Abstract
The immunogenicity and protective efficacy of four versions of recombinant C-terminal 19-kDa epidermal growth factor-like region of the major surface protein 1 (rMSP119) of Plasmodium falciparum was studied in Aotus monkeys. Vaccination with each of the four rMSP119 constructs elicited high levels of antibodies to MSP119 but only one construct, the 19-kDa fragment expressed as a secreted fusion protein from Saccharomyces cerevisiae (yP30P2MSP119), induced a high degree of protective immunity in Aotus nancymai against lethal P. falciparum challenge. Protective formulation required Freund's adjuvant; vaccination with yP30P2MSP119 in six other adjuvants that are suitable for human use induced lower levels of antibody response and no protection. These results emphasize the need to continue the search for an adjuvant that is comparable to Freund's adjuvant in potency and is safe for use in humans.
The major protein of mature blood-stage schizonts of malaria parasites is sequentially processed during merozoite release and erythrocyte (RBC) reentry into a series of fragments collectively referred to as major merozoite surface protein 1 (MSP1) (15). Of particular interest among these fragments are the cysteine-rich 19-kDa C terminus which contains two epidermal growth factor (EGF)-like domains (MSP119) and a C-terminal 42 kDa fragment (MSP142) which includes MSP119 (4). A large body of evidence suggests that MSP1 is a target of protective immunity against asexual blood-stage malaria parasites. Several monoclonal antibodies (MAbs) that recognize the cysteine-rich EGF-like domains on MSP119 (2, 3) and polyclonal antibodies raised against MSP142 (8) inhibit the invasion of erythrocytes by Plasmodium falciparum merozoites. However, the most direct evidence establishing MSP1 as a vaccine candidate comes from in vivo challenge studies conducted in murine and monkey models. More than a decade ago it was demonstrated that immunization with native MSP1 confers protection against lethal P. yoelii in mice (16) and against P. falciparum in monkeys (22). Also, MAb 302, which recognizes an epitope in the first EGF-like domain of P. yoelii MSP119, protects mice against lethal parasite challenge (7). In more recent years, several laboratories have demonstrated that immunization with recombinant MSP142 (rMSP142) or rMSP119 protects mice against P. yoelii (10, 20, 23, 24) and monkeys against P. falciparum (9, 19).
We have previously reported the expression of MSP119 as a His6-tagged fusion protein with two universal helper T-cell epitopes of tetanus toxoid, P30P2 (yP30P2MSP119). This fusion protein is secreted from Saccharomyces cerevisiae and elicits protection in Aotus nancymai from an otherwise lethal challenge infection (19). The protective formulation included complete Freund's adjuvant (CFA). These findings warranted new studies to further evaluate and develop yP30P2MSP119 as a vaccine candidate and to search for a delivery system that is appropriate as a constituent of vaccine for human use. Here we report additional studies with yP30P2MSP119 in various adjuvant formulations as well as in comparison with three additional rMSP119 constructs in Aotus monkeys.
MATERIALS AND METHODS
Construction of recombinant plasmids and protein purification.
A total of four constructs based on rMSP119 were used in this study.
(i) yP30P2MSP119.
The recombinant protein was produced as both the FVO and 3D7 versions of P. falciparum.
(ii) yP30P2MSP119 FVO.
The NH2 terminus of the MSP119 gene of the FVO strain of P. falciparum was fused to two helper T-cell epitopes, P30 and P2, of tetanus toxoid and expressed as a secreted, His6-tagged protein in yeast. Construction of this recombinant plasmid, expression, and protein purification have been previously described (19).
(iii) yP30P2MSP119 3D7.
For this construct, a gene fragment encoding amino acids Asn1631 to Ser1723 was PCR amplified from the genomic DNA of the 3D7 strain of P. falciparum. The primers used for this purpose were sense oligonucleotide 5′-CACCTCGAGAACATTTCACAACACCAA-3′ and antisense oligonucleotide 5′-CCACTAGTGGTGGTGGTGGTGGTGACTGCAGAAAATACCATC-3′. The PCR-amplified gene fragment was ligated to a plasmid containing P30P2 sequences. The P30P2MSP119 cassettes were eventually ligated into pIXY 154, a derivative of pADH2 (21) that was kindly provided by V. Price, Immunex, Inc., Seattle, Wash. The resulting plasmid was transformed into S. cerevisiae strain 2905/6 by electroporation. The Trp+ recombinant cells were grown at 30°C in the presence of Trp-selective medium. Recombinant yeast was induced for 6 to 18 h with ethanol, and the secreted recombinant protein was microfiltered, ultrafiltered, and diafiltered with a 10-kDa spiral fiber filter. The His6-tagged protein was purified on a Ni-nitrilotriacetic acid column, and protein was eluted with sodium acetate, pH 4.5 (17).
Both the FVO and 3D7 versions of the yP30P2MSP119 used for NIH trial 2 (described below) were produced using good manufacturing practices (GMPs) in the Department of Biologics Research (DBR) at the Walter Reed Army Institute of Research (WRAIR). The products were purified from yeast fermentation ultrafiltrate by sequential nickel agarose and size exclusion chromatography followed by concentration and aseptic filtration and stored at −75°C.
(iv) yEVE-MSP119.
The construct consists of amino acids E, V, and E on the NH2 terminus of the MSP119 sequence with a terminal His6 tag. To create yEVE-MSP119, the gene fragment for MSP119 was PCR amplified from the genomic DNA of the FVO strain of the parasite, using sense (5′-CTGGTACC TTTGGATAAAAGAGAAGTAGAAAACATTTCACAACACCAATGT-3′) and antisense 5′-CCACTAGTGGTGGTGGTGGTGACTGCAGAAAATACCATC-3′) primers. The PCR-amplified gene fragment was ligated to pIXY 154 into KpnI-SpeI restriction sites, and the recombinant plasmid was electroporated into S. cerevisiae 2905/6 cells. The recombinant protein was expressed in a clean room, using the procedure described above.
(v) yMSP119.
A nonfused version of MSP119 of the FVO strain of P. falciparum was expressed as a His6-tagged protein in S. cerevisiae (yMSP119). The procedures for plasmid construction, expression, and protein purification were described earlier (18). This protein was used as a capture antigen in enzyme-linked immunosorbent assay (ELISA).
(vi) bGST-MSP119.
The protein was expressed in E. coli as a fusion between Schistosoma japonicum glutathione S-transferase (GST) and MSP119 of the FVO strain of P. falciparum (bGST-MSP119). The procedures for the plasmid construction, protein expression, and purification have been described earlier (5).
Adjuvants.
The following adjuvants were used in this study: CFA and Freund's incomplete adjuvant (IFA), Rehydragel alum (Reheis, Inc.), SBAS2 (SmithKline Beecham Pharmaceuticals, Inc., Rixensart, Belgium), Montanide ISA 720 (Seppic, Inc.), lipid A in liposomes (lipid A/liposomes), polyphosphazine (Avant Immunotherapeutics, Inc., Needham, Mass.) and microspheres (Immunex).
