Abstract
The onset of puberty has become earlier over the decades, and nutrients and diet are related to the timing of puberty onset. Hence, we aimed to investigate the association between diet or nutrients in infancy, childhood and early puberty. PubMed, Embase, and Cochrane library were searched systematically up to 15 April 2022. The pooled relative risks (RRs) or regression coefficients (beta) were estimated using the random-effect model or fixed-effect model according to the heterogeneity between studies. Twenty-two articles on diet or nutrients in childhood and six about breastfeeding in infancy were included. The prolonged breastfeeding duration in infancy could reduce the risk of early menarche (beta 0.31, 95% CI: 0.01, 0.60, p = 0.045). The high intake of yogurt was associated with a 35% reduction in the risk of earlier menarche (RR 0.65, 95% CI: 0.47, 0.89, p = 0.008). Girls with severe food insecurity experienced later menarche (RR 0.81, 95% CI: 0.67, 0.98, p = 0.027). Conversely, due to the high intake of protein, the risk of early menarche increased by 8% (RR 1.08, 95% CI: 1.01, 1.16, p = 0.016). High intake of yogurt, longer duration of breastfeeding, and food insecurity decreased the possibility of earlier menarche, while high intake of protein increased that risk. As a modifiable factor, diet and nutrients in infancy and childhood provide new insights into the future prevention of early puberty.
Keywords: diet, nutrients, early puberty, infancy, childhood
1. Introduction
Puberty is a pivotal period in the lifecycle. During this period, people experience the transformation from children to adults, attaining both somatic growth and reproductive maturity. For boys, genital growth, pubic hair development, and voice break are recognized as major puberty milestones. For girls, breast growth, pubic hair development, and menarche are the main secondary sexual characteristics in puberty [1]. There is a trend that the prevalence of early puberty is increasing worldwide, particularly in girls [2,3]. Early puberty increases many subsequent physical and psychological unhealthy conditions or diseases. Indeed, several studies showed a significant association between early puberty and endocrine diseases, for example, early menopause, breast cancer, and prostate cancer [4,5,6].
The timing of puberty is determined by numerous risk factors with their complex interplay. Genes partly determine when an individual’s puberty begins. For instance, makorin ring finger protein 3 (MKRN3) and delta-like noncanonical Notch ligand 1 (DLK1) are key genetic drivers in the progression of central precocious puberty [7,8]. Obesity also plays a critical role [9]. Ferrari et al. [10] indicated that girls with higher BMI had a higher risk of early menarche and breast development. In addition, an incomplete family structure may contribute to early puberty [11].
As a modifiable factor, the influence of diet and nutrients on the timing of puberty onset receives keen attention. However, the findings showed an inconsistent association. Rogers et al. [12] found that the high intake of meat predisposed girls to earlier menarche in British, while this positive association was not observed by Wu et al. [13]. Likewise, the influence of carbohydrate intake on early menarche was also controversial [14,15]. Several studies pointed out that the duration of breastfeeding was associated with the timing of menarche and breast development [16,17]. Moreover, increasing evidence indicates that food insecurity also affects the age of menarche [18]. The previous studies mainly focused on children’s nutritional status and subsequent menarche onset, leaving gaps in understanding other pubertal milestones and nutritional status in early life. Therefore, this systematic review and meta-analysis aimed to comprehensively clarify the influence of diet and nutrients in infancy and childhood on early puberty, providing new insights into formulating policies for the prevention of early pubertal development.
2. Methods
We performed this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. This study was registered with PROSPERO (CRD42022349856).
2.1. Search Strategy
We searched comprehensively in PubMed, Embase, and Cochrane library for relevant studies published from inception to 15 April 2022. We did not impose any language restrictions nor limit the research types. To ensure a thorough literature search, the following keywords were used, food, diet, breastfeeding, milk, meat, dairy, carbohydrate, fat, protein, food insecurity, early puberty, precocious puberty, premature, thelarche, and menarche. Boolean operators “OR” and “AND” were used to combine these search terms. To retrieve articles more accurately, we limited the age of the research subjects to less than 18 years old, as puberty generally ends at this age. We also reviewed references of included articles to identify relevant literature.
2.2. Inclusion and Exclusion Criteria
To be eligible, the published articles should meet the following criteria: (a) They were epidemiological studies, including cross-sectional, case-control, or cohort studies. (b) Different levels of diet or nutrient intake were considered as exposure. The way of assessment was food frequency questionnaires, food diaries, or dietary recall interviews. The outcome of interest was the number of children at different pubertal stages and/or the age at pubertal milestones. Pubertal indicators were obtained by clinical diagnosis, questionnaire, or self-reporting. (c) Studies investigated the association between early puberty and diet or nutrient intake. The adjusted odds ratio (OR), relative risk (RR), hazard ratio (HR), or regression coefficients (beta), as well as its 95% confidence interval (CI), were provided.
Studies were excluded if they (a) did not study specific diet or nutrients but focused on only dietary patterns; (b) selected subjects with medical conditions or diseases that would cause early puberty, such as adrenal dysfunction, pituitary tumor, thyroid hypofunction; (c) did not assess pubertal outcomes (breast, pubic hair, and testicular development) according to the Tanner staging; (d) were the only one article to show the relationship and could not be combined using meta-analysis.
2.3. Literatures Screening and Data Extraction
The studies obtained through the search strategy were screened to rule out duplicates. Then, the review of the title and abstract was performed. Among the full-text articles, ineligible articles were removed according to the exclusion criteria. For all included studies, the following characters were extracted, including first author, year of publication, country, study design, health status, age, gender, sample size, type of diet or nutrients, the pubertal outcome of interest, ORs, RRs, HRs or beta with 95% CIs for the relationship between diet or nutrients and early puberty. Confounding factors were taken into consideration.
2.4. Quality Assessment
The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of cohort and case-control studies. It assessed the risk of bias from three domains of selection, comparability, and outcome, giving scores ranging from 0 to 9. In this article, we defined the criterion controlling important confounding factors: maternal age at menarche, maternal education level, household income, weight, and height. Referring to the previous meta-analysis [19], the studies were deemed to be low, moderate, and high quality according to the score “0–3”, “4–6”, and “7–9”, respectively. The Agency for Healthcare Research and Quality (AHRQ) scale was used to assess cross-sectional studies. Scores of “0–3”, “4–7”, and “8–11” received through an 11-item checklist were considered as low, moderate, and high quality, respectively.
2.5. Statistical Analysis
The effect size measures used to report the relationship between diet or nutrients and puberty outcome were ORs, RRs, HRs, or beta with 95% CIs. We selected the risk estimates for the highest exposure group compared to the lowest or reference group with the adjusted model. Given that the relevant articles are scarcely available, the ORs and HRs were deemed equivalent to RRs and then pooled together directly [20]. The pooled risk estimates were depicted graphically with forest plots. Additionally, I2 and p statistical variables were used to evaluate the heterogeneity between studies. A random-effect model was used in the presence of significant heterogeneity (I2 > 50% or p < 0.1), otherwise, the fixed-effect model was selected. For the issues with more than three articles, sensitivity analysis was performed by omitting one study sequentially to assess the robustness of the results. Besides, Egger’s test was conducted to inspect the publication bias. Unfortunately, we were unable to conduct the subgroup analysis to explore possible sources of heterogeneity between studies owing to the limited number of studies. All statistical analyses were performed using Stata 16.0 (Stata Corp LLC, College Station, TX, USA).
