Abstract
It is generally accepted that cellular, but not humoral immunity, plays an important role in host defense against intracellular bacteria. However, studies of some of these pathogens have provided evidence that antibodies can provide immunity if present during the initiation of infection. Here, we examined immunity against infection by Ehrlichia chaffeensis, an obligate intracellular bacterium that causes human monocytic ehrlichiosis. Studies with mice have demonstrated that immunocompetent strains are resistant to persistent infection but that SCID mice become persistently and fatally infected. Transfer of immune serum or antibodies obtained from immunocompetent C57BL/6 mice to C57BL/6 scid mice provided significant although transient protection from infection. Bacterial clearance was observed when administration occurred at the time of inoculation or well after infection was established. The effect was dose dependent, occurred within 2 days, and persisted for as long as 2 weeks. Weekly serum administration prolonged the survival of susceptible mice. Although cellular immunity is required for complete bacterial clearance, the data show that antibodies can play a significant role in the elimination of this obligate intracellular bacterium during active infection and thus challenge the paradigm that humoral responses are unimportant for immunity to such organisms.
Cellular immune responses have long been considered to be a hallmark of immunity to intracellular bacterial pathogens (reviewed in references 17 and 32). Classical studies of well-characterized intracellular bacterial pathogens such as Listeria monocytogenes and Mycobacterium tuberculosis have provided clear evidence for a critical role for cellular immunity in host defense (19, 22, 26). Indeed, it has often been difficult to demonstrate a significant role for humoral immunity during intracellular bacterial infection, although exceptions exist (12, 28). The failure in many studies to observe a role for antibodies has led to general acceptance of the tenet that antibodies play little or no role in host defense during intracellular bacterial infection, although antibodies are well known to exert neutralizing effects during virus infections. Moreover, when interpreted as part of the Th1/Th2 paradigm, humoral immune responses have often been considered to be antagonistic to protective cellular responses during intracellular bacterial infections (3). However, accumulating evidence from both older and more recent studies indicates that humoral immunity may be important for immunity to a number of intracellular bacterial and fungal parasites (reviewed in reference 6). These data suggest that both cellular and humoral immune responses can contribute to immunity to intracellular bacterial pathogens.
To further address the role of cellular and humoral immunity during intracellular bacterial infection, we have examined the immunological basis of resistance and susceptibility to infection by Ehrlichia chaffeensis, an obligate intracellular bacterium that infects cells of the monocyte/macrophage lineage. E. chaffeensis is the etiologic agent of human monocytic ehrlichiosis (HME), an emerging tick-borne disease that resembles toxic shock syndrome (13). The bacterium is transmitted by the tick Amblyomma americanum, commonly found in the southeastern and mid-Atlantic regions of the United States. HME is characterized by a number of nonspecific symptoms including malaise and myalgia, as well as specific hematological abnormalities such as leukopenia and thrombocytopenia (36). Little is known of the host factors that influence susceptibility and resistance to HME, although some studies have suggested that humoral immune responses might play an important role during infections by related ehrlichiae (18, 33).
Inbred mice have been shown to be susceptible to experimental infection by E. chaffeensis (35, 38). Our previous studies showed that immunocompetent mice (e.g., BALB/c and C57BL/6) developed only transient infection and inflammation and cleared the ehrlichiae within about 2 weeks (38). However, immunocompromised SCID mice, which lack T and B lymphocytes, developed persistent infection and disease and became moribund within 3 weeks postinfection. To determine if a B-cell-derived antibody provided protection from infection, immune serum from C57BL/6 mice was transferred to susceptible SCID mice prior to or during active infection. A significant protective effect was observed after passive transfer of immune serum, and the active component was determined to be the antibody. The transferred antibodies caused bacterial elimination and ameliorated disease, even when administered to mice well after infection had been established. Furthermore, mice deficient for α/β T cells or both α/β and γ/δ T cells, although persistently infected, remained healthy, presumably due to the presence of B cells. Thus, although both cellular and humoral immune responses are involved in host defense, antibodies, in the absence of lymphocytes, can contribute to the elimination of this intracellular pathogen during an active infection. These data therefore support a model for immunity to intracellular bacteria that includes roles for both cellular and humoral immune responses.
MATERIALS AND METHODS
Animals.
All mice were obtained from the Jackson Laboratories, Bar Harbor, Maine, or were bred in the Animal Care Facility at the Wadsworth Center under microisolator conditions in accordance with institutional guidelines for animal welfare. All strains in the study were carried on the C57BL/6 genetic background.
Bacteria and inoculations.
The Arkansas isolate of E. chaffeensis was used for the infections described in this study (4). The bacteria were cultured in the canine histiocyte cell line DH82, as described previously (38). Six- to 12-week-old sex-matched mice were inoculated with E. chaffeensis-infected DH82 cells (106 to 2 × 106 cells/animal; >90% infected) or with a homogenate of infected splenocytes obtained from a C57BL/6 scid mouse by peritoneal injection. Quantitative PCR (QPCR) analyses later estimated that at the time of inoculation the infected DH82 cells harbored 250 to 500 bacteria per cell, although the number of viable organisms may have been lower (G. M. Winslow and M. Reilly, unpublished data). Tissues were excised from infected mice at various times after infection and were stored at −70° prior to DNA extraction and QPCR analyses.
Quantitation of bacteria in mouse tissue.
DNA was prepared from mouse tissue and analyzed by semiquantitative PCR or QPCR for the 16S ribosomal DNA rDNA of E. chaffeensis. Semiquantitative PCR was performed as described previously (38) and relied on a comparison of the relative intensities of ethidium bromide-stained PCR products in agarose gels. PCR products were scored visually and assigned an infectivity index based on a scale of 1 to 6, where a score of 1 indicated products at the limit of detection and a score of 6 indicated products at saturation. This method was highly sensitive and was used routinely for bacterial quantitation, although loss of linearity of the assay has been observed at high levels of bacterial infection. Test samples were normalized by comparison to E. chaffeensis DNA standards. In some cases, densitometry was used to provide further quantitation of the PCR products. The gels were photographed, and the band intensities were quantitated using a scanning densitometer (Scanalytics, Billerica, Mass.). To provide more-accurate bacterial quantitation, representative data from most experiments were analyzed by QPCR. In all cases, the QPCR analyses reflected the results obtained using the semiquantitative assay. The QPCR or densitometry data from representative animals are presented in the figures. The semiquantitative PCR data from selected experiments are presented in the accompanying tables. Data from the semiquantitative analyses of mice from replicate experiments were analyzed for statistical significance. P values were computed from Wilcoxon's ranked-sum statistic, which permits testing small data sets without utilizing distributional assumptions (37).
