domain of a CAR consists most often of an antibody-derived single-chain variable fragment (scFv) [47] but can also be composed of a natural ligand or receptor domain [48]. Similar to TCRs, the scFv variable heavy and light chains both also contain three CDRs flanked by framework residues. Despite the general similarities in terms of architecture and formation through genetic rearrangement of the variable portion of both scFv-based CARs and TCRs [46,49], more detailed studies pointed out that compared to antibodies, TCRs display longer CDR3 loops [50], a higher number of negatively charged amino acids in CDR1 and CDR2 [51] and structurally more variable loops [49]. Besides this, conventional CARs do not target processed proteins presented via MHC molecules but instead bind to unprocessed antigens expressed on the cell surface, including proteins, glycolipids and carbohydrates. With the goal to broaden the range of CAR targets by also including intracellular proteins, some work was also focused on generating pMHC-directed CARs, so-called TCR-mimicking CARs [52,53,54,55,56]. Moreover, several approaches aimed at engineering TCRs attached to antibody-derived antigen-binding domains to achieve HLA independence [57,58,59]. This underlines the steady interchange of new learning between TCRs and CARs, allowing for the implementation of advantageous features at both ends. TCR signaling requires not only engagement with the pMHC but also, with rare exceptions, interaction with the co-receptor CD4 or CD8 [60,61,62] and always an intracellular association with the CD3 complex [63], consisting of the three dimers CD3εδ, CD3εγ and CD3ζζ [64,65]. The cytoplasmic tails of CD3ε,γ,δ and CD3ζ contain one and three immunoreceptor tyrosine-based activation motifs (ITAMs), respectively [26]. ITAM phosphorylation upon TCR engagement with pMHC leads to intracellular signal amplification (more detailed in Section 2.3.1 Proximal Signaling) [26]. Except for CARs harboring a CD3ζ transmembrane domain [66], CARs are usually unable to associate with the endogenous CD3 complex and are therefore equipped with a single CD3ζ signaling domain in their intracellular part. This means that a conventional CAR only displays 3 ITAMs, whereas the TCR/CD3 complex contains a total of 10 ITAMs [26]. It has been postulated that increasing the number of ITAMs can lead to higher sensitivity and potency, thereby minimizing the required number of engaged receptors for an equivalent response [26,67]. However, in vivo studies demonstrated that CD19 CARs harboring a mutated CD3ζ signaling domain with only one functional ITAM located proximally to the membrane and ablation of the second and third ITAMs (1XX) outperformed the standard CARs containing a full CD3ζ chain, while inactivation of the two N-terminal ITAMs (XX3) demonstrated decreased efficiency [68,69]. Despite those advances regarding optimal ITAM positioning and number in the CD3ζ chain, the diverse roles of the other CD3 chains are currently extensively studied, not only in regard to the TCR but also as additional or alternative domains in the CAR [70]. Additional incorporation of the CD3ε chain was, for instance, reported to allow for a more balanced CAR response with enhanced persistence and reduced cytokine secretion, tonic signaling and exhaustion [70].
The first generation of CARs is characterized by an extracellular scFv, linked to a transmembrane domain via a spacer region, followed by the intracellular CD3ζ signaling domain [71]. This build-up was insufficient to produce potent and persistent T cell responses and was further optimized and step-wise extended through additional domains to increase in vivo efficacy. Integration of a co-stimulatory domain derived, e.g., from CD28 or 4-1BB led to the development of second-generation CARs (here referred to as 28ζ and BBζ CAR, respectively) with the ability to produce IL-2 and remain functional upon repeated antigen encounter [72]. Second-generation CARs were the first format approved by the FDA in 2017 [73,74]. Third-generation CARs are characterized by a combination of two co-stimulatory domains, while fourth- and fifth-generation CARs additionally incorporate a transgene for pro-inflammatory cytokine production or a JAK-STAT3/5 pathway-activating domain, respectively [75,76,77]. The idea of providing a CD3ζ domain, a co-stimulatory domain and also cytokines was adopted from natural T cell signaling, which requires three signals for full activation and effector function: first, stable pMHC binding, second, co-stimulation and, third, signaling through cytokine receptors [78].
Up to now, each of the domains in the previously described second-generation CAR has been exchanged or modified, displaying certain characteristics and different effects on functionality [79]. However, one optimal composition or the “magic bullet”, which would be suitable in all settings, has not yet been found [80]. Despite modifications of the CAR endodomains, it was shown that CAR functionality is strongly affected by the length and type of the spacer, linking the transmembrane domain and scFv [81]. Depending on scFv affinity, target epitope location or distance to the cell surface, it was shown that linker length influences CAR T cell activation, tonic signaling, phenotype, migration and overall potency [82,83,84,85]. This leads to the conclusion that every CAR molecule needs to be constructed individually and all of the CAR domains should consequently be adjusted to one another while considering tumor cell type as well as the target antigen size and density [81,82,86,87].
2.2. TCR versus CAR: Activation upon Stimulation
2.2.1. Antigen Engagement for Initiation of Activation
T cell signaling through TCRs is initiated upon recognition of antigenic peptides presented on MHC molecules and is further strengthened through the binding of co-receptors CD4 or CD8 to MHC [88,89]. In contrast, CAR signaling is induced after the engagement of unprocessed antigens in an MHC-independent manner [47]. However, how the antigen recognition by TCRs and CARs is transmitted into intracellular signaling remains incompletely understood. There are three main theories describing signaling mechanisms triggered by the TCR: (1) nanoclustering, (2) kinetic segregation model and (3) mechanosensing. First, despite the fact that a single TCR complex can act as a predominant receptor driving ligand recognition [90], it has been observed that the nanoclustering of TCR is a process of antigen discrimination [91]. This is regulated by the differentiation state of T cells, as memory T cells, which have increased antigen sensitivity compared to naïve T cells and tend to form dense signaling-competent TCR clusters [92]. Second, the kinetic segregation model describes a size-dependent protein segregation mechanism. After T cells encounter a high-affinity cognate antigen, molecules containing shorter ectodomains such as TCRs and co-receptors can still diffuse to the “close-contact zone” at the interface between the T cell and antigen-presenting cell (APC), whereas molecules with longer ectodomains, such as CD45 tyrosine phosphatase, are excluded from the interface. This leads to a change in the kinase:phosphatase balance in the proximity of the TCR complex, thereby initiating TCR phosphorylation [93,94,95]. Third, increasing evidence indicates that the TCR acts as an anisotropic mechanosensor, which can discriminate antigens in a force-dependent manner. TCR:pMHC binding exerts force leading to structural transitions of the TCR complex and local cytoskeleton rearrangement, eventually leading to downstream signaling [96,97,98,99]. These three theories have shed light on different aspects and are not necessarily mutually exclusive. Instead, they could all contribute to kinetic proof-reading, a process of signaling accumulation to discriminate self from foreign ligands prior to dissociation of the TCR:pMHC complex [100]. Compared to TCRs, knowledge about signaling initiation upon CARs still remains to be elucidated. There is evidence suggesting that receptor clustering is an important step to initiate signaling [101] and supporting that the proof-reading kinetics of CARs resemble those of TCRs [102].
The sensitivity of TCRs was described to be up to 100-fold higher than that of CARs [25,103]. Although TCRs are generally characterized by low-affinity binding, it was shown that already a single pMHC complex was enough to trigger TCR signaling [18,19]. In contrast, CAR signaling requires clustering, which consequently means that a higher and sufficient number of antigens needs to be expressed on the target cell surface [20,24,57,104]. Hence, CAR and TCR signaling both depend on the optimal balance between affinity and antigen density. The comparison of CARs and transgenic HLA-independent TCRs, generated to target the same surface antigen, demonstrated that the structure of the TCR and its association with the complete CD3 complex is indeed the key to the higher sensitivity [58,59,105]. More precisely, it is thought that several structural features, such as the number and positioning of ITAMs [26,67], the interaction with the co-receptor CD4 or CD8 [106] and the interaction with co-stimulatory and adhesion molecules such as CD2 and LFA-1 [27], account for the more efficient signal transduction in TCRs [28], resulting in higher sensitivity and a lowered activation threshold [28].
2.2.2. Formation of the Immunological Synapse
The immunological synapse (IS) is the structure at the interface between the T cell and its target cell or the APC, and is formed within minutes of TCR:pMHC or CAR:antigen binding. Upon IS formation, molecular interactions, cytoskeletal rearrangement and dynamic regulation take place, and they are essential for subsequent cell activation.
Typically, the TCR-IS is characterized as a well-organized structure. Kupfer and colleagues named this radially symmetric compartment the supramolecular activation cluster (SMAC), and it consists of three parts. The central part (cSMAC) includes TCR-MHC, intracellular signaling molecules and co-stimulatory (CD28, ICOS, 4-1BB, etc.)/co-inhibitory (PD-1/TIM-3/LAG-3, etc.) interaction clusters. The peripheral ring (pSMAC) surrounds the cSMAC and is formed by adhesion molecules. Finally, the distal ring (dSMAC) contains molecules with larger ectodomains such as CD45 [33]. This “bull’s eye” pattern has been observed in certain T cell subsets including cytotoxic T lymphocytes (CTLs) [107,108], naïve CD4 [109], Th1 cells and when contacting B cells, tumor cells and the artificial planar lipid bilayer. However, “non-classical ISs”, as well as motile kinapse (a transient and dynamic structure at the interface), have also been observed under various conditions [110]. For instance, “multifocal ISs”, characterized by adhesion molecules dispersed among multiple TCR:MHC accumulations, have been reported in Th2 cells [111,112] at the interface between T cells and dendritic cells [32] or upon contact between immature double-positive thymocytes and the artificial lipid bilayer [113]. Thus, the formation of the bull’s eye structure with well-defined cSMACs and pSMACs is not a requirement for T cell activation. Initially, it was proposed that the proper accumulation of TCR-proximal molecules in the cSMAC is required to initiate signaling [114], yet the variations on SMACs indicate that this is not the case. Moreover, phosphorylated signaling proteins such as lymphocyte-specific protein tyrosine kinase (Lck) and Zeta-chain-associated protein kinase 70 (ZAP-70) are found prior to the formation of mature ISs, and the majority of phosphorylated signaling proteins are localized in the pSMAC [33,115]. Using an artificial planar lipid bilayer has demonstrated that small TCR microclusters were firstly found at the periphery of the interface, which was associated with signaling molecules including Lck, ZAP-70, the linker for activation of T cells (LAT) and SH2 domain containing leukocyte protein of 76kDa (SLP-76). Meanwhile, tyrosine phosphorylation and calcium signaling also take place in pSMAC. However, as TCR microclusters migrate toward the cSMAC, TCR-proximal signaling molecules including CD28 and protein kinase C-θ dissociate from the microclusters [116,117], indicating that TCR signaling is initiated and sustained in the pSMAC. In addition, rearrangement of the actin cytoskeleton also occurs during IS formation [118,119], which is required for the centripetal movement of microclusters, as interfering with actin rearrangement through myosin IIa inhibition led to diminished proximal TCR signaling including the phosphorylation of ZAP-70 and LAT, indicating that actin polymerization is essential for proximal signaling [120]. Thus, cSMAC may rather play a role in the downregulation of signaling. Cemerski and colleagues’ study revealed that, depending on the stimulation strength, both signaling initiation and downregulation can occur in cSMAC [121]. Upon stimulation of weak agonists, signaling from cSMAC can be detected shortly after signaling events from pSMAC occur [121]. Indeed, accumulating evidence supports the idea that cSMAC plays a dual role in both sustaining and terminating the TCR-dependent signaling [119,122]. Cbl-b, a ubiquitin ligase and strong ubiquitin signal were found to concentrate in the cSMAC, supporting that cSMAC is associated with the internalization and degradation of the TCR complex [123]. Moreover, cSMAC formation is important for the cytolytic function of CTLs, meaning site-directed secretion of cytolytic granules to target the membrane through the polarization of the microtubule cytoskeleton [108,124].
