Abstract
Nontypeable Haemophilus influenzae (NTHI) is a major pathogen of otitis media. One of the outer membrane proteins of NTHI, P6, is an antigen common to all strains and is considered as a candidate for mucosal vaccine. To elucidate the possibility of developing a nasal vaccine against nontypeable Haemophilus influenzae (NTHI) and to investigate mucosal immune responses in the middle ear, mice were immunized intranasally with the P6 outer membrane protein of NTHI, and P6-specific immune responses in the middle ear mucosa were examined. Mice were given with P6 and cholera toxin intranasally as an adjuvant on days 0, 7, and 14 and were killed on day 21. The P6-specific immunoglobulin A (IgA) antibody titer in ear wash was significantly elevated. Mononuclear cells were isolated from middle ear mucosa, and an increase in P6-specific IgA-producing cells was shown with an enzyme-linked immunospot assay. In addition, an increase in memory T cells in middle ear mucosa was detected with flow cytometric analysis after intranasal immunization. Moreover, in vitro stimulation with P6 resulted in proliferation of purified CD4+ T cells from immunized mice, and these T cells expressed Th2 cytokine mRNA. These results indicate that P6-specific IgA–B-cell immune responses and selected Th2 cytokine expressing Th cells were induced in middle ear mucosa by intranasal immunization. These findings suggest that a nasal vaccine is useful for preventing otitis media with effusion.
Nontypeable Haemophilus influenzae (NTHI) is a major pathogen of otitis media with effusion (OME) and other upper respiratory tract diseases (10, 30). In patients with OME, this bacterium is frequently isolated from the nasopharynx, as well as from middle ear effusions, and the inhibition of NTHI colonization in the upper respiratory tract is considered effective in preventing OME. Due to the increase of antibiotic-resistant strains of NTHI in recent years, the development of a vaccine against this bacterium is considered an important goal for public health. Since NTHI lacks capsular antigens, the chief antigenicity is present in the outer membrane proteins (OMPs). One of the OMPs of NTHI, P6, is a common antigen to all strains and is considered as a candidate for mucosal vaccine (7, 9, 10, 11, 30, 31).
In the mucosal surface, secretory immunoglobulin A (IgA) plays a major role in protective immunity. We previously demonstrated that intranasal immunization was an effective regimen for inducing mucosal IgA immune responses in the upper respiratory tract (26) and that the nasal mucosal IgA immune responses induced by intranasal immunization were effective for the clearance of bacteria in the nasopharynx.
The mucosal immune system is considered as a separate functional entity quite independent of the systemic immune system because the mucosal immune system possesses unique anatomic features and is composed of specialized subsets of lymphoid cells (21, 27, 34). Despite the recent emphasis on a better understanding of molecular and cellular aspects of the mucosal immune system, little information is currently available regarding the middle ear mucosa (MEM). Several histologic studies have indicated that the MEM has a function as a mucosal effector site, as does the nasal mucosa (19, 29, 36, 37, 41); however, immune responses by mucosal lymphocytes in the middle ear have not been studied because of the difficulty in isolating cells from the MEM. Recently, we established a method for isolating lymphocytes from the MEM and analyzed mucosal lymphocytes at the single cell level in the middle ear of normal mice. Results of that study showed that MEM has characteristics of a mucosal effector site (S. Suenaga, S. Kodama, S. Veyama, M. Suzuki, and G. Mogi, submitted for publication).
Several studies concerning the prevention of OME by mucosal immunization have been reported, and these reports have suggested that intranasal immunization with P6 is effective for the prevention of OME (7, 9, 12, 18, 35). However, studies with mice have not investigated immune responses in the middle ear (18), and studies using chinchilla models have not analyzed immunological aspects (3, 12, 35). In the present study, we investigated antigen-specific immune responses in the middle ear by intranasal immunization for the ultimate purpose of developing a mucosal vaccine for preventing OME. P6-specific T- and B-cell immune responses in the MEM were examined at the cellular and transcriptional levels.
MATERIALS AND METHODS
Animals.
BALB/c mice were purchased from Charles River Japan (Atsugi, Japan). The mice were maintained under specific-pathogen-free conditions. Young adult mice between 6 and 8 weeks of age were used in the experiments.
Preparation of P6 from NTHI.
P6 OMP was purified from NTHI (strain 76) in our laboratory according to a previously reported method (22, 33). Briefly, NTHI was grown on chocolate agar plates and suspended in phosphate-buffered saline (PBS). The suspension was sonicated and centrifuged at 21,000 × g for 30 min at room temperature. The pellet was resuspended in 1% sodium dodecyl sulfate with 0.1 M Tris, 0.5 M NaCl, and 0.1% 2-mercaptoethanol (buffer B, pH 8.0) with RNase (10 mg/ml), sonicated, incubated, and centrifuged. This procedure was repeated twice. The pellet was then suspended in buffer B without RNase, sonicated, incubated, and centrifuged again. The pellet was finally suspended in buffer A (0.01 M Tris, 0.15 M NaCl; pH 7.4) and incubated at 65°C for 30 min. The insoluble material was removed by centrifugation at 100,000 × g for 60 min at 30°C. The protein contained in the supernatant was thought to be pure P6. The protein concentration was determined by use of a protein assay kit (Bio-Rad Laboratories, Richmond, Calif.). Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified protein demonstrated a single band with a molecular mass of approximately 16,000 Da. The level of endotoxin in the P6 preparations was measured by use of Toxicolor System (Seikagaku Corp., Tokyo, Japan) and Endospecy (Seikagaku Corp.) according to the manufacturer's directions. Lipooligosaccharide was found to be present only at a concentration of 0.13 ng/mg of protein.
For immunofluorescence studies, P6 was labeled with fluorescein isothiocyanate (FITC) according to a previously reported method (41).
Immunization.
Mice were immunized intranasally on days 0, 7, and 14 with 10 μl of PBS containing a mixture of 10 μg of P6 and 1 μg of cholera toxin (CT; Sigma, St. Louis, Mo.) as a mucosal adjuvant (P6+CT-immunized group) or 10 μl of PBS containing 1 μg of CT alone (CT-immunized group). Control mice were given PBS without antigen. Mice were killed on day 21.
P6-specific antibody assays.
On day 21, ear wash, nasal wash, saliva, and serum were collected from mice under deep anesthesia by introperitoneal injection with 0.1 ml of PBS containing 2 mg of ketamine (Sigma) and 0.2 mg of xylazine (Sigma). Saliva samples were obtained following intraperitoneal injection with 0.1 ml of PBS containing 100 μg of pilocarpine (Sigma) to induce salivary secretion. Blood samples were collected by orbital puncture. After blood was collected, the mice were killed. The mice wer then perfused transcardially with PBS to avoid contamination of the peripheral blood. Ear wash samples were collected by washing the tympanic cavity with 10 μl of cold PBS. Nasal wash samples were collected by washing the nasal cavity with 200 μl of cold PBS according to a previously reported method (26).
P6-specific antibody titers in ear wash, nasal wash, saliva, and serum were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (26, 33). Briefly, 96-well plates (Nunc, Roskilde, Denmark) were coated with an optimal concentration of P6 (100 μl of a 5-μg/ml concentration of P6) in PBS. Plates were incubated overnight at 4°C in a humidified atmosphere and washed three times with PBS. Wells were blocked with 200 μl of PBS containing 1% bovine serum albumin (BSA; Gibco BRL, Gaithersburg, Md.) for 1 h at 37°C. After an extensive washing, serial dilutions of the ear wash, the nasal wash, the saliva, or the serum were added and incubated for 4 h at room temperature. After the incubation and washing, 100 μl of a 1:1,000-diluted biotinylated goat anti-mouse IgM, IgG, or IgA (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was added to the wells. A detection solution containing a 1:2,000 dilution of horseradish peroxidase-conjugated streptavidin (Gibco BRL) was added. The plates were incubated overnight at 4°C. After washing, the color reaction was developed at room temperature with 100 μl of 1.1 mM ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)] in 0.1 M citrate-phosphate buffer (pH 4.2) containing 0.01% H2O2 (Moss, Inc., Pasadena, Calif.) after a 15-min incubation. Endpoint titers were expressed as the reciprocal log2 of the last dilution, which gave an optical density at 414 nm (OD414) of >0.1 OD units above the OD414 value of negative control samples obtained from control mice.
Isolation of MNCs.
Mononuclear cells (MNCs) were isolated from the MEM, nasal passage (NP), nasal-associated lymphoid tissue (NALT), cervical lymph node (CLN), and spleen (SP). For the isolation of MNCs from the MEM, we established a dissociation method (Suenaga et al., submitted). Briefly, the skin was carefully removed from the head region, and the bulla was isolated from the temporal bone. Then, the bulla was dissected, and the MEM (tympanic mucosa) was detached from the bone under microscopy. MNCs were isolated from the MEM after enzymatic digestion at 37°C for 10 min with 0.5 mg of collagenase type IV (Sigma) per ml. MNCs were isolated from NALT, NP, CLN, and SP by gently passing them through a steel mesh according to a previously reported method (2, 16, 26). Then, MNCs were suspended in complete medium (RPMI 1640 supplemented with 10 ml per liter of nonessential amino acid solution, 1 mM HEPES, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 40 μg of gentamicin per ml, and 10% fetal calf serum).
Enumeration of immunoglobulin-producing cells.
For the enumeration of P6-specific immunoglobulin-producing cells, the numbers of P6-specific IgA-, IgG-, and IgM-producing cells in MEM, NP, NALT, CLN, and SP were determined with enzyme-linked immunospot (ELISPOT) assay as previously described (16, 26, 33). Briefly, 96-well filtration plates with a nitrocellulose base (Millititer HA; Millipore Corp., Bedford, Mass.) were coated with 5 μg of P6 per ml and incubated overnight at 4°C. Plates were washed three times with PBS and then blocked with complete medium for 1 h. The blocking medium was removed, and test cells in complete medium were added at various concentrations and were cultured at 37°C in air with 5% CO2 and 95% humidity for 4 h. After the incubation, the plates were washed thoroughly with PBS and then with PBS containing Tween solution (0.05%; PBS-Tween). For the capture of antibody-producing cells, 1 μg of horseradish peroxidase-labeled affinity-purified goat anti-mouse IgM, IgG, or IgA (Southern Biotechnology Associates, Inc.) per ml in PBS-Tween was added. After overnight incubation at 4°C, the plates were washed five times with PBS-Tween, and the spots were developed at room temperature with 100 μl of 1.6 mM 3-amino-9-ethylcarbazole in 0.1 M sodium acetate buffer (pH 5.0) containing 0.05% H2O2 (Moss, Inc.) after a 30-min incubation. The plates were washed with water and dried; with the aid of a stereomicroscope, red-brown-colored spots were counted as P6-specific antibody-forming cells.
Immunohistochemistry and confocal laser scanning microscopy.
For histological observation, the mice were killed under deep anesthesia on day 21 and then perfused transcardially with PBS, followed by perfusion with 10% neutral buffered formalin. The heads were immersed in the same fixative for 6 h and decalcified with 0.12 M EDTA (pH 7.0) for 2 weeks. After dehydration, the tissues were embedded in paraffin for immunohistochemistry and in Tissue-Tek O.C.T. compound (Sakura Finetek USA, Inc., Torrance, Calif.) for confocal laser scanning microscopy.
