Abstract
There is growing interest in the use of metformin to extend lifespan and prevent the onset of age‐related disorders in non‐diabetic individuals. The impact of metformin on lifespan and aging has been studied in several model organisms, with varying effects. We conducted a systematic review of studies that performed laboratory experiments investigating the effect of metformin on overall lifespan in healthy Mus musculus mice and in Caenorhabditis elegans nematodes. Lifespan results for mice and nematodes were analyzed in separate meta‐analyses, and there was a significant amount of heterogeneity across experiments within each species. We found that metformin was not significantly associated with an overall lifespan‐prolonging effect in either mice or nematodes. For nematodes, however, there was a lifespan‐prolonging effect in experiments using live OP50 Escherichia coli as a food source, an effect that was larger when metformin was started earlier in life. Our work highlights the importance of testing compounds in a diversity of model organisms. Moreover, in all species, including humans, it may be necessary to study the effect of metformin on aging in both younger and older cohorts.
Keywords: aging, lifespan, metformin, mice, nematodes, senescence, survival
Across studies, metformin seems to prolong lifespan in nematodes fed a specific bacterial diet but not in mice and may have a larger effect if started early in life.

Abbreviations
- AFT
accelerated failure time
- CAMARADES
Collaborative Approach to Meta‐Analysis and Review of Animal Data in Experimental Stroke
- C. elegans
Caenorhabditis elegans
- DL
DerSimonian‐Laird
- FUdR
fluorodeoxyuridine
- HR
hazard ratio
- PH
proportional hazards
- SJ
Sidik‐Jonkman
- TAME
Targeting Aging with Metformin
- TJL
Jackson laboratory
- UM
University of Michigan laboratory
- UT
University of Texas laboratory
1. INTRODUCTION
The biguanide metformin has been used for years as a first‐line treatment for type 2 diabetes mellitus. However, there is growing interest in the potential use of metformin to prevent age‐related disease in individuals without diabetes (Barzilai et al., 2016; Kulkarni et al., 2020). The rationale for this is supported by prior work showing that metformin can prevent diabetes (Knowler, 2002) and various age‐related cancers (Bodmer et al., 2010; Landman et al., 2010). Further proof‐of‐principle was recently suggested by clinical trials showing that other diabetes medications, semaglutide and tirzepatide, can augment weight loss in overweight adults without diabetes (Jastreboff, 2022; Wilding, 2021). No randomized placebo‐controlled trial has supported metformin's use as a pro‐longevity intervention in normoglycemic humans, although epidemiological studies have suggested that metformin may prolong lifespan and have beneficial impacts on health beyond its antihyperglycemic effect (Campbell et al., 2017). Ultimately, definitive evidence regarding potential benefits of metformin in those without diabetes or prediabetes may be obtained from the Targeting Aging with Metformin trial (TAME) (Barzilai et al., 2016), which has been approved by the Food and Drug Administration and is among the first trials to focus on aging as a composite endpoint rather than a single disease‐oriented outcome.
The effects of metformin on longevity are challenging to study in human trials, and in prior work (including TAME), the only practical option has been to focus on biomarkers or composite endpoints as proxies for longevity and aging. On the contrary, mean and maximum lifespan have frequently been measured under controlled laboratory conditions in model organisms, such as the mouse and nematode. Studies in these organisms have demonstrated effects of metformin on multiple biochemical pathways relevant to aging (Kulkarni et al., 2020), showing that metformin activates AMPK and inhibits mTORC1 (Howell et al., 2017; Kalender et al., 2010; Martin‐Montalvo et al., 2013), activates SIRT1 (Cuyàs et al., 2018), decreases IGF‐1 levels (Sarfstein et al., 2013), reduces inflammation, attenuates cellular senescence (Moiseeva et al., 2013), enhances autophagy (Bharath et al., 2020), and reduces formation of reactive oxygen species by inhibiting mitochondrial complex I (Algire et al., 2012). Nevertheless, the effects of metformin on lifespan have varied among studies, with some showing a significant favorable effect (Anisimov et al., 2005), some showing no significant effect (Palliyaguru et al., 2020), and some showing a significant negative effect (Zhu et al., 2021).
The effects of an anti‐aging intervention on lifespan in model organisms can vary considerably between experiments, even though experiments are generally performed in controlled laboratory settings. The effects of dietary restriction in mice, for example, are highly sensitive to strain, with more robust and favorable effects seen in hybrid mice compared to inbred mice (Swindell, 2012), and overall diminution of favorable effects in wild‐derived mice (Harper et al., 2006). Likewise, the effects of rapamycin on lifespan in mice, although apparently more robust than those of caloric restriction, are more favorable in females compared to males (Swindell, 2017). These patterns have been discernable through meta‐analyses that compare findings across laboratories. Such work, for instance, has been performed to better understand the effects of rapamycin on laboratory mouse lifespan (Swindell, 2017) and the effect of resveratrol on longevity across many species, including mice and nematodes (Hector et al., 2012). To our knowledge, however, no prior meta‐analyses have been performed to evaluate the effects of metformin on lifespan in model organisms.
In this study, we aimed to quantify the impact of metformin on lifespan in the laboratory mouse Mus musculus (Kunstyr & Leuenberger, 1975) and the nematode Caenorhabditis elegans (C. elegans) (Olsen et al., 2006). We performed a systematic review and meta‐analysis of controlled experiments in which metformin was given to laboratory mice or to C. elegans and survival data was reported. We sought to systematically integrate studies to estimate overall effect sizes, determine if significant heterogeneity was present among experiments, and to identify experimental factors associated with such heterogeneity.
2. METHODS
2.1. Overall design of systematic review
This review was carried out following the PRISMA 2020 checklist for systematic reviews and meta‐analyses (Page et al., 2021) and previously published recommendations for systematic reviews of preclinical studies (Sena et al., 2014). The quality of studies was assessed using a modified version of the Collaborative Approach to Meta‐Analysis and Review of Animal Data in Experimental Stroke (CAMARADES) checklist (Macleod et al., 2004; Vesterinen et al., 2014). This study was prospectively registered with the PROSPERO database (https://www.crd.york.ac.uk/prospero/). This study was not funded. The authors have no competing interests. All data are available from the corresponding author (AJP) upon request.
2.2. Search and study extraction
We systematically searched PubMed, Web of Science, and Google Scholar for articles examining the impact of metformin on lifespan in model organisms; the specific search strategies used are summarized in Appendix 1. We also hand‐searched references of all included articles to find additional studies. The final search was performed on August 1, 2022. Both authors screened each abstract. Articles were excluded if they did not report novel experimental results with survival data (either summary statistics such as hazard ratios (HRs) or median lifespans, raw survival curves, or both); were studies in humans or organisms other than mice and C. elegans; used disease model mice (such as stroke models, see e.g., Winkler et al., 2001); administered metformin to mice via injection instead of oral intake; or were repeats of already included studies.