It has been proposed that the quality of CAR-IS can predict CAR T cell efficiency [125]. However, in contrast with TCR-IS, CAR-IS is not well studied along with a lot of open questions needed to be addressed. Davenport and colleagues performed a cell-based side-by-side comparison of IS formation between HER2-28ζ CAR and OTI-TCR on CD8+ CTLs [29]. They observed a disorganized CAR-IS structure with a multifocal pattern, where elements of cSMAC and pSMAC are not separated but rather merged together, and no ring structure of adhesion molecules surrounding CAR clusters was detected in CAR-IS. This might be due to the fact that the ectodomain is larger in CARs than in TCRs [126]. In addition, both proximal (Lck and ZAP-70) and distal (ERK, cytotoxic granule delivery) signaling was induced and decreased faster in CAR-IS than TCR-IS, and the CAR-IS formation was less dependent on LFA-1:ICAM-1 interaction as compared to the TCR. The non-classical CAR-IS structure was further confirmed using an artificial planar lipid bilayer and a third-generation CAR (CD19-28-BBζ) [21], where segregation of CD45 from CAR clusters was observed. Moreover, they showed that LAT, an essential scaffold protein for TCR signaling, is not necessarily required for the downstream SLP-76 phosphorylation. In addition, the overall binding strength, or the avidity between the CAR and its target, is a critical factor determining CAR T cell response. Differential requirement of avidity has been demonstrated by comparing a liquid tumor (CD19+ or BCMA+) and glioblastoma. Unlike a liquid tumor, engagement of the IFNγ receptor was required for treating a solid tumor [127], indicating that a certain avidity threshold needs to be reached for sufficient CAR T cell response. However, it is still not clear whether these findings are unbiased or based on certain bias introduced by model selection and assay design. Indeed, further investigation is needed to elucidate how other molecules such as co-stimulatory/co-inhibitory elements are involved in CAR-IS formation or stabilization, whether different IS structures are formed in certain T cell subsets and how the cytoskeleton is regulated and its consequence. As the cSMAC of TCR-IS plays a dual role in both sustaining and terminating signaling, it is important to study the impact of cSMAC absence in CAR-IS.
2.3. TCR versus CAR: Signaling Cascade
Signaling events are triggered upon antigen engagement and during immunological synapse/kinapse formation. The subsequent signaling cascades then lead to the activation of T cells. The utilization of TCR intracellular pathways to drive T cell activation is the fundament of CAR design. As the TCR/CD3 complex and the conventional CARs both have the CD3ζ intracellular domain, it is expected that some signaling cascades are shared for T cell activation and to induce a desired anti-tumor response. However, differences in signaling prevail due to the artificial constitution of CARs [23,128].
2.3.1. Proximal Signaling
TCR signaling is largely initiated by a set of protein tyrosine kinases (PTKs) including the Src family tyrosine kinases Lck and Fyn. Lck is basally active [129], and it exists in soluble, membrane-anchored and coreceptor-bound forms [130,131]. After antigen engagement, Lck is recruited to the TCR complex and phosphorylates the ITAMs of CD3 subunits [129,132]. ITAM phosphorylation leads to the recruitment of ZAP-70 and subsequently phosphorylation and activation of ZAP-70. Once phosphorylated, ZAP-70 is able to initiate a series of phosphorylation cascades resulting in the assembly and activation of signaling complexes, which are important for propagating the TCR/PTK signal into late signaling outcomes. Two adaptor proteins are the most important targets of phosphorylated ZAP-70: the LAT and SLP-76 [133]. When phosphorylated by ZAP-70, LAT and SLP-76 can bind to specific signaling proteins through SH2 domains and form oligomeric signalosomes [134,135]. Multiple signaling proteins can be recruited to phosphorylated LAT, including phospholipase C gamma (PLCγ), the adapter growth factor receptor-bound protein 2 (GRB2) and GRB2-related adapter downstream of Shc (Gads). In addition, SLP-76 is indirectly associated with phosphorylated LAT through Gads. Recruitment of PLCγ activates the calcium and Ras/MAPK pathway [136], whereas the multivalent interactions between LAT, Gads and SLP-76 enable the reversible assembly of a protein cluster or the LAT signalosome, which enhances actin polymerization [137,138,139] and triggers Ras, Rac and Rho GTPas activation. In addition, TCR and CD28 co-stimulatory molecules activate the PI3K pathway, which is also associated with LAT and SLP-76 [140] and leads to calcium (Ca2+) influx and subsequent activation of the NFAT pathway.
Although signaling elements for proximal signaling of CARs and TCRs are similar, they differ in some aspects due to their artificial design in combining activating and co-stimulatory elements in one construct. Furthermore, there is a different requirement in antigen engagement, since TCRs have higher sensitivity to antigens than CARs, even though CARs have significantly higher affinity to antigens than TCRs [24], which has been shown to be associated with less efficient Lck recruitment and recycling to CARs than to TCRs [28]—probably due to the high redundancy of ITAMs in the TCR complex and less efficient CD4/CD8 coreceptor recruitment to CARs. Conventional CARs have the CD3ζ intracellular domain containing three ITAMs to transduce the signal for T cell activation. However, whether this finding can be translated to other systems remains to be elucidated. Upon the antigen binding of CARs, ZAP-70, SLP-76 and PLCγ are phosphorylated [126,141]. In contrast with TCRs, LAT might not be essential for CAR signaling as the loss of LAT had no impact on microcluster formation, actin remodeling and downstream signaling [21]. Depending on the CAR design, differences in kinetics and signaling magnitude have been observed. By comparing 28ζ and BBζ CARs targeting CD19 and ROR1, 28ζ CARs induced more rapid and intense phosphorylation of signaling intermediates and demonstrated a more effector-cell-like phenotype than BBζ CARs, which can be explained by the increased basal phosphorylation of the CD3ζ domain as well as greater Lck recruitment to 28ζ CARs [141]. Moreover, BBζ CARs have been shown to recruit the Themis-SHP1 phosphatase complex, which attenuates phosphorylation signaling [142].
2.3.2. Downstream Signaling and Outcome
In principle, TCR and CAR downstream signaling is supposed to be similar, since the goal of introducing CARs is to redirect T cell specificity against the tumor and induce T cell effector function including proliferation, differentiation, and cytotoxicity through lytic granules and production of cytokines to achieve tumor clearance and sustained control [143]. However, optimal T cell activation requires not only signaling from the TCR receptor (signal 1) but also signaling from co-stimulatory molecules such as CD28 (signal 2) to prevent anergy, as well as soluble molecules such as cytokines to obtain full effector function (signal 3). Cell response is a consequence of signaling interplay across different pathways. Given the modular architecture of CARs and depending on the signaling domains integrated into the CAR, the signaling outcome can be remarkably different. It has been confirmed by different studies that changes in the signaling domain can lead to various CAR T cell responses with the mechanisms not fully understood to date. Here, we summarize the main downstream signaling pathways for T cell activation and modulation of the TCR and the corresponding knowledge about CARs.
2.3.3. Calcium/NFAT Pathway
The second messenger Ca2+ plays an important role in T cell activation. Phosphorylated PLCγ cleaves phosphatidylinositol bisphosphate into inositol triphosphate (IP3) and diacylglycerol. IP3 then binds to the IP3/Ca2+ channel on the endoplasmic reticulum leading to Ca2+ release from the endoplasmic reticulum to the cytosol and promoting Ca2+ influx from extracellular through calcium-release-activated Ca2+ channels (CRAC) with a process termed store-operated Ca2+ entry (SOCE). The elevated Ca2+ can enable variable T cell effector functions in a magnitude- and duration-dependent manner [144]. Upon transient cytosolic Ca2+ increase, T cell motility, release of cytolytic granules by CTLs [145,146] and mitochondria translocations are induced [147]. Prolonged Ca2+ signaling can lead to the activation of the nuclear factor of activated T cells (NFAT), a key transcriptional regulator of the IL-2 gene and other cytokine genes, as well as subsequent cellular response including proliferation, cytokine production and differentiation [148,149,150,151].
A recent study on CD19-BBζ CAR T cells revealed the benefit of inhibiting calcium influx by using SOCE inhibitor BTP-2 both in vitro and in vivo. As calcium signaling was hyperactivated via sustained tonic signaling in these CAR T cells, treatment with BTP-2 rendered CAR T cells less exhausted and terminally differentiated with a metabolic profile of downregulated glycolysis [152].
2.3.4. Ras/ERK/AP-1 and NF-κB Pathway
Diacylglycerol and cytosolic Ca2+ induce the activation of protein kinase C. Diacylglycerol can activate Ras guanyl-nucleotide-releasing proteins (RasGRPs) either directly through recruitment mechanisms or indirectly through PKC-mediated phosphorylation [153]. Subsequently, RasGRPs and SOS activate the MAPK/ERK pathway which then induces the activation of the transcription factor activator protein-1 (AP-1), a transcriptional complex formed by c-Jun and c-Fos, as well as B-cell lymphoma 2 (Bcl-2), which are involved in the cell cycle, cytokine production and cell apoptosis [154].
On the other hand, activated protein kinase C-ζ phosphorylates caspase recruitment domain-containing membrane-associated guanylate kinase protein-1 (CARMA1) leading to the recruitment of B cell lymphoma 10 (BCL10), mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1) and tumor necrosis TNF receptor associated factor 6 (TRAF6) and the formation of the CARMA1-BCL10-MALT1 complex [155,156]. This complex activates the IκB kinase (IKK) complex and leads to subsequent phosphorylation and ubiquitination of IκB and release of nuclear factor-κB (NF-κB), which regulates numerous genes critical for survival, proliferation, differentiation and cytokine production [157,158].
CARs with 4-1BB as a co-stimulatory domain have outperformed CD28 co-stimulated CARs in persistence and enrichment in a central memory-like state, indicating that distinct proliferation and survival signals are mediated by these two molecules [159,160]. Indeed, it has been shown only BBζ CARs activated noncanonical NF-κB (ncNF-κB) signaling after ligand engagement, and interfering with this pathway resulted in the reduced expansion and survival of CD19-BBζ T cells as well as accumulation of pro-apoptotic protein Bim [161], providing new possibility in manipulating CAR T cell responses.
2.3.5. PI3K/AKT/mTOR Pathway
The phosphoinositide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of the rapamycin (mTOR) pathway is a key regulator of cell proliferation. P85 is a subunit of PI3K, which can associate with both LAT and SLP-76 [162,163] and triggers activation of the PI3K pathway [140]. In addition, upon ligation of CD28 to B7.1 and B7.2 molecules, CD28 is phosphorylated by Src family tyrosine kinase, leading to the binding and activation of AKT by p85 and subsequent mTOR activation [164]. In addition, PI3K and Akt pathways are required for T cells to increase their glycolytic rate upon stimulation [165].
