Abstract
The agents of Lyme disease (Borrelia burgdorferi) and human granulocytic ehrlichiosis (Ehrlichia phagocytophila) are both transmitted by the tick Ixodes scapularis. In nature, ticks are often infected with both agents simultaneously. We studied whether previous infection with either Borrelia or Ehrlichia in ticks would affect acquisition and transmission of a second pathogen. Ehrlichia-infected I. scapularis nymphs were fed upon Borrelia-infected mice, and Borrelia-infected I. scapularis nymphs were fed upon Ehrlichia-infected mice. The efficiency with which previously infected nymphal ticks acquired a second pathogen from infected hosts was compared to that of uninfected ticks. An average of 51% ± 15% of ticks acquired Ehrlichia from infected mice regardless of their prior infection status with Borrelia. An average of 85% ± 10% of ticks acquired Borrelia from infected mice regardless of their prior infection status with Ehrlichia. Also, we assessed the efficiency with which individual nymphs could transmit either agent alone, or both agents simultaneously, to individual susceptible hosts. An average of 76% ± 9% of Borrelia-infected ticks and 84% ± 10% of Ehrlichia-infected ticks transmitted these agents to mice regardless of the presence of the other pathogen. There was no evidence of interaction between the agents of Lyme disease and human granulocytic ehrlichiosis in I. scapularis ticks. The presence of either agent in the ticks did not affect acquisition of the other agent from an infected host. Transmission of the agents of Lyme disease and human granulocytic ehrlichiosis by individual ticks was equally efficient and independent. Dually infected ticks transmitted each pathogen to susceptible hosts as efficiently as ticks infected with only one pathogen.
The black-legged tick Ixodes scapularis is a vector of Borrelia burgdorferi, the etiologic agent of Lyme disease, as well as Ehrlichia phagocytophila, the etiologic agent of human granulocytic ehrlichiosis (HGE) (2, 4, 31, 32). In nature, nymphal and adult ticks are often infected with both agents simultaneously (3, 16, 29, 33). Nymphal or adult ticks can acquire both pathogens simultaneously from a single coinfected host during either larval or nymphal feeding (M. Levin, unpublished data). Alternatively, adult ticks may have acquired pathogens consecutively—one during larval feeding and a second during nymphal feeding. In nature, the prevalence of either pathogen in ticks increases significantly from the nymphal to the adult stage, and consequently, the prevalence of coinfection in questing adult ticks can be 7 to 10 times higher than in nymphs (16). This observation suggests that consecutive acquisition of different pathogens by individual ticks may happen more frequently than simultaneous acquisition.
Simultaneous infection with these two agents has also been documented in humans and rodents (16, 18–20). Infection with both agents may result from the bite of a single coinfected tick. However, there is no experimental evidence for simultaneous transmission of Ehrlichia and Borrelia by individual ticks. Moreover, the efficiency with which infected ticks can transmit either E. phagocytophila or B. burgdorferi to susceptible hosts has not been studied in detail. A recent study found that some laboratory mice fed upon by small numbers of I. scapularis ticks infected with E. phagocytophila failed to acquire infection, suggesting that E. phagocytophila may be transmitted less efficiently than B. burgdorferi (4). However, all transmission studies of B. burgdorferi published to date also involve groups of infected ticks (27, 28), and it is therefore not known if all individual ticks are capable of Borrelia transmission. Evidence from studies of tick-borne encephalitis suggests that the efficiency of transmission by a population of infected Ixodes persulcatus ticks is considerably less than 100% (10).
We questioned whether previous infection with either B. burgdorferi or E. phagocytophila in ticks would affect acquisition and/or transmission of a second pathogen. In order to determine this, we measured the efficiency with which previously infected nymphal ticks acquired a second pathogen from infected hosts and compared it to the efficiency of acquisition by uninfected ticks. We also measured the efficiency with which individual nymphs could transmit either agent alone or both agents simultaneously to individual susceptible hosts.
MATERIALS AND METHODS
The white-footed mouse (Peromyscus leucopus) is known to be a major reservoir for B. burgdorferi. It also has been shown to be susceptible to infection with E. phagocytophila (5, 16, 21, 32). Therefore, we used white-footed mice as hosts in our experiments. Two-month-old mice were derived from a specific-pathogen-free P. leucopus colony maintained in our laboratory. The maintenance and care of experimental animals complied with the National Institutes of Health guidelines for the humane use of laboratory animals. The mice were not exposed to ticks or pathogens prior to the experiments.
