Abstract
Human alveolar macrophages (HAM) are the major resident phagocytic cells of the gas-exchanging areas of the lung. Following contact with macrophages, bacteria enter phagosomes, which gradually acquire the characteristics of terminal phagolysosomes, with incorporation of lysosome-associated membrane protein (LAMP). We measured the binding of type 1 Streptococcus pneumoniae to the surface of HAM and then measured subsequent internalization and phagosomal incorporation of LAMP-1 under various opsonic conditions. Opsonization with serum containing immunoglobulin resulted in significantly greater binding of pneumococci to HAM compared with opsonization with immunoglobulin G (IgG)-depleted serum containing complement, which in turn resulted in marginally increased binding over that observed in the absence of opsonization. Internalization of opsonized S. pneumoniae gradually increased to a maximum of 20% of bound bacteria by 120 min of warm incubation, with 20% of internalized pneumococci being localized within LAMP-containing compartments by 80 min. Internalization of opsonized S. pneumoniae by HAM correlated with a reduction of bacterial viability. When inocula were adjusted so that pneumococcal binding under different conditions was equalized, subsequent internalization, trafficking to LAMP-containing compartments, and reduction of bacterial viability were less efficient in the absence of opsonization than that observed following opsonization with adsorbed or IgG-replete adsorbed serum. Once bound to the surface of HAM, pneumococci opsonized with adsorbed serum with or without IgG were internalized, processed, and killed equally well. In conclusion, binding, intracellular trafficking, and killing of S. pneumoniae by HAM are each significantly increased by opsonization with serum containing immunogloblin and/or complement.
Pneumonia caused by Streptococcus pneumoniae is a consequence of failure by the host to clear or kill pneumococci inhaled into the lung. The alveolar macrophage is the resident phagocyte of the alveoli of normal human lungs and is responsible for the removal of pathogens that enter gas-exchanging areas. Pathogens can be recognized by a variety of receptors on the surface of the macrophage, including Fc receptors (which recognize the Fc component of cognate immunoglobulin bound to bacteria in immune individuals), complement receptors CR1, CR3, and CR4 (which recognize C3b stabilized on the bacterial surface), and other receptors, including the macrophage scavenger receptor, platelet-activating factor receptor, CD14 (5, 18), and possibly members of the Toll family (29).
Following binding to the surface, macrophages will phagocytize both opsonized and unopsonized pathogens, which are internalized and killed in a sequence of discrete stages. Studies with Mycobacterium spp. (6), Leishmania spp. (26), Listeria monocytogenes (24), and Neisseria meningitidis (21) have shown that microorganisms enter phagosomes, which mature into terminal phagolysosomes coincident with acquisition of numerous proteins, including the late endosome/lysosome-associated membrane proteins LAMP-1 and LAMP-2 (3), from the endocytic network.
Human alveolar macrophages (HAM) have been demonstrated by radioisotopic methods (16) to phagocytize S. pneumoniae. Capsulate S. pneumoniae organisms have been found to bind poorly to human macrophages (compared to noncapsulate organisms), and therefore their subsequent internalization has been difficult to study. Although opsonization of pathogens markedly increases binding of particles to the surface of macrophages, other organisms, such as Staphylococcus aureus, have been demonstrated to bind to and be phagocytized by HAM in the absence of opsonization (14).
We determined the kinetics of internalization and trafficking of a capsulate type 1 S. pneumoniae strain to HAM compartments containing LAMP-1 and measured these kinetics under conditions that might be operating in nonimmune humans. We questioned whether attachment of S. pneumoniae to the surface of HAM in the absence of cognate immunoglobulin or complement results in intracellular trafficking with benign consequences for the pathogen.
MATERIALS AND METHODS
Harvesting of alveolar macrophages.
Healthy volunteers gave informed consent to bronchoscopy and bronchoalveolar lavage (BAL). None had a recent history of viral infection or antibiotic use. Lavage with 200 ml of warm sterile saline was carried out after the bronchoscope was lodged in a middle-lobe subsegmental bronchus under midazolam sedation, and macrophages were derived from BAL fluid using standard methods. This procedure was approved by the South Sheffield Research Ethics Committee (96/270). Briefly, BAL samples were filtered using a coarse porcelain sieve and transferred into 50-ml centrifuge tubes. A pellet was obtained using a short spin (102 × g for 5 min) and was resuspended in serum-free RPMI 1640 medium (Gibco BRL, Life Technologies, Paisley, United Kingdom [U.K.]) containing antibiotics (penicillin [40 IU/ml], streptomycin [75 IU/ml], gentamicin [80 IU/ml], and amphotericin B [0.5 IU/ml]) and incubated at 37°C for 60 min without agitation. Following incubation, the cells were washed and resuspended in RPMI medium containing 2 mM l-glutamine and 10% newborn calf serum (both from Gibco BRL, Life Technologies). Cells were then counted and diluted to 106 cells/ml. One milliliter of cell suspension was pipetted into tissue culture wells in 24-well plates (Nunc, Paisley, U.K.) with or without 13-mm glass coverslips (BDH, Poole, England) and incubated at 37°C in 5% CO2 until use on the third day of incubation. At this point, no antibiotic activity was demonstrated in the lysed cells by bioassay (data not shown).
