Abstract
The gut microbiome (GM) has emerged in the last few years as a main character in several diseases. In pediatric oncological patients, GM has a role in promoting the disease, modulating the effectiveness of therapies, and determining the clinical outcomes. The therapeutic course for most pediatric cancer influences the GM due to dietary modifications and several administrated drugs, including chemotherapies, antibiotics and immunosuppressants. Interestingly, increasing evidence is uncovering a role of the GM on drug pharmacokinetics and pharmacodynamics, defining a bidirectional relationship. Indeed, the pediatric setting presents some contrasts with respect to the adult, since the GM undergoes a constant multifactorial evolution during childhood following external stimuli (such as diet modification during weaning). In this review, we aim to summarize the available evidence of pharmacomicrobiomics in pediatric oncology.
Keywords: gut microbiota, oncology, pharmacomicrobiomics, chemotherapy, antibiotics, pediatrics
1. Introduction
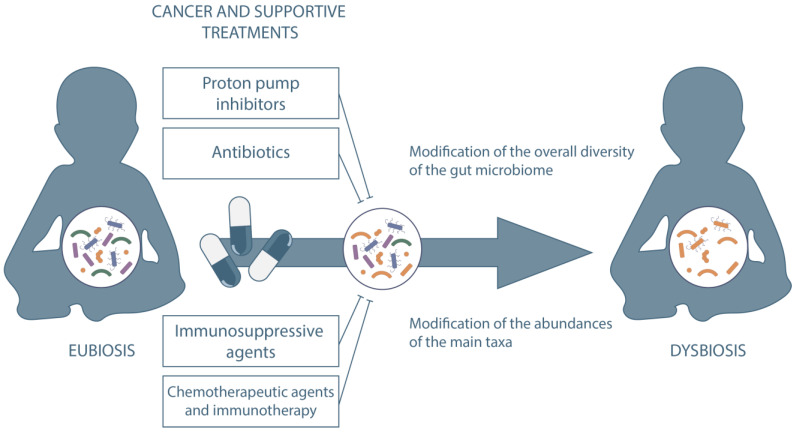
The gut microbiome (GM) is considered a major determinant in human health with a pivotal role in the modulation of the immune system [1]. In oncology, the GM has been demonstrated to contribute in the pathogenesis of cancer [2,3] and to play a major role during anticancer therapy. GM dysbiosis, characterized by reduced ecosystem diversity, loss of health-related commensals and overgrowth of potentially pathogenic bacterial species, occurs frequently in cancer patients [2,4]. These GM modifications have been associated with clinical outcomes in cancer patients. For example, intestinal domination, defined as the presence of a single bacterial taxon comprising more than 30% of the entire GM, of Enterococcus is associated with increased mortality in the acute leukemia chemotherapy population [5]. Moreover, GM composition correlates with response and toxicity following anti-CD19 CAR T cell therapy [6] and anti-PD1 immunotherapy [7]. Most of the current literature addresses the role of the GM during hematopoietic stem cell transplantation (HSCT). The intestinal ecosystem undergoes a significant disruption during the procedure due to a combination of upsetting events [8,9]. In particular, intestinal mucosal barrier damage associated with the conditioning regimen, dietary alterations and the use of broad-spectrum antibiotics profoundly injures the GM [4,10,11,12]. The resulting alterations in the GM architecture are correlated with clinical outcomes. In recent years, new methodologies for studying the GM have extended our comprehension of the host-microbiome interaction, shedding light on bacterial functional properties such as metabolomics and metagenomics [13,14]. Drug-metabolizing activity of human gut bacteria has emerged as an increasingly interesting function. About two thirds of drugs could be metabolized by at least one strain in the GM [15]. Compound modifications by gut microorganisms via enzymatic transformation alter bioavailability, bioactivity and toxicity, potentially leading to intestinal and systemic pharmacological effects [16]. Chemical transformation by microorganisms (biotransformation) is not the only mechanism implied. Bioaccumulation, consisting of bacteria storing the drug intracellularly without chemically modifying it, has also recently emerged as a common mechanism that alters drug availability with potential implications for pharmacokinetics, side effects and drug responses [17]. The GM could therefore influence an individual’s response to a specific drug. However, the interaction between drugs and the GM is bidirectional, given that drugs could influence microbial composition. Cancer patients are commonly at high risk of infectious complications making antimicrobial therapy an essential component of their management. Numerous findings exist about the impact of antimicrobial drugs on intestinal ecosystem [18]. More recently, scientific interest has increased about the relationship between GM and other drugs that could shape microbial composition [16]. Some of these drugs are routinely used in the oncological setting, including chemotherapeutic agents, immunosuppressive agents, steroids, protonic pump inhibitors and biliary acids [19,20]. The interaction between these drugs and the GM is one of the key components of the complex interplay between the intestinal ecosystem and the host during anticancer therapies. This bidirectional relationship between GM and cancer presents with distinctive features in pediatric patients, considering the rapidly and continuously evolving GM community, the strong impact of complications in growing subjects and the different subsets of diseases that require specific therapies [21,22]. Therefore, the pediatric setting deserves a specific focus when addressing pharmacomicrobiomics. In this paper, we aim to provide a complete overview on the bidirectional relationship between GM and drugs commonly used in the clinical practice in pediatric patients with cancer (Figure 1).
Figure 1.
Impact on GM of drugs commonly used in clinical practice in pediatric patients with cancer.
The main studies addressing the relationship between drugs commonly used in pediatric patients and gut microbiota are summarized in Table 1.
Table 1.
Studies addressing the relationship between drugs commonly used in pediatric patients and gut microbiota. ↑, increased; ↓, decreased.
| Drug Name | Year | First Author | Setting | Sample Size | Interaction with Gut Microbiome | Ref |
|---|---|---|---|---|---|---|
| Irinotecan (CPT-11) | 2015 | Wallace, BD | Preclinical | / | Intestine bacteria producing β-glucuronidase can convert non-toxic CPT-11 metabolite (SN-38-G) to toxic metabolite (SN-38), causing diarrhea. | [23] |
| Irinotecan (CPT-11) | 2008 | Stringer, AM | Preclinical, rats | / | ↑ number of β-glucuronidases-expressing species. | [24] |
| Cyclophosphamide | 2013 | Viaud, S | Preclinical | / | Translocation of specific Gram-positive bacteria from the intestine to secondary lymphoid organs was critical for the differentiation of CD4+ T cells into Th1 and Th17 cells. | [25] |
| Cyclophosphamide | 2015 | Xu, X | Preclinical | / | ↑ Firmicutes, ↓ Bacteroidetes. | [26] |
| L-asparaginase | 2021 | Dunn, KA | Pediatric ALL | 12 patients | ↑ Escherichia in the community if decreased-activity, ↑ Bacteroides and Streptococcus in the community if increased-activity. | [27] |
| Anti-PD1 | 2018 | Gopalakrishnan, V | Adults, melanoma | 112 patients | ↑ α-diversity of responders to anti-PD1 therapy. Higher proportion of Ruminococcaceae, Faecalibacterium, and Bifidobacterium spp. reported in responders. | [28] |
| Anti-PD1 | 2018 | Routy, B | Mice, Adults | Mice, 249 treated | ↑ Akkermansia, Ruminococcus spp., Alistipes spp., and Eubacterium spp in responders. ↓ Bifidobacterium adolescentis, B. longum, and Parabacteroids distasonis in responders. |
[29] |
| Cyclosporine | 2019 | Jia et al. | Preclinical | 8 treated | ↑ gut microbial richness, Enterobacteriaceae. ↓ F. prausnitzii, Clostridium clusters I and XIV. | [30] |
| Cyclosporine | 2020 | O Reilly et al. | Adults | 6 ex vivo, 8 in vivo | No significant α and β diversity before and after treatment. | [31] |
| Tacrolimus | 2017 | Zhang et al. | Mice | 8 treated | No change in bacterial richness and diversity. ↑ genera Allobaculum, Bacteroides and Lactobacillus. ↓ Clostridiales, Ruminococcaceae, Rikenella, Ruminococcaceae and Oscillospira. |
[32] |
| Tacrolimus | 2017 | Bhat et al. | Mice | 5 treated | ↓ Mollicutes, Micrococcaceae, Actinomycetales, Roseburia, Oscillospira, Rothia and Staphylococcus. ↑ A. muciniphila. | [33] |
| Tacrolimus | 2018 | Toral et al. | Mice | 8 treated | ↓ microbial diversity. ↑ Firmicutes/Bacteroidetes ratio. |
[34] |
| Tacrolimus | 2018 | Jiang et al. | Mice | 8 high dosage, 8 medium dosage, 8 low dosage | Intermediate dose: ↑ Bifidobacterium, Faecalibacterium prausnitzii ↓ less Enterobacteriaceae, Bacteroides-Prevotella Low and high doses: ↑ Enterobacteriaceae ↓ Bifidobacterium, Faecalibacterium prausnitzii. |
[35] |
| MMF | 2018 | Flannigan et al. | Mice | 9 treated | ↓ overall diversity ↑ Proteobacteria (Escherichia/Shigella), Deferribacteres, Firmicutes ↓ Bacteroidetes and Verrucomicrobia phyla, Akkermansia, Parabacteroides and Clostridium genera. |
[36] |
| Rapamycin | 2017 | Bhat et al., | Mice | 5 treated | ↓ bacterial diversity. ↓ Roseburia, Oscillospira, Mollicutes, Rothia, Micrococcaceae, Acninomycetales and Staphylococcus. |
[33] |
| Rapamycin | 2016 | Jung et al. | Mice | 5 treated | ↓ Turicibacter, unclassified Marinilabiliaceae, Alloprevotella. ↑ Ruminococcus. | [37] |
| Alemtuzumab | 2013 | Li et al. | Monkeys | 15 treated | ↑ Enterobacteriales order and Prevotella genus. ↓ Lactobacillales order. |
[38] |
| Steroids | 2014 | Lee et al. | Humans | 4 treated | ↓ Clostridiales ↑ Erysipelotrichales. |
[39] |
| Steroids | 2016 | Tourret et al. | Mice | 8–10 treated | ↑ Firmicutes/Bacteroidetes ratio ↓ Clostridium sensu stricto. |
[40] |
| Steroids | 2017 | Wu et al. | Mice | 30 lower dose, 30 higher dose | ↓ bacterial richness and diversity. ↓ Firmicutes, Bacteroides, Actinobacteria, α and γ Proteobacteria, Clostridiales and Lactobacillus. ↑ Proteobacteria. |
[41] |
| Steroids | 2019 | He et al. | Mice | 10 treated | ↓ Proteobacteria, Deferribacteres, Rikenella, Mucispirillum, Oscillospira and Bilophila. ↑ Prevotella and Anaerostipes. |
[42] |
| Steroids | 2020 | Vich Vila et al. | Adults | 17 treated | ↑ Methanobrevibacter smithii and Streptococcus salivarius. | [43] |
| PPI | 2016 | Jackson et al. | Adults | 1827 | ↓ diversity in PPI users. ↑ Lactobacillales order, families Micrococcaceae and Streptococcaceae, genera Rothia and Streptococcus, species Rothia mucilaginosa and Streptococcus anginosus. ↓ families Erysipelotrichaceae, Lachnospiraceae, Ruminococcaceae, genera Firmicutes, species Erysipelotrichales and Clostridiales. |
[44] |
| PPI | 2015 | Imhann et al. | Adults | 99 treated | ↓ species richness and ↓ Shannon diversity, although not significant. ↑ Gammaproteobacteria class, Actinomycetales order, families Streptococcaceae and Micrococcaceae, genera Rothia, Streptococcus and Veilonella, species Lactobacillus salivarius. |
[45] |
| PPI | 2015 | Freedberg et al. | Adults | 12 treated | No changes in diversity. ↑ families Enterococcaceae, Streptococcaceae, Micrococcaceae and Staphylococcaceae. ↓ Clostridiales. |
[46] |