Immunization.
During the course of this study, three vaccine trials were conducted at two different locations. Two of these trials were carried out at the animal facility of the National Institutes of Health (NIH; Bethesda, Md.) (NIH trials 1 and 2), and the third trial was conducted at the Chamblee campus of the Centers for Disease Control and Prevention (CDC trial). The vaccination schedule and parasite challenge for NIH trial 2 was synchronized with the CDC trial with the goal of comparing the protective adjuvant effect of CFA with six other adjuvants that are suitable for human use. Blood was drawn from each monkey on the day of each immunization and on the day of, and in most instances 4 weeks after, the parasite challenge. Serum samples were separated and stored frozen at −70°C until used.
NIH trial 1.
The purpose of the first trial was to confirm the protective efficacy of yP30P2MSP119 and to test the vaccine efficacy of yEVE-MSP119 and bGST-MSP119 in CFA. All three vaccines contained the MSP119 sequences from the FVO parasite. The protective efficacy of yP30P2MSP119 (lot 940826) was tested in A. nancymai and A. vociferans. yEVE-MSP119 (lot 941107) and bGST-MSP119 (lot 941130) vaccines were tested in A. nancymai. Aotus monkeys of both sexes were used in this study. The monkeys were housed at the Primate Research Facility, NIH, according to the NIH guideline for the care and use of laboratory animals. Monkeys were stratified according to sex and then assigned to each group by card draw. The immunization and parasite challenge parts of the study were performed blinded to the investigators who were actively involved in the process. Aotus monkeys, two in each group, were immunized with 0.5 ml of an emulsion containing 250 μg of the recombinant protein of interest comprised of equal volumes of the antigen in phosphate-buffered saline (PBS) and CFA. The same immunization regimen was repeated on days 21 and 42 in IFA.
NIH trial 2.
The second trial was conducted to determine the efficacy of clinical-grade yP30P2MSP119 against homologous and heterologous P. falciparum challenge. A. nancymai monkeys, four in each group, were immunized with 250 μg of yP30P2MSP119 containing FVO (lot 0195) and 3D7 (lot 0207) MSP1 sequences produced using GMPs. The recombinant proteins were emulsified in an equal volume of CFA as in NIH trial 1. Immunizations were repeated on days 21 and 42 with IFA. The control monkeys were immunized with a transmission-blocking vaccine candidate antigen, Pfs25, produced in yeast under GMP conditions and delivered in CFA followed by two injections in IFA.
In this trial, we also compared the adjuvant efficacy of Montanide ISA 720 with Freund's adjuvant delivered with the FVO version of yP30P2MSP119. A. nancymai monkeys, four in each group, were immunized with 250 μg of yP30P2MSP119 delivered in Montanide ISA 720 by an immunization regimen similar to that followed with Freund's adjuvant. Control monkeys, four in each group, received TBV25H in Freund's adjuvant or Montanide ISA 720. Blood was drawn from each monkey on the day of each immunization and on the day of, and in most instances 4 weeks after, the parasite challenge. Serum samples were separated and stored frozen at −70°C until used.
CDC trial.
Six adjuvant formulations of yP30P2MSP119 vaccine were tested for the ability to induce protection in A. nancymai. All vaccines were formulated from the same lot of bulk antigen (lot 0195) produced by the DBR at WRAIR. Except as noted below, each group contained three animals, each of which received 0.8 ml of vaccine subcutaneously containing 200 μg of antigen IM on days 0, 28, and 56.
Alum-adsorbed vaccine was formulated for a phase I human trial by the DBR using aluminum hydroxide (Rehydragel LV) to contain antigen (200 μg/ml) and aluminum (800 μg/ml); 88% of the protein was bound to alum. The vaccine is designated yP30P2MSP119 FVO (lot 0245, BPR no. BPR-139-00). Animals received 1 ml of vaccine in each injection. Due to variation in alum and antigen concentrations discovered among other vials of the same lot after the study was completed, animals may have received more or less than the calculated dose. However, no variation in opacity among the vials used for the study was noted.
SBAS2-adjuvanted vaccine was formulated at SmithKline Beecham Pharmaceuticals by Natalie Garcon to contain 250 μg of antigen per ml of vaccine.
Montanide ISA 720-adjuvanted vaccine was formulated at the Laboratory of Parasitic Diseases, NIH, by David Kaslow to contain 250 μg of antigen per ml of vaccine. It was emulsified by 10× expulsion through a 21-gauge needle and stored under nitrogen.
Vaccine adjuvanted with lipid A (Salmonella enterica serovar Minnesota R595) in liposomes was formulated in the Department of Membrane Biochemistry at the WRAIR by Roberta Owens to contain 250 μg of antigen per ml of vaccine.
Polyphosphazine-adjuvanted vaccine was formulated at the Avant Immunotherapeutics to contain 250 μg of antigen per ml of vaccine.
Vaccine in microspheres with granulocyte-macrophage colony-stimulating factor was formulated at the Immunex by Dean Pettet and at the Laboratory of Parasitic Diseases, NIH, by David Kaslow, immediately before administration. Animals were given a single immunization subcutaneously.
Parasite challenge.
The FVO strain of P. falciparum was used to challenge Aotus monkeys. In all three studies, the challenge inoculum was obtained by infection of naive monkeys inoculated with a frozen stock of FVO parasites. Aliquots of the same frozen stabilate were used for NIH trial 2 and the CDC trial. In all the trials, 2 weeks after the third immunization, monkeys were challenged by the intravenous route with 104 P. falciparum parasitized RBCs with the exception of the group of monkeys immunized with the yP30P2MSP119 microsphere formulation (CDC trial). In this group, monkeys were challenged 58 days after a single-dose immunization. Three days after the challenge infection, blood smears were taken daily and parasitemias were quantified following staining with Giemsa stain and counting with the help of light microscopy. Immunized Aotus monkeys that required treatment with mefloquine to control their parasitemia were considered as nonprotected. The criterion for treatment was if the parasitemia in the challenged monkeys exceeded 5% or the hematocrit dropped below 25%. Monkeys were treated with 50 mg of mefloquine at the NIH facility and at with 20 mg of mefloquine and 50 mg of quinine sulfate at the CDC facility.
Serologic assays. (i) ELISA.
For ELISA, 96-well microtiter plates were coated with 0.1 μg of yMSP119 per 0.1 ml in each well. The plates were incubated overnight at 4°C, washed three times with PBS–0.05% Tween 20, and then blocked with PBS–1% bovine serum albumin (BSA) for 1 h at 37°C. Dilutions of test sera were added to wells and incubated for 1 h at 37°C. Following washings, alkaline phosphatase conjugated anti-human immunoglobulin (IgG; Promega) was added to wells as secondary antibody and incubated for 1 h at 37°C. After washings, solubilized phosphatase tablets (Sigma Chemical Co., St. Louis, Mo.) were added as substrate. Following incubation at room temperature, the plates were read at an absorbance of 410 nm.
Indirect immunofluorescence (IIF).
P. falciparum (FVO) parasites, obtained directly from infected Aotus monkeys or from in vitro cultures, were air dried on toxoplasmosis slides (Bellco Glass, Vineland, N.J.) and fixed with ice-cold methanol for 15 min before use. The slides were washed with PBS and then blocked with PBS–3% BSA for 1 h at room temperature. A 1:100 dilution of Aotus sera in PBS–1% BSA was preabsorbed with washed human RBCs for 1 h at room temperature. The preabsorbed sera were added to the wells and incubated overnight at 4°C in a humid chamber. After washing with PBS, fluorescein isothiocyanate-conjugated goat anti-human IgG (Cappel, West Chester, Pa.) at a 1:200 dilution in PBS was added to wells and incubated for 2 h at room temperature in the dark. After washings, slides were mounted with a coverslip using Fluoromont solution (Southern Biotechnology) and were read by using a fluorescent light microscope.
MSP1 secondary processing inhibition by Aotus sera.
The effects of the Aotus sera on secondary processing of MSP1 on the surface of intact merozoites were analyzed using a modification of the assay described previously (3). Naturally released merozoites of the P. falciparum T9/96 clone were isolated (1) and washed twice in serum-free RPMI 1640 medium. Washed merozoites were resuspended in ice-cold 50 mM Tris-HCl (pH 7.5) containing 10 mM CaCl2 and 2 mM MgCl2, supplemented with protease inhibitors antipain, leupeptin, and aprotinin at 10 μg/ml and tosyl-l-lysyl choromethyl ketone (TLCK) at 74 μg/ml (reaction buffer). Aliquots of approximately 109 merozoites were dispensed into 1.5-ml Eppendorf tubes on ice in 18 μl of reaction buffer. Each aliquot was supplemented with 2 μl of serum. Control samples were supplemented with reaction buffer only or with phenylmethylsulfonyl fluoride (1 mM, final concentration), which is a potent inhibitor of the processing activity. Samples were mixed, incubated on ice for 15 min, and then centrifuged at 12,000 × g at 4°C for 10 min. Half of the supernatant was removed and then the merozoites were transferred to 37°C for 1 h for processing to proceed. Samples were then solubilized by the addition of 20 μl of 2× sodium dodecyl sulfate (SDS) sample buffer (nonreducing), heated, and clarified, and the extracts were analyzed by Western blotting using the anti-MSP-133 MAb X509 (4). Antibody binding was detected using horseradish peroxidase-conjugated goat anti-human IgG (Bio-Rad) and enhanced chemiluminescence (SuperSignal Western blotting substrate; Pierce). Sera were tested in two independent assays.
RESULTS
This study had two major objectives. First, we compared the protective efficacies of four different versions of rMSP119 vaccines. Second, we tested whether yP30P2MSP119 could induce protective immunity when delivered in adjuvant formulations suitable for use in humans.
Two of these recombinant proteins, yP30P2MSP119 and bGST-MSP119, have been described before (5, 19). The protective yP30P2MSP119 construct was expressed in S. cerevisiae as a histidine-tagged, secreted protein. The recombinant plasmid was designed to express MSP119 as a fusion with the P30 and P2 epitopes that contain 30 amino acid residues. However, the NH2-terminal sequencing of GMP-produced yP30P2MSP119 determined by automated Edman degradation (Biological Resource Branch, National Institute of Allergy and Infectious Diseases) revealed that the major product is truncated, with only three C-terminus residues of P2P30 sequences, EVE, remaining for lot 940826 and eight residues, FIGITEVE, for lot 0245. Thus, the resultant recombinant protein is heterogeneous, making it difficult to define the protective immunogen used for vaccination. As an approach to circumvent this problem, we fused three additional amino acids, EVE, at the NH2 terminus of MSP119, creating the construct yEVE-MSP119 expressed as a His6-tagged, secreted protein in S. cerevisiae. Analysis of yEVE-MSP119 by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and by Western blot analysis indicated the presence of a band of the predicted molecular weight (data not shown). Furthermore, automated Edman degradation amino acid sequencing of the NH2 terminus of the purified protein revealed that EVE-MSP119 was expressed as a full-length protein.
In this study, we also constructed and expressed the 3D7 version of yP30P2MSP119 as a His6-tagged fusion protein in S. cerevisiae in secreted form under GMPs (lot 0207). Analysis of yP30P2MSP119 3D7 by SDS-PAGE, Western blotting, and NH2-terminal sequencing reveal this protein to be truncated in the same manner as the FVO product, having FIGITEVE as its NH2 terminus.
The immunogenicity and efficacy of rMSP119 vaccines were determined in three independent trials in the P. falciparum-Aotus challenge model. Two of these trials were conducted at the NIH, and the third was done at the CDC.
NIH trial 1.
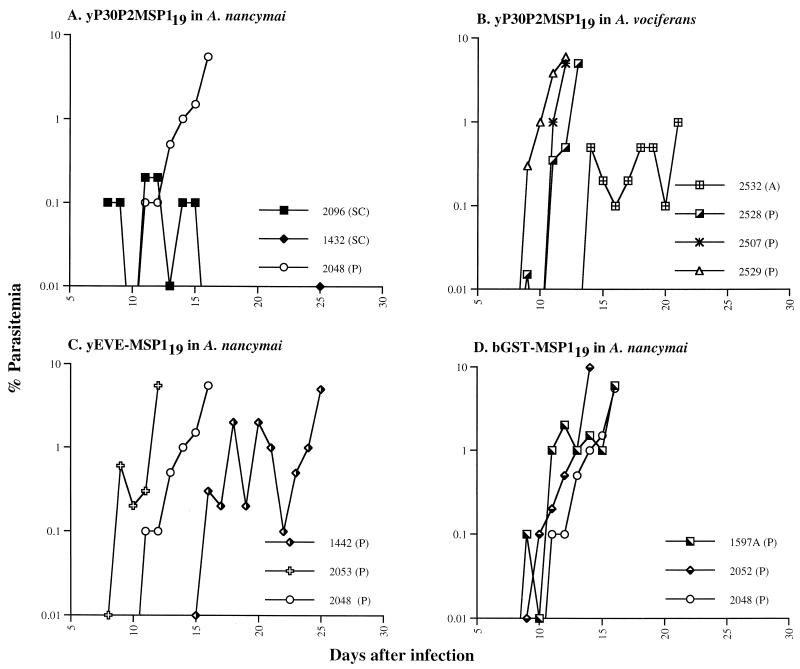
In this trial, vaccines representing the MSP119 sequence from the FVO strain of P. falciparum were compared in the P. falciparum-Aotus challenge model. Monkeys were stratified according to species and sex and randomly assigned to one of the following groups. Two controls, one A. nancymai and one A. vociferans, received CFA in the absence of any immunogen. Two A. nancymai and two A. vociferans monkeys received the yP30P2MSP119 FVO emulsified in CFA. In the other two groups, A. nancymai, two in each group, received yEVE-MSP119 or bGST-MSP119 vaccine emulsified in CFA. For the first immunization, the vaccines were emulsified in CFA; subsequent injections were given in IFA on days 21 and 42, and 2 weeks later animals were challenged with 104 parasitized RBCs of the FVO strain of P. falciparum. Following challenge infection, an infection control A. nancymai (2048), vaccinated with CFA alone, developed a peak parasitemia of 5.5% on day 16 postchallenge and received chemotherapy. Both of the A. nancymai monkeys vaccinated with yP30P2MSP119 (2096 and 1432) were able to resolve their infection after reaching a peak parasitemia of 0.2% (Fig. 1 and Table 1). In comparison, immunization with yEVE-MSP119 did not protect against FVO challenge, and both A. nancymai monkeys (1442 and 2053) required chemotherapy. The two A. nancymai monkeys (1597A and 2052) immunized with bGST-MSP119 were also not protected and required treatment by day 16. The courses of their parasitemia were very similar to that for the control A. nancymai 2048 (Fig. 1). Thus, in this trial, among the three rMSP119 vaccines tested, only yP30P2MSP119 was able to induce protective immunity in A. nancymai. In another group, two A. vociferans monkeys (2532 and 2528) immunized with yP30P2MSP119 in CFA were not protected. Upon challenge infection, one immunized A. vociferans 2528 had almost the same course of parasitemia as A. vociferans 2507, immunized with CFA alone, and unimmunized A. vociferans 2529. One immunized A. vociferans monkey, 2532, had a delayed prepatency and was treated on day 21 at 1.0% parasitemia for hematocrit below 25% (Fig. 1 and Table 1). Thus, none of the vaccinated A. vociferans monkeys were able to resolve their infection and required chemotherapy. This finding is consistent with our previous results showing that with the immunization schedule used in this study, the yP30P2MSP119-CFA formulation induced protection in A. nancymai but not in A. vociferans (19).
FIG. 1.
Parasitemia curves for infections of the FVO strain of P. falciparum in A. nancymai or A. vociferans monkeys immunized with yP30P2MSP119 (A and B), yEVE-MSP119 (C), or bGST-MSP119 (D). rMSP119 vaccines were delivered in Freund's adjuvant. SC, self-cleared infection; A, treated for severe anemia (hematocrit below 25%); P, treated for parasitemia above 5%.
TABLE 1.
NIH trial 1: antibody responses and outcome of P. falciparum challenge in Aotus monkeys immunized with rMSP119 vaccines in CFA
| Vaccine |
Aotus
|
Prepatent periodb | % Peak parasitemiac | Day of treatmentd | Outcome of infectione | IIFf | Estimated ELISA titerg
|
||
|---|---|---|---|---|---|---|---|---|---|
| No. | Speciesa | Pre | Post | ||||||
| yP30P2MSP119 | 2096 | N | 8 | 0.2 | None | Self-clear | 106 | 35,157 | 28,716 |
| 1432 | N | 25 | 0.01 | None | Self-clear | 106 | 35,610 | 44,670 | |
| 2532 | V | 14 | 1.0 | 21 (A) | Virulent | 106 | 26,420 | ND | |
| 2528 | V | 9 | 5.0 | 13 (P) | Virulent | 105 | 16,000 | 8,528 | |
| yEVE-MSP119 | 1442 | N | 15 | 5.0 | 25 (P) | Virulent | 106 | 4,815 | 11,084 |
| 2053 | N | 8 | 5.5 | 12 (P) | Virulent | 105 | 4,727 | 35,962 | |
| bGST-MSP119 | 1597A | N | 9 | 6.0 | 16 (P) | Virulent | 106 | 37,036 | 55,897 |
| 2052 | N | 9 | 9.9 | 14 (P) | Virulent | 106 | 59,859 | 51,222 | |
| CFA | 2048 | N | 11 | 5.5 | 16 (P) | Virulent | <102 | 12 | 52 |
| 2507 | V | 11 | 5.0 | 12 (P) | Virulent | ND | ND | ND | |
| Unimmunized | 2529 | V | 9 | 6.0 | 12 (P) | Virulent | ND | ND | ND |
N, A. nancymai; V, A. vociferans.
Day of the first appearance of detectable parasitemia after parasite challenge.
Highest percentage of infected RBCs observed following parasite challenge.
Day monkeys received treatment with mefloquine to cure parasitemia. (A), Treatment given for severe anemia (hematocrit below 25%); (P), treatment given for parasitemia above 25%.
Self-clear, infection cleared after reaching a low-grade parasitemia; virulent, above 5% parasitemia requiring treatment.
Reaction against the FVO strain of P. falciparum. Sera were taken on day 14 after the third immunization (the day of parasite challenge).
Determined at A410 of 0.5, using MSP119 QKNG as capture antigen. Pre, sera taken on the day of parasite challenge; Post, sera taken 4 weeks after the parasite challenge; ND, not determined.
Immunization with all three versions of rMSP119 vaccines induced high levels of anti-MSP119 IgG responses as measured by IIF and ELISA (Table 1). However, among the three MSP119 vaccines compared, yEVE-MSP119 induced the lowest IgG ELISA titers (Table 1). Following FVO challenge, only yEVE-MSP119-immunized monkeys showed boosted IgG responses; postchallenge sera from Aotus monkeys immunized with yP30P2MSP119 or bGST-MSP119 had no significant boosting in IgG titers. Interestingly, pre- or postchallenge IgG titers did not correlate with protective immunity.
Having established that immunization with yP30P2MSP119 in CFA is capable of inducing protective immunity in A. nancymai, we next wanted to (i) search for an alternative protective adjuvant formulation that is suitable for use in humans and (ii) to determine whether yP30P2MSP119 vaccine will protect against challenge with a heterologous strain of P. falciparum. To address these questions, we conducted two concurrent vaccine trials, one at the NIH (NIH trial 2) and the other at the CDC.
NIH trial 2.
The purposes of NIH trial 2 were to (i) compare the adjuvant efficacy of Montanide ISA 720 with CFA and (ii) determine the effect of heterologous parasite challenge on yP30P2MSP119 vaccine efficacy. A. nancymai monkeys, four in each group, were given three immunizations with either the FVO or 3D7 version of yP30P2MSP119 in CFA. Four control A. nancymai received TBV25H in CFA. In addition, four A. nancymai monkeys received yP30P2MSP119 FVO in Montanide ISA 720 and four control A. nancymai monkeys were immunized with TBV25H in Montanide ISA 720. The regimen of immunizations with these vaccines was similar to that described for the NIH trial 1.
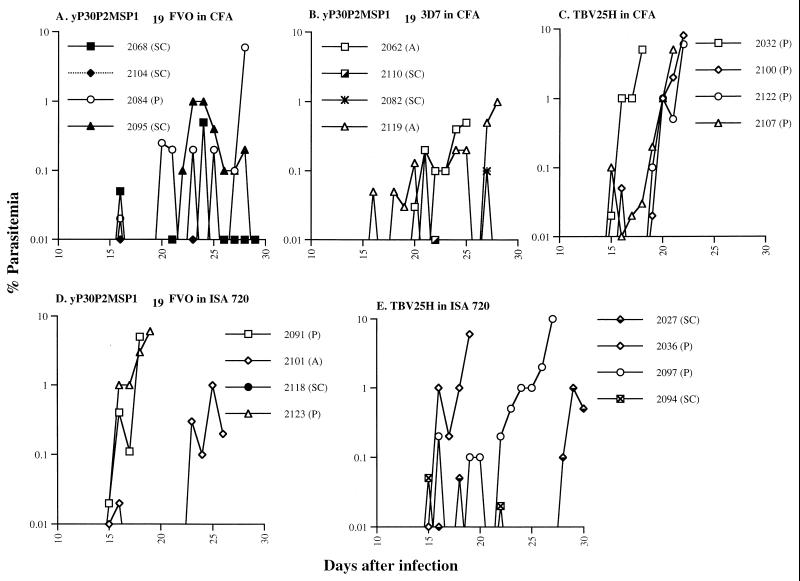
Following FVO challenge, four control A. nancymai monkeys (2032, 2100, 2122, and 2107) immunized with TBV25H in CFA became parasitemic and required chemotherapy on or before day 22 (Fig. 2). Three (2068, 2104, and 2095) of the four A. nancymai monkeys immunized with yP30P2MSP119 FVO in CFA were protected after reaching peaks parasitemia of 0.5, 0.1, and 1%, respectively. A. nancymai 2084 could not control its infection and received treatment on day 28 (Fig. 2 and Table 2). In comparison, two of the four A. nancymai monkeys (2062, 2110, 2082, and 2119) immunized with yP30P2MSP119 3D7 were protected; A. nancymai 2110 and 2082 self-resolved their infection after reaching peak parasitemias of 0.1 and 0.2%, respectively (Fig. 2). A. nancymai monkeys 2062 and 2119 received chemotherapy at low parasitemias of 0.5 and 1%, respectively (Fig. 2 and Table 2), because of severe anemia (hematocrit below 25%), suggesting that rMSP119 vaccine induced partial protection against heterologous parasite challenge.
FIG. 2.
Parasitemia curves for infections of the FVO strain of P. falciparum in A. nancymai monkeys immunized with the FVO or 3D7 version of yP30P2MSP119 or TBV25H in CFA (A to C) and yP30P2MSP119 or TBV25H in Montanide ISA 720 (D and E). SC, self-cleared infection; A, treated for severe anemia (hematocrit below 25%); P, treated for parasitemia above 5%.
TABLE 2.
NIH trial 2: antibody responses and the outcome of P. falciparum challenge in A. nancymai immunized with the 3D7 or FVO version of yP30P2MSP119 in CFA or Montanide ISA 720
| Vaccine | Aotus no. | Prepatent perioda | % Peak parasitemiab | Day of treatmentc | Outcome of infectiond | IIFe | Estimated ELISA titerf
|
|
|---|---|---|---|---|---|---|---|---|
| Pre | Post | |||||||
| yP30P2MSP119 | 2068 | 16 | 0.5 | None | Self-clear | 106 | 15,205 | 24,658 |
| FVO in CFA | 2104 | 16 | 0.1 | None | Self-clear | 106 | 58,155 | 68,384 |
| 2084 | 16 | 6.0 | 28 (P) | Virulent | 105 | 16,292 | 8,750 | |
| 2095 | 22 | 1.0 | None | Self-clear | 106 | 2,407 | 2,137 | |
| yP30P2MSP119 | 2062 | 20 | 0.5 | 25 (A) | Virulent | 106 | 19,499 | 38,249 |
| 3D7 in CFA | 2110 | 22 | 0.1 | None | Self-clear | 105 | 33,101 | 29,279 |
| 2082 | 21 | 0.2 | None | Self-clear | 106 | 26,978 | 36,514 | |
| 2119 | 16 | 1.0 | 28 (A) | Virulent | 106 | 29,077 | 21,510 | |
| TBV25H in CFA | 2032 | 18 | 5.0 | 18 (P) | Virulent | <102 | 15 | 120 |
| 2100 | 22 | 8.0 | 22 (P) | Virulent | <102 | 24 | 28 | |
| 2122 | 22 | 6.0 | 22 (P) | Virulent | <102 | 12 | 26 | |
| 2107 | 21 | 5.0 | 27 (P) | Virulent | <102 | 8 | 22 | |
| yP30P2MSP119 | 2091 | 15 | 5.0 | 18 (P) | Virulent | 103 | 143 | 0 |
| FVO in Montanide ISA 720 | 2101 | 15 | 1.0 | 26 (A) | Virulent | 104 | 776 | 688 |
| 2118 | None | 0.0 | None | Self-clear | 103 | 3,493 | 1,911 | |
| 2123 | 15 | 6.0 | 19 (P) | Virulent | 103 | 3 | 3 | |
| TBV25H in Montanide ISA 720 | 2027 | 16 | 1.0 | None | Self-clear | <102 | ND | ND |
| 2036 | 15 | 6.0 | 19 (P) | Virulent | <102 | ND | ND | |
| 2097 | 16 | 10.0 | 27 (P) | Virulent | <102 | ND | ND | |
| 2094 | 15 | 0.05 | None | Self-clear | <102 | ND | ND | |
Day of the first appearance of detectable parasitemia after parasite challenge.
Highest percentage of infected RBCs observed following parasite challenge.
Day monkeys received treatment with mefloquine to cure parasitemia. (A), treatment given for severe anemia (hematocrit below 25%); (P), treatment given for parasitemia above 25%.
Self-clear, infection cleared after reaching a low-grade parasitemia; virulent, above 5% parasitemia requiring treatment.
Reaction against the FVO strain of P. falciparum. Sera were taken on day 14 after the third immunization (the day of parasite challenge).
Determined at A410 of 0.5 using yMSP119 QKNG as capture antigen. Pre, sera taken on the day of parasite challenge; Post, sera taken 4 weeks after the parasite challenge; ND, not determined.
Immunization with yP30P2MSP119 in Montanide ISA 720 resulted in protection of only one of four A. nancymai monkeys. A. nancymai 2091 and 2123 developed parasitemias of 5 and 6%, respectively, and received chemotherapy. A. nancymai 2101 maintained low-grade parasitemia but failed to clear infection and had to be treated at 1.0% parasitemia on day 26 because its hematocrit dropped below 25%. A. nancymai 2118 was protected and had no detectable parasitemia for the 60-day observation period (Fig. 2 and Table 2). Among the four A. nancymai (2027, 2036, 2097, and 2094) immunized with the TBV25H control vaccine in ISA 720, two (2036 and 2097) of four developed high parasitemia and received chemotherapy on or before day 27 (Fig. 2). A. nancymai monkeys 2027 and 2094 self-cleared their infection after reaching peak parasitemias of 1 and 0.05%, respectively (Fig. 2 and Table 2). These data suggest that Montanide ISA 720 has some nonspecific protective effect against erythrocyte-stage malaria and is less potent than Freund's adjuvant in its ability to induce MSP119-specific protective immune responses.
There were no significant differences in the IgG titers of the sera from A. nancymai immunized with either the 3D7 or FVO version of yP30P2MSP119 in CFA. More significantly, the protected and nonprotected A. nancymai had similar IgG titers (Table 2). However, immunization with yP30P2MSP119 in Montanide ISA 720 induced substantially lower antibody titers (Table 2), suggesting that CFA is more potent in inducing anti-MSP119 antibody responses. No significant boosting in MSP1-specific ELISA IgG titers were seen in the Aotus sera immunized with either vaccine-adjuvant formulation following parasite challenge (Table 2).
CDC trial.
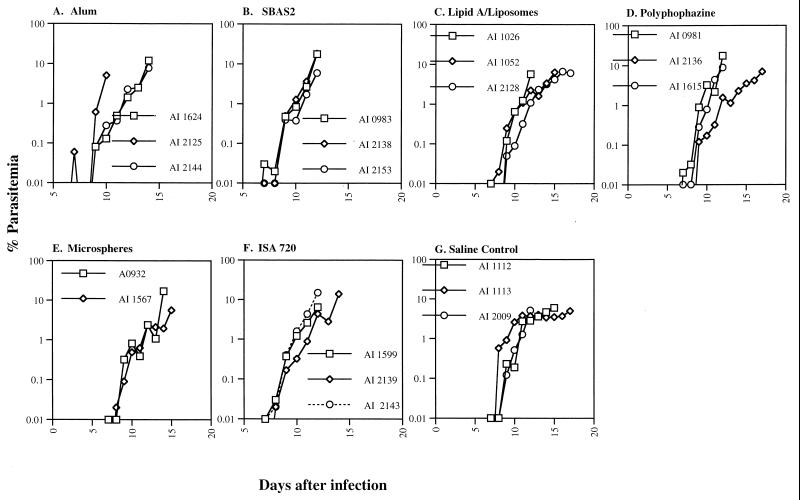
This trial was conducted in an attempt to find a potent adjuvant comparable to CFA in its ability to induce protective immunity when delivered with rMSP119. With this aim, the following six adjuvants were tested: alum, SBAS2, Montanide ISA 720, lipid A/liposomes, polyphosphazine, and microspheres. A. nancymai monkeys were immunized with yP30P2MSP119 FVO formulated in each of these adjuvants. Control A. nancymai monkeys received normal saline injection. Each group included three A. nancymai monkeys with the exception of microsphere-vaccine formulation group, which had two. Upon FVO challenge, the three control A. nancymai monkeys (AI1112, AI1113, and AI2009) developed patent infections by day 7 and were treated on or before day 18. In the vaccinated groups, none of the A. nancymai monkeys immunized with yP30P2MSP119 delivered in either of the six adjuvant formulations were protected (Fig. 3). The course of parasitemias in the immunized A. nancymai were essentially similar to that in the control A. nancymai and required chemotherapy on or before day 17 after the challenge infection (Fig. 3). Thus, it appears that none of the six adjuvants tested were capable of inducing a MSP119-specific protective immune response in the P. falciparum-Aotus challenge model.
FIG. 3.
Parasitemia curves for infections of the FVO strain of P. falciparum in A. nancymai monkeys immunized with yP30P2MSP119 delivered in alum (A), SBAS2 (B), lipid A/liposomes (C), polyphosphazine (D), microspheres (E), or Montanide ISA 720 (F). (G) normal saline without antigen.
Immunization with these yP30P2MSP119-adjuvant formulations induced moderate to low levels of anti-MSP119 ELISA and IIF IgG responses (Table 3). In general, these responses were significantly lower than for rMSP119-CFA vaccine formulations (Tables 1 and 2 versus 3).
TABLE 3.
CDC trial: antibody responses in A. nancymai immunized with the yP30P2MSP119 FVO vaccine delivered in six non-Freund's adjuvants that are suitable for use in humansa
| Vaccine formulation | Aotus no. | IIFb | Estimated ELISA titerc
|
|
|---|---|---|---|---|
| Pre | Post | |||
| yP30P2MSP119 in: | ||||
| Alum | AI1624 | 103 | 156 | 256 |
| AI2125 | 10 | 550 | 662 | |
| AI2144 | 102 | 258 | 826 | |
| SBAS2 | AI2138 | 105 | 3,918 | 16,780 |
| AI2153 | 104 | 1,658 | 4,678 | |
| AI0983 | 103 | 2,407 | 2,137 | |
| Lipid A/liposomes | AI1026 | 103 | 145 | 1,555 |
| AI1052 | 104 | 579 | 5,504 | |
| AI2128 | 105 | 606 | 6,056 | |
| Polyphazine | AI0981 | 103 | 422 | 881 |
| AI1615 | 104 | 647 | 742 | |
| AI2136 | 105 | 1,000 | 3,440 | |
| Microspheres | AI0932 | 103 | 6,439 | ND |
| AI1567 | 105 | 584 | 3,926 | |
| Montanide ISA 720 | AI1599 | 103 | 1,319 | 2,339 |
| AI2139 | 105 | 1,044 | 2,000 | |
| AI2143 | 103 | |||
| Saline control | AI1112 | <10 | 23 | 38 |
| AI1113 | <10 | 28 | 36 | |
| AI2009 | <10 | 46 | 58 | |
Following P. falciparum (FVO) challenge, all monkeys in this trial reached a parasitemia of more than 5% and required treatment.
Reaction against the FVO strain of P. falciparum. Sera were taken on the day of parasite challenge.
Determined at A410 of 0.5 using yMSP119 QKNG as capture antigen. Pre, sera taken on the day of parasite challenge; Post, sera taken 4 weeks after the parasite challenge; ND, not determined.
Correlation between MSP1 processing inhibition and protection.
To investigate the mechanism(s) of protective immunity, sera from protected and nonprotected Aotus were tested for the ability to inhibit the secondary processing of MSP142 on P. falciparum merozoites in a semiquantitative MSP1 processing inhibition assay (13). Four levels of inhibition were observed: just detectable, significant, strong, and apparently complete. Sera from Aotus monkeys vaccinated with three different rMSP119 vaccines (yP30P2MSP119 3D7 and FVO; bGST-MSP119FVO) delivered in Freund's adjuvant were tested in two independent assays.
Prechallenge sera from six protected (1432, 2096, 2068, 2104, 2110, and 2082) and three nonprotected (2084, 2119, and 2062) A. nancymai monkeys vaccinated with the yP30P2MSP119 immunogen had apparently complete or strong inhibitory effect in at least one secondary processing inhibition assay (Table 4). Serum from one protected yP30P2MSP119-vaccinated A. nancymai monkey (2095) had no significant inhibition of processing. Sera from two nonprotected yP30P2MSP119-vaccinated A. vociferans monkeys (2528 and 2532) significantly inhibited MSP142 processing. Both of the A. nancymai monkeys (1597A and 2052) vaccinated with bGST-MSP119 had apparently complete processing inhibition although neither was protected (Table 4). No processing-inhibitory activity was detected in any sera from animals immunized with TBV25H. Processing inhibition directly correlated with ELISA and IIF titers in the sera of vaccinated Aotus monkeys. Sera that had anti-MSP119 ELISA titers of more than 16,000 (determined at an optical density of 0.5 at A410) or endpoint IIF titer of 106 showed apparently complete or strong inhibition of MSP142 processing (Table 4).
TABLE 4.
Effect of Aotus sera on the inhibition of the secondary processing of MSP1 and its relationship between antibody response and protection
| Vaccinea |
Aotus
|
MSP1 processing inhibitionc
|
IIFd | Estimated ELISA titere | ||
|---|---|---|---|---|---|---|
| No. | Speciesc | Assay 1 | Assay 2 | |||
| yP30P2MSP119 FVO | 1432b | N | ++(+) | ++(+) | 106 | 35,610 |
| 2096b | N | +++ | +++ | 106 | 35,157 | |
| 2068b | N | ++ | +++ | 106 | 15,205 | |
| 2104b | N | +++ | +++ | 106 | 58,155 | |
| 2084 | N | + | ++ | 105 | 16,292 | |
| 2095b | N | +/− | +/− | 106 | 2,407 | |
| 2532 | V | + | ++ | 106 | 26,420 | |
| 2528 | V | + | + | 105 | 16,000 | |
| yP30P2MSP119 3D7 | 2062 | N | ++ | +++ | 106 | 19,499 |
| 2110b | N | ++ | ++ | 105 | 33,101 | |
| 2082 | N | +++ | +++ | 106 | 26,978 | |
| 2119 | N | +++ | +++ | 106 | 29,077 | |
| bGST-MSP119 | 1597A | N | +++ | +++ | 106 | 37,036 |
| 2052 | N | +++ | +++ | 106 | 59,859 | |
| TBV25H | 2032 | N | +/− | − | <102 | 15 |
| 2100 | N | − | − | <102 | 24 | |
| 2122 | N | − | − | <102 | 12 | |
| 2107 | N | <102 | 8 | |||
rMSP119 or control TBV25H vaccine was delivered in CFA. Sera used for these assays were taken on day 14 after the third immunization (the day of parasite challenge).
Self-cleared infection.
N, A. nancymai; V, A. vociferans. −, no detectable processing inhibition; +/−, just detectable processing inhibition; +, significant processing inhibition; ++, strong processing inhibition; +++, apparently complete processing inhibition; ++(+),
Endpoint IIF titer against the FVO strain of P. falciparum parasites.
Determined at A410 of 0.5 using yMSP119 QKNG as capture antigen.
DISCUSSION
We have previously demonstrated that vaccination with the yeast-produced 19-kDa carboxyl terminus of MSP1 fused to two universal helper T-cell epitopes of tetanus toxoid emulsified in CFA protected A. nancymai against the virulent FVO strain of P. falciparum (19). This raised the prospect of a MSP1-based vaccine that will protect humans against erythrocyte-stage malaria parasites. However, several issues remained to be resolved to guide vaccine formulation for human use. First, the major immunogen used for the vaccination was not full length but consisted of cleaved products that contained only three to eight amino acid residues from the tetanus toxoid sequences. Second, the protective formulation included CFA, which is not suitable for use in humans. Third, it is not clear whether a MSP119-based vaccine that contains sequence representing a single MSP1 allele will protect humans in the field, where infection with parasites having one or more allelic forms of MSP1 is common.
In this study, we tested the immunogenicity and in vivo protective efficacy of four rMSP119 constructs in the P. falciparum-Aotus model. All rMSP119 vaccines when delivered in CFA were highly immunogenic and, with the exception of yEVE-MSP119 vaccine, induced comparable levels of antibody responses (Tables 1 and 2). In NIH trial 1, among the three rMSP119 vaccines, yP30P2MSP119, yEVE-MSP119, and bGST-MSP119, only yP30P2MSP119 protected A. nancymai against FVO challenge (Fig. 1 and 2). Together, in two trials reported here, five of six A. nancymai monkeys self-resolved their infection without antimalarial chemotherapy. yEVE-MSP119 and bGST-MSP119 had no detectable protective effect. Furthermore, under similar vaccination conditions, yeast-produced nonfused MSP119 also failed to induce protective immunity in A. nancymai against FVO challenge (A. Egan and D. C. Kaslow, unpublished data). Previously, vaccination with bGST-MSP119 adjuvanted with liposomes and alum failed to protect A. nancymai against virulent FVO challenge (6). In this context, it is important to mention that in the P. yoelii murine model, both bGST-MSP119 (10, 24) and yeast-produced nonfused MSP119 (yPyMSP119) (14) induced immunity in mice against lethal parasite challenge, although immunization with yPyMSP119 induced a greater degree of protection (Chun and D. C. Kaslow, unpublished data).
It is not clear why yP30P2MSP119 truncated to EVE-MSP119 (19) but not nontruncated yEVE-MSP119 (this report) induces protection. It is possible that the minor fraction of purified full-length protein present in the immunogen contributed to immunity. Another, more likely possibility is that prior to cleavage, the P30P2 sequences influenced the conformation of rMSP119. Three lines of evidence suggest a complex relationship between conformational folding of MSP119 and protective immunity. First, MAbs that inhibit parasite growth in vitro recognize epitopes that are reduction sensitive (2). Second, alkylation abolishes the protective effect of P. yoelii bGST-MSP119 (20). Third, reduced MSP1 gave better T-cell responses than nonreduced in parasite-exposed populations (12).
Using the three-dose immunization regimen described, yP30P2MSP119 in Freund's adjuvant formulation failed to protect A. vociferans monkeys. This result is in agreement with our previous study in which three vaccinations with yP30P2MSP119 in Freund's adjuvant did not protect A. vociferans, suggesting that immunity is host species dependent (19). However, A. Egan et al. recently found (12a) that a fourth intramuscular injection with immunogen in the absence of adjuvant protected A. vociferans. Whether or not an rMSP119-based vaccine will induce protective immune responses in a HLA allele-diverse human populations remains to be seen.
The allelic diversity of MSP119 raises a question about the effectiveness of an MSP119-based vaccine encoding only one allelic form. In this study, both the FVO and 3D7 versions of yP30P2MSP119 induced high levels of IIF and ELISA titers in A. nancymai (Table 2). Nonetheless, monkeys that received vaccine from 3D7 sequences appeared to have a slightly lower degree of protective immunity against FVO parasites (Fig. 2). The effect that MSP1 sequence diversity will have on the outcome of an MSP1-based vaccine in the field is not known. It is plausible that it may be necessary to incorporate MSP1 sequences from multiple parasite clones that are prevalent in the area where the vaccine is administered.
