Abstract
Sublethal infection of mice with recombinant Listeria monocytogenes expressing a model epitope in either secreted or nonsecreted form results in similar CD8+ T-cell priming. Since nonsecreted bacterial proteins have no obvious access to the endogenous major histocompatibility complex (MHC) class I presentation pathway, presentation of these antigens requires destruction of the bacterium to reveal the nonsecreted molecules to an exogenous MHC class I presentation pathway. Gamma interferon (IFN-γ), a cytokine made by multiple cell types in response to L. monocytogenes infection, could be required for exogenous presentation of nonsecreted bacterial antigens via its capacity to upregulate the expression of molecules involved in antigen presentation, its capacity to activate macrophages to kill bacteria to expose nonsecreted molecules or both. IFN-γ knockout (KO) mice were used to address the requirement for IFN-γ in CD8+ T-cell priming against (i) a model exogenous antigen and (ii) secreted and nonsecreted L. monocytogenes antigens. We demonstrate that IFN-γ KO mice are capable of cross-presenting the model exogenous antigen ovalbumin to prime CD8+ T-cell responses that are only slightly weaker than that in wild-type (WT) mice. Despite their extreme susceptibility to primary L. monocytogenes infection, previously immunized and naive IFN-γ KO mice were able to generate CD8+ T-cell responses against both secreted and nonsecreted L. monocytogenes antigens which were similar to responses of WT mice. Interestingly, IFN-γ KO mice were as capable as WT mice in mediating the characteristic drop in bacterial load in the liver at 4 h postinfection, although the IFN-γ KO mice have exacerbated bacterial loads as early as 24 h postinfection. These results demonstrate that the regulatory functions of IFN-γ are not required for priming of CD8+ T cells by cross-presentation of a model exogenous antigen or in response to a nonsecreted L. monocytogenes antigen. In addition, the capacity of IFN-γ to activate the microbicidal activities of macrophages is not required for the very early innate immune response to L. monocytogenes or priming of CD8+ T cells against a nonsecreted bacterial antigen.
Listeria monocytogenes, a gram-positive facultative intracellular bacterial pathogen, can enter and multiply within both phagocytic and nonphagocytic cells. L. monocytogenes is able to productively infect phagocytic cells due to its ability to disrupt the membrane-bound phagosome after infection (14). Although escape from the vacuole allows a subset of bacteria to avoid the bactericidal effects of phagolysosomal fusion, entry into the host cell cytoplasm provides a route for bacterial antigens to enter the endogenous pathway of major histocompatibility complex (MHC) class I antigen processing and presentation to CD8+ T cells (9).
The endogenous pathway of MHC class I antigen presentation is initiated by the degradation of cytosolic proteins, either self or pathogen derived, by host cell proteases such as the proteasome (31). The resulting peptides are transported into the endoplasmic reticulum by the transporters associated with antigen processing (TAP). In the endoplasmic reticulum, the peptides bind to MHC class I molecules and are transported to the cell surface where the MHC class I-peptide complex can be recognized by CD8+ T cells. However, antigens without access to the cytoplasm and the endogenous pathway (exogenous antigens) can also be presented by MHC class I molecules. In vitro experiments suggest multiple routes for the presentation of exogenous model antigens by MHC class I molecules including cytosol-dependent and -independent pathways (1, 2, 22, 28, 32, 37). These studies have generally implicated professional antigen-presenting cells (APC) such as macrophages and dendritic cells (DC) in the presentation of exogenous antigens to prime CD8+ T-cell responses. Recent evidence suggests that in vivo cross-presentation of model exogenous antigens to prime CD8+ T-cell responses is dependent on CD40L-CD40 interactions (5, 36).
Once L. monocytogenes enters the cytoplasm, secreted L. monocytogenes proteins are accessible to the endogenous MHC class I antigen presentation pathway (31), whereas nonsecreted L. monocytogenes proteins are sequestered within the bacterial cell. However, it has been demonstrated that recombinant L. monocytogenes which express an H-2Ld-restricted epitope from the nucleoprotein (NP118-126) of lymphocytic choriomeningitis virus (LCMV), as either a secreted or a nonsecreted fusion protein, are able to prime similar CD8+ T-cell responses (38). Since the nonsecreted fusion protein is not exposed when L. monocytogenes is in the host cell cytoplasm, presentation of the nonsecreted epitope requires destruction of L. monocytogenes in the phagosome to reveal nonsecreted L. monocytogenes antigens to an exogenous MHC class I presentation pathway.
Gamma interferon (IFN-γ), a cytokine produced by NK cells during the initial phase of L. monocytogenes infection, activates the microbicidal activities of macrophages, increasing destruction of L. monocytogenes in the phagosome (33). As such, IFN-γ is critical in resistance to primary infection with virulent L. monocytogenes (10, 17, 24, 27). IFN-γ also plays multiple regulatory roles in the host immune response including the upregulation of molecules such as MHC class I, TAP, LMP2, and LMP7 that enhance antigen presentation to CD8+ T cells (19). Previous work has demonstrated that IFN-γ is not required for detectable priming of CD8+ T-cell responses against secreted L. monocytogenes proteins that are presented by the endogenous MHC class I presentation pathway (24). However, the requirement for IFN-γ, as a regulatory molecule in priming of CD8+ T cells against exogenous antigens or as a molecule that enhances priming of CD8+ T cells in response to nonsecreted bacterial antigens by increasing the microbicidal activities of macrophages, is unknown.
In this study, we used IFN-γ knockout (KO) mice to examine the requirement for IFN-γ, through its immune regulatory or microbicidal actions, for in vivo priming of CD8+ T cells against a model exogenous antigen or a nonsecreted L. monocytogenes antigen.
MATERIALS AND METHODS
Mice.
BALB/c (H-2d MHC), C57BL/6 (B6; H-2b MHC), and CB6 (H-2bxd) mice were purchased from the National Cancer Institute. IFN-γ KO mice on the B6 background (18) were purchased from The Jackson Laboratory. IFN-γ KO mice on the BALB/c background have been described elsewhere (24). H-2bxd IFN-γ KO mice were generated by crossing the parental IFN-γ KO strains.
Bacteria.
Bacteria used in this study were virulent L. monocytogenes strain 10403s (6), virulent recombinant L. monocytogenes strain XFL303 (referred to as LM-NPs), expressing a secreted fusion protein containing the LCMV NP118-126 epitope, recombinant L. monocytogenes XFL304 (referred to as LM-NPns), expressing a nonsecreted fusion protein containing the LCMV NP118-126 epitope (38), and attenuated recombinant L. monocytogenes strain DP-L1942, which carries an in-frame deletion in the actA gene (8). Growth and maintenance of all L. monocytogenes strains were as described elsewhere (24). Actual numbers of CFU injected were determined for each experiment by plate count.