Infected I. scapularis nymphs were produced by allowing larval ticks to feed upon white-footed mice previously infected with either B. burgdorferi or E. phagocytophila. Both agents originated from nymphal ticks collected in Westchester County (N.Y.) and were maintained separately in a laboratory tick-mouse cycle. The identities of the agents had been previously confirmed by indirect immunofluorescence assay and by DNA sequencing of amplified PCR products (4, 14).
Infection with B. burgdorferi and E. phagocytophila in ticks and mice was determined by PCR. For PCR, individual nymphal or adult ticks or pools of engorged larvae were placed in sterile 1.5-cm3 plastic vials, deep frozen in liquid nitrogen, ground with a sterile plastic pestle, and resuspended in 100 μl of Tris-borate buffer. DNA was extracted from the ticks with an IsoQuick nucleic acid extraction kit (ORCA Research Inc., Bothell, Wash.) to maximize sensitivity (30). Briefly, guanidine thiocyanate, a proprietary extraction matrix, and sodium dodecyl sulfate solution were added to a suspension, and the mixture was incubated at 65°C for 10 min. After separation of phases by centrifugation, the DNA was precipitated with sodium acetate and isopropanol and washed with 70% ethanol. The final DNA pellet was resuspended in 50 μl of RNase-free water, and 1 2.5-μl aliquot was used for each PCR test. Primers EHR521 (5′-TGT AGO CGG TTC GOT AAG TTA AAG-3′) and EHR747 (5′-GCA CTC ATC GTT TAC AGC GTG-3′) were used to amplify a 247-bp fragment of 16S ribosomal DNA from E. phagocytophila (24). Primers FLA297 (5′-CGG CAC ATA TTC AGA TGC AGA CAG-3′) and FLA652 (5′-CCT GTT GAA CAC CCT CTT GAA CC-3′), based on the published nucleotide sequence (6), were used to amplify a 378-bp fragment of the flagellin gene of B. burgdorferi. The amplification products were visualized in 2% agarose gels.
Acquisition experiment.
Ten mice were each infected with Borrelia by allowing 10 I. scapularis nymphs from a B. burgdorferi-infected cohort to feed on them. Another 10 mice were each similarly infected with Ehrlichia by allowing 10 nymphs from an E. phagocytophila-infected cohort to feed on them. The infection in the nymphal cohorts prior to the investigation was assessed by testing representative samples of 25 ticks. The prevalence of infection in the Borrelia-infected cohort was 44.0% ± 10.1%, and the prevalence of infection in the Ehrlichia-infected cohort was 40.0% ± 10.0%.
Two weeks later, 25 nymphs from the B. burgdorferi-infected cohort were placed on each of five mice previously infected with Ehrlichia. The other five Ehrlichia-infected mice were each fed upon by 25 uninfected nymphs. Similarly, five B. burgdorferi-infected mice were infested with 25 nymphs from the Ehrlichia-infected cohort, and 25 uninfected nymphs fed upon the other five B. burgdorferi-infected mice. The engorged nymphs were collected and kept at 22°C and 98% relative humidity until they molted. Freshly moiled adult ticks were individually tested by PCR for infection.
Transmission experiment.
Single-infected and coinfected nymphs were produced by allowing larval ticks to feed upon white-footed mice singly or simultaneously infected with B. burgdorferi and E. phagocytophila in the course of the previous experiment. These nymphs were placed individually on single naive mice and allowed to feed to repletion. The resulting engorged nymphs were collected and individually tested by PCR for infection.
Two weeks after the feeding by infected nymphs, the mice were infested with uninfected larval ticks for xenodiagnosis. The infection status of individual mice was assessed using 20 engorged xenodiagnostic larvae per mouse (four pools of five ticks). The tick pools were tested for both pathogens by PCR. Our previous study had shown that feeding density influences the acquisition of B. burgdorferi in larval I. scapularis (15). Therefore, the mice were infested with a large number of larvae (approximately 200) in order to maximize the sensitivity of xenodiagnosis. The xenodiagnostic larvae were derived from a colony of I. scapularis maintained in our laboratory by allowing them to feed on uninfected mice and rabbits for several generations. Representative samples of ticks from the colony are regularly tested to ensure that the colony is free of both tick-borne pathogens. Xenodiagnosis was performed only on mice from which individual replete nymphs that tested positive for either pathogen were collected.