Studies on CAR signaling have demonstrated a more rapid and stronger early response of 28ζ CARs associated with PI3K signaling and signal transducer and activator of transcription 3 (STAT3) as compared to BBζ CARs [126,141], which might be due to differentiation of 28ζ CAR T cells to the short-lived, terminally differentiated effector state. In addition, in solid tumors, IL-2 secretion from 28ζ CAR T cells has been shown to promote Treg proliferation and thereby suppress the CAR T cell response. Modification on the Lck binding moiety in the intracellular part of CD28 demonstrated reduced IL-2 production and enhanced antitumor activity in the presence of Tregs [166]. 28ζ CAR bearing this modification outperformed BBζ CAR against prostate cancer [167], suggesting that excessive CD28-derived proximal signaling limited CAR T cell persistence, but adequate CD28 signaling could be a better option compared to 4-1BB in specific cases.
2.3.6. Endosomal Trafficking and Lysosomal Degradation
Maintenance of a certain surface expression level of TCR complexes and CARs is critical for a sustained T cell response and is regulated by endocytosis, a process by which cells absorb external material by engulfing it with the cell membrane and is involved in the recycling and degradation of receptors. Coordinated by different Rab GTPases, several mechanisms of endocytosis have been characterized, which can be divided into two categories: clathrin-dependent endocytosis (CDE) and clathrin-independent endocytosis (CIE) [168]. The CDE pathway has been comprehensively analyzed with clathrin and its adaptor AP2 as major players [169]. Five CIE-independent pathways have been proposed including FEME (fast endophilin-mediated endocytosis), caveolae-associated endocytosis, CLIP/GEEC endocytosis and Arf6-mediated and flotillin-mediated endocytosis [34]. In contrast with CDE, processes involved in CIE remain to be fully elucidated. The internalized vesicles undergo homotypic fusion to form early endosomes (EEs); the EEs accumulate and subsequently form the sorting endosome, where the cargos are selected for recycling, lysosomal degradation or the trans-Golgi network [34]. Depending on the condition, the same receptor often conducts several internalization pathways at once [170,171]. Internalization is an essential process to regulate T cell response, as its deficiency could lead to hyperactivation and rapid exhaustion of T cells [123]. The molecular mechanisms of TCR endocytosis have been under intensive investigation, whereas studies about CARs are emerging.
In the resting state, constitutive internalization and recycling of the TCR complex take place, with CDE as the main mechanism. AP-2 is recruited and binds to AP-2 binding motifs on the ITAMs of CD3 subunits, with higher efficiency to CD3δ [172], or to the di-leucine motif present on the intracellular domain of the CD3γ chain [173,174]. Upon activation, internalization of both antigen-engaged and bystander TCRs is induced. Bystander TCRs convey CDE for internalization and recycling [175], where the cargos are delivered to recycling endosomes for return to the plasma membrane. On the other hand, CIE has been identified as the main pathway of internalization for engaged TCRs, which guides engaged TCRs to late endosomes (LEs), at least partially, for lysosomal degradation [35,176]. Thus, it has been proposed that the balance between recycling and degradation seems to be regulated by the strength of activation [34]. However, which CIE mechanisms are specifically involved in TCR internalization remains unclear. It has been demonstrated that TC21 (Rras2) is associated with TCR and is necessary for TCR internalization from the IS through a RhoG-dependent mechanism [177]. Lysosomal degradation of the receptor is carried out through TCR ubiquitination, which requires two RING finger E3 ubiquitin ligases c-Cbl and Cbl-b, ubiquitous Rab GTPases, and recruitment of endosomal sorting complexes required for transport (ESCRT) [177]. In addition, TC21 and RhoG have also been shown to play a role in TCR trogocytosis [177], a process by which plasma membrane fragments from target cells or APCs are transferred to lymphocytes [178]. Initially, TCR internalization was considered to terminate TCR signaling. However, increasing evidence support the notion that the internalized receptor continues to signal from specialized endosomes and is critical to sustain TCR signaling [179,180] suggesting a dual role of TCR endocytosis [181].
The trafficking of CARs during activation has been investigated by Li et al. on the CD19-BBζ CAR, and it was shown that engagement of tumor antigens induced rapid ubiquitination of CARs, causing CAR downmodulation followed by lysosomal degradation. By mutating all lysine in the cytoplasmic domain of the CAR to arginine, they could successfully repress CAR degradation while enhancing the recycling of internalized CARs to the plasma membrane. This approach has demonstrated both in vitro and in vivo to promote long-term killing capacity and persistence, and to maintain CAR T cells in a less differentiated state with a metabolic profile enriched in oxidative phosphorylation. This finding also underscores the difference between TCRs and CARs in endosomal trafficking and regulation of signaling. A very recent study has shed light on the relevant mechanism, and a correlation between scFv affinity and trogocytosis has been demonstrated, where low-affinity CAR T cells seemed to be more beneficial as compared to high-affinity CAR T cells for prolonged persistence, as antigen transfer by trogocytosis and subsequent fratricide was reduced by low-affinity CAR T cells [37].
3. Part II—A Change in Perspective: From “TCR versus CAR” to “TCR and CAR”
While the previous section was focused on side-by-side comparing TCRs and CARs in order to better understand their mechanism of action and to find possible solutions for current challenges and limitations, the following section serves as a change in perspective: from “TCR versus CAR” to “TCR and CAR”. Indeed, during the last five years, more and more studies aimed at combining TCR and CAR technologies, on the one hand, to understand potential interactions and dependencies between TCR and CAR and, on the other hand, as an approach to strengthen the overall anti-cancer T cell response. The latter is based on the hypothesis that in a combinatorial approach, TCR and CAR could counteract their limitations and eventually harmonize through a synergistic interplay.
3.1. Combination of an Endogenous TCR and a CAR
The crucial role of the native TCR for CAR T cell signaling and efficacy was already discussed more than ten years ago [66]. More recent studies have shown that knock-out of the endogenous TCR resulted in reduced persistence of CD19-BBζ CAR T cells in vivo, although early CAR T cell response and signaling were not affected by the lack of the native TCR [182]. On a side note, other studies demonstrated that a lack of the endogenous TCR upon CAR knock-in into the TCR locus even resulted in improved functionality [183]. It was hypothesized that stimulation of the endogenous TCR through xenogeneic mouse tissue (displayed by clinical signs of GvHD) or infectious stimuli, might cause physiological activation of the TCR-positive CAR T cell, thereby prolonging survival and tumor control [182]. Instead of removing the native TCR, Cliona M. Rooney’s group aimed for the opposite: actively engaging and activating the native TCR in CAR T cells by using virus-specific T cells as a source for CAR T cell manufacturing [184,185,186,187]. After it was shown that in vitro stimulation of virus-specific TCRs in CAR T cells prolonged survival and was superior compared to polyclonal activation through CD3-specific antibodies [184], this combination of native TCR plus CAR stimulation was soon implemented in several clinical trials (NCT00085930 [188], NCT00840853 [186,189]). Multi-virus-specific CD19-28ζ CAR T cells were shown to rapidly expand in a virus-load-dependent manner, and CAR T cell proliferation was significantly lower in patients without pre-existing EBV, CMV or adenovirus infection [186]. On a side note, this clinical study was successfully realized without prior lymphodepletion, and not even the two HLA-mismatched patients experienced GvHD, which was explained by the strongly reduced TCR repertoire of the infused CAR T cells. The presence of virus infection and stimulation of the native, virus-specific TCR were hence key to therapeutic success, and instead of leaving it to chance, subsequent approaches aimed at controlled, vaccination-induced native TCR activation in CAR T cells [185,186]. It was reported that in vitro vaccination with VZV peptide mix-loaded DCs even helped to partially recover third-generation GD2-28-OX40ζ CAR T cells, which were already exhausted through a previous CAR target antigen encounter [185]. This is particularly surprising given the fact that repetitive signaling via the native TCR or a CAR is generally thought to cause chronic exhaustion, caspase activation and programmed cell death [190]. The most obvious approach to achieve recovery of exhausted CAR T cells would consequently be to reduce signaling, instead of adding a second stimulus such as via the endogenous TCR. In line with that, the FDA-approved Src kinase inhibitor dasatinib was, for instance, successfully applied to enforce a transient rest in CAR signaling, thereby inducing epigenetic reprogramming and restoring functionality of exhausted CAR T cells [191]. Recent work has shown that the revival of exhausted CAR T cells via the endogenous TCR seems to be dependent on several factors:
First, it was observed that the outcome of native TCR activation was dependent on the CAR structure. Native TCR engagement in virus-specific 4-1BB-bearing CAR T cells led to stronger activation marker expression than in CD28 co-stimulated CAR T cells, ultimately causing TCR downregulation and apoptosis [187]. In accordance with this, several studies showed the beneficial effect of stimulating the virus-specific endogenous TCR in CAR T cells containing a CD28 co-stimulatory domain, verified for different scFvs and also for second- and third-generation constructs [185,186,187]. This is especially surprising as CD28 co-stimulated CARs are generally linked to stronger and more rapid effector function, while 4-1BB is thought to promote more durable CAR T cell response and long-term persistence [159,160,192]. Nonetheless, expression of transgenic full-length CD28 was shown to inhibit activation-induced cell death (AICD) through the upregulation of anti-apoptotic proteins and suppression of CD95L [193,194]. This anti-apoptotic effect was also reported for CARs, co-stimulated through CD28 [195].
The second factor, which seems to influence CAR T cell re-activation during concomitant native TCR stimulation, is the level of TCR antigen and the type of stimulus. In two different syngeneic mouse models, it was observed that dual activation of a CAR and endogenous TCR, led to the upregulation of pro-apoptotic and inhibitory receptor genes in CD8+ T cells, consequently resulting in exhaustion and strongly reduced tumor clearance. The CD4+ subset demonstrated increased expansion, although persistence of CAR T cells was not increased [196]. Before jumping to conclusions regarding the role of the T cell subset, it is important to have a closer look into the type of TCR stimulus. Since this in vivo work was performed using CD19-28ζ CAR T cells with TCR specificity for HY (male minor histocompatibility antigen) or ovalbumin [196], it was hypothesized that the continuous expression of those non-viral TCR antigens might have caused more repetitive stimulation and consequently the observed exhausted phenotype [196,197]. On the contrary, the previously mentioned studies [184,185,186,187] aimed at engaging the native TCR through vaccination, e.g., via virus-peptide-pulsed DCs or pre-existing virus, instead of endogenous antigen. The very latest study in this context demonstrated that also oncolytic viruses can be used for the stimulation of CD8+ CAR T cells via the native TCR. CAR T cells loaded with vesicular stomatitis virus or reovirus exhibited enhanced trafficking, infiltration, persistence and functionality in vivo [197]. Additionally, epitope spreading was stated to cause further expansion of the endogenous tumor-specific potential, thereby protecting against re-challenge with CAR target-negative tumor cells. Similar findings were reported in the context of extensive in vivo studies with transgenic mice, expressing HER2-28ζ CAR and gp100 TCR [198]. The latter was specifically activated through gp100-encoding vaccinia virus, thereby significantly increasing the CAR T cell expansion, persistence and the cytotoxic potential against HER2-expressing target cells. Furthermore, long-term surviving mice also developed immunity against tumor antigens other than HER2, which was attributed to epitope spreading and memory formation.