Immunization with ovalbumin-loaded splenocytes.
Age- and sex-matched mice were immunized intravenously with 25 × 106 splenocytes that had been loaded with ovalbumin as described previously (11). Briefly, 120 × 106 splenocytes were depleted of red blood cells by incubating in ACK lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA) for 5 min at room temperature. After being washed twice in Hanks' balanced salt solution, the splenocytes were incubated for 10 min at 37°C in 1 ml of a freshly prepared solution of ovalbumin (10 mg/ml) in hypertonic medium (0.5 M sucrose, 10% polyethylene glycol 1000, 10 mM HEPES). Splenocytes were then diluted to 15 ml with warm hypotonic media (60% RPMI 1640, 40% water) and incubated for an additional 2 min at 37°C. Cells were washed twice in Hanks' balanced salt solution and then resuspended at 50 × 106 splenocytes/ml. Splenocytes were irradiated (10 Gy) prior to injection in 0.5 ml.
Infection with L. monocytogenes.
Eight- to ten-week-old age- and sex-matched BALB/c or IFN-γ KO mice were immunized by intravenous injection with 106 CFU of ActA mutant L. monocytogenes strain DP-L1942 and were challenged 28 to 33 days later by intravenous injection with 3 × 105 CFU of virulent LM-NPs or LM-NPns. Naive IFN-γ KO mice were infected with 25, 50, or 100 CFU of virulent recombinant L. monocytogenes by intravenous injection. Naive BALB/c mice were infected with 103 CFU.
In vitro stimulation of effector cells.
Spleen cells (40 × 106) from mice immunized or infected 7 days previously were incubated for 5 days with 25 × 106 irradiated (30 Gy) APC from either B6 (ovalbumin injections) or BALB/c (L. monocytogenes infections) mice. Irradiated splenocytes were pulsed with 1 nM appropriate peptide (OVA257-264 or NP118-126, respectively) for 1 h at 37°C and then washed extensively prior to coculture with splenocytes from responder mice. Cultures were grown at 37°C in RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, and antibiotics (RP10 [23]).
51Cr release assays.
OVA257-264-specific responses were determined in standard 4-h 51Cr release assays using EL-4 (H-2b MHC) targets with or without 10 nM OVA257-264 peptide. NP118-126-specific responses were determined in standard 4-h 51Cr release assays using P815 (H-2d MHC) targets with or without 10 nM NP118-126 peptide. In all assays, 104 51Cr-labeled target cells were plated in each well of 96-well microtiter plates in 100 μl of RP10. Effector cells were added in 100 μl to generate a series of effector-to-target ratios. In all assays, total release was determined by incubating targets in 0.5% Triton X-100. Spontaneous release was determined by incubating targets in media alone. Percent specific release was determined as (experimental release − spontaneous release)/(total release − spontaneous release) × 100.
Intracellular cytokine staining of splenocytes.
Intracellular cytokine staining was performed using a Cytofix/Cytoperm Plus kit (Pharmingen). Briefly, 20 × 106 splenocytes from each mouse were treated with ACK lysis buffer for 5 min at room temperature to remove red blood cells. Splenocytes were washed twice in RP10 and resuspended in 1 ml of RP10 supplemented with 5% rat concanavalin A supernatant and 50 mM α-methylmannoside. Cells (200 μl) were incubated for 4 to 5 h at 37°C with medium alone or with 100 nM either NP118-126, OVA257-264, or LL091-99 peptide, all in the presence of brefeldin A. Cells were washed twice in FACS (fluorescence-activated cell sorting) buffer (phosphate buffered saline supplemented with 1% fetal calf serum and NaN3) and were incubated with antibody directed against the FcγII/III receptors (2.4G2) diluted 1:100 (to block Fc receptors) and fluorescein isothiocyanate-labeled anti-CD8 (1:100) on ice for 15 to 30 min. The cells were washed twice with FACS buffer and then fixed and permeabilized by incubating for 10 to 20 min in 250 μl of Cytofix/Cytoperm. The splenocytes were then washed twice in Perm/wash solution and stained with phycoerythrin-conjugated anti-tumor necrosis factor (TNF) (1:100) for 30 min on ice. Cells were washed twice in perm/wash solution and resuspended in 250 μl of FACS buffer for analysis by flow cytometry (FACScan; Becton Dickinson). The stimulated and unstimulated splenocytes from each mouse were compared to each other for analysis. The gate for TNF+ cells was selected such that the percentage of TNF+ cells in the unstimulated sample for each mouse was 0.2% or less of the CD8+ splenocytes; this level has been subtracted from the peptide-stimulated splenocytes to determine the level of response above background. The total number of CD8+ cells in the spleen of each mouse was determined by multiplying the percentage of CD8+ cells for each mouse by the total number of splenocytes. The number of CD8+ cells was then multiplied by the percentage of TNF+ cells in the CD8+ population in order to determine the number of TNF+ CD8+ cells in each spleen. The number of TNF+ cells obtained in the unstimulated samples was subtracted from the number of TNF+ cells obtained in the stimulated sample from each mouse.
CFU assays.
BALB/c or IFN-γ KO mice were infected with 105 CFU of L. monocytogenes strain 10403s. At various times following infection, CFU were counted in organ homogenates of spleen and livers as described elsewhere (24). The results are expressed as mean CFU/organ ± standard deviation (SD). Statistical analysis was performed using Student's t test.
RESULTS
IFN-γ is not required for in vivo CD8+ T-cell priming against a model exogenous antigen.
IFN-γ enhances multiple aspects of MHC class I antigen presentation including MHC gene expression, TAP expression, and proteasome configuration (19). Although IFN-γ may enhance the efficiency of MHC class I presentation in some systems, it is not required to prime CD8+ T-cell responses against some viruses (21) and secreted L. monocytogenes antigens (24), both of which are accessible to the endogenous MHC class I presentation pathway. However, the requirement for IFN-γ in presentation of exogenous model antigens or nonsecreted bacterial antigens, both of which require processing by exogenous MHC class I presentation pathways, has not been addressed.