3. Results
3.1. Literature Screening and Characteristics of Included Studies
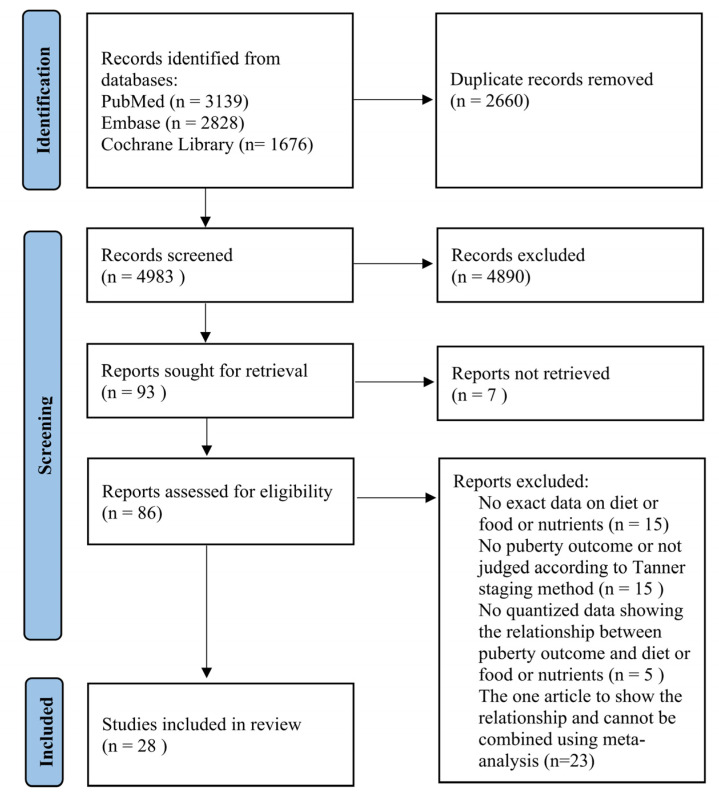
The process of the systematic search is shown in Figure 1. We obtained 7643 potentially eligible articles from PubMed, Embase and Cochrane Library, of which 2660 duplicates were removed. After further reviewing the title and abstract, 86 articles were eligible for full-text assessment. Owing to irrelevance to the inclusion criteria, 58 articles were ruled out, and the excluded reasons were presented in Supplementary Table S1. Finally, 28 articles were included in this systemic review and meta-analysis.
Figure 1.
Flow chart for study selection in this systemic review and meta-analysis.
The characteristics of the 28 included articles are presented in Table 1. Briefly, 22 articles investigated the association between childhood diet or nutrients and early puberty. In addition, six articles [16,21,22,23,24,25] provided the effects of breastfeeding duration in infancy on the timing of menarche and breast development. Among the included studies, five were cross-sectional one was a case-control study [26], and 22 were cohort studies. These studies were performed in different countries around the world with a large sample of 162,073. Studies evaluating the risk of childhood diet and nutrients primarily used hazard ratios, providing benefits to test the causal relationship.
Table 1.
Characteristics of 28 included studies.
| First Author | Year | Country | Study Design | Gender | Age (Years) a |
Sample Size | Diet or Nutrients | Diet/Nutrients Assessment |
Puberty Outcome | Quality b |
|---|---|---|---|---|---|---|---|---|---|---|
| Gaskins [27] | 2017 | Chile | C | F | 3–4 | 389 | dairy, milk, yogurt | DR | Age at menarche | 8/9 |
| Jansen [28] | 2015 | Colombia | C | F | 8.4 | 456 | red meat | FSQ | Age at menarche | 7/9 |
| Burris [29] | 2020 | USA | CS | F | 13.2 | 40 | food insecurity | FFQ | Age at menarche | 7/11 |
| Jansen [30] | 2015 | Colombia | CS | F | 13.9 | 15,441 | food insecurity | Age at menarche | 8/11 | |
| Carwile [31] | 2015 | USA | C | F | 9–14 | 5583 | milk, low-fat milk, dairy, high-fat dairy, meat, red meat | FFQ | Age at menarche | 7/9 |
| Ramezani Tehrani [32] | 2013 | Iran | C | F | 8.9 | 134 | milk, yogurt | DR | Age at menarche | 6/9 |
| Belachew [33] | 2011 | Ethiopia | C | F | 14.8 | 900 | food insecurity | FSQ | Age at menarche | 6/9 |
| Rogers [12] | 2015 | UK | C | F | 7 | 3298 | total energy, protein, meat | FFQ/DR | Age at menarche | 8/9 |
| Koprowski [15] | 1999 | USA | C | F | 10.6 | 1378 | total energy, carbohydrate, protein, fat | FFQ | Age at menarche | 7/9 |
| Wiley [34] | 2011 | USA | C | F | 9–12 | 3665 | milk | DR | Age at menarche | 6/9 |
| Moisan [26] | 1990 | Canada | CC | F | 10.7 | 666 | total energy, protein, carbohydrate, fat, vitamin | DR | Age at menarche | 7/9 |
| Maclure [35] | 1991 | USA | C | F | 10 | 194 | total energy, protein, fat, vitamin | FFQ | Age at menarche | 7/9 |
| Meyer [36] | 1990 | Canada | C | F | 10.9 | 109 | total energy, carbohydrate, protein, fat | DR | Age at menarche | 4/9 |
| Xu [37] | 2022 | China | C | M/F | M:7.1 F:7 |
5920 | fat | DR | Age at genital stage 2, breast stage 2, menarche, voice break | 6/9 |
| Koo [38] | 2002 | Canada | C | F | 6–14 | 637 | total energy | FFQ | Age at menarche | 8/9 |
| Meng [14] | 2017 | China | C | F | 6–18 | 3199 | total energy, carbohydrate, protein, fat | DR | Age at menarche | 7/9 |
| Cheng [39] | 2022 | UK | C | M/F | 1 or 7 | 7730 | total energy, carbohydrate, protein, fat | FFQ/FD | Age at menarche, genital stage 2, breast stage 2, voice break | 8/9 |
| Villamor [40] | 2017 | Colombia | C | F | 8.6 | 1464 | food insecurity | FSQ | Age at menarche | 6/9 |
| English [21] | 2020 | UK | C | M/F | 1 | 4217 | breastfeeding duration | Q | Age at menarche, voice break | 8/9 |
| Morris [22] | 2010 | UK | C | F | 46 | 81,606 | breastfeeding duration | Q | Age at menarche | 5/9 |
| Al-Mathkoori [23] | 2018 | Kuwait | CS | F | 16.8 | 496 | breastfeeding duration | TI | Age at menarche | 7/11 |
| Hvidt [24] | 2021 | Denmark | C | M/F | 1 | 13,511 | breastfeeding duration | TI | Age at genital stage 2,3,4,5; pubic hair stage 2,3,4,5; breast stage 2,3,4,5; menarche; voice break development | 8/9 |
| Segovia-Siapco [41] | 2014 | USA | CS | F | 15 | 327 | soy intake | FFQ | Age at menarche | 8/11 |
| Xiong [42] | 2022 | China | C | M/F | M:7.3 F:7.2 |
4781 | soy intake | FFQ | Age at menarche, breast stage 2, gonadal growth stage 2, voice break | 9/9 |
| Kale [16] | 2015 | USA | C | F | 6–8 | 1237 | breastfeeding duration | Q/TI | Age at breast onset, age at pubic hair onset | 8/9 |
| Aghaee [25] | 2019 | USA | C | F | 1 | 3331 | breastfeeding duration | Q | Age at breast onset, age at pubic hair onset | 9/9 |
| Wolff [43] | 2008 | USA | CS | F | 9.5 | 186 | lignans, flavonols | FFQ | Age at breast, pubic hair development (stage = 1; 2+) | 9/11 |
| Mervish [44] | 2013 | USA | C | F | 7.3 | 1178 | lignans, flavonols | DR | Age at breast stage 2, pubic hair stage 2 | 8/9 |
Note: CS, cross-sectional study; C, cohort study; CC, case-control study; M, male; F, female; Q, questionnaire; FFQ, food frequency questionnaire; DR, dietary recall; FD, food diary; FSQ, food security questionnaire; TI, telephone interview. a Age (years): the age at baseline, mean or range. b Quality: The quality of cohort studies and case-control studies was evaluated with the Newcastle–Ottawa Scale (NOS). The quality of cross-sectional studies was assessed with the Agency for Healthcare Research and Quality (AHRQ) scale.