For each study, both authors in parallel extracted study characteristics including year of publication, specific mouse or C. elegans variant used, age metformin treatment was started, method of metformin delivery, and any special experimental characteristics. For mouse studies, we recorded the sex of mice used for each experiment and noted whether the strain was inbred or non‐inbred (hybrid/outbred). For nematode studies, we extracted the strain of Escherichia coli used for feeding in each experiment and whether it was alive or UV treated. We also extracted whether 5′‐fluorodeoxyuridine (FUdR) was used to prevent reproduction in these experiments (Wang et al., 2019). For each study, we extracted any reported lifespan data, including median or mean lifespans and Cox proportional HRs. In addition to summary data, we extracted any raw survival data; if Kaplan–Meier survival curves were available, they were converted to x‐y data using Plot Digitizer (http://plotdigitizer.sourceforge.net/) which was then transformed into survival data using R code (Wei & Royston, 2017). Additionally, the authors of all included studies were contacted and asked to provide raw survival data; whenever such data was made available, it was used for all calculations.
2.3. Outcomes
The primary outcome was the Cox proportional hazards (PH) HR (Bender et al., 2005). We also calculated as a secondary outcome the accelerated failure time (AFT) deceleration factor (percent increase or decrease in survivorship) using the Weibull distribution (Swindell, 2009). For each experiment, appropriateness of the Cox PH model was assessed using proportional hazard tests and log–log plots (Grambsh & Therneau, 1994). For both Cox PH and AFT models, censoring information was incorporated whenever available. All survival calculations were made using the survival package in R (Therneau, 2022). To check the accuracy of our algorithm for converting published survival curves to hazard ratio data, we used the simsurv package in R (Brilleman et al., 2021) to generate random survival datasets and each author independently estimated hazard ratio data from these datasets; these results are included in Table S1.
2.4. Meta‐analysis methods
Given a large amount of expected heterogeneity and the goal to generalize results beyond the specific experiments included in the meta‐analysis, we utilized random effects meta‐analysis. Random effects meta‐analysis was carried out using the Sidik‐Jonkman (SJ) estimator which has lower type I error rates than the DerSimonian‐Laird (DL) estimator (IntHout et al., 2014). Both included outcomes (hazard ratios and deceleration factors) were log‐transformed before meta‐analysis. Heterogeneity was estimated with the Higgins and Thompson's I2 statistic and Cochran's Q test (Sedgwick, 2012; von Hippel, 2015). Meta‐analysis calculations were performed using the meta package in R (Balduzzi et al., 2019). The possibility of publication bias or small study effects (Lin et al., 2020) was assessed using funnel plots and the Egger test for funnel plot asymmetry (Sterne et al., 2011); funnel plot analysis was performed using the metafor package in R (Viechtbauer, 2010).
One mouse study and four nematode studies contained two or more experiments that used a shared control group, yielding non‐independent effect size estimates. For the mouse study, the experiment with the larger sample size was included in calculations; for the nematode studies, as all experiments had similar sample sizes, the experiment with the most frequently used metformin dose across studies (50 mM) was used for calculations. For funnel plot analyses, in studies with multiple experiments, the single experiment with the largest sample size was selected as representative of the study. For all statistical tests, a nominal type I error rate was set at α = 0.05. The Benjamini–Hochberg method was used to adjust for multiple hypothesis testing across meta‐analysis subgroups (Benjamini & Hochberg, 1995). Version 4.0.0 of the R programming language was used for all calculations (R Core Team, 2020).
3. RESULTS
Our search strategy yielded 1068 studies. Of these, 446 (41.8%) were cancer studies, 167 (15.6%) were human studies, 163 (15.3%) were cell studies, 120 (11.2%) were reviews or commentaries without new data, 92 (8.6%) did not report any survival data related to aging, 28 (2.6%) were excluded disease models, 9 (0.9%) were repeats of other already included studies, and 8 (0.7%) exposed animals to both metformin and another molecule in the experimental group. Of the remaining 33 studies, 14 were excluded since they involved other organisms, and one was excluded since it was a mouse study that used subcutaneous injection of metformin. This left 10 mouse studies and 10 C. elegans studies. The PRISMA flow diagram is shown in Figure S1.
From the 10 mouse studies, we extracted 20 independent lifespan experiments, and from the 10 C. elegans studies, we extracted 31 independent lifespan experiments. The baseline properties of the studies and lifespan experiments are summarized in Table S2 for mice and Table S3 for C. elegans. Of the 10 mouse studies, one author provided survival data (Strong et al., 2016); of the 10 C. elegans studies, two authors provided survival data (Cabreiro et al., 2013; Wu et al., 2016).
3.1. Dosing and administration of metformin
For mice, we defined a “high dose” of metformin as an effective weight‐based dose of 500 mg/kg or higher; this was used in 3 of 20 experiments (15%). Mice received metformin either via drinking water (n = 10, 50%) or mixed in with chow (n = 10, 50%). In most experiments, mice received metformin daily (17 experiments, 85%). Alternate dosing schedules included giving metformin every other week daily (1 experiment), 5 days per week with 2 days off (1 experiment), and 5 days per month with the remaining days off (1 experiment).
We defined “early” administration of metformin as metformin dosing starting before 12 weeks (84 days) for mice; in turn, we defined early as dosing starting before Day 5 of adulthood for nematodes. These values were chosen based upon the distribution of start times among the available studies for each species, with the chosen times corresponding to gaps in the ordered sequence of start times. For mice, 6/20 (30%) experiments used early administration; for nematodes, 25/31 (81%) experiments used early administration.
For nematode experiments, 19 used OP50 E. coli as a food source (61%), three used HT115 E. coli (10%), and the remaining nine experiments (29%) used a variety of other E. coli strains (BL21G, CS180, CS2429, GD1, HB101, OP50‐MR, or OP50‐R26), Bacillus subtilis, or did not have a bacterial food source (axenic culture) (Lenaerts et al., 2008; Reinke et al., 2010). Of the 31 experiments, 19 used FUdR to prevent reproduction (61%).