Preparation and opsonization of S. pneumoniae.
Type 1 S. pneumoniae (WHO reference laboratory strain SSISP 1/1 from Statens Seruminstitut, Copenhagen, Denmark) was grown to mid-log phase in brain heart infusion (Oxoid, Unipath Ltd., Basingstoke, U.K.) with 10% fetal calf serum (Bioclear Ltd., Wiltshire, U.K.). Aliquots of this broth were prepared monthly and stored at −80°C. Opsonizing serum was prepared from four individuals 3 months following vaccination with the 23-valent pneumococcal vaccine. Each serum produced a protective antibody response, which was confirmed by the WHO Reference Laboratory, and the four were pooled. To remove cognate antibody, the serum was then adsorbed with the type 1 reference strain of S. pneumoniae at 4°C for 30 min. To then replace immunoglobulin (i.e., to make immunoglobulin G [IgG]-replete adsorbed serum), the eluted IgG from a protein A column over which had been passed the same volume of complete immune serum was added to adsorbed serum. Complete immune serum and IgG-replete adsorbed serum deposited C3b and IgG onto the surface of opsonized pneumococci, as demonstrated by fluorescein isothiocyanate (FITC)-conjugated anti-IgG Fc and anti-C3b monoclonal antibodies (Sigma Chemical Co., St. Louis, Mo.), while adsorbed serum deposited C3b but no IgG. On thawing, aliquots of bacterial suspensions were spun to a pellet three times at 2,000 × g for 3 min, resuspended in 1 ml of phosphate-buffered saline (PBS) to wash, and then resuspended in RPMI medium plus 10% opsonizing serum (either whole serum, adsorbed serum, or IgG-replete adsorbed serum) or 10% PBS (control) and incubated at 37°C for 15 min on a rotating rack. Bacteria were then spun and washed twice in PBS and resuspended in enriched RPMI medium (with 2 mM l-glutamine and 10% fetal calf serum) at 4°C at various concentrations. Bacteria were vortexed vigorously in the presence of glass beads (BDH Laboratories) for 30 s to disrupt clumps of bacteria.
Experimental infection of alveolar macrophages.
Alveolar macrophages were washed once with warm enriched RPMI medium, which was then replaced with enriched RPMI medium plus 10% bovine serum albumin (BSA) to restrict bacterial adherence to the plastic surfaces of wells. HAM were incubated with BSA medium for 30 min and then cooled to 4°C. The medium was replaced with 250 μl of the prepared bacterial suspension and incubated at 4°C for 60 min (at this temperature, bound bacteria are not internalized). To compare the effects of different conditions of opsonization on intracellular trafficking, inocula were adjusted so that bacteria subject to each treatment became bound to the macrophage surface at approximately five pneumococci per cell. After this, the suspension was aspirated and the wells were washed once with 1 ml of warm enriched RPMI medium, which was then aspirated and replaced with 250 μl of enriched RPMI medium and the whole was incubated at 37°C in 5% CO2. HAM were fixed for immunofluorescence using 4% paraformaldehyde immediately after the 60-min cold incubation and at intervals over the period of warm incubation (during which internalization could proceed).
Measurement of internalization and colocalization with LAMP-1.
After fixation, HAM were washed three times by flooding with 1 ml of PBS and were then incubated for 10 min with 200 μl of PBS containing a 1:20 dilution of rabbit antipneumococcal (type 1) antibody (Statens Seruminstitut). Cells were then washed and incubated for 10 min with PBS containing a 1:20 dilution of FITC-conjugated goat anti-rabbit IgG Fc in 1:20 goat serum. HAM were then washed and incubated for 15 min with 400 μl of a 1:10 dilution in RPMI of mouse anti-human LAMP-1 antibody (H4A3; American Type Culture Collection, Manassas, Va.) in the presence of 0.01% saponin (Sigma), followed by PBS washing and incubation for 15 min with 400 μl of a 1:200 dilution of CY-3 conjugated sheep anti-mouse IgG antibody (Sigma). This antibody was diluted in stock solution containing 100 μl of Triton X-100 and 200 μl of 10% sodium dodecyl sulfate (both from BDH Laboratories), 9 ml of PBS, 500 μl of sheep serum (Sigma), and 4 μl of DAPI (4′,6′-diamidino-2-phenylindole) (Molecular Probes, Leiden, The Netherlands). Following this incubation, wells were washed once with PBS and twice with distilled water, aspirated, dried, and mounted on glass slides in Vectashield with DAPI (Vector Laboratories, Burlingame, Calif.). Viewing was carried out with a Leica DMRB fluorescent microscope using three suitable filters and a triple filter.
First, the percentage of HAM associated with bacteria was calculated. Then, the number of internalized bacteria was estimated by subtracting the number of extracellular bacteria (FITC associated) from the total number of bacteria (exhibiting DAPI fluorescence) per macrophage. From the first 100 HAM with associated bacteria, the number of bacteria associated per cell, the number of bacteria internalized per cell, and the number of bacteria colocalizing with LAMP were counted. From these data, the mean number of bacteria bound per cell was calculated, along with the percentage of bound bacteria internalized and the percentage of internalized bacteria colocalized with LAMP. For assessment of binding, cells were also Gram stained.
