Abstract
Aims
Prescribed aerobic-based exercise training is a low-risk fundamental component of cardiac rehabilitation (CR). Secondary prevention therapeutic strategies following a spontaneous coronary artery dissection (SCAD) or aortic dissection (AD) should include CR. Current exercise guidance for post-dissection patients recommends fundamental training components including target heart rate zones are not warranted. Omitting fundamental elements from exercise prescriptions risks safety and makes it challenging for both clinicians and patients to understand and implement recommendations in real-world practice. We review the principles of exercise prescription for CR, focusing on translating guidelines and evidence from well-studied high-risk CR populations to support the recommendation that exercise testing and individualized exercise prescription are important for patients following a dissection.
Methods and results
When patients self-perceive exercise intensity there is a tendency to underestimate intensities within metabolic domains that should be strictly avoided during routine exercise training following a dissection. However, exercise testing associated with CR enrolment has gained support and has not been linked to adverse events in optimally medicated post-dissection patients. Graded heart rate and blood pressure responses recorded throughout exercise testing provide key information for developing an exercise prescription. An exercise prescription that is reflective of medical history, medications, and cardiorespiratory fitness optimizes patient safety and yields improvements in blood pressure control and cardiorespiratory fitness, among other benefits.
Conclusion
This clinical practice and education article demonstrates how to develop and manage a CR exercise prescription for post-acute dissection patients that can be safe and effective for maintaining blood pressure control and improving cardiorespiratory fitness pre–post CR.
Keywords: SCAD, exercise training, fibromuscular dysplasia, Type 1 SCAD, Type 2 SCAD, Type 3 SCAD, Type A aortic dissection, Type B aortic dissection
Introduction
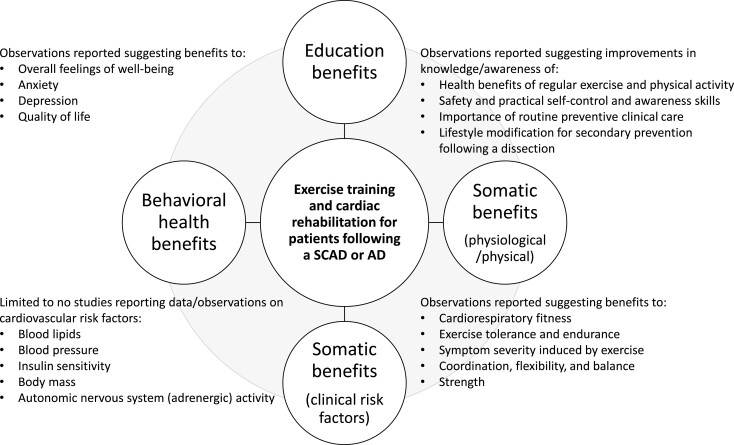
Blood flow that enters and collects between arterial wall tissue layers can quickly lead to the development of a dissection where the accumulation of blood forms a pressurized false lumen wedging between the inner two-thirds and the outer third layer of the tunica media. In some cases, dissection expansion in the radial and axial directions leads to inward compression of the true lumen and obstructed blood flow. In other cases, the thin outer third tunica media and adventitia layers containing the dissection rupture. While urgent care involving aggressive anti-pressor pharmacological therapy is necessary for any acute dissection due to high fatality rates that worsen as time to treatment increases, not all dissections require the emergency open heart surgery that is nearly always recommended for acute Type A aortic dissections (AD).1–4 Non-surgical conservative medical care is appropriate as an acute management option for some dissections, an approach generally favoured in cases involving a spontaneous coronary artery dissection (SCAD).5–7 Moreover, after patients stabilize and leave the hospital, the contemporary view is that in addition to the prognostic benefits of adhering to anti-pressor pharmacological regimens for the lifelong maintenance of heart rate and blood pressure control, post-acute dissection patients of either type also benefit from incorporating non-pharmacological lifestyle therapies such as exercise-based cardiac rehabilitation (CR) into the secondary prevention therapeutic strategy (Figure 1).5–17
Figure 1.
The proposed benefits associated with incorporating exercise training and cardiac rehabilitation as a core component of the secondary prevention therapeutic strategy for stable patients recovering from a spontaneous coronary artery dissection or aortic dissection.
A modern focus of CR advocates has been the drive to extend support for exercise-based CR as a medically necessary intervention for treating and managing high-risk cardiovascular conditions not meeting traditional CR qualifying criteria.5–17 Support for these efforts has been guided mainly by expert opinion,5–7,13,14,16 but also aligns with a key reason why exercise-based CR is recognized as a comprehensive secondary prevention strategy for improving cardiovascular prognosis among traditional qualifiers.18–22 In particular, exercise-based CR prioritizes the need to satisfy the principle of specificity of training, inclusive of patient medical history, pharmacological regimen, familiarity with exercise training, motivation and goals, and cardiorespiratory fitness level.18,19,23 Thus, an exercise prescription developed for CR can easily avoid recommending the type of quick, impulsive, and high-intensity exercise types that act as the concerning mechanical stressors that need to be intentionally avoided among individuals who in the weeks-to-months prior experienced a life-threatening SCAD or AD.5,8–12,14–19,23
Other than general information that is available on the benefits of physical activity, exercise, and CR, there is a paucity of widespread practitioner understanding of processes involved in how to provide post-acute dissection patients individualized guidance on exercise training for secondary prevention that is appropriate for their level of risk.5–7,13,15–17 To date, a lack of randomized clinical trial data coming from exercise-based CR studies on dissection survivors combined with unclear information on how to translate traditional CR guidelines to these high-risk patients has meant most individuals and their families do not receive personalized education on the beneficial role exercise training and CR can play in recovery.5–17 When education on exercise-based CR has not occurred by the time of routine outpatient follow-up, this also typically means no referral has been or will be placed for post-acute dissection patients to enrol in centre-based CR.10–13,15,16 An exact number of patients who could benefit from post-dissection CR is not known, but recent data suggest that the incidence of SCAD may be higher than previously estimated, and there are no signs suggesting that the incidence of AD has lessened since early reports of the 1980s (Table 1).2,24–29
Table 1.
Key epidemiology, clinical features, and risk factors of patients diagnosed with spontaneous coronary artery dissection (SCAD) or aortic dissection (AD)
| SCAD | AD | |
|---|---|---|
| Estimated prevalence | 1%–4% of all acute coronary syndrome cases | 4.4 per 100 000 person yearsa |
| Mortality | Lowb | 21.3 per 1 millionc |
| Sex | ||
| Women (overall) | ↑ | ↓ |
| Early-to-middle adulthood | ↑ | ↓ |
| Middle-to-late adulthood | ↓ | ↑ |
| Men (overall) | ↓ | ↑ |
| Early-to-middle adulthood | ↓ | ↓ |
| Middle-to-late adulthood | ↓ | ↑ |
| Predisposing risk factors | ||
| Pregnancy/postpartum/pre-eclampsia | ++ | ++ |
| Fibromuscular dysplasia | ++ | |
| Genetics/connective tissue disorders | ++ | ++ |
| Autoimmune/inflammatory diseases | ++ | ++ |
| Sex hormone disruptions/therapy | ++ | |
| Stimulating risk factors | ||
| Extreme/severe intensity exercise | ++ | ++ |
| Extreme/severe intensity physical activity | ++ | ++ |
| Extreme/severe physical torque movements | ++ | ++ |
| Intense psychological stress | ++ | ++ |
| Excessive Valsalva engagement | ++ | ++ |
| Recreational amphetamine use | ++ | ++ |
| Cardiovascular risk factors | ||
| Atherosclerosis | + | ++ |
| Hypertension | + | ++ |
| Dyslipidemia | + | ++ |
| Smoker/ex-smoker | + | ++ |
| Type II diabetes | + | + |
| Overweight/obese | + | ++ |
Overall age- and sex-adjusted incidence 1995 to 2015 (DeMartino et al.27).