3.2. Overall and subgroup analyses for studies in mice
Across all mouse experiments, metformin was not associated with a statistically significant reduction in hazard of death (HR = 0.98, 0.77–1.24, p = 0.84, n = 20), an effect with significant heterogeneity (I 2 = 87%, 82%–91%, p < 0.0001) (Figure 1). There was no significant effect of metformin on lifespan in several subgroups, including early start (HR = 0.70, 0.48–1.02, p = 0.32, n = 6), low dose experiments (HR = 0.87, 0.72–1.05, p = 0.40, n = 17), experiments with non‐inbred mice (HR = 0.88, 0.73–1.07, p = 0.40, n = 11) or experiments with only male mice (HR = 1.09, 0.72–1.65, p = 0.76, n = 9) or only female mice (HR = 0.89, 0.68–1.17, p = 0.58, n = 11). Metformin also did not significantly prolong lifespan in early start experiments with female mice (HR = 0.63, 0.44–0.90, p = 0.12, n = 5).
FIGURE 1.
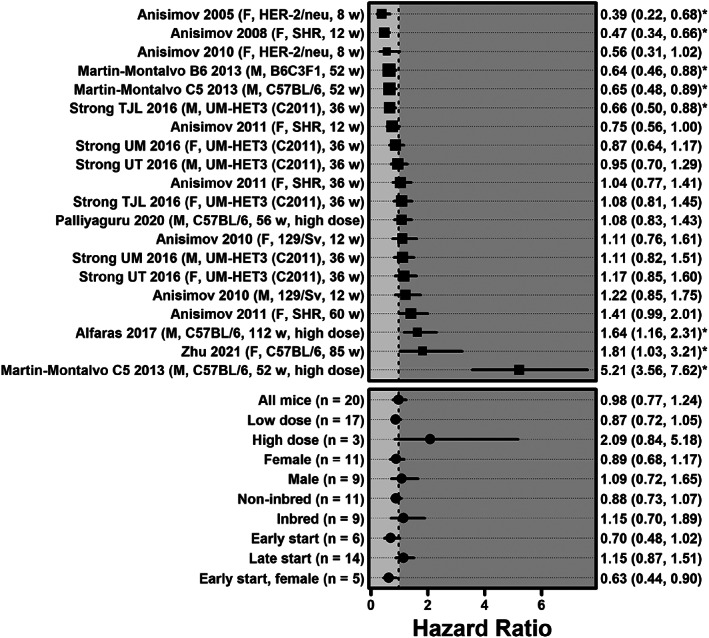
Forest plot of hazard ratios for all mouse experiments, as well as specific subgroups. The description on the left includes the primary author and publication year of the study including the experiment, as well as the sex, genotype, and age in weeks at which metformin was first administered. For the NIA ITP data from (Strong et al., 2016) the study site is also included (TJL = Jackson laboratory, UM = University of Michigan, UT = University of Texas). HRs and 95% CIs are listed on the right (* indicates p < 0.05). Larger square symbols are used for experiments with larger sample sizes. The meta‐analysis summary effects for all experiments (n = 20) as well as for specific subgroups are shown at the bottom.
In univariate meta‐regression, there was no statistically significant difference between mice that received low dose metformin and mice that received high dose metformin (HR = 0.87 vs. 1.86, meta‐regression p = 0.056). There was no significant difference in survival according to start time (early HR = 0.70 vs. 1.15, meta‐regression p = 0.13), gender (male HR 0.89 vs. 1.09, meta‐regression p = 0.40), or genotype (non‐inbred HR = 0.88 vs. 1.15, meta‐regression p = 0.36). In bivariate meta‐regression controlling for early start and gender or early start and dose, early start did not have a significant effect on survival (p = 0.17, p = 0.17); however, when controlling for early start and genotype, early start was associated with improved survival (p = 0.042). Notably, none of the above results changed significantly after removing the Martin‐Montalvo 2013 high dose experiment (see Table S4). Mirroring hazard ratio results, metformin was not associated with a statistically significant AFT deceleration factor across all experiments (D = 1.01, 0.97–1.05, p = 0.66, n = 20), in early start experiments (D = 1.08, 0.98–1.18, p = 0.35, n = 6), low dose experiments (D = 1.03, 0.99–1.07, p = 0.36, n = 17), experiments with non‐inbred mice (D = 1.04, 0.98–1.09, p = 0.38, n = 11), male mouse experiments (D = 0.99, 0.94–1.05, p = 0.65, n = 9) female mouse experiments (HR = 1.03, 0.98–1.09, p = 0.48, n = 11), or early start experiments with female mice (D = 1.11, 1.01–1.21, p = 0.22, n = 5).
3.3. Overall and subgroup analyses for studies in nematodes
Across all C. elegans experiments, metformin was not associated with a statistically significant reduction in hazard of death (HR = 0.78, 0.53–1.13, p = 0.19, n = 31), an effect with significant heterogeneity (I 2 = 96%, 95%–97%, p < 0.0001) (Figure 2). Early start experiments showed a significant reduction in mortality (HR = 0.61, 0.41–0.91, p = 0.045, n = 25), while late start experiments were associated with increased mortality (HR = 2.08, 1.20–3.61, p = 0.041, n = 6).
FIGURE 2.
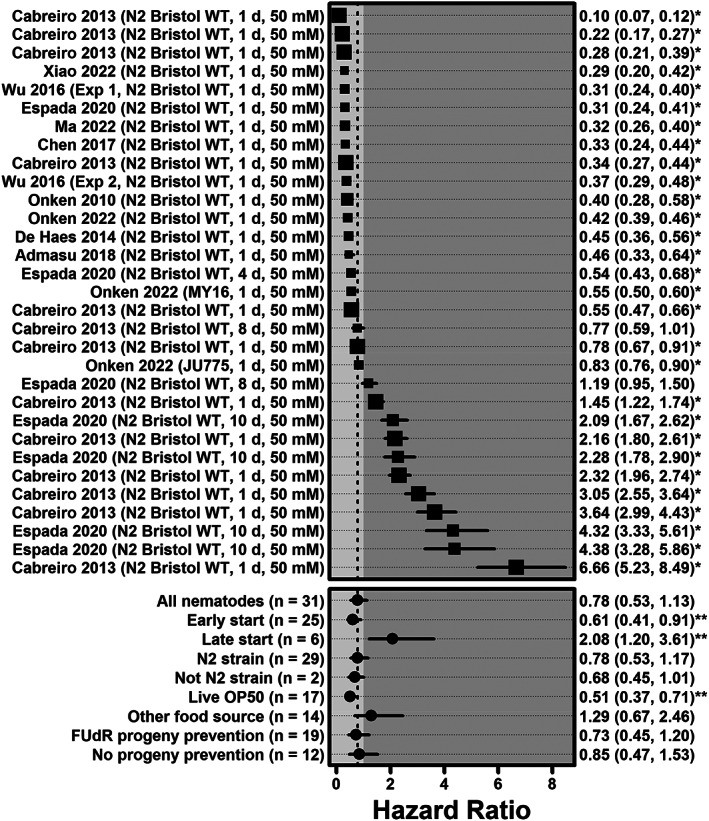
Forest plot of hazard ratios for all Caenorhabditis elegans experiments and specific subgroups. The description on the left includes the primary author and publication year of the study including the experiment, as well as the strain, age in days at which metformin was first administered, and the metformin dose. HRs and 95% CIs are listed on the right (* indicates p < 0.05). Larger square symbols are used for experiments with larger sample sizes. Meta‐analysis summary effects for all experiments (n = 31) as well as for specific subgroups are shown at the bottom (** indicates p < 0.05 after the Benjamini–Hochberg correction for multiple comparisons).