3.2. Quality Assessment
The details of quality assessment based on NOS are shown in Supplementary Table S2. The studies evaluated from three domains of selection, comparability, and outcome were all moderate or high quality. The self-report of menarche was the main cause of the quality decline. Age at menarche could not be obtained by the clinical examination like other pubertal milestones, such as the Tanner stage of breast development. In addition, the inadequacy of cohort follow-up and lack of adjustments for confounding factors would lower the scores. Similarly, six studies evaluated by AHRQ were graded as moderate or high quality.
3.3. Findings from the Studies
3.3.1. Association of Breastfeeding Duration and Early Puberty
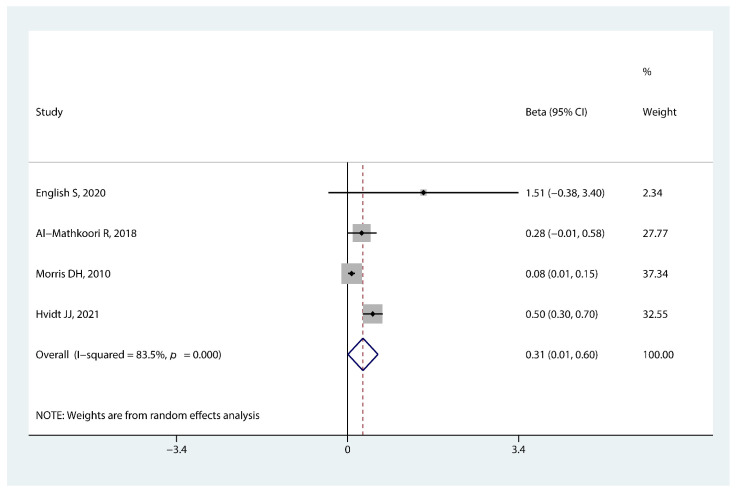
As illustrated in Figure 2, there was a significant association between breastfeeding duration in infancy and timing of menarche (beta 0.31, 95% CI: 0.01, 0.60, p = 0.045). The combined beta indicated 0.31 months later timing of menarche onset for every additional month of breastfeeding duration in girls.
Figure 2.
Forest plot of the association between breastfeeding duration in infancy and early menarche onset [21,22,23,24].
3.3.2. Association of Total Energy and Macronutrients Intake and Early Puberty
We did not observe an association between early menarche and total energy intake (RR 1.09, 95% CI: 0.92, 1.29, p = 0.296, Figure 3a), carbohydrate intake (RR 0.88, 95% CI: 0.76, 1.02, p = 0.072, Figure 3b), and fat intake (RR 1.00, 95% CI: 0.86, 1.17, p = 0.965, Figure 3d). However, protein intake increased the risk of early menarche onset by 8% (RR 1.08, 95% CI: 1.01, 1.16, p = 0.016, Figure 3c).
Figure 3.
Forest plot of relative risk of early menarche onset for (a) total energy intake, (b) carbohydrate intake, (c) protein intake, and (d) fat intake [12,14,15,26,35,36,37,38,39].
3.3.3. Association of Micronutrients and Phytochemicals Intake and Early Puberty
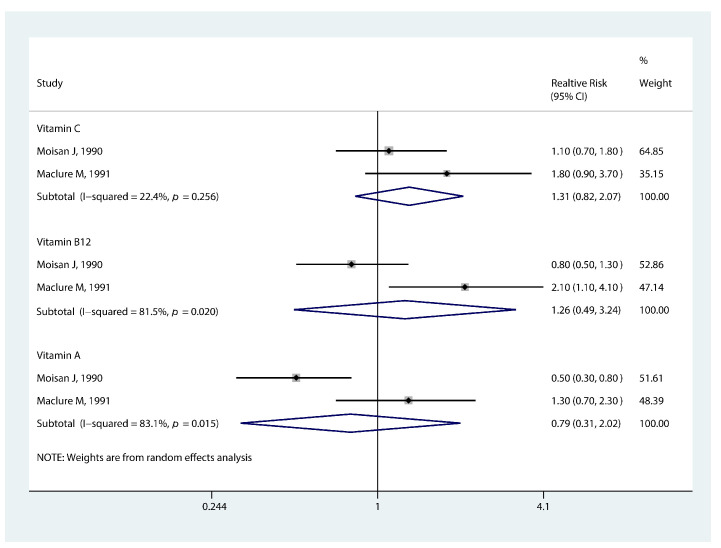
The pooled RR for the association between vitamins and earlier menarche onset is shown in Figure 4. The highest vitamin C intake was not significantly linked to earlier menarche compared to the lowest level (RR 1.31, 95% CI: 0.82, 2.07, p = 0.254). A similar relationship was observed for vitamin A (RR 0.79, 95% CI: 0.31, 2.02, p = 0.629) and vitamin B12 (RR 1.26, 95% CI: 0.49, 3.24, p = 0.630). Besides, Wolff et al. [43] and Mervish et al. [44] found lignan intake was irrelevant to the risk of earlier breast growth and pubic hair development; flavonols intake was not related to the development of pubic hair. While, Mervish et al. [44] discovered a relationship between breast growth and flavonols intake (HR 0.74, 95% CI: 0.61, 0.91).
Figure 4.
Forest plot of relative risk of early menarche onset for vitamins [26,35].
3.3.4. Association of Milk, Dairy, Yogurt Intake, and Early Puberty
We found that yogurt may reduce the risk of early menarche (RR 0.65, 95% CI: 0.47, 0.89, p = 0.008, Figure 5c). The pooled RR corresponded to an approximately 35% reduction in the risk of earlier menarche for girls. We did not observe a significant association between earlier menarche and milk (RR 0.91, 95% CI: 0.67, 1.23, p = 0.527, Figure 5a) or dairy intake (RR 1.00, 95% CI: 0.88, 1.14, p = 0.965, Figure 5b).
Figure 5.
Forest plot of relative risk of early menarche onset for (a) milk intake, (b) dairy intake, and (c) yogurt intake [27,31,32,34].
3.3.5. Association of Meat Intake and Early Puberty
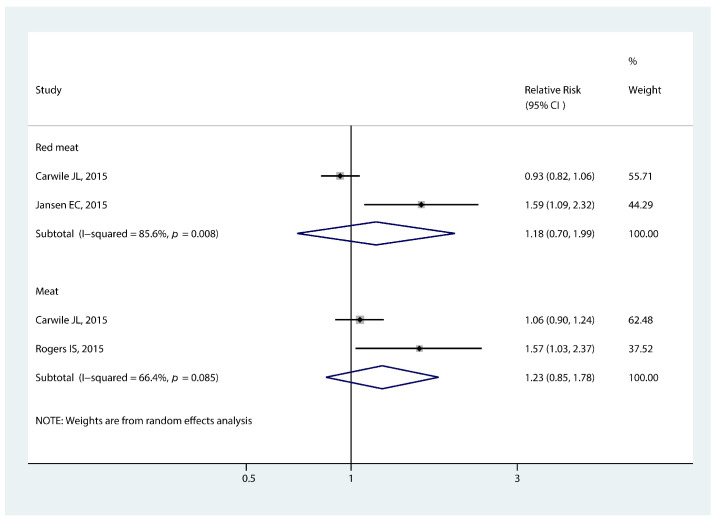
We did not observe a significant association between meat intake and earlier menarche (RR 1.23, 95% CI: 0.85, 1.78, p = 0.280, Figure 6). There was a similar relationship between red meat intake and the timing of menarche (RR 1.18, 95% CI: 0.70, 1.99, p = 0.536, Figure 6).
