Abstract
The pathology observed during Chlamydia infection is due initially to localized tissue damage caused by the infection itself, followed by deleterious host inflammatory responses that lead to permanent scarring. We have recently reported that the infection by Chlamydia in vitro results in apoptosis of epithelial cells and macrophages and that infected monocytes secrete the proinflammatory cytokine interleukin-1β. At the same time, proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) can also trigger apoptosis of susceptible cells. To study the possible relationship between Chlamydia trachomatis infection and apoptosis in vivo, we used the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling technique to determine whether infection may cause apoptosis in the genital tract of mice and, conversely, whether cytokines produced during the inflammatory response may modulate the level of apoptosis. Our results demonstrate that infected cells in the endocervix at day 2 or 7 after infection are sometimes apoptotic, although there was not a statistically significant change in the number of apoptotic cells in the endocervix. However, large clumps of apoptotic infected cells were observed in the lumen, suggesting that apoptotic cells may be shed from the endocervix. Moreover, there was a large increase in the number of apoptotic cells in the uterine horns and oviducts after 2 or 7 days of infection, which was accompanied by obvious signs of upper tract pathology. Interestingly, depletion of TNF-α led to a decrease in the level of apoptosis in the uterine horns and oviducts of animals infected for 7 days, suggesting that the inflammatory cytokines may exert part of their pathological effect via apoptosis in infected tissues.
Apoptosis is a key phenomenon in the regulation of cell population size and cell life span (18, 52). This process of cell death plays an important role in normal tissue homeostasis and in certain pathological conditions, including cancer. A growing number of studies over the last few years have shown that intracellular microbes can also modulate apoptosis of the host cell, either inhibiting or promoting cell death, and it has been proposed that the persistence and pathogenesis of several pathogenic microbes may be related to their ability to dysregulate apoptosis (2).
Although microbe-induced apoptosis has been extensively characterized for viral infections (2, 6, 47), apoptosis has also been observed during infections in vivo by bacteria or protozoan parasites, such as for Escherichia coli, Yersinia pseudotuberculosis, Trypanosoma cruzi, Shigella flexneri, Bordetella pertussis, Salmonella enterica serovar Typhimurium, and Toxoplasma gondii (13, 21, 26, 27, 34, 40, 59). Apoptosis due to infection by these pathogens may allow the pathogens to exit from infected cells, eliminate potentially dangerous phagocytic cells, and/or evade the host immune response or stimulate inflammatory responses (2, 5, 28, 30, 59). We recently reported that Chlamydia psittaci induces apoptosis in infected epithelial cells and macrophages in vitro (33), although we did not evaluate whether the infection has any effect on host cell viability in vivo.
In humans, the most common consequence of chlamydial genital infection is salpingitis, which can lead to tubal obstruction and infertility (4). In controlled studies in guinea pigs and mice (3, 9, 38), bacteria are initially detected in the cervical epithelium, but the pathology ascends in most animals to the endometrium and the oviducts within 7 to 9 days after intravaginal inoculation, culminating often in infertility. Most of the damage attributable to Chlamydia is due not to the infection itself but to the inflammation and fibrosis that follow the infection (4).
Polymorphonuclear leukocytes are typically observed in the cervix as early as 2 days after infection, and acute inflammation in the uterine horns and oviducts follows within 5 to 7 days after infection (4). A number of inflammatory mediators are present during infection, and these could contribute to tissue damage and fibrosis. Two predominant cytokines usually produced during inflammation are interleukin-1 (IL-1) and tumor necrosis factor (TNF-α), which activate polymorphonuclear leukocytes and contribute to fibrosis due to enhanced production of prostaglandins and collagen and increased expression of integrin, as well as secretion of IL-6, IL-8, and transforming growth factor β (32, 51, 58). TNF-α has in fact been detected in the fallopian tubes of women infected with Chlamydia (48) and in secretions from Chlamydia-infected mice and guinea pigs (7, 8, 54). Results based on studies using mice that display mouse strain-dependent variations in the pathological outcome of Chlamydia genital infection and correspondingly different levels of TNF-α production have suggested that while TNF-α and other inflammatory cytokines may aid in eradicating Chlamydia infection, they may also promote long-term tissue damage (7).
The preferential target tissue of sexually transmitted chlamydial infections in females is the columnar epithelium of the cervix (4, 29), but monocytes and macrophages can also be infected (23) and may aid in disseminating the infection by certain serovars of Chlamydia. As macrophages undergoing apoptosis secrete IL-1 (15), it is conceivable that apoptosis of these cells during Chlamydia infection may contribute to the inflammatory response. Conversely, cytokines such as TNF-α are able to induce apoptosis of some target cells (12), suggesting that the inflammation following Chlamydia infection may also directly trigger apoptosis.
We have therefore investigated apoptosis in the genital tract of mice using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) method, which reveals early DNA breaks during apoptosis (42), allowing identification of apoptotic cells that may have evaded detection by conventional histological techniques. The potential effect of the inflammatory response on apoptosis was evaluated by measuring apoptosis in the uterine horns and oviducts in infected mice whose TNF-α levels had been depleted with antibodies.
MATERIALS AND METHODS
Cells and animals.