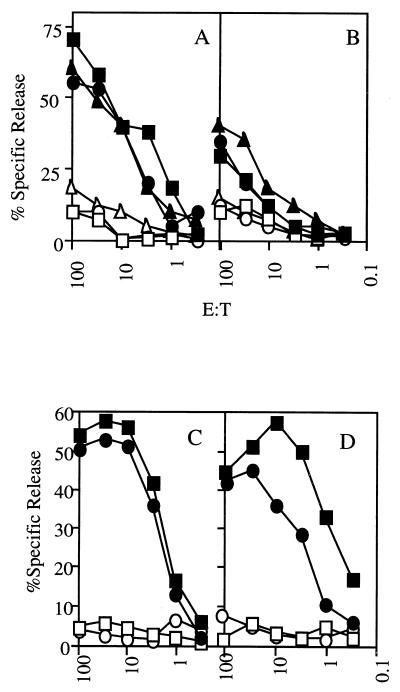
As a model to determine if IFN-γ was required for the function of exogenous MHC class I presentation pathways in vivo, we used cross-presentation of ovalbumin-loaded spleen cells (29). CB6 (H-2bxd, wild type [WT]) or H-2bxd IFN-γ KO mice were immunized with ovalbumin-loaded splenocytes derived from BALB/c (H-2d) mice. Priming of a H-2Kb-restricted CD8+ T-cell response to the OVA257-264 epitope in this situation requires cross-presentation since the antigen-loaded BALB/c splenocytes lack the presenting MHC class I molecule. The ability of naive or immunized animals to generate a CD8+ T-cell response to the H-2Kb-restricted OVA257-264 peptide was determined by 51Cr release assay 5 days after in vitro restimulation of splenocytes from each mouse. Immunized WT and IFN-γ KO mice were able to generate a CD8+ T-cell response against the OVA257-264 peptide presented via cross-presentation (Fig. 1A and B) under in vitro restimulation conditions which failed to induce a response from naive WT or IFN-γ KO mice (data not shown). Although clearly detectable, the level of response was lower in the IFN-γ KO mice than in WT mice. Similar results were obtained after immunization with ovalbumin-loaded B6 (H-2b) splenocytes (Fig. 2). CD8+ T-cell priming did not depend on IFN-γ produced by the immunizing splenocytes since H-2bxd IFN-γ KO mice immunized with ovalbumin-loaded, H-2d MHC IFN-γ KO (24) splenocytes were able to generate a specific CD8+ T-cell response against the OVA257-264 epitope (Fig. 1C and D). These results indicate that IFN-γ it is not an absolute requirement for in vivo priming of CD8+ T-cell responses against a model exogenous antigen.
FIG. 1.
Cross-presentation of OVA257-264 does not require IFN-γ. H-2bxd WT (A and C) and IFN-γ KO (B and D) mice were immunized with ovalbumin-loaded H-2d BALB/c splenocytes (A and B) or H-2d IFN-γ KO splenocytes (C and D). Seven days postimmunization, spleen cells were stimulated with OVA257-264 peptide in vitro and the CD8+ T-cell response to the H-2Kb-restricted OVA257-264 epitope was tested in a standard 51Cr release assay after 5 days in culture. Each pair of symbols represents the ability of an individual mouse to recognize either EL-4 (H-2b) targets pulsed with OVA257-264 (filled symbols) or the same targets without peptide (open symbols). A representative experiment of six is shown. E:T, effector/target cell ratio.
FIG. 2.
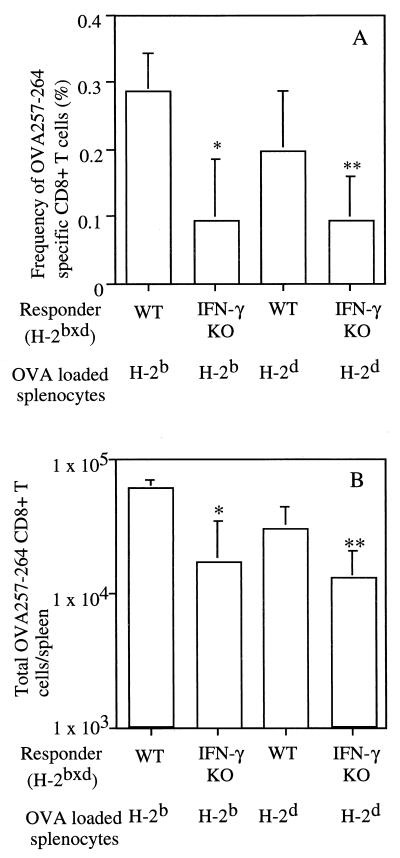
An increased number of CD8+ T cells respond to OVA257-264 in WT mice. (A) The percent OVA257-264-responsive CD8+ T cells in groups of H-2bxd WT or IFN-γ KO mice was determined by intracellular cytokine staining for TNF at 7 days after immunization with BALB/c H-2d or B6 (H-2b) splenocytes loaded with ovalbumin. (B) The total number of TNF+ CD8+ cells in the spleen of each mouse was determined as described in Materials and Methods. Data are presented as mean ± SD for groups of five mice. ∗, P < 0.05; ∗∗, P < 0.005 by Student's t test. A representative experiment of three is shown.
The frequency and total number of OVA257-264-specific CD8+ T cells are reduced in IFN-γ KO mice.
The bulk in vitro restimulation and 51Cr release assay does not provide a quantitative assessment of the in vivo OVA257-264 specific CD8+ T-cell response generated by cross-presentation. Recently, several new techniques including staining with MHC class I tetramers, intracellular IFN-γ staining and IFN-γ enzyme-linked immunospot analysis, have been shown to provide similar estimates of CD8+ T-cell priming after infection (30). CD8+ T cells also produce TNF after antigen stimulation, and detection of TNF has been used as a measure of antigen specificity in several applications including the expression cloning of MHC class I-restricted tumor antigens (40). Since staining for IFN-γ is not useful in the analysis of cells from IFN-γ KO mice, we performed intracellular TNF staining on spleen cells to determine the frequency and total number of OVA257-264-specific CD8+ T cells in immunized mice. In other experiments, we recently demonstrated that intracellular staining for TNF provides a reliable measure of the frequency of antigen-specific CD8+ T cells although staining for TNF detects only a subset of the cells (∼50% in comparisons of three different epitopes) detected by intracellular staining for IFN-γ (2a). H-2bxd WT and IFN-γ KO mice were immunized with ovalbumin-loaded splenocytes from either B6 or BALB/c mice, and their spleen cells were analyzed 7 days later. WT mice exhibited similar frequency of OVA257-264 specific CD8+ T cells after immunization with BALB/c- or B6-loaded splenocytes, representing 0.2 to 0.28% of CD8+ T cells, respectively (Fig. 2A), although the absolute number of OVA257-264-specific CD8+ T cells was ∼2-fold-higher in mice immunized with B6-loaded splenocytes (Fig. 2B). Furthermore, the OVA257-264-specific CD8+ T-cell response in WT mice was increased in frequency (two- to threefold [Fig. 2A]) and absolute number (two- to fourfold [Fig. 2B]) compared to that observed in IFN-γ KO mice immunized with ovalbumin-loaded BALB/c or B6 splenocytes. These results demonstrate that IFN-γ plays a quantitative role in the efficiency of CD8+ T-cell priming but further confirm that IFN-γ is not required to generate a CD8+ T-cell response via cross-presentation of a model exogenous antigen.