In-hospital and 30-day outcomes, 1 death reported out of 750 cases (Saw et al.28). Overall mortality rate among all patients in the general population uncertain.
Overall age-adjusted mortality rate in 2019 (Nazir et al.24).
+Unlikely causative associations.
++Reported among case/observational data and current expert opinion suggests either known associations or possible associations of high clinical/research interest among patients.
↑ More likely to be observed among patients.
↓ Less likely to be observed among patients.
Clarifying what should be expected of CR and exercise prescription and training guidance as part of secondary prevention care for post-acute dissection patients remains an unmet clinical need. Current exercise and physical activity recommendations are too general and challenging to translate for clinical implementation and patient understanding.1,5–7,13,16,30 There is a lack of clarity in how to prescribe patients individualized exercise plans that can be both safe and effective. Neither practitioners nor patients are able to clearly interpret recommendations suggesting that CR and exercise training are important following a dissection, but implementing classical training elements such as target heart rate zones when prescribing exercise lacks utility and can be largely counterproductive.6,7 The scientific and clinical basis supporting the need for clinical exercise testing and prescribing individualized exercise training as fundamental components of CR for patients following a dissection are discussed in this review.
Overview of pathogenesis and risk factors
Specific stimulating factors have been identified as explaining certain cases of SCAD or AD as briefly outlined in Table 1.1–3,5–7,14,26,29 Mechanical stress associated with general exercise participation has traditionally been highlighted as being particularly concerning for patients at risk for a first dissection, and among those who are survivors practicing secondary prevention. However, despite concerns over the causal association between exercise and dissection, the existing evidence does not support nor suggest exercise training performed to the specifications expected of CR is the primary underlying cause of original events or event recurrence (Table 2).5,7–12,17,28,31–36 There are multiple lines of ongoing exploratory research aimed at identifying and clarifying phenotype, genetic, and clinical traits that may make individuals susceptible to SCAD or AD (Table 1).1,2,5–7,25,26,29
Table 2.
Overview of studies on clinical exercise testing and/or cardiac rehabilitation participation in patients recovering from spontaneous coronary artery dissection or aortic dissection
| Study | Participants No./%Female | Indication | Exercise test BP (mmHg) | CR exercise sessions | Aerobic training intensity | Exercise-related SAE |
|---|---|---|---|---|---|---|
| Corone et al., 200910 | 26/NR | De Bakey Type I | NR | 3–5 weekly | Avg. 11 RPE | None |
| Prospective (France) | Avg. total: 18 (range: 5–50) | Avg. SBP: 150 mmHg | ||||
| Delsart et al., 201632 | 105/30.5 | Stanford A and B | Cycle: Avg. BP at AT: 151/77 | NA | NA | None |
| Prospective (France) | ||||||
| Fuglsang et al., 201711 | 10/40 | Stanford A | Cycle: Pre-CR | 3 weekly over 12 weeks (36 sessions) | Referenced AHA/EAPC guidelines | None |
| Retrospective (Denmark) | Max Avg.: 200/95 | |||||
| Post CR | ||||||
| Max Avg.: 207/99 | Avg. total NR | |||||
| Hornsby et al., 202033 | 28/14 | Stanford A | 3.6 mo post-surgery | NA | NA | None |
| Retrospective (USA) | ||||||
| Treadmill: Max Avg.: 156/70 | ||||||
| Norton et al., 202134 | 21/14 | Stanford A | Treadmill: 3 mo post-surgery | NA | NA | None |
| Prospective (USA) | ||||||
| Max Avg.: 170/68 | ||||||
| 15 mo post-surgery | ||||||
| Max Avg.: 167/75 | ||||||
| Delsart et al., 202131 | 165/29.7 | Stanford A and B | Cycle: Max Avg.: 183/85 | NA | NA | None |
| Prospective (France) | ||||||
| Silber et al., 20159 | 9/100 | SCAD | NR | 1–3 weekly | 60%–70% HRR | None |
| Prospective (USA) | Avg. total: 28 (range: 5–39) | 12–14 RPE | ||||
| Chou et al., 20168 | 48/100 | SCAD | NR | 1 weekly | 50%–70% HRR | None |
| Prospective (Canada) | Avg. total: 12.4 | 12–14 RPE | ||||
| Krittanawong et al., 201612 | 269/97 | SCAD | NR | 1–3 weekly | NR | None |
| Retrospective (USA) | Avg. total: 18 | |||||
| Imran et al., 201817 | 10/80 | SCAD | Modality NR | 3 weekly over 12 weeks (36 sessions) | 70%–85% HRmax | None |
| Retrospective (USA) | Symptom limited up to SBP/DBP 140 and/or 90 | Exercise BP <140/90 mmHg | ||||
| 2 of 10 patients pre-planned to complete only 20 sessions | Rest to exercise rise in SBP ≤20 mmHg | |||||
| Avg. end-exercise BP NR | 4–6/10 RPE scale |
AT, anaerobic threshold; BP, blood pressure; CR, cardiac rehabilitation; HRmax, maximal heart rate achieved on pre-CR stress test; HRR, heart rate reserve; NA, not applicable/studied; NR, not reported; RPE, rating of perceived exertion (Borg, 6–20 scale); SAE, serious adverse events; SCAD, spontaneous coronary artery dissection.
Medically stable post-acute dissection patients should not look to avoid participating in individualized aerobic exercise training because of generalized concern that exercise of any type, intensity, and modality provokes inappropriate Valsalva engagement, physical strain, and mechanical stress leading to excessive hemodynamic shear stress and pressure development. Committing to a sedentary lifestyle out of fear of potential exercise implications should not be considered an option for patients since participating in chronic physical inaction increases the risk of an atherosclerotic cardiovascular disease event over the middle term.37,38 The type of cardiovascular risk predicted by chronic physical inactivity could be reasonably compounded further in patients at risk of AD or among AD survivors practicing secondary prevention since hypertension and multimorbidity are risk factors for AD and are commonly observed among individuals who live sedentary lifestyles.1,2 Moreover, although the current body of evidence does not implicate traditional cardiovascular risk factors as major causal factors for SCAD,5–7 the prevalence of traditional cardiovascular risk factors among SCAD survivors has recently been suggested to be more common than previously thought and likely comparable to age- and sex-matched populations (Table 1).28
Exercise prescription for CR and secondary prevention
Prescription-based exercise training is a hallmark of CR and secondary cardiovascular disease prevention. The personalized approach to educating patients on how to exercise for their individualized goal of improving heart health while participating in routine exercise training over the course of CR is well-established, promotes safety, and yields favourable effects on multiple inter-related health parameters.18–23,39–43 Contemporary clinical guidelines also provide the class I recommendation that it is generally safe and medically appropriate for most patients to undergo maximal effort clinical exercise testing as a core component of enrolling in CR and developing an exercise prescription.18,19,44,45 Consensus support for these complementary clinical exercise models for improving heart health and risk assessment represents guidance that has evolved from the early years of CR when only post-acute myocardial infarction patients had been allowed to participate in exercise-based CR. Not only is exercise-based CR currently recommended as part of the secondary prevention medical strategy for treating high-risk CR eligible patients with multimorbidity, but modern guidelines also strongly recommend all stable patients, even those deemed high risk, not delay enrolment in CR beyond the ideal post-hospital discharge window in order to optimize therapeutic effects and improve cardiovascular prognosis.18,19,46
Prescribed exercise training following a major heart event regardless of whether it is performed in CR or self-supervised is meant to largely focus on stimulating aerobic metabolic pathways to yield improvements in cardiovascular responses to both submaximal and maximal levels of metabolic demand, cardiorespiratory fitness, and cardiovascular risk.18,19,23 Typically, aiming to achieve these CR focused exercise training goals means initially prescribing continuous duration cardio-style exercise that encourages well-controlled work intensities no greater than moderate-to-somewhat hard exertion based on both subjective and objective scaling (Tables 3 and 4).18,19,23,47 As medically appropriate and as risk tolerance allows, it is further expected that CR staff guide careful patient progression in one or more principle components of the exercise prescription over time (Tables 3 and 4).18,19,23,47 The cumulative results of this purposefully prescriptive dynamic approach to initiating and progressing exercise training under the directed guidance of CR professionals are gradual developments in whole body adaptations proven to benefit clinical markers of cardiovascular prognosis. Patients enrolled in CR because of traditional qualifying indications commonly demonstrate at program completion sustainable improvements in blood pressure control and cardiorespiratory fitness,18,20,21,47–49 factors directly relevant to managing the post-acute dissection risk of a secondary event.
