Abstract
Plant miRNAs are powerful regulators of gene expression at the post-transcriptional level, which was repeatedly proved in several model plant species. miRNAs are considered to be key regulators of many developmental, homeostatic, and immune processes in plants. However, our understanding of plant miRNAs is still limited, despite the fact that an increasing number of studies have appeared. This systematic review aims to summarize our current knowledge about miRNAs in spring barley (Hordeum vulgare), which is an important agronomical crop worldwide and serves as a common monocot model for studying abiotic stress responses as well. This can help us to understand the connection between plant miRNAs and (not only) abiotic stresses in general. In the end, some future perspectives and open questions are summarized.
Keywords: gene expression, regulation, barley, miRNAs, plants, environmental stress
1. Introduction
MicroRNAs (miRNAs) are important players in post-transcriptional gene expression regulation in multicellular species. miRNAs can modify/decrease the expression of fully or partially complementary mRNA molecules [1,2]. Original reports about RNA silencing, where miRNAs belong, date back to the 1990s [3] when the first attempt to introduce a chimeric chalcone synthase using Agrobacterium tumefaciens vector led to the decrease or complete loss of anthocyanin pigmentation in petals (flowers) of Petunia hybrida [4]. A similar observation was in 1992 documented also for the Neurospora crassa where the transformation using plasmids containing artificial constructs led to the albino phenotype [5]. Another experiment in 1993 carried out by Victor Ambros, Rosalind Lee and Rhonda Feinbaum resulted in the final revelation that the lin-4 gene (involved in the regulation of Caenorhabditis elegans developmental events) codes not for a protein, but for small RNA with regulatory function [6], and such RNAs were later called miRNAs and their nomenclature was established [7]. Today, miRNAs are considered to be master regulators of many cell differentiation, developmental, and homeostatic processes in animals [8] and plants [9,10], and are also widely accepted as an important component of the cellular immune system, which is documented even for plant species [11,12,13,14].
Despite the fact that the number of studies dealing with plant miRNAs during the past few years has steeply increased (from 11 articles in 2002 to the current 928 in 2021), our complex understanding of their role in environmental stress responses remains limited, see, e.g., current review dealing with miRNA regulation and stress adaptation in plants [15]. Our specific focus on barley stems from the importance of this agronomical crop, which was one of the first cultivated grains as early as 10,000 years ago [16]. Barley is currently ranked 4th in worldwide production after wheat, maize, and rice, and is extensively used in food production, for feeding cattle, or brewery worldwide [17,18]. Additionally, barley plants serve as an important commodity with potential health benefits—from hail to barley grass food supplements. Effects on the gut microbiota and suppression of already developed chronic diseases including obesity, diabetes, circulatory disorders, and cancer were documented or at least hypothesized [19,20]. An additional and equally important application of barley seedlings is its use as a monocot model species (often considered as a model plant for the whole Triticeae tribe). Barley has a short cultivation period from seed planting to sampling the material (14 days) and does not require complex growth conditions which makes it a suitable species for a wide range of experiments [21,22,23]. Lastly, barley has a sequenced reference genome (cultivar Morex, NCBI ID: GCF_904849725.1) of a length of 4.27 Gbps consisting of 7 chromosomes, and circular chloroplastic DNA [24]. More than one-half of all 58,438 predicted genes (53%; total of 31,449) are protein-coding genes, while approximately one-tenth are pseudogenes (9.9%; total of 5778) [25]. Most importantly, almost one-third of the barley genes are coding for small RNAs (30.3%; total of 17,729) [25] which further highlights their functional relevance in gene expression regulation and justifies the need to properly understand their involvement in physiological and stress-related processes. The rest of the genes are uncharacterized (6%; a total of 3481) [25]. For the above-mentioned reasons, we decided to perform a review focused specifically on spring barley (Hordeum vulgare) miRNAs. In the following chapters, we will briefly discuss plant miRNAs biogenesis and regulatory potential in general, then we will move to specific roles of miRNAs in barley physiology and stress responses, conserved barley miRNAs and their high-confidence mRNA targets, and finally give some possible future directions of research in this field, with focus on barley.
2. Plant miRNAs—Biogenesis and Regulatory Potential
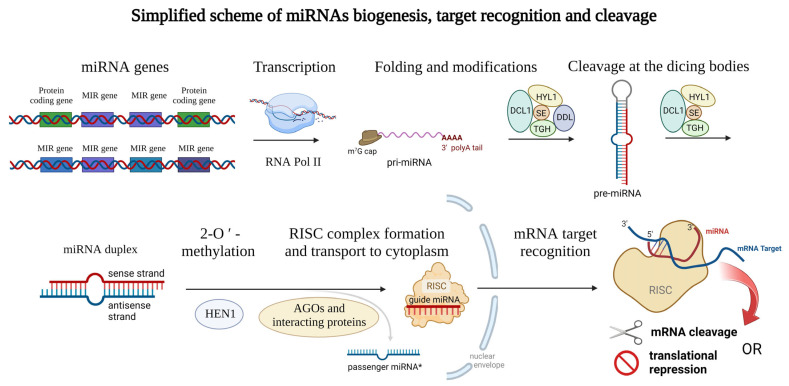
Mature plant miRNAs are 19–25-nucleotide-long ribonucleic acids that can have either intergenic (miRNA gene is localized between two protein-coding sequences of the DNA) or intragenic origin [26], where miRNAs are cleaved from the mRNA sequences during the splicing (also called intron-derived miRNAs [27]). Specifically, in barley, more than 75% of miRNAs are transcribed from intergenic loci [28]. The biogenesis of miRNAs is ensured by the DNA-dependent RNA polymerase II which is responsible for the biosynthesis itself [2]. In some cases, multiple plant miRNAs are synthesized all at once (multiple miRNAs localized in one long transcript) [29] and often form a miRNA family, which is a group of miRNAs derived from a common ancestor [30]. Emerging miRNAs can be modified co-transcriptionally, or post-transcriptionally. Similar to other transcripts, a 7-methylguanosine (m7G) cap is attached to the 5′ end of the miRNA, and the 3′ end is polyadenylated (or can be spliced) [31]. Later, the transcript encoding miRNA (or multiple miRNAs) is folded to the stem-loop structure which is called pri-miRNA [2] (meaning primary miRNA transcript). Such pri-miRNAs are further cleaved by the dicing bodies. Dicing bodies consists of several proteins including DICER-LIKE 1 (DCL1), DAWDLE (DDL), HYL1, TGH, and SE [32,33], resulting in miRNA duplex formation which can be later 2′-O-methylated by the HEN1 methylase [34] and incorporated into the RNA-induced silencing complex (RISC) [2,35]. The complex issue of further proteins involved in plant miRNA biogenesis is reviewed in Li et al., 2021 [36]. miRNAs of both origins (intragenic as well as intergenic) lead to the formation of a mature RISC with incorporated mature miRNA. In most cases, only the sense/guide miRNA strand is incorporated into the RISC, while the antisense/passenger miRNA (miRNA*) strand is disrupted, but recently also the regulation potential of the passenger miRNA became the center of interest [14,37,38]. For a clear summary of miRNA biogenesis see Figure 1 below.
Figure 1.
Schematic representation of the miRNAs biogenesis. Genes encoding miRNAs are transcribed by RNA polymerase II and modified on their ends (m7G cap and polyA tail) and thus the primary microRNA (pri-miRNA) arise. Then, the typical stem-loop structure is formed by complementary base pairing and cleaved at the dicing bodies (consisting of several proteins including DCL1, HYL1, SE, TIGH, and DDL) resulting in miRNA duplex formation which can be later 2′-O-methylated (ensured by the HEN1 protein). Guide miRNA is incorporated into the RISC consisting of several proteins, and transported into the cytoplasm, where mRNA target recognition and cleavage can take place while the passenger miRNA is released away. Proteins from the Argonaute family (AGOs) can modify the stability of the miRNAs and also affect the interaction with target mRNAs. This figure was created using BioRender (https://biorender.com/; accessed on 20 June 2022).
miRNAs interact with their target mRNAs mostly at their 3′ UTRs, but interactions occurring in the 5′ UTRs or coding regions were documented as well [39,40]. RISC is directed to the complementary mRNA transcript, whereby the Watson–Crick base-pairing aligns guide miRNA and target mRNA transcript, and depending on the central miRNA region complementarity, mRNA is cleaved (usually when there is perfect base-pair complementarity), or translation repression occurs (central miRNA region is not completely complementary to mRNA) [41]. Moreover, in the case the target mRNA is cleaved, so-called phased secondary small interfering RNAs (phasiRNAs) can arise [42]. phasiRNAs are 21 or 24-nucleotide-long siRNAs having important roles in plant stress responses [42], development [43], and reproduction [44].
Similar to the other genes, miRNA transcription is precisely fine-tuned. This is assured mainly by transcription factors binding [45] and methylation status of DNA [46], both heavily influenced by endogenous and exogenous stimuli. In 2018, protein WHIRLY1 was found to be involved in increased levels of nuclear miRNAs in high-light conditions in barley. It was therefore proposed that WHIRLY1 can bind to RNA and it might be a general factor influencing the biogenesis and/or stability of various miRNAs [47].
An additional level of miRNA complexity is their dynamic stability [33,48]. It was documented that the processes such as 3′-end modifications and interaction with Argonaute proteins (AGOs) can both reduce and increase the stability of miRNAs depending on the actual needs of the plant. For example, AGO1 from Arabidopsis thaliana was proposed to stabilize miRNAs, and miRNA–mRNA target interaction [2].
Besides post-transcriptional gene silencing (PTGS), miRNAs can regulate plant genes via RNA-induced methylation of DNA [49,50]. Such a process was in detail described in the Arabidopsis thaliana, where miRNAs (miR165, miR166) regulate the methylation status of PHB and PHV genes [51], and are responsible for the determination of the abaxial and adaxial leaf side. Similarly, the miRNA-induced gene methylation was described even in the Oryza sativa where the miR1873 ensures the methylation of its own gene [50]. To make our understanding of miRNAs-based regulation of gene expression more challenging, the stimulative effect of miRNAs on gene expression was observed and documented as well [52].
Last but not least, in 2015 it was proposed that plant pri-miRNAs are capable of encoding small functional peptides [53,54] described as miPEPs. The best-characterized miPEPs (miPEP171d, miPEP172c, and miPEP858a) were found in plant species including Arabidopsis thaliana (miPEP165a [53], miPEP858 [55]), Medicago truncatula (miPEP171b [41]), Glycine max (miPEP172c [56]), and Vitis vinifera (miPEP171d1 [57]). The mechanism of miPEPs molecular function is still largely unclear, but generally, miPEPs positively affect the accumulation of their associated miRNAs [54]. It is also likely that many of miPEPs will be species-specific [57].
3. miRNAs in Barley Physiology and Stress Responses
miRNAs in plants are important regulators of various physiological processes including shoot apical meristem development [58], leaf growth [59], flower formation [60], seed production [61], and root expansion [59]. It was found that miRNA171 in barley is responsible for the regulation of shoot meristem development through three independent pathways, i.e., firstly through the down-regulation of SCARECROW-LIKE (SCL) transcription factors, secondly via up-regulation of miRNA156 and repressing vegetative phase transitions (a possibly monocotyledon-specific mechanism), and thirdly by repressing expression of TRD and HvPLA1 genes [62]. Additionally, flower development in grasses including barley is tightly regulated by miRNAs. It was found that miRNA159, miRNA171, miRNA172, and miRNA396 regulate the expression of floral organ identity genes in barley, rice, and maize [63]. In barley, cleistogamous flowering (i.e., shedding its pollen before opening) arises from the suppression of the AP2 transcription factor via miR172, originally thought to be a result of target mRNA cleavage [64], but later it was proved that miR172-mediated AP2 regulation occurs at the translational level [65]. It is also known that the expression of barley miR393 is active in the developmental period, and its misexpression affects seedling growth and stomatal density [66]. In 2018, it was found that miR160 in barley simultaneously targets class II ARF members which are functionally involved in developmental stages by regulating the auxin-mediated genes [67]. Figure 2 illustratively depicts some of the most known barley miRNAs (and their targets) that play important roles in developmental processes.
Figure 2.
miRNAs play important roles also in the developmental processes. In spring barley (Hordeum vulgare), specific miRNAs were linked with the targets involved in the regulation of flowering, root development, seed germination, and also with stomata development. Inhibition is indicated by the red ┴ mark, while positive effect by the green arrow. This figure was created using BioRender (https://biorender.com/; accessed on 20 June 2022).
Besides the non-stress conditions, miRNAs play key roles in gene expression regulation in response to a variety of abiotic stimuli, including several stress responses. In plants, their involvement in many abiotic stress responses including heat stress responses, low-temperature responses, drought exposure responses, carbon dioxide responses, light stress responses, or gamma radiation responses was reported [68,69,70,71,72]. Specifically in barley, miRNAs responsive to salinity stress [73,74,75,76], drought [77,78,79,80,81], nitrogen [82], boron [83], phosphorus [84,85], aluminum [74,86,87], cadmium [88], cold deacclimation [89], heat stress [90], and possibly to light [21] were identified till date. A chronological summary of the most impactful miRNA studies in barley (starting from 2010) can be found below in Table 1.
Table 1.
Chronological summary of studies dealing with miRNAs in barley species and most important results obtained.
| Title of the Study and Reference | Barley Cultivars Inspected |
Year of Publication |
Most Important Findings |
|---|---|---|---|
| Regulation of barley miRNAs upon dehydration stress correlated with target gene expression [79] | Hordeum vulgare | 2010 | A total of 28 potential miRNAs were identified using bioinformatic approaches (BLASTn of known plant miRNAs and barley expressed sequence tags (ESTs), and RNA folding algorithms). |
| Discovery of barley miRNAs through deep sequencing of short reads [91] | Hordeum vulgare cultivars Golden Promise and Pallas | 2011 | The first large-scale study of miRNAs in Hordeum Vulgare, 100 miRNAs were identified (only 56 of them had orthologs in wheat, rice, or Brachypodium) and 3 candidates were validated in vitro using a Northern blot assay. |
| Identification and Characterization of MicroRNAs from Barley (Hordeum vulgare L.) by High-Throughput Sequencing [92] | Hordeum vulgare L. | 2012 | 126 conserved miRNAs (belonging to 58 families), and 133 novel miRNAs (50 families) were identified in this study. |
| miRNA regulation in the early development of barley seed [61] | Hordeum vulgare | 2012 | 84 known miRNAs and 7 new miRNAs together with 96 putative miRNA target genes were identified during the early development of barley seeds (first 15 days post anthesis). |
| Developmentally regulated expression and complex processing of barley pri-microRNAs [93] | Hordeum vulgare cultivar Rolap | 2013 | miRNA genes in barley often contain introns which may play important role in miRNA processing. |
| A Comprehensive Expression Profile of MicroRNAs and Other Classes of Non-Coding Small RNAs in Barley Under Phosphorous-Deficient and -Sufficient Conditions [84] | Hordeum vulgare L., cultivar Pallas | 2013 | 221 conserved miRNAs and 12 novel miRNAs were identified, many of them were phosphorus condition-specific. A total of 47 miRNAs were significantly differentially expressed between the two phosphorus treatments. |
| Boron Stress Responsive MicroRNAs and Their Targets in Barley [83] | Hordeum vulgare L. cultivar Sahara | 2013 | 31 known and 3 new miRNAs were identified in barley, and 25 of them were found to respond to boron treatment. |
| Transcriptionally and post-transcriptionally regulated microRNAs in heat stress response in barley [90] | Hordeum vulgare cultivar Rolap | 2014 | Four heat stress up-regulated barley miRNAs were found (miR160a, miR166a, miR167h, and miR5175a). |
| Differential expression of microRNAs and other small RNAs in barley between water and drought conditions [80] | Hordeum vulgare cultivar Golden Promise | 2014 | Three novel miRNAs, designated as hvu-miRX33, hvu-miRX34, and hvu-miRX35 were identified. hvu-miRX34 had no homologous miRNA in wheat. |
| The miR9863 Family Regulates Distinct Mla Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling [94] | Hordeum vulgare L. | 2014 | The key role of the miR9863 family in the immune response to the pathogen (powdery mildew fungus, Blumeria graminis f. sp. hordei) was proposed |
| Polycistronic artificial miRNA-mediated resistance to Wheat dwarf virus in barley is highly efficient at low temperature [95] | Artificially transformed Hordeum vulgare cultivar Golden Promise | 2015 | Polycistronic artificial miRNA in plasmid vector was successfully transformed into barley embryos and mediated resistance to Wheat dwarf virus. |
| Global Identification of MicroRNAs and Their Targets in Barley under Salinity Stress [73] | Hordeum vulgare cultivar Morex | 2015 | Authors identified 152 miRNAs (142 conserved and 10 novel ones), and 44 miRNAs (39 conserved and 5 novel ones) were found to be salinity-responsive. |
| Characterization of microRNAs and their targets in wild barley (Hordeum vulgare subsp. spontaneum) using deep sequencing [96] | Hordeum vulgare subsp. spontaneum | 2016 | A total of 70 known miRNAs and 18 novel miRNA candidates were identified and many of them were predicted to target mRNAs encoding transcription factors. |
| Developmental changes in barley microRNA expression profiles coupled with miRNA target analysis [97] | Hordeum vulgare cultivar Rolap | 2016 | miRNA transcriptomes of five barley developmental stages were inspected. Overall, miR168-3p and miR1432-5p levels increased while the 5′U-miR156-5p level decreased during barley development. |
| miR393-Mediated Auxin Signaling Regulation is Involved in Root Elongation Inhibition in Response to Toxic Aluminum Stress in Barley [86] | Hordeum vulgare cultivar Golden Promise | 2017 | Barley miR393 was functionally characterized. It regulates root sensitivity to aluminum through the alteration of auxin signaling. |
| Differential expression of microRNAs and potential targets under drought stress in barley [78] | Hordeum vulgare L. cultivars Commander, Fleet, Hindmarsh, and breeding line WI4304 | 2017 | miRNA regulation under drought stress in barley is genotype-specific. |
| microRNAs participate in gene expression regulation and phytohormone cross-talk in barley embryo during seed development and germination [98] | Hordeum vulgare cultivar Golden Promise | 2017 | A total of 1324 known miRNAs and 448 novel miRNA candidates were identified. miR393-mediated auxin response regulation significantly affected grain development. |
| Small RNA Activity in Archeological Barley Shows Novel Germination Inhibition in Response to Environment [99] | Ancient Hordeum vulgare | 2017 | Sequencing of miRNAs obtained from archeological barley samples (600–900 years BP) revealed their local adaptation to an agrarian environment around the river Nile. |
| Genome-wide analysis of the SPL/miR156 module and its interaction with the AP2/miR172 unit in barley [100] | Hordeum vulgare L. | 2018 | The study identified 17 barley SPL genes, and 7 of them contain a putative miR156 target site. |
| Identification of microRNAs in response to aluminum stress in the roots of Tibetan wild barley and cultivated barley [87] | Hordeum vulgare Al-sensitive Golden Promise and Tibetan wild barley (Al-tolerant XZ29) | 2018 | 50 miRNAs responsive to aluminum stress were detected, and some of them were found to be exclusively expressed in Al-tolerant XZ29. |
| Identification of microRNAs responding to salt stress in barley by high-throughput sequencing and degradome analysis [76] | Tibetan wild barley accession XZ16; Hordeum vulgare cultivar Golden Promise | 2019 | miR393a, miR156d, and miR172b (regulating HvAFB2/HvTIR1, UGTs, and HvAP2) are responsible for salt tolerance in barley roots. |
| Genotypic difference of cadmium tolerance and the associated microRNAs in wild and cultivated barley [88] | Hordeum vulgare cultivar Golden Promise and wild barley WB-1 | 2019 | 216 conserved miRNAs (in 59 miRNA families) and 87 novel miRNAs were identified. Authors suggest that miRNAs may play critical roles underlying the genotypic difference of cadmium tolerance in barley. |
| Genome-Wide Identification and Characterization of Drought Stress Responsive microRNAs in Tibetan Wild Barley [81] | Tibetan wild barley Hordeum vulgare L. ssp. Spontaneum | 2020 | 69 conserved miRNAs and 1574 novel miRNAs were identified, some of them were differentially expressed in drought conditions. |
| Barley microRNAs as metabolic sensors for soil nitrogen availability [82] | Hordeum vulgare cultivar Golden Promise | 2020 | Authors identified 13 barley miRNAs that are nitrogen excess responsive with the possible function of metabolic sensors for soil nitrogen availability. |
| The Impact of Zinc Oxide Nanoparticles on Cytotoxicity, Genotoxicity, and miRNA Expression in Barley (Hordeum vulgare L.) Seedlings [101] | Hordeum vulgare L. var. Abava | 2020 | ZnO nanoparticles significantly changed the expression of barley miR156a, miR159a, and miR159c in a dosage-dependent manner. |
| Identification of microRNAs in response to low potassium stress in the shoots of Tibetan wild barley and cultivated [102] | A Tibetan wild barley accession (XZ153) and a cultivar (ZD9) differing in low K tolerance | 2021 | A total of 1088 miRNAs were identified in the two barley genotypes under low potassium conditions. 65 of them were significantly differentially expressed. |
| Barley Seeds miRNome Stability during Long-Term Storage and Aging [103] | Hordeum vulgare cultivar Damazy | 2021 | miRNome of barley seeds harvested in 1972 was inspected. 61 known and 81 novel miRNA were identified pointing to the fact that miRNAs in dry seeds are extremely stable. |
| Identification microRNAs and target genes in Tibetan hulless barley to BLS infection [104] | Hordeum vulgare L. variety nudum Hook. f. | 2021 | A total of 36 conserved and 56 novel miRNAs were identified, some of them were differentially expressed between BLS (barley leaf stripe fungal disease)-sensitive and BLS-tolerant barley genotypes. |
| Pi-starvation induced transcriptional changes in barley revealed by a comprehensive RNA-Seq and degradome analyses [85] | Hordeum vulgare L. | 2021 | Authors suggest that barley adapts to inorganic phosphate (Pi)-starvation also via differential expression of several miRNAs. |
| Identification of microRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots [74] | Hordeum vulgare cultivar Golden Promise | 2021 | 525 miRNAs (198 known and 327 novel miRNAs) were identified through high-throughput sequencing. 31 miRNAs were differentially expressed under inspected stresses. |
| An miR156-regulated nucleobase-ascorbate transporter 2 confers cadmium tolerance via enhanced anti-oxidative capacity in barley [105] | Hordeum vulgare genotypes Zhenong8 (ZN8) (Cd-tolerant genotype) and W6nk2 (Cd-sensitive genotype) | 2022 | miR156g-3p_3 targets a novel nucleobase-ascorbate transporter gene (HvNAT2). HvNAT2 evolved from the Zygnematales in Streptophyte algae and positively regulates cadmium tolerance → genetic engineering of NAT in plants may have potential in the remediation of soil/water cadmium pollution |
| Regulation of Phenolic Compound Production by Light Varying in Spectral Quality and Total Irradiance [21] | Hordeum vulgare L. cultivar Bojos | 2022 | Several barley miRNAs were differentially expressed in response to the spectral quality of incident light. |
From the above-mentioned studies, it is evident that barley miRNAs play a complex role in responses to various abiotic and biotic stresses or stimuli, which is schematically depicted in Figure 3.
Figure 3.
miRNAs form a complex regulatory network in barley (Hordeum vulgare). Environmental cues, both abiotic (i.e., spectral quality and intensity of the incident light, growth temperature, drought, high salinity, heavy metals exposure, etc.) and biotic (for example pathogens) can affect the expression of miRNAs and thus also their target genes. This figure was created using BioRender (https://biorender.com/; accessed on 20 June 2022).
4. Target Transcripts of Barley miRNAs
Several web-based tools, resources, and databases related to small RNAs comprising miRNAs in plants exist [106]. To browse miRNAs identified in barley to date, mainly PNRD [107], PmiREN [108], miRBase [109], Plant small RNA genes [110], or the integrative miRNEST database [111] can be used. As these databases use slightly different methods of required miRNAs evidence or data sources, overall counts of miRNAs deposited here differ. Total counts of barley miRNAs in each of the databases together with direct links and other useful information are listed in Table 2.
Table 2.
User-friendly online databases comprising barley miRNAs.
| Database Name | Direct Link | The Overall Count of Barley miRNAs | Notes |
|---|---|---|---|
| Plant Non-coding RNA Database (PNRD) | http://structuralbiology.cau.edu.cn/PNRD/index.php | 71 | 58 of them were experimentally validated |
| Plant MicroRNA Encyclopedia (PmiREN) | https://www.pmiren.com/ | 178 | Divided into 94 miRNA families |
| miRBase | https://www.mirbase.org/summary.shtml?org=hvu | 69 | / |
| Plant small RNA genes | https://plantsmallrnagenes.science.psu.edu/ | 49 | Contain also 118 entities similar to miRNAs |
| miRNEST | http://rhesus.amu.edu.pl/mirnest/copy/browse.php | 398 | An integrative miRNA resource |
According to TarDB: A miRNA Target Database in Plants [112] (http://www.biosequencing.cn/TarDB/browse.html, accessed 20 June 2022), there are currently 20 conserved miRNAs in barley (Table 3). It is worth mentioning that TarDB uses relatively strict parameters to identify high-confidence plant miRNAs and their targets based on cross-species conservation filter, degradome, and sRNA-seq data, so Table 3 below is rather illustrative than exhaustive. It is supposed that the overall number of functional miRNAs in barley is much higher, according to a study published in November 2021 [113], a total of 156 miRNAs including 35 known and 121 novel miRNAs experimentally identified in Tibetan hull-less barleys, targeting over 1200 genes (nonetheless it was done by computational prediction, only selected targets were also in vitro verified using RLM-5′ RACE method) [113].
Table 3.
High-confidence miRNA targets in Hordeum vulgare together with their experimentally verified or supposed biological functions in higher plants.
| miRNA | mRNA Target(s) in Hordeum vulgare | Known Biological Function(s) of miRNA in Plant Species and Further Notes | References |
|---|---|---|---|
| miR156a | SBP-box gene family member | Inflorescence morphogenesis regulation in tomato (Solanum lycopersicum) plants; male fertility regulation in thale cress (Arabidopsis thaliana) plants | [114,115,116] |
| miR156b | |||
| miR159a | MYB family transcription factor;lectin-like receptor kinase | Ensure normal growth via regulation of GAMYB genes | [117,118,119] |
| miR159b | MYB family transcription factor; | ||
| miR166a | START domain-containing protein; MATE domain-containing protein; class III HD-Zip protein 8 | Shoot apical meristem and vascular differentiation, leaf and root development; evolutionarily conserved stress biomarker in land plants—drought, salinity, temperature, biotic stress | [120,121] |
| miR166b | |||
| miR166c | |||
| miR168-5p | receptor-like protein kinase 5 precursor | Function in plants is unclear but targets many important mammalian transcripts (123 in total), including the gene for Low-density lipoprotein receptor adaptor protein 1 (LDLRAP1, also known as ARH)) | [122] |
| miR171-3p | scarecrow transcription factor family protein | Regulation of germination and seedling growth in Tibetan hull-less barley (Hordeum vulgare L. var. nudum); drought tolerance by regulation of flavonoid biosynthesis genes in rice | [113,123] |
| miR397a | laccase precursor protein; transporter family protein; | Plant development; circadian regulation and plant flowering; cold response in thale cress (Arabidopsis thaliana) | [124,125] |
| miR399 | rp1; ubiquitin-conjugating enzyme protein; pentatricopeptide | Salt stress response and flowering regulation in thale cress (Arabidopsis thaliana) | [126,127] |
| miR444a | FAD-binding domain of DNA photolyase domain-containing protein; DnaK family protein; alpha-taxilin; MADS-box family gene with MIKCc type-box; pentatricopeptide; WD domain, G-beta repeat domain-containing protein | Regulation of nitrate signaling pathway in nitrate-dependent root growth, nitrate accumulation, and phosphate-starvation responses in rice (Oryza sativa); antiviral pathway in rice; regulation of brassinosteroids synthesis in rice | [128,129,130] |
| miR444b | MADS-box family gene with MIKCc type-box; methyltransferase; zinc finger, C3HC4 type domain-containing protein | ||
| miR1120 | An enzyme of the cupin superfamily protein; retrotransposon protein; tesmin/TSO1-like CXC domain-containing protein; WD domain, G-beta repeat domain-containing protein; CCR4-NOT transcription factor; glycosyltransferase family 43 protein; amine oxidase-related; Divergent PAP2 family domain-containing protein | Early anther development in wheat (Triticum aestivum). miR1120 in barley has many diverse mRNA targets, however, it is questionable, if this miR1120 is a true miRNA (originating from hairpin RNA precursor), as the miR1120 gene region in barley displays almost 80% sequence similarity to the short transposon element DNA/TcMar-Stowaway | [93,131] |
| miR1436 | pseudogene | Various stress responses in Cestrum nocturnum L. and Cestrum diurnum L. | [132] |
| miR5048a | cysteine-rich receptor-like protein kinase precursor | Wheat (Triticum aestivum) grains development regulation | [133] |
| miR5049c | modifier of rudimentary protein; auxin-induced protein 5NG4; Spc97/Spc98 family protein; protein kinase domain-containing protein; OsWAK receptor-like protein kinase | Hormone, stress (heat, drought, salinity, and excess boron), and light responsiveness in barley (Hordeum vulgare L.) | [67] |
| miR5049f | resistance protein; transcription factor-related; WD domain, G-beta repeat domain-containing protein; TBC domain-containing protein; | Regulation of salt adaptation in Hordeum bulbosum | [75] |
| miR6197 | DUF26 kinase; exosome complex exonuclease rrp4 | Boron stress response regulation in barley (Hordeum vulgare) | [83] |
| miR6201 | C4-dicarboxylate transporter/malic acid transport protein | Cadmium stress response regulation in wheat (Triticum aestivum) | [134] |
Many barley miRNAs are targeting mRNA transcripts encoding transcription factors. This is maybe not too surprising, as it was previously known that most of the plant miRNA targets are transcription factors that regulate plant growth and development [135]. In Figure 4, known barley miRNA targets from TarDB [112] are grouped according to their gene ontologies (GOs), separately for ‘all conserved miRNA targets’ (dataset containing 92 mRNAs) and ‘degradome-supported miRNA targets’ (dataset containing 37 mRNAs, 15 of them are common with the first subset of ‘all conserved miRNA targets’). GO terms for these datasets were acquired using PLAZA Workbench [136,137]. It can be seen that most miRNA targets participate in diverse biological processes, comprising metabolic, developmental, regulatory, and reproductive processes. This fact may be in good aggreement with general observations and knowledge from miRNA studies not only in barley plants. From the point of molecular functions view, barley miRNA targets are employed mainly in binding processes (e.g., organic cyclic compound binding, protein binding, nucleic acid binding, etc.) and some targets possess catalytic activity (Figure 4). It is worth noting that the above-mentioned GOs are more general than specific, and they deserve more detailed analysis in the future. Moreover, it may be interesting that many miRNA targets (once translated into proteins) can bind DNA and theoretically act as transcriptional activators or repressors influencing the expression of their superior miRNA genes, thus forming another regulatory layer (or feedback loop) [138]. This issue could certainly serve as a potential theme for further research in the field of plant development and stress responses.
Figure 4.
Biological processes and Molecular functions of miRNA targets in barley. In the upper half of the image, the most abundant GOs of all conserved miRNA targets in barley are shown. In the lower half of the image, the most abundant GOs of degradome-supported miRNA targets are depicted. Blue bar plots stand for biological processes, whereas the orange ones correspond to molecular functions.
It is essential to bear in mind that a particular miRNA can interact with many different mRNA molecules [139], and that particular miRNA targets can be relatively quickly acquired through plant evolution [140]. A good example is miR168a from sweet orange (Citrus sinensis L. Osbeck), where besides its original target (AGO1 mRNA) it gained a novel target, CUC2 mRNA [141]. Another specific case was observed in rice (Oryza sativa japonica cv. Nipponbare), where miR159 triggers MAP kinase 8 mRNA, in addition to its original target (MYB mRNA) [142]. Interestingly, miRNA activity can be regulated by bait in the form of long non-coding RNAs (lncRNAs), where such lncRNAs mimic the target mRNAs and sequester specific miRNAs (preventing them from interacting with their mRNA targets)—this phenomenon is usually described as (mi)RNA decoy [143] or Target Mimics [144]. In barley, there is a study from 2020 where authors identified about 8000 lncRNAs and found a total of 32 endogenous target mimics that may potentially decoy 18 different miRNAs [145].
As an illustrative example of miRNAs targets diversity, we depicted all computationally predicted mRNA targets of single barley miRNA, particularly miR5049c (Figure 5). According to TarDB [112], this miRNA has the potential to target 17 different mRNAs originating from various genes across the whole barley genome (Chromosomes 1 to 7). The molecular and biological functions of proteins encoded by these mRNAs are also very diverse, and some of them participate in response to external stimuli, e.g., HSP20-like chaperones superfamily protein (by homology) [146]. The fact that one miRNA can bind multiple mRNA targets is relatively well-known for many years [147,148]. Obviously, at the same time, a single miRNA molecule can bind only a single mRNA target, therefore one may imagine that the relative accessibility of particular mRNA to a particular miRNA determines the proportion of specific mRNA-miRNA interactions. The cell usually produces only a fraction of all possible mRNAs, and therefore such a mechanism of regulation would seem efficient.
Figure 5.
Barley pri-miR5049c structure together with miR5049 putative mRNA targets. pri-miRNA structure was computed via RNAfold web server [149] and visualized in the form of a Forna diagram [150]. Nucleotides in blue circles correspond to the mature 21nt-long miRNA region. Grey lines depict inhibition of specific mRNA targets (if the line is full and the description is in red, mRNA cleavage was predicted, according to TarDB: “Cleavage is predicted if miRNA 5′ positions 9–11 have the perfect match”). Chromosome numbers correspond to the location of genes encoding particular mRNAs, Un stands for Unplaced locus.
5. Conclusions and Future Directions
This review gives a basic overview of a rapidly growing amount of miRNA studies in barley (Hordeum vulgare). From what we know, it is clear that miRNAs play an important role in many developmental processes as well as in a variety of stress-induced molecular and biological responses. It is likely that more and more putative miRNAs will be discovered in barley, and many of them will be linked to abiotic or biotic stresses, including drought, cold, high temperatures, high salinity, micronutrient excess or deficiency in the soil, spectral quality of incident light, or infectious agents. Identification of plant miRNA targets on a large scale has traditionally been made mainly by bioinformatic approaches [151,152,153,154,155,156]. On the other hand, experimental validation is needed to verify predicted mRNA targets—historically, this has been done using laboratory-intensive in vitro methods like the 5′ RACE assay [157], but nowadays, the high-throughput degradome sequencing technique can be employed to validate (at least partially) predicted miRNA targets. Nonetheless, four criteria (according to a nice review by Giulia Riolo et al. [158]) should ideally be fulfilled when validating novel miRNAs:
-
(a)
Showing co-expression of miRNA and target mRNA in vivo;
-
(b)
Proving interaction between miRNA and a specific site within target mRNA;
-
(c)
Demonstrating miRNA-mediated effects on target protein expression;
-
(d)
Demonstrating miRNA effects on biological function.
What is quite difficult for our complex understanding of miRNA mechanisms is that even different genotypes/cultivars of barley tend to express unique miRNA patterns. This may point to rapid miRNA evolution allowing gene expression fine-tuning in a dynamically changing environment and agriculture. In addition, different plant tissues may express a different ‘miRNome’ in response to various stress signals [159].
Several studies have discussed the possibility of miRNA-based technology to improve plant resistance to abiotic factors [160,161]. In 2017, Jannatul Ferdous et al. published a study where the drought-inducible expression of miR827 enhanced drought tolerance in transgenic barley [162]. In maize, the knock-down of miR166 using short tandem target mimics technology resulted in enhanced abiotic stress resistance, abscisic acid level elevation, and indole acetic acid level reduction [163]. As miR166 is conserved also in barley, it would be interesting to identify whether its knock-down would have similar effects. Another promising possibility offers CRISPR/Cas technology already utilized for miRNA gene editing in rice [164,165] and Arabidopsis thaliana [166], further reviewed in [167]. Finally, there is an increasing effort to use exogenous/artificially made miRNAs (or siRNAs) in modern plant protection and improvement, and such RNA interference technology is usually considered GMO-free [168]. Among the various options, chitosan nanoparticles bearing miRNAs seem to be particularly attractive [169].
Barley miRNAs could also be efficiently used as molecular markers. In 2020, researchers proposed selected miRNAs as a tool to monitor the barley response to soil compaction [170].
Below, several outstanding questions are summarized:
-
(1)
Are some of the barley miRNAs tissue/developmental, or stage-specific? Are we able to catalog it in some integrative and user-friendly way? For this purpose, it would be beneficial to have something like a barley miRNA atlas (similar to PmiRExAt, where wheat, rice, maize, and Arabidopsis miRNAs in multiple tissues and developmental stages can be found) [171].
-
(2)
Which barley miRNAs have the potential to become a useful stress biomarker? In other words, do some stress-specific miRNAs exist?
-
(3)
Is barley miRNome rather complete, or not? Compared to rice, wheat, and Arabidopsis, the total number of known barley miRNAs is still lack behind, and bona fide many discoveries waiting for us!
To better depict the above-mentioned perspectives in barley miRNAs research, we have created a diagram where particular future aims are divided into two categories, i.e., work to be done either using dry-lab or wet-lab methods, together with possible future applications (Figure 6).
Figure 6.
Future goals in miRNAs research in barley, divided into dry-lab and wet-lab categories, and possible future applications.
All in all, even though a lot is known about miRNAs in barley, much remains to be resolved. Aristotle said “the more you know, the more you realize you don’t know”, and complex miRNAs problematics in barley (and generally in plants) could definitely fit this quote.
Author Contributions
Conceptualization, A.V. and M.B.; resources, M.B. and A.V.; writing—original draft preparation, A.V. and M.B.; writing—review and editing, V.Š., P.P. and J.Č.; visualization, A.V. and M.B.; supervision, J.Č.; project administration, J.Č.; funding acquisition, J.Č. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study.
Funding Statement
This research was funded by the Czech Science Foundation (GACR 21-18532S to V.Š. and A.V.), the University of Ostrava (SGS11/PřF/2022 to A.V.; SGS10/PřF/2022 to P.P.). Participation of V.Š. was also supported by the Ministry of Education, Youth and Sports of the Czech Republic, project “SustES—Adaptation strategies for sustainable ecosystem services and food security under adverse environmental conditions” (CZ.02.1.01/0.0/0.0/16_019/0000797). J.Č., P.P. and M.B. were supported by the National Agency for Agricultural Research (NAZV) of the Czech Republic grant no. QK1810391 “Utilization of genomic and transcriptomic approaches to create genetic resources and breeding materials of poppy with specific traits”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lam J.K.W., Chow M.Y.T., Zhang Y., Leung S.W.S. SiRNA versus MiRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J., Mei J., Ren G. Plant MicroRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019;10:360. doi: 10.3389/fpls.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen G.L., Blau H.M. A Brief History of RNAi: The Silence of the Genes. FASEB J. 2006;20:1293–1299. doi: 10.1096/fj.06-6014rev. [DOI] [PubMed] [Google Scholar]
- 4.Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in Trans. Plant Cell. 1990;2:279–289. doi: 10.2307/3869076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romano N., Macino G. Quelling: Transient Inactivation of Gene Expression in Neurospora Crassa by Transformation with Homologous Sequences. Mol. Microbiol. 1992;6:3343–3353. doi: 10.1111/j.1365-2958.1992.tb02202.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V., Bartel B., Bartel D.P., Burge C.B., Carrington J.C., Chen X., Dreyfuss G., Eddy S.R., Griffiths-Jones S., Marshall M., et al. A Uniform System for MicroRNA Annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebert L.F.R., MacRae I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia D. A MiRacle in Plant Development: Role of MicroRNAs in Cell Differentiation and Patterning. Semin. Cell Dev. Biol. 2008;19:586–595. doi: 10.1016/j.semcdb.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Millar A.A. The Function of MiRNAs in Plants. Plants. 2020;9:198. doi: 10.3390/plants9020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammad T., Zhang F., Zhang Y., Liang Y. RNA Interference: A Natural Immune System of Plants to Counteract Biotic Stressors. Cells. 2019;8:38. doi: 10.3390/cells8010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Galili G. Tuning the Orchestra: MiRNAs in Plant Immunity. Trends Plant Sci. 2019;24:189–191. doi: 10.1016/j.tplants.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Cui C., Wang J.-J., Zhao J.-H., Fang Y.-Y., He X.-F., Guo H.-S., Duan C.-G. A Brassica MiRNA Regulates Plant Growth and Immunity through Distinct Modes of Action. Mol. Plant. 2020;13:231–245. doi: 10.1016/j.molp.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Mengistu A.A., Tenkegna T.A. The Role of MiRNA in Plant–Virus Interaction: A Review. Mol. Biol. Rep. 2021;48:2853–2861. doi: 10.1007/s11033-021-06290-4. [DOI] [PubMed] [Google Scholar]
- 15.Pagano L., Rossi R., Paesano L., Marmiroli N., Marmiroli M. MiRNA Regulation and Stress Adaptation in Plants. Environ. Exp. Bot. 2021;184:104369. doi: 10.1016/j.envexpbot.2020.104369. [DOI] [Google Scholar]
- 16.Nevo E. Advance in Barley Sciences. Springer; Dordrecht, The Netherlands: 2013. Evolution of Wild Barley and Barley Improvement; pp. 1–23. [Google Scholar]
- 17.Pourkheirandish M., Komatsuda T. The Importance of Barley Genetics and Domestication in a Global Perspective. Ann. Bot. 2007;100:999–1008. doi: 10.1093/aob/mcm139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ullrich S.E. Barley: Production, Improvement, and Uses. John Wiley & Sons; New York, NY, USA: 2010. [Google Scholar]
- 19.Tosh S.M., Bordenave N. Emerging Science on Benefits of Whole Grain Oat and Barley and Their Soluble Dietary Fibers for Heart Health, Glycemic Response, and Gut Microbiota. Nutr. Rev. 2020;78:13–20. doi: 10.1093/nutrit/nuz085. [DOI] [PubMed] [Google Scholar]
- 20.Lahouar L., El-Bok S., Achour L. Therapeutic Potential of Young Green Barley Leaves in Prevention and Treatment of Chronic Diseases: An Overview. Am. J. Chin. Med. 2015;43:1311–1329. doi: 10.1142/S0192415X15500743. [DOI] [PubMed] [Google Scholar]
- 21.Pech R., Volná A., Hunt L., Bartas M., Červeň J., Pečinka P., Špunda V., Nezval J. Regulation of Phenolic Compound Production by Light Varying in Spectral Quality and Total Irradiance. Int. J. Mol. Sci. 2022;23:6533. doi: 10.3390/ijms23126533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood W.A. Barley. Humana Press; New York, NY, UAS: 2019. An Introduction to Barley: The Crop and the Model; pp. 1–5. [DOI] [PubMed] [Google Scholar]
- 23.Sato K. History and Future Perspectives of Barley Genomics. DNA Res. 2020;27:dsaa023. doi: 10.1093/dnares/dsaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saski C., Lee S.-B., Fjellheim S., Guda C., Jansen R.K., Luo H., Tomkins J., Rognli O.A., Daniell H., Clarke J.L. Complete Chloroplast Genome Sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and Comparative Analyses with Other Grass Genomes. Theor. Appl. Genet. 2007;115:571–590. doi: 10.1007/s00122-007-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoch C.L., Ciufo S., Domrachev M., Hotton C.L., Kannan S., Khovanskaya R., Leipe D., Mcveigh R., O’Neill K., Robbertse B. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database. 2020;2020:baaa062. doi: 10.1093/database/baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato F., Tsuchiya S., Meltzer S.J., Shimizu K. MicroRNAs and Epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 27.Shapulatov U., van Hoogdalem M., Schreuder M., Bouwmeester H., Abdurakhmonov I.Y., van der Krol A.R. Functional Intron-Derived MiRNAs and Host-Gene Expression in Plants. Plant Methods. 2018;14:83. doi: 10.1186/s13007-018-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X., Hornyik C., Bayer M., Marshall D., Waugh R., Zhang R. In Silico Identification and Characterization of Conserved Plant MicroRNAs in Barley. Open Life Sci. 2014;9:841–852. doi: 10.2478/s11535-014-0308-z. [DOI] [Google Scholar]
- 29.Baldrich P., Hsing Y.-I.C., San Segundo B. Genome-Wide Analysis of Polycistronic MicroRNAs in Cultivated and Wild Rice. Genome. Biol. Evol. 2016;8:1104–1114. doi: 10.1093/gbe/evw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Q., Mao Y., Hu L., Wu Y., Ji Z. MiRClassify: An Advanced Web Server for MiRNA Family Classification and Annotation. Comput. Biol. Med. 2014;45:157–160. doi: 10.1016/j.compbiomed.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S., Dou Y., Li S., Ren G., Chevalier D., Zhang C., Yu B. DAWDLE Interacts with DICER-LIKE Proteins to Mediate Small RNA Biogenesis. Plant Physiol. 2018;177:1142–1151. doi: 10.1104/pp.18.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Xiang Y., Chen S., Shi M., Jiang X., He Z., Gao S. Mechanisms of MicroRNA Biogenesis and Stability Control in Plants. Front. Plant Sci. 2022;13:844149. doi: 10.3389/fpls.2022.844149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu B., Yang Z., Li J., Minakhina S., Yang M., Padgett R.W., Steward R., Chen X. Methylation as a Crucial Step in Plant MicroRNA Biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djami-Tchatchou A.T., Sanan-Mishra N., Ntushelo K., Dubery I.A. Functional Roles of MicroRNAs in Agronomically Important Plants—Potential as Targets for Crop Improvement and Protection. Front. Plant Sci. 2017;8:378. doi: 10.3389/fpls.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M., Yu B. Recent Advances in the Regulation of Plant MiRNA Biogenesis. RNA Biol. 2021;18:2087–2096. doi: 10.1080/15476286.2021.1899491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medley J.C., Panzade G., Zinovyeva A.Y. MicroRNA Strand Selection: Unwinding the Rules. Wiley Interdiscip. Rev. RNA. 2021;12:e1627. doi: 10.1002/wrna.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijer H.A., Smith E.M., Bushell M. Regulation of MiRNA Strand Selection: Follow the Leader? Biochem. Soc. Trans. 2014;42:1135–1140. doi: 10.1042/BST20140142. [DOI] [PubMed] [Google Scholar]
- 39.Vimalraj S., Selvamurugan N. MicroRNAs: Synthesis, Gene Regulation and Osteoblast Differentiation. Curr. Issues Mol. Biol. 2013;15:7–18. doi: 10.21775/cimb.015.007. [DOI] [PubMed] [Google Scholar]
- 40.Forman J.J., Coller H.A. The Code within the Code: MicroRNAs Target Coding Regions. Cell Cycle. 2010;9:1533–1541. doi: 10.4161/cc.9.8.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu B., Wang H. Translational Inhibition by MicroRNAs in Plants. Prog. Mol. Subcell. Biol. 2010;50:41–57. doi: 10.1007/978-3-642-03103-8_3. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Teng C., Xia R., Meyers B.C. PhasiRNAs in Plants: Their Biogenesis, Genic Sources, and Roles in Stress Responses, Development, and Reproduction. Plant Cell. 2020;32:3059–3080. doi: 10.1105/tpc.20.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araki S., Le N.T., Koizumi K., Villar-Briones A., Nonomura K.-I., Endo M., Inoue H., Saze H., Komiya R. MiR2118-Dependent U-Rich PhasiRNA Production in Rice Anther Wall Development. Nat. Commun. 2020;11:3115. doi: 10.1038/s41467-020-16637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia R., Chen C., Pokhrel S., Ma W., Huang K., Patel P., Wang F., Xu J., Liu Z., Li J., et al. 24-Nt Reproductive PhasiRNAs Are Broadly Present in Angiosperms. Nat. Commun. 2019;10:627. doi: 10.1038/s41467-019-08543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Z., Khanna K., Ruan S. Expression of MicroRNAs and Its Regulation in Plants. Semin. Cell Dev. Biol. 2010;21:790–797. doi: 10.1016/j.semcdb.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhabra R. MiRNA and Methylation: A Multifaceted Liaison. ChemBioChem. 2015;16:195–203. doi: 10.1002/cbic.201402449. [DOI] [PubMed] [Google Scholar]
- 47.Świda-Barteczka A., Krieger-Liszkay A., Bilger W., Voigt U., Hensel G., Szweykowska-Kulinska Z., Krupinska K. The Plastid-Nucleus Located DNA/RNA Binding Protein WHIRLY1 Regulates MicroRNA-Levels during Stress in Barley (Hordeum vulgare L.) RNA Biol. 2018;15:886–891. doi: 10.1080/15476286.2018.1481695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y., Mo B., Chen X. Mechanisms That Impact MicroRNA Stability in Plants. RNA Biol. 2012;9:1218–1223. doi: 10.4161/rna.22034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallego-Bartolomé J. DNA Methylation in Plants: Mechanisms and Tools for Targeted Manipulation. New Phytol. 2020;227:38–44. doi: 10.1111/nph.16529. [DOI] [PubMed] [Google Scholar]
- 50.Wu L., Zhou H., Zhang Q., Zhang J., Ni F., Liu C., Qi Y. DNA Methylation Mediated by a MicroRNA Pathway. Mol. Cell. 2010;38:465–475. doi: 10.1016/j.molcel.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Bao N., Lye K.-W., Barton M.K. MicroRNA Binding Sites in Arabidopsis Class III HD-ZIP MRNAs Are Required for Methylation of the Template Chromosome. Dev. Cell. 2004;7:653–662. doi: 10.1016/j.devcel.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Vasudevan S. Posttranscriptional Upregulation by MicroRNAs. WIREs RNA. 2012;3:311–330. doi: 10.1002/wrna.121. [DOI] [PubMed] [Google Scholar]
- 53.Lauressergues D., Couzigou J.-M., Clemente H.S., Martinez Y., Dunand C., Bécard G., Combier J.-P. Primary Transcripts of MicroRNAs Encode Regulatory Peptides. Nature. 2015;520:90–93. doi: 10.1038/nature14346. [DOI] [PubMed] [Google Scholar]
- 54.Prasad A., Sharma N., Prasad M. Noncoding but Coding: Pri-MiRNA into the Action. Trends Plant Sci. 2021;26:204–206. doi: 10.1016/j.tplants.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Sharma A., Badola P.K., Bhatia C., Sharma D., Trivedi P.K. Primary Transcript of MiR858 Encodes Regulatory Peptide and Controls Flavonoid Biosynthesis and Development in Arabidopsis. Nat. Plants. 2020;6:1262–1274. doi: 10.1038/s41477-020-00769-x. [DOI] [PubMed] [Google Scholar]
- 56.Couzigou J.-M., André O., Guillotin B., Alexandre M., Combier J.-P. Use of MicroRNA-Encoded Peptide MiPEP172c to Stimulate Nodulation in Soybean. New Phytol. 2016;211:379–381. doi: 10.1111/nph.13991. [DOI] [PubMed] [Google Scholar]
- 57.Chen Q., Deng B., Gao J., Zhao Z., Chen Z., Song S., Wang L., Zhao L., Xu W., Zhang C., et al. A MiRNA-Encoded Small Peptide, Vvi-MiPEP171d1, Regulates Adventitious Root Formation. Plant Physiol. 2020;183:656–670. doi: 10.1104/pp.20.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong C.E., Zhao Y.-T., Wang X.-J., Croft L., Wang Z.-H., Haerizadeh F., Mattick J.S., Singh M.B., Carroll B.J., Bhalla P.L. MicroRNAs in the Shoot Apical Meristem of Soybean. J. Exp. Bot. 2011;62:2495–2506. doi: 10.1093/jxb/erq437. [DOI] [PubMed] [Google Scholar]
- 59.Choudhary A., Kumar A., Kaur H., Kaur N. MiRNA: The Taskmaster of Plant World. Biologia. 2021;76:1551–1567. doi: 10.1007/s11756-021-00720-1. [DOI] [Google Scholar]
- 60.Waheed S., Zeng L. The Critical Role of MiRNAs in Regulation of Flowering Time and Flower Development. Genes. 2020;11:319. doi: 10.3390/genes11030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curaba J., Spriggs A., Taylor J., Li Z., Helliwell C. MiRNA Regulation in the Early Development of Barley Seed. BMC Plant Biol. 2012;12:120. doi: 10.1186/1471-2229-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curaba J., Talbot M., Li Z., Helliwell C. Over-Expression of MicroRNA171 Affects Phase Transitions and Floral Meristem Determinancy in Barley. BMC Plant Biol. 2013;13:6. doi: 10.1186/1471-2229-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smoczynska A., Szweykowska-Kulinska Z. MicroRNA-Mediated Regulation of Flower Development in Grasses. Acta Biochim. Pol. 2016;63:687–692. doi: 10.18388/abp.2016_1358. [DOI] [PubMed] [Google Scholar]
- 64.Nair S.K., Wang N., Turuspekov Y., Pourkheirandish M., Sinsuwongwat S., Chen G., Sameri M., Tagiri A., Honda I., Watanabe Y., et al. Cleistogamous Flowering in Barley Arises from the Suppression of MicroRNA-Guided HvAP2 MRNA Cleavage. Proc. Natl. Acad. Sci. USA. 2010;107:490–495. doi: 10.1073/pnas.0909097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anwar N., Ohta M., Yazawa T., Sato Y., Li C., Tagiri A., Sakuma M., Nussbaumer T., Bregitzer P., Pourkheirandish M., et al. MiR172 Downregulates the Translation of Cleistogamy 1 in Barley. Ann. Bot. 2018;122:251–265. doi: 10.1093/aob/mcy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan W., Suo J., Shi B., Zhou C., Bai B., Bian H., Zhu M., Han N. The Barley MiR393 Has Multiple Roles in Regulation of Seedling Growth, Stomatal Density, and Drought Stress Tolerance. Plant Physiol. Biochem. 2019;142:303–311. doi: 10.1016/j.plaphy.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 67.Tombuloglu H. Genome-Wide Analysis of the Auxin Response Factors (ARF) Gene Family in Barley (Hordeum vulgare L.) J. Plant Biochem. Biotechnol. 2019;28:14–24. doi: 10.1007/s13562-018-0458-6. [DOI] [Google Scholar]
- 68.Shriram V., Kumar V., Devarumath R.M., Khare T.S., Wani S.H. MicroRNAs as Potential Targets for Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2016;7:817. doi: 10.3389/fpls.2016.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barczak-Brzyżek A., Brzyżek G., Koter M., Siedlecka E., Gawroński P., Filipecki M. Plastid Retrograde Regulation of MiRNA Expression in Response to Light Stress. BMC Plant Biol. 2022;22:150. doi: 10.1186/s12870-022-03525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subburaj S., Ha H.-J., Jin Y.-T., Jeon Y., Tu L., Kim J.-B., Kang S.-Y., Lee G.-J. Identification of γ-Radiation-Responsive MicroRNAs and Their Target Genes in Tradescantia (BNL Clone 4430) J. Plant Biol. 2017;60:116–128. doi: 10.1007/s12374-016-0433-5. [DOI] [Google Scholar]
- 71.Visentin I., Pagliarani C., Deva E., Caracci A., Turečková V., Novák O., Lovisolo C., Schubert A., Cardinale F. A Novel Strigolactone-MiR156 Module Controls Stomatal Behaviour during Drought Recovery. Plant Cell Environ. 2020;43:1613–1624. doi: 10.1111/pce.13758. [DOI] [PubMed] [Google Scholar]
- 72.Saminathan T., Alvarado A., Lopez C., Shinde S., Gajanayake B., Abburi V.L., Vajja V.G., Jagadeeswaran G., Raja Reddy K., Nimmakayala P., et al. Elevated Carbon Dioxide and Drought Modulate Physiology and Storage-Root Development in Sweet Potato by Regulating MicroRNAs. Funct. Integr. Genom. 2019;19:171–190. doi: 10.1007/s10142-018-0635-7. [DOI] [PubMed] [Google Scholar]
- 73.Deng P., Wang L., Cui L., Feng K., Liu F., Du X., Tong W., Nie X., Ji W., Weining S. Global Identification of MicroRNAs and Their Targets in Barley under Salinity Stress. PLoS ONE. 2015;10:e0137990. doi: 10.1371/journal.pone.0137990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuang L., Yu J., Shen Q., Fu L., Wu L. Identification of MicroRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots. Plants. 2021;10:2754. doi: 10.3390/plants10122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu B., Sun G. Micro RNA s Contribute to Enhanced Salt Adaptation of the Autopolyploid Hordeum bulbosum Compared with Its Diploid Ancestor. Plant J. 2017;91:57–69. doi: 10.1111/tpj.13546. [DOI] [PubMed] [Google Scholar]
- 76.Kuang L., Shen Q., Wu L., Yu J., Fu L., Wu D., Zhang G. Identification of MicroRNAs Responding to Salt Stress in Barley by High-Throughput Sequencing and Degradome Analysis. Environ. Exp. Bot. 2019;160:59–70. doi: 10.1016/j.envexpbot.2019.01.006. [DOI] [Google Scholar]
- 77.Smoczynska A., Pacak A.M., Nuc P., Swida-Barteczka A., Kruszka K., Karlowski W.M., Jarmolowski A., Szweykowska-Kulinska Z. A Functional Network of Novel Barley MicroRNAs and Their Targets in Response to Drought. Genes. 2020;11:488. doi: 10.3390/genes11050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferdous J., Sanchez-Ferrero J.C., Langridge P., Milne L., Chowdhury J., Brien C., Tricker P.J. Differential Expression of MicroRNAs and Potential Targets under Drought Stress in Barley. Plant Cell Environ. 2017;40:11–24. doi: 10.1111/pce.12764. [DOI] [PubMed] [Google Scholar]
- 79.Kantar M., Unver T., Budak H. Regulation of Barley MiRNAs upon Dehydration Stress Correlated with Target Gene Expression. Funct. Integr. Genom. 2010;10:493–507. doi: 10.1007/s10142-010-0181-4. [DOI] [PubMed] [Google Scholar]
- 80.Hackenberg M., Gustafson P., Langridge P., Shi B.-J. Differential Expression of MicroRNAs and Other Small RNAs in Barley between Water and Drought Conditions. Plant Biotechnol. J. 2015;13:2–13. doi: 10.1111/pbi.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiu C.-W., Liu L., Feng X., Hao P.-F., He X., Cao F., Wu F. Genome-Wide Identification and Characterization of Drought Stress Responsive MicroRNAs in Tibetan Wild Barley. Int. J. Mol. Sci. 2020;21:2795. doi: 10.3390/ijms21082795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grabowska A., Smoczynska A., Bielewicz D., Pacak A., Jarmolowski A., Szweykowska-Kulinska Z. Barley MicroRNAs as Metabolic Sensors for Soil Nitrogen Availability. Plant Sci. 2020;299:110608. doi: 10.1016/j.plantsci.2020.110608. [DOI] [PubMed] [Google Scholar]
- 83.Ozhuner E., Eldem V., Ipek A., Okay S., Sakcali S., Zhang B., Boke H., Unver T. Boron Stress Responsive MicroRNAs and Their Targets in Barley. PLoS ONE. 2013;8:e59543. doi: 10.1371/journal.pone.0059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hackenberg M., Huang P.-J., Huang C.-Y., Shi B.-J., Gustafson P., Langridge P. A Comprehensive Expression Profile of MicroRNAs and Other Classes of Non-Coding Small RNAs in Barley Under Phosphorous-Deficient and -Sufficient Conditions. DNA Res. 2013;20:109–125. doi: 10.1093/dnares/dss037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sega P., Kruszka K., Bielewicz D., Karlowski W., Nuc P., Szweykowska-Kulinska Z., Pacak A. Pi-Starvation Induced Transcriptional Changes in Barley Revealed by a Comprehensive RNA-Seq and Degradome Analyses. BMC Genom. 2021;22:165. doi: 10.1186/s12864-021-07481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai B., Bian H., Zeng Z., Hou N., Shi B., Wang J., Zhu M., Han N. MiR393-Mediated Auxin Signaling Regulation Is Involved in Root Elongation Inhibition in Response to Toxic Aluminum Stress in Barley. Plant Cell Physiol. 2017;58:426–439. doi: 10.1093/pcp/pcw211. [DOI] [PubMed] [Google Scholar]
- 87.Wu L., Yu J., Shen Q., Huang L., Wu D., Zhang G. Identification of MicroRNAs in Response to Aluminum Stress in the Roots of Tibetan Wild Barley and Cultivated Barley. BMC Genom. 2018;19:560. doi: 10.1186/s12864-018-4953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu J., Wu L., Fu L., Shen Q., Kuang L., Wu D., Zhang G. Genotypic Difference of Cadmium Tolerance and the Associated MicroRNAs in Wild and Cultivated Barley. Plant Growth Regul. 2019;87:389–401. doi: 10.1007/s10725-019-00479-1. [DOI] [Google Scholar]
- 89.Chen F., He J., Jin G., Chen Z.-H., Dai F. Identification of Novel MicroRNAs for Cold Deacclimation in Barley. Plant Growth Regul. 2020;92:389–400. doi: 10.1007/s10725-020-00646-9. [DOI] [Google Scholar]
- 90.Kruszka K., Pacak A., Swida-Barteczka A., Nuc P., Alaba S., Wroblewska Z., Karlowski W., Jarmolowski A., Szweykowska-Kulinska Z. Transcriptionally and Post-Transcriptionally Regulated MicroRNAs in Heat Stress Response in Barley. J. Exp. Bot. 2014;65:6123–6135. doi: 10.1093/jxb/eru353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schreiber A.W., Shi B.-J., Huang C.-Y., Langridge P., Baumann U. Discovery of Barley MiRNAs through Deep Sequencing of Short Reads. BMC Genom. 2011;12:129. doi: 10.1186/1471-2164-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lv S., Nie X., Wang L., Du X., Biradar S.S., Jia X., Weining S. Identification and Characterization of MicroRNAs from Barley (Hordeum vulgare L.) by High-Throughput Sequencing. Int. J. Mol. Sci. 2012;13:2973–2984. doi: 10.3390/ijms13032973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kruszka K., Pacak A., Swida-Barteczka A., Stefaniak A.K., Kaja E., Sierocka I., Karlowski W., Jarmolowski A., Szweykowska-Kulinska Z. Developmentally Regulated Expression and Complex Processing of Barley Pri-MicroRNAs. BMC Genom. 2013;14:34. doi: 10.1186/1471-2164-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J., Cheng X., Liu D., Xu W., Wise R., Shen Q.-H. The MiR9863 Family Regulates Distinct Mla Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling. PLoS Genet. 2014;10:e1004755. doi: 10.1371/journal.pgen.1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kis A., Tholt G., Ivanics M., Várallyay É., Jenes B., Havelda Z. Polycistronic Artificial MiRNA-Mediated Resistance to Wheat Dwarf Virus in Barley Is Highly Efficient at Low Temperature. Mol. Plant Pathol. 2016;17:427–437. doi: 10.1111/mpp.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng P., Bian J., Yue H., Feng K., Wang M., Du X., Weining S., Nie X. Characterization of MicroRNAs and Their Targets in Wild Barley (Hordeum vulgare Subsp. Spontaneum) Using Deep Sequencing. Genome. 2016;59:339–348. doi: 10.1139/gen-2015-0224. [DOI] [PubMed] [Google Scholar]
- 97.Pacak A.M., Kruszka K., Świda-Barteczka A., Nuc P., Karlowski W., Jarmołowski A., Szweykowska-Kulińska Z. Developmental Changes in Barley MicroRNA Expression Profiles Coupled with MiRNA Targets Analysis. Acta Biochim. Pol. 2016;63:799–809. doi: 10.18388/abp.2016_1347. [DOI] [PubMed] [Google Scholar]
- 98.Bai B., Shi B., Hou N., Cao Y., Meng Y., Bian H., Zhu M., Han N. MicroRNAs Participate in Gene Expression Regulation and Phytohormone Cross-Talk in Barley Embryo during Seed Development and Germination. BMC Plant Biol. 2017;17:150. doi: 10.1186/s12870-017-1095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith O., Palmer S.A., Clapham A.J., Rose P., Liu Y., Wang J., Allaby R.G. Small RNA Activity in Archeological Barley Shows Novel Germination Inhibition in Response to Environment. Mol. Biol. Evol. 2017;34:2555–2562. doi: 10.1093/molbev/msx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tripathi R.K., Bregitzer P., Singh J. Genome-Wide Analysis of the SPL/MiR156 Module and Its Interaction with the AP2/MiR172 Unit in Barley. Sci. Rep. 2018;8:7085. doi: 10.1038/s41598-018-25349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Plaksenkova I., Kokina I., Petrova A., Jermaļonoka M., Gerbreders V., Krasovska M. The Impact of Zinc Oxide Nanoparticles on Cytotoxicity, Genotoxicity, and MiRNA Expression in Barley (Hordeum vulgare L.) Seedlings. Sci. World J. 2020;2020:6649746. doi: 10.1155/2020/6649746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye Z., Zeng J., Long L., Ye L., Zhang G. Identification of MicroRNAs in Response to Low Potassium Stress in the Shoots of Tibetan Wild Barley and Cultivated. Curr. Plant Biol. 2021;25:100193. doi: 10.1016/j.cpb.2020.100193. [DOI] [Google Scholar]
- 103.Puchta M., Groszyk J., Małecka M., Koter M.D., Niedzielski M., Rakoczy-Trojanowska M., Boczkowska M. Barley Seeds MiRNome Stability during Long-Term Storage and Aging. Int. J. Mol. Sci. 2021;22:4315. doi: 10.3390/ijms22094315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao X., Wang Y., Yao Y., Bai Y., Wu K., Qiao Y. Identification MicroRNAs and Target Genes in Tibetan Hulless Barley to BLS Infection. Agron. J. 2021;113:2273–2292. doi: 10.1002/agj2.20649. [DOI] [Google Scholar]
- 105.Wang N.-H., Zhou X.-Y., Shi S.-H., Zhang S., Chen Z.-H., Ali M.A., Ahmed I.M., Wang Y., Wu F. An MiR156-Regulated Nucleobase-Ascorbate Transporter 2 Confers Cadmium Tolerance via Enhanced Anti-Oxidative Capacity in Barley. J. Adv. Res. 2022 doi: 10.1016/j.jare.2022.04.001. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liao P., Li S., Cui X., Zheng Y. A Comprehensive Review of Web-Based Resources of Non-Coding RNAs for Plant Science Research. Int. J. Biol. Sci. 2018;14:819–832. doi: 10.7150/ijbs.24593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yi X., Zhang Z., Ling Y., Xu W., Su Z. PNRD: A Plant Non-Coding RNA Database. Nucleic Acids Res. 2015;43:D982–D989. doi: 10.1093/nar/gku1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo Z., Kuang Z., Zhao Y., Deng Y., He H., Wan M., Tao Y., Wang D., Wei J., Li L. PmiREN2.0: From Data Annotation to Functional Exploration of Plant MicroRNAs. Nucleic Acids Res. 2022;50:D1475–D1482. doi: 10.1093/nar/gkab811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kozomara A., Birgaoanu M., Griffiths-Jones S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019;47:D155–D162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lunardon A., Johnson N.R., Hagerott E., Phifer T., Polydore S., Coruh C., Axtell M.J. Integrated Annotations and Analyses of Small RNA–Producing Loci from 47 Diverse Plants. Genome Res. 2020;30:497–513. doi: 10.1101/gr.256750.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szcześniak M.W., Makalowska I. MiRNEST 2.0: A Database of Plant and Animal MicroRNAs. Nucleic Acids Res. 2014;42:D74–D77. doi: 10.1093/nar/gkt1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J., Liu X., Zhang S., Liang S., Luan W., Ma X. TarDB: An Online Database for Plant MiRNA Targets and MiRNA-Triggered Phased SiRNAs. BMC Genom. 2021;22:348. doi: 10.1186/s12864-021-07680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dou X., Zhou Z., Zhao L. Identification and Expression Analysis of MiRNAs in Germination and Seedling Growth of Tibetan Hulless Barley. Genomics. 2021;113:3735–3749. doi: 10.1016/j.ygeno.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 114.Xing S., Salinas M., Höhmann S., Berndtgen R., Huijser P. MiR156-Targeted and Nontargeted SBP-Box Transcription Factors Act in Concert to Secure Male Fertility in Arabidopsis. Plant Cell. 2010;22:3935–3950. doi: 10.1105/tpc.110.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui L., Zheng F., Wang J., Zhang C., Xiao F., Ye J., Li C., Ye Z., Zhang J. MiR156a-Targeted SBP-Box Transcription Factor SlSPL13 Regulates Inflorescence Morphogenesis by Directly Activating SFT in Tomato. Plant Biotechnol. J. 2020;18:1670–1682. doi: 10.1111/pbi.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J., Cheng X., Liu P., Sun J. MiR156-Targeted SBP-Box Transcription Factors Interact with DWARF53 to Regulate TEOSINTE BRANCHED1 and BARREN STALK1 Expression in Bread Wheat. Plant Physiol. 2017;174:1931–1948. doi: 10.1104/pp.17.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Millar A.A., Lohe A., Wong G. Biology and Function of MiR159 in Plants. Plants. 2019;8:255. doi: 10.3390/plants8080255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Csukasi F., Donaire L., Casañal A., Martínez-Priego L., Botella M.A., Medina-Escobar N., Llave C., Valpuesta V. Two Strawberry MiR159 Family Members Display Developmental-Specific Expression Patterns in the Fruit Receptacle and Cooperatively Regulate Fa-GAMYB. New Phytol. 2012;195:47–57. doi: 10.1111/j.1469-8137.2012.04134.x. [DOI] [PubMed] [Google Scholar]
- 119.da Silva E.M., Silva G.F.F.E., Bidoia D.B., da Silva Azevedo M., de Jesus F.A., Pino L.E., Peres L.E.P., Carrera E., López-Díaz I., Nogueira F.T.S. Micro RNA 159-Targeted Sl GAMYB Transcription Factors Are Required for Fruit Set in Tomato. Plant J. 2017;92:95–109. doi: 10.1111/tpj.13637. [DOI] [PubMed] [Google Scholar]
- 120.Yadav A., Kumar S., Verma R., Lata C., Sanyal I., Rai S.P. MicroRNA 166: An Evolutionarily Conserved Stress Biomarker in Land Plants Targeting HD-ZIP Family. Physiol. Mol. Biol. Plants. 2021;27:2471–2485. doi: 10.1007/s12298-021-01096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen H., Fang R., Deng R., Li J. The OsmiRNA166b-OsHox32 Pair Regulates Mechanical Strength of Rice Plants by Modulating Cell Wall Biosynthesis. Plant Biotechnol. J. 2021;19:1468–1480. doi: 10.1111/pbi.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Javed M., Solanki M., Sinha A., Shukla L.I. Position Based Nucleotide Analysis of MiR168 Family in Higher Plants and Its Targets in Mammalian Transcripts. MicroRNA. 2017;6:136–142. doi: 10.2174/2211536606666170215154151. [DOI] [PubMed] [Google Scholar]
- 123.Um T., Choi J., Park T., Chung P.J., Jung S.E., Shim J.S., Kim Y.S., Choi I.-Y., Park S.C., Oh S.-J. Rice MicroRNA171f/SCL6 Module Enhances Drought Tolerance by Regulation of Flavonoid Biosynthesis Genes. Plant Direct. 2022;6:e374. doi: 10.1002/pld3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang S., Zhou J., Gao L., Tang Y. Plant MiR397 and Its Functions. Funct. Plant Biol. 2020;48:361–370. doi: 10.1071/FP20342. [DOI] [PubMed] [Google Scholar]
- 125.Dong C.-H., Pei H. Over-Expression of MiR397 Improves Plant Tolerance to Cold Stress in Arabidopsis Thaliana. J. Plant Biol. 2014;57:209–217. doi: 10.1007/s12374-013-0490-y. [DOI] [Google Scholar]
- 126.Pegler J.L., Oultram J.M., Grof C.P., Eamens A.L. Molecular Manipulation of the MiR399/PHO2 Expression Module Alters the Salt Stress Response of Arabidopsis Thaliana. Plants. 2020;10:73. doi: 10.3390/plants10010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim W., Ahn H.J., Chiou T.-J., Ahn J.H. The Role of the MiR399-PHO2 Module in the Regulation of Flowering Time in Response to Different Ambient Temperatures in Arabidopsis Thaliana. Mol. Cells. 2011;32:83–88. doi: 10.1007/s10059-011-1043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan Y., Wang H., Hamera S., Chen X., Fang R. MiR444a Has Multiple Functions in the Rice Nitrate-Signaling Pathway. Plant J. 2014;78:44–55. doi: 10.1111/tpj.12446. [DOI] [PubMed] [Google Scholar]
- 129.Wang H., Jiao X., Kong X., Hamera S., Wu Y., Chen X., Fang R., Yan Y. A Signaling Cascade from MiR444 to RDR1 in Rice Antiviral RNA Silencing Pathway. Plant Physiol. 2016;170:2365–2377. doi: 10.1104/pp.15.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiao X., Wang H., Yan J., Kong X., Liu Y., Chu J., Chen X., Fang R., Yan Y. Promotion of BR Biosynthesis by MiR444 Is Required for Ammonium-Triggered Inhibition of Root Growth. Plant Physiol. 2020;182:1454–1466. doi: 10.1104/pp.19.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun L., Sun G., Shi C., Sun D. Transcriptome Analysis Reveals New MicroRNAs-Mediated Pathway Involved in Anther Development in Male Sterile Wheat. BMC Genom. 2018;19:333. doi: 10.1186/s12864-018-4727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bano N., Fakhrah S., Nayak S.P., Bag S.K., Mohanty C.S. Identification of MiRNA and Their Target Genes in Cestrum nocturnum L. and Cestrum diurnum L. in Stress Responses. Physiol. Mol. Biol. Plants. 2022;28:31–49. doi: 10.1007/s12298-022-01127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li T., Ma L., Geng Y., Hao C., Chen X., Zhang X. Small RNA and Degradome Sequencing Reveal Complex Roles of MiRNAs and Their Targets in Developing Wheat Grains. PLoS ONE. 2015;10:e0139658. doi: 10.1371/journal.pone.0139658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y., Wang X., Yuan L., Liu Y., Shen T., Zhang Y. Comparative Small RNA Profiling and Functional Exploration on Wheat with High-and Low-Cadmium Accumulation. Front. Genet. 2021;12:635599. doi: 10.3389/fgene.2021.635599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Samad A.F.A., Sajad M., Nazaruddin N., Fauzi I.A., Murad A.M.A., Zainal Z., Ismail I. MicroRNA and Transcription Factor: Key Players in Plant Regulatory Network. Front. Plant Sci. 2017;8:565. doi: 10.3389/fpls.2017.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van Bel M., Diels T., Vancaester E., Kreft L., Botzki A., Van de Peer Y., Coppens F., Vandepoele K. PLAZA 4.0: An Integrative Resource for Functional, Evolutionary and Comparative Plant Genomics. Nucleic Acids Res. 2018;46:D1190–D1196. doi: 10.1093/nar/gkx1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Bel M., Silvestri F., Weitz E.M., Kreft L., Botzki A., Coppens F., Vandepoele K. PLAZA 5.0: Extending the Scope and Power of Comparative and Functional Genomics in Plants. Nucleic Acids Res. 2022;50:D1468–D1474. doi: 10.1093/nar/gkab1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H.-M., Kuang S., Xiong X., Gao T., Liu C., Guo A.-Y. Transcription Factor and MicroRNA Co-Regulatory Loops: Important Regulatory Motifs in Biological Processes and Diseases. Brief. Bioinform. 2015;16:45–58. doi: 10.1093/bib/bbt085. [DOI] [PubMed] [Google Scholar]
- 139.Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread Changes in Protein Synthesis Induced by MicroRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 140.Baldrich P., Beric A., Meyers B.C. Despacito: The Slow Evolutionary Changes in Plant MicroRNAs. Curr. Opin. Plant Biol. 2018;42:16–22. doi: 10.1016/j.pbi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y., Wang L., Chen D., Wu X., Huang D., Chen L., Li L., Deng X., Xu Q. Genome-Wide Comparison of MicroRNAs and Their Targeted Transcripts among Leaf, Flower and Fruit of Sweet Orange. BMC Genom. 2014;15:695. doi: 10.1186/1471-2164-15-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baldrich P., Campo S., Wu M.-T., Liu T.-T., Hsing Y.-I.C., Segundo B.S. MicroRNA-Mediated Regulation of Gene Expression in the Response of Rice Plants to Fungal Elicitors. RNA Biol. 2015;12:847–863. doi: 10.1080/15476286.2015.1050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Banks I.R., Zhang Y., Wiggins B.E., Heck G.R., Ivashuta S. RNA Decoys. Plant Signal. Behav. 2012;7:1188–1193. doi: 10.4161/psb.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma X., Liu C., Gu L., Mo B., Cao X., Chen X. TarHunter, a Tool for Predicting Conserved MicroRNA Targets and Target Mimics in Plants. Bioinformatics. 2018;34:1574–1576. doi: 10.1093/bioinformatics/btx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Unver T., Tombuloglu H. Barley Long Non-Coding RNAs (LncRNA) Responsive to Excess Boron. Genomics. 2020;112:1947–1955. doi: 10.1016/j.ygeno.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Yu J., Cheng Y., Feng K., Ruan M., Ye Q., Wang R., Li Z., Zhou G., Yao Z., Yang Y., et al. Genome-Wide Identification and Expression Profiling of Tomato Hsp20 Gene Family in Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2016;7:1215. doi: 10.3389/fpls.2016.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lewis B.P., Burge C.B., Bartel D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 148.Ni W.-J., Leng X.-M. Dynamic MiRNA–MRNA Paradigms: New Faces of MiRNAs. Biochem. Biophys. Rep. 2015;4:337–341. doi: 10.1016/j.bbrep.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gruber A.R., Lorenz R., Bernhart S.H., Neuböck R., Hofacker I.L. The Vienna RNA Websuite. Nucleic Acids Res. 2008;36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kerpedjiev P., Hammer S., Hofacker I.L. Forna (Force-Directed RNA): Simple and Effective Online RNA Secondary Structure Diagrams. Bioinformatics. 2015;31:3377–3379. doi: 10.1093/bioinformatics/btv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jones-Rhoades M.W., Bartel D.P. Computational Identification of Plant MicroRNAs and Their Targets, Including a Stress-Induced MiRNA. Mol. Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 152.Sun Y.-H., Lu S., Shi R., Chiang V.L. RNAi and Plant Gene Function Analysis. Humana Press; Totowa, NJ, USA: 2011. Computational Prediction of Plant MiRNA Targets; pp. 175–186. [DOI] [PubMed] [Google Scholar]
- 153.Dai X., Zhuang Z., Zhao P.X. Computational Analysis of MiRNA Targets in Plants: Current Status and Challenges. Brief. Bioinform. 2011;12:115–121. doi: 10.1093/bib/bbq065. [DOI] [PubMed] [Google Scholar]
- 154.Fahlgren N., Carrington J.C. Plant MicroRNAs. Humana Press; Totowa, NJ, USA: 2010. MiRNA Target Prediction in Plants; pp. 51–57. [DOI] [PubMed] [Google Scholar]
- 155.Pandey P., Srivastava P.K., Pandey S.P. Plant MicroRNAs. Humana Press; Totowa, NJ, USA: 2019. Prediction of Plant MiRNA Targets; pp. 99–107. [DOI] [PubMed] [Google Scholar]
- 156.Rhoades M.W., Reinhart B.J., Lim L.P., Burge C.B., Bartel B., Bartel D.P. Prediction of Plant MicroRNA Targets. Cell. 2002;110:513–520. doi: 10.1016/S0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 157.Llave C., Xie Z., Kasschau K.D., Carrington J.C. Cleavage of Scarecrow-like MRNA Targets Directed by a Class of Arabidopsis MiRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- 158.Riolo G., Cantara S., Marzocchi C., Ricci C. MiRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2020;4:1. doi: 10.3390/mps4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patel P., Yadav K., Ganapathi T.R., Penna S. Plant MiRNAome: Cross Talk in Abiotic Stressful Times. In: Rajpal V.R., Sehgal D., Kumar A., Raina S.N., editors. Genetic Enhancement of Crops for Tolerance to Abiotic Stress: Mechanisms and Approaches, Vol. I. Sustainable Development and Biodiversity; Springer International Publishing; Cham, Switzesland: 2019. pp. 25–52. [Google Scholar]
- 160.Zhang B., Wang Q. MicroRNA-Based Biotechnology for Plant Improvement. J. Cell. Physiol. 2015;230:1–15. doi: 10.1002/jcp.24685. [DOI] [PubMed] [Google Scholar]
- 161.Zhang B. MicroRNA: A New Target for Improving Plant Tolerance to Abiotic Stress. J. Exp. Bot. 2015;66:1749–1761. doi: 10.1093/jxb/erv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferdous J., Whitford R., Nguyen M., Brien C., Langridge P., Tricker P.J. Drought-Inducible Expression of Hv-MiR827 Enhances Drought Tolerance in Transgenic Barley. Funct. Integr. Genom. 2017;17:279–292. doi: 10.1007/s10142-016-0526-8. [DOI] [PubMed] [Google Scholar]
- 163.Li N., Yang T., Guo Z., Wang Q., Chai M., Wu M., Li X., Li W., Li G., Tang J., et al. Maize MicroRNA166 Inactivation Confers Plant Development and Abiotic Stress Resistance. Int. J. Mol. Sci. 2020;21:9506. doi: 10.3390/ijms21249506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chung P.J., Chung H., Oh N., Choi J., Bang S.W., Jung S.E., Jung H., Shim J.S., Kim J.-K. Efficiency of Recombinant CRISPR/RCas9-Mediated MiRNA Gene Editing in Rice. Int. J. Mol. Sci. 2020;21:9606. doi: 10.3390/ijms21249606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hua K., Tao X., Zhu J.-K. Expanding the Base Editing Scope in Rice by Using Cas9 Variants. Plant Biotechnol. J. 2019;17:499–504. doi: 10.1111/pbi.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ó’Maoiléidigh D.S., van Driel A.D., van Singh A., Sang Q., LeBec N., Vincent C., de Olalla E.B.G., Vayssières A., Branchat M.R., Severing E., et al. Systematic Analyses of the MIR172 Family Members of Arabidopsis Define Their Distinct Roles in Regulation of APETALA2 during Floral Transition. PLoS Biol. 2021;19:e3001043. doi: 10.1371/journal.pbio.3001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deng F., Zeng F., Shen Q., Abbas A., Cheng J., Jiang W., Chen G., Shah A.N., Holford P., Tanveer M., et al. Molecular Evolution and Functional Modification of Plant MiRNAs with CRISPR. Trends Plant Sci. 2022;27:890–907. doi: 10.1016/j.tplants.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 168.Dalakouras A., Wassenegger M., Dadami E., Ganopoulos I., Pappas M.L., Papadopoulou K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020;182:38–50. doi: 10.1104/pp.19.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mujtaba M., Wang D., Carvalho L.B., Oliveira J.L., Espirito Santo Pereira A.D., Sharif R., Jogaiah S., Paidi M.K., Wang L., Ali Q., et al. Nanocarrier-Mediated Delivery of MiRNA, RNAi, and CRISPR-Cas for Plant Protection: Current Trends and Future Directions. ACS Agric. Sci. Technol. 2021;1:417–435. doi: 10.1021/acsagscitech.1c00146. [DOI] [Google Scholar]
- 170.Ražná K., Rataj V., Macák M., Galambošová J. MicroRNA-Based Markers as a Tool to Monitor the Barley (Hordeum vulgare L.) Response to Soil Compaction. Acta Fytotech. Zootech. 2020;23:139–146. doi: 10.15414/afz.2020.23.03.139-146. [DOI] [Google Scholar]
- 171.Gurjar A.K.S., Panwar A.S., Gupta R., Mantri S.S. PmiRExAt: Plant MiRNA Expression Atlas Database and Web Applications. Database. 2016;2016:baw060. doi: 10.1093/database/baw060. [DOI] [PMC free article] [PubMed] [Google Scholar]