The development of the QPCR assay will be described in detail elsewhere. Briefly, an internal standard PCR probe was generated for quantitation by deletion of nucleotides between the MluI (5′ ACGCGT 3′) and PpuMI (5′ GGGGACCC 3′) restriction endonuclease sites in the 16S rDNA of E. chaffeensis, concomitant with insertion of a double-stranded DNA fragment generated using the following complementary oligonucleotides from plasmid pBR327 (16): 5′ CGCGTACGTTCCTCTACCGCGGGTTGTCAGGG 3′ (sense strand) and 5′ GTCCCCTGACAACCCGCGGTAGAGGAACGTA 3′ (antisense strand). The double-stranded DNA fragment contained ends compatible with those generated by restriction endonuclease digestion of the plasmid containing the 16S rDNA. The inserted DNA was joined to the vector with T4 DNA ligase, and the resulting plasmid was used to transform Escherichia coli XL-1 Blue cells (Stratagene, Inc., La Jolla, Calif.). The plasmid was sequenced to verify that the correct construct was recovered. The wild-type (pCR16S) and mutant (pCR16SΔ) plasmid DNAs were purified using the miniprep method (Wizard; Promega Corp., Madison, Wis.), and the DNA was further purified by microfiltration using a Centricon 100 filter apparatus (Millipore, Inc., New Bedford, Mass.). DNA concentrations were determined by spectroscopy.
PCR analyses were performed as described previously (10) using the oligonucleotides 5′ CAATTGCTTATAACCTTTTGGT 3′ and 5′ CCCTATTAGGAGGGATACGACCTT 3′, which bind to the 5′ and 3′ regions of the E. chaffeensis 16S rDNA, respectively. To allow microtiter plate capture, the latter primer was synthesized with a 5′ biotin nucleotide derivative (5′-biotin phosphoramidite; Glen Research, Sterling, Va.) following the protocols supplied by the manufacturer. Methods used to prepare the tissue DNA for analysis and the PCR conditions have been described previously (38). Each QPCR mixture contained DNA isolated from infected tissues (100 to 500 ng/reaction) and included in addition predetermined quantities of the internal standard DNA (pCR16SΔ; 500 to 1,500 copies). The PCRs were performed under noncompetitive conditions, such that amplification of the bacterial DNA was largely unaffected by the presence of the internal standard DNA.
Quantitation was performed by binding the biotinylated PCR products to streptavidin-coated microtiter wells (Roche Molecular Biochemicals, Indianapolis, Ind.) in 1× SSC (0.015 M sodium citrate [pH 7.0] plus 0.15 M NaCl) containing 0.5% Tween 20 for at least 1 h. The wells were washed with 1× SSC, the DNA was denatured by incubation with 0.1 N NaOH for 10 min and washed, and the bound DNA was hybridized with digoxigenilated oligonucleotide probes (10 pM in hybridization buffer containing 1× SSC, 20 mM HEPES [pH 7.0], 2 mM EGTA, and 0.1% Tween 20). The hybridization probes specifically bound PCR products generated from either the bacterial DNA or the internal standard DNA. The digoxigenilated hybridization probes were generated using terminal deoxynucleotide transferase and digoxigenin-dUTP in accordance with protocols described by the supplier (Roche Molecular Biochemicals). After the microtiter plate was washed to remove unbound oligonucleotides, the bound digoxigenilated oligonucleotides were detected using alkaline phosphatase-conjugated antidigoxigenin antibodies (Roche Molecular Biochemicals) and p-nitrophenyl phosphate as the substrate (Sigma Chemicals, St. Louis, Mo.). Optical densities were determined at 405 nm using an MR5000 plate reader (Dynatech Laboratories, Chantilly, Va.). To quantitate the masses of the bound products, the optical densities were compared with those of DNA standards generated from wild-type and deletion mutant template DNAs by PCR. The copy number of the bacterial DNA was determined by comparison with the internal standard, and the number of copies of ehrlichia DNA per gram of tissue was determined. Liver tissue contained on average 7.3 mg of cellular DNA per g of tissue. The QPCR assay could detect as few as 5 × 105 organisms per g of liver tissue. Sensitivity of the assay was apparently limited by contaminating host cell DNA and the enzyme-linked immunosorbent assay (ELISA) reagents used for quantitation.
Administration of immune serum and antibodies.
E. chaffeensis-infected DH82 cells (106 to 2 × 106) were administered intraperitoneally to C57BL/6 mice on day 0, the mice were boosted with the same inoculum on day 14, and serum was harvested 5 days later. Serum was aliquoted and stored at −20°C. A typical in vivo administration was performed using 0.1 ml of serum.
Immunofluorescence assay for E. chaffeensis.
Assays were performed using E. chaffeensis-infected DH82 cells which were attached to microscope slides. The slides were fixed in methanol, blocked in 10% normal goat serum, and incubated with serial dilutions of mouse antisera. Serum samples and secondary antibodies were diluted in phosphate-buffered saline (PBS) containing 2% fetal calf serum. Primary antibodies were detected using fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (Ig) (heavy and light chain specific; Fisher Scientific, Springfield, N.J.). Slides were observed under immunofluorescence using a Zeiss Axioplan microscope. The titration end point was determined to be the lowest serum dilution that allowed detection of bacteria.
Western analysis.
E. chaffeensis antigens for Western analyses were obtained from infected DH82 cells. Infected cells were harvested by centrifugation and lysed by homogenization using a disposable tissue grinder (Sage Products, Inc., Crystal Lake, Ill.). The crude lysate was centrifuged at 600 × g for 5 min, and the low-speed supernatant was then passed through a 5-μM-pore-size syringe filter (Micron Separations Inc., Westborough, Mass.) to remove debris. The filtrate was centrifuged at 15,000 rpm for 5 min in a microcentrifuge, and the pellet was stored at −70°C. The pellets were resuspended in sample buffer containing 2% sodium dodecyl sulfate (SDS) and 2% β-mercaptoethanol, electrophoresed in an 8 to 20% acrylamide gradient SDS-polyacrylamide gel electrophoresis gel, and blotted to polyvinyl difluoride blotting membranes. The membranes were blocked with 1% nonfat dry milk in PBS, and probed with human or mouse antiserum at a 100-fold dilution in blocking solution. Bound antibodies were detected using horseradish peroxidase-conjugated goat anti-human Ig and goat anti-mouse Ig secondary reagents, and the blots were developed using chemiluminescence (ECL Plus; Amersham-Pharmacia Biotech, Piscataway, N.J.). The human E. chaffeensis immune serum was obtained from an infected patient and was provided by S. Wong, Wadsworth Center, New York State Department of Health.
Antibody purification.