HeLa 299 cells, McCoy cells, and L cells (American Type Culture Collection, Manassas, Va.) were maintained in a humidified incubator at 37°C with 5% CO2 in Dulbecco modified Eagle medium (Life Technologies, Inc., Rockville, Md.) supplemented with 10% heat-inactivated fetal calf serum and 2 mM l-glutamine. The Chlamydia species used here, the mouse pneumonitis strain (MoPn) of C. trachomatis (3, 45), was obtained from Roger Rank (University of Arkansas) and was grown in L cells and purified by Renografin as previously described for C. psittaci (14). The quantity of bacterial inclusion-forming units (IFU) was determined by incubating the chlamydiae with McCoy cells for 1 day and revealing the presence of bacteria by immunofluorescence with fluorescein isothiocyanate-labeled anti-Chlamydia antibodies (lipopolysaccharide [LPS]-specific monoclonal antibody clone C4; Argene, Varilhes, France). Four-week-old female C57BL/6 mice were purchased from IFFA CREDO (L'Arbresle, France).
Infections.
For infections in vitro, HeLa 229 cells growing at about 70% confluence on tissue culture flasks (Costar) were infected with a multiplicity of infection (MOI) of 6.6 as previously described for C. psittaci (32) and incubated during the indicated times at 37°C.
For infections in vivo, mice first received 2.5 mg of medroxyprogesterone acetate (Sigma Chemical Co., St. Louis, Mo.; or Upjohn, Kalamazoo, Mich.) subcutaneously at 3 and 10 days before infection (7). Infection was initiated by placing 50 μl of SPG buffer (250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid [pH 7.2]), containing 50 μg of gentamicin per ml and 106 IFU of C. trachomatis MoPn into the vaginal vault of the mice, corresponding to a 50% infectious dose of 4 × 102 (unpublished observations). Uninfected control mice were inoculated intravaginally with the same volume of SPG buffer and gentamicin. For these experiments, two uninfected mice and six infected mice were sacrificed after 2 days, and two uninfected mice and seven infected mice were sacrificed after 7 days.
For TNF-α depletion experiments, 4- to 6-week-old C57BL/6 mice were purchased from Jackson Laboratories (West Grove, Pa.). To study the effect of TNF-α depletion, the mice received 2.5 mg of medroxyprogesterone acetate subcutaneously 7 days before vaginal infection and were injected intravaginally with 150 μg of polyclonal anti-mouse TNF-α antibodies (Endogen, Woburn, Mass.) twice a day on days 0, 2, 4, and 6 of infection with 107 IFU of C. trachomatis MoPn in SPG and 50 μg of gentamicin per ml (50% infectious dose of 4 × 103). Control mice were injected intravaginally with 150 μg of normal rabbit immunoglobulin G (Endogen).
It should be noted that previous studies have used 107 IFU to infect mice (20, 50). In our case, we observed that 106 and 107 IFU gave similar apoptosis and TNF-α depletion results.
FACS analysis of apoptosis.
Quantitative measurement of apoptosis was performed by cytofluorimetry of detergent-permeabilized propidium iodide-stained cells as described elsewhere (10). Both adherent cells and cells in the supernatant were collected for analysis. The cells were transferred into 12- by 75-mm FALCON 2052 fluorescence-activated cell sorting (FACS) tubes (Becton Dickinson, San Jose, Calif.). Data from 10,000 HeLa cells were collected on a FACScan flow cytometer (Becton Dickinson) with an argon ion laser tuned to 488 nm. Apoptosis was measured in the FL2 range.
DNA fragmentation assay.
Infected or uninfected HeLa cells were washed with phosphate-buffered saline and centrifuged (1,200 rpm for 5 min), and the pellet was incubated for 1 h at 37°C with 2 ml of lysis buffer containing 0.6% sodium dodecyl sulfate, 10 mM EDTA, 10 mM Tris, and 20 μg of RNase A per ml. Two hundred microliters of 5 M NaCl was then added, and this solution was incubated for 40 min on ice and finally centrifuged for 30 min at 13,000 × g. The DNA was extracted from the supernatant with phenol-chloroform-isoamyl alcohol (25:24:1), precipitated with absolute ethanol, and treated with RNase A (40 μg/ml) at 37°C for 30 min. Finally, the amount of DNA from each sample was normalized by measuring the optical density at 260 nm, and the DNA was loaded onto a 1.8% agarose gel containing 0.4 μg of ethidium bromide per ml.
Histological procedures.
Mice were sacrificed 2 and 7 days after vaginal infection. The entire genital tract was removed, fixed in 4% paraformaldehyde, and embedded in 37°C paraffin. Longitudinal 4-μm sections were cut, stained with hematoxylin and eosin (Shandon) or with an unconjugated anti-C. trachomatis monoclonal antibody (1:100 dilution; Biogene Sis), and incubated with a peroxidase-conjugated anti-mouse immunoglobulin antibody (Dako). Infected cells were revealed in red with the AEC dimethyl formamide substrate (Sigma) on eosin-counterstained tissue.
To identify apoptotic cells, the cuts were double stained with a murine anti-MoPn immune serum (1:500 dilution) obtained from infected mice (7) and the TUNEL method, using a cell death detection kit from Boehringer Mannheim (Meylan, France) as instructed by the manufacturer. The infected cells were revealed with the unconjugated anti-MoPn immune serum followed by incubation with trimethyl rhodamine isothiocyanate-conjugated rabbit anti-mouse immunoglobulins (DAKO SA, Trappes, France), and the apoptotic cells appeared in green due to the fluorescein-labeled dUTP. Samples were examined with a Zeiss Axiophot microscope attached to a cooled charge-coupled device camera (Photometrics), and images were acquired and analyzed with the IPLab spectrum program (Signal Analytics Corporation, Vienna, Va.).