Previously immunized WT and IFN-γ KO mice demonstrate similar primary responses to secreted and nonsecreted bacterial antigens.
In addition to its role in antigen presentation, IFN-γ is important in host defense against L. monocytogenes infection (3, 10, 17, 24, 27). IFN-γ activates the microbicidal activities of macrophages, resulting in destruction of L. monocytogenes in the phagosome (33). Thus, priming of CD8+ T cells against the nonsecreted L. monocytogenes antigen may be dependent on the antimicrobial action of IFN-γ. The preceding studies (Fig. 1 and 2) demonstrate that the regulatory functions of IFN-γ are not required for MHC class I presentation of a model exogenous antigen. This result permits the analysis of the impact of the antimicrobial activities of IFN-γ on CD8+ T-cell priming against nonsecreted L. monocytogenes antigens.
The application of IFN-γ KO mice to this question is complicated by the finding these mice are extremely susceptible to infection with virulent LM (24). The previously determined 50% lethal dose (LD50) of virulent L. monocytogenes for IFN-γ KO mice is approximately 10 organisms (24), and thus a survivable immunizing dose of 0.1 LD50 would require the accurate administration of a single organism. However, IFN-γ KO mice survive high levels of infection (LD50 of ∼106.5 CFU) with an attenuated L. monocytogenes strain, DP-L1942, that lacks the ActA virulence factor and cannot spread between cells (24). After immunization with DP-L1942, IFN-γ KO mice develop acquired immunity to subsequent challenge with virulent L. monocytogenes that is dependent on CD8+ T cells and is similar to that generated by immunization of WT mice (24). To determine whether IFN-γ is required to generate a response against nonsecreted bacterial antigens, WT and IFN-γ KO mice were first immunized with DP-L1942 and then challenged with recombinant L. monocytogenes expressing the H-2Ld-restricted NP118-126 epitope from LCMV as a secreted (strain XFL303, referred to as LM-NPs) or nonsecreted (strain XFL304, referred to as LM-NPns) fusion protein (38).
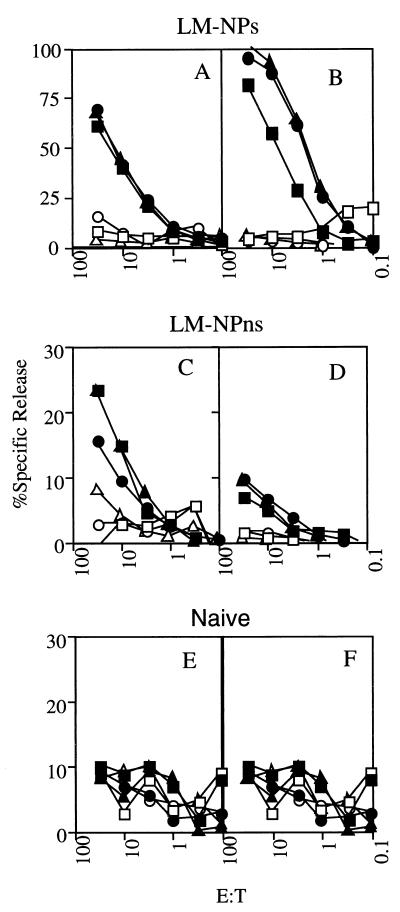
In this experimental scenario, the previously immunized IFN-γ KO mice survive the infection with virulent recombinant L. monocytogenes due to the secondary response against endogenous, shared L. monocytogenes antigens. However, the CD8+ T-cell response against the NP118-126 epitope, which is not expressed by the immunizing L. monocytogenes, represents a primary response and was tested in a 51Cr release assay following in vitro restimulation. WT and IFN-γ KO mice previously immunized with attenuated L. monocytogenes are able to respond to NP118-126 expressed as a secreted fusion protein (Fig. 3A and B). Naive WT mice also mount a substantial CD8+ T-cell response against the NP118-126 expressed as either a secreted or a nonsecreted fusion protein (see Fig. 5A and B). In contrast, the response by both the previously immunized WT and IFN-γ KO mice to NP118-126 expressed as a nonsecreted fusion protein is greatly reduced, although it is still detectable in all animals (Fig. 3C and D), compared to the complete lack of response observed with cells from uninfected WT and IFN-γ KO mice (Fig. 3E and F). The decreased response to the nonsecreted fusion protein in previously immunized mice is not due to failure to infect the mice with a high dose of LM-NPns since the recall responses to LLO91-99, an antigen shared by DP-L1942 and the recombinant L. monocytogenes, are similar in LM-NPns- and LM-NPs-challenged mice (Fig. 4). The decreased response against the nonsecreted antigen is not due to the absence of IFN-γ since it is also seen in WT mice and is thus a consequence of prior immunization. These results suggest that prior immunization has a differential impact on the primary response to a secreted L. monocytogenes antigen compared to a primary response to the same antigen expressed in nonsecreted form. In addition, these results demonstrate that IFN-γ is not required to generate a low-level CD8+ T-cell response to the nonsecreted L. monocytogenes antigen.
FIG. 3.
Prior immunization has differential impact on CD8+ T-cell responses to secreted or nonsecreted L. monocytogenes antigens in both WT and IFN-γ KO mice. The response generated against the NP118-126 epitope was determined by in vitro restimulation of spleen cells from WT (A, C, and E) or IFN-γ KO (B, D, and F) mice at 7 days after infection with recombinant L. monocytogenes. WT BALB/c mice were immunized with 106 CFU of attenuated L. monocytogenes DP-L1942 and challenged 30 days later with 3 × 105 CFU of virulent recombinant L. monocytogenes LM-NPs (A) or LM-NPns (C). IFN-γ KO mice were immunized with 106 CFU of attenuated L. monocytogenes DP-L1942 and challenged 30 days later with 3 × 105 CFU of virulent recombinant L. monocytogenes LM-NPs (B) or LM-NPns (D). Spleen cells from naive WT (E) or IFN-γ KO mice (F) were also analyzed. Each pair of symbols represents the ability of in vitro-restimulated spleen cells from an individual mouse to recognize P815 (H-2d) targets pulsed with NP118-126 (filled symbols) or the same targets without peptide (open symbols). The y axes for panels A and B are different from those for the other panels. A representative experiment of three is shown. E:T, effector/target cell ratio.
FIG. 5.