Table 3.
Key principles, components, and recommendations to be considered for developing an individualized exercise prescription for patients recovering from a spontaneous coronary artery dissection or aortic dissection
| Foundational principles of exercise prescription: F.I.T.T.—V.P. |
|
V̇O2, oxygen uptake; heart rate reserve (= HRrest + [HRpeak − HRrest] · X%); VT1, first ventilatory threshold/lactate threshold; VT2, second ventilatory threshold/respiratory compensation point.
Table 4.
Individualized exercise testing and exercise prescription recommendations for stable patients recovering from a spontaneous coronary artery dissection or aortic dissection
| Exercise training type(s) | Clinical exercise testing | Main exercise prescription features |
|---|---|---|
| Aerobic training: | Maximal effort CPET is the optimal clinical exercise test | Exercise training should be performed while on optimal rate-limiting and anti-pressor medications. |
|
|
|
| Training intensities should correspond to exercise BP <150/<90 mmHg. Avoid intensities causing >10 mmHg rise in DBP above rest. | ||
| Examples to consider: | Exercise stress testing is acceptable when CPET is unavailable. | |
| ||
| A dedicated period of ≥8–10 min for both warm up and active cool down are required. | ||
| Prescribe an initial low intensity phase (Min: 4 weeks, longer as needed): | ||
| All measurements should be acquired while patient is on optimal rate-limiting and anti-pressor medications: |
|
|
| Examples to avoid: | Peak exercise: | |
|
|
|
| Maintain an active lifestyle on non-scheduled training days. | VT1 and VT2 domains | If exercise BP remains controlled, progress to moderate intensity phase, as tolerated: |
|
|
|
| Lifting and intensity precautions should always be followed, for example: | CPET data should be recorded throughout testing and reviewed while interpreting both submaximal and peak response data. | |
| ||
| Where appropriate, other CPET data and variables in addition to those listed above should be considered when developing the exercise prescription. | ||
| Strength, resistance, and other types of training | ||
|
|
|
| Modalities to consider: | ||
| ||
| Examples to avoid: | ||
| ||
CPET, cardiopulmonary exercise testing; DBP, diastolic blood pressure; HR, heart rate; HRR, heart rate reserve (= HRrest + [HRpeak − HRrest] · X%); RPE, rating of perceived exertion, 6–20 scale; SBP, systolic blood pressure; V̇O2peak, peak exercise oxygen uptake; VT1, first ventilatory threshold/lactate threshold; VT2, second ventilatory threshold/respiratory compensation point.
Exercise training and blood pressure control
A common clinical benefit that follows the proven CR approach to a prescribed exercise training intervention includes improved blood pressure control.18,47,49–52 For most patients, although yet to be confirmed in those following a dissection, the predominant underlying mechanism among the many contributing factors responsible for lowered blood pressure following an exercise training intervention is suggested to be a coordinated fall in systemic vascular resistance associated with improved regulation of autonomic nervous system control and decreased tonic sympathetic adrenergic drive activity.53 Other relevant, but not yet widely clinically established moderating mechanisms influential to the blood pressure lowering effects of exercise training are suggested to stem from improvements in endothelial function and related pathways of nitric oxide-dependent dilation and arterial stiffness regulation.53,54 More clinically observable correlates of exercise training and improved blood pressure control are known to include increased cardiorespiratory fitness and weight loss, both of which have been independently linked to favourable effects on the mechanisms underlying blood pressure control.53,55
Among patients with primary hypertension, it is estimated that an aerobic exercise training intervention conducted in the style of CR yields an average lowering effect on resting systolic and diastolic blood pressure at least equal to 7.6/4.7 mmHg,47 and 3.2/2.7 mmHg in daytime ambulatory blood pressure.56 The antihypertensive effects of exercise training alone are strong enough to potentially be more powerful than the independent effects of traditional pharmacological therapy.52 When prescribed and taken in combination, exercise training effects can also work to further strengthen the therapeutic benefits of antihypertensive medications.52 Thus, although randomized control trial exercise training studies have not been conducted in adults with primary hypertension following a dissection, the therapeutic effects of prescribed exercise training on lowering blood pressure and managing primary hypertension are particularly relevant for the secondary prevention of SCAD since these patients are not known to demonstrate classical cardiovascular multimorbidity as the main cause of the initial event.
In patients diagnosed with the higher risk, severe form of resistant hypertension, the aerobic exercise training lowering effect on blood pressure is suggested to be even more powerful than that observed in primary hypertension.47,50,51 As a result of 12 weeks of supervised aerobic exercise training performed thrice weekly for up to 40 min per session, patients with resistant hypertension have been observed to safely demonstrate mean reductions of at least 6.2/4.4 and 7.3/5.0 mmHg in 24-h ambulatory and daytime ambulatory systolic and diastolic blood pressure, respectively.50 The sizeable and clinically relevant reductions in ambulatory blood pressure following an intervention of up to 36 sessions of prescribed exercise training are achievable while keeping patients safe from exercise-related adverse events and from engaging in excessive physical exertion.47,50,51
Constraining exercise session workload intensities to heart rate and/or work-rate levels no greater than ranges roughly equivalent to 50%–70% of maximal/peak exercise oxygen uptake (V̇O2) and limiting session durations to avoid training to exhaustion are appropriate prescription features for high-risk post-dissection patients, similar to what has been prescribed for patients with resistant hypertension.50 Even greater exercise prescription precision may be achievable when setting heart rate and/or work-rate intensities based on the first ventilatory threshold (VT1) and second ventilatory threshold (VT2) landmarks identified on cardiopulmonary exercise testing.19 For most high-risk patients who are entering CR or beginning any exercise training intervention, regardless of past experiences with exercise, it would not be uncommon to prescribe an initial training run-in period lasting at least several weeks where heart rate or work-rate zones are set at levels falling well-below the metabolic equivalent of the VT1 point identified on cardiopulmonary exercise testing (Table 4). Exercise training intensity less than the VT1 point is typically considered very low risk physical and hemodynamic stress, well-tolerated by traditional CR qualifying participants, and permits patients to set a baseline level of fitness that safely allows for the natural progression in training intensity and duration to occur over the course of a multiple month training intervention.18,19,23,47
The high likelihood that the exercise training effect on improving blood pressure control over a wide range of hypertension grades can occur in the absence of stimulating excessive hemodynamic stress and provoking exercise-related adverse events directly addresses key secondary prevention concerns following a dissection. Prescribed exercise training may even provide an effective stand-alone alternative therapeutic strategy for chronically managing blood pressure control in post-acute dissection patients with pre-existing hypertension who may demonstrate minimal responsiveness or intolerance to traditional pharmacological regimens.47,50–52 Patients exhibiting the worst baseline levels of blood pressure control are known to typically experience the strongest blood pressure lowering effects of exercise training.47,50,51
Exercise training and cardiorespiratory fitness
Cardiorespiratory fitness represents an important modifiable clinical marker of cardiovascular prognosis among patients who meet traditional qualifying criteria for CR enrolment. Historically among CR graduates, the typical improvement in cardiorespiratory fitness in the approximate range of 0.7–1.0 metabolic equivalents (METS) as a direct consequence of exercise-based CR is sizeable and not achievable through the effects caused by a standard pharmacological regimen alone.18–22,41 Patients of nearly any disease severity and baseline cardiorespiratory fitness level who are able to improve cardiorespiratory fitness pre-to-post CR not only benefit from increased exercise tolerance that translates well to carrying out activities of daily living, but they can also expect related reductions in major adverse cardiovascular event risk over the short-to-middle term.18,19,21,22 As small as a 1.0 mL/kg/min rise in peak V̇O2 is associated with reducing both cardiac and any-cause mortality risk.18,19,21
In contrast to what is known of the direct link between cardiorespiratory fitness, exercise-based CR, and cardiovascular prognosis among patients with acute coronary syndrome or heart failure with reduced ejection fraction, there is not a well-developed body of evidence confirming similar associations among patients following a dissection. To date, a limited number of observational data from exercise training studies following a dissection are encouraging in suggesting cardiorespiratory fitness is modifiable and can improve as a result of participating in exercise-based CR (Table 2).