Early start of metformin administration was associated with significantly improved survival compared with later start (HR = 0.61 vs. 2.08, meta‐regression p = 0.017). There was no significant difference between experiments using N2 strains and other strains (HR = 0.78 vs. 0.68, meta‐regression p = 0.85), or between experiments that used FUdR for progeny prevention and those that did not (HR = 0.73 vs. 0.85, meta‐regression p = 0.85). However, experiments using live OP50 E. coli were associated with improved survival compared with those using other food sources (HR = 0.51 vs. 1.29, meta‐regression p = 0.017).
For nematodes, metformin was not associated with a significant AFT deceleration factor across all experiments (D = 1.07, 0.98–1.17, p = 0.15, n = 31), or in early start experiments (D = 1.13, 1.02–1.25, p = 0.057, n = 25), or late start experiments (D = 0.85, 0.74–0.97, p = 0.057). Metformin was associated with a significant deceleration factor in experiments using live OP50 E. coli (D = 1.20, 1.10–1.31, p = 0.0009, n = 17).
For nematodes, there was a significant, inverse dose–response relationship between HR and day of treatment start, with each additional day increasing the log hazard ratio by 0.15 (p = 0.0034); this relationship remained after adjusting for strain, food source and use of FUdR (β = 0.15, p = 0.0099). For mice, there was no significant relationship between HR and week of treatment start (β = 0.003, p = 0.88), including after adjusting for dose, sex, and strain (β = −0.011, p = 0.64).
3.4. Study quality and publication bias
The quality of studies varied, with a median CAMARADES quality sum of 4 (IQR: 3–5) for mouse studies and 3 (IQR: 2–3) for nematode studies, out of a total of 9 (see Figure S2). Of note, no mouse study described blinding, and only one (10%) described allocation concealment. Similarly, only two nematode studies (20%) described blinding, and none described allocation concealment. Three of the 10 mouse studies (30%) described sample size calculations, and only one of the 10 nematode studies (10%) did so.
Using funnel plots and Egger's test for funnel plot asymmetry, we did not find significant evidence of publication bias; these results are summarized in Figure 3 below.
FIGURE 3.
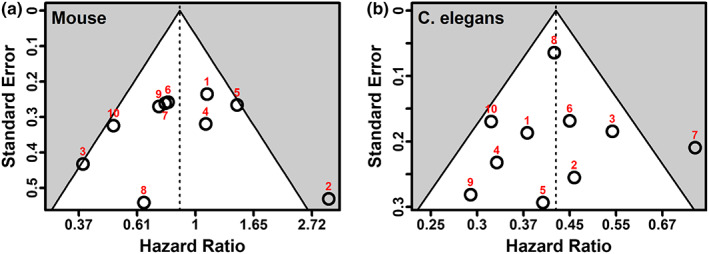
Left: Funnel plot for 10 mouse studies; Egger's p = 0.92. Right: Funnel plot for 10 Caenorhabditis elegans studies; Egger's p = 0.98. Both plots show standard error versus log (hazard ratio) for the largest experiment from that publication; vertical lines show the pooled random effects estimate for each set of experiments (HR = 0.81 for mice, 0.42 for nematodes). Including all 20 mouse experiments resulted in an Egger's p = 0.72; including all 31 C. elegans experiments resulted in an Egger's p = 0.59. For the list of associated studies, see Tables S5a,b.
4. DISCUSSION
Studies of purported anti‐aging molecules in model organisms provide important data for delineating mechanisms of activity and assessing translatability to humans. In addition to growing epidemiological evidence, a variety of preclinical studies in model organisms have connected metformin to beneficial effects in both healthspan and lifespan (Cabreiro et al., 2013; Crimmins, 2015; Piskovatska et al., 2019). The anti‐aging mechanisms of metformin are still being deciphered, but there are likely diverse pathways involved, with differential effects among model organisms. In this systematic review and meta‐analysis, we found that metformin has heterogenous effects on lifespan within species as well as differing effects between mice and nematodes.
There was no significant association between metformin supplementation and lifespan in mice; in subgroup analyses, we found no significant difference in outcomes between experiments with male and female mice or between inbred and non‐inbred mouse strains. Prior published studies are also non‐definitive regarding sex‐specific effects of metformin on mouse longevity, with some studies demonstrating benefit in only female mice (Anisimov et al., 2010) and others demonstrating benefit in only males (Zhu et al., 2021). In humans, metformin was reported to lower fasting plasma glucose and 2‐h postprandial glucose more in females compared to males (Li et al., 2021), and one large‐scale study showed that female diabetics treated with metformin had a lower incidence of cardiovascular events than male diabetics (Raparelli et al., 2020). Further studies will be needed to understand whether sex moderates the effect of metformin on mouse longevity. Overall, the longevity results reported here for metformin differ from previous results for rapamycin in mice (Swindell, 2017), which was found to robustly decrease mortality across experiments. Similar to rapamycin, we found a larger survival benefit of metformin when started early in life or when administered to female mice, however, neither of these associations were statistically significant in the current study.
Higher metformin doses in mice were associated with less survival benefit, potentially due to toxic effects, such as lactic acidosis, vitamin B12 deficiency, or nephrotoxicity (Alfaras et al., 2017). Unfortunately, it is difficult to separate the impact of higher doses from later start times, as all the high dose experiments included in this review also had a late start time for metformin supplementation. Notably, redoing the analyses after removing all high dose experiments did not significantly change our results. In humans, however, metformin is contraindicated when eGFR declines below 30, and some loss of renal function is expected with age in mice, particularly inbred as compared with non‐inbred mice (Hackbarth & Harrison, 1982). It is thus possible that attenuation of metformin benefits at higher doses and later start times reflects emergent metformin toxicity secondary to age‐related renal decline.