Figure 6.
Forest plot of relative risk of early menarche onset for meat and red meat [12,30,31].
3.3.6. Association of Soy Intake and Early Puberty
As shown in Figure 7, the pooled RR showed a nonsignificant association between soy intake and earlier menarche (RR 0.95, 95% CI: 0.72, 1.26, p = 0.730).
Figure 7.
Forest plot of relative risk of early menarche onset for soy intake [41,42].
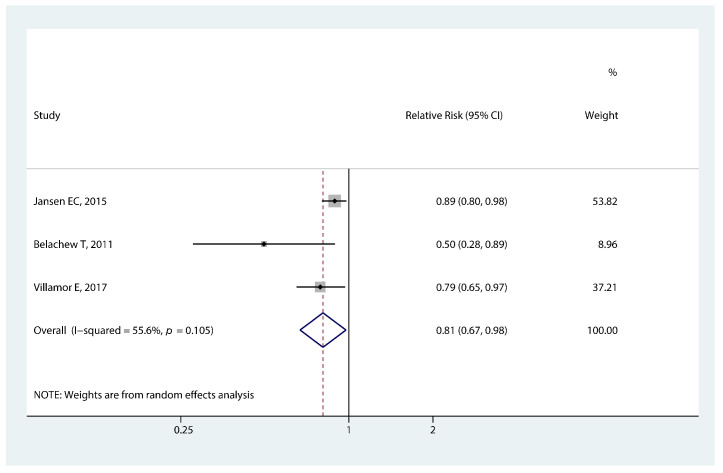
3.3.7. Association of Food Insecurity and Early Puberty
The significant association between food insecurity in developing countries and the timing of menarche is presented in Figure 8. Girls who experienced food insecurity in childhood had a lower risk of early menarche (RR 0.81, 95% CI: 0.67, 0.98, p = 0.027).
Figure 8.
Forest plot of relative risk of early menarche onset for food insecurity [30,33,40].
3.4. Sensitivity Analysis and Publication Bias
The sensitivity analysis confirmed the robust association between the delay of age at menarche and prolonged breastfeeding duration or food insecurity (Supplementary Figures S1 and S2). However, the exclusion of the Rogers et al. study [12] altered the association between protein intake and earlier menarche to insignificant (RR 1.00, 95% CI: 0.91 to 1.11, Supplementary Figure S3c). Conversely, omitting Koprowski et al. study [15] materially changed the association between total energy intake and earlier menarche (RR 1.16, 95% CI: 1.01, 1.33, Supplementary Figure S3a). Likewise, the association between carbohydrate intake and earlier menarche became significant after removing Meyer et al. study [36] (RR 0.85, 95% CI: 0.73, 0.98, Supplementary Figure S3b) or Moisan et al. study [26] (RR 0.85, 95% CI: 0.73, 0.99, Supplementary Figure S3b). Publication bias was only detected for the studies of carbohydrate (Egger’s test: p = 0.006) and fat intake (Egger’s test: p = 0.000).
4. Discussion
In this systematic review and meta-analysis, our findings suggested that prolonged breastfeeding duration and high intake of yogurt had protective effects on earlier menarche. In addition, we observed a significant association between food insecurity and delayed menarche onset. In contrast, girls with a high intake of protein were more likely to experience earlier menarche. However, we did not observe significant associations of early puberty with total energy intake, carbohydrate, fat, milk, dairy, meat, vitamin, lignan, flavonols, and soy intake.
4.1. Prolonged Breastfeeding Duration and Delayed Puberty
The present meta-analysis suggested that delayed timing of menarche is related to breastfeeding duration early in life. Studies have also pointed out a preventive effect of prolonged breastfeeding duration on earlier breast development [16,25]. Insulin-like growth factor-1 (IGF1) may be of major importance in this association, as it can promote pubertal development by alleviating the inhibitory effect of dynorphin (DYN) on gonadotropin-releasing hormone (GnRH) [45]. The evidence is that the protein content of formula milk is much higher than human milk, resulting in a higher level of IGF1 [8,46]. Moreover, the breastfed infants presented a subsequent lower risk of obesity or overweight after four months postnatal [47]. Studies focusing on early-life growth discovered a negative correlation between weight in late infancy and the timing of menarche [48,49]. Furthermore, the early adiposity rebound (EAR) facilitates understanding this association. Adiposity rebound occurring before five years old is an established risk factor for downstream obesity [50]. Children who breastfed for more than four months were less likely to experience the EAR, reported in a large longitudinal study [51]. The risk of early puberty caused by obesity is, therefore, lower in children avoiding experiencing EAR. Hence, breastfeeding for at least six months, as recommended by the latest Dietary Guidelines for Americans [52], is feasible and imperative.
4.2. High Yogurt Intake and Delayed Puberty
Girls with high yogurt intake might have a lower likelihood of earlier menarche onset. Obesity is a recognized cause of early puberty, and several studies have focused on yogurt protein and anti-obesity, with mixed findings [53,54]. Meanwhile, insulin resistance, via its obesity-inducing impact, has a vital impact on advancing menarche onset [55]. According to a national cohort study in the USA, children who had yogurt regularly had a healthier insulin profile [56], but this impact might be influenced by the amount of fat in yogurts [57]. Additionally, preliminary studies suggested that probiotic bacteria in yogurt might prevent obesity by altering the gut microbiome [58,59].
4.3. Food Insecurity and Delayed Puberty
As defined, food insecurity is limited or uncertain availability of nutritionally adequate and safe foods or limited or uncertain ability to acquire acceptable foods in socially acceptable ways [60]. In underdeveloped nations and areas, food insecurity mainly refers to hunger and undernutrition, which has become a prevalent worry [61,62]. Infants and children experiencing food insecurity are prone to thinness and stunting [63,64]. Girls who are stunted or thin have later menarche onset in developing countries [65,66]. Taken together, these findings support the possible causal relationship between food insecurity and delayed menarche that we discovered. Noteworthy, this connection was denied by a cross-sectional study conducted in the US [29]. This discrepancy might be ascribed to the fact that in affluent countries, food insecurity is more related to unhealthy food like fried food and sugared drinks.
4.4. High Protein Intake and Earlier Puberty
High protein intake during childhood might be an effective predictor of early menarche. As previously stated, insulin resistance is related to earlier puberty onset. The long-term consumption of high-protein diets brings a higher risk of insulin resistance [67]. With this background, it can be hypothesized that insulin resistance is an intermediary factor linking high protein intake to earlier menarche. However, in boys, pubertal development seems not to be linked to protein intake [39]. This gender gap can be explained by the fact that girls are intrinsically more prone to insulin resistance than boys [68]. Protein intake can also promote pubertal development mediated by the increasing IGF1 level [8]. Studies have reported that girls with higher IGF1 levels experienced earlier onset of thelarche and menarche [69,70]. Of note, the type of protein intake interferes with the association with circulating IGF1 concentrations [71], thereby having diverse impacts on puberty. Thus, further studies are warranted to shed light on the relationship between different types of protein intake and early puberty.
