Abstract
Salmonella is a type of common foodborne pathogen of global concern, seriously endangering human health. In molecular biological detection of Salmonella, the method of amplifying DNA often faces the problem of aerosol pollution. In this study, a microfluidic chip was developed to integrate loop-mediated isothermal amplification (LAMP) and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas12a system to detect Salmonella. The LAMP reaction solution was initially injected into the chamber to amplify at 65 °C for 20 min; the CRISPR/Cas12a reaction solution was subsequently injected to mix with the amplicons for fluorescent signal production at 43 °C for 30 min. Then, the results can be confirmed by naked eyes under 495 nm light or by a fluorescence immunochromatographic reader. The detection limit of this method for Salmonella DNA was 118 pg/μL. The sensitivity and specificity of this method was 100%. Furthermore, this method was used to detect Salmonella after enrichment for 4 h in salmon and chicken samples spiked with 30 CFU/25 g, and was verified to have a stable detection capability in real samples. The microfluidic chip integrated with the LAMP and CRISPR/Cas12a system not only provides a possibility of highly sensitive endpoint fluorescent visual detection of a foodborne pathogen, but also greatly eliminates the risk of aerosol contamination.
Keywords: Salmonella, LAMP, CRISPR/Cas12a, visual detection
1. Introduction
Salmonella is an important pathogen that poses a substantial threat to human health. Approximately 86% of the described salmonellosis cases were caused by foodborne infections [1]. Aquatic products, especially salmon eaten without heat treatment, are easily contaminated with Salmonella and more likely cause harm to human health [2]. Human infection by Salmonella strains may result in fever, vomiting, abdominal pain, nausea, hemorrhagic enteritis, and other symptoms [3,4,5]. Therefore, a rapid and accurate method to detect Salmonella contamination in food is required.
Loop-mediated isothermal amplification (LAMP) has been developed for its simplicity, high amplification capacity, and rapidity [6,7,8]. LAMP requires Bst DNA polymerase and a set 4–6 primers, which can be completed at a constant temperature (60–65 °C) without an expensive thermocycler [9]. Furthermore, LAMP can amplify 109-fold target sequence copies within 15–60 min [10,11]. The amplification capacity of LAMP is 10–100 times higher than that of traditional polymerase chain reaction (PCR) [12]. On the basis of these advantages, LAMP has been widely applied in the field of foodborne pathogen detection [13].
The clustered regularly interspaced short palindromic repeats (CRISPR)/Cas system can specifically bind and cleave target nucleic acids under the guidance of CRISPR RNA (crRNA), which identifies and captures target DNA by base-complementary pairing [14,15,16]. The CRISPR/Cas system has shown the potential to improve the specificity and accuracy of genetic detection [17,18]. CRISPR/Cas12a, from the type V-A CRISPR system, exhibits site-specific dsDNA cutting and nonspecific ssDNA trans-cleavage ability, which provides an efficient signal amplification tool for molecular diagnostics [19].
LAMP-binding CRISPR/Cas system has been applied in the field of disease diagnosis and foodborne pathogen detection [20,21,22]. The reaction process of the LAMP-binding CRISPR/Cas12a system requires two separate steps because the high temperature in the course of LAMP may reduce the activity of Cas12a. However, opening tubes and transferring LAMP solution into CRISPR/Cas reaction solution would lead to aerosol leakage [21,23]. Reports have shown that LAMP amplicons can contaminate reagents in the laboratory by aerosol for several weeks, and they can be promptly used as templates in subsequent reactions, thereby jeopardizing the results of the reactions. When contamination begins, the false-positive rate of the test results would increase sharply, unless all reagents and primers were replaced [24]. Herein, we designed a microfluidic chip as the platform to integrate LAMP and the CRISPR/Cas12a system for the accurate detection of Salmonella.
2. Materials and Methods
2.1. Bacterial Strains and DNA Extraction
The standard bacterial strains and clinically isolated strains were from the Jiangxi Province Center for Disease Control and Prevention. Detailed information of all strains is shown in Table 1. All bacterial strains were cultured in trypticase soy broth (TSB, Land Bridge Technology Co., Ltd., Beijing, China) on a shaking incubator at 180 rpm and 37 °C overnight. One milliliter of each bacterial culture in TSB was centrifuged at 8000 rpm/min for 5 min. Then, the pellet was washed with sterilized 1× PBS (Solarbio Co., Ltd., Beijing, China) for three times and resuspended with 1 mL 1× PBS. Resuspension was boiled for lysing bacteria cells, and then centrifuged at 8500 rpm for 5 min. The supernatants were collected to obtain genomic DNA. The concentration of DNA was determined by Micro-Spectrophotometer K5600 (Kaiao Co., Ltd., Beijing, China).
Table 1.
Sources and identification numbers of strains.
| Bacteria | Source | Identification Number |
|---|---|---|
| Salmonella Enteritidis | CMCC | CMCC 50041 |
| Salmonella Enteritidis | Jiangxi CDC | Kalado |
| Salmonella Enteritidis | Jiangxi CDC | 14S39 |
| Salmonella Rissen | Jiangxi CDC | 15S2 |
| Salmonella Enteritidis | Jiangxi CDC | 15S50 |
| Salmonella Kottbus | Jiangxi CDC | 15S59 |
| Salmonella Thompson | Jiangxi CDC | 16S24 |
| Salmonella Litchfield | Jiangxi CDC | 17S38 |
| Salmonella Newport | Jiangxi CDC | 17S40 |
| Salmonella London | Jiangxi CDC | 17S68 |
| Salmonella Derby | Jiangxi CDC | 18S10 |
| Salmonella Give | Jiangxi CDC | 18S49 |
| Salmonella Orion | Jiangxi CDC | 18S60 |
| Pseudomonas aeruginosa | ATCC | ATCC 27853 |
| Pseudomonas aeruginosa | CMCC | CMCC(B) 10104 |
| Pseudomonas aeruginosa | BNCC | CGMCC 1.1785 |
| Pseudomonas aeruginosa | BNCC | ATCC 9027 |
| Staphylococcus aureus | CMCC | CMCC 26002 |
| Staphylococcus aureus | Jiangxi CDC | JP-1 |
| Listeria monocytogenes | CMCC | CMCC 54001 |
| Listeria monocytogenes | Jiangxi CDC | DZ-JX-1 |
| Bacillus cereus | CMCC | CMCC 63303 |
| Bacillus cereus | Jiangxi CDC | LY-FC-1 |
| Escherichia coli | CMCC | CMCC 44496 |
| Escherichia coli | CMCC | CMCC 44350 |
2.2. LAMP Primers, crRNA, and Report DNA Design
invA, a virulence gene, is chromosomally located and conserved in almost all the Salmonella serotypes [25,26]. The sequence of invA (GenBank No. M90846.1) was used to design LAMP primers. LAMP primers, including the forward inner primer (FIP), the backward inner primer (BIP), the outer forward primer (F3), the outer backward primer (B3), and additional loop primers (FL and BL) were designed via Primer Explorer software Version 5 (http://primerexplorer.jp/lampv5e/index.html accessed on 20 June 2021). The crRNA for specifically recognizing the specific amplicon was designed through the Benchling website (https://www.benchling.com/crispr/ accessed on 3 July 2021). Report DNA was designed as a short ssDNA (5 nt), labeled with fluorophore (6-FAM) and quencher (BHQ1) groups at both ends [27]. The primers, crRNA, and report DNA were synthesized by Sangon Co., Ltd. (Shanghai, China); the detailed sequence information is listed in Table 2.
Table 2.
The sequences of primers, crRNA, and report DNA.
| Name | Sequence (5′-3′) |
|---|---|
| F3 | GCGAAGCGTACTGGAAAGG |
| B3 | TCAACAATGCGGGGATCTG |
| FIP | ATGATGCCGGCAATAGCGTCAC-AAAGCCAGCTTTACGGTTCC |
| BIP | GTGGGGATGACTCGCCATGG-ACCATCACCAATGGTCAGC |
| LF | AAACTTCATCGCACCGTCAAA |
| LB | TATGGATTTGTCCTCCGCCCT |
| crRNA | UAAUUUCUACUAAGUGUAGAUAAUACCGCCAAUAAAGUUCA |
| Report DNA | 6-FAM-TTATT-BHQ1 |
2.3. LAMP Assay
LAMP solution was prepared according to New England Biolabs (NEB) instruction in a total volume of 25 μL, containing 3.5 μL of 10 mM dNTP mix (Vazyme Co., Ltd., Nanjing, China), 2.5 μL of 10× isothermal amplification buffer, 1.5 μL of 100 mM MgSO4, 1 μL of 800 U/mL Bst 2.0 DNA polymerase (New England Biolabs, Ipswich, MA, USA), 1 μL of 40 μM FIP/BIP primers, 1 μL of 5 μM F3/B3 primers, 1 μL of 10 μM FL/BL primers, and 2 μL DNA template. Then, 13 μL of mixture was incubated in thermostatic metal bath TU-10 (Yiheng Co., Ltd., Shanghai, China) at 65 °C for 5, 10, 15, 20, and 25 min and terminated at 80 °C for 10 min. The product of LAMP was characterized by QIAxcel advanced capillary electrophoresis (Qiagen, Hilden, Germany).
2.4. LAMP–CRISPR/Cas12a Detection System
The LAMP assay was performed according to Section 2.3. The Cas12a-mediated detection system contained 1.95 μL 10× NEBuffer 2.1, 1.3 μL of Cas12a (1, 2, 3, and 4 μM) (New England Biolabs, Ipswich, MA, USA), 1.95 μL of crRNA (0.5, 1.0, 1.5, 2.0, and 2.5 μM), 1.3 μL of report DNA (20, 40, 60, 80, and 100 μM), and 13 μL of LAMP amplicons as an activator. Then, 19.5 μL of mixture was incubated a thermostatic metal bath at 25 °C, 31 °C, 37 °C, 43 °C, and 49 °C for 30 min. The products of LAMP cleaved by Cas12a were characterized by capillary electrophoresis.
2.5. Detection of Salmonella by Microfluidic Chip Integrated with LAMP and CRISPR/Cas12a System
The polydimethylsiloxane (PDMS) chip was designed to be a reaction and observation platform, with one observation chamber and two narrow channels for injection of reaction solution. Thirteen microliters of LAMP components were initially injected into the chamber by microinjectors (Anting Co., Ltd., Shanghai, China) with smart syringe pump XMSP-C (Ximai Co., Ltd., Nanjing, China) at the speed of 15 μL/min. Then, the chip was stored at 65 °C for amplification, and subsequently at 80 °C for enzyme inactivation. After amplification, 6.5 μL of CRISPR/Cas12a components were injected into the chamber at the speed of 15 μL/min to mix with LAMP amplicons by another channel, and then the chip was stored at 43 °C. The fluorescence could be observed by the naked eyes under 495 nm light and measured by a fluorescence immunochromatographic reader (Helmen Co., Ltd., Hangzhou, China). Actual devices and the effect diagrams are shown in Figure S1.
2.6. Evaluation of Detection Limit
Typically, the detection limit is determined by setting a threshold, which can be calculated from the average fluorescence intensity and standard deviation of the negative control. The value of the threshold equals the average intensity and three times the standard deviation [28].
Extracted genomic DNA that served as the template was performed a 10-fold gradient dilution from 10−2 to 10−4 with 1× PBS to evaluate the detection limit of this method. The DNA templates of each concentration were amplified by LAMP. Then, amplicons were employed to activate the CRISPR/Cas12a cleaving system. The fluorescence intensity of these results was measured by a fluorescence immunochromatographic reader.
2.7. Sensitivity and Specificity
One Salmonella standard strain, twelve Salmonella clinical strains, nine non-Salmonella standard strains, and three non-Salmonella clinical strains were selected to test the sensitivity and specificity of this method. DNA extracted from these strains was amplified by LAMP. Then, amplicons were employed for activating the CRISPR/Cas12a cleaving system. The fluorescence intensity of these results was measured by a fluorescence immunochromatographic reader.
2.8. Detection of Salmonella in Artificially Contaminated Salmon and Chicken
Salmon and chicken samples were purchased from a local supermarket. Twenty-five grams of salmon and chicken samples, which were verified to be Salmonella-free, were made into homogenates and spiked with Salmonella at a final concentration of 30 CFU/25 g, whereas un-spiked samples were tested to evaluate the specificity of this method in real samples [29]. Then, samples were placed into TSB media (225 mL of each) and incubated at 37 °C with shaking at 180 rpm. Two milliliters of culture solution were aspirated every hour from 0 to 6 h. Solutions collected were divided into two groups. One group was tested by the LAMP–CRISPR/Cas12a system, and another group was used for colony count by Salmonella chromogenic medium (CHRO Magar, Paris, France).
To check the effect of the matrix of real samples on the test efficiency in comparing with the buffer solution, 25 g of salmon and chicken samples were made into homogenates with TSB medium, and all samples were irradiated under a UV lamp (15 W) for 30 min to ensure sterility. Preprocessed samples and the other TSB medium with no sample were spiked with Salmonella at final concentrations of 4 × 101, 4 × 102, 4 × 103, 4 × 104 CFU/mL, respectively, and then tested by the LAMP–CRISPR/Cas12a system.
3. Results
3.1. Principle and Operation of the Detection System
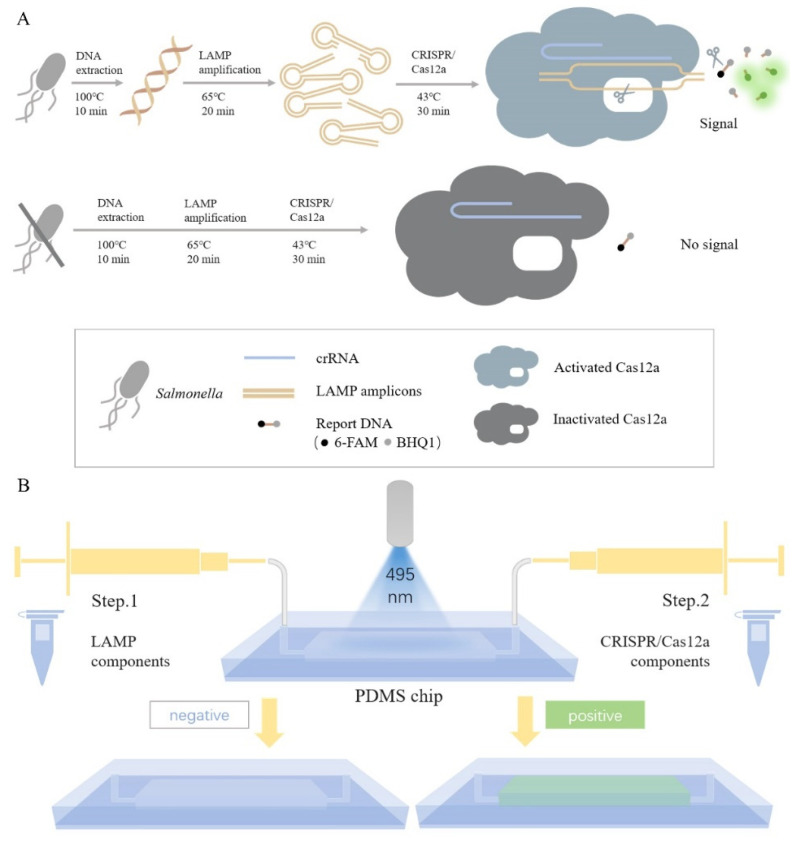
The principle and operation of Salmonella detection by the microfluidic chip integrated with the LAMP and CRISPR/Cas12a system are shown in Scheme 1. In summary, crude genomic DNA of Salmonella extracted using the heated lysis method was rapidly amplified by LAMP. The amplicons could be recognized and captured by crRNA, and then the CRISPR/Cas12a system could cleave LAMP amplicons with site-specific dsDNA cutting ability and report DNA with nonspecific ssDNA trans-cleavage ability. The cleavage of report DNA, which was labeled with fluorophore 6-FAM and quencher BHQ1, resulted in the appearance of fluorescence, as shown in Scheme 1A.
Scheme 1.
Schematics of (A) principle and (B) operation of LAMP–CRISPR/Cas12a detection system on a chip to detect Salmonella.
PDMS chip was selected as the detection platform to eliminate the risk of aerosol contamination. LAMP components with or without DNA of Salmonella were initially injected into the chamber through one channel, and CRISPR/Cas12a components were injected into the chamber through another channel after LAMP was completed, as shown in Scheme 1B. Then, the result could be confirmed by the naked eyes under 495 nm light or by a fluorescence immunochromatographic reader because of the suitable size of chip pre-designed.
The risk of aerosol leakage was eliminated in this process owing to the leakproofness of the chip, thereby protecting the detection environment and reagents from aerosol pollution. With this chip, the false-positive results are greatly avoided, providing a platform for more accurate detection results in subsequent detection.
3.2. Analysis of LAMP–CRISPR/Cas12a Detection System
The extracted genomic DNA of Salmonella was amplified by LAMP. The capillary electrophoresis image (Figure S2) showed the bands, which were the LAMP product and the Cas12a-cleaved LAMP product. The difference between the two sets of bands indicated the occurrence of the cleavage.
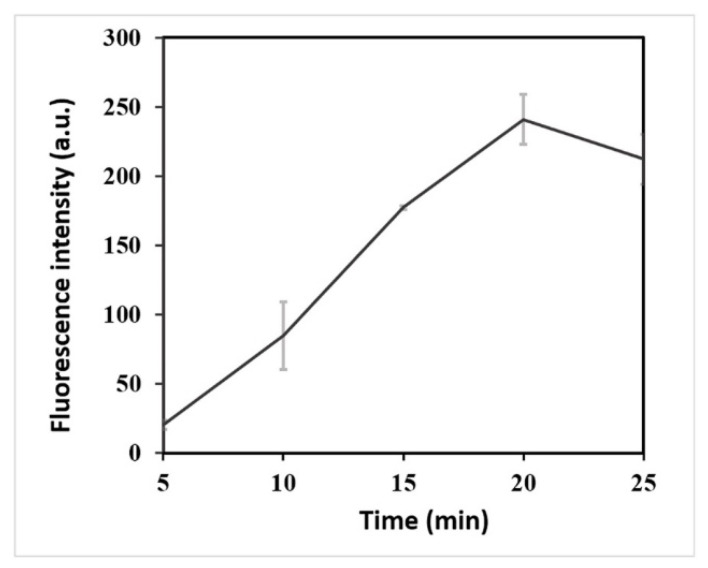
LAMP amplicons were employed for activating the CRISPR/Cas12a cleaving system. More LAMP amplicons were contributed to obtain a higher fluorescence intensity of the CRISPR/Cas12a detection system. After 20 min, the LAMP amplification system generated ample target molecules for the CRISPR/Cas12a detection system (Figure 1). The optimal reaction time of LAMP to improve the detection efficiency was 20 min.
Figure 1.
Determination of optimal action time of LAMP. Fluorescence intensity with different reaction time (5, 10, 15, 20, and 25 min) of LAMP.
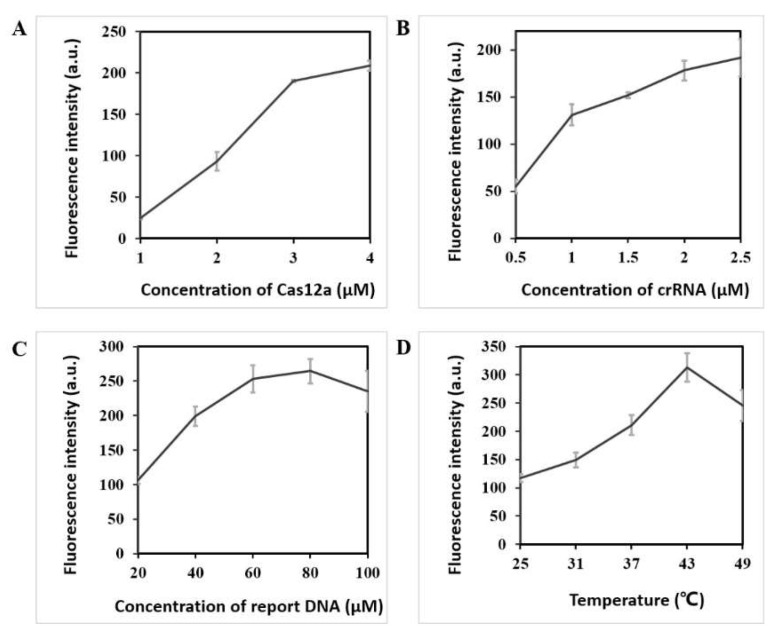
The experimental factors, including the concentration of CRISPR/Cas12a components and reaction temperature, were investigated to reduce the detection limit. First, the fluorescence intensity increased as the concentration of Cas12a increased, as shown in Figure 2A. When the concentration of Cas12a ranged 3–4 μM, the fluorescence intensity did not increase considerably. Therefore, the optimal concentration of Cas12a was 3 μM. Second, the concentration of crRNA was optimized. As the concentration of crRNA increased, the fluorescence intensity increased significantly initially, and then the increase magnitude gradually decreased, as shown in Figure 2B. Thus, 2 μM was finally selected as the optimal concentration of crRNA. Third, as the concentration of report DNA increased, the fluorescence intensity peaked at 80 μM and then decreased, as shown in Figure 2C. Hence, 80 μM was selected as the optimal concentration. Fourth, five temperatures from 25 °C to 49 °C were selected for the experiment. The fluorescence intensity peaked at 43 °C, and the fluorescence intensity began to decrease as the temperature continued to increase, as shown in Figure 2D. This result might be due to the effect of higher temperature on the activity of the enzyme. Therefore, 43 °C was selected as the optimal reaction temperature.
Figure 2.
Optimization of LAMP–CRISPR detection system for Salmonella detection. (A) Fluorescence intensity using different concentrations (1, 2, 3, and 4 μM) of Cas12a; (B) Fluorescence intensity using different concentrations (0.5, 1.0, 1.5, 2.0, and 2.5 μM) of crRNA; (C) Fluorescence intensity using different concentrations (20, 40, 60, 80, and 100 μM) of report DNA; (D) Fluorescence intensity under different temperatures (25 °C, 31 °C, 37 °C, 43 °C, and 49 °C) of CRISPR/Cas12a system.
3.3. Detection Limit of the Proposed Method
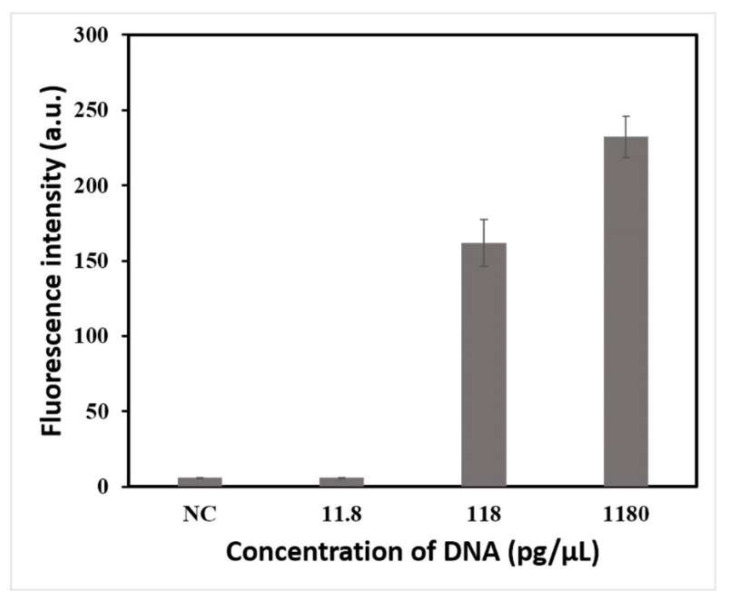
After optimizing the conditions, this method was established for Salmonella detection. A 10-fold dilution series of extracted genomic DNA from 10−2 to 10−4 was used to determine the detection limit of this method, and the original concentration of the DNA was 118 ng/μL. The results are shown in Figure 3. The values of fluorescence intensity were 6, 162, and 232 when the concentrations of DNA were 11.8, 118, and 1180 pg/μL, respectively. The threshold was calculated as 6; thus, 118 pg/μL was regarded as the detection limit of the proposed method.
Figure 3.
Detection limit test results of LAMP–CRISPR/Cas12a detection system for Salmonella detection using 10-fold serial dilutions of crude genomic DNA in ddH2O (diluted from 1180 pg/μL to 11.8 pg/μL).
3.4. Sensitivity and Specificity
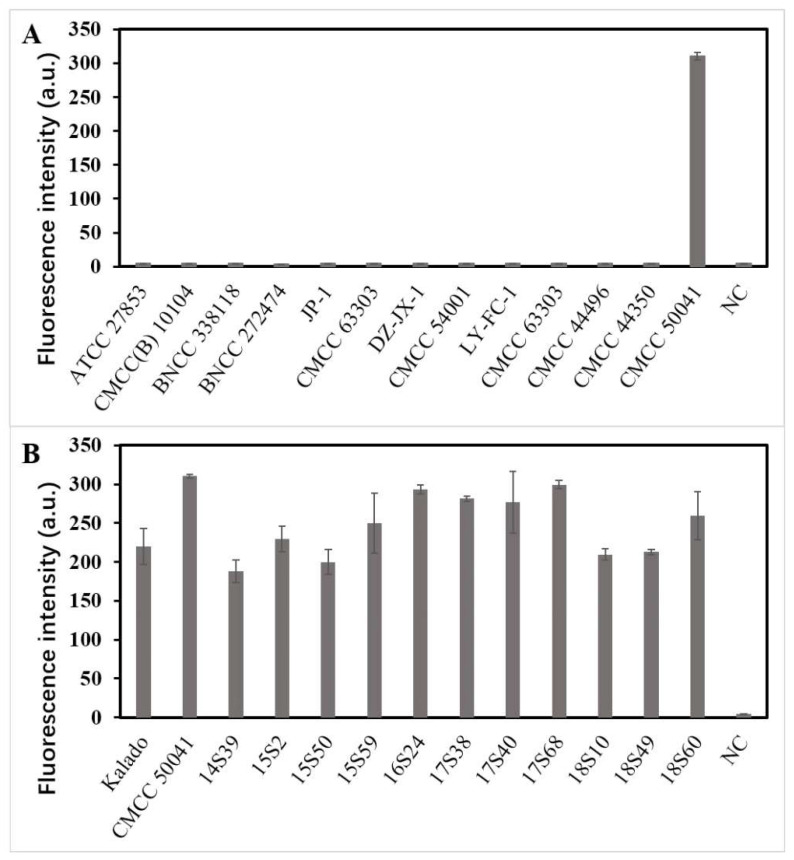
As shown in Figure 4, compared with the threshold (5), all twelve non-Salmonella strains had no evident fluorescence signal (5, 5, 5, 4, 5, 5, 4, 4, 5, 4, 5, and 5), thereby confirming that the detection method established in this study had high specificity. Meanwhile, all thirteen Salmonella strains had evident fluorescence signals (220, 310, 188, 200, 250, 293, 281, 277, 299, 210, 213, 230, and 259, respectively), confirming that the detection method had high sensitivity.
Figure 4.
Estimating the specificity and sensitivity of LAMP–CRISPR detection system for Salmonella detection. (A) Fluorescence intensity using non-Salmonella standard strains for specific evaluation. (B) Fluorescence intensity using Salmonella standard strains for sensitive evaluation.
3.5. Detection of Salmonella in Salmon and Chicken Using the Proposed Method
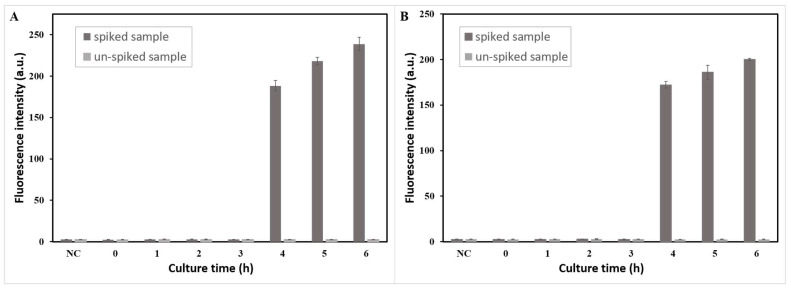
Furthermore, the performance of the LAMP–CRISPR/Cas12a detection system was evaluated by salmon and chicken samples spiked with Salmonella (30 CFU/25 g), and un-spiked samples were tested to evaluate the specificity of this method in real samples. DNA extracted from bacteria with different culture times (0, 1, 2, 3, 4, 5, and 6 h) was detected by this method. As Figure 5 shows, Salmonella in a spiked salmon sample cultured at 4 h was detected with a fluorescence intensity of 188 when the threshold was 3, and Salmonella in a spiked chicken sample cultured at 4 h was detected with a fluorescence intensity of 172. The results indicated that Salmonella in both salmon and chicken samples could be detected by this system after only 4 h of enrichment. Figure S3 shows that after 4 h of enrichment, the concentration of Salmonella was approximately 5 × 102 CFU/mL in the salmon sample and approximately 4.2 × 102 CFU/mL in the chicken sample (100 μL of culture solution was used for colony count). Meanwhile, the groups of un-spiked samples after 6 h of culture did not show positive results even with large amounts of Escherichia coli, indicating that this method had high specificity in real samples.
Figure 5.
Minimum detection time test results of LAMP–CRISPR detection system for Salmonella detection in the (A) salmon sample and (B) chicken sample spiked and un-spiked.
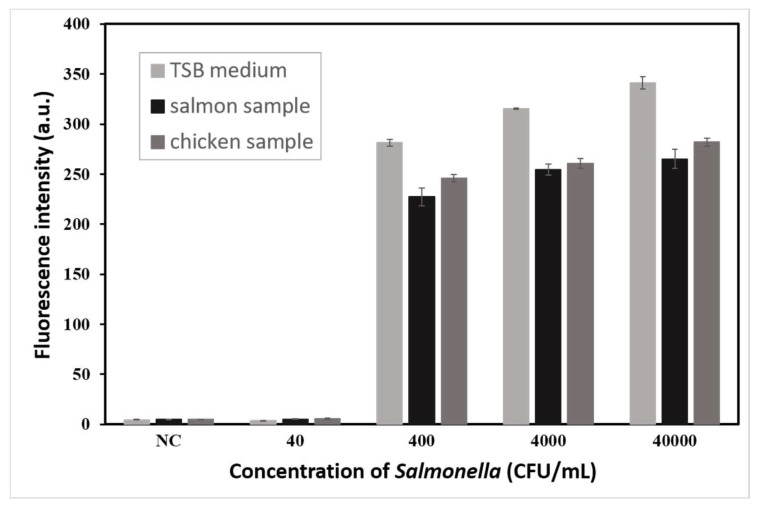
To check the effect of the matrix of real samples on the test efficiency, TSB medium, salmon sample, and chicken sample were spiked with Salmonella (4 × 101, 4 × 102, 4 × 103, and 4 × 104 CFU/mL). As Figure 6 shows, 400 CFU/mL of Salmonella could be detected in TSB medium, salmon sample, and chicken sample with fluorescence intensities of 281, 227, and 246, respectively. The results indicated that the matrix of real sample did not affect the detection efficiency of this method, but only slightly weakened the fluorescence intensity. Therefore, this method had a stable detection capability in real samples.
Figure 6.
Matrixes test results of LAMP–CRISPR detection system for Salmonella detection in TSB medium, salmon sample, and chicken sample spiked with Salmonella (4 × 101, 4 × 102, 4 × 103, and 4 × 104 CFU/mL).
3.6. Discussion
Due to inadequate storage temperatures or inadequate cooking, foods can be a source of pathogen infections, causing tremendous harm to human health. Therefore, pathogens including Salmonella should be controlled in foods. Mukama et al. [20] reported a simple, inexpensive, and ultrasensitive DNA probe based LFB with CRISPR/Cas and LAMP, which achieved high sensitivity and specificity both in pure and complex samples. However, opening tubes and transferring LAMP products into the CRISPR/Cas reaction solution would lead to aerosol leakage, which would seriously jeopardize the results of the subsequent detection. Therefore, we need a leakproof and observable reaction platform. In an attempt to establish a more rapid and accurate method to detect Salmonella, we developed a LAMP combined with CRISPR/Cas12a integrated into a microfluidic chip system.
First, we optimized the reaction conditions of the LAMP and CRISPR/Cas12a system to reduce the detection limit. Then, we evaluated the performance of this method. The results showed this method had high sensitivity and specificity, which were consistent with previous reports [21]. Its excellent performance was due to two sets of pre-designed specific sequences used in the system. Six primers of LAMP made the amplification process have high sensitivity and efficiency. The designed crRNA could specifically recognize the LAMP amplicons for secondary confirmation, which further improved the accuracy of the detection. Furthermore, the performance of the LAMP–CRISPR/Cas12a detection system was evaluated by salmon and chicken samples spiked with Salmonella. The results showed the good detection performance for Salmonella in real samples, affirming the practical application potential of this method.
Further research aimed at optimizing the design and reducing the cost of the microfluidic chip may enhance the applicability of the assay. This method can also be applied to the detection of other pathogens, providing great potential as a universal platform for pathogen detection. Therefore, exploring the possibility of high-throughput detection on a single chip will also be of great interest.
4. Conclusions
In summary, this study developed a LAMP combined CRISPR/Cas12a integrated into a microfluidic chip system for Salmonella detection. Two separate liquid additions were performed through two narrow channels to achieve two-step reactions on one chip, eliminating the risk of aerosol contamination and cross-contamination that could result from opening the cap of a centrifuge tube, which is a typical problem associated with LAMP. The designed chip can be used as a reaction platform as well as a platform for reading the results directly. The size of the chip was designed to be suitable to allow the experimental results to be read by a portable fluorescence immunochromatographic reader or directly interpreted by the naked eyes under the irradiation of a certain wavelength of ultraviolet light. The detection limit of the proposed method could reach 118 pg/μL of crude genomic DNA, and the entire detection process could be completed within 50 minutes. Furthermore, this method was used to detect Salmonella after enrichment for 4 h in salmon and chicken samples spiked with 30 CFU/25 g, and was verified to have a stable detection capability in real samples. At the same time, the detection method had high sensitivity and specificity for detecting twelve Salmonella strains and thirteen non-Salmonella strains. The results showed that the proposed detection system was suitable for on-site rapid Salmonella detection with a low detection limit. Its high performance provides great potential as a universal platform for pathogen detection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11233887/s1, Figure S1: Devices and the effect diagrams. (A) PDMS chip model marked with actual size. (B) Smart syringe pumps and microinjectors for injection. (C) Fluorescence immunochromatographic reader. (D) Results of naked eyes observation of Salmonella detection on PDMS chip, negative and positive results, respectively. (E) Negative and positive results read by fluorescent immunochromatographic reader; Figure S2: Electropherogram of negative control, LAMP products, and LAMP products cleaved by Cas12a; Figure S3: Culture results of bacteria solution collected from (A) spiked salmon sample, (B) un-spiked salmon sample, (C) spiked chicken sample and (D) un-spiked chicken sample at different times on the Salmonella chromogenic medium, in the matrix test. Purple colonies circled were Salmonella anthropogenically added, and blue colonies were Escherichia coli from sample.
Author Contributions
Investigation, Conceptualization, Data curation, Software, Writing—original draft: Y.L.; Methodology: S.S.; Chip design: S.W.; Resources: D.L. and W.L.; Project administration: J.L.; Funding acquisition: D.L.; Writing—review and editing: W.L and S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Natural Science Foundation of China (82003467), Key Research and Development Program of Jiangxi Province (20192BBGL70053, 20192BBG70069), General Project of Jiangxi Natural Science Foundation (20202BAB206066), Science and Technology Fund Plan of Jiangxi Provincial Health Commission (202130977).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’Brien S.J., Jones T.F., Fazil A., Hoekstra R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Friesema I., de Jong A., Hofhuis A., Heck M., Kerkhof H.V.D., de Jonge R., Hameryck D., Nagel K., van Vilsteren G., van Beek P., et al. Large outbreak of Salmonella Thompson related to smoked salmon in the Netherlands, August to December 2012. Eurosurveillance. 2014;19:20918. doi: 10.2807/1560-7917.ES2014.19.39.20918. [DOI] [PubMed] [Google Scholar]
- 3.Chiu C.-H., Su L.-H., Chu C. Salmonella enterica serotype Choleraesuis: Epidemiology, pathogenesis, clinical disease, and treatment. Clin. Microbiol. Rev. 2004;17:311–322. doi: 10.1128/CMR.17.2.311-322.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi X., Li P., Xu X., Yuan Y., Bu S., Lin D. Epidemiological and Molecular Investigations on Salmonella Responsible for Gastrointestinal Infections in the Southwest of Shanghai from 1998 to 2017. Front. Microbiol. 2019;10:2025. doi: 10.3389/fmicb.2019.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia K., André B., Ulrich S., Burkhard M., Madeleine P., Amir A. Establishment and validation of a loop-mediated isothermal amplification (LAMP) assay targeting the ttrRSBCA locus for rapid detection of Salmonella spp. in food. Food Control. 2021;126:107973. doi: 10.1016/j.foodcont.2021.107973. [DOI] [Google Scholar]
- 6.Shang Y., Ye Q., Cai S., Wu Q., Pang R., Yang S., Xiang X., Wang C., Zha F., Ding Y., et al. Loop-mediated isothermal amplification (LAMP) for rapid detection of Salmonella in foods based on new molecular targets. LWT—Food Sci. Technol. 2021;142:110999. doi: 10.1016/j.lwt.2021.110999. [DOI] [Google Scholar]
- 7.Ye H., Nowak C., Liu Y., Li Y., Zhang T., Bleris L., Qin Z. Plasmonic LAMP: Improving the Detection Specificity and Sensitivity for SARS-CoV-2 by Plasmonic Sensing of Isothermally Amplified Nucleic Acids. Small. 2022;18:2107832. doi: 10.1002/smll.202107832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notomi T., Mori Y., Tomita N., Kanda H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015;53:1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- 9.Heithoff D.M., Barnes L., Mahan S.P., Fox G.N., Arn K.E., Ettinger S.J., Bishop A.M., Fitzgibbons L.N., Fried J.C., Low D.A., et al. Assessment of a Smartphone-Based LAMP Assay for Detection of SARS-CoV-2 and Influenza Viruses. JAMA Netw. Open. 2022;5:e2145669. doi: 10.1001/jamanetworkopen.2021.45669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe S., Okubo A., Miyajima Y., Satoh K., Makimura K. Specific detection of Trichophyton rubrum and Trichophyton interdigitale based on loop-mediated isothermal amplification (LAMP) from onychomycosis specimens. J. Dermatol. 2019;46:1179–1183. doi: 10.1111/1346-8138.15092. [DOI] [PubMed] [Google Scholar]
- 11.Liao W., Long D., Huang Q., Wei D., Liu X., Wan L., Feng Y., Zhang W., Liu Y. Rapid Detection to Differentiate Hypervirulent Klebsiella pneumoniae (hvKp) From Classical K. pneumoniae by Identifying peg-344 With Loop-Mediated Isothermal Amplication (LAMP) Front. Microbiol. 2020;11:1189. doi: 10.3389/fmicb.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Sun L., Sun H., Hong Y., Xiao Z., Pang X., Piao Z., Feng J., Liang Y. A loop-mediated isothermal DNA amplification (LAMP) assay for detection of the clubroot pathogen Plasmodiophora brassicae. Plant Dis. 2021;106:1730–1735. doi: 10.1094/PDIS-11-21-2430-RE. [DOI] [PubMed] [Google Scholar]
- 13.Sivakumar R., Dinh V.P., Lee N.Y. Ultraviolet-induced in situ gold nanoparticles for point-of-care testing of infectious diseases in loop-mediated isothermal amplification. Lab Chip. 2021;21:700–709. doi: 10.1039/D1LC00019E. [DOI] [PubMed] [Google Scholar]
- 14.Swarts D.C., Jinek M. Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol. Cell. 2019;73:589–600.e4. doi: 10.1016/j.molcel.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Z., Li R., Zhang H., Wang J., Lu Y., Zhang D., Yang L. PAM-free loop-mediated isothermal amplification coupled with CRISPR/Cas12a cleavage (Cas-PfLAMP) for rapid detection of rice pathogens. Biosens. Bioelectron. 2022;204:114076. doi: 10.1016/j.bios.2022.114076. [DOI] [PubMed] [Google Scholar]
- 16.Liu P., Wang X., Liang J., Dong Q., Zhang J., Liu D., Wang S., Bi J., Liu W., Wang Z., et al. A Recombinase Polymerase Amplification-Coupled Cas12a Mutant-Based Module for Efficient Detection of Streptomycin-Resistant Mutations in Mycobacterium tuberculosis. Front. Microbiol. 2022;12:796916. doi: 10.3389/fmicb.2021.796916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W., Jin T., Dai Y., Liu C.C. Surpassing the detection limit and accuracy of the electrochemical DNA sensor through the application of CRISPR Cas systems. Biosens. Bioelectron. 2020;155:112100. doi: 10.1016/j.bios.2020.112100. [DOI] [PubMed] [Google Scholar]
- 18.Xia X., Ma B., Zhang T., Lu Y., Khan M.R., Hu Y., Lei C., Deng S., He Q., He G., et al. G-Quadruplex-Probing CRISPR-Cas12 Assay for Label-Free Analysis of Foodborne Pathogens and Their Colonization In Vivo. ACS Sens. 2021;6:3295–3302. doi: 10.1021/acssensors.1c01061. [DOI] [PubMed] [Google Scholar]
- 19.Zhou R., Li Y., Dong T., Tang Y., Li F. A sequence-specific plasmonic loop-mediated isothermal amplification assay with orthogonal color readouts enabled by CRISPR Cas12a. Chem. Commun. 2020;56:3536–3538. doi: 10.1039/D0CC00397B. [DOI] [PubMed] [Google Scholar]
- 20.Mukama O., Wu J., Li Z., Liang Q., Yi Z., Lu X., Liu Y., Liu Y., Hussain M., Makafe G.G., et al. An ultrasensitive and specific point-of-care CRISPR/Cas12 based lateral flow biosensor for the rapid detection of nucleic acids. Biosens. Bioelectron. 2020;159:112143. doi: 10.1016/j.bios.2020.112143. [DOI] [PubMed] [Google Scholar]
- 21.Mukama O., Yuan T., He Z., Li Z., Habimana J.D., Hussain M., Li W., Yi Z., Liang Q., Zeng L. A high fidelity CRISPR/Cas12a based Lateral flow biosensor for the detection of HPV16 and HPV18. Sens. Actuators B Chem. 2020;316:128119. doi: 10.1016/j.snb.2020.128119. [DOI] [Google Scholar]
- 22.Zhang T., Zhao W., Zhao W., Si Y., Chen N., Chen X., Zhang X., Fan L., Sui G. Universally Stable and Precise CRISPR-LAMP Detection Platform for Precise Multiple Respiratory Tract Virus Diagnosis Including Mutant SARS-CoV-2 Spike N501Y. Anal. Chem. 2021;93:16184–16193. doi: 10.1021/acs.analchem.1c04065. [DOI] [PubMed] [Google Scholar]
- 23.Bai L., Wang L., Huang S., Bai R., Lv X., Sun L., Zhang F., Xu X. Rapid, Visual, and Sequence-Specific Detection of Salmonella in Egg Liquid with vis-NEAA, a CRISPR/Cas12 Empowered New Strategy. J. Agric. Food Chem. 2022;70:2401–2409. doi: 10.1021/acs.jafc.1c06715. [DOI] [PubMed] [Google Scholar]
- 24.Wong Y.-P., Othman S., Lau Y.-L., Radu S., Chee H.-Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018;124:626–643. doi: 10.1111/jam.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Y., Liu Y., He S., Xu X., Cao X., Ye Y., Zheng H. Ultrasensitive electrochemical DNA sensor for virulence invA gene of Salmonella using silver nanoclusters as signal probe. Sens. Actuators B Chem. 2018;272:53–59. doi: 10.1016/j.snb.2018.05.133. [DOI] [Google Scholar]
- 26.Chaudhary J.H., Nayak J.B., Brahmbhatt M.N., Makwana P.P. Virulence genes detection of Salmonella serovars isolated from pork and slaughterhouse environment in Ahmedabad, Gujarat. Vet. World. 2015;8:121–124. doi: 10.14202/vetworld.2015.121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J.S., Ma E., Harrington L.B., Costa M.D., Tian X., Palefsky J.M., Doudna J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan S., Liu D., Guo Q., Wu S., Chen R., Luo K., Hu L., Xiong Y., Lai W. Sensitive detection of Escherichia coli O157:H7 based on cascade signal amplification in ELISA. J. Dairy Sci. 2016;99:7025–7032. doi: 10.3168/jds.2016-11320. [DOI] [PubMed] [Google Scholar]
- 29.Li C., Wang Y., Wang J., Wang X. Properties of a Novel Salmonella Phage L66 and Its Application Based on Electrochemical Sensor-Combined AuNPs to Detect Salmonella. Foods. 2022;11:2836. doi: 10.3390/foods11182836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.