Serum fractionation was performed by precipitation with 40% ammonium sulfate, followed by centrifugation and resuspension in PBS. Protein A- and protein G-Sepharose (Amersham-Pharmacia Biotech) chromatography was performed using standard methods. An ELISA assay of the purified Igs revealed that IgM and all IgG mouse antibody subclasses were present after purification. One hundred micrograms of affinity-purified antibodies and 200 μg of salt-fractionated antibodies were used for in vivo injections.
RESULTS
Adoptive transfer of immune serum abrogated infection.
It has been demonstrated previously that immunocompetent C57BL/6 mice cleared E. chaffeensis infection within about 2 weeks but that immunodeficient C57BL/6 scid mice became persistently infected and moribund within 3 weeks postinfection (38). The infected SCID mice exhibited a number of abnormalities, including liver inflammation and necrosis, splenomegaly, and thrombocytopenia (38). To determine the basis of immunological resistance to infection, serum was obtained from immunocompetent C57BL/6 mice after E. chaffeensis infection and was transferred to susceptible C57BL/6 scid mice coincident with E. chaffeensis infection or 3 days postinfection, at a time when the infection was active. Tissues from the infected mice were subjected to visual observation and histological analysis, as described previously (38) and were analyzed for the presence of bacteria by QPCR. Previous studies demonstrated that many tissues become colonized in SCID mice, but liver tissue was chosen for the analyses because it was a principal site of bacterial colonization.
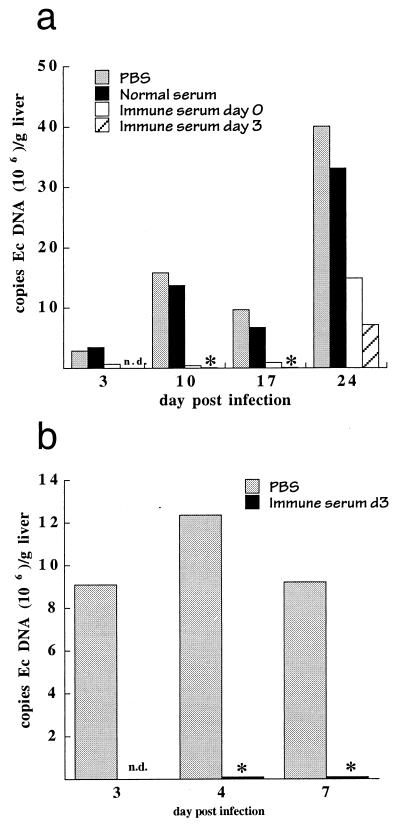
Adoptive transfer of C57BL/6 immune serum, but not normal serum, provided a marked protection of C57BL/6 scid mice from infection (Fig. 1a, Table 1). In several experiments, no significant changes in bacterial loads were observed after transfer of buffer or normal C57BL/6 serum. A reduction in the number of bacteria in liver tissue was observed for as long as 2 weeks following immune serum administration. Of particular importance was the observation of a reduction in bacterial numbers because this indicated that humoral immunity could provide protection after infection had been established. The limit of detection of the QPCR assay was approximately 5 × 105 bacteria per g of liver tissue, so it was not possible to determine if complete bacterial clearance occurred using the QPCR assay. Bacterial colonization was restored within 3 weeks of antibody administration (Fig. 1a), however, indicating that serum administration was not completely effective in the liver or that the liver was colonized by bacteria from tissues unaffected by the serum treatment. The transfer of immune serum also protected mice from disease, as determined by the lack of visible liver lesions that have been characteristically observed in SCID mice within 10 to 17 days postinfection (data not shown). In addition, histological analyses of liver sections performed as early as 48 h and as late as 2 weeks following serum transfer demonstrated inflammatory infiltration and coagulative necrosis in control mice but not in the mice that received the immune serum.
FIG. 1.
Administration of immune serum protected mice from ehrlichia infection. (a) Transient bacterial elimination in susceptible SCID mice. C57BL/6 scid mice were infected intraperitoneally with 106 to 2 × 106 E. chaffeensis-infected DH82 cells (day 0), followed by subcutaneous injection of 0.1 ml of PBS, normal C57BL/6 serum, or immune serum on day 0 or of immune serum on day 3 postinfection, as indicated. Liver tissue was harvested on the indicated days postinfection and was analyzed for the presence of E. chaffeensis by QPCR for E. chaffeensis 16S rDNA, as described in Materials and Methods. Immune serum was obtained from C57BL/6 mice that had been inoculated with E. chaffeensis-infected DH82 cells. Similar observations of the immune serum protection were made in at least three independent experiments using a semiquantitative PCR assay (Table 1). The lower bacterial titers observed on day 17 postinfection, compared to those on days 10 and 24, were not observed in other experiments (see also Fig. 4b) and most likely represent experimental variability. In all cases, the immune serum group was significantly different from controls. ∗, bacteria were not detected in the assays. Ec, E. chaffeensis. (b) Bacterial elimination in immunocompetent C57BL/6 mice. The mice were infected as described for panel a, and PBS or immune serum was administered via the peritoneum on day 3 postinfection. Tissues were harvested at the time of serum administration and 1 or 4 days later, and representative mice from each group were analyzed by QPCR. The experiment was performed in triplicate, and semiquantitative PCR analysis, which is more sensitive than QPCR, also failed to detect bacteria in spleen or liver tissue of any of the mice that received immune serum (not shown). P values obtained from the semiquantitative analyses were 0.09 and 0.001 for mice analyzed on day 4 and day 7, respectively. n.d., not determined.
TABLE 1.
Serum-mediated bacterial elimination in SCID micea
| Treatment | Admin. day | Analysis day p.i. | Infectivity index for expt:
|
||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Buffer | 0 | 3 | 2 | 2 | 3 |
| 0 | 10 | 3 | 5 | 5 | |
| 0 | 17 | 5 | 5 | 0 | |
| 0 | 24 | 6 | 5 | 5 | |
| Normal serum | 0 | 3 | 2 | 1 | 3 |
| 0 | 10 | 5 | 0 | 0 | |
| 0 | 17 | 5 | 3 | 4 | |
| 0 | 24 | 6 | 3 | nd | |
| Immune serum | 0 | 3 | 0 | 0 | 0 |
| 0 | 10 | 0 | 0 | 0 | |
| 0 | 17 | 1 | 1 | 0 | |
| 0 | 24 | 6 | 2 | 6 | |
| Immune serum | 3 | 3 | nd | nd | nd |
| 3 | 10 | 0 | nd | 0 | |
| 3 | 17 | 1 | nd | 0 | |
| 3 | 24 | 5 | nd | 5 | |
Data were derived from semiquantitative PCR analyses of E. chaffeensis 16S rDNA, as described previously (38). The infectivity index was determined by analysis of the PCR products, which were assigned a score from 1 to 6 on the basis of a visual estimation of band intensities. Samples from different experiments were normalized to standardized quantities of E. chaffeensis DNA obtained from cell culture. The semiquantitative analyses in general underestimated high bacterial loads. P values were determined by combining the control and serum-treated groups from each analysis day and were as follows: 0.012 (day 3), 0.002 (day 10), 0.004 (day 17), 0.5, (day 24). For details, see Materials and Methods. nd, not determined; p.i., postinfection; admin., administration.