4.5. No Association between Early Puberty and Some Other Diets or Nutrients Intake
Controversy remains as to whether the total energy and carbohydrate intake affect the pubertal onset. We did not find the effects of energy and carbohydrate intake on early puberty in our study, but this finding was not robustly supported by sensitivity analysis. A previous meta-analysis suggested that girls with a higher level of total energy and carbohydrate intake were prone to earlier menarche onset [72]. Besides, a recent study found that the total energy intake was related to the timing of voice breaks in boys [39]. We infer that the real connection might be concealed by the study year, considering the marked change in the total energy intake in the past 30 years. Although we did not demonstrate the risk of earlier menarche for high fat intake, several possible mechanisms have been proposed. Leptin appears to link fat intake to the hypothalamic-pituitary-gonadal (HPG) axis. High fat intake can cause elevated leptin levels and even leptin resistance [73]. Leptin stimulates GnRH secretion via the regulation of Kisspeptin neurons [74], followed by accelerated puberty startup. High fat intake also initiates early puberty by inducing obesity [75,76]. Interestingly, high-fat diets can induce precocious puberty independent of obesity and leptin levels [77]. However, different types of fatty acids have divergent impacts on pubertal development directly [8] or through obesity [67], which deserves further research.
The impacts of micronutrients and phytochemicals on pubertal milestones remain poorly studied. Although a prior meta-analysis indicated a link between vitamin C and earlier menarche [72], evidence of the underlying mechanism is lacking. The intermediate impact of obesity may be a feasible research direction. However, much of the literature concerning the relationship between vitamins and obesity was controversial or contradictory [78,79]. Lignan, one kind of phytochemical, is defined as phytoestrogen due to its estrogenic and antiestrogenic properties [80,81]. As one kind of polyphenol [82], flavonols have a lot of effects against obesity via their antioxidant or anti-inflammatory properties [83]. However, we did not find their relationship with pubertal development.
Despite a positive relationship between milk consumption and IGF1 [84,85], we found that total milk intake was not correlated to menarche onset. The marginal result on low-fat milk and the uncorrelation result on total milk deserves attention. We boldly speculate that there may be a milk component associated with early puberty, but the milk fat renders this relationship indeterminate. With its similar structure to 17B-estradiol (E2), isoflavones interact with the HPG axis as phytoestrogens [86], affecting puberty progression [87]. As the main source of isoflavones, soy consumption had no effect on menarche time. This null finding can mainly be explained by the divergent soy intake habit and consumption in China and the USA.
4.6. Strengths and Limitations
Some strengths deserve to be highlighted. To date, this is the first meta-analysis to simultaneously investigate the association between diet or nutrient intakes during infancy and childhood and subsequent puberty timing. Second, the sample size of studies in our meta-analysis was relatively large. Ten studies were conducted in developing countries, and eighteen were done in developed countries. Since socioeconomic and demographic characteristics could be controlled to some extent, the findings were more generalizable. Third, the risk of early puberty was predominantly estimated with hazard ratios through prospective design. The above provided strong evidence for the causal relationship between early puberty and diet or nutrients. Furthermore, notwithstanding significant heterogeneity shown by several results, the quality of included studies was all moderate or high.
Some limitations also merit attention. We chose the risk estimates using the most-adjusted model, but the confounding factors adjusted in the included studies were still diverse. Besides, we selected the risk of early menarche in the highest intake group compared with the lowest or reference group, while the classification criteria of exposure groups were divergent in each research. Moreover, several methodologies were used to assess diet or nutrient intake, raising concerns about measurement accuracy in practice. Hence, the high heterogeneity would inevitably flaw the robustness of the final results. Unfortunately, we could not perform the subgroup analysis because of the scarcer studies. In addition, due to a lack of information to transform, the risk estimates (ORs, RRs, HRs) were directly described as the RRs. When the ORs are pooled with RRs and HRs, the risk of early menarche is overestimated.
5. Conclusions
Prolonged duration of breastfeeding, high intake of yogurt, and food insecurity had inverse associations with earlier menarche. In contrast, girls with a high intake of protein were more likely to experience earlier menarche. To sum up, the diet and nutrients in infancy and childhood may play a relevant role in pubertal development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14235004/s1, Figure S1: Sensitivity analysis of studies for breastfeeding duration; Figure S2: Sensitivity analysis of studies for food insecurity; Figure S3: Sensitivity analysis of studies for (a) total energy intake, (b) carbohydrate intake, (c) protein intake, and (d) fat intake; Figure S4: Sensitivity analysis of studies for total milk intake; Table S1: Characteristics of 58 excluded studies; Table S2: Newcastle–Ottawa Scale for quality assessment of cohort studies and case-control studies.
Author Contributions
J.T. drafted the manuscript; J.T., P.X. and X.H. collected and extracted the data; J.T. conducted statistical analysis; and S.L. and C.L. supervised and revised the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant numbers 81872637 and 82173534, Project of “Unveiling the Top” for Sanya Women and Children Hospital [SYFY-JBGS-202201], and Zhongshan City Social Welfare Science and Technology Research Project [2019B1017].
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farello G., Altieri C., Cutini M., Pozzobon G., Verrotti A. Review of the Literature on Current Changes in the Timing of Pubertal Development and the Incomplete Forms of Early Puberty. Front. Pediatr. 2019;7:147. doi: 10.3389/fped.2019.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert-Lind C., Busch A.S., Petersen J.H., Biro F.M., Butler G., Bräuner E., Juul A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls. JAMA Pediatr. 2020;174:e195881. doi: 10.1001/jamapediatrics.2019.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bräuner E.V., Busch A.S., Eckert-Lind C., Koch T., Hickey M., Juul A. Trends in the Incidence of Central Precocious Puberty and Normal Variant Puberty Among Children in Denmark, 1998 to 2017. JAMA Netw. Open. 2020;3:e2015665. doi: 10.1001/jamanetworkopen.2020.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra G.D., Pandeya N., Dobson A.J., Chung H.-F., Anderson D., Kuh D., Sandin S., Giles G.G., Bruinsma F., Hayashi K., et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum. Reprod. 2017;32:679–686. doi: 10.1093/humrep/dew350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P.-J., Hou M.-F., Ou-Yang F., Tsai E.-M., Wang T.