Abstract
In October 2020, KDIGO (Kidney Disease: Improving Global Outcomes) published its first clinical practice guideline directed specifically to the care of patients with diabetes and chronic kidney disease (CKD). This commentary presents the views of the KDOQI (Kidney Disease Outcomes Quality Initiative) work group for diabetes in CKD, convened by the National Kidney Foundation to provide an independent expert perspective on the new guideline. The KDOQI work group believes that the KDIGO guideline takes a major step forward in clarifying glycemic targets and use of specific antihyperglycemic agents in diabetes and CKD. The purpose of this commentary is to carry forward the conversation regarding optimization of care for patients with diabetes and CKD. Recent developments for prevention of CKD progression and cardiovascular events in people with diabetes and CKD, particularly related to sodium/glucose cotransporter 2 (SGLT2) inhibitors, have filled a longstanding gap in nephrology’s approach to the care of persons with diabetes and CKD. The multifaceted benefits of SGLT2 inhibitors have facilitated interactions between nephrology, cardiology, endocrinology, and primary care, underscoring the need for innovative approaches to multidisciplinary care in these patients. We now have more interventions to slow kidney disease progression and prevent or delay kidney failure in patients with diabetes and kidney disease, but methods to streamline their implementation and overcome barriers in access to care, particularly cost, are essential to ensuring all patients may benefit.
Introduction
In October of 2020, KDIGO (Kidney Disease: Improving Global Outcomes) published its first clinical practice guideline dedicated to the management of diabetes in chronic kidney disease (CKD) since the initial KDOQI (Kidney Disease Outcomes Quality Initiative) publication in 2007.1,2 The nearly decade-long gap between guideline updates reflects the prior scarcity of novel therapies for patients with diabetes and CKD. The emergence of new and highly efficacious drug treatments beneficial to patients with diabetes and CKD were reason enough to update existing guidelines, but this new guideline goes well beyond an expansion of therapeutic options. As conveyed by the title of the guideline, which refers to diabetes management in CKD, KDIGO not only covers glycemia and blood pressure targets, but also provides recommendations on nutrition, exercise, and self-management, underscoring the complexities and unique challenges in caring for the patient with diabetes and CKD. The commentary discusses the importance of multidisciplinary care models to include primary care providers, nephrologists, cardiologists, diabetologists, nurses, dietitians, and social workers in forming a holistic team approach for treating CKD. The emphasis on multidisciplinary care models reflects a shift in the US health care system toward more collaborative multidisciplinary care, represented by the emergence of “accountable care organizations” and “value-based care.” Indeed, the KDIGO recommendations are timely for this transformative period in health care.
It is important to highlight that the KDIGO Guideline uses the term “diabetes and CKD,” rather than “diabetic kidney disease (DKD)” or “diabetic nephropathy,” which historically referred to patients with longstanding diabetes and overt proteinuria (urinary albumin-creatinine ratio [UACR] ≥300 mg/g). The KDOQI work group agrees that use of “diabetes and CKD” reflects “the current clinical approach of treating most presentations of diabetes and CKD similarly and avoids the connotation that CKD is caused by traditional diabetes physiology.”2 Some are concerned, however, that “diabetes and CKD” may be imprecise and raise confusion at this time when nephrology clinical practice and research enters the world of precision medicine. While DKD is the culmination of multiple and incompletely defined disease processes that differ among individuals, it is not “diabetes and autosomal-dominant polycystic kidney disease,” nor is it “diabetes and immunoglobulin A nephropathy,” to name 2 examples. The diagnosis of DKD connotes a broad but relatively conserved set of molecular and pathologic changes that have been confirmed by multiple genome-wide transcriptomic and other “omic” analyses,3–7 which present in clinically recognized patterns and result in a relatively limited set of clinical and renal structural alterations. Given the prevalence of diabetes, it is not unusual for patients to have clinical and pathologic amalgams of DKD and other (nondiabetic) CKDs, especially in patients with unusual clinical presentations. The preference of our workgroup would be to state that the KDIGO guideline pertains to patients with “DKD,” which would include all patients in whom the clinical, pathologic, and molecular analyses support the diabetic milieu as the etiology of CKD. This management guideline may also apply to other patients with nondiabetic CKD occurring in the context of diabetes, but those decisions will require careful consideration of the underlying kidney and systemic disease processes. Nevertheless, to maintain consistency with the KDIGO guideline, this commentary will use the terminology “diabetes and CKD” throughout the document.
This KDIGO guideline has also taken a new approach to guideline format, moving away from guideline “statements” with varying strengths of evidence (including ungraded statements). Guideline statements have been replaced by “recommendations,” which continue to be graded based on the current evidence, and “practice points,” defined as guidance to be interpreted and applied according to the given clinical scenario. Moreover, the guideline is rendered not only as text, but also as tables, figures, and algorithms, to be succinct in their format. The KDOQI work group supports the adoption of this new format, which represents a 20-year trend8,9 toward adopting a uniform set of standards intended to facilitate easy comparisons between guidelines from different societies as well as rapid updates.
Review and Approval Process for the KDOQI Commentary
The KDOQI leaders selected cochairs, who then identified individuals with expertise in clinical care and research related to diabetes and CKD and invited them to participate in this KDOQI commentary. The cochairs then divided the commentary workgroup members into groups of 3-4 individuals (2 leads and 1-2 reviewers) to provide a detailed commentary on each section of the KDIGO guideline. The commentaries were then assembled into a single document, with discussion via teleconference to address areas without unanimous agreement, and reviewed and edited by the full KDOQI work group. The final document was reviewed and approved by the KDOQI leadership and the National Kidney Foundation (NKF) Scientific Advisory Board.
For each section of the guideline that is highlighted herein, the text is organized into subsections providing a general commentary (including context for the recommendation, the extent of the KDOQI work group’s agreement with the recommendation, and any areas not taken into consideration by KDIGO), followed by discussion of clinical utility, implementation, and challenges. This commentary also discusses topics relevant to patients with diabetes and CKD that were not addressed in the KDIGO guideline—for example, the care of diabetes and CKD in adolescents and young adults. In addition, suggestions are made regarding issues that need more research or were felt to be important to be included in policy decisions. All guideline materials are reproduced with permission of KDIGO.
Guideline Statements and Commentary
Comprehensive Diabetes and CKD Management
The first chapter of the KDIGO guideline begins with an important practice point, the introduction of the concept of comprehensive care for the successful management of CKD in patients with type 1 and type 2 diabetes with emphasis on the multidisciplinary aspects of reducing kidney and cardiovascular risk. The KDIGO work group makes a point to not weigh in on aspects of care that are covered in other guidelines within KDIGO or other professional organizations but addresses the multisystem complications that impact patients with diabetes and CKD. Specifically, they call out the importance of antiplatelet therapies primarily for secondary prevention of cardiovascular events. The benefits of multifactorial intervention are reviewed and care models are more explicitly addressed in the fifth chapter.
Commentary
The KDOQI work group agrees there is great complexity in caring for persons living with diabetes and CKD and agree with the need for multidisciplinary care involving primary care providers and multiple related subspecialties, as well as the important roles of other patient care team members, including educators, dietitians, pharmacists, laboratory technicians, and family members. This strategy has now been endorsed by several professional organizations, including in the 2021 American Diabetes Association (ADA) Standards of Medical Care,10 the 2019 ADA–European Association for the Study of Diabetes (EASD) Consensus,11 the 2020 American College of Cardiology (ACC) Expert Consensus Decision Pathway,12 and the American Association of Clinical Endocrinology (AACE) and American College of Endocrinology (ACE) Consensus Statement.13 These organizations support the need for multidisciplinary approaches that promote lifestyle changes as the foundation for disease management in patients with diabetes and all severities of CKD, as well as those receiving kidney replacement therapy (KRT), including dialysis and kidney transplantation.2,14 This concept is illustrated in Fig 1 (adapted from the KDIGO guideline), wherein more specific therapies are targeted to patients at higher risk for CKD progression and cardiovascular events.
Figure 1.
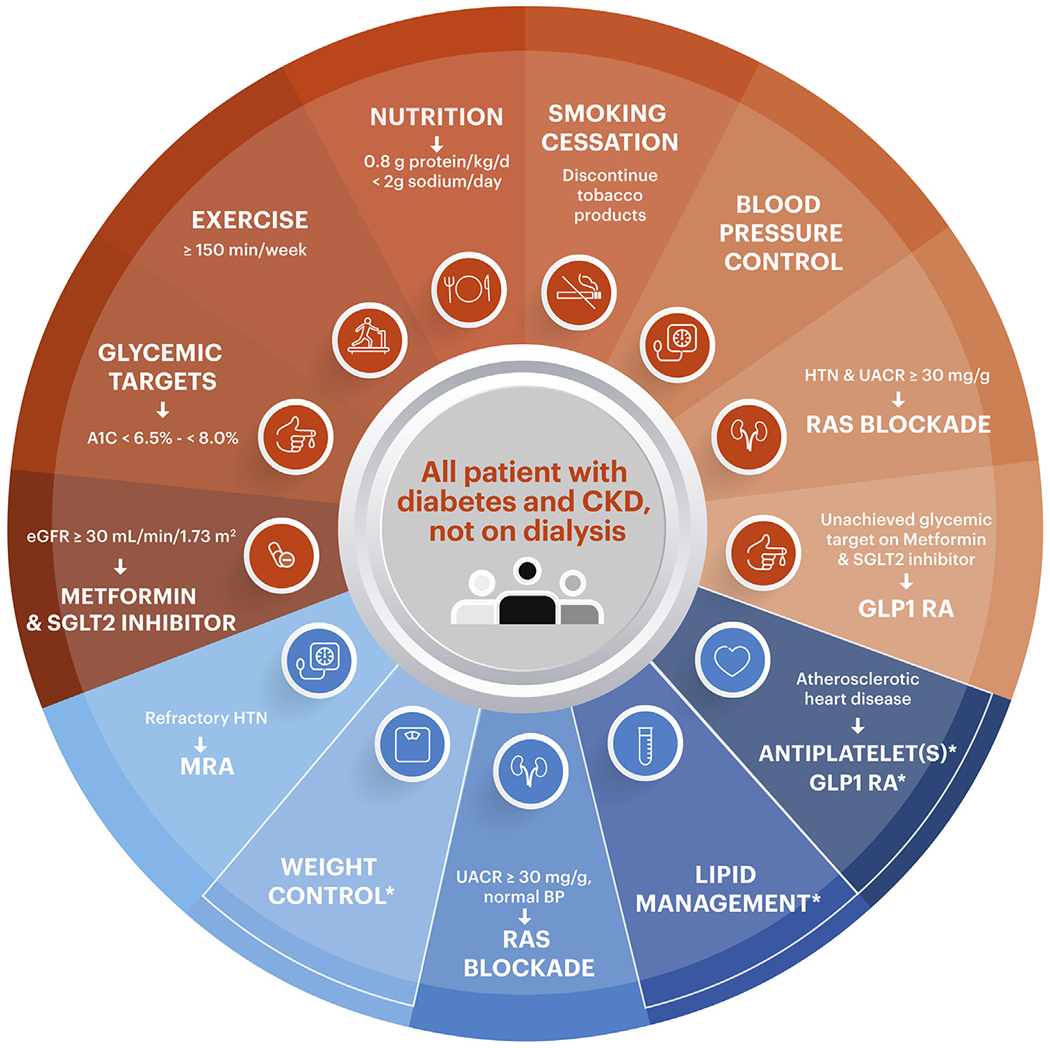
Schematic for comprehensive care of the patient with type 2 diabetes and CKD. Recommendations from KDIGO (Kidney Disease: Improving Global Outcomes) are represented in orange. Practice points from KDIGO are represented in blue. Asterisk refers to recommendations not specifically addressed by the original schematic in the KDIGO guidelines but deemed important worthy of inclusion by the KDOQI work group. Abbreviations: CKD, chronic kidney disease; HTN, hypertension; GLP-1 RA, glucagon-like peptide 1 receptor agonist; RAS, renin-angiotensin system; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium/glucose transporter 2; UACR, urinary albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; BP, blood pressure.
The issue of CKD care in children and adolescents with diabetes was not addressed by the KDIGO work group. The incidences of both type 1 and type 2 diabetes are increasing among children and adolescents,15 resulting in the growing problem of microvascular complications in youth and young adults.16–20 It is likely that many general pediatricians and nephrologists may be unaware of the rising prevalence of CKD risk factors among children and adolescents, and lack of screening and aggressive management heightens their lifetime risks of CKD due to the potential longer exposure to the diabetic milieu. To address this, programs and clinical trials directed at multidisciplinary care and transition of care for younger patients with diabetes and CKD could include patients still under the care of pediatric practitioners who often follow their patients into early adulthood.