Although the effect of immune serum administration was readily apparent in immunocompromised SCID mice, it was not known if the protective effect also occurred in immunocompetent mice. Immunocompetent C57BL/6 mice typically develop transient infection after bacterial inoculation and clear the bacteria to undetectable levels within 10 to 17 days postinfection (38). To determine if immune serum could mediate bacterial clearance in these mice, serum was administered 3 days postinfection, after bacterial infection had been established. The effect of serum administration was analyzed 1 to 3 days after serum administration. Immune serum eliminated the bacteria from the livers of the C57BL/6 mice in less than 3 days (Fig. 1b), compared to the 1 to 2 weeks required for clearance in control C57BL/6 mice, indicating that immune serum was also effective in immunocompetent mice.
The protective effects of immune serum are specific for E. chaffeensis.
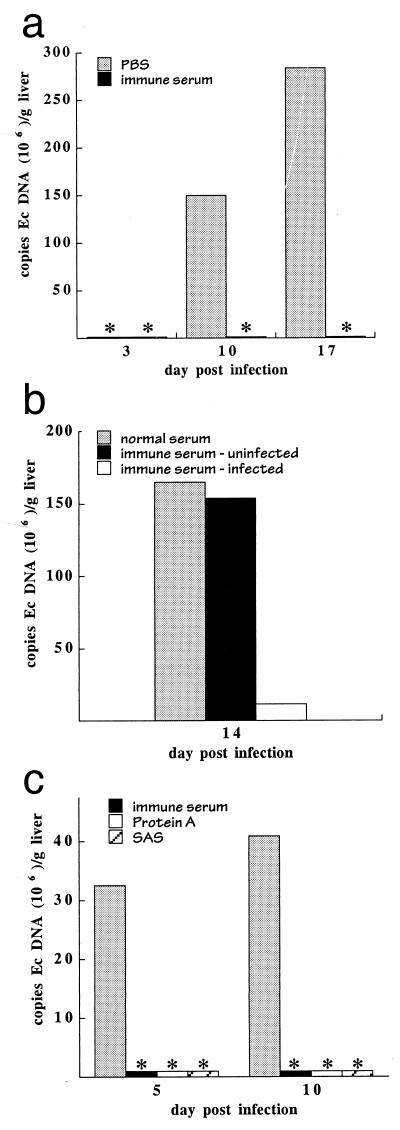
E. chaffeensis is routinely cultured in vitro in the canine histiocyte cell line DH82, and the mice used for both experimentation and production of antisera were inoculated using the ehrlichia-infected DH82 cells. It has been previously demonstrated that E. chaffeensis can infect and multiply in mouse tissues (38), so it was unlikely that the observed effect of the immune serum could be attributed to the DH82 cells which harbor the bacteria at the time of infection. However, to eliminate the possibility that anti-DH82 antibodies might be responsible for the observed bacterial clearance, two experiments were performed. It has been demonstrated previously that infection could occur upon transfer of infected mouse splenocytes obtained from SCID mice to uninfected SCID recipients (38). Immune serum administration effectively eliminated bacteria when performed after inoculation of mice with infected mouse splenocytes (Fig. 2a), indicating that the effect of the serum was not dependent on the cells used to culture the ehrlichiae. Furthermore, transfer of immune serum obtained from mice immunized with uninfected DH82 cells had no effect on bacterial proliferation or disease in SCID mice that had been inoculated with E. chaffeensis-infected DH82 cells (Fig. 2b). Thus, the effect of the immune serum administration was due to its antibacterial activity.
FIG. 2.
Bacterial clearance was E. chaffeensis specific and was mediated by antibodies. (a) C57BL/6 scid mice were infected by transfer of E. chaffeensis-infected splenocytes obtained from a SCID mouse 17 days postinfection. Immune serum was obtained from C57BL/6 mice that had been inoculated with E. chaffeensis-infected DH82 cells and was administered at the time of bacterial infection. Bacterial loads were determined by QPCR. ∗, bacteria were not detected in the infected mice. Each histogram bar represents a single mouse. Ec, E. chaffeensis. (b) Mice were infected as described for Fig. 1 and were administered on day 10 postinfection normal mouse serum or serum obtained from C57BL/6 mice that had been inoculated with either uninfected DH82 cells (immune serum-uninfected) or with E. chaffeensis-infected DH82 cells (immune serum-infected). Liver tissue was harvested on day 14 for QPCR analyses. QPCR analyses of representative individual mice are shown. The observations were confirmed in a separate experiment (not shown) where 16 mice (in three groups) were analyzed over a period of 24 days. The semiquantitative data from both experiments where serum was administered on day 14 postinfection were normalized and combined (a total of four mice for each group), and the means and standard deviations were as follows: normal serum, 5.0 ± 0.82; serum from mice inoculated with uninfected cells, 4.3 ± 1.1; immune serum, 1.8 ± 1.8. (c) C57BL/6 scid mice were infected on day 0 with E. chaffeensis-infected DH82 cells, followed by intraperitoneal administration of 0.1 ml of PBS or C57BL/6 immune serum, 200 μg of ammonium sulfate-fractionated immune serum (SAS), or 100 μg of protein A affinity-purified antibodies 3 days postinfection. The presence of E. chaffeensis antibodies in each of the preparations was confirmed by immunofluorescence assay. Mice were harvested 5 and 10 days postinfection, and liver tissue from representative individual mice was analyzed by QPCR. Semiquantitative analyses of a total of 16 mice from two experiments revealed significant differences between the buffer- and antibody-treated mice (mean ± standard deviation): PBS, 3.8 ± 0.83; immune serum, 1.0 ± 1.2; protein A-purified antibodies, 0.5 ± 0.87; ammonium sulfate-purified antibodies, 1.3 ± 1.3. ∗, bacteria were not detected in the QPCR assays.
To demonstrate that the effect of the immune serum was due to serum antibodies, Igs from immune serum were fractionated by precipitation with 40% ammonium sulfate and were further purified by protein A affinity chromatography. The purified antibodies were tested for their ability to mediate clearance of E. chaffeensis in vivo. The purified antibodies mediated bacterial clearance in SCID mice, which indicated that the activity in the serum was due to antibodies (Fig. 2c). ELISA analyses revealed that the affinity-purified antibody preparation contained antibodies of all mouse isotypes (data not shown), so it was not possible to draw any conclusions regarding the efficacies of particular antibody classes and subclasses during bacteria clearance.