-N. Association of early-onset breast cancer with body mass index, menarche, and menopause in Taiwan. BMC Cancer. 2022;22:259. doi: 10.1186/s12885-022-09361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Célind J., Bygdell M., Martikainen J., Styrke J., Damber J.-E., Kindblom J.M., Ohlsson C. Timing of the Pubertal Growth Spurt and Prostate Cancer. Cancers. 2021;13:6238. doi: 10.3390/cancers13246238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts S.A., Kaiser U.B. Genetics in Endocrinology: Genetic etiologies of central precocious puberty and the role of imprinted genes. Eur. J. Endocrinol. 2020;183:R107–R117. doi: 10.1530/EJE-20-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calcaterra V., Verduci E., Magenes V.C., Pascuzzi M.C., Rossi V., Sangiorgio A., Bosetti A., Zuccotti G., Mameli C. The Role of Pediatric Nutrition as a Modifiable Risk Factor for Precocious Puberty. Life. 2021;11:1353. doi: 10.3390/life11121353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deardorff J., Reeves J.W., Hyland C., Tilles S., Rauch S., Kogut K., Greenspan L.C., Shirtcliff E., Lustig R.H., Eskenazi B., et al. Childhood Overweight and Obesity and Pubertal Onset Among Mexican-American Boys and Girls in the CHAMACOS Longitudinal Study. Am. J. Epidemiol. 2022;191:7–16. doi: 10.1093/aje/kwab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari V., Stefanucci S., Ferrari M., Ciofi D., Stagi S., Milanesi A., Cecchi R., Fiore R., Pierattelli M., Pittari A.M., et al. Retrospective longitudinal analysis of the effects of postnatal weight gain on the timing and tempo of puberty and menarche in a cohort of Italian girls. Ital. J. Pediatr. 2022;48:20. doi: 10.1186/s13052-022-01222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katagiri A., Nawa N., Fujiwara T. Association Between Paternal Separation During Early Childhood and Pubertal Timing Among Girls Using Longitudinal Birth Cohort in Japan. Front. Endocrinol. 2021;12:766728. doi: 10.3389/fendo.2021.766728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers I.S., Northstone K., Dunger D.B., Cooper A.R., Ness A.R., Emmett P.M. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 2010;13:2052–2063. doi: 10.1017/S1368980010001461. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Gu Q., Cui X., Zhu Z., Zang J., Wang Z., Wu F., Shen X. Higher poultry consumption was associated with an earlier age at menarche. Acta Paediatr. 2021;110:889–895. doi: 10.1111/apa.15554. [DOI] [PubMed] [Google Scholar]
- 14.Meng X., Li S., Duan W., Sun Y., Jia C. Secular Trend of Age at Menarche in Chinese Adolescents Born From 1973 to 2004. Pediatrics. 2017;140:e20170085. doi: 10.1542/peds.2017-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koprowski C., Ross R.K., Mack W.J., Henderson B.E., Bernstein L. Diet, body size and menarche in a multiethnic cohort. Br. J. Cancer. 1999;79:1907–1911. doi: 10.1038/sj.bjc.6690303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kale A., Deardorff J., Lahiff M., Laurent C., Greenspan L.C., Hiatt R.A., Windham G., Galvez M.P., Biro F.M., Pinney S.M., et al. Breastfeeding Versus Formula-Feeding and Girls’ Pubertal Development. Matern. Child Health J. 2014;19:519–527. doi: 10.1007/s10995-014-1533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blell M., Pollard T.M., Pearce M.S. Predictors of Age at Menarche in the Newcastle Thousand Families Study. J. Biosoc. Sci. 2008;40:563–575. doi: 10.1017/S0021932007002696. [DOI] [PubMed] [Google Scholar]
- 18.Burris M.E., Wiley A.S. Marginal Food Security Predicts Earlier Age at Menarche Among Girls From the 2009-2014 National Health and Nutrition Examination Surveys. J. Pediatr. Adolesc. Gynecol. 2021;34:462–470. doi: 10.1016/j.jpag.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Yuan S.-Q., Liu Y.-M., Liang W., Li F.-F., Zeng Y., Liu Y.-Y., Huang S.-Z., He Q.-Y., Quach B., Jiao J., et al. Association Between Eating Speed and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Nutr. 2021;8:700936. doi: 10.3389/fnut.2021.700936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che T., Yan C., Tian D., Zhang X., Liu X., Wu Z. The Association Between Sleep and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021;12:773646. doi: 10.3389/fendo.2021.773646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English S., Wright I., Ashburn V., Ford G., Caramaschi D. Prenatal anxiety, breastfeeding and child growth and puberty: Linking evolutionary models with human cohort studies. Ann. Hum. Biol. 2020;47:106–115. doi: 10.1080/03014460.2020.1751286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris D.H., Jones M., Schoemaker M., Ashworth A., Swerdlow A. Determinants of age at menarche in the UK: Analyses from the Breakthrough Generations Study. Br. J. Cancer. 2010;103:1760–1764. doi: 10.1038/sj.bjc.6605978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Mathkoori R., Albatineh A.N., Al-Shatti M., Al-Taiar A. Is age of menarche among school girls related to breastfeeding during infancy? Am. J. Hum. Biol. 2018;30:e23122. doi: 10.1002/ajhb.23122. [DOI] [PubMed] [Google Scholar]
- 24.Hvidt J.J., Brix N., Ernst A., Lunddorf L.L., Ramlau-Hansen C.H. Breast feeding and timing of puberty in boys and girls: A nationwide cohort study. Paediatr. Périnat. Epidemiol. 2021;35:578–589. doi: 10.1111/ppe.12767. [DOI] [PubMed] [Google Scholar]
- 25.Aghaee S., Deardorff J., Greenspan L.C., Quesenberry C.P., Jr., Kushi L.H., Kubo A. Breastfeeding and timing of pubertal onset in girls: A multiethnic population-based prospective cohort study. BMC Pediatr. 2019;19:277. doi: 10.1186/s12887-019-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moisan J., Meyer F., Gingras S. A Nested Case-Control Study of the Correlates of Early Menarche. Am. J. Epidemiol. 1990;132:953–961. doi: 10.1093/oxfordjournals.aje.a115738. [DOI] [PubMed] [Google Scholar]
- 27.Gaskins A.J., Pereira A., Quintiliano D., Shepherd J., Uauy R., Corvalán C., Michels K.B. Dairy intake in relation to breast and pubertal development in Chilean girls. Am. J. Clin. Nutr. 2017;105:1166–1175. doi: 10.3945/ajcn.116.150359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen E.C., Marín C., Mora-Plazas M., Villamor E. Higher Childhood Red Meat Intake Frequency Is Associated with Earlier Age at Menarche. J. Nutr. 2015;146:792–798. doi: 10.3945/jn.115.226456. [DOI] [PubMed] [Google Scholar]
- 29.Burris M., Miller E., Romero-Daza N., Himmelgreen D. Food Insecurity and Age at Menarche in Tampa Bay, Florida. Ecol. Food Nutr. 2020;59:346–366. doi: 10.1080/03670244.2020.1727464. [DOI] [PubMed] [Google Scholar]
- 30.Jansen E.C., Herrán O.F., Villamor E. Trends and correlates of age at menarche in Colombia: Results from a nationally representative survey. Econ. Hum. Biol. 2015;19:138–144. doi: 10.1016/j.ehb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Carwile J.L., Willett W.C., Wang M., Rich-Edwards J., Frazier A.L., Michels K.B. Milk Consumption after Age 9 Years Does Not Predict Age at Menarche. J. Nutr. 2015;145:1900–1908. doi: 10.3945/jn.115.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tehrani F.R., Moslehi N., Asghari G., Gholami R., Mirmiran P., Azizi F. Intake of Dairy Products, Calcium, Magnesium, and Phosphorus in Childhood and Age at Menarche in the Tehran Lipid and Glucose Study. PLoS ONE. 2013;8:e57696. doi: 10.1371/journal.pone.0057696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belachew T., Hadley C., Lindstrom D., Getachew Y., Duchateau L., Kolsteren P. Food insecurity and age at menarche among adolescent girls in Jimma Zone Southwest Ethiopia: A longitudinal study. Reprod. Biol. Endocrinol. 2011;9:125. doi: 10.1186/1477-7827-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley A.S. Milk Intake and Total Dairy Consumption: Associations with Early Menarche in NHANES 1999-2004. PLoS ONE. 2011;6:e14685. doi: 10.1371/journal.pone.0014685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maclure M., Travis L.B., Willett W., MacMahon B. A prospective cohort study of nutrient intake and age at menarche. Am. J. Clin. Nutr. 1991;54:649–656. doi: 10.1093/ajcn/54.4.649. [DOI] [PubMed] [Google Scholar]
