Abstract
The purpose of current research was to assess the apoptotic effects of biofabrication silver nanoparticles (AgNPs) mediated by the aqueous extract of Phlomis armeniaca on human breast cancer cells (MCF-7 and MDA-MB-231) in monolayer (2D) and spheroid (3D) cultures. The biosynthesized AgNPs were characterized by UV–Vis spectrophotometer (the peaks of resonances at 432 nm), scanning electron microscopy (SEM) and energy-dispersive X-ray spectrometry (EDS). 1–20 µM/mL AgNPs were applied to MCF-7 and MDA-MB-231 cell lines to determine IC50 values at 24, 48 and 72nd h and were found to be 10 µM/mL for both cell lines. Immunohistochemical staining results of BrdU, TUNEL, caspase-3 and Endo G in both 2D and 3D cultures and gene expression levels of caspases (caspase-3, -8 and -9) and Endo G were evaluated. Moreover, the total oxidant/antioxidant status (TOS–TAS) due to AgNPs application in both cell culture mediums was evaluated. AgNPs treatment results in both cell lines in both 2D and 3D cultures showed a significant decrease in the BrdU labeling index, while large amounts of cells were labelled with TUNEL and Endo G. In 2D culture, Endo G expression increased in MCF-7 cells at 48 and 72nd hours, while it increased significantly in MDA-MB-231 cells at all hours. OSI results show that ROS production is increased in cell medium treated with AgNPs. In conclusion, AgNPs mediated by Phlomis armeniaca, synthesized by a green method, successfully induced damage to mitochondria, resulting in cell cycle arrest and consequent cell proliferation blockade and death in both MCF-7 and MDA-MB-231 cells.
Keywords: Green synthesis, Silver nanoparticles, MCF-7 cells, MDA-MB-231 cells, Monolayer (2D) culture, Spheroid (3D) culture
Introduction
The breast cancer is the 2nd most commonly occurring in females globally, with only non-melanoma skin cancer being more prevalent (Waks and Winer 2019). In fact, approximately one in every eight women will be diagnosed with breast cancer during their lifetime (Yedjou et al. 2019). The currently available breast cancer treatments are considered to be quite effective, especially if the early detection of disease (Berumen et al. 2018). However, in recent years, researchers have started to assess the use of treatment approaches more commonly used in other types of cancer studies. One such treatment approach is nanomedicine, which has capable of producing alternatives for the prevention of diseases at the cellular degree, treatment and diagnosis (Saxena et al. 2020). The applications of nanotechnology in the field of medicine include the manufacture of assorted anti-viral, anti-bacterial, anti-fungal, anti-plasmodial, anti-inflammatory, anti-diabetic, anti-cancer and anti-oxidant agents (Javed et al. 2020; Pandit et al. 2022).
For the last several decades, biomedical research have focused on the use of different parts of herbs, either in powder formation or as an extract, for nanoparticle (NP) production processes (Dikshit et al. 2021). Biogenic silver nanoparticles (AgNPs) synthesised as a therapeutic material in metal nanoparticles offer the advance of pharmaceutical and biomedical implementation with minimal systemic toxicity as well as improved catalytic activity and stability (Venugopal et al. 2017; Bagur et al. 2020). As each extract used in bioreduction with plants contains different combinations of reducing agents at different concentrations, the properties of the biosynthesised metal NPs (e.g., size, shape and yield) are determined by the content of the plant extract (Khan et al. 2019; Martínez-Cabanas et al. 2021). Phlomis armeniaca Willd., a member of the Lamiaceae family, is an endemic medicinal plant that grows in Turkey. Prior studies have shown that Phlomis armeniaca contains a rich mixture of phytochemicals that provide comprehensive protection against reactive oxygen species (ROS) and oxidants (Konczak et al. 2014; Dalar et al. 2015). Indeed, it has previously been stated that Phlomis armeniaca is a natural antioxidant and antimutagenic source (Yumrutas and Saygideger 2012; Sarikurkcu et al. 2015). ROS formation plays a significant act in the modulation of cellular signalling related to cell proliferation, differentiation and death. It is also relevant to metallic NP-induced toxicity (Abdal Dayem et al. 2017).