Weight loss was not specifically addressed by the KDIGO guideline. The obesity pandemic has translated to approximately 44% of patients with CKD having obesity.21 Moreover, obesity exacerbates major risk factors for CKD progression, including glycemic and blood pressure control, and is likely itself a mediating factor for CKD pathogenesis.22–24 Obesity also complicates treatment options for kidney failure, such as suitability for transplantation and feasibility of arteriovenous fistula placement.25 Weight management is recommended by the ADA, and the KDOQI work group suggests that nephrologists be trained in lifestyle and medical therapies for mitigation of obesity in diabetes and CKD.2,10
Clinical Utility
Interdisciplinary care models and shared decision-making structures embracing effective, individualized education have demonstrated improved outcomes including reduction of cardiovascular events, decreased hospitalization rates, and lower risk of all-cause mortality, especially in advanced CKD (stages 4-5).26–28 There are significant racial and ethnic differences in the burden of diabetes and CKD in children, adolescents, and adults.15,29–31 The early institution of lifestyle changes in these and other at-risk groups that include moderate to vigorous exercise for 60 minutes per day, limiting computer screen time to no more than 2 hours per day, and dietary referrals are important consideration, and were proved to be effective in younger age groups.32,33
Implementation and Challenges
Despite the documented benefits of advancing patient education and multidisciplinary care for patients with diabetes and CKD, the current US health care system and its payment structure may not fully facilitate these practices.34 Most patients receive fragmented care from clinicians in different locations and health systems, and communication between providers remains poor.35–37 The growing therapeutic choices for prevention of diabetes complications may aggravate this problem, further underscoring the importance of patient-centered, unified, multidisciplinary approaches. Moreover, communication barriers are greatest for the most marginalized populations, who often suffer the worst health outcomes.38 While advances in electronic health records and portals for interprovider and patient-provider connections have improved communications, these tools are often not available to patients with low educational attainment and/or income or those residing in remote/rural areas. Consideration of regional infrastructure, involvement of community health workers,39,40 and improvements in practical, cultural, and financial incentives could help advance widespread and impactful achievements in this forum. Further complicating the delivery of comprehensive care to patients with diabetes is the universally poor implementation of clinical practice guideline recommendations based on scientifically proven clinical interventions to reduce morbidity.41 In the United States, while only 12%-39% of patients with CKD are receiving angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) therapy,42,43 up to 25% receive medications that are potentially harmful, such as the use of nonsteroidal anti-inflammatory drugs or proton pump inhibitors.43 Some of the recognized barriers can be differentiated into education (lack of provider awareness and familiarity with the guidelines and their recommendations), guideline-related (guideline complexity, layout, accessibility, and applicability), and external factors (organizational constraints such as lack of standardization of processes and procedures, time restrictions, heavy workload, and cost).44 Another significant barrier is low patient awareness.45 The net result of poor CKD awareness, limited education, as well as personal, financial, and institutional barriers, is the low understanding of the health risks associated with CKD, poor prioritization of CKD management, and reduced adherence to recommended care.46,47
The use of “high points” and condensed versions of lengthy practice recommendations tailored for busy clinicians may improve their dissemination, acceptance, and implementation. To reduce potential conflicts between professional societies (such as the American Society of Nephrology, NKF, American Heart Association, ADA, and American College of Physicians), it is important to make a concerted effort to harmonize clinical practice standards. The incorporation of decision support algorithms with a quick reference guide for the treatment of CKD into the electronic medical record system has demonstrated the potential to provide further support for primary care providers.48
Ideally, centralized multidisciplinary outpatient clinics offering ready access to nephrologists, cardiologists, endocrinologists, and other relevant specialty providers for patients with diabetes and CKD will provide more focused, efficient, and integrated clinical care than the current siloed care that contributes to poor outcomes. While creation of more ideal models remains aspirational in many countries and regions, a more achievable approach is the use of digital consult platforms, which may be particularly feasible and needed in rural settings where subspecialists may be physically distanced. This approach was demonstrated in a 1-year pilot study that compared digital consult access to nephrology care by primary care physicians versus traditional referral processes. The iKinect Project, comprising a network of 160 virtual community primary care physicians, demonstrated that digital consults resulted in improved care delivery, enhanced patient experiences, reduced nephrology care gaps, and greater health care utilization.49 In summary, a collaborative effort to change policies governing health care and innovative modifications to the processes of health care delivery may provide a path toward reductions in barriers to implementation of recommended approaches.
Renin-Angiotensin System Blockade
Recommendation 1.2.1: We recommend that treatment with an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB) be initiated in patients with diabetes, hypertension, and albuminuria, and that these medications be titrated to the highest approved dose that is tolerated (1B).
Commentary and Clinical Utility
The bulk of this chapter is dedicated to the therapeutic use and implications of renin-angiotensin system (RAS) inhibitors. Current KDIGO recommendations specifically endorse the initiation and subsequent titration of RAS inhibitors (ACEIs or ARBs) to maximally tolerated doses in patients with diabetes, hypertension, and increased albuminuria to achieve at least a 30% reduction in albuminuria, as this may be associated with lower CKD progression and risk of kidney failure.50 Notably, the quality of evidence from randomized controlled trials (RCTs) on the effect of ACEIs compared to either placebo or standard of care on critical clinical outcomes (all-cause mortality, progression of moderately to severely increased albuminuria, and doubling of serum creatinine) from systematic review was considered as moderate.51 The KDOQI work group agrees that, based on available evidence, ACEIs and ARBs are well tolerated and are indicated to slow kidney function decline in patients with diabetes, hypertension, and persistent mild/moderate or severe albuminuria. However, the evidence for preservation of kidney function in albuminuric patients with well-preserved baseline kidney function is quite limited. The use of ACEIs and ARBs in the setting of albuminuria but normal blood pressure was suggested as a “consideration” in a practice point, possibly due to lack of obvious benefit.52 However, ACEI/ARB use in patients with hypertension and estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 but without albuminuria appears to be of little or no benefit over blood pressure control with other agents. Both the KDIGO and ADA guidelines discourage RAS inhibition in normoalbuminuric, normotensive patients with diabetes.2,53
KDIGO applies the recommendation of ACEI/ARB use in people with diabetes and CKD to kidney transplant recipients. ACEI/ARB use in kidney transplant recipients at large has not been found to improve life or graft survival, even among people with proteinuria54; however, no studies have specifically addressed this question in transplant patients with diabetes and albuminuria. The KDOQI work group agrees that, while there are no data for or against ACEI/ARB use in this population, it is reasonable to use RAS blockade in kidney transplant recipients with diabetes and hypertension and albuminuria. KDIGO also specified that ACEI/ARB use is not indicated in patients on dialysis except as antihypertensives, and we agree with this sentiment.
Several practice points are offered to troubleshoot potential adverse effects with the use of ACEIs/ARBs. Practice point 1.2.6, which suggests that ACEIs or ARBs should only be reduced or discontinued as a last resort in patients with hyperkalemia, is supported by several studies. Discontinuation of ACEIs/ARBs in progressive kidney disease is a clinical dilemma being addressed in the STOP-ACEi RCT.55 While awaiting outcomes of this trial, a recent retrospective cohort study found a higher risk of death and the composite outcome of cardiovascular death, myocardial infarction, and ischemic stroke after discontinuation of ACEI or ARB therapy in individuals who reached eGFR <30 mL/min/1.73 m2.56 Studies addressing another clinical practice dilemma, continuation of RAS blockade after acute kidney injury, demonstrated that patients who continued to receive RAS blockade after an acute kidney injury event had a lower risk of death at 1 and 2 years after hospitalization.56,57 However, continuation of ACEIs/ARBs was associated with more hospital admissions for kidney causes, pointing to the need for close monitoring.56 Since RAS inhibitors are associated with an increased risk of hyperkalemia, monitoring for hyperkalemia within 1-2 weeks after initiation remains appropriate. Furthermore, although serum creatinine concentrations often increase acutely with RAS inhibitor initiation, an initial increase of ≤30% followed by stabilization within 2 months is associated with long-term preservation of kidney function.58 Therefore, every effort to maintain adequate dosing of RAS inhibitors, even in individuals more likely to develop hyperkalemia, could be useful. An approach to maintain adequate RAS inhibition may be facilitated by dietary restrictions and/or potassium binders as outlined in the KDIGO guideline. The guideline also notes that the concurrent use of ACEIs and ARBs or inclusion of direct renin inhibitors in conjunction with an ACEI or ARB increases adverse outcomes and should be avoided, and the KDOQI work group agrees with this.
The use of mineralocorticoid receptor antagonists (MRAs) for patients with diabetes and CKD is mentioned briefly in a practice point. It focuses primarily on their use in resistant hypertension, while acknowledging their antiproteinuric qualities. In several studies, an MRA significantly reduced albuminuria/proteinuria in patients with diabetes and CKD stages 1-4 who were already receiving an ACEI/ARB.59 However, KDIGO placed little emphasis on this drug class due to the lack of long-term clinical trials demonstrating efficacy in cardiac or renal outcomes. The KDOQI work group agreed that MRAs are certainly advantageous in the setting of resistant hypertension60 and to reduce proteinuria in some circumstances such as nephrotic-range proteinuria.61
Since the publication of the KDIGO guideline, clinical trial data have emerged demonstrating the benefits of the novel nonsteroidal MRA finerenone on primary renal outcomes. The FIDELIO-DKD study demonstrated that, in people with type 2 diabetes and albuminuric CKD treated with finerenone over 2.6 years, there was a slowed progression of CKD (a composite of kidney failure, sustained decrease in eGFR of at least 40%, or death from kidney failure), with a hazard ratio (HR) of 0.86 (95% CI, 0.75-0.99).62 The availability of another drug class to treat diabetes and CKD provides optimism for the future of this patient population, and we await consideration of this new drug class in future KDIGO updates.
Implementation and Challenges
Although ACEIs and ARBs have remained the mainstay of first-line therapy for patients with diabetes and CKD, observational studies have shown that their use in the United States is suboptimal.42,43 While not specific to patients with diabetes, data from the National Health and Nutrition Epidemiology Survey (NHANES) showed that only 39% of people with UACR ≥30 mg/g were receiving an ACEI or ARB.63 In contrast, data from Europe suggest that 75%-88% of patients with UACR ≥30 mg/g were receiving this important therapy.64 The basis for such poor uptake of ACEI/ARB therapy in patients with albuminuric CKD in the United States is unclear and would benefit from further research.
Smoking Cessation
Recommendation 1.3.1: We recommend advising patients with diabetes and CKD who use tobacco to quit using tobacco products (1D).
Commentary and Clinical Utility
The endorsement of smoking cessation and avoidance of tobacco products in patients with diabetes and CKD was deemed a strong recommendation based on smoking as a strong risk factor for development and progression of CKD.65,66 While there is a lack of direct evidence to support the strategy in this specific patient group, the plethora of evidence for the totality of the population makes this a highly useful recommendation. Of note, the recommendation is also supported by the AACE, ACE, ADA, and EASD.10,11,13
Glycemic Monitoring
Recommendation 2.1.1: We recommend hemoglobin A1C (HbA1c) to monitor glycemic control in patients with diabetes and CKD (1C).
Commentary
Glycemic monitoring in patients with diabetes and CKD has a well-defined role, ie, to balance the benefits of individualizing glycemic control while avoiding hypoglycemic events, which are associated with increased cardiovascular mortality in patients with diabetes and CKD.67 How to best monitor glycemia in people with type 2 diabetes and CKD remains controversial. The KDIGO guideline presents observational evidence as to the strength of correlation between tests that measure ambient glycemia (ie, HbA1c, glycated albumin, and fructosamine) with plasma glucose concentration, concluding that, although all correlations weaken as CKD progresses, HbA1c appears to correlate the best across the spectrum of CKD. Importantly, all measures of ambient glycemia are affected by the CKD environment. For example, glycated albumin will be falsely low (implying good glycemic control) when the serum albumin is <3 g/dL or when proteinuria is in the nephrotic range. Similarly, fructosamine levels will be biased in the presence of hypoalbuminemia. On the other hand, HbA1c levels will be affected by factors interfering with red cell turnover: worsening kidney function, anemia, transfusions, erythropoietin-stimulating agents, and iron supplementation, creating the potential for false negatives and, less frequently, false positives (eg, due to hemoglobin carbamylation). Continuous glucose monitoring (CGM) and self-monitoring of blood glucose (SMBG), which directly measure interstitial and blood glucose, provide measures that are not known to be affected by CKD and its treatment. A weakness of SMBG is that it may not detect the wide and often unpredictable glucose excursions common in patients with diabetes and CKD.68 However, the inconvenience and costs of these approaches are substantially higher than the other glycemic monitoring methods. Currently, there are no RCTs to recommend alternative measures of glycemic control over HbA1c in these patients.