In contrast to our findings in mice, across several experiments and subgroups metformin had a significant effect on the lifespan of C. elegans. This disparity may be due to the significant differences in biology between the two organisms. For example, metformin may uniquely impact C. elegans lifespan via alteration of E. coli metabolism, a central food source for laboratory‐maintained C. elegans, leading to downstream methionine restriction; notably, metformin was found to reduce lifespan in C. elegans grown on axenic cultures without E. coli (Cabreiro et al., 2013). Food source significantly altered the effect of metformin on C. elegans lifespan in our meta‐analysis, although the majority of experiments using a food source other than live OP50 E. coli came from one study (Cabreiro et al., 2013). Espada et al. (2020) used UV treated OP50 and HT115 E. coli but only with metformin started late in life. Although metformin may alter the intestinal microbiome (Brunkwall & Orho‐Melander, 2017), such effects of metformin on methionine metabolism may be less prominent in mice. Future studies of metformin's effect in mice with or without a methionine‐restricted diet may be worthwhile to evaluate whether dietary methionine is required for positive effects on mouse lifespan.
Early administration of metformin was associated with a significantly larger beneficial effect on lifespan for nematodes; but this effect was not statistically significant for mice. Espada et al. (2020) found metformin to be harmful if started late in the nematode lifespan, finding that although metformin activated specific longevity promoting pathways in young nematodes it led to ATP exhaustion and cell death in older nematodes. Possible mechanisms for an age‐dependent effect of metformin on lifespan in mice have yet to be elucidated. Importantly, none of the studies included in this review included experiments where metformin was started in both young and old cohorts of mice under otherwise similar experimental conditions. However, a two‐by‐two factorial experiment to evaluate the effects of start time (early vs. late) and sex (male vs. female), and the interaction between these factors, would be feasible to perform in mice. Our results highlight this experiment as an important direction for future work. Additionally, further studies of metformin's effect on the lifespan of C. elegans to investigate the interaction between food source and start time will be important.
Although the overall absence of a significant effect on lifespan in mice compared with C. elegans on an OP50 diet may be due to biological differences, it also possible that in mice, healthspan may be increased without a significant impact on lifespan due to competing risks (Garmany et al., 2021; Koller et al., 2012). Indeed, certain studies included in this review which did not show a significant prolongation of lifespan nevertheless found improved insulin sensitivity, reduced hepatic steatosis, and other beneficial health effects in mice (Alfaras et al., 2017). Similarly, a study in Fischer‐344 rats found metformin to have beneficial effects on body weight and adipose tissue, without any appreciable impact on lifespan (Smith et al., 2010). Additionally, even if metformin has lifespan‐prolonging effects, such effects may be smaller than healthspan effects and require larger sample sizes to detect (Richardson et al., 2016).
4.1. Limitations
Our study has several limitations. First, we were only able to obtain original survival curve or hazard ratio data from the authors of one of 10 mouse studies and two of 10 nematode studies. For the remainder, the survival data were reconstructed from the published survival curves, which invariably introduces noise into effect size estimates. To mitigate this, effect size estimates were produced by both authors separately and averaged; the estimates produced by both authors aligned closely, differing by 5% or less for most experiments.
Second, by only including three databases we may have systematically missed unpublished or non‐English language data, although we attempted to reduce this risk by searching gray literature (via Google Scholar) as well as manual searches of references. In our analysis, we did not find significant evidence of small study effects through funnel plot analysis, although the power of these tests is limited given the small number of included studies (Sterne et al., 2011).
Notably, survival curves of 7 of 20 mouse experiments (35%) and 15 of 31 nematode experiments (48%) showed evidence of nonproportional hazards, determined via visual inspection of log–log plots. However, given the similar trends obtained from the meta‐analyses of deceleration factors, it is unlikely that nonproportionality significantly biased outcomes.
Finally, the identified studies for both mice and nematodes were generally of low quality, with almost none reporting methods of allocation concealment or blinding and less than a quarter reporting sample size calculations; only half reported randomization methods. The lack of these design features may have biased the reported effects (Hirst et al., 2014).
5. CONCLUSION
There is increasing interest in the possible use of metformin to increase healthspan and prevent onset of age‐related disorders in non‐diabetic individuals. In this systematic review and meta‐analysis, we found metformin to have no significant overall effect on the lifespan of healthy mice, nor any significant beneficial effect in studied subgroups. In contrast, we found significant evidence of lifespan prolonging effects of metformin in C. elegans nematodes fed a diet of OP50 E. coli and starting metformin supplementation earlier in the nematode lifespan led to more beneficial effects on lifespan. Important next steps for preclinical research into metformin's potential role as a geroprotector would include mouse experiments of daily metformin supplementation started at different points in the natural lifespan. Such studies can be formulated as factorial designs to also study the interaction between metformin start time and sex, which should yield insights into the relationship between these two factors and associated mechanisms. A greater understanding of this could in turn have important implications for clinical research in humans and may indicate a need for trials to be run in both younger and older cohorts.
AUTHOR CONTRIBUTIONS
Austin J. Parish conceived and designed analysis, collected data, performed analysis, and wrote and edited manuscript. William R. Swindell collected data, performed analysis, and wrote and edited manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
The graphical abstract was created with BioRender.com.
APPENDIX 1.
SEARCH STRATEGIES USED IN THIS STUDY
PubMed Search Strategy
(metformi*[Title] OR biguanid*[Title] OR “N‐N‐dimethylbiguanide”[Title] OR glucophage[Title]) AND ((lifespan[Title] OR “life span”[Title] OR “life‐span”[Title] OR survival[Title] OR healthspan[Title/Abstract] OR “health‐span”[Title/Abstract] OR longevity[Title/Abstract] OR aging[Title/Abstract] OR ageing[Title/Abstract]) OR aging[majr])
Web of Science Search Strategy
TI = ((metform* OR biguanid* OR “N‐N‐dimethylbiguanide” OR glucophag*) AND (lifespan OR “life span” OR “life‐span” OR survival OR healthspan OR “health‐span” OR longevity OR aging OR ageing OR senescen*)).
Google Scholar Search Strategy
allintitle: metformin (lifespan OR “life span” OR “life‐span” OR longevity OR aging OR ageing OR healthspan OR “health span” OR “health‐span” OR “survival”).
Parish, A. J. , & Swindell, W. R. (2022). Metformin has heterogeneous effects on model organism lifespans and is beneficial when started at an early age in Caenorhabditis elegans: A systematic review and meta‐analysis. Aging Cell, 21, e13733. 10.1111/acel.13733
DATA AVAILABILITY STATEMENT
All data utilized in this study will be made publicly available upon publication via the Open Science Framework (osf.io).