Quantification of apoptotic cells in vivo.
The IPLab spectrum program was used to quantify the relative number of apoptotic cells in each tissue. To distinguish fluorescently positive cells from nonapoptotic cells, a threshold of fluorescence intensity was defined using a sample that had been stained with the TUNEL kit without the terminal deoxynucleotidyltransferase enzyme. Under these conditions, the minimal threshold of fluorescence intensity that excludes the false-positive cells was defined. To identify the apoptotic cells in images, the IPLab spectrum program was used to add a green color to the cells with a fluorescence intensity under the threshold level (nonapoptotic cells) and a red color to the cells with a fluorescence intensity higher than the threshold level (apoptotic cells). The relative number of apoptotic cells per image was then counted, and 10 fields were analyzed per tissue.
RESULTS
Apoptosis of epithelial cells in vitro during infection with the murine strain of C. trachomatis.
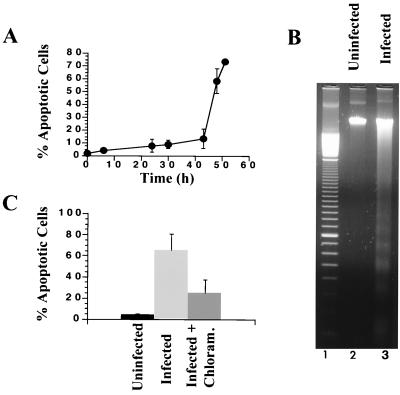
We have recently reported that the guinea pig inclusion conjunctivitis (GPIC) serovar of C. psittaci induces apoptosis of epithelial cells, with apoptosis being measurable after a 1-day infection (33). To determine whether the murine strain of C. trachomatis, MoPn, shares this property, we measured the time course of apoptosis of the epithelial cell line, HeLa, by cytofluorimetry using iodide propidium-stained, detergent-permeabilized cells as described in Materials and Methods. We observed few apoptotic cells during short infection times, but significant levels were detected after a 42-h infection, at which point release of MoPn elementary bodies begins to occur (not shown), and most of the cells were apoptotic after a 2-day infection (Fig. 1A). This time course is reminiscent of the apoptosis previously observed during infection in vitro with C. psittaci (33), although the kinetics were slightly slower for MoPn-induced apoptosis and the MOI required for MoPn (6.6) was higher than that required for GPIC (1.0).
FIG. 1.
Death of epithelial cells infected with C. trachomatis in vitro. HeLa cells were infected at various times with an MOI of 6.6. (A) Time course of C. trachomatis-induced apoptosis, measured by cytofluorimetry. (B) DNA fragmentation of uninfected cells (lane 2) or cells infected with C. trachomatis for 48 h (lane 3). After extraction, DNA was loaded onto agarose gels containing ethidium bromide. Lane 1 shows a 100-bp DNA ladder size marker. (C) Effect of chloramphenicol on Chlamydia-induced apoptosis. HeLa cells were infected during 48 h with or without chloramphenicol, and apoptosis was measured by cytofluorimetry. Each experiment was performed with three different preparations of HeLa cells infected on separate days.
To confirm the apoptotic nature of the host cell death, we studied DNA fragmentation of infected cells by DNA electrophoresis on agarose gels. While there was no DNA fragmentation in uninfected HeLa cells, there was a high level of fragmentation in cells that had been infected for 48 h (Fig. 1B). The sizes of the DNA fragments were integral values of 200 bp, which is a characteristic feature of intranucleosomal cleavage due to activated endonucleases during the apoptotic process (57).
To evaluate whether chlamydial growth is required for this type of apoptosis, we infected cells with C. trachomatis for 48 h in the presence or absence of chloramphenicol, an antibiotic that inhibits prokaryotic protein synthesis without altering protein synthesis of the eukaryotic host cell (49, 53). In one set of experiments (Fig. 1C), more than half of the apoptosis due to an infection with C. trachomatis MoPn for 48 h was inhibited by incubation with chloramphenicol, and larger inhibitory effects were observed in other experiments, suggesting that chlamydial protein synthesis is necessary for efficient triggering of apoptosis of infected cells. This interpretation is reinforced by the observation that there is no apoptosis if chlamydiae are first inactivated by exposure to UV irradiation (reference 33 and not shown). As we have previously shown that C. psittaci also induces apoptosis in vitro (33), these results suggest that infection may lead to apoptosis regardless of the Chlamydia species or serovar. In addition, infection with C. trachomatis MoPn also induced apoptosis of McCoy cells (not shown), implying that other cell types may also be susceptible to Chlamydia-induced apoptosis.
Apoptosis in the murine genital tract during infection with C. trachomatis.
To establish whether C. trachomatis may also induce apoptosis in vivo, we used the murine model of C. trachomatis MoPn genital infection (3). After injection of progesterone to synchronize the estrus cycle, female C57BL/6 mice were infected intravaginally with chlamydiae. Tissues for histological analysis were obtained by sacrificing mice after 2 or 7 days of infection. The entire genital tract was excised en bloc, fixed in paraformaldehyde, and processed for histology or TUNEL as described in Materials and Methods. The cervix, uterine horn, and oviduct were then assessed separately for the presence of inflammation and apoptosis.