Antibody production in C57BL/6 mice.
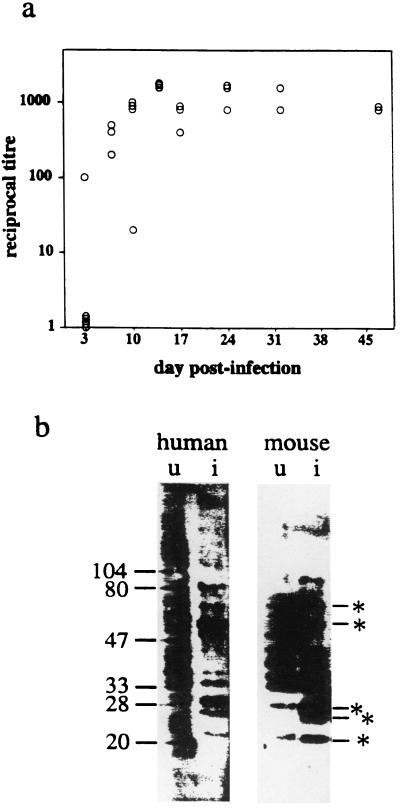
Serum from normal mice was ineffective at bacterial clearance, so it was likely that the effect of the serum obtained from immunized mice was due to the presence of anti-E. chaffeensis antibodies. To demonstrate that the C57BL/6 mice generated antiehrlichia antibodies, serum from infected C57BL/6 mice was examined by immunofluorescence assay and Western blotting. Anti-E. chaffeensis antibodies were detected in serum from C57BL/6 mice within 7 days following inoculation and for as long as 48 days postinfection, indicating that a potent humoral response was made in the C57BL/6 mice (Fig. 3a).
FIG. 3.
E. chaffeensis antibody responses in C57BL/6 mice. (a) C57BL/6 mice were inoculated with 2 × 106 infected DH82 cells, serum was harvested on the indicated days postinfection, and E. chaffeensis antibody titers were determined by immunofluorescence assay using a secondary antibody specific for mouse Ig. Each data point represents the serum titer of one mouse. (b) Western analysis of murine and human E. chaffeensis antisera. Bacterial antigens were obtained from uninfected (u) or E. chaffeensis-infected (i) DH82 cells. The samples were Western blotted and probed with mouse or human antisera, followed by a species-specific horseradish peroxidase-conjugated secondary antibody and chemiluminescence development. ∗, E. chaffeensis molecules that were detected by both the mouse and human antibodies. Molecular mass standards, in kilodaltons, are at the left of the gel.
To identify candidate bacterial antigens that were possible targets of the protective mouse antibodies, Western blots of E. chaffeensis antigens obtained from infected DH82 cells were probed with C57BL/6 immune serum. Several E. chaffeensis antigens were detected by the mouse antibodies, including proteins of 22, 27, 28, 54, 73, and 88 kDa (Fig. 3b). The antigens were in many cases identical to those detected using serum from an infected human (Fig. 3b). The 28-kDa antigen was immunodominant in several sera from mice and humans and is probably the previously described E. chaffeensis outer membrane protein (OMP) (30, 39).
The effect of antibodies administered during an established infection.
The onset of liver disease is typically observed in SCID mice within 10 days postinfection, and the mice are usually moribund within 3 weeks (38). In Fig. 1 it was demonstrated that serum administration on day 3 postinfection was effective at bacterial clearance. To determine if administration of immune serum could mediate bacterial clearance in SCID mice later than 3 days after infection, serum was administered 10 and 17 days postinfection and bacterial loads in the liver were quantitated 1 and 2 weeks later. Bacterial clearance was observed when serum was administered either 10 or 17 days postinfection, and the effect persisted for as long as 2 weeks (Fig. 4a; Table 2). Histological analyses 1 week after serum administration revealed that the mice that received the immune serum did not exhibit necrotic liver lesions and tissue inflammation (data not shown).
FIG. 4.
Clearance of bacteria during active infection. (a) C57BL/6 scid mice received PBS on day 10 or immune serum on day 10 or day 17 postinfection and were analyzed by QPCR 1 and 2 weeks later. Ec, E. chaffeensis. (b) SCID mice received PBS or serum on day 10 postinfection and were analyzed 1, 3, 7, and 10 days later. (c) SCID mice were administered serum on day 17 postinfection and were analyzed 1 and 3 days later. The control mice shown in panels b and c are identical. (d) Infected SCID mice received 0.1 ml of PBS or dilutions of immune serum on day 3 postinfection and were analyzed 7 or 14 days later. The values in the key to the bars are the reciprocal titers of the immune serum that was administered, as determined by immunofluorescence assay. In all experiments, mice were analyzed only where indicated by the histograms. ∗, bacteria were not detected. The data obtained by semiquantitative analyses of the experiments shown in panels a to c, as well as data from an additional experiment, are shown in Table 2.
TABLE 2.
Bacterial clearance during established infectiona
| Expt | Treatment | Tissue | Admin. day | Analysis day p.i. | Infectivity index |
|---|---|---|---|---|---|
| 2 | None | Liver | 10 | 4 | |
| 1 | None | Liver | 10 | 5 | |
| 1 | None | Spleen | 10 | 5 | |
| 2 | None | Liver | 11 | 4 | |
| 2 | None | Liver | 13 | 4 | |
| 1 | None | Liver | 17 | 5 | |
| 2 | None | Liver | 17 | 5 | |
| 1 | None | Spleen | 17 | 5 | |
| 2 | None | Liver | 18 | 5 | |
| 2 | None | Liver | 20 | 5 | |
| 3 | None | Liver | 21 | 4 | |
| 3 | None | Liver | 21 | 4 | |
| 3 | None | Liver | 21 | 5 | |
| 1 | None | Liver | 24 | 5 | |
| 1 | None | Spleen | 24 | 5 | |
| 1 | ISb | Liver | 3 | 10 | 0 |
| 1 | IS | Spleen | 3 | 10 | 0 |
| 1 | IS | Liver | 3 | 17 | 1 |
| 1 | IS | Spleen | 3 | 17 | 1 |
| 2 | IS | Liver | 10 | 11 | 5 |
| 2 | IS | Liver | 10 | 13 | 3 |
| 1 | IS | Liver | 10 | 17 | 2 |
| 2 | IS | Liver | 10 | 17 | 2 |
| 1 | IS | Spleen | 10 | 17 | 2 |
| 2 | IS | Liver | 10 | 20 | 1 |
| 1 | IS | Liver | 10 | 24 | 2 |
| 1 | IS | Spleen | 10 | 24 | 2 |
| 2 | IS | Liver | 17 | 18 | 5 |
| 2 | IS | Liver | 17 | 20 | 2 |
| 3 | IS | Liver | 17 | 21 | 0 |
| 3 | IS | Liver | 17 | 21 | 2 |
| 1 | IS | Liver | 17 | 24 | 3 |
| 1 | IS | Spleen | 17 | 24 | 3 |
| 1 | IS | Liver | 17 | 31 | 3 |
| 1 | IS | Spleen | 17 | 31 | 3 |
Semiquantitative PCR data from three experiments were normalized and sorted as shown. The QPCR data from experiments 1 and 2 are shown in Fig. 4a and 4b and c, respectively. Both liver and spleen tissues were analyzed in experiment 1. Comparison of the combined data from the control and serum-treated groups revealed high statistical significance (P = 0.0001). Admin., administration; p.i. postinfection.