Finally, recent studies indicate that the early-differentiation status during CAR T cell manufacturing might exhibit a determining factor, as native TCR stimulation through pre-existing virus infection has been shown to be advantageous only in virus-specific CAR T cells displaying a central memory but not an effector memory phenotype [186,189]. This is in line with the generally accepted characteristics of central memory T cells, namely the robust proliferative potential and the long-term persistence [199,200,201]. However, as previously described, functional recovery of already exhausted, and consequently terminally differentiated, CAR T cells was also successfully achieved in vitro using peptide mix-loaded DCs [185]. This contradictory result might have again been influenced through the type of TCR stimulus used.
The discussed studies (summarized in Table 2) underline the potential of dual stimulation through CARs and native TCRs, not only raising the question of whether CAR T cells could also be activated through vaccination targeting a transgenic TCR but also, in general, whether there is a beneficial or even synergistic effect of combining a CAR with a tumor-cell-targeting transgenic TCR.
Table 2.
Overview of studies combining endogenous TCR and CAR.
| Combination of Endogenous TCR and CAR | |||||
|---|---|---|---|---|---|
| CAR | TCR Specificity |
TCR Stimulus |
Study | Main Result | Citation |
| GD2-ζ | EBV- specific TCR |
Patients with EBV pre-infection | NCT000 85930 |
Prolonged survival and expansion compared to anti-CD3 antibody activation |
Pule et al., 2008 [184] Louis et al., 2011 [188] |
| CD19-28ζ | EBV- specific TCR |
EBV-transformed lymphoblastoid B cell lines | NCT008 40853 |
Stimulation of native TCR increased CAR T cell expansion; T cells were donor-derived after allogeneic HSCT (no GVHD) |
Cruz et al., 2013 [189] |
| GD2-28-OX40ζ | VZV- specific TCR |
VZV peptide mix-loaded DCs | In vitro | Exhausted and dysfunctional CAR T cells recovered upon stimulation of native TCR | Tanaka et al., 2017 [185] |
| CD19-28ζ | HY- specific TCR |
Male bone-marrow-derived cells (HY) | In vivo | Dual stimulation led to exhaustion and apoptosis in CD8+ (not in CD4+) CAR T cells | Yang et al., 2017 [196] |
| Her2-28ζ | gp100- specific TCR |
Recombinant vaccinia virus encoding gp100 | In vivo | Increased expansion, persistence, tumor infiltration and functionality upon native TCR stimulation | Slaney et al., 2017 [198] |
| (1) GD2-ζ (2) GD2-28ζ (3) GD2-BBζ |
VZV-/EBV- specific TCR |
VZV or EBV peptide mix-loaded DCs | In vitro | TCR stimulation led to increased expansion and functionality in GD2-28ζ (but not in GD2-BBζ) CAR T cells | Omer et al., 2018 [187] |
| CD19-28ζ | EBV- specific TCR |
Patients with EBV pre-infection | NCT008 40853 |
Virus load-dependent increase in CAR T cell expansion |
Lapteva et al., 2019 [186] |
| EGFRvIII-28-BBζ | Oncolytic VSV or reovirus |
Oncolytic virus co-administered with CAR T cell | In vivo | Enhanced trafficking, infiltration and functionality; long-term effects through in vivo reactivation with TCR-directed oncolytic virus | Evgin et al., 2022 [197] |
3.2. Combination of a Transgenic TCR and a CAR
As reviewed in the previous section, the approach of dual native TCR and CAR stimulation primarily aimed at increasing efficacy and persistence of the CAR T cells, while the paramount objective of combining a transgenic TCR and a CAR has been targeting multiple antigens and hence reducing the risk for immune escape [202,203]. The relevance of this is underlined by the fact that although CD19-targeting CAR T cell therapy has led to extremely promising responses, relapse was observed in half of the patients, primarily due to loss of CD19 surface expression [7]. As hypothesized by Carl June, the likelihood of tumor escape through both downregulation of the MHC and loss of the CAR target antigen is expected to be very low, which ultimately means that in dual-specific T cells at least either one, transgenic TCR or CAR, would still work [204]. In 2016, it was shown for the first time that a CAR and a transgenic TCR can be functionally co-expressed in the very same T cell [202]. The CD8+ T cells, which were transiently transfected with a CSPG4-28ζ CAR and a gp100 TCR, led to antigen-specific cytokine secretion and target cell lysis in vitro. The functionality was similar to CAR- or TCR-only T cells, suggesting that co-expression of the CAR and the transgenic TCR did not cause reciprocal inhibition. It is important to highlight that simultaneous stimulation of the CAR and the transgenic TCR resulted in increased cytotoxicity. Interestingly, this effect was shown to be dependent on the co-existence of both receptors on the very same T cells, as this was not achieved with a 1:1 mixture of CAR T cells and TCR T cells (normalized to the total cell count). On the contrary, subsequent work with dual-specific T cells expressing the very same receptors demonstrated reduced cytolytic activity compared to T cells only expressing CSPG4 CAR or gp100 TCR [203]. It is not clear whether this opposed outcome was due to the different types of TCR delivery (lentiviral transduction instead of transient-electroporation-based transfection) or to general variations in the experimental set-up such as the use of another target cell line. However, both studies showed that dual-specific CAR- and transgenic-TCR-expressing T cells specifically recognized and lysed single- and double-positive target cells, representing a potential solution against tumor escape.
Furthermore, several studies demonstrated the beneficial effects of combining a transgenic TCR with a non-classical, modified CAR [205,206,207]. In vivo experiments, for example, displayed that expansion and efficacy of NY-ESO-1 TCR-transduced T cells can be enhanced through co-expression of scFv-lacking 4-1BBζ CAR [205]. In this regard, it was hypothesized that the CAR, albeit not engaging any target antigen, induced a certain trans co-stimulation, thereby enhancing transgenic TCR signaling. Another study demonstrated that the serial killing potential and in vivo anti-tumor response through both native or transgenic TCR was increased by co-expression of CD19 CARs with 4-1BB co-stimulation but lacking the CD3ζ signaling domain [207].
To sum this up, the conclusion can be drawn that a CAR and a transgenic TCR cannot only be functionally co-expressed in the very same T cell, thereby enabling dual specificity and counteracting tumor escape, but might also synergize in a mutually reinforcing way. To this end, further studies evaluating the combination of a transgenic TCR and a CAR (summarized in Table 3) will be crucial to deepen the understanding in regard to the influence on signaling, activation, exhaustion and persistence. While therapy-associated toxicities such as cytokine release syndrome and immune effector-cell-associated neurotoxicity syndrome are major risks in CAR T cell immunotherapy, such strong immune activation is generally not expected during treatment with TCR T cells [208,209]. This explains why additive effects of cytokine-related toxicity are unlikely, although the combination of a transgenic TCR and a CAR results in dual and potentially simultaneous stimulation. Therefore, further studies should rather be focused on exhaustion and persistence, instead of abnormal immune activation. However, clinical guidelines for the management and prevention of cytokine storms are well established [210]. Off-target effects due to the mispairing of transgenic and endogenous TCR chains and the potential risk for uncontrolled formation of new specificities were described as safety problems during TCR gene therapy [211]. Knock-out or replacement of the endogenous TCR by the transgenic TCR represent possible solutions, which might also be applied in a combinatorial approach with CARs and transgenic TCRs [183,212,213].
Table 3.
Overview of studies combining transgenic TCR and CAR.
| Combination of Transgenic TCR and CAR | |||||
|---|---|---|---|---|---|
| CAR | TCR Specificity |
CAR/TCR Stimulus |
Study | Main Result | Citation |
| CSPG4-28ζ (transient) |
gp100 (transient) |
Target cell line | In vitro | Functionally co- expressed, without reciprocal inhibition |
Uslu et al., 2016 [202] |
| CSPG4-28ζ (transient) |
gp100 (stable) |
Target cell line | In vitro | Functionally co- expressed; reduced cytotoxicity compared to TCR T cells |
Simon et al., 2019 [203] |
| BBζ (lacking scFv) |
NY-ESO-1 | TCR-target- expressing cell line |
In vitro/ in vivo |
Increased proliferation and tumor regression upon single and repeated TCR stimulation | Miyao et al., 2018 [205] |
| (1) CD19-28 (2) CD19-BB (3) CD19-28-OX40 (lacking signaling domain) |
Survivin | Target cell line | In vitro/ in vivo |
Enhanced apoptosis with CD19-BB CAR; CD19-28-OX40 (not CD19-28) increased repeated killing and prolonged tumor control in vivo |
Omer et al., 2022 [206] |
4. Conclusions
Adoptive T cell therapies represent a powerful tool in modern medicine with a so far unrivaled potential to cure patients suffering from advanced hematological malignancies and solid cancer as shown in several clinical trials (CAR T cell trials NCT02228096, NCT02445248, NCT02348216, NCT03391466; TCR T cell trials NCT03159585, NCT02280811, NCT02992743). Interestingly, in the context of this clinical success, a clear trend emerged quite early demonstrating that inherent features of TILs and TCR-engineered T cells resulted in durable and complete responses when treating solid cancer [11,15,214,215,216], while, for instance, the “poster child for CAR therapies” [217], the CD19-directed CAR, led to complete remissions of >88% when treating leukemia and lymphoma in clinical trials. Therefore, aiming to better understand this discrepancy among both technologies, we specifically discussed the current knowledge about TCR and CAR signaling and encouraged a change in perspective from “TCR versus CAR” to “TCR and CAR” as a next step to further ameliorate the potential of genetically modified T cell therapies by counteracting the limitations of CARs and TCRs, respectively. The generation of such T cell products, however, still requires a deeper understanding of T cell signaling and T cell biology, especially in the context of genetically engineered T cells. Accordingly, further dedicated studies including advanced multi-omics analyses are indispensable to further evolve adoptive T cell therapies toward successful treatment of a broad range of cancers.
Acknowledgments
A.K. is participant in the BIH-Charité Advanced Clinician Scientist Pilotprogram funded by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health. We acknowledge financial support from the Open Access Publication Fund of Charité – Universitätsmedizin Berlin and the German Research Foundation (DFG).