Clinical Utility
Certification and assay standardization activities over the past 20 years have resulted in HbA1c measurements being mostly, but not universally, free of measurement bias when measured in the laboratory.69 Due to its low cost and availability, it is the preferred method for glycemic monitoring in CKD. It is reasonable to measure HbA1c twice a year in stable patients with diabetes and up to 4 times a year if the individualized glycemic target has not been met and/or therapy is adjusted (practice point 2.1.1). Accuracy and precision of HbA1c will decline with worsening kidney function (practice point 2.1.2),70,71 and thus one may miss substantial hyperglycemia or excessive hypoglycemia when eGFR declines below 30 mL/min/1.73 m2 or in patients receiving dialysis. Risk of hypoglycemia is particularly high in patients with diabetes and CKD who are treated with insulin regimens that involve multiple injections per day or with sulfonylureas.72 There is merit in considering and implementing direct assessment of capillary (SMBG) or interstitial (CGM) glucose in patients with discordant HbA1c and measured plasma glucose levels or those with frequent hypoglycemia (practice points 2.1.3 and 2.1.4).
Implementation and Challenges
Implementing the KDIGO guideline recommendation to use HbA1c to monitor glycemic control is straightforward: the test is widely available and can easily be added to a basic metabolic panel to provide a rough, individualized correlation with the prevailing level of plasma glucose. While the frequency of testing also appears to be straightforward, the declining performance of the HbA1c as kidney function worsens implies that one may need more frequent assessments, particularly in those individuals with rapidly declining kidney function.68 In such circumstances, as kidney function declines, so will HbA1c, creating a false sense of improving glycemic control. This appears particularly problematic in patients who have an apparent “acceptable” range of HbA1c between 6.5% and 8%. Neither glycated albumin nor fructosamine has been sufficiently validated to support their use over HbA1c in CKD.54 SMBG and CGM may offer distinct advantages in this population, especially when these devices are coupled with mobile apps or telemedicine that can identify patients at risk for hypoglycemia. However, SMBG may be inconvenient, CGMs are not currently approved for use in patients receiving dialysis, and insurance coverage or copays limit the wide deployment of these modalities.
Glycemic Targets
Recommendation 2.2.1: We recommend an individualized HbA1C target ranging from <6.5% to <8.0% in patients with diabetes and CKD not treated with dialysis [callout to guideline figure 9 omitted] (1C).
Commentary
Landmark trials have clearly demonstrated the benefits of glycemic control in diabetes for preventing microvascular disease.73–77 Nevertheless, the notion that a universal HbA1c goal was suitable for all patients with diabetes was challenged by a series of trials that showed either lack of benefit or increase in harm for macrovascular outcomes and mortality when strict HbA1c goals (<6.5%) were imposed.67,78,79 The increased rate of harm was particularly notable for people with diabetes and CKD, with 31% higher all-cause mortality and 41% higher cardiovascular mortality in those randomized to intensive glycemic control.80 Taken together, it has become evident that weighing risks and benefits for each patient supersede the pursuit of a set HbA1c value; hence, individualized HbA1c goals can be advantageous.
The KDIGO guideline presents evidence that an HbA1c goal <6.5% may be suitable for some patients with diabetes and CKD, whereas an HbA1c goal <8% may be appropriate for others. While they included kidney transplant patients in their recommendations, they clearly state that appropriate goals for HbA1c in dialysis patients are unknown, and the KDOQI work group agreed with this. KDIGO suggests lower HbA1c goals (<6.5%) among lower-risk patients with diabetes, such as early CKD stage 1-2, few other health issues, at low hypoglycemic risk, and with good support systems. A weakness of this strategy is the assumption that HbA1c is equally reflective of blood glucose in all individuals. However, there are factors outside of HbA1c that may vary between individuals beyond glycemia, such as red blood cell turnover and hemoglobin glycation, that are still not well understood.
Safe achievement of optimal HbA1c targets may be facilitated by CGM or SMBG and by selection of antihyperglycemic agents that are not associated with hypoglycemia (practice points 2.1.5 and 2.2.1). Patients with significant comorbidities (diabetes-related or otherwise) and/or limited life expectancy may be candidates for higher HbA1c (eg, <8%), especially if they are taking insulin, sulfonylureas, or glinides, in order to balance avoidance of hyper- and hypoglycemic crises. CGM metrics, such as time in the glycemic range and duration of hypoglycemia, may be considered as alternatives to HbA1c for defining glycemic targets for patients in whom individualized HbA1c has been deemed inadequate to prevent significant hypo- or hyperglycemia and/or their complications (practice points 2.1.6 and 2.2.2).
Clinical Utility
Standardization of the HbA1c assay has facilitated rapid uptake, avoiding the need for overnight fasting or oral glucose tolerance testing to assess glycemic control.81 Setting lower limits for HbA1c helps prevent hypoglycemic events,77 whereas use of CGM with low glucose alert settings can prevent hypoglycemia in real time. Setting upper limits for glucose control helps prevent complications, even with and including CKD.82 It is a central focus of the KDIGO guideline to remind readers that glucose control remains central when caring for patients with diabetes.
Implementation and Challenges
Implementing individualized HbA1c goals should be straightforward, based on recommendations from the KDIGO guideline. A critical first step is shared decision-making between the patient and his/her provider(s) as to what the HbA1c goal should be. A clear second step is in monitoring to determine whether or not the goal has been met, with subsequent discussion about ways to achieve the goal or change the goal. Education, activation, and involvement of patients in shared decision-making remains vital to diabetes care and achieving HbA1c goals.83 Primary care providers are most likely to be responsible for implementing individualized HbA1c goals early in the diabetes and CKD course. Referral to endocrinology or nephrology may be recommended, particularly for patients with type 1 diabetes, when the primary care provider deems the diabetes or diabetes-related CKD, respectively, to be poorly controlled or outside the scope of their expertise. Once care with an endocrinologist has been established, setting and seeking of the HbA1c goal is then the combined responsibility of the endocrinologist and primary care provider in partnership with the patient, and close communication among all providers is important. It may be valuable for nephrology to communicate the status of the patient’s kidney function, recent laboratory tests, and capillary glucose measures during dialysis when applicable. At that time, endocrinology may alter the HbA1c goal and/or recommend SMBG and/or CGM surveillance of glycemic control in conjunction with HbA1c if not already initiated.
Protein Intake
Recommendation 3.1.1: We suggest maintaining a protein intake of 0.8 g protein/kg (weight)/d for those with diabetes and CKD not treated with dialysis (2C).
Commentary
The 2012 KDIGO CKD guideline suggested maintaining a protein intake of 0.8 g/kg/d in adults with and without diabetes and eGFR <30 mL/min/ 1.73 m2.84 The 2020 KDIGO diabetes and CKD guideline expands this recommendation to all patients (regardless of eGFR) with CKD not receiving KRT and diabetes. Despite this expansion, the 2020 KDIGO guideline does not differ significantly from recommended protein intake for all adults by other organizations.85,86 In contrast, in 2020, KDOQI updated its clinical practice guideline for nutrition in CKD, which now recommends a slightly lower goal of 0.6-0.8 g/kg/d, specifically in patients with CKD stage 3-5 and diabetes (not on dialysis).87 The KDOQI work group felt the difference between a range of 0.6-0.8 versus 0.8 g/kg/d is unlikely to be statistically, clinically, or practically different (given the day-to-day variation in diet), and it is unlikely that trials to undertake this question will ever be done. The 0.8-g/kg/d definition of “moderate protein intake” derives from data in the general and CKD populations, which have found potential for malnutrition with lower protein intake and associations of higher protein intake with worsening CKD, overweight/obesity, and cardiovascular disease. Specific to diabetes and CKD is the observation that high-protein diets in a rodent model can exacerbate hyperfiltration and subsequent interstitial fibrosis, processes that are intrinsic to the natural history of diabetic glomerulosclerosis.88
Additional studies may be warranted to support the KDIGO recommendation for moderate protein intake in people with diabetes and CKD. Results of observational studies and clinical trials of protein restriction are highly variable, and it is difficult to draw definitive conclusions due to significant heterogeneity in study design, participant characteristics, definition of “low” protein diet, and study duration. Some of the variability in results may be related to whether the studies were conducted before or after ACEI/ARB use became standard of care in diabetes and CKD. There are data to suggest that protein restriction has similar mechanisms to RAS inhibition, and it has been hypothesized that protein restriction in combination with ACEIs/ARBs could have an additive beneficial effect, although this remains to be proven.89 Moreover, safety data for protein restriction is equivocal, as studies of protein restriction to <0.8 g/kg are sparse, with evaluation of nutritional status focusing on serum albumin or prealbumin, which are of questionable validity.2
Practice point 3.1.2 states that protein intake in patients with diabetes on dialysis may need to be somewhat higher (1.0-1.2 g/kg) than for nondialysis patients, which is consistent with the 2020 KDOQI nutrition guideline in CKD.87 While there is little hard evidence for this, the guideline arises from the knowledge of a higher risk for malnutrition in dialysis patients who frequently suffer low serum albumin levels, particularly those on peritoneal dialysis.2
In addition to the total intake of protein, there is increasing awareness of considering the origin and quality of protein, which influence its digestibility, advanced glycation end-product intake, and dietary acid load. The KDIGO guideline does not make a specific recommendation for increasing vegetable sources of protein due to the lack of clinical trials in this space. However, the guideline did reference its legitimacy in a practice point that is supported with observational data.2 The guideline suggests that patients with diabetes and CKD consume a diet high in vegetables, fruits, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, with lower intake of processed meats, but a detailed review of evidence for this practice point is not provided. In animal studies, intake of acid-inducing foods (rich in animal proteins) versus base-inducing foods (fruits and vegetables) is associated with kidney disease progression.90–92 In humans, vegetable and fruit supplementation in patients with advanced CKD may preserve eGFR.90,91 Notably, dietary patterns can be modified to prevent the onset of CKD.93,94 In a large community-based prospective cohort study, high intake of nonfermented vegetables significantly decreased the risk for incident proteinuria by 32% and the risk for incident eGFR <60 mL/min/1.73 m2 by 14% compared to the lowest intake during an average follow-up of 8 years.94 In another large population-based cohort study of health-related behaviors in the northern Netherlands, dietary patterns characterized by a high intake of eggs, dairy products, fruits, vegetables, and legumes and low intake of meat and sweets was associated independently with a lower risk of eGFR decline.93 Moreover, these studies suggest that health behaviors and health education should not remain confined to those who already developed CKD but should be part of the therapeutic approach in patients at risk for CKD.
Clinical Utility
As stated in the KDIGO guideline, there is little evidence for clinical utility of protein restriction in patients with diabetes and CKD. The most rigorous appraisal of the evidence was a 2009 Cochrane systematic review that concluded that restricting protein intake in patients with diabetes and CKD may slow progression to kidney failure, but to a nonsignificant degree.95 A more recent meta-analysis included a few additional trials and found heterogeneity by diabetes type, with the type 1 diabetes subgroup (as well as nondiabetic patients) seeing a greater benefit than the type 2 diabetes subgroup.96 The impact on mortality and kidney failure has only been evaluated by a single, small (N = 82) study in people with long-duration type 1 diabetes, but it did find a statistically significant benefit.97 While there is little evidence for significant benefit from moderating protein intake and switching to vegetable protein sources, there is also no harm imposed.2
Implementation and Challenges
Implementation of restricting protein in addition to carbohydrate, salt, and lipids while maintaining an adequate nutritional intake requires significant lifestyle changes that must be maintained indefinitely for optimal results. The complexity of such changes may overwhelm both clinicians, who need to be aware of various sources of macronutrients, and patients, who will navigate the availability, cost, and preparation when adopting these changes. The cost of following the KDIGO dietary recommendations is not insignificant,2 particularly when taken in the context of high prescription drug costs for patients with diabetes and CKD. Food insecurity is common, occurring in 12% of adults with diabetes, and is associated with worse HbA1c control and adherence to medications, indices important to kidney and cardiovascular health.98,99 There are very few data regarding food insecurity specific to the population with diabetes and CKD, an area that could benefit from greater attention. Moreover, the rapid pace inherent to US culture has led to the pervasive multi-billion-dollar fast food industry and “ready-made meals” that offer limited quantities of the recommended nutritional values100,101 that may be important to people living with diabetes and CKD. This obstacle will be difficult to overcome. However, a major, well-organized, well-financed, and unified approach from the medical community, similar to that taken in antismoking campaigns, may be helpful.
It is unknown what proportion of individuals with diabetes and CKD adhere to a moderate protein restriction and whether this has changed over time. Appropriately, the current KDIGO practice points recommend individualized nutrition education at diagnosis of diabetes and yearly nutrition education to help build self-management skills in patients with longstanding diabetes and CKD.102 While the KDOQI work group agrees with these suggestions, dietary counseling by registered dietitians, accredited nutrition providers, behavioral therapists, or diabetes education programs will incur additional health care costs that, in some countries, is covered by health care systems, while, in other countries, is shouldered by the patient. In the United States, for the approximately 34 million people with diabetes, there are 102,000-106,000 registered dietitian and dietitian nutritionists, a group whose composition lacks diversity in both sex and race/ethnicity.103 Notably, broadening the diversity of dietitians may be effective in reaching and impacting the population with diabetes and CKD, who are notably quite diverse.47 Moreover, less than 25% of this workforce is found in outpatient care centers, schools, residential care facilities, or physician offices,103 suggesting an insufficient and inefficient referral system. Much patient education is shifting online, where patients with diabetes can find tools and programs for lifestyle changes; however, these are rarely tailored for CKD and are inaccessible to people without access to the internet.
Sodium Intake
Recommendation 3.1.2: We suggest that sodium intake be <2 g of sodium per day (or <90 mmol of sodium per day, or <5 g of sodium chloride per day) in patients with diabetes and CKD (2C).
Commentary
This recommendation is unchanged from that of the 2012 KDIGO guideline for CKD of all causes84; the 2020 KDIGO diabetes and CKD guideline elaborates on the lack of studies specific to this patient group. Accordingly, the basis for this guideline is extrapolated from the general population or people with CKD in general. The updated 2020 KDOQI clinical practice guideline for nutrition in CKD recommends a slightly higher sodium allowance, up to 2.3 g (100 mmol) per day, only in patients with CKD stage 3 or higher, and indicated specific utility for not only blood pressure and volume control, but also to reduce proteinuria.87 The KDOQI work group felt that there is unlikely to be a clinically meaningful difference with a salt intake upper limit of 2 g versus 2.3 g per day. Furthermore, while salt reduction has clear short-term clinical benefits, studies on risks associated with long-term salt restriction particularly in diabetes and hypertension have not reported consistent findings,104,105 and the impact of salt restriction in patients taking a sodium/glucose cotransporter 2 (SGLT2) inhibitor has not been assessed. Thus, the relative risks and benefits have not been completely determined for this population.
Clinical Utility
RCTs have indicated that moderate salt restriction significantly reduces blood pressure, fluid overload, albuminuria, and cardiac hypertrophy in patients with CKD.106 The effects of sodium reduction are assumed to have greater importance in persons with than without CKD,106 as the former experience higher prevalence of hypertension, a major risk factor for worsening kidney and cardiovascular outcomes. Further, CKD is associated with increased sodium sensitivity and inadequate blood pressure regulation by the renin-angiotensin-aldosterone system, and, in advanced CKD and/or comorbid heart failure, volume overload from high salt is common, often necessitating hospitalization.107 Still, long-term effects on kidney outcomes and mortality remain unclear. Two meta-analyses published after February 2020,107,108 and therefore not included by the KDIGO work group, confirm the beneficial effects of lowering sodium intake starting from early stages of CKD, and suggest that moderate dietary salt restriction, defined as <2.5 g sodium per day, significantly reduces blood pressure and proteinuria with few adverse effects. Still, results are conflicting, and there is significant heterogeneity of study designs and follow-up time, and important shortcomings to measurement of salt intake. Controlled clinical trials with long follow-up time, pre-specified clinical end points, and sound statistical analyses for multifactorial causality may be valuable to define the optimal level of salt intake at which progression of CKD may be ameliorated in patients with diabetes.
Implementation and Challenges
The average sodium intake in the United States is 3,400 mg/d, well above the updated KDIGO recommendations. Of those encouraged to limit their sodium intake, virtually all exceed the recommended limit on a daily basis.109,110 A review of studies evaluating the effect of urinary sodium excretion on kidney failure and cardiovascular outcomes found that less than one third of those with CKD consumed <2 g per day.108 These studies indicate that implementation of low-sodium diets is challenging, particularly given the numerous other nutritional restrictions in patients with diabetes and CKD, including carbohydrates, protein, and, in more advanced CKD, potassium, magnesium, and phosphorus. Innovative approaches could be advantageous to ease patients’ control of their diets. Suggested methods for self-monitoring salt intake include urine chloride strips and web-based self-management programs, though the utility of these interventions is limited, depending on educational attainment, income, and access to technology. An online aid is available to clinicians for developing and implementing sodium reduction programs, policies, and initiatives aimed at lowering sodium intake.111
Physical Activity
Recommendation 3.2.1: We recommend that patients with diabetes and CKD be advised to undertake moderate-intensity physical activity for a cumulative duration of at least 150 minutes per week, or to a level compatible with their cardiovascular and physical tolerance (1D).
Commentary and Clinical Utility
Physical activity is a cornerstone of diabetes management, as it improves insulin sensitivity and management of hyperglycemia, blood pressure, hepatic steatosis, depression, and strength and mobility, adding disability-free years. This guideline recommendation is similar to the previous guidance on this issue in the 2012 KDIGO blood pressure and CKD guidelines,84,112 as well as the 2019 guideline from the American College of Cardiology and the American Heart Association on the primary prevention of cardiovascular diseases113 and the 2021 guideline from the ADA.114 Because of a paucity of reliable studies on the role of physical activity in patients with diabetes and CKD, this recommendation was largely derived from studies in the general population or those at high risk for cardiovascular disease that sometimes included relatively small numbers of participants with diabetes and CKD. The KDOQI work group agrees that the guideline is reasonably derived from evidence in other populations; however, additional studies may be warranted to support the KDIGO conclusion. Indeed, a recent meta-analysis of RCTs indicated that, while exercise training among nondialysis CKD patients improved physical and walking capacity, it had no significant effect on all-cause mortality and kidney function relative to usual care.115 The specific target of 150 minutes of moderate-intensity physical activity per week is poorly supported for patients with diabetes and CKD.115 The prevalence of disability is higher in people with CKD than without CKD, with 26%-31% reporting difficulties in lower-extremity mobility and general physical activity and limitations in their ability to work (22%) and in the type of work they perform (34%).116 Since light-intensity physical activity has been shown to improve outcomes in CKD patients and has not been shown to be inferior to moderate-intensity physical activity,117 the KDIGO guideline suggests light or moderate-intensity physical activity for patients with diabetes and CKD to a level compatible with their cardiovascular and physical tolerance.
Implementation and Challenges
Even though the long-term benefits of encouraging regular physical activity remain uncertain among patients with diabetes and CKD, a sedentary lifestyle increases cardiovascular risk,116 and therefore recommending any degree of regular physical activity can have important physical, psychological, and social benefits.118,119 Implementation of the recommendation for physical activity, with or without intentional weight loss, will rely on a multidisciplinary network of physicians and health care providers to assess baseline activity level, identify suitable physical activities, personalize lifestyle programs, and monitor the clinical effectiveness of interventions. It is therefore valuable to increase awareness of CKD and CKD-related lifestyle interventions among physicians, podiatrists, and advanced practice providers from multiple specialties.
The practice points developed by KDIGO are derived from the overall guideline and focus on its implementation. The focus on individualizing care in each of the practice points is based on limited data and is reasonable but leaves the practicing clinician without clear direction.2 Practice point 3.2.1 mentions patient ethnicity as a consideration in physical activity recommendations; in fact, there is no evidence to vary the amount of physical activity because of ethnic differences,2 and, as such, ethnicity should not be a factor in its determination. Further, patients with diabetes and CKD are at increased risk for physical activity–related adverse events, including falls, dehydration, hypoglycemia, and hypotension, that may offset the benefits of maintaining an active lifestyle. These events could be mitigated through self-management of and directed education about fluid consumption, glycemic control before and after physical activity, and foot care, among other issues.120
Metformin
Recommendation 4.1.1: We recommend treating patients with T2D, CKD, and an eGFR ≥30 mL/min per 1.73 m2 with metformin (1B).
Commentary
Metformin is an appropriate first-line agent for the treatment of type 2 diabetes because of its proven efficacy in improving glycemia, its cardiovascular benefits as shown in the UKPDS,121 long-term safety data,122 and its low cost.123 The KDIGO recommendation is in line with guidelines from the ADA124 and the AACE,125 which recommend metformin plus lifestyle modification as initial therapy. However, the European Society of Cardiology (ESC) recently recommended that a glucagon-like peptide 1 receptor agonist (GLP-1 RA) or an SGLT2 inhibitor be given instead of metformin as an initial agent in drug-naïve patients with high or very high cardiovascular risk, and that metformin be limited to those without evidence of cardiovascular disease.126 This ESC recommendation was based on several cardiovascular outcome trials (CVOTs) showing significant benefits of GLP-1 RAs and SLGT2 inhibitors, but, in fact, in all of these studies, the investigational drug was added to a base therapy with metformin.
Data supporting the choice of metformin as a first-line agent come from several studies spanning over 2 decades. In addition to the cardiovascular benefit of metformin demonstrated by the UKPDS,121 a retrospective cohort study of Korean patients with type 2 diabetes and stage 3 CKD showed metformin to decrease all-cause mortality as well as progression to kidney failure.127 Likewise, 2 systematic reviews and meta-analyses also showed decreased progression to kidney failure and reduced all-cause mortality with metformin use in patients with diabetes and CKD stage 3.128,129
The KDIGO guideline discusses the change in the Federal Drug Administration (FDA) warning regarding the risk for lactic acidosis and mentions the recommendation that metformin can be used safely without an increased risk of lactic acidosis down to an eGFR of 30 mL/min/1.73 m2.130,131 Additional more recent large studies showing a lack of risk for lactic acidosis above this eGFR cutoff now support this recommendation.45,127,130 While the FDA does not explicitly state that dose adjustments are required above an eGFR of 30 mL/min/1.73 m2, we agree with the KDIGO guideline, which offers a practice point to halve the dose for an eGFR <45 mL/min/1.73 m2. Not mentioned by KDIGO is the FDA recommendation that metformin not be initiated in people with an eGFR <45 mL/min/1.73 m2, based upon the notion that these patients are likely to progress to an eGFR <30 mL/min/1.73 m2, wherein metformin would be contraindicated. KDIGO does, however, provide a suggestion to reduce the dose of metformin, even when the eGFR is 45-59 mL/min/1.73 m2, in the presence of conditions that predispose patients to hypoperfusion and hypoxemia. These transient conditions generally are seen in the inpatient setting.2 Other inpatient factors that could cause problems include radiology dye-induced acute kidney injury and sepsis. We agree with the recommendation of the ADA and the AACE that metformin should be discontinued when most patients are admitted to the hospital because of their increased risks for these conditions that also increase the risk for lactic acidosis.132
Clinical Utility
The KDIGO guideline includes several practice points that are particularly useful and worth reiterating. These include (1) the use of metformin in kidney transplant patients with the same eGFR cut point of 30 mL/min/1.73 m2,133–135 (2) the suggestion for more than annual monitoring of kidney function when patients reach CKD stage 3 because of the possibility of a more rapid fall in eGFR possibly necessitating modification of metformin dosing,2 (3) a reduction in dosing of metformin to 1,000 mg/d when the eGFR falls to <45 mL/min/1.73 m2,2 and (4) the need to monitor for vitamin B12 deficiency with use of metformin for more than 4 years.136,137
While the KDOQI work group agrees with these statements, it should be noted that the KDIGO practice point regarding the need to reduce the dose of metformin to a maximum of 1,000 mg/d when the eGFR is <45 mL/min/1.73 m2 is based upon expert opinion rather than being data-driven. It may be important to determine from existing databases and prospective studies whether such a recommendation is actually needed.
The KDIGO practice point about the use of metformin in transplant patients is based primarily upon registry and pharmacy claims data and 1 small prospective study. Additional data from large, prospective studies may be valuable to confirm the efficacy and safety of metformin in such patients.
Implementation and Challenges
Primary care and diabetes clinicians often prescribe metformin as the initial pharmacological therapy in patients with type 2 diabetes.138 The change in the FDA guideline from using serum creatinine levels to eGFR calculations with a cutoff of 30 mL/min/1.73 m2 may reduce the challenges for clinicians in determining when to stop metformin, as most laboratories now report the actual eGFR when it is <60 mL/min/1.73 m2.139 The KDIGO practice point about measuring the eGFR more frequently than annually when the eGFR is <60 mL/min/1.73 m2 is reasonable.2 However, it may require education on the part of nonnephrology clinicians, highlighting the importance of concerted efforts to improve uptake of this guideline. Moreover, other antihyperglycemic agents also have caveats with respect to their use in CKD and transplant, issues often overlooked by all clinicians. Table 1 summarizes the salient features of antihyperglycemic agents not otherwise discussed by the KDIGO guideline and issues important to their use in patients with CKD.
Table 1.
Commonly Used Antihyperglycemic Agents Used for Type 2 Diabetes With HbA1c Above Target in the Setting of Current Use, Contraindication, or Intolerance With Combination Metformin, SGLT2 Inhibitors, and GLP-1 RA
| Antihyperglycemic Class | Mechanism (Range of HbA1c Change) | Risks/Drawbacks | Benefits | Factors to Consider in CKD/Transplant/Dialysis |
|---|---|---|---|---|
| Sulfonylureas (glipizide, glyburide, glimepiride, gliclazide) | Increased insulin secretion (0.8%-1.5%) | Hypoglycemia, weight gain | Inexpensive | - Glipizide and gliclazide are safer in CKD; not renally cleared, no active metabolites185,186 - Glyburide and glimepiride pose increased hypoglycemia risk - Glyburide and glimepiride should not be used with eGFR <60 and <30 mL/min/1.73 m2, respectively187,188 - Glyburide and glimepiride effectively increase cyclosporine levels; glipizide does not189,190 - Cotrimoxazole combined with glipizide increases risk for hypoglycemia191 |
| DPP-4 inhibitors (linagliptin, sitagliptin, saxagliptin, alogliptin) | Decreased breakdown of GLP-1 (0.5%-0.8%) | Do not use with GLP-1 RA | None | - Only linagliptin does not need dose adjustment in CKD192 - Cyclosporine and itraconazole increase saxagliptin levels, so dose should be reduced193 |
| Thiazolidinediones (pioglitazone) | Increased insulin sensitivity (0.5%-1.4%) | Fluid retention194; fracture risk194,195 | Inexpensive, decreased insulin needs | - No dose adjustments required188,196 - Safe in kidney transplantation197 |
| Glinides (nateglinide, repaglinide) | Rapid, short-lived insulin secretion with meals (0.5%-0.8%) | Hypoglycemia, weight gain | Reduces postprandial hyperglycemia | - Repaglinide safer in CKD; not renally cleared, but if eGFR <30 mL/min/1.73 m2, use with caution198,199 - Nateglinide has active metabolites that are renally cleared, so if eGFR <60 mL/min/1.73 m2, increased hypoglycemia risk198,199 - Nateglinide cleared by hemodialysis198 - Cyclosporine and itraconazole increase repaglinide levels200 |
| α-Glucosidase inhibitors (acarbose, miglitose) | Delays carbohydrate absorption in small intestine (0.5%-0.8%) | Flatulence, diarrhea | Reduces postprandial hyperglycemia | - Miglitol renally excreted, so if eGFR <60 mL/min/1.73 m2, not recommended186,201 - Acarbose minimally absorbed, but metabolites renally cleared, so if eGFR <30 mL/min/1.73 m2, not recommended186,201 |
| Insulin | Exogenous insulin | Hypoglycemia, injectable, weight gain | None, no dose limitations or ceiling on HbA1c reduction | - Renally metabolized and doses should be adjusted with decreasing eGFR, particularly rapid-acting insulin202,203 - Rapid-acting insulins given after meal may be helpful in patients with gastroparesis |
Abbreviations: CKD, chronic kidney disease; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; RA, receptor agonist; HbA1c, hemoglobin A1c.
SGLT2 Inhibitors
Recommendation 4.2.1: We recommend treating patients with T2D, CKD, and an eGFR ≥30 mL/min per 1.73 m2 with an SGLT2i (1A).
Commentary
The discovery of the cardiorenal protective effects of SGLT2 inhibitors has been a turning point for the treatment and prognosis of patients with type 2 diabetes and CKD. The magnitude of their mitigating effect on cardiorenal end points and general consistency of findings across SGLT2 inhibitor trials is a rarity in clinical research, and thus the scientific and academic communities have embraced this long-awaited innovation.11,14,126 It is important to mention that the CVOT with ertugliflozin was published soon after the KDIGO guideline became available and did not meet statistical significance for its primary cardiovascular or kidney outcomes.140 The effect of ertugliflozin on heart failure, however, was statistically significant and in line with other SGLT2 inhibitor CVOTs. Moreover, study design and outcome definitions differed between the trials, and post hoc analysis showed that ertugliflozin did lower the risk of the kidney outcome defined as a composite of sustained 40% decline from baseline eGFR, KRT, or kidney disease–related death (HR, 0 66; 95% CI, 0.50-0.88).141 Given this new information, the panel felt that, when initiating patients on therapy, providers should consider the beneficial class effect of SGLT2 inhibitors,142 the specific drug choices that are evidence-based, using where possible the most effective drug for the individual patient, according to trial eligibility criteria, trial design, and subgroup analyses outcomes.
The KDIGO guideline reviews the data demonstrating the cardiorenal benefits of SGLT2 inhibitors for type 2 diabetes, including (1) reduction in composite kidney end points of doubling of creatinine, kidney failure, and death from kidney failure and (2) reduction in composite major adverse cardiovascular outcomes of cardiovascular death, nonfatal myocardial infarction and stroke, and hospitalization for congestive heart failure. The impact of this drug class on cardiovascular as well as kidney outcomes is of particular importance to patients with type 2 diabetes and CKD given that the majority of people with type 2 diabetes and CKD are more likely to die of cardiovascular causes than to progress to advanced CKD or kidney failure.143 Furthermore, the notion that diabetes increases the risk of cardiovascular death has recently been found to be predominantly mediated by the presence of CKD.143,144
An important practice point underscores the lack of data for efficacy or safety in kidney transplant recipients, particularly given their immunosuppressed status and the potential increased risk for infection. There are, in fact, a few small studies suggesting that their safety is similar to that in the native CKD population. Kidney transplant patients suffer high rates of congestive heart failure,145 cardiovascular events,146 death with a functioning graft,147 and graft loss due to nonimmunologic causes (ie, graft CKD progression148). A clinical trial with sufficient power to address efficacy in this growing population could be highly impactful.
Notably, KDIGO does not mention albuminuria as a criterion in their recommendation for use of SGLT2 inhibitors in type 2 diabetes and CKD. The rationale for this omission is not specifically addressed, but may be based on a benefit in kidney outcomes even in the SGLT2 inhibitor CVOTs, in which roughly half of participants had normoalbuminuria and another 30% had moderate albuminuria.149 Additionally, a post hoc analysis of the CANVAS-R trial analyzed eGFR slope, stratifying by severity of albuminuria (mild, moderate, or severe), and found that, while the greatest numeric reduction was in the severe albuminuria group (3.0 [95% CI, 2.0-4.0] mL/min/1.73 m2 per year), reductions in the moderate and mild albuminuria groups were also statistically significant (1.0 [95% CI, 0.6-1.4] and 1.0 [95% CI, 0.9-1.3] mL/min/1.73 m2 per year, respectively).150 The difference in eGFR slope in the mild albuminuria group, however, was driven by a positive direction in the canagliflozin group, which has not been replicated by other studies.
The most convincing evidence that the presence or magnitude of albuminuria is unnecessary for the benefit of SGLT2 inhibitors comes from recent post hoc analyses of the EMPA-REG and CANVAS studies,151,152 which analyzed cardiorenal outcomes, stratifying participants according to KDIGO CKD risk categories. While the absolute risk reductions were greater for higher risk categories, the relative risk reductions for both cardiac and kidney outcomes were similar across KDIGO CKD risk categories. Particularly noteworthy is that even participants in the low-risk category (eGFR ≥90 mL/min/1.73 m2 and UACR <30 mg/g) experienced a decreased risk for incident or worsening nephropathy and the composite end point. This finding has great implications given the much greater numbers of individuals with earlier CKD stages who stand to benefit.
The KDIGO guideline specifies a lower limit eGFR of 30 mL/min/1.73 m2 for initiation of SGLT2 inhibitor but maintains that it is safe to continue until reaching the need for KRT. Available evidence supports this statement, as the majority of trials have used this minimum eGFR as an inclusion criterion.153 Notably, however, 2 trials published shortly after the release of the KDIGO guideline, DAPA-CKD and EMPEROR, enrolled patients down to an eGFR of 25 and 20 mL/min/1.73 m2, respectively. EMPA-KIDNEY (ClinicalTrials.gov identifier NCT03594110)154 is enrolling patients down to an eGFR of 20 mL/min/1.73 m2, and thus may provide additional evidence of safety and efficacy in patients with CKD stage 4. It may be appropriate in patients with UACR >1,000 mg/g who are at high risk for rapid decline in eGFR or have a history of rapid eGFR decline to initiate an SGLT2 inhibitor at an eGFR of 25 mL/min/1.73 m2, as the DAPA-CKD trial is directly applicable to patients with CKD.
Clinical Utility
Evidence supports the additional practice points provided by the KDIGO guideline, which encompass the addition of an SGLT2 inhibitor to existing antihyperglycemic agents unless there is concern for hypoglycemia, in which case it is recommended to reduce the dose or discontinue antihyperglycemic medications other than metformin.2 The rationale for maintaining metformin is that the vast majority of evidence for SGLT2 inhibitor cardiorenal efficacy in type 2 diabetes is based on trials adding SGLT2 inhibitor to metformin unless the patient is intolerant. The KDOQI work group agrees with this sentiment; however, there may be room for exceptions. While an infrequent situation, the patient with type 2 diabetes and CKD on metformin monotherapy with HbA1c in the low or normal range (eg, <7.0%) might not be initiated on an SGLT2 inhibitor based on the KDIGO guideline, thus not reaping the benefits of this therapeutic class. Approximately 18%-25% of participants in 3 large SGLT2 inhibitor CVOTs155–157 and 50% in the CREDENCE trial158 were not on metformin at baseline. There are no post hoc analyses comparing outcomes according to baseline metformin therapy; however, such information may be helpful to inform clinical decisions. Moreover, SGLT2 inhibitor cardiorenal outcomes have been similar in diabetic and nondiabetic strata, strongly suggesting there is potential benefit regardless of the use of metformin.159 Therefore, in some cases, it may be reasonable to reduce or discontinue metformin in order to safely utilize an SGLT2 inhibitor from a glycemic perspective.
The KDOQI work group agreed with other practice points, including withholding of SGLT2 inhibitors during hospitalization or periods of fasting when risk for diabetic ketoacidosis is higher, and to consider decreasing or holding diuretics in euvolemic individuals with initiation of SGLT2 inhibitor to reduce the risk for hypovolemia.2 It is particularly important to underscore the practice point regarding the expected and benign initial decline in eGFR with SGLT2 inhibitor initiation.160
Implementation and Challenges
KDIGO offers a clinical practice point to prioritize agents with proven cardiorenal benefit, while also acknowledging that choice of therapy will mostly depend upon insurance formulary and availability of public and private patient assistance programs. While not all agents have met statistical significance for individual and even composite cardiorenal outcomes, meta-analyses have demonstrated consistency in end point results and show that benefits are most likely a class effect.142,161,162
The primary implementation challenge for the recommended usage of SGLT2 inhibitor in patients with type 2 diabetes and CKD will likely be the wide adoption of this recommendation.163 As an example, despite the evidence of cardiorenal benefit of ACEI/ARB therapy for patients with type 2 diabetes, in the United States, only 21%-50% of people with a clear indication are currently receiving these important medications.42,43 This is in contrast to Europe, wherein 75%-80% of eligible patients are receiving ACEI/ARB therapy.64 To avoid similar experience with SGLT2 inhibitors, it may be advantageous to place a greater emphasis on the effective delivery of clinical practice guidelines across specialties. This effort may include concise discussion on the tolerability, adverse effects, as well as the risk-benefit ratio, with clear guidance on how to mitigate undesired harmful effects.
We agree with the KDIGO guideline that reasons for the limited implementation of proven therapies in the United States are complex and multifactorial, and include physician and patient awareness and appreciation of the impact of CKD on cardiovascular morbidity and mortality, physician awareness and harmonization of clinical practice guidelines, physician inertia, competing health issues, polypharmacy, and differences in cost by country location.44 The majority of clinical practice guidelines, KDIGO included, have taken steps to harmonize recommendations for SGLT2 inhibitor initiation.11,14 The complex and multifactorial issues may only be addressed with intentional, multidisciplinary efforts at local, regional, and national levels.164
Glucagon-Like Peptide 1 Receptor Agonists
Recommendation 4.3.1: In patients with T2D and CKD who have not achieved individualized glycemic targets despite use of metformin and SGLT2i, or who are unable to use those medications, we recommend a long-acting GLP-1 RA (1B).
Commentary
This KDIGO recommendation is reasonable given the very high cardiovascular risk in patients with type 2 diabetes who have CKD.143 However, the KDIGO recommendation does not specify that all GLP-1 RAs are equal with respect to cardiovascular or kidney benefits. In their discussion, they do note that cardiovascular benefit has been shown for liraglutide, dulaglutide, injectable semaglutide, and albiglutide (not available in the United States) and the lack of cardiovascular benefit with lixisenatide and exenatide and oral semaglutide. It can be noted that, in the oral semaglutide PIONEER 6 study, which was not powered to show cardiovascular benefit, there were significant reductions in cardiovascular and all-cause mortality.165 A full postmarketing cardiovascular outcome study for oral semaglutide is ongoing. Thus, the cardiovascular benefit is not truly a class effect, and a suggested modification of the guideline recommendation would be “…we recommend a long-acting GLP-1 RA with proven cardiovascular benefits.”
In their discussion of kidney benefits, KDIGO notes that the composite kidney outcome benefit for liraglutide was driven primarily by the reduction in new severely increased albuminuria. However, they omitted this proviso when discussing the composite kidney outcome benefits for semaglutide and dulaglutide. They do mention that the exploratory analyses show possible preservation of eGFR with dulaglutide in the REWIND study when using eGFR reductions of 40% and 50% from baseline and acknowledge that these will need confirmation, as the primary analysis of a sustained reduction of 30% from baseline did not show this.166 In the discussion of harms of GLP-1 RAs, they recognize that “Treatment with GLP-1 RA may be used to prevent end-organ damage (heart and kidney) as well as manage hyperglycemia.” Since there is no proven benefit of this class on eGFR preservation outside the possible effect of dulaglutide,167 we feel that this statement that includes “kidney” may be too strong.
Clinical Utility
The following guideline practice points are especially worthy of mention: (1) the prioritization of GLP-1 RAs to those agents with documented cardiovascular benefits, (2) starting with a low dose and titrating upward slowly to minimize gastrointestinal side effects, (3) not using GLP-1 RAs with dipeptidyl peptidase 4 (DPP-4) inhibitors, and (4) pointing out the lack of hypoglycemia risk with this class unless they are being used with insulin or sulfonylureas. These practice points are of particular importance to CKD patients who are more susceptible to hypoglycemia.68 In the AWARD-7 trial, participants treated with dulaglutide experienced significantly lower rates of hypoglycemia (blood glucose ≤70 mg/dL) compared to insulin glargine.168 Specifically, liraglutide, semaglutide, and dulaglutide do not require dose adjustment for low eGFR.169–172 Recommendations are the same for kidney transplant recipients as for other CKD patients; however, experience in patients requiring dialysis is lacking, but pharmacokinetic studies showed no excess of side effects or changes in efficacy in patients on peritoneal dialysis.173,174
KDIGO also briefly mentions the weight-loss benefits of GLP1 agonists; however, given the rising prevalence of obesity and its independent effect on CKD and CVD pathogenesis, we feel this effect should be highlighted. Studies have shown a 5%-10% body weight loss with GLP-1 RAs in obese individuals, which is often maintained over the long term. This benefit is far-reaching, impacting not only cardiovascular and kidney health, but also the potential for improvements in other chronic illnesses and quality of life.175
Implementation and Challenges
The lack of uniformity of cardiovascular benefits among the approved drugs in this class precludes concluding that the cardiovascular benefits are a class effect. While insurance and pharmacy benefit manager policies may dictate which drug can be used for a given patient, it may be important for clinicians to recognize this lack of class effect, and it may take additional effort with prior approvals to specify use of a GLP-1 RA with proven cardiovascular benefit.
Although the AWARD-7 trial of patients with stage 3 CKD treated with dulaglutide suggested that there was some preservation of eGFR,168 the subgroup with stage 3 CKD in the larger REWIND trial did not show a significant benefit on the sustained decline of eGFR of >30%.166 The FLOW trial may help clarify the role of the injectable semaglutide on reduction of kidney disease progression as a primary outcome.176
Self-Management Education Programs
Recommendation 5.1.1: We recommend that a structured self-management educational program be implemented for care of people with diabetes and CKD [callout to guideline figure 28 omitted] (1C).
Commentary and Clinical Utility
In its final chapter, the KDIGO guideline addresses the issue of self-management in patients with diabetes and CKD as well as the related topic of implementing team-based integrated care.2 The content is similar to recommendations issued in a recent consensus report issued by the ADA and other professional groups entitled “Diabetes Self-Management Education and Support in Adults With Type 2 Diabetes”177 and rely, to a large extent, on similar analyses of supporting literature.
The general recommendations and practice point guidance presented in the guideline are supported by available evidence.2 The KDOQI work group agrees that the goal of self-management would be embraced by patients, at least in most situations.178 This was the major reason KDIGO raised the guideline to the level of 1C (ie, a recommendation, instead of 2C [suggestion]). A systematic review supports the efficacy of well-structured self-management educational programs in improving surrogate outcomes, such as blood pressure and glycemic control,179 in a generally cost-effective manner.180 However, we agree with the KDIGO guideline that the evidence supporting this recommendation is relatively weak, largely due to the lack of reporting of critical outcomes and relatively low quality of evidence for surrogate end points. Finally, it may be beneficial to use multiple educational modalities for effective self-management education.
Implementation
The KDIGO guideline authors noted some concern regarding cost-effectiveness of telehealth approaches; however, this conclusion was based on mostly low-quality studies performed 10-15 years ago in which costs of implementing telemedicine education were significantly higher than other approaches.180 It is unlikely that such a cost differential still exists. Results from a more recent pragmatic RCT on the cost-effectiveness of home telemonitoring in patients with CKD have not yet been reported.181 It is expected that the COVID-19 pandemic has facilitated much of the infrastructure and cultural acceptance of telehealth, particularly regarding individual and group-based educational sessions and peer support groups. Moreover, strategies to best develop, implement, and evaluate effective programs tailored to the complex and diverse population of patients with diabetes and CKD may not be universally applicable. Embedded in a practice point, KDIGO suggests that health care systems take on this role; however, it seems the main challenge to this will be the cost of such programs and difficulty in assessing their cost-effectiveness.
Team-Based Integrated Care
Recommendation 5.2.1: We suggest that policymakers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD (2B).
Practice Point 5.2.1: Team-based integrated care, supported by decision-makers, should be delivered by physicians and nonphysician personnel (eg, trained nurses and dietitians, pharmacists, health care assistants, community workers, and peer supporters) preferably with knowledge of CKD [callout to guideline figure 33 omitted].
Commentary
KDIGO recommendation 5.2.1 reflects the well-established practice of team-based approaches to the management of diabetes, adding in the management of CKD.2 Importantly, the other impetus for this recommendation is the recognition of the worldwide pandemic of diabetes and that improved management of the millions of patients with diabetes and CKD will likely require a systematic, team-based integrated care system.182 Studies of patients with both CKD and diabetes are limited, but implementing a team-based approach in this group will likely not be inherently different than in other chronic disease populations. The KDOQI work group feels that the guideline as written is vague in delineating which policy makers and institutional decision-makers they are calling on and what, specifically, their role would be. While these details could be considered beyond the scope of international clinical guidelines, the lack of such specifics could potentially impede progress in designing impactful care models for patients with diabetes and CKD. State governments, academic medical centers, public health departments, and pharmaceutical organizations may collaborate to (1) identify regions with unacceptably high rates of diabetes and CKD, (2) set benchmarks for reductions in disease prevalence, and (3) design, implement, and test results of team-based programs, propagating those with greatest efficacy to each and every targeted locale.
Clinical Utility
Much of the literature supporting the clinical efficacy of such approaches comes from the team-based management of general diabetes populations.182 Widespread data specific to the population with diabetes and CKD is lacking; nevertheless, the potential clinical and economic impact of such programs is best represented by the Special Diabetes Program for American Indians, which resulted in a decreased incidence in diabetes-related kidney failure by 54% between 2000 and 2013.183
Implementation and Challenges
The institution of a team-based integrative care strategy for patients with diabetes and CKD worldwide faces challenges, including changes to payment systems, processes of care, cultures within the health care system and access to care, and structural racism and classism, which is not better illustrated than in the space of diabetes and CKD. Most importantly, there is no commitment worldwide to devote the resources needed for this approach. Until such a commitment is made, this recommendation will likely remain aspirational for most countries. Nations with the economic and political resources and, perhaps most importantly, the necessary advocacy can at least start to pave the road. In the United States, one approach to addressing these challenges is the Advancing American Kidney Health Initiative, which set a benchmark to reduce the incidence of all kidney failure by 25% by the year 2030.184
Conclusions
We are at a pivotal moment in the care of people with diabetes and CKD. Never before has there been so much attention paid and resources provided to this important disease state. While many challenges remain in this field (Box 1), KDIGO has made a significant step forward in providing a comprehensive clinical practice guideline dedicated to diabetes and CKD. Importantly, this guideline is in sync with those of most other major national and international professional organizations, providing clarity for providers of varying subspecialties. While some recommendations are quite clear, such as early incorporation of RAS and SGLT2 inhibition in the care of patients with diabetes and CKD, others raise questions, such as how to integrate multidisciplinary and comprehensive care models into the current structure of health care in the United States. There is much work to be done to improve the health and quality of life for patients with diabetes and CKD, but KDIGO has now provided a platform from which the research, policy, and clinical communities can unite to make further impactful changes.
Box 1. Areas Identified That Are Complementary and Supplemental to Those Described in KDOQI Guideline for Further Research and Policy Changes to Improve Care of People With Diabetes and CKD.
Research
Uptake of CKD screening and treatment for diabetes complications in adolescents and young adults
Optimal approaches to transition from pediatric to adult diabetes care and risk for complications
Medical therapies for weight loss in children with obesity and diabetes
Medical therapies for weight loss in people with diabetes and CKD
Improvements in and testing of electronic health record/patient portals with best practice advisory to both patients and providers
Efficacy of community health workers in improving risk factors slowing progression of diabetes complications
Identifying practical, cultural, political, and financial incentives to improve multidisciplinary care models
Benefits of ACEI/ARBs in nonalbuminuric CKD and diabetes
Quantitative and qualitative studies regarding lack of prescribing ACEI/ARBs in diabetes and CKD
Precision medicine approaches to targeting specific drugs based on individual pathogenetic etiology, eg, ACEIs, ARBs, SGLT2 inhibitors, GLP-1 RAs, MRAs
Identifying optimal, individualized glycemic control parameters in diabetes and CKD
Policy Changes
Expanding access to continuous glucose monitors for patients with type 2 diabetes and CKD
Expanding access to SGLT2 inhibitors and GLP-1 RAs for inadequately insured patients with diabetes at high risk of CKD and cardiovascular events
Incentivizing and forming individual and group educational forums for diabetes care and complications
Incentivizing subspecialty care in rural areas with high rates of diabetes and/or providing transportation for high-risk patients with poor access to subspecialty care
Incentivizing multidisciplinary/shared clinics between subspecialties and e-consults for primary care providers
Improved access to healthy foods for people with diabetes and food insecurity
Broadening the diversity of dietitians and training specific to diabetes, CKD, and weight loss
Expanding access to exercise trainers for people with diabetes, CKD, and obesity
Broadening the use of social workers to provide guidance to providers and patients regarding social support networks and access to medications
Abbreviations: CKD, chronic kidney disease; ACEI, angiotensin II converting enzyme inhibitor; ARB, angiotensin II receptor blocker; SGLT2, sodium/glucose transporter 2; GLP-1 RA, glucagon-like peptide 1 receptor agonist; RAS, renin-angiotensin system; MRA, mineralocorticoid receptor antagonist.
Because they are designed to reflect the views and recommendations of the responsible KDOQI Commentary work group and they are reviewed and approved by KDOQI and NKF leadership, KDOQI Commentaries are not peer reviewed by AJKD. This article was prepared by a KDOQI Commentary work group comprising the authors and cochaired by Drs Amy Mottl and Susanne Nicholas. It was reviewed and approved by the NKF Scientific Advisory Board and the KDOQI Chair and Vice Chair.
Acknowledgements:
The authors thank Dr Meda Pavkov for her substantive review and edits to this work. The authors also thank Debra Taylor, Jessica Joseph, and the NKF for their assistance with the commentary. Guideline recommendations included in this article originally were published in Kidney International, are ©2020 KDIGO, and were reproduced with permission from KDIGO.
Support:
No financial support was received for the development of this commentary.
Financial Disclosure:
Dr Mottl has received consulting fees from Bayer and honoraria from UpToDate. Dr Agryropoulos has received consulting fees from Baxter and HSAG as well as research support from Dialysis Clinic Inc. Dr Mauer has received consulting fees from Novo Nordisk. Dr Molitch has received consulting fees from Merck, Pfizer, Tiburio, and VA hospitals as well as research grants from Bayer, Novartis, Chiasma, Crinetics, Strongbridge, and NIH. Dr Perreault has received honoraria from Novo Nordisk, Sanofi, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Merck, and Janssen. The remaining authors declare that they have no relevant financial interests.
References
- 1.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(suppl 2):S12–S154. [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO): KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(suppl):S1–S115. [DOI] [PubMed] [Google Scholar]
- 3.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem RM, Todd JN, Sandholm N, et al. Genome-wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J Am Soc Nephrol. 2019;30:2000–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan M, Keaton JM, Dimitrov L, et al. Genome-wide association study identifies novel loci for type 2 diabetes-attributed end-stage kidney disease in African Americans. Hum Genomics. 2019;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zuydam NR, Ahlqvist E, Sandholm N, et al. A genome-wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes. 2018;67:1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar SK, Abboud HE, Goddard KA, et al. Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: the family investigation of nephropathy and diabetes (FIND). Diabetes. 2007;56:1577–1585. [DOI] [PubMed] [Google Scholar]
- 8.Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–677. [DOI] [PubMed] [Google Scholar]
- 9.Terracciano L, Brozek J, Compalati E, Schünemann H. GRADE system: new paradigm. Curr Opin Allergy Clin Immunol. 2010;10:377–383. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S40–S52. [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26:107–139. [DOI] [PubMed] [Google Scholar]
- 14.de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98:839–848. [DOI] [PubMed] [Google Scholar]
- 15.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metabol. 2011;96:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahkoska AR, Isom S, Divers J, et al. The early natural history of albuminuria in young adults with youth-onset type 1 and type 2 diabetes. J Diabetes Complications. 2018;32:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. [DOI] [PubMed] [Google Scholar]
- 20.Westreich KD, Isom S, Divers J, et al. Trajectories in estimated glomerular filtration rate in youth-onset type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Diabetes Complications. 2021;35:107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8:293–300. [DOI] [PubMed] [Google Scholar]
- 24.Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman AN, Kaplan LM, le Roux CW, Schauer PR. Management of obesity in adults with CKD. J Am Soc Nephrol. 2021;32:777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Kidney Foundation. Chronic kidney disease change package, 2020. Accessed May 2, 2020. https://www.kidney.org/contents/chronic-kidney-disease-change-package
- 27.Narva AS, Norton JM, Boulware LE. Educating patients about CKD: the path to self-management and patient-centered care. Clin J Am Soc Nephrol. 2016;11:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y, Xiong J, Chen Y, et al. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai R, Patel U, Parekh T, et al. Nationwide trends in prevalent cardiovascular risk factors and diseases in young adults: differences by sex and race and in-hospital outcomes. South Med J. 2020;113:311–319. [DOI] [PubMed] [Google Scholar]
- 30.Vart P, Powe NR, McCulloch CE, et al. National trends in the prevalence of chronic kidney disease among racial/ethnic and socioeconomic status groups, 1988-2016. JAMA Netw Open. 2020;3:e207932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Sidell MA, Arterburn D, et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care. 2019;42:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt M, Nahari A, Wang PW, et al. The quality of clinical practice guidelines for management of pediatric type 2 diabetes mellitus: a systematic review using the AGREE II instrument. Syst Rev. 2018;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha E, Paul S, Braxter BJ, Umpierrez G, Faulkner MS. Dietary behaviors and glucose metabolism in young adults at risk for type 2 diabetes. Diabetes Educ. 2018;44:158–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busink E, Canaud B, Schröder-Bäck P, et al. Chronic kidney disease: exploring value-based healthcare as a potential viable solution. Blood Purif. 2019;47:156–165. [DOI] [PubMed] [Google Scholar]
- 35.Chung S, Huang Q, LaMori J, Doshi D, Romanelli RJ. Patient-reported experiences in discussing prescribed medications with a health care provider: evidence for racial/ethnic disparities in a large health care delivery system. Popul Health Manag. 2020;23:78–84. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor SJ. Fragmentation is a prominent feature of the American healthcare landscape. J Healthc Manag. 2014;59:1–2. [PubMed] [Google Scholar]
- 37.Walker AQ, Blake CE, Moore JB, Wilcox S, DuBois K, Watkins KW. Experiences of midlife and older African American men living with type 2 diabetes. Ethn Health. 2021:1–15. [DOI] [PubMed] [Google Scholar]
- 38.Harding K, Mersha TB, Vassalotti JA, Webb FA, Nicholas SB. Current state and future trends to optimize the care of chronic kidney disease in African Americans. Am J Nephrol. 2017;46:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collinsworth AW, Vulimiri M, Schmidt KL, Snead CA. Effectiveness of a community health worker-led diabetes self-management education program and implications for CHW involvement in care coordination strategies. Diabetes Educ. 2013;39:792–799. [DOI] [PubMed] [Google Scholar]
- 40.Wolf MS, Seligman H, Davis TC, et al. Clinic-based versus outsourced implementation of a diabetes health literacy intervention. J Gen Intern Med. 2014;29:59–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–1230. [DOI] [PubMed] [Google Scholar]
- 42.Murphy DP, Drawz PE, Foley RN. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol. 2019;30:1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD Registry. JAMA Netw Open. 2019;2:e1918169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Veer SN, Tomson CR, Jager KJ, van Biesen W. Bridging the gap between what is known and what we do in renal medicine: improving implementability of the European Renal Best Practice guidelines. Nephrol Dial Transplant. 2014;29:951–957. [DOI] [PubMed] [Google Scholar]
- 45.Chu PY, Hackstadt AJ, Chipman J, et al. Hospitalization for lactic acidosis among patients with reduced kidney function treated with metformin or sulfonylureas. Diabetes Care. 2020;43:1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crews DC, Bello AK, Saadi G. Burden, access and disparities in kidney disease. Clin Kidney J. 2019;12:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2021. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 48.Carroll JK, Pulver G, Dickinson LM, et al. Effect of 2 clinical decision support strategies on chronic kidney disease outcomes in primary care: a cluster randomized trial. JAMA Netw Open. 2018;1:e18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ong SW, Kaushal A, Pariser P, Chan CT. An integrated kidney care econsult practice model: results from the iKinect Project. Am J Nephrol. 2019;50:262–271. [DOI] [PubMed] [Google Scholar]
- 50.Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128–139. [DOI] [PubMed] [Google Scholar]
- 51.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;2006:Cd00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. Br Med J. 2016;352:i438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S40–S52. [DOI] [PubMed] [Google Scholar]
- 54.Hiremath S, Fergusson DA, Fergusson N, Bennett A, Knoll GA. Renin-angiotensin system blockade and long-term clinical outcomes in kidney transplant recipients: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2017;69:78–86. [DOI] [PubMed] [Google Scholar]
- 55.Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med. 2018;178:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gayat E, Hollinger A, Cariou A, et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intens Care Med. 2018;44:598–605. [DOI] [PubMed] [Google Scholar]
- 58.Ohkuma T, Jun M, Rodgers A, et al. Acute increases in serum creatinine after starting angiotensin-converting enzyme inhibitor-based therapy and effects of its continuation on major clinical outcomes in type 2 diabetes mellitus. Hypertension. 2019;73:84–91. [DOI] [PubMed] [Google Scholar]
- 59.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;2014:Cd007004. [DOI] [PubMed] [Google Scholar]
- 60.Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. [DOI] [PubMed] [Google Scholar]
- 62.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 63.Foti KE, Wang D, Chang AR, et al. Potential implications of the 2021 KDIGO blood pressure guideline for adults with chronic kidney disease in the United States. Kidney Int. 2021;99:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eder S, Leierer J, Kerschbaum J, et al. Guidelines and clinical practice at the primary level of healthcare in patients with type 2 diabetes mellitus with and without kidney disease in five European countries. Diab Vasc Dis Res. 2019;16:47–56. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura K, Nakagawa H, Murakami Y, et al. Smoking increases the risk of all-cause and cardiovascular mortality in patients with chronic kidney disease. Kidney Int. 2015;88:1144–1152. [DOI] [PubMed] [Google Scholar]
- 66.Pan A, Wang Y, Talaei M, Hu FB. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 68.Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;41:756–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Little RR, Rohlfing C, Sacks DB. The National Glycohemoglobin Standardization Program: over 20 years of improving hemoglobin A(1c) measurement. Clin Chem. 2019;65:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 2010;30:72–79. [DOI] [PubMed] [Google Scholar]
- 71.Jung M, Warren B, Grams M, et al. Performance of non-traditional hyperglycemia biomarkers by chronic kidney disease status in older adults with diabetes: Results from the Atherosclerosis Risk in Communities Study. J Diabetes. 2018;10:276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174:259–268. [DOI] [PubMed] [Google Scholar]
- 73.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 74.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Boer IH, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 77.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. [DOI] [PubMed] [Google Scholar]
- 78.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 80.Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649–659. [DOI] [PubMed] [Google Scholar]
- 81.National Glycohemoglobin Standardization Program. Harmonizing hemoglobin testing. A better A1C test means better diabetes care; 2010. Accessed March 20, 2021. http://ngsp.org/
- 82.Ruospo M, Saglimbene VM, Palmer SC, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev. 2017;6:Cd010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 85.Dwyer JT. Recommended dietary allowance; 2013. Accessed March 20, 2021. https://www.sciencedirect.com/topics/food-science/recommended-dietary-allowance
- 86.USDA, Department of Health and Human Services. Dietary guidelines for Americans; 2020. Accessed March 30, 2021. https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials
- 87.Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(suppl 1):S1–S107. [DOI] [PubMed] [Google Scholar]
- 88.Hostetter TH, Meyer TW, Rennke HG, Brenner BM. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int. 1986;30:509–51. [DOI] [PubMed] [Google Scholar]
- 89.Koppe L, Fouque D. The role for protein restriction in addition to renin-angiotensin-aldosterone system inhibitors in the management of CKD. Am J Kidney Dis. 2019;73:248–25. [DOI] [PubMed] [Google Scholar]
- 90.Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86:1031–1038. [DOI] [PubMed] [Google Scholar]
- 92.Rebholz CM, Coresh J, Grams ME, et al. Dietary acid load and incident chronic kidney disease: results from the ARIC Study. Am J Nephrol. 2015;42:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai Q, Dekker LH, Bakker SJL, de Borst MH, Navis GJ. Dietary patterns based on estimated glomerular filtration rate and kidney function decline in the general population: the Lifelines Cohort Study. Nutrients. 2020;12:1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jhee JH, Kee YK, Park JT, et al. A diet rich in vegetables and fruit and incident CKD: a community-based prospective cohort study. Am J Kidney Dis. 2019;74:491–500. [DOI] [PubMed] [Google Scholar]
- 95.Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2007;2007:Cd002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rughooputh MS, Zeng R, Yao Y. Protein diet restriction slows chronic kidney disease progression in non-diabetic and in type 1 diabetic patients, but not in type 2 diabetic patients: a meta-analysis of randomized controlled trials using glomerular filtration rate as a surrogate. PLoS One. 2015;10:e0145505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002;62:220–228. [DOI] [PubMed] [Google Scholar]
- 98.Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36:3093–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silverman J, Krieger J, Kiefer M, Hebert P, Robinson J, Nelson K. The relationship between food insecurity and depression, diabetes distress and medication adherence among low-income patients with poorly-controlled diabetes. J Gen Intern Med. 2015;30:1476–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lawrence MA, Baker PI. Ultra-processed food and adverse health outcomes. Br Med J. 2019;365:l2289. [DOI] [PubMed] [Google Scholar]
- 101.Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). Br Med J. 2019;365:l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson CAM, Nguyen HA. Nutrition education in the care of patients with chronic kidney disease and end-stage renal disease. Semin Dial. 2018;31:115–121. [DOI] [PubMed] [Google Scholar]
- 103.Rogers D. Report on the Academy/Commission on Dietetic Registration 2020 needs satisfaction survey. J Acad Nutr Diet. 2021;121:134–138. [DOI] [PubMed] [Google Scholar]
- 104.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tseng E, Appel LJ, Yeh HC, et al. Effects of the dietary approaches to stop hypertension diet and sodium reduction on blood pressure in persons with diabetes. Hypertension. 2021;77:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suckling RJ, He FJ, Macgregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev. 2010;2010:Cd006763. [DOI] [PubMed] [Google Scholar]
- 107.Overwyk KJ, Quader ZS, Maalouf J, et al. Dietary sodium intake and health indicators: a systematic review of published literature between January 2015 and December 2019. Adv Nutr. 2020;11:1174–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borrelli S, Provenzano M, Gagliardi I, et al. Sodium intake and chronic kidney disease. Int J Mol Sci. 2020;21:4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Usual sodium intakes compared with current dietary guidelines – United States, 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:1413–1417. [PubMed] [Google Scholar]
- 110.National Academy of Medicine. Vital directions for health and health care; 2017. Accessed March 20, 2021. https://nam.edu/wp-content/uploads/2018/02/Vital-Directions-for-Health-and-Health-Care-Final-Publication-022718.pdf
- 111.Centers for Disease Control and Prevention. Sodium reduction toolkit: a global opportunity to reduce population-level sodium intake; 2016. Accessed March 20, 2021. https://www.carpha.org/Portals/0/Knowledge%20Bank/C/CDC%20Toolkit%20Salt%20reduction.pdf
- 112.KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. Kidney Int. 2012;2:337–414. [DOI] [PubMed] [Google Scholar]
- 113.Arnett DK, Khera A, Blumenthal RS. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: part 1, lifestyle and behavioral factors. JAMA Cardiol. 2019;4:1043–1044. [DOI] [PubMed] [Google Scholar]
- 114.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl):S15–S33. [DOI] [PubMed] [Google Scholar]
- 115.Nakamura K, Sasaki T, Yamamoto S, Hayashi H, Ako S, Tanaka Y. Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: a systematic review and meta-analysis. Sci Rep. 2020;10:18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention. Chronic Kidney Disease Initiative; 2020. Accessed March 20, 2021. https://www.cdc.gov/kidneydisease/index.html
- 117.Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol. 2015;10:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kosmadakis GC, John SG, Clapp EL, et al. Benefits of regular walking exercise in advanced pre-dialysis chronic kidney disease. Nephrol Dial Transplant. 2012;27:997–1004. [DOI] [PubMed] [Google Scholar]
- 119.Stefanović V, Milojković M. Effects of physical exercise in patients with end stage renal failure, on dialysis and renal transplantation: current status and recommendations. Int J Artif Organs. 2005;28:8–15. [DOI] [PubMed] [Google Scholar]
- 120.Centers for Disease Control and Prevention. Get Active!; 2018. Accessed May 2, 2021. https://www.cdc.gov/diabetes/managing/active.html
- 121.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 122.Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klam C, Neher JO, Mayo H, Lo V. Clinical inquiries. What is the best medical therapy for new-onset type 2 diabetes? J Fam Pract. 2006;55:998–1000. [PubMed] [Google Scholar]
- 124.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111–S124. [DOI] [PubMed] [Google Scholar]
- 125.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2018 executive summary. Endocr Pract. 2018;24:91–120. [DOI] [PubMed] [Google Scholar]
- 126.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 127.Kwon S, Kim YC, Park JY, et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care. 2020;43:948–955. [DOI] [PubMed] [Google Scholar]
- 128.Crowley MJ, Diamantidis CJ, McDuffie JR, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu Y, Lei M, Ke G, et al. Metformin use and risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease-a systematic review and meta-analysis. Front Endocrinol. 2020;11:559446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. 2018;178:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shin JI, Sang Y, Chang AR, et al. The FDA metformin label change and racial and sex disparities in metformin prescription among patients with CKD. J Am Soc Nephrol. 2020;31:1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol. 2014;40:546–553. [DOI] [PubMed] [Google Scholar]
- 134.Vest LS, Koraishy FM, Zhang Z, et al. Metformin use in the first year after kidney transplant, correlates, and associated outcomes in diabetic transplant recipients: a retrospective analysis of integrated registry and pharmacy claims data. Clin Transplant. 2018;32:e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alnasrallah B, Goh TL, Chan LW, Manley P, Pilmore H. Transplantation and diabetes (Transdiab): a pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation. BMC Nephrol. 2019;20:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP Jr. Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2012;35:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. Br Med J. 2010;340:c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6:e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Orloff J, Min JY, Mushlin A, Flory J. Safety and effectiveness of metformin in patients with reduced renal function: a systematic review. Diabetes Obes Metab. 2021;23:2035–204. [DOI] [PubMed] [Google Scholar]
- 140.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. [DOI] [PubMed] [Google Scholar]
- 141.Cherney DZI, Charbonnel B, Cosentino F, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johansen ME, Argyropoulos C. The cardiovascular outcomes, heart failure and kidney disease trials tell that the time to use sodium glucose cotransporter 2 inhibitors is now. Clin Cardiol. 2020;43:1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lenihan CR, Liu S, Deswal A, Montez-Rath ME, Winkelmayer WC. De novo heart failure after kidney transplantation: trends in incidence and outcomes. Am J Kidney Dis. 2018;72:223–233. [DOI] [PubMed] [Google Scholar]
- 146.Israni AK, Snyder JJ, Skeans MA, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant. 2010;10:338–353. [DOI] [PubMed] [Google Scholar]
- 147.Awan AA, Niu J, Pan JS, Erickson KF, et al. Trends in the causes of death among kidney transplant recipients in the United States (1996–2014). Am J Nephrol. 2018;48:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lam NN, Tonelli M, Lentine KL, et al. Albuminuria and post-transplant chronic kidney disease stage predict transplant outcomes. Kidney Int. 2017;92:470–478. [DOI] [PubMed] [Google Scholar]
- 149.Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2018;10:88–89. [DOI] [PubMed] [Google Scholar]
- 150.Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS Program. J Am Soc Nephrol. 2019;30:2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levin A, Perkovic V, Wheeler DC, et al. Empagliflozin and cardiovascular and kidney outcomes across KDIGO risk categories: post hoc analysis of a randomized, double-blind, placebo-controlled, multinational trial. Clin J Am Soc Nephrol. 2020;15:1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neuen BL, Ohkuma T, Neal B, et al. Relative and absolute risk reductions in cardiovascular and kidney outcomes with canagliflozin across KDIGO risk categories: findings from the CANVAS program. Am J Kidney Dis. 2021;77:23–34.e1. [DOI] [PubMed] [Google Scholar]
- 153.Rhee JJ, Jardine MJ, Chertow GM, Mahaffey KW. Dedicated kidney disease-focused outcome trials with sodium-glucose cotransporter-2 inhibitors: lessons from CREDENCE and expectations from DAPA-HF, DAPA-CKD, and EMPA-KIDNEY. Diabetes Obes Metab. 2020;22(suppl 1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.ClinicalTrials.gov. EMPA-KIDNEY (The Study of Heart and Kidney Protection with Empagliflozin). US National Library of Medicine, 2021. Accessed September 8, 2021. https\\www.clinicaltrials.gov/ct2/show/NCT03594110
- 155.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 156.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 157.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 158.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 159.Schubert M, Hansen S, Leefmann J, Guan K. Repurposing antidiabetic drugs for cardiovascular disease. Front Physiol. 2020;11:568632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oshima M, Jardine MJ, Agarwal R, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021;99:999–1009. [DOI] [PubMed] [Google Scholar]
- 161.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237–1250. [DOI] [PubMed] [Google Scholar]
- 162.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 163.Schernthaner G, Shehadeh N, Ametov AS, et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tuttle KR, Brosius FC III, Cavender MA, et al. SGLT2 Inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021;77:94–109. [DOI] [PubMed] [Google Scholar]
- 165.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. [DOI] [PubMed] [Google Scholar]
- 166.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. [DOI] [PubMed] [Google Scholar]
- 167.Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. 2021;131:e142243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. [DOI] [PubMed] [Google Scholar]
- 169.Victoza® (liraglutide) injection [package insert]. Plainsboro NJ: Novo Nordisk; 2020. [Google Scholar]
- 170.Basgen JM, Nicholas SB, Mauer M, Rozen S, Nyengaard JR. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp Nephrol. 2006;103:e139–e148. [DOI] [PubMed] [Google Scholar]
- 171.Najafian B, Crosson JT, Kim Y, Mauer M. Glomerulotubular junction abnormalities are associated with proteinuria in type 1 diabetes. J Am Soc Nephrol. 2006;17(suppl):S53–S60. [DOI] [PubMed] [Google Scholar]
- 172.Trulicity® (dulaglutide) injection [package insert]. Indianapolis, IN: Eli Lilly and Company; 2020. [Google Scholar]
- 173.Granhall C, Søndergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57:1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grill HJ. A role for GLP-1 in treating hyperphagia and obesity. Endocrinology. 2020;161:bqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17:339–352. [DOI] [PubMed] [Google Scholar]
- 177.Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care and Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care. 2020;43:1636–1649. [DOI] [PubMed] [Google Scholar]
- 178.Steinsbekk A, Rygg L, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zimbudzi E, Lo C, Misso ML, Ranasinha S, Kerr PG, Teede HJ, Zoungas S. Effectiveness of self-management support interventions for people with comorbid diabetes and chronic kidney disease: a systematic review and meta-analysis. Syst Rev. 2018;7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Teljeur C, Moran PS, Walshe S, et al. Economic evaluation of chronic disease self-management for people with diabetes: a systematic review. Diabet Med. 2017;34:1040–1049. [DOI] [PubMed] [Google Scholar]
- 181.Thilly N, Chanliau J, Frimat L, et al. Cost-effectiveness of home telemonitoring in chronic kidney disease patients at different stages by a pragmatic randomized controlled trial (eNephro):rationale and study design. BMC Nephrol. 2017;18:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Epping-Jordan JE, Pruitt SD, Bengoa R, Wagner EH. Improving the quality of health care for chronic conditions. Qual Saf Health Care. 2004;13:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Burrows NR, Zhang Y, Hora I, et al. Sustained lower incidence of diabetes-related end-stage kidney disease among American Indians and Alaska Natives, Blacks, and Hispanics in the U.S., 2000-2016. Diabetes Care. 2020;43:2090–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.U.S. Department of Health and Human Services. Advancing American Kidney Health. U.S. Department of Health and Human Services, 2021. Accessed March 20, 2021. https://aspe.hhs.gov/system/files/pdf/262046/AdvancingAmericanKidneyHealth.pdf
- 185.Alsahli M, Gerich JE. Hypoglycemia in patients with diabetes and renal disease. J Clin Med. 2015;4:948–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17:365–370. [DOI] [PubMed] [Google Scholar]
- 187.Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rosenkranz B, Profozic V, Metelko Z, Mrzljak V, Lange C, Malerczyk V. Pharmacokinetics and safety of glimepiride at clinically effective doses in diabetic patients with renal impairment. Diabetologia. 1996;39:1617–1624. [DOI] [PubMed] [Google Scholar]
- 189.Islam SI, Masuda QN, Bolaji OO, Shaheen FM, Sheikh IA. Possible interaction between cyclosporine and glibenclamide in posttransplant diabetic patients. Ther Drug Monit. 1996;18:624–626. [DOI] [PubMed] [Google Scholar]
- 190.Sagedal S, Asberg A, Hartmann A, Bergan S, Berg KJ. Glipizide treatment of post-transplant diabetes does not interfere with cyclosporine pharmacokinetics in renal allograft recipients. Clin Transplant. 1998;12:553–556. [PubMed] [Google Scholar]
- 191.Tan A, Holmes HM, Kuo YF, Raji MA, Goodwin JS. Coadministration of co-trimoxazole with sulfonylureas: hypoglycemia events and pattern of use. J Gerontol A Biol Sci Med Sci. 2015;70:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Graefe-Mody U, Friedrich C, Port A, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*). Diabetes Obes Metab. 2011;13:939–946. [DOI] [PubMed] [Google Scholar]
- 193.Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab. 2010;12:648–658. [DOI] [PubMed] [Google Scholar]
- 194.Home P. Safety of PPAR agonists. Diabetes Care. 2011;34(suppl 2):S215–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. [DOI] [PubMed] [Google Scholar]
- 196.Thompson-Culkin K, Zussman B, Miller AK, Freed MI. Pharmacokinetics of rosiglitazone in patients with end-stage renal disease. J Int Med Res. 2002;30:391–399. [DOI] [PubMed] [Google Scholar]
- 197.Luther P, Baldwin D Jr. Pioglitazone in the management of diabetes mellitus after transplantation. Am J Transplant. 2004;4:2135–2138. [DOI] [PubMed] [Google Scholar]
- 198.Inoue T, Shibahara N, Miyagawa K, et al. Pharmacokinetics of nateglinide and its metabolites in subjects with type 2 diabetes mellitus and renal failure. Clin Nephrol. 2003;60:90–95. [DOI] [PubMed] [Google Scholar]
- 199.Schumacher S, Abbasi I, Weise D, Hatorp V, Sattler K, Sieber J, Hasslacher C. Single- and multiple-dose pharmacokinetics of repaglinide in patients with type 2 diabetes and renal impairment. Eur J Clin Pharmacol. 2001;57:147–152. [DOI] [PubMed] [Google Scholar]
- 200.Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005;78:388–399. [DOI] [PubMed] [Google Scholar]
- 201.Reuser AJ, Wisselaar HA. An evaluation of the potential side-effects of alpha-glucosidase inhibitors used for the management of diabetes mellitus. Eur J Clin Invest. 1994;24(suppl 3):19–24. [DOI] [PubMed] [Google Scholar]
- 202.Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med. 2003;20:642–645. [DOI] [PubMed] [Google Scholar]
- 203.Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27:351–35. [DOI] [PubMed] [Google Scholar]