IS, immune serum.
To determine the time required to observe bacterial clearance after serum administration, SCID mice were infected, immune serum was administered 10 or 17 days postinfection, and the mice were analyzed at various intervals thereafter. Bacterial numbers were reduced to low or undetectable levels in the liver within 2 days of serum administration (Fig. 4b and c; Table 2). The data demonstrated that antibodies mediated rapid clearance of bacteria from infected tissues well after infection and disease had been established.
To determine the effective serum dosage, graded amounts of immune serum were administered to SCID mice 3 days postinfection and liver tissue was analyzed 7 and 14 days later. Administration of serum (0.1 ml) with an effective reciprocal titer of 100 or higher on day 3 postinfection resulted in the clearance of bacteria from the liver (Fig. 4d). The effects of the serum titration were also evident when tissue was analyzed 17 days postinfection, at which time bacterial recovery was dose dependent (Fig. 4d).
Repeated serum administration prolonged survival.
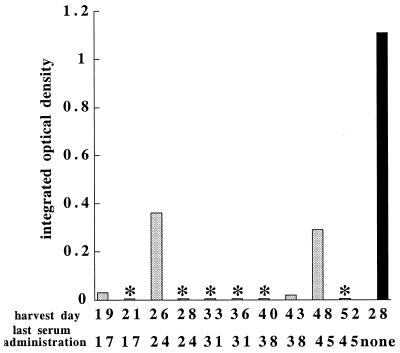
A variety of explanations could account for the transient nature of the serum protection. To determine if repeated serum administration could provide prolonged immunity, immune serum was injected at weekly intervals beginning 10 days postinfection and mice were analyzed 2 to 4 days after each serum administration. Untreated SCID mice were moribund within 3 to 4 weeks, but the treated mice exhibited reduced bacterial loads and were free of disease when serum administration was continued at weekly intervals, up to 52 days postinfection (Fig. 5). Cessation of serum treatment resulted in the recovery of bacterial numbers and the onset of liver disease within 7 to 14 days (data not shown). Therefore, antibodies provided extended immunity when they were administered at weekly intervals, although they did not support complete bacterial clearance.
FIG. 5.
Repeated serum administration results in prolonged immunity. Infected C57BL/6 scid mice received PBS or weekly injections of serum beginning 10 days postinfection. Liver tissue was harvested at 2- and 4-day intervals following each serum administration (shaded histograms) or 28 days after infection of a mouse that received no serum (black histogram). The bacterial loads were determined using semiquantitative PCR. The data indicate the integrated optical densities of ethidium bromide-stained PCR products in agarose gels, as determined by densitometry. Semiquantitative PCR was utilized because bacterial loads in the mice that received the antibodies were below the limit of detection using QPCR. Two additional mice that did not receive serum died and were not analyzed. ∗, PCR products were not detectable in agarose gels. Based on the assumption that the 2 SCID mice that did not survive exhibited levels of bacterial infection similar to that of the surviving mouse, a statistical comparison of 10 treated mice that exhibited low bacterial loads with 3 untreated mice indicated a P value of 0.004 by Fisher's exact test (14). In two additional experiments 13 mice that received two or more injections of immune serum all survived longer than 31 days postinfection (not shown).
The involvement of T cells in controlling infection.
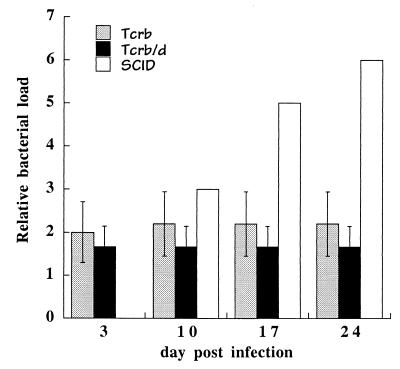
The failure of immune serum to completely eliminate bacteria in SCID mice suggested that other mechanisms were responsible for the apparently complete clearance observed in immunocompetent mice. To determine the requirement for T cells in bacterial clearance, T-cell receptor β-chain-deficient mice, which lack αβ T cells, were infected with E. chaffeensis (23, 24). αβ T cells were required for complete bacterial clearance, because in their absence persistent bacterial infection was observed for as long as 24 days postinfection (Fig. 6). However, unlike infection of SCID mice, where bacterial growth was uncontrolled and led to morbidity, infection in the αβ-T-cell-deficient mice was maintained at relatively low levels. Protection from infection was not due to the presence of γδ T cells in the αβ-T-cell-deficient mice, because mice doubly deficient for both αβ and γδ T cells also exhibited only low or undetectable infection (Fig. 6). The T-cell-deficient mice did not exhibit any signs of disease at any time during the postinfection period and in other experiments survived as long as 72 days. Therefore, T cells were required for complete bacterial clearance, but in their absence other cells, most likely B cells, were able to provide significant immunity against infection.
FIG. 6.
Persistent infection in T-cell-deficient mice. T-cell receptor β-chain-deficient (TCRb), TCRβ/TCRδ-chain deficient (TCRb/d), and C57BL/6 scid (SCID) mice were infected with E. chaffeensis, and bacterial colonization was monitored by semiquantitative PCR on the indicated days postinfection. PCR analyses of C57BL/6 scid mice are shown as a basis for comparison. Normalized data from three experiments are shown and represent the averages of semiquantitative PCR analyses of three to four mice from each of the gene-targeted strains. Error bars indicate standard deviations. Data from the analyses of the SCID mice were obtained from a single animal on the indicated days. Semiquantitative PCR was utilized because it was not possible to detect the bacteria in the T-cell-deficient mice by QPCR. The T-cell-deficient mice were maintained on the C57BL/6 genetic background (strains C57BL/6 tcrb and C57BL/6 tcrb/tcrd). No sign of disease was noted in any of the T-cell-deficient mice. ∗, PCR products were not detectable in agarose gels.
DISCUSSION
Immunity to E. chaffeensis.