The novelty of this research lies in the molecular-level evaluation of the anticancer effects of plant-based biogenic AgNPs, which are considered a potential anticancer candidate, using immunohistochemical and gene expression techniques via monolayer (2D) and spheroid (3D) cultures of human breast cancer cell lines.
Materials and methods
Materials
The commercial dried Phlomis armeniaca used in this study was supplied by the traditional herbal market in Isparta, Turkey. The analytical-grade silver nitrate salt (AgNO3) was supplied by Sigma-Aldrich Chemical Pvt. Ltd. Moreover, aqueous solutions were all prepared with distilled water.
Phlomis armeniaca aqueous extract preparation
To prepare aqueous extract, 2 g of dried Phlomis armeniaca leaves are mixed with 100 mL of distilled water and boiled in a microwave (50 Hz–1200 W) oven for 1 min. Finally, plant leaves were filtered by filter paper and the obtaining filtrate was stored in an amber bottle at 4 °C in the dark until subsequent use.
Green synthesis of the AgNPs
AgNPs prepared by the following procedure: 5 mL of the Phlomis armeniaca leaf aqueous extract was mixed with 95 mL of silver salt (5 mM AgNO3) solution in a flask. The flask was heated in a microwave (50 Hz–1200 W) oven for 1 min. Reaction process was continued by monitoring the modification of the time-dependent colour, which indicated the production of AgNPs. The mixture was stored in the refrigerator for the further analysis.
Characterisation techniques
As mentioned above, during the biosynthesis process, the solution colour changed from yellow to dark brown represented the fabrication of AgNPs. The biosynthesised AgNPs were examined using ultraviolet–visible (UV–Vis) spectroscopy (SHIMADZU® UV-1801; Shimadzu Corp.). The absorption spectrum of the solution was scanned in the wavelength ranging from 300 to 700 nm. The morphology of the AgNPs were characterized by the scanning electron microscopy (SEM) and chemical composition of the biosynthesized AgNPs was determined using energy-dispersive X-ray (EDS) analysis.
Experimental design and cell culture
The present experimental study was conducted under in vitro conditions. The experiments were performed in the Histology and Embryology Department of Suleyman Demirel University’s Faculty of Medicine using both the research laboratory and cell culture laboratory facilities.
The breast cancer cell lines (MCF-7 and MDA-MB-231) acquired from the German Collection of Microorganisms and Cell Cultures (DSMZ) were used in the study. Inactivated 10% fetal calf serum (FCS) (Biological Industries, Israel) and Roswell Park Memorial Institute (RPMI) 1640 (Biological Industries, Israel) containing 100 IU/mL penicillin/streptomycin were used as the medium for the breast cancer cells (MCF-7 and MDA-MB-231). The required cells were produced in 25 cm2 and 75 cm2 flasks containing this medium by placing them in a 37 °C incubator (Heal Force, China) with 5% CO2, 95% air mixture and humidity and routine passages three times a week.
To determine the effective concentration, a total of 5 × 105 cells from both cell lines were cultured live on each of the six-well plates into which 5 mL of the RPMI 1640 medium was added. AgNPs were applied to the MCF-7 and MDA-MB-231 cells at concentrations of 1–20 µM/mL. For each dose, the seeding was performed in three wells for all of the groups at 24, 48 and 72nd h and they were incubated at 37 °C in a 5% CO2 air mixture in a humid environment. At the end of each period, the cells were collected and treated with the trypan blue. The cytotoxicity of the cell lines and the IC50 (half-maximum inhibitory concentration) of AgNPs were determined by a hemocytometer-based trypan blue dye exclusion cell count. Based on the average cell count were obtained after counting the cells by three different individuals. Then, two groups were formed for both the MCF-7 and MDA-MB-231 cells, namely, the control group and the AgNPs group. Next, bromodeoxyuridine (BrdU) labelling, terminal deoxynucleotidyl transferase (tdt)-mediated dUTP nick-end labeling (TUNEL), endonuclease G (Endo G) and active caspase-3 immunohistochemical staining were performed in the two-dimensional (lamellar cultivation) and three-dimensional (spheroidal model) culture media, which were then evaluated using light microscopy. In addition, the expression levels of the caspase-3, caspase-8, caspase-9 and Endo G genes were measured. Moreover, the total antioxidant status (TAS), total oxidant status (TOS) and oxidative stress index (OSI) levels in the cell culture medium were measured.
