Abstract
A green and simple method was proposed for the synthesis of silver nanoparticles (Ag-NPs) using Piper cubeba seed extract as a reducing agent for the first time. The prepared Ag-NPs were characterized using different spectroscopic and microscopic techniques. The obtained Ag-NPs showed an emission band at 320 nm when excited at 280 nm and exhibited strong green fluorescence under UV-light. The produced Ag-NPs were used as fluorescent nanosensors for the spectrofluorimetric determination of ornidazole (ONZ) and miconazole nitrate (MIZ) based on their quantitative quenching of Ag-NPs native fluorescence. The current study introduces the first spectrofluorimetric method for the determination of the studied drugs using Ag-NPs without the need for any pre-derivatization steps. Since the studied drugs don't exhibit native fluorescent properties, the importance of the proposed study is magnified. The proposed method displayed a linear relationship between the fluorescence quenching and the concentrations of the studied drugs over the range of 5.0–80.0 µM and 20.0–100.0 µM with limits of detection (LOD) of 0.35 µM and 1.43 µM for ONZ and MIZ, respectively. The proposed method was applied for the determination of ONZ and MIZ in different dosage forms and human plasma samples with high % recoveries and low % RSD values. The developed method was validated according to ICH guidelines. Moreover, the synthesized Ag-NPs demonstrated significant antimicrobial activities against three different bacterial strains and one candida species. Therefore, the proposed method may hold potential applications in the antimicrobial therapy and related mechanism research.
Subject terms: Analytical chemistry, Fluorescent probes
Introduction
Piper cubeba is a plant cultivated in Java and Sumatra for its essential oil. It was used for the treatment of abdominal pain and dysentery. Piper cubeba has many active constituents including tannins, glycosides, and polyphenols as flavonoids that is responsible for its antioxidant and reducing activities1.
The synthesis of silver nanoparticles (Ag-NPs) have attracted the interest of many researchers because of their different applications, including environmental2,3, industrial4,5, biomedical6,7, and pharmaceutical applications8,9. Ag-NPs synthesis could be performed by two methods, either “top to down” or “bottom to top”. Top to down approach includes the starting material size reduction where nanoparticles usually made by several physical techniques10,11. Bottom to top method is performed using a reducing and/or capping agent that could be chemical or natural. The main drawback of using chemical agents was their potential toxicity that made researchers directed to the green synthesis of nanoparticles using either animal metabolites12,13, bacteria14, fungus15, algae16, or plants17.
Ag-NPs were found to have strong antimicrobial activity against various microorganisms including, fungi, virus, and bacteria18,19. Due to their antibacterial activities, Ag-NPs have recently been used extensively in a variety of disciplines including agriculture20, water disinfection21, textiles and paints22,23, and as an antimicrobial agent in combination with other antibiotics24.
Ornidazole (ONZ) is a nitroimidazole drug that has been used to treat bacterial and protozoal infections. ONZ has been also used for treating the infections of the intestine, stomach, and uro-genital tract. The chemical name of ONZ is 1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole (Fig. 1a)25. Miconazole nitrate (MIZ) is an imidazole antifungal drug that has been used for the treatment of skin infections and superficial candidiasis. MIZ could also be taken orally or as oral gel for treating intestinal and oropharyngeal candidiasis. Chemically, it is 1‐[2,4‐Dichloro‐b‐(2,4‐dichlorobenzyloxy) phenylethyl]‐1H‐imidazole (Fig. 1b)26. Both ONZ and MIZ belong to anti-microbial drugs.
Figure 1.
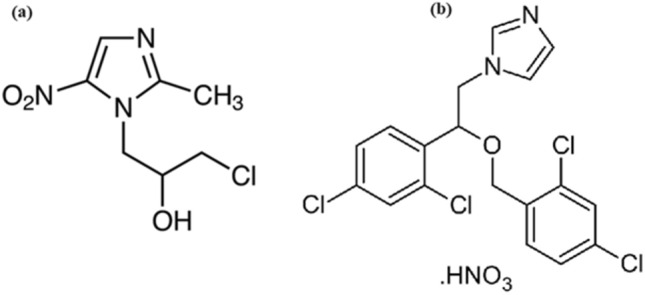
Chemical structure of ONZ (a) and MIZ (b).
Many techniques have been reported in the literature for determining ONZ and MIZ in pharmaceutical dosage forms. The reported techniques for ONZ were spectrophotometry27–29, spectrofluorimetry30, HPLC31,32, TLC33, electrochemistry34–37, gas chromatography38, and capillary electrophoresis39,40, while for MIZ the reported methods included spectrophotometry41,42, gas chromatography38,43, capillary zone electrophoresis44, HPLC45–48, and HPTLC49. Till now, there haven’t been any reported spectrofluorimetric methods for the determination of either ONZ or MIZ using Ag-NPs and no spectrofluorimetric methods at all were reported for MIZ determination.
The current study aimed to discuss the ecofriendly synthesis of Ag-NPs using Piper cubeba seed extract as a green reducing agent for the first time. The characterization of Ag-NPs optical properties and morphology was carried out. Antimicrobial activities had also been investigated. In addition, the quenching of fluorescence intensity by ONZ and MIZ was examined and used to design a simple and green spectrofluorimetric method for their estimation. Since the studied drugs don't exhibit native fluorescent properties, the importance of the proposed study is magnified as it presents a simple, green, and sensitive spectrofluorimetric approach for their analysis in different dosage forms and human plasma samples. Compared to the proposed method, the reported spectrofluorimetric method for ONZ30 required complicated and time consuming steps. This method is considered the first spectrofluorimetric one for MIZ.
Experimental
Materials and reagents
Ornidazole (99.16%) was provided by Organopharma (Cairo, Egypt) and miconazole nitrate (99.95%) was kindly provided by Amriya Pharmaceutical industries (Alexandria, Egypt). Astranida® coated tablets (500 mg ONZ/tablet, Batch No. 19052A), Daktarin® cream (20 mg MIZ/1 g, Batch No. LBE0841), and Daktarin® oral gel (20 mg miconazole/1 g, Batch No. LIB2G00) were purchased from local Pharmacy. Piper cubeba seeds were purchased from local market. Human plasma samples were provided by Mansoura University Hospitals (Mansoura, Egypt), and they were stored at − 80 °C until used after thawing. Silver nitrate, phosphoric acid, methanol, boric acid, and glacial acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Britton–Robinson buffer (0.02 M, pH 2–12) solutions were freshly prepared. All reagents and chemicals were of analytical grade and the experiments were performed using double distilled water.
Preparation of piper cubeba extract
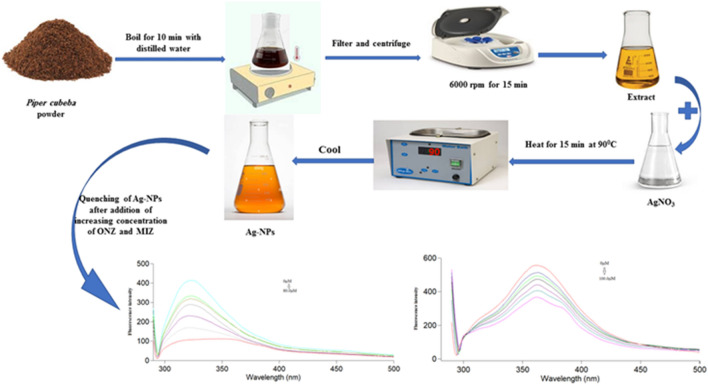
Piper cubeba powder (5 g) was weighed and boiled for 10 min in 100 mL double distilled water. After cooling, the resultant solution was then filtered and centrifuged at 6000 rpm for 15 min then the supernatant was kept in refrigerator until used (Fig. 2). All experiments on Piper cubeba seeds were performed in accordance with relevant guidelines and regulations and were approved by the Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt.
Figure 2.
Scheme for the synthesis of Ag-NPs and application for determination of ONZ and MIZ.
Synthesis of Ag-NPs
Ag-NPs were prepared using a green synthesis approach by mixing 10 mL (1.0 mM) AgNO3 with 5 mL Piper cubeba extract followed by heating for 15 min using water bath maintained at 90 °C. After cooling, the color turned dark brown indicating the formation of Ag-NPs. The prepared solution was stored in the refrigerator for later use (Fig. 2).
Instrumentation
Fluorescence measurements were performed using Cary Eclipse Fluorescence Spectrophotometer with Xenon flash lamp from Agilent Technologies (United States). It was adjusted at 800 V and the slit width was set at 5 nm. A double beam spectrophotometer (PG Instrument, UK) was used to carry out the spectrophotometric measurements. The Fourier Transform Infrared (FT-IR) spectra of Ag-NPs were detected using FT-IR spectrophotometer (IS10 Nicolet, USA). All measurements were carried out in the range of 4000–1000 cm−1 and recorded at 4 cm−1 resolution as 32 scans. The morphology of Ag-NPs was investigated using high-resolution transmission electron microscope (HRTEM) operated at 200 kV (JEOL, JEM-2100, Tokyo). Size of Ag-NPs was also confirmed by using the dynamic light scattering (DLS) nanoparticle size analyzer (Brookhaven, USA). As well, elemental analysis of Ag-NPs was performed using energy-dispersive X-ray (EDX) spectroscopy attached to scanning electron microscope (Jeol, JSM-IT100LA, Japan). Centrifugation was performed using Centrifuge, model 2-16P, (Germany). For pH measurements, a Jenway 3510 pH meter (Jenway, UK) was used. Vortex mixer (IVM-300p, Taiwan) and ultrasonic bath (SS 101H 230, USA) were also used.
Antimicrobial study
The antimicrobial activity of Ag-NPs synthesized from Piper cubeba extract was investigated using agar disc diffusion method against four different pathogenic organisms including, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Candida albicans. The synthesized Ag-NPs (50.0 µg/mL) were tested for antimicrobial activity in comparison with the Piper cubeba extract and 1.0 mM of each of AgNO3, ONZ, and MIZ. The method used the Lysogenia Broth (LB) media where 100 µL of liquid culture were spread on, and then tested organisms were cultured on the LB agar plates. Filter paper discs containing the tested compounds were placed on the agar surface, and after incubation overnight at 27 °C, the germination and growth of the tested microorganisms were inhibited and the diameters of inhibition zones (mm) were measured and recorded.
Standard solutions
Standard solution of each of ONZ and MIZ (1.0 mM) was prepared separately by dissolving 0.011 g and 0.024 g, respectively, in methanol in 50 mL volumetric flask. Serial dilution of the standard solution was performed using double distilled water to prepare working solutions in the range of 5.0–80.0 μM for ONZ and 20.0–100.0 μM for MIZ. The standard solutions of both drugs were stable for at least one week when kept at 4 °C.
Spectrofluorimetric measurements
Factors that could affect the sensing of the studied drugs fluorescence were optimized by adding 0.5 mL of Ag-NPs to 20.0 μM of ONZ or 50.0 μM of MIZ solution. The intensity of fluorescence was determined at 320 nm using an excitation wavelength of 280 nm. The fluorescence intensities of increased concentrations of ONZ and MIZ in the range of 5.0–80.0 µM and 20.0–100.0 µM, respectively were investigated at room temperature, at pH of 4 and 9 using 2 mL and 1 mL Britton–Robinson buffer, respectively. The calibration curves were plotted using fluorescence quenching vs. the drugs concentrations (μM) then the regression equations were derived.
Method optimization
Experimental parameters that could affect the quenching of the fluorescence intensity of Ag-NPs by ONZ and MIZ were studied. Such parameters included the buffer pH, buffer volume, incubation time, and temperature. The pH effect was studied using Britton–Robinson buffer over the pH range of 2–12. Buffer volume in the range of (250.0 µL–2.0 mL) for ONZ and (200.0 µL–1.5 mL) for MIZ was also investigated. The incubation time effect was studied at different time intervals from one to sixty minutes. Finally, the temperature was studied in the range of (25–60 °C) for ONZ and (25–40 °C) for MIZ.
Method validation
The proposed method was validated according to ICHQ2 (R1) guidelines50. Validation parameters including linearity, limit of detection, limit of quantitation, accuracy, precision, robustness, and method selectivity were studied. Linearity was studied using six standard solutions (n = 3) over the range of (5.0–80.0 μM) and (20.0–100.0 μM) for ONZ and MIZ, respectively. The calibration curves were obtained by plotting fluorescence quenching (F0 − F) versus different drug concentrations in μM, and the data obtained was analyzed. Limits of detection (LOD) and quantitation (LOQ) are a measure of method sensitivity, and they could be calculated using the following Equations51:
| 1 |
| 2 |
where Sa is the standard deviation of the intercept of the regression line and b is its slope.
The method accuracy was also investigated by comparing the results obtained by the proposed method to those obtained by the reported methods29,42. Method precision was also evaluated using three replicates of three different concentrations in the same day (intra-day precision) and during three consecutive days (inter-day precision). After that, the analytical data was statically analyzed using Student t-test and Variance ratio F test for accuracy and precision, respectively.
Robustness of the developed method was studied to find out if small changes in the experimental parameters could influence the quenching of the fluorescence intensities of Ag-NPs by the studied drugs. For ONZ, these changes involved: volume of Ag-NPs (500.0 μL ± 5), Britton–Robinson buffer pH (4 ± 0.2), and volume of buffer (2 mL ± 0.1). While for MIZ, these changes involved: volume of Ag-NPs (500.0 μL ± 5), Britton–Robinson buffer pH (9 ± 0.2), and volume of buffer (1 mL ± 0.1).
Finally, the selectivity was investigated by studying the ability of the proposed method to detect the studied drugs in their commercial preparations, spiked human plasma, and in the presence of other antifungal and common co-administered drugs.
Assay of tablets
Ten tablets of Astranida® (500 mg ONZ/tablet) were weighed and ground. An equivalent amount of powder to 10.98 mg of ONZ was mixed with 30 mL of methanol and sonicated for about 20 min. The mixture was then filtered, transferred to 50 mL volumetric flask and completed with methanol to the mark. Working solutions were prepared by dilution with double distilled water using 10 mL volumetric flasks. The procedure described under “Spectrofluorimetric measurements” section was then followed and the corresponding regression equation was used to determine the nominal contents of tablets.
Analysis of cream and gel dosage forms
An accurately weighed amounts of Daktarin® cream (20 mg MIZ/1 g) and Daktarin® oral gel (20 mg miconazole/1 g) equivalent to 23.95 mg and 20.81 mg of miconazole nitrate and miconazole, respectively, were added separately to 40 mL of methanol and sonicated for 20 min at 60 °C. The resulting solutions were cooled for 2 min to solidify the base, then filtered, washed and completed to 50 mL with methanol using 50 mL volumetric flask. Different concentrations within the concentration range of MIZ were prepared after dilution with double distilled water. The procedure described under “Spectrofluorimetric measurements” section was then followed and the corresponding regression equation was used to determine the nominal contents of the cream and gel dosage forms.
Procedure for human plasma
Into a series of 15-mL Falcon tubes, 1 mL aliquots of ONZ standard solution were added to 1 mL human plasma and vortex mixed for 2 min. Then 0.5 mL of Ag-NPs and 2 mL Britton–Robinson buffer (pH 4) were added. Protein precipitation was achieved by completing to 5 mL with methanol. The final concentration was in the range of 20.0–40.0 μM ONZ. The solution was then vortex mixed for 2 min and centrifuged at 6000 rpm for 20 min. The supernatant (3.5 mL) was filtered using syringe filter 0.45 μm and fluorescence measurements were performed. Blank plasma was prepared in a similar way. The concentration measurements were performed as described under “Spectrofluorimetric measurements” section and the corresponding regression equation was derived. All experiments were performed in accordance with the Institutional Ethics Approval of the relevant University Committee (Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt).
Fluorescence quantum yield measurements
The quantum yield of Ag-NPs was calculated using the following Eq. 52:
where Φ represents the quantum yield, F is the integrated fluorescence intensity of Ag-NPs and the standard (St) after excitation at 280 nm, A is the absorbance at 280 nm, and η is the refractive index of solvent (η = 1.33 for both solvents). 2-amino pyridine in 0.1 M H2SO4 was used as a standard with ΦSt equals 0.6. The absorbance (ASt and AAg-NPs) was kept less than 0.1 in order to minimize the absorption effect.
Ethics declarations
All experiments were performed in accordance with relevant guidelines and regulations and this work was approved by the Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt.
Informed consent
A waiver for the informed consent for the current study was obtained from the Committee of Research Ethics in the Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt.
Results and discussion
Characterization of Ag-NPs
In this study, the green synthesis of Ag-NPs was carried out using Piper cubeba seed extract for the first time (Fig. 2). Under UV lamp, the synthesized Ag-NPs solution showed strong green fluorescence (Fig. S1). They were characterized using UV–visible spectrophotometry, spectrofluorimetry, DLS, EDX, FT-IR spectrometry, and HRTEM.
Figure S2 shows the UV–visible absorption spectra of each of AgNO3 and Ag-NPs. As observed, Ag-NPs showed maximum UV absorption peak at 390 nm53. Fluorescence spectra of Ag-NPs are presented in Fig. 3a, as Ag-NPs were excited at 280 nm, the maximum emission peak appeared at 320 nm. The emission showed excitation wavelength dependency. For detecting the optimum excitation wavelength (λex) that result in the optimal emission, λex was changed from 260 to 300 nm and 280 nm was found to be the optimum λex (Fig. 3b).
Figure 3.
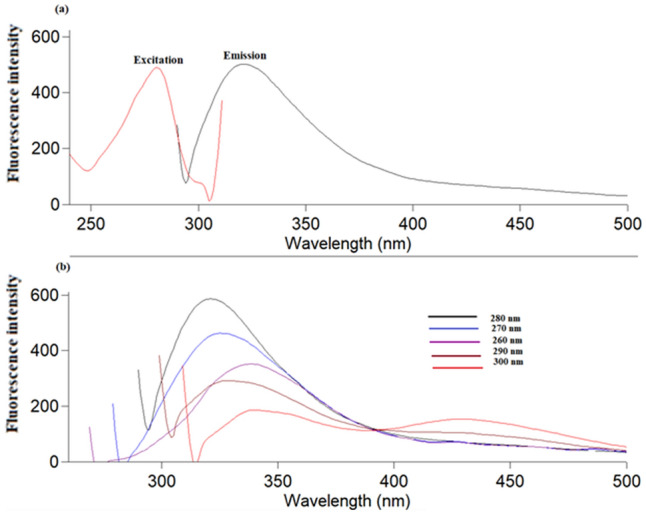
(a) Fluorescence emission spectra of Ag-NPs at 320 nm after excitation at 280 nm, (b) Fluorescence emission spectra of the Ag-NPs at different excitation wavelengths (260–300 nm).
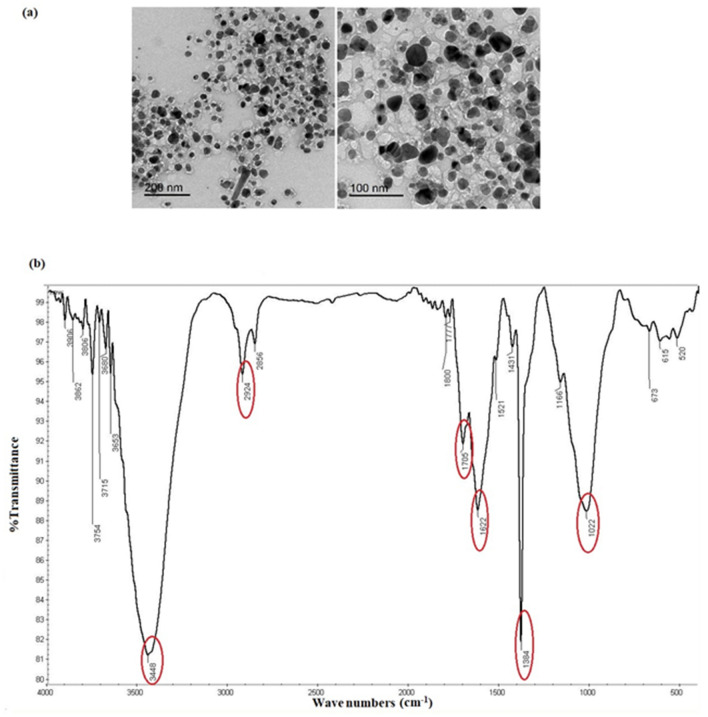
The morphology of Ag-NPs was examined using HRTEM which revealed that Ag-NPs were well separated with spherical shape (Fig. 4a). As well, DLS was used to measure the particle size distribution of Ag-NPs, which had a main average size of about 88 nm and polydispersity of 0.202 (Fig. S3a). EDX spectroscopy was also performed to investigate the elemental composition of Ag-NPs, which showed absorption peaks of silver at 3 kV confirming the formation of Ag-NPs (Fig. S3b). Other peaks also appeared as C, O, Cl, and K that could be due to their presence in the Piper cubeba extract.
Figure 4.
(a) HRTEM images of Ag-NPs, (b) FT-IR spectra of Ag-NPs.
FT-IR was used to study the surface functional groups of Ag-NPs. The obtained spectra showed different functional groups including N–H (3448 cm−1), C–H (2924 cm−1), C=O (1705 cm−1), C=C (1622 cm−1), C–N (1384 cm−1), and C–O (1022 cm−1)54 as shown in Fig. 4b.
The quantum yield of Ag-NPs was also evaluated using 2-amino pyridine as a standard and they showed a good quantum yield of about 0.45.
Antimicrobial activity of Ag-NPs
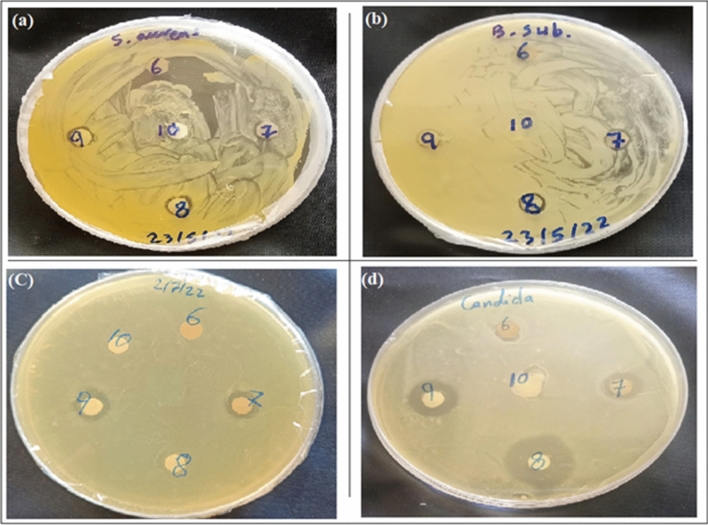
The antimicrobial activity of Ag-NPs was investigated against various pathogenic organisms including, gram positive bacteria (B. subtilis, S. aureus), gram negative bacteria (E. coli), and Candida albicans fungi. Upon comparing Ag-NPs with ONZ and Piper cubeba extract, Ag-NPs were found to have greater antimicrobial activity against candida albicans (11.21 mm), B. subtilis (9.12 mm), S. aureus (8.86 mm), and E. coli (7.41 mm) in contrast to the extract and ONZ that have less antifungal activity (Piper cubeba extract, 9.25 mm and ONZ, 9.46 mm) and no antibacterial action. In addition, the prepared Ag-NPs were also compared with AgNO3 and MIZ, and it was found that, all the four pathogens were susceptible for MIZ and AgNO3 except for B. subtilis which was more susceptible for Ag-NPs (Table 1, Fig. 5).
Table 1.
Antimicrobial activity of Ag-NPs using agar disc diffusion method.
| Inhibition zone diameter (mm) | ||||
|---|---|---|---|---|
| Candida albicans | S. aureus | B. subtilis | E. coli | |
| Piper cubeba extract | 9.25 | 0.00 | 0.00 | 0.00 |
| Ag-NPs (50.0 µg/mL) | 11.21 | 8.86 | 9.12 | 7.41 |
| AgNO3 (1.0 mM) | 12.31 | 9.38 | 8.59 | 7.09 |
| ONZ (1.0 mM) | 9.46 | 0.00 | 0.00 | 0.00 |
| MIZ (1.0 mM) | 15.14 | 9.42 | 8.99 | 9.91 |
Figure 5.
Antimicrobial activity of Piper cubeba extract (6), Ag-NPs (50.0 µg/mL) (7), MIZ (1.0 mM) (8), AgNO3 (1.0 mM) (9), and ONZ (1.0 mM) (10) against (a) S. aureus, (b) B. subtilis, (c) E. coli, and (d) C. albicans.
Mechanism of fluorescence quenching
Figure 6 shows the fluorescence spectra of Ag-NPs in presence of each of ONZ and MIZ with different concentrations. The fluorescence quenching of Ag-NPs increases quantitatively by increasing the concentration of the studied drugs.
Figure 6.
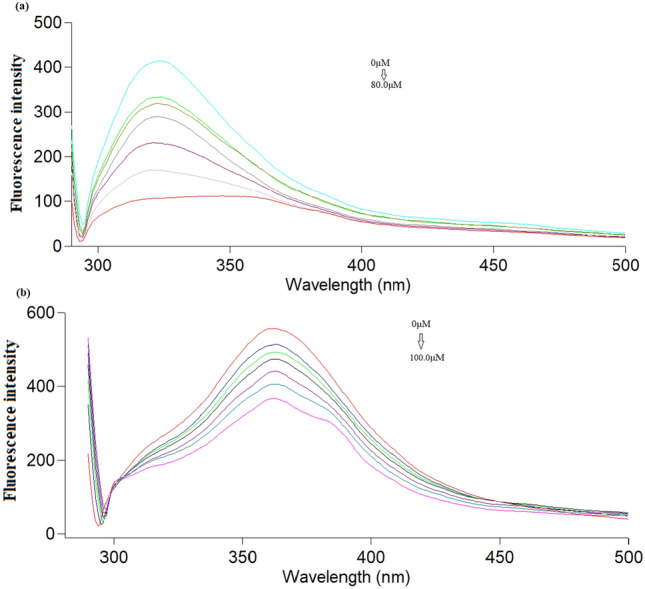
Fluorescence emission spectra of Ag-NPs upon the addition of different concentrations of (a) ONZ (from top to bottom: 0, 5.0, 10.0, 20.0, 40.0, 60.0, 80.0 μM), (b) MIZ (from top to bottom: 0, 20.0, 30.0, 40.0, 60.0, 80.0, 100.0 μM).
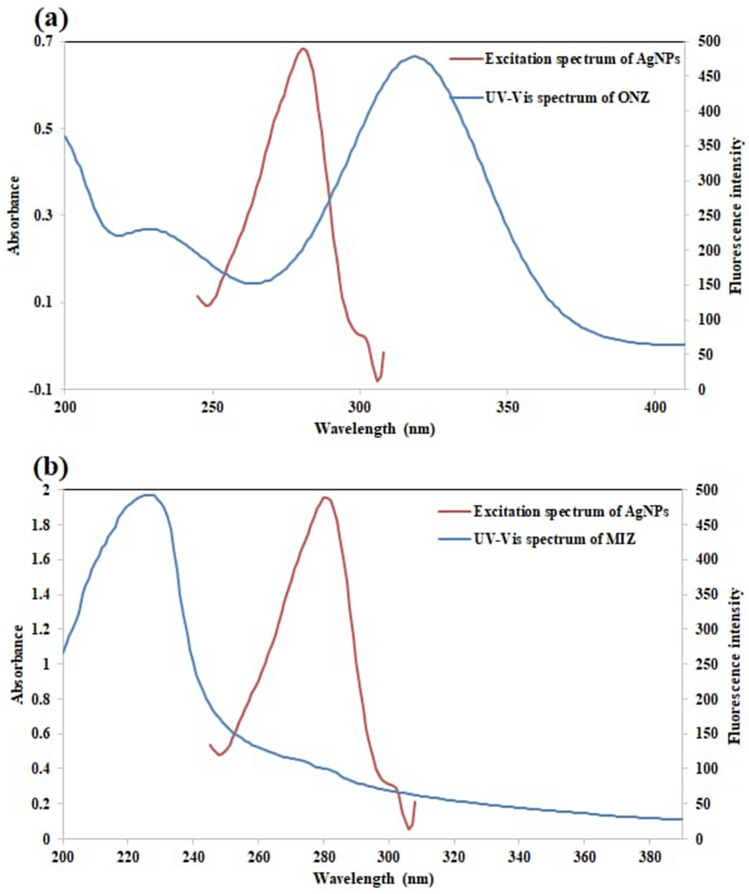
Generally, quenching of fluorescence has different mechanisms, including inner filter effect (IFE), dynamic quenching, and static quenching55. Since there was an overlapping between the excitation spectra of Ag-NPs and the UV–visible absorption spectra of ONZ, IFE could occur (Fig. 7a). In contrary, no overlapping occurred between MIZ absorption spectra and Ag-NPs excitation spectra, and that resulted in excluding the IFE in this case (Fig. 7b).
Figure 7.
(a) A co-plot showing the overlapping between the fluorescence excitation spectrum of Ag-NPs and the UV–Vis absorption spectrum of ONZ, (b) A co-plot showing the absence of overlapping between the fluorescence excitation spectrum of Ag-NPs and the UV–Vis absorption spectrum of MIZ.
For prospective IFE, the intensity of the fluorescence of Ag-NPs was corrected following the addition of ONZ in increasing concentrations using Eq. (3):
| 3 |
where Fcorr and Fobs are the fluorescence intensity corrected and observed after excluding the IFE from the Fobs., Aex and Aem refer to the drug absorbance at the excitation and emission wavelengths, respectively.
The suppressed efficiency (%E) was calculated for both the corrected and observed fluorescence according to Eq. (4):
| 4 |
where %E is the suppressed efficiency, F is the Fcorr or Fobs, F0 is the blank fluorescence intensity.
The %E of both corrected and observed fluorescence intensities of Ag-NPs were separately plotted against ONZ and MIZ concentrations in µM. From Fig. 8 we can conclude that the IFE plays an important role in the quenching of Ag-NPs by ONZ, while for MIZ there was no effect.
Figure 8.
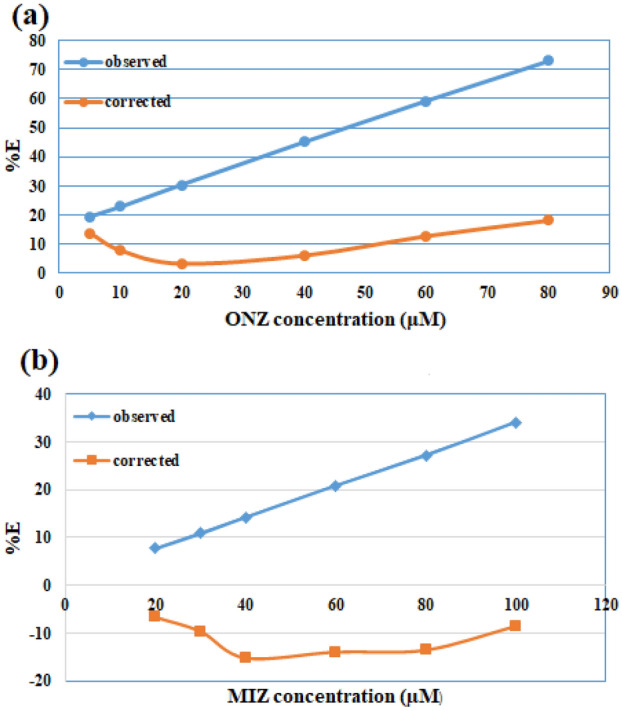
%E of observed and corrected fluorescence of Ag-NPs after the addition of various concentrations of (a) ONZ (5.0, 10.0, 20.0, 40.0, 60.0, 80.0 μM), (b) MIZ (20.0, 30.0, 40.0, 60.0, 80.0, 100.0 μM).
In addition to the IFE, other mechanisms might occur like static or dynamic quenching. The static quenching occurs due to the ground state complex formation. While, in dynamic quenching, the quencher (ONZ or MIZ) diffuses in the excited state to the fluorophore and contacts with it56. Another difference between the two prescribed mechanisms is the dependency on temperature. In the dynamic quenching, increasing the temperature increases the Stern–Volmer constant, while decreases it in the static one57. The possible quenching mechanisms were investigated using Stern–Volmer Eq. (5):
| 5 |
where (F0) and (F) are the fluorescence intensities before and after adding the drug, KSV is the Stern–Volmer (S–V) constant, [Q] is the concentration of quencher56.
The proposed method was performed at different temperature settings (298, 308, 318 K) and S–V equation was adopted where Fo/F was plotted against the concentration of each drug in µM (Fig. 9). For ONZ, the KSV values were 18.2 × 103, 17.05 × 103, and 16.65 × 103, while for MIZ the KSV values were 4.65 × 103, 3.3 × 103, and 0.95 × 103 at 298, 308, 318 K, respectively. As a result, in addition to the previously discussed IFE, the mechanism of quenching for both ONZ and MIZ is the static quenching since KSV decreased by increasing the temperature58–60.
Figure 9.
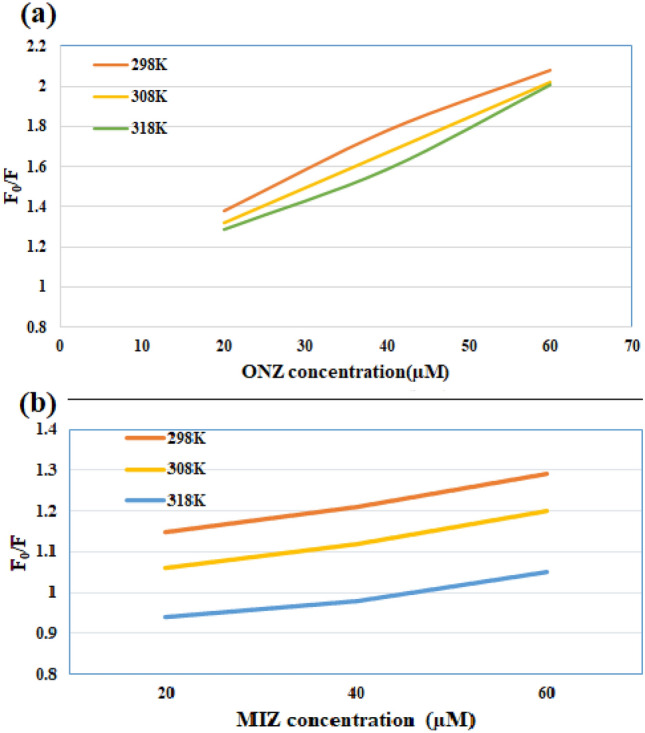
Stern–Volmer plots for the quenching of Ag-NPs fluorescence at three different temperature settings (298, 308, and 318 K) by different concentrations of (a) ONZ (20.0, 40.0, 60.0 μM), (b) MIZ (20.0, 40.0, 60.0 μM).
Optimization of experimental parameters
Effect of temperature on Ag-NPs
In order to confirm the maximum formation of Ag-NPs, the effect of temperature on Ag-NPs synthesis was studied by heating the mixture at different temperature settings in the range of 30–90 °C. It was found that the absorbance (λmax = 390 nm) increased by increasing the temperature, which indicated the maximum formation of Ag-NPs (Fig. S4). This was supported by the change of the color into dark brown and also was compatible with the results of most of the reported studies that investigated the effect of temperature on Ag-NPs61,62. Accordingly, 90 °C was used as the optimal temperature for the synthesis of Ag-NPs.
Effect of pH and buffer volume
Upon studying the effect of pH and buffer volume, Britton–Robinson buffers with pH 4 and pH 9 were found to result in a subsequent increase in the fluorescence quenching of Ag-NPs by ONZ and MIZ, respectively (Fig. S5a). In addition, the optimum buffer volumes were 2 mL and 1 mL for ONZ and MIZ, respectively (Fig. S5b).
Effect of incubation time
It was found that quenching of the fluorescence intensity of Ag-NPs by both drugs was stable for about 10 min then the quenching effect slightly decreased with increasing the incubation time (Fig. S5c).
Effect of temperature
Increasing the temperature was found to result in a significant decrease in the fluorescence quenching with the studied drugs (Fig. S5d). As a result, the spectrofluorimetric measurements were performed at room temperature.
Method validation
The proposed method was validated according to ICHQ2 (R1) guidelines50.
Linearity and range
The calibration curve was obtained by plotting fluorescence quenching (F0 − F) versus different drug concentrations in μM which was linear over the range of (5.0–80.0 μM) and (20.0–100.0 μM) for ONZ and MIZ, respectively. The correlation coefficient (r) for both drugs was 0.9999. Linear regression analysis was represented using the following equations:
| 6 |
| 7 |
where F0 is the native fluorescence intensity of Ag-NPs, F is the Ag-NPs fluorescence intensity in presence of ONZ or MIZ, C is the concentration of the studied drugs in μM, and r is the correlation coefficient. The developed method’s analytical performance data was summarized in Table 2.
Table 2.
Analytical performance data of the proposed method.
| Parameters | ONZ | MIZ |
|---|---|---|
| Linear concentration range (µM) | 5.0–80.0 | 20.0–100.0 |
| LOD (µM) | 0.35 | 1.43 |
| LOQ (µM) | 1.06 | 4.35 |
| Regression equation | (F0 − F) = 2.9667C + 66.05 | (F0 − F) = 1.8221C + 6.4803 |
| Correlation coefficient (r) | 0.9999 | 0.9999 |
| S.D. of the residuals, Sy/x | 1.11 | 0.88 |
| S.D. of the intercept, Sa | 0.31 | 0.79 |
| S.D. of the slope, Sb | 0.02 | 0.01 |
| Percentage relative standard deviation (% RSD) | 1.93 | 0.82 |
| Percentage relative error (% Error) | 0.78 | 0.34 |
LOD and LOQ
LOD and LOQ are a measure of method sensitivity that were 0.35 and 1.06 μM, respectively for ONZ and 1.43 and 4.35 μM, respectively for MIZ. The measured LOD and LOQ values proved that the proposed method is adequately sensitive (Table 2).
Accuracy and precision
Upon comparing the results obtained by the proposed method with those obtained by the reported methods29,42, the analytical data was statically analyzed using both Variance ratio F-test and Student t-test which showed that there was no marked difference between the precision and accuracy of the proposed and reported methods (Table 3). The intra and inter-day precisions were also evaluated and the investigated drugs showed acceptable % error and %RSD, as presented in Table S1.
Table 3.
Application of the proposed method for the determination of the studied drugs in raw materials.
| Parameter | ONZ | MIZ | ||||
|---|---|---|---|---|---|---|
| Conc. taken (μM) | Conc. found (μM) | % Recoverya | Conc. taken (μM) | Conc. found (μM) | % Recoverya | |
| 5.0 | 4.87 | 97.44 | 20.0 | 20.23 | 101.16 | |
| 10.0 | 9.79 | 97.99 | 30.0 | 29.73 | 99.11 | |
| 20.0 | 19.87 | 99.36 | 40.0 | 40.11 | 100.27 | |
| 40.0 | 40.69 | 101.74 | 60.0 | 60.21 | 100.35 | |
| 60.0 | 60.17 | 100.29 | 80.0 | 79.26 | 99.07 | |
| 80.0 | 79.59 | 99.48 | 100.0 | 100.46 | 100.46 | |
| Mean | 99.13 | 100.07 | ||||
| ± SD | 1.92 | 0.82 | ||||
| % RSD | 1.93 | 0.82 | ||||
| % Error | 0.78 | 0.34 | ||||
| Comparison method29 | Comparison method42 | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | 98.57 ± 1.33 | 99.61 ± 0.51 | ||||
| Nc | 3 | 3 | ||||
| t value | 0.77 (2.36)b | 0.87 (2.36)b | ||||
| F value | 1.37 (19.30)b | 2.59 (19.30)b | ||||
aMean of three determinations.
bValues in parenthesis are the tabulated t and F values at p = 0.0551.
cNumber of samples.
Robustness
As described under “Experimental” section, the robustness of the developed method was studied to find out if small changes in the experimental parameters could influence the quenching of the fluorescence intensities of Ag-NPs by ONZ and MIZ. The obtained results indicated that, small changes in experimental conditions did not significantly affect the quenching of the Ag-NPs fluorescence intensity by both drugs, as shown in Table S2.
Selectivity
The selectivity of the proposed method for determining ONZ and MIZ was verified in their commercial dosage forms with low %RSD (less than 1.59%) and high % recovery (98.0–101.04%) without any interference from the existed excipients. The possible interfering excipients as lactose, maltose, mannitol, dextrin, and citric acid were studied in details and confirmed the high selectivity of the method, since they almost did not affect the fluorescence intensity of the Ag-NPs (Fig. S6). Similarly, the method selectivity was proved, by its ability to detect the studied drugs in the presence of different metal ions like Na+, K+, Ca+2, Mg+2, and Ba+2 without any interference (Fig. S7). The proposed method was also able to determine ONZ and MIZ in the presence of other antifungal and common co-administered drugs such as clotrimazole, ketoconazole, terconazole, ciprofloxacin, ofloxacin, and hydrocortisone sodium succinate. The tolerance limit of the drugs was determined as the concentration that result in 2% or less relative error, which was 25.0 µM for clotrimazole, 10.0 µM for both ketoconazole and terconazole, 1.0 µM for both ciprofloxacin and ofloxacin, and 25.0 µM for hydrocortisone sodium succinate60. In addition, the method could determine ONZ in spiked human plasma with low %RSD and high % recoveries demonstrating the absence of interference from plasma components. Accordingly, the developed method showed excellent selectivity for determination of ONZ and MIZ without any interference.
Method applications
Analysis of ONZ and MIZ in commercial dosage forms
The studied drugs were successfully determined in their commercial dosage forms including, Astranida® tablets for ONZ, Daktarin® cream, and Daktarin® oral gel for MIZ with high % recovery (98.0–101.04%) and low %RSD values (less than 1.59%). Table 4 shows that the results of the proposed method were in an acceptable agreement with those obtained by the reported methods29,42. Statistical analysis of the resultant data by Variance ratio F-test and Student t-test indicated that the method has good accuracy and precision, respectively.
Table 4.
Application of the proposed method for the determination of the studied drugs in commercial dosage forms.
| Parameter | Astranida® film-coated tablets (ONZ 500 mg / tablet) | |||||
|---|---|---|---|---|---|---|
| Conc. Taken (μM) | Conc. Found (μM) | % recoverya | ||||
| 40.0 | 39.33 | 98.33 | ||||
| 50.0 | 49.14 | 98.28 | ||||
| 60.0 | 60.62 | 101.04 | ||||
| Mean | 99.22 | |||||
| ± SD | 1.58 | |||||
| % RSD | 1.59 | |||||
| Comparison method 29 | ||||||
|---|---|---|---|---|---|---|
| Mean ± SD | 100.24 ± 0.95 | |||||
| Nc | 3 | |||||
| t-value | 0.302 (2.77)b | |||||
| F-value | 2.66 (19.00)b | |||||
| Parameter | Daktarin® cream (MIZ 20 mg / 1 g) | Daktarin® oral gel (Miconazole 20 mg / 1 g) | ||||
|---|---|---|---|---|---|---|
| Conc. taken (μM) | Conc. found (μM) | % recoverya | Conc. taken (μM) | Conc. found (μM) | % Recoverya | |
| 20.0 | 19.66 | 98.28 | 20.0 | 20.11 | 100.56 | |
| 40.0 | 39.2 | 98.0 | 40.0 | 39.98 | 99.96 | |
| 60.0 | 59.17 | 98.62 | 60.0 | 59.53 | 99.21 | |
| Mean | 98.63 | 99.91 | ||||
| ± SD | 0.71 | 0.68 | ||||
| % RSD | 0.72 | 0.68 | ||||
| Comparison method 42 | Comparison method 42 | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | 98.08 ± 0.58 | 99.32 ± 1.83 | ||||
| Nc | 3 | 3 | ||||
| t-value | 0.57 (2.78)b | 0.52 (2.78)b | ||||
| F-value | 3.43 (19.0)b | 7.33 (19.0)b | ||||
aMean of three determinations.
bValues in parenthesis are the tabulated t- and F- values at p = 0.05 51.
cNumber of samples.
Determination of ONZ in human plasma
The selectivity and high sensitivity of the proposed analytical method allowed its application to analyze and/or detect ONZ in spiked human plasma, since the mean maximum plasma concentration of ONZ was 10.9 μg/ml (49.63 µM) when a dose of 750 mg was administered63. Linear relationship was found in plasma samples spiked with ONZ after plotting the quenching of fluorescence (F0 − F) against the concentrations of ONZ in µM. It was demonstrated that the method has sufficient ability to analyze the studied drug with high mean percentage recoveries and low SD values (100.08% ± 1.75), indicating that endogenous plasma components did not interfere, which pointed out to a negligible matrix effect (Table 5, Fig. S8). Linear regression analysis is represented by the following Eq. (8):
| 8 |
Table 5.
Application of the proposed method for the determination of ONZ in spiked human plasma.
| Parameter | Conc. taken (μM) | Conc. found (μM) | % Recoverya |
|---|---|---|---|
| 20.0 | 20.29 | 101.43 | |
| 30.0 | 29.43 | 98.10 | |
| 40.0 | 40.29 | 100.71 | |
| Mean | 100.08 | ||
| ± SD | 1.75 | ||
| % RSD | 1.75 | ||
| % Error | 1.01 |
aMean of three determinations.
Comparison with other methods
In comparison to the other reported methods, the proposed method provides a simple, fast, and an ecofriendly spectrofluorimetric approach for the estimation of ONZ and MIZ instead of the expensive, complicated, and tedious HPLC, capillary electrophoresis, and other chromatographic techniques. The proposed method is the first spectrofluorimetric method for the determination of MIZ and the first one used Ag-NPs as a fluorescent probe for the estimation of ONZ without the need for any pre-derivatization steps. Since the studied drugs don't exhibit native fluorescent properties, the importance of the proposed study is magnified. Compared to the reported spectrofluorimetric method for ONZ30, the proposed method is almost as sensitive as the reported one, which was not green, time consuming, and more complicated.
Conclusion
The current study provides a green, simple, rapid, and economic spectrofluorimetric method for the estimation of ONZ and MIZ. This method is the first spectrofluorimetric one for the determination of the studied drugs using Ag-NPs without the need for any pre-derivatization steps. Since the studied drugs don't exhibit native fluorescent properties, the importance of the proposed study is magnified. The developed method depends on the ecofriendly synthesis of Ag-NPs using Piper cubeba seed extract. The produced Ag-NPs were characterized using UV–Vis spectroscopy, spectrofluorimetry, FTIR, DLS, EDX, and HRTEM. The synthesized Ag-NPs showed a strong green fluorescence, which was quantitatively quenched by the studied drugs. Antimicrobial activity of Ag-NPs was examined using the disc diffusion method. The synthesized nanoparticles exhibited marked antimicrobial activity against various strains of gram negative and gram positive bacteria in addition to Candida albicans fungi. Therefore, the proposed method may hold potential applications in the antimicrobial therapy and related mechanism research. The developed method was applied for determining the studied drugs in commercial dosage forms with high % recoveries and low %RSD values. The proposed method was also used for the sensitive detection and determination of ONZ in the spiked human plasma.
Supplementary Information
Abbreviations
- Ag-NPs
Silver nanoparticles
- DLS
Dynamic light scattering
- EDX
Energy-dispersive X-ray
- FTIR
Fourier transform infrared
- HPLC
High performance liquid chromatography
- HPTLC
High performance thin layer chromatography
- HRTEM
High-resolution transmission electron microscope
- IFE
Inner filter effect
- LOD
Limit of detection
- LOQ
Limit of quantitation
- MIZ
Miconazole nitrate
- ONZ
Ornidazole
- RSD
Relative standard deviation
- TLC
Thin layer chromatography
Author contributions
G.M.: Conceptualization, Methodology, Data curation, Investigation, Supervision, Writing-review & editing. E.A.: Conceptualization, Methodology, Formal analysis, Validation, Writing-original draft. R.A.E.: Investigation, Supervision, Writing-review & editing. F.B.: Conceptualization, Resources, Investigation, Supervision, Writing-review & editing. All authors approved the manuscript for publication.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25830-x.
References
- 1.Nahak G, Sahu R. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J. Appl. Pharm. Sci. 2011;1:153. [Google Scholar]
- 2.Yu S-J, Yin Y-G, Liu J-F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts. 2013;15:78–92. doi: 10.1039/C2EM30595J. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann MG, Rosa CH, Rosa GR, Dias D. Biogenic synthesis of gold and silver nanoparticles used in environmental applications: A review. Trends. Environ. Anal. Chem. 2021;30:e00129. doi: 10.1016/j.teac.2021.e00129. [DOI] [Google Scholar]
- 4.Haider A, Kang I-K. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Adv. Mater. Sci. Eng. 2015;2015:165257. doi: 10.1155/2015/165257. [DOI] [Google Scholar]
- 5.Yaqoob AA, Umar K, Ibrahim MNM. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020;10:1369–1378. doi: 10.1007/s13204-020-01318-w. [DOI] [Google Scholar]
- 6.Burdușel A-C, et al. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials. 2018;8:681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNamara K, Tofail SA. Nanoparticles in biomedical applications. Adv. Phys. 2017;2:54–88. doi: 10.1039/c5cp00831j. [DOI] [PubMed] [Google Scholar]
- 8.Jouyban A, Rahimpour E. Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta. 2020;217:121071. doi: 10.1016/j.talanta.2020.121071. [DOI] [PubMed] [Google Scholar]
- 9.Mathur P, Jha S, Ramteke S, Jain N. Pharmaceutical aspects of silver nanoparticles. Artificells Nanomed. Biotechnol. 2018;46:115–126. doi: 10.1080/21691401.2017.1414825. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013;31:346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Akintayo G, et al. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2020. p. 012041. [Google Scholar]
- 13.Lateef A, Ojo SA, Elegbede JA. The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol. Rev. 2016;5:601–622. doi: 10.1515/ntrev-2016-0049. [DOI] [Google Scholar]
- 14.Sadowski Z, Maliszewska I, Grochowalska B, Polowczyk I, Kozlecki T. Synthesis of silver nanoparticles using microorganisms. Mater. Sci. Poland. 2008;26:419–424. [Google Scholar]
- 15.Alavi M. Bacteria and fungi as major bio-sources to fabricate silver nanoparticles with antibacterial activities. Expert Rev. Anti-infect. Ther. 2022;20:1–10. doi: 10.1080/14787210.2022.2045194. [DOI] [PubMed] [Google Scholar]
- 16.Adelere, I. A. & Lateef, A. Microalgal nanobiotechnology and its applications—A brief overview. Microb. Nanobiotechnol. 233–255 (2021).
- 17.Ahmad S, et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019;14:5087. doi: 10.2147/IJN.S200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathishkumar R, et al. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 2019;23:1180–1191. doi: 10.1016/j.jscs.2019.07.008. [DOI] [Google Scholar]
- 19.Hasanin M, Elbahnasawy MA, Shehabeldine AM, Hashem AH. Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities. Biometals. 2021;34:1313–1328. doi: 10.1007/s10534-021-00344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Upadhyaya CP, Singh A, Abd-Elsalam KA, Prasad R. Nanobiotechnology Applications in Plant Protection 247–265. Springer; 2018. [Google Scholar]
- 21.Deshmukh SP, Patil S, Mullani S, Delekar S. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguda O, Lateef A. Novel biosynthesis of silver nanoparticles through valorization of Parkia biglobosa fermented-seed wastewater: Antimicrobial properties and nanotextile application. Environ. Technol. Innov. 2021;24:102077. doi: 10.1016/j.eti.2021.102077. [DOI] [Google Scholar]
- 23.Lateef A, et al. Biogenic synthesis of silver nanoparticles using a pod extract of Cola nitida: Antibacterial and antioxidant activities and application as a paint additive. J. Taibah Univ. Sci. 2016;10:551–562. doi: 10.1016/j.jtusci.2015.10.010. [DOI] [Google Scholar]
- 24.Vazquez-Muñoz R, et al. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE. 2019;14:e0224904. doi: 10.1371/journal.pone.0224904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh P, Mittal R, Sharma G, Singh S, Singh A. Ornidazole: Comprehensive profile. Profiles Drug Subst Excip Relat Methodol. 2003;30:123–184. doi: 10.1016/S0099-5428(03)30007-3. [DOI] [PubMed] [Google Scholar]
- 26.Al-Badr AA. Miconazole nitrate: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2005;32:3–65. doi: 10.1016/S0099-5428(05)32001-6. [DOI] [PubMed] [Google Scholar]
- 27.Darwish KM, Salama I, Mostafa S, El-Sadek M. Extractional spectrophotometric analysis of metronidazole, tinidazole, ornidazole and secnidazole bases through acid-dye complexation using bromothymol blue dye. Pak. J. Pharm. Sci. 2012;25:207–217. [PubMed] [Google Scholar]
- 28.Khalile S, Elqudaby H, Ali F, Eid SM. Spectrophotometric determination of ornidazole, secnidazole and tinidazole in pharmaceutical preparations based on formation of dyes. J. Pharm. Res. 2011;4:4352–4354. [Google Scholar]
- 29.Rana M, et al. Spectrophotometric method development and determination of ornidazole in bulk and tablet dosage form. Int. J. Pharm Tech Res. 2011;3:153–156. [Google Scholar]
- 30.Mehrzad-Samarin M, Faridbod F, Ganjali MR. A luminescence nanosensor for ornidazole detection using graphene quantum dots entrapped in silica molecular imprinted polymer. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019;206:430–436. doi: 10.1016/j.saa.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Shekar MS, Sagar JV, Narsaiah N, An R, Krishna D. Validated HPLC method for the determination of ornidazole in human serum and urine. Indian J. Pharm. Sci. 2005;67:302. [Google Scholar]
- 32.Bind B, Lokhande R, Munigela N, Kolhal S, Gupta A. RP-HPLC method for the simultaneous determination of metronidazole, tinidazole, ornidazole, secnidazole and ofloxacin in bulk and pharmaceutical dosage form. Int. J. Pharm. Sci. Rev. Res. 2015;34:61–67. [Google Scholar]
- 33.Shovkova OV, Klimenko LY, Shovkova ZV, Ulanova VA, Shpychak OS. Application of thin layer chromatography in analysis of secnidazole, ornidazole, tinidazole and nimorazole. J. Pharm. Sci. Res. 2018;10:3411–3416. [Google Scholar]
- 34.Özkan S, Şenturk Z, Biryol I. Determination of ornidazole in pharmaceutical dosage forms based on reduction at an activated glassy carbon electrode. Int. J. Pharm. 1997;157:137–144. doi: 10.1016/S0378-5173(97)00208-1. [DOI] [PubMed] [Google Scholar]
- 35.Turan S, Durmus Z, Kiliç E. Electrochemical behavior of ornidazole and its adsorptive stripping determination in pharmaceuticals. Curr. Pharm. Anal. 2009;5:416–423. doi: 10.2174/157341209789649140. [DOI] [Google Scholar]
- 36.Ettadili F, et al. Electrochemical determination of ornidazole at silver electrode: Analytical application in human blood. Chem. Data Collect. 2022;39:100850. doi: 10.1016/j.cdc.2022.100850. [DOI] [Google Scholar]
- 37.Fonseca JL, Rivera MG, Monteagudo JG. Electrochemistry of ornidazole. Anal. Lett. 1993;26:109–124. doi: 10.1080/00032719308016799. [DOI] [Google Scholar]
- 38.Phatak H, Vaidya V. A rapid gas chromatography-mass spectroscopy method for simultaneous quantification of ornidazole and miconazolefrom cream formulations: Development, validation and application. Int. J. Pharm. Sci. Res. 2016;7:2976. [Google Scholar]
- 39.See KL, Elbashir AA, Saad B, Ali ASM, Aboul-Enein HY. Simultaneous determination of ofloxacin and ornidazole in pharmaceutical preparations by capillary zone electrophoresis. Biomed. Chromatogr. 2009;23:1283–1290. doi: 10.1002/bmc.1251. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Zhang Z, Wu K. In vivo and real time determination of ornidazole and tinidazole and pharmacokinetic study by capillary electrophoresis with microdialysis. J. Pharm. Biomed. Anal. 2006;41:1453–1457. doi: 10.1016/j.jpba.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Göğer NG, Gökçen L. Quantitative determination of miconazole in creams by second order derivative spectrophotometry. Anal. Lett. 1999;32:2595–2602. doi: 10.1080/00032719908542990. [DOI] [Google Scholar]
- 42.Kumar N, et al. Analytical method development and validation for the estimation of miconazole nitrate in bulk and marketed topical formulation. Res. J. Pharm. Technol. 2019;12:4999–5003. doi: 10.5958/0974-360X.2019.00866.7. [DOI] [Google Scholar]
- 43.Ashour S, Kattan N. Simultaneous determination of miconazole nitrate and metronidazole in different pharmaceutical dosage forms by gas chromatography and flame ionization detector (GC-FID) Int. J. Biomed. Sci. 2010;6:13–18. [PMC free article] [PubMed] [Google Scholar]
- 44.Korany MA, Maher HM, Galal SM, Ragab MA. Development and optimization of a capillary zone electrophoresis technique for simultaneous determination of miconazole nitrate and hydrocortisone acetate in a cream pharmaceutical formulation. J. AOAC. Int. 2013;96:1295–1301. doi: 10.5740/jaoacint.11-343. [DOI] [PubMed] [Google Scholar]
- 45.Akay C, Özkan SA, Şentürk Z, Cevheroğlu Ş. Simultaneous determination of metronidazole and miconazole in pharmaceutical dosage forms by RP-HPLC. Il Farmaco. 2002;57:953–957. doi: 10.1016/S0014-827X(02)01296-X. [DOI] [PubMed] [Google Scholar]
- 46.Heneedak HM, Salama I, Mostafa S, El-Sadek M. HPLC and chemometric methods for the simultaneous determination of miconazole nitrate and nystatin. J. Chromatogr. Sci. 2012;50:855–861. doi: 10.1093/chromsci/bms127. [DOI] [PubMed] [Google Scholar]
- 47.Hermawan D, Sulaeman U, Istiqomah A, Aboul-Enein H. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. p. 012011. [Google Scholar]
- 48.Kobylińska M, Kobylińska K, Sobik B. High-performance liquid chromatographic analysis for the determination of miconazole in human plasma using solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 1996;685:191–195. doi: 10.1016/0378-4347(96)00125-9. [DOI] [PubMed] [Google Scholar]
- 49.Patel KG, Shah PM, Shah PA, Gandhi TR. Validated high-performance thin-layer chromatographic (HPTLC) method for simultaneous determination of nadifloxacin, mometasone furoate, and miconazole nitrate cream using fractional factorial design. J. Food Drug Anal. 2016;24:610–619. doi: 10.1016/j.jfda.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ICH, I. Q2 (R1): Validation of analytical procedures: Text and methodology. In: International Conference on Harmonization, Geneva (2005).
- 51.Miller J, Miller JC. Statistics and Chemometrics for Analytical Chemistry. London: Pearson education; 2018. [Google Scholar]
- 52.Rurack K. Standardization and Quality Assurance in Fluorescence Measurements I 101–145. Springer; 2008. [Google Scholar]
- 53.Soto KM, et al. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. Lwt. 2019;103:293–300. doi: 10.1016/j.lwt.2019.01.023. [DOI] [Google Scholar]
- 54.Smith BC. Fundamentals of Fourier Transform Infrared Spectroscopy. CRC Press Florida; 2011. [Google Scholar]
- 55.Abd Elhaleem SM, Elsebaei F, Shalan S, Belal F. Turn-off fluorescence of nitrogen and sulfur carbon quantum dots as effective fluorescent probes for determination of imatinib. Application to biological fluids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022;272:120954. doi: 10.1016/j.saa.2022.120954. [DOI] [PubMed] [Google Scholar]
- 56.Muttannavar V, et al. Effect of hydrogen bonding on ffluorescence quenching of quinolin-8-ol-analysis using negative stern–volmer plots. Int. J. Life Sci. Pharma Res. 2018;8:16–23. [Google Scholar]
- 57.Zu F, et al. The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Michrochim. Acta. 2017;184:1899–1914. doi: 10.1007/s00604-017-2318-9. [DOI] [Google Scholar]
- 58.Magdy G, Hakiem AFA, Belal F, Abdel-Megied AM. Green one-pot synthesis of nitrogen and sulfur co-doped carbon quantum dots as new fluorescent nanosensors for determination of salinomycin and maduramicin in food samples. Food Chem. 2021;343:128539. doi: 10.1016/j.foodchem.2020.128539. [DOI] [PubMed] [Google Scholar]
- 59.El-Malla SF, Elshenawy EA, Hammad SF, Mansour FR. N-doped carbon dots as a fluorescent nanosensor for determination of colchicine based on inner filter effect. J. Fluoresc. 2021;31:675–684. doi: 10.1007/s10895-021-02698-0. [DOI] [PubMed] [Google Scholar]
- 60.Magdy G, Al-enna AA, Belal F, El-Domany RA, Abdel-Megied AM. Application of sulfur and nitrogen doped carbon quantum dots as sensitive fluorescent nanosensors for the determination of saxagliptin and gliclazide. R. Soc. Open Sci. 2022;9:220285. doi: 10.1098/rsos.220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chunfa D, et al. Rapid and green synthesis of monodisperse silver nanoparticles using mulberry leaf extract. Rare Metal Mater. Eng. 2018;47:1089–1095. doi: 10.1016/S1875-5372(18)30125-5. [DOI] [Google Scholar]
- 62.Khan M, et al. Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Trans. 2018;47:11988–12010. doi: 10.1039/C8DT01152D. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz D, Jeunet F. Comparative pharmacokinetic studies of ornidazole and metronidazole in man. Chemotherapy. 1976;22:19–29. doi: 10.1159/000221906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.