The data presented in this study demonstrate that both cellular and humoral mechanisms contribute to resistance to E. chaffeensis infection in the mouse. A role for T cells was not surprising, because cellular immune responses have long been considered to be essential for the elimination of intracellular bacteria (32). However, the finding that antibodies, in the absence of T and B cells, could cause elimination of an obligate intracellular bacterium was unexpected, because humoral immunity has generally been considered to be relatively ineffective against many intracellular bacteria. A role for humoral immune responses at the onset of intracellular infection has been suggested from studies of other ehrlichiae. Passive transfer of antibodies against Ehrlichia risticii protected mice from disease (18), and administration of immune sera partially protected C3H/HeN mice from infection with the agent of human granulocytic ehrlichiosis (aoHGE) (33). These studies demonstrated the efficacy of serum when administered prior to bacterial infection, so one possible interpretation was that serum antibodies directly neutralized the bacteria or blocked bacterial invasion of phagocytes. In the data presented here, antibodies were shown to exert their effects well after infection had been established in tissues, demonstrating a physiological role for antibodies after the initiation of an active infection.
Laboratory mice can be experimentally infected with E. chaffeensis after intraperitoneal, subcutaneous, and intradermal inoculation using infected cells or cell-free ehrlichiae (38; E. Yager and G. M. Winslow, unpublished data). These routes of administration clearly differ from the normal route of tick transmission, but it has not yet been determined whether or how different routes of administration affect antibody responses or bacterial clearance. In our studies, immune serum administration was effective long after infection had been established in many tissues, so it is unlikely that the protective effects observed were entirely an artifact of experimental inoculation. This conclusion is supported by observations that mice were protected from infection by the aoHGE when bacteria were administered by either needle or tick inoculation (33).
The protective effect of a single administration of immune serum persisted for as long as 14 days postadministration. The transient nature of the protection was likely due to the depletion of the transferred antibodies. This conclusion is supported by the analysis of mice that received repeated administration of immune serum, where it was shown that protection could be maintained for as long as 45 days postinfection and, presumably, indefinitely. Although bacteria were often not detected in liver tissue by QPCR after serum administration, bacterial clearance was incomplete, because the bacteria were able to eventually recolonize the liver and cause disease. The recovery of bacteria may have originated from low levels of persistent bacteria in the tissue or may have resulted from bacterial emigration from tissues that may have been inaccessible to the antibodies.
The observation that antibodies alone failed to eliminate the bacteria in SCID mice suggested that cellular immunity was required for the apparently complete clearance observed in immunocompetent C57BL/6 mice. T-cell-deficient mice developed a low-level chronic infection, indicating that T cells were required for complete clearance. T cells might be necessary to induce macrophage ehrlichiacidal activities, to directly kill infected cells, or to provide help to B cells for antibody production. T-cell-deficient mice were susceptible to only low-level infection and did not exhibit disease, so it is possible that the partial protection observed was due to T-cell-independent B-cell responses, although other differences between SCID and T-cell-deficient mice might also affect resistance. In an immunocompetent mouse, the T-cell-independent antibody responses might act to control the disease during the early stages of infection, prior to the development of a full T-cell response.
E. chaffeensis antigens recognized by the mouse.
Western analyses indicated that several E. chaffeensis antigens that may be targets of the protective antibodies were recognized by the mouse. These included proteins of 22, 27, 28, 54, 73, and 88 kDa. The 28-kDa protein, which was highly immunodominant in several different mouse sera, is likely one of a family of E. chaffeensis OMPs that have been described previously (27, 30). Immunization of immunocompetent mice with the p28 OMP (also known as ORF5) protected immunocompetent mice from infection (27), suggesting that cellular or humoral responses to this protein may be important during host defense. The p28 OMPs identified in several E. chaffeensis isolates were genetically diverse (39), suggesting that antigenic variation in the OMP may provide a mechanism for immune system evasion.
Humans are well known to make strong antibody responses to E. chaffeensis (8, 31). In this study, most of the antigens recognized by the mouse were also recognized using serum from an infected human and the human antibody specificities observed were largely reminiscent of those in previously published reports (8, 9). Because similar humoral responses have been observed, it is likely that the underlying mechanisms of antibody-mediated immunity in mice and humans are quite similar.
Mechanisms of humoral immunity to E. chaffeensis.
Because antibodies have not generally been considered to have an important role during host defense against intracellular bacteria, it will be important to identify the mechanisms whereby antibodies are able to clear E. chaffeensis from infected tissues. Antibodies might, for example, act to facilitate the cytolysis of organisms via the classical pathway of complement deposition and/or by opsonization. However, this explanation would require that the ehrlichiae be exposed to the extracellular milieu, perhaps during intercellular migration. Although E. chaffeensis is known to be an obligate intracellular bacterium, and thus requires host cells for replication, it is possible that during their life cycle the bacteria are found outside of cells, where they would be susceptible to antibodies. Antibody-mediated bacterial clearance was observed to occur within 2 days of antibody administration, so this explanation would require that most resident bacteria be exposed extracellularly during this relatively brief period. The closely related ehrlichia, the aoHGE, is granulocytotropic, a tropism that would likely require these ehrlichia to migrate rapidly among these short-lived cells. Such a life-style might also be characteristic of E. chaffeensis. The particular sensitivity of the ehrlichiae to antibodies, which presumably act extracellularly, also suggests that there are aspects of the life-styles of these obligate intracellular pathogens that have previously gone unappreciated.
The failure of antibodies to mediate complete bacterial clearance suggests that resident intracellular bacteria may be resistant to antibody-mediated clearance. Complete bacterial clearance has been shown to require T cells. However, antibodies might also contribute to intracellular elimination, perhaps by mediating the cytolysis of infected macrophages or of bacteria within infected macrophages (15). Preliminary studies have indicated that natural killer cell-mediated antibody-dependent cell cytotoxicity was not involved in antibody-mediated immunity, because serum-mediated clearance occurred in SCID mice after antibody-mediated natural killer cell depletion (G. M. Winslow and E. Yager, unpublished data). It is also possible that antibodies or immune complexes trigger ehrlichiacidal activities in infected macrophages. In support of this hypothesis, in vitro studies have indicated that immune complexes of E. chaffeensis induced inflammatory cytokine production in a human macrophage cell line (20). Administration of Fab fragments of serum antibodies proved not to be effective at bacterial clearance (E. Volk and G. M. Winslow, unpublished data), but the interpretation of the experiments was complicated by possible differences in the half-lives of the fragmented antibodies. Another hypothesis is that antibodies might directly affect bacterial viability within intracellular compartments (2). Some studies have suggested that antibodies can neutralize viruses inside infected cells (21), so a role for intracellular antibodies during intracellular bacterial infection is possible. Further studies will be required to distinguish between these mechanisms.
Humoral immunity and intracellular pathogens.