Author Contributions
Conceptualization, K.T., X.W., K.A., A.K. and D.L.; investigation, K.T. and X.W.; writing—original draft preparation, K.T. and X.W.; writing—review and editing, K.T., X.W., D.L., C.E., K.A. and A.K.; visualization, K.T. and X.W.; supervision, D.L., C.E., K.A. and A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg S.A., Spiess P., Lafreniere R. A New Approach to the Adoptive Immunotherapy of Cancer with Tumor-Infiltrating Lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R., et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using Transfer Immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zacharakis N., Huq L.M. Breast Cancers Are Immunogenic: Immunologic Analyses and a Phase II Pilot Clinical Trial Using Mutation-Reactive Autologous Lymphocytes. J. Clin. Oncol. 2022;40:1741–1754. doi: 10.1200/JCO.21.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg S.A., Packard B.S., Aebersold P.M., Solomon D., Topalian S.L., Toy S.T., Simon P., Lotze M.T., Yang J.C., Seipp C.A., et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 5.Wang S., Sun J., Chen K., Ma P., Lei Q., Xing S., Cao Z., Sun S., Yu Z., Liu Y., et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 2021;19:140. doi: 10.1186/s12916-021-02006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A., Watkins R., Vilgelm A.E. Cell Therapy With TILs: Training and Taming T Cells to Fight Cancer. Front. Immunol. 2021;12:690499. doi: 10.3389/fimmu.2021.690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei F., Cheng X.X., Xue J.Z., Xue S.A. Emerging Strategies in TCR-Engineered T Cells. Front. Immunol. 2022;13:850358. doi: 10.3389/fimmu.2022.850358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengsayadeth S., Savani B.N., Oluwole O., Dholaria B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. eJHaem. 2022;3:6–10. doi: 10.1002/jha2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterner R.C., Sterner R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M., et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Wang L. The Emerging World of TCR-T Cell Trials Against Cancer: A Systematic Review. Technol. Cancer Res. Treat. 2019;18:1533033819831068. doi: 10.1177/1533033819831068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K., Goloubeva O., Vogl D.T., Lacey S.F., Badros A.Z., Garfall A., Weiss B., Finklestein J., et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angelo S.P., Druta M., Liebner D.A., Schuetze S., Somaiah N., Tine B.A.V., Tap W.D., Pulham T., Chagin K., Norry E., et al. Pilot study of NY-ESO-1c259 T cells in advanced myxoid/round cell liposarcoma. J. Clin. Oncol. 2018;36((Suppl. 15)):3005. doi: 10.1200/JCO.2018.36.15_suppl.3005. [DOI] [Google Scholar]
- 15.Robbins P.F., Kassim S.H., Tran T.L.N., Crystal J.S., Morgan R.A., Feldman S.A., Yang J.C., Dudley M.E., Wunderlich J.R., Sherry R.M., et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive receptor: Long-term follow-up and correlates with response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins P.F., Morgan R.A., Feldman S.A., Yang J.C., Sherry R.M., Dudley M.E., Wunderlich J.R., Nahvi A.V., Helman L.J., Mackall C.L., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafer P., Kelly L.M., Hoyos V. Cancer Therapy With TCR-Engineered T Cells: Current Strategies, Challenges, and Prospects. Front. Immunol. 2022;13:835762. doi: 10.3389/fimmu.2022.835762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J., Brameshuber M., Zeng X., Xie J., Li Q.-J., Chien Y.-H., Valitutti S., Davis M.M. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013;39:846–857. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykulev Y., Joo M., Vturina I., Tsomides T.J., Eisen H.N. Evidence that a Single Peptide–MHC Complex on a Target Cell Can Elicit a Cytolytic T Cell Response. Immunity. 1996;4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 20.Anikeeva N., Panteleev S., Mazzanti N.W., Terai M., Sato T., Sykulev Y. Efficient killing of tumor cells by CAR-T cells requires greater number of engaged CARs than TCRs. J. Biol. Chem. 2021;297:101033. doi: 10.1016/j.jbc.2021.101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong R., Libby K.A., Blaeschke F., Fuchs W., Marson A., Vale R.D., Su X. Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR) EMBO J. 2020;39:e104730. doi: 10.15252/embj.2020104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L., Brzostek J., Sankaran S., Wei Q., Yap J., Tan T.Y.Y., Lai J., MacAry P.A., Gascoigne N.R.J. Targeting CAR to the Peptide-MHC Complex Reveals Distinct Signaling Compared to That of TCR in a Jurkat T Cell Model. Cancers. 2021;13:867. doi: 10.3390/cancers13040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner S.E., Johnson S.M., Brown C.E., Wang L.D. Chimeric antigen receptor signaling: Functional consequences and design implications. Sci. Adv. 2020;6:eaaz3223. doi: 10.1126/sciadv.aaz3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K., Kuramitsu S., Posey A.D., June C.H. Expanding the Therapeutic Window for CAR T Cell Therapy in Solid Tumors: The Knowns and Unknowns of CAR T Cell Biology. Front. Immunol. 2018;9:2486. doi: 10.3389/fimmu.2018.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salter A.I., Rajan A. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Sci. Signal. 2021;14:eabe2606. doi: 10.1126/scisignal.abe2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kersh E.N., Shaw A.S., Allen P.M. Fidelity of T Cell Activation Through Multistep T Cell Receptor ζ Phosphorylation. Science. 1998;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 27.Burton J., Siller-Farfán J., Pettmann J., Salzer B., Kutuzov M., van der Merwe A., Dushek O. Inefficient exploitation of accessory receptors reduces the sensitivity of chimeric antigen receptors. bioRxiv. 2021 doi: 10.1101/2021.10.26.465853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gudipati V., Rydzek J., Doel-Perez I., Goncalves V.D.R., Scharf L., Konigsberger S., Lobner E., Kunert R., Einsele H., Stockinger H., et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 2020;21:848–856. doi: 10.1038/s41590-020-0719-0. [DOI] [PubMed] [Google Scholar]
- 29.Davenport A.J., Cross R.S., Watson K.A., Liao Y., Shi W., Prince H.M., Beavis P.A., Trapani J.A., Kershaw M.H., Ritchie D.S., et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA. 2018;115:E2068–E2076. doi: 10.1073/pnas.1716266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wachsmann T.L.A., Wouters A.K., Remst D.F.G., Hagedoorn R.S., Meeuwsen M.H., van Diest E., Leusen J., Kuball J., Falkenburg J.H.F., Heemskerk M.H.M. Comparing CAR and TCR engineered T cell performance as a function of tumor cell exposure. Oncoimmunology. 2022;11:2033528. doi: 10.1080/2162402X.2022.2033528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thauland T.J., Koguchi Y., Wetzel S.A., Dustin M.L., Parker D.C. Th1 and Th2 cells form morphologically distinct immunological synapses. J. Immunol. 2008;181:393–399. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brossard C., Feuillet V., Schmitt A., Randriamampita C., Romao M., Raposo G., Trautmann A. Multifocal structure of the T cell—Dendritic cell synapse. Eur. J. Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 33.Freiberg B.A., Kupfer H., Maslanik W., Delli J., Kappler J., Zaller D.M., Kupfer A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 34.Evnouchidou I., Caillens V., Koumantou D., Saveanu L. The role of endocytic trafficking in antigen T cell receptor activation. Biomed. J. 2022;45:310–320. doi: 10.1016/j.bj.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monjas A., Alcover A., Alarcón B. Engaged and bystander T cell receptors are down-modulated by different endocytotic pathways. J. Biol. Chem. 2004;279:55376–55384. doi: 10.1074/jbc.M409342200. [DOI] [PubMed] [Google Scholar]
- 36.Li W., Qiu S., Chen J., Jiang S., Chen W., Jiang J., Wang F., Si W., Shu Y., Wei P., et al. Chimeric Antigen Receptor Designed to Prevent Ubiquitination and Downregulation Showed Durable Antitumor Efficacy. Immunity. 2020;53:456–470.e456.. doi: 10.1016/j.immuni.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Olson M.L., Vander Mause E.R., Radhakrishnan S.V., Brody J.D., Rapoport A.P., Welm A.L., Atanackovic D., Luetkens T. Low-Affinity CAR T Cells Exhibit Reduced Trogocytosis, Preventing Fratricide and Antigen-Negative Tumor Escape While Preserving Anti-Tumor Activity. Leukemia. 2022;36:1943–1946. doi: 10.1038/s41375-022-01585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kettleborough C.A., Saldanha J., Heath V.J., Morrison C.J., Bendig M.M. Humanization of a mouse monoclonal antibody by CDR–grafting: The importance of framework residues on loop conformation. Protein Eng. Des. Sel. 1991;4:773–783. doi: 10.1093/protein/4.7.773. [DOI] [PubMed] [Google Scholar]
- 39.Gaciarz A., Ruddock L.W. Complementarity determining regions and frameworks contribute to the disulfide bond independent folding of intrinsically stable scFv. PLoS ONE. 2017;12:e0189964. doi: 10.1371/journal.pone.0189964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang J., Sha Y., Jia Z., Prasad L., Delbaere L.T. Framework residues 71 and 93 of the chimeric B72.3 antibody are major determinants of the conformation of heavy-chain hypervariable loops. J. Mol. Biol. 1995;253:385–390. doi: 10.1006/jmbi.1995.0560. [DOI] [PubMed] [Google Scholar]
- 41.Rammensee H.-G., Falk K., Rötzschke O. MHC molecules as peptide receptors. Curr. Opin. Immunol. 1993;5:35–44. doi: 10.1016/0952-7915(93)90078-7. [DOI] [PubMed] [Google Scholar]
- 42.Davis M.M., Bjorkman P.J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph M.G., Stanfield R.L., Wilson I.A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 44.Klein J. The major histocompatibility complex of the mouse. Science. 1979;203:516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- 45.Crux N.B., Elahi S. Human Leukocyte Antigen (HLA) and Immune Regulation: How Do Classical and Non-Classical HLA Alleles Modulate Immune Response to Human Immunodeficiency Virus and Hepatitis C Virus Infections? Front. Immunol. 2017;8:832. doi: 10.3389/fimmu.2017.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janeway C.A., Travers P., Walport M., Shlomchik M.J. Immunobiology: The Immune System in Health and Disease. 5th ed. Garland Science; New York, NY, USA: 2001. [Google Scholar]
- 47.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branella G.M., Spencer H.T. Natural Receptor- and Ligand-Based Chimeric Antigen Receptors: Strategies Using Natural Ligands and Receptors for Targeted Cell Killing. Cells. 2021;11:21. doi: 10.3390/cells11010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong W.K., Leem J., Deane C.M. Comparative Analysis of the CDR Loops of Antigen Receptors. Front. Immunol. 2019;10:2454. doi: 10.3389/fimmu.2019.02454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rock E.P., Sibbald P.R., Davis M.M., Chien Y.H. CDR3 length in antigen-specific immune receptors. J. Exp. Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blevins S.J., Pierce B.G., Singh N.K., Riley T.P., Wang Y., Spear T.T., Nishimura M.I., Weng Z., Baker B.M. How structural adaptability exists alongside HLA-A2 bias in the human αβ TCR repertoire. Proc. Natl. Acad. Sci. USA. 2016;113:E1276–E1285. doi: 10.1073/pnas.1522069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarmarkovich M., Marshall Q.F., Warrington J.M., Premaratne R., Farrel A., Groff D., Li W., di Marco M., Runbeck E., Truong H., et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature. 2021;599:477–484. doi: 10.1038/s41586-021-04061-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Zhang G., Wang L., Cui H., Wang X., Zhang G., Ma J., Han H., He W., Wang W., Zhao Y., et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci. Rep. 2014;4:3571. doi: 10.1038/srep03571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H., Xu Y., Xiang J., Long L., Green S., Yang Z., Zimdahl B., Lu J., Cheng N., Horan L.H., et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2017;23:478–488. doi: 10.1158/1078-0432.CCR-16-1203. [DOI] [PubMed] [Google Scholar]