Differences in prevalence of infection were analyzed using χ2 and analysis of variance (ANOVA) statistics.
RESULTS AND DISCUSSION
Acquisition experiment.
An average of 19 (12 to 23) nymphal ticks fed to repletion on each of the 20 infected mice and were tested for both agents as adults. When nymphs from the Borrelia-infected cohort fed upon five mice infected with Ehrlichia, 39 of the resulting adult ticks tested PCR positive and 47 tested PCR negative for B. burgdorferi (Table 1). The prevalence of Borrelia infection in adult ticks (45.3% ± 10.6%) did not differ from that in the same cohort of nymphs tested prior to feeding (44.0% ± 19.9%). When nymphs from the Ehrlichia-infected cohort fed upon five mice infected with B. burgdorferi, 48 of the resulting adult ticks tested PCR positive and 47 tested PCR negative for Ehrlichia (Table 2). Again, the difference in Ehrlichia infection between nymphal ticks prior to feeding (40.0% ± 19.6%) and the resulting adult ticks (50.5% ± 10.1%) was not statistically significant.
TABLE 1.
Acquisition of E. phagocytophila by ticks infected with B. burgdorferi and by uninfected ticks
| Mouse | No. of ticks infected with B. burgdorferi
|
No. of uninfected ticks
|
Pχ2a | ||
|---|---|---|---|---|---|
| Tested | Acquiring E. phagocytophila (%) | Tested | Acquiring E. phagocytophila (%) | ||
| PI-387 | 11 | 6 (54.5) | 9 | 4 (44.4) | 0.65 |
| PI-388 | 6 | 3 (50.0) | 6 | 3 (50.0) | 1.00 |
| PI-389 | 8 | 5 (62.5) | 12 | 8 (75.0) | 0.85 |
| PI-390 | 7 | 4 (57.1) | 7 | 3 (42.9) | 0.59 |
| PI-391 | 7 | 2 (28.6) | 13 | 5 (38.5) | 0.67 |
| Total | 39 | 20 (51.3 ± 15.9)b | 47 | 24 (51.1 ± 14.4)b | 0.98 |
Pχ2, probability for a chi-square test distribution.
±95% confidence interval.
TABLE 2.
Acquisition of B. burgdorferi by ticks infected with E. phagocytophila and by uninfected ticks
| Mouse | No. of ticks infected with E. phagocytophila
|
No. of uninfected ticks
|
Pχ2a | ||
|---|---|---|---|---|---|
| Tested | Acquiring B. burgdorferi (%) | Tested | Acquiring B. burgdorferi (%) | ||
| PI-397 | 6 | 4 (66.7) | 14 | 10 (71.4) | 0.83 |
| PI-398 | 8 | 7 (87.5) | 9 | 7 (77.8) | 0.60 |
| PI-399 | 11 | 11 (100.0) | 7 | 7 (100.0) | 1.00 |
| PI-400 | 12 | 11 (91.7) | 8 | 8 (100.0) | 0.40 |
| PI-401 | 11 | 8 (72.7) | 9 | 8 (88.9) | 0.39 |
| Total | 48 | 41 (85.4 ± 10.0)b | 47 | 40 (85.1 ± 10.3)b | 0.97 |
Pχ2, probability for a chi-square test distribution.
±95% confidence interval.
Nymphs may be able to acquire pathogens not only from an infectious host but also from infected ticks during cofeeding (7, 22, 23, 25). However, transmission by cofeeding did not increase the prevalence of either B. burgdorferi or Ehrlichia in our experiment. Therefore, we assume that the ticks which tested positive for B. burgdorferi after feeding on mice (Table 1) were infected with B. burgdorferi prior to feeding. The same assumption applies to the adult ticks that tested positive for Ehrlichia (Table 2).
A total of 44 ticks from the Borrelia-infected cohort acquired Ehrlichia during feeding upon five infected mice (Table 1). The efficiency of Ehrlichia acquisition by nymphal ticks from the same cohort varied among individual mice but did not differ between ticks that were or were not previously infected with B. burgdorferi (Pχ2 = 0.98). On the average, approximately 50% of nymphs from the Borrelia-infected cohort acquired Ehrlichia from infected mice regardless of their prior infection status with B. burgdorferi (Table 1).