The particular sensitivity of E. chaffeensis to antibodies may reflect features unique to the ehrlichiae. However, a growing body of old and new evidence suggests that antibodies can contribute to immunity to several other intracellular bacterial as well as fungal pathogens (6). Antibodies that provide protection against infection by intracellular pathogens such as Salmonella spp. (12, 28), M. tuberculosis (34), Legionella pneumophila (5), Brucella abortus (11, 29), Rickettsia typhi (15) and Cryptococcus neoformans (7, 25) have been identified. Unlike what was done in the present study, however, antibody efficacy was generally evaluated by experimental administration of antibodies prior to infection with these agents, so it was not possible to judge the role of antibodies after the infections were initiated. Nevertheless, the data challenge the notion that immunity to intracellular pathogens is solely the domain of cellular immune responses. Humoral responses might previously have gone unappreciated during intracellular infections, in part due to the choice of experimental organism, host genetics, and experimental design. In other cases, a contribution for humoral immunity during intracellular infection might have been overlooked when the activities of protective antibodies were masked by nonprotective antibodies (40).
The findings that antibodies can contribute to host defense have important implications for the design of vaccines and therapies to eliminate intracellular bacterial pathogens, i.e., it may be desirable to elicit both cellular and humoral immune responses to obtain maximum efficacy. Indeed, the efficacy of a Salmonella enterica serovar Typhi capsular polysaccharide vaccine presumably owes its effectiveness to the ability to elicit humoral immune responses (1).
ACKNOWLEDGMENTS
We thank Frank Abbruscato, Melissa Reilly, and Michelle Tackley for excellent technical assistance, and Donal Murphy for critical review of the manuscript, and Arturo Casadevall and Harry Taber for helpful discussion. We recognize the contribution of the Wadsworth Center's Immunology and Molecular Biology Core facilities and the Department of Anatomical Pathology.
This work was supported in part by U.S. Public Health Service grant CA69710-02 and by Centers for Disease Control and Prevention grant U50/CCU213698-01-1.
REFERENCES
- 1.Acharya I L, Lowe C U, Thapa R, Gurubacharya V L, Shrestha M B, Cadoz M, Schulz D, Armand J, Bryla D A, Birger Trollfors M P H, Cramton T, Schneerson R, Robbins J B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. N Engl J Med. 1987;317:1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 2.Alarcón-Segovia D, Ruiz-Argüelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–164. doi: 10.1016/s0167-5699(96)90258-3. [DOI] [PubMed] [Google Scholar]
- 3.Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieland J K, Heath L A, Huffnagle G B, Remick D G, McClain M S, Hurley M C, Kunkel R K, Fantone J C, Engleberg C. Humoral immunity and regulation of intrapulmonary growth of Legionella pneumophila in the immunocompetent host. J Immunol. 1996;157:5002–5008. [PubMed] [Google Scholar]
- 6.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S-M, Cullman L C, Walker D H. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and Ehrlichia canis. Clin Infect Dis. 1997;4:731–735. doi: 10.1128/cdli.4.6.731-735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S-M, Dumler J S, Feng H-M, Walker D H. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1994;50:52–58. [PubMed] [Google Scholar]
- 10.Chu F K. Rapid and sensitive PCR-based detection and differentiation of etiologic agents of human granulocytic and monocytotropic ehrlichioses. Mol Cell Probes. 1998;12:93–99. doi: 10.1006/mcpr.1998.0150. [DOI] [PubMed] [Google Scholar]
- 11.Cloeckaert A, Jacques I, De Wergifosse P, Dubray G, Limet J N. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect Immun. 1992;60:312–315. doi: 10.1128/iai.60.1.312-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenstein T K, Killar L M, Sultzer B M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984;150:425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 13.Fichtenbaum C J, Peterson L R, Weil G J. Ehrlichiosis presenting as a life-threatening illness with features of toxic shock syndrome. Am J Med. 1993;95:351–357. doi: 10.1016/0002-9343(93)90302-6. [DOI] [PubMed] [Google Scholar]
- 14.Fisher R A. The logic of inductive reference. J R Stat Soc. 1935;98:39–54. [Google Scholar]
- 15.Gambrill M R, Wisseman C L J. Mechanisms of immunity in typhus infections. III. Influence of human immune serum and complement on the fate of Rickettsia mooseri within human macrophages. Infect Immun. 1973;8:631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenthner P C, Hart C E. Quantitative, competitive PCR assay for HIV-1 infection using a microplate-based detection system. BioTechniques. 1998;24:810–816. doi: 10.2144/98245dt01. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann S H E. Immunity to intracellular bacteria. Adv Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 18.Kaylor P S, Crawford T B, McElwain T F, Palmer G H. Passive transfer of antibody to Ehrlichia risticii protects mice from ehrlichiosis. Infect Immun. 1991;59:2058–2062. doi: 10.1128/iai.59.6.2058-2062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane F C, Unanue E R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972;135:1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E, Rikihisa Y. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB-α and activation of NF-κB. Infect Immun. 1997;65:2890–2897. doi: 10.1128/iai.65.7.2890-2897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 22.Miki K, Mackaness G B. The passive transfer of acquired resistance to Listeria monocytogenes. J Exp Med. 1964;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S H E. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 24.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang L, Ichikawa Y, Jaenisch R, Hooper M L, Tonewaga S. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North R J. T-cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974;10:66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of E. chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornellas E P, Roantree R J, Steward J P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970;121:113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- 29.Plommet M, Plommet A-M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983;41:97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 31.Rikihisa Y, Ewing S A, Fox J C. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingi infections in dogs and humans. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaible U E, Collins H L, Kaufmann S H E. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–377. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 33.Sun W, Ijdo J W, Telford III S R, Hodzic E, Zhang Y, Barthold S W, Fikrig E. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J Clin Investig. 1997;100:3014–3018. doi: 10.1172/JCI119855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins J B, Unanue E, Casadevall A, Bloom B R. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Telford S R, III, Dawson J E. Persistent infection of C3H/HeJ mice by Ehrlichia chaffeensis. Vet Microbiol. 1996;52:103–112. doi: 10.1016/0378-1135(96)00064-8. [DOI] [PubMed] [Google Scholar]
- 36.Walker D H, Dumler J S. Human monocytic and granulocytic ehrlichioses. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 37.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- 38.Winslow G, Yager E, Shilo K, Collins D N, Chu F K. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect Immun. 1998;66:3892–3899. doi: 10.1128/iai.66.8.3892-3899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X J, McBride J, Walker D H. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J Clin Microbiol. 1999;37:1137–1143. doi: 10.1128/jcm.37.4.1137-1143.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan R, Casadevall A, Spira G, Scharff M D. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans to a protective antibody. J Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]