Immunohistochemical labelling with bromodeoxyuridine
Deoxyribonucleic acid (DNA) synthesis (S phase) analyses of the immunohistochemical labelling were performed using BrdU methods. The AgNPs were treated with coverslip cultivation in a 24-well plate using a 10 µM/mL dose (as calculated during the dose determination experiment). At the end of all times (24, 48 and 72nd hours), the cells were incubated with BrdU for 60 min at 37 °C. The cells were then fixed by means of incubation with phosphate buffer saline (PBS) and 70% ethanol for the monolayer culture. The spheroids were removed, fixed and washed using tap water for the spheroid culture. Then, it was incubated with ethyl alcohol batches and xylene, buried in paraffin, cut using a microtome and applied to a poly-l-lysine-coated slide. The remaining treatments were carried out as defined by Bayram et al. (2017) for both the monolayer and spherical cultures.
Immunohistochemical labelling with caspase-3
Round coverslips were fitted in a 24-well plate and 1 × 105 cells per well were seeded onto the coverslips. The AgNP dose calculated as 10 µM/mL was used in dose determination studies. At the end of all times (24, 48 and 72nd hours), the cells were incubated with BrdU at 37 °C for 60 min. The cells were fixed by incubation with PBS and 70% ethanol for monolayer cultures, respectively. Cells incubated with PBS, washed with distilled water and inhibited in 3% hydrogen peroxide in methyl alcohol. 10 min after this application, cells were incubated for 30 min in 4N-hydrochloric acid (HCl). The remaining procedure were performed as described in Bayram et al. (2018) publication for both monolayer and spherical cultures with using primary antibody (active caspase-3) (Invitrogen Co. Canada), secondary antibody (Biotinylated Goat Anti-Mouse).
Immunohistochemical labelling with Endo G
MCF-7/MDA-MB-231 cells (1 × 105 cells per well) were seeded in 24-well plate and treated with 10 µM/mL AgNPs. This procedure was performed similar to the protocol for staining with activated Caspase-3. Differently, Endo G primary antibody was used in the primary antibody application step for monolayer culture. The protocol for spherical culture of immunohistochemical marking with active caspase-3 was applied to the sections taken on separate slides for the 24, 48 and 72nd hours. Differently, Endo-G primary antibody was used at the stage of primary antibody application.
Terminal deoxynucleotidyl transferase (TDT)-mediated dUTP nick-end labelling (TUNEL) assay
The TUNEL method is used for detecting apoptosis. “ApopTag Plus Peroxidase in situ apoptosis kit (Millipore)” was used for this procedure. MCF-7/MDA-MB-231 cells (1 × 105 cells per well) were seeded in 24-well plate and treated with 10 µM/mL AgNPs. The medium was then removed and cells were pre-fixed with paraformaldehyde and incubated into 2:1 ethyl alcohol–acetic acid at − 20 °C for 5 min. The procedure for spheroid cultures was the same as described above. The remaining procedure was performed as described in Bayram et al. (2018) publication for both monolayer and spherical cultures.
Histological analysis (H&E staining) for spheroid culture
The cell samples were separated by microtome, were de-paraffinized in xylol, then were stained with Hematoxylin–Eosin (H&E).
Gene expression
Total ribonucleic acid (RNA) isolation was accomplished using PureZOL (Trizol, Bio-Rad) from 1 × 106 cells obtained without trypsin. Concentration and purity of RNA were measured in Multiskan Go (Thermo Fisher Scientific, USA). RNA purity was between 1.8 and 2.0 (OD260/OD280) and the integrity was evaluated by agarose gel electrophoresis as Çelik et al. (2020) described. 1 µg of RNA for cDNA was used and synthesized cDNA was diluted 1/20 for each reaction. cDNA synthesis was performed using the iScript Reverse Transcription Supermix cDNA Synthesis Kit (Bio-Rad). Then, the kit protocol was applied in the thermal cycler. Expression levels of the caspase-3, caspase-8, caspase-9 and Endo G genes were measured on the Qiagen RotorGene Q real-time PCR instrument with the iTaq SYBR Green Supermix kit (Bio-Rad). Reaction optimization was performed according to the ct values of the PUM1 reference (housekeeping) gene. Each sample was run in three replications.