In three separate trials (reference 19 and the present study), we have shown that immunization with yP30P2MSP119 when delivered in Freund's adjuvant protected seven of eight A. nancymai monkeys against virulent P. falciparum challenge. Finding a protective adjuvant formulation that is suitable for human use still remains a formidable challenge. With this goal in mind, we formulated the protective yP30P2MSP119 immunogen in six different adjuvants and compared their levels of immunogenicity and protective efficacy in A. nancymai. Compared to Freund's adjuvant, all of these adjuvants suitable for human use induced lower levels of anti-MSP119 antibodies as measured by ELISA and against asexual stage P. falciparum parasites (Tables 1 and 2 versus 3). Among the six adjuvants tested, MSP119-alum induced the lowest levels of antibody response (Table 3). Although none of the non-Freund's adjuvant-vaccine formulations protected A. nancymai against FVO challenge, it remains to be seen whether these rMSP1-non-Freund's adjuvant formulations will be able to induce protective immune responses in humans, especially those previously exposed to malaria parasites.
The mechanism of MSP1-induced immunity in the P. falciparum-Aotus model is still unclear. In our previous study, prechallenge sera from MSP119 vaccinated Aotus monkeys that survived challenge with virulent FVO failed to block parasite growth in vitro, suggesting that neutralizing antibodies may not be the only mechanism of immunity (19). Blackman and colleagues have demonstrated that protective anti-MSP119 MAbs that inhibit merozoite invasion also prevent the secondary processing of MSP1 on the surface of extracellular merozoites at or around the time of invasion (3). On the other hand, some MAbs that have no effect on parasite invasion (nonprotective) negate the effect of antibodies that inhibit MSP1 processing (3, 13). In the present study, the majority of the sera from the rMSP119-vaccinated monkeys effectively inhibited the secondary processing of MSP1, but their inhibitory effect had no correlation with protective immunity (Table 4). Sera from both protected and nonprotected Aotus monkeys had similar inhibitory effects, suggesting that the presence of these antibodies may not be sufficient to control parasitemia. Thus, the mechanism(s) of MSP1-induced immunity in the Aotus model remains largely unclear. In our studies (this report [NIH trials 1 and 2] and reference 19), there were no obvious differences in the prepatency (the time of the first appearance of parasitemia) between the protected and nonprotected monkeys. This suggests that antibodies present at the time of challenge that block the merozoite invasion were not the primary mediator of immunity and other factors contributed to parasite clearance. In the P. yoelii-murine model, the mechanism of anti-MSP119 immunity has been shown to be antibody dependent. In these studies, prechallenge sera from vaccinated mice transfer protection into naive recipients (11, 24; Chun and Kaslow, unpublished data). The sera from immune mice had predominantly IgG1 subclass, a product of the Th2-type response (14, 24).
An rMSP119 vaccine that will successfully protect humans against P. falciparum malaria may require the use of a potent non-Freund's adjuvant-based formulation. Research is in progress in several laboratories to search for an alternative adjuvant(s) that would induce protective immune responses in humans when delivered with rMSP1 or other malaria vaccine candidate antigens.
ACKNOWLEDGMENTS
Numerous people are gratefully acknowledged for support and assistance: Ginny Price for providing the yeast expression plasmid; Ken Eckles, Brian Bell, and Jay Wood for production of clinical-grade vaccine; David Haynes and Kathy Moch for providing FVO parasites for immunofluorescence assays; Trevor Jones for help with statistical analysis; Cherise Fenton, Charles Hatcherson, JoAnn Sullivan, and Carla Morris for Aotus work; and Louis Miller for scientific interaction and support. Special thanks are due to our collaborators who gave access to adjuvants and formulated experimental vaccines: Joe Cohen at SmithKline Beecham; Bryan Roberts at Avant Immunotherapeutics; Jean Pierre Planchot at Seppic, Inc.; Dean Pettit at Immunex; and Carl Alving and Robyn Owens at the Walter Reed Army Institute of Research. We thank Traci Callahan for production of the final version of the manuscript.
REFERENCES
- 1.Blackman M J. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell Biol. 1994;45:213–220. doi: 10.1016/s0091-679x(08)61853-1. [DOI] [PubMed] [Google Scholar]
- 2.Blackman M J, Heidrich H G, Donachie S, McBride J S, Holder A A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackman M J, Whittle H, Holder A A. Processing of the Plasmodium falciparum merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 5.Burghaus P A, Holder A A. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Burghaus P A, Wellde B T, Hall T, Richards R L, Egan A F, Riley E M, Ballou W R, Holder A A. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect Immun. 1996;64:3614–3619. doi: 10.1128/iai.64.9.3614-3619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns J M, Jr, Parke L A, Daly T M, Cavacini L A, Long C A. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malaria parasite Plasmodium yoelii. J Immunol. 1989;143:2670–2676. [PubMed] [Google Scholar]
- 8.Chang S P, Gibson H L, Lee-Ng C T, Barr P J, Hui G S N. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- 9.Chang S P, Case S E, Gosnell W L, Hashimoto A, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee-Ng C T, Barr P J, Yokota B T, Hui G N S. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly T M, Long C A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 12.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognization of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Egan A F, Blackman M J, Kaslow D C. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive, -exposed, and/or -rechallenged Aotus vociferans monkeys. Infect Immun. 2000;68:1418–1427. doi: 10.1128/iai.68.3.1418-1427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guevara Patiño J A, Holder A A, McBride J S, Blackman M J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J Exp Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirunpetcharat C, Tian J H, Kaslow D C, Rooijen N V, Kumar S, Berzofsky J A, Miller L H, Good M F. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 15.Holder A A. The precursors to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- 16.Holder A A, Freeman R R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 17.Kaslow D C, Shiloach J. Production, purification and immunogenicity of malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. BioTechnology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow D C, Hui G, Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP119) variants secreted from Saccharomyces cerevisiae. Mol Biochem Parasitol. 1994;63:283–289. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Yadava A, Keister D B, Tian J H, Ohl M, Perdue-Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 20.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 21.Price V, Mochizuki D, March C J, Cosman D, Deeley M C, Klinke R, Clevenger W, Gillis S, Baker P, Urdal D. Expression, purification and characterization of recombinant murine granulocyte-macrophage-colony-stimulating factor and bovine interleukin-2 from yeast. Gene. 1987;55:287–293. doi: 10.1016/0378-1119(87)90288-5. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui W A, Tam L Q, Kramer K J, Hui G S N, Case S E, Yamaga K M, Chang S-P, Chan E B, Kan S C. Merozoite surface coat precursor completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian J H, Kumar S, Kaslow D C, Miller L H. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian J H, Miller L H, Kaslow D C, Ahlers J, Good M F, Alling D W, Berzofsfy J A, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]