REFERENCES
- Alfaras, I. , Mitchell, S. J. , Mora, H. , Lugo, D. R. , Warren, A. , Navas‐Enamorado, I. , Hoffmann, V. , Hine, C. , Mitchell, J. R. , Le Couteur, D. G. , Cogger, V. C. , Bernier, M. , & de Cabo, R. (2017). Health benefits of late‐onset metformin treatment every other week in mice. NPJ Aging and Mechanisms of Disease, 3, 16. 10.1038/s41514-017-0018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algire, C. , Moiseeva, O. , Deschênes‐Simard, X. , Amrein, L. , Petruccelli, L. , Birman, E. , Viollet, B. , Ferbeyre, G. , & Pollak, M. N. (2012). Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prevention Research, 5(4), 536–543. 10.1158/1940-6207.CAPR-11-0536 [DOI] [PubMed] [Google Scholar]
- Anisimov, V. N. , Egormin, P. A. , Bershtein, L. M. , Zabezhinskii, M. A. , Piskunova, T. S. , Popovich, I. G. , & Semenchenko, A. V. (2005). Metformin decelerates aging and development of mammary tumors in HER‐2/neu transgenic mice. Bulletin of Experimental Biology and Medicine, 139(6), 721–723. 10.1007/s10517-005-0389-9 [DOI] [PubMed] [Google Scholar]
- Anisimov, V. N. , Piskunova, T. S. , Popovich, I. G. , Zabezhinski, M. A. , Tyndyk, M. L. , Egormin, P. A. , Yurova, M. V. , Rosenfeld, S. V. , Semenchenko, A. V. , Kovalenko, I. G. , Poroshina, T. E. , & Berstein, L. M. (2010). Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging, 2(12), 945–958. 10.18632/aging.100245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi, S. , Rücker, G. , & Schwarzer, G. (2019). How to perform a meta‐analysis with R: A practical tutorial. Evidence‐Based Mental Health, 22(4), 153–160. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai, N. , Crandall, J. P. , Kritchevsky, S. B. , & Espeland, M. A. (2016). Metformin as a tool to target aging. Cell Metabolism, 23(6), 1060–1065. 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, R. , Augustin, T. , & Blettner, M. (2005). Generating survival times to simulate cox proportional hazards models. Statistics in Medicine, 24(11), 1713–1723. 10.1002/sim.2059 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bharath, L. P. , Agrawal, M. , McCambridge, G. , Nicholas, D. A. , Hasturk, H. , Liu, J. , Jiang, K. , Liu, R. , Guo, Z. , Deeney, J. , Apovian, C. M. , Snyder‐Cappione, J. , Hawk, G. S. , Fleeman, R. M. , Pihl, R. , Thompson, K. , Belkina, A. C. , Cui, L. , Proctor, E. A. , … Nikolajczyk, B. S. (2020). Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging‐associated inflammation. Cell Metabolism, 32(1), 44–55.e6. 10.1016/j.cmet.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer, M. , Meier, C. , Krähenbühl, S. , Jick, S. S. , & Meier, C. R. (2010). Long ‐term metformin use is associated with decreased risk of breast cancer. Diabetes Care, 33(6), 1304–1308. 10.2337/dc09-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilleman, S. L. , Wolfe, R. , Moreno‐Betancur, M. , & Crowther, M. J. (2021). Simulating survival data using the simsurv R package. Journal of Statistical Software, 97(3), 1–27. 10.18637/jss.v097.i03 [DOI] [Google Scholar]
- Brunkwall, L. , & Orho‐Melander, M. (2017). The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: From current human evidence to future possibilities. Diabetologia, 60(6), 943–951. 10.1007/s00125-017-4278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro, F. , Au, C. , Leung, K. Y. , Vergara‐Irigaray, N. , Cochemé, H. M. , Noori, T. , Weinkove, D. , Schuster, E. , Greene, N. D. , & Gems, D. (2013). Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell, 153(1), 228–239. 10.1016/j.cell.2013.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. M. , Bellman, S. M. , Stephenson, M. D. , & Lisy, K. (2017). Metformin reduces all‐cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta‐analysis. Ageing Research Reviews, 40, 31–44. 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Crimmins, E. M. (2015). Lifespan and healthspan: Past, present, and promise. The Gerontologist, 55(6), 901–911. 10.1093/geront/gnv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuyàs, E. , Fernández‐Arroyo, S. , Verdura, S. , García, R. Á. , Stursa, J. , Werner, L. , Blanco‐González, E. , Montes‐Bayón, M. , Joven, J. , Viollet, B. , Neuzil, J. , & Menendez, J. A. (2018). Metformin regulates global DNA methylation via mitochondrial one‐carbon metabolism. Oncogene, 37(7), 963–970. 10.1038/onc.2017.367 [DOI] [PubMed] [Google Scholar]
- Espada, L. , Dakhovnik, A. , Chaudhari, P. , Martirosyan, A. , Miek, L. , Poliezhaieva, T. , Schaub, Y. , Nair, A. , Döring, N. , Rahnis, N. , Werz, O. , Koeberle, A. , Kirkpatrick, J. , Ori, A. , & Ermolaeva, M. A. (2020). Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans . Nature Metabolism, 2(11), 1316–1331. 10.1038/s42255-020-00307-1 [DOI] [PubMed] [Google Scholar]
- Garmany, A. , Yamada, S. , & Terzic, A. (2021). Longevity leap: Mind the healthspan gap. NPJ Regenerative Medicine, 6(1), 57. 10.1038/s41536-021-00169-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsh, P. M. , & Therneau, T. M. (1994). Proportional hazards tests and diagnostics based on weighted residuals. Biometrika, 81(3), 515–526. 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- Hackbarth, H. , & Harrison, D. E. (1982). Changes with age in renal function and morphology in C57BL/6, CBA/HT6, and B6CBAF1 mice. Journal of Gerontology, 37(5), 540–547. 10.1093/geronj/37.5.540 [DOI] [PubMed] [Google Scholar]