Total oxidant/antioxidant status assay
Samples taken from the media of cells that were not treated with silver nanoparticles in consecutive hours (24, 48 and 72nd hours) are the control of samples taken from the media of the cells that were treated. The kits (Rel Assay Diagnostics kit, Mega Tip, Gaziantep, Turkey) were used to measure TOS and TAS parameters in the specimens taken from the cell culture medium. Following the manufacturer's instructions, the medium on the cells (liquid medium collected from the cells) was transferred to the falcon and stored at − 80 °C and studied directly (such as serum) (Erel 2004, 2005). The parameters were performed by spectrophotometric method using Epoch 2 microplate reader (Biotek® Instruments, USA) in accordance with the producer's instructions. TOS results were expressed in μmol H2O2 equivalent/L. TAS results were expressed as mmol Trolox equivalent/L. An indicative parameter of the oxidative stress level, oxidative stress index (OSI), is the ratio of TOS to TAS and is calculated from next equation:
Statistical analysis
Experimental data were offered as mean ± standard error/standard deviation. The Kruskal–Wallis analysis of variance test was used to evaluate the significance of the difference between the groups in SPSS 18 software. The difference between two independent groups consisting of continuous variable values was determined with the Mann–Whitney U test. The limit of significance was accepted as p < 0.05. Real-time qPCR data were evaluated using the REST2009 program, normalizing to the reference gene and using the ΔΔCt method. One-way ANOVA was used for intergroup comparison in TAS and TOS analyses.
Results
Evaluation of biosynthesis and characterization of AgNPs
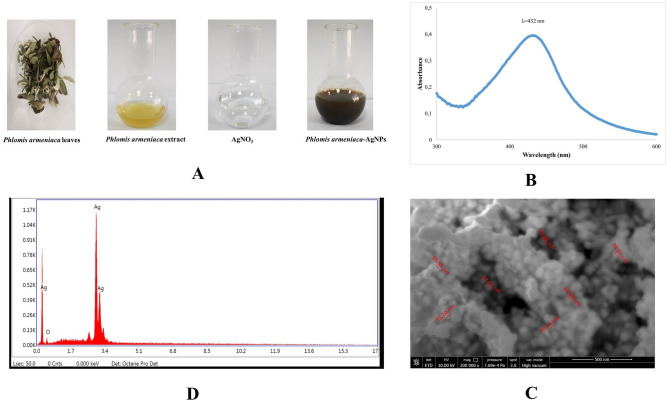
AgNPs were synthesized through the bioreduction of AgNO3 solution by aqueous extract of Phlomis armeniaca leaves. Figure 1A shows the change in colour from yellow to dark brown due to the reduction of Ag2+ to Ag0 as time-dependent formation of silver nanoparticles. The broad absorption peak was monitored at 432 nm (Fig. 1B), in the evaluation of the absorption scanning of AgNPs ranging in the 300–700 nm with UV–Vis spectrophotometer. In Fig. 1C, the different particle size and cubic/square surface structure of AgNPs labelled in the 38.20–62.88 nm range are demonstrated by SEM. According to EDS, the presence of Ag ion peaks in the elemental composition of biosynthesised AgNPs was detected. The EDS spectrum from AgNPs was showed a multiple characteristic energy line corresponding to Ag at the largest 3.4 keV (Fig. 1D).
Fig. 1.