In an early study from France which prospectively studied the benefit of CR in patients following surgery for De Bakey type I AD (mean days after surgery program initiated, 27 ± 21) (Table 2), those who completed pre–post maximal effort exercise testing (n = 13) exhibited a mean increase in maximal exercise Watts achieved from 62.7 to 91.6 (P = 0.002) at program completion.10 Others have reported in a Danish retrospective study of patients (n = 10) following surgical treatment for a Stanford type A AD (pre-CR clinical exercise testing occurred 6–12 weeks after surgery), a mean increase in peak exercise V̇O2 of 22% (23.5 mL/kg/min vs. 29.0 mL/kg/min) occurred at the conclusion of 12 weeks of CR (Table 2).11 Likewise, in the first prospective study of CR in patients following SCAD from the United States (program starting an average of 12.3 days after event), participants (n = 4 of 9 completed pre–post clinical exercise testing) demonstrated an average increase of 18% in peak V̇O2 at program completion (Δ4.4 mL/kg/min; 25.4 ± 4.1 mL/kg/min vs. 28.2 ± 3.0 mL/kg/min) (Table 2).9 Similar cardiorespiratory fitness benefits have been reported from a Canadian prospective study of CR in post-acute SCAD patients (n = 70; program started a median 0.6 years after event) in which they observed the estimated peak exercise METS achieved increased from 10.1 ± 3.3 to 11.5 ± 3.5 (P < 0.001) at program completion (Table 2).8
Mechanistic reasons that can explain how cardiorespiratory fitness changes and why this type of whole-body training adaptation in response to exercise training is likely to be clinically beneficial following a dissection have yet to be elucidated. The absence of this evidence base while important to recognize, has also not been countered with a body of evidence that suggests improving cardiorespiratory fitness is not important and yields no prognostic benefits among patients following a dissection. There is also a lack of evidence that supports a pharmacological-only secondary prevention strategy following a dissection that relegates the addition of lifestyle interventions such as exercise-based CR as yielding null effects on cardiovascular prognosis and ineffective for benefitting biomarkers of cardiovascular health, such as cardiorespiratory fitness. There is likely a low probability that most post-acute SCAD or AD patients are able to self-acquire the knowledge, comprehension, and ability to develop a safe exercise prescription that yields improved cardiorespiratory fitness and maintained heart rate and blood pressure control consistent with what could be accomplished in CR.
Exercise training and weight management
In addition to blood pressure management, weight management represents another core component included in the individual treatment plan developed for patients at the time of CR enrolment. However, the ability to achieve clinically meaningful weight loss (e.g. ≥1.0 kg57) as a result of exercise-based CR has not been directly evaluated or observed in studies of patients who participated in CR following an AD. The observations that have been reported suggesting exercise-based CR could benefit body weight and mass changes among enrollees recovering from SCAD are also limited in number.9,17
In the first prospective study of exercise-based CR among post-acute SCAD patients, participants (n = 7) demonstrated from pre-to-post CR an average total body weight loss of 1.5 ± 1.5 kg, but also benefitted from gains in lean mass totalling on average 0.4 ± 1.5 kg.9 Among the four patients who completed pre-to-post CR cardiopulmonary exercise testing, the one patient who exhibited a nominal change in peak V̇O2 (0.1 mL/kg/min) also experienced increases in total body weight and fat mass, but not lean mass.9 The other three patients who demonstrated clinically meaningful pre-to-post CR increases in peak V̇O2 (>1.0 mL/kg/min) each exhibited decreased total body weight and increased lean mass.9 Similarly, in the only other study that evaluated the effects of exercise-based CR on body mass changes among post-acute SCAD participants (n = 10), cardiorespiratory fitness increased (Δ0.8 ± 0.04 peak METS) and body mass index decreased (25.3–24.4 kg/m2) pre-to-post CR; although the fall in body mass index did not achieve statistical significance.17 Viewed together, the modest body weight and mass changes observed among these limited number of observations in post-acute SCAD CR participants cannot be interpreted to imply causation, but are nonetheless not unexpected.
Total training volume per session and per week over the course of a CR program among post-acute SCAD or AD participants can be expected to be purposefully less than levels expected of most traditional CR qualifiers due to the unique need to conservatively taper-in exercise duration, intensity, and frequency progressions (Table 4). The overall deployment of exercise-based CR taking such an approach should typically mean for most post-acute dissection patients that much of the required caloric deficit needed to lose weight over the early-to-middle weeks of CR participation could be expected to stem from diet and nutrition modification. Indeed, not only is diet and nutrition counselling another core component of the required CR individual treatment plan, but it is consistently reported in weight loss studies across the health spectra that the combined effects exercise training and diet/nutrition modification yield the strongest and longest lasting effects on clinically meaningful weight loss and maintenance.58 The opportunity to ramp-up the contributions from exercise training to largely account for the caloric deficit required for enhanced weight loss during the course of a CR program is not a realistic option for post-acute dissection patients since this would require inappropriate sized increases and progressions in exercise session training intensity, frequency, and/or duration to meet the weekly training volume demand reported to be effective for enhanced weight loss among overweight patients with coronary artery disease eligible for CR.59 A top priority for post-acute SCAD or AD patients participating in exercise-based CR should not be the achievement of bulk or enhanced levels of weight loss by program completion. Instead, patients should be guided towards focusing on the long-term achievement of gradual weight loss of up to 0.5 lb per week coupled with the continued maintenance of body weight upon reaching goal, which are weight management targets more consistent with the moderated post-dissection approach to exercise training.