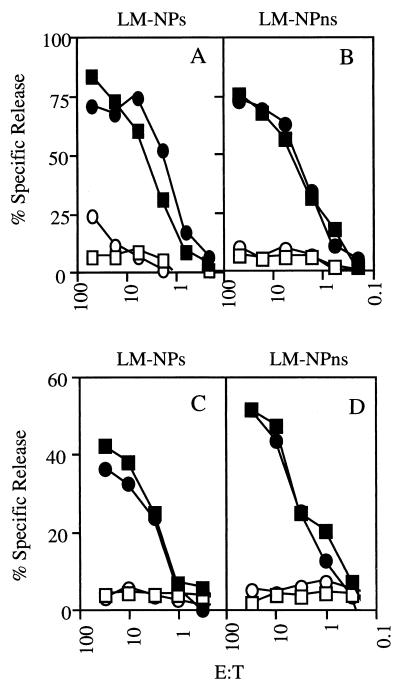
IFN-γ KO mice respond to both secreted and nonsecreted bacterial antigens. The response generated against the NP118-126 epitope by naive WT (A and B) or IFN-γ KO (C and D) mice which were infected with LM-NPs (A and C) or LM-NPns (B and D) was tested in a standard 51Cr release assay. WT mice were infected with 103 bacteria; IFN-γ KO mice were infected with 25, 50, or 100 bacteria. All mice infected with 50 or 100 CFU of either recombinant failed to survive to day 7. IFN-γ KO mice which received 25 bacteria and survived 7 days were used for this analysis. Each pair of symbols represents the ability of in vitro-restimulated spleen cells from an individual mouse to recognize either P815 targets pulsed with NP118-126 (filled symbols) or the same targets without peptide (open symbols). The experiment shown is representative of two experiments in which mice survived to 7 days. E:T, effector/target cell ratio.
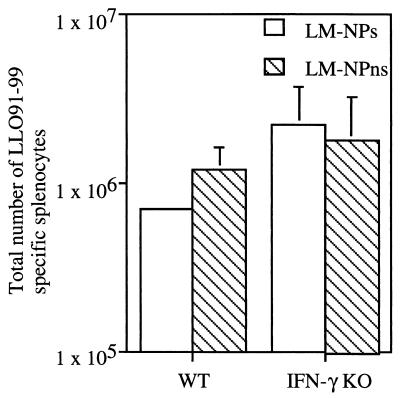
FIG. 4.
Recall response of LLO91-99-specific CD8+ T cells. WT or IFN-γ KO mice were infected with 106 CFU of DP-L1942 (ActA−); 30 days later, groups of three mice were infected with 3 × 105 CFU of either LM-NPs or LM-NPns. The CD8+ T-cell response to LLO91-99 was determined by intracellular cytokine staining for TNF at 7 days after infection with LM-NPs or LM-NPns. Data are presented as mean ± SD of the total number of LLO91-99-specific CD8+ T cells/spleen. A representative experiment of three is shown.
IFN-γ is not required to generate an immune response against nonsecreted bacterial antigens.
The previous results suggested that IFN-γ is not required to mount a CD8+ T-cell response to a nonsecreted LM antigen. We further evaluated this notion by analyzing the NP118-126-specific CD8+ T-cell response in IFN-γ KO mice after primary infection with recombinant L. monocytogenes. In these experiments, we infected IFN-γ KO mice with 25, 50, or 100 CFU of recombinant L. monocytogenes and analyzed surviving mice for NP118-126-specific CD8+ T-cell responses at 7 days postinfection by in vitro restimulation and 51Cr release assays. Although the majority of animals succumbed to infection before analysis, surviving IFN-γ KO mice responded equally well to either the secreted or nonsecreted NP118-126 epitope (Fig. 5C and D). Furthermore, the response against the secreted and nonsecreted NP118-126 by IFN-γ KO mice was similar to the response by WT mice (Fig. 5A and B). Therefore, the ability to generate CD8+ T-cell responses to nonsecreted bacterial antigens is not dependent on the antimicrobial activities of IFN-γ.
IFN-γ KO and WT mice exhibit similar patterns of response against secreted and nonsecreted LM antigens.
Since the level of response detected after in vitro restimulation and 51Cr release assays does not necessarily correlate with the number of responding CD8+ T cells in vivo, intracellular TNF staining was performed to determine the frequency and total number of NP118-126-responsive CD8+ T cells in WT and IFN-γ KO mice immunized with recombinant L. monocytogenes. As reported for naive WT mice infected with recombinant L. monocytogenes (38) (Fig. 6A and B), the frequency of NP118-126-specific CD8+ T cells observed after infection of IFN-γ KO mice with recombinant L. monocytogenes expressing the secreted epitope was increased (∼5-fold) compared to mice immunized with recombinant L. monocytogenes expressing the nonsecreted epitope (Fig. 6C). Similarly, the total number of NP118-126-specific CD8+ T cells per spleen was increased ∼7-fold in mice challenged with recombinant L. monocytogenes that secrete the epitope (Fig. 6D). Thus, IFN-γ KO mice respond to both secreted and nonsecreted NP118-126 similarly to WT mice. This result demonstrates that IFN-γ-dependent processes are not required for presentation of nonsecreted L. monocytogenes antigens and do not significantly affect the relative efficiency of CD8+ T-cell priming against secreted and nonsecreted L. monocytogenes antigens.
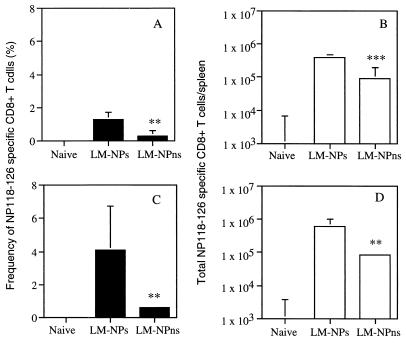
FIG. 6.
The absence of IFN-γ does not alter the relative magnitude of the primary response to secreted and nonsecreted LM antigens. WT BALB/c mice were infected with 103 CFU of recombinant L. monocytogenes; IFN-γ KO mice were infected with 25 CFU. The frequency of NP118-126-specific CD8+ T cells from 7-day-infected naive WT BALB/c (A) and IFN-γ KO (C) mice was determined by intracellular cytokine staining for TNF as described in the legend to Fig. 4. The number of NP118-126-specific CD8+ T cells in the spleen of WT (B) and IFN-γ KO mice (D) was calculated. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005 by Student's t test. A representative experiment of two is shown.
IFN-γ KO mice eliminate L. monocytogenes with kinetics similar to those for WT mice early in infection.