- 36.Meyer F., Moisan J., Marcoux D., Bouchard C. Dietary and Physical Determinants of Menarche. Epidemiology. 1990;1:377–381. doi: 10.1097/00001648-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y., Xiong J., Gao W., Wang X., Shan S., Zhao L., Cheng G. Dietary Fat and Polyunsaturated Fatty Acid Intakes during Childhood Are Prospectively Associated with Puberty Timing Independent of Dietary Protein. Nutrients. 2022;14:275. doi: 10.3390/nu14020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo M.M., Rohan T.E., Jain M., McLaughlin J.R., Corey P.N. A cohort study of dietary fibre intake and menarche. Public Health Nutr. 2002;5:353–360. doi: 10.1079/PHN2002261. [DOI] [PubMed] [Google Scholar]
- 39.Cheng T.S., Sharp S.J., Brage S., Emmett P.M., Forouhi N.G., Ong K.K. Longitudinal associations between prepubertal childhood total energy and macronutrient intakes and subsequent puberty timing in UK boys and girls. Eur. J. Nutr. 2021;61:157–167. doi: 10.1007/s00394-021-02629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villamor E., Marín C., Mora-Plazas M., Oliveros H. Micronutrient status in middle childhood and age at menarche: Results from the Bogotá School Children Cohort. Br. J. Nutr. 2017;118:1097–1105. doi: 10.1017/S0007114517003130. [DOI] [PubMed] [Google Scholar]
- 41.Segovia-Siapco G., Pribis P., Messina M., Oda K., Sabaté J. Is soy intake related to age at onset of menarche? A cross-sectional study among adolescents with a wide range of soy food consumption. Nutr. J. 2014;13:54. doi: 10.1186/1475-2891-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong J., Xu Y., Liu X., Wang X., Shan S., Crabbe M.J.C., Zhao L., Fang H., Cheng G. Prospective association of dietary soy and fibre intake with puberty timing: A cohort study among Chinese children. BMC Med. 2022;20:145. doi: 10.1186/s12916-022-02320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff M.S., Britton J.A., Boguski L., Hochman S., Maloney N., Serra N., Liu Z., Berkowitz G., Larson S., Forman J. Environmental exposures and puberty in inner-city girls. Environ. Res. 2008;107:393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mervish N.A., Gardiner E.W., Galvez M.P., Kushi L.H., Windham G.C., Biro F.M., Pinney S.M., Rybak M.E., Teitelbaum S.L., Wolff M.S. Dietary flavonol intake is associated with age of puberty in a longitudinal cohort of girls. Nutr. Res. 2013;33:534–542. doi: 10.1016/j.nutres.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dees W.L., Hiney J.K., Srivastava V.K. IGF-1 Influences Gonadotropin-Releasing Hormone Regulation of Puberty. Neuroendocrinology. 2021;111:1151–1163. doi: 10.1159/000514217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larnkjaer A., Mølgaard C., Michaelsen K.F. Early nutrition impact on the insulin-like growth factor axis and later health consequences. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:285–292. doi: 10.1097/MCO.0b013e328351c472. [DOI] [PubMed] [Google Scholar]
- 47.Agosti M., Tandoi F., Morlacchi L., Bossi A. Nutritional and metabolic programming during the first thousand days of life. La Pediatr. Med. E Chir. 2017;39:157. doi: 10.4081/pmc.2017.157. [DOI] [PubMed] [Google Scholar]
- 48.Aris I.M., Perng W., Dabelea D., Ganiban J.M., Liu C., Marceau K., Robertson O.C., Hockett C.W., Mihalopoulos N.L., Kong X., et al. Analysis of Early-Life Growth and Age at Pubertal Onset in US Children. JAMA Netw. Open. 2022;5:e2146873. doi: 10.1001/jamanetworkopen.2021.46873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei J., Liu S., Cheng Y., Yang W., Zhu Z., Zeng L. Association of Infant Physical Development and Rapid Growth With Pubertal Onset Among Girls in Rural China. JAMA Netw. Open. 2021;4:e216831. doi: 10.1001/jamanetworkopen.2021.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolland-Cachera M.F., Deheeger M., Maillot M., Bellisle F. Early adiposity rebound: Causes and consequences for obesity in children and adults. Int. J. Obes. 2006;30((Suppl. S4)):S11–S17. doi: 10.1038/sj.ijo.0803514. [DOI] [PubMed] [Google Scholar]
- 51.Lin D., Chen D., Huang J., Li Y., Wen X., Wang L., Shi H. Pre-Birth and Early-Life Factors Associated With the Timing of Adiposity Peak and Rebound: A Large Population-Based Longitudinal Study. Front. Pediatr. 2021;9:742551. doi: 10.3389/fped.2021.742551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips J.A. Dietary Guidelines for Americans, 2020–2025. Work. Health Saf. 2021;69:395. doi: 10.1177/21650799211026980. [DOI] [PubMed] [Google Scholar]
- 53.Razmpoosh E., Zare S., Fallahzadeh H., Safi S., Nadjarzadeh A. Effect of a low energy diet, containing a high protein, probiotic condensed yogurt, on biochemical and anthropometric measurements among women with overweight/obesity: A randomised controlled trial. Clin. Nutr. ESPEN. 2020;35:194–200. doi: 10.1016/j.clnesp.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Zheng M., Yu H.-J., He Q.-Q., Heitmann B.L., Rangan A., McNaughton S.A., Campbell K.J. Protein Intake During Infancy and Subsequent Body Mass Index in Early Childhood: Results from the Melbourne InFANT Program. J. Acad. Nutr. Diet. 2021;121:1775–1784. doi: 10.1016/j.jand.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Di Sessa A., Grandone A., Marzuillo P., Umano G.R., Cirillo G., del Giudice E.M. Early menarche is associated with insulin-resistance and non-alcoholic fatty liver disease in adolescents with obesity. J. Pediatr. Endocrinol. Metab. 2021;34:607–612. doi: 10.1515/jpem-2020-0684. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y., Wang H., Hollis J.H., Jacques P.F. The associations between yogurt consumption, diet quality, and metabolic profiles in children in the USA. Eur. J. Nutr. 2015;54:543–550. doi: 10.1007/s00394-014-0735-7. [DOI] [PubMed] [Google Scholar]
- 57.Slurink I.A.L., Voortman T., Ochoa-Rosales C., Ahmadizar F., Kavousi M., Kupper N., Smeets T., Soedamah-Muthu S.S. Dairy Product Consumption in Relation to Incident Prediabetes and Longitudinal Insulin Resistance in the Rotterdam Study. Nutrients. 2022;14:415. doi: 10.3390/nu14030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez-Moreno M., Perez-Herrera A., Locia-Morales D., Dizzel S., Meyre D., Stearns J.C., Cruz M. Association of gut microbiome with fasting triglycerides, fasting insulin and obesity status in Mexican children. Pediatr. Obes. 2020;16:e12748. doi: 10.1111/ijpo.12748. [DOI] [PubMed] [Google Scholar]
- 59.Carrizales-Sánchez A.K., García-Cayuela T., Hernández-Brenes C., Senés-Guerrero C. Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects. Gut Microbes. 2021;13:1960135. doi: 10.1080/19490976.2021.1960135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson S.A. Core Indicators of Nutritional State for Difficult-to-Sample Populations. J. Nutr. 1990;120((Suppl. S11)):1559–1600. doi: 10.1093/jn/120.suppl_11.1555. [DOI] [PubMed] [Google Scholar]