| PPI | 2015 | Tsuda et al. | Adults | 18 treated | No changes in α diversity, increased β diversity. ↓ genus Faecalibacterium. |
[47] |
| PPI | 2020 | Vich Vila et al. | Adults | 108 treated | ↑ species Veillonella parvula, Streptococcus salivarius, Streptococcus parasanguinis, Streptococcus vestibularis, Bifidobacterium dentium, Haemophilus parainfluenzae. | [43] |
| PPI | 2021 | Simakachorn et al. | Pediatrics | 20 treated | No significant change in α and β diversity. No change in total number of species-level taxonomy categories. |
[48] |
| UDCA | 2018 | Pearson et al. | Adults | 661 treated | No change in microbial richness. ↑ Streptocuccus, Escherichia and Bilophila spp., Faecalibacterium prausnitzii; ↓ Fusobacterium spp., Ruminococcus gnavus. |
[20] |
| UDCA | 2018 | Tang et al. | Adults | 60 treated | ↑ Enterobacteriaceae. | [49] |
2. Antibiotics
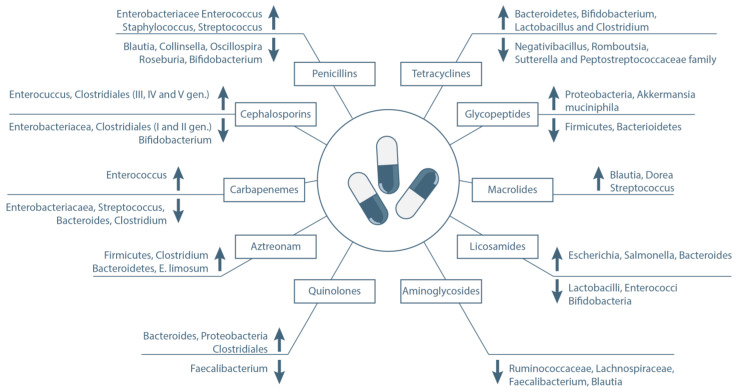
The advances in the understanding of the role of GM in human health have changed our views on antibiotic use in the last few years, particularly during chemotherapy or in immunocompromised patients. Antibiotic use in these patients could represent a double-edged sword. Other than the anti-infective function, antibiotics are known to be strong negative modulators of the GM [50]. Their detrimental role was already known, as their use is associated with metabolic alterations and with the emergence of pathogenic strains, such as C. Difficile [51]. However, the emerging field of microbiota research has revealed deep and complex antibiotic-related modifications in the gut flora that result in various positive or detrimental clinical effects [50,52]. This seems to be mediated by a selective pressure by the antibiotics on the GM resulting in different patterns of modification depending on the antibacterial activity spectrum. Moreover, it has been demonstrated that antibiotic-driven modifications of the GM can last long after treatment with relevant later consequences [53]. Considering the high rate of antibiotics administrated during anticancer therapies, the effect on GM is prominent in these and should be considered in clinical decision algorithms [54]. While the topic should deserve a specific focus considering the extent of the available literature (systematic review can be found in reference [55]), we herein report the effect of the main antibacterial molecules on GM with a particular focus on bacteria with a proven effect on outcome for cancer patients (Figure 2).
Figure 2.
Impact of main antibacterial molecules on clinically relevant gut bacterial species.
2.1. β-Lactams
β-lactams are frequently used as first line antibiotic therapy for febrile neutropenia in children with cancer [56,57]. Recent ECIL8 guidelines recommend the use of an antipseudomonal non-carbapenem β-lactam/β-lactamase inhibitor combination for clinically stable patients, whereas carbapenems are reserved for clinically unstable conditions [58]. β-lactams are characterized by a wide antibacterial spectrum, appropriate for empirical therapies. Following their use, changes in microbial composition persist long after the end of the therapy. A long term alteration has been reported that lasts up to 12 weeks after treatment, with an incomplete restoration of microbial composition and emergence of antibiotic-resistant strains [59]. However, other authors reported complete recovery of GM and resistome composition at baseline levels one week after a short-term antibiotic course [60]. All β-lactams have an effect of modification in the GM in terms of overall α and β diversity and at a taxonomic scale, but different types present particular effects. β-lactams have generally been reported to both increase and decrease the relative abundance of different strains, even if the decrease is more common [55]. Amoxicillin has been associated with an increased abundance of the family of Enterobacteriacee [61] and an increase of pathogenic genera such as Enterococcus, Staphylococcus and Streptococcus [62]. In contrast, other health promoting genera have been reported to be reduced in abundance, namely, Blautia, Collinsella, Oscillospira and Roseburia [63]. Some studies also reported a small increase in Clostridiales clostridium [64], an order known to be associated with positive clinical outcomes in an onco-hematological setting, as a producer of short chain fatty acids (SCFA), such as butyrate [65]. One study directly addressed the effect of penicillin administration on SCFA and did not find any changes in fecal concentrations during treatment [66].
Cephalosporins are broadly used in pediatric patients as well, often as the first line treatment for neutropenic fever [57,67]. Most studies reported a decrease of Enterobacteriacea and an increase of Enterococcus following their administration [68]. Enterococcus has been associated with negative clinical outcomes both in leukemia and in HSCT patients [5,69]. Interestingly, the effect of cephalosporin on Clostridiales depends on the generation of the antibiotic. First and second generation cephalosporins have been associated with a decrease, while third, fourth and fifth generation with an increase of Clostridiales [70]. SCFA have been measured in one study showing a decrease after ceftriaxone administration [71]. The genus of Bifidobacterium is commonly decreased after treatment with penicillins and cephalosporins [72]. This represent an important genus in pediatric cancer patients, being far more present in children rather than in adults [8], and alterations in Bifidobacterium abundances can last up to 6 weeks following treatment [73]. Cefepime is frequently used in this setting and was specifically addressed in several studies [74]. In a cohort of HSCT recipients, cefepime was found not to exert an effect on intestinal diversity suggesting a potential protective role [75]. From a compositional point of view, cefepime has been associated with a decrease in E. coli and bifidobacteria abundances and an increase in Bacteroides spp. and C. difficile [76].
Carbapenems represent a β-lactams subclass with a narrower antimicrobial spectrum, usually used as second line therapy. Their use has been associated with a decrease in the abundance of Enterobacteriacaea, Streptococcus spp., Bacteroides spp., Clostridium spp. and an increased abundance of Enterococcus spp. [77].
Among monobactams, aztreonam is the most frequently used. Recently its use has been associated with positive outcome in HSCT recipients, suggesting a little suppressive effect on GM [51]. Two studies addressed the effect of aztreonam on GM showing an increase in Firmicutes, Clostridium, Bacteroidetes and E. limosum [78,79]. Interestingly, this latter has been associated with a reduced incidence of relapse after HSCT [80].
2.2. Quinolones
Quinolones inhibit the ligase activity of the type II topoisomerases, DNA gyrase and topoisomerase IV. They are used mainly as prophylactic agents during chemotherapy and HSCT [81,82]. They are also used as step-down therapy following episodes of febrile neutropenia [83]. The effect of quinolones on gut microbiota is variable, with reports of increase in the Bacteroides, Proteobacteria family and members of the Clostridiales order as well as decrease in Faecalibacterium [84,85,86]. Quinolone-induced modifications can last for a variable period, from 1 month up to one year [84,85].
2.3. Tetracyclines
Tetracyclines inhibit protein synthesis by binding to the 30S and 50S subunit of microbial ribosomes. Their use in pediatric patients is quite limited considering their negative effect of dental discoloration; however, they can be used in particular situation in pediatric cancer patients [87]. Patients receiving tetracyclines presents an increase of Bacteroidetes and a decrease of Bifidobacterium, Lactobacillus and Clostridium [88]. Moreover, a decrease of Negativibacillus, Romboutsia, Sutterella and Peptostreptococcaceae family was reported [63].
2.4. Glycopeptides
Glycopeptides are characterized by the ability to inhibit synthesis of the cell wall in susceptible microbes by inhibiting peptidoglycan synthesis. They are particularly used in neutropenic patients when Gram-positive bacteria are suspected. Glycopeptides are associated with a reduced fecal microbial diversity, with a decrease of Gram-positive bacteria, particularly Firmicutes, compensated by Gram-negative bacteria, mainly Proteobacteria [85], confirmed also in murine models [89,90]. In other studies the depletion of Gram-positive bacteria has been replaced by Akkermansia muciniphila [91], a strain associated with longer neutropenic fever in pediatric allogeneic HSCT (allo-HSCT) recipients [92] and worse outcome in cancer patients [52].
2.5. Macrolides
Macrolide antibiotics are protein synthesis inhibitors, binding reversibly to the P site on the 50S subunit of the bacterial ribosome. They are mainly used for the treatment of intracellular bacteria. The effect of macrolide on GM is variable, impacting the abundance of many taxa and reducing the α-diversity [55,93]. Interestingly, its use is often associated with colonization of Gram-positive bacteria. In particular, the bacterial depletion mediated by macrolide has been reported to be occupied by Gram-positive anaerobes, predominantly Blautia and Dorea, intestinal commensal organisms within the bacterial class Clostridia, particularly important in pediatric cancer patients in which Clostridia has been associated with lower complications rates [3]. These modifications seem to be persistent after the antibiotic discontinuation [94]. Macrolides have also been associated with increased abundances of Streptococcus [95], associated with higher infectious complications in pediatric patients [96]. Interestingly, recent evidence showed that the GM of pediatric patients prior to allo-HSCT is enriched with genes coding for drug resistance to macrolides [14].
2.6. Other Antibiotics
Other less studied antibiotics have been associated to different extents with microbiota modifications. Licosamides in healthy volunteers affected the GM substantially, resulting in a reduction of Lactobacilli, Enterococci and Bifidobacteria [85]. In hospitalized patients, clindamycin treatment was associated with increases in Escherichia spp., Salmonella spp. and Bacteroides spp. [72]. Aminoglycosides treatment in healthy volunteers has been associated with a decrease in Ruminococcaceae, Lachnospiraceae, Faecalibacterium spp. and Blautia spp. [97].