The capacity of IFN-γ KO mice to mount a CD8+ T-cell response against a nonsecreted L. monocytogenes antigen suggests the existence of IFN-γ-independent mechanisms to kill the organism and reveal these molecules to the host processing machinery. Previous studies have demonstrated that the number of L. monocytogenes in the liver decreases, as a result of bacterial destruction by neutrophils (12, 35), between 4 and 8 h after infection of WT mice and is followed by an exponential increase in the number of bacteria over the next several days (12, 35). In the spleen, however, the number of L. monocytogenes increases continuously. To determine whether this early control of infection was independent of IFN-γ, we compared the number of L. monocytogenes detected in the organs of IFN-γ KO and WT mice over the first 24 h postinfection. During the early course of infection, the IFN-γ KO mice exhibit the same capacity to limit L. monocytogenes growth in the liver as WT mice (Fig. 7A), whereas bacterial growth is unchecked in the spleen (Fig. 7B). However, by approximately 24 h postimmunization, the IFN-γ KO mice demonstrate a reduced ability to control L. monocytogenes infection in both organs. This early control of L. monocytogenes in the liver occurred even though the challenge dose used to allow detection of L. monocytogenes shortly after infection represents 10,000 times the LD50 of virulent L. monocytogenes in IFN-γ KO mice. These results demonstrate the existence of IFN-γ-independent mechanisms of bacterial killing in vivo that may affect CD8+ T-cell priming against nonsecreted L. monocytogenes antigens.
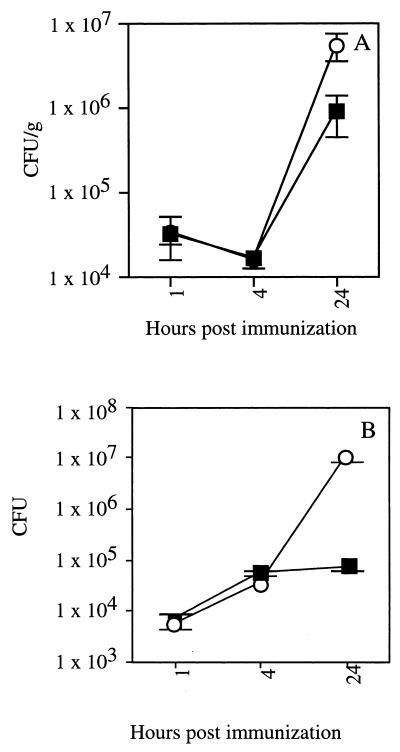
FIG. 7.
Bacterial burdens in IFN-γ KO and WT mice are similar at early time points after infection. CFU in the liver (A) and the spleen (B) of WT (filled symbols) and IFN-γ KO (open symbols) mice were determined at the indicated times after infection with 105 CFU of virulent L. monocytogenes strain 10403s. Data are from a representative of two independent experiments and are presented as mean CFU ± SD from three mice per group. CFU per organ at 1 and 4 h were not significantly different between WT and IFN-γ KO mice. CFU per liver (P < 0.01) and spleen (P < 0.005) were significantly higher in IFN-γ KO mice at 24 h postinfection.
DISCUSSION
In previous studies (38) and in data presented in here (Fig. 3 and 6), sublethal infection with recombinant L. monocytogenes expressing a nonsecreted form of a model epitope results in a CD8+ T-cell response that is only slightly less vigorous than that observed after sublethal infection with recombinant L. monocytogenes expressing the same epitope in secreted form. Processing and presentation of the nonsecreted L. monocytogenes epitope requires bacterial lysis, a process that generally occurs in the phagosomes of professional APC and neutrophils. Since these nonsecreted molecules originate in an extracytosolic compartment, their processing occurs by an exogenous pathway of MHC class I presentation. In contrast, secreted L. monocytogenes molecules are freely accessible to the widely distributed endogenous MHC class I presentation pathway that originates in the cytosol (9). Interestingly, epitope-specific protective immunity was observed only against recombinant L. monocytogenes expressing the secreted antigen (38). These results reveal a striking dichotomy between CD8+ T-cell priming and protective immunity based on antigen location and suggest that exogenous and endogenous MHC class I presentation pathways perform nonoverlapping functions in the host response to infection.
Although several pathways have been identified for MHC class I presentation of exogenous model antigens in vitro (22, 28, 32, 37), the exact pathway for in vivo presentation of the nonsecreted L. monocytogenes antigen is unknown. IFN-γ KO mice were used in an initial attempt test the hypothesis that the listeriacidal activity associated with IFN-γ-mediated activation of professional APC (33) is required for destruction of the organism in the phagosome and presentation of the nonsecreted antigen by infected APC to prime CD8+ T-cell responses.
In addition to its role as an important effector of innate immunity to L. monocytogenes infection (10, 24, 27), IFN-γ is a critical regulator of expression of a number of immunologically relevant gene products, some of which, such as LMP7 and TAP, are involved in MHC class I antigen presentation (19). In order to dissociate the antimicrobial activities of IFN-γ from its regulatory function, we first determined whether IFN-γ was required for exogenous presentation of a model MHC class I-restricted antigen. In vivo cross-presentation of the H-2Kb-restricted ovalbumin epitope (OVA257-264) to CD8+ T cells is dependent on CD4+ T-cell help (4) and has recently been shown to require CD40L-CD40 interaction (5). By definition, cross-presentation is dependent on an exogenous pathway of MHC class I presentation since the antigen expressing cells cannot directly present the epitope to host T cells. Our studies with IFN-γ KO mice demonstrate that in contrast to mice lacking CD40-mediated signals, IFN-γ KO mice are able to cross-present this model exogenous antigen to prime a significant CD8+ T-cell response. However, IFN-γ enhances the frequency of the CD8+ T-cell response against exogenous antigens ∼2- to 3-fold, as determined by intracellular cytokine staining (Fig. 2). This result is similar to that reported by Geginat et al. for CD8+ T-cell priming against murine cytomegalovirus in mice treated with anti-IFN-γ monoclonal antibody (20). Although IFN-γ contributes to the efficiency of cross-priming, our results clearly demonstrate that the cytokine is not required for cross-presentation of a model antigen to prime CD8+ T-cell responses in vivo. This result allows direct analysis of the requirement for the microbicidal activities of IFN-γ in exogenous presentation of the nonsecreted L. monocytogenes antigen.