When a cohort of exclusively uninfected nymphs fed upon the second group of five mice infected with Ehrlichia, a total of 43 of 96 resulting adult ticks (44.8% ± 10.0%) acquired the infection. Individual mice transmitted Ehrlichia to 30.0 to 60.1% of feeding ticks. The difference in acquisition of Ehrlichia by a cohort of B. burgdorferi-infected nymphs and a cohort of uninfected nymphs was not statistically significant (PANOVA = 0.22).
A total of 81 ticks from the Ehrlichia-infected cohort acquired Borrelia during feeding upon five infected mice (Table 2). The efficiency of B. burgdorferi acquisition by nymphal ticks from the same cohort varied among individual mice but did not differ between ticks that were or were not previously infected with Ehrlichia (Pχ2 = 0.97). An average of 85.3% ± 7.2% of nymphs from the Ehrlichia-infected cohort acquired B. burgdorferi from infected mice regardless of their prior infection status with Ehrlichia (Table 2).
When a cohort of exclusively uninfected nymphs fed upon an additional five mice infected with B. burgdorferi, a total of 88 of 105 resulting adult ticks (83.8% ± 7.1%) acquired the infection. Individual mice transmitted B. burgdorferi to 73.9 to 90.5% of feeding ticks. The difference in acquisition of B. burgdorferi by a cohort of Ehrlichia-infected nymphs and a cohort of uninfected nymphs was not statistically significant (PANOVA = 0.37).
Thus, previous infection with B. burgdorferi or E. phagocytophila in nymphal I. scapularis did not affect the ability of the ticks to acquire a second pathogen from infected hosts.
Transmission experiment.
A total of 98 mice were successfully fed upon by individual nymphal ticks. Of those 98 nymphs, 89 were infected with either B. burgdorferi or E. phagocytophila or both as detected by PCR performed on the engorged ticks (Table 3). Xenodiagnostic results showed that 31 of 38 (81.6%) ticks infected with only B. burgdorferi transmitted the spirochete to mice compared to 70% (21 of 30) transmission success when ticks were simultaneously infected with both Borrelia and Ehrlichia (Table 3). This difference between the two groups of ticks was not statistically significant (Pχ2 = 0.27). When ticks were infected with Ehrlichia only, 18 of 21 (85.7%) transmitted it to susceptible mice, as determined by xenodiagnosis (Table 3). Of 30 dually infected ticks, 18 (83.3%) transmitted Ehrlichia. Thus, there also was no difference in the efficiency of transmission of E. phagocytophila between ticks infected with one or both pathogens (Pχ2 = 0.82). The efficiency of transmission did not differ significantly between B. burgdorferi and Ehrlichia either in ticks infected with one pathogen (Pχ2 = 0.69) or in dually infected ticks (Pχ2 = 0.22).
TABLE 3.
Transmission of B. burgdorferi and E. phagocytophila to mice by individual I. scapularis nymphs
| Infection in nymphs | No. of ticks fed | Transmitting B. burgdorferia
|
Transmitting E. phagocytophilaa
|
|---|---|---|---|
| No. (%) | No. (%) | ||
| B. burgdorferi only | 38 | 31 (81.6 ± 12.5) | |
| B. burgdorferi and E. phagocytophila | 30 | 21 (70.0 ± 16.7) | 25 (83.3 ± 13.6) |
| E. phagocytophila only | 21 | 18 (85.7 ± 15.3) |
± 95% confidence interval.
In another study of pathogen transmission by individual I. scapularis ticks, six of seven nymphs that fed to repletion transmitted B. burgdorferi to hamsters (26). However, the ticks themselves were not examined, and it was not known whether the nontransmitting ticks were infected. Our data show that only 70 to 81% of infected I. scapularis nymphs transmit B. burgdorferi to susceptible hosts even when fed to repletion. The efficiency of transmission of E. phagocytophila by infected ticks is 83 to 86% and is not significantly different from that of B. burgdorferi. The differential infectivity of ticks is likely to be related to the variability of pathogen concentration among infected ticks (1, 11–13, 17). This has been shown to occur in ticks transmitting spring-summer tick-borne encephalitis virus (8, 9).