- 55.Rafiq S., Purdon T.J., Daniyan A.F., Koneru M., Dao T., Liu C., Scheinberg D.A., Brentjens R.J. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms Tumor 1 antigen. Leukemia. 2017;31:1788–1797. doi: 10.1038/leu.2016.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuberth P.C., Jakka G., Jensen S.M., Wadle A., Gautschi F., Haley D., Haile S., Mischo A., Held G., Thiel M., et al. Effector memory and central memory NY-ESO-1-specific re-directed T cells for treatment of multiple myeloma. Gene Ther. 2013;20:386–395. doi: 10.1038/gt.2012.48. [DOI] [PubMed] [Google Scholar]
- 57.Baeuerle P.A., Ding J., Patel E., Thorausch N., Horton H., Gierut J., Scarfo I., Choudhary R., Kiner O., Krishnamurthy J., et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 2019;10:2087. doi: 10.1038/s41467-019-10097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y., Liu G., Wang J., Zheng Z.-Y., Jia L., Rui W., Huang D., Zhou Z.-X., Zhou L., Wu X., et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 2021;13:eabb5191. doi: 10.1126/scitranslmed.abb5191. [DOI] [PubMed] [Google Scholar]
- 59.Mansilla-Soto J., Eyquem J., Haubner S., Hamieh M., Feucht J., Paillon N., Zucchetti A.E., Li Z., Sjostrand M., Lindenbergh P.L., et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 2022;28:345–352. doi: 10.1038/s41591-021-01621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davari K., Holland T., Prassmayer L., Longinotti G., Ganley K.P., Pechilis L.J., Diaconu I., Nambiar P.R., Magee M.S., Schendel D.J., et al. Development of a CD8 co-receptor independent T-cell receptor specific for tumor-associated antigen MAGE-A4 for next generation T-cell-based immunotherapy. J. Immunother. Cancer. 2021;9:e002035. doi: 10.1136/jitc-2020-002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehrotra S., Al-Khami A.A., Klarquist J., Husain S., Naga O., Eby J.M., Murali A.K., Lyons G.E., Li M., Spivey N.D., et al. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J. Immunol. 2012;189:1627–1638. doi: 10.4049/jimmunol.1103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams C.M., Schonnesen A.A., Zhang S.-Q., Ma K.-Y., He C., Yamamoto T., Eckhardt S.G., Klebanoff C.A., Jiang N. Normalized Synergy Predicts That CD8 Co-Receptor Contribution to T Cell Receptor (TCR) and pMHC Binding Decreases As TCR Affinity Increases in Human Viral-Specific T Cells. Front. Immunol. 2017;8:894. doi: 10.3389/fimmu.2017.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmadi M., King J.W., Xue S.-A., Voisine C., Holler A., Wright G.P., Waxman J., Morris E., Stauss H.J. CD3 limits the efficacy of TCR gene therapy in vivo. Blood. 2011;118:3528–3537. doi: 10.1182/blood-2011-04-346338. [DOI] [PubMed] [Google Scholar]
- 64.Schamel W.W.A. The stoichiometry of the T cell antigen receptor and its implications for the signal transduction mechanism. Signal Transduct. 2007;7:311–319. doi: 10.1002/sita.200600123. [DOI] [Google Scholar]
- 65.Wucherpfennig K.W., Gagnon E., Call M.J., Huseby E.S., Call M.E. Structural biology of the T-cell receptor: Insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a005140. doi: 10.1101/cshperspect.a005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bridgeman J.S., Hawkins R.E., Bagley S., Blaylock M., Holland M., Gilham D.E. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J. Immunol. 2010;184:6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 67.James J.R. Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. Sci. Signal. 2018;11:eaan1088. doi: 10.1126/scisignal.aan1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feucht J., Sun J., Eyquem J., Ho Y.J., Zhao Z., Leibold J., Dobrin A., Cabriolu A., Hamieh M., Sadelain M. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duan Y., Chen J., Meng X., Liu L., Shang K., Wu X., Wang Y., Huang Z., Liu H., Huang Y., et al. Balancing activation and costimulation of CAR tunes signaling dynamics and enhances therapeutic potency. bioRxiv. 2022 doi: 10.1101/2022.03.01.482445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu W., Zhou Q., Masubuchi T., Shi X., Li H., Xu X., Huang M., Meng L., He X., Zhu H., et al. Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy. Cell. 2020;182:855–871.e823.. doi: 10.1016/j.cell.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 71.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J., et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor. Nat. Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 73.Krause A., Guo H.F., Latouche J.B., Tan C., Cheung N.K., Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 1998;188:619–626. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kagoya Y., Tanaka S., Guo T., Anczurowski M., Wang C.H., Saso K., Butler M.O., Minden M.D., Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chmielewski M., Abken H. CAR T Cells Releasing IL-18 Convert to T-Bet(high) FoxO1(low) Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017;21:3205–3219. doi: 10.1016/j.celrep.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 77.Avanzi M.P., Yeku O., Li X., Wijewarnasuriya D.P., van Leeuwen D.G., Cheung K., Park H., Purdon T.J., Daniyan A.F., Spitzer M.H., et al. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep. 2018;23:2130–2141. doi: 10.1016/j.celrep.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gutcher I., Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feucht J., Sadelain M. Function and evolution of the prototypic CD28ζ and 4-1BBζ chimeric antigen receptors. Immuno Oncol. Technol. 2020;8:2–11. doi: 10.1016/j.iotech.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strebhardt K., Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 81.Alabanza L., Pegues M., Geldres C., Shi V., Wiltzius J.J.W., Sievers S.A., Yang S., Kochenderfer J.N. Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol. Ther. 2017;25:2452–2465. doi: 10.1016/j.ymthe.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watanabe N., Bajgain P., Sukumaran S., Ansari S., Heslop H.E., Rooney C.M., Brenner M.K., Leen A.M., Vera J.F. Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology. 2016;5:e1253656. doi: 10.1080/2162402X.2016.1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guest R.D., Hawkins R.E., Kirillova N., Cheadle E.J., Arnold J., O’Neill A., Irlam J., Chester K.A., Kemshead J.T., Shaw D.M., et al. The Role of Extracellular Spacer Regions in the Optimal Design of Chimeric Immune Receptors: Evaluation of Four Different scFvs and Antigens. J. Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 84.Hudecek M., Lupo-Stanghellini M.-T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C., Riddell S.R. Receptor Affinity and Extracellular Domain Modifications Affect Tumor Recognition by ROR1-Specific Chimeric Antigen Receptor T Cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kunkele A., Johnson A.J., Rolczynski L.S., Chang C.A., Hoglund V., Kelly-Spratt K.S., Jensen M.C. Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent AICD. Cancer Immunol. Res. 2015;3:368–379. doi: 10.1158/2326-6066.CIR-14-0200. [DOI] [PubMed] [Google Scholar]
- 86.Textor A., Grunewald L., Anders K., Klaus A., Schwiebert S., Winkler A., Stecklum M., Rolff J., Henssen A.G., Hopken U.E., et al. CD28 Co-Stimulus Achieves Superior CAR T Cell Effector Function against Solid Tumors Than 4-1BB Co-Stimulus. Cancers. 2021;13:1050. doi: 10.3390/cancers13051050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drent E., Poels R., Ruiter R., van de Donk N., Zweegman S., Yuan H., de Bruijn J., Sadelain M., Lokhorst H.M., Groen R.W.J., et al. Combined CD28 and 4-1BB Costimulation Potentiates Affinity-tuned Chimeric Antigen Receptor-engineered T Cells. Clin. Cancer Res. 2019;25:4014–4025. doi: 10.1158/1078-0432.CCR-18-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Artyomov M.N., Lis M., Devadas S., Davis M.M., Chakraborty A.K. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc. Natl. Acad. Sci. USA. 2010;107:16916–16921. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palacios E.H., Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 90.Brameshuber M., Kellner F., Rossboth B.K., Ta H., Alge K., Sevcsik E., Göhring J., Axmann M., Baumgart F., Gascoigne N.R.J., et al. Monomeric TCRs drive T cell antigen recognition. Nat. Immunol. 2018;19:487–496. doi: 10.1038/s41590-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pageon S.V., Tabarin T., Yamamoto Y., Ma Y., Nicovich P.R., Bridgeman J.S., Cohnen A., Benzing C., Gao Y., Crowther M.D., et al. Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination. Proc. Natl. Acad. Sci. USA. 2016;113:E5454–E5463. doi: 10.1073/pnas.1607436113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar R., Ferez M., Swamy M., Arechaga I., Rejas M.T., Valpuesta J.M., Schamel W.W.A., Alarcon B., van Santen H.M. Increased Sensitivity of Antigen-Experienced T Cells through the Enrichment of Oligomeric T Cell Receptor Complexes. Immunity. 2011;35:375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Burroughs N.J., Lazic Z., van der Merwe P.A. Ligand Detection and Discrimination by Spatial Relocalization: A Kinase-Phosphatase Segregation Model of TCR Activation. Biophys. J. 2006;91:1619–1629. doi: 10.1529/biophysj.105.080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis S.J., van der Merwe P.A. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 95.James J.R., Vale R.D. Biophysical Mechanism of T Cell Receptor Triggering in a Reconstituted System. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim S.T., Takeuchi K., Sun Z.-Y.J., Touma M., Castro C.E., Fahmy A., Lang M.J., Wagner G., Reinherz E.L. The αβ T Cell Receptor Is an Anisotropic Mechanosensor. J. Biol. Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Judokusumo E., Tabdanov E., Kumari S., Dustin M.L., Kam L.C. Mechanosensing in T Lymphocyte Activation. Biophys. J. 2012;102:L5–L7. doi: 10.1016/j.bpj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das D.K., Feng Y., Mallis R.J., Li X., Keskin D.B., Hussey R.E., Brady S.K., Wang J.-H., Wagner G., Reinherz E.L., et al. Force-dependent transition in the T-cell receptor β-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc. Natl. Acad. Sci. USA. 2015;112:1517–1522. doi: 10.1073/pnas.1424829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng Y., Brazin K.N., Kobayashi E., Mallis R.J., Reinherz E.L., Lang M.J. Mechanosensing drives acuity of αβ T-cell recognition. Proc. Natl. Acad. Sci. USA. 2017;114:E8204–E8213. doi: 10.1073/pnas.1703559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yousefi O.S., Günther M., Hörner M., Chalupsky J., Wess M., Brandl S.M., Smith R.W., Fleck C., Kunkel T., Zurbriggen M.D., et al. Optogenetic control shows that kinetic proofreading regulates the activity of the T cell receptor. eLife. 2019;8:e42475. doi: 10.7554/eLife.42475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang Z.L., Lorenzini M.H., Chen X., Tran U., Bangayan N.J., Chen Y.Y. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tischer D.K., Weiner O.D. Light-based tuning of ligand half-life supports kinetic proofreading model of T cell signaling. eLife. 2019;8:e42498. doi: 10.7554/eLife.42498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harris D.T., Hager M.V., Smith S.N., Cai Q., Stone J.D., Kruger P., Lever M., Dushek O., Schmitt T.M., Greenberg P.D., et al. Comparison of T Cell Activities Mediated by Human TCRs and CARs That Use the Same Recognition Domains. J. Immunol. 2018;200:1088–1100. doi: 10.4049/jimmunol.1700236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J., Zhang X., Zhou Z., Liu Y., Yu L., Jia L., Yang J., Li J., Yu H., Li W., et al. A novel adoptive synthetic TCR and antigen receptor (STAR) T-Cell therapy for B-Cell acute lymphoblastic leukemia. Am. J. Hematol. 2022;97:992–1004. doi: 10.1002/ajh.26586. [DOI] [PubMed] [Google Scholar]