A complication to these experiments is the finding that IFN-γ plays an important role in the innate immune response to primary L. monocytogenes infection, as shown by in vivo depletion studies (10) and the extreme susceptibility of IFN-γ KO (24) and IFN-γ receptor KO (27) mice to virulent L. monocytogenes infection. The recombinant L. monocytogenes strains expressing the NP118-126 epitope are highly virulent in IFN-γ KO mice (see the legend to Fig. 5), and thus infecting these animals with a survivable dose of recombinant L. monocytogenes was technically challenging. To circumvent this problem, we first made use of an earlier observation that IFN-γ KO mice can be vaccinated with a high dose of an attenuated L. monocytogenes strain (one that lacks the ActA virulence factor) and develop acquired, CD8+ T-cell immunity to L. monocytogenes infection that is similar to that achieved by vaccination of WT mice (24). To assess the requirement for IFN-γ in CD8+ T-cell priming against the nonsecreted LM antigen, we first vaccinated IFN-γ KO and WT mice with the ActA− L. monocytogenes strain and then challenged the immune mice with a high dose of recombinant L. monocytogenes expressing the NP118-126 epitope as a secreted or nonsecreted fusion protein. In this experimental scenario, the mice survive the challenge infection due to the secondary CD8+ T-cell response to endogenous L. monocytogenes antigens, such as LLO91-99 or p60217-225 (24), which are shared by all L. monocytogenes strains. However, responses to the NP118-126 epitope are primary responses, occurring in the context of a secondary response. These studies revealed low levels of CD8+ T-cell priming against the nonsecreted L. monocytogenes antigen in both WT and IFN-γ KO mice, suggesting that the prior immunization, and not the lack of IFN-γ, affected the priming against the nonsecreted antigen.
The impact of prior immunization on the response to a newly introduced L. monocytogenes antigen has recently been addressed by two groups with disparate results. Using a frequency analysis after in vitro restimulation with the nonspecific mitogen concanavalin A, Bouwer et al. found that existing immunity to L. monocytogenes did not inhibit the ability to develop a CD8+ T-cell response against a secreted bacterial antigen introduced during a recall response to L. monocytogenes (7). In contrast, Vijh et al. used a peptide-specific enzyme-linked immunospot assay on direct ex vivo splenocytes and found that the response to newly introduced secreted bacterial antigens was reduced during a recall response compared to the response generated during primary infection (41). In our experiments with 51Cr release assays performed 5 days after in vitro restimulation, the response against the secreted NP118-126 fusion protein introduced during a recall infection did not appear to differ significantly from that found in a primary immune response (Fig. 3). However, the response against the nonsecreted epitope was substantially reduced (Fig. 3). These findings suggest that prior immunization substantially impairs the response to a newly introduced nonsecreted bacterial antigen but has less impact on the response to a newly introduced secreted antigen. When the responses to the secreted and nonsecreted epitopes were tested using a more quantitative analysis directly ex vivo, it was found that the responses to both the secreted and nonsecreted NP118-126 epitope were weaker than the response generated during a primary infection (data not shown). The disparity found using these different methods is consistent with the notion that bulk in vitro restimulation assays may underestimate differences in initial precursor frequencies. The decreased response to newly introduced antigens may result from elimination of infected cells before effective CD8+ T-cell priming against the new antigen can occur. For example, perforin-dependent elimination of LCMV infected APC by memory cytotoxic T lymphocytes is thought to control the T-cell response against LCMV variants given in subsequent challenges (27a). Thus, the prior immunization scheme used in these experiments adds a level of complexity to the interpretation of the results.
To eliminate this complication, we infected naive IFN-γ KO mice with graded low doses (25 to 100 CFU) of the NP118-126-expressing recombinant L. monocytogenes strains and measured the NP118-126-specific CD8+ T-cell response in surviving mice at 7 days postinfection. All mice infected with 50 or 100 CFU of either strain succumbed to infection prior to analysis, confirming the lack of resistance to primary L. monocytogenes infection in the absence of IFN-γ. Some mice that received 25 CFU survived 7 days after infection with virulent recombinant L. monocytogenes. Bulk in vitro restimulation of spleen cells from these mice revealed no significant differences in the level of CD8+ T-cell priming in IFN-γ KO mice infected with recombinant L. monocytogenes expressing the secreted or nonsecreted NP118-126 epitope. Frequency analysis by intracellular TNF staining revealed that the three- to fivefold increased CD8+ T-cell priming against the secreted epitope observed after infection of WT BALB/c mice was also observed in H-2d MHC IFN-γ KO mice. These results demonstrate that the microbicidal activities of IFN-γ are not required for CD8+ T-cell priming against the nonsecreted L. monocytogenes antigen.
In contrast to the decreased CD8+ T-cell priming in IFN-γ KO mice in response to ovalbumin, no difference in the magnitude of the CD8+ T-cell response to the nonsecreted NP epitope was seen between the WT and IFN-γ KO mice. This finding is complicated because the antigen load given in the two sets of experiments differs between the WT and IFN-γ KO mice. In the studies in which mice were immunized with ovalbumin, the WT and IFN-γ KO mice were given the same number of antigen-loaded splenocytes from the same preparation. In the studies in which IFN-γ KO mice were given recombinant L. monocytogenes, even 25 organisms caused a lethal infection associated with high bacterial numbers for these mice. Further studies are required to examine the magnitude of CD8+ T-cell priming in WT and IFN-γ KO mice. However, the finding that the relative levels of CD8+ T-cell priming against the secreted and nonsecreted epitopes in WT and IFN-γ KO mice are similar suggests that IFN-γ-mediated microbicidal activity has very little impact on the CD8+ T-cell response to nonsecreted antigens.
Although IFN-γ is required for innate immunity against L. monocytogenes, the number of organisms present in the spleens and livers of IFN-γ KO mice was similar to that of WT mice at 1 and 4 h after high-dose infection. During this time, the number of bacteria decreases in the liver as previously demonstrated (12, 13, 35). This decrease in the number of L. monocytogenes in the liver in WT mice is due, at least in part, to the action of neutrophils since depletion of these cells prior to infection results in a continuous increase in the number of organisms seen in the liver (12). Further evidence for neutrophil-mediated elimination of L. monocytogenes in the liver is provided by the fact that neutrophils are found in close proximity with infected hepatocytes but not with infected splenocytes (13). As early as 24-h postinfection, the IFN-γ KO mice have increased numbers of bacteria in both the spleen and liver compared to the WT animals. Similar exacerbation of infection at 48 h after infection of IFN-γ receptor KO mice have been reported (17). These results demonstrate the presence of IFN-γ-independent pathways of L. monocytogenes killing and are consistent with the CD8+ T-cell priming results.
The precise mechanism by which nonsecreted L. monocytogenes antigens prime CD8+ T-cell responses in vivo is not known. In one scenario, infected APC such as macrophages or DC may kill some L. monocytogenes in the phagosome and activate one of the previously described exogenous MHC class I presentation pathways. It is intriguing to speculate that expression of the pore-forming listeriolysin O (LLO) molecule by L. monocytogenes may facilitate a phagosome to cytosol pathway of exogenous presentation, even after bacterial destruction. Support for this notion comes from experiments where in vitro presentation of a model CD8+ T-cell antigen expressed in Escherichia coli is greatly enhanced by coexpression of low levels of LLO even though the LLO is not secreted and the bacteria are killed in the phagosome (26). An additional possibility is that bacteria which escape to the host cell cytoplasm die or are killed in this location. A possible candidate for the killing of cytoplasmically located L. monocytogenes is ubiquicidin, which is located in the cytoplasm and has microbicidal activity (25). Construction of LLO-deficient recombinant L. monocytogenes expressing the nonsecreted epitope should address this issue. However, any pathway of CD8+ T-cell priming that depends on killing of L. monocytogenes in the phagosome of an infected APC would be predicted to exhibit some dependence on the microbicidal activities of IFN-γ. Our results demonstrating that IFN-γ is not required as an effector of microbicidal activity for CD8+ T-cell priming against a nonsecreted L. monocytogenes antigen are not consistent with this scenario.
DC are the most potent APC in priming T-cell responses (39), and recent in vitro results show that DC are capable of cross-presenting antigens obtained from virus infected cells that are undergoing apoptosis (1, 2). Such a pathway may be operative to present nonsecreted L. monocytogenes antigens to prime CD8+ T-cell responses in vivo. In this case, killing of L. monocytogenes would have to be carried out by an IFN-γ-independent pathway, and the effector cell would need to undergo apoptosis. Our results demonstrate the existence of IFN-γ-independent mechanisms for killing of L. monocytogenes that are consistent with a previously demonstrated role for neutrophils in innate resistance to L. monocytogenes infection. Since the neutrophil is capable of killing L. monocytogenes and also undergoes spontaneous apoptosis after a short life span vivo (13, 15, 16, 34), it is an attractive candidate as the substrate for DC-mediated priming of CD8+ T cells against nonsecreted L. monocytogenes antigens. A second potential route by which DC may acquire nonsecreted L. monocytogenes epitopes for antigen presentation is from infected hepatocytes, which appear to undergo L. monocytogenes-induced apoptosis (34). However, L. monocytogenes are not killed by the hepatocytes as they undergo apoptosis (34). Therefore, lysis of the bacteria to expose the nonsecreted antigen may occur during the process of phagocytosis by the DC. Resolution of these issues will require identification of cells that present the nonsecreted epitope after in vivo infection. The recombinant L. monocytogenes expressing the same model CD8+ T-cell epitope in secreted and nonsecreted form (38) provide elegant probes to dissect the function of exogenous and endogenous MHC class I presentation pathways in the context of in vivo bacterial infection.
ACKNOWLEDGMENTS
We thank Lori Gorton and Gail Mayfield for excellent technical assistance and Stanley Perlman for critical review of the manuscript.
This work was supported by NIH grants AI36864 and AI42767 to J.T.H. A.R.T. is supported by USPHS training grant T32 AI07511.
REFERENCES
- 1.Albert M L, Pearce S F A, Francisco L M, Sauter B, Pampa R, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2a.Badovinac, V. P., and J. T. Harty. Intracellular staining for TNF and IFN-gamma detects frequencies of antigen-specific CD8+ T cells. J. Immunol. Methods, in press. [DOI] [PubMed]
- 3.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 4.Bennett S R, Carbone F R, Karamalis F, Miller J F A P, Heath W R. Induction of a CD8 cytotoxic T lymphocyte response by cross-priming requires cognate CD4 help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett S R M, Carbone F R, Karamalis F, Flavell R A, Miller J F A P, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 6.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 7.Bouwer H G A, Shen H, Fan X, Miller J F, Barry R A, Hinrichs D J. Existing antilisterial immunity does not inhibit the development of a Listeria monocytogenes-specific primary cytotoxic T-lymphocyte response. Infect Immun. 1999;67:253–258. doi: 10.1128/iai.67.1.253-258.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunt L M, Portnoy D A, Unanue E R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 10.Buchmeier N A, Schreiber R D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci USA. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone F R, Bevan M J. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlan J W. Critical role of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez D J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czuprynski C J, Brown J F, Maroushek N, Wagner R D, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 16.Czuprynski C J, Henson P M, Campbell P A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984;35:193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Dai W J, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-γ receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 18.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 19.Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon γ. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 20.Geginat G, Ruppert T, Hengel H, Holtappels R, Koszinowski U H. IFN-gamma is a prerequisite for optimal antigen processing of viral peptides in vivo. J Immunol. 1997;158:3303–3310. [PubMed] [Google Scholar]
- 21.Graham M B, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon-γ gene. J Exp Med. 1993;178:1725–1744. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding C V, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 23.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harty J T, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:107–119. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 25.Hiemstra P S, van den Barselaar M T, Roest M, Nibbering P H, van Furth R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J Leukoc Biol. 1999;66:423–428. doi: 10.1002/jlb.66.3.423. [DOI] [PubMed] [Google Scholar]
- 26.Higgins D E, Shastri N, Portnoy D A. Delivery of protein to the cytosol of macrophages using Escherichia coli K-2. Mol Microbiol. 1999;31:1631–1641. doi: 10.1046/j.1365-2958.1999.01272.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 27a.Klenerman P, Zinkernagel R M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:483–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 28.Kovacsovics-Bankowski M, Rock K L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 29.Moore M W, Carbone F R, Bevan M J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 30.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Stansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 31.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 33.Portnoy D A, Schreiber R D, Connelly P, Tilney L G. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989;170:2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen H, Gordon S, North R J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenberger S P, Toes R E M, van der Voort E I H, Offringa R, Melief C J M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 37.Schrimbeck R, Reimann J. ‘Empty’ Ld molecules capture peptides from endocytosed hepatitis B surface antigen particles for major histocompatibility complex class I restricted presentation. Eur J Immunol. 1996;26:2812–2822. doi: 10.1002/eji.1830261204. [DOI] [PubMed] [Google Scholar]
- 38.Shen H, Miller J F, Fan X, Kolwyck D, Ahmed R, Harty J T. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 39.Steinman R M, Pack M, Inaba K. Dendritic cell development and maturation. Adv Exp Med Biol. 1997;417:1–6. doi: 10.1007/978-1-4757-9966-8_1. [DOI] [PubMed] [Google Scholar]
- 40.Viale O, van der Bruggen P, Meuer E, Kunzmann R, Kohler H, Mertelsmann R, Boon T, Fisch P. Recognition by human V gamma9/Vdelta2 T cells of melanoma cells upon fusion with Daudi cells. Immunogenetics. 1996;45:27–34. doi: 10.1007/s002510050163. [DOI] [PubMed] [Google Scholar]
- 41.Vijh S, Pilip I M, Pamer E G. Noncompetitive expansion of cytotoxic T lymphocytes specific for different antigens during bacterial infection. Infect Immun. 1999;67:1303–1309. doi: 10.1128/iai.67.3.1303-1309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]