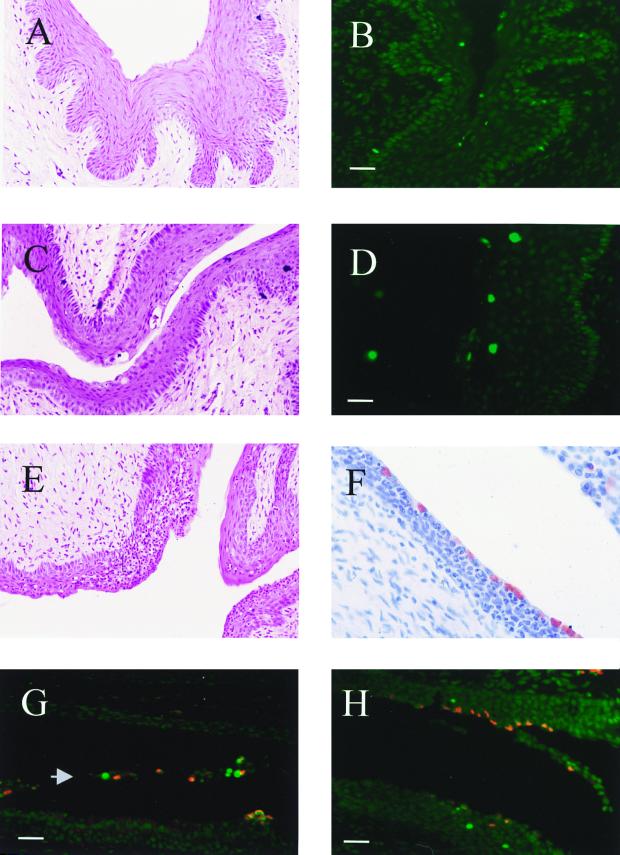
Two days after infection, the infected cervix had visibly damaged cells, some of which were condensed, and there was a low level of infiltration of inflammatory cells (Fig. 2C). Most of the damaged cells were present in the stratified squamous epithelium of the vagina. The inflammatory response became significantly enhanced after 7 days of infection, with a heavy infiltration of mono- and polymorphonuclear cells in the uterine mucosa (Fig. 2E). As a control, there was no inflammation nor chlamydial staining in uninfected mice (Fig. 2A and data not shown).
FIG. 2.
Histological characterization of the lower genital tract during infection with C. trachomatis. The lower tract, uninfected (A) or infected for 2 (C) or 7 (E) days, was prepared for histology as described in Materials and Methods and counterstained with hematoxylin and eosin. Infected tissues (C and E) contain damaged cells in the stratified squamous epithelium, as well as an infiltrate of mono- and polymorphonuclear cells. TUNEL staining of uninfected tissue (B) and tissue that had been infected for 7 days (D) was observed by fluorescence microscopy. Cells with apoptotic nuclei appear in green. (F) Immunolabeling of Chlamydia inclusions (red) in tissue that had been infected for 7 days and counterstained with hematoxylin. (G and H) Tissues that had been infected for 7 days, double labeled with anti-Chlamydia serum (red) and the TUNEL technique (green). Representative images show infected apoptotic cells that are released in the lumen of the lower uterus (G; arrowhead) and uninfected apoptotic cells in the epithelium and occasional apoptotic cells that are also infected (G and H). Bars = 8 μm.
We attempted to evaluate whether the chlamydiae are able to directly induce apoptosis in infected cells or indirectly in neighboring cells. Thus, we used the TUNEL method combined with anti-Chlamydia staining to observe simultaneously apoptotic and infected cells. This approach revealed that apoptosis occurred in both infected and uninfected cells (Fig. 2G and H), with the majority of apoptotic cells during infection being localized in the lining cells of the mucosa of the lower uterus and endocervix. We did not find a significant change in the number of apoptotic cells in uninfected mice that were not pretreated with progesterone (not shown). In addition, we observed that infection leads to detachment of apoptotic and infected cells from the epithelium (Fig. 2G). Taken together, these results suggest that the chlamydiae may induce apoptosis directly. However, ostensibly uninfected cells were also apoptotic (Fig. 2H). These may be the same cells as the spontaneously apoptotic cells found in uninfected tissues, but the results also raise the possibility that the chlamydiae may induce apoptosis indirectly, via locally produced cytokines secreted during initial phases of the infection. Finally, although an early feature of apoptosis is detachment of dying cells from neighboring cells, detachment of cells also leads to apoptosis, and we cannot exclude the possibility that infection may first cause detachment, followed by apoptosis.
The number of apoptotic cells during infection was quantified as described in Materials and Methods. After a 2- or 7-day infection, there was no significant change in the number of apoptotic cells in the endocervix and lower uterus of infected mice compared to uninfected mice (Fig. 2B and D and data not shown). Hence, it is possible that infection by C. trachomatis in vivo does not lead directly to apoptosis. Alternatively, as suggested by Fig. 2G, it is conceivable that the dying lining epithelial cells may be quickly shed into the lumen of the uterus, as these cells were not observed in the lumen of uninfected mice. However, it was not possible to quantify the apoptosis in the lumen due to the large number of apoptotic cells found in clumps, and they were therefore not taken into account when determining the level of apoptosis in the lower genital tract.