- 61.Shahraki S.H., Amirkhizi F., Amirkhizi B., Hamedi S. Household Food Insecurity Is Associated with Nutritional Status among Iranian Children. Ecol. Food Nutr. 2016;55:473–490. doi: 10.1080/03670244.2016.1212710. [DOI] [PubMed] [Google Scholar]
- 62.Kastorini C.-M., Markaki I., Tsiampalis T., Critselis E., Petralias A., Linos A., the DIATROFI Program Research Team Dietary patterns and food insecurity of students participating in a food aid programme: The Mediterranean perspective. Eur. J. Public Health. 2021;31:143–150. doi: 10.1093/eurpub/ckaa178. [DOI] [PubMed] [Google Scholar]
- 63.Agho K.E., Mukabutera C., Mukazi M., Ntambara M., Mbugua I., Dowling M., Kamara J.K. Moderate and severe household food insecurity predicts stunting and severe stunting among Rwanda children aged 6–59 months residing in Gicumbi district. Matern. Child Nutr. 2019;15:e12767. doi: 10.1111/mcn.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moradi S., Mirzababaei A., Mohammadi H., Moosavian S.P., Arab A., Jannat B., Mirzaei K. Food insecurity and the risk of undernutrition complications among children and adolescents: A systematic review and meta-analysis. Nutrition. 2019;62:52–60. doi: 10.1016/j.nut.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 65.Svefors P., Pervin J., Khan A.I., Rahman A., Ekström E., El Arifeen S., Selling K.E., Persson L. Stunting, recovery from stunting and puberty development in the MINIMat cohort, Bangladesh. Acta Paediatr. 2020;109:122–133. doi: 10.1111/apa.14929. [DOI] [PubMed] [Google Scholar]
- 66.Patil S.N., Joglekar C.V., Mohite R.B., Surve M.M., Sonawane S., Chavan R. Menarche among rural adolescent girls in Dervan (Data from the KONKAN region of the state of Maharashtra, India) J. Fam. Med. Prim. Care. 2020;9:5276–5281. doi: 10.4103/jfmpc.jfmpc_509_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wali J., Solon-Biet S., Freire T., Brandon A. Macronutrient Determinants of Obesity, Insulin Resistance and Metabolic Health. Biology. 2021;10:336. doi: 10.3390/biology10040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy M.J., Metcalf B.S., Voss L.D., Jeffery A.N., Kirkby J., Mallam K.M., Wilkin T.J. Girls at Five Are Intrinsically More Insulin Resistant Than Boys: The Programming Hypotheses Revisited—The EarlyBird Study (EarlyBird 6) Pediatrics. 2004;113:82–86. doi: 10.1542/peds.113.1.82. [DOI] [PubMed] [Google Scholar]
- 69.Upners E.N., Busch A.S., Almstrup K., Petersen J.H., Assens M., Main K.M., Jensen R.B., Juul A. Does height and IGF-I determine pubertal timing in girls? Pediatr. Res. 2021;90:176–183. doi: 10.1038/s41390-020-01215-6. [DOI] [PubMed] [Google Scholar]
- 70.Biro F.M., Huang B., Wasserman H., Gordon C.M., Pinney S.M. Pubertal Growth, IGF-1, and Windows of Susceptibility: Puberty and Future Breast Cancer Risk. J. Adolesc. Health. 2021;68:517–522. doi: 10.1016/j.jadohealth.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watling C.Z., Kelly R.K., Tong T.Y., Piernas C., Watts E.L., Tin S.T., Knuppel A., Schmidt J.A., Travis R.C., Key T.J., et al. Associations of circulating insulin-like growth factor-I with intake of dietary proteins and other macronutrients. Clin. Nutr. 2021;40:4685–4693. doi: 10.1016/j.clnu.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen N., Fan H.-Y., Tsai M.-C., Tung T.-H., Huynh Q., Huang S.-Y., Chen Y. Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis. Nutrients. 2020;12:2544. doi: 10.3390/nu12092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendoza-Herrera K., Florio A.A., Moore M., Marrero A., Tamez M., Bhupathiraju S.N., Mattei J. The Leptin System and Diet: A Mini Review of the Current Evidence. Front. Endocrinol. 2021;12:749050. doi: 10.3389/fendo.2021.749050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruno C., Vergani E., Giusti M., Oliva A., Cipolla C., Pitocco D., Mancini A. The “Adipo-Cerebral” Dialogue in Childhood Obesity: Focus on Growth and Puberty. Physiopathological and Nutritional Aspects. Nutrients. 2021;13:3434. doi: 10.3390/nu13103434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X., Zhuo S., Zhu T., Yao P., Yang M., Mei H., Li N., Ma F., Wang J.M., Chen S., et al. Fpr2 Deficiency Alleviates Diet-Induced Insulin Resistance Through Reducing Body Weight Gain and Inhibiting Inflammation Mediated by Macrophage Chemotaxis and M1 Polarization. Diabetes. 2019;68:1130–1142. doi: 10.2337/db18-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mishra A., Mirzaei H., Guidi N., Vinciguerra M., Mouton A., Linardic M., Rappa F., Barone R., Navarrete G., Wei M., et al. Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat. Metab. 2021;3:1342–1356. doi: 10.1038/s42255-021-00469-6. [DOI] [PubMed] [Google Scholar]
- 77.Ullah R., Raza A., Rauf N., Shen Y., Zhou Y.-D., Fu J. Postnatal Feeding With a Fat Rich Diet Induces Precocious Puberty Independent of Body Weight, Body Fat, and Leptin Levels in Female Mice. Front. Endocrinol. 2019;10:758. doi: 10.3389/fendo.2019.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang W., Zhan W., Wei M., Chen Q. Associations Between Different Dietary Vitamins and the Risk of Obesity in Children and Adolescents: A Machine Learning Approach. Front. Endocrinol. 2021;12:816975. doi: 10.3389/fendo.2021.816975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kardaş F., Yücel A.D., Kendirci M., Kurtoğlu S., Hatipoğlu N., Akın L., Gül Ü., Gökay S., Üstkoyuncu P.S. Evaluation of micronutrient levels in children and adolescents with obesity and their correlation with the components of metabolic syndrome. Turk. J. Pediatr. 2021;63:48–58. doi: 10.24953/turkjped.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Rodríguez-García C., Sánchez-Quesada C., Toledo E., Delgado-Rodríguez M., Gaforio J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules. 2019;24:917. doi: 10.3390/molecules24050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aehle E., Müller U., Eklund P.C., Willför S.M., Sippl W., Dräger B. Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry. 2011;72:2396–2405. doi: 10.1016/j.phytochem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12:457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pérez-Torres I., Castrejón-Téllez V., Soto M., Rubio-Ruiz M., Manzano-Pech L., Guarner-Lans V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021;22:1786. doi: 10.3390/ijms22041786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grenov B., Larnkjær A., Mølgaard C., Michaelsen K.F. Role of Milk and Dairy Products in Growth of the Child. Nestlé Nutr. Inst. Workshop Ser. 2020;93:77–90. doi: 10.1159/000503357. [DOI] [PubMed] [Google Scholar]
- 85.Wiley A.S., Joshi S.M., Lubree H.G., Bhat D.S., Memane N.S., Raut D.A., Yajnik C.S. IGF-I and IGFBP-3 concentrations at 2 years: Associations with anthropometry and milk consumption in an Indian cohort. Eur. J. Clin. Nutr. 2018;72:564–571. doi: 10.1038/s41430-018-0108-z. [DOI] [PubMed] [Google Scholar]
- 86.Domínguez-López I., Yago-Aragón M., Salas-Huetos A., Tresserra-Rimbau A., Hurtado-Barroso S. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients. 2020;12:2456. doi: 10.3390/nu12082456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J., Kim S., Huh K., Kim Y., Joung H., Park M. High serum isoflavone concentrations are associated with the risk of precocious puberty in Korean girls. Clin. Endocrinol. 2011;75:831–835. doi: 10.1111/j.1365-2265.2011.04127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.