A Biosynthesis of AgNPs (time dependent colour change), B UV–Vis spectrum of biosynthesized AgNPs, C SEM image of biosynthesized AgNPs, D EDX spectrum of AgNPs
Cytotoxicity and cell proliferation assays
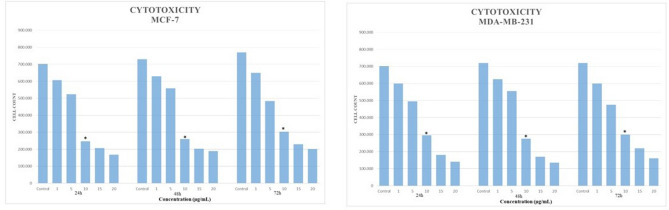
After the evaluation by recording the total cell counts at 24, 48 and 72nd hours, the IC50 value for AgNPs applied to MCF-7 and MDA-MB-231 cell lines was determined as 10 µM/mL (Fig. 2). Representative data are presented by repeating all experimental studies 3 times. The cell count increased in the control group proportionally as a function of the 72 h incubation time. AgNPs administered groups inhibited cell proliferation of MCF-7 and MDA-MB-231 cells for all time intervals compared with the control group (p < 0.05) (Fig. 3).
Fig. 2.
Cytotoxicity of AgNPs in MCF-7 and MDA-MB-231 cell lines. *Statistically significant when compared to the internal control group
Fig. 3.
Cell proliferation data over time for MCF-7 and MDA-MB-231 cells
DNA synthesis analysis of monolayer (2D) cultures
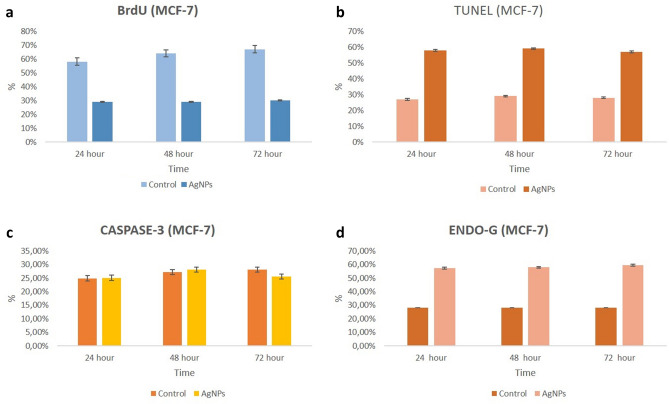
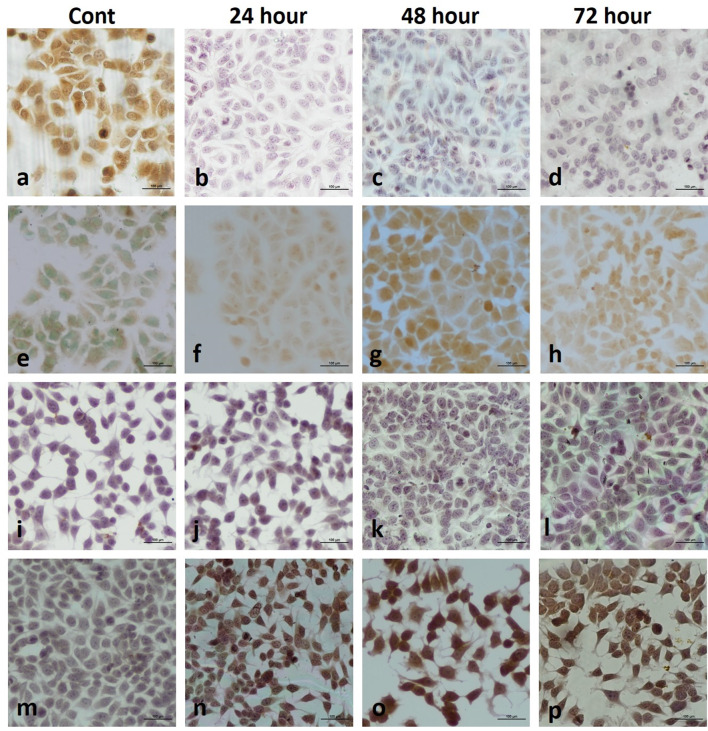
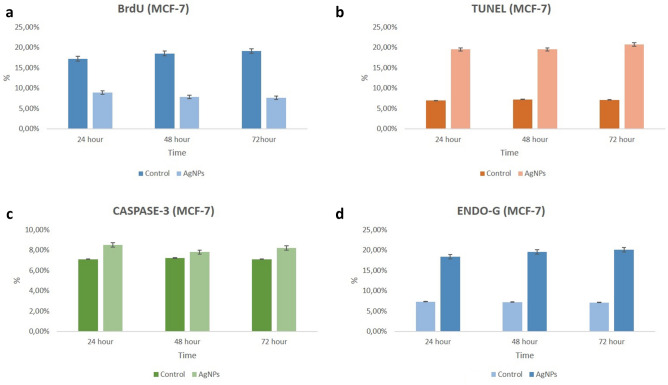