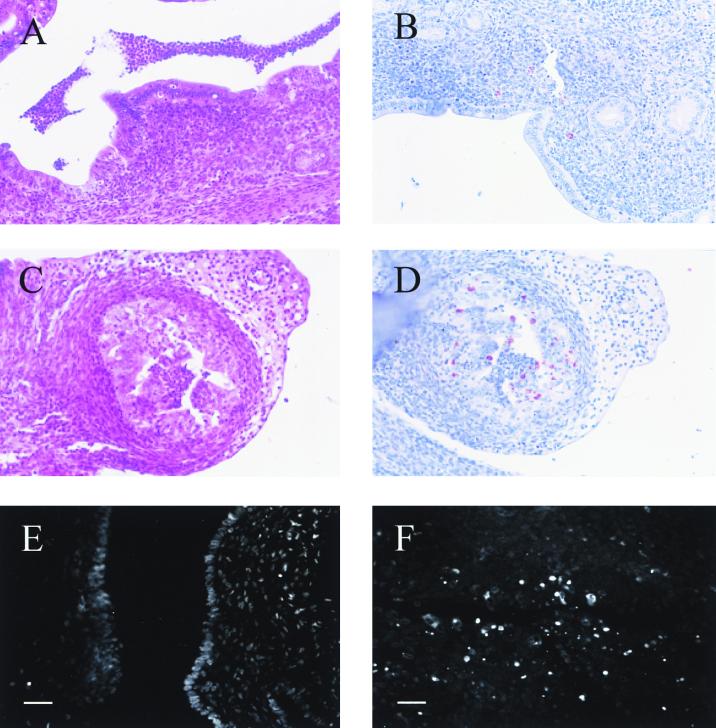
In the uterine horns and oviducts, we also observed an inflammatory response that was often accompanied by an accumulation of nucleated cells in the lumen (Fig. 3A and C). At these infection times, we found lining epithelial cells with large cytoplasmic Chlamydia inclusions in the uterine horns and oviducts, as well as infected cells in the submucosa of the uterine horns and the underlying stroma (Fig. 3B and D).
FIG. 3.
Histological characterization of the upper genital tract during infection with C. trachomatis. The uterine horns (A and B) and oviducts (C and D) were infected for 7 days; histological samples were counterstained with hematoxylin and eosin (A and C) or were stained with anti-Chlamydia serum to reveal Chlamydia inclusions (red) and counterstained with hematoxylin (B and D). An uninfected uterine horn (E) or a uterine horn that had been infected for 7 days (F) was stained with the TUNEL technique and observed by fluorescence microscopy as described in Materials and Methods. Bars = 8 μm.
In contrast to the endocervix, it was easier to demonstrate a difference in the level of apoptosis in the uterine horns and oviducts due to the low physiological level of apoptosis in the absence of infection. The number of apoptotic cells in the uterine horns and oviducts increased significantly in mice infected for 2 or 7 days compared to uninfected controls (Fig. 3E and F; Fig. 4 [see below]). As in the case of the endocervix, however, both infected and uninfected cells were apoptotic (not shown).
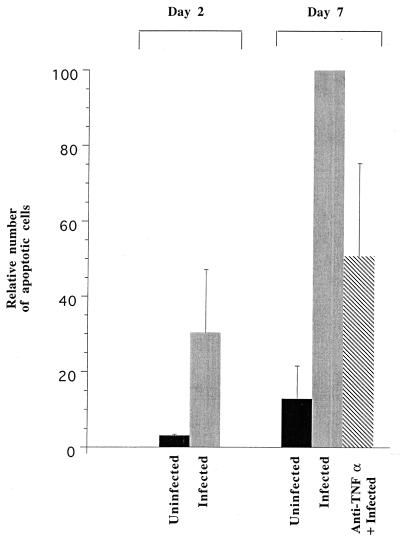
FIG. 4.
Apoptosis in the upper genital tract during C. trachomatis infection and the effect of TNF-α depletion. A low level of spontaneous apoptosis is observed in the oviducts of uninfected mice. There was a large increase in the number of apoptotic cells after a 2-day infection with C. trachomatis and a further increase after a 7-day infection. The increase after a 7-day infection was partially inhibited in mice that had been pretreated with antibodies against TNF-α. Samples were stained with the TUNEL technique as shown in Fig. 3E and F, and the number of apoptotic cells was determined as described in Materials and Methods. Two uninfected mice for each group, three infected mice at day 2, ten infected mice at day 7, and eight TNF-α-depleted mice were used in the analysis.
Role of TNF-α in modulating apoptosis in the uterine horns and oviducts.
In C57BL/6 mice, TNF-α has been detected in genital tract secretions of animals during the first week of infection with C. trachomatis MoPn (7, 8). TNF-α is also known to trigger apoptosis of a diverse range of cells in vitro (12). We therefore depleted TNF-α levels in vivo by injecting mice with a polyclonal anti-mouse TNF-α before and during infection with C. trachomatis, as described in Materials and Methods.
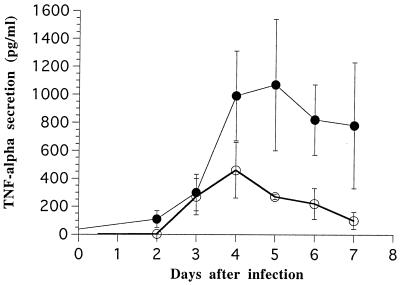
TNF-α levels in endocervical secretions from mice injected with anti-TNF-α antibodies, determined by enzyme-linked immunosorbent assay (ELISA), were significantly lower than in controls (P < 0.001 by two-way analysis of variance). The Tukey test revealed significant decreases on days 4 through 7 (not shown). Thus, TNF-α levels on day 5 were 1,070 ± 470 pg/ml in controls and 270 ± 22 pg/ml in the antibody-treated group. On day 7, TNF-α levels were 780 ± 450 pg/ml in controls and 100 ± 60 pg/ml in the antibody-treated group (Fig. 5). Treatment with anti-TNF-α antibodies had no effect on levels of gamma interferon and IL-1β (not shown).
FIG. 5.
TNF-α secretion in the genital tract after depletion with anti-TNF-α antibodies. TNF-α secretion was measured by ELISA as described in Materials and Methods in untreated mice (●) or mice that had been injected with antibodies against TNF-α (○). The secretions were measured in duplicate on ELISA, and six mice were used for each group.
The number of apoptotic cells was quantified with the TUNEL technique and image analysis software as described in Materials and Methods. As seen in Fig. 4, there was a significant decrease in the number of apoptotic cells in mice infected for 7 days whose TNF-α levels were diminished by administration of anti-TNF-α antibodies, suggesting that TNF-α produced during Chlamydia infection contributes to apoptosis in the upper genital tract.
DISCUSSION
We have previously reported that epithelial cells and macrophages die through apoptosis during infection with C. psittaci in vitro (33). To determine whether apoptosis may be induced by infection in vivo, we used the TUNEL technique to quantify the number of apoptotic cells in the genital tract of female mice during infection with C. trachomatis, having verified first that C. trachomatis infection leads to apoptosis in vitro with similar characteristics as observed during C. psittaci-induced apoptosis.
We observed a large increase in the number of apoptotic cells in the uterine horns and oviducts of mice infected with C. trachomatis, and the apoptotic cells were present in both the epithelium and submucosa. However, the results were ambiguous in the case of the lower genital tract, which already had a large number of spontaneously apoptotic cells in uninfected mice. Infected cells that were also apoptotic were in fact observed in the endocervix of infected mice, but we did not observe a quantitative difference in the levels of apoptosis between infected and uninfected mice. Clusters of apoptotic infected cells were found in the lumen of the vagina after a 2-day infection, and it is thus possible that the apoptotic cells may be shed from the surface epithelial cells, as has been shown for shedding of apoptotic cells during menstruation (44). In this respect, shedding of apoptotic cells may be a general phenomenon provoked by infection with different Chlamydia species and strains, since it had previously been reported that dying infected cells with a condensed nucleus are released from the cervical epithelium of guinea pigs infected with the GPIC strain of C. psittaci (43). Alternatively, or simultaneously, since apoptotic cells in vivo are normally recognized and degraded by neighboring phagocytic cells (36), it is conceivable that the infected cells undergoing apoptosis may be rapidly cleared from the tissue by phagocytes.
During apoptosis of epithelial cells in vitro, the majority of the apoptotic cells were infected, although many uninfected apoptotic cells were also observed (33). We had therefore proposed that chlamydiae may induce apoptosis directly through infection, but that locally produced cytokines secreted during initial phases of the infection may also cause apoptosis of uninfected cells. Several cytokines are in fact secreted by cells infected by Chlamydia both in vitro and in vivo. Infection of an epithelial cell line causes release of the proinflammatory cytokine IL-1α (39), and incubation of dendritic cells and monocytes with chlamydiae in vitro results in secretion of TNF-α and IL-1β, respectively (31, 33). In vivo, an acute inflammatory response is the dominant host response in the early stages of the infection and is observed in the endometrium and oviducts within a few days of infection (4). In mice, TNF-α has been detected in the genital tract secretions of animals inoculated with C. trachomatis MoPn. High levels were measured within 2 days after intravaginal inoculation, peaked at 6 days, and decreased after a week (7). It had been proposed that TNF-α may aid the host to eradicate the chlamydiae, thus preventing infection of the oviduct (7). Hence, TNF-α, in conjunction with gamma interferon, transforming growth factor β, and IL-1 (25, 37, 55, 56), may be a factor responsible for the inflammatory response and consequent fibrosis.
While TNF-α is a pleiotropic cytokine that is cytocidal for tumor cells, it also has effects on untransformed cells (1, 24, 35, 41), and it has been proposed that TNF-α may serve as the local signal contributing to the processes of shedding and bleeding in humans during menstruation (46). Favoring the likelihood that TNF-α could also trigger apoptosis during Chlamydia infection, we have observed a large increase in the number of apoptotic cells in the uterine horns and oviducts, which was partially inhibited by depletion with antibodies against TNF-α. In addition, the number of apoptotic cells in the upper tract of TNF-α-depleted mice that had been infected for 7 days is close to the number observed in untreated mice that had been infected for 2 days, further reinforcing the possibility that the increased level of apoptosis in untreated mice infected for 7 days may be due to proinflammatory cytokines, since the inflammatory response in oviducts of C57 mice infected with C. trachomatis MoPn does not begin until at least 3 days of infection (7).
