Abstract
The field of targeted protein degradation (TPD) is a rapidly developing therapeutic modality with the promise to tame disease-relevant proteins in ways that are difficult or impossible to tackle with other strategies. While we move into the third decade of TPD, multiple degrader drugs have entered the stage of the clinic and many more are expected to follow. In this review, we provide an update on the most recent advances in the field of targeted degradation with insights into possible clinical implications for cancer prevention and treatment.
Keywords: cancer, targeted protein degradation, degrader molecules, clinical trials
1. Introduction
Cancer is a major public health problem. Each year nearly 20 million new cases are diagnosed and almost 10 million deaths from cancer are registered [1,2]. The standard of care for patients includes not only surgery and radiotherapy but also chemotherapy; poor selectivity and acquired resistance limit its clinical application. Tumors harbor different underlying genetic causes and express different proteins in each patient. This heterogeneity of cancer paves the ground for precision and personalized medicine. In the past two decades, the focus of novel drug development has thus shifted towards the identification and targeting of molecular differences/drivers between tumors. A major class of targeted therapies employs monoclonal antibodies and small molecules that act by blocking the activity of pathogenic proteins that drive tumorigenesis [3]. To date, the therapeutic applicability of monoclonal antibodies is restricted to cell surface proteins [4]. Both multi-targeted and highly selective small-molecule compounds are applied in advanced treatment-resistant tumors, many being approved for early clinical settings as adjuvant therapies or as first-line for metastatic or recurrent disease [5]. Despite their success, the response is not durable due to development of resistance (i) by increasing the abundance of the target protein; (ii) by rewiring survival pathways in the complex cell-signaling milieu; or (iii) by acquisition of new mutations within the target protein that allow the malignant cell to escape the inhibitory effects of the compound. A broad range of targets such as transcription factors, scaffolding and regulatory proteins have remained undruggable due to the lack of suitable binding pockets that directly modulate protein function. Another limitation is presented by high drug concentrations to ensure active-site occupancy and to sustain clinical benefit in vivo, which in turn increases the risk of off-target effects [6]. One way to reduce intracellular protein concentrations is through gene-editing technologies using therapeutic nucleic acids including antisense oligonucleotides (ASO), small interfering RNA (siRNA) and CRISPR-Cas9 [6]. To date ten ASO therapeutics [7] and four siRNA-based therapies [8] have been approved by the Food and Drug Administration (FDA) for their use in different disease conditions, but none has been approved to treat cancers. Most of the current trials using CRISPR-based treatments are still in early stages. Although the potential uses of these technologies seem unlimited, their safe use in vivo has been limited by dependency on protein half-life, challenges in delivery, nonspecific activation of the immune system and engagement of off-targets (with an increase of adverse events) [6,9,10,11,12].
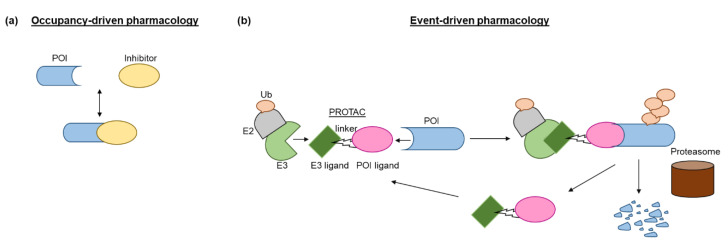
Inspired by the fact that cells employ the ubiquitin-proteasome system (UPS) to maintain intracellular proteostasis, interest has focused on an alternative approach that aims to control protein function by regulating expression levels of a target protein rather than its activity. Such efforts have evolved from the discovery that proteasome inhibitors that block protein degradation have tumoricidal activity. Two examples approved for the treatment of multiple myeloma are carfilzomib and bortezomib [13]. Newer strategies involve the pharmacological hijacking of the cellular quality-control system to posttranslationally eliminate oncoproteins. Such a modality was first developed rather accidentially. Thalidomide, a drug historically more infamous than famous, was prescribed as a sedative to treat pregnancy-related morning sickness, which led to birth of thousands of children with severe defects in late 1950s/early 1960s [14,15]. After more than 60 years, the underlying molecular basis has been deciphered: thalidomide acts by promoting destruction of proteins needed for normal fetal development [15]. In the 1980s it gained a second life as an antiangiogenic drug, mitigating the growth of blood vessels in tumors. As such, it has been repurposed for treating multiple myeloma, and derivatives such as lenalidomide and pomalidomide have been developed to treat hematological malignancies. Thalidomide and its analogs are collectively referred to immunomodulatory drugs (IMiDs) and act as molecular glues that mediate ubiquitination of a protein of interest (POI) by promoting a protein-protein interaction between POI and an E3 ligase, cereblon (CRBN) being its direct binding target [16]. Thus, they lack affinity for the POI in the absence of the E3 ligase. Another modality that hijacks the UPS consists of PROTACs (PROteolysis TArgeting Chimeras). PROTACs have evolved from cell-impermeable peptide-small molecule chimeras to orally bioavailable compounds that tag unwanted proteins for destruction via ubiquitination in patients. They are heterobifunctional small molecules with two covalently linked ligands: one binds a POI, while the other one simultaneously recruits an E3 ubiquitin ligase. This transient binding event results in polyubiquitination of the POI and its destruction by the 26S proteasome. The PROTAC is then recycled to attack another copy of the POI, resulting in substoichiometric activity. This event-driven action is catalytic and eliminates the need to maintain high drug levels, both characteristics that distinguish PROTACs from classical occupancy-driven pharmacology of small-molecule inhibitors (Figure 1, Table 1). With the discovery of PROTACs, targeted protein degradation (TPD) expands the reach of drug development by enabling the degradation of targets that have been stigmatized as undruggable by traditional inhibitor-based tools. This progress has fueled the identification of additional TPD approaches that hijack endolysosomal and macroautophagic degradation pathways rather than the UPS system. While the proteasome-based technologies are limited to proteins with cytoplasmic domains, LYTACs (Lysosome TArgeting Chimeras) and AUTACs (Autophagy Targeting Chimeras)/ATTECs (Autophagy Tethering Compounds) promote the targeted destruction of extracellular proteins or entire organelles and protein aggregates, respectively. The non-UPS platforms are rather conceptual frameworks; much more biophysical/structural study is warranted. In the last years, other strategies for proximity-based therapeutic modalities beyond degradation have also been described including targeted phosphorylation using phosphorylation-inducing chimeric small molecules, targeted dephosphorylation and targeted deubiquitination followed by targeted protein stabilization [17,18,19].
Figure 1.
Different strategies of protein suppression. (a) A small-molecule inhibitor binds to the active site of a protein of interest (POI) to inhibit its enzymatic functions. Most enzyme inhibitors bind noncovalently and reversibly. (b) PROTAC directs E3 ligase to a POI and mediates the polyubiquitination of the POI by an E2 conjugating enzyme. The modified POI is recognized and degraded by the 26S proteasome. The PROTAC can then be recycled to induce the next round of the POI degradation. PROTAC: PROteolysis TArgeting Chimera; POI: protein of interest; Ub: ubiquitin.
Table 1.
Characteristics of protein suppressive strategies. PO: per oral; IV: intravenous injection; SC: subcutaneous injection.
| Main Approaches To Suppress Target Of Interest | ||||
|---|---|---|---|---|
| PROTAC | Monoclonal Antibody | Small Molecule Inhibitor | Gene-Editing Via Nucleic Acids |
|
| Selectivity | +++ | ++ | + | ++ |
| Route Of Administration | PO/IV/SC | IV/SC | PO/IV/SC | IV/SC |
| Target Of Interest | Protein on cell surface and inside a cell | Protein on cell surface | Protein on cell surface and inside a cell | DNA or RNA |
| Metabolic Stability | ++ | -/+ | ++ | -/+ |
| Tissue/Cell Penetration | + | -/+ | ++ | -/+ |
| Concentrations Required | Substoichiometric | N/A | Stoichiometric | N/A |
| Active Binding Site | - (Can target undruggable and mutated proteins) |
+++ (Can target undruggable proteins) |
+++ (Cannot target mutated proteins) |
- (Can target undruggable proteins) |
| Inhibitory Outcome | Blockade of both enzymatic and scaffolding functions | N/A | Impaired enzymatic function | N/A |
| Elimination Of POI | +++ | - | - | +++ |
| Catalytic Mechanism Of Action | +++ | - | - | +++ |
| Systemic Delivery | +++ | +++ | +++ | - |
In this review we will reflect on different targeted protein degradation approaches and focus on the current status of clinical translation of TPD in cancer treatment.
2. Targeted Protein Degradation Approaches
Targeted protein degradation (TPD) has received a lot of attention as a novel and innovative chemical tool and therapeutic modality. By harnessing protein degradation pathways, TPD mediates depletion of a protein of interest from within or outside the cell (Figure 2, Table 2). Two main types of chemical degraders consist of (i) bifunctional molecules that carry a ligand binding to POI connected by a linker to another ligand binding to a component of the degradation machinery (e.g., the ubiquitin system, autophagy or the lysosomal system) and (ii) molecular glues that induce protein association of proteins that do not interact in the absence of these ligands [20]. TPD acts on a kinetic resolution, enabling near-complete target removal within minutes up to few hours [20]. Such progress expands the druggable space beyond small-molecule inhibitors.
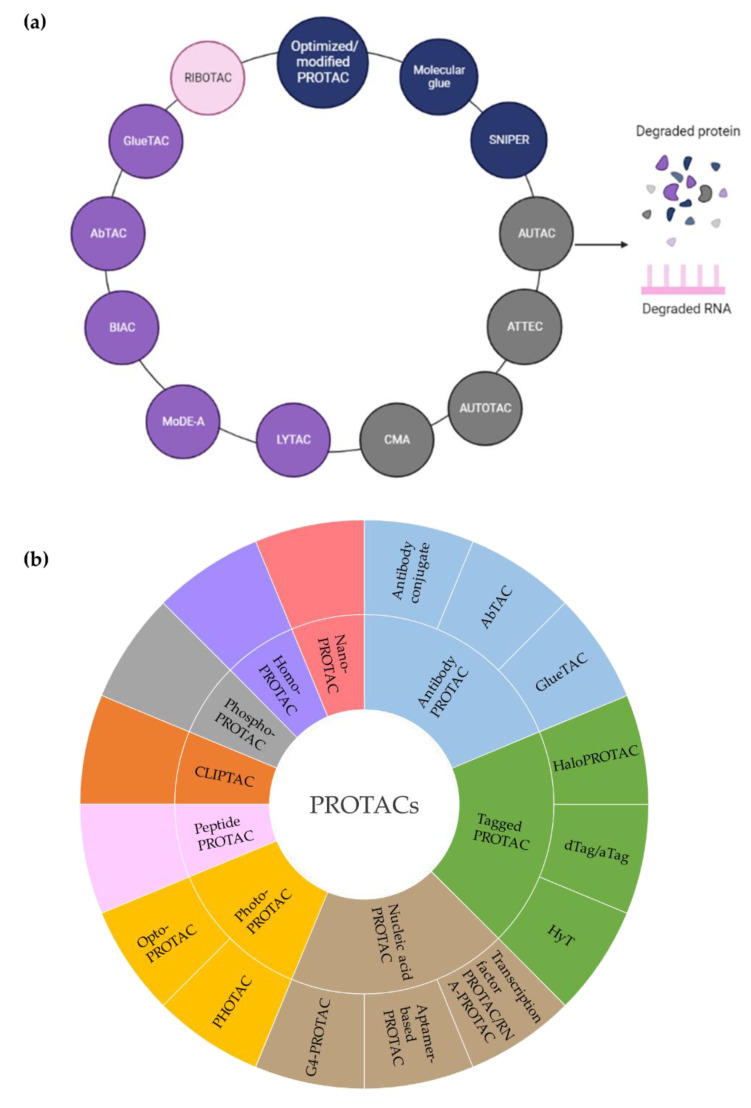
Figure 2.
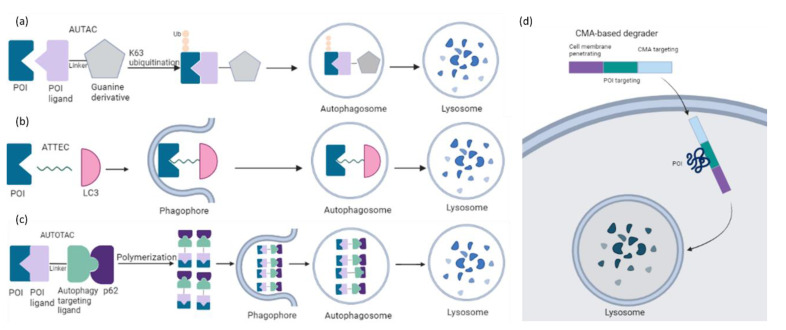
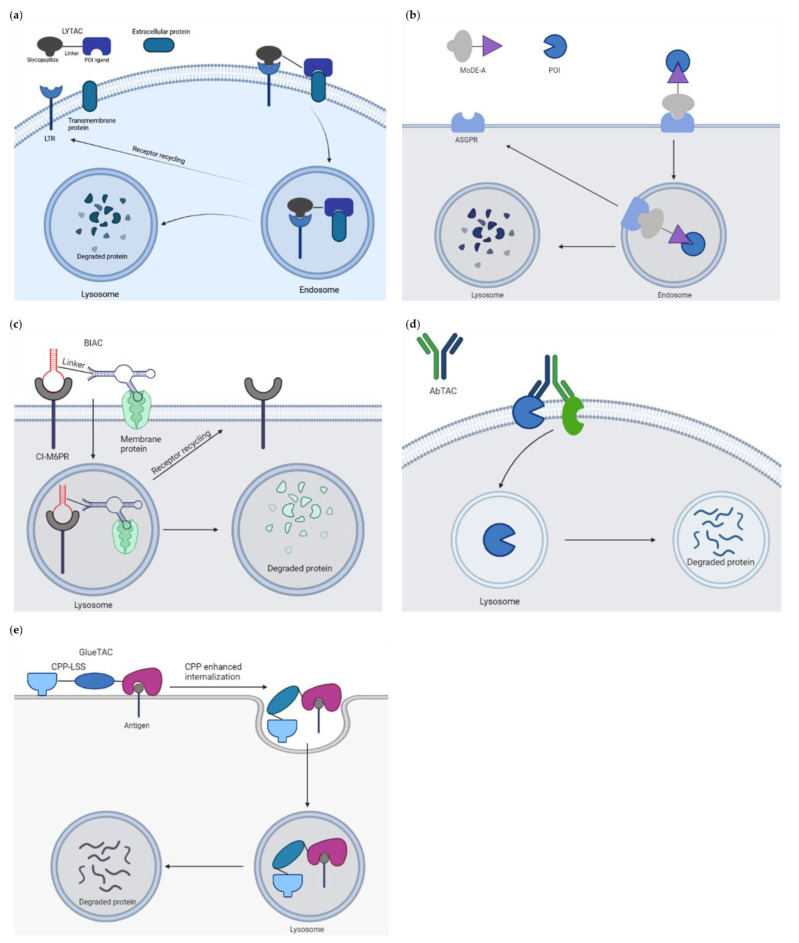
Overview of therapeutic modalities based on targeted degradation. (a) Targeted protein degradation approaches hijack either the ubiquitin-proteasome system or the autophagy or lysosomal pathways. RIBOTAC uses RNA-targeting small molecules and RNase L to eliminate intracellular oncogenic RNAs. Blue: ubiquitin-proteasome system; gray: autophagy pathway; violet: lysosomal pathway; pink: targeted RNA degradation. (b) Different PROTAC-based strategies are summarized. AbTac; antibody-based PROTAC; aTAG: AchillesTag; ATTEC: autophagy tethering compound; AUTAC: Autophagy Targeting Chimera; AUTOTAC: AUTOphagy TArgeting Chimera; BIAC: bispecific aptamer chimera; CLIPTAC: in-cell-click-formed proteolysis targeting chimera; CMA: chaperone-mediated autophagy; dTAG: degrader tag; GlueTac; GlueBody Chimera; G4:G-quadruplex; HyT: hydrophobic tagging; LYTAC: LYsosome TArgeting Chimera; MoDE-A; molecular degraders of extracellular proteins through the ASGPR; PROTAC: PROteolysis TArgeting Chimera; RIBOTAC: RIBOnuclease TArgeting Chimera; PHOTAC: PHOtochemically TArgeting Chimera; RNA: ribonucleic acid; SNIPER: Specific and non-genetic inhibitors of apoptosis protein (IAP)-dependent protein eraser.
Table 2.
Comparison of different degrader systems. AbTac: antibody-based PROTAC; ATTEC: autophagy tethering compound; AUTAC: Autophagy Targeting Chimera; AUTOTAC: AUTOphagy TArgeting Chimera; BIAC: bispecific aptamer chimera; CMA: chaperone-mediated autophagy; GlueTac; GlueBody Chimera; LYTAC: LYsosome TArgeting Chimera; Mode-A; molecular degraders of extracellular proteins through the ASGPR; PROTAC: PROteolysis TArgeting Chimera; RIBOTAC: RIBOnuclease TArgeting Chimera; RNA: ribonucleic acid; SNIPER: Specific and non-genetic inhibitors of apoptosis protein (IAP)-dependent protein eraser.
| Degradation Pathways | Degradation Systems | Degradable Targets |
Advantages | Limitations | Highest Phase |
|---|---|---|---|---|---|
| Ubiquitin-proteasome | PROTAC | Intracellular proteins | In vivo, oral, improved selectivity and efficiency, does not require tight binding, catalytic and substoichiometric, definite structure and mechanism | High molecular weight, high surface area | Phase II |
| Molecular glue | Intracellular proteins | Good pharmacology, specific to ligase and substrate | Mainly relies on accidential discovery | Approved | |
| SNIPER | Intracellular proteins | Catalytic and substoichiometric, definite structure and mechanism | Dependent on E3 ligase IAPs | Exploratory | |
| Autophagy | AUTAC | Cytoplasmic proteins, fragmented organelle | Broader targets | Lack of detailed mechanism for K63 ubiquitination | Exploratory |
| ATTEC | Intracellular proteins, nonproteins | Broader targets, low molecular weight, good transmembrane activity, better pharmacokinetics | Lack of detailed interaction between LC3 and degraders, high molecular design costs, low versatility | Exploratory | |
| AUTOTAC | Degradation-resistant aggregates | Broader targets | Unclarified mechanism | Exploratory | |
| CMA-based | Transmembrane proteins, aggregates | High specificity | Low stability and delivery efficiency, dependent on cell penetrating peptides, limited therapeutic effects | Exploratory | |
| Lysosomal | LYTAC | Extracellular proteins, membrane-associated proteins | Broader targets | Limited clinical applicability, required an antibody or a small synthetic peptide to maintain its characteristics, difficult to determine the optimal linking site, high molecular weight, induced immunogenicity | Exploratory |
| MoDE-A | Extracellular proteins | Small in size, monodisperse and nonprotein based, well-tolerated immunogenicity | Relies on highly expressed ASGPR on hepatocytes | Exploratory | |
| BIAC | Membrane-associated proteins | Costless and easily synthesized | In the early stages | Exploratory | |
| AbTAC | Transmembrane proteins | Utilizes two specific binding sites of bispecific antibodies to recruit E3, simple optimization of binding properties, built of human parts, reduced immune response | High molecular weight | Exploratory | |
| GlueTAC | Cell surface proteins | Cell-type independent degradation strategy, high specificity and efficiency | In the early stages | Exploratory | |
| Ribonuclease | RIBOTAC | RNA | Favorable pharmacokinetic profile, low concentration, catalytic | High molecular weight, slow cellular uptake | Exploratory |
2.1. Hijacking the UPS
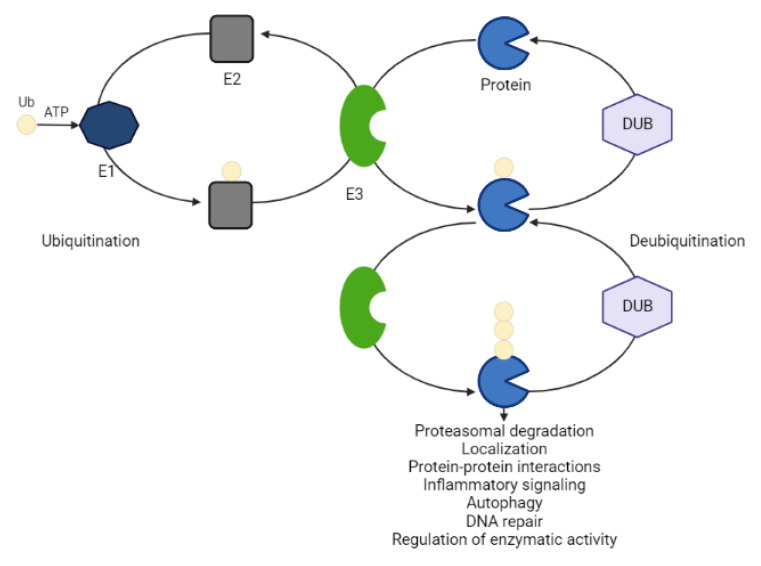
The ubiquitin-proteasome system (UPS) is an essential pathway in the cell that processes the ablation of misfolded or damaged proteins involved in the pathogenesis of different diseases [21]. This tightly regulated process centers around an 8.6 kDA stable protein, so-called ubiquitin. The covalent attachment of ubiquitin to a lysine residue within the POI occurs in a concerted action of three ubiquitin enzymes (activating E1, conjugating E2, ligase E3) at the expense of ATP. These three enzymes act sequentially. Ubiquitin is first activated by E1 and then transferred onto E2. E3 interacts simultaneously with a ubiquitin-loaded E2 and the target POI by mediating isopeptide bond formation between the ubiquitin and a substrate lysine [22]. The structural variation of ubiquitin chains establishes a code that directs different cellular fates of the substrate (e.g., protein degradation, localization, protein-protein interactions, inflammatory signaling, autophagy, DNA repair and regulation of enzymatic activity) [22,23]. While K48-linked polyubiquitin chains mainly label proteins for 26S proteasome-mediated recognition and degradation, K63-linked polyubiquitination mainly signals for lysosomal degradation [21,24,25,26]. E3 ligases play a key role in the entire process of ubiquitination due to their specificity for substrates. There are approximately 1000 annotated E3 ligases that are categorized into the homology to E6AP C terminus (HECT) domain-containing E3s, the RING-between-RING (RBR) family E3s and the really interesting new gene (RING) finger domain-containing E3. Protein ubiquitination is a dynamic and reversible process (Figure 3). Deubiquitinating enzymes (DUBs) cleave the attached ubiquitin moieties from substrates, preventing degradative pathways [23]. In the human genome, more than 100 functional DUBs have been identified, which can be divided into eight families: ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), ovarian tumor proteases (OTUs), Jab1/MPN domain-associated metallopeptidase (JAMM) domain proteins, Josephin or Machado–Joseph disease protein domain proteases (MJDs), the monocyte chemotactic protein-induced protein (MCPIP) family, the motif interacting with Ub-containing novel DUB family (MINDY), and Zn-finger and UFSP domain proteins (ZUFSPs). DUB modifications have been implicated in tumorigenesis at multiple levels and inhibitors targeting DUBs are attracting increased attention from pharmaceutical companies [23].
Figure 3.
The process of ubiquitination and deubiquitination. Ubiquitin binds to the target protein through the sequential action of activating E1, conjugating E2 and ligase E3 enzymes at the expense of ATP. Deubiquitinating enzymes (DUBs) remove ubiquitin from the substrate by cleaving the isopeptide bond. The balance between ubiquitination and deubiquitination regulates a variety of cellular processes. ATP: adenosine triphosphate; Ub: ubiquitin.
Active ubiquitination and degradation of tumor suppressors (e.g., p53) are at the root of maintaining cancer cell proliferation. Here, targeted protein stabilization (TPS) instead of degradation would prove therapeutically beneficial [27]. Recently, heterobifunctional small molecules consisting of a recruiter of a DUB linked to a protein-targeting ligand were constructed to stabilize the levels of specific proteins that are otherwise actively degraded in a ubiquitin-dependent manner [19]. The proof of concept for the DUBTAC (deubiquitinase-targeting chimeras) platform has been provided in human cystic fibrosis bronchial epithelial cells and in hematoma cells. While its clinical translation remains to be seen, there are many fields other than cancer that could benefit from targeted deubiquitination and stabilization, including immunooncology, diabetes, Gaucher’s disease or Parkinson’s disease [19]. In diseases caused by haploinsufficiency, where loss of one gene copy is responsible for the pathology, DUBTACs may slow down the turnover rate of the POI to elevate its levels to attenuate disease progression [19].
2.1.1. PROTACs (PROteolysis TArgeting Chimeras)
The PROTAC technology has experienced three generations of development. The first generation required peptide sequences for E3 ligase recognition and cell penetration. The first PROTAC was applied in Xenopus egg extract to target methionine aminopeptidase 2 to the Skp1-Cullin-F box complex for ubiquitination and proteasomal degradation [28]. The same approach was further employed to ubiquitinate and deplete the androgen and estrogen receptors (AR and ER), two cancer-associated targets involved in the progression of prostate and breast cancers, respectively [29]. Another peptide-based PROTAC engaged the von Hippel-Lindau (VHL) as an E3 ligase to degrade POI in intact cells [30]. However, these chimeric compounds exhibited low cellular activity at least partially due to poor cell permeability and chemical stability, limiting their clinical applicability. The second generation of PROTAC sought to design non-peptidic E3 ligase ligands to pave the ground for all small-molecule PROTACs. This concept succeeded by development of a compound consisting of a selective AR modulator tethered to nutlin, which is a ligand for the E3 ligase MDM2 and hinders MDM2 binding to its substrate p53 [31]. This PROTAC recruited AR to MDM2 for ubiquitination and degradation in HeLa cells. Despite advances in degradation efficiency, there are still some limitations including potential off-target specificity, high molecular weight and cytotoxicity. The third generation focuses on controllable PROTAC including phosphate-dependent and light-controlled PROTACs, which stimulate protein degradation via activated kinase signaling signal or visible light, respectively [32,33,34,35,36], and will be discussed in depth below.
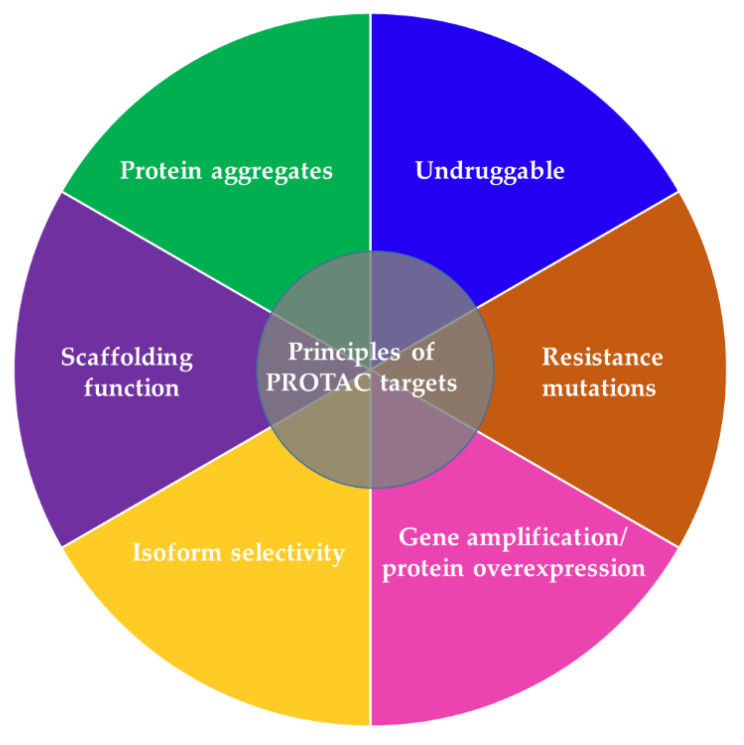
PROTACs have potential advantages compared to other traditional approaches to suppressing a POI, providing them with a greater chance for faster clinical development against a wider range of targets in multiple cancer types (Table 1, Table 2, Figure 4) [37,38]. Due to the catalytic mode of action (as reflected in the continuous and rapid ablation of the POI) PROTACs are required at significantly lower concentrations (in a nanomolar or picomolar range) to exert a biological effect and unlike traditional small-molecule inhibitors, they tolerate low affinity binding to the POI [37]. Previous reports thus suggested that ineffective inhibitors with weak kinase interactions may still have clinical relevance in PROTAC design and application [37,38,39]. PROTACs exhibit higher tissue and target selectivity toward mutant over wild-type protein both in vitro and in vivo (Figure 4), and thus provide a promising modality to treat cancer and other diseases [16,37,38]. Another advantage of PROTACs is their enhanced protein isoform selectivity (Figure 4). Winter and colleagues described selective destruction of the cell cycle kinase CDK6 (cyclin-dependent kinase 6) by a PROTAC while sparing its close homolog CDK4 [40]—a feature that distinguishes PROTAC treatment from approved CDK4/6 kinase inhibitors that fail to discriminate between these two kinases. Similar observations were reported on isoform specific SGK3 (serum/glucocorticoid regulated kinase family member 3) PROTAC degrader over SGK1/2 [41]. Both studies exemplify the benefit of the PROTAC approach in targeting kinase signaling pathways with enhanced efficacy and selectivity, more than is possible with conventional inhibitors [40,41]. Selectively attacking oncogenic proteins in diseased cells while sparing their homologs in healthy tissue may help reducing the off-target effects in patients. Linker composition and E3 complex/POI interactions that create a favorable ternary complex may at least partially explain wild-type/mutant and homolog specificity of PROTACs. Future studies will shed light on the exact mechanisms of selectivity evident for degraders.
Figure 4.
Overview of main targets of PROTAC degraders.
Unlike small-molecule inhibitors that function via binding to catalytic or allosteric sites, PROTACs derived from a binder (e.g., peptide, chemical entities, etc.) on any POI surface pocket with reasonable affinity may cause target destruction [37,38]. This distinct mechanism of action allows PROTACs to attack clinically relevant targets that are traditionally considered undruggable such as transcription factors lacking catalytic sites for small-molecule inhibitors (Figure 4). PROTACs are able to mitigate not only catalytic activity of target enzymes but also eliminate other functions of the POI such as scaffolding functions that often remain intact despite attenuated enzymatic activity (Figure 4) [38]. This is of potential clinical relevance because unimpaired scaffolding functions are prone to induce rewiring of signal transduction which damages efficacy and manifests as resistance as seen with small-molecule inhibitors [38]. Of clinical importance, PROTACs can offer a thorough elimination of multi-protein complexes because they degrade both the target and the interacting subunit, whereas small-molecule inhibitors often attenuate the function of a target POI while sparing others [38,42,43].
PROTACs are also able to eradicate mutated targets (Figure 4). In fact, they can bypass acquired resistance to kinase inhibitors by targeting an alternative ligand-binding site. This is exemplified by the finding that by targeting an alternative RAS-binding domain of BRAF, a rigosertib-derived PROTAC promotes degradation of mutant BRAF and overcomes the mutation-mediated drug resistance [44]. Although administration of a PROTAC at a lower dosage mitigates the likelihood of resistant mutations emerging in the target, PROTAC treatment is still prone to elicit resistance. Causative alterations are not limited to the neo-substrates but can also arise within the E3 ligases [40,45,46,47,48]. To date the majority of reported PROTACs use either CRBN or VHL as the hijacked E3 ligase, with >30 proteins being destructed through CRBN and >20 via VHL [49,50,51,52]. Acquired resistance to these two E3 ligases has been reported in cancer cells following chronic treatment [48]. Unlike many targeted therapeutics, resistance did not result from secondary mutations that influence compound binding to the target POI but it was primarily caused by genomic alterations within the core components of the degradation machinery. In line with this, Winter and colleagues recently established a haploid genetics-based pipeline to profile the landscape of resistance mechanisms to small-molecule degraders [47]. They deciphered functional hotspots in the E3 ligases CRBN and VHL that tumor cells can use to evade degrader molecules. In fact, a number of identified hotspots were disrupted in patients that relapsed from degrader treatment, supporting the potential clinical relevance of their assay. This pipeline only covers copy number loss and splicing defects and mimics the scenario of homozygous mutations, whereas patients may harbor heterozygous mutations. Future data on clinical trials of degraders will provide additional insight into clinically relevant functional hotspots. A critical step in PROTAC development is the formation of a stabile ternary complex: although CRBN and VHL have a wide palette of substrates, some protein-ligase pairs do not endure long enough for the E3 ligase to ubiquitinate [53]. This however could be ensured by alternative E3 ligases. Among those are MDM2, DCAF15, DCAF16, RNF4, RNF114, FEM1B, KEAP1, AhR, cIAP1 and XIAP, which pose several distinct advantages including specificity for tissue, tumor, cell type or cell state, and synergistic tumoricidial effects through activation of pro-apoptotic cell cycle regulatory proteins [54].
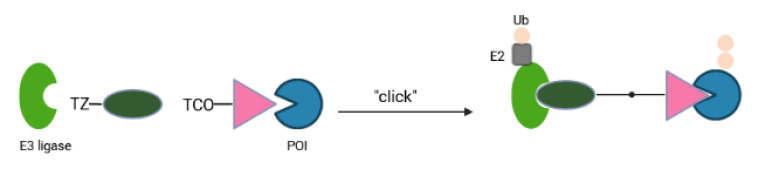
Although protein degradation has revolutionized drug discovery platforms, current PROTACs come with several shortcomings that are likely to restrict their potential as therapeutics. These heterobifunctional molecules require linker optimization, possess high molecular weight and a high polar surface area that is normally associated with poor cellular penetration, solubility and other drug-like properties. An alternative has been proposed using the idea of CLIPTACs (in-cell-click-formed proteolysis targeting chimeras) in which two small precursor molecules with the ability to click intracellularly will pass through cellular membranes more easily than one large compound (Figure 5). Heightman and colleagues generated a tetrazine-tagged E3 ligand and a trans-cyclooctene-tagged POI ligand as the precursors [55]. Via the click reaction between tetrazine and trans-cyclooctene, forming a covalent six-membered ring moiety, these two precursors created integrated PROTACs (CLIPTAC) in cells that successfully depleted oncogenic BRD4 or ERK1/2 in a CRBN E3 ligase- and proteasome-dependent manner [55]. The main advantage of CLIPTAC is a significant reduction of the molecular weight and polar surface area of the separate partners compared to the pre-assembled PROTAC molecule. Tuning the click reaction to proceed at a slower rate may help to avoid the possibility that the bioorthogonal combination reaction occurs outside the cells, resulting in a heterobifunctional molecule with high molecular weight and polar surface area that fails to penetrate into cells.
Figure 5.
Schematic diagram of CLIPTACs. CLIPTACs consist of cell permeable precursors that join together via click-chemistry to form an active molecule in cells. POI: protein of interest; TCO: trans-cyclooctene; TZ: tetrazine; Ub: ubiquitin.
High bioavailability can be achieved by orally bioavailable prodrug PROTACs derived from CRBN ligands [56]. Wei et al. reported the first prodrug PROTAC based on the structure of CDK4/6 inhibitor ribociclib’s derivative, with the oral bioavailability up to 68% [56]. In melanoma cells, the degrader could not only degrade CDK2/4/6 simultaneously and effectively, but also induced cell cycle arrest and apoptosis of malignant cells. Another tool to increase bioavailability is offered by computer-aided drug design softwares to in silico predict the solubility and ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties of molecules before design [51]. Furthermore, the cell/tissue permeability of PROTACs may be enhanced by using long flexible linkers to generate intramolecular hydrogen bonds to at least partially reduce polarity [57]. A further way is to attach cell-permeable peptides to E3 ligands [58]. Finally, identification of highly specific POI/E3 ligands is crucial for the design and development of potent PROTACs with minimal off-target effects.
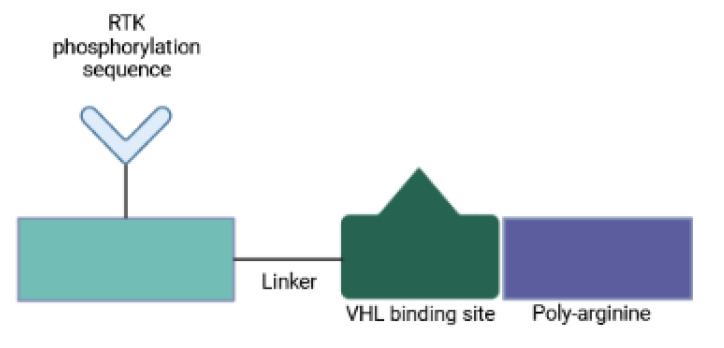
Since PROTACs operate in a catalytic manner and enable systemic protein knockdown, their off-target toxicity is a major concern. One strategy to circumvent systemic undesired effects involves the use of designed peptides, so-called phosphoPROTACs, that can be conditionally activated via phosphorylation by specific growth-factor stimuli [32]. A recent study has described two phosphoPROTAC molecules that coupled the tyrosine phosphorylation sequences of the nerve growth factor receptor, TrkA, or the neuregulin receptor, ErbB3, with a peptide ligand for the VHL E3 ligase [32] (Figure 6). These phosphoPROTACs then recruited either the neurotrophic signaling effector FRS2α or the survival promoting PI3K, respectively, to be ubiquitinated and depleted upon activation of specific receptor tyrosine kinases and phosphorylation of the phosphoPROTACs. Anticancer properties were demonstrated both in vitro and in vivo. Activation of phosphoPROTACs was dependent on their kinase-mediated phosphorylation: (i) phospho-null variants remained inactive; and (ii) stimulation of unrelated growth factor receptors did not induce target protein knockdown. This approach provides not only a time- and dose-dependent control but also cell type selectivity, all features that distinguish phosphoPROTACs from nucleic acid-based strategies. Furthermore, conditional activation makes phosphoPROTACs suitable for the selective treatment of malignant cells. Another strength is that it is not likely to arouse drug-resistant mutants [32]. Current tyrosine kinase inhibitors mitigate cell proliferation by blocking enzymatic function, which in turn signals for a selective pressure for target mutations that evade inhibitor binding and preserve kinase activity. PhosphoPROTACs on the other hand require dysregulated kinase function to stop cellular signaling and tumor growth. Therefore, changes within the kinase structure to prevent phosphoPROTAC activation are anticipated to cause loss of kinase signaling too [32]. By changing the autophosphorylation sequence of a phosphoPROTAC, other receptor tyrosine kinase/effector pairings can be investigated.
Figure 6.
Schematic diagram of phosphoPROTACs. PhosphoPROTAC consists of an RTK phosphorylation sequence, VHL binding sequence and a poly-D-Arginine sequence connected by a linker. When the RTK is activated, the tyrosine of the RTK phosphorylation sequence is phosphorylated, recruits and activates POI with PTB and SH2 domains. The peptidic VHL binding sequence following hydroxylation of the proline residue recruits the VHL E3 ligase to mediate polyubiquitination and degradation of the POI, thereby inactivating tyrosine kinase signaling. RTK: receptor tyrosine kinase; VHL: von Hippel-Lindau.
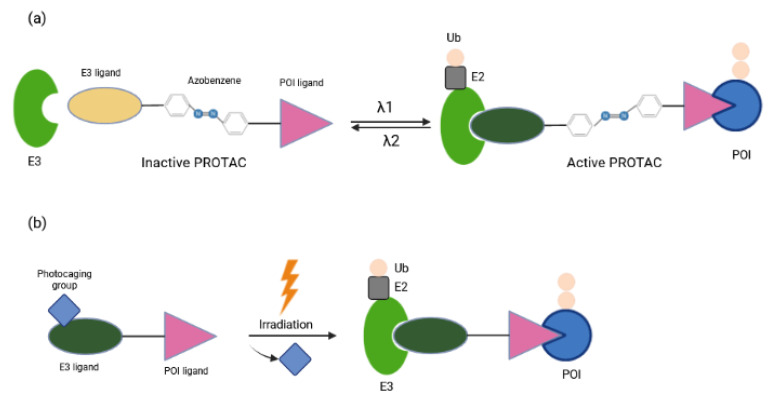
Other approaches for tissue/cell specific degradation of POIs by PROTAC while avoiding deleterious effects elsewhere employ light stimuli [59,60]. Optical control can be achieved with caged compounds [61], with genetically engineered photoreceptors [62], or with synthetic photoswitches whose activity can be changed through a combination of photochemical isomerization and thermal relaxation [63,64]. Recently, several reports have focused on light-induced control of protein destruction [34,35,36]. By incorporating azobenzene photoswitches into PROTACs, Trauner and colleagues have described a novel strategy to control TPD with the spatiotemporal precision that light provides [34]. These so-called PHOTACs (PHOtochemically TArgeting Chimeras) are trifunctional molecules compromised of a ligand for an E3 ligase, a photoswitch and a ligand for POI (Figure 7a). They are inactive as degraders in the dark and become active under blue-violet light (380–440 nm). Activated PHOTACs gradually lose their activity through thermal relaxation, or can be quickly inactivated photochemically [34]. Thus, their inactivation is much less dependent on dilution, clearance, or metabolism. As a proof of principle they were used to tame BET (Bromo- and extra-terminal) family proteins or FKBP12 by binding CRBN E3 ligase complex and mediating proteolysis in a light-dependent manner, which in turn suppressed viability of lymphoblastic leukemia cells. The concentrations needed for maximum photo effect were in the nanomolar range, minimizing possible off-target effects. Another strategy for light-inducible control of PROTAC activity has been provided by Wei and colleagues [36]. In the dark, nitroveratryloxycarbonyl (NVOC) labeling on the CRBN E3 ligand as photolabile blocking group hindered the association between PROTAC and E3 ligase, whereas upon UV irradiation the photocaging group was released from PROTAC, facilitating the formation of the POI-PROTAC-E3 complex (Figure 7b). These so-called opto-PROTAC molecules were applied for restricted degradation of cancer-related proteins of IKZF1/3, BRDs and ALK fusion proteins at a specific time and rate by UVA illumination. Tumoricidal properties were reflected in reduced cancer cell proliferation in an optically-controlled manner. Despite their advantages, photoPROTAC technologies still come with some limitations. Considering UVA irradiation might cause DNA damage and is mainly applied in blood, skin and lung cancers due to its inefficiency in penetrating tissues, it would be more appropriate to develop alternative methods that do not require UVA exposure [36]. Caging groups with absorption within the near-infrared region may enhance tissue penetration and improve clinical output. In vivo experiments using mouse models are definitely crucial to validate the functions of these molecules.
Figure 7.
Schematic diagram of photochemical controls of PROTAC. (a) The PHOTAC molecule switches between an inactive (orange) and an active form (dark green) under blue-violet light. (b) The photocaged-PROTAC is activated in a UVA-dependent manner. The photocage group is then released, enabling the degradation of POI in a controllable manner. POI: Protein of interest; Ub: Ubiquitin.
Conventional small-molecule PROTACs generally display unfavorable pharmacokinetics and lack of tumor specificity, which may contribute to systemic toxicity due to their nonspecific distribution in normal tissues. One way to achieve tumor specific delivery of PROTACs has been proposed by using gold nanoparticle (GNP)-based multi-headed PROTACs [65]. Cer/Pom-PEG@GNPs composed of interconnected ligands for POI and E3 ligase promoted effective ALK degradation in a dose- and time-dependent manner and hindered proliferation of a lung adenocarcinoma cell line with minor off-target toxicity. Although in vivo data is missing, Cer/Pom-PEG@GNPs as a nano-based drug carrier promises prolonged circulation and specific delivery of drugs to tumor regions, and can be beneficial in patients resistant to ALK kinase inhibitors [65]. Furthermore, Yu and colleagues reported polymeric PROTAC (POLY-PROTAC) nanotherapeutics for tumor specific targeted degradation [66]. The POLY-PROTACs self-assemble into micellar nanoparticles and sequentially respond to extracellular matrix metalloproteinase-2, intracellular acidic and reductive tumor microenvironments. The POLY-PROTAC nanoparticles carry azide groups for bioorthogonal click reaction-amplified PROTAC delivery to the tumor region. Tumor specific BRD4 depletion via the POLY-PROTAC nanoplatform combined with photodynamic therapy resulted in tumor regression in a mouse xenograft model of MDA-MB-231 breast cancer [66]. For the successful translation of the so-called nanoPROTACs into the clinical setting, safety analysis and optimization of a chemical and manufacturing control procedure are required [67].
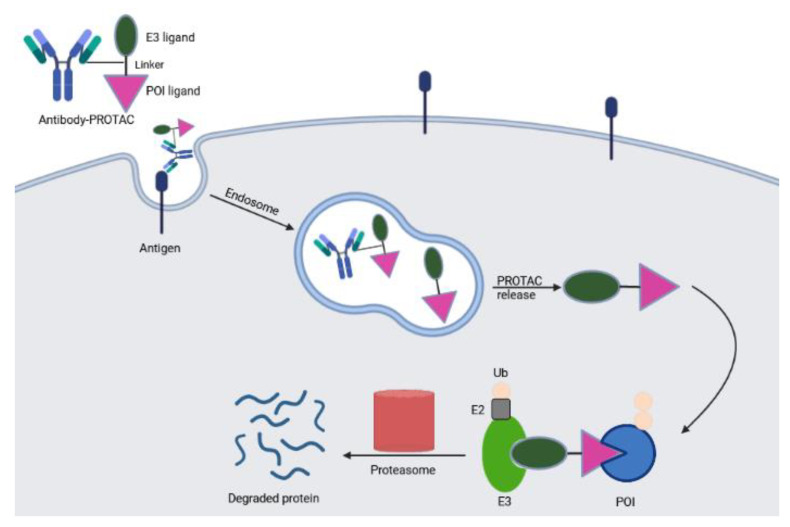
Selective delivery of a broad spectrum PROTAC into specific cell types is also feasible by an antibody-PROTAC conjugate. Maneiro et al. described a trastuzumab-PROTAC conjugate in which E3 ligase-catalyzed degrader activity is caged with an antibody linker which can be hydrolyzed upon antibody-PROTAC internalization, releasing the active PROTAC and stimulating protein destruction [68] (Figure 8). Proof of principle was provided by degradation of BRD4 only in HER2 positive breast cancer cells but not in HER2 negative background. The event required proteasome activity: incubation with bortezomib, a proteasome inhibitor, prevented antibody-PROTAC mediated BRD4 depletion. Other studies expanded the target spectrum and applied this concept to different tumor models [69,70,71,72].
Figure 8.
Schematic depiction of degrader-antibody conjugates. These molecules allow cell specific delivery of the degrader. Endocytosis of the conjugates mediates the release of active PROTAC which marks the POI for proteasomal destruction.
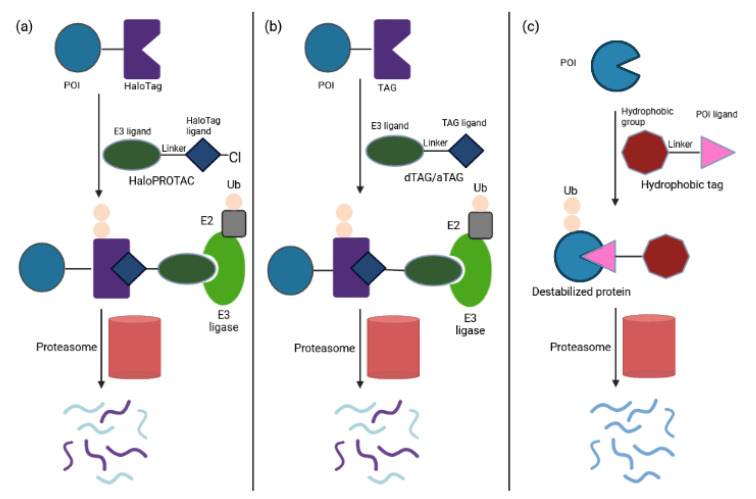
2.1.2. Tag-Based Chemical Degraders
The selection of appropriate E3 ligase/E3 ligand systems is crucial for the progress of PROTAC research. Application of PROTACs in an endogenous setting is mainly limited to target proteins with available ligands. An advancing solution has been provided by tag-directed chemical degrader systems in which the tag-POI fusion protein was expressed in cells and the universal PROTAC was administered to attract the candidate E3 ligase and the tag of the tag-POI fusion [51]. Measuring the abundance of tag-POI complex validated whether the candidate E3 ligase could mediate POI destruction. The most-widely used tag-based strategies are HaloPROTAC (Figure 9a) and degrader tag (dTAG) (Figure 9b) [73,74,75,76]. HaloPROTACs degrade HaloTag7 fusion proteins by combining chloroalkane HaloTag ligands with a small molecule ligand for the VHL E3 substrate receptor, and exhibit favorable potency and kinetics [73]. Proof of concept was demonstrated by depletion of cytoplasmic proteins such as ERK1 and MEK1. Tovell et al. improved HaloPROTAC degrader probes by combining them with CRISPR-Cas9 technology [74]. The lead probe induced reversible depletion of two endosomally localized proteins, SGK3 and VPS34, thereby blocking downstream signaling.
Figure 9.
Schematic depiction of tag-based degraders. (a) The HaloPROTAC utilizes recognition of hexyl chloride tags and promotes destruction of HaloTAG-fused POI. The modality simplifies the optimization of PROTACs. (b) The dTAG molecule is composed of an E3 ligase ligand linked to a POI ligand, which facilitates formation a ternary complex between the fusion protein and E3 ligase, leading to polyubiquitination and degradation of the POI. The key difference between the dTAG and aTAG technologies is the identity of the TAG protein used. (c) Misfolded proteins are degraded through recognition of hydrophobic patches.
The dTAG system uses PROTACs to degrade target proteins that have been genetically fused to a mutant isoform of FKBP12 [75,76]. Both CRBN- or VHL-recruiting dTAG molecules have been proven suitable for in vivo use. Recently, Fischer and colleagues reported a novel degradation tag BRD4BD1L94V along with the corresponding CRBN-based heterofunctional degrader utilizing a bump-and-hole approach [77]. They also proved the compatibility of simultaneous application of the BRD4BD1L94V system and the dTAG system. This strategy complements currently available degradation tags to attack disease co-dependencies.
Furthermore, Veits et al. recently reported a novel degradation methodology on fusing a POI to the small protein MTH1 (MutT homolog-1), which serves as a ligand-binding tag [78].This AchillesTag (aTAG) can be paired with different heterobifunctional degraders that recruit the CRBN E3 ligase into close proximity, leading to ablation of any aTAG-fused POI (Figure 9b). Proof of principle has been provided by selectively controlling Chimeric Antigen Receptor (CAR) protein levels [78]. Treatment with aTAG degraders attenuated CAR-mediated target tumor cell killing and T-cell activation/cytokine release. These effects were rapid and reversible as CAR-T activity was restored upon drug removal. Six CAR-T cell products are already in the market for the treatment of B-cell acute lymphoblastic leukemia, lymphomas and multiple myeloma: Kymriah (Tisagenlecleucel), Yescarta (Axicabtagene ciloleucel), Tecartus (Brexucabtagene autoleucel), Breyanzi (Lisocabtagene maraleucel), Abecma (Idecabtagene vicleucel) and Carvykti (Ciltacabtagene autoleucel). However, these therapies have been associated with unique adverse events including cytokine-release syndrome, neurologic events and immune effector cell-associated neurotoxicity [78,79]. Therefore, fine tuning of protein expression in CAR-T therapy provides clinical benefit. To date a suicide switch in the CAR has been proposed to improve the safety of T cell adoptive immunotherapy for lymphomas, which however resulted in the irreversible loss of treatment [80]. The aTAG degradation system was also efficacious in vivo with favorable pharmacokinetic properties. Altogether, the aTAG model provides another layer in the drug discovery efforts to address adverse consequences and define desired in vitro and in vivo properties of a degrader therapeutic.
Unfolded or misfolded proteins expose hydrophobic regions that signal for recruitment of E3 ligases to degrade unwanted POIs in a proteasome-dependent fashion. Several studies have mimicked protein unfolding by labeling specific targets with low molecular weight hydrophobic tags to promote their destruction [81,82,83,84,85]. This modality has been applied to degrade the pseudokinase Her3, an undruggable target implicated in breast, ovarian and non-small cell lung cancers, by bifunctional molecules consisting of a covalent Her3 targeting small molecule linked to a hydrophobic adamantane moiety [82]. The resulting silencing of Her3 hindered productive heterodimerization of Her3/Her2 and Her3/c-Met, and reduced proliferation of Her3-dependent cell lines. In addition, Ma et al. developed an EZH2 inhibitor-based degrader using hydrophobic tagging (HyT) strategy by linking an EZH2 inhibitor to a bulky adamantyl group to degrade EZH2 [84]. This target protein is the main enzymatic subunit of the PRCs complex; high expression levels are associated with poor prognosis in multiple types of cancer. Using hydrophobic tagging, the lead degrader compound selectively killed triple negative breast cancer cells in vitro and in vivo, whereas existing EZH2 inhibitors fail to do so [84]. Another hydrophobic tagged dimeric molecule was shown to reduce cellular levels of SRC-1, a transcription co-activator, which in turn suppressed cancer cell migration and invasion [83]. A further successful application of this approach has been demonstrated by Gustafson et al. [85]. Molecules containing hydrophobic degrons linked to small-molecule AR ligands induced AR ablation, decreased expression of AR target genes and attenuated proliferation of androgen-dependent prostate cancer cell lines. The toxic effects were similar to those seen with enzalutamide, an FDA-approved inhibitor of AR signaling. Remarkably, this drug also retained its antiproliferative properties in cells resistant to current standard of care drugs for castration-resistant prostate cancer [85]. Altogether, the hydrophobic tagging strategy (Figure 9c) adds to the emerging paradigm of targeted protein degradation as a therapeutic strategy. It holds the promise to develop peptide-based degraders with improved cell permeability and metabolic stability in order to manipulate disease-relevant proteins and drug-resistant mutants that have been insensitive to traditional approaches.
2.1.3. Degrader Systems Based on PROTACs
Homo-PROTACs as Suicide Molecules
The human genome comprises approximately 1000 predicted E3 ubiquitin ligases that are important not only in normal cellular physiology but also in diseased states, making them attractive targets for drug discovery. Overexpression of E3 ligases is associated with poor clinical prognosis or drug resistance in cancer cells. These enzymes do not exhibit deep and druggable active sites for binding to small molecules; their inhibition is generally achieved by targeting protein-protein interactions. Only few potent compounds have been described that bind to the E3 substrate recognition site. However, competition with high-affinity endogenous substrates may increase unspecific cytotoxicity. Further, E3 ligases are multi-domain and multi-subunit enzymes [86]. Thus, targeting an individual binding site leaves other interactions functional, resulting in ineffective blockade of the enzyme. To overcome these obstacles, interest has focused on the design of bivalent compounds to dimerize an E3 ligase to trigger its suicide-type chemical knockdown inside the cells. The so-called homo-PROTACs are a unique type of PROTACs comprised by two identical molecules linked together. Proof of concept has been provided by several VHL- and CRBN-based homo-PROTACs with no in vivo therapeutic potency addressed [50,86,87,88].
Recently, He et al. extended this principle to MDM2, an oncogenic E3 ligase and antitumor target [89]. A homo-PROTAC for an effective disruption of MDM2-p53 interaction may lead to a distinct strategy in cancer therapy. The lead homo-PROTAC compound dimerized MDM2 with high binding activity and induced its proteasomal self-degradation [89]. As a consequence, p53 expression was increased in a dose-dependent manner, triggering apoptosis in A549 non-small cell lung cancer cells. In vivo tumoricidal activity was demonstrated in a xenograft mouse model, making this study the first example of a homo-PROTAC with in vivo therapeutic potency. This approach offers a new avenue to overcome the bottleneck of the dose-related adverse effects including the risk for hematological diseases [90,91] of MDM2-p53 small-molecule inhibitors observed during clinical studies.
Nucleic Acid-Based PROTACs
RNA-PROTACs
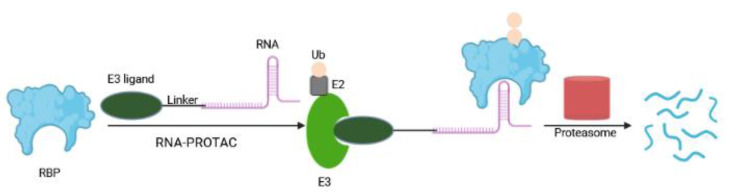
A new type of PROTAC, so-called RNA-PROTAC, aims to degrade RNA binding proteins (RBPs), a class that until now has been proven difficult to target pharmacologically [92]. RBPs interact with target RNAs in a sequence- and structure-dependent fashion via their unique RNA binding domain. Their dysregulation (upon genetic alteration, epigenetic change, noncoding RNA-mediated regulation, and posttranslational modifications) are at the origin of many diseases ranging from neuronal disorders to cancers [93,94,95]. Altered RBPs influence several steps in the development and progression of cancer, including sustained cell proliferation, evasion of apoptosis, avoiding immune surveillance, inducing angiogenesis, and activating metastasis [93]. RNA-PROTAC chimeric structures employ small RNA mimics as targeting groups that dock the RNA-binding site of the RBP, whereby a conjugated E3-recruiting peptide derived from the HIF-1-alpha protein directs the RBP for proteasomal degradation (Figure 10). The first RNA-PROTACs target the stem cell factor and oncoprotein Lin28 and the splicing factor RBFOX1 in cancer cell lines [92]. This strategy has some shortcomings including the instability of RNA oligomer and the requirement of RNA secondary structure for its proper interaction with RBPs [96]. RNA-PROTACs have large molecular weights, making their clinical applicability challenging [97]. Here, cellular uptake may be improved by employing the CLIPTAC approach [55] to generate smaller RNA-PROTAC precursors. Furthermore, the synthesis of RNA-PROTACs and their permeability may be advanced by using water-soluble, non-peptidic linkers and E3 ligands; and application of nanoscale delivery systems may improve their efficiency in vivo [97,98].
Figure 10.
Schematic depiction of RNA-PROTACs. An RNA-PROTAC with a short oligonucleotide binds the RNA binding domain of RBP and directs it for degradation. RBP: RNA binding protein; Ub: ubiquitin.
Transcription Factor PROTACs
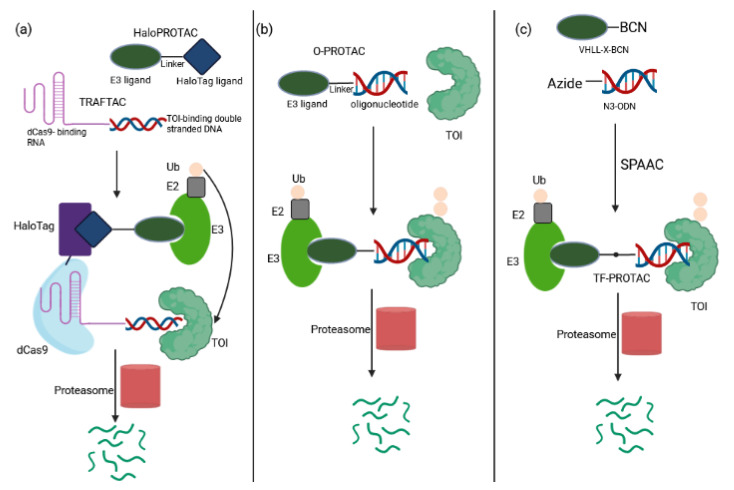
Transcription factors (TF) are central in numerous diseases, yet remain incurable due to the lack of enzymatic activity and ligandable sites. In a recent study, Crews and colleagues have co-opted the DNA-binding capability of TF to develop TRAnscription Factor TArgeting Chimeras (TRAFTACs) [99]. The TRAFTAC is an oligonucleotide with a short, double-stranded DNA TF recognition sequence that can simultaneously bind to a transcription factor of interest (TOI) and VHL-E3 ligase via an intermediary HaloTag fused dCas9 protein (dCas9HT7) (Figure 11a). This multicomponent approach (a TRAFTAC, dCas9HT7 and a HaloPROTAC) resulted in proteasomal depletion of two disease-causing TFs: NF-κB (a key player in cell proliferation and overactivated in many cancers and inflammatory diseases) and brachyury (involved in tumor migration, invasion, and metastasis, and not expressed in normal adult human cells). In vivo efficacy was proven in a zebrafish model. Similar to PROTACs, TRAFTACs exhibit an event-driven pharmacology, and require a brief interaction of TOI with the chimeric oligo to induce TF destruction in a catalytic fashion. The multicomponent complex can bind to another TOI molecule after completing the first ubiquitination cycle. These features and their increased in vivo stability distinguish TRAFTACs from oligonucleotide-derived decoy elements [100]. Thus, the TRAFTAC strategy offers a creative tool to advance drugging TFs with a known DNA-binding sequence via the engagement of endogenous substrate mimics [99,100]. A major hurdle of the multicomponent nature is the limited bioavailability. The success of its utility relies on the ectopic expression of the Cas9 protein in cells. An efficient delivery strategy may enhance the translational possibilities for TRAFTACs in patients [99,100].
Figure 11.
Overview of degradation of transcription factors. (a) TRAFTAC, a heterobifunctional dsDNA/CRISPR-RNA chimera, recruits E3 ligase complex through dCas9-HT7 in the presence of HaloPROTAC. TRAFTAC binds to dCas9-HT7 via its RNA moiety while dsDNA portion of the chimera binds to the transcription factor of interest (TOI). Addition of HaloPROTAC recruits VHL E3 ligase complex to the TOI, thereby targeting it for ubiquitination and proteasomal degradation. (b) In the O-PROTAC model the dsDNA is incorporated in the TOI binding ligand of the PROTAC. (c) A BCN-modified VHL ligand (VHLL-X-BCN) is incorporated onto an azide-modified DNA oligomer (N3-ODN) via a copper-free strain-promoted azide–alkyne cycloaddition (SPAAC) reaction, forming a TF-PROTAC to recruit the VHL E3 enzyme, thereby triggering ubiquitination and degradation of the TOI by the 26S proteasome. dsDNA: double-stranded DNA; Ub: ubiquitin.
A complementary drug discovery and development platform has been proposed by the O-PROTAC model, in which a double-stranded oligonucleotide is incorporated as a TOI binding moiety in PROTAC [101] (Figure 11b). This modality has been successfully applied to destruct ERG, a TF overexpressed in 50% of both primary and metastatic prostate cancer [102], and LEF1, another cancer-related TF involved in migration and invasion, with potent efficacy in cultured cells. O-PROTAC offers straight-forward predictability, reprogrammability and superior stability.
The field evolves at a rapid pace as highlighted by a recent report of TF-PROTAC that links an DNA oligonucleotide to an E3 ligase ligand via a click reaction, to selectively degrade the TOI (Figure 11c). Here, commercially available azide-modified DNA oligomers are conjugated to the bicyclooctyne (BCN)-modified VHL ligands with various linkers (VHLL-X-BCN) via a copper-free strain-promoted azide–alkyne cycloaddition reaction [96]. The selectivity of TF-PROTACs depends on the DNA oligonucleotides used that can be specific to the TOI. Proof of concept has been demonstrated by targeted degradation of two cancer-relevant TFs, NF-κB and E2F, in a VHL E3 ligase and proteasome-dependent manner, thereby inhibiting cellular proliferation.
Altogether, compared with RNA, the DNA oligomer is more stable and the DNA binding specificity of TFs is better defined than the RNA binding specificity of RBPs. Therefore, TF-targeting platforms expand the druggable target spectrum with therapeutic benefits for patients with cancer and other diseases.
G4-PROTACs
G-quadruplexes (G4s) are four-stranded nucleic acid structures of DNA or RNA rich in guanine bases that are enriched in gene promoter regions. Many G4s harbor physicochemical and structural properties that render them favorable for drug design. Transcriptional repression of pathogenic proteins through stabilization of G4 structures or telomerase inhibition by telomeric-G4s have been suggested as novel antitumor strategies; a first-in-class G4-interacting compound has reached phase II trials in neuroendrocine/carcinoid tumors (NCT00780663) [103]. Recently, Patil et al. reported the use of G4 as a PROTAC warhead to CRBN and VHL small molecule ligand, respectively, for targeted degradation of a G4-binding protein (RHAU/DHX36) in HeLA and K562cancer cell lines [104].
Aptamer-PROTACs
Aptamers are single-stranded DNA or RNA oligonucleotides that bind to the target protein with high specificity and affinity. They possess favorable in vivo safety profiles without potential immunogenicity, establishing them as targeted therapeutics in oncology [105,106,107,108]. Sheng and colleagues described a strategy for modifying PROTACs with an aptamer to overcome the limitations of conventional PROTACs such as cell type selectivity [109]. The first aptamer-PROTAC was designed by conjugating a BET-directed PROTAC to the nucleic acid aptamer AS1411 via a cleavable linker. Compared to the unmodified BET PROTAC, the designed molecule improved tumor targeting specificity, leading to enhanced in vivo BET degradation and antitumor potency in a breast cancer xenograft model, and reduced toxicity. Hence, this technology holds the promise to improve the drug-likeness of conventional PROTACs.
Recently, Tan and colleagues provided the first proof of concept evidence using nucleic acid aptamer as a targeting ligand [110]. The designed molecule ZL216 promoted the formation of nucleolin-ZL216-VHL ternary complex by using AS1411 as a ligand for binding to nucleolin, which potently eliminated nucleolin in breast cancer cells in vitro and in vivo, and inhibited proliferation and migration of breast cancer cells in vitro [110]. Although ZL216 confers enhanced water solubility and tumor-selective binding, the therapeutic potential and pharmacokinetic features in vivo require further evaluation.
2.1.4. Molecular Glues
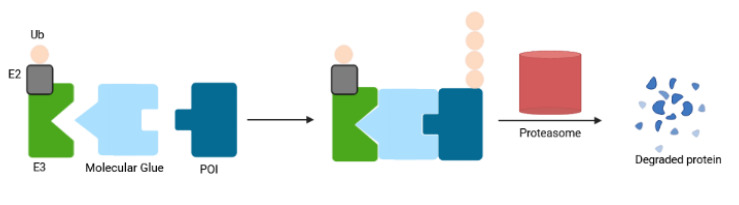
Molecular glue degraders are small, drug-like monovalent compounds that induce interactions between an E3 ligase and a target protein, promoting the degradation of the latter in a proteasome-dependent manner [111] (Figure 12). Unlike PROTACs, these molecules lack a linker within their structure, resulting in lower molecular weight. Molecular glues do not depend on a binding pocket on their target for action, making them suitable for depletion of undruggable proteins. The microbial products rapamycin, FK506 (tacrolimus) and cyclosporin A (sandimmune) with immunosuppressive properties are among the first molecular glues described [112,113]. Auxin represents another example for natural monovalent degraders. This pivotal phytohormone involved in plant growth and development mediates TIR1 ubiquitin ligase-catalyzed degradation of the AUX/IAA family of transcription repressors [114]. The auxin-inducible degron (AID) system enables its applicability in nonplant cells [115]. However, the need to express TIR1 and the enhanced risk of immunogenicity when engineered in mammalian cells limit its therapeutic potential.
Figure 12.
Schematic depiction of molecular glue degraders. These molecules acts as a protein-protein-interaction inducers to enhance or stimulate interactions between E3 ligase and the POI, thereby triggering ubiquitination-mediated proteasomal degradation of the POI. Ub: ubiquitin.
Similarly, the clinically approved thalidomide and its analogs known as immunomodulatory drugs (IMiDs) exert their therapeutic activity via a molecular glue mechanism by reprogramming the target spectrum of the E3 substrate receptor CRBN. IMiD binding to CRBN resulted in proteasomal depletion of the IKAROS family members IKZF1 (Ikaros) and IKZF3 (Aiolos) that are the lymphoid transcription factors crucial in myeloma cell survival [116,117,118]. Lenalidomide eliminated myelodysplastic syndrome cells with deletion of chromosome 5q by promoting the ubiquitination and degradation of CK1α [119]. Several cellular proteins have been unraveled to be potential neo-substrates of IMiDs, which may facilitate development of novel IMiDs [15,120,121]. IMiDs have been also modified to generate chemically inducible degradation systems [122,123,124]. When tagged with an IMiD degron, drug treatment induced rapid CRBN-dependent destruction without the need for an exogenous ubiquitin ligase, as is required by the AID system [122,123,124]. In vivo potency was demonstrated with the IKZF3 degrons [122,123]. This IMiD-dependent inducible system has been applied to control CAR-T cell therapy in mice. A mouse xenotransplant model for acute lymphoblastic leukemia verified the ability of a degron-tagged CAR to target and kill CD19-positive cells, triggering complete control/clearance of the tumor. The activity of CAR19-degron could also be regulated in vivo by dosing the clinically approved lenalidomide [122]. These results indicate that degron tagging will not only allow on/off switching of CAR-T activity, but also that in vivo fine tuning of CAR-T activity could be feasible by adjusting lenalidomide dosing or dose regimen over the course of CAR-T therapy. This strategy thus holds the promise to mitigate potential toxicities associated with CAR-T therapy as described above.
Similar to the IMiD pharmacology, the arylsulfonamide indisulam and its analogs E7820 and tasisulam function via a molecular glue mechanism to drive the degradation of the essential RNA-binding protein RBM39 and the closely related splicing factor RBM23 (because of the high sequence conversation between their RBM domains involved in indisulam-induced molecular recognition) by chemically reprogramming the substrate DCAF5 [125,126,127]. The majority of molecular glues have been discovered serendipitously for a given target. Recent studies report experimental strategies for a rational design of such compounds, revealing a series of novel β-catenin and cyclin K degraders among others [47,128,129,130].
Despite the therapeutic efficacy of drug-induced destruction of transcription factors and other cancer targets, a subset of proteins remain resistant to targeted degradation using existing approaches. An alternative mechanism has been described in which a small molecule induced the highly specific, reversible polymerization of a POI, followed by its sequestration into cellular foci and subsequent depletion [131]. The small molecule BI-3802 induced formation of BCL6 filaments which led to ubiquitination of BCL6, a transcriptional repressor critical for the tumorigenesis of germinal center B cells, by the E3 ubiquitin ligase SIAH1, thereby triggering its degradation [131]. Recently, another BCL6-degrader with tumoricidal activity in a lymphoma xenograft mouse model following oral dosing has been reported but the mode of degradation has not been deciphered [132].
Altogether, molecular glue degraders open highly appealing avenues for the development of antitumor therapeutic agents and synthetic biology.
2.1.5. SNIPERs
Specific and non-genetic inhibitors of apoptosis protein (IAP)-dependent protein erasers (SNIPERs) are chimeric molecules designed to induce IAP-mediated ubiquitination and proteasomal degradation of POI. They recruit the IAP family of RING-type E3 ubiquitin ligases—cIAP1, cIAP2 and XIAP with antiapoptotic properties. Cancer cells often overexpress IAPs to evade apoptosis with a concomitant increase in resistance to cancer therapy, making these proteins attractive drug targets. The chemical structure of a SNIPER consists of a selective IAP antagonist such as bestatin, MV1 and LCL161, a PEG linker and a peptide- or small-molecule-based POI specific component [133,134,135]. Unlike the chimeric molecules that recruit CRBN and VHL ligases, SNIPERs promote simultaneous destruction of IAPs along with the target protein, which may display synergistic effects on induction of apoptotic cell death [136]. Among the target proteins successfully depleted by SNIPERs at nanomolar concentrations are AR, ER, BCR-ABL, BRD4, Notch I and others [133,134].
2.2. Hijacking the Non-UPS
2.2.1. (Macro)autophagy Degradation Targeting Chimeras
The capacity of the dominating TPD platforms such as PROTAC is limited to soluble intracellular proteins because of their dependence on the UPS. Harnessing an alternative intracellular degradation mechanism such as macroautophagy may broaden the target spectrum including aggregated proteins, non-protein biomolecules, and organelles. The first autophagy-mediated degraders are chimeric molecules called AUTACs (Autophagy Targeting Chimeras), that consist of a S-guanylation-inspired degradation tag and a specific binder of an intracellular target of interest [137,138] (Figure 13a). Of note, S-guanylation is still dependent on K63-linked ubiquitination of the target. AUTAC degraded not only proteins but also fragmented mitochondria. Mitochondria-targeted AUTAC accelerated both the removal of dysfunctional fragmented mitochondria and the biogenesis of functionally normal mitochondria in Down Syndrome-derived fibroblast cells. The generality of the modality has been demonstrated by specific AUTACs against methionine aminopeptidase 2, FKBP prolyl isomerase 1A and BET family proteins followed by target clearance in HeLa cells. These data open a new window in research on autophagy-based agents with cargo specificity. Its applicability in cancer treatment requires further investigation.
Figure 13.
Concepts of degrader technologies hijacking autophagy pathways. (a) AUTACs bind to the POI and attach a degradation tag mimicking S-guanylation, a posttranslational modification that triggers K63 ubiquitination of the POI. The POI is recognized by the autophagy receptor SQSTM1/p62 and is recruited to the selective autophagy pathway for depletion. (b) ATTECs interact with both the POI and LC3, tethering the POI to the phagophores or autophagosomes for subsequent autophagic degradation. (c) The central mode of action in AUTOTAC is the ability of the p62-binding ligand to induce a conformational activation of otherwise inactive p62 into an autophagy-compatible version. Upon binding to the p62 moiety, p62 exposes PB1 and LIR domains, promoting p62 self-polymerization in complex with targets and its interaction with LC3 on autophagic membranes. (d) The CMA-based degrader first enters the cell, then binds the target protein via the POI binding sequence and is then transported to the lysosome for degradation. POI: protein of interest; Ub: ubiquitin.
A complementary approach was proposed by autophagy tethering compounds (ATTECs) to target non-protein biomolecules or relevant organelles [139,140]. These glues tether the POI with autophagosomes through direct binding to the POI and the key autophagosome-associated protein LC3 (Figure 13b). Li et al. have described that ATTECs against mutant huntingtin (mHTT) directed the protein to the autophagosome for clearance in vitro and in vivo, thereby rescuing phenotypes associated with Huntington’s disease [139]. The autophagy activity per se remained unchanged. A recent study reported autophagic degradation of lipid droplets (LD) via ATTEC targeting [140]. LDs are lipid-storing cellular structures, which are abnormally accumulated in many diseases. LD-ATTEC compounds were generated by connecting LC3-binding molecules to LD-binding probes via a linker. Their application resulted in almost complete clearance of LD and rescued LD-associated phenotypes in cells and in two independent mouse models with hepatic lipidosis. This study provides evidence that all autophagy substrates could be targeted for degradation by designed ATTECs.
Another chemical platform called AUTOphagy TArgeting Chimera (AUTOTAC) employs bifunctional molecules composed of target-binding ligands linked to autophagy-targeting ligands [141]. AUTOTACs bind the ZZ domain of the otherwise inactive autophagy receptor p62/Sequestosome-1/SQSTM1, which is activated into oligomeric bodies in complex with targets for their sequestration and degradation (Figure 13c). These chimeras were used to deplete a variety of oncoproteins and degradation-resistant aggregates in neurodegeneration at nanomolar concentrations in vitro and in vivo. AUTOTACs thus provide a direct tool to target not only the monomeric but also the oligomeric and aggregated species of the pathological hallmark proteins. There are several points to be fully investigated in future studies: (i) the off-target and selectivity features, (ii) how AUTOTACs can be recycled for multiple rounds of degradation, and (iii) whether they act catalytically and/or escape the lysosome.
Unlike AUTAC/ATTEC/AUTOTAC which utilize macroautophagy, CMA-based degraders harness chaperone-mediated autophagy (CMA) for protein degradation [142]. These molecules carry three functional groups: a cell membrane penetration sequence, a POI-binding sequence and a CMA-targeting motif (KFERQ) (Figure 13d). This strategy has been used to target numerous cytosolic proteins in neuronal cultures [143]. In a similar attempt, Xu and colleagues described that the polypeptide motif (MDFSGLSLIKLKKQ) on HIP1R (Huntingtin-interacting protein 1–related) possessed similar lysosomal targeting activity like the KFERQ motif and could be applied to lysosomal degradation of PD-L1 in cancer cells [144]. This discovery offers a novel path in immunotherapy. However, the low stability and delivery efficiency are among the obstacles for their druggability.
2.2.2. Harnessing Endolysosomal Pathways
The cytosolic localization of the UPS and targetable autophagy machinery restricts these approaches to POI with cytosolic domains and requires degraders to be cell permeable. To expand the scope of TPD to extracellular and membrane associated proteins that make up to 40% of all protein-encoding genes and involve growth factors, cytokines and other key agents in cancer and other diseases, a novel platform called LYTAC (LYsosome TArgeting Chimeras) has been developed (Figure 14a). These molecules bind and direct secreted and membrane proteins to lysosomes [145]. The first generation of LYTAC was composed of a target protein binder (a small molecule or an antibody) conjugated to a synthetic oligopeptide ligand, mannose-6-phosphonate (M6Pn). The first LYTACs used the cation-independent mannose-6-phosphonate receptor (CI-M6PR) as the endogenous lysosome-trafficking receptor. The M6Pn-LYTACs promoted internalization and lysosomal degradation of several therapeutically relevant proteins including apolipoprotein E4, epidermal growth factor receptor, CD71 and programmed death-ligand 1 by bridging them with CI-M6PR in HepG2 cells [145]. One drawback of this tool limiting its clinical applicability is that CI-M6PR is expressed in most tissues. To achieve a tissue-specific LYTAC activity, the second generation engaged the liver cell-specific asialoglycoprotein receptor (ASGPR) as the shuttling receptor [146]. An antibody or a small synthetic peptide was used as a binder linked to a triantenerrary N-acetylgalactosamine ligand that engaged ASGPR to drive the degradation of EGFR, HER2 and integrins, respectively, resulting in antiproliferative effects in hepatocellular carcinoma cells. These site-specific LYTACs improved pharmacokinetics in vivo. In a parallel study, Tang and colleagues similarly utilized ASGPR as the internalizing receptor for lysosomal induced degradation of several protein targets [147]. These reports hence establish LYTACs as a protein degradation modality with the ability to restrict degradation to a specific cell type expressing a given lysosome-targeting receptor.
Figure 14.
Concepts of degrader technologies hijacking lysosomal pathway. (a) LYTACs utilize a glycan tag to mark an extracellular POI for intracellular lysosomal degradation following shuttling receptor-mediated internalization. (b) Mechanism of action of MoDE-A bifunctional molecules. MoDE-A brings the target protein to ASGPR on hepatocytes for lysosomal degradation. (c) Bispecific aptamer chimeras use DNA aptamers to target CI-M6PR and transmembrane POI. (d) AbTAC recruits RNF43 to internalize cell surface proteins. (e) GlueTAC consists of a single-domain antibody, a cell-penetrating peptide and a lysosome-sorting sequence. The single-domain antibody is responsible for targeting POI and CPP-induced endocytosis of the complex followed by lysosomal degradation. ASGPR: asialoglycoprotein receptor; CI-M6PR: cation-independent mannose-6-phosphonate receptor; CPP-LSS: cell-penetrating peptide and lysosome-sorting sequence; LTR: lysosomal targeted receptor; POI: protein of interest; RNF43: RING finger protein 43.
In a similar attempt, Caianiello et al. developed a class of modular, bifunctional synthetic molecules termed MoDE-As (molecular degraders of extracellular proteins through the ASGPR) to drive the degradation of extracellular proteins [148]. MoDE-A molecules bridge POI to ASGPR on liver cells for endocytosis and lysosomal degradation (Figure 14b). MoDE-A induced depletion of both antibody and proinflammatory cytokine proteins in vitro and in vivo. MoDE-As possess several advantages compared to other TPD platforms: they are relatively small in size, monodispersed and nonprotein based. ASGPR is immunologically tolerogenic, reducing the likeliness of autoimmune responses to targeted proteins.
Recently, Han and colleagues constructed bispecific aptamer chimeras (BIAC) for lysosomal degradation of targeted membrane-associated proteins such as mesenchymal epithelial transition (Met) receptor and membrane receptor tyrosine protein kinase-like 7 (PTK-7), both known therapeutic cancer targets [149] (Figure 14c).
Wells and colleagues reported the development of an antibody-based PROTAC (AbTAC) that recruits membrane-bound E3 ligase RNF43 for the depletion of the cell-surface immune checkpoint protein programmed death-ligand 1 (PD-L1) [150] (Figure 14d). The event occurred in a lysosomal-dependent manner: incubation with bafilomycin, a lysosome acidification inhibitor, mitigated the degradation of PD-L1, whereas the proteasome inhibitor MG-132 did not. The lead AbTac AC-1 is a fully recombinant bispecific IgG, allowing not only for its rapid and renewable generation, but also for simple optimization of binding properties. It is built of human parts, limiting the chances to evoke an immune response. Thus, the technology holds the promise to expand the PROTAC field to target challenging membrane proteins. Another antibody-derived PROTAC strategy has been described by Zhang et al. [151] (Figure 14e). By conjugating with a cell-penetrating peptide and a lysosomal-sorting sequence, the resulting GlueTAC promoted the internalization and degradation of programmed death-ligand 1 (PD-L1) in vitro and in vivo, leading to sustained T cell activation and attenuated tumor growth in mice. This data provides an additional angle to degrade cell surface proteins.
2.2.3. RIBOTACs
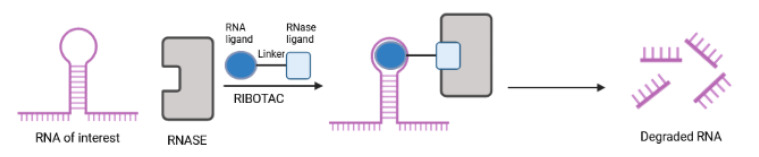
Another new strategy to combat cancer has been proposed through RIBOnuclease TArgeting Chimeras (RIBOTACs), which use RNA-targeting small molecules and RNase L, an otherwise latent ribonuclease, to accomplish the degradation of intracellular oncogenic RNAs (Figure 15). The first demonstration of a RIBOTAC as a potential cancer therapy involved selective cleavage of the miR-96 precursor in cancer cells in a catalytic and substoichiometric fashion [152]. Silenced miR-96 derepressed pro-apoptotic FOXO1 transcription factor, triggering apoptosis in breast cancer, but not in healthy breast cells [153]. Another small molecule called Targapremir-210 has been described to tackle triple negative breast cancer [154]. Here, silenced miR-210, an essential microRNA for cancer survival in hypoxic niches, derepressed glycerol-3-phosphate dehydrogenase 1-like enzyme (GPD1L), a hypoxia-associated protein, decreased HIF-1α, and triggered apoptotic cell death of diseased cells (MDA-MB-231) only under hypoxic conditions that are critical to the metastatic and invasive characteristics of cancer. In line with this, the module impaired the metastatic nature of these cells. Antiproliferative effects of Targapremir-210 could be reflected in a mouse xenograft model of hypoxic triple negative breast cancer. Compared to oligonucleotide-based/occupancy-based therapeutics, RIBOTACs offer many advantages [155]: (i) more favorable pharmacokinetic properties; (ii) catalytic nature (a RIBOTAC can recycle to another RNA); and (iii) low concentrations. Major drawbacks include challenging and time-consuming steps in design and development as well as slow cellular uptake due to high molecular weights [155]. Nevertheless, efforts to develop RIBOTACs for other disease-relevant RNAs that affect cellular responses to environmental conditions are anticipated in the near future.
Figure 15.
Schematic depiction of RIBOnuclease TArgeting Chimeras. The bivalent RIBOTAC molecules consist of an RNA-binding ligand (dark blue) and a ribonuclease (RNase) recruitment ligand (light blue) joined by a linker (black line). Upon binding a target RNA, RIBOTACs recruit an RNase in close proximity of the target, thereby promoting its destruction.
3. Clinical Advances of Chemical Degraders in Oncology
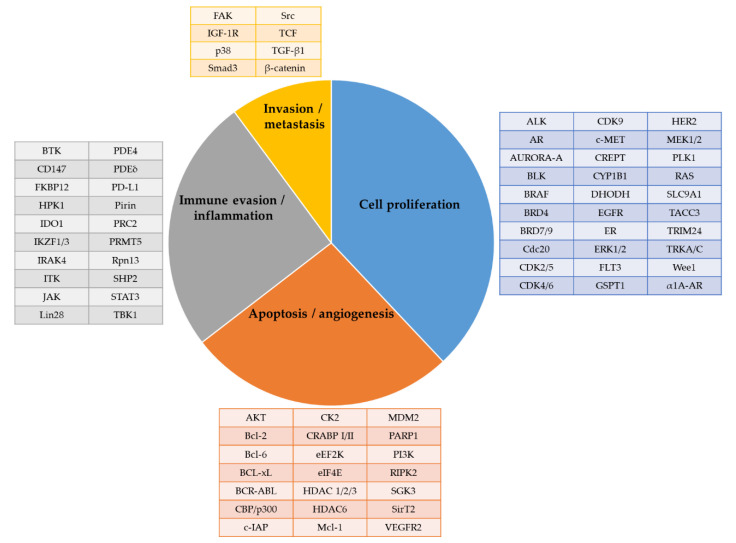
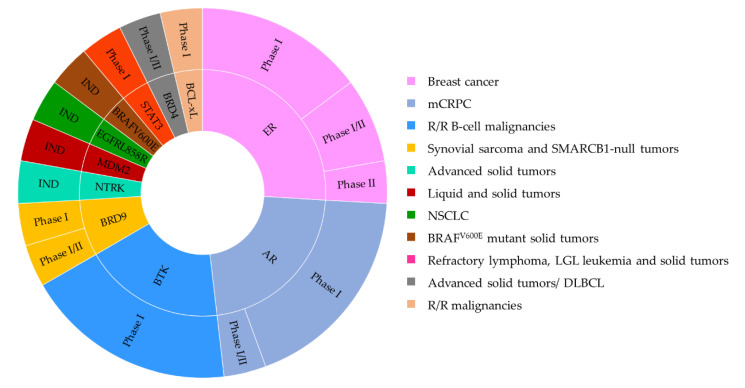
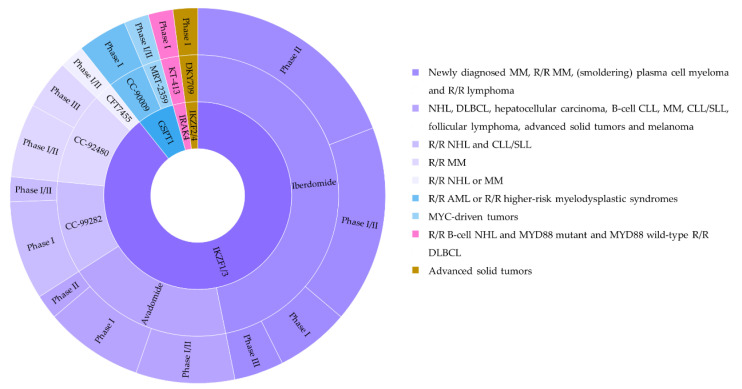
The first wave of protein degraders has focused on the oncology fields. The target proteins can be categorized into the following groups: (i) those involved in cancer cell proliferation; (ii) in apoptosis; (iii) in angiogenesis; (iv) in immune evasion or inflammation; and (v) in cancer invasion and metastasis (Figure 16) [50,51,156,157]. Among those, several have progressed into clinical activities for multiple disease indications (Table 3 and Table 4).
Figure 16.
Summary of protein degradation target candidates involved in hallmarks of cancer. ALK: anaplastic lymphoma kinase; AR: androgen receptor; Bcl: B-cell lymphoma; BCL-xL: B-cell lymphoma-extra large; BRAF: v-raf murine sarcoma viral oncogene homolog B1; BRD: bromodomain-containing protein; BTK: Bruton’s tyrosine kinase; Cdc20: cell division cycle 20; CDK: cyclin-dependent kinase; cIAP: cellular inhibitor of apoptosis; CK2: casein kinase 2; CRABP I/II: cellular retinoic acid-binding protein 1/2; CREPT: cell cycle related and expression elevated protein in tumor; CYP1B1: cytochrome p450 family 1 subfamily B member 1; DHODH: dihydroorotate dehydrogenase; eEF2K: eukaryotic elongation factor 2 kinase; EGFR: epidermal growth factor receptor; eIF4E: eukaryotic initiating factor 4E; ER: estrogen receptor; ERK: extracellular signal-regulated kinase; FAK: focal adhesion kinase; FKBP: FK506-binding proteins; FLT3: fms-like tyrosine kinase 3; GSPT1: G1 to S phase transition 1; HDAC: histone deacetylase; HER: human epidermal growth factor receptor; HPK1: hematopoietic progenitor kinase 1; IDO1: indoleamine 2,3-dioxygenase 1; IGF-1R: insulin-like growth factor 1 receptor; IKZF: IKAROS zinc finger family; IRAK4: interleukin-1 receptor-associated kinase 4; ITK: interleukin-2-inducible T-cell kinase; JAK: janus kinase; Mcl-1: myeloid cell leukemia-1; MDM2: mouse double minute 2; MEK: mitogen-activated protein kinase kinase; PARP1: poly [ADP-ribose] polymerase 1; PDE: phosphodiesterase; PD-L1: programmed death-ligand 1; PI3K: phosphoinositide 3-kinases; PLK1: polo-like kinase 1; PRC: polycomb repressive complex; PRMT5: protein arginine methyltransferase 5; RAS: rat sarcoma; RIPK2: receptor interacting serine/threonine kinase 2; SGK3: serum/Glucocorticoid regulated kinase family member 3; SHP2: src homology 2 domain containing protein tyrosine phosphatase-2; SirT2: Sirtuin 2; SLC9A1: solute carrier family 9 member A1; STAT3: signal transducer and activator of transcription 3; TACC3: transforming acidic coiled-coil containing protein 3; TBK1: TANK-binding kinase 1; TCF: T-cell factor; TGF-β1: transforming growth factor beta 1; TRIM24: tripartite motif containing 24; TRKA/C: tropomyosin receptor kinase A/C; VEGFR: vascular endothelial growth factor receptor; α1A-AR: alpha-1A adrenergic receptor.
Table 3.
Summary of PROTACs under clinical evaluation for cancer therapy. Yellow: recruiting; violet: not yet recruiting. AR: androgen receptor; BCL-xL: B-cell lymphoma-extra large; BRAF: v-raf murine sarcoma viral oncogene homolog B1; BRD: bromodomain-containing protein; BTK: Bruton’s tyrosine kinase; DLBCL: diffuse large B cell lymphoma; EGFR: epidermal growth factor receptor; ER: estrogen receptor; IND: investigational new drug; LGL: large granular lymphocyte; mCRPC: metastatic castration-resistant prostate cancer; MDM2: mouse double minute 2; NSCLC: non-small cell lung cancer; R/R: relapsed/refractory; STAT3: signal transducer and activator of transcription 3; NTRK: neurotrophic tyrosine receptor kinase; 2H2022: Second half of the year 2022.
| Time | Degrader | Target | Indication | NCT Number | Phase |
|---|---|---|---|---|---|
| 2019 2022 |
ARV-110 | AR | mCRPC |
NCT03888612
NCT05177042 |
Phase I/II Phase I |
| 2021 | ARV-766 | AR | mCRPC | NCT05067140 | Phase I |
| 2020 | CC-94676 | AR | mCRPC | NCT04428788 | Phase I |
| 2022 | HP518 | AR | mCRPC | NCT05252364 | Phase I |
| 2022 | AC176 | AR | mCRPC | NCT05241613 | Phase I |
| 2021 | DT2216 | BCL-xL | R/R malignancies | NCT04886622 | Phase I |
| 2022 | RNK05047 | BRD4 | Advanced solid tumors/ DLBCL | NCT05487170 | Phase I/II |
| 2022 | CFT8634 | BRD9 | Synovial sarcoma and SMARCB1-null tumors | NCT05355753 | Phase I/II |
| 2021 | FHD-609 | BRD9 | Advanced synovial sarcoma or advanced SMARCB1-null tumors | NCT04965753 | Phase I |
| 2021 | NX-2127 | BTK | R/R B-cell malignancies | NCT04830137 | Phase I |
| 2021 | NX-5948 | BTK | R/R B-cell malignancies | NCT05131022 | Phase I |
| 2021 2022 |
BGB-16673 | BTK | B-cell malignancies |
NCT05006716
NCT05294731 |
Phase I Phase I |
| 2021 | HSK29116 | BTK | R/R B-cell malignancies | NCT04861779 | Phase I |
| 2019 2022 2022 2022 2022 |
ARV-471 | ER | ER+/HER2- locally advanced or metastatic breast cancer |
NCT04072952
NCT05501769 NCT05463952 NCT05549505 NCT05548127 |
Phase I/II Phase I Phase I Phase II Phase I/II |
| 2021 2022 |
AC682 | ER | ER+/HER2- locally advanced or metastatic breast cancer |
NCT05080842
NCT05489679 |
Phase I Phase I |
| 2022 | KT-333 | STAT3 | Refractory lymphoma, LGL leukemia and solid tumors | NCT05225584 | Phase I |
| 2022 | CFT1946 | BRAFV600E | BRAF-V600E mutant solid tumors | IND | |
| Planned for (2H2022) | CFT8919 | EGFRL858R | NSCLC | IND | |
| Planned for (2H2022) | KT-253 | MDM2 | Liquid and solid tumors | IND | |
| 2022 | CG001419 | NTRK | Advanced solid tumors | IND |
Table 4.
Summary of molecular glues under clinical evaluation for cancer therapy. Red: active, not recruiting; yellow: recruiting; blue: completed; violet: not yet recruiting. AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B cell lymphoma; GSPT1: G1 to S phase transition 1; IKZF: IKAROS zinc finger family; IRAK4: interleukin-1 receptor-associated kinase 4; MM: multiple myeloma; MyD88: myeloid differentiation primary response 88; NHL: non-Hodgkin’s lymphoma; NSCLC: non-small cell lung cancer; R/R: relapsed/refractory; SCLC: small cell lung cancer; SLL: small lymphocytic lymphoma.
| Time | Degrader | Target | Indication | NCT Number | Phase |
|---|---|---|---|---|---|
| 2011 2014 2015 2015 2015 2016 2017 2017 2017 |
Avadomide | IKZF1/3 | NHL, DLBCL, hepatocellular carcinoma, B-cell CLL, MM, CLL/SLL, follicular lymphoma, advanced solid tumors and melanoma |
NCT01421524
NCT02031419 NCT02417285 NCT02406742 NCT02509039 NCT02859324 NCT03310619 NCT03283202 NCT03834623 |
Phase I Phase I Phase I Phase I/II Phase I Phase I/II Phase I/II Phase I/II Phase II |
| 2016 2020 |
CC-90009 | GSPT1 | R/R AML or R/R higher-risk myelodysplastic syndromes |
NCT02848001
NCT04336982 |
Phase I Phase I |
| 2017 2019 2022 2022 2022 |
CC-92480 | IKZF1/3 | R/R MM |
NCT03374085
NCT03989414 NCT05372354 NCT05519085 NCT05552976 |
Phase I/II Phase I/II Phase I/II Phase III Phase III |
| 2017 2019 2020 2021 2021 |
CC-99282 | IKZF1/3 | R/R NHL and CLL/SLL |
NCT03310619
NCT03930953 NCT04434196 NCT04884035 NCT05169515 |
Phase I/II Phase I Phase I Phase I Phase I |
| 2021 | CFT7455 | IKZF1/3 | R/R NHL or MM | NCT04756726 | Phase I/II |
| 2019 | DKY709 | IKZF2/4 | Advanced solid tumors | NCT03891953 | Phase I |
| 2016 2017 2020 2020 2020 2021 2021 2021 2021 2021 2021 2021 2022 2022 2022 2022 2022 2022 2022 2022 2022 2022 |
Iberdomide | IKZF1/3 | Newly diagnosed MM, R/R MM, (smoldering) plasma cell myeloma and R/R lymphoma |
NCT02773030
NCT03310619 NCT04392037 NCT04464798 NCT04564703 NCT04776395 NCT04855136 NCT04998786 NCT04884035 NCT04934475 NCT04975997 NCT05169515 NCT05177536 NCT05199311 NCT05272826 NCT05289492 NCT05392946 NCT05354557 NCT05434689 NCT05527340 NCT05558319 NCT05560399 |
Phase I/II Phase I/II Phase II Phase I/II Phase II Phase II Phase I/II Phase II Phase I Phase III Phase III Phase I Phase II Phase I/II Phase II Phase I/II Phase I/II Phase II Phase I/II Phase II Phase II Phase I |
| 2022 | KT-413 | IRAK4 | R/R B-cell NHL and MYD88 mutant and MYD88 wild-type R/R DLBCL | NCT05233033 | Phase I |
| 2022 | MRT-2359 | GSPT1 | NSCLC, SCLC, high-grade neuroendocrine cancer of any primary site, DLBCL and tumors with L-MYC or N-MYC amplification | NCT05546268 | Phase I/II |
3.1. PROTAC-Based Clinical Trials
3.1.1. AR PROTAC
The androgen receptor (AR) is a key driver of castration-resistant prostate cancer during the transition from a localized to a metastatic disease. Targeting the AR signaling axis with abiraterone, enzalutamide, darolutamide and apalutamide has demonstrated an overall survival benefit in the castration-sensitive state [158]. However, the response is short-lived; the disease remains invariably fatal. The majority of patients progressing on enzalutamide or abiraterone exhibit genetic alterations in the AR locus, either in the form of amplifications or point mutations in the AR gene [159,160]. Thus, destroying AR—and not simply inhibiting it—may change the treatment paradigm for this lethal disease.
ARV-110 (Bavdegalutamide) is the first-in-class PROTAC against AR. The treatment led to near-complete clearance of AR and suppressed AR-associated gene expression in vivo. ARV-110 inhibited the synthesis of prostate-specific antigen (PSA) and AR-dependent cancer cell proliferation by inducing apoptotic cell death. It also exhibited activity in enzalutamide refractory/resistant prostate cancer xenograft models with AR amplification and mutations with the exception of ARL702H mutation and AR-V7 variance [159,160,161]. In phase I trials, patients with metastatic castration-resistant prostate cancer (mCRPC) who showed progression following at least two prior therapies (enzalutamide and/or abiraterone) received ARV-110 orally once or twice daily in sequential cohorts. Enhanced activity was observed in patients with specific molecular profiles including ART878 and ARH875 mutations [162]. In phase II trials to assess antitumor activity, patients with mCRPC and ≥1 prior novel hormonal agent and/or chemotherapy were divided into 3 biomarker-specific groups: (i) ART878 and/or ARH875 mutations; (ii) ARL702H mutation or AR-V7; and (iii) wild-type AR or other AR alterations. A fourth subgroup involved patients based with a clinical history of less prior treatment strategy: ≤1 therapy for mCRPC, 1 novel hormonal agent, and no chemotherapy [162]. ARV-110 was applied at 420 mg once daily. The best PSA declines were achieved in patients with ART878A/S and/or ARH875Y mutations. Of 7 RECIST (Response Evaluation Criteria in Solid Tumors)-evaluable patients with ART878A/S and/or ARH875Y mutations, 6 exhibited tumor shrinkage. Among the most common treatment-related adverse events were nausea, fatigue, vomiting, decreased appetite, diarrhea and alopecia; none were grade ≥4 (NCT03888612). In a parallel study, the combination of ARV-110 with abiraterone will be assessed in patients with metastatic prostate cancer with PSA increase on abiraterone (NCT05177042). ARV-110 oral tablets in combination with abiraterone and a corticosteroid will be administered daily in 28-day cycles. ARV-766 is another oral AR PROTAC with a different profile than ARV-110 [163]. A phase I clinical trial is underway to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics in patients with mCRPC. The compound will be given once or twice daily in escalating doses (NCT05067140). Another phase I trial employs the oral PROTAC CC-94676 (AR-LDD) in patients with mCRPC who progressed on androgen deprivation therapy and at least one prior secondary hormonal therapy approved for CRPC (NCT04428788). CC-94676 displays similar preclinical activity as ARV-110 in regard to potent AR protein degradation, favorable pharmacokinetic properties, and sustained suppression of tumor growth in VCaP CRPC mouse models [161]. Yet it was not compared directly with ARV-110 for tumoricidal activity in a clinical setting. HP518, an oral PROTAC, has high activity of degrading wild-type AR and variant AR resistant to enzalutamide. An open-label study will evaluate its pharmacokinetics, safety and antitumor activity in patients with mCRPC. Participant are enrolled based on the following criteria: (i) metastatic disease at study entry proven by ≥2 bone lesions on bone scan or by soft tissue disease observed by CT/MRI; (ii) disease progression while receiving any androgen deprivation therapy, androgen biosynthesis inhibitors or second-generation AR inhibitors; (iii) recovery from toxicities related to any prior treatments; and (iv) ongoing androgen deprivation therapy with LHRH agonist/antagonist therapy or history of bilateral orchiectomy (NCT05252364). A further clinical trial in phase I is under way to evaluate the orally bioavailable AC176 in patients with mCRPC who have progressed on ≥2 prior systemic therapies (NCT05241613). In addition, a PROTAC degrader targeting AR-V7 splice variant and full-length AR is in development as a potential CRPC therapy.
3.1.2. ER PROTAC
Around 80% of all newly diagnosed cases of breast cancer are estrogen receptor positive (ER+). While approved treatments have achieved great success in this patient population, many ER+ breast cancers develop resistance to therapy [164]. Fulvestrant, a selective estrogen receptor degrader (SERD), is the standard of care for ER+ metastatic breast cancer following anti-estrogen therapy. Even though fulvestrant has verified the importance of ER degradation as a therapeutic intervention, up to 50% of ER can remain when compared to baseline levels after six months of treatment. Several ER-directed PROTAC degraders are being tested in preclinical and/or clinical settings [165,166,167,168]. Unlike fulvestrant, which is administered via intramuscular injection, the ER-directed PROTAC ARV-471 is an oral therapy for women with ER+ metastatic breast cancer. In preclinical studies, ARV-471 robustly degraded ER in ER+ breast cancer cell lines, suppressed expression of ER-target genes (PR, GREB1, TFF) and inhibited cell proliferation of ER-dependent cell lines (MCF7, T47D) [167]. ARV-471 also affected clinically relevant ESR1 variants (Y537S and D538G) and suppressed growth of cell lines expressing those variants. ARV-471 showed in vivo activity in immature rat uterotrophic model, MCF7/E2 xenograft model, tamoxifen-resistant MCF7 mouse model, and ESR1 Y537S PDX model. ARV-471 exhibited improved in vivo activity compared to fulvestrant, which was further augmented by its combination with CDK4/6 inhibitors such as palbociclib.
Several clinical trials with ARV-471 are underway. A phase I, open-label study will recruit Japanese patients with ER+/HER2- (human epidermal growth factor 2 negative) locally advanced or metastatic breast cancer to investigate the safety, tolerability and pharmacokinetics of ARV-471 (NCT05463952). The drug will be administered orally once daily with food in continuous dosing over 28-day cycles. A phase II neoadjuvant study will evaluate ARV-471 or anastrozole, a nonsteroidal aromatase inhibitor, in post-menopausal women with ER+/HER2- localized breast cancer amenable to surgical resection to address the biological activity of respective compound (NCT05549505). ARV-471 will be administered once daily and 1 mg anastrozole will be given once daily until surgery, which will take place approximately 5.5 months after starting treatment. The safety, tolerability and clinical activity of ARV-471 alone or in combination with palbociclib are addressed in the ongoing dose escalation and cohort expansion study in patients with ER+/HER2- advanced or metastatic breast cancer following prior chemotherapy or hormonal therapy (NCT04072952). The phase I part utilized traditional 3 + 3 dose escalation with ARV-471 oral tablets once daily for 28-day cycles in 21 heavily pre-treated patients with poorer prognosis. 48% of patients had visceral metastatic disease often in the liver and lung. All patients received previous CDK4/6 inhibitors; 71% of patients received fulvestrant; 38% of patients had chemotherapy; and 24% of patients had other selective ER degraders in the clinical trial setting. They had a median of 4 previous lines of therapy for their advanced or metastatic breast cancer. ARV-471 was well tolerated, the highest dose being 360 mg, and no grade 3/4 adverse events were observed. In paired tumor biopsy samples before and after treatment with ARV-471, mean ER degradation was significantly higher than with fulvestrant and has been observed in patients with either wild-type ER or mutant ERY537S, ERY537N, and ERD538G in their tumors. A further study is recruiting to assess the combination of ARV-471 and everolimus, an mTOR inhibitor, in patients with advanced or metastatic ER+/HER2- breast cancer who have received a prior CDK4/6 inhibitor and endocrine therapy in the advanced/metastatic setting (NCT05501769). Post-menopausal women or pre-/peri-menopausal women with ovarian suppression will receive ARV-471 oral tablets in combination with everolimus administered daily in 28-day cycles. A new sub-study aims to evaluate the safety and effects of ARV-471 when given together with the CDK4/6 inhibitor abemaciclib for the treatment of advanced or metastatic breast cancer (NCT05548127). The participants are enrolled based on the following criteria: (i) tumor is advanced or metastatic and cannot be fully treated by surgery or radiation therapy; (ii) tumor is sensitive to hormonal therapy; and (iii) tumor is nonresponsive to previous treatments. ARV-471 will be administered orally once daily, while abemaciclib will be given orally twice a day. The medication will continue until the tumors are not responding or side effects become too severe.
Other phase I clinical trials focus on AC682, an orally available ER degrader given as a single agent in 28-day cycles to address safety/tolerability, pharmacokinetics and antineoplastic activity in patients with ER+/HER2− locally advanced or metastatic breast cancer (NCT05080842, NCT05489679). These studies either involve post-menopausal patients with life expectancy ≥3 months (NCT05080842) or those with ≥1 prior endocrine therapy regimen, concomitant use of CDK4/6 inhibitor being allowed (NCT05489679).
3.1.3. BTK PROTAC
Bruton’s tyrosine kinase (BTK) plays a crucial role in B cell development, differentiation and signaling. BTK is closely associated with chronic B-cell receptor (BCR) activation, and is critical for the survival of B-cell neoplasms. Ibrutinib, a first-in-class covalent BTK inhibitor, has been approved for the treatment of several types of Non-Hodgkin’s lymphoma, specifically relapsed/refractory mantle cell lymphoma, chronic lymphocytic leukemia and Waldenström macroglobulinaemia. However, resistance eventually develops, highlighting the dire need for other strategies.
NX-2127 is not only a BTK PROTAC but also a molecular glue for IKZF1/3 degradation due to its cereblon binding activity to recruit ubiquitin ligase complex for BTK degradation [169]. The compound efficiently depleted not only wild-type (ibrutinib-sensitive) but also mutant BTK (ibrutinib-resistant BTK-C481S) with antitumor activity in vitro and in vivo. Major off-targets of ibrutinib including ITK, EGFR, and TEC were not affected upon PROTAC administration. The clinical safety and activity of NX-2127 are being evaluated in an ongoing phase 1a/1b study in adults with advanced B-cell malignancies who have received ≥ two prior lines of therapy (or one for patients with Waldenström macroglobulinaemia) and for whom no other therapies are known for clinical benefit (NCT04830137). A patient with R/R DLBCL (who had 4 prior lines of therapy) receiving 300 mg daily of NX-2128 experienced a complete response at 8 weeks which was confirmed at 16 weeks and remains ongoing [170]. The complete response included dramatic reductions in lymph node size and resolution of abnormal metabolic activity to background levels. The clinical response was assigned to degradation of BTK and IKZF1/3. In a similar vein, a dose escalation and cohort expansion study is recruiting participants to assess the safety and preliminary efficacy of the oral BTK PROTAC NX-5948 for treatment of advanced B-cell malignancies (NCT05131022). Further dose escalation and expansion trials of the oral degrader BGB-16673 are underway in patients with B-cell malignancies (NCT05006716, NCT05294731). HSK29116, another oral degrader, is currently being evaluated in a phase 1a/1b multi-center study to assess its safety and antineoplastic activity in patients with relapsed/refractory B-cell malignancies (NCT04861779).
3.1.4. BRD4 PROTAC
BRD4, a member of the bromo- and extra-terminal (BET) family of proteins, recruits transcriptional regulatory complexes to acetylated chromatin, thereby controlling specific networks of genes critical for proliferation and cell cycle progression [171]. Alterations in regulation of BRD4 activities have been allied with cancer and inflammatory diseases. The catalytic role of BRD proteins in transcription led to the development of small-molecule inhibitors against BRDs. JQ1, the first BRD inhibitor reported [172], marks a success story of BRD4 as a novel therapeutic vulnerability, and paved the way for several BRD inhibitors with some being under clinical evaluation [171,173,174,175,176,177,178,179]. However, early phase clinical trials show only modest clinical activity as single agents in patients with advanced cancer [171,179]. This can be explained by the assumption that small molecule BRD inhibitors may only block their chromatin binding function but spare other functional domains [171]. This highlights the unmet need for more effective strategies to treat diseases with BRDs as their Achilles’ heel. To date several structurally different BRD4-directed PROTAC degraders have been described [109,180,181,182,183,184,185,186,187,188,189,190,191,192]. RNK05047 is a BRD4 degrader in a phase I/II study in patients with advanced solid tumors including diffuse large B-cell lymphoma (NCT05487170) [184]. The compound will be administered intravenously to assess its safety, tolerability, pharmacokinetics, pharmacodynamics and clinical activity.
3.1.5. BRD9 PROTAC
Bromodomain-containing protein 9 (BRD9), a specific component of the non-canonical mammalian SWI/SNF chromatin remodelling complex, is essential to maintain the transformed phenotype of acute myeloid leukemia and the growth of synovial sarcoma [161,193]. This makes BRD9 protein an appealing drug target. However, BRD9 has been considered undruggable using currently available modalities: patients with advanced synovial sarcoma benefit from very limited therapeutic options with median overall survival of only 18 months. CFT8634 is an orally bioavailable degrader against BRD9 for the treatment of synovial sarcoma and SMARCB1-deleted solid malignancies [194]. It is superior to existing BRD inhibitors due to high specificity towards BRD9 over BRD4/7 and improved toxicity profile. CFT8634 demonstrated potent antitumor activity in both cell line and patient-derived xenograft models of synovial sarcoma when administered orally [194]. The safety, tolerability, pharmacokinetics, pharmacodynamics, and tumoricidal activity of CFT8634 are assessed in an ongoing phase 1/2 study in patients with locally advanced or metastatic SMARCB1-perturbed cancers, including synovial sarcoma and SMARCB1-null tumors with unresectable or metastatic disease, who received ≥1 prior line of standard of care systemic therapy and for whom no other therapies are known to confer clinical benefit (NCT05355753). FHD-609 is another BRD9 PROTAC that is currently under clinical evaluation in a phase I dose escalation and expansion study in patients with advanced synovial sarcoma (NCT04965753). Unlike the oral CFT8634, FHD-609 is given intravenously every 2 weeks. Initial clinical data from 2 patients showed degradation of BRD9 in on-treatment metastatic tumor biopsies.
3.1.6. STAT3 PROTAC
STAT3, a protein that has been historically undruggable, is a transcriptional regulator allied with numerous cancers and other inflammatory and autoimmune disorders. Under steady state conditions, the STAT3 protein is activated by cytokines and growth factors, thereby triggering transcriptional regulation of many cellular functions, while under stress conditions such as cancer, STAT3 activity becomes dysregulated, resulting in persistent activation of STAT3, which is associated with poor prognosis. Selective degraders targeting STAT3 thus have the potential to provide an intriguing solution to address multiple STAT3-dependent pathologies. KT-333 is a first-in-class PROTAC of STAT3. The FDA has granted orphan drug designation to KT-333 for the treatment of peripheral T-cell lymphoma, a disease entity with dysregulated STAT3 for which no approved therapies are available [195]. An open-label study enrolls adults with refractory lymphoma, large granular lymphocytic leukemia and solid tumors to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of KT-333 when administered intravenously weekly in 28-day cycles (NCT05225584).
3.1.7. BCL-xL PROTAC
B-cell lymphoma extra large (BCL-xL) is a well-validated cancer target. However, the on-target and dose-limiting thrombocytopenia limits the use of BCL-XL inhibitors, such as orally bioavailable ABT263, as safe and effective anticancer agents. DT2216 is a selective BCL-xL degrader utilizing ABT263 and a VHL E3 ligase binder [196]. DT2216 demonstrated superior potency against various BCL-xL-dependent leukemia and cancer cells but considerably less toxicity to platelets than ABT263 in vitro due to the lack of significant VHL expression in platelets. Anticancer activity was verified in several xenograft tumors as a single agent (MOLT-4 T-ALL xenograft) or in combination with other therapeutic agents (H146 SCLC xenograft, MDA-MB231 breast cancer xenograft, and T-ALL PDX models), without causing significant thrombocytopenia. DT2216 selectively killed various BCL-xL-dependent T-cell lymphomas (TCL) cells including MyLa cells in vitro [197]. DT2216 alone was highly effective against MyLa TCL xenografts in mice without causing appreciable thrombocytopenia or other toxicity. Furthermore, DT2216 combined with ABT199, a selective Bcl-2 inhibitor, synergistically reduced disease burden and improved survival in a TCL PDX mouse model dependent on both Bcl-2 and BCL-xL. A recent study reported that BCL-xL is highly expressed within the tumor-infiltrating (TI)-Treg population from renal cell carcinoma and several other human cancers. Proteasomal degradation of BCL-xL using two independent PROTACs (DT2166 and PZ15227 with a CRBN E3 ligase ligand) induced apoptosis of TI-Tregs and the activation of TI-CD8+ T cells [198]. These activities resulted in a potent suppression of syngeneic tumor growth with no detectable damage within several normal tissues or thrombocytopenia in immunocompetent, but not in immunodeficient or CD8+ T cell-depleted mice. Thus, BCL-xL destruction represents a novel avenue for cancer immunotherapy. Based on these promising preclinical findings, DT2216 is under clinical evaluation in a dose escalation and cohort expansion study for its safety, tolerability, and activity in patients with relapse and refractory solid tumors and hematologic malignancies when administered intravenously in 28-day cycles (NCT04886622).
3.1.8. Other PROTACs
CFT1946 is an orally bioavailable mutant selective degrader of BRAFV600X, which accounts for approximately 50,000 cancer diagnoses annually, to offer more durable responses. The bifunctional CFT1946 received “the study may proceed letter” from the FDA to initiate a phase I/II clinical study in patients with BRAFV600 mutant solid cancers including lung, colorectal and melanoma [199]. The trial initiation is expected by the end of 2022. CFT1946 is active in vitro and in vivo in models with BRAFV600E-driven disease and in the escape mutant BRAFV600E/NRASQ61K-driven model [200]. In preclinical settings, BRAFV600E degradation by CFT1946 caused loss of MAPK signaling superior to inhibition alone and attenuated viability of BRAFV600E cells but not wild-type BRAF cells. Another PROTAC which has been approved for the investigational new drug (IND) application is the world’s first-in-class NTRK degrader (neurotrophic tyrosine receptor kinase; CG001419) for the treatment of advanced solid tumors [201]. One PROTAC that is in late preclinical development and planned for an IND application submission is CFT8919, an orally bioavailable mutant selective degrader of EGFRL858R in non-small cell lung cancer [202]. Preclinical data show that CFT8919 induced tumor regression in mouse models resistant to first and third generation EGFR inhibitors. CFT8919 also exhibited intracranial activity, reducing tumor burden in a brain metastasis model. This indicates its potential to be active in the central nervous system. A further molecule planned to be filed for an IND application in late 2022 is KT-253, a first-in-class MDM2 degrader. Degradation of MDM2, rather than inhibition, has the ability to block the feedback loop which upregulates MDM2 production and effectively drives MDM2-dependent tumor cells to rapid apoptosis by robust p53 stabilization [203]. KT-253 exhibited improved potency relative to reversible small-molecule inhibitors leading to potent in vitro and in vivo efficacy that is superior to all clinically active agents. Sustained tumor regression was achieved in vivo in leukemia models following just a single dose [203]. As wild-type p53 is present in >50% of tumors, KT-253 represents another program with broad potential in liquid and solid tumors.
3.2. Molecular Glue-Based Clinical Trials
IMiDs are among the standard of care for treatment of multiple myeloma and have shown activity in some non-Hodgkin’s lymphoma subtypes. Given that many patients treated with these agents often develop disease progression, an unmet need remains. New generation IMiDs have been designed for better clinical efficacy [121]. Avadomide (CC-122) displays antitumor properties and modulation of immune cells [119,204]. It binds CRBN to degrade IKZF1 and IKZF3, triggering apoptosis of DLBCL cell lines and blocking tumor growth in xenograft mouse models. Avadomide is in clinical trials for non-Hodgkin’s lymphoma, DLBCL, hepatocellular carcinoma, B-cell chronic lymphocytic leukemia, multiple myeloma, chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, primary liver cancer, advanced solid tumors and melanoma as a single agent or in combination with other agents (NCT03283202, NCT03834623, NCT03310619, NCT02509039, NCT02859324, NCT02417285, NCT02406742, NCT02323906, NCT01421524, NCT02031419). Iberdomide (CC-220) induces CRBN catalyzed proteasomal degradation of IKZF1 and IKFZ3 in monocytes, B and T cells. The drug is under clinical evaluation as monotherapy or in combination with other agents for the treatment of newly diagnosed multiple myeloma, relapsed or refractory multiple myeloma, (smoldering) plasma cell myeloma and relapsed or refractory lymphoma (NCT05560399, NCT04998786, NCT05354557, NCT04564703, NCT05177536, NCT04392037, NCT05392946, NCT05527340, NCT04776395, NCT05169515, NCT04975997, NCT05434689, NCT05199311, NCT05289492, NCT04884035, NCT04855136, NCT05272826, NCT04464798, NCT05558319, NCT03310619, NCT04934475, NCT02773030). CC-99282 is a novel, oral IKZF1/3 targeting CRBN E3 ligase modulator agent with an improved substrate degradation profile compared with other IKZF1/3 degrading agents such as lenalidomide, avadomide, and iberdomide. CC-99282 showed enhanced antiproliferative, apoptotic and immune-stimulatory activity in a range of follicular lymphoma and DLBCL models, including those with chemoresistance [205,206]. It displayed a robust distribution profile that favors target tissues such as lymphoid organs. CC-99282 acted synergistically in combination with anti-CD20 monoclonal antibody rituximab treatment [206]. Tumoricidal activity was validated in vivo, resulting in improved tumor regression and tumor-free animals in several lymphoma xenograft models, including an intracranial xenograft model [206]. A recent analysis in >20 DLBCL cell lines showed that loss of IKZF1/3 is necessary but not sufficient for CC-99282 efficacy [207]. An explanation has been provided by the observation that IKZF1 regulated gene expression via histone modifications, including polycomb repressive complex PRC2-mediated histone H3 lysine 27 trimethylation [207]. Consistently, pathway analysis upon exposure to CC-99282 or tazemetostat, an EZH2 inhibitor, demonstrated high overlap between pathways altered by both agents. Combination treatment led to additive and/or synergistic antiproliferative and apoptotic effects in DLBCL cell lines. No alterations were observed in IKZF1 degradation or overall H3K27me3 status but downstream effects were enhanced. Combination treatment yielded durable responses and tumor-free animals [207]. CC-99282 is currently under investigation in phase I clinical studies in patients with relapsed/refractory non-Hodgkin’s lymphomas and chronic lymphocytic leukemia/small lymphocytic lymphoma as monotherapy or in combination with other agents (NCT04434196, NCT04884035, NCT03930953, NCT05169515, NCT03310619). CFT7455 is another orally available next-generation IKZF1/3 degrader for the treatment of hematological malignancies. Rapid and deep degradation of these targets from malignant B cells resulted in tumor cell death, and depletion from the tumor microenvironment resulted in T-cell activation [208,209]. In in vitro and in vivo models of multiple myeloma and non-Hodgkin’s lymphoma, including anaplastic large cell lymphoma, diffuse large B-cell lymphoma, and mantle cell lymphoma, CFT7455 demonstrated higher binding affinity to CRBN E3 ligase complex and greater activity than other investigational and approved agents in similar classes [208,209]. A first-in-human, phase I dose escalation and expansion study is currently enrolling patients with relapsed/refractory non-Hodgkin’s lymphoma or multiple myeloma (NCT04756726). The purpose of the trial is to characterize the safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of CFT7455 administered orally according to different dosing schedules as a single agent and in combination with dexamethasone. Exploratory objectives include characterization of target engagement and IKZF1/3 degradation and assessing the immunomodulatory effects of CFT7455. CC-92480 is another oral CRBN E3 ligase modulator currently under clinical investigation in patients with newly diagnosed and/or refractory/relapsed multiple myeloma (NCT03374085, NCT05552976, NCT05519085, NCT03989414, NCT05372354). CC-92480 induces rapid degradation of the transcription factors IKZF1/3, leading to immune-stimulatory effects and apoptosis of myeloma cells, including those resistant to lenalidomide and pomalidomide [210,211,212,213]. CC-92480 in combination with dexamethasone and the proteasome inhibitor bortezomib appears to be safe and well tolerated with encouraging preliminary efficacy in patients with refractory/relapsed multiple myeloma. The overall response rate across all doses per investigator assessment was 73.7% (14/19 pts), including 3 stringent complete responses and 1 complete response [211]. Parallel trials are ongoing to determine the recommended phase II dose of CC-92480 in combination with standard treatments including carfilzomib, daratumumab, elotuzumab, isatuximab or with novel inhibitors tazemetostat, BMS-986158 and trametinib.
Interleukin-1 Receptor-Associated Kinase 4 (IRAK4) is a key mediator of innate immunity. It is found hyperactivated in a variety of autoimmune diseases. Multiple small-molecule kinase inhibitors of IRAK4 have been developed to block its kinase activity in autoimmune, inflammatory and oncological diseases, with the most advanced being in phase II clinical trials. However, some reports have indicated a nonkinase function for IRAK4 in several cell types, pointing out the need of alternative therapeutic interventions [214]. KT-413 is a novel IRAKIMiD degrader of IRAK4 and IMiD substrates. An early trial is under way to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of KT-413 in patients with MYD88 mutant and MYD88 wild-type relapsed or refractory DLBCL when administered intravenously (NCT05233033). The trial will also explore target (IRAK4/IKZF1/IKZF3) knockdown and downstream effects in peripheral blood mononuclear cells and tumors.
Malignant tumors must evade the immune system for continuous growing, a hurdle some malignancies may overcome by attracting immune-suppressive regulatory T cells (Tregs). The zinc finger transcription factor IKZF2 (Helios) is critical for the activity and stability of Tregs; its deficiency enhances immune responses to tumors in vivo, indicating IKZF2 may be an appealing target for cancer immunotherapy. DKY709 is the first CRBN modulator agent targeting IKZF2/4 with no activity towards IKZF1/3. Upon exposure to DKY709, human Tregs showed reduced suppressive activity [215]. Oral treatment with DKY709 drove a rapid and sustained degradation of IKZF2 including in humans and led to delayed tumor growth in mice with humanized immune systems and enhanced immunization responses in monkeys [215]. DKY709 is currently being investigated in a phase I trial in patients with advanced solid tumors as an immune-enhancing agent for cancer immunotherapy alone or in combination with other immunotherapy agents (NCT03891953).
Other CRBN modulators including CC-885, CC-90009, ZXH-1-161 and oral bioavailable candidates SJ6986 and MRT-2359 exhibit therapeutic potential for GSPT1 degradation [216,217,218,219,220]. CC-90009 is being tested in clinical trials for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome (NCT02848001 and NCT04336982), while a phase I/II clinical trial testing MRT-2359 in MYC-driven tumors is under way (NCT05546268).
4. Conclusions
Over the last two decades, the field of targeted protein degradation has evolved successfully, but almost all of this progress has been catalyzed through PROTACs alone. PROTACs have demonstrated excellent preclinical activities in multiple types of cancers by robust and selective depletion of their targets. Potent tumoricidal activities could also be validated in some in vivo models that were resistant to the counterpart small-molecule inhibitors of PROTACs. Despite their success, there are still some challenges concerning optimal druggability and evaluation of biological activity. Artificial intelligence and virtual drug screening platforms may improve identification of highly specific POI/E3 ligands. Molecular dynamic simulations, X-Ray crystallography and cryo-electron microscopy may be effective techniques for determining better ternary complex structures of PROTAC with POI and E3 ligase [221]. Drug-like properties may be improved by cell permeable precursors or by computer aided drug design softwares [51,55]. Clinical delivery and metabolic stability may be advanced by application of prodrug PROTACs, antibody-drug conjugates, folate-PROTACs, aptamer-PROTAC conjugates and nanoparticle based PROTACs to penetrate deep into tumors [56,65,66,67,68,109,222].
Targeted protein degradation is one of the fastest growing fields. Within a relatively short time, the platform is already impacting medicine as multiple therapeutic degraders have entered the stage of practical application from conceptual frameworks (Figure 17 and Figure 18). Clinical PROTAC degraders of AR and ER with minor off-target toxicities harbor favorable pharmacokinetic profiles and are well-tolerated by heavily pretreated patients with advanced/refractory prostate or ER+/HER2- breast cancer, respectively. Although degrader drugs that are under clinical investigation (PROTAC and molecular glues, see Table 3 and Table 4, Figure 17 and Figure 18) or already on the market (IMiDs) attack a variety of cancers, other disease indications may also benefit from this emerging drug paradigm (Table 5). A topical AR-PROTAC compound (GT20029) in phase I clinical trials could treat patients with androgenetic alopecia and acne (NCT05428449). An orally bioavailable heterobifunctional IRAK4 degrader (KT-474) in phase I clinical trials (NCT04772885) could treat patients with various immuno-inflammatory diseases, such as rheumatoid arthritis, atopic dermatitis or hidradenitis suppurativa. A pilot study suggested a favorable benefit/risk ratio in systemic lupus erythematosus for iberdomide, a drug with an immunomodulatory mechanism of action, supporting further clinical investigation (NCT02185040 and NCT03161483) [223]. Other clinical studies explore whether iberdomide and its analog avadomide are safe and effective in patients with severe renal impairment (NCT04933747, NCT03097016). With the discontinuation of the phase III trial of tominersen, a huntingtin protein (HTT)-silencing antisense oligonucleotide therapy, targeted degrader modalities could become the first disease-modifying therapy for the neurodegenerative Huntington disease: two TPD strategies have already been reported to reduce mHTT levels [139,224]. Targeted degraders could also emerge as potential antiviral agents: a RIBOTAC molecule binds and degrades a functional structure within the SARS-CoV-2′s RNA genome, the causative agent of the global pandemic COVID-19, thereby triggering the inhibition of viral propagation [225].
Figure 17.
Overview of PROTAC degraders in clinical trials for cancer treatment. AR: androgen receptor; BCL-xL: B-cell lymphoma-extra large; BRAF: v-raf murine sarcoma viral oncogene homolog B1; BRD: bromodomain-containing protein; BTK: Bruton’s tyrosine kinase; DLBCL: diffuse large B cell lymphoma; EGFR: epidermal growth factor receptor; ER: estrogen receptor; IND: investigational new drug, LGL: large granular lymphocyte; mCRPC: metastatic castration-resistant prostate cancer; MDM2: mouse double minute 2; NSCLC: non-small cell lung cancer; NTRK: neurotrophic tyrosine receptor kinase; R/R: relapsed/refractory; SMARCB1: SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1; STAT3: signal transducer and activator of transcription 3.
Figure 18.
Overview of molecular glues in clinical trials for cancer treatment. AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; DLBCL: diffuse large B cell lymphoma; IKZF: IKAROS zinc finger family; IRAK4: interleukin-1-receptor-associated kinase 4; GSPT1: G1 to S phase transition 1; MM: multiple myeloma; MyD88: myeloid differentiation primary response 88; NHL: non-Hodgkin’s lymphoma; R/R: relapsed/refractory; SLL: small lymphocytic lymphoma.
Table 5.
Summary of degrader drugs under clinical evaluation in other disease entities. Red: active, not recruiting; yellow: recruiting; blue: completed; violet: not yet recruiting. AD: atopic dermatitis; AGA: androgenetic alopecia; AR: androgen receptor; HS: hidradenitis suppurativa; IKZF: IKAROS zinc-finger; IRAK4: interleukin-1 receptor-associated kinase 4; SLE: systemic lupus erythematosus.
| Time | Degrader | Target | Indication | NCT Number | Phase |
|---|---|---|---|---|---|
| 2017 2021 |
Avadomide | IKZF1/3 | Renal insufficiency Critical illness and sepsis |
NCT03097016
NCT05083520 |
Phase I |
| 2022 | GT20029 | AR | Acne vulgaris and AGA | NCT05428449 | Phase I |
| 2014 2017 2019 2021 2021 |
Iberdomide | IKZF1/3 | SLE SLE Hepatic impairment Renal insufficiency Critical illness and sepsis |
NCT02185040
NCT03161483 NCT03824678 NCT04933747 NCT05083520 |
Phase II Phase II Phase I Phase I |
| 2021 | KT-474 | IRAK4 | AD and HS | NCT04772885 | Phase I |
Results of ongoing clinical trials are expected to establish the clinical significance of degrader drugs in oncology and other disease entities. Evidence of long-term effects or potent toxicity is still missing. Future clinical investigation will put more emphasis on the assessment of the clinical efficacy and adverse events in larger cohorts, exploration of potential resistance mechanisms, defining biomarkers for optimal selection of patients and exploration of potential synergy with other cancer therapeutics. In fact, early clinical investigations suggest that combination of chemical degraders either with targeted inhibitors or with chemotherapy/other agents may represent a robust alternative path for cancer therapy: ARV-471 produced a synergistic tumoricidal effect when combined with the CDK4/6 kinase inhibitor.
Potentials of targeted degradation applications go well beyond what was thought to be possible. PROTACs could be used as immune-enhancing agents. In combination with immune checkpoint inhibitors, they could augment the ability of the immune system to recognize and erase tumors. Degrader systems against immune checkpoint proteins and those that modulate T-cell receptor function have already been developed [144,150,151,221,226,227]. These may show improved pharmacokinetic and toxicity profiles when compared to antibody-based inhibitors. Two degrader agents are under clinical investigation as a novel avenue for cancer immunotherapy (NCT04886622 and NCT03891953). A recent study described the first tumor microenvironment-reprogramming nanoPROTAC strategy synergized with phototherapy that specifically revived antitumor immunity [228]. Such molecules in combination with other cancer treatments (e.g., chemo-/targeted/radiotherapy) could be utilized to intervene with immune-associated signaling pathways including hypoxia, lipid metabolism, glycolysis, glutaminolysis and adenosine signaling.
The incorporation of new elements into PROTAC technologies may help taming the undruggable drug targets and thus broaden the landscape of targeted degradation. This is highlighted by recent reports of utilizing RNA and DNA as respective recruiting elements to eliminate unwanted targets for cancer prevention and treatment [92,96,99,100,101]. In addition, a range of additional TPD approaches independent of the proteasomal pathway fuels the expansion of the drug target spectrum, although further investigations are warranted [137,138,139,140,141,145,146,147,148]. Thus, targeted degradation holds enormous promise to deliver medicine to the clinic in ways that are impossible with other modalities. We believe that the field of TPD will continue progressing with its rapid pace to maximize the benefits for patients and change the course of many deadly disease entities.
Acknowledgments
We thank Herwig P. Moll for his input and critical reading of the manuscript.
Abbreviations
| 2H2022 | Second half of the year 2022 |
| AbTAC | Antibody-based PROTAC |
| AD | Atopic dermatitis |
| ADMET | Absorption, distribution, metabolism, excretion and toxicity |
| AGA | Androgenetic alopecia |
| AhR | Aryl hydrocarbon receptor |
| AID | Auxin-inducible degron |
| ALK | Anaplastic lymphoma kinase |
| AML | Acute myeloid leukemia |
| AR | Androgen receptor |
| ASGPR | Asialoglycoprotein receptor |
| ASO | Antisense oligonucleotide |
| aTAG ATP |
AchillesTag Adenosine triphosphate |
| ATTEC | Autophagy tethering compound |
| AUTAC | Autophagy targeting chimera |
| AUTOTAC | Autophagy targeting chimera |
| Bcl | B-cell lymphoma |
| BCL-xL | B-cell lymphoma-extra large |
| BCR | Breakpoint cluster region protein |
| BET | Bromo- and extra-terminal family |
| BIAC | Bispecific aptamer chimera |
| BRAF | V-raf murine sarcoma viral oncogene homolog B1 |
| BRD | Bromodomain-containing protein |
| BTK | Bruton’s tyrosine kinase |
| CAR | Chimeric antigen receptor |
| CDC20 | Cell division cycle 20 |
| CDK | Cyclin-dependent kinase |
| Cer | Ceritinib |
| cIAP | Cellular inhibitor of apoptosis |
| CI-M6PR | Cation-independent mannose-6-phosphonate receptor |
| CK1α | Casein kinase 1α |
| CK2 | Casein kinase 2 |
| CLIPTAC | In-cell-click-formed proteolysis targeting chimera |
| CLL | Chronic lymphocytic leukemia |
| CMA | Chaperone-mediated autophagy |
| CPP-LSS | Cell-penetrating peptide and lysosome-sorting sequence |
| CRABP I/II | Cellular retinoic acid-binding protein 1/2 |
| CRBN | Cereblon |
| CREPT | Cell cycle-related and expression elevated protein in tumor |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CT | Computed tomography |
| CYP1B1 DCAF5 |
Cytochrome p450 family 1 subfamily B member 1 DDB1 and CUL4 associated factor 5 |
| DCAF | DDB1 and CUL4 associated factor |
| DHODH | Dihydroorotate dehydrogenase |
| DHX36 | DEAH-box-protein 36 |
| DLBCL DNA |
Diffuse large B cell lymphoma Deoxyribonucleid acid |
| dsDNA | Double-stranded DNA |
| dTAG | Degrader tag |
| DUB | Deubiquitinating enzyme |
| DUBTAC | Deubiquitinase-targeting chimera |
| eEF2K | Eukaryotic elongation factor 2 kinase |
| EGFR | Epidermal growth factor receptor |
| eIF4E | Eukaryotic initiating factor 4E |
| EML4 | Echinoderm microtubule-associated protein-like 4 |
| ER | Estrogen receptor |
| ErbB3 | Erb-b2 receptor tyrosine kinase 3 |
| ERK | Extracellular signal-regulated kinase |
| ESR1 | Estrogen receptor 1 |
| EZH2 | Enhancer of zeste homolog 2 |
| FAK | Focal adhesion kinase |
| FDA | Food and drug administration |
| FEM1B | Feminization-1 homolog b |
| FKBP | FK506-binding proteins |
| FLT3 | Fms-like tyrosine kinase 3 |
| FOXO1 GlueTAC |
Forkhead box protein O1 GlueBody Chimera |
| GNPs | Gold nanoparticles |
| GPD1L | Glycerol-3-phosphate dehydrogenase 1 like protein |
| GREB1 | Growth regulating estrogen receptor binding 1 |
| GSTP1 G4 |
G1 to S phase transition 1 G-quadruplex |
| HDAC | Histone deacetylase |
| HECT | Homology to E6AP C terminus |
| HER | Human epidermal growth factor receptor |
| HIF-1-alpha | Hypoxia-inducible factor 1-alpha |
| HIP1R | Huntingtin-interacting protein 1–related |
| HPK1 | Hematopoietic progenitor kinase 1 |
| HS HyT IAP |
Hidradenitis suppurativa Hydrophobic tagging Inhibitors of apoptosis protein |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IKZF | IKAROS zinc finger family |
| IMiD | Immunomodulatory drug |
| IND | Investigational new drug |
| IRAK4 | Interleukin-1 receptor-associated kinase 4 |
| ITK IV |
Interleukin-2-inducible T-cell kinase Intravenous injection |
| JAK | Janus kinase |
| JAMM | Jab1/MPN domain-associated metallopeptidase |
| KEAP1 | Kelch-like ECH associated protein 1 |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LD | Lipid droplets |
| LEF1 | Lymphoid enhancer binding factor 1 |
| LGL | Large granular lymphocyte |
| LHRH | Luteinizing hormone-releasing hormone |
| LIR | LC3-interacting region |
| LTR | Lysosomal targeted receptor |
| LYTAC | Lysosome targeting chimera |
| MCL | Mantle cell lymphoma |
| Mcl-1 | Myeloid cell leukemia-1 |
| MCPIP | Monocyte chemotactic protein-induced protein |
| mCRPC | Metastatic castration-resistant prostate cancer |
| MDM2 | Mouse double minute 2 |
| MEK | Mitogen-activated protein kinase kinase |
| Met | Mesenchymal epithelial transition |
| mHTT | Mutant huntingtin |
| MINDY | Motif interacting with Ub-containing novel DUB family |
| miR-96 | MicroRNA 96 |
| MJD | Machado–Joseph disease protein domain |
| MM | Multiple myeloma |
| MoDE-A | Molecular degrader of extracellular proteins through the ASGPR |
| MRI | Magnetic resonance imaging |
| MTH1 | MutT homolog-1 |
| MyD88 | Myeloid differentiation primary response 88 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHL | Non-Hodgkin’s lymphoma |
| NRAS | Neuroblastoma RAS viral oncogene homolog |
| NSCLC | Non-small cell lung cancer |
| NTRK | Neurotrophic tyrosine receptor kinase |
| NVOC | Nitroveratryloxycarbonyl |
| OTU | Ovarian tumor protease |
| PARP1 | Poly [ADP-ribose] polymerase 1 |
| PB1 | Phox and Bem1 domain |
| PDE | Phosphodiesterase |
| PD-L1 | Programmed death-ligand 1 |
| PDX | Patient derived xenograft |
| PEG | Polyethylene glycol |
| PHOTAC | Photochemically targeting chimera |
| PI3K | Phosphoinositide 3-kinases |
| PLK1 PO |
Polo-like kinase 1 Per os |
| POI | Protein of interest |
| POLY-PROTAC | Polymeric PROTAC |
| Pom | Pomalidomide |
| PR | Progesterone receptor |
| PRC | Polycomb repressive complex |
| PRMT5 | Protein arginine methyltransferase 5 |
| PROTACs | Proteolysis targeting chimeras |
| PTB | Phosphotyrosine-binding |
| PTK-7 | Protein kinase-like 7 |
| R/R | Relapsed/refractory |
| RAS | Rat sarcoma |
| RBFOX1 | RNA binding Fox-1 homolog 1 |
| RBM23 | RNA binding motif protein 23 |
| RBM39 | RNA binding motif protein 39 |
| RBP | RNA binding protein |
| RBR | RING-between-RING |
| RHAU | RNA helicase associated with AU-rich element |
| RIBOTAC | Ribonuclease Targeting chimera |
| RING | Really interesting new gene |
| RIPK2 RNA |
Receptor interacting serine/threonine kinase 2 Ribonucleic acid |
| RNF | RING finger protein |
| RTK SC |
Receptor tyrosine kinase Subcutaneous injection |
| SCLC | Small cell lung cancer |
| SERD | Selective estrogen receptor degrader |
| SGK | Serum/glucocorticoid regulated kinase family member |
| SH2 | Src homology 2 |
| SHP2 | Src homology 2 domain containing protein tyrosine phosphatase-2 |
| siRNA | Small interfering RNA |
| SirT2 | Sirtuin 2 |
| SLC9A1 | Solute carrier family 9 member A1 |
| SLE | Systemic lupus erythematosus |
| SLL | Small lymphocytic lymphoma |
| SMARCB1 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1 |
| SNIPER | Specific and non-genetic inhibitors of apoptosis protein (IAP)-dependent protein eraser |
| SPAAC | Strain-promoted azide–alkyne cycloaddition |
| SRC-1 | Steroid receptor coactivator-1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TACC3 | Transforming acidic coiled-coil containing protein 3 |
| TBK1 | TANK-binding kinase 1 |
| TCF | T-cell factor |
| TCL | T-cell lymphomas |
| TCO | Trans-cyclooctene |
| TF | Transcription factor |
| TFF | Trefoil factor |
| TGF-β1 | Transforming growth factor beta 1 |
| TI | Tumor-infiltrating |
| TOI | Transcription factor of interest |
| TPD | Targeted protein degradation |
| TPS | Targeted protein stabilization |
| TRAFTAC | Transcription factor targeting Chimera |
| Treg | Regulatory T cell |
| TRIM24 | Tripartite motif containing 24 |
| TrkA | Tropomyosin receptor kinase A |
| TZ | Tetrazine |
| Ub | Ubiquitin |
| UCH | Ubiquitin C-terminal hydrolase |
| UPS | Ubiquitin-proteasome system |
| USP | Ubiquitin-specific protease |
| UV | Ultraviolet |
| VEGFR | Vascular endothelial growth factor receptor |
| VHL | Von Hippel-Lindau |
| WM | Waldenström macroglobulinaemia |
| XIAP | X-linked inhibitor of apoptosis protein |
| ZUFSP | Zn-finger and UFSP domain protein |
| α1A-AR | Alpha-1A adrenergic receptor |
Author Contributions
I.Z.U. was the principal investigator and takes primary responsibility for the paper; A.K.A.A.S., M.V.T., E.C. and I.Z.U. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Fund of City of Vienna for innovative, interdisciplinary cancer research (IZU), by the Austrian Science Fund (FWF) Doc Fund DOC59-B33 (E.C.), the FWF standalone grants P 33430 and P 32900 (E.C.), and by the City of Vienna Fund for innovative, interdisciplinary Cancer Research (E.C.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 3.Salami J., Crews C.M. Waste disposal-An attractive strategy for cancer therapy. Science. 2017;355:1163–1167. doi: 10.1126/science.aam7340. [DOI] [PubMed] [Google Scholar]
- 4.Mir O., Broutin S., Desnoyer A., Delahousse J., Chaput N., Paci A. Pharmacokinetics/Pharmacodynamic (PK/PD) relationship of therapeutic monoclonal antibodies used in oncology: What’s new? Eur. J. Cancer. 2020;128:103–106. doi: 10.1016/j.ejca.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Bedard P.L., Hyman D.M., Davids M.S., Siu L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395:1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 6.Lai A.C., Crews C.M. Induced protein degradation: An emerging drug discovery paradigm. Nat. Rev. Drug Discov. 2017;16:101–114. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migliorati J.M., Liu S., Liu A., Gogate A., Nair S., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X.-B. Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs. Drug Metab. Dispos. 2022;50:888–897. doi: 10.1124/dmd.121.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padda I.S., Mahtani A.U., Parmar M. Small Interfering RNA (SiRNA) Based Therapy. StatPearls Publishing; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 9.Marques J.T., Williams B.R.G. Activation of the mammalian immune system by siRNAs. Nat. Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 10.Dahlman J.E., Kauffman K.J., Langer R., Anderson D.G. Nanotechnology for in vivo targeted siRNA delivery. Adv. Genet. 2014;88:37–69. doi: 10.1016/B978-0-12-800148-6.00003-1. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 12.Fedorov Y., Anderson E.M., Birmingham A., Reynolds A., Karpilow J., Robinson K., Leake D., Marshall W.S., Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teicher B.A., Tomaszewski J.E. Proteasome inhibitors. Biochem. Pharmacol. 2015;96:1–9. doi: 10.1016/j.bcp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Vitale G. Molecular glues begin to stick. C EN Glob. Enterp. 2022;100:20–24. doi: 10.1021/cen-10029-cover. [DOI] [Google Scholar]
- 15.Donovan K.A., An J., Nowak R.P., Yuan J.C., Fink E.C., Berry B.C., Ebert B.L., Fischer E.S. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. Elife. 2018;7:e38430. doi: 10.7554/eLife.38430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Békés M., Langley D.R., Crews C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022;21:181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazoe S., Tom J., Fu Y., Wu W., Zeng L., Sun C., Liu Q., Lin J., Lin K., Fairbrother W.J., et al. Heterobifunctional Molecules Induce Dephosphorylation of Kinases-A Proof of Concept Study. J. Med. Chem. 2020;63:2807–2813. doi: 10.1021/acs.jmedchem.9b01167. [DOI] [PubMed] [Google Scholar]
- 18.Siriwardena S.U., Godage D.N.P.M., Shoba V.M., Lai S., Shi M., Wu P., Chaudhary S.K., Schreiber S.L., Choudhary A. Phosphorylation-Inducing Chimeric Small Molecules. J. Am. Chem. Soc. 2020;142:14052–14057. doi: 10.1021/jacs.0c05537. [DOI] [PubMed] [Google Scholar]
- 19.Henning N.J., Boike L., Spradlin J.N., Ward C.C., Liu G., Zhang E., Belcher B.P., Brittain S.M., Hesse M.J., Dovala D., et al. Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat. Chem. Biol. 2022;18:412–421. doi: 10.1038/s41589-022-00971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels D.L., Winter G.E. Degrading boundaries to break new ground in chemical biology. Curr. Res. Chem. Biol. 2022;2:100033. doi: 10.1016/j.crchbi.2022.100033. [DOI] [Google Scholar]
- 21.Nam T., Han J.H., Devkota S., Lee H.-W. Emerging Paradigm of Crosstalk between Autophagy and the Ubiquitin-Proteasome System. Mol. Cells. 2017;40:897–905. doi: 10.14348/molcells.2017.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popovic D., Vucic D., Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 23.Antao A.M., Tyagi A., Kim K.-S., Ramakrishna S. Advances in Deubiquitinating Enzyme Inhibition and Applications in Cancer Therapeutics. Cancers. 2020;12:1579. doi: 10.3390/cancers12061579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komander D., Rape M. The Ubiquitin Code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 25.Alabi S.B., Crews C.M. Major advances in targeted protein degradation: PROTACs, LYTACs, and MADTACs. J. Biol. Chem. 2021;296:100647. doi: 10.1016/j.jbc.2021.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costales M.G., Aikawa H., Li Y., Childs-Disney J.L., Abegg D., Hoch D.G., Velagapudi S.P., Nakai Y., Khan T., Wang K.W., et al. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc. Natl. Acad. Sci. USA. 2020;117:2406–2411. doi: 10.1073/pnas.1914286117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabapathy K., Lane D.P. Therapeutic targeting of p53: All mutants are equal, but some mutants are more equal than others. Nat. Rev. Clin. Oncol. 2018;15:13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto K.M., Kim K.B., Kumagai A., Mercurio F., Crews C.M., Deshaies R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto K.M., Kim K.B., Verma R., Ransick A., Stein B., Crews C.M., Deshaies R.J. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol. Cell. Proteom. 2003;2:1350–1358. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Cyrus K., Wehenkel M., Choi E.-Y., Swanson H., Kim K.-B. Two-headed PROTAC: An effective new tool for targeted protein degradation. Chembiochem. 2010;11:1531–1534. doi: 10.1002/cbic.201000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneekloth A.R., Pucheault M., Tae H.S., Crews C.M. Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg. Med. Chem. Lett. 2008;18:5904–5908. doi: 10.1016/j.bmcl.2008.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hines J., Gough J.D., Corson T.W., Crews C.M. Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs. Proc. Natl. Acad. Sci. USA. 2013;110:8942–8947. doi: 10.1073/pnas.1217206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z.-W., Liu Y., Zhu X. PhotoPROTACs: A Novel Biotechnology for Cancer Treatment. Trends Cell Biol. 2020;30:749–751. doi: 10.1016/j.tcb.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Reynders M., Matsuura B.S., Bérouti M., Simoneschi D., Marzio A., Pagano M., Trauner D. PHOTACs enable optical control of protein degradation. Sci. Adv. 2020;6:934–951. doi: 10.1126/sciadv.aay5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaff P., Samarasinghe K.T.G., Crews C.M., Carreira E.M. Reversible Spatiotemporal Control of Induced Protein Degradation by Bistable PhotoPROTACs. ACS Cent. Sci. 2019;5:1682–1690. doi: 10.1021/acscentsci.9b00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Chen H., Ma L., He Z., Wang D., Liu Y., Lin Q., Zhang T., Gray N., Kaniskan H.Ü., et al. Light-induced control of protein destruction by opto-PROTAC. Sci. Adv. 2020;6:969–986. doi: 10.1126/sciadv.aay5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham H. The mechanism of action and clinical value of PROTACs: A graphical review. Cell. Signal. 2022;99:110446. doi: 10.1016/j.cellsig.2022.110446. [DOI] [PubMed] [Google Scholar]
- 38.Yang X., Yin H., Kim R.D., Fleming J.B., Xie H. Preclinical and Clinical Advances of Targeted Protein Degradation as a Novel Cancer Therapeutic Strategy: An Oncologist Perspective. Target. Oncol. 2021;16:1–12. doi: 10.1007/s11523-020-00782-2. [DOI] [PubMed] [Google Scholar]
- 39.Bondeson D.P., Smith B.E., Burslem G.M., Buhimschi A.D., Hines J., Jaime-Figueroa S., Wang J., Hamman B.D., Ishchenko A., Crews C.M. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem. Biol. 2018;25:78. doi: 10.1016/j.chembiol.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brand M., Jiang B., Bauer S., Donovan K.A., Liang Y., Wang E.S., Nowak R.P., Yuan J.C., Zhang T., Kwiatkowski N., et al. Homolog-Selective Degradation as a Strategy to Probe the Function of CDK6 in AML. Cell Chem. Biol. 2019;26:300–306.e9. doi: 10.1016/j.chembiol.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tovell H., Testa A., Zhou H., Shpiro N., Crafter C., Ciulli A., Alessi D.R. Design and Characterization of SGK3-PROTAC1, an Isoform Specific SGK3 Kinase PROTAC Degrader. ACS Chem. Biol. 2019;14:2024–2034. doi: 10.1021/acschembio.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potjewyd F., Turner A.-M.W., Beri J., Rectenwald J.M., Norris-Drouin J.L., Cholensky S.H., Margolis D.M., Pearce K.H., Herring L.E., James L.I. Degradation of Polycomb Repressive Complex 2 with an EED-Targeted Bivalent Chemical Degrader. Cell Chem. Biol. 2020;27:47–56.e15. doi: 10.1016/j.chembiol.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farnaby W., Koegl M., Roy M.J., Whitworth C., Diers E., Trainor N., Zollman D., Steurer S., Karolyi-Oezguer J., Riedmueller C., et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat. Chem. Biol. 2019;15:672–680. doi: 10.1038/s41589-019-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H., Chen F., Pei S., Gou S. Pomalidomide hybrids act as proteolysis targeting chimeras: Synthesis, anticancer activity and B-Raf degradation. Bioorg. Chem. 2019;87:191–199. doi: 10.1016/j.bioorg.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Jiang B., Gao Y., Che J., Lu W., Kaltheuner I.H., Dries R., Kalocsay M., Berberich M.J., Jiang J., You I., et al. Discovery and resistance mechanism of a selective CDK12 degrader. Nat. Chem. Biol. 2021;17:675–683. doi: 10.1038/s41589-021-00765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gosavi P.M., Ngan K.C., Yeo M.J.R., Su C., Li J., Lue N.Z., Hoenig S.M., Liau B.B. Profiling the Landscape of Drug Resistance Mutations in Neosubstrates to Molecular Glue Degraders. ACS Cent. Sci. 2022;8:417–429. doi: 10.1021/acscentsci.1c01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanzl A., Barone E., Bauer S., Yue H., Nowak R.P., Hahn E., Pankevich E.V., Koren A., Kubicek S., Fischer E.S., et al. E3-specific degrader discovery by dynamic tracing of substrate receptor abundance. bioRxiv. 2022 doi: 10.1101/2022.10.10.511612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Riley-Gillis B., Vijay P., Shen Y. Acquired Resistance to BET-PROTACs (Proteolysis-Targeting Chimeras) Caused by Genomic Alterations in Core Components of E3 Ligase Complexes. Mol. Cancer Ther. 2019;18:1302–1311. doi: 10.1158/1535-7163.MCT-18-1129. [DOI] [PubMed] [Google Scholar]
- 49.He M., Cao C., Ni Z., Liu Y., Song P., Hao S., He Y., Sun X., Rao Y. PROTACs: Great opportunities for academia and industry (an update from 2020 to 2021) Signal Transduct. Target. Ther. 2022;7:181. doi: 10.1038/s41392-022-00999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan S., He Y., Zhang X., Yuan Y., Pu S., Kong Q., Zheng G., Zhou D. PROteolysis TArgeting Chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene. 2020;39:4909–4924. doi: 10.1038/s41388-020-1336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Pu W., Zheng Q., Ai M., Chen S., Peng Y. Proteolysis-targeting chimeras (PROTACs) in cancer therapy. Mol. Cancer. 2022;21:99. doi: 10.1186/s12943-021-01434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bricelj A., Steinebach C., Kuchta R., Gütschow M., Sosič I. E3 Ligase Ligands in Successful PROTACs: An Overview of Syntheses and Linker Attachment Points. Front. Chem. 2021;9:707317. doi: 10.3389/fchem.2021.707317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadd M.S., Testa A., Lucas X., Chan K.-H., Chen W., Lamont D.J., Zengerle M., Ciulli A. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat. Chem. Biol. 2017;13:514–521. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer L.T., Zhang X. Expanding the landscape of E3 ligases for targeted protein degradation. Curr. Res. Chem. Biol. 2022;2:100020. doi: 10.1016/j.crchbi.2022.100020. [DOI] [Google Scholar]
- 55.Lebraud H., Wright D.J., Johnson C.N., Heightman T.D. Protein Degradation by In-Cell Self-Assembly of Proteolysis Targeting Chimeras. ACS Cent. Sci. 2016;2:927–934. doi: 10.1021/acscentsci.6b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei M., Zhao R., Cao Y., Wei Y., Li M., Dong Z., Liu Y., Ruan H., Li Y., Cao S., et al. First orally bioavailable prodrug of proteolysis targeting chimera (PROTAC) degrades cyclin-dependent kinases 2/4/6 in vivo. Eur. J. Med. Chem. 2021;209:112903. doi: 10.1016/j.ejmech.2020.112903. [DOI] [PubMed] [Google Scholar]
- 57.Matsson P., Kihlberg J. How Big Is Too Big for Cell Permeability? J. Med. Chem. 2017;60:1662–1664. doi: 10.1021/acs.jmedchem.7b00237. [DOI] [PubMed] [Google Scholar]
- 58.Schneekloth J.S., Fonseca F.N., Koldobskiy M., Mandal A., Deshaies R., Sakamoto K., Crews C.M. Chemical genetic control of protein levels: Selective in vivo targeted degradation. J. Am. Chem. Soc. 2004;126:3748–3754. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- 59.Salerno A., Seghetti F., Caciolla J., Uliassi E., Testi E., Guardigni M., Roberti M., Milelli A., Bolognesi M.L. Enriching Proteolysis Targeting Chimeras with a Second Modality: When Two Are Better Than One. J. Med. Chem. 2022;65:9507–9530. doi: 10.1021/acs.jmedchem.2c00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng S., Zhang H., Shen Z., Huang W. Photopharmacology of Proteolysis-Targeting Chimeras: A New Frontier for Drug Discovery. Front Chem. 2021;10:9:639176. doi: 10.3389/fchem.2021.639176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva J.M., Silva E., Reis R.L. Light-triggered release of photocaged therapeutics—Where are we now? J. Control. Release. 2019;298:154–176. doi: 10.1016/j.jconrel.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lerch M.M., Hansen M.J., van Dam G.M., Szymanski W., Feringa B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016;55:10978–10999. doi: 10.1002/anie.201601931. [DOI] [PubMed] [Google Scholar]
- 64.Hüll K., Morstein J., Trauner D. In Vivo Photopharmacology. Chem. Rev. 2018;118:10710–10747. doi: 10.1021/acs.chemrev.8b00037. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Han L., Liu F., Yang F., Jiang X., Sun H., Feng F., Xue J., Liu W. Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras. Colloids Surf. B: Biointerfaces. 2020;188:110795. doi: 10.1016/j.colsurfb.2020.110795. [DOI] [PubMed] [Google Scholar]
- 66.Gao J., Hou B., Zhu Q., Yang L., Jiang X., Zou Z., Li X., Xu T., Zheng M., Chen Y.-H., et al. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumour-specific protein degradation and precise cancer therapy. Nat. Commun. 2022;13:4318. doi: 10.1038/s41467-022-32050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Juan A., del Mar Noblejas-López M., Arenas-Moreira M., Alonso-Moreno C., Ocaña A. Options to Improve the Action of PROTACs in Cancer: Development of Controlled Delivery Nanoparticles. Front. Cell Dev. Biol. 2022;9:3912. doi: 10.3389/fcell.2021.805336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maneiro M., Forte N., Shchepinova M.M., Kounde C.S., Chudasama V., Baker J.R., Tate E.W. Antibody—PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chem. Biol. 2020;15:1306–1312. doi: 10.1021/acschembio.0c00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pillow T.H., Adhikari P., Blake R.A., Chen J., Del Rosario G., Deshmukh G., Figueroa I., Gascoigne K.E., Kamath A.V., Kaufman S., et al. Antibody Conjugation of a Chimeric BET Degrader Enables in vivo Activity. ChemMedChem. 2020;15:17–25. doi: 10.1002/cmdc.201900497. [DOI] [PubMed] [Google Scholar]
- 70.Dragovich P.S., Adhikari P., Blake R.A., Blaquiere N., Chen J., Cheng Y.-X., den Besten W., Han J., Hartman S.J., He J., et al. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα) Bioorg. Med. Chem. Lett. 2020;30:126907. doi: 10.1016/j.bmcl.2019.126907. [DOI] [PubMed] [Google Scholar]
- 71.Dragovich P.S., Pillow T.H., Blake R.A., Sadowsky J.D., Adaligil E., Adhikari P., Bhakta S., Blaquiere N., Chen J., Dela Cruz-Chuh J., et al. Antibody-Mediated Delivery of Chimeric BRD4 Degraders. Part 1: Exploration of Antibody Linker, Payload Loading, and Payload Molecular Properties. J. Med. Chem. 2021;64:2534–2575. doi: 10.1021/acs.jmedchem.0c01845. [DOI] [PubMed] [Google Scholar]
- 72.Dragovich P.S., Pillow T.H., Blake R.A., Sadowsky J.D., Adaligil E., Adhikari P., Chen J., Corr N., Cruz-Chuh J.D., Del Rosario G., et al. Antibody-Mediated Delivery of Chimeric BRD4 Degraders. Part 2: Improvement of In Vitro Antiproliferation Activity and In Vivo Antitumor Efficacy. J. Med. Chem. 2021;64:2576–2607. doi: 10.1021/acs.jmedchem.0c01846. [DOI] [PubMed] [Google Scholar]
- 73.Buckley D.L., Raina K., Darricarrere N., Hines J., Gustafson J.L., Smith I.E., Miah A.H., Harling J.D., Crews C.M. HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins. ACS Chem. Biol. 2015;10:1831–1837. doi: 10.1021/acschembio.5b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tovell H., Testa A., Maniaci C., Zhou H., Prescott A.R., Macartney T., Ciulli A., Alessi D.R. Rapid and Reversible Knockdown of Endogenously Tagged Endosomal Proteins via an Optimized HaloPROTAC Degrader. ACS Chem. Biol. 2019;14:882–892. doi: 10.1021/acschembio.8b01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nabet B., Roberts J.M., Buckley D.L., Paulk J., Dastjerdi S., Yang A., Leggett A.L., Erb M.A., Lawlor M.A., Souza A., et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 2018;14:431–441. doi: 10.1038/s41589-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nabet B., Ferguson F.M., Seong B.K.A., Kuljanin M., Leggett A.L., Mohardt M.L., Robichaud A., Conway A.S., Buckley D.L., Mancias J.D., et al. Rapid and direct control of target protein levels with VHL-recruiting dTAG molecules. Nat. Commun. 2020;11:4687. doi: 10.1038/s41467-020-18377-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowak R.P., Xiong Y., Kirmani N., Kalabathula J., Donovan K.A., Eleuteri N.A., Yuan J.C., Fischer E.S. Structure-Guided Design of a “Bump-and-Hole” Bromodomain-Based Degradation Tag. J. Med. Chem. 2021;64:11637–11650. doi: 10.1021/acs.jmedchem.1c00958. [DOI] [PubMed] [Google Scholar]
- 78.Veits G.K., Henderson C.S., Vogelaar A., Eron S.J., Lee L., Hart A., Deibler R.W., Baddour J., Elam W.A., Agafonov R.V., et al. Development of an AchillesTAG degradation system and its application to control CAR-T activity. Curr. Res. Chem. Biol. 2021;1:100010. doi: 10.1016/j.crchbi.2021.100010. [DOI] [Google Scholar]
- 79.Adkins S. CAR T-Cell Therapy: Adverse Events and Management. J. Adv. Pract. Oncol. 2019;10:21–28. doi: 10.6004/jadpro.2019.10.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Budde L.E., Berger C., Lin Y., Wang J., Lin X., Frayo S.E., Brouns S.A., Spencer D.M., Till B.G., Jensen M.C., et al. Combining a CD20 Chimeric Antigen Receptor and an Inducible Caspase 9 Suicide Switch to Improve the Efficacy and Safety of T Cell Adoptive Immunotherapy for Lymphoma. PLoS ONE. 2013;8:e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tae H.S., Sundberg T.B., Neklesa T.K., Noblin D.J., Gustafson J.L., Roth A.G., Raina K., Crews C.M. Identification of hydrophobic tags for the degradation of stabilized proteins. ChemBioChem. 2012;13:538–541. doi: 10.1002/cbic.201100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie T., Lim S.M., Westover K.D., Dodge M.E., Ercan D., Ficarro S.B., Udayakumar D., Gurbani D., Tae H.S., Riddle S.M., et al. Pharmacological targeting of the pseudokinase Her3. Nat. Chem. Biol. 2014;10:1006–1012. doi: 10.1038/nchembio.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi S.R., Wang H.M., Shin M.H., Lim H.-S. Hydrophobic Tagging-Mediated Degradation of Transcription Coactivator SRC-1. Int. J. Mol. Sci. 2021;22:6407. doi: 10.3390/ijms22126407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma A., Stratikopoulos E., Park K.S., Wei J., Martin T.C., Yang X., Schwarz M., Leshchenko V., Rialdi A., Dale B., et al. Discovery of a first-in-class EZH2 selective degrader. Nat. Chem. Biol. 2020;16:214. doi: 10.1038/s41589-019-0421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gustafson J.L., Neklesa T.K., Cox C.S., Roth A.G., Buckley D.L., Tae H.S., Sundberg T.B., Stagg D.B., Hines J., McDonnell D.P., et al. Small-Molecule-Mediated Degradation of the Androgen Receptor through Hydrophobic Tagging. Angew. Chem. Int. Ed. 2015;54:9659–9662. doi: 10.1002/anie.201503720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maniaci C., Hughes S.J., Testa A., Chen W., Lamont D.J., Rocha S., Alessi D.R., Romeo R., Ciulli A. Homo-PROTACs: Bivalent small-molecule dimerizers of the VHL E3 ubiquitin ligase to induce self-degradation. Nat. Commun. 2017;8:830. doi: 10.1038/s41467-017-00954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinebach C., Lindner S., Udeshi N.D., Mani D.C., Kehm H., Köpff S., Carr S.A., Gütschow M., Krönke J. Homo-PROTACs for the Chemical Knockdown of Cereblon. ACS Chem. Biol. 2018;13:2771–2782. doi: 10.1021/acschembio.8b00693. [DOI] [PubMed] [Google Scholar]
- 88.Steinebach C., Kehm H., Lindner S., Vu L.P., Köpff S., Mármol Á.L., Weiler C., Wagner K.G., Reichenzeller M., Krönke J., et al. PROTAC-mediated crosstalk between E3 ligases. Chem. Commun. 2019;55:1821–1824. doi: 10.1039/C8CC09541H. [DOI] [PubMed] [Google Scholar]
- 89.He S., Ma J., Fang Y., Liu Y., Wu S., Dong G., Wang W., Sheng C. Homo-PROTAC mediated suicide of MDM2 to treat non-small cell lung cancer. Acta Pharm. Sin. B. 2021;11:1617–1628. doi: 10.1016/j.apsb.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wagner A.J., Banerji U., Mahipal A., Somaiah N., Hirsch H., Fancourt C., Johnson-Levonas A.O., Lam R., Meister A.K., Russo G., et al. Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2017;35:1304–1311. doi: 10.1200/JCO.2016.70.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iancu-Rubin C., Mosoyan G., Glenn K., Gordon R.E., Nichols G.L., Hoffman R. Activation of p53 by the MDM2 inhibitor RG7112 impairs thrombopoiesis. Exp. Hematol. 2014;42:137–145.e5. doi: 10.1016/j.exphem.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 92.Ghidini A., Cléry A., Halloy F., Allain F.H.T., Hall J. RNA-PROTACs: Degraders of RNA-Binding Proteins. Angew. Chem. Int. Ed. 2021;60:3163–3169. doi: 10.1002/anie.202012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong S. RNA Binding Protein as an Emerging Therapeutic Target for Cancer Prevention and Treatment. Eur. J. Cancer Prev. 2017;22:203–210. doi: 10.15430/JCP.2017.22.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerstberger S., Hafner M., Tuschl T. A census of human RNA-binding proteins. Nat. Rev. Genet. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang E.T., Taliaferro J.M., Lee J.-A., Sudhakaran I.P., Rossoll W., Gross C., Moss K.R., Bassell G.J. Dysregulation of mRNA Localization and Translation in Genetic Disease. J. Neurosci. 2016;36:11418–11426. doi: 10.1523/JNEUROSCI.2352-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J., Chen H., Kaniskan H.Ü., Xie L., Chen X., Jin J., Wei W. TF-PROTACs Enable Targeted Degradation of Transcription Factors. J. Am. Chem. Soc. 2021;143:8902–8910. doi: 10.1021/jacs.1c03852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X., Pu W., Chen S., Peng Y. Therapeutic targeting of RNA-binding protein by RNA-PROTAC. Mol. Ther. 2021;29:1940–1942. doi: 10.1016/j.ymthe.2021.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roberts T.C., Langer R., Wood M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020;19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samarasinghe K.T.G., Jaime-Figueroa S., Burgess M., Nalawansha D.A., Dai K., Hu Z., Bebenek A., Holley S.A., Crews C.M. Targeted degradation of transcription factors by TRAFTACs: TRAnscription Factor TArgeting Chimeras. Cell Chem. Biol. 2021;28:648–661.e5. doi: 10.1016/j.chembiol.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ng C.S.C., Banik S.M. Taming transcription factors with TRAFTACs. Cell Chem. Biol. 2021;28:588–590. doi: 10.1016/j.chembiol.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 101.Shao J., Yan Y., Ding D., Wang D., He Y., Pan Y., Yan W., Kharbanda A., Li H., Huang H. Destruction of DNA-binding proteins by programmable O’PROTAC: Oligonucleotide-based PROTAC. Adv. Sci. (Weinh). 2021;20:e2102555. doi: 10.1002/advs.202102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perner S., Mosquera J.-M., Demichelis F., Hofer M.D., Paris P.L., Simko J., Collins C., Bismar T.A., Chinnaiyan A.M., De Marzo A.M., et al. TMPRSS2-ERG fusion prostate cancer: An early molecular event associated with invasion. Am. J. Surg. Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 103.Balasubramanian S., Hurley L.H., Neidle S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patil K.M., Chin D., Seah H.L., Shi Q., Lim K.W., Phan A.T. G4-PROTAC: Targeted degradation of a G-quadruplex binding protein. Chem. Commun. 2021;57:12816–12819. doi: 10.1039/D1CC05025G. [DOI] [PubMed] [Google Scholar]
- 105.Thiel K.W., Giangrande P.H. Intracellular delivery of RNA-based therapeutics using aptamers. Ther. Deliv. 2010;1:849–861. doi: 10.4155/tde.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou J., Rossi J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017;16:181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenberg J.E., Bambury R.M., Van Allen E.M., Drabkin H.A., Lara P.N., Harzstark A.L., Wagle N., Figlin R.A., Smith G.W., Garraway L.A., et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs. 2014;32:178–187. doi: 10.1007/s10637-013-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yazdian-Robati R., Bayat P., Oroojalian F., Zargari M., Ramezani M., Taghdisi S.M., Abnous K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020;155:1420–1431. doi: 10.1016/j.ijbiomac.2019.11.118. [DOI] [PubMed] [Google Scholar]
- 109.He S., Gao F., Ma J., Ma H., Dong G., Sheng C. Aptamer-PROTAC Conjugates (APCs) for Tumor-Specific Targeting in Breast Cancer. Angew. Chem. Int. Ed. 2021;60:23299–23305. doi: 10.1002/anie.202107347. [DOI] [PubMed] [Google Scholar]
- 110.Zhang L., Li L., Wang X., Liu H., Zhang Y., Xie T., Zhang H., Li X., Peng T., Sun X., et al. Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin. Mol. Ther. Nucleic Acids. 2022;30:66–79. doi: 10.1016/j.omtn.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Y., Erb M.A. Enabling cancer target validation with genetically encoded systems for ligand-induced protein degradation. Curr. Res. Chem. Biol. 2021;1:100011. doi: 10.1016/j.crchbi.2021.100011. [DOI] [Google Scholar]
- 112.Schreiber S.L. The Rise of Molecular Glues. Cell. 2021;184:3–9. doi: 10.1016/j.cell.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 113.Geiger T.M., Schäfer S.C., Dreizler J.K., Walz M., Hausch F. Clues to molecular glues. Curr. Res. Chem. Biol. 2022;2:100018. doi: 10.1016/j.crchbi.2021.100018. [DOI] [Google Scholar]
- 114.Tan X., Calderon-Villalobos L.I.A., Sharon M., Zheng C., Robinson C.V., Estelle M., Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 115.Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Method. 2009;6:917–922. doi: 10.1038/nmeth.1401. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y., Hsu C.-P., Lu J.-F., Kuchimanchi M., Sun Y.-N., Ma J., Xu G., Zhang Y., Xu Y., Weidner M., et al. FLT3 and CDK4/6 inhibitors: Signaling mechanisms and tumor burden in subcutaneous and orthotopic mouse models of acute myeloid leukemia. J. Pharmacokinet. Pharmacodyn. 2014;41:675–691. doi: 10.1007/s10928-014-9393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krönke J., Udeshi N.D., Narla A., Grauman P., Hurst S.N., McConkey M., Svinkina T., Heckl D., Comer E., Li X., et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jan M., Sperling A.S., Ebert B.L. Cancer therapies based on targeted protein degradation—Lessons learned with lenalidomide. Nat. Rev. Clin. Oncol. 2021;18:401–417. doi: 10.1038/s41571-021-00479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krönke J., Fink E.C., Hollenbach P.W., MacBeth K.J., Hurst S.N., Udeshi N.D., Chamberlain P.P., Mani D.R., Man H.W., Gandhi A.K., et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature. 2015;523:183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sievers Q.L., Petzold G., Bunker R.D., Renneville A., Słabicki M., Liddicoat B.J., Abdulrahman W., Mikkelsen T., Ebert B.L., Thomä N.H. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science. 2018;362:eaat0572. doi: 10.1126/science.aat0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao S., Wang S., Song Y. Novel immunomodulatory drugs and neo-substrates. Biomark. Res. 2020;8:2. doi: 10.1186/s40364-020-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carbonneau S., Sharma S., Peng L., Rajan V., Hainzl D., Henault M., Yang C., Hale J., Shulok J., Tallarico J., et al. An IMiD-inducible degron provides reversible regulation for chimeric antigen receptor expression and activity. Cell Chem. Biol. 2021;28:802–812.e6. doi: 10.1016/j.chembiol.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 123.Koduri V., McBrayer S.K., Liberzon E., Wang A.C., Briggs K.J., Cho H., Kaelin W.G. Peptidic degron for IMiD-induced degradation of heterologous proteins. Proc. Natl. Acad. Sci. USA. 2019;116:2539–2544. doi: 10.1073/pnas.1818109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamanaka S., Shoya Y., Matsuoka S., Nishida-Fukuda H., Shibata N., Sawasaki T. An IMiD-induced SALL4 degron system for selective degradation of target proteins. Commun. Biol. 2020;3:515. doi: 10.1038/s42003-020-01240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bussiere D.E., Xie L., Srinivas H., Shu W., Burke A., Be C., Zhao J., Godbole A., King D., Karki R.G., et al. Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex. Nat. Chem. Biol. 2020;16:15–23. doi: 10.1038/s41589-019-0411-6. [DOI] [PubMed] [Google Scholar]
- 126.Faust T.B., Yoon H., Nowak R.P., Donovan K.A., Li Z., Cai Q., Eleuteri N.A., Zhang T., Gray N.S., Fischer E.S. Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nat. Chem. Biol. 2020;16:7–14. doi: 10.1038/s41589-019-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Du X., Volkov O.A., Czerwinski R.M., Tan H., Huerta C., Morton E.R., Rizzi J.P., Wehn P.M., Xu R., Nijhawan D., et al. Structural Basis and Kinetic Pathway of RBM39 Recruitment to DCAF15 by a Sulfonamide Molecular Glue E7820. Structure. 2019;27:1625–1633.e3. doi: 10.1016/j.str.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 128.Simonetta K.R., Taygerly J., Boyle K., Basham S.E., Padovani C., Lou Y., Cummins T.J., Yung S.L., von Soly S.K., Kayser F., et al. Prospective discovery of small molecule enhancers of an E3 ligase-substrate interaction. Nat. Commun. 2019;10:1402. doi: 10.1038/s41467-019-09358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Słabicki M., Kozicka Z., Petzold G., Li Y.-D., Manojkumar M., Bunker R.D., Donovan K.A., Sievers Q.L., Koeppel J., Suchyta D., et al. The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature. 2020;585:293–297. doi: 10.1038/s41586-020-2374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayor-Ruiz C., Bauer S., Brand M., Kozicka Z., Siklos M., Imrichova H., Kaltheuner I.H., Hahn E., Seiler K., Koren A., et al. Rational discovery of molecular glue degraders via scalable chemical profiling. Nat. Chem. Biol. 2020;16:1199–1207. doi: 10.1038/s41589-020-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Słabicki M., Yoon H., Koeppel J., Nitsch L., Burman S.S.R., Di Genua C., Donovan K.A., Sperling A.S., Hunkeler M., Tsai J.M., et al. Small-molecule-induced polymerization triggers degradation of BCL6. Nature. 2020;588:164–168. doi: 10.1038/s41586-020-2925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellenie B.R., Cheung K.-M.J., Varela A., Pierrat O.A., Collie G.W., Box G.M., Bright M.D., Gowan S., Hayes A., Rodrigues M.J., et al. Achieving In Vivo Target Depletion through the Discovery and Optimization of Benzimidazolone BCL6 Degraders. J. Med. Chem. 2020;63:4047–4068. doi: 10.1021/acs.jmedchem.9b02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H., Dong J., Cai M., Xu Z., Cheng X.D., Qin J.J. Protein degradation technology: A strategic paradigm shift in drug discovery. J. Hematol. Oncol. 2021;14:138. doi: 10.1186/s13045-021-01146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Naito M., Ohoka N., Shibata N. SNIPERs—Hijacking IAP activity to induce protein degradation. Drug Discov. Today Technol. 2019;31:35–42. doi: 10.1016/j.ddtec.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 135.Naito M. Targeted protein degradation and drug discovery. J. Biochem. 2022;172:61–69. doi: 10.1093/jb/mvac041. [DOI] [PubMed] [Google Scholar]
- 136.Ma Z., Ji Y., Yu Y., Liang D. Specific non-genetic IAP-based protein erasers (SNIPERs) as a potential therapeutic strategy. Eur. J. Med. Chem. 2021;216:113247. doi: 10.1016/j.ejmech.2021.113247. [DOI] [PubMed] [Google Scholar]
- 137.Takahashi D., Moriyama J., Nakamura T., Miki E., Takahashi E., Sato A., Akaike T., Itto-Nakama K., Arimoto H. AUTACs: Cargo-Specific Degraders Using Selective Autophagy. Mol. Cell. 2019;76:797–810.e10. doi: 10.1016/j.molcel.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 138.Takahashi D., Arimoto H. Targeting selective autophagy by AUTAC degraders. Autophagy. 2020;16:765–766. doi: 10.1080/15548627.2020.1718362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z., Wang C., Wang Z., Zhu C., Li J., Sha T., Ma L., Gao C., Yang Y., Sun Y., et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds. Nature. 2019;575:203–209. doi: 10.1038/s41586-019-1722-1. [DOI] [PubMed] [Google Scholar]
- 140.Fu Y., Chen N., Wang Z., Luo S., Ding Y., Lu B. Degradation of lipid droplets by chimeric autophagy-tethering compounds. Cell Res. 2021;31:965–979. doi: 10.1038/s41422-021-00532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ji C.H., Kim H.Y., Lee M.J., Heo A.J., Park D.Y., Lim S., Shin S., Ganipisetti S., Yang W.S., Jung C.A., et al. The AUTOTAC chemical biology platform for targeted protein degradation via the autophagy-lysosome system. Nat. Commun. 2022;13:904. doi: 10.1038/s41467-022-28520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao L., Zhao J., Zhong K., Tong A., Jia D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022;7:113. doi: 10.1038/s41392-022-00966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang T., Liu C., Li W., Kuang J., Qiu X., Min L., Zhu L. Targeted protein degradation in mammalian cells: A promising avenue toward future. Comput. Struct. Biotechnol. J. 2022;20:5477–5489. doi: 10.1016/j.csbj.2022.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H., Yao H., Li C., Shi H., Lan J., Li Z., Zhang Y., Liang L., Fang J.-Y., Xu J. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat. Chem. Biol. 2019;15:42–50. doi: 10.1038/s41589-018-0161-x. [DOI] [PubMed] [Google Scholar]
- 145.Banik S.M., Pedram K., Wisnovsky S., Ahn G., Riley N.M., Bertozzi C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature. 2020;584:291–297. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahn G., Banik S.M., Miller C.L., Riley N.M., Cochran J.R., Bertozzi C.R. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 2021;17:937–946. doi: 10.1038/s41589-021-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Y., Teng P., Montgomery N.T., Li X., Tang W. Development of Triantennary N-Acetylgalactosamine Conjugates as Degraders for Extracellular Proteins. ACS Cent. Sci. 2021;7:499–506. doi: 10.1021/acscentsci.1c00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Caianiello D.F., Zhang M., Ray J.D., Howell R.A., Swartzel J.C., Branham E.M.J., Chirkin E., Sabbasani V.R., Gong A.Z., McDonald D.M., et al. Bifunctional small molecules that mediate the degradation of extracellular proteins. Nat. Chem. Biol. 2021;17:947–953. doi: 10.1038/s41589-021-00851-1. [DOI] [PubMed] [Google Scholar]
- 149.Miao Y., Gao Q., Mao M., Zhang C., Yang L., Yang Y., Han D. Bispecific Aptamer Chimeras Enable Targeted Protein Degradation on Cell Membranes. Angew. Chem. Int. Ed. 2021;60:11267–11271. doi: 10.1002/anie.202102170. [DOI] [PubMed] [Google Scholar]
- 150.Cotton A.D., Nguyen D.P., Gramespacher J.A., Seiple I.B., Wells J.A. Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. J. Am. Chem. Soc. 2021;143:593–598. doi: 10.1021/jacs.0c10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H., Han Y., Yang Y., Lin F., Li K., Kong L., Liu H., Dang Y., Lin J., Chen P.R. Covalently Engineered Nanobody Chimeras for Targeted Membrane Protein Degradation. J. Am. Chem. Soc. 2021;143:16377–16382. doi: 10.1021/jacs.1c08521. [DOI] [PubMed] [Google Scholar]
- 152.Santiago L., Daniels G., Wang D., Deng F.-M., Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am. J. Cancer Res. 2017;7:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 153.Costales M.G., Matsumoto Y., Velagapudi S.P., Disney M.D. Small Molecule Targeted Recruitment of a Nuclease to RNA. J. Am. Chem. Soc. 2018;140:6741–6744. doi: 10.1021/jacs.8b01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Costales M.G., Suresh B., Vishnu K., Disney M.D. Targeted Degradation of a Hypoxia-Associated Non-coding RNA Enhances the Selectivity of a Small Molecule Interacting with RNA. Cell Chem. Biol. 2019;26:1180–1186.e5. doi: 10.1016/j.chembiol.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dey S.K., Jaffrey S.R. RIBOTACs: Small Molecules Target RNA for Degradation. Cell Chem. Biol. 2019;26:1047–1049. doi: 10.1016/j.chembiol.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 156.Bozilovic J., Eing L., Berger B.-T., Adhikari B., Weckesser J., Berner N.B., Wilhelm S., Kuster B., Wolf E., Knapp S. Novel, highly potent PROTACs targeting AURORA-A kinase. Curr. Res. Chem. Biol. 2022;2:100032. doi: 10.1016/j.crchbi.2022.100032. [DOI] [Google Scholar]
- 157.Uras I.Z., Moll H.P., Casanova E. Targeting KRAS mutant non-small-cell lung cancer: Past, present and future. Int. J. Mol. Sci. 2020;21:4325. doi: 10.3390/ijms21124325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Westaby D., de Los Dolores Fenor de La Maza M., Paschalis A., Jimenez-Vacas J.M., Welti J., de Bono J., Sharp A. A New Old Target: Androgen Receptor Signaling and Advanced Prostate Cancer. Annu. Rev. Pharmacol. Toxicol. 2022;62:131–153. doi: 10.1146/annurev-pharmtox-052220-015912. [DOI] [PubMed] [Google Scholar]
- 159.Neklesa T., Snyder L.B., Willard R.R., Vitale N., Pizzano J., Gordon D.A., Bookbinder M., Macaluso J., Dong H., Ferraro C., et al. ARV-110: An oral androgen receptor PROTAC degrader for prostate cancer. J. Clin. Oncol. 2019;37:259. doi: 10.1200/JCO.2019.37.7_suppl.259. [DOI] [Google Scholar]
- 160.Snyder L.B., Neklesa T.K., Chen X., Dong H., Ferraro C., Gordon D.A., Macaluso J., Pizzano J., Wang J., Willard R.R., et al. Abstract 43: Discovery of ARV-110, a first in class androgen receptor degrading PROTAC for the treatment of men with metastatic castration resistant prostate cancer. Cancer Res. 2021;81:43. doi: 10.1158/1538-7445.AM2021-43. [DOI] [Google Scholar]
- 161.Xie H., Liu J., Glison D.M.A., Fleming J.B. The clinical advances of proteolysis targeting chimeras in oncology. Explor. Target. Anti Tumor Ther. 2021;2:511–521. doi: 10.37349/etat.2021.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao X., Burris H.A., III, Vuky J., Dreicer R., Sartor A.O., Sternberg C.N., Percent I.J., Hussain M.H.A., Kalebasty A.R., Shen J., et al. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2022;40:17. doi: 10.1200/JCO.2022.40.6_suppl.017. [DOI] [Google Scholar]
- 163.Arvinas Releases Interim Clinical Data Further Demonstrating the Powerful Potential of PROTAC® Protein Degraders ARV-471 and ARV-110|Arvinas. [(accessed on 3 October 2022)]. Available online: https://ir.arvinas.com/news-releases/news-release-details/arvinas-releases-interim-clinical-data-further-demonstrating/
- 164.McAndrew N.P., Finn R.S. Management of ER positive metastatic breast cancer. Semin. Oncol. 2020;47:270–277. doi: 10.1053/j.seminoncol.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 165.Gonzalez T.L., Hancock M., Sun S., Gersch C.L., Larios J.M., David W., Hu J., Hayes D.F., Wang S., Rae J.M. Targeted degradation of activating estrogen receptor α ligand-binding domain mutations in human breast cancer. Breast Cancer Res. Treat. 2020;180:611–622. doi: 10.1007/s10549-020-05564-y. [DOI] [PubMed] [Google Scholar]
- 166.Hu J., Hu B., Wang M., Xu F., Miao B., Yang C.-Y., Wang M., Liu Z., Hayes D.F., Chinnaswamy K., et al. Discovery of ERD-308 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Estrogen Receptor (ER) J. Med. Chem. 2019;62:1420–1442. doi: 10.1021/acs.jmedchem.8b01572. [DOI] [PubMed] [Google Scholar]
- 167.Flanagan J., Qian Y., Gough S., Andreoli M., Bookbinder M., Cadelina G., Bradley J., Rousseau E., Willard R., Pizzano J., et al. Abstract P5-04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Res. 2019;79:P5-04-18. doi: 10.1158/1538-7445.SABCS18-P5-04-18. [DOI] [Google Scholar]
- 168.He W., Zhang H., Perkins L., Bouza L., Liu K., Qian Y., Fan J. Abstract PS18-09: Novel chimeric small molecule AC682 potently degrades estrogen receptor with oral anti-tumor efficacy superior to fulvestrant. Cancer Res. 2021;81:PS18-09. doi: 10.1158/1538-7445.SABCS20-PS18-09. [DOI] [Google Scholar]
- 169.Sun Y., Zhao X., Ding N., Gao H., Wu Y., Yang Y., Zhao M., Hwang J., Song Y., Liu W., et al. PROTAC-induced BTK degradation as a novel therapy for mutated BTK C481S induced ibrutinib-resistant B-cell malignancies. Cell Res. 2018;28:779–781. doi: 10.1038/s41422-018-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nurix Therapeutics I. Nurix Therapeutics Reports Case Study of Patient with Aggressive Non-Hodgkin’s Lymphoma (NHL) Showing a Complete Clinical Response to NX-2127 at the 5th Annual Targeted Protein Degradation (TPD) Summit. [(accessed on 19 October 2022)]. Available online: https://www.globenewswire.com/en/news-release/2022/10/26/2541639/0/en/Nurix-Therapeutics-Reports-Case-Study-of-Patient-with-Aggressive-Non-Hodgkin-s-Lymphoma-NHL-Showing-a-Complete-Clinical-Response-to-NX-2127-at-the-5th-Annual-Targeted-Protein-Degra.html.
- 171.Wang C., Zhang Y., Yang S., Chen W., Xing D. PROTACs for BRDs proteins in cancer therapy: A review. J. Enzym. Inhib. Med. Chem. 2022;37:1694–1703. doi: 10.1080/14756366.2022.2081164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I., et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ameratunga M., Braña I., Bono P., Postel-Vinay S., Plummer R., Aspegren J., Korjamo T., Snapir A., de Bono J.S. First-in-human Phase 1 open label study of the BET inhibitor ODM-207 in patients with selected solid tumours. Br. J. Cancer. 2020;123:1730–1736. doi: 10.1038/s41416-020-01077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang L., Chen Y., Mi Y., Qiao J., Jin H., Li J., Lu Z., Wang Q., Zou Z. ATF2 inhibits ani-tumor effects of BET inhibitor in a negative feedback manner by attenuating ferroptosis. Biochem. Biophys. Res. Commun. 2021;558:216–223. doi: 10.1016/j.bbrc.2020.08.113. [DOI] [PubMed] [Google Scholar]
- 175.Bui M.H., Lin X., Albert D.H., Li L., Lam L.T., Faivre E.J., Warder S.E., Huang X., Wilcox D., Donawho C.K., et al. Preclinical Characterization of BET Family Bromodomain Inhibitor ABBV-075 Suggests Combination Therapeutic Strategies. Cancer Res. 2017;77:2976–2989. doi: 10.1158/0008-5472.CAN-16-1793. [DOI] [PubMed] [Google Scholar]
- 176.Leal A.S., Williams C.R., Royce D.B., Pioli P.A., Sporn M.B., Liby K.T. Bromodomain inhibitors, JQ1 and I-BET 762, as potential therapies for pancreatic cancer. Cancer Lett. 2017;394:76–87. doi: 10.1016/j.canlet.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 177.Doroshow D.B., Eder J.P., LoRusso P.M. BET inhibitors: A novel epigenetic approach. Ann. Oncol. 2017;28:1776–1787. doi: 10.1093/annonc/mdx157. [DOI] [PubMed] [Google Scholar]
- 178.Shapiro G.I., Dowlati A., LoRusso P.M., Eder J.P., Anderson A., Do K.T., Kagey M.H., Sirard C., Bradner J.E., Landau S.B. Abstract A49: Clinically efficacy of the BET bromodomain inhibitor TEN-010 in an open-label substudy with patients with documented NUT-midline carcinoma (NMC) Mol. Cancer Ther. 2015;14:A49. doi: 10.1158/1535-7163.TARG-15-A49. [DOI] [Google Scholar]
- 179.Tontsch-Grunt U., Traexler P.-E., Baum A., Musa H., Marzin K., Wang S., Trapani F., Engelhardt H., Solca F. Therapeutic impact of BET inhibitor BI 894999 treatment: Backtranslation from the clinic. Br. J. Cancer. 2022;127:577–586. doi: 10.1038/s41416-022-01815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu J., Qian Y., Altieri M., Dong H., Wang J., Raina K., Hines J., Winkler J.D., Crew A.P., Coleman K., et al. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem. Biol. 2015;22:755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zengerle M., Chan K.-H., Ciulli A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem. Biol. 2015;10:1770–1777. doi: 10.1021/acschembio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kounde C.S., Shchepinova M.M., Saunders C.N., Muelbaier M., Rackham M.D., Harling J.D., Tate E.W. A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chem. Commun. 2020;56:5532–5535. doi: 10.1039/D0CC00523A. [DOI] [PubMed] [Google Scholar]
- 183.Peter B., Eisenwort G., Sadovnik I., Bauer K., Willmann M., Rülicke T., Berger D., Stefanzl G., Greiner G., Hoermann G., et al. BRD4 degradation blocks expression of MYC and multiple forms of stem cell resistance in Ph+ chronic myeloid leukemia. Am. J. Hematol. 2022;97:1215–1225. doi: 10.1002/ajh.26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ranok Therapeutics Ranok Therapeutics Announces Initiation of Patient Dosing in a Phase 1/2 Clinical Trial of RNK05047, a First-in-Class BRD4-Targeting CHAMPTM Protein Degrader|Ranok Therapeutics Co., Ltd. [(accessed on 19 October 2022)]. Available online: https://ranoktherapeutics.com/newsdetail.html?aid=43.
- 185.Winter G.E., Mayer A., Buckley D.L., Erb M.A., Roderick J.E., Vittori S., Reyes J.M., di Iulio J., Souza A., Ott C.J., et al. BET Bromodomain Proteins Function as Master Transcription Elongation Factors Independent of CDK9 Recruitment. Mol. Cell. 2017;67:5–18.e19. doi: 10.1016/j.molcel.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bai L., Zhou B., Yang C.-Y., Ji J., McEachern D., Przybranowski S., Jiang H., Hu J., Xu F., Zhao Y., et al. Targeted Degradation of BET Proteins in Triple-Negative Breast Cancer. Cancer Res. 2017;77:2476–2487. doi: 10.1158/0008-5472.CAN-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qin C., Hu Y., Zhou B., Fernandez-Salas E., Yang C.-Y., Liu L., McEachern D., Przybranowski S., Wang M., Stuckey J., et al. Discovery of QCA570 as an Exceptionally Potent and Efficacious Proteolysis Targeting Chimera (PROTAC) Degrader of the Bromodomain and Extra-Terminal (BET) Proteins Capable of Inducing Complete and Durable Tumor Regression. J. Med. Chem. 2018;61:6685–6704. doi: 10.1021/acs.jmedchem.8b00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang J., Chen P., Zhu P., Zheng P., Wang T., Wang L., Xu C., Zhou J., Zhang H. Development of small-molecule BRD4 degraders based on pyrrolopyridone derivative. Bioorg. Chem. 2020;99:103817. doi: 10.1016/j.bioorg.2020.103817. [DOI] [PubMed] [Google Scholar]
- 189.Bemis T.A., La Clair J.J., Burkart M.D. Traceless Staudinger ligation enabled parallel synthesis of proteolysis targeting chimera linker variants. Chem. Commun. 2021;57:1026–1029. doi: 10.1039/D0CC05395C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hu R., Wang W.-L., Yang Y.-Y., Hu X.-T., Wang Q.-W., Zuo W.-Q., Xu Y., Feng Q., Wang N.-Y. Identification of a selective BRD4 PROTAC with potent antiproliferative effects in AR-positive prostate cancer based on a dual BET/PLK1 inhibitor. Eur. J. Med. Chem. 2022;227:113922. doi: 10.1016/j.ejmech.2021.113922. [DOI] [PubMed] [Google Scholar]
- 191.Min J., Mayasundari A., Keramatnia F., Jonchere B., Yang S.W., Jarusiewicz J., Actis M., Das S., Young B., Slavish J., et al. Phenyl-Glutarimides: Alternative Cereblon Binders for the Design of PROTACs. Angew. Chem. Int. Ed. 2021;60:26663–26670. doi: 10.1002/anie.202108848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ohoka N., Ujikawa O., Shimokawa K., Sameshima T., Shibata N., Hattori T., Nara H., Cho N., Naito M. Different Degradation Mechanisms of Inhibitor of Apoptosis Proteins (IAPs) by the Specific and Nongenetic IAP-Dependent Protein Eraser (SNIPER) Chem. Pharm. Bull. 2019;67:203–209. doi: 10.1248/cpb.c18-00567. [DOI] [PubMed] [Google Scholar]
- 193.Weisberg E., Chowdhury B., Meng C., Case A.E., Ni W., Garg S., Sattler M., Azab A.K., Sun J., Muz B., et al. BRD9 degraders as chemosensitizers in acute leukemia and multiple myeloma. Blood Cancer J. 2022;12:110. doi: 10.1038/s41408-022-00704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.C4 Therapeutics US Securities and Exchange Commission. [(accessed on 4 October 2022)]; Available online: https://www.sec.gov/Archives/edgar/data/1662579/000119312520243234/d772024ds1.htm.
- 195.Kymera Therapeutics Receives FDA Orphan Drug Designation. [(accessed on 4 October 2022)]. Available online: https://www.globenewswire.com/en/news-release/2022/06/01/2454307/0/en/Kymera-Therapeutics-Receives-FDA-Orphan-Drug-Designation-for-KT-333-a-First-in-Class-Investigational-STAT3-Degrader-for-the-Treatment-of-Peripheral-T-Cell-Lymphoma.html.
- 196.Khan S., Zhang X., Lv D., Zhang Q., He Y., Zhang P., Liu X., Thummuri D., Yuan Y., Wiegand J.S., et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 2019;25:1938–1947. doi: 10.1038/s41591-019-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.He Y., Koch R., Budamagunta V., Zhang P., Zhang X., Khan S., Thummuri D., Ortiz Y.T., Zhang X., Lv D., et al. DT2216-a Bcl-xL-specific degrader is highly active against Bcl-xL-dependent T cell lymphomas. J. Hematol. Oncol. 2020;13:95. doi: 10.1186/s13045-020-00928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kolb R., De U., Khan S., Luo Y., Kim M.-C., Yu H., Wu C., Mo J., Zhang X., Zhang P., et al. Proteolysis-targeting chimera against BCL-XL destroys tumor-infiltrating regulatory T cells. Nat. Commun. 2021;12:1281. doi: 10.1038/s41467-021-21573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.C4 Therapeutics C4 Therapeutics Receives Study May Proceed Letter from U.S. [(accessed on 5 October 2022)]. Available online: https://www.globenewswire.com/news-release/2022/09/29/2525008/0/en/C4-Therapeutics-Receives-Study-May-Proceed-Letter-from-U-S-FDA-to-Initiate-Phase-1-2-Clinical-Trial-of-CFT1946-an-Orally-Bioavailable-BiDAC-Degrader-in-BRAF-V600-Mutant-Solid-Cance.html.
- 200.Sowa M.E., Kreger B., Baddour J., Liang Y., Simard J.R., Poling L., Li P., Yu R., Hart A., Agafonov R.V., et al. Abstract 2158: Preclinical evaluation of CFT1946 as a selective degrader of mutant BRAF for the treatment of BRAF driven cancers. Cancer Res. 2022;82:2158. doi: 10.1158/1538-7445.AM2022-2158. [DOI] [Google Scholar]
- 201.Cullgen Announces Chinese NMPA Allowance of Investigational New Drug Application of TRK Degrader to Begin Clinical Trials. [(accessed on 18 October 2022)]. Available online: https://www.cullgen.com/news.
- 202.C4 Therapeutics C4 Therapeutics Presents Pre-clinical Data on CFT8919, A Selective Degrader of EGFR L858R, at Keystone Symposium on Targeted Protein Degradation—C4 Therapeutics. [(accessed on 5 October 2022)]. Available online: https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-presents-pre-clinical-data-cft8919-selective/
- 203.Chutake Y., Mayo M., Chen D., Enerson B., Cho P., Filiatrault J., Brown C., Placke M., Adams M., Karnik R., et al. Abstract 3934: KT-253, a highly potent and selective heterobifunctional MDM2 degrader for the treatment of wildtype p53 tumors with superior potency and differentiated biological activity compared to small molecule inhibitors (SMI) Cancer Res. 2022;82:3934. doi: 10.1158/1538-7445.AM2022-3934. [DOI] [Google Scholar]
- 204.Hagner P.R., Man H.-W., Fontanillo C., Wang M., Couto S., Breider M., Bjorklund C., Havens C.G., Lu G., Rychak E., et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood. 2015;126:779–789. doi: 10.1182/blood-2015-02-628669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lopez-Girona A., Groocock L., Mo Z., Narla R.K., Janardhanan P., Wood S., Mendy D., Barnes L., Peng S., Jankeel D., et al. CC-99282 IS a Novel Cereblon E3 Ligase Modulator (Celmod) Agent with Potent and Broad Antitumor Activity in Preclinical Models of Diffuse Large B-Cell Lymphoma (DLBCL) Hematol. Oncol. 2021;39:315–316. doi: 10.1002/hon.144_2880. [DOI] [Google Scholar]
- 206.Carrancio S., Groocock L., Janardhanan P., Jankeel D., Galasso R., Guarinos C., Narla R.K., Groza M., Leisten J., Pierce D.W., et al. CC-99282 is a Novel Cereblon (CRBN) E3 Ligase Modulator (CELMoD) Agent with Enhanced Tumoricidal Activity in Preclinical Models of Lymphoma. Blood. 2021;138:1200. doi: 10.1182/blood-2021-148068. [DOI] [Google Scholar]
- 207.Carrancio S., Fontanillo C., Galasso R., Colombo M., Wood S., Guarinos C., Panjkovich A., Jankeel D., Blattler A., Janardhanan P., et al. Abstract 3932: Pathway interaction and mechanistic synergy of CC-99282, a novel cereblon E3 ligase modulator (CELMoD) agent, with enhancer of zeste homolog 2 inhibitors (EZH2is) Cancer Res. 2022;82:3932. doi: 10.1158/1538-7445.AM2022-3932. [DOI] [Google Scholar]
- 208.Berdeja J., Ailawadhi S., Horwitz S.M., Matous J.V., Mehta-Shah N., Martin T., Muchtar E., Richardson P.G., Richard S., Bhutani M., et al. A Phase 1 Study of CFT7455, a Novel Degrader of IKZF1/3, in Multiple Myeloma and Non-Hodgkin Lymphoma. Blood. 2021;138:1675. doi: 10.1182/blood-2021-153575. [DOI] [Google Scholar]
- 209.C4 Therapeutics C4 Therapeutics Presents Pre-clinical Data on CFT7455, a Novel IKZF1/3 Degrader for the Treatment of Hematologic Malignancies, at the 16th Annual International Conference on Malignant Lymphoma—C4 Therapeutics. [(accessed on 4 October 2022)]. Available online: https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-presents-pre-clinical-data-cft7455-novel-ikzf13/
- 210.Hansen J.D., Correa M., Nagy M.A., Alexander M., Plantevin V., Grant V., Whitefield B., Huang D., Kercher T., Harris R., et al. Discovery of CRBN E3 Ligase Modulator CC-92480 for the Treatment of Relapsed and Refractory Multiple Myeloma. J. Med. Chem. 2020;63:6648–6676. doi: 10.1021/acs.jmedchem.9b01928. [DOI] [PubMed] [Google Scholar]
- 211.Richardson P.G., Ocio E., Raje N.S., Gregory T., White D., Oriol A., Sandhu I., Raab M.-S., LeBlanc R., Rodriguez C., et al. CC-92480, a Potent, Novel Cereblon E3 Ligase Modulator (CELMoD) Agent, in Combination with Dexamethasone (DEX) and Bortezomib (BORT) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Preliminary Results from the Phase 1/2 Study CC-92480-MM-002. Blood. 2021;138:2731. doi: 10.1182/blood-2021-147812. [DOI] [Google Scholar]
- 212.Richardson P.G., Vangsted A.J., Ramasamy K., Trudel S., Martínez J., Mateos M.-V., Otero P.R., Lonial S., Popat R., Oriol A., et al. First-in-human phase I study of the novel CELMoD agent CC-92480 combined with dexamethasone (DEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) J. Clin. Oncol. 2020;38:8500. doi: 10.1200/JCO.2020.38.15_suppl.8500. [DOI] [Google Scholar]
- 213.Wong L., Lamba M., Nunez M.D.J., Bauer D., Richardson P.G., Bahlis N.J., Vangsted A.J., Ramasamy K., Trudel S., Martinez-Lopez J., et al. Dose- and Schedule-Dependent Immunomodulatory Effects of the Novel CELMoD Agent CC-92480 in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:47–48. [Google Scholar]
- 214.Nunes J., McGonagle G.A., Eden J., Kiritharan G., Touzet M., Lewell X., Emery J., Eidam H., Harling J.D., Anderson N.A. Targeting IRAK4 for Degradation with PROTACs. ACS Med. Chem. Lett. 2019;10:1081–1085. doi: 10.1021/acsmedchemlett.9b00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Solomon J., Bonazzi S., d’Hennezel E., Beckwith R., Xu L., Fazal A., Magracheva A., Ramesh R., Cernijenko A., Antonakos B., et al. Targeted degradation of IKZF2 for cancer immunotherapy. Biol. Sci. 2022 doi: 10.21203/RS.3.RS-1531006/V1. [DOI] [Google Scholar]
- 216.Matyskiela M.E., Lu G., Ito T., Pagarigan B., Lu C.-C., Miller K., Fang W., Wang N.-Y., Nguyen D., Houston J., et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature. 2016;535:252–257. doi: 10.1038/nature18611. [DOI] [PubMed] [Google Scholar]
- 217.Nishiguchi G., Keramatnia F., Min J., Chang Y., Jonchere B., Das S., Actis M., Price J., Chepyala D., Young B., et al. Identification of Potent, Selective, and Orally Bioavailable Small-Molecule GSPT1/2 Degraders from a Focused Library of Cereblon Modulators. J. Med. Chem. 2021;64:7296–7311. doi: 10.1021/acs.jmedchem.0c01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Powell C.E., Du G., Che J., He Z., Donovan K.A., Yue H., Wang E.S., Nowak R.P., Zhang T., Fischer E.S., et al. Selective Degradation of GSPT1 by Cereblon Modulators Identified via a Focused Combinatorial Library. ACS Chem. Biol. 2020;15:2722–2730. doi: 10.1021/acschembio.0c00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hansen J.D., Correa M., Alexander M., Nagy M., Huang D., Sapienza J., Lu G., LeBrun L.A., Cathers B.E., Zhang W., et al. CC-90009: A Cereblon E3 Ligase Modulating Drug That Promotes Selective Degradation of GSPT1 for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2021;64:1835–1843. doi: 10.1021/acs.jmedchem.0c01489. [DOI] [PubMed] [Google Scholar]
- 220.Gavory G., Ghandi M., d’Alessandro A.-C., Bonenfant D., Chicas A., Delobel F., Demarco B., Flohr A., King C., Laine A.-L., et al. Abstract 3929: Identification of MRT-2359 a potent, selective and orally bioavailable GSPT1-directed molecular glue degrader (MGD) for the treatment of cancers with Myc-induced translational addiction. Cancer Res. 2022;82:3929. doi: 10.1158/1538-7445.AM2022-3929. [DOI] [Google Scholar]
- 221.Bond M.J., Crews C.M. Proteolysis targeting chimeras (PROTACs) come of age: Entering the third decade of targeted protein degradation. RSC Chem. Biol. 2021;2:725–742. doi: 10.1039/D1CB00011J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu J., Chen H., Liu Y., Shen Y., Meng F., Kaniskan H.Ü., Jin J., Wei W. Cancer Selective Target Degradation by Folate-Caged PROTACs. J. Am. Chem. Soc. 2021;143:7380–7387. doi: 10.1021/jacs.1c00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Furie R.A., Hough D.R., Gaudy A., Ye Y., Korish S., Delev N., Weiswasser M., Zhan X., Schafer P.H., Werth V.P. Iberdomide in patients with systemic lupus erythematosus: A randomised, double-blind, placebo-controlled, ascending-dose, phase 2a study. Lupus Sci. Med. 2022;9:e000581. doi: 10.1136/lupus-2021-000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tomoshige S., Nomura S., Ohgane K., Hashimoto Y., Ishikawa M. Discovery of Small Molecules that Induce the Degradation of Huntingtin. Angew. Chem. Int. Ed. 2017;56:11530–11533. doi: 10.1002/anie.201706529. [DOI] [PubMed] [Google Scholar]
- 225.Haniff H.S., Tong Y., Liu X., Chen J.L., Suresh B.M., Andrews R.J., Peterson J.M., O’Leary C.A., Benhamou R.I., Moss W.N., et al. Targeting the SARS-CoV-2 RNA genome with small molecule binders and ribonuclease targeting chimera (RiboTAC) degraders. ACS Cent. Sci. 2020;6:1713–1721. doi: 10.1021/acscentsci.0c00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cheng B., Ren Y., Cao H., Chen J. Discovery of novel resorcinol diphenyl ether-based PROTAC-like molecules as dual inhibitors and degraders of PD-L1. Eur. J. Med. Chem. 2020;199:112377. doi: 10.1016/j.ejmech.2020.112377. [DOI] [PubMed] [Google Scholar]
- 227.Wang Y., Zhou Y., Cao S., Sun Y., Dong Z., Li C., Wang H., Yao Y., Yu H., Song X., et al. In vitro and in vivo degradation of programmed cell death ligand 1 (PD-L1) by a proteolysis targeting chimera (PROTAC) Bioorgan. Chem. 2021;111:104833. doi: 10.1016/j.bioorg.2021.104833. [DOI] [PubMed] [Google Scholar]
- 228.Zhang C., He S., Zeng Z., Cheng P., Pu K. Smart Nano-PROTACs Reprogram Tumor Microenvironment for Activatable Photo-Metabolic Cancer Immunotherapy. Angew. Chem. 2022;134:e202114957. doi: 10.1002/ange.202114957. [DOI] [PubMed] [Google Scholar]