- 105.Stone J.D., Harris D.T., Soto C.M., Chervin A.S., Aggen D.H., Roy E.J., Kranz D.M. A novel T cell receptor single-chain signaling complex mediates antigen-specific T cell activity and tumor control. Cancer Immunol. Immunother. CII. 2014;63:1163–1176. doi: 10.1007/s00262-014-1586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morch A.M., Balint S., Santos A.M., Davis S.J., Dustin M.L. Coreceptors and TCR Signaling—The Strong and the Weak of It. Front. Cell Dev. Biol. 2020;8:597627. doi: 10.3389/fcell.2020.597627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Potter T.A., Grebe K., Freiberg B., Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stinchcombe J.C., Bossi G., Booth S., Griffiths G.M. The Immunological Synapse of CTL Contains a Secretory Domain and Membrane Bridges. Immunity. 2001;15:751–761. doi: 10.1016/S1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 109.Reichardt P., Dornbach B., Rong S., Beissert S., Gueler F., Loser K., Gunzer M. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 110.Thauland T.J., Parker D.C. Diversity in immunological synapse structure. Immunology. 2010;131:466–472. doi: 10.1111/j.1365-2567.2010.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balamuth F., Leitenberg D., Unternaehrer J., Mellman I., Bottomly K. Distinct Patterns of Membrane Microdomain Partitioning in Th1 and Th2 Cells. Immunity. 2001;15:729–738. doi: 10.1016/S1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 112.Jackman R.P., Balamuth F., Bottomly K. CTLA-4 Differentially Regulates the Immunological Synapse in CD4 T Cell Subsets. J. Immunol. 2007;178:5543–5551. doi: 10.4049/jimmunol.178.9.5543. [DOI] [PubMed] [Google Scholar]
- 113.Hailman E., Burack W.R., Shaw A.S., Dustin M.L., Allen P.M. Immature CD4+CD8+ Thymocytes Form a Multifocal Immunological Synapse with Sustained Tyrosine Phosphorylation. Immunity. 2002;16:839–848. doi: 10.1016/S1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 114.Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 115.Lee K.-H., Holdorf A.D., Dustin M.L., Chan A.C., Allen P.M., Shaw A.S. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 116.Yokosuka T., Kobayashi W., Sakata-Sogawa K., Takamatsu M., Hashimoto-Tane A., Dustin M.L., Tokunaga M., Saito T. Spatiotemporal Regulation of T Cell Costimulation by TCR-CD28 Microclusters and Protein Kinase C θ Translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yokosuka T., Sakata-Sogawa K., Kobayashi W., Hiroshima M., Hashimoto-Tane A., Tokunaga M., Dustin M.L., Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 118.Campi G., Varma R., Dustin M.L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varma R., Campi G., Yokosuka T., Saito T., Dustin M.L. T Cell Receptor-Proximal Signals Are Sustained in Peripheral Microclusters and Terminated in the Central Supramolecular Activation Cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ilani T., Vasiliver-Shamis G., Vardhana S., Bretscher A., Dustin M.L. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat. Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cemerski S., Das J., Giurisato E., Markiewicz M.A., Allen P.M., Chakraborty A.K., Shaw A.S. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alarcón B., Mestre D., Martínez-Martín N. The immunological synapse: A cause or consequence of T-cell receptor triggering? Immunology. 2011;133:420–425. doi: 10.1111/j.1365-2567.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vardhana S., Choudhuri K., Varma R., Dustin M.L. Essential Role of Ubiquitin and TSG101 Protein in Formation and Function of the Central Supramolecular Activation Cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stinchcombe J.C., Majorovits E., Bossi G., Fuller S., Griffiths G.M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 125.Xiong W., Chen Y., Kang X., Chen Z., Zheng P., Hsu Y.-H., Jang J.H., Qin L., Liu H., Dotti G., et al. Immunological Synapse Predicts Effectiveness of Chimeric Antigen Receptor Cells. Mol. Ther. 2018;26:963–975. doi: 10.1016/j.ymthe.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramello M.C., Benzaïd I., Kuenzi B.M., Lienlaf-Moreno M., Kandell W.M., Santiago D.N., Pabón-Saldaña M., Darville L., Fang B., Rix U., et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci. Signal. 2019;12:eaap9777. doi: 10.1126/scisignal.aap9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Larson R.C., Kann M.C., Bailey S.R. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature. 2022;604:563–570. doi: 10.1038/s41586-022-04585-5. [DOI] [PubMed] [Google Scholar]
- 128.Wu L., Wei Q., Brzostek J., Gascoigne N.R.J. Signaling from T cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cell. Mol. Immunol. 2020;17:600–612. doi: 10.1038/s41423-020-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nika K., Soldani C., Salek M., Paster W., Gray A., Etzensperger R., Fugger L., Polzella P., Cerundolo V., Dushek O., et al. Constitutively Active Lck Kinase in T Cells Drives Antigen Receptor Signal Transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ehrlich L.I.R., Ebert P.J.R., Krummel M.F., Weiss A., Davis M.M. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17:809–822. doi: 10.1016/S1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- 131.Kim P.W., Sun Z.-Y.J., Blacklow S.C., Wagner G., Eck M.J. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science. 2003;301:1725–1728. doi: 10.1126/science.1085643. [DOI] [PubMed] [Google Scholar]
- 132.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 133.Smith-Garvin J.E., Koretzky G.A., Jordan M.S. T cell activation. Annu. Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Samelson L.E. Signal transduction mediated by the T cell antigen receptor: The role of adapter proteins. Annu. Rev. Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 135.Lo W.-L., Shah N.H., Ahsan N., Horkova V., Stepanek O., Salomon A.R., Kuriyan J., Weiss A. Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT. Nat. Immunol. 2018;19:733–741. doi: 10.1038/s41590-018-0131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang W., Trible R.P., Zhu M., Liu S.K., McGlade C.J., Samelson L.E. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J. Biol. Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 137.Liu S.K., Fang N., Koretzky G.A., McGlade C.J. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 1999;9:67–75. doi: 10.1016/S0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 138.Su X., Ditlev J.A., Hui E., Xing W., Banjade S., Okrut J., King D.S., Taunton J., Rosen M.K., Vale R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 2016;352:595–599. doi: 10.1126/science.aad9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang W.Y.C., Yan Q., Lin W.-C., Chung J.K., Hansen S.D., Christensen S.M., Tu H.-L., Kuriyan J., Groves J.T. Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. Proc. Natl. Acad. Sci. USA. 2016;113:8218–8223. doi: 10.1073/pnas.1602602113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shim E.K., Jung S.H., Lee J.R. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. J. Immunol. 2011;186:2926–2935. doi: 10.4049/jimmunol.1001785. [DOI] [PubMed] [Google Scholar]
- 141.Salter A.I., Ivey R.G., Kennedy J.J., Voillet V., Rajan A., Alderman E.J., Voytovich U.J., Lin C., Sommermeyer D., Liu L., et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 2018;11:eaat6753. doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun C., Shou P., Du H., Hirabayashi K., Chen Y., Herring L.E., Ahn S., Xu Y., Suzuki K., Li G., et al. THEMIS-SHP1 Recruitment by 4-1BB Tunes LCK-Mediated Priming of Chimeric Antigen Receptor-Redirected T Cells. Cancer Cell. 2020;37:216–225.e216.. doi: 10.1016/j.ccell.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Askonas B.A. T-cell differentiation and effector functions. Immunology. 1988;64((Suppl. 1)):51–52. [PubMed] [Google Scholar]
- 144.Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 145.Beal A.M., Anikeeva N., Varma R., Cameron T.O., Vasiliver-Shamis G., Norris P.J., Dustin M.L., Sykulev Y. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pores-Fernando A.T., Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol. Rev. 2009;231:160–173. doi: 10.1111/j.1600-065X.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 147.Joseph N., Reicher B., Barda-Saad M. The calcium feedback loop and T cell activation: How cytoskeleton networks control intracellular calcium flux. Biochim. Biophys. Acta Biomembr. 2014;1838:557–568. doi: 10.1016/j.bbamem.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 148.Karttunen J., Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc. Natl. Acad. Sci. USA. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewis R.S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 150.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 151.Macian F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 152.Shao M., Teng X., Guo X., Zhang H., Huang Y., Cui J., Si X., Ding L., Wang X., Li X., et al. Inhibition of Calcium Signaling Prevents Exhaustion and Enhances Anti-Leukemia Efficacy of CAR-T Cells via SOCE-Calcineurin-NFAT and Glycolysis Pathways. Adv. Sci. 2022;9:e2103508. doi: 10.1002/advs.202103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stone J.C. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Cancer. 2011;2:320–334. doi: 10.1177/1947601911408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roskoski R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 155.Roche M.I., Ramadas R.A., Medoff B.D. The Role of CARMA1 in T Cells. Crit. Rev. Immunol. 2013;33:219–243. doi: 10.1615/CritRevImmunol.2013007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schulze-Luehrmann J., Ghosh S. Antigen-Receptor Signaling to Nuclear Factor κB. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 157.Sun W., Yang J. Molecular basis of lysophosphatidic acid-induced NF-κB activation. Cell. Signal. 2010;22:1799–1803. doi: 10.1016/j.cellsig.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X., Yang J., Zhang X., Lu X.-A., Xiong M., Zhang J., Zhou X., Qi F., He T., Ding Y., et al. Efficacy and Safety of CD28- or 4-1BB-Based CD19 CAR-T Cells in B Cell Acute Lymphoblastic Leukemia. Mol. Ther. Oncolytics. 2020;18:272–281. doi: 10.1016/j.omto.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ying Z., He T., Wang X., Zheng W., Lin N., Tu M., Xie Y., Ping L., Zhang C., Liu W., et al. Parallel Comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin’s Lymphoma. Mol. Ther. Oncolytics. 2019;15:60–68. doi: 10.1016/j.omto.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Philipson B.I., O’Connor R.S., May M.J., June C.H., Albelda S.M., Milone M.C. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci. Signal. 2020;13:eaay8248. doi: 10.1126/scisignal.aay8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gibbins J.M., Briddon S., Shutes A., van Vugt M.J., van de Winkel J.G., Saito T., Watson S.P. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor gamma-chain and linker for activitor of T cells (LAT) in platelets stimulated by collagen and convulxin. J. Biol. Chem. 1998;273:34437–34443. doi: 10.1074/jbc.273.51.34437. [DOI] [PubMed] [Google Scholar]
