Abstract
BALB/c and strain 129 mice infected intranasally with Chlamydia pneumoniae displayed a moderate-to-severe inflammation in the lungs and produced interleukin-12 (IL-12), gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-10, with peak levels on days 1 to 3 postinfection (p.i.), returning to basal levels by day 16 p.i. Anti-IL-12 treatment resulted in less-severe pathological changes but higher bacterial titers on days 3 and 7 p.i. By day 16 p.i., the inflammatory responses of control antibody-treated mice subsided. The bacterial titers of both anti-IL-12- and control antibody-treated mice decreased within 3 weeks to marginally detectable levels. Anti-IL-12 treatment significantly reduced lung IFN-γ production and in vitro spleen cell IFN-γ production in response to either C. pneumoniae or concanavalin A. In γ-irradiated infected mice, cytokine production was delayed, and this delay correlated with high bacterial titers in the lungs. Following C. pneumoniae infection, 129 mice lacking the IFN-γ receptor α chain gene (G129 mice) produced similar IL-12 levels and exhibited similarly severe pathological changes but had higher bacterial titers than 129 mice. However, by day 45 p.i., bacterial titers became undetectable in both wild-type 129 and G129 mice. Thus, during C. pneumoniae lung infection, IL-12, more than IFN-γ, plays a role in pulmonary-cell infiltration. IFN-γ and IL-12, acting mostly through its induction of IFN-γ and Th1 responses, play an important role in controlling acute C. pneumoniae infection in the lungs, but eventually all mice control the infection to undetectable levels by IL-12- and IFN-γ-independent mechanisms.
Chlamydia pneumoniae is a highly specialized gram-negative intracellular bacterium with a biphasic life cycle, in which the spore-like elementary bodies facilitate transit between cells and the metabolically active reticulate bodies are responsible for intracellular replication (30, 59). C. pneumoniae infection is by far the most common human chlamydial infection, with seropositivity in at least 50% of the general population over age 20 in the United States and other countries (12, 17, 19, 26, 38, 43, 58). Although most of the acute infections are probably asymptomatic, the clinical syndromes most frequently associated with C. pneumoniae are pneumonia, pharyngitis, sinusitis, and bronchitis (13, 17, 18, 20, 39, 58). In a series of studies, 10% of cases of pneumonia and approximately 5% of cases of bronchitis, sinusitis and, in Finland, pharyngitis have been attributed to the organism (30). In addition, C. pneumoniae has been proposed to be a risk factor for immune-reactive disorders such as adult-onset asthma, reactive arthritis, and acute chest syndrome of sickle cell anemia (21, 40). Patients with human immunodeficiency virus infection or other immunosuppressive diseases have increased incidences of seropositivity and isolation of bacteria from the lungs, although the role of C. pneumoniae as an opportunistic pathogen in immunocompromised persons is not well defined (1, 14). Persistently elevated titers of antibody to C. pneumoniae have been observed in patients with sarcoidosis and lung cancer (33). Recently, increasing evidence has implicated C. pneumoniae in the pathogenesis of coronary artery disease, as suggested by seroepidemiological studies and by direct demonstration of such organisms in disease lesions of patients (4, 31, 47, 48, 51, 53). Studies in apolipoprotein E-deficient transgenic mice and in rabbits also showed that C. pneumoniae infection leads to inflammatory changes in the aorta (11, 32, 41). More recently, Bachmaier et al. (2) reported that a peptide from the murine heart muscle-specific α myosin heavy chain that has sequence homology to the 60-kDa cysteine-rich outer membrane proteins of C. pneumoniae, Chlamydia psittaci, and Chlamydia trachomatis was able to induce autoimmune inflammatory heart disease in mice, suggesting that chlamydial infections and heart disease are linked through antigenic mimicry.
C. pneumoniae, like other species of Chlamydia (5, 35, 46, 50, 60), is a potent inducer of the cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, as shown in a human monocytic cell line (22) and in freshly isolated human peripheral blood mononuclear cells (27). Very recently, Penttila et al. (44) reported that lung-derived mononuclear cells from infected mice show gamma interferon (IFN-γ) responses upon in vitro restimulation with inactivated C. pneumoniae, whereas cells isolated from naive or primary-challenged mice do not. However, the immune mechanisms involved in C. pneumoniae resistance and pathogenesis, and the role of key immune regulatory factors such as IL-12 and IFN-γ, remain largely unknown. IL-12 is a heterodimeric cytokine composed of a 40-kDa and a 35-kDa chain (28) produced primarily by phagocytic cells and dendritic cells, in response to infections by various bacteria, viruses, protozoa, and fungi, and to other inflammatory or immunological stimuli (9, 34, 57). IL-12 augments NK cell and T-lymphocyte cytotoxic activity, favors Th1 differentiation, and induces the production of IFN-γ and other cytokines (28, 56). IL-12 and IL-12-induced IFN-γ participate in the regulation of adaptive immune responses and the activation of macrophages, thus playing an important role in innate resistance and immunity (55, 57).
To examine C. pneumoniae infection-mediated cytokine production and to analyze the mechanisms of host resistance against this infection, we focused on the ability of C. pneumoniae to induce pulmonary IL-12 and IFN-γ production in vivo and investigated the roles of these cytokines in resistance to C. pneumoniae infection.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old male BALB/c mice were obtained from Harlan Sprague-Dawley (Indianapolis, Ind.). IFN-γ receptor α chain gene-deficient G129 mice and wild-type 129 mice were obtained from M. Aguet (Lausanne, Switzerland) and bred in the pathogen-free animal breeding facility at The Wistar Institute (Philadelphia, Pa.). All mice were fed a regular mouse chow diet ad libitum and housed under biosafety level 2 conditions. In some experiments, mice were γ-irradiated at a sublethal dose (450 rads) using a Mark 1 irradiator (J. L. Shepherd & Associates, Glendale, Calif.) 6 to 8 h before bacterial infection (3).
C. pneumoniae strain, inoculum preparation, and infection of mice.
C. pneumoniae strain TW 183, a pharyngeal isolate, was purchased from the American Type Culture Collection (ATCC, Manassas, Va.) and was propagated in McCoy cells (ATCC) in Dulbecco's modified Eagle medium (Gibco BRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum, 0.3 mg of l-glutamine/ml, 0.5% glucose, 4 mM HEPES, 25 μg of gentamicin/ml, and 1 μg of cycloheximide/ml in a 35°C, 5% CO2-saturated humidified incubator (15). Infected cells were harvested on day 3 or 4 and were disrupted by two cycles of freeze-thawing and ultrasonification; different harvests were pooled. After centrifugation at 500 × g for 5 min to remove cell debris, bacteria were concentrated by high-speed centrifugation at 25,000 × g for 25 min. Pellets were resuspended in phosphate-buffered saline (PBS), pH 7.4, mixed with an equal volume of sucrose-phosphate-glutamic acid buffer (0.22 M sucrose, 10 mM NaH2PO4, 3.8 mM KH2PO4, 5 mM glutamic acid [pH 7.4]), aliquoted, and frozen at −70°C until use.
Chlamydial titers were determined by an immunofluorescence assay in which McCoy cells were infected with 100 μl of serial dilutions of bacterial stock or lung suspensions in triplicate in 24-well culture plates, incubated for 72 h, fixed with methanol-acetone (1:1), and stained with an anti-major outer membrane protein (anti-MOMP) antibody (immunoglobulin G3 [IgG3] mouse monoclonal antibody [MAb] against C. pneumoniae) (DAKO, Cambridgeshire, England), followed by a fluorescein isothiocyanate-labeled secondary antibody [goat F(ab′)2 anti-mouse IgG] (Sigma Chemical Co., St. Louis, Mo.), to identify chlamydial inclusions. After inclusions were counted under a fluorescence microscope and corrected for dilution factors, bacterial titers were expressed as inclusion-forming units (IFU) per milliliter. The detection limit of this assay is 103 IFU/lung.
Heat-inactivated (HI) bacteria were prepared by heating a viable bacterial suspension with a known concentration for 45 min at 75°C. Infectivity was abrogated, as judged by the undetectable titer.
Before infection, mice were mildly sedated by intraperitoneal injection of 180 to 200 μl of sodium pentobarbital (60 mg/ml) (Butler Controlled Drug, Columbus, Ohio). Mice were then inoculated intranasally either with 2 × 104 to 4 × 104 IFU of C. pneumoniae suspended in 50 μl of PBS, with the equivalent amount of HI bacteria, or with 50 μl of PBS. In initial experiments, mock infection was performed using preparations derived from uninfected McCoy cell cultures, which were treated exactly as for the preparation of bacterial suspensions. Because no cytokine induction was ever observed in these mock-infected animals, 50 μl of PBS was used for mock infection in later experiments. All chlamydial inocula were free of mycoplasmal contamination as determined by PCR and Hoechst staining (Cell Center, The University of Pennsylvania, Philadelphia, Pa.).
Lung histopathology and in situ hybridization.
Mice anesthetized with sodium pentobarbital were sacrificed by axillary bleeding. Lungs were removed in toto, including trachea and bronchi, and immersed in periodate-lysine-paraformaldehyde fixative for 48 h before paraffin embedding. Serial 6-μm-thick sections, with 30 μm between sections, were made randomly, followed by hematoxylin and eosin (H & E) staining for histological analysis or in situ hybridization for detection of bacterial DNA or IL-12 p40 mRNA. A total of 10 sections from each lung were evaluated. Pathological changes were scored based on the percentage of lung parenchyma involved, as follows: 1, <25% of lung parenchyma involved; 2, <50% but >25% involved; 3, >50% involved.
In situ hybridization was performed as described previously (54) with some modifications. Briefly, C. pneumoniae MOMP probes were generated by amplifying the gene in two fragments, MOMP A (631 bases) and MOMP B (546 bases), by PCR. Primer sequences for MOMP A were 5′-TCT-AGG-TAC-CTA-AGC-ATA-ATC-TTT-AGA-GGT-3′ (MOMP/A 5′) and 5′-TCT-AGA-ATT-CAG-CTC-CCA-AAG-TTG-CAC-AAC-3′ (MOMP/A 3′). Primer sequences for MOMP B were 5′-TCT-AGA-ATT-CCA-ATA-TGC-ACA-GTC-CAA-ACC-3′ (MOMP/B 5′) and 5′-TCT-AGG-GCC-CTT-AGA-ATC-TGA-ACT-GAC-CAG-3′ (MOMP/B 3′). A murine IL-12 p40 probe (275 bases between nucleotide positions 35 and 280 of the published murine p40 cDNA sequence [49]) was cloned into the PCR II/TA cloning vector (Invitrogen, San Diego, Calif.) and amplified using transfected Escherichia coli. The MOMP/A and MOMP/B primer mix and the IL-12 p40 probes were labeled with 35S by nick translation (Gibco BRL), purified by passage through quick-spin columns (Boehringer-Ingelheim, Ridgefield, Conn.), and dissolved at a working concentration of 1 ng of labeled probe per 15 μl of hybridization solution (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 45% formamide, 10 mM Tris [pH 7.4], 1 mM EDTA, 1× Denhardt's solution, 10% dextran sulfate, and 1 mg of salmon sperm DNA/ml). After treatment with 1 μg of proteinase K/ml (Sigma), tissue sections were hybridized overnight at 50°C with the appropriate probe. Slides were then washed under stringent conditions (at 45°C in 2× SSC–45% formamide–1 mM Tris [pH 7.0]–1 mM EDTA) to remove unhybridized and nonspecifically bound probe, autoradiographed with Kodak NT/B2 emulsion, developed, fixed, and counterstained with H & E stain.
Cytokines and anticytokine antibodies.
Murine cytokines (IL-12, kindly provided by Stan Wolf [Genetics Institute, Cambridge, Mass.], TNF-α, a gift from Gianni Garotta [Human Genome Sciences, Rockville, Md.], IFN-γ [R & D Systems, Minneapolis, Minn.], IL-10 [Endogen, Woburn, Mass.], and IL-4 [Endogen]) were used as standards in cytokine assays.
Antibodies were produced and characterized in our laboratory unless otherwise noted. Rat anti-mouse cytokine antibodies used in this study were as follows: C17.8 (IgG2a) and C17.15 (IgG1) (both anti-IL-12 p40); C18.2 (IgG2a) (anti-IL-12 p35); AN18 (IgG1) (anti-IFN-γ; from Gianni Garotta); Jes5-2A5 (IgG1) (anti-IL-10) (DNAX, Palo Alto, Calif.); XMG 6 (IgG1) (anti-IFN-γ) and XT22 (IgG1) (anti-TNF-α) (both from Alan Sher, National Institute of Allergy and Infectious Diseases, Bethesda, Md.); 11B11 (IgG1) and BVD6-24G2 (IgG2) (both anti-IL-4) (ATCC); and Jes-16E3 (IgG2b) (anti-IL-10) (Pharmingen, San Diego, Calif.). Polyclonal anti-TNF-α antibodies were obtained from Pharmingen. Normal rat IgG2a was purchased from Sigma.
The anti-IL-12 p40 MAb C17.8 was purified by ammonium sulfate precipitation and used for neutralization of endogenous IL-12 as described previously by our laboratory (61). Briefly, mice were injected intraperitoneally with 1.0 mg of antibody per mouse on the day before infection and with 0.5 mg per mouse on days 5, 10, and 15 after infection. Control mice were injected with normal rat IgG2a according to the same schedule.
Cytokine assays.
Mouse lungs were minced with scissors and mechanically homogenized in 2 ml of RPMI-1640 medium using sterile Pyrex glass tissue grinders (Fisher Scientific, Fair Lawn, N.J.). Homogenized lungs were centrifuged at 10,000 rpm in a microcentrifuge for 5 min, and the supernatants were collected and stored frozen at −70°C until they were assayed for cytokine content.
Cytokine production by spleen cells from infected animals was analyzed in vitro using single-cell suspensions (16). Briefly, spleen fragments were pressed through stainless-steel meshes in RPMI-1640 medium (Sigma) supplemented with HI fetal calf serum (10%) (Gibco BRL), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Whittaker Bioproducts, Walkersville, Md.). Erythrocytes were lysed by treatment with Tris-NH4Cl solution (pH 7.2). Cells were washed, counted, placed in 24-well flat-bottom tissue culture plates (Costar, Cambridge, Mass.) at a concentration of 2 × 106 cells/ml, and stimulated with concanavalin A (ConA) (2 μg/ml; Sigma), viable C. pneumoniae (2 × 104 IFU/ml), or an equivalent amount of HI C. pneumoniae organisms for 48 h at 37°C in a 5% CO2 atmosphere. Murine IL-12 p70, IFN-γ, TNF-α, IL-10, and IL-4 were detected in cell supernatants by a two-site radioimmunoassay with the antibody pairs C18.2–C17.15, AN18–XMG6, XT22–polyclonal anti-TNF-α, Jes 5-2A5–Jes-16E3, and 11B11–BVD6, respectively.
Analysis of bacterial titers in lungs.
Infectivity assays were performed essentially as described by Moazed et al. (41). Homogenized lung suspensions were centrifuged at 500 × g for 5 min at 4°C to remove tissue debris and were frozen at −70°C until tested. Infectious titers expressed as IFU per lung were determined as described for the C. pneumoniae stocks by titration of tissue homogenates in McCoy cells.
Statistical analysis.
Data are represented as means (± standard deviations [SD]) from mice of each experimental group. Differences between groups were compared using an unpaired, two-tailed Student's t test. A P value of <0.05 was considered significant.
RESULTS
Symptoms and lung pathology associated with C. pneumoniae infection.
BALB/c mice treated with an anti-IL-12 antibody or a control isotype-matched antibody, wild-type 129 mice, and G129 mice were inoculated intranasally with 50 μl of C. pneumoniae (2 × 104 IFU) or PBS. Mice were evaluated for clinical symptoms for 2 to 3 weeks. In each group, five to six mice were sacrificed on days 3, 7, and 16 postinfection (p.i.), body and lung weights were measured, and lungs were preserved for histological study.
Mice in all C. pneumoniae-infected groups showed signs of sickness, including minor lethargy, slightly ruffled fur, decreased body weight, and increased lung weight (data not shown). These symptoms were still present on day 16 p.i., when most of the experimental mice were sacrificed. In C. pneumoniae-infected BALB/c mice, the lung weight was significantly increased on days 3 and 7 p.i. under both anti-IL-12 and control antibody treatment conditions, but on day 16 p.i. it was significantly increased only under the anti-IL-12 treatment condition. In both G129 and wild-type 129 mice, significantly increased lung weight was not observed until day 7 p.i. with C. pneumoniae, but unlike in BALB/c mice, a striking increase in lung weight was still present at day 16 p.i. in both the former strains (data not shown). On day 45 p.i., the lung weight was still increased in infected 129 mice, and the lungs of infected G129 mice (0.52 ± 0.05 g) were significantly heavier than those of infected wild-type mice (0.35 ± 0.05 g).
Pulmonary histopathological study revealed interstitial pneumonitis, bronchitis, and bronchiolitis with a patchy, multilobar condition on days 3, 7, and 16 p.i. in both control antibody (Fig. 1B)- and anti-IL-12 (Fig. 1C)-treated infected mice. Interalveolar spaces were widened due to edema, congestion, and predominantly mononuclear inflammatory cell infiltration. Occasionally, multinucleated giant cells were present in alveolar spaces. Focal inflammation and accumulation of polymorphonuclear leukocytes in alveolar, bronchial, and bronchiolar epithelial walls and lumina were observed, as well as perivascular lymphocytic cuffing and peribronchial and peribronchiolar accumulations of lymphocytes. There was evidence of vessel wall necrosis and inflammation consistent with vasculitis. Infected lungs also demonstrated large numbers of macrophages, desquamated alveolar epithelial cells, and hyperplasia of septal-lining epithelial cells. Infected BALB/c mice depleted of endogenous IL-12 displayed the above-mentioned inflammation associated with histopathological changes, but to a lesser extent on day 7 p.i. compared to control antibody-treated infected BALB/c mice. However, by day 16, the lungs of anti-IL-12-treated infected mice showed pathology scores similar to, or even slightly higher than, those for control antibody-treated infected mice (data not shown).
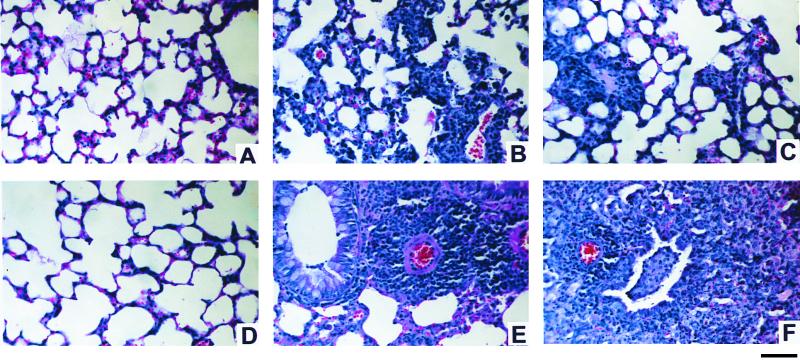
FIG. 1.
Histological comparison of lungs of mice at 7 days after C. pneumoniae infection or mock infection. All infected mice (B, C, E, and F) received 50 μl (2 × 104 IFU) of C. pneumoniae intranasally. Anti-IL-12-treated mice (A and C) were injected with an IL-12-neutralizing MAb 1 day before infection and on day 5 p.i. Seven days after C. pneumoniae inoculation, lungs were harvested and prepared for histological study, as described in Materials and Methods. (A) Section from an anti-IL-12-treated uninfected BALB/c mouse, representing typical histology of uninfected BALB/c mice (treated with anti-IL-12 or control antibody). (B and C) Typical pneumonitis observed during infection in control antibody- and anti-IL-12-treated BALB/c mice, respectively. (D) Section from a G129 mouse, representing typical histology of uninfected wild-type 129 and G129 mice. (E) Typical perivascular cuffing in the lung of an infected 129 mouse. (F) Small vascular cuffing and bronchial infiltration in the lung of an infected G129 mouse. Bar, 100 μm.
The duration of inflammation in the lungs of both wild-type 129 mice and IFN-γ receptor gene-deficient G129 mice that were infected with C. pneumoniae was longer than that in BALB/c mice, with a greater percentage of the lung affected and more-numerous perivascular and peribronchial infiltrations (Fig. 1E and F). Thus, 129 mice appear to be more prone to C. pneumoniae-induced inflammation than BALB/c mice. The histopathological changes were more extensive in G129 mice than in wild-type 129 mice. No significant histopathological changes were observed in any uninfected or PBS mock-infected mice (Fig. 1A and D).
Cytokine production during C. pneumoniae infection.
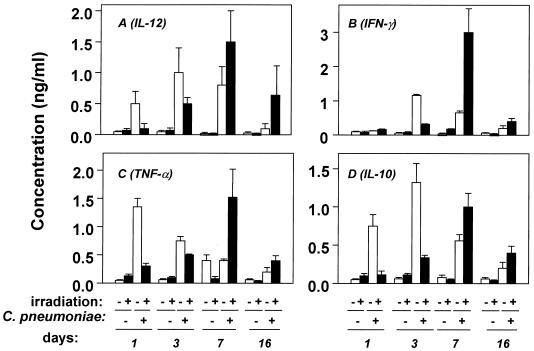
C. pneumoniae infection induced IL-12 production in the lungs of γ-irradiated and nonirradiated BALB/c mice (Fig. 2A). This infection also induced production of IFN-γ (Fig. 2B). In nonirradiated mice, both IL-12 and IFN-γ reached peak levels of production on day 3 p.i. and returned to basal levels on day 16 p.i. In addition, significant levels of TNF-α, with a peak on day 1 p.i. (Fig. 2C), and IL-10, peaking on day 3 p.i. (Fig. 2D), were also detected. IL-4 production never exceeded background levels (∼80 pg/ml) (data not shown). In γ-irradiated mice, cytokine production was delayed and did not reach peak levels until day 7 p.i. (Fig. 2).
FIG. 2.
Pulmonary cytokine levels in nonirradiated and γ-irradiated BALB/c mice during C. pneumoniae infection. Immunocompetent (open bars) and temporarily immunosuppressed (450 rads of γ-irradiation) (filled bars) BALB/c mice were inoculated intranasally with 4 × 104 IFU of C. pneumoniae. On the indicated days p.i., mice were sacrificed and homogenized lung supernatants were collected, as described in Materials and Methods, to determine levels of the indicated cytokines in the lungs. Data are presented as means ± SD for five to six mice in one representative experiment out of three.
Replication of C. pneumoniae appeared to be necessary for the in vivo induction of cytokines, since infection of BALB/c mice with equal amounts of HI C. pneumoniae organisms induced IL-12, IFN-γ, and IL-10 to levels representing only 9.7, 9.2, and 6.2%, respectively, of those induced by viable C. pneumoniae organisms on day 3 p.i., a time when infection-mediated cytokine production was at peak levels. Like the viable bacteria, inactivated C. pneumoniae bacteria induced peak levels of TNF-α (mean, 455 ng/ml) on day 1 p.i., but this level represented only 32% of that induced by viable bacteria (data not shown).
Effects of endogenous IL-12 depletion on bacterial titers in the lungs and in situ bacterial DNA expression.
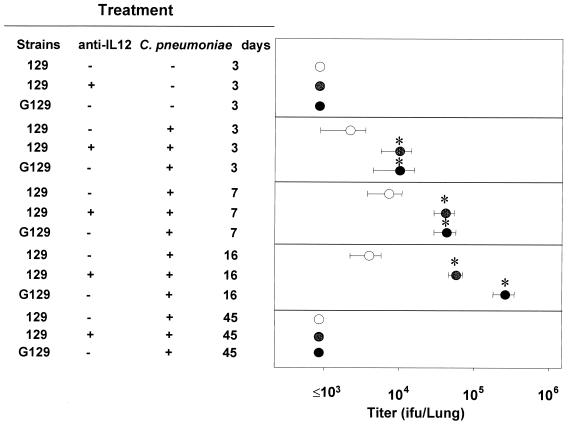
Based on our observations that C. pneumoniae infection mediates the production of IL-12 and in light of the known essential role of IL-12 in activating a protective immune response against many intracellular pathogens (55, 56), we evaluated the role of endogenous IL-12 in the host defense against chlamydial infection in depletion experiments. BALB/c mice treated with an IL-12-neutralizing antibody or isotype-matched control antibody were infected with C. pneumoniae, and the titers of bacteria in the lung homogenates were determined. In control antibody-treated infected mice, bacterial titers were low (2 × 103 to 4 × 103/lung) at all times, whereas in anti-IL-12-treated infected mice, titers were significantly elevated (3.1 × 104 ± 0.8 × 104 and 6.2 × 104 ± 1.0 × 104) on days 3 and 7 p.i., respectively (Fig. 3), suggesting an important role for endogenous IL-12 in early host resistance to this infection. At 2 to 3 weeks p.i., titers in anti-IL-12-treated mice returned to undetectable levels (<103 IFU). A similar role for endogenous IL-12 in early antichlamydial resistance was observed in 129 mice (Fig. 4). Titers of C. pneumoniae were also increased in G129 mice compared to those in wild-type 129 mice (Fig. 4), indicating the role of IFN-γ in the early control of this infection.
FIG. 3.
Growth of C. pneumoniae in lungs of BALB/c mice treated with an isotype control MAb (open circles) or anti-IL-12 MAb (filled circles). Mice were injected with antibody 1 day before infection and every 5 days during the course of infection. Results (means ± SD for four mice) are from one experiment representative of three independent experiments. Asterisks indicate significant differences (P < 0.05).
FIG. 4.
Titers of C. pneumoniae recovered from lungs of control antibody-treated or anti-IL-12-treated 129 mice and from lungs of G129 mice. After infection at 4 × 104 IFU/mouse, mice were sacrificed on various days and homogenized lung supernatants were collected to determine pulmonary bacterial titers, as described in Materials and Methods. Results (means ± SD for five mice) are from one experiment representative of three independent experiments. Asterisks indicate significant differences (P < 0.05) between control (isotype-matched control MAb-treated 129 mice) and experimental (G129 mice and anti-IL-12-treated 129 mice) groups.
The effects of IL-12 and IFN-γ during C. pneumoniae infection were further demonstrated by in situ hybridization analysis. C. pneumoniae-specific DNA was detected in the lungs of anti-IL-12-treated infected BALB/c and G129 mice at 7 days p.i. These signals appeared to localize in the cytoplasm of bronchial epithelial cells, alveolar cells, and some mononuclear inflammatory cells in the lung tissues of infected animals, primarily in areas demonstrating inflammatory changes (data not shown). Lower levels of signal were detected in control antibody-treated infected mice during the course of infection. Lung sections from uninfected animals were all negative for hybridization signals.
Effects of IL-12-neutralizing antibody on cytokine production mediated by C. pneumoniae infection.
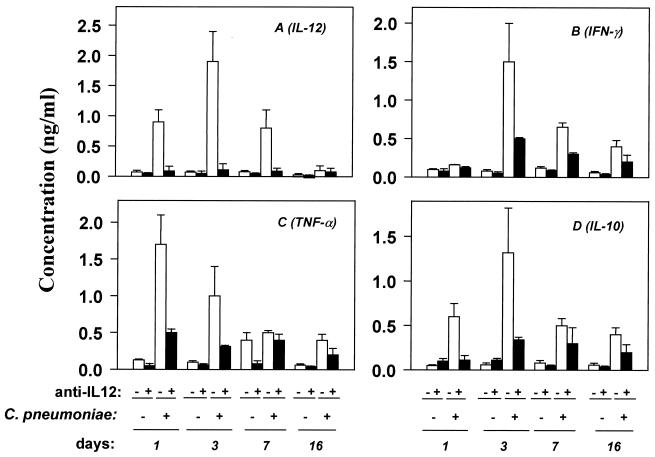
To investigate the regulatory effect of IL-12 on other cytokines in vivo, we measured the pulmonary cytokine levels of both control antibody- and anti-IL-12-treated BALB/c mice following C. pneumoniae infection. IL-12 levels were essentially undetectable in anti-IL-12-treated mice (Fig. 5A), indicating that the MAb efficiently neutralized chlamydial infection-mediated IL-12 production. Anti-IL-12 significantly reduced the levels of infection-mediated production of IFN-γ (Fig. 5B), TNF-α (Fig. 5C), and IL-10 (Fig. 5D). IL-4 was undetectable in both control antibody- and anti-IL-12-treated mice (data not shown).
FIG. 5.
Effects of anti-IL-12 treatment on C. pneumoniae infection-mediated cytokine production. BALB/c mice were treated with anti-IL-12 at 1 mg/mouse 1 day before bacterial inoculation and subsequently every 5 days at 0.5 mg/mouse during the course of the experiment. Mice were sacrificed on various days p.i. Pulmonary cytokine levels were measured as described in Materials and Methods. Open and filled bars represent control MAb- and anti-IL-12-treated animals, respectively. Data (means ± SD) are from one experiment representative of three independent experiments each with determinations from three to five animals.
In vitro analysis of splenocytes from 15-day-infected mice (treated with control antibody or anti-IL-12) revealed that, following stimulation with the mitogen ConA or with live or HI chlamydiae, IFN-γ levels in culture supernatants from anti-IL-12-treated mice were significantly lower than those from control antibody-treated infected mice (mean decreases, 77.7, 54.2, and 70.4%, respectively) (data not shown). These data, together with the in vivo observation, indicate the importance of IL-12 in priming for IFN-γ production and Th1 responses during C. pneumoniae infection.
Effects of IFN-γ signaling.
To determine whether the protective effect of endogenous IL-12 during early infection is mediated through its IFN-γ-inducing function and to determine the relative importance of IFN-γ in host defense against chlamydial infection, cytokine levels and bacterial titers in the lungs of G129 mice and 129 mice inoculated intranasally with 50 μl (4 × 104 IFU) of C. pneumoniae were assessed. Infected mice were sacrificed on days 3, 7, and 16 p.i. (four to six mice in each experimental group).
Because G129 mice do not express the IFN-γ receptor on the cell surface, all IFN-γ signaling-related functions are impaired. However, these mice produced levels of IL-12, IL-10, and TNF-α similar to those produced in wild-type 129 mice, whereas IFN-γ levels in G129 mice were higher than those in 129 mice (data not shown). In situ analysis showed that IL-12 p40 mRNA was expressed at the same intensity and with the same kinetics in the lungs of infected G129 mice as in 129 mouse lungs, i.e., it was detectable at day 3 p.i., peaked at day 7 p.i. and returned to background level at day 16 p.i. (data not shown).
A titration assay revealed lung bacterial loads in G129 mice that were 0.5 and 1.5 log units higher on days 7 and 16 p.i., respectively, than those in 129 mice. C. pneumoniae replication in the lungs of G129 mice was confirmed by in situ hybridization analysis. High levels of C. pneumoniae DNA signals were detected in the lungs of bacterium-infected G129 mice, whereas the lungs of infected 129 mice showed much lower levels of signal (data not shown). These results clearly indicate the importance of IFN-γ in early host resistance against chlamydial infection, although the bacterial infection was eventually decreased to unmeasurable levels in G129 mice also, at day 45 p.i.
Bacterial clearance in immunocompetent and immunosuppressed mice.
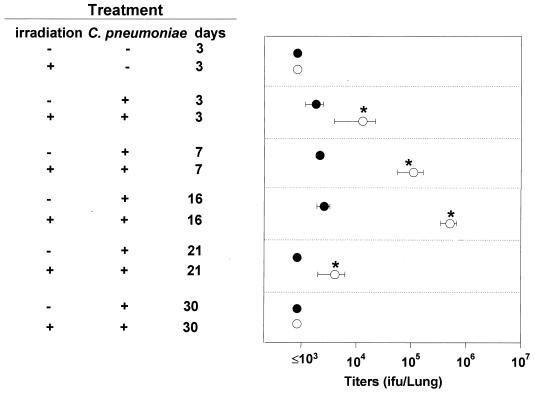
To investigate the relationship between the delayed cytokine response to infection in mice irradiated before the infection and the ability of these mice to clear the infection (Fig. 2), we compared bacterial titers in nonirradiated and γ-irradiated BALB/c mice. C. pneumoniae titers were much higher in the lungs of irradiated mice than in those of nonirradiated mice through day 16 p.i. (Fig. 6). Delayed induction of IL-12 and IFN-γ resulting from temporary immune suppression induced by γ-irradiation may have contributed to the impaired protection against chlamydial replication in the lung. At 30 days p.i., C. pneumoniae titers were at undetectable levels (<103 IFU) in both groups.
FIG. 6.
Titers of C. pneumoniae recovered from lungs of immunocompetent (filled circles) and temporarily immunosuppressed (γ-irradiated) (open circles) BALB/c mice. Infectious titers expressed as IFU per lung were assayed by titration of lung homogenates in McCoy cells as described in Materials and Methods. Data (means ± SD for four to five mice) are from one experiment representative of three independent experiments. Asterisks indicate significant differences (P < 0.05) between titers of nonirradiated and irradiated mice.
DISCUSSION
IL-12 is essential in the host defense against many intracellular pathogens, including Mycobacterium tuberculosis, Brucella abortus, and species of Listeria, Toxoplasma, Candida, and Leishmania (55, 56). Our present data indicate that endogenous IL-12 also plays a crucial role in controlling acute C. pneumoniae infection, as indicated by the increase in replication and DNA signals in the lungs of mice treated to ablate IL-12. One of the mechanisms of protection provided by IL-12 could be its IFN-γ-inducing function, since depletion of endogenous IL-12 drastically reduced the level of infection-mediated IFN-γ production. Furthermore, G129 mice lacking the IFN-γ receptor gene had prolonged pulmonary infection and were unable to control the acute infection effectively. On the other hand, both IL-12-depleted and IFN-γ receptor gene-deficient mice did clear the pulmonary infection eventually, suggesting a role for other effector mechanisms in host defense. Similar effects of IL-12 and IFN-γ have been reported during genital-tract infection with C. trachomatis, where IL-12-depleted mice displayed minimal bacterial clearance for 1 month p.i. but eventually resolved the infection completely (45). Cotter et al. (7) found that IFN-γ knockout mice exhibit more-disseminated and longer-lasting genital-tract infections with C. trachomatis than wild-type mice. In another study (25), IFN-γ receptor-deficient mice exhibited severe ascending primary genital-tract infections of prolonged duration despite strong local antibody responses. In our study of C. pneumoniae infection in mice, we observed a series of inflammation-associated phenomena, including infiltration of lymphoid cells in the affected areas of the lungs of BALB/c and 129 mice. Such inflammatory responses were less severe in anti-IL-12-treated BALB/c mice 7 days p.i., with only small inflammatory foci, whereas bacterial replication was more extensive. This observation suggests the importance of IL-12 in recruiting lymphoid cells to the affected areas of lungs. The overall infiltration and inflammatory response in G129 mice was stronger than that in 129 mice, yet G129 mice showed much higher bacterial titers and DNA signals. Thus, IFN-γ might not be required for the recruitment of lymphoid cells during C. pneumoniae infection, and inflammatory-cell infiltration alone might not be sufficient for controlling the infection.
Although anti-IL-12 treatment of C. pneumoniae-infected mice inhibited infection-mediated IFN-γ production by more than 75% on day 3 p.i., the residual IFN-γ levels were still significantly higher than those in the lungs of mock-infected animals (∼400 to 500 ng/ml versus <100 pg/ml). This observation raises the possibility that an IL-12-independent mechanism of IFN-γ production is operative during chlamydial infection. The residual IFN-γ in the lungs of anti-IL-12-treated infected mice might be sufficient to induce macrophage effector functions, such as activation of inducible nitric oxide synthase, and induction of nitric oxide and other reactive nitrogen intermediates (10, 42), which might contribute to the elimination of the bacteria. The existence of an IL-12-independent IFN-γ-producing pathway is further suggested by the in vitro studies, in which splenocytes from anti-IL-12-treated infected mice still produced significant levels of IFN-γ following stimulation either with a nonspecific mitogen (ConA) or with a specific antigen (viable or heat-killed chlamydiae). IL-12-independent IFN-γ expression has been noted previously. For example, Magram et al. (36) found that IFN-γ production was only partially reduced in mice genetically deficient in IL-12 during endotoxemia, and Cooper et al. (6) observed delayed but normal levels of IFN-γ mRNA expression in the livers of IL-12 gene-disrupted mice infected with M. tuberculosis, which was associated with full recovery of macrophage activation. One possible candidate underlying IL-12-independent IFN-γ production is IL-18 (also known as IFN-γ-inducing factor), a cytokine that shares many functions with IL-12 and synergizes with it (29). IL-18 production is markedly induced in the livers of both wild-type and IL-12-deficient mice in postsystemic mycobacterial infection (29). Whether IL-18 is induced during C. pneumoniae infection remains to be investigated.
As in other disease systems (9), IFN-γ production induced by C. pneumoniae infection is mediated largely in an IL-12-dependent fashion, since neutralization of endogenous IL-12 decreased C. pneumoniae-induced IFN-γ production. The high titers of bacteria recovered from the lungs of G129 mice further confirmed the importance of IL-12-induced IFN-γ in host defense. Although G129 mice do not express the IFN-γ receptor and are defective in IFN-γ–IFN-γ receptor signaling (24), the ability of those mice to produce IL-12 (and TNF-α) following C. pneumoniae infection was not impaired, indicating that IFN-γ is not essential for IL-12 induction in vivo in these mice. The elevated IFN-γ levels in IFN-γ receptor gene-deficient mice, a phenomenon also observed by Dai et al. (8), could be due to the inability of those mice to utilize the cytokine.
IL-12 is critical in the development of CD4+ T-helper type-1 (Th1) responses (23, 37). Th1 and Th2 CD4+ T-cell subsets mutually down-regulate the activity of each subset. Thus, in vivo neutralization of IL-12 might be expected to prevent the development of a Th1 response, leading to the emergence of a Th2 response and the production of IL-4 and IL-10. Administration of anti-IL-12 to chlamydia-infected mice not only significantly reduced in vivo production of IFN-γ and TNF-α but also reduced levels of IL-10, suggesting that IL-12 may be important in vivo for infection-induced production of these cytokines. However, IL-4 was not detected after C. pneumoniae infection in either anti-IL-12-treated or control antibody-treated BALB/c mice, or in G129 and 129 mice. This observation contrasts with the cytokine profile in listeriosis, in which IL-4 is produced in the absence of strong Th1 responses (52).
It has been suggested (1, 14) that immune-suppressed patients are at a higher risk of C. pneumoniae infection. Therefore, we included sublethally γ-irradiated immunosuppressed mice in our study. We observed that sublethal γ-irradiation delayed, but did not eliminate, the ability of BALB/c mice to produce cytokines, and C. pneumoniae titers in γ-irradiated mice returned to undetectable levels 4 weeks p.i. Nevertheless, the γ-irradiated mice did exhibit progressively elevated bacterial titers during the first 2 weeks of C. pneumoniae infection, suggesting that temporary immune suppression provided a window of opportunity for C. pneumoniae to proliferate in the lung. The higher peak levels of IL-12 and IFN-γ in the lungs of γ-irradiated mice may be due to the larger amount of bacteria, as a result of a period of uninhibited proliferation, encountered by the regenerated immune-competent cells.
Since its discovery in the late 1980s, C. pneumoniae has attracted attention as a common pulmonary pathogen and a possible cause of coronary artery disease (30, 59). Our study defines the role of the immune mechanism, specifically, the central role of IL-12 and IFN-γ–IFN-γ receptor signaling in the pathogenesis and host defense against early acute C. pneumoniae infection. However, it remains important to determine whether such mechanisms contribute to the development of coronary artery disease associated with C. pneumoniae infection. Such information might be useful in developing effective treatments and designing prophylactic measures to control and prevent chlamydial infection.
ACKNOWLEDGMENTS
We thank Kati Hugdus for technical assistance, Marion Sacks for editorial assistance, and Elsa Aglow at the Wistar Histology Core Facility for tissue sectioning and H & E staining. M. Wysocka organized the breeding of G129 mice and provided helpful discussions.
This work is supported in part by a grant from the W. W. Smith Charitable Trust and by Public Health Service grants CA10815, CA32898, and AI34412. Yuemei Geng was supported by NIH training grant T32CA 09140.
REFERENCES
- 1.Augenbraun M H, Roblin P M, Chirgwin K, Landman D, Hammerschlag M R. Isolation of Chlamydia pneumoniae from the lungs of patients infected with the human immunodeficiency virus. J Clin Microbiol. 1991;29:401–402. doi: 10.1128/jcm.29.2.401-402.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmaier K, Neu N, de la Maza L, Pal S, Hessel A, Penninger J M. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 3.Berencsi K, Endresz V, Klurfeld D, Kari L, Kritchevsky D, Gonczol E. Early atherosclerotic plaques in the aorta following cytomegalovirus infection of mice. Cell Adhes Commun. 1998;5:39–47. doi: 10.3109/15419069809005597. [DOI] [PubMed] [Google Scholar]
- 4.Campbell L A, O'Brien E R, Cappuccio A L, Kuo C C, Wang S P, Stewart D, Patton D L, Cummings P K, Grayston J T. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues [see comments] J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 5.Carlin J M, Weller J B. Potentiation of interferon-mediated inhibition of Chlamydia infection by interleukin-1 in human macrophage cultures. Infect Immun. 1995;63:1870–1875. doi: 10.1128/iai.63.5.1870-1875.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai W J, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 9.D'Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 11.Fong I W, Chiu B, Viira E, Fong M W, Jang D, Mahony J. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997;35:48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsey T, Darougar S, Treharne J D. Prevalence in human beings of antibodies to Chlamydia IOL-207, an atypical strain of chlamydia. J Infect. 1986;12:145–152. doi: 10.1016/s0163-4453(86)93608-x. [DOI] [PubMed] [Google Scholar]
- 13.Gaydos C A, Eiden J J, Oldach D, Mundy L M, Auwaerter P, Warner M L, Vance E, Burton A A, Quinn T C. Diagnosis of Chlamydia pneumoniae infection in patients with community-acquired pneumonia by polymerase chain reaction enzyme immunoassay. Clin Infect Dis. 1994;19:157–160. doi: 10.1093/clinids/19.1.157. [DOI] [PubMed] [Google Scholar]
- 14.Gaydos C A, Fowler C L, Gill V J, Eiden J J, Quinn T C. Detection of Chlamydia pneumoniae by polymerase chain reaction-enzyme immunoassay in an immunocompromised population. Clin Infect Dis. 1993;17:718–723. doi: 10.1093/clinids/17.4.718. [DOI] [PubMed] [Google Scholar]
- 15.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng Y, Savage S M, Razanai-Boroujerdi S, Sopori M L. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- 17.Grayston J T. Infections caused by Chlamydia pneumoniae strain TWAR [see comments] Clin Infect Dis. 1992;15:757–761. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- 18.Grayston J T, Aldous M B, Easton A, Wang S P, Kuo C C, Campbell L A, Altman J. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J Infect Dis. 1993;168:1231–1235. doi: 10.1093/infdis/168.5.1231. [DOI] [PubMed] [Google Scholar]
- 19.Grayston J T, Campbell L A, Kuo C C, Mordhorst C H, Saikku P, Thom D H, Wang S P. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 20.Grayston J T, Diwan V K, Cooney M, Wang S P. Community- and hospital-acquired pneumonia associated with Chlamydia TWAR infection demonstrated serologically. Arch Intern Med. 1989;149:169–173. [PubMed] [Google Scholar]
- 21.Hahn D L, Dodge R W, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma [see comments] JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 22.Heinemann M, Susa M, Simnacher U, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 25.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanamoto Y, Ouchi K, Mizui M, Ushio M, Usui T. Prevalence of antibody to Chlamydia pneumoniae TWAR in Japan. J Clin Microbiol. 1991;29:816–818. doi: 10.1128/jcm.29.4.816-818.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaukoranta-Tolvanen S S, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–846. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 30.Kuo C C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo C C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 32.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurila A L, Von Hertzen L, Saikku P. Chlamydia pneumoniae and chronic lung diseases. Scand J Infect Dis Suppl. 1997;104:34–36. [PubMed] [Google Scholar]
- 34.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 35.Magee D M, Smith J G, Bleicker C A, Carter C J, Bonewald L F, Schachter J, Williams D M. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect Immun. 1992;60:1217–1220. doi: 10.1128/iai.60.3.1217-1220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 37.Manetti R, Parronchi P, Giudizi M G, Piccinni M P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marin A, Karolyi A, Szalka A. Prevalence of Chlamydia pneumoniae antibodies in Hungary. Eur J Clin Microbiol Infect Dis. 1992;11:139–142. doi: 10.1007/BF01967065. [DOI] [PubMed] [Google Scholar]
- 39.Marrie T J, Grayston J T, Wang S P, Kuo C C. Pneumonia associated with the TWAR strain of Chlamydia. Ann Intern Med. 1987;106:507–511. doi: 10.7326/0003-4819-106-4-507. [DOI] [PubMed] [Google Scholar]
- 40.Miller S T, Hammerschlag M R, Chirgwin K, Rao S P, Roblin P, Gelling M, Stilerman T, Schachter J, Cassell G. Role of Chlamydia pneumoniae in acute chest syndrome of sickle cell disease. J Pediatr. 1991;118:30–33. doi: 10.1016/s0022-3476(05)81839-6. [DOI] [PubMed] [Google Scholar]
- 41.Moazed T C, Kuo C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 42.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 43.Montes M, Cilla G, Alcorta M, Perez-Trallero E. High prevalence of Chlamydia pneumoniae infection in children and young adults in Spain [see comments] Pediatr Infect Dis J. 1992;11:972–973. [PubMed] [Google Scholar]
- 44.Penttila J M, Anttila M, Puolakkainen M, Laurila A, Varkila K, Sarvas M, Makela P H, Rautonen N. Local immune responses to Chlamydia pneumoniae in the lungs of BALB/c mice during primary infection and reinfection. Infect Immun. 1998;66:5113–5118. doi: 10.1128/iai.66.11.5113-5118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 46.Rothermel C D, Schachter J, Lavrich P, Lipsitz E C, Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun. 1989;57:2705–2711. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saikku P, Leinonen M, Mattila K, Ekman M R, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 48.Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman M R, Manninen V, Manttari M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study [see comments] Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 49.Schoenhaut D S, Chua A O, Wolitzky A G, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 50.Shemer-Avni Y, Wallach D, Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988;56:2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shor A, Kuo C C, Patton D L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 52.Szalay G, Ladel C H, Blum C, Kaufmann S H. IL-4 neutralization or TNF-alpha treatment ameliorate disease by an intracellular pathogen in IFN-gamma receptor-deficient mice. J Immunol. 1996;157:4746–4750. [PubMed] [Google Scholar]
- 53.Thom D H, Wang S P, Grayston J T, Siscovick D S, Stewart D K, Kronmal R A, Weiss N S. Chlamydia pneumoniae strain TWAR antibody and angiographically demonstrated coronary artery disease. Arterioscler Thromb. 1991;11:547–551. doi: 10.1161/01.atv.11.3.547. [DOI] [PubMed] [Google Scholar]
- 54.Tournier I, Bernuau D, Poliard A, Schoevaert D, Feldmann G. Detection of albumin mRNAs in rat liver by in situ hybridization: usefulness of paraffin embedding and comparison of various fixation procedures. J Histochem Cytochem. 1987;35:453–459. doi: 10.1177/35.4.3546490. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 56.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 58.Wang S-P, Grayston J T. Population prevalence of antibody to Chlamydia pneumoniae, strain TWAR. In: Bowie W R, Caldwell H D, Jones R P, Mardh P-A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 402–405. [Google Scholar]
- 59.Ward M E. The immunobiology and immunopathology of chlamydial infections. Acta Pathol Microbiol Immunol Scand. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 60.Williams D M, Magee D M, Bonewald L F, Smith J G, Bleicker C A, Byrne G I, Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990;58:1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]