Exercise safety and perceived exertion
Both the safety and success of the ‘exercise is medicine’ paradigm require dedicated patient education and specific guidance focused on aerobic exercise training to contain physiological training zones reflective of current medical history, physical capabilities, cardiorespiratory fitness level, and cardiovascular medications.18,19,23 Using vague descriptive words for intensity such as ‘moderate’, ‘brisk’, or ‘vigorous’ in the absence of concurrently providing patients with clear and concise exercise information inclusive of individualized target heart rate zones for aerobic-based training carries a likelihood of leading to unnecessary risk/danger, concern, confusion, inconsistency, and lack of results since individuals are forced to train based solely on an often times naïve subjective perception of exertion. Patient reported rating of perceived exertion is commonly lower than actual intensity, highly variable between and within days, and sensitive to confounding by unknown non-cardiovascular factors.60–63
Patients with heart conditions of various aetiologies and severities who are naïve to structured exercise or have not recently participated in exercise demonstrate a propensity to subjectively underestimate the intensity of physical exertion as actual exercise physiological stress reaches, and then surpasses, moderate metabolic intensity.60 Not only does such tendency occur during rising levels of graded exercise stress, but patients can further be expected to subjectively underestimate what moderate or greater physiological exertion should feel like during more continuous bouts of exertion, such as over-ground self-paced walking or during CR.61,63 Moreover, the presence of an optimal β-blocker therapy regimen, which is not uncommon among CR enrollees, cannot be expected to offset the subjective tendency to repetitively perform more intense exercise than what is needed to yield improved cardiorespiratory fitness as a result of CR.62,63 An over-abundance of self-reliance on subjective scoring of exertion can be dangerous in post-dissection patients since the unpredictable disconnect between perceived exertion and physiological exertion results in patient guesswork and regular training at physical stress levels that are far higher than what is recommended. Therefore, those who are deemed medically stable in the outpatient setting should be encouraged to undergo periodic (e.g. annual) maximal effort individualized clinical exercise testing while on optimal rate-limiting and anti-pressor medications in order to acquire comprehensive information on graded heart rate and blood pressure responses to be referenced for creating the exercise prescription (Tables 3 and 4, Figure 2). An accumulating body of evidence inclusive of data from both post-SCAD and AD studies suggests it is safe and not medically unreasonable for stable patients to participate in graded exercise testing in a controlled clinical environment for the purpose of developing an exercise prescription for CR (Table 2).8–11,31–34 No exercise-related serious adverse events have been reported in studies of maximal effort clinical exercise testing when conducted in as little as 7 days following SCAD, or 10 days following AD (Table 2).9,10
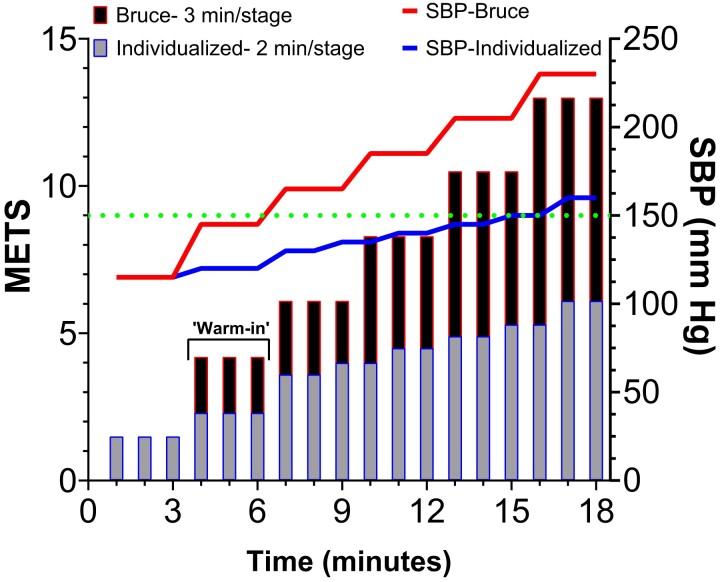
Figure 2.
Importance of individualizing the clinical exercise test. Schematic illustrating for a typical stable patient following a spontaneous coronary artery dissection on optimal pharmacological rate-limiting therapy how differences in clinical exercise testing protocols can affect stage-to-stage increases in systolic blood pressure (SBP) relative to the level of metabolic equivalent of task (METS) performed. In the absence of cardiopulmonary exercise testing which is the preferred clinical test, but less readily available than exercise stress testing, METS achieved in this example are estimated using the FRIEND73 equation relying on treadmill belt velocity and grade. The first 3 min is pre-test for both protocols at an estimated 1.5 METS and SBP of 115 mmHg. The Bruce protocol consists of 3-min length stages where treadmill belt velocity and grade both change each stage. The starting treadmill belt velocity is 1.7 mph at a grade of 10%. Alternatively, the individualized protocol consists of an initial 3-min ‘warm-in’ phase at 1.0 mph and grade of 0.0%; thereafter including 2-min length stages where velocity remains constant at 2.0 mph while grade progressively increases beginning at the second stage. The horizontal dotted line is set at SBP = 150 mmHg and represents the upper level which should not be surpassed while performing continuous duration aerobic exercise training on a routine basis. The figure illustrates that by having patients participate in the conventional Bruce protocol, they would achieve the threshold SBP within 3 min at an estimated MET level of 4.2. Conventional exercise training criteria based on the conventional use of the Bruce protocol would recommend patients perform aerobic exercise training at intensities no greater than the first stage of the Bruce protocol, possibly explaining why patients might express frustration and feel discouraged in maintaining a routine.
Individualized clinical exercise testing and exercise intensity assessment
Due to the clinical importance of needing to phenotype the cardiovascular response at each level of metabolic stress, not just peak exertion, special attention should be given to ensuring that the clinical exercise test is individualized to best reflect medical history, physical capabilities, and recent training history since an inappropriate testing protocol and/or modality can serve as an appreciable source of variance affecting the perception of exercise intensity and actual cardiovascular responses (Figure 2).60,64–66 When available, the cardiopulmonary exercise test is the optimal customizable clinical exercise physiological assessment tool to precisely characterize the cardiovascular and cardiopulmonary responses during each distinct metabolic domain, commonly identifiable via the non-invasive determination of the VT1 and VT2 landmarks.19 In instances where cardiopulmonary exercise testing is unavailable the acceptable clinical alternative is the exercise stress test performed to volitional fatigue.
Included in any exercise test protocol customization as illustrated in our example in Figure 2, patients should be afforded a proper ‘warm-in’ unloaded phase (e.g. 0.0% grade, 0.0 Watts, etc.) of at least 3-min immediately prior to performing any externally loaded exercise as this will not only help ease anxiety-driven hyperventilatory coupled cardiovascular responses, but it will also allow the cardiovascular system reach a homeostatic state and thereby avoid the rapid and sizeable exercise-induced rise in heart rate and blood pressure that are observed to coincide with impulsive loaded exercise,67 something that always needs to be avoided by post-dissection patients. A protocol that is too aggressive at the start will also typically yield a truncated duration test not directly attributable to cardiovascular limitations and under-represented peak exercise cardiovascular responses that will result in overly conservative calculated training zones (Figure 2), possibly explaining comments6,7 suggesting patients express frustration and experience lack of results when given target heart rate training zones to follow.
Since the absolute cardiorespiratory fitness value achieved as a result of clinical exercise testing can be expected to vary greatly across individuals due to basic human features, even in the setting of meticulous protocol selection and cardiopulmonary exercise testing it is important that factors such as age, sex at birth, body size, and weight be accounted for when interpreting test data for developing the exercise prescription.18,19,68,69 Dismissing the relevance of such detail when interpreting the clinical exercise test could have direct clinical implications, particularly since unique age periods of incidence and sex are such prominent features of SCAD and AD patient phenotypes (Table 1).1–3,5,70 Individuals who experience SCAD are more likely to be women in young-to-middle adulthood;5,6,14,35,71 whereas AD more commonly occurs in middle-to-late adulthood, affecting women later in life than men up until 75 years of age where incidence rates are then similar across sexes (Table 1).2,3,70,72 These age- and sex-related trends represent relevant information that should aid the understanding of exercise physiological responses of both SCAD and AD patients because for example, it is generally recognized that sex differences alone will always dictate lower cardiorespiratory fitness will be achieved by women than men with all else being held equal, and age-related declines in cardiorespiratory fitness start to accelerate more rapidly upon reaching the fourth decade of life for both sexes.68,69
Training recommendations should reflect knowledge and an in-depth understanding of how to translate both absolute and relative cardiorespiratory fitness levels to developing an exercise prescription, and prescribed training zones referencing energy expenditure in units of METS should always take into account both age and sex effects.18,19 Broadly recommending1,16,30 a training range of 3–5 METS for post-acute dissection patients should be avoided since for example, a patient who achieves a true peak exercise METS level of 6 would be routinely exercising in-excess at a METS level of 5 (Figure 2). The possibility where low cardiorespiratory fitness confounds the appropriate use of non-individualized training ranges among post-acute dissection patients is unlikely to be a rare occurrence since these individuals typically demonstrate below normal levels of relative cardiorespiratory fitness.8–11,31–34 Thus, it is clear that the observed exercise physiological information that can be gained by participating in individualized clinical exercise testing proves invaluable for constructing each component of the individualized aerobic-based exercise training prescription regardless of whether it is carried out in CR or self-supervised.
Concerns and other considerations
Concerns have arisen about the usefulness, and possible counterproductive effects, of providing patients with prescriptive information, such as target heart rate training zones.6,7 Why this may be a barrier to routine exercise participation in recovering dissection patients has not been clearly explained to the extent where it is clinically reasonable to dismiss the importance and real-world application of this type of classical exercise training information.6,7 When properly derived, there are only upsides to providing patients with clear and concise individualized objective guidance on how to routinely approach aerobic exercise training.18,19,23 Allowing post-acute dissection patients to self-judge descriptions (e.g. ‘moderate’) of recommended exercise intensities on a routine basis runs a high likelihood of inadvertent overexertion to levels that have implications beyond the feelings of fatigue and shortness of breath. Instituting standard operating procedures consistent with secondary prevention exercise guidelines for other high cardiovascular risk patient groups can greatly lessen concerns of whether individualized recommendations such as exercise heart rate zones are reasonably safe to achieve on a routine basis and reflective of current physical conditioning levels and effects from cardiovascular medications.18,19,23 This also means under no circumstance should exercise heart rate training zones be determined based on any type of predicted maximal heart rate equation in post-acute dissection patients. The heart rate and blood pressure responses provided by patients while on optimal rate-limiting and anti-pressor medications during individualized clinical exercise testing should be primarily used to determine target heart rate and/or work-rate training zones for aerobic-based continuous duration exercise (Tables 3 and 4, Figure 2). Other important information such as cardiorespiratory fitness should be used to characterize current physical conditioning and motivation levels to help shape appropriate target heart rate and/or work-rate training zones.
Patient education
The final component that must be involved in developing the exercise prescription and target heart rate/work-rate zones for post-dissection patients is a detailed discussion on how individuals are to apply recommendations to routine aerobic-based exercise and how rate-limiting and anti-pressor medications may affect day-to-day exercise training responses. Such discussions are effective for clarifying and reinforcing to patients what exercises are appropriate for using target heart rate zones and what types of high-risk exercises and physical activities should be avoided. Patients should be made to clearly understand that target heart zones meant for cardio-style exercise training should not be used to guide intensity of strength/resistance training. Isometric exercise (e.g. planks), exercise and physical activities involving severe upper and/or lower body torsional movements, and high-intensity interval training must be avoided (Tables 3 and 4). These type of exercise precautions naturally require that training and competition for sport and athletic performance also be discontinued.
Conclusion
When patients recovering from SCAD or AD receive clear and concise individualized council on how to participate in routine exercise training responsibly, and why this is important for lifelong heart rate and blood pressure control and secondary prevention risk management, the integrative health benefits gained are likely to be multifactorial and consistent with what is observed for those who are encouraged to enter CR because of traditional cardiovascular indications. Current evidence and expert recommendations suggest continuity of care following SCAD or AD that is inclusive of prescribed exercise training and CR yields patient improvements in psychosocial well-being, symptom severity, blood pressure control, and cardiorespiratory fitness at CR program completion.5–15,17,23
Contributor Information
Erik H Van Iterson, Section of Preventive Cardiology and Rehabilitation, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Miller Family Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Ave., Desk JB-1, Cleveland, OH 44195, USA.
Luke J Laffin, Section of Preventive Cardiology and Rehabilitation, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Miller Family Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Ave., Desk JB-1, Cleveland, OH 44195, USA.
Lars G Svensson, Department of Thoracic and Cardiovascular Surgery, Miller Family Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Ave., Desk J4-1, Cleveland, OH 44195, USA.
Leslie Cho, Section of Preventive Cardiology and Rehabilitation, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Miller Family Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Ave., Desk JB-1, Cleveland, OH 44195, USA.
Lead author biography
 Dr Van Iterson is the Director of Cardiac Rehabilitation and member of the Professional Staff in the Department of Cardiovascular Medicine and Section of Preventive Cardiology and Rehabilitation in the Heart, Vascular, and Thoracic Institute at the Cleveland Clinic, Cleveland, OH, USA. His other professional responsibilities include serving as the Director of the Cardiopulmonary Exercise Testing CORE Laboratory in the C5 Research center in the Heart, Vascular, and Thoracic Institute. He is also the Quality Director of the Metabolic Exercise Testing Center in the Heart, Vascular, and Thoracic Institute. His academic and research activities focus on the areas of cardiac rehabilitation, cardiopulmonary exercise testing, clinical exercise physiology, exercise is medicine, and preventive cardiology.
Dr Van Iterson is the Director of Cardiac Rehabilitation and member of the Professional Staff in the Department of Cardiovascular Medicine and Section of Preventive Cardiology and Rehabilitation in the Heart, Vascular, and Thoracic Institute at the Cleveland Clinic, Cleveland, OH, USA. His other professional responsibilities include serving as the Director of the Cardiopulmonary Exercise Testing CORE Laboratory in the C5 Research center in the Heart, Vascular, and Thoracic Institute. He is also the Quality Director of the Metabolic Exercise Testing Center in the Heart, Vascular, and Thoracic Institute. His academic and research activities focus on the areas of cardiac rehabilitation, cardiopulmonary exercise testing, clinical exercise physiology, exercise is medicine, and preventive cardiology.
Data availability
No new data were generated in support of the article.
Funding
None.
Conflict of interest: Dr Laffin reported receiving personal fees from Medtronic, Gordy Health, LucidAct Health, and Eli Lilly Pharmaceuticals and grants from AstraZeneca and Mineralys Therapeutics outside the submitted work. No other disclosures were reported.