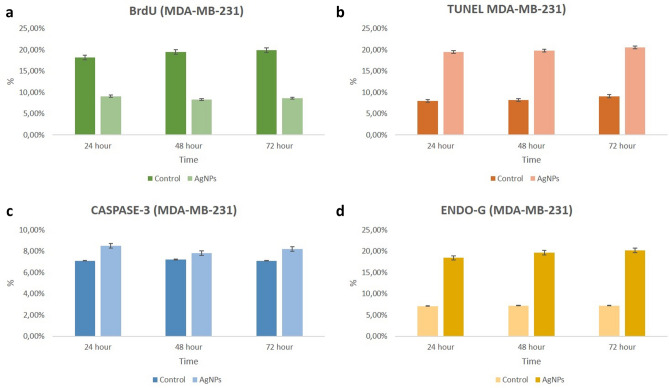
Analysis of DNA synthesis (S-phase of cell cycle) in human breast cancer cells was evaluated by BrdU labelling. To be considered BrdU-positive, there must be brown-stained nucleated cells representing BrdU uptake. Accordingly, MCF-7 and MDA-MB-231 cell nuclei were evaluated as positive for BrdU at all hours (24, 48 and 72nd hours) in the control group. BrdU labeling index decreased significantly at all hours (24, 48 and 72nd hours) in both MCF-7 and MDA-MB-231 cells treated with AgNPs compared to the control group (p < 0.05) (Figs. 4, 5, 6, 7).
Fig. 4.
Two-dimensional cell culture staining in MCF-7 cells a BrdU; b TUNEL; c Caspase-3; d Endo G
Fig. 5.
Two-dimensional cell culture in MCF-7 cells. BrdU staining (a control, AgNPs-treated groups; b 24 h, c 48 h, d 72 h); TUNEL staining (e control, AgNPs-treated groups; f 24 h, g 48 h, h 72 h); Caspase-3 staining (i control, AgNPs-treated groups; j 24 h, k 48 h, l 72 h); Endo G staining (m control, AgNPs-treated groups; n 24 h, o 48 h, p 72 h). One picture as a representative for internal controls were used for all three time intervals
Fig. 6.