It has previously been reported that Chlamydia infections in vitro induce apoptosis toward the end of the infection cycle (reference 33 and this work) and protect infected cells against apoptosis due to external ligands (11). During infections in vivo, most of the cells dying as a result of the inflammatory response in the upper genital tract at day 7 do not appear to be infected and thus should not be protected. Alternatively, infected cells in vivo may be protected only partially, compared to the results previously reported with cell lines in vitro.
The observation that TNF-α can modulate apoptosis during infection with C. trachomatis is reminiscent of the role played by this cytokine during infections with Mycobacterium tuberculosis, which leads to apoptosis of alveolar macrophages in vivo. Addition of TNF-α increases the cytotoxicity, while treatment of infected macrophages with pentoxifylline or anti-TNF-α antibodies increase host cell survival (19). Extensive apoptosis was also detected within caseating granulomas from lung tissue samples from clinical tuberculosis cases (19), suggesting that TNF-α-dependent apoptosis during M. tuberculosis infection could contribute to the pathology in a clinical setting.
Part of the TNF-α production during bacterial infections could result from LPS stimulation of host immune cells. Thus, in vivo administration of Porphyromonas gingivalis LPS induced apoptosis in the spleen, lymph nodes, and thymus (17). Serum TNF-α levels were higher than in untreated controls, and recombinant TNF-α also caused apoptosis (17), as was previously shown for LPS from Escherichia coli. Although it has been reported that Chlamydia LPS or whole chlamydiae are weak inducers of TNF-α secretion from isolated whole blood cells (16), it is possible that a small population of effector cells in the genital tract mucosa, such as dendritic cells (31), could be responsible for the high levels of TNF-α observed during Chlamydia infection in vivo (7, 8).
In conclusion, we propose that Chlamydia-induced release of IL-1 and TNF-α, possibly acting in concert with other inflammatory cytokines, could lead to apoptosis of directly infected and/or neighboring cells. Finally, as macrophages and monocytes undergoing apoptosis also release IL-1 (15), the cells secreting the proinflammatory cytokines, as well as the cells undergoing Chlamydia-induced apoptosis in vivo, need to be identified in order to evaluate their contribution to the pathology of Chlamydia infections.
ACKNOWLEDGMENTS
We are grateful to Roger Rank (University of Arkansas) for helpful suggestions during the course of the work and for constructive criticisms of the manuscript and to Huot Khun for assistance in preparing histological sections.
This investigation was financed by funds from the Institut Pasteur, CNRS, and Ligue Nationale Contre le Cancer (Comité de Paris) for purchase of the CCD camera and by NIH grant R01 AI43337-01A1.
REFERENCES
- 1.Abreu-Martin M T, Vidrich A, Lynch D H, Targan S R. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-α and ligation of Fas antigen. J Immunol. 1995;155:4147–4154. [PubMed] [Google Scholar]
- 2.Ameisen J C, Estaquier J, Idziorek T. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol Rev. 1994;142:9–51. doi: 10.1111/j.1600-065x.1994.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 3.Barron A L, White H J, Rank R G, Soloff B L, Moses E B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 4.Bavoil P M, Hsia R-C, Rank R G. Prospects for a vaccine against Chlamydia genital disease. I. Microbiology and pathogenesis. Bull Inst Pasteur. 1996;94:5–54. [Google Scholar]
- 5.Chinnaiyan A M, Woffendin C, Dixit V M, Nabel G J. The inhibition of pro-apoptotic ICE-like proteases enhances HIV replication. Nat Med. 1997;3:333–337. doi: 10.1038/nm0397-333. [DOI] [PubMed] [Google Scholar]
- 6.Collins M. Potential roles of apoptosis in viral pathogenesis. Am J Respir Crit Care Med. 1995;152:S20–S24. doi: 10.1164/ajrccm/152.4_Pt_2.S20. [DOI] [PubMed] [Google Scholar]
- 7.Darville T, Andrews C W, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darville T, Laffoon K K, Kishen L R, Rank R G. Tumor necrosis factor alpha activity in genital tract secretions of guinea pigs infected with chlamydiae. Infect Immun. 1995;63:4675–4681. doi: 10.1128/iai.63.12.4675-4681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas R S, Tarshis A D, Pletcher C H, Nowell P C, Moore J S. A simplified method for the coordinate examination of apoptosis and surface phenotype of murine lymphocytes. J Immunol Methods. 1995;188:219–228. doi: 10.1016/0022-1759(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 11.Fan T, Lu H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golstein P, Ojcius D M, Young J D. Cell death mechanisms and the immune system. Immunol Rev. 1991;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 13.Gueirard P, Druilhe A, Pretolani M, Guiso N. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun. 1998;66:1718–1725. doi: 10.1128/iai.66.4.1718-1725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez-Martin C B, Ojcius D M, Hsia R-C, Hellio R, Bavoil P M, Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- 15.Hogquist K A, Nett M A, Unanue E R, Chaplin D D. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isogai E, Isogal H, Kimura K, Fujii N, Takagi S, Hirose K, Hayashi M. In vivo induction of apoptosis and immune responses in mice by administration of lipopolysaccharide from Porphyromonas gingivalis. Infect Immun. 1996;64:1461–1466. doi: 10.1128/iai.64.4.1461-1466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 19.Keane J, Balcewicz-Sablinska M K, Remold H G, Chupp G L, Meek B B, Fenton M J, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan I A, Matsuura T, Kasper L H. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 22.Klahr S, Morrissey J J. The role of growth factors, cytokines, and vasocative compounds in obstructive nephropathy. Semin Nephrol. 1998;18:622–632. [PubMed] [Google Scholar]
- 23.La Verda D, Byrne G I. Interactions between macrophages and chlamydiae. Immunol Ser. 1994;60:381–399. [PubMed] [Google Scholar]
- 24.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann P G, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 25.Magee D M, Smith J G, Bleicker C A, Carter C J, Bonewald L F, Schachter J, Williams D M. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect Immun. 1992;60:1217–1220. doi: 10.1128/iai.60.3.1217-1220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McOrist S, Roberts L, Jasni S, Rowland A C, Lawson G H, Gebhart C J, Bosworth B. Developed and resolving lesions in porcine proliferative enteropathy: possible pathogenetic mechanisms. J Comp Pathol. 1996;115:35–45. doi: 10.1016/s0021-9975(96)80026-0. [DOI] [PubMed] [Google Scholar]
- 27.Monack D M, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulder J W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojcius D M, Bravo de Alda Y, Kanellopoulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 32.Ojcius D M, Degani H, Mispelter J, Dautry-Varsat A. Enhancement of ATP levels and glucose metabolism during an infection by Chlamydia. NMR studies of living cells. J Biol Chem. 1998;273:7052–7058. doi: 10.1074/jbc.273.12.7052. [DOI] [PubMed] [Google Scholar]
- 33.Ojcius D M, Souque P, Perfettini J-L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 34.Pai C H, Kelly J K, Meyers G L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peralta Soler A, Mullin J M, Knudsen K A, Marano C W. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am J Physiol. 1996;270:869–879. doi: 10.1152/ajprenal.1996.270.5.F869. [DOI] [PubMed] [Google Scholar]
- 36.Platt N, da Silva R P, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 37.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rank R G, Sanders M M. Pathogenesis of endometritis and salpingitis in a guinea pig model of chlamydial genital infection. Am J Pathol. 1992;140:927–936. [PMC free article] [PubMed] [Google Scholar]
- 39.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robaye B, Mosselmans R, Fiers W, Dumont J E, Galand P. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am J Pathol. 1991;138:447–453. [PMC free article] [PubMed] [Google Scholar]
- 42.Sgonc R, Wick G. Methods for the detection of apoptosis. Int Arch Allergy Immunol. 1994;105:327–332. doi: 10.1159/000236777. [DOI] [PubMed] [Google Scholar]
- 43.Soloff B L, Rank R G, Barron A L. Electron microscopic observations concerning the in vivo uptake and release of the agent of guinea-pig inclusion conjunctivitis (Chlamydia psittaci) in guinea-pig exocervix. J Comp Pathol. 1985;95:335–344. doi: 10.1016/0021-9975(85)90037-4. [DOI] [PubMed] [Google Scholar]
- 44.Spencer S J, Cataldo N A, Jaffe R B. Apoptosis in the human female reproductive tract. Obstet Gynecol Surv. 1996;51:314–323. doi: 10.1097/00006254-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 45.Stephenson E H, Storz J. Protein profiles of dense-centered forms of five chlamydial strains of animal origin. Am J Vet Res. 1975;36:881–887. [PubMed] [Google Scholar]
- 46.Tabibzadeh S. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod. 1996;2:77–92. doi: 10.1093/molehr/2.2.77. [DOI] [PubMed] [Google Scholar]
- 47.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 48.Toth M, Jeremias J, Ledger W J, Witkin S S. In vivo tumor necrosis factor production in women with salpingitis. Surg Gynecol Obstet. 1992;174:359–362. [PubMed] [Google Scholar]
- 49.Tribby I I, Friis R R, Moulder J W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973;127:155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- 50.Tseng C-T, Rank R. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–5875. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Berg W B. Lessons for joint destruction from animal models. Curr Opin Rheumatol. 1997;9:221–228. doi: 10.1097/00002281-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Vaux D L, Korsmeyer S J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 53.Vining L C, Stuttard C. Chloramphenicol. Bio/Technology. 1995;28:505–530. doi: 10.1016/b978-0-7506-9095-9.50028-9. [DOI] [PubMed] [Google Scholar]
- 54.Williams D M, Bonewald L F, Roodman G D, Byrne G I, Magee D M, Schachter J. Tumor necrosis factor alpha is a cytotoxin induced by murine Chlamydia trachomatis infection. Infect Immun. 1989;57:1351–1355. doi: 10.1128/iai.57.5.1351-1355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams D M, Grubbs B G, Park-Snyder S, Rank R G, Bonewald L F. Activation of latent transforming growth factor beta during Chlamydia trachomatis-induced murine pneumonia. Res Microbiol. 1996;147:251–262. doi: 10.1016/0923-2508(96)81385-4. [DOI] [PubMed] [Google Scholar]
- 56.Williams D M, Magee D M, Bonewald L F, Smith J G, Bleicker C A, Byrne G I, Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990;58:1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyllie A H, Kerr J F R, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 58.Zhang K, Phan S H. Cytokines and pulmonary fibrosis. Biol Signals. 1996;5:232–239. doi: 10.1159/000109195. [DOI] [PubMed] [Google Scholar]
- 59.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]