- 163.Shim E.K., Moon C.S., Lee G.Y., Ha Y.J., Chae S.-K., Lee J.R. Association of the Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) with the p85 subunit of phosphoinositide 3-kinase. FEBS Lett. 2004;575:35–40. doi: 10.1016/j.febslet.2004.07.090. [DOI] [PubMed] [Google Scholar]
- 164.Laplante M., Sabatini D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., Thompson C.B. The CD28 Signaling Pathway Regulates Glucose Metabolism. Immunity. 2002;16:769–777. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 166.Kofler D.M., Chmielewski M., Rappl G., Hombach A., Riet T., Schmidt A., Hombach A.A., Wendtner C.-M., Abken H. CD28 Costimulation Impairs the Efficacy of a Redirected T-cell Antitumor Attack in the Presence of Regulatory T cells Which Can Be Overcome by Preventing Lck Activation. Mol. Ther. 2011;19:760–767. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alzubi J., Dettmer-Monaco V., Kuehle J., Thorausch N., Seidl M., Taromi S., Schamel W., Zeiser R., Abken H., Cathomen T., et al. PSMA-Directed CAR T Cells Combined with Low-Dose Docetaxel Treatment Induce Tumor Regression in a Prostate Cancer Xenograft Model. Mol. Ther. Oncolytics. 2020;18:226–235. doi: 10.1016/j.omto.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 169.Schmid E.M., McMahon H.T. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 170.Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P.P. Clathrin-Mediated Internalization Is Essential for Sustained EGFR Signaling but Dispensable for Degradation. Dev. Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 171.Boucrot E., Ferreira A.P.A., Almeida-Souza L., Debard S., Vallis Y., Howard G., Bertot L., Sauvonnet N., McMahon H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517:460–465. doi: 10.1038/nature14067. [DOI] [PubMed] [Google Scholar]
- 172.Szymczak A.L., Vignali D.A.A. Plasticity and Rigidity in Adaptor Protein-2-Mediated Internalization of the TCR:CD3 Complex. J. Immunol. 2005;174:4153–4160. doi: 10.4049/jimmunol.174.7.4153. [DOI] [PubMed] [Google Scholar]
- 173.Dietrich J., Hou X., Wegener A.M., Geisler C. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.von Essen M., Menné C., Nielsen B.L., Lauritsen J.P.H., Dietrich J., Andersen P.S., Karjalainen K., Ødum N., Geisler C. The CD3 gamma leucine-based receptor-sorting motif is required for efficient ligand-mediated TCR down-regulation. J. Immunol. 2002;168:4519–4523. doi: 10.4049/jimmunol.168.9.4519. [DOI] [PubMed] [Google Scholar]
- 175.San José E., Borroto A., Niedergang F., Alcover A., Alarcón B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/S1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 176.Alcover A., Alarcón B., Di Bartolo V. Cell Biology of T Cell Receptor Expression and Regulation. Annu. Rev. Immunol. 2018;36:103–125. doi: 10.1146/annurev-immunol-042617-053429. [DOI] [PubMed] [Google Scholar]
- 177.Martínez-Martín N., Fernández-Arenas E., Cemerski S., Delgado P., Turner M., Heuser J., Irvine D.J., Huang B., Bustelo X.R., Shaw A., et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–222. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Joly E., Hudrisier D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 179.Yudushkin I.A., Vale R.D. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3ζ in the endosomal compartment. Proc. Natl. Acad. Sci. USA. 2010;107:22128–22133. doi: 10.1073/pnas.1016388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Willinger T., Staron M., Ferguson S.M., De Camilli P., Flavell R.A. Dynamin 2-dependent endocytosis sustains T-cell receptor signaling and drives metabolic reprogramming in T lymphocytes. Proc. Natl. Acad. Sci. USA. 2015;112:4423–4428. doi: 10.1073/pnas.1504279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Onnis A., Baldari C.T. Orchestration of Immunological Synapse Assembly by Vesicular Trafficking. Front. Cell Dev. Biol. 2019;7:110. doi: 10.3389/fcell.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stenger D., Stief T.A., Käuferle T., Willier S., Rataj F., Schober K., Vick B., Lotfi R., Wagner B., Grunewald T., et al. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood. 2020;136:1407–1418. doi: 10.1182/blood.2020005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gonen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z., et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tanaka M., Tashiro H., Omer B., Lapteva N., Ando J., Ngo M., Mehta B., Dotti G., Kinchington P.R., Leen A.M., et al. Vaccination Targeting Native Receptors to Enhance the Function and Proliferation of Chimeric Antigen Receptor (CAR)-Modified T Cells. Clin. Cancer Res. 2017;23:3499–3509. doi: 10.1158/1078-0432.CCR-16-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lapteva N., Gilbert M., Diaconu I., Rollins L.A., Al-Sabbagh M., Naik S., Krance R.A., Tripic T., Hiregange M., Raghavan D., et al. T-Cell Receptor Stimulation Enhances the Expansion and Function of CD19 Chimeric Antigen Receptor–Expressing T Cells. Clin. Cancer Res. 2019;25:7340–7350. doi: 10.1158/1078-0432.CCR-18-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Omer B., Castillo P.A., Tashiro H., Shum T., Huynh M.T.A., Cardenas M., Tanaka M., Lewis A., Sauer T., Parihar R., et al. Chimeric Antigen Receptor Signaling Domains Differentially Regulate Proliferation and Native T Cell Receptor Function in Virus-Specific T Cells. Front. Med. 2018;5:343. doi: 10.3389/fmed.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cruz C.R., Micklethwaite K.P., Savoldo B., Ramos C.A., Lam S., Ku S., Diouf O., Liu E., Barrett A.J., Ito S., et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vardhana S.A., Hwee M.A., Berisa M., Wells D.K., Yost K.E., King B., Smith M., Herrera P.S., Chang H.Y., Satpathy A.T., et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 2020;21:1022–1033. doi: 10.1038/s41590-020-0725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weber E.W., Parker K.R., Sotillo E., Lynn R.C., Anbunathan H., Lattin J., Good Z., Belk J.A., Daniel B., Klysz D., et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372:eaba1786. doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.van der Stegen S.J., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burr J.S., Savage N.D., Messah G.E., Kimzey S.L., Shaw A.S., Arch R.H., Green J.M. Cutting edge: Distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 194.Kerstan A., Armbruster N., Leverkus M., Hunig T. Cyclosporin A abolishes CD28-mediated resistance to CD95-induced apoptosis via superinduction of caspase-3. J. Immunol. 2006;177:7689–7697. doi: 10.4049/jimmunol.177.11.7689. [DOI] [PubMed] [Google Scholar]
- 195.Zhong X.S., Matsushita M., Plotkin J., Riviere I., Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang Y., Kohler M.E., Chien C.D., Sauter C.T., Jacoby E., Yan C., Hu Y., Wanhainen K., Qin H., Fry T.J. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci. Transl. Med. 2017;9:eaag1209. doi: 10.1126/scitranslmed.aag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Evgin L., Kottke T. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 2022;14:eabn2231. doi: 10.1126/scitranslmed.abn2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Slaney C.Y., von Scheidt B., Davenport A.J., Beavis P.A., Westwood J.A., Mardiana S., Tscharke D.C., Ellis S., Prince H.M., Trapani J.A., et al. Dual-specific Chimeric Antigen Receptor T Cells and an Indirect Vaccine Eradicate a Variety of Large Solid Tumors in an Immunocompetent, Self-antigen Setting. Clin. Cancer Res. 2017;23:2478–2490. doi: 10.1158/1078-0432.CCR-16-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 200.Klebanoff C.A., Gattinoni L., Torabi-Parizi P., Kerstann K., Cardones A.R., Finkelstein S.E., Palmer D.C., Antony P.A., Hwang S.T., Rosenberg S.A., et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kueberuwa G., Gornall H., Alcantar-Orozco E.M., Bouvier D., Kapacee Z.A., Hawkins R.E., Gilham D.E. CCR7(+) selected gene-modified T cells maintain a central memory phenotype and display enhanced persistence in peripheral blood in vivo. J. Immunother. Cancer. 2017;5:14. doi: 10.1186/s40425-017-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Uslu U., Schuler G., Dörrie J., Schaft N. Combining a chimeric antigen receptor and a conventional T-cell receptor to generate T cells expressing two additional receptors (TETARs) for a multi-hit immunotherapy of melanoma. Exp. Dermatol. 2016;25:872–879. doi: 10.1111/exd.13095. [DOI] [PubMed] [Google Scholar]
- 203.Simon B., Harrer D.C., Schuler-Thurner B., Schuler G., Uslu U. Arming T Cells with a gp100-Specific TCR and a CSPG4-Specific CAR Using Combined DNA- and RNA-Based Receptor Transfer. Cancers. 2019;11:696. doi: 10.3390/cancers11050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Garber K. Driving T-cell immunotherapy to solid tumors. Nat. Biotechnol. 2018;36:215–219. doi: 10.1038/nbt.4090. [DOI] [PubMed] [Google Scholar]
- 205.Miyao K., Terakura S., Okuno S., Julamanee J., Watanabe K., Hamana H., Kishi H., Sakemura R., Koyama D., Goto T., et al. Introduction of Genetically Modified CD3ζ Improves Proliferation and Persistence of Antigen-Specific CTLs. Cancer Immunol. Res. 2018;6:733–744. doi: 10.1158/2326-6066.CIR-17-0538. [DOI] [PubMed] [Google Scholar]
- 206.Thakur A., Scholler J., Kubicka E., Bliemeister E.T., Schalk D.L., June C.H., Lum L.G. Bispecific Antibody Armed Metabolically Enhanced Headless CAR T Cells. Front. Immunol. 2021;12:690437. doi: 10.3389/fimmu.2021.690437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Omer B., Cardenas M.G., Pfeiffer T., Daum R., Huynh M., Sharma S., Nouraee N., Xie C., Tat C., Perconti S., et al. A Costimulatory CAR Improves TCR-based Cancer Immunotherapy. Cancer Immunol. Res. 2022;10:512–524. doi: 10.1158/2326-6066.CIR-21-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morris E.C., Neelapu S.S., Giavridis T., Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Reviews. Immunol. 2022;22:85–96. doi: 10.1038/s41577-021-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Siegler E.L., Kenderian S.S. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies. Front. Immunol. 2020;11:1973. doi: 10.3389/fimmu.2020.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu Y., Yan X., Zhang F., Zhang X., Tang F., Han Z., Li Y. TCR-T Immunotherapy: The Challenges and Solutions. Front Oncol. 2021;11:794183. doi: 10.3389/fonc.2021.794183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Morton L.T., Reijmers R.M., Wouters A.K., Kweekel C., Remst D.F., Pothast C.R., Falkenburg J.F., Heemskerk M.H. Simultaneous Deletion of Endogenous TCRαβ for TCR Gene Therapy Creates an Improved and Safe Cellular Therapeutic. Mol. Therapy. 2020;28:64–74. doi: 10.1016/j.ymthe.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Müller T.R., Jarosch S., Hammel M., Leube J., Grassmann S., Bernard B., Effenberger M., Andrä I., Chaudhry M.Z., Käuferle T., et al. Targeted T cell receptor gene editing provides predictable T cell product function for immunotherapy. Cell Rep. Med. 2021;2:100374. doi: 10.1016/j.xcrm.2021.100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Besser M.J., Shapira-Frommer R., Itzhaki O., Treves A.J., Zippel D.B., Levy D., Kubi A., Shoshani N., Zikich D., Ohayon Y., et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: Intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 215.Johnson L.A., Heemskerk B., Powell D.J., Cohen C.J., Morgan R.A., Dudley M.E., Robbins P.F., Rosenberg S.A. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Andersen R., Donia M., Ellebaek E., Borch T.H., Kongsted P., Iversen T.Z., Hölmich L.R., Hendel H.W., Met Ö., Andersen M.H., et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 217.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G., Plotkin J., Sadelain M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]