- Harper, J. M. , Leathers, C. W. , & Austad, S. N. (2006). Does caloric restriction extend life in wild mice? Aging Cell, 5(6), 441–449. 10.1111/j.1474-9726.2006.00236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector, K. L. , Lagisz, M. , & Nakagawa, S. (2012). The effect of resveratrol on longevity across species: A meta‐analysis. Biology Letters, 8(5), 790–793. 10.1098/rsbl.2012.0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst, J. A. , Howick, J. , Aronson, J. K. , Roberts, N. , Perera, R. , Koshiaris, C. , & Heneghan, C. (2014). The need for randomization in animal trials: An overview of systematic reviews. PLoS One, 9(6), e98856. 10.1371/journal.pone.0098856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, J. J. , Hellberg, K. , Turner, M. , Talbott, G. , Kolar, M. J. , Ross, D. S. , Hoxhaj, G. , Saghatelian, A. , Shaw, R. J. , & Manning, B. D. (2017). Metformin inhibits hepatic mTORC1 signaling via dose‐dependent mechanisms involving AMPK and the TSC complex. Cell Metabolism, 25(2), 463–471. 10.1016/j.cmet.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout, J. , Ioannidis, J. P. , & Borm, G. F. (2014). The Hartung‐Knapp‐Sidik‐Jonkman method for random effects meta‐analysis is straightforward and considerably outperforms the standard DerSimonian‐Laird method. BMC Medical Research Methodology, 14, 25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff, A. M. , Aronne, L. J. , Ahmad, N. N. , Wharton, S. , Connery, L. , Alves, B. , Kiyosue, A. , Zhang, S. , Liu, B. , Bunck, M. C. , Stefanski, A. , & SURMOUNT‐1 Investigators . (2022). Tirzepatide once weekly for the treatment of obesity. The New England Journal of Medicine, 387(3), 205–216. 10.1056/NEJMoa2206038 [DOI] [PubMed] [Google Scholar]
- Kalender, A. , Selvaraj, A. , Kim, S. Y. , Gulati, P. , Brûlé, S. , Viollet, B. , Kemp, B. E. , Bardeesy, N. , Dennis, P. , Schlager, J. J. , Marette, A. , Kozma, S. C. , & Thomas, G. (2010). Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase‐dependent manner. Cell Metabolism, 11(5), 390–401. 10.1016/j.cmet.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler, W. C. , Barrett‐Connor, E. , Fowler, S. E. , Hamman, R. F. , Lachin, J. M. , Walker, E. A. , Nathan, D. M. , & Diabetes Prevention Program Research Group . (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine, 346(6), 393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller, M. T. , Raatz, H. , Steyerberg, E. W. , & Wolbers, M. (2012). Competing risks and the clinical community: Irrelevance or ignorance? Statistics in Medicine, 31(11–12), 1089–1097. 10.1002/sim.4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, A. S. , Gubbi, S. , & Barzilai, N. (2020). Benefits of metformin in attenuating the hallmarks of aging. Cell Metabolism, 32(1), 15–30. 10.1016/j.cmet.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstyr, I. , & Leuenberger, H. G. (1975). Gerontological data of C57BL/6J mice. I. Sex differences in survival curves. Journal of Gerontology, 30(2), 157–162. 10.1093/geronj/30.2.157 [DOI] [PubMed] [Google Scholar]
- Landman, G. W. , Kleefstra, N. , van Hateren, K. J. , Groenier, K. H. , Gans, R. O. , & Bilo, H. J. (2010). Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC‐16. Diabetes Care, 33(2), 322–326. 10.2337/dc09-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts, I. , Walker, G. A. , Van Hoorebeke, L. , Gems, D. , & Vanfleteren, J. R. (2008). Dietary restriction of Caenorhabditis elegans by axenic culture reflects nutritional requirement for constituents provided by metabolically active microbes. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63(3), 242–252. 10.1093/gerona/63.3.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Li, J. , Shan, Z. , Yang, W. , Liu, J. , Tian, H. , Zhou, Z. , Ji, Q. , Weng, J. , Jia, W. , Lu, J. , Liu, J. , Xu, Y. , & Yang, Z. (2021). Gender‐differential effects on blood glucose levels between acarbose and metformin in Chinese patients with newly diagnosed type 2 diabetes: A sub‐analysis of the MARCH trial. Endocrine Journal, 68(1), 69–79. 10.1507/endocrj.EJ20-0006 [DOI] [PubMed] [Google Scholar]
- Lin, L. , Shi, L. , Chu, H. , & Murad, M. H. (2020). The magnitude of small‐study effects in the Cochrane database of systematic reviews: An empirical study of nearly 30 000 meta‐analyses. BMJ Evidence‐Based Medicine, 25(1), 27–32. 10.1136/bmjebm-2019-111191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod, M. R. , O'Collins, T. , Howells, D. W. , & Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke, 35(5), 1203–1208. 10.1161/01.STR.0000125719.25853.20 [DOI] [PubMed] [Google Scholar]
- Martin‐Montalvo, A. , Mercken, E. M. , Mitchell, S. J. , Palacios, H. H. , Mote, P. L. , Scheibye‐Knudsen, M. , Gomes, A. P. , Ward, T. M. , Minor, R. K. , Blouin, M. J. , Schwab, M. , Pollak, M. , Zhang, Y. , Yu, Y. , Becker, K. G. , Bohr, V. A. , Ingram, D. K. , Sinclair, D. A. , Wolf, N. S. , … de Cabo, R. (2013). Metformin improves healthspan and lifespan in mice. Nature Communications, 4, 2192. 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva, O. , Deschênes‐Simard, X. , St‐Germain, E. , Igelmann, S. , Huot, G. , Cadar, A. E. , Bourdeau, V. , Pollak, M. N. , & Ferbeyre, G. (2013). Metformin inhibits the senescence‐associated secretory phenotype by interfering with IKK/NF‐κB activation. Aging Cell, 12(3), 489–498. 10.1111/acel.12075 [DOI] [PubMed] [Google Scholar]
- Olsen, A. , Vantipalli, M. C. , & Lithgow, G. J. (2006). Using Caenorhabditis elegans as a model for aging and age‐related diseases. Annals of the New York Academy of Sciences, 1067, 120–128. 10.1196/annals.1354.015 [DOI] [PubMed] [Google Scholar]
- Page, M. J. , Moher, D. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … McKenzie, J. E. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372, n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliyaguru, D. L. , Minor, R. K. , Mitchell, S. J. , Palacios, H. H. , Licata, J. J. , Ward, T. M. , Abulwerdi, G. , Elliott, P. , Westphal, C. , Ellis, J. L. , Sinclair, D. A. , Price, N. L. , Bernier, M. , & de Cabo, R. (2020). Combining a high dose of metformin with the SIRT1 activator, SRT1720, reduces life span in aged mice fed a high‐fat diet. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(11), 2037–2041. 10.1093/gerona/glaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskovatska, V. , Stefanyshyn, N. , Storey, K. B. , Vaiserman, A. M. , & Lushchak, O. (2019). Metformin as a geroprotector: Experimental and clinical evidence. Biogerontology, 20(1), 33–48. 10.1007/s10522-018-9773-5 [DOI] [PubMed] [Google Scholar]
- Raparelli, V. , Elharram, M. , Moura, C. S. , Abrahamowicz, M. , Bernatsky, S. , Behlouli, H. , & Pilote, L. (2020). Sex differences in cardiovascular effectiveness of newer glucose‐lowering drugs added to metformin in type 2 diabetes mellitus. Journal of the American Heart Association, 9(1), e012940. 10.1161/JAHA.119.012940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- Reinke, S. N. , Hu, X. , Sykes, B. D. , & Lemire, B. D. (2010). Caenorhabditis elegans diet significantly affects metabolic profile, mitochondrial DNA levels, lifespan and brood size. Molecular Genetics and Metabolism, 100(3), 274–282. 10.1016/j.ymgme.2010.03.013 [DOI] [PubMed] [Google Scholar]