Assay of loss of viability of S. pneumoniae.
The killing of pneumococci by HAM was estimated by expressing the viable count from wells containing live HAM as a percentage of the “expected” bacterial viability observed in wells containing paraformaldehyde-fixed cells. All HAM were cultured as described above and washed with warm enriched RPMI. Then, cells in control wells were fixed by a 2-min incubation with 2% paraformaldehyde, after which all wells were washed twice with enriched RPMI and incubated at 37°C with enriched RPMI plus BSA, as described above. After 30 min, plates were placed on ice and the medium in each well was replaced with 250 μl of an appropriate (to equalize binding between opsonic treatments) bacterial suspension. Wells containing live or fixed cells and bacteria were all incubated at 4°C for 60 min. At the end of this incubation, the preparations were washed with 1 ml of warm enriched RPMI medium and incubated for 3 h at 37°C in 5% CO2 in 250 μl of warm enriched RPMI medium. At this point, and after 1, 2, and 3 h of warm incubation, HAM were lysed using 2% saponin (750 μl) with vigorous agitation. A viable count of S. pneumoniae was taken from the lysate by a dilutional technique. The expected bacterial viability was calculated as follows. First, the viable count after 1, 2, and 3 h of warm incubation was expressed as a multiple of the viable count at the beginning of warm incubation. Then the value obtained from live wells was expressed as a percentage (expected bacterial viability) of that from control wells.
Statistical analysis.
For comparisons between opsonic conditions, data were subjected to Kruskal-Wallis analysis. The area under the curve (AUC) was used as a summary statistic where data were collected at multiple intervals over a period of incubation. Where the null hypothesis was rejected, statistical significance was assessed by the Mann-Whitney U test. These tests were carried out using Stata 5 (Statacorp).
RESULTS
Binding of S. pneumoniae to HAM.
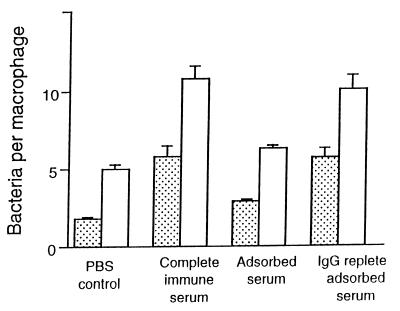
Binding of S. pneumoniae to HAM at 4°C is indicated in Fig. 1. Cells were challenged for 60 min with 1 × 107 CFU (multiplicity of infection [MOI], 10) or 2 × 107 CFU (MOI, 20) for 60 min. As expected, significantly greater binding was observed with S. pneumoniae opsonized with complete immune serum than with nonopsonized pneumococci. Opsonization with adsorbed serum marginally increased binding of S. pneumoniae over that of nonopsonized pneumococci (which was significant at an MOI of 10 but not at an MOI of 20). Opsonization with IgG-replete adsorbed serum restored binding to the level observed after opsonization with complete immune serum. The binding observed was proportional to the size of the inoculum. By adjusting the inocula of opsonized and nonopsonized S. pneumoniae, the number of bacteria bound to the surface of alveolar macrophages after 60 min of incubation at 4°C could be equalized. The inocula required to deposit a mean of five pneumococci onto the macrophage surface were as follows: (i) nonopsonization, 4 × 107 CFU; (ii) adsorbed serum, 3 × 107 CFU; (iii) IgG-replete adsorbed serum, 1 × 107 CFU; and (iv) complete immune serum, 1 × 107 CFU.
FIG. 1.
Influence of opsonization on binding of S. pneumoniae to human macrophages. Inocula: 1 × 107 CFU (MOI = 10) (stippled columns) and 2 × 107 CFU (MOI = 20) (open columns). For an MOI of 10: opsonization with PBS versus complete immune serum, P = 0.001; with PBS versus adsorbed serum, P = 0.04; with adsorbed versus complete immune serum, P = 0.01; and with adsorbed versus IgG-replete adsorbed serum, P = 0.01. For an MOI of 20: opsonization with PBS versus complete immune serum, P = 0.001; with PBS versus adsorbed serum, P = 0.09; with adsorbed versus complete immune serum, P = 0.01; and with adsorbed versus IgG-replete adsorbed serum, P = 0.001. (Kruskal-Wallis analysis, Mann-Whitney U test, eight different human donors.)
Trafficking of opsonized S. pneumoniae to LAMP-containing intracellular compartments.