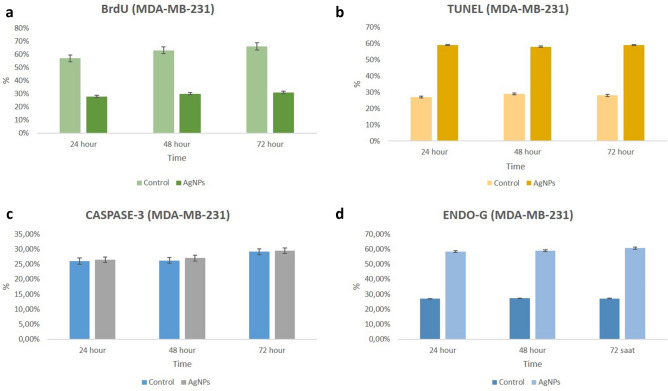
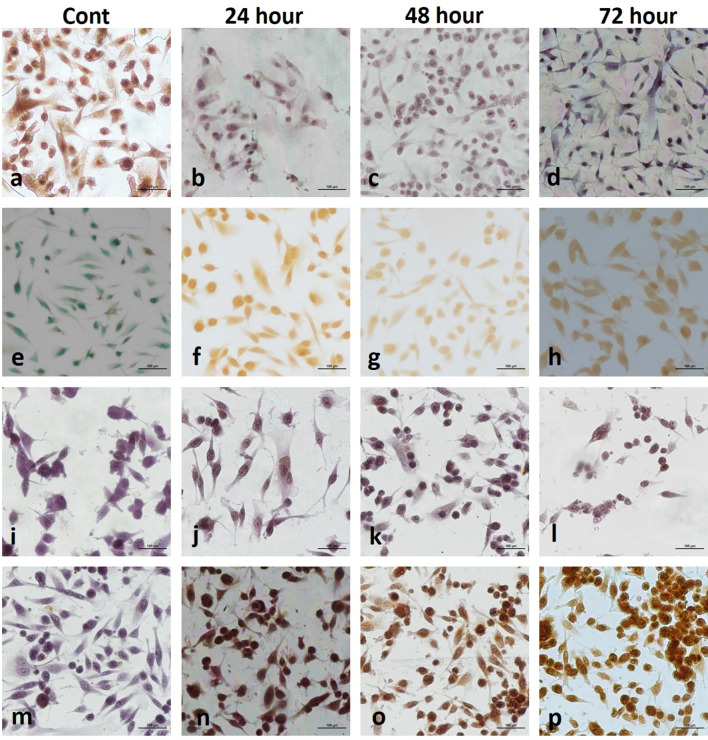
Two-dimentional cell culture staining in MDA-MB-231 cells a BrdU; b TUNEL; c Caspase-3; d Endo G
Fig. 7.
Two-dimensional cell culture in MDA-MB-231 cells. BrdU staining (a control, AgNPs-treated groups; b 24 h, c 48 h, d 72 h); TUNEL staining (e control, AgNPs-treated groups; f 24 h, g 48 h, h 72 h); Caspase-3 staining (i control, AgNPs-treated groups; j 24 h, k 48 h, l 72 h); Endo G staining (m control, AgNPs-treated groups; n 24 h, o 48 h, p 72 h). One picture as a representative for internal controls were used for all three time intervals
H&E staining for 3D cell culture
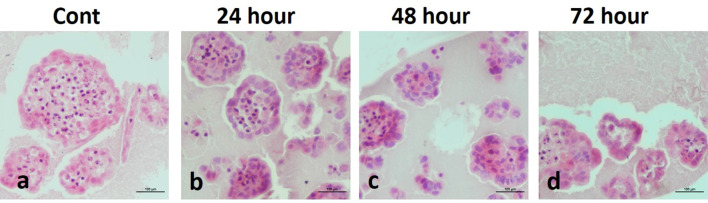
The spheroid structures of the control group were observed in a very smooth appearance at all hours (24, 48, 72nd hours) in the light microscopic examination (Figs. 8, 9). On the other hand, in the AgNPs groups, scattered spheroid structures with distorted borders were observed besides the smooth-looking spheroids. In addition, degeneration was observed in some spheroid structures (Figs. 8, 9).
Fig. 8.
H–E staining in MCF-7 cells (a control, AgNPs-treated groups; b 24 h, c 48 h, d 72 h) (×20)
Fig. 9.
H–E staining in MDA-MB-231 cells (a control, AgNPs-treated groups; b 24 h, c 48 h, d 72 h) (×20)
DNA synthesis analysis of spheroid (3D) cultures
According to the BrdU labeling index evaluations performed in three-dimensional multicellular spheroid model culture, in the control group (both MCF-7 and MDA-MB-231 cells), a large number of labeling was observed at the periphery of the spheroids, and a small number of labeling was observed in the spheroid centre. Consistent with our two-dimensional culture results, a large number of cells were labelled with BrdU (Figs. 10, 11, 12, 13).
Fig. 10.
Three-dimentional cell culture staining in MCF-7 cells a BrdU; b TUNEL; c Caspase-3; d Endo G
Fig. 11.