For detection of IgA-positive cells in the middle ear and the nasophaynx, horizontal, serial, 6-μm paraffin sections were prepared for light microscopic examination. Specimens were dehydrated through a graded series of ethanol and treated with 3% hydrogen peroxide in absolute methanol for 20 min. Sections were exposed to a 5% normal goat serum in PBS for 30 min and then incubated for 24 h with biotinylated goat anti-mouse IgA antibody (diluted in 1% BSA-PBS. After a rinsing with PBS, sections were incubated with ABC reagent (Vector Laboratories, Burlingame, Calif.) for 1 h and developed in 0.05% 3,3′-diaminobenzidine–0.01% H2O2 substrate medium in 0.1 M phosphate buffer for 8 min.
For detection of P6-specific IgA-producing cells in the middle ear, confocal laser scanning microscopy was also performed. Cryostat sections of middle ear (10 μm) were incubated with FITC-labeled P6 followed by Texas red-conjugated goat anti-mouse IgA (EY Laboratories, San Mateo, Calif.). After being rinsed in PBS, sections processed for immunofluorescence were examined with an OS/Inter Vision Lazer Confocal Imaging System (NORAN Instruments, Inc., Middleton, Wis.).
Analysis and purification of T cells in MEM.
For the characterization of T cells from MEM, three-color flow cytometric analysis was performed (24, 25). For staining of memory or naive Th cells, FITC-conjugated anti-CD4 (anti-L3T4; H129.19), phycoerythrin (PE)-conjugated anti-CD45RB (16A), and Cy-Chrome-conjugated anti-CD3ɛ (145-2C11) monoclonal antibodies (MAbs) were used. MAbs were purchased from Pharmingen (San Diego, Calif.). The frequencies of memory and naive Th cells were determined by incubation with a combination of FITC-conjugated anti-CD4, PE-conjugated anti-CD45RB, and Cy-Chrome-conjugated anti-CD3. These samples were then subjected to flow cytometoric analysis with a FACSCalibur (Becton Dickinson Corp., Sunnyvale, Calif.). Each analysis was performed at least three times to verify the results.
For purification of CD4+ T cells from the MEM, MNCs from the MEM were incubated with FITC-conjugated anti-CD4 (anti-L3T4; H129.19) MAbs, and CD4+ T cells were sorted with a cell sorter (EPICS Elite; Coulter, Hialeah, Fla.).
P6-specific proliferation assay.
The proliferative responses to P6 of CD4+ T cells from MEM were determined by [3H]thymidine (TdR) uptake. Sorted CD4+ T cells (2 × 104/well) from MEM were incubated with feeder cells that were prepared from splenocytes of control mice and were subjected to 30 Gy of gamma irradiation and P6 at various concentrations for 96 h at 37°C in a humidified atmosphere of 5% CO2 in air. During the last 6 h of incubation, [3H]TdR was added, and [3H]TdR incorporation was assessed with a scintillation counter.
P6-specific cytokine assay.
For the detection of gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, IL-5, IL-6, IL-10, and transforming growth factor β (TGF-β)-specific mRNA in P6-specific CD4+ T cells isolated from MEM, a modified standard reverse transcription-PCR (RT-PCR) amplification protocol was employed (15, 16). CD4+ T cells from MEM (2 × 104/well) of P6+CT-immunized mice were incubated with feeder cells (2 × 104/well) and P6 (10 μg/ml) for 96 h at 37°C.
Control cells were CD4+ T cells of P6+CT-immunized mice incubated without P6. The total RNA was extracted by the guanidinium thiocyanate procedure (6). RT and PCR were performed (6, 15, 16). In general, 10 ng of total RNA was reverse transcribed with 2.5 μM oligo(dT)12–16 (Gibco BRL) and 2.5 mM concentrations of each deoxynucleoside triphosphate at 42°C for 1 h. The reaction tube was incubated at 70°C for 10 min to inactivate RT enzyme activity. The cDNA (an applied cDNA was equivalent to transcription from 10 ng of total RNA) was amplified by PCR by using cytokine-specific primers purchased from Clontech (Sunnyvale, Calif.). Briefly, after heating at 94°C for 2 min, cDNAs were amplified for 35 cycles, each cycle consisting of 94°C for 2 min, cDNAs were amplified for 35 cycles, each cycle consisting of 94°C for 45 s, 60°C for 45 s, and 72°C for 2 min, followed by a final extension step of 72°C for 7 min. PCR products were then stored at 4°C until analyzed. PCR products were separated by electrophoresis on 1.8% agarose gels and visualized by UV light illumination after ethidium bromide (0.5 μg/ml) staining.
T-helper cell assay.
For the elucidation of helper functions of MEM CD4+ T cells for NALT B cells, in vitro B-cell culture assay was performed (16, 20). Briefly, MNCs were isolated from the NALT of P6+CT-immunized mice, and cells were incubated for 30 min at 4°C with a cytolytic monoclonal anti-T-cell cocktail containing anti-CD4 (GK1.5), anti-CD8 (53.6-72) and anti-Thy-1.2 (30-H12), followed by incubation with anti-rat κ chain (MAR18.5) (24). Cells were then treated with baby rabbit complement (Pel-Freeze Biological, Rogers, Ariz.) for 30 min at 37°C. NALT B cells prepared by this procedure were >97% surface immunoglobulin positive according to flow cytometric analysis. Purified CD4+ T cells from MEM (2 × 104/well), feeder cells (2 × 104/well), and P6 (10 μg/ml) were then added to individual NALT B-cell cultures (105/well). These cultures were incubated for 4 days at 37°C in an atmosphere of 5% CO2. Supernatants were harvested from individual cultures, and the titer of P6-specific IgA antibody was determined by ELISA as described above.
Statistics.
Student's t test was used to determine significance of the data. Differences of P <0.05 were considered significant.
RESULTS
Intranasal immunization induced P6-specific immune responses in MEM.
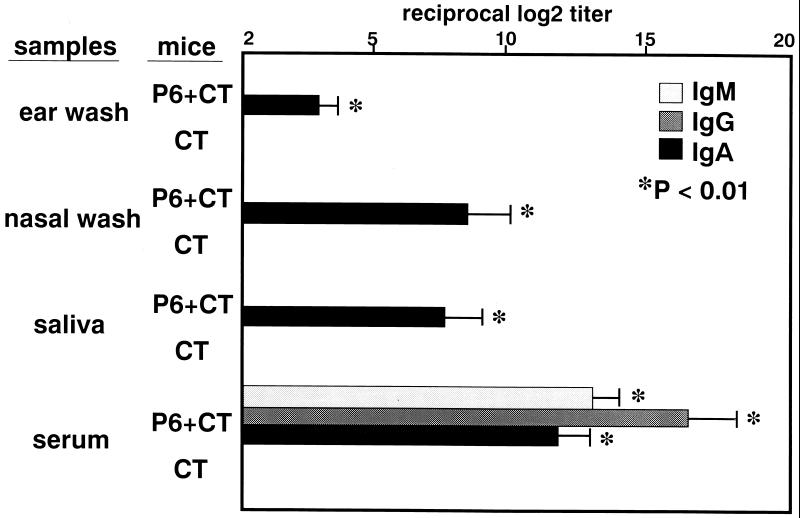
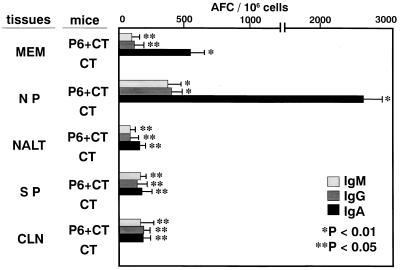
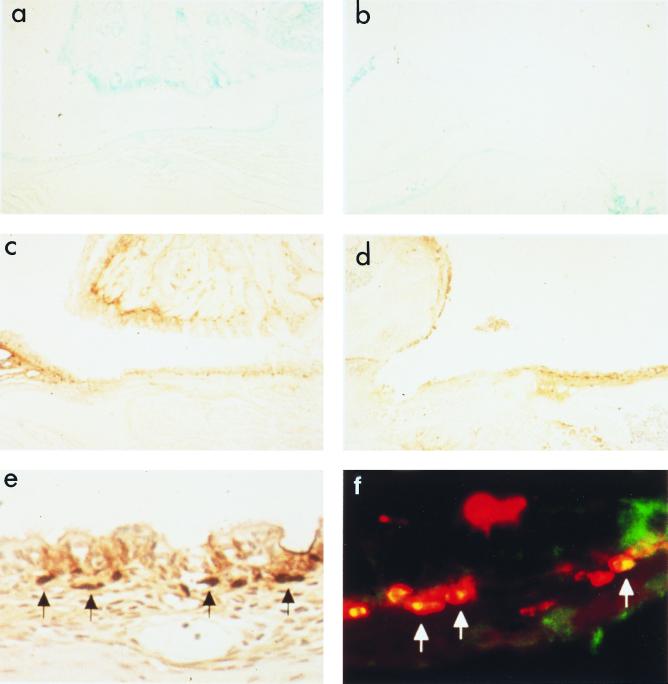
P6-specific immune responses were induced by intranasal immunization with P6 and CT. The levels of IgA antibodies in ear wash, nasal wash, and saliva were significantly elevated after intranasal immunization with P6 and CT. However, P6-specific immune responses were not induced by intranasal immunization with CT alone. The levels of IgM, IgG, and IgA antibodies in serum were also higher in P6+CT-immunized mice than in CT-immunized mice (Fig. 1). The specific IgA antibody-producing cells in P6+CT-immunized mice were increased in the MEM and NP, which are considered mucosal effector sites (Fig. 2). However, the increase of the specific IgA antibody-producing cells was not observed in CT-immunized mice. In P6+CT-immunized mice, there were fewer antibody-producing cells in NALT, which is considered a mucosal inductive site, than in MEM or NP. The dominant isotype of P6-specific immunoglobulin-producing cells in MEM was IgA, followed by small numbers of IgG- and IgM-producing cells. Thus, MEM tissues are considered to be the major IgA effector site in the middle ear cavity. This finding was also confirmed by immunohistologic examination of the MEM. IgA-positive cells detected in the MEM, as well as in the nasopharynx, of P6+CT-immunized mice (Fig. 3c, d, and e) were more numerous than those detected in control mice (Fig. 3a and b). Confocal laser scanning examination also showed P6-specific IgA-producing cells in the MEM of P6+CT mice (Fig. 3f). These results indicate that P6-specific immune responses were induced in MEM and NP by NALT-targeted immunization with P6 and CT. These results indicate that MEM is an important mucosal effector site containing a high frequency of IgA-producing cells for the production of secretory IgA for NTHI-specific immunity in the middle ear.
FIG. 1.
Intranasal immunization with P6 and CT induced P6-specific immune responses. The antibody titers were determined by ELISA. The levels of P6-specific IgA antibodies in ear wash, nasal wash, and saliva were significantly elevated after intranasal immunization with P6 and CT (P < 0.01). The levels of IgM, IgG, and IgA antibodies in serum were also elevated (P < 0.01). The levels of P6-specific antibodies were not elevated after intranasal immunization with CT alone. These results are expressed as the mean ± the standard error (SE) and were obtained from a total of five experiments with 10 mice per group.
FIG. 2.
Intranasal immunization with P6 and CT induced P6-specific antibody-producing cells in mucosal effector sites. P6-specific antibody-producing cells were determined with the ELISPOT assay. The specific IgA antibody-producing cells were increased in the MEM and NP of P6+CT immunized mice (P < 0.01). The number of antibody-producing cells in the NALT was smaller than those in the MEM and NP. The specific IgA antibody-producing cells were not detected in CT-immunized mice. These results are expressed as the mean ± the SE and were obtained from a total of three experiments with 20 mice per group.
FIG. 3.
Immunohistochemistry and confocal laser scanning imaging: a and b, control (a, nasopharynx; b, middle ear); c, d, e, and f; P6+CT-immunized (c, nasopharynx; d and e, middle ear; f, confocal laser scanning imaging of the middle ear). Magnifications: a, b, c, and d, × 100; e and f, × 400. Large numbers of IgA-positive cells are shown in the MEM and the nasopharynx of P6+CT-immunized mice. The black arrows indicate IgA-positive cells (e). Confocal laser scanning imaging shows P6-specific IgA-producing cells in the middle ear of P6+CT-immunized mice. The white arrows indicate P6-specific IgA-producing cells (f).
Memory Th cells increased in MEM after intranasal immunization.
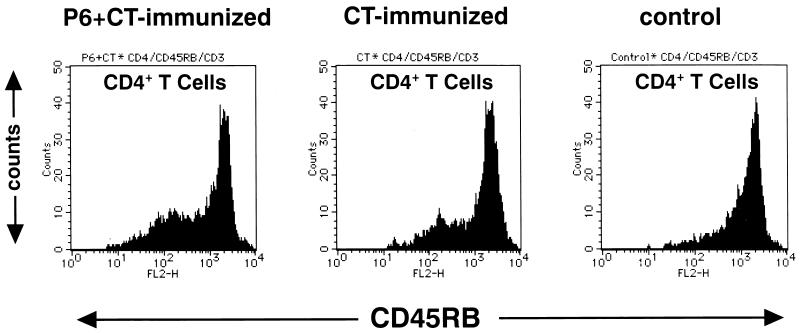
The populations of lymphocytes in MEM, NP, and NALT were characterized by fluorescence-activated cell sorting (FACS). Figure 4 shows the alteration of memory Th cell populations after intranasal immunization. CD45RBlow CD4+ CD3+ T cells were memory Th cells, and CD45RBhigh CD4+ CD3+ T cells were naive Th cells. Increases of memory Th cells were observed in the MEM, NP, and NALT of P6+CT-immunized mice (Table 1). Although increases of memory Th cells were also observed in CT-immunized mice, the frequency of memory Th cells in CT-immunized mice was lower than that in P6+CT-immunized mice. These results demonstrate that memory Th cells were induced in the MEM, as well as in NP and NALT, by intranasal immunization.
FIG. 4.
The alterations of memory Th cell populations in MEM analyzed by FACS. The histogram shows the frequency of CD45RB-stained cells among CD4+ CD3+ T cells. CD45RBlow CD4+ CD3+ T cells are memory Th cells. Intranasal immunization induced the increase of memory Th cells in the MEM. The increase of memory Th cells in P6+CT-immunized mice was greater than that in the CT-immunized mice. Each analysis was performed at least three times to verify the results.
TABLE 1.
Alterations of memory Th cell populations after intranasal immunization
| Mouse group | Frequency (%) of CD45RBlow cells from thea:
|
||
|---|---|---|---|
| MEM | NP | NALT | |
| P6+CT immunized | 26.3 ± 4.2*,† | 67.2 ± 3.3*,† | 32.1 ± 2.8*,† |
| CT immunized | 17.6 ± 5.1* | 49.5 ± 4.5* | 26.7 ± 4.1* |
| Control | 6.7 ± 2.4 | 24.2 ± 3.7 | 14.3 ± 3.8 |
Frequency of CD45RBlow cells among CD4+ CD3+ T cells. Each value is expressed as the mean ± the SE and was obtained from a total of three experiments with 20 mice per group. *, P < 0.01 compared to the control; †, P < 0.05 compared to the CT-immunized group.
CD4+ T cells isolated from MEM are biologically functional cells.
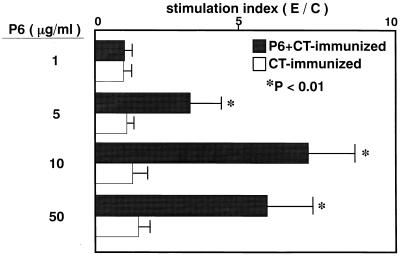
To examine whether CD4+ T cells isolated from MEM of intranasally immunized mice are biologically capable of responding to P6, MEM CD4+ T cells from P6+CT-immunized mice or CT-immunized mice were incubated with feeder cells in various concentrations of P6. In P6+CT-immunized mice, high levels of proliferative responses were induced in MEM CD4+ T cells by P6 (Fig. 5). In CT-immunized mice, although memory Th cells increased in MEM, CD4+ T cells did not respond to P6. These results show that after intranasal immunization with P6+CT, MEM CD4+ T cells are capable of responding to P6.
FIG. 5.
Proliferative responses of MEM T cells against P6. MEM T cells isolated from P6+CT-immunized mice or CT-immunized mice were incubated with feeder cells and P6. The control cultures were incubated without P6. In P6+CT-immunized mice, high levels of proliferative responses were induced in MEM CD4+ T cells by P6 (P < 0.01). Data are expressed as a stimulation index, where E/C = the cpm of [3H]TdR incorporated with P6/cpm of [3H]TdR incorporated without P6. These results are expressed as the mean ± the SE and were obtained from a total of three experiments with 20 mice per group.
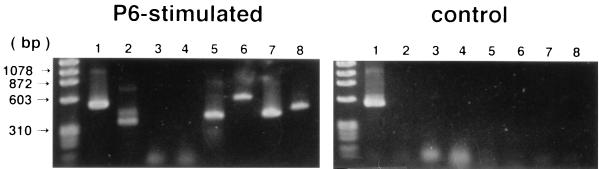
RT-PCR analysis for P6-specific Th1 and Th2 cytokine mRNA expression by MEM CD4+ T cells.
To characterize P6-specific Th1 and Th2 cytokine mRNA expression by MEM CD4+ T cells, IFN-γ-, IL-2-, IL-4-, IL-5-, IL-6-, IL-10-, and TGF-β-specific RT-PCRs were performed. RNA preparations from MEM CD4+ T cells of P6+CT-immunized mice incubated with 10 μg of P6 per ml contained mRNA for Th1 and Th2 cytokines, including IFN-γ, IL-5, IL-6, IL-10, and TGF-β (Fig. 6). Thus, 365-, 424-, 638-, 455-, and 525-bp bands corresponding to these respective cytokines were readily detected after electrophoresis of the agar gels (Fig. 6). In contrast, mRNA expression of Th1 and Th2 cytokines was not detected for MEM CD4+ T cells that were not given P6 (Fig. 6). Th1 and Th2 cytokine mRNA expression was also not detected for RNA preparations from MEM CD4+ T cells of CT-immunized mice or control mice incubated with 10 μg of P6 (data not shown) per ml. These results show that MEM CD4+ T cells expressing P6-specific Th1 and Th2 cytokine mRNA, which is important for the induction of the specific secretory IgA immune responses, were induced in MEM after intranasal immunization with P6 and CT.
FIG. 6.
RT-PCR analysis for the expression of P6-specific cytokine mRNA by MEM T cells. RNA preparations from MEM T cells of P6+CT-immunized mice incubated with P6 contained mRNA for Th1 and Th2 cytokines, including IFN-γ, IL-5, IL-6, IL-10, and TGF-β. Control cells were CD4+ T cells of P6+CT-immunized mice incubated without P6. Each analysis was performed at least three times to verify the results. Lanes: 1, β-actin (540 bp); 2, IFN-γ (365 bp); 3, IL-2 (413 bp); 4, IL-4 (357 bp); 5, IL-5 (424 bp); 6, IL-6 (638 bp); 7, IL-10 (455 bp); 8, TGF-β (525 bp).
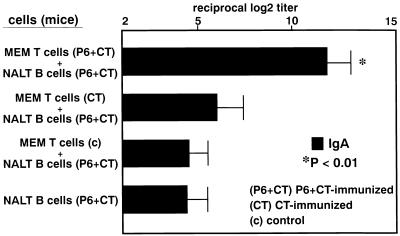
MEM CD4+ T cells possess helper function for P6-specific IgA responses of NALT B cells.
Our current study provides an evidence that MEM CD4+ T cells from P6+CT-immunized mice are programmed to produce IgA-enhancing cytokines. It was important to examine whether MEM CD4+ T cells can support NALT B-cell responses for the production of IgA antibodies. As shown in Fig. 7, MEM CD4+ T cells from P6+CT-immunized mice enhanced P6-specific IgA responses of NALT B cells since higher levels of P6-specific IgA antibody were noted in these cultures than in cultures of MEM CD4+ T cells from CT-immunized mice, control mice, or the control culture of only NALT B cells. These results indicate that intranasal immunization induced P6-specific Th cells in the MEM for the specific IgA B-cell responses. Our results suggest that antigen-specific IgA+ B cells primed in NALT might home to the MEM and that these cells differentiate to IgA-producing plasma cells in the MEM with the help of MEM Th2 T cells.
FIG. 7.
Helper function of MEM T cells. MEM CD4+ T cells from P6+CT-immunized mice enhanced P6-specific IgA responses of NALT B cells since higher levels of P6-specific IgA antibody were noted in these cultures than in cultures of MEM CD4+ T cells from CT-immunized mice, control mice, or the control culture of only NALT B cells (P < 0.01). These results are expressed as the mean ± the SE and were obtained from a total of three experiments with 20 mice per group.
DISCUSSION
Intranasal immunization is now considered an effective vaccination route for the induction of IgA responses. Previous studies showed that specific antibodies induced by intranasal immunization were detected not only in nasal wash but also in saliva (18, 26, 38, 43, 44, 46). Our previous study demonstrated that intranasal immunization is an effective regimen for the induction of antigen-specific mucosal IgA responses in the upper respiratory tract (26). However, to our knowledge no prior studies investigated the mucosal immune response in the middle ear after intranasal immunization. An understanding of the antigen-specific mucosal immunity in the middle ear is needed to aid in the development of a mucosal vaccine to prevent OME. An important aspect of the present study is the direct demonstration of the generation of P6-specific IgA-producing cells and the specific Th2 cytokine mRNA expressing T cells in the MEM of P6+CT-immunized mice. Our present study is the first report to analyze the precise antigen-specific immune responses in the middle ear at the cellular and transcriptional levels.
The nasal tract is equipped with a mucosal inductive site in the NALT for the priming of immunocompetent cells to induce antigen-specific mucosal immune responses, such as Peyer's patch in the intestinal tract (2, 14, 16, 24, 43). Thus, IgA precursor B cells in NALT are primed and activated by intranasal immunization and then disseminate throughout the mucosal effector sites, including the nasal mucosa and the MEM, via the common mucosal immune system (21, 27, 34, 43, 46). Our preliminary study showed that inoculation of the dye into the nose of mice did not result in staining of the middle ear, suggesting that the antigens did not contact the middle ear via intranasal immunization (S. Kodama, M. Suzuki, and G. Mogi, unpublished data). In the present study, many P6-specific IgA-producing cells were detected not only in the NP but also in the MEM of P6+CT-immunized mice; however, fewer P6-specific IgA-producing cells were noted in the NALT. This suggests that P6-specific IgA-precursor B cells primed in the NALT migrate into the MEM and that these cells differentiate into IgA-producing plasma cells in the MEM.
CD4+ T cells are known to be essential for IgA B-cell responses (45). The helper T cells that preferentially produce IL-4, IL-5, IL-6, and IL-10, the Th2 subset of CD4+ T cells, promote IgA responses (15, 27, 34). Our prior results indicate that intranasal immunization of normal mice with the combined OMPs and CT vaccine induced Th2-type cells in the IgA mucosal response (26). A severe impairment of IgA responses is observed in anti-CD4-treated and athymic mice, which also have reduced germinal center development and few IgA-producing cells in the intestinal lamina propria (1, 28). The interaction between CD4+ Th2 cells and B cells that are induced to undergo isotype switching normally occurs in germinal centers (23) where the interaction between CD40L on the activated CD4+ T cells and the CD40 molecule on B cells also initiates a signal needed for the isotype-switching process (23). A special subset of the CD4+ T cells is thus essential for the induction of systemic IgA responses. Specifically, MEM CD4+ T cells can secrete IL-5 and IL-6, which are key cytokines for inducing secretory IgA+ B cells to differentiate into IgA plasma cells (4, 5). IgA-producing cells are also greatly reduced in intestinal tissues of IL-5Rα−/− (17) and IL-6−/− mice (39). MEM CD4+ T cells can also secrete TGF-β, which serves as an IgA isotype switch (8). Mucosal IgA responses are also reduced in TGF-β−/− mice (40). T-cell-derived cytokines, including IFN-γ, can induce secretory component in epithelial cells and major histocompatibility complex class II expression on antigen-presenting cells (1, 15). All of these cytokines can be produced by MEM CD4+ T cells. In the present study, P6-specific Th cells, which could produce these cytokines, were induced in the MEM by intranasal immunization with P6 and CT. Moreover, these MEM CD4+ T cells enable secretory IgA+ B cells derived from the NALT to differentiate into IgA plasma cells in the MEM.
Secretory IgA (IgA) is known to have an inhibitory effect on bacterial adherence (25, 42). Salivary secretory IgA has been shown to possess inhibitory activity to various species of Streptococcus, and the inhibitory activity of secretory IgA correlates with the agglutinating activity (32, 42). The adherence of NTHI to human nasopharyngeal epithelial cells is markedly reduced by nasopharyngeal secretions containing NTHI-specific secretory IgA antibodies (25). Clinical investigation also showed that the decreased nasopharyngeal colonization of NTHI is related to the occurrence of P6-specific secretory IgA antibody in nasopharyngeal secretions (13). In our previous study, the clearance of NTHI from the nasopharynx was shown to be enhanced by intranasal immunization. In the present study, P6-specific IgA antibodies after intranasal immunization increased not only in the nasal wash but also in the ear wash. These results indicate that intranasal immunization with P6 and CT may enhance the clearance of NTHI not only from the nasopharynx but also from the middle ear cavity.
In summary, our present results demonstrate that P6-specific IgA and Th2 immune responses are induced in the middle ear by intranasal immunization. This finding indicates that nasal vaccination might be a useful strategy in preventing OME.
ACKNOWLEDGMENTS
This work was supported by Grant-in-Aid for Basic Scientific Research (A) from the Ministry of Education, Science, Sports, and Culture of Japan (10307040).
We thank Y. Kurono, T. Hiroi, and H. Kiyono for helpful advice; Y. Takenaka for assistance with the preparation of P6; and A. Miura and Y. Hirai for assistance in preparing the manuscript.
REFERENCES
- 1.Aicher W K, Fujihashi K, Yamamoto M, Kiyono H, McGhee J R. Effect of the lpr/lpr mutation on T and B cell populations in the lamina propria of small intestine, a mucosal effector site. Int Immunol. 1992;4:959–968. doi: 10.1093/intimm/4.9.959. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma H, Thompson A H, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 3.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modified experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beagley K W, Eldridge J H, Kiyono H, Everson M P, Koopman W J, Honjo T, McGhee J R. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988;141:2035–2042. [PubMed] [Google Scholar]
- 5.Beagley K W, Eldridge J H, Lee F, Kiyono H, Everson M P, Koopman W J, Hirano T, Kishimoto T, McGhee J R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high-rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Cripps A W, Taylor D C, Wallace F J, Clancy R L. Respiratory immunity stimulated by intestinal immunization with purified nontypeable Haemophilus influenzae antigens. J Infect Dis. 1992;165:199–201. doi: 10.1093/infdis/165-supplement_1-s199. [DOI] [PubMed] [Google Scholar]
- 8.Defrance T, Vanvervliet B, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor B cooperate to induce anti-CD40-activated native human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMaria T F D, Murwin M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faden H, Brodsky L, Bernstein J, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children: local immune responses to nontypeable Haemophilus influenzae. Infect Immun. 1989;57:3555–3559. doi: 10.1128/iai.57.11.3555-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnehm H E S, Pelton I, Gulati S, Rice P A. Characterization of antigens from nontypeable Haemophilus influenzae recognized by human bacterial antibodies. J Clin Investig. 1985;75:1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green B A, Vazquez M E, Zlotnick G W, Quigley-Peape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harabuchi Y, Faden H, Yamanaka N, Duffy L, Wolf J, Krystofik D Tonawanda/Williamsville Pediatrics. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. J Infect Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- 14.Heritage P L, Underdown B J, Arsenault A L, Snider D P, McDermott M R. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am J Respir Crit Care Med. 1997;156:1256–1262. doi: 10.1164/ajrccm.156.4.97-03017. [DOI] [PubMed] [Google Scholar]
- 15.Hiroi T, Fujihashi K, McGhee J R, Kiyono H. Polarized Th2 cytokine expression by both mucosal γδ and αβ T cells. Eur J Immunol. 1995;25:2743–2751. doi: 10.1002/eji.1830251005. [DOI] [PubMed] [Google Scholar]
- 16.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28:3346–3353. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Hiroi T, Yanagita M, Iijima H, Iwatani K, Yoshida T, Takatsu K, Kiyono H. Deficiency of IL-5 receptor α-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosa-associated tissues. J Immunol. 1999;162:821–828. [PubMed] [Google Scholar]
- 18.Hotomi M, Saito T, Yamanaka N. Specific mucosal immunity and enhanced nasopharyngeal clearance of nontypeable Haemophilus influenzae after intranasal immunization with outer membrane protein P6 and cholera toxin. Vaccine. 1998;16:1950–1956. doi: 10.1016/s0264-410x(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 19.Ichimiya I, Kawauti H, Mogi G. Analysis of immunocompetent cells in the middle ear mucosa. Arch Otolaryngol Head Neck Surg. 1990;116:324–330. doi: 10.1001/archotol.1990.01870030088015. [DOI] [PubMed] [Google Scholar]
- 20.Kelsoe G. B cell diversification and differentiation in the periphery. J Exp Med. 1994;180:5–11. doi: 10.1084/jem.180.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyono H, Bienenstock J. Features of inductive and effector sites to consider in mucosal immunization and vaccine development. Reg Immunol. 1992;4:54–62. [PubMed] [Google Scholar]
- 22.Kodama H, Faden H. Cellular immunity to the P6 outer membrane protein of nontypeable Haemophilus influenzae. Infect Immun. 1995;63:2467–2472. doi: 10.1128/iai.63.7.2467-2472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopman G, Pals S T. Cellular interactions in the germinal center: role of adhesion receptors and significance for the pathogenesis of AIDS and malignant lymphoma. Immunol Rev. 1992;126:21–26. doi: 10.1111/j.1600-065x.1992.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuper C F, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–223. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 25.Kurono Y, Shimamura K, Shigemi H, Mogi G. Inhibition of bacterial adherence by nasopharyngeal secretions. Ann Otol Rhinol Laryngol. 1991;100:455–458. doi: 10.1177/000348949110000605. [DOI] [PubMed] [Google Scholar]
- 26.Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, McGhee J R, Kiyono H. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180:122–132. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 27.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamential concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 28.Mega J, Fujihashi K, Kiyono H, McGhee J R. Regulation of mucosal immune responses by T lymphocytes: the effect of chronic CD4+ T cell deficiency on IgA synthesis. Reg Immunol. 1992;4:70–78. [PubMed] [Google Scholar]
- 29.Mogi G, Maeda S, Watanabe N. The development of mucosal immunity in guinea pig middle ears. Int J Pediatr Otorhinolaryngol. 1980;1:331–349. doi: 10.1016/0165-5876(80)90007-5. [DOI] [PubMed] [Google Scholar]
- 30.Murphy T F, Apicella M A. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune responses to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Murphy T F, Bartos L C, Rice P A, Nelson M B, Dudas K C, Apicella M A. Identification of a 16,600-Dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Investig. 1986;78:1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto N, Kurono Y, Suzuki M, Kerakawauchi H, Mogi G. Immune responses of adenoidal lymphocytes specific to Haemophilus influenzae in the nasopharynx. Laryngoscope. 1998;108:1036–1041. doi: 10.1097/00005537-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Staats H F R, Jackson J, Marinaro M, Takahashi I, Kiyono H, McGhee J R. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immun. 1994;6:572–583. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Bakaletz L O. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun. 1994;62:1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi M, Kanai N, Watanabe A. Lymphocyte subsets in immune-mediated otitis media with effusion. Eur Arch Otorhinolaryngol. 1992;249:24–27. doi: 10.1007/BF00175666. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Peppard J, Harris J P. Immunohistochemical study of murine middle ear and eustachian tube. Acta Otorhinolaryngol. 1989;107:97–103. doi: 10.3109/00016488909127485. [DOI] [PubMed] [Google Scholar]
- 38.Tamura S, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 39.van der Poll T C, Keogh V, Guirao X, Buurman W A, Kopf M, Lowry S F. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 40.van Ginkel F W, Wahl S M, Kearney J F, Kweon M, Fujihashi K, Burrows P D, Kiyono H, McGhee J R. Partial IgA-deficiency with increased Th2-type cytokine in TGF-β1 knockout mice. J Immunol. 1999;163:1951–1957. [PubMed] [Google Scholar]
- 41.Watanabe N, Yoshimura H, Mogi G. Induction of antigen-specific IgA-forming cells in the middle ear mucosa. Arch Otolaryngol Head Neck Surg. 1988;114:758–762. doi: 10.1001/archotol.1988.01860190062024. [DOI] [PubMed] [Google Scholar]
- 42.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunogloblin A: a mechanism of antigen disposal. Science. 1972;77:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- 43.Wu H Y, Nikolova E B, Beagley K W, Russell M W. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu-Amano J, Kiyono H, Jackson R J, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subset for immunoglobulin A response: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto M, Briles D E, Yamamoto S, Ohmura M, Kiyono H, McGhee J R. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998;161:4115–4121. [PubMed] [Google Scholar]