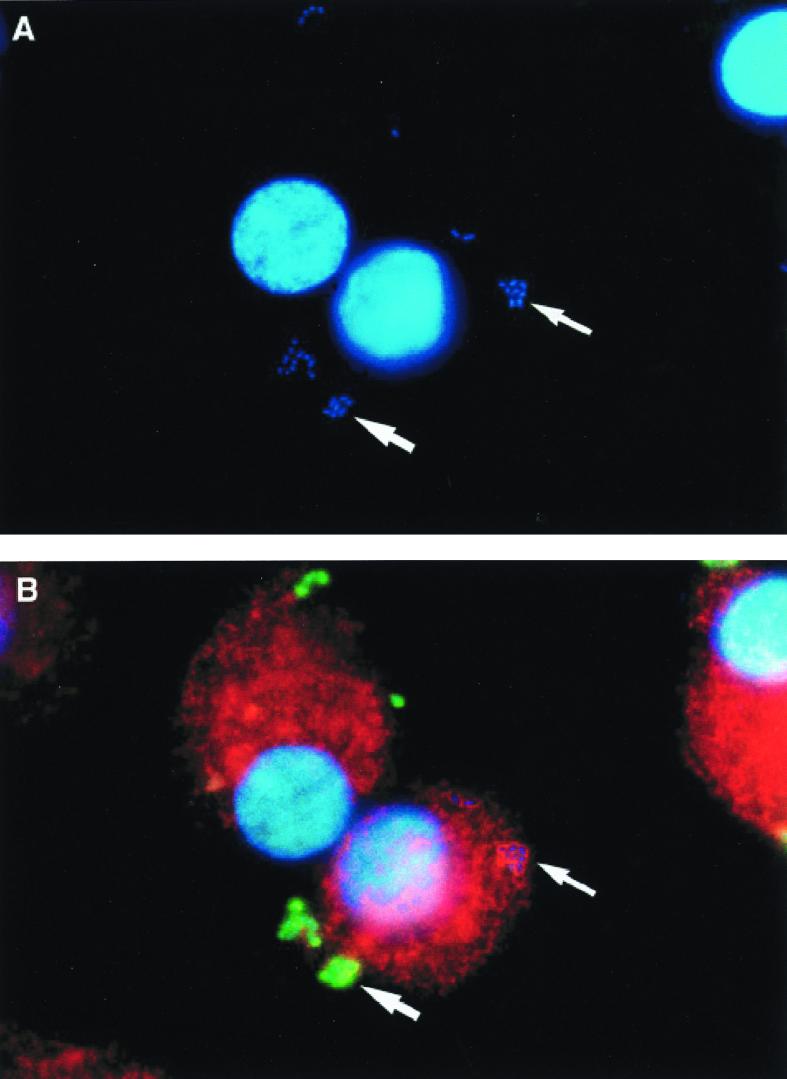
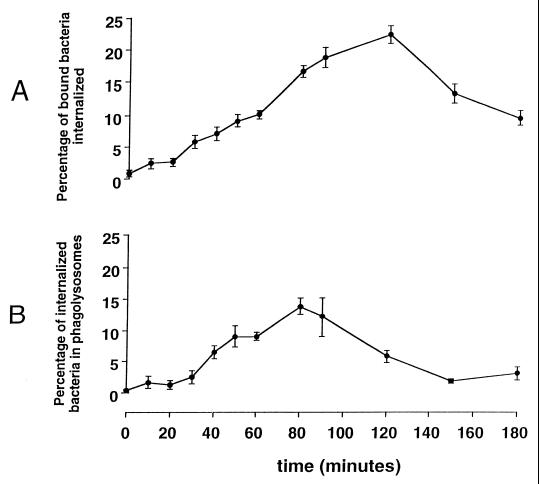
Following the period of cold incubation of HAM with S. pneumoniae opsonized with complete immune serum and warming of macrophages to 37°C, internalization of S. pneumoniae by HAM was permitted. Following internalization, phagosomes containing S. pneumoniae colocalized with LAMP-1 (Fig. 2). The kinetics of internalization of S. pneumoniae are demonstrated in Fig. 3. Under these conditions, the proportion of associated pneumococci that were identified as internalized within alveolar macrophages gradually rose to a maximum of 20% by 120 min of warm incubation. The proportion of internalized S. pneumoniae colocalizing with LAMP-1 gradually increased to a maximum of 20% by 80 min and then declined.
FIG. 2.
Immunofluorescence of HAM infected with S. pneumoniae. The two panels are identical fields of view of cells after 80 min of warm incubation. (A) Through the UV filter, DAPI-labeled bacteria are shown in association with macrophages. (B) Through the triple filter, some pneumococci are seen to fluoresce green because they are accessible to FITC-labeled antipneumococcal antibody (larger arrow). This indicates that they have not been internalized. Other pneumococci, however, do not fluoresce green, and some are seen to colocalize with the CY-3-immunolabeled LAMP, which forms red rings around them, indicating that they are within late endosomal or lysosomal compartments (smaller arrow).
FIG. 3.
Intracellular trafficking of S. pneumoniae opsonized with complete immune serum. Data are the means ± the standard errors of the means for nine different human donors.
Effect of opsonization on intracellular trafficking and loss of viability of S. pneumoniae.
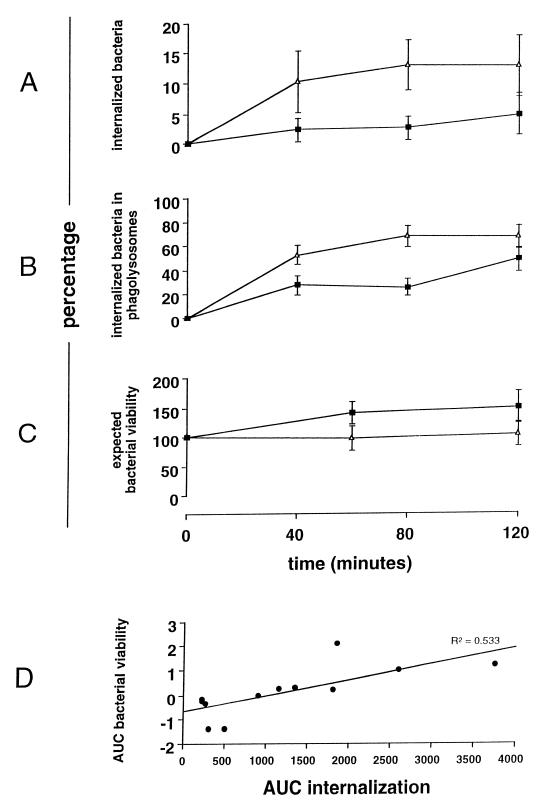
Internalization of bacteria by HAM and their colocalization with LAMP-1 are shown in Fig. 4A and B. Inocula were adjusted to equalize binding after 60 min of initial incubation at 4°C. Following warming to 37°C, there was more rapid internalization and trafficking to terminal phagolysosomes of S. pneumoniae opsonized with complete immune serum than of nonopsonized S. pneumoniae.
FIG. 4.
Comparison of intracellular trafficking of opsonized and unopsonized pneumococci and correlation with loss of viability. Data for pneumococci opsonized with complete immune serum (Δ) and nonopsonized bacteria (i.e., PBS control) (■) are shown. Data in panels A to C are the means ± the standard errors of the means for nine different human donors. (A) Percentage of bound bacteria internalized (z = 2.3; P = 0.02); (B) percentage of internalized bacteria colocalized with LAMP-1 (z = 2.3; P = 0.02; (C) percentage of expected bacterial viability of S. pneumoniae (z = −2.3, P = 0.02; Mann-Whitney test for AUC); (D) correlation of the AUCs of internalization and loss of viability of S. pneumoniae from 12 experiments in which internalization and viability were measured at the same point.
The percentage of expected growth of S. pneumoniae incubated with live compared to fixed alveolar macrophages under the conditions of opsonization with complete immune serum or no opsonization is shown in Fig. 4C. There was a significantly greater loss of expected bacterial viability of pneumococci opsonized with complete serum at 60 min, despite equivalent binding (five bacteria/macrophage) following adjustment of the inoculum.
Internalization correlated with killing of S. pneumoniae.
To correlate internalization with killing of S. pneumoniae, data for each were summarized using the AUC summary statistic of data for each chase. For each volunteer's cells, the AUC (internalization) was plotted against the loss of expected bacterial viability (difference between AUC[fixed cells] and AUC[live alveolar macrophages]). There was a correlation of 0.71 (R2 = 0.5; P = 0.006) between killing and internalization in these assays (Fig. 4D).
Intracellular trafficking and killing of S. pneumoniae in the absence of immunoglobulin.
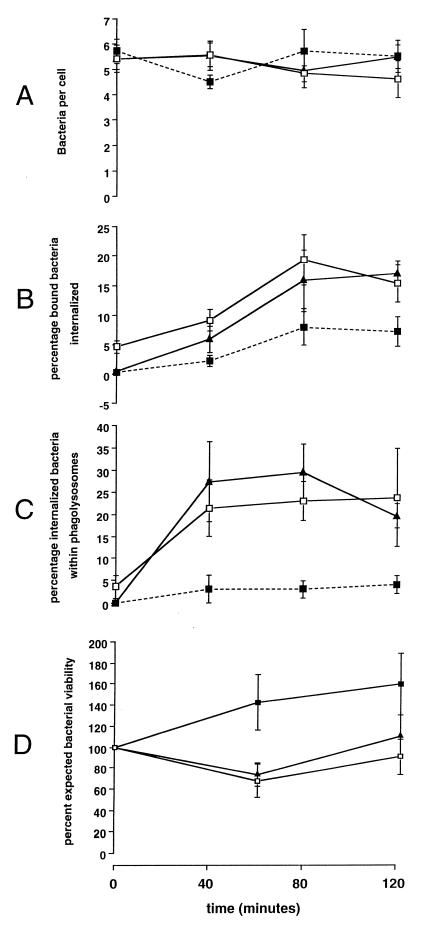
In separate experiments, the intracellular trafficking and killing of S. pneumoniae under conditions of opsonization with adsorbed serum or IgG-replete adsorbed serum or of no opsonization were compared after equalization of binding (Fig. 5).
FIG. 5.
Binding, internalization, percentage of internalized bacteria within terminal phagolysosomes, and loss of viability of S. pneumoniae under various conditions of opsonization. ■, no opsonization; ▴, opsonization with adsorbed serum; □, opsonization with IgG-replete adsorbed serum (data are means ± standard errors of the means for six different human donors). (A) Association of bacteria with human alveolar macrophages. (B) Percentage of associated bacteria that were internalized (Kruskal-Wallis analysis for AUC; P = 0.02): adsorbed serum versus no opsonization, P = 0.05; IgG-replete adsorbed serum verus no opsonization, P = 0.03 (Mann-Whitney U test). (C) Percentage of internalized bacteria that were seen within terminal phagolysosomes (Kruskal-Wallis analysis for AUC, P = 0.01): IgG-depleted serum versus no opsonization, P = 0.03; IgG-replete adsorbed serum versus no opsonization, P = 0.04 (Mann-Whitney U test). (D) Loss of viability of S. pneumoniae: nonopsonized versus adsorbed serum, z = −1.95, P = 0.05; nonopsonized versus replete serum, z = 2.17, P = 0.03; adsorbed versus IgG-replete adsorbed serum, z = −1.12, P = 0.26 (Mann-Whitney U test, n = six different human donors).
The number of bacteria initially bound to HAM after incubation at 4°C for 1 h in each case was five. The subsequent association of bacteria with alveolar macrophages remained relatively constant over the 120-min warm incubation period (Fig. 5A). When the rates of internalization of pneumococci that either were not opsonized or were opsonized with adsorbed or IgG-replete adsorbed serum were compared (Fig. 5B), it was shown that nonopsonized bacteria were internalized relatively slowly, with only 5% of associated bacteria being internalized by 120 min. Bacteria opsonized with adsorbed serum or IgG-replete adsorbed serum were internalized approximately three times as quickly over this period, though there was no difference in the rate of internalization between these two conditions. The proportion of internalized bacteria seen to colocalize with LAMP-1 (Fig. 5C) was four to five times greater following opsonization with adsorbed or IgG-replete serum than the proportion of nonopsonized organisms. Nonetheless, a small proportion of nonopsonized organisms colocalized with LAMP. There was no significant difference between the rate of LAMP-1 colocalization of phagosomes containing S. pneumoniae opsonized with adsorbed or IgG-replete adsorbed serum. Likewise, both conditions resulted in increased loss of bacterial viability compared to nonopsonized pneumococci over 120 min (Fig. 5D). In separate experiments, supernatants of washed paraformaldehyde-fixed HAM did not inhibit growth of S. pneumoniae (data not shown).
DISCUSSION
In this study, we demonstrated that S. pneumoniae binds to HAM and can be internalized and processed to phagosomes which gradually acquire the late endosomal/lysosomal marker LAMP-1. In parallel with these events, there is a loss of viability of bacteria that correlates with internalization. Although binding of S. pneumoniae to HAM is increased by opsonization, it is apparent that opsonization also results in more rapid internalization of pneumococci and maturation of phagosomes. These results suggest that non-Fc and non-CR macrophage receptors available to bind S. pneumoniae to HAM transduce internalization and intracellular processing at lower levels of efficiency. In nonimmune individuals, binding of S. pneumoniae to HAM is likely less efficient than it is in immune individuals, but if complement (or possibly other opsonins) is deposited onto S. pneumoniae, internalization and processing to terminal phagolysosomes of those organisms that are successfully bound should subsequently proceed with the efficiency that is observed in people with antibody. Significant amounts of IgG and complement are found in the alveolar fluid of the immune host (22); in the nonimmune host, bacteria will be opsonized for macrophage phagocytosis by complement and other soluble factors, including surfactant protein A (25).
Previous work has demonstrated that opsonization enhances intracellular processing and killing of other streptococcal species (group B streptococcus [27] and Streptococcus suis [1]) by mouse macrophages. Our work now confirms that this is the case for S. pneumoniae with the relevant human phagocyte (HAM) and also that HAM can phagocytize and kill opsonized type I S. pneumoniae similar to serotypes 3, 6, and 14 (16).
Although phagocytosis via the Fc receptors is a constitutive property of macrophages, complement receptor-mediated phagocytosis is developmentally regulated but can be activated readily during adherent culture or exposure to extracellular matrix (20). Our observation that opsonization with adsorbed serum results in equivalent rates of internalization of S. pneumoniae by HAM compared to opsonization in the presence of IgG is curious but is consistent with previous reports; Shaw and Griffin demonstrated that both complement and Fc receptor-mediated internalization of IgG and C3-coated particles occurs at equivalent rates (23), and Newman et al. (19) showed that phagocytosis following binding of sheep erythrocytes to Fc or complement receptors of human monocyte-derived macrophages results in comparable rates of internalization, though there are subtle differences in the cytoskeletal assembly associated with internalization. Caron and Hall have demonstrated that Fc and complement receptors employ distinct biochemical mechanisms of phagocytosis but transduce internalization of particles at similar rates (4). Fc receptors activate Cdc42 and Rac GTPases. On the other hand, CR3-mediated phagocytosis is dependent on Rho GTPase alone. The present study, of a relevant pathogen-human cell interaction, is consistent with this and suggests that incorporation of LAMP-1 into pneumococcal phagosomes may also be unaffected by the different signals transduced by Fc and complement receptor-mediated uptake.
Previous models of the interaction between HAM and S. pneumoniae have been designed to answer questions relating to opsonophagocytosis following vaccination (12, 13) and the role of the capsule in resisting phagocytosis (16, 17). In these studies, high efficiency of internalization of Staphylococcus aureus, nontypeable Haemophilus influenzae, and noncapsulate S. pneumoniae, and low (less than 10%) efficiency of internalization of capsulate pneumococci (with no detectable loss of viability) were observed. Our findings are consistent with these studies and further suggest that S. pneumoniae is sluggishly ingested by macrophages and possibly retards the maturation of its phagosome compared to other extracellular bacteria, e.g., N. meningitidis (for which osponization confers efficient [approximately 75% of bound bacteria] phagocytosis and trafficking of 50 to 75% of ingested organisms to LAMP-1 positive late endosomes or lysosomes [21]).
The importance of complement in phagocytic defense against pneumococci was described by Winkelstein and Drachman (28). The classic experiments of Hosea, Brown, and Frank (2, 15) using a guinea pig model of pneumococcal bacteremia demonstrated the importance of complement in innate immunity against S. pneumoniae; depletion of complement components C3 to C9 resulted in lethal failure of clearance of S. pneumoniae bacteremia compared to immune and nonimmune animals. The poor internalization and killing by HAM of S. pneumoniae not opsonized with complement that we describe here reflect these observations.
In the present study, the initial hour of incubation was conducted at 4°C to permit binding of pneumococci to the surface of HAM without permitting phagocytosis, so that we could equalize binding under the different opsonizing conditions. On warming, synchronous internalization of all pneumococci in contact with relevant receptors was permitted, allowing comparison of the kinetics of internalization between treatments. There is a possibility that the use of cold incubation may have differential effects on macrophages from individual hosts or, alternatively, influence interactions between the polysaccharide capsule and surface receptors. We removed immunoglobulin from the opsonizing serum by adsorbing with the same strain of S. pneumoniae used in the study (7). Adsorption of serum at 4°C resulted in no loss of complement activity within the adsorbed serum. It is possible that removal of IgG via adsorption may have also removed a number of lectins (including mannose-binding lectin) and this may have resulted in an underestimation of the differential internalization efficiencies after opsonization with adsorbed or replete serum.
Human macrophages kill other microorganisms using a combination of reactive oxygen metabolites, nitric oxide, and microbicidal peptides acquired by maturing phagosomes (10). To our knowledge, this is the first confirmation that S. pneumoniae trafficks to LAMP-containing compartments (LAMP is enriched in late endosomes and lysosomes). This is evidence that alveolar macrophage killing of this organism is not exclusively extracellular or due to nonphagolysosomal mechanisms and is consistent with observations made with noncapsulate pneumococci (16). Classical intracellular pathogens are well-known to be capable of subverting the maturation of phagosomes, either by inhibiting the incorporation of LAMP into the phagosome (by Mycobacterium tuberculosis) (6) or by escaping from the phagosome to avoid killing (e.g., by L. monocytogenes), thus making the macrophage a protective niche. Some extracellular pathogens, such as Neisseria, have also been shown to colocalize with LAMP and are capable of frustrating macrophage microbicidal mechanisms by reducing the rate of incorporation of LAMP into phagosomes (21).
The fact that summary statistics describing internalization (measured by immunofluorescence) and bacterial viability showed a good fit in linear regression analysis was evidence that internalization was necessary for at least some macrophage bactericidal activity, as our assay of loss of bacterial viability did not distinguish extracellular and intracellular killing by macrophages. Both immunofluorescence and the assay of loss of viability showed that alveolar macrophages were actively microbicidal against complete serum-opsonized pneumococci, with incorporation of LAMP-1 into phagosomes for at least the first hour of incubation, but thereafter activity declined dramatically. This observation could be due to a number of factors, including exhaustion of macrophages, necrosis, or serum-dependent apoptosis. Macrophages are known to survive for several months in vivo and are thought to be capable of multiple phagocytic episodes. It may be that following ingestion of a number of pneumococci, the macrophage ceases to ingest and redirects its effort to the production of proinflammatory cytokines. Production of tumor necrosis factor and gamma interferon has been shown to increase dramatically after several hours' stimulation of alveolar macrophages by M. tuberculosis (11). Alternatively, pneumococci may induce apoptosis in macrophages, as has been shown in vitro for Mycobacterium avium (9) and Shigella flexneri (8). These different possibilities were not addressed in our study.
In summary, we have demonstrated that HAM ingest S. pneumoniae, which are processed to intracellular compartments which colocalize with LAMP-1. We have also shown that opsonization by serum containing cognate immunoglobulin results in enhanced binding compared to that by serum containing C3 but no IgG, though the rate of internalization and processing through to terminal phagolysosomes of bound pneumococci is no different between these two conditions. Binding of S. pneumoniae in the absence of opsonization results in relatively poor binding, but internalization can still occur, albeit at a lower rate, and to compartments that are relatively deficient in LAMP-1.
ACKNOWLEDGMENTS
This study was supported by the Wellcome Trust (Clinical Tropical Medicine Training Fellowship to S.B.G.), the Ralph Sutcliffe Fund and the National Meningitis Trust (R.C.R.), and the Peel Trust (G.R.B.I.).
We thank Joel D. Ernst for helpful comments and suggestions.
REFERENCES
- 1.Brazeau C, Gottschalk M, Vincelette S, Martineau-Doizé B. In vitro phagocytosis and survival of Streptococcus suis capsular type 2 inside murine macrophages. Microbiology. 1996;142:1231–1237. doi: 10.1099/13500872-142-5-1231. [DOI] [PubMed] [Google Scholar]
- 2.Brown E J, Hosea S W, Frank M M. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev Infect Dis. 1983;5:S797–S805. doi: 10.1093/clinids/5.supplement_4.s797. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson S R, Roth J, Pilfer G, Fukuda M. Isolation and characterisation of human lysosomal membrane glycoproteins H-LAMP-1 and H-LAMP-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988;263:18911–18919. [PubMed] [Google Scholar]
- 4.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Ro GTP-ases. Science. 1998;282:1717–1722. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 5.Cauwels A, Wan E, Leismann M, Tuomanen E. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–3260. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens D L, Horwitz M E. Characterisation of the Mycobacterium tuberculosis phagosome and evidence that phagosome maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelfand J A, Fauci A S, Green I, Frank M M. A simple method for the determination of complement bearing mononuclear cells. J Immunol. 1976;116:3595–3598. [PubMed] [Google Scholar]
- 8.Guichon A, Zychlinksy A. Clinical isolates of Shigella species induce apoptosis in macrophages. J Infect Dis. 1997;175:470–473. doi: 10.1093/infdis/175.2.470. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Catanzaro A, Rao S P. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun. 1997;65:5262–5271. doi: 10.1128/iai.65.12.5262-5271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiemstra S, van Furth R. Antimicrobial mechanisms: antimicrobial polypeptides of mononuclear phagocytes. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker Inc.; 1994. pp. 197–202. [PubMed] [Google Scholar]
- 11.Hirsch C S, Ellner J J, Russell D G, Rich E A. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- 12.Hof D G, Repine J E, Giebank G S, Hoidal J R. Production of opsonins that facilitate phagocytosis of Streptococcus pneumoniae by human alveolar macrophages or neutrophils after vaccination with pneumococcal polysaccharide. Am Rev Respir Dis. 1981;124:193–195. doi: 10.1164/arrd.1981.124.2.193. [DOI] [PubMed] [Google Scholar]
- 13.Hof D G, Repine J E, Peterson P K, Hoidal J R. Phagocytosis by human alveolar macrophages and neutrophils: qualitative differences in the opsonic requirements for uptake of Staphylococcus aureus and Streptococcus pneumoniae by human alveolar macrophages or neutrophils after vaccination with pneumococcal polysaccharide. Am Rev Respir Dis. 1980;121:65–71. doi: 10.1164/arrd.1980.121.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Hoidal J R, Schmeling D, Peterson P K. Phagocytosis, bacterial killing and metabolism by human lung phagocytes. J Infect Dis. 1981;144:61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- 15.Hosea S W, Brown E J, Frank M M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980;142:903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson S, Musher D M, Chapman A, Goree A, Lawrence E C. Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J Infect Dis. 1985;152:4–13. doi: 10.1093/infdis/152.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson S, Musher D M, Goree A, Lawrence E C. Human alveolar lining material and antibacterial defenses. Am Rev Respir Dis. 1986;133:136–140. doi: 10.1164/arrd.1986.133.1.136. [DOI] [PubMed] [Google Scholar]
- 18.Mosser D M. Receptors on phagocytic cells involved in microbial recognition. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker; 1994. pp. 99–114. [PubMed] [Google Scholar]
- 19.Newman S L, Mikus L K, Tucci M A. Differential requirements for cellular cytoskeleton in human macrophage complement receptor and Fc receptor-mediated phagocytosis. J Immunol. 1991;146:967–974. [PubMed] [Google Scholar]
- 20.Newman S L, Tucci M A. Regulation of human monocytes/macrophage function by extracellular matrix. J Clin Investig. 1986;86:703–714. doi: 10.1172/JCI114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Read R C, Zimmerli S, Broaddus V C, Sanan D A, Stephens D S, Ernst J D. The (α2→8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64:3210–3217. doi: 10.1128/iai.64.8.3210-3217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds H Y. Normal and defective respiratory host defenses. In: Pennington J E, editor. Respiratory infections; diagnosis and management. 3rd ed. New York, N.Y: Raven Press; 1994. pp. 2–33. [Google Scholar]
- 23.Shaw D R, Griffin F M. Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981;289:409–411. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- 24.Tilney L G, Portnoy D A. Actin filaments in the growth, movement, and spread of the intracellular parasite Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tino M J, Wright J R. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:677–688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 26.Titus R G, Theodos C M, Shankar A H, Hall L R. Interactions between Leishmania major and macrophages. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker; 1994. pp. 437–460. [PubMed] [Google Scholar]
- 27.Valentin-Wiegand P, Benkel P, Rohde M, Chhatwal G S. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkelstein J A, Drachman R H. Deficiency of pneumococcal serum opsonizing activity in sickle-cell disease. N Engl J Med. 1968;279:459–466. doi: 10.1056/NEJM196808292790904. [DOI] [PubMed] [Google Scholar]
- 29.Wright S D. A new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]