3. Chemotherapeutic Agents
The relationship between GM and chemotherapy drug metabolism represent a rapidly expanding field of research [98]. The reciprocal targeted modulation has demonstrated to affect both efficacy and adverse drug reactions of several drugs commonly used as part of pediatric antineoplastic treatments.
3.1. Irinotecan
Irinotecan is an antineoplastic agent used primarily for the treatment of soft tissue sarcomas, bone tumors and neuroblastoma in children [99,100]. It is administered as a prodrug, CPT-11, which requires enzymatic conversion by carboxylesterase. SN-38 is the active metabolite that acts as a topoisomerase I inhibitor, causing single-strand DNA breaks and ultimately cell death [101]. The SN-38 is then glucuronidated in the liver by UDP-glucuronosyltransferase into its less toxic derivate, SN-38-G, and later transported in the bile and excreted to the intestinal lumen. Some symbiotic species of GM can produce β-glucuronidase that reverts SN-38-G to its active and more toxic form, SN-38, increasing irinotecan intestinal toxicity. These bacteria include Escherichia coli, Bacteroides vulgatus, and Clostridium ramosum [23,102]. The main adverse effect associated with irinotecan is diarrhea, which can be acute or delayed [103]. Delayed-type diarrhea can be severe and potentially dose-limiting [104]. The exact mechanism is still unknown but probably directly mediated by high concentration of intraluminal SN-38 [105]. Interestingly, selective inhibition of β-glucuronidase activity has proven successful in alleviating intestinal toxicity in mice [102]. Furthermore, Irinotecan treatment itself can modify the host GM composition, increasing the number of β-glucuronidases-expressing species, such as E. coli, Staphylococcus spp., and Clostridium spp. [24], which could potentially amplify this effect. Considering the role of GM components on irinotecan metabolite-induced diarrhea, the potential utility of antibiotics coadministration with irinotecan has been studied, with positive results. The administration penicillin/streptomycin, to irinotecan-treated rats resulted in decreased levels of SN-38 in the feces and reduced diarrhea [105]. Although some early studies suggested a role of neomycin in reducing irinotecan-induced delayed-diarrhea [106], some later evidence mitigated these results [107]. Despite its possible utility, the use of concomitant prophylactic antibiotics with chemotherapy is controversial, due to possible emergence of antibiotic resistance and impact on GM composition. More specific strategies to target β-glucuronidase activity have been investigated, including the “old” drugs such as Amoxapine to inhibit β-glucuronidases [108]. 3D X-ray crystallographic data are also under investigation in order to rationally design a β-glucuronidase inhibitor [102]. More recent pharmacological compounds have been tested with positive results [109], but their application in clinical practice has not been yet validated.
3.2. Cyclophosphamide
Cyclophosphamide (CP) is an alkylating agent with immunosuppressant and anticancer effects. It is widely used in the treatment of immune dysregulatory conditions and malignancies. CP is a prodrug, which requires metabolic activation. Its active form exerts its therapeutic activity through several mechanisms. The main active metabolite is phosphoramide mustard, which forms irreversible DNA crosslinks both between and within DNA strands at guanine N-7 positions, leading to cell apoptosis [110]. CP also promotes the differentiation of antitumor Th1 and Th17 cells [111], depletes oncogenic regulatory T-cells and induces the production of pro-apoptotic cytokines, promoting immune-driven cancer cell death [112]. Viaud et al. demonstrated that germ-free mice, treated with broad-spectrum antibiotics, showed a significantly reduced anticancer response after CP administration [25]. CP administration resulted in increased IL-17 levels in specific-pathogen-free (SPF) mice against germ-free (GF) mice. The translocation of specific Gram-positive bacteria (such as Lactobacillus johnsonii and Enterococcus hirae) from the intestine to secondary lymphoid organs was critical for the differentiation of CD4+ T cells into Th1 and Th17 cells. Furthermore, CP and vancomycin cotreatment resulted in overall worse anticancer response in their animal model, altering CTX-induced Th17 differentiation, which is mandatory for the tumoricidal activity of chemotherapy [25]. More recent studies confirmed the synergic interplay between specific bacterial species (such as Enterococcus hirae and Barnesiella intestinihominis) and CP, facilitating its antitumoral activity [113]. Moreover, CP treatment has an impact on the GM composition. When comparing the GM of both CP-treated and CP-naïve mice, the treatment reduced fecal bacterial diversity, increased the proportion of Firmicutes, and decreased the proportion of Bacteroidetes bacteria [26]. Recent studies investigated the potential modulation of various polysaccharides compounds on the effects of CP on immune modulation, intestinal permeability, and microbial communities in the mouse with mixed results [26,114,115,116,117].
3.3. L-Asparaginase
L-Asparaginase (ASNase) is a critical chemotherapeutic compound of many acute lymphoblastic leukemia and lymphoma protocols [118,119]. ASNase breaks extracellular asparagine, an amino acid required for protein assembly of leukemic cells. ASNase treatment is often accompanied by severe adverse reactions. Since ASNase is a non-self-protein, antibodies may develop against it which can lead to hypersensitivity reactions occurring in 25% of patients and undetected inactivation of ASNase, which correlates with a poor response to treatment [120]. Two ASNase formulations are currently available, polyethylene-glycolated (PEG) form of the E. coli ASNase (PEG-ASNase) and Erwinia crysanthemi-derived ASPase [121]. Endogenous GM strains, such as Salmonella or Shigella flexneri, naturally produce the L-asparaginase periplasmic enzymes that are similar to PEG-ASNase for 96 and 99.1% of their molecular structure, respectively [118,122]. A recent study demonstrated that specific GM communities were associated with different ASNase activity levels in treated children. Escherichia predominated in the decreased-activity community while Bacteroides and Streptococcus predominated in the increased-activity community [27]. Furthermore, microbial ASNS was significantly negatively correlated with change in serum ASNase activity, although the mechanism remains unknown [27].
3.4. Other Chemotherapeutic Drugs
Other chemotherapeutic agents have shown interaction with GM composition. For example, the alkylating agent Melphalan has been found to be metabolized by “super metabolizer” bacterial strains such as Bacteroides dorei and Clostridium spp. [15]. In recent years, evidence emerged about the inactivation through deglycosylation of the widely adopted anthracycline Doxorubicin mediated by specific gut bacterial strains [123,124,125], but further studies are needed to confirm its clinical relevance in vivo.
4. Anti-Programmed Cell Death Proteins
In recent years, immunotherapies and immune checkpoint inhibitors emerged as alternatives or complementary to conventional chemotherapy in the treatment of various malignancies. The interaction between PD-L1 and PD-1 is intended as an immune checkpoint in various physiological situations, such as immune tolerance in pregnancy, to prevent self-rejection and minimize the inflammatory response. However, during many carcinogenic processes, the activation of the PD-L1/PD-1 signaling cascade results in decreased T-cell activation, leading to a reduced anticancer immune response [126,127]. A wide variety of anti-PD-1/PD-L1 antibodies have been developed to treat multiple malignancies including Hodgkin lymphoma, sarcomas, melanoma, and small cell lung cancers. However, the rise of anti-PD-1 therapy is accompanied by significant variability in patient response to these inhibitors. Given the recent understanding of the complex interaction between the GM and the immune response, researchers examined the interaction of the host gut microbial community with anti-PD-1/PD-L1 inhibitors. Sivan and colleagues firstly showed that commensal Bifidobacteria have a positive association with antitumor T-cell response and Bifidobacterium-treated mice showed a significant improvement in tumor control [128]. Studies on human stool samples from metastatic melanoma patients were also conducted. Through the integration of 16S ribosomal RNA gene sequencing, metagenomic shotgun sequencing, and quantitative polymerase chain reaction, a significant association between commensal microbial composition and clinical response was demonstrated, with Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium more abundant in stool samples of responders [129]. Moreover, the anti-PD-1 responders had presented greater levels of α-diversity and higher proportions of Ruminococcaceae, Faecalibacterium, and Bifidobacterium species [28]. Microbiome composition alters anti-PD1 response, with Akkermansia, Ruminococcus spp., Alistipes spp., and Eubacterium spp. being more represented in drug responders, while under-representation was found for Bifidobacterium adolescentis, B. long and Parabacteroids distasonis in drug responders [29]. Furthermore, avoiding antibiotics during anti-PD-1 treatment could increase patients’ positive responses from 25% up to 40%. In the setting of advanced melanoma, tumor cells may become resistant to anti-PD-1 agents. Davar et al. proposed fecal microbiota transplantation (FMT) as a strategy to promote a positive and durable gut microbiome perturbation. Responders exhibited an increased abundance of taxa that were previously shown to be associated with response to anti-PD-1, increased CD8+ T cell activation, and decreased frequency of interleukin-8-expressing myeloid cells. This reprogrammed tumor microenvironment did overcome resistance to anti-PD-1 in this subset of PD-1 advanced melanoma [7]. Notably, Tanoue et al. performed a phase 1/2 trial with an oral microbial product (VE800) that contains 11 clonal commensal bacterial strains from healthy human donor feces and that is capable of robustly inducing interferon-γ-producing CD8 T cells in the intestine. The study showed that colonization of mice with the 11-strain mixture enhances both host resistance against Listeria monocytogenes infection and the therapeutic efficacy of immune checkpoint inhibitors in syngeneic tumor models [130]. The impact of dietary habits and commercially available probiotic supplements on fecal microbiota profiles of patients treated with anti-PD-1 for melanoma, has also been investigated. 128 patients with sufficient dietary fiber intake showed a significant improvement in progression free survival compared to insufficient fiber intake, with no impact of probiotic administration [131]. Other microbiome-modifying interventions have been proposed to enhance immune checkpoint inhibitor antitumor activity, such as natural polyphenols [132] and ketogenic diet [133], but more evidence is needed to implement those interventions in routine clinical practice [22]. Similarly, anti-CTLA-4 antibodies are used as checkpoint inhibitors in many malignancies. Notably, GM composition seems to present a strong influence also on the efficacy of this form of immunotherapy [134]. These findings suggest that the GM has a significant impact on the efficacy of immune checkpoint inhibitors in a variety of malignancy models [135,136,137], especially in specific subsets of patients that show low response to this treatment.
5. Immunosuppressive Agents
The interaction between GM and host’s immune system is bidirectional. Microbiota plays a major role in the development and regulation of the immune system, while the immune system controls the microbiota through the production and secretion of antimicrobial peptides and secretory IgA [40,138].
5.1. Cyclosporine
Cyclosporine (CSA) is a calcineurin inhibitor able to bind to immunophilins called cyclophilins, leading to an increased cyclophilin affinity to calcineurin, a calmodulin-activated serine phosphatase that associates with NFAT (nuclear factor of activated T cells) and initiates events involved in T-cell activation. The complex cyclosporine-cyclophillin binds and inhibits calcineurin, blocking the synthesis of proinflammatory cytokines and interrupting the downstream sequence of events leading to rejection [139]. O’Reilly et al. noticed no significant difference in α or β diversity with CSA use. CSA decreases the viability of B. distasonis, but it does not affect Lactobacillus or Bifidobacterium species [31]. On the other hand, Junjun Jia et al. demonstrated in rats an improved diversity of the intestinal microbiota and a richness of species, with an enrichment of Enterobacteriaceae and a decrease of F. prausnitzii and Clostridium clusters I and XIV, with CSA use [30].
5.2. Tacrolimus
Similar to CSA, tacrolimus is also a calcineurin inhibitor, that binds to different immunophilins called FK-binding proteins, with a block of T-cell activation [139]. Tacrolimus in rats decreases microbial diversity and increases the Firmicutes/Bacteroidetes ratio [34]. Tacrolimus does not change the bacterial richness and diversity of GM, without difference at the phylum level, but with an increase in the relative abundance of Allobaculum, Bacteroides and Lactobacillus [32]. In rats, a decreased abundance of Mollicutes, Micrococcaceae, Actinomycetales, Roseburia, Oscillospira, Rothia and Staphylococcus and increased A. muciniphila was also observed [33]. Tacrolimus triggers a gut dysbiosis that is analogous to that observed in metabolic diseases, with increased Firmicutes/Bacteroides ratio [138]. Interestingly, in a rat model, Jiang at al showed that an intermediate dose was associated with an increase in beneficial bacteria (Bifidobacterium, F. prausnitzii) and a decrease in less beneficial bacteria (Enterobacteriaceae, Bacteroides-Prevotella). On the other hand, lower and higher doses were associated with increased abundance of Enterobacteriaceae and decrease of Bifidobacterium and F. prausnitzii [35]. Patients who required high doses of tacrolimus harbored a higher relative abundance of F. prausnitzii in their GM, and F. prausnitzii abundance at 1 week after kidney transplant was positively correlated with future dosing of tacrolimus at 1 month [140]. Some gut bacteria, such as F. prausnitzii or Clostridiales, seem to transform tacrolimus into a 15-fold less active metabolite in vitro.
5.3. Other Immunosuppressive Drugs
Mycophenolate mofetil (MMF) is a pro-drug that is converted into the active metabolite mycophenolic acid (MPA), which inhibits inosine monophosphate dehydrogenase and suppresses the proliferation of T and B lymphocytes [36]. MMF causes GI toxicity in 30–50% of patients, ranging from nausea, vomiting, diarrhea, abdominal pain to a colitis that resembles IBD [36,138]. In mice, MMF treatment causes a decrease in microbiota richness, with a reduction in Bacteroidetes and Verrucomicrobia phyla and in the genera Akkermansia, Parabacteroides and Clostridium, and increased Proteobacteria (mainly Escherichia/Shigella), Deferribacters and Firmicutes. These changes result in a shift of the microbiota toward one with greater pathogenic potential [36]. MMF has a narrow therapeutic index and blood concentrations of MPA are highly variable, probably also depending on MPA enterohepatic recirculation (EHR), with patients with higher HER having a better immunosuppression but also more concentration-dependent toxicities. Thus Saqr et al. studied how bacteria influence EHR, showing that MPA EHR is positively correlated with B. vulgatus and B. thetaiotaomicron and negatively correlated with Blautia hydrogenotrophica.
Rapamycin, also known as sirolimus, inhibits mTOR, a protein kinase that regulates cell growth, proliferation and survival, thus interfering with lymphocyte proliferation [138]. Bhat et al. showed in a rat model that bacterial diversity is significantly decreased with the use of rapamycin, with decreased Roseburia, Oscillospira, Mollicutes, Rothia, Micrococcaceae, Acninomycetales and Staphylococcus. Decreased Turicibacter, unclassified Marinilabiliaceae, Alloprevotella, unclassified Porphyromonadaceae, Ruminococcus, Bifidobacterium, Marvinbryantia, Helicobacter and Coprobacillus in rapamycim-treated mice, and increased Ruminococcus were also reported [37].
Alemtuzumab is a monoclonal antibody that targets CD52 expressed on T and B lymphocytes, natural killer cells and monocytes, inducing rapid depletion of T cells from peripheral blood. In a monkey model, Li et al. showed that alemtuzumab treatment causes reduced Lactobacillales, and increased Enterobacteriales and Prevotella. In the GM, they also noticed an increase in the Clostridiales order and a decrease in Faecalibacterium genus [38].
6. Steroids
Glucocorticoids are the mainstays in the treatment of inflammatory and autoimmune pathologies, and they are used as immunosuppressants following organ transplantation and as lympholytics in chemotherapeutic regimens. Glucocorticoids reduce inflammation by suppressing pro-inflammatory cytokine expression through inhibition and upregulation of gene transcription [141]. In a mouse model, glucocorticoids decreased bacterial richness and diversity, reduced relative abundance of Firmicutes, Bacteroides, Actinobacteria, alfa and gamma Proteobacteria, and decreased Clostridiales and Lactobacillus. On the other hand, steroids increased abundance of Proteobacteria, closely related to a proinflammatory state [40,41,42]. A decrease of Deferribacteres, Rikenella, Mucispirillum, Oscillospira and Bilophila, and an increase in Prevotella, Methanobrevibacter smithii and Anaerostipes were also reported [39,42,43]. In the gut, Clostridium scindens converts endogenous glucocorticoids into androgens [142]. It has been demonstrated that dexamethasone is metabolized into androgens by Clostridium scindens in vivo, with potential implications also for other steroids [15].
7. Protonic Pump Inhibitors
Protonic pump inhibitors (PPIs) are among the most commonly used drugs worldwide [16,143]. PPIs are prodrugs that need to be activated by addition of two protons and, once they are activated, they can inactivate the proton pump by binding to the H+-K+-ATPase that normally creates a 1 million-fold gradient in H+ concentration from inside the parietal cell to the gastric lumen in return for inward transport of K+ [143]. A first report on 20 children treated with PPI found no significant changes in overall number of species-level taxonomy categories but with an increase in the phylum Firmicutes in some subgroups [48]. Other studies demonstrated that PPI use is associated with an altered composition of the GM, with an increase in the Lactobacillales order and in particular the family Streptococcaceae, which has been associated with increased risk for C. difficile infection (CDI) [43,44,45,46,144]. A strong tendency of a reduction of Faecalibacterium, which is known to possess anti-inflammatory properties, was also reported [47]. Characteristic of the gut microbiome of PPI users are species highly prevalent in the oral microbiome, such as Streptococcus parasanguinis [43]. Higher dosages are associated with larger microbial changes and functional changes, such as an increase of fatty acid and lipid biosynthesis, fermentation NAD metabolism and biosynthesis of L-arginine and purine deoxyribonucleoside degradation [43]. These changes could result from downward movement of upper tract commensals due to removal of the gastric acid barrier by PPI, causing an “oralisation” of the GM in PPI users. However, PPI also have a direct effect on the GM, potentially generated through binding of PPIs to bacterial H+/K+ ATPases [16,145]. A trend toward reduced diversity was also reported in some reports, even if not always significant [44,45]. Other studies showed no major changes in diversity [46,47,48].
8. Ursodeoxycholic Acid
Ursodeoxycholic acid is a naturally occurring bile acid that is used to treat a variety of hepatic and gastrointestinal diseases and also specifically in HSCT, for prevention of hepatic complications. Bile acids have recently emerged as important regulators of the intestinal microbiota; thus, it is interesting to see how ursodiol modifies the microbiota, but not many studies have investigated this [146]. Studies conducted on patients with primary biliary cholangitis show that UDCA causes an increased abundance of the Enterobacteriaceae family [49]. Pearson et al. showed in patients with a story of colorectal adenomans that UDCA causes a shift in microbial community composition, with an increase in species of Streptococcus, Escherichia and Bilophila and decrease in Fusobacterium, and in particular an overrepresentation of Faecalibacterium prausnitzii and an underrepresentation of Ruminococcus gnavus [20]. In mice, ursodeoxycholic acid increases several key bile acid species, that in turn alter directly or indirectly the gut microbial composition [147].
9. Conclusions
We provided an outline of the interaction between commonly used drugs in pediatric oncology and the GM. Pharmacomicrobiomics is an emerging field of study that could change the outlook of research both regarding pharmacology and microbiome studies. The intestinal flora composition is an issue to be considered in the individual variability of pharmacokinetics, response to therapy and adverse event rate, other than the usual studied genetic polymorphism. The impact of drugs on the GM should be taken into consideration in the future among the factors that can influence trial design and drug prescribing, with potential implications related to GM modulation [148]. In the near future, testing of the microbiome may provide a tool to help guide initial dose selection and dose adjustments of selected drugs, such as in the case of MMF. Moreover, it could help in the personalized follow up of patients at higher risk of treatment-related toxicity or treatment failure. Thanks to this field of research, targeting the microbiome could be an interesting future perspective in order to reduce the number of poor responders or patients experiencing severe adverse events in selected cases. A deeper understanding of the biological mechanism underpinning this complex interplay is needed before translating the presented findings to clinical research. Moreover, dissecting the effect of single drugs in the human setting is complex, because in oncology different molecules are often administered together and pharmacological interactions are a key component in the management of pediatric cancer patients. A better knowledge on the impact of GM on drug metabolism could lead to fascinating results, potentially translating to clinical practice.