References
- 1. Salameh MJ, Ratchford EV. Aortic dissection. Vasc Med 2016;21:276–280. [DOI] [PubMed] [Google Scholar]
- 2. Sen I, Erben YM, Franco-Mesa C, DeMartino RR. Epidemiology of aortic dissection. Semin Vasc Surg 2021;34:10–17. [DOI] [PubMed] [Google Scholar]
- 3. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM; American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American College of R, American Stroke A, Society of Cardiovascular A, Society for Cardiovascular A, Interventions, Society of Interventional R, Society of Thoracic S and Society for Vascular M . 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 4. Malaisrie SC, Szeto WY, Halas M, Girardi LN, Coselli JS, Sundt TM III, Chen EP, Fischbein MP, Gleason TG, Okita Y, Ouzounian M, Patel HJ, Roselli EE, Shrestha ML, Svensson LG, Moon MR; Surgery ACPSCAC . 2021 The American Association for Thoracic Surgery expert consensus document: surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg 2021;162:735–758 e2. [DOI] [PubMed] [Google Scholar]
- 5. Adlam D, Alfonso F, Maas A, Vrints C, Writing C. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular D, Council on Clinical C, Council on C, Stroke N, Council on G, Precision M, Stroke C . Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, Rose CH. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol 2020;76:961–984. [DOI] [PubMed] [Google Scholar]
- 8. Chou AY, Prakash R, Rajala J, Birnie T, Isserow S, Taylor CM, Ignaszewski A, Chan S, Starovoytov A, Saw J. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol 2016;32:554–560. [DOI] [PubMed] [Google Scholar]
- 9. Silber TC, Tweet MS, Bowman MJ, Hayes SN, Squires RW. Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev 2015;35:328–333. [DOI] [PubMed] [Google Scholar]
- 10. Corone S, Iliou MC, Pierre B, Feige JM, Odjinkem D, Farrokhi T, Bechraoui F, Hardy S, Meurin P; Cardiac Rehabilitation Working Group of the French Society of C . French Registry of cases of type I acute aortic dissection admitted to a cardiac rehabilitation center after surgery. Eur J Cardiovasc Prev Rehabil 2009;16:91–95. [DOI] [PubMed] [Google Scholar]
- 11. Fuglsang S, Heiberg J, Hjortdal VE, Laustsen S. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J 2017;51:99–105. [DOI] [PubMed] [Google Scholar]
- 12. Krittanawong C, Tweet MS, Hayes SE, Bowman MJ, Gulati R, Squires RW, Hayes SN. Usefulness of cardiac rehabilitation after spontaneous coronary artery dissection. Am J Cardiol 2016;117:1604–1609. [DOI] [PubMed] [Google Scholar]
- 13. Tweet MS, Olin JW, Bonikowske AR, Adlam D, Hayes SN. Physical activity and exercise in patients with spontaneous coronary artery dissection and fibromuscular dysplasia. Eur Heart J 2021;42:3825–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pristera N, Chaudhury P, Van Iterson EH, Cho LS. Spontaneous coronary artery dissection: principles of management. Cleve Clin J Med 2021;88:623–630. [DOI] [PubMed] [Google Scholar]
- 15. Chacin-Suarez AS, Bonikowske AR, Medina-Inojosa JR, Gulati R, Best PJ, Hayes SN, Tweet MS. Physical activity and exercise patterns after spontaneous coronary artery dissection: insights from a large multinational registry. Front Cardiovasc Med 2021;8:642739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwaab B, Rauch B, Völler H, Benzer W, Schmid J-P. Beyond randomised studies: recommendations for cardiac rehabilitation following repair of thoracic aortic aneurysm or dissection. Eur J Prev Cardiol 2021;28:e17–e19. [DOI] [PubMed] [Google Scholar]
- 17. Imran H, Gaw A, Stabile L, Shah N, Choudhary G, Wu WC. Safety and outcomes of cardiac rehabilitation for patients with spontaneous coronary artery dissection. J Rehabil Med Clin Commun 2018;1:1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ambrosetti M, Abreu A, Corra U, Davos CH, Hansen D, Frederix I, Iliou MC, Pedretti RF, Schmid JP, Vigorito C, Voller H, Wilhelm M, Piepoli MF, Bjarnason-Wehrens B, Berger T, Cohen-Solal A, Cornelissen V, Dendale P, Doehner W, Gaita D, Gevaert AB, Kemps H, Kraenkel N, Laukkanen J, Mendes M, Niebauer J, Simonenko M, Zwisler AO. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;28:460–495. [DOI] [PubMed] [Google Scholar]
- 19. Hansen D, Abreu A, Ambrosetti M, Cornelissen V, Gevaert A, Kemps H, Laukkanen JA, Pedretti R, Simonenko M, Wilhelm M, Davos CH, Doehner W, Iliou MC, Krankel N, Voller H, Piepoli M. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: why and how: a position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2022;29:230–245. [DOI] [PubMed] [Google Scholar]
- 20. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, Whellan D, O'Connor C, Keteyian SJ, Coats A, Davos CH, Dalal HM, Dracup K, Evangelista LS, Jolly K, Myers J, Nilsson BB, Passino C, Witham MD, Yeh GY; ExTra MIIC . Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol 2019;73:1430–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dibben G, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2021;11:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Squires RW, Kaminsky LA, Porcari JP, Ruff JE, Savage PD, Williams MA. Progression of exercise training in early outpatient cardiac rehabilitation: AN OFFICIAL STATEMENT FROM THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATION. J Cardiopulm Rehabil Prev 2018;38:139–146. [DOI] [PubMed] [Google Scholar]
- 24. Nazir S, Ariss RW, Minhas AMK, Issa R, Michos ED, Birnbaum Y, Moukarbel GV, Ramanathan PK, Jneid H. Demographic and regional trends of mortality in patients with aortic dissection in the United States, 1999 to 2019. J Am Heart Assoc 2022;11:e024533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Persu A, Lopez-Sublet M, Al-Hussaini A, Pappaccogli M, Radhouani I, Van der Niepen P, Adair W, Beauloye C, Brillet PY, Chan N, Chenu P, Devos H, Escaned J, Garcia-Guimaraes M, Hammer F, Jackson R, Jebri S, Kotecha D, Macaya F, Mahon C, Natarajan N, Neghal K, Nicol ED, Parke KS, Premawardhana D, Sajitha A, Wormleighton J, Samani NJ, McCann GP, Adlam D. Prevalence and disease Spectrum of extracoronary arterial abnormalities in spontaneous coronary artery dissection. JAMA Cardiol 2022;7:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adlam D, Garcia-Guimaraes M, Maas A. Spontaneous coronary artery dissection: no longer a rare disease. Eur Heart J 2019;40:1198–1201. [DOI] [PubMed] [Google Scholar]
- 27. DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, Greason K, Kalra M, Johnstone J, Shuja F, Harmsen WS, Macedo T, Mandrekar J, Chamberlain AM, Weiss S, Goodney PP, Roger V. Population-based assessment of the incidence of aortic dissection, intramural hematoma, and penetrating ulcer, and its associated mortality from 1995 to 2015. Circ Cardiovasc Qual Outcomes 2018;11:e004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, Brass N, Lavoie A, Bishop H, Lavi S, Pearce C, Renner S, Madan M, Welsh RC, Lutchmedial S, Vijayaraghavan R, Aymong E, Har B, Ibrahim R, Gornik HL, Ganesh S, Buller C, Matteau A, Martucci G, Ko D, Mancini GBJ. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J 2019;40:1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baechler CJ, Witt DR, Lohese O, Benson G. Spontaneous coronary artery dissection and evidence-based medicine. Am J Cardiol 2022;171:65–68. [DOI] [PubMed] [Google Scholar]
- 30. Chaddha A, Kline-Rogers E, Woznicki EM, Brook R, Housholder-Hughes S, Braverman AC, Pitler L, Hirsch AT, Eagle KA. Cardiology patient page. Activity recommendations for postaortic dissection patients. Circulation 2014;130:e140–e142. [DOI] [PubMed] [Google Scholar]
- 31. Delsart P, Delahaye C, Devos P, Domanski O, Azzaoui R, Sobocinski J, Juthier F, Vincentelli A, Rousse N, Mugnier A, Soquet J, Loobuyck V, Koussa M, Modine T, Jegou B, Bical A, Hysi I, Fabre O, Pontana F, Matran R, Mounier-Vehier C, Montaigne D. Prognostic value of aerobic capacity and exercise oxygen pulse in postaortic dissection patients. Clin Cardiol 2021;44:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delsart P, Maldonado-Kauffmann P, Bic M, Boudghene-Stambouli F, Sobocinski J, Juthier F, Domanski O, Coisne A, Azzaoui R, Rousse N, Fayad G, Modine T, Haulon S, Vincentelli A, Mounier-Vehier C, Montaigne D. Post aortic dissection: gap between activity recommendation and real life patients aerobic capacities. Int J Cardiol 2016;219:271–276. [DOI] [PubMed] [Google Scholar]
- 33. Hornsby WE, Norton EL, Fink S, Saberi S, Wu X, McGowan CL, Brook RD, Jones LW, Willer CJ, Patel HJ, Eagle KA, Lavie CJ, Rubenfire M, Yang B. Cardiopulmonary exercise testing following open repair for a proximal thoracic aortic aneurysm or dissection. J Cardiopulm Rehabil Prev 2020;40:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Norton EL, Wu KH, Rubenfire M, Fink S, Sitzmann J, Hobbs RD, Saberi S, Willer CJ, Yang B, Hornsby WE. Cardiorespiratory fitness after open repair for acute type A aortic dissection – a prospective study. Semin Thorac Cardiovasc Surg 2022;34:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GB. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–655. [DOI] [PubMed] [Google Scholar]
- 36. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 37. Loprinzi PD. Sedentary behavior and predicted 10-yr risk for a first atherosclerotic cardiovascular disease (ASCVD) event using the pooled cohort risk equations among US adults. Int J Cardiol 2016;203:443–444. [DOI] [PubMed] [Google Scholar]
- 38. Patterson R, McNamara E, Tainio M, de Sa TH, Smith AD, Sharp SJ, Edwards P, Woodcock J, Brage S, Wijndaele K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rognmo O, Moholdt T, Bakken H, Hole T, Molstad P, Myhr NE, Grimsmo J, Wisloff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012;126:1436–1440. [DOI] [PubMed] [Google Scholar]
- 40. Pack QR, Dudycha KJ, Roschen KP, Thomas RJ, Squires RW. Safety of early enrollment into outpatient cardiac rehabilitation after open heart surgery. Am J Cardiol 2015;115:548–552. [DOI] [PubMed] [Google Scholar]
- 41. Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev 2017;4:CD012264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ennis S, Lobley G, Worrall S, Evans B, Kimani PK, Khan A, Powell R, Banerjee P, Barker T, McGregor G. Effectiveness and safety of early initiation of poststernotomy cardiac rehabilitation exercise training: the SCAR randomized clinical trial. JAMA Cardiol 2022;7:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lai AF, Braverman AC. Endurance exercise following ascending thoracic aortic aneurysm resection in bicuspid aortic valve aortopathy. JAMA Cardiol 2022;7:772–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Squires RW, Allison TG, Johnson BD, Gau GT. Non-physician supervision of cardiopulmonary exercise testing in chronic heart failure: safety and results of a preliminary investigation. J Cardiopulm Rehabil 1999;19:249–253. [DOI] [PubMed] [Google Scholar]
- 45. Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation 2012;126:2465–2472. [DOI] [PubMed] [Google Scholar]
- 46. Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, Pack Q, Sanderson BK, Wang TY. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. J Am Coll Cardiol 2018;71:1814–1837. [DOI] [PubMed] [Google Scholar]
- 47. Hanssen H, Boardman H, Deiseroth A, Moholdt T, Simonenko M, Krankel N, Niebauer J, Tiberi M, Abreu A, Solberg EE, Pescatello L, Brguljan J, Coca A, Leeson P. Personalized exercise prescription in the prevention and treatment of arterial hypertension: a Consensus Document from the European Association of Preventive Cardiology (EAPC) and the ESC Council on Hypertension. Eur J Prev Cardiol 2022;29:205–215. [DOI] [PubMed] [Google Scholar]
- 48. Squires RW, Montero-Gomez A, Allison TG, Thomas RJ. Long-term disease management of patients with coronary disease by cardiac rehabilitation program staff. J Cardiopulm Rehabil Prev 2008;28:180–186, quiz 187–188. [DOI] [PubMed] [Google Scholar]
- 49. Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J 2011;162:571–584 e2. [DOI] [PubMed] [Google Scholar]
- 50. Lopes S, Mesquita-Bastos J, Garcia C, Bertoquini S, Ribau V, Teixeira M, Ribeiro IP, Melo JB, Oliveira J, Figueiredo D, Guimaraes GV, Pescatello LS, Polonia J, Alves AJ, Ribeiro F. Effect of exercise training on ambulatory blood pressure among patients with resistant hypertension: a randomized clinical trial. JAMA Cardiol 2021;6:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blumenthal JA, Hinderliter AL, Smith PJ, Mabe S, Watkins LL, Craighead L, Ingle K, Tyson C, Lin PH, Kraus WE, Liao L, Sherwood A. Effects of lifestyle modification on patients with resistant hypertension: results of the TRIUMPH randomized clinical trial. Circulation 2021;144:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pescatello LS, Wu Y, Gao S, Livingston J, Sheppard BB, Chen MH. Do the combined blood pressure effects of exercise and antihypertensive medications add up to the sum of their parts? A systematic meta-review. BMJ Open Sport Exerc Med 2021;7:e000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005;46:667–675. [DOI] [PubMed] [Google Scholar]
- 54. Lopes S, Afreixo V, Teixeira M, Garcia C, Leitao C, Gouveia M, Figueiredo D, Alves AJ, Polonia J, Oliveira J, Mesquita-Bastos J, Ribeiro F. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens 2021;39:214–222. [DOI] [PubMed] [Google Scholar]
- 55. Valenzuela PL, Carrera-Bastos P, Gálvez BG, Ruiz-Hurtado G, Ordovas JM, Ruilope LM, Lucia A. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol 2021;18:251–275. [DOI] [PubMed] [Google Scholar]
- 56. Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens 2013;31:639–648. [DOI] [PubMed] [Google Scholar]
- 57. Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Thomas RJ, Squires RW, Allison TG. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil 2008;15:336–340. [DOI] [PubMed] [Google Scholar]
- 58. Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis 2018;61:206–213. [DOI] [PubMed] [Google Scholar]
- 59. Ades PA, Savage PD, Toth MJ, Harvey-Berino J, Schneider DJ, Bunn JY, Audelin MC, Ludlow M. High-calorie-expenditure exercise: a new approach to cardiac rehabilitation for overweight coronary patients. Circulation 2009;119:2671–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whaley MH, Brubaker PH, Kaminsky LA, Miller CR. Validity of rating of perceived exertion during graded exercise testing in apparently healthy adults and cardiac patients. J Cardiopulm Rehabil 1997;17:261–267. [DOI] [PubMed] [Google Scholar]
- 61. Joo KC, Brubaker PH, MacDougall A, Saikin AM, Ross JH, Whaley MH. Exercise prescription using resting heart rate plus 20 or perceived exertion in cardiac rehabilitation. J Cardiopulm Rehabil 2004;24:178–184, quiz 185–186. [DOI] [PubMed] [Google Scholar]
- 62. Zanettini R, Centeleghe P, Ratti F, Benna S, Di Tullio L, Sorlini N. Training prescription in patients on beta-blockers: percentage peak exercise methods or self-regulation? Eur J Prev Cardiol 2012;19:205–212. [DOI] [PubMed] [Google Scholar]
- 63. Shea MG, Headley S, Mullin EM, Brawner CA, Schilling P, Pack QR. Comparison of ratings of perceived exertion and target heart rate-based exercise prescription in cardiac rehabilitation: a randomized controlled pilot study. J Cardiopulm Rehabil Prev 2022;42:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bonzheim SC, Franklin BA, DeWitt C, Marks C, Goslin B, Jarski R, Dann S. Physiologic responses to recumbent versus upright cycle ergometry, and implications for exercise prescription in patients with coronary artery disease. Am J Cardiol 1992;69:40–44. [DOI] [PubMed] [Google Scholar]
- 65. Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol Respir Environ Exerc Physiol 1983;55:1558–1564. [DOI] [PubMed] [Google Scholar]
- 66. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA; American Heart Association Exercise CR, Prevention Committee of the Council on Clinical Cardiology CoNPA, Metabolism CoC, Stroke N, Council on E and Prevention . Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 67. Haouzi P, Chenuel B, Chalon B. Effects of body position on the ventilatory response following an impulse exercise in humans. J Appl Physiol (1985) 2002;92:1423–1433. [DOI] [PubMed] [Google Scholar]
- 68. Jackson AS, Sui X, Hebert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med 2009;169:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005;112:674–682. [DOI] [PubMed] [Google Scholar]
- 70. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM, Oxford Vascular S. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 72. Melvinsdottir IH, Lund SH, Agnarsson BA, Sigvaldason K, Gudbjartsson T, Geirsson A. The incidence and mortality of acute thoracic aortic dissection: results from a whole nation study. Eur J Cardiothorac Surg 2016;50:1111–1117. [DOI] [PubMed] [Google Scholar]
- 73. Kokkinos P, Kaminsky LA, Arena R, Zhang J, Myers J. New generalized equation for predicting maximal oxygen uptake (from the fitness registry and the importance of exercise national database). Am J Cardiol 2017;120:688–692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated in support of the article.