- Richardson, A. , Fischer, K. E. , Speakman, J. R. , de Cabo, R. , Mitchell, S. J. , Peterson, C. A. , Rabinovitch, P. , Chiao, Y. A. , Taffet, G. , Miller, R. A. , Rentería, R. C. , Bower, J. , Ingram, D. K. , Ladiges, W. C. , Ikeno, Y. , Sierra, F. , & Austad, S. N. (2016). Measures of healthspan as indices of aging in mice‐a recommendation. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(4), 427–430. 10.1093/gerona/glv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfstein, R. , Friedman, Y. , Attias‐Geva, Z. , Fishman, A. , Bruchim, I. , & Werner, H. (2013). Metformin downregulates the insulin/IGF‐I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53‐dependent or ‐independent manners. PLoS One, 8(4), e61537. doi: 10.1371/journal.pone.0061537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick, P. (2012). Meta‐analyses: Tests of heterogeneity. BMJ, 344, e3971. 10.1136/bmj.e3971 [DOI] [Google Scholar]
- Sena, E. S. , Currie, G. L. , McCann, S. K. , Macleod, M. R. , & Howells, D. W. (2014). Systematic reviews and meta‐analysis of preclinical studies: Why perform them and how to appraise them critically. Journal of Cerebral Blood Flow and Metabolism, 34(5), 737–742. 10.1038/jcbfm.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. L., Jr. , Elam, C. F., Jr. , Mattison, J. A. , Lane, M. A. , Roth, G. S. , Ingram, D. K. , & Allison, D. B. (2010). Metformin supplementation and life span in Fischer‐344 rats. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65(5), 468–474. 10.1093/gerona/glq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. , Sutton, A. J. , Ioannidis, J. P. , Terrin, N. , Jones, D. R. , Lau, J. , Carpenter, J. , Rücker, G. , Harbord, R. M. , Schmid, C. H. , Tetzlaff, J. , Deeks, J. J. , Peters, J. , Macaskill, P. , Schwarzer, G. , Duval, S. , Altman, D. G. , Moher, D. , & Higgins, J. P. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ, 343, d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- Strong, R. , Miller, R. A. , Antebi, A. , Astle, C. M. , Bogue, M. , Denzel, M. S. , Fernandez, E. , Flurkey, K. , Hamilton, K. L. , Lamming, D. W. , Javors, M. A. , de Magalhães, J. P. , Martinez, P. A. , McCord, J. M. , Miller, B. F. , Müller, M. , Nelson, J. F. , Ndukum, J. , Rainger, G. E. , … Harrison, D. E. (2016). Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α‐glucosidase inhibitor or a Nrf2‐inducer. Aging Cell, 15(5), 872–884. 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell, W. R. (2009). Accelerated failure time models provide a useful statistical framework for aging research. Experimental Gerontology, 44(3), 190–200. 10.1016/j.exger.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell, W. R. (2012). Dietary restriction in rats and mice: A meta‐analysis and review of the evidence for genotype‐dependent effects on lifespan. Ageing Research Reviews, 11(2), 254–270. 10.1016/j.arr.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell, W. R. (2017). Meta‐analysis of 29 experiments evaluating the effects of rapamycin on life span in the laboratory mouse. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(8), 1024–1032. 10.1093/gerona/glw153 [DOI] [PubMed] [Google Scholar]
- Therneau, T. (2022). A package for survival analysis in R. R package version 3.3. https://CRAN.R‐project.org/package=survival
- Vesterinen, H. M. , Sena, E. S. , Egan, K. J. , Hirst, T. C. , Churolov, L. , Currie, G. L. , Antonic, A. , Howells, D. W. , & Macleod, M. R. (2014). Meta‐analysis of data from animal studies: A practical guide. Journal of Neuroscience Methods, 221, 92–102. 10.1016/j.jneumeth.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- von Hippel, P. T. (2015). The heterogeneity statistic I(2) can be biased in small meta‐analyses. BMC Medical Research Methodology, 15, 35. 10.1186/s12874-015-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Zhao, Y. , & Zhang, Z. (2019). Age‐dependent effects of floxuridine (FUdR) on senescent pathology and mortality in the nematode Caenorhabditis elegans . Biochemical and Biophysical Research Communications, 509(3), 694–699. 10.1016/j.bbrc.2018.12.161 [DOI] [PubMed] [Google Scholar]
- Wei, Y. , & Royston, P. (2017). Reconstructing time‐to‐event data from published Kaplan‐Meier curves. The Stata Journal, 17(4), 786–802. [PMC free article] [PubMed] [Google Scholar]
- Wilding, J. , Batterham, R. L. , Calanna, S. , Davies, M. , Van Gaal, L. F. , Lingvay, I. , McGowan, B. M. , Rosenstock, J. , Tran, M. , Wadden, T. A. , Wharton, S. , Yokote, K. , Zeuthen, N. , Kushner, R. F. , & STEP 1 Study Group . (2021). Once‐weekly Semaglutide in adults with overweight or obesity. The New England Journal of Medicine, 384(11), 98–1002. 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- Winkler, D. T. , Bondolfi, L. , Herzig, M. C. , Jann, L. , Calhoun, M. E. , Wiederhold, K. H. , Tolnay, M. , Staufenbiel, M. , & Jucker, M. (2001). Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. The Journal of Neuroscience, 21(5), 1619–1627. 10.1523/JNEUROSCI.21-05-01619.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Zhou, B. , Oshiro‐Rapley, N. , Li, M. , Paulo, J. A. , Webster, C. M. , Mou, F. , Kacergis, M. C. , Talkowski, M. E. , Carr, C. E. , Gygi, S. P. , Zheng, B. , & Soukas, A. A. (2016). An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell, 167(7), 1705–1718.e1. 10.1016/j.cell.2016.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Shen, W. , Liu, Z. , Sheng, S. , Xiong, W. , He, R. , Zhang, X. , Ma, L. , & Ju, Z. (2021). Effect of metformin on cardiac metabolism and longevity in aged female mice. Frontiers in Cell and Development Biology, 8, 626011. 10.3389/fcell.2020.626011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
All data utilized in this study will be made publicly available upon publication via the Open Science Framework (osf.io).