Author Contributions
Writing—original draft preparation, D.L., F.V., F.B., S.C. and E.M.; critical review and editing, P.B., A.P. (Andrea Pession), A.P. (Arcangelo Prete) and R.M.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived due to design of the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This paper was supported by the Cassa di Risparmio di Bologna (CARISBO) (Bando di Ricerca medica traslazionale e clinica 2019, code 2020CARISBO_MASETTI_R).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schluter J., Peled J.U., Taylor B.P., Markey K.A., Smith M., Taur Y., Niehus R., Staffas A., Dai A., Fontana E., et al. The Gut Microbiota Is Associated with Immune Cell Dynamics in Humans. Nature. 2020;588:303–307. doi: 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullin N., Azevedo Antunes C., Straussman R., Stein-Thoeringer C.K., Elinav E. Microbiome and Cancer. Cancer Cell. 2021;39:1317–1341. doi: 10.1016/j.ccell.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Masetti R., Muratore E., Leardini D., Zama D., Turroni S., Brigidi P., Esposito S., Pession A. Gut Microbiome in Pediatric Acute Leukemia: From Predisposition to Cure. Blood Adv. 2021;5:4619–4629. doi: 10.1182/bloodadvances.2021005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shono Y., Van Den Brink M.R.M. Gut Microbiota Injury in Allogeneic Haematopoietic Stem Cell Transplantation. Nat. Rev. Cancer. 2018;18:283–295. doi: 10.1038/nrc.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messina J.A., Tan C.Y., Ren Y., Hill L., Bush A., Lew M., Andermann T., Peled J.U., Gomes A., van den Brink M.R.M., et al. Enterococcus Intestinal Domination Is Associated with Increased Mortality in the Acute Leukemia Chemotherapy Population. Clin. Infect. Dis. 2021:ciab1043. doi: 10.1093/cid/ciab1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith M., Dai A., Ghilardi G., Amelsberg K.V., Devlin S.M., Pajarillo R., Slingerland J.B., Beghi S., Herrera P.S., Giardina P., et al. Gut Microbiome Correlates of Response and Toxicity Following Anti-CD19 CAR T Cell Therapy. Nat. Med. 2022;28:713–723. doi: 10.1038/s41591-022-01702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal Microbiota Transplant Overcomes Resistance to Anti-PD-1 Therapy in Melanoma Patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masetti R., Zama D., Leardini D., Muratore E., Turroni S., Prete A., Brigidi P., Pession A. The Gut Microbiome in Pediatric Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Pediatr. Blood Cancer. 2020;67:e28711. doi: 10.1002/pbc.28711. [DOI] [PubMed] [Google Scholar]
- 9.Henig I., Yehudai-Ofir D., Zuckerman T. The Clinical Role of the Gut Microbiome and Fecal Microbiota Transplantation in Allogeneic Stem Cell Transplantation. Haematologica. 2021;106:933–946. doi: 10.3324/haematol.2020.247395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zama D., Gori D., Muratore E., Leardini D., Rallo F., Turroni S., Prete A., Brigidi P., Pession A., Masetti R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2021;27:e1–e180. doi: 10.1016/j.jtct.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Weber D., Jenq R.R., Peled J.U., Taur Y., Hiergeist A., Koestler J., Dettmer K., Weber M., Wolff D., Hahn J., et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017;23:845–852. doi: 10.1016/j.bbmt.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingham A.C., Kielsen K., Cilieborg M.S., Lund O., Holmes S., Aarestrup F.M., Müller K.G., Pamp S.J. Specific Gut Microbiome Members Are Associated with Distinct Immune Markers in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation. Microbiome. 2019;7:131. doi: 10.1186/s40168-019-0745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masetti R., Zama D., Leardini D., Muratore E., Turroni S., Brigidi P., Pession A. Microbiome-Derived Metabolites in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2021;22:1197. doi: 10.3390/ijms22031197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amico F., Soverini M., Zama D., Consolandi C., Severgnini M., Prete A., Pession A., Barone M., Turroni S., Biagi E., et al. Gut Resistome Plasticity in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation. Sci. Rep. 2019;9:5649. doi: 10.1038/s41598-019-42222-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann M., Zimmermann-Kogadeeva M., Wegmann R., Goodman A.L. Mapping Human Microbiome Drug Metabolism by Gut Bacteria and Their Genes. Nature. 2019;570:462–467. doi: 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weersma R.K., Zhernakova A., Fu J. Interaction between Drugs and the Gut Microbiome. Gut. 2020;69:1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klünemann M., Andrejev S., Blasche S., Mateus A., Phapale P., Devendran S., Vappiani J., Simon B., Scott T.A., Kafkia E., et al. Bioaccumulation of Therapeutic Drugs by Human Gut Bacteria. Nature. 2021;597:533–538. doi: 10.1038/s41586-021-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn M.A., Eden G., Ryan F.J., Bensalem J., Wang X., Blake S.J., Choo J.M., Chern Y.T., Sribnaia A., James J., et al. The Composition of the Gut Microbiota Following Early-Life Antibiotic Exposure Affects Host Health and Longevity in Later Life. Cell Rep. 2021;36:109564. doi: 10.1016/j.celrep.2021.109564. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W., An Y., Qin X., Wu X., Wang X., Hou H., Song X., Liu T., Wang B., Huang X., et al. Gut Microbiota-Derived Metabolites in Colorectal Cancer: The Bad and the Challenges. Front. Oncol. 2021;11:739648. doi: 10.3389/fonc.2021.739648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson T., Caporaso J.G., Yellowhair M., Bokulich N.A., Padi M., Roe D.J., Wertheim B.C., Linhart M., Martinez J.A., Bilagody C., et al. Effects of Ursodeoxycholic Acid on the Gut Microbiome and Colorectal Adenoma Development. Cancer Med. 2019;8:617–628. doi: 10.1002/cam4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotz S.J., Dandoy C.E. The Microbiome in Pediatric Oncology. Cancer. 2020;126:3629–3637. doi: 10.1002/cncr.33030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muratore E., Leardini D., Baccelli F., Venturelli F., Prete A., Masetti R. Nutritional Modulation of the Gut Microbiome in Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Front. Nutr. 2022;9:993668. doi: 10.3389/fnut.2022.993668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace B.D., Roberts A.B., Pollet R.M., Ingle J.D., Biernat K.A., Pellock S.J., Venkatesh M.K., Guthrie L., O’Neal S.K., Robinson S.J., et al. Structure and Inhibition of Microbiome β-Glucuronidases Essential to the Alleviation of Cancer Drug Toxicity. Chem. Biol. 2015;22:1238–1249. doi: 10.1016/j.chembiol.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringer A.M., Gibson R.J., Logan R.M., Bowen J.M., Yeoh A.S.J., Keefe D.M.K. Faecal Microflora and Beta-Glucuronidase Expression Are Altered in an Irinotecan-Induced Diarrhea Model in Rats. Cancer Biol. Ther. 2008;7:1919–1925. doi: 10.4161/cbt.7.12.6940. [DOI] [PubMed] [Google Scholar]
- 25.Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillère R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Zhang X. Effects of Cyclophosphamide on Immune System and Gut Microbiota in Mice. Microbiol. Res. 2015;171:97–106. doi: 10.1016/j.micres.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Dunn K.A., Connors J., Bielawski J.P., Nearing J.T., Langille M.G.I., Van Limbergen J., Fernandez C.V., MacDonald T., Kulkarni K. Investigating the Gut Microbial Community and Genes in Children with Differing Levels of Change in Serum Asparaginase Activity during Pegaspargase Treatment for Acute Lymphoblastic Leukemia. Leuk. Lymphoma. 2021;62:927–936. doi: 10.1080/10428194.2020.1850718. [DOI] [PubMed] [Google Scholar]
- 28.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 30.Jia J., Tian X., Jiang J., Ren Z., Lu H., He N., Xie H., Zhou L., Zheng S. Structural Shifts in the Intestinal Microbiota of Rats Treated with Cyclosporine A after Orthotropic Liver Transplantation. Front. Med. 2019;13:451–460. doi: 10.1007/s11684-018-0675-3. [DOI] [PubMed] [Google Scholar]
- 31.O’Reilly C., O’Sullivan Ó., Cotter P.D., O’Connor P.M., Shanahan F., Cullen A., Rea M.C., Hill C., Coulter I., Paul Ross R. Encapsulated Cyclosporine Does Not Change the Composition of the Human Microbiota When Assessed Ex Vivo and In Vivo. J. Med. Microbiol. 2020;69:854–863. doi: 10.1099/jmm.0.001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Liu L., Tang H., Jiao W., Zeng S., Xu Y., Zhang Q., Sun Z., Mukherjee A., Zhang X., et al. Immunosuppressive Effect of the Gut Microbiome Altered by High-Dose Tacrolimus in Mice. Am. J. Transplant. 2018;18:1646–1656. doi: 10.1111/ajt.14661. [DOI] [PubMed] [Google Scholar]
- 33.Bhat M., Pasini E., Copeland J., Angeli M., Husain S., Kumar D., Renner E., Teterina A., Allard J., Guttman D.S., et al. Impact of Immunosuppression on the Metagenomic Composition of the Intestinal Microbiome: A Systems Biology Approach to Post-Transplant Diabetes. Sci. Rep. 2017;7:10277. doi: 10.1038/s41598-017-10471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toral M., Romero M., Rodríguez-Nogales A., Jiménez R., Robles-Vera I., Algieri F., Chueca-Porcuna N., Sánchez M., de la Visitación N., Olivares M., et al. Lactobacillus Fermentum Improves Tacrolimus-Induced Hypertension by Restoring Vascular Redox State and Improving ENOS Coupling. Mol. Nutr. Food Res. 2018;62:1–13. doi: 10.1002/mnfr.201800033. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J.W., Ren Z.G., Lu H.F., Zhang H., Li A., Cui G.Y., Jia J.J., Xie H.Y., Chen X.H., He Y., et al. Optimal Immunosuppressor Induces Stable Gut Microbiota after Liver Transplantation. World J. Gastroenterol. 2018;24:3871–3883. doi: 10.3748/wjg.v24.i34.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flannigan K.L., Taylor M.R., Pereira S.K., Rodriguez-Arguello J., Moffat A.W., Alston L., Wang X., Poon K.K., Beck P.L., Rioux K.P., et al. An Intact Microbiota Is Required for the Gastrointestinal Toxicity of the Immunosuppressant Mycophenolate Mofetil. J. Heart Lung Transplant. 2018;37:1047–1059. doi: 10.1016/j.healun.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Jung M.J., Lee J., Shin N.R., Kim M.S., Hyun D.W., Yun J.H., Kim P.S., Whon T.W., Bae J.W. Chronic Repression of MTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-Induced Obese Mice. Sci. Rep. 2016;6:30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q.R., Wang C.Y., Tang C., He Q., Li N., Li J.S. Reciprocal Interaction between Intestinal Microbiota and Mucosal Lymphocyte in Cynomolgus Monkeys after Alemtuzumab Treatment. Am. J. Transplant. 2013;13:899–910. doi: 10.1111/ajt.12148. [DOI] [PubMed] [Google Scholar]
- 39.Lee J.R., Muthukumar T., Dadhania D., Toussaint N.C., Ling L., Pamer E., Suthantiran M. Gut Microbial Community Structure and Complications Following Kidney Transplantation: A Pilot Study. Transplantation. 2014;98:697–705. doi: 10.1097/TP.0000000000000370.Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tourret J., Willing B.P., Dion S., MacPherson J., Denamur E., Finlay B.B. Immunosuppressive Treatment Alters Secretion of Ileal Antimicrobial Peptides and Gut Microbiota, and Favors Subsequent Colonization by Uropathogenic Escherichia coli. Transplantation. 2017;101:74–82. doi: 10.1097/TP.0000000000001492. [DOI] [PubMed] [Google Scholar]
- 41.Wu T., Yang L., Jiang J., Ni Y., Zhu J., Zheng X., Wang Q., Lu X., Fu Z. Chronic Glucocorticoid Treatment Induced Circadian Clock Disorder Leads to Lipid Metabolism and Gut Microbiota Alterations in Rats. Life Sci. 2018;192:173–182. doi: 10.1016/j.lfs.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 42.He Z., Kong X., Shao T., Zhang Y., Wen C. Alterations of the Gut Microbiota Associated with Promoting Efficacy of Prednisone by Bromofuranone in MRL/Lpr Mice. Front. Microbiol. 2019;10:978. doi: 10.3389/fmicb.2019.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vich Vila A., Collij V., Sanna S., Sinha T., Imhann F., Bourgonje A.R., Mujagic Z., Jonkers D.M.A.E., Masclee A.A.M., Fu J., et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020;11:362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson M.A., Goodrich J.K., Maxan M.E., Freedberg D.E., Abrams J.A., Poole A.C., Sutter J.L., Welter D., Ley R.E., Bell J.T., et al. Proton Pump Inhibitors Alter the Composition of the Gut Microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imhann F., Bonder M.J., Vila A.V., Fu J., Mujagic Z., Vork L., Tigchelaar E.F., Jankipersadsing S.A., Cenit M.C., Harmsen H.J.M., et al. Proton Pump Inhibitors Affect the Gut Microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedberg D.E., Toussaint N.C., Chen S.P., Ratner A.J., Whittier S., Wang T.C., Wang H.H., Abrams J.A. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149:883–885.e9. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuda A., Suda W., Morita H., Takanashi K., Takagi A., Koga Y., Hattori M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin. Transl. Gastroenterol. 2015;6:e89. doi: 10.1038/ctg.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simakachorn L., Tanpowpong P., Chanprasertyothin S., Thongpradit S., Treepongkaruna S. Gut Microbiota Characteristics in Children after the Use of Proton Pump Inhibitors. Turkish J. Gastroenterol. 2021;32:70–75. doi: 10.5152/tjg.2020.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang R., Wei Y., Li Y., Chen W., Chen H., Wang Q., Yang F., Miao Q., Xiao X., Zhang H., et al. Gut Microbial Profile Is Altered in Primary Biliary Cholangitis and Partially Restored after UDCA Therapy. Gut. 2018;67:534–571. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 50.Ianiro G., Tilg H., Gasbarrini A. Antibiotics as Deep Modulators of Gut Microbiota: Between Good and Evil. Gut. 2016;65:1906–1915. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 51.Theriot C.M., Young V.B. Interactions Between the Gastrointestinal Microbiome and Clostridium Difficile. Annu. Rev. Microbiol. 2015;69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C., Lieberman S.R., Bhatt A.S., Jenq R.R., Xu K., Gomes C., Gyurkocza B., Moss E.L., Jay H.V., Calarfiore M., et al. Increased GVHD-Related Mortality with Broad-Spectrum Antibiotic Use after Allogeneic Hematopoietic Stem Cell Transplantation in Human Patients and Mice. Sci. Transl. Med. 2016;8:339ra71. doi: 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jernberg C., Löfmark S., Edlund C., Jansson J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiology. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 54.Muratore E., Baccelli F., Leardini D., Campoli C., Belotti T., Viale P., Prete A., Pession A., Masetti R., Zama D. Antimicrobial Stewardship Interventions in Pediatric Oncology: A Systematic Review. J. Clin. Med. 2022;11:4545. doi: 10.3390/jcm11154545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nel Van Zyl K., Matukane S.R., Hamman B.L., Whitelaw A.C., Newton-Foot M. Effect of Antibiotics on the Human Microbiome: A Systematic Review. Int. J. Antimicrob. Agents. 2022;59:106502. doi: 10.1016/j.ijantimicag.2021.106502. [DOI] [PubMed] [Google Scholar]
- 56.Livadiotti S., Milano G.M., Serra A., Folgori L., Jenkner A., Castagnola E., Cesaro S., Rossi M.R., Barone A., Zanazzo G., et al. A Survey on Hematology-Oncology Pediatric AIEOP Centers: Prophylaxis, Empirical Therapy and Nursing Prevention Procedures of Infectious Complications. Haematologica. 2012;97:147–150. doi: 10.3324/haematol.2011.048918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zama D., Masetti R., Baccelli F., Leardini D., Muratore E., Abram N., Vendemini F., Biffi A., Perruccio K., D’Amico M.R., et al. Antibiotic Prophylaxis and Management of Infections in Pediatric Hematopoietic Stem Cell Transplantation: A Survey from the Stem Cell Transplant and the Infectious Disease Working Groups of the AIEOP Network. Bone Marrow Transplant. 2022;57:1851–1853. doi: 10.1038/s41409-022-01793-5. [DOI] [PubMed] [Google Scholar]
- 58.Lehrnbecher T., Averbuch D., Castagnola E., Cesaro S., Ammann R.A., Garcia-Vidal C., Kanerva J., Lanternier F., Mesini A., Mikulska M., et al. 8th European Conference on Infections in Leukaemia: 2020 Guidelines for the Use of Antibiotics in Paediatric Patients with Cancer or Post-Haematopoietic Cell Transplantation. Lancet Oncol. 2021;22:e270–e280. doi: 10.1016/S1470-2045(20)30725-7. [DOI] [PubMed] [Google Scholar]
- 59.Raymond F., Ouameur A.A., Déraspe M., Iqbal N., Gingras H., Dridi B., Leprohon P., Plante P.L., Giroux R., Bérubé È., et al. The Initial State of the Human Gut Microbiome Determines Its Reshaping by Antibiotics. ISME J. 2016;10:707–720. doi: 10.1038/ismej.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacPherson C.W., Mathieu O., Tremblay J., Champagne J., Nantel A., Girard S.A., Tompkins T.A. Gut Bacterial Microbiota and Its Resistome Rapidly Recover to Basal State Levels after Short-Term Amoxicillin-Clavulanic Acid Treatment in Healthy Adults. Sci. Rep. 2018;8:11192. doi: 10.1038/s41598-018-29229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pallav K., Dowd S.E., Villafuerte J., Yang X., Kabbani T., Hansen J., Dennis M., Leffler D.A., Newburg D.S., Kelly C.P. Effects of Polysaccharopeptide from Trametes Versicolor and Amoxicillin on the Gut Microbiome of Healthy Volunteers: A Randomized Clinical Trial. Gut Microbes. 2014;5:458–467. doi: 10.4161/gmic.29558. [DOI] [PubMed] [Google Scholar]
- 62.Brismar B., Edlund C., Nord C.E. Impact of Cefpodoxime Proxetil and Amoxicillin on the Normal Oral and Intestinal Microflora. Eur. J. Clin. Microbiol. Infect. Dis. 1993;12:714–719. doi: 10.1007/BF02009388. [DOI] [PubMed] [Google Scholar]
- 63.Zaura E., Brandt B.W., de Mattos M.J.T., Buijs M.J., Caspers M.P.M., Rashid M.U., Weintraub A., Nord C.E., Savell A., Hu Y., et al. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. MBio. 2015;6:e01693-15. doi: 10.1128/mBio.01693-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De La Cochetière M.F., Durand T., Lepage P., Bourreille A., Galmiche J.P., Doré J. Resilience of the Dominant Human Fecal Microbiota upon Short-Course Antibiotic Challenge. J. Clin. Microbiol. 2005;43:5588–5592. doi: 10.1128/JCM.43.11.5588-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romick-Rosendale L.E., Haslam D.B., Lane A., Denson L., Lake K., Wilkey A., Watanabe M., Bauer S., Litts B., Luebbering N., et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2018;24:2418–2424. doi: 10.1016/j.bbmt.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 66.Heimdahl A., Nord C.E. Effect of Phenoxymethylpenicillin and Clindamycin on the Oral, Throat and Faecal Microflora of Man. Scand. J. Infect. Dis. 1979;11:233–242. doi: 10.3109/inf.1979.11.issue-3.11. [DOI] [PubMed] [Google Scholar]
- 67.Calitri C., Ruberto E., Castagnola E. Antibiotic Prophylaxis in Neutropenic Children with Acute Leukemia: Do the Presently Available Data Really Support This Practice? Eur. J. Haematol. 2018;101:721–727. doi: 10.1111/ejh.13162. [DOI] [PubMed] [Google Scholar]
- 68.Rashid M.-U., Rosenborg S., Panagiotidis G., Löfdal K.S., Weintraub A., Nord C.E. Ecological Effect of Ceftazidime/Avibactam on the Normal Human Intestinal Microbiota. Int. J. Antimicrob. Agents. 2015;46:60–65. doi: 10.1016/j.ijantimicag.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 69.Stein-Thoeringer C.K., Nichols K.B., Lazrak A., Docampo M.D., Slingerland A.E., Slingerland J.B., Clurman A.G., Armijo G., Gomes A.L.C., Shono Y., et al. Lactose Drives Enterococcus Expansion to Promote Graft-versus-Host Disease. Science. 2019;366:1143–1149. doi: 10.1126/science.aax3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmermann P., Curtis N. The Effect of Antibiotics on the Composition of the Intestinal Microbiota—A Systematic Review. J. Infect. 2019;79:471–489. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Meijer-Severs G.J., Van Santen E., Meijer B.C. Short-Chain Fatty Acid and Organic Acid Concentrations in Feces of Healthy Human Volunteers and Their Correlations with Anaerobe Cultural Counts during Systemic Ceftriaxone Administration. Scand. J. Gastroenterol. 1990;25:698–704. doi: 10.3109/00365529008997595. [DOI] [PubMed] [Google Scholar]
- 72.Pérez-Cobas A.E., Artacho A., Knecht H., Ferrús M.L., Friedrichs A., Ott S.J., Moya A., Latorre A., Gosalbes M.J. Differential Effects of Antibiotic Therapy on the Structure and Function of Human Gut Microbiota. PLoS ONE. 2013;8:e80201. doi: 10.1371/journal.pone.0080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zwittink R.D., Renes I.B., van Lingen R.A., van Zoeren-Grobben D., Konstanti P., Norbruis O.F., Martin R., Groot Jebbink L.J.M., Knol J., Belzer C. Association between Duration of Intravenous Antibiotic Administration and Early-Life Microbiota Development in Late-Preterm Infants. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:475–483. doi: 10.1007/s10096-018-3193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Payne L.E., Gagnon D.J., Riker R.R., Seder D.B., Glisic E.K., Morris J.G., Fraser G.L. Cefepime-Induced Neurotoxicity: A Systematic Review. Crit. Care. 2017;21:276. doi: 10.1186/s13054-017-1856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee S.E., Lim J.Y., Bin Ryu D., Kim T.W., Park S.S., Jeon Y.W., Yoon J.H., Cho B.S., Eom K.S., Kim Y.J., et al. Alteration of the Intestinal Microbiota by Broad-Spectrum Antibiotic Use Correlates with the Occurrence of Intestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019;25:1933–1943. doi: 10.1016/j.bbmt.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Bächer K., Schaeffer M., Lode H., Nord C.E., Borner K., Koeppe P. Multiple Dose Pharmacokinetics, Safety, and Effects on Faecal Microflora, of Cefepime in Healthy Volunteers. J. Antimicrob. Chemother. 1992;30:365–375. doi: 10.1093/jac/30.3.365. [DOI] [PubMed] [Google Scholar]
- 77.Pletz M.W.R., Rau M., Bulitta J., De Roux A., Burkhardt O., Kruse G., Kurowski M., Nord C.E., Lode H. Ertapenem Pharmacokinetics and Impact on Intestinal Microflora, in Comparison to Those of Ceftriaxone, after Multiple Dosing in Male and Female Volunteers. Antimicrob. Agents Chemother. 2004;48:3765–3772. doi: 10.1128/AAC.48.10.3765-3772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Limaye P.B., Renaud H.J., Klaassen C.D. Effect of Various Antibiotics on Modulation of Intestinal Microbiota and Bile Acid Profile in Mice. Toxicol. Appl. Pharmacol. 2014;277:138–145. doi: 10.1016/j.taap.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu S.-L., Gong Y., Zhang J., Chen Y., Wu Z., Xu Q., Fang Y., Wang J., Tang L.-L. Effect of the Short-Term Use of Fluoroquinolone and β-Lactam Antibiotics on Mouse Gut Microbiota. Infect. Drug Resist. 2020;13:4547–4558. doi: 10.2147/IDR.S281274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peled J.U., Devlin S.M., Staffas A., Lumish M., Khanin R., Littmann E.R., Ling L., Kosuri S., Maloy M., Slingerland J.B., et al. Intestinal Microbiota and Relapse after Hematopoietic-Cell Transplantation. J. Clin. Oncol. 2017;35:1650–1659. doi: 10.1200/JCO.2016.70.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alexander S., Fisher B.B.T.B.T., Gaur A.H.H., Dvorak C.C.C., Villa Luna D., Dang H., Chen L., Green M., Nieder M.L.L., Fisher B.B.T.B.T., et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children With Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation. JAMA. 2018;320:995. doi: 10.1001/jama.2018.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leardini D., Muratore E., Abram N., Baccelli F., Belotti T., Prete A., Gori D., Masetti R., Oncology P., Unit H., et al. Effectiveness of Quinolone Prophylaxis in Pediatric Acute Leukemia and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2022:ofac594. doi: 10.1093/ofid/ofac594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olson J., Mehra S., Hersh A.L., Thorell E.A., Stoddard G.J., Maese L., Barnette P.E., Lemons R.S., Pavia A.T., Knackstedt E.D. Oral Step-Down Therapy With Levofloxacin for Febrile Neutropenia in Children With Cancer. J. Pediatr. Infect. Dis. Soc. 2021;10:27–33. doi: 10.1093/jpids/piaa015. [DOI] [PubMed] [Google Scholar]
- 84.Stewardson A.J., Gaïa N., François P., Malhotra-Kumar S., Delémont C., Martinez de Tejada B., Schrenzel J., Harbarth S., Lazarevic V., Vervoort J., et al. Collateral Damage from Oral Ciprofloxacin versus Nitrofurantoin in Outpatients with Urinary Tract Infections: A Culture-Free Analysis of Gut Microbiota. Clin. Microbiol. Infect. 2015;21:e1–e344. doi: 10.1016/j.cmi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 85.Rashid M.U., Zaura E., Buijs M.J., Keijser B.J.F., Crielaard W., Nord C.E., Weintraub A. Determining the Long-Term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin. Infect. Dis. 2015;60:S77–S84. doi: 10.1093/cid/civ137. [DOI] [PubMed] [Google Scholar]
- 86.Pérez-Cobas A.E., Gosalbes M.J., Friedrichs A., Knecht H., Artacho A., Eismann K., Otto W., Rojo D., Bargiela R., Von Bergen M., et al. Gut Microbiota Disturbance during Antibiotic Therapy: A Multi-Omic Approach. Gut. 2013;62:1591–1601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jothishankar B., Di Raimondo C., Mueller L., Zain J., Parekh V., Abdulla F. Primary Cutaneous Marginal Zone Lymphoma Treated with Doxycycline in a Pediatric Patient. Pediatr. Dermatol. 2020;37:759–761. doi: 10.1111/pde.14165. [DOI] [PubMed] [Google Scholar]
- 88.Thompson K.G., Rainer B.M., Antonescu C., Florea L., Mongodin E.F., Kang S., Chien A.L. Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients. Ann. Dermatol. 2020;32:21–30. doi: 10.5021/ad.2020.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vrieze A., Out C., Fuentes S., Jonker L., Reuling I., Kootte R.S., van Nood E., Holleman F., Knaapen M., Romijn J.A., et al. Impact of Oral Vancomycin on Gut Microbiota, Bile Acid Metabolism, and Insulin Sensitivity. J. Hepatol. 2014;60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 90.Hwang I., Park Y.J., Kim Y.-R., Kim Y.N., Ka S., Lee H.Y., Seong J.K., Seok Y.-J., Kim J.B. Alteration of Gut Microbiota by Vancomycin and Bacitracin Improves Insulin Resistance via Glucagon-like Peptide 1 in Diet-Induced Obesity. FASEB J. 2015;29:2397–2411. doi: 10.1096/fj.14-265983. [DOI] [PubMed] [Google Scholar]
- 91.Hansen C.H.F., Krych L., Nielsen D.S., Vogensen F.K., Hansen L.H., Sørensen S.J., Buschard K., Hansen A.K. Early Life Treatment with Vancomycin Propagates Akkermansia Muciniphila and Reduces Diabetes Incidence in the NOD Mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 92.Masetti R., D’Amico F., Zama D., Leardini D., Muratore E., Ussowicz M., Fraczkiewicz J., Cesaro S., Caddeo G., Pezzella V., et al. Febrile Neutropenia Duration Is Associated with the Severity of Gut Microbiota Dysbiosis in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Cancers. 2022;14:1932. doi: 10.3390/cancers14081932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doan T., Arzika A.M., Ray K.J., Cotter S.Y., Kim J., Maliki R., Zhong L., Zhou Z., Porco T.C., Vanderschelden B., et al. Gut Microbial Diversity in Antibiotic-Naive Children after Systemic Antibiotic Exposure: A Randomized Controlled Trial. Clin. Infect. Dis. 2017;64:1147–1153. doi: 10.1093/cid/cix141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abeles S.R., Jones M.B., Santiago-Rodriguez T.M., Ly M., Klitgord N., Yooseph S., Nelson K.E., Pride D.T. Microbial Diversity in Individuals and Their Household Contacts Following Typical Antibiotic Courses. Microbiome. 2016;4:39. doi: 10.1186/s40168-016-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nikolaou E., Kamilari E., Savkov D., Sergeev A., Zakharova I., Vogazianos P., Tomazou M., Antoniades A., Shammas C. Intestinal Microbiome Analysis Demonstrates Azithromycin Post-Treatment Effects Improve When Combined with Lactulose. World J. Pediatr. 2020;16:168–176. doi: 10.1007/s12519-019-00315-6. [DOI] [PubMed] [Google Scholar]
- 96.Hakim H., Dallas R., Wolf J., Tang L., Schultz-Cherry S., Darling V., Johnson C., Karlsson E.A., Chang T.C., Jeha S., et al. Gut Microbiome Composition Predicts Infection Risk during Chemotherapy in Children with Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2018;67:541–548. doi: 10.1093/cid/ciy153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heinsen F.A., Knecht H., Neulinger S.C., Schmitz R.A., Knecht C., Kühbacher T., Rosenstiel P.C., Schreiber S., Friedrichs A.K., Ott S.J. Dynamic Changes of the Luminal and Mucosaassociated Gut Microbiota during and after Antibiotic Therapy with Paromomycin. Gut Microbes. 2015;6:243–254. doi: 10.1080/19490976.2015.1062959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park E.M., Chelvanambi M., Bhutiani N., Kroemer G., Zitvogel L., Wargo J.A. Targeting the Gut and Tumor Microbiota in Cancer. Nat. Med. 2022;28:690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 99.Yohe M.E., Heske C.M., Stewart E., Adamson P.C., Ahmed N., Antonescu C.R., Chen E., Collins N., Ehrlich A., Galindo R.L., et al. Insights into Pediatric Rhabdomyosarcoma Research: Challenges and Goals. Pediatr. Blood Cancer. 2019;66:e27869. doi: 10.1002/pbc.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bagatell R., London W.B., Wagner L.M., Voss S.D., Stewart C.F., Maris J.M., Kretschmar C., Cohn S.L. Phase II Study of Irinotecan and Temozolomide in Children with Relapsed or Refractory Neuroblastoma: A Children’s Oncology Group Study. J. Clin. Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawato Y., Aonuma M., Hirota Y., Kuga H., Sato K. Intracellular Roles of SN-38, a Metabolite of the Camptothecin Derivative CPT-11, in the Antitumor Effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 102.Wallace B.D., Wang H., Lane K.T., Scott J.E., Orans J., Koo J.S., Venkatesh M., Jobin C., Yeh L.A., Mani S., et al. Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gibson R.J., Stringer A.M. Chemotherapy-Induced Diarrhoea. Curr. Opin. Support. Palliat. Care. 2009;3:31–35. doi: 10.1097/SPC.0b013e32832531bb. [DOI] [PubMed] [Google Scholar]
- 104.McQuade R.M., Stojanovska V., Donald E.L., Rahman A.A., Campelj D.G., Abalo R., Rybalka E., Bornstein J.C., Nurgali K. Irinotecan-Induced Gastrointestinal Dysfunction Is Associated with Enteric Neuropathy, but Increased Numbers of Cholinergic Myenteric Neurons. Front. Physiol. 2017;8:391. doi: 10.3389/fphys.2017.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takasuna K., Hagiwara T., Hirohashi M., Kato M., Nomura M., Nagai E., Yokoi T., Kamataki T. Inhibition of Intestinal Microflora β-Glucuronidase Modifies the Distribution of the Active Metabolite of the Antitumor Agent, Irinotecan Hydrochloride (CPT-11) in Rats. Cancer Chemother. Pharmacol. 1998;42:280–286. doi: 10.1007/s002800050818. [DOI] [PubMed] [Google Scholar]
- 106.Modulation of Irinotecan-Induced Diarrhea by Cotreatment with Neomycin in Cancer Patients—PubMed. [(accessed on 23 April 2022)]; Available online: https://pubmed.ncbi.nlm.nih.gov/11350876/ [PubMed]
- 107.De Jong F.A., Kehrer D.F.S., Mathijssen R.H.J., Creemers G.-J., de Bruijn P., van Schaik R.H.N., Planting A.S.T., van der Gaast A., Eskens F.A.L.M., Janssen J.T.P., et al. Prophylaxis of Irinotecan-Induced Diarrhea with Neomycin and Potential Role for UGT1A1*28 Genotype Screening: A Double-Blind, Randomized, Placebo-Controlled Study. Oncologist. 2006;11:944–954. doi: 10.1634/theoncologist.11-8-944. [DOI] [PubMed] [Google Scholar]
- 108.Kong R., Liu T., Zhu X., Ahmad S., Williams A.L., Phan A.T., Zhao H., Scott J.E., Yeh L.A., Wong S.T.C. Old Drug New Use--Amoxapine and Its Metabolites as Potent Bacterial β-Glucuronidase Inhibitors for Alleviating Cancer Drug Toxicity. Clin. Cancer Res. 2014;20:3521–3530. doi: 10.1158/1078-0432.CCR-14-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yue B., Gao R., Lv C., Yu Z., Wang H., Geng X., Wang Z., Dou W. Berberine Improves Irinotecan-Induced Intestinal Mucositis Without Impairing the Anti-Colorectal Cancer Efficacy of Irinotecan by Inhibiting Bacterial β-Glucuronidase. Front. Pharmacol. 2021;12:2880. doi: 10.3389/fphar.2021.774560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Emadi A., Jones R.J., Brodsky R.A. Cyclophosphamide and Cancer: Golden Anniversary. Nat. Rev. Clin. Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 111.Viaud S., Flament C., Zoubir M., Pautier P., LeCesne A., Ribrag V., Soria J.C., Marty V., Vielh P., Robert C., et al. Cyclophosphamide Induces Differentiation of Th17 Cells in Cancer Patients. Cancer Res. 2011;71:661–665. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]
- 112.Sistigu A., Viaud S., Chaput N., Bracci L., Proietti E., Zitvogel L. Immunomodulatory Effects of Cyclophosphamide and Implementations for Vaccine Design. Semin. Immunopathol. 2011;33:369–383. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 113.Daillère R., Vétizou M., Waldschmitt N., Yamazaki T., Isnard C., Poirier-Colame V., Duong C.P.M., Flament C., Lepage P., Roberti M.P., et al. Enterococcus Hirae and Barnesiella Intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Wang J., Li M., Gao Y., Li H., Fang L., Liu C., Liu X., Min W. Effects of Exopolysaccharides from Lactiplantibacillus Plantarum JLAU103 on Intestinal Immune Response, Oxidative Stress, and Microbial Communities in Cyclophosphamide-Induced Immunosuppressed Mice. J. Agric. Food Chem. 2022;70:2197–2210. doi: 10.1021/acs.jafc.1c06502. [DOI] [PubMed] [Google Scholar]
- 115.Bai Y., Zeng Z., Xie Z., Chen G., Chen D., Sun Y., Zeng X., Liu Z. Effects of Polysaccharides from Fuzhuan Brick Tea on Immune Function and Gut Microbiota of Cyclophosphamide-Treated Mice. J. Nutr. Biochem. 2022;101:108947. doi: 10.1016/j.jnutbio.2022.108947. [DOI] [PubMed] [Google Scholar]
- 116.Han C., Wang Y., Liu R., Ran B., Li W. Structural Characterization and Protective Effect of Lonicerae Flos Polysaccharide on Cyclophosphamide-Induced Immunosuppression in Mice. Ecotoxicol. Environ. Saf. 2022;230:113174. doi: 10.1016/j.ecoenv.2022.113174. [DOI] [PubMed] [Google Scholar]
- 117.Ying M., Yu Q., Zheng B., Wang H., Wang J., Chen S., Nie S., Xie M. Cultured Cordyceps Sinensis Polysaccharides Modulate Intestinal Mucosal Immunity and Gut Microbiota in Cyclophosphamide-Treated Mice. Carbohydr. Polym. 2020;235:115957. doi: 10.1016/j.carbpol.2020.115957. [DOI] [PubMed] [Google Scholar]
- 118.Masetti R., Pession A. First-Line Treatment of Acute Lymphoblastic Leukemia with Pegasparaginase. Biol. Targets Ther. 2009;3:359–368. doi: 10.2147/BTT.S3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pession A., Valsecchi M.G., Masera G., Kamps W.A., Magyarosy E., Rizzari C., Van Wering E.R., Lo Nigro L., Van Der Does A., Locatelli F., et al. Long-Term Results of a Randomized Trial on Extended Use of High Dose L-Asparaginase for Standard Risk Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2005;23:7161–7167. doi: 10.1200/JCO.2005.11.411. [DOI] [PubMed] [Google Scholar]
- 120.Silverman L.B., Gelber R.D., Dalton V.K., Asselin B.L., Barr R.D., Clavell L.A., Hurwitz C.A., Moghrabi A., Samson Y., Schorin M.A., et al. Improved Outcome for Children with Acute Lymphoblastic Leukemia: Results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.V97.5.1211. [DOI] [PubMed] [Google Scholar]
- 121.Asselin B.L., Whitin J.C., Coppola D.J., Rupp I.P., Sallan S.E., Cohen H.J. Comparative Pharmacokinetic Studies of Three Asparaginase Preparations. J. Clin. Oncol. 1993;11:1780–1786. doi: 10.1200/JCO.1993.11.9.1780. [DOI] [PubMed] [Google Scholar]
- 122.George D.T., Mathesius U., Behm C.A., Verma N.K. The Periplasmic Enzyme, AnsB, of Shigella Flexneri Modulates Bacterial Adherence to Host Epithelial Cells. PLoS ONE. 2014;9:e94954. doi: 10.1371/journal.pone.0094954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Westman E.L., Canova M.J., Radhi I.J., Koteva K., Kireeva I., Waglechner N., Wright G.D. Bacterial Inactivation of the Anticancer Drug Doxorubicin. Chem. Biol. 2012;19:1255–1264. doi: 10.1016/j.chembiol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 124.Blaustein R.A., Seed P.C., Hartmann E.M. Biotransformation of Doxorubicin Promotes Resilience in Simplified Intestinal Microbial Communities. mSphere. 2021;6:e0006821. doi: 10.1128/mSphere.00068-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan A., Culp E., Perry J., Lau J.T., Macneil L.T., Surette M.G., Wright G.D. Transformation of the Anticancer Drug Doxorubicin in the Human Gut Microbiome. ACS Infect. Dis. 2018;4:68–76. doi: 10.1021/acsinfecdis.7b00166. [DOI] [PubMed] [Google Scholar]
- 126.Sunshine J., Taube J.M. PD-1/PD-L1 Inhibitors. Curr. Opin. Pharmacol. 2015;23:32–38. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bie F., Tian H., Sun N., Zang R., Zhang M., Song P., Liu L., Peng Y., Bai G., Zhou B., et al. Research Progress of Anti-PD-1/PD-L1 Immunotherapy Related Mechanisms and Predictive Biomarkers in NSCLC. Front. Oncol. 2022;12:331. doi: 10.3389/fonc.2022.769124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., Luke J.J., Gajewski T.F. The Commensal Microbiome Is Associated with Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanoue T., Morita S., Plichta D.R., Skelly A.N., Suda W., Sugiura Y., Narushima S., Vlamakis H., Motoo I., Sugita K., et al. A Defined Commensal Consortium Elicits CD8 T Cells and Anti-Cancer Immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 131.Spencer C.N., McQuade J.L., Gopalakrishnan V., McCulloch J.A., Vetizou M., Cogdill A.P., Wadud Khan M.A., Zhang X., White M.G., Peterson C.B., et al. Dietary Fiber and Probiotics Influence the Gut Microbiome and Melanoma Immunotherapy Response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Messaoudene M., Pidgeon R., Richard C., Ponce M., Diop K., Benlaifaoui M., Nolin-Lapalme A., Cauchois F., Malo J., Belkaid W., et al. A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti-PD-1 Resistance through Effects on the Gut Microbiota. Cancer Discov. 2022;12:1070–1087. doi: 10.1158/2159-8290.CD-21-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferrere G., Alou M.T., Liu P., Goubet A.G., Fidelle M., Kepp O., Durand S., Iebba V., Fluckiger A., Daillère R., et al. Ketogenic Diet and Ketone Bodies Enhance the Anticancer Effects of PD-1 Blockade. JCI Insight. 2021;6:e145207. doi: 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller P.L., Carson T.L. Mechanisms and Microbial Influences on CTLA-4 and PD-1-Based Immunotherapy in the Treatment of Cancer: A Narrative Review. Gut Pathog. 2020;12:43. doi: 10.1186/s13099-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dees K.J., Koo H., Humphreys J.F., Hakim J.A., Crossman D.K., Crowley M.R., Nabors L.B., Benveniste E.N., Morrow C.D., McFarland B.C. Human Gut Microbial Communities Dictate Efficacy of Anti-PD-1 Therapy in a Humanized Microbiome Mouse Model of Glioma. Neuro-Oncology Adv. 2021;3:vdab023. doi: 10.1093/noajnl/vdab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peng Z., Cheng S., Kou Y., Wang Z., Jin R., Hu H., Zhang X., Gong J.F., Li J., Lu M., et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020;8:1251–1261. doi: 10.1158/2326-6066.CIR-19-1014. [DOI] [PubMed] [Google Scholar]
- 137.Zheng Y., Wang T., Tu X., Huang Y., Zhang H., Tan D., Jiang W., Cai S., Zhao P., Song R., et al. Gut Microbiome Affects the Response to Anti-PD-1 Immunotherapy in Patients with Hepatocellular Carcinoma. J. Immunother. Cancer. 2019;7:193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gabarre P., Loens C., Tamzali Y., Barrou B., Jaisser F., Tourret J. Immunosuppressive Therapy after Solid Organ Transplantation and the Gut Microbiota: Bidirectional Interactions with Clinical Consequences. Am. J. Transplant. 2021;22:1014–1030. doi: 10.1111/ajt.16836. [DOI] [PubMed] [Google Scholar]
- 139.Kapturczak M.H., Meier-Kriesche H.U., Kaplan B. Pharmacology of Calcineurin Antagonists. Transplant. Proc. 2004;36:S25–S32. doi: 10.1016/j.transproceed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 140.Lee J.R., Muthukumar T., Dadhania D., Taur Y., Jenq R.R., Toussaint N.C., Ling L., Pamer E., Suthanthiran M. Gut Microbiota and Tacrolimus Dosing in Kidney Transplantation. PLoS ONE. 2015;10:e0122399. doi: 10.1371/journal.pone.0122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cain D.W., Cidlowski J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ridlon J.M., Ikegawa S., Alves J.M.P., Zhou B., Kobayashi A., Iida T., Mitamura K., Tanabe G., Serrano M., De Guzman A., et al. Clostridium Scindens: A Human Gut Microbe with a High Potential to Convert Glucocorticoids into Androgens. J. Lipid Res. 2013;54:2437–2449. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ward R.M., Kearns G.L. Proton Pump Inhibitors in Pediatrics: Mechanism of Action, Pharmacokinetics, Pharmacogenetics, and Pharmacodynamics. Pediatr. Drugs. 2013;15:119–131. doi: 10.1007/s40272-013-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jackson M.A., Verdi S., Maxan M.E., Shin C.M., Zierer J., Bowyer R.C.E., Martin T., Williams F.M.K., Menni C., Bell J.T., et al. Gut Microbiota Associations with Common Diseases and Prescription Medications in a Population-Based Cohort. Nat. Commun. 2018;9:2655. doi: 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Masetti R., Biagi E., Zama D., Muratore E., D’Amico F., Leardini D., Turroni S., Prete A., Brigidi P., Pession A. Early Modifications of the Gut Microbiome in Children with Hepatic Sinusoidal Obstruction Syndrome after Hematopoietic Stem Cell Transplantation. Sci. Rep. 2021;11:14307. doi: 10.1038/s41598-021-93571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Winston J.A., Rivera A., Cai J., Patterson A.D., Theriot C.M. Secondary Bile Acid Ursodeoxycholic Acid Alters Weight, the Gut Microbiota, and the Bile Acid Pool in Conventional Mice. PLoS ONE. 2021;16:e0246161. doi: 10.1371/journal.pone.0246161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pession A., Zama D., Muratore E., Leardini D., Gori D., Guaraldi F., Prete A., Turroni S., Brigidi P., Masetti R. Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J. Pers. Med. 2021;11:100. doi: 10.3390/jpm11020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.