Three-dimentional cell culture staining in MCF-7 cells. BrdU staining (a control, AgNPs-treated groups; b 24 h, c 48 h, d 72 h); TUNEL staining (e control, AgNPs-treated groups; f 24 h, g 48 h, h: 72 h); Caspase-3 staining (i control, AgNPs-treated groups; j 24 h, k 48 h, l 72 h); Endo G staining (m control, AgNPs-treated groups; n 24 h, o 48 h, p 72 h). One picture as a representative for internal controls were used for all three time intervals
Fig. 12.
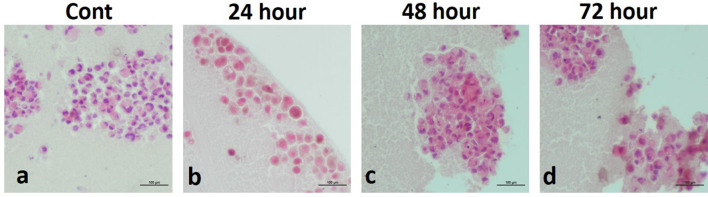
Three-dimentional cell culture staining in MDA-MB-231 cells a BrdU; b TUNEL; c Caspase-3; d Endo G
Fig. 13.
Three-dimentional cell culture staining in MDA-MB-231 cells. BrdU staining (a control, AgNPs-treated groups; b 24 h c 48 h, d 72 h); TUNEL staining (e control, AgNPs-treated groups; f 24 h, g 48 h, h 72 h); Caspase-3 staining (i control, AgNPs-treated groups; j 24 h, k 48 h, l 72 h); Endo G staining (m control, AgNPs-treated groups; n 24 h o 48 h, p 72 h). One picture as a representative for internal controls were used for all three time intervals
In the AgNPs group, as in the lamellar groups, there was a significant decrease in BrdU labeling index at all hours in all experimental groups compared to the control group (p < 0.05) (Figs. 10, 11, 12, 13).
Caspase-3 level analysis for monolayer (2D) cultures
In the immunohistochemical staining with Caspase-3 at the end of the 24th, 48th and 72nd hours in accordance with the experimental plan; Caspase-3 labeling was observed to be very weak in the control group at all hours (Figs. 4, 5, 6, 7).
In the groups applied 10 mM/mL AgNPs; it was observed that a small number of cells were marked with Caspase-3 in both cell lines at all hours. In this case, no significant difference was observed when compared with the control group (Figs. 4, 5, 6, 7).
Caspase-3 level analysis for spheroid (3D) cultures
In the three-dimensional spheroid culture, in accordance with our two-dimensional culture results; very few cells in the control groups were labelled with Caspase-3 (p < 0.05) (Figs. 10, 11, 12, 13).
In the AgNPs groups given for all times (24, 48 and 72nd hours), a smaller mean number of cells were marked with Caspase-3, similar to the control group, in both MCF-7 and MDA-MB-231 cells. There was no significant difference between the control and experimental groups in both cell lines (Fig. 10, 11, 12, 13).
Endo G level analysis for monolayer (2D) cultures
In the immunohistochemical staining with Endo G at the end of the 24, 48 and 72nd hours in two-dimensional culture; very few cells in the control group were observed to be marked with Endo-G at all hours (Figs. 4, 5, 6, 7).
In the groups applied 10 mM/mL AgNPs; it was determined that more cells were marked with Endo G (p < 0.05) in both cell lines compared to the control at all hours (Figs. 4, 5, 6, 7).
Endo G level analysis for spheroid (3D) cultures
In the three-dimensional spheroid culture, in accordance with our two-dimensional culture results; very few cells in the control groups (MCF-7 and MDA-MB-231 cells) were labelled with Endo G (p < 0.05) (Figs. 10, 11, 12, 13).
AgNPs groups treated with all cell lines for 24, 48 and 72nd hours showed more cells labelling with Endo G compared to the control group (p < 0.05), which was also consistent with the cells in the two-dimensional medium (Figs. 10, 11, 12, 13).
TUNEL analysis for monolayer (2D) cultures
Monolayer cultures were used to identify the susceptibility variations in the molecular process induced by AgNPs for 24, 48 and 72nd hours to confirm the mechanism of cell death in the cell lines (MCF-7 and MDA-MB-231). TUