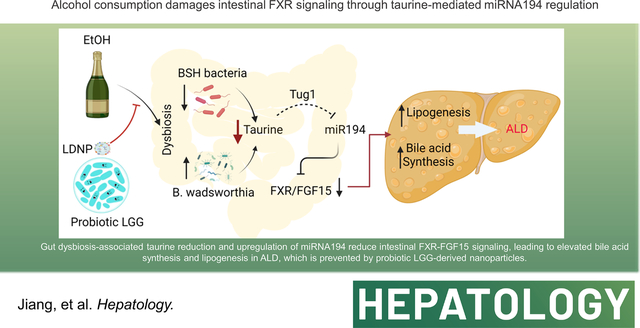
Abstract
Objective:
Intestinal farnesoid X receptor (FXR) plays a critical role in alcohol-associated liver disease (ALD). We aimed to investigate whether alcohol-induced dysbiosis increased intestinal miR194 that suppressed Fxr transcription, and whether Lactobacillus rhamnosus GG-derived exosome-like nanoparticles (LDNPs) protected against ALD through regulation of intestinal miR194-FXR signaling in mice.
Methods and results
Binge-on-chronic alcohol exposure mouse model was utilized. In addition to the decreased ligand-mediated FXR activation, alcohol feeding repressed intestinal Fxr transcription and increased miR194 expression. This transcriptional suppression of Fxr by miR194 was confirmed in intestinal epithelial Caco-2 cells and mouse enteriods. The alcohol feeding-reduced intestinal FXR activation was further demonstrated by the reduced FXR reporter activity in fecal samples and by the decreased FGF15 mRNA in intestine and protein levels in the serum, which caused an increased hepatic bile acid synthesis and lipogeneses. We further demonstrated that alcohol feeding increased-miR194 expression was mediated by taurine upregulated gene 1 (Tug1) through gut microbiota regulation of taurine metabolism. Importantly, three-day oral administration of LDNPs increased bile salt hydrolase (BSH)-harboring bacteria that decreased conjugated bile acids and increased gut taurine concentration, which upregulated Tug1 leading to a suppression of intestinal miR194 expression and recovery of FXR activation. Activated FXR upregulated FGF15 signaling and subsequently reduced hepatic bile acid synthesis and lipogenesis and attenuated ALD. This protective effects of LDNPs were eliminated in intestinal FxrΔIEC and Fgf15−/− mice. We further showed that miR194 was upregulated, whereas BSH activity and taurine levels were decreased in fecal samples of patients with ALD.
Conclusion:
Our results demonstrated that gut microbiota-mediated miR194 regulation contributes to ALD pathogenesis and to the protective effects of LDNPs through modulating intestinal FXR signaling.
Graphical Abstract
INTRODUCTION
Patients with alcohol-associated liver disease (ALD) often exhibit manifestations of cholestasis, a liver pathology characterized by accumulation of hepatic bile acids (BAs). Excess BAs can be toxic and can be an important causative factor in liver injury and hepatocyte death (1). BAs are end products of cholesterol catabolism and are made and released by the liver and stored in the gallbladder. Due to their detergent-like functions, BAs play critical roles in solubilization and absorption of cholesterol, dietary lipids, and fat-soluble vitamins in the intestine. BAs can also act as signaling molecules through activation of several receptors, including farnesoid X receptor (FXR), Takada-G-protein receptor 5 (TGR5), and sphingosine-1-phosphate receptor 2 (S1PR2) (2). FXR, highly expressed in the liver and intestine, is a BA-sensing nuclear receptor that regulates many biological functions, including BA homeostasis and lipogenesis.
BA synthesis is regulated by FXR in the liver and intestine. In the liver, BAs activate FXR and upregulate small heterodimer partner (SHP), which functions as a suppressor of gene expression of genes encoding cholesterol 7α-hydroxylase (Cyp7a1) and sterol 12α-hydroxylase (Cyp8b1), resulting in decreased BA de novo synthesis. In the intestine, BA-activated FXR upregulates fibroblast growth factor (FGF) 15/19 (mouse/human) and promotes FGF15/19 secretion into the portal vein, which leads to suppression of Cyp7a1 transcription and BA synthesis. Global FXR knockout mice have increased hepatic BA levels and liver injury (3). However, hepatocyte-specific FXR deletion does not change the BA pool and the enzymes for BA de novo synthesis (4), suggesting that intestinal FXR is a major player in regulating hepatic BA synthesis. Indeed, administration of intestinal FXR agonists reduced hepatic BA levels in murine models of ALD (5, 6).
While the FXR ligand activation has been well-studied, how FXR gene expression is regulated remains incompletely understood. Previous research demonstrated that hepatic microRNA194 (miR194) regulates Fxr mRNA expression in a mouse model of non-alcoholic fatty liver disease (NAFLD) (7). However, it is unclear whether miR194 regulates intestinal Fxr in ALD.
Probiotics have been used as interventions in the management of ALD in patients and in experimental animal models (8, 9). Our previous studies demonstrated that Lactobacillus rhamnosus GG (LGG) supplementation decreases hepatic BAs by increasing intestinal FXR–FGF15 signaling pathway–mediated suppression of BA de novo synthesis and enhancing BA excretion in mice, which have undergone bile duct ligation (BDL), and in multidrug resistance protein 2 knockout (Mdr2−/−) mice (10). Most recently, we found that LGG-derived exosome-like nanoparticles (LDNPs) were protective against ALD in a mouse model (11). However, whether LDNPs regulate BA homeostasis in ALD is unknown.
The present study was designed to investigate the how the intestinal Fxr gene is regulated and FXR is activated by alcohol in experimental ALD in mice. Our findings demonstrated that alcohol feeding increases intestinal miR194 through gut microbiota-mediated altered taurine metabolism, resulting in a suppressed Fxr gene expression and a decreased BA-mediated FXR activation, which leads to BA accumulation and increased lipogenesis and injury in the liver, and this can be attenuated by LDNP treatment.
MATERIAL AND METHODS
Animal Study
Male C57BL/6J mice (8 weeks of age) were obtained from Jackson Laboratory (Bar Harbor, ME). All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Louisville. Fgf15−/− and FxrΔIEC mice (7 weeks of age) were used as previously described (12). They were maintained at 22°C with a 12-hour:12-hour light/dark cycle and had free access to normal chow diet and sterile water.
Mice were fed the Lieber DeCarli Diet containing 5% alcohol (w/v) (Alcohol-fed, AF) or isocaloric maltose dextrin (Pair-fed, PF). For the AF groups, mice were initially fed the control Lieber-DeCarli liquid diet (Bio-Serve, Flemington, NJ) for 5 days to acclimate them to the liquid diet. The content of alcohol in the liquid diet was gradually increased from 1.6% (w/v) to 5% (w/v) in the next 6 days and remained at 5% for the subsequent 10 days. Mice in PF group were fed isocaloric maltose dextrin in substitution for alcohol in the liquid diet. On experimental Day 10, a bolus of EtOH (5 g/kg body weight) was given to AF mice by gavage 9 hours before harvesting, while mice in PF groups received a gavage of isocaloric maltose dextrin (10D+1B model). LDNPs were administered to mice in the last 3 days by daily gavage of 200 μL of LDNPs (50 μg protein content).
Statistical Analysis
Statistical analyses were performed using the statistical computer package GraphPad Prism, version 9 (GraphPad Software Inc., San Diego, CA). Results are expressed as means ± SEM. Statistical comparisons were made using two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test, one-way ANOVA with Tukey’s post hoc test, or Student t test, where appropriate. Differences were considered to be significant at p≤0.05. Significance is noted as *p≤0.05, **p≤0.01, ***p≤0.001 between groups.
Please see additional methods in the Supporting Information.
RESULTS
LDNP Treatment Reversed Alcohol-induced Fatty Liver and Liver Injury
Three-day LDNP treatment protected against alcohol-induced liver injury in mice with NIAAA binge-on-chronic alcohol feeding model (Fig. 1A–1C). We further showed that LDNPs’ effect on the reduction of liver fat accumulation is, at least in part, by inhibiting lipogenesis. Hepatic mRNA and nuclear levels of Srebp-1c was significantly increased by alcohol (Fig. 1D &1E)). The Srebp-1c target lipogenesis genes, Acc, Fasn and Scd-1, were markedly increased by alcohol feeding and decreased by LDNP treatment (Fig. 1F).
Figure 1. LDNP treatment reversed alcohol-induced liver injury.
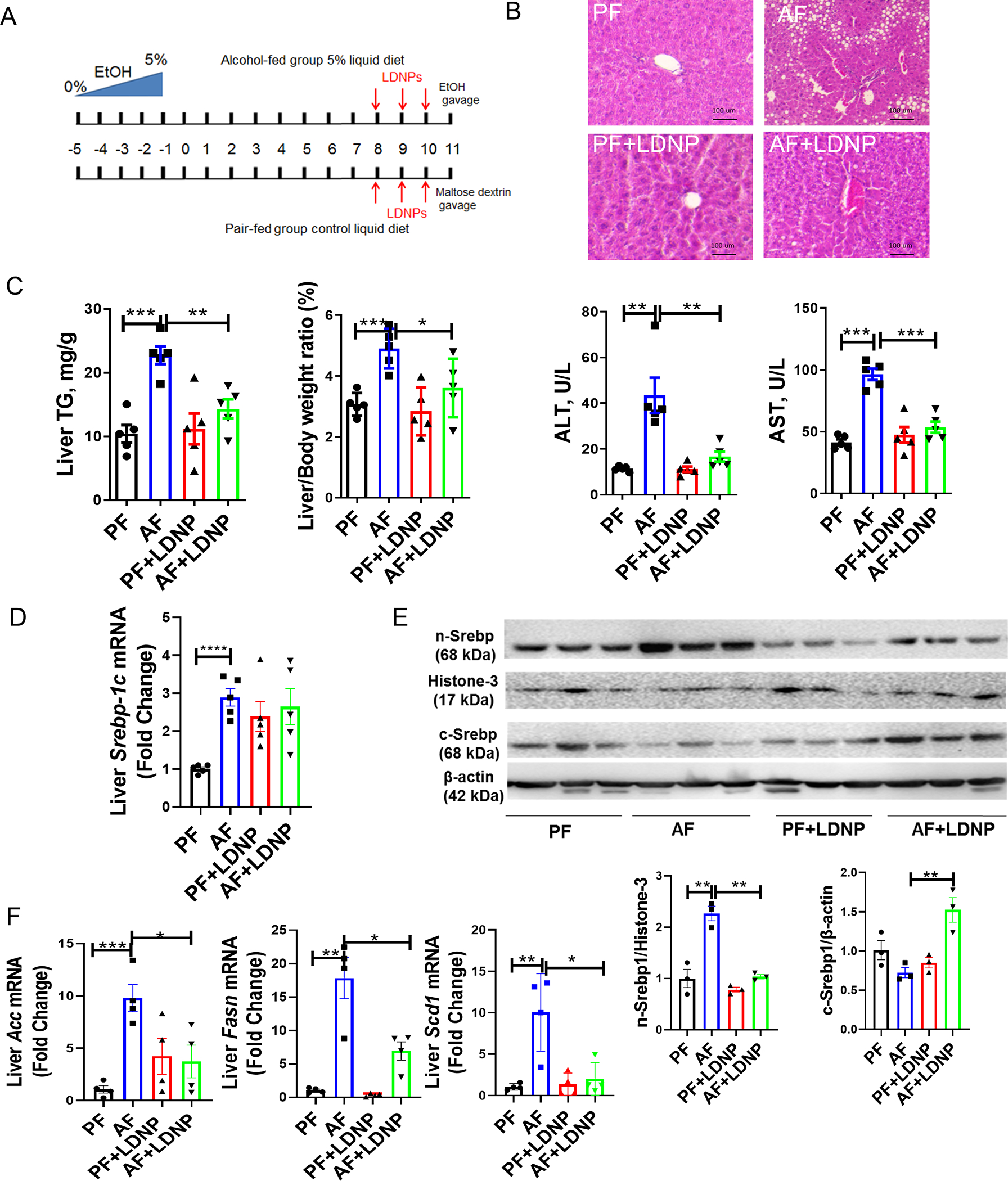
(A) Experimental design of animal treatment. (B) Representative microphotographs of H&E-stained mouse liver sections. (C) Hepatic TG levels, liver to body weight ratio and serum ALT and AST levels. (D) Hepatic mRNA levels of Srebp-1c. (E) Nuclear (n-Srebp1) and cytoplasmic (c-Srebp1) protein levels of liver Srebp1c. (F) Liver Acc, Fasn and SCD1 mRNA levels. n = 3–5/group.
Alcohol Feeding and LDNP Treatment Altered Hepatic BA Metabolism and Intestinal FXR-FGF15 Signaling Pathway
To investigate the involvement of BA metabolism in the effects of alcohol and LDNP treatment, liver and circulating BA levels were determined. Hepatic, serum (Fig. 2A) and fecal BAs were significantly increased in AF mice and were markedly reduced by LDNP treatment (Fig. 2A). In the liver, the relative proportion of lithocholic acid (LCA), which is the most toxic BA in liver (13), was increased by alcohol (Fig. S1B). Serum 12α-Hydroxylated BA, which can induce hepatic steatosis (14), was significantly upregulated by alcohol (Fig. S1C). Importantly, alcohol feeding caused a robust elevation of the serum 7α-hydroxy-4-cholesten-3-one (C4) level, a surrogate marker of BA synthesis (Fig. 2A). Alcohol-fed mice had elevated hepatic mRNA expression and protein levels of Cyp7a1, the major enzyme in the classic BA synthesis pathway (Fig. 2A & 2B). Importantly, LDNP treatment significantly reduced all of them (Fig. 2A & 2B). mRNA levels of additional P450 enzymes involved in BA synthesis, such as Cyp8b1 and sterol 27-hydroxylase (Cyp27a1), were not altered by alcohol exposure or by LDNP treatment (Fig. S2A). However, mRNA expression of oxysterol 7α-hydroxylase (Cyp7b1) was decreased by alcohol and further reduced after LDNP treatment (Fig. S2B). Additionally, as shown in Fig S2C and S2D, we also observed the alterations in those BA transporters by alcohol feeding and LDNP treatment.
Figure 2. BA synthesis regulation by alcohol and LDNPs.
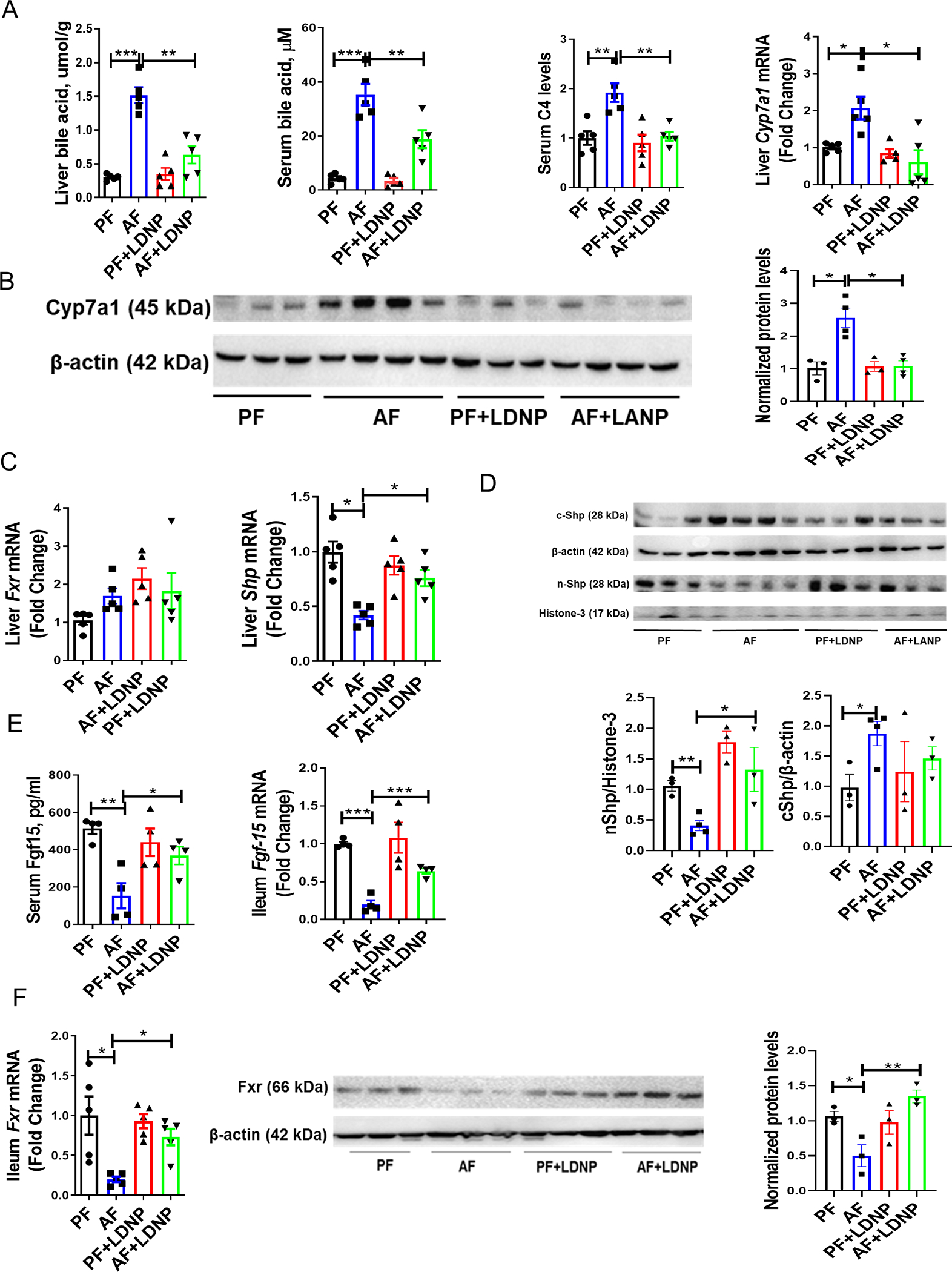
(A) Liver BA levels, serum BA levels, serum C4 levels and hepatic Cyp7a1 mRNA levels. (B) Hepatic protein expression of Cyp7a1. (C) Hepatic mRNA expression of Fxr and Shp. (D) Nuclear (nShp) and cytoplasmic (cShp) protein expression of liver Shp. (E) Serum Fgf15 levels and ileum mRNA expression of Fgf15. (F) Ileum mRNA and protein expression of Fxr. n = 3–5/group.
Hepatic mRNA expression of Fxr remained unchanged by alcohol feeding or LDNP treatment groups (Fig. 2C). However, we found that hepatic levels of chenodeoxycholic acid (CDCA) and deoxycholic acid (DCA), potent FXR agonists, were significantly decreased in alcohol-fed mice, but were not altered by LDNP treatment (Fig. S3A). In addition, no changes were found for hepatic cholic acid (CA) (FXR agonist) and T-α/βMCA (FXR antagonists) concentrations in the liver (Fig. S3). SHP is an orphan receptor and is regulated by FXR and by FGF15 derived from the intestine (15). Alcohol feeding decreased hepatic Shp mRNA expression, which was markedly increased by LDNP treatment (Fig. 2C). Consistent with previous studies (16, 17), we showed that nuclear Shp levels were substantially reduced by alcohol and increased by LDNP (Fig. 2D). Additionally, the specificity of Shp antibody was verified by liver Shp KO mice as shown in Fig. S3B. Taken together, these data suggest that LDNP treatment suppressed BA synthesis in alcohol-fed mice through a hepatic SHP-CYP7A1-mediated pathway.
It is known that intestine-derived FGF15 plays a pivotal role in hepatic BA (18). To understand the effects of LDNPs on BA metabolism in ALD, we measured intestinal FXR-FGF15 signaling. Alcohol feeding caused a significant reduction of circulating Fgf15 protein levels, which was restored by LDNP treatment (Fig. 2E). Similarly, mRNA levels of Fgf15 in ileal tissues were decreased by alcohol exposure and increased by LDNP treatment (Fig. 2E). Ileal mRNA and protein levels of Fxr were significantly reduced by alcohol exposure and normalized by LDNP treatment (Fig. 2F). These results suggest that alcohol exposure reduced the ileal FXR-FGF15 signaling pathway, which was restored by LDNP treatment. The activation of intestinal FXR-FGF15 signaling could contribute to the beneficial effects of LDNPs on the inhibition of BA synthesis in the liver in ALD.
Alcohol Feeding Increased Ileal miR194 Expression
To evaluate how the Fxr gene is regulated at the transcriptional level, we performed a miRNA microarray assay in ileal samples (Fig. S4). Among the changed miRNAs, we observed that alcohol feeding markedly upregulated miR194 and miR192 (Fig. 3A), which suppressed Fxr gene expression by binding 3′-UTR of the Nr1h4 gene (Fxr) (7). We further confirmed the ileal upregulation of miR194 and miR192 by real-time qPCR (Fig. 3B). Notably, alcohol exposure significantly increased hepatic miR192 levels, but not miR194 (Fig. 3B), implying that the action of alcohol on miR194 regulation is likely intestine-specific. Recent studies showed that serum exosomal miR192 is a biomarker of ALD (19). Consistent with this study, we found that alcohol feeding increased serum exosomal miR192, but not miR194 levels (Fig. 3C), further suggesting a local regulation of ileal miR194 by alcohol. We also found that the expression of both pri-miR194–2 and pri-miR194–1 in the ileum was increased by alcohol feeding, but the increase of pri-miR194–2 was more pronounced (Fig.3C), which is the major contributor to the expression of miR194 in the gastrointestinal tract (20). These results suggest partial transcriptional regulation of miR194 expression in the intestine by alcohol. Interestingly, intestinal mucus-derived exosomes contain miR194, and it was significantly increased by alcohol feeding (Fig. S5A). However, the crypt-derived exosomal miR194 was unchanged (Fig. S5B), indicating that alcohol upregulates miR194 in the intestinal epithelium. Importantly, LDNP treatment significantly reduced the alcohol-upregulated mature miR194 and pri-miR194–2 in the ileum (Fig. 3D). miRNAs are normal constituents of murine and human feces, and most fecal miRNAs are derived from host gut epithelial cells (21). Fecal miRNAs have been used as biomarkers for screening and diagnosis of multiple intestinal diseases (22). Thus, we performed a miRNA microarray assay in fecal samples. As shown in Fig. S6A–B, alcohol feeding induced a significant dysregulation of fecal miRNA expression. Similarly, the fecal miR194 level was also significantly elevated by alcohol (Fig. S6C). These data unambiguously demonstrate that alcohol exposure increases intestinal epithelium-derived miR194 expression, which is suppressed by LDNP treatment.
Figure 3. The effects of alcohol and LDNPs on intestinal microRNA expression and validation of miR194 regulation of FXR.
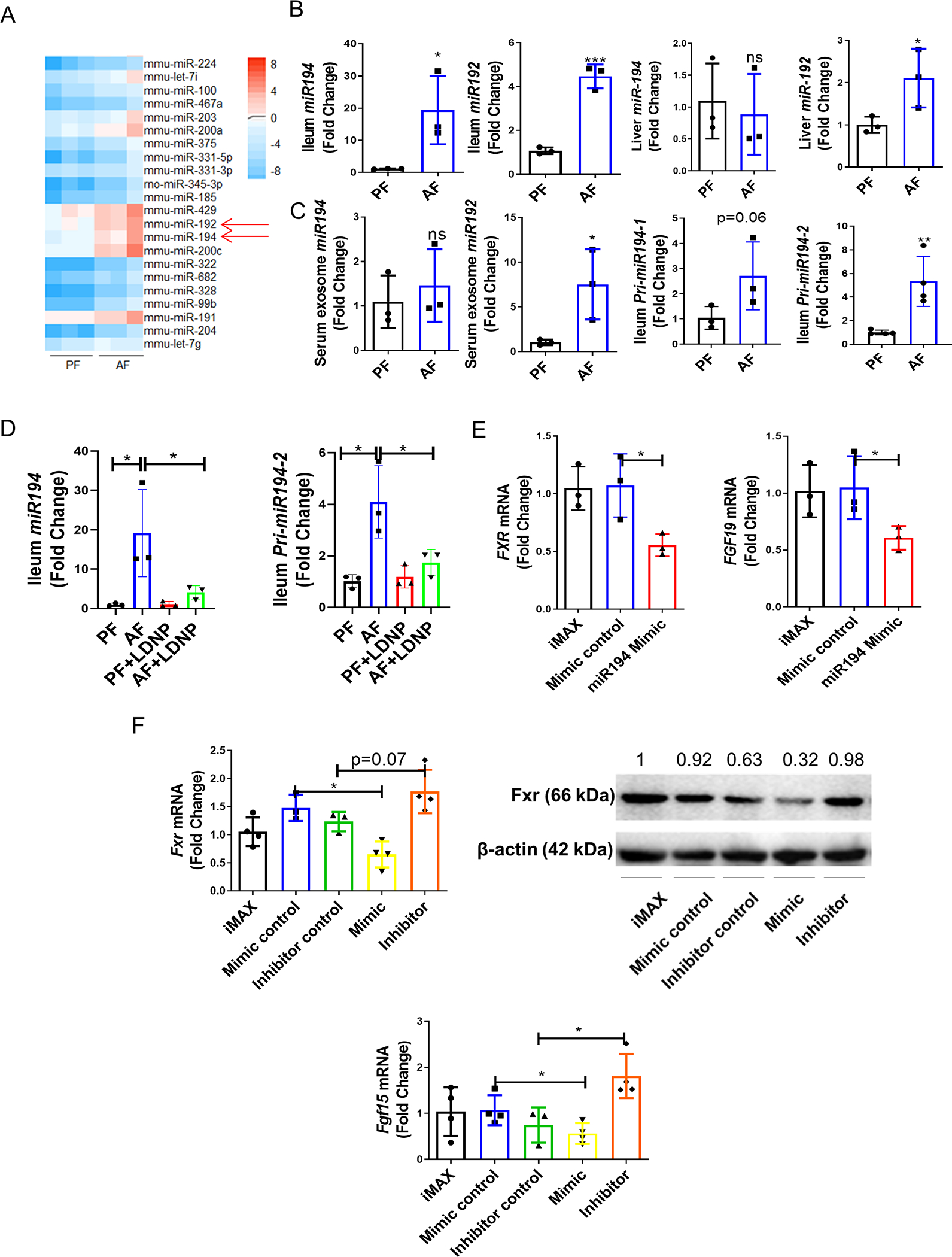
(A) Heat map of the most upregulated miRNAs by a microRNA array assay. (B) Validation of two dysregulated miRNAs (miR194 and miR192) in ileum samples by RT-q-PCR and hepatic miR194 and miR192 expressions. (C)Serum exosomal miR194/192 levels and ileum Pri-miR194–1/2 mRNA expression. (D) Ileum miR194 and Pri-miR194–2 expression. (E) miR194 mimic on FXR and FGF19 expressions in human Caco-2 cells (F) miR194 mimic and inhibitor on Fxr (upper panel) and FGF15 (lower panel) mRNA and protein expression in mouse intestinal organoids. iMAX: lipofectamine RNAiMAX. Data are expressed as mean ± SEM. n = 3–5/group.
To further investigate the effect of miR194 on FXR expression, human intestinal epithelial Caco-2 cells were transfected with 100 nM miR194 mimic for 24 hours. As shown in Fig. 3E, FXR mRNA levels were suppressed by miR194 mimic. Moreover, the mRNA levels of FXR- target gene, FGF19, were also reduced by miR194 mimic (Fig. 3E). Next, we examined the effect of miR194 on Fxr expression in mouse 3D intestinal organoids (23) (Fig. S6D). The organoid culture was transfected with 100 nM miR194 mimic or anti-miR194 inhibitor for 48 hours. miR194 mimic reduced, and miR194 inhibitor increased intestinal Fxr mRNA and protein levels (Fig. 3F). Similar results were obtained for Fgf15 mRNA and serum Fgf15 proteins (Fig. 3F). We also performed an ex vivo ileal culture experiment to examine the effect of miR194 on the Fxr-Fgf15 pathway. As shown in Fig. S7, miR194 mimic significantly reduced Fxr and Fgf15 mRNA expression in this ex vivo study. These data demonstrate that miR194 suppresses FXR gene expression in the intestinal tissue. In addition, alcohol or LDNPs had no direct effects on the expression levels of miR194, FXR and FGF15 in Caco-2 cells (Fig. S8), indicating that the effects of alcohol and LDNPs observed in vivo may be regulated indirectly through mediators such as the modification of microbiota.
Alcohol Feeding Decreased Fecal Taurine Concentrations and TUG1 Expression
Previous studies (24) demonstrated that miR194 was regulated by taurine-upregulated gene 1 (TUG1), a long non-coding RNA, which is upregulated by taurine. TUG1 induced an epigenetic regulator that enhances zeste homolog 2 (EZH2)-associated promoter methylation, and can directly bind to and act as a biological sponge to reduce miR194 expression (25). Our results showed that ileal TUG1 expression was significantly decreased by alcohol and increased after LDNP administration (Fig. 4A). Similarly, fecal taurine concentrations were also reduced by alcohol feeding and restored by LDNP treatment (Fig. 4A). The reduction of fecal taurine concentration by alcohol feeding was also demonstrated in other alcohol feeding models (26). Notably, taurine treatment increased TUG1 expression and reduced miR194 levels in mouse 3D intestinal organoids (Fig. 4B). Moreover, taurine pre-treatment restored ileum TUG1 and suppressed miR194 levels in alcohol-fed mice (Fig. 4C).
Figure 4. Alcohol Feeding Increased miR194 Expression though Taurine-TUG1 pathway.
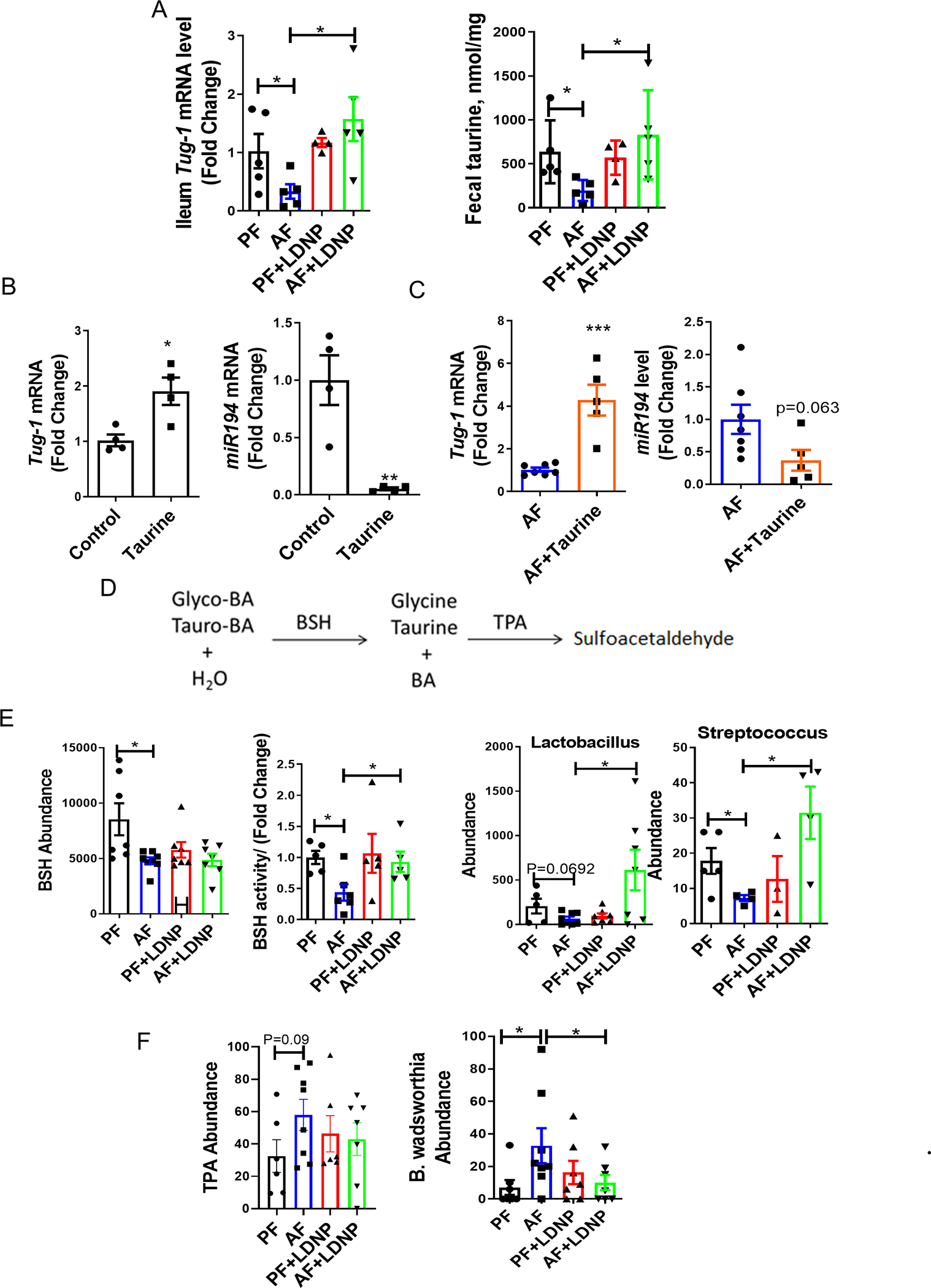
(A) Ileum TUG1 mRNA levels and fecal taurine concentration. (B) Effect of taurine on Tug1 (left panel) and miR194 (right panel) expressions in mouse intestinal organoids. Organoids were stimulated with taurine (150 mM) or PBS for 72 hours. (C) Ileum Tug1 (left panel) and miR194 (right panel) levels under taurine treatment. Mice were treated as described in the Fig 1, and taurine was added to the drinking water or diet at a dose of 2g/kg bw/mouse for total 25 days. (D) Conjugated bile acid metabolism in intestine. (E) OTUs of BSH, fecal BSH activity and Lactobacillus/Streptococcus levels. (F) OTUs of TPA (left panel) and fecal B. wadsworthia change (right panel). n = 3–8/group.
Free taurine is released from tauro-BA by gut bacteria and choloylglycine hydrolase (bile salt hydrolase, BSH, classified as EC 3.5.1.24) and BSH is responsible for the deconjugation (deamidation) of conjugated (amidated) BAs (Fig. 4D). Of note, deltaproteobacteria convert sulfonated taurine (2-aminoethanesulfonate) to ammonia, acetate, and sulfite via taurine-pyruvate aminotransferase (EC 2.6.1.77 encoded by TPA). Previous studies have identified that B. wadsworthia use taurine as a substrate (27). Therefore, alcohol feeding caused-gut bacterial dysbiosis may contribute to the taurine regulation of intestinal miR194.
To evaluate gut microbiota alteration, we performed 16s metagenomics analysis in murine fecal samples. Fig. S9A is a snapshot for the α-rarefaction curves for the number of observed OTUs indicating that the PF group had the highest number of observed OTUs while AF group had the smallest number of observed OTUs. Principal covariant analysis (PCoA) showed that the mean distances between PF, AF and AF+LDNP were significantly different (p < 0.05) (Fig. S9B). At the phylum level, alcohol feeding led to a remarkable increase in the abundance of Proteobacteria and Firmicutes and a decrease in Bacteroidetes and Verrucomicrobia. Interestingly, Akkermansia muciniphila, a Gram-negative intestinal commensal belonging to Verrucomicrobia phylum, has been shown to promote barrier function partly by enhancing mucus production (28). Importantly, LDNP treatment prevented the ethanol-induced expansion of the Proteobacteria and the ethanol-decreased Verrucomicrobia (Fig. S9C).
Importantly, our results showed that bacterial genomic DNA encoding BSH was significantly reduced by alcohol feeding but not altered after LDNP treatment (Fig. 4E). Fecal BSH activity was markedly reduced after alcohol feeding and significantly increased by LDNP treatment (Fig. 4E). Streptococcus and Lactobacillus, the well-defined BSH-containing bacterial genera (29), were decreased by alcohol, and increased after LDNP treatment (Fig. 4E). Additionally, the bacterial genomic DNA encoding TPA was increased by alcohol feeding and decreased by LDNP, although without statistical significance (Fig. 4F). However, the taurine-consuming bacteria species, B. wadsworthia, was significantly increased by alcohol and reduced by LDNP treatment (Fig. 4F).
Taken together, these results suggest that alcohol feeding causes intestinal dysbiosis that decreases gut taurine concentration, which in turn, results in a reduced TUG1 expression and consequently, an increased miR194 expression, and this dysregulation can be reversed by LDNP treatment.
Alcohol Feeding Altered BA Profile and FXR Activity
Liver-derived BAs flow into the intestine from the gallbladder and undergo de-conjugation and detoxification by gut bacteria. Conjugated and deconjugated BAs often possess different FXR agonism activities (30). As shown in Fig. 5A, isodeoxycholic acid (iso-DCA, * labeled), a BA that is formed via epimerization of DCA by intestinal bacteria and inhibits FXR activity (31), was the major form of BA in feces. Iso-DCA levels were increased in AF mice and reduced by LDNP treatment. Similarly, LCA (^ labeled)was also increased by alcohol feeding. ω-muricholic acid (ω-MCA, ^^ labeled), which is the second most abundant form of BAs in the feces and acts as an FXR agonist in intestine (32), was decreased by alcohol feeding. 12-ketolithocholic acid and cholic acid-7-sulfate (CA7S) were also decreased by alcohol feeding. Importantly, LDNP treatment had minimal effect on the BA profile in PF mice, but markedly reversed the changes in AF mice.
Figure 5. Effects of alcohol and LDNP on FXR activity.
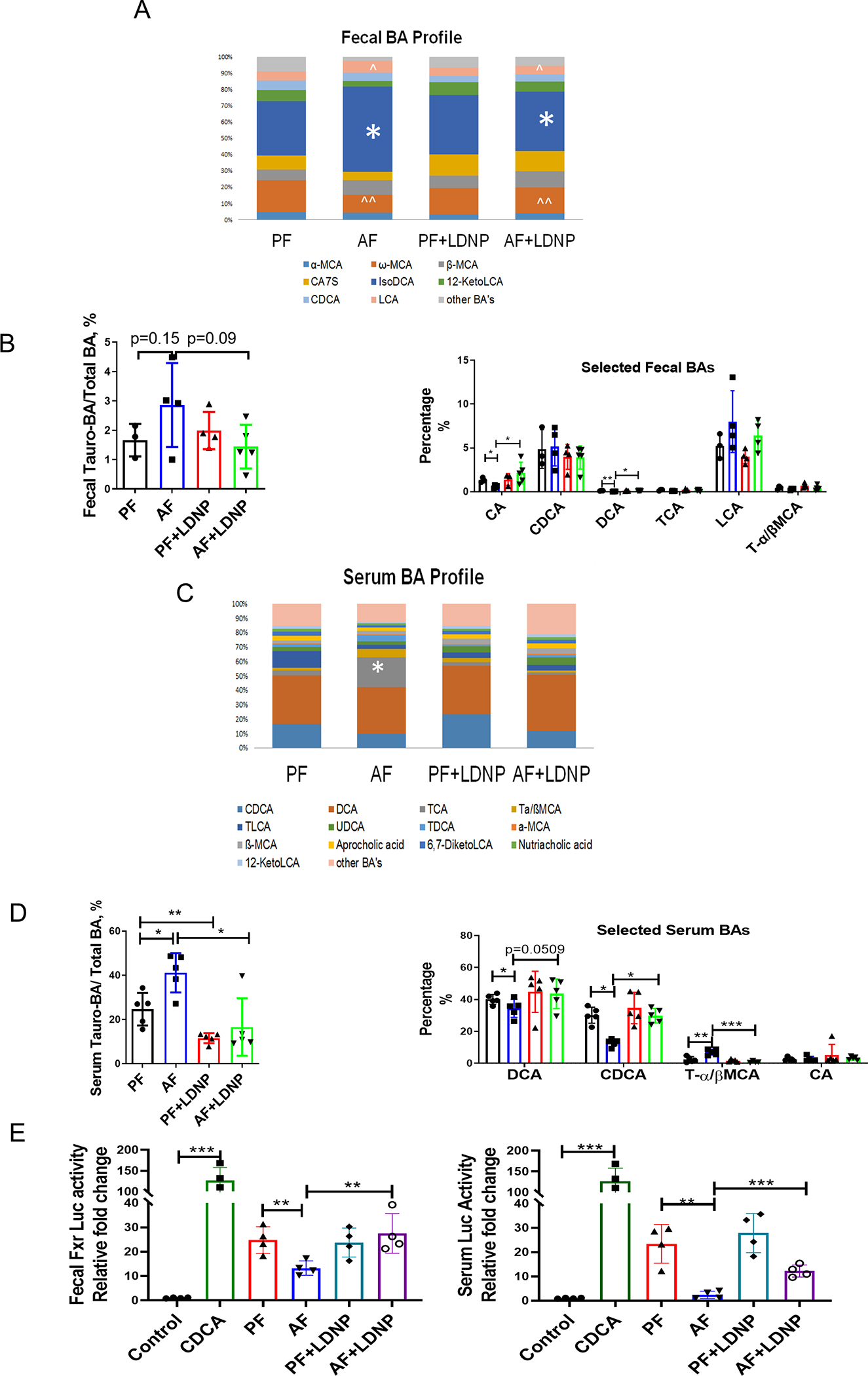
(A) Fecal bile acid composition ratios. (B) Fecal tauro-BA/total BA ratios and selected fecal BA concentrations. (C) Serum BA composition ratios. (D) Serum tauro-BA/total BA ratios and selected serum BA concentrations. (E) Luciferase reporter assay of FXR activities of fecal extracts (left panel) and serum (right panel). Luciferase reporter assay was performed in HEK293 cells transfected with FXR expression plasmid, FXR reporter plasmid, and β-galactosidase expression plasmid. Cells were stimulated with CDCA (20 μM) alone, fecal supernatant or serum for 6 hours. n = 3–5/group.
Fecal total BA profiling is shown in Fig. S10A. Interestingly, the tauro-BA/total BA ratio was slightly increased by alcohol and decreased by LDNP treatment (Fig. 5B). Previous research showed that tauro-BA down-regulated intestinal FXR-FGF15 signaling (33). Next, we analyzed the selected BAs that reportedly have FXR ligand activity. Fecal levels of CA and DCA were significantly decreased in alcohol-fed mice, and this effect was reversed by LDNPs. Alcohol feeding increased, and LDNPs decreased, LCA levels. There was no significant effect on fecal CDCA and T-α/βMCA levels by either alcohol or LDNP treatment (Fig. 5B).
Most gut microbiota-manipulated BAs are reabsorbed by the intestine into the circulation. We thus measured the serum BA profile. In contrast to fecal BAs, the major forms of BAs in the serum were DCA and CDCA. TCA levels were low in PF mice but dramatically increased by alcohol-feeding and decreased by LDNPs (Fig. 5C, * labeled). Tauro-BA accounted for about 20% of the total BA in the serum of PF mice, and that was increased to 40% by alcohol feeding. LDNP treatment significantly reduced the tauro-BA/total BA ratio in both PF and AF mice (Fig. 5D). Alcohol-increased tauro-BAs may contribute to the decrease in free taurine, as demonstrated previously (Fig. 4A).
Serum total BA levels were significantly increased by alcohol and decreased by LDNPs (Fig. 2A). This was mainly due to the changes in conjugated BAs, since the unconjugated BA level was insignificantly altered by either alcohol or LDNPs (Fig. S10B). Interestingly, serum FXR agonists, DCA and CDCA, were reduced by alcohol feeding and restored by LDNP treatment. The levels of CA, another FXR agonist, were low and not altered by alcohol or LDNP treatment. Interestingly, T-α/βMCA, FXR antagonist, was increased by alcohol feeding but markedly reduced by LDNP treatment (Fig. 5D).
To further determine FXR activity, we transfected HEK293 cells with an FXR expressing plasmid and an FXR luciferase reporter plasmid. The cells were treated with fecal supernatants or serum samples, and FXR agonist CDCA was used as a positive controlSerum (Fig. 5E left panel) and fecal supernatants (Fig. 5E right panel) from PF mice also had significantly increased FXR-luciferase activity, which was markedly reduced in alcohol feeding samples. Importantly, LDNP treatment improved the alcohol-suppressed FXR-luciferase activity (Fig. 5E).
Taken together, in addition to the transcriptional regulation, alcohol feeding causes dysregulation of BAs that reduced FXR activation, and LDNP treatment positively modified gut microbiota and BA metabolism that led to an increased FXR activity in ALD mice.
Intestinal FXR Deficiency Abolished the Protective Effects of LDNP against Alcohol-induced Liver Injury
Our results demonstrated that LDNPs increased intestinal FXR activity in alcohol-fed mice. We thus hypothesized that the lack of intestinal FXR would diminish the protective effects of LDNPs. To test this hypothesis, we used intestinal epithelial cell-specific Fxr knockout mice (FxrΔIEC). The knockout efficiency of FXR was tested as shown in Fig. S11A. FxrΔIEC and Fxrfl/fl mice were fed Lieber DeCarli liquid diet in a binge-on-chronic alcohol feeding model as described in Fig 1, and LDNPs were given to the mice once daily for the last three days. Agreeing with previous studies (12), alcohol feeding increased hepatic fat accumulation and liver injury in the FxrΔIEC mice compared to Fxrfl/fl mice (Fig. 6A–C). Importantly, the beneficial effects of LDNP treatment on alcohol-induced fatty liver and liver injury were diminished in the FxrΔIEC mice but not Fxrfl/fl mice (Fig. 6A–C). LDNP treatment was no longer effective on the reduction of BA levels in the serum and liver in the FxrΔIEC mice (Fig. 6D). Liver Cyp7a1 mRNA and protein levels and serum Fgf15 protein levels were also unchanged by LDNPs (Fig. 6E–F & S12). Taken together, these data indicate that intestinal FXR plays a major role in the beneficial effects of LDNP on alcohol-induced liver injury.
Figure 6. The beneficial effect of LDNPs was abolished in FxrΔIEC mice.
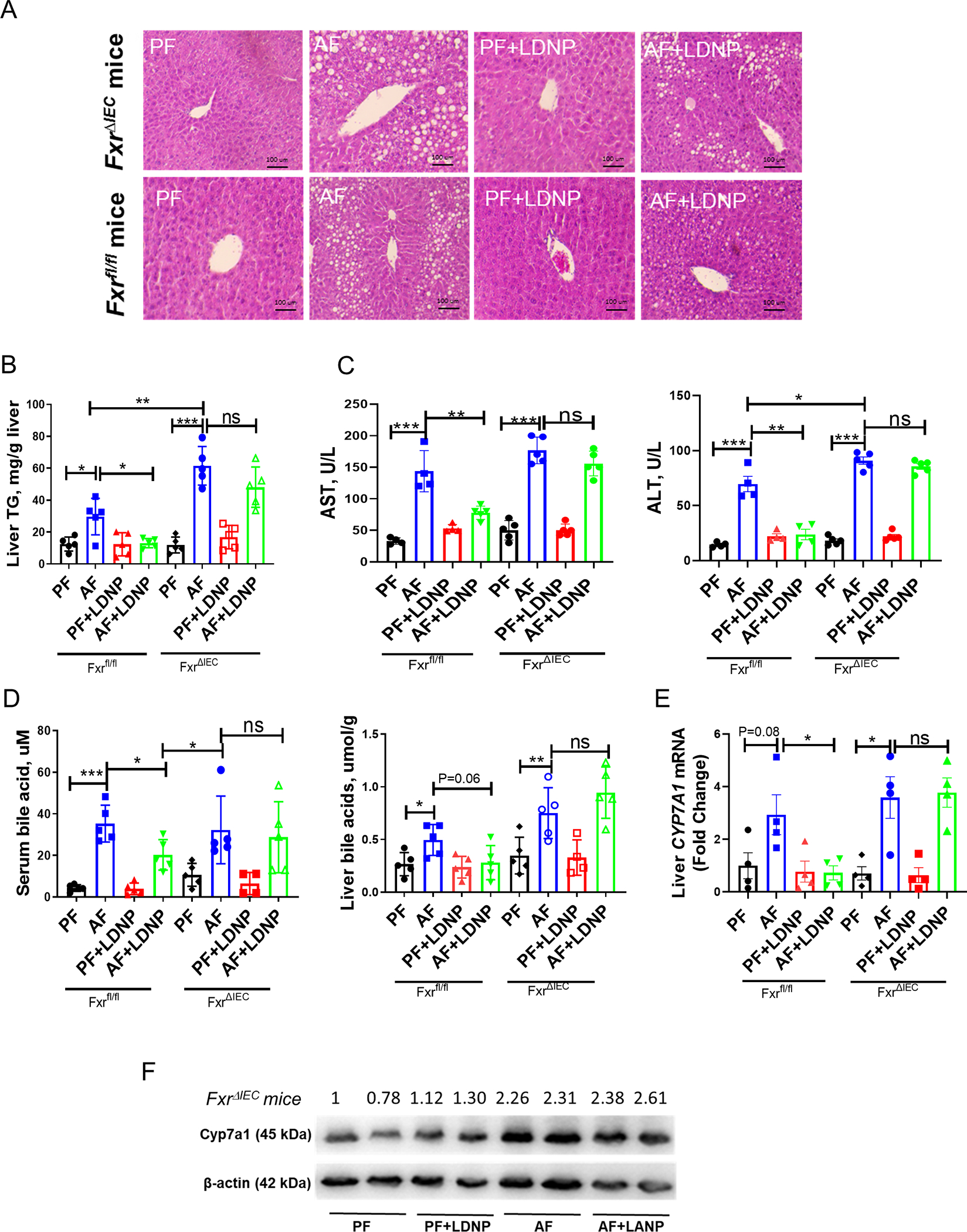
(A) Representative microphotographs of H&E-stained mouse liver sections. (B) Hepatic TG levels. (C) Serum ALT and AST levels. (D) Serum and liver BA levels. (E) Hepatic mRNA expressions of Cyp7a1. (F) Hepatic protein levels of Cyp7a1. n = 3–5/group.
Protective Effect of LDNPs Against Alcohol-Induced Liver Injury was Diminished in Fgf15 KO Mice
We further examined the effects of LDNPs in FXR-FGF15 pathways in Fgf15−/− mice. FGF15 is an endocrine hormone mainly expressed in the intestine in mice, while human FGF19 is expressed in both intestine and liver under disease conditions (34, 35). Thus, using whole-body knockout of Fgf15 is appropriate to dissect the role of Fgf15 in liver pathology in mice. The KO efficiency of Fgf15 was validated as shown in Fig. S11B. Notably, the protective effects of LDNPs against alcohol-induced liver steatosis and injury, the beneficial effects of LDNPs on BA reduction in the serum and liver, and Cyp7a1 mRNA expression in WT mice were completely abolished in the Fgf15−/− mice (Fig. 7A–F). Taken together, those data indicate that intestinal FXR-FGF15 signaling is required for the beneficial effects of LDNPs against alcohol-induced liver injury.
Figure 7. The beneficial effect of LDNPs was abolished in Fgf15 KO mice.
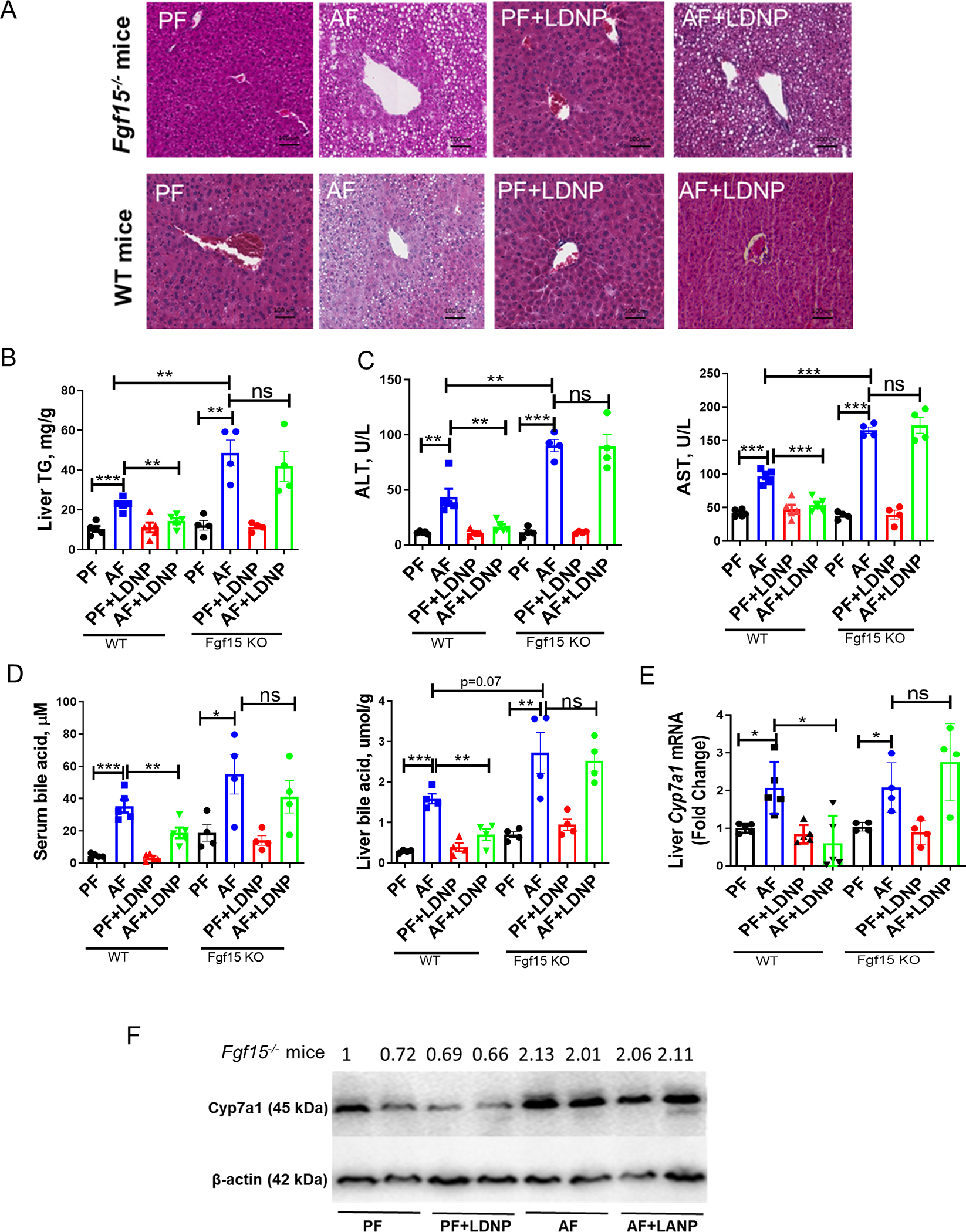
(A) Representative microphotographs of H&E-stained mouse liver sections. (B) Hepatic TG levels. (C) Serum ALT and AST levels. (D) Serum and liver BA levels. (E) Hepatic mRNA expressions of Cyp7a1. (F) Hepatic protein levels of Cyp7a1. n = 3–5/group.
Patients with Alcohol-Associated Hepatitis had Elevated Fecal Bile Acids and miR194 but Decreased Taurine Levels and BSH Activity
We further examined the miR194-FXR signaling in samples from patients with AH. Agreeing with previous studies (1), serum BA concentrations in AH patients were significantly higher than in healthy controls (data not shown). Fecal levels of total BAs were also significantly increased in AH patients (Fig. 8A). Importantly, fecal miR194 level was elevated in AH patients compared to healthy controls (Fig. 8B). We further showed fecal taurine concentration was lower in AH patients compared to healthy controls, and it negatively correlated with AST level (Fig. 8C & 8D). Additionally, patients’ fecal BSH activity was also significantly decreased and negatively correlated with serum AST level (Fig. 8E & 8F). Previous research demonstrated a gut dysbiosis in the AH patients (36) and in experimental ALD in mice (37). The dysregulated taurine homeostasis may be a result of decreased BSH-harboring bacteria, such as Lactobacillus, which contributes to the FXR activation.
Figure 8. Fecal levels of miR194, bile acids and taurine in AH patients.
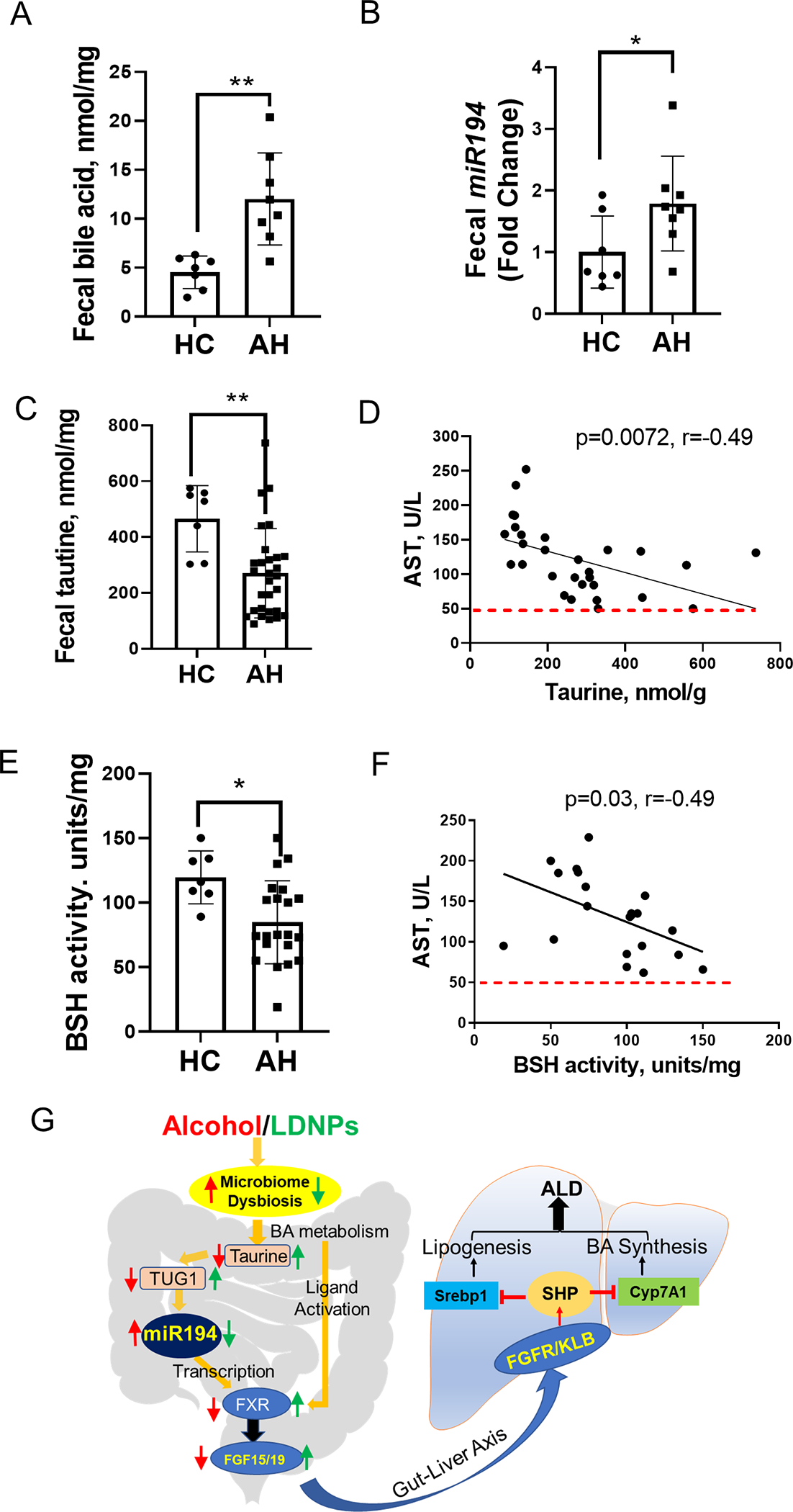
Stool samples were lyophilized before analyses. (A) Fecal bile acids and miR194 levels. (C) Fecal taurine concentrations and correlations between fecal taurine concentrations and serum AST levels. (D) Fecal BSH activity and correlation between BSH activity and serum AST levels. (E) A proposed model of LDNPs prevention against ALD through modulation of intestinal miR194-FXR-FGF15 signaling.
DISCUSSION
Cholestasis is a frequent feature of ALD, and the accumulation of toxic BAs is the major contributor (38). Reducing hepatic BA overload is a therapeutic goal in the management of ALD (39). Clinical studies have demonstrated that patients with AH have an increased BA synthesis even in the face of cholestasis and increased BA levels (40, 41). BA synthesis is regulated by hepatic and intestinal FXR signaling. Although it is well-known that BAs are endogenous ligands of FXR, how intestinal FXR is regulated at the transcriptional level in ALD is unknown. In this study we showed that intestinal miR194 suppressed intestinal Fxr mRNA expression in alcohol-fed mice. Alcohol feeding increased intestinal miR194 and decreased intestinal Fxr expression which led to a decreased FXR-FGF15 signaling in the enterohepatic axis. We confirmed that miR194 suppressed FXR gene expression in an intestinal epithelial Caco-2 cell line, a mouse intestinal organoid culture and ex vivo ileum tissue culture, and we showed that miR194 mimic inhibited, whereas miR194 inhibitor increased, FXR mRNA expression. In line with previous studies (1), we found that alcohol feeding significantly changed BA profiles in both feces and serum samples. Both the fecal and serum BAs that have potential FXR antagonistic activity were increased, while those BAs that activate FXR were decreased by alcohol feeding. The combinatorial effects of alcohol-induced transcriptional suppression and BA-mediated inhibition of FXR contribute to the defective enterohepatic FXR-FGF15 signaling that is critical in the regulation of hepatic BA homeostasis and lipogenesis in ALD.
How is intestinal miR194 regulated in mice with experimental ALD? Ethanol at physiological concentrations had no effect on miR194 expression in Caco-2 cells and ileal organoids, indicating an indirect regulation that likely involved gut microbiota. We demonstrated that alcohol-caused gut dysbiosis decreased gut taurine concentration thatconsequently caused a reduction of TUG1 expression resulting in the upregulation of miR194. This taurine-depended regulation of TUG1 and miR194 was further demonstrated in ileal organoids and in mice. We further demonstrated that fecal taurine concentrations, along with miR194, were reduced in AH patients and were negatively correlated with serum AST levels. Previous studies have demonstrated that taurine supplementation reduced experimental ALD (42). Our study provided further mechanistic insights into the protective role of taurine. Altering gut microbiota to increase intestinal taurine availability, thereby suppressing miR194-mediated reduction of FXR-FGF15 signaling is a plausible strategy for inhibition of ALD development and progression.
Management of ALD is challenging. Previous studies demonstrated that the administration of probiotics to animals (8) or humans with ALD was beneficial (9). Probiotic treatment restricted the growth of harmful bacteria, prevented gut leakiness and attenuated liver injury. Our recent studies further showed that the supernatant from the probiotic, LGG, was effective in the prevention of ALD in animal models (43). We further showed that the effect of the supernatant was mediated by probiotic-derived nanoparticles (LDNPs) through intestinal AhR-IL22 pathway (11). In this study, we further showed that LDNP administration suppresses the alcohol-induced intestinal miR194 expression and BA dysregulation, and subsequently increases intestinal FXR-FGF15 signaling and suppresses BA synthesis.
In addition to Fxr transcriptional regulation, gut microbiota changes by alcohol and LDNPs play a critical role in the BA transformation that regulates FXR ligand activity. Interestingly, T-α/βMCA, a significant FXR antagonist, were increased by alcohol and decreased by LDNPs in serum. Of note, a previous study showed that chronic alcohol feeding decreased tauro-BAs (5). The discrepancy may be a result of different alcohol feeding models. In addition, it is unknown how FXR activity was changed in above-mentioned study (5). Different BA species and abundance can have a profound impact on FXR activation. Importantly, the alteration of FXR activities by alcohol and LDNP treatment was confirmed by FXR reporter assay.
The regulation of intestinal miR194-mediated FXR-FGF15 signaling by LDNPs causes a reduction of BA de novo synthesis. Gut-derived FGF15 binds hepatic FGFR4 and starts a signaling cascade resulting in a phosphorylation of SHP that promotes its nuclear translocation and subsequent activation. The regulation of SHP activation not only inhibits Cyp7a1 to suppress BA de novo synthesis but also inhibits lipogenesis resulting in attenuation of fatty liver. We further demonstrate that the inhibitory effects of LDNPs on fatty liver and BA accumulation in mice with ALD require intestinal FXR and FGF15. Intestinal epithelial FXR knockout and FGF15 depletion abolished the beneficial effects of LDNPs.
As with most studies, the present study has some limitations. We used the 10D+1B mouse model. Human ALD has a broad spectrum, and various animal models recapitulate different stages of human ALD, although animal models that mimic severe ALD are still lacking. The results in the present study need to be evaluated in different models of ALD. Second, the reduction of intestinal FXR and FGF15 and elevation of miR194 by alcohol need to be validated in intestinal specimens from patients with ALD.
In conclusion, the current study provides a novel mechanism by which intestinal miR194 regulates FXR activation in ALD, and LDNP treatment inhibits ALD through intestinal miR194-FXR-FGF15 signaling pathway (Fig. 8E). We demonstrate that alcohol consumption causes gut dysbiosis that leads to a reduced gut taurine concentration, and consequently a decreased TUG1 expression and an increased miR194 expression. Increased intestinal miR194 leads to reduced FXR transcription and dysregulated BA profiles that, in turn, lead to decreased FXR ligand activation, which reduces intestinal FGF15 expression. This defective enterohepatic FXR-FGF15 signaling results in an increased hepatic BA synthesis and lipogenesis and liver injury. Of note, we administrated LDNPs only in the last three days of our model, suggesting a treatment potential for LDNP in ALD and other BA-associated liver diseases.
Supplementary Material
Acknowledgments
We thank Dr. Sayeepriyadarshini Anakk from University of Illinois Urbana-Champaign for providing Shp KO mouse tissue and Jeffrey Warner for support in organoid culture. We thank Mrs. Marion McClain for manuscript proofreading.
Financial support:
This study was supported by NIH grants R01AA023190 (W.F.), U01AA026934, U01AA026936, U01AA026980, R01AA023681, P20GM113226, and P50AA024337 (C.J.M.). S10OD020106 (X.Z.), R01AA028435 and R01AA021434 (S-Y.C), K23AA029198 (V.V.), R01GM135258 and R21ES029258 (G.L.G.), and F31AA028725-02 (M.J.). Support was also provided by the VA grants I01BX002996 (C.J.M), I01BX002741 (G.L.G.) and IK6BX004199 (Research Career Award, H-G. Z.).
REFERENCES
- 1.Tung BY, Carithers RL Jr. Cholestasis and alcoholic liver disease. Clin Liver Dis 1999;3:585–601. [DOI] [PubMed] [Google Scholar]
- 2.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 2008;7:678–693. [DOI] [PubMed] [Google Scholar]
- 3.Kong B, Zhang M, Huang M, Rizzolo D, Armstrong LE, Schumacher JD, Chow MD, et al. FXR deficiency alters bile acid pool composition and exacerbates chronic alcohol induced liver injury. Dig Liver Dis 2019;51:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Kong B, Huang M, Wan R, Armstrong LE, Schumacher JD, Rizzolo D, et al. FXR deletion in hepatocytes does not affect the severity of alcoholic liver disease in mice. Dig Liver Dis 2018;50:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, Wang L, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018;67:2150–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iracheta-Vellve A, Calenda CD, Petrasek J, Ambade A, Kodys K, Adorini L, Szabo G. FXR and TGR5 Agonists Ameliorate Liver Injury, Steatosis, and Inflammation After Binge or Prolonged Alcohol Feeding in Mice. Hepatol Commun 2018;2:1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie H, Song C, Wang D, Cui S, Ren T, Cao Z, Liu Q, et al. MicroRNA-194 inhibition improves dietary-induced non-alcoholic fatty liver disease in mice through targeting on FXR. Biochim Biophys Acta Mol Basis Dis 2017;1863:3087–3094. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol 2011;179:2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 2008;42:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, et al. Probiotic Lactobacillus rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology 2020;71:2050–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Z, Li F, Liu Y, Jiang M, Zhang L, He L, Wilkey DW, et al. Exosome-Like Nanoparticles From Lactobacillus rhamnosusGG Protect Against Alcohol-Associated Liver Disease Through Intestinal Aryl Hydrocarbon Receptor in Mice. Hepatol Commun 2021;5:846–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Kong B, Zhang M, Rizzolo D, Armstrong LE, Schumacher JD, Chow MD, et al. Enhanced alcoholic liver disease in mice with intestine-specific farnesoid X receptor deficiency. Lab Invest 2020;100:1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song P, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile Acid concentrations and hepatotoxicty in mice. Toxicol Sci 2011;123:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Shimizu H, Hagio M, Fukiya S, Watanabe M, Tanaka Y, Joe GH, et al. 12alpha-Hydroxylated bile acid induces hepatic steatosis with dysbiosis in rats. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158811. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2005;2:217–225. [DOI] [PubMed] [Google Scholar]
- 16.Kim YC, Seok S, Zhang Y, Ma J, Kong B, Guo G, Kemper B, et al. Intestinal FGF15/19 physiologically repress hepatic lipogenesis in the late fed-state by activating SHP and DNMT3A. Nat Commun 2020;11:5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao J, Xiao Z, Kanamaluru D, Min G, Yau PM, Veenstra TD, Ellis E, et al. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev 2009;23:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem 2009;284:11110–11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med 2015;13:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Wang W, Zhang P, Xiao J, Wang J, Huang C. Discrimination of the expression of paralogous microRNA precursors that share the same major mature form. PLoS One 2014;9:e90591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, et al. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016;19:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashid H, Hossain B, Siddiqua T, Kabir M, Noor Z, Ahmed M, Haque R. Fecal MicroRNAs as Potential Biomarkers for Screening and Diagnosis of Intestinal Diseases. Front Mol Biosci 2020;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–265. [DOI] [PubMed] [Google Scholar]
- 24.Gu XX, Xu XX, Liao HH, Wu RN, Huang WM, Cheng LX, Lu YW, et al. Dexmedetomidine hydrochloride inhibits hepatocyte apoptosis and inflammation by activating the lncRNA TUG1/miR-194/SIRT1 signaling pathway. J Inflamm (Lond) 2021;18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G, Zhou H, Yao W, Meng L, Lang B. lncRNA TUG1 Promotes Cisplatin Resistance by Regulating CCND2 via Epigenetically Silencing miR-194–5p in Bladder Cancer. Mol Ther Nucleic Acids 2019;16:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L, Li F, Yin X, Bohman P, Kim S, McClain CJ, Feng W, et al. Profiling of Polar Metabolites in Mouse Feces Using Four Analytical Platforms to Study the Effects Of Cathelicidin-Related Antimicrobial Peptide in Alcoholic Liver Disease. J Proteome Res 2019;18:2875–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natividad JM, Lamas B, Pham HP, Michel ML, Rainteau D, Bridonneau C, da Costa G, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun 2018;9:2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018;67:891–901. [DOI] [PubMed] [Google Scholar]
- 29.Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y, Kalavagunta PK, et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 2019;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009;50:1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020;581:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tveter KM, Villa-Rodriguez JA, Cabales AJ, Zhang L, Bawagan FG, Duran RM, Roopchand DE. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res Care 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang F, Zheng X, Ma X, Jiang R, Zhou W, Zhou S, Zhang Y, et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun 2019;10:4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009;49:1228–1235. [DOI] [PubMed] [Google Scholar]
- 35.Stroeve JH, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest 2010;90:1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciocan D, Voican CS, Wrzosek L, Hugot C, Rainteau D, Humbert L, Cassard AM, et al. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol Ther 2018;48:961–974. [DOI] [PubMed] [Google Scholar]
- 37.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013;8:e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, Coulter S, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol 2018;69:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matye DJ, Li Y, Chen C, Chao X, Wang H, Ni H, Ding WX, et al. Gut-restricted apical sodium-dependent bile acid transporter inhibitor attenuates alcohol-induced liver steatosis and injury in mice. Alcohol Clin Exp Res 2021;45:1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol 2014;306:G929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthiah MD, Smirnova E, Puri P, Chalasani N, Shah VH, Kiani C, Taylor S, et al. Development of Alcohol-Associated Hepatitis Is Associated With Specific Changes in Gut-Modified Bile Acids. Hepatol Commun 2022;6:1073–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology 2009;49:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 2012;303:G32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