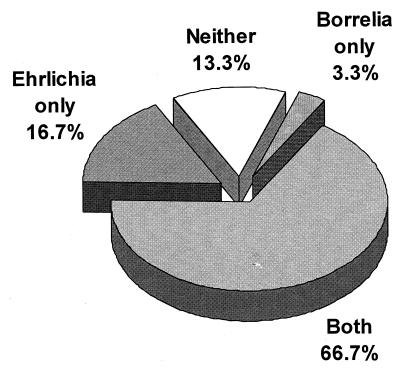
Of 30 ticks infected with Borrelia and Ehrlichia simultaneously, 20 successfully transmitted both pathogens while 4 failed to transmit either (Fig. 1). Our results suggest that transmission of the agents of Lyme disease and HGE by individual ticks is equally efficient and independent. Simultaneous infection with the agents of Lyme disease and HGE has been observed both in human patients and in wild animals (16, 18–20). Mixed infections in hosts may originate either from the bite of a single tick infected with two pathogens or from multiple bites of singly infected ticks. Simultaneous transmission of B. burgdorferi and Babesia microti by individual I. scapularis nymphs has been previously reported (26). The present study provides evidence that dually infected ticks are capable of simultaneous transmission of B. burgdorferi and E. phagocytophila and that infection of ticks with one of these pathogens does not interfere with transmission of the other.
FIG. 1.
Transmission of B. burgdorferi and E. phagocytophila by individual I. scapularis nymphs simultaneously infected with both pathogens.
There was no evidence of interaction between the agents of Lyme disease and HGE in I. scapularis. The presence of either agent in ticks did not interfere with acquisition of the other agent from an infected host. Transmission of the agents of Lyme disease and HGE by individual ticks was equally efficient and independent. Dually infected ticks transmitted each pathogen to susceptible hosts as efficiently as ticks infected with only one pathogen, and most dually infected ticks were able to transmit both pathogens to a susceptible host.
ACKNOWLEDGMENTS
This research was sponsored by grants from the G. Harold and Leila Y. Mathers Charitable Foundation, the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI28956), and USDA cooperative agreement 58-1265-5023.
REFERENCES
- 1.Brunet L R, Spielman A, Telford S R., III Density of Lyme disease spirochetes within deer ticks collected from zoonotic sites. Am J Trop Med Hyg. 1995;53:300–302. doi: 10.4269/ajtmh.1995.53.300. [DOI] [PubMed] [Google Scholar]
- 2.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels T J, Falco R C, Schwartz I, Varde S, Robbins R G. Deer ticks (Ixodes scapularis) and the agents of Lyme disease and human granulocytic ehrlichiosis in a New York City park. Emerg Infect Dis. 1997;3:353–355. doi: 10.3201/eid0303.970312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Des Vignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking Ixodes scapularis (Acari: Ixodidae) in southern New York state. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 5.Des Vignes F, Levin M L, Fish D. Comparative vector competence of Dermacentor variabilis and Ixodes scapularis (Acari: Ixodidae) for the agent of human granulocytic ehrlichiosis. J Med Entomol. 1999;36:182–185. doi: 10.1093/jmedent/36.2.182. [DOI] [PubMed] [Google Scholar]
- 6.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 7.Gern L, Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) J Med Entomol. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Korenberg E I, Bannova G G, Kovalevskii Y V, Karavanov A S. Intrapopulation differences in the infectivity of adult Ixodes persulcatus P. Sch. with the tick-borne encephalitis virus and an assessment of its total content in ticks. Vopr Virusol. 1988;33:456–461. . (In Russian.) [PubMed] [Google Scholar]
- 9.Korenberg E I, Kovalevskii Y V. Variation in parameters affecting risk of human disease due to TBE virus. Folia Parasitol (Prague) 1995;42:307–312. [PubMed] [Google Scholar]
- 10.Kovalevskii Y V, Korenberg E I. Factors that determine the possibility of tick-borne encephalitis infection. 3. The probability of human contact with an infected vector in the central taiga forests of Khabarovsk Territory. Med Parazitol (Moscow) 1990;3:5–8. . (In Russian.) [PubMed] [Google Scholar]
- 11.Kovalevskii Y V, Korenberg E I. Differences in Borrelia infections in adult Ixodes persulcatus and Ixodes ricinus ticks (Acari: Ixodidae) in populations of north-western Russia. Exp Appl Acarol. 1995;19:19–29. doi: 10.1007/BF00051934. [DOI] [PubMed] [Google Scholar]
- 12.Kovalevskii Y V, Korenberg E I, Kutlina T V, Ustinova O A. Standards for reviewing preparations in the study of ixodid tick nymphs by dark-field microscopy in borreliosis foci. Med Parazitol (Moscow) 1996;4:18–21. . (In Russian.) [PubMed] [Google Scholar]
- 13.Kovalevskii Y V, Korenberg E I, Nikitochkin I G. The optimization of a method for assessing the contagiosity and the degree of individual infectiousness of ticks with Borrelia. Med Parazitol (Moscow) 1991;3:18–21. . (In Russian.) [PubMed] [Google Scholar]
- 14.Levin M, Levine J F, Apperson C S, Norris D E, Howard P B. Reservoir competence of the rice rat (Rodentia: Cricetidae) for Borrelia burgdorferi. J Med Entomol. 1995;32:138–142. doi: 10.1093/jmedent/32.2.138. [DOI] [PubMed] [Google Scholar]
- 15.Levin M, Papero M, Fish D. Feeding density influences acquisition of Borrelia burgdorferi in larval Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 1997;34:569–572. doi: 10.1093/jmedent/34.5.569. [DOI] [PubMed] [Google Scholar]
- 16.Levin M L, des Vignes F, Fish D. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg Infect Dis. 1999;5:204–208. doi: 10.3201/eid0502.990203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin M L, Kovalevskii Y V, Piskunova A Y, Schegoleva T V. Evaluation of individual tick infection rate with Lyme disease agent by microscopic examination of fixed smears. In: Korenberg E I, editor. Problems of tick-borne borrelioses. Academy of Medical Sciences; 1993. pp. 157–162. of Russia, Moscow, Russia. [Google Scholar]
- 18.Magnarelli L A, Anderson J F, Stafford III K C, Dumler J S. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme disease in white-footed mice. J Wildl Dis. 1997;33:466–473. doi: 10.7589/0090-3558-33.3.466. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadelman R B, Horowitz H W, Hsieh T C, Wu J M, Aguero-Rosenfeld M E, Schwartz I, Nowakowski J, Varde S, Wormser G P. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson W L, Muir S, Sumner J W, Childs J E. Serologic evidence of infection with Ehrlichia spp. in wild rodents (Muridae: Sigmodontinae) in the United States. J Clin Microbiol. 1998;36:695–700. doi: 10.1128/jcm.36.3.695-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuttall P A. Displaced tick-parasite interactions at the host interface. Parasitology. 1998;116:S65–S72. doi: 10.1017/s003118200008495x. [DOI] [PubMed] [Google Scholar]
- 23.Ogden N H, Nuttall P A, Randolph S E. Natural Lyme disease cycles maintained via sheep by cofeeding ticks. Parasitology. 1997;115:591–599. doi: 10.1017/s0031182097001868. [DOI] [PubMed] [Google Scholar]
- 24.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Jr, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 25.Patrican L A. Acquisition of Lyme disease spirochetes by cofeeding Ixodes scapularis ticks. Am J Trop Med Hyg. 1997;57:589–593. doi: 10.4269/ajtmh.1997.57.589. [DOI] [PubMed] [Google Scholar]
- 26.Piesman J, Hicks T C, Sinsky R J, Obiri G. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. J Clin Microbiol. 1987;25:2012–2013. doi: 10.1128/jcm.25.10.2012-2013.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piesman J, Mather T N, Sinsky R J, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piesman J, Maupin G O, Campos E G, Happ C M. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J Infect Dis. 1991;163:895–897. doi: 10.1093/infdis/163.4.895. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz I, Fish D, Daniels T J. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz I, Varde S, Nadelman R B, Wormser G P, Fish D. Inhibition of efficient polymerase chain reaction amplification of Borrelia burgdorferi DNA in blood-fed ticks. Am J Trop Med Hyg. 1997;56:339–342. doi: 10.4269/ajtmh.1997.56.339. [DOI] [PubMed] [Google Scholar]
- 31.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey county. Emerg Infect Dis. 1998;4:97–99. doi: 10.3201/eid0401.980113. [DOI] [PMC free article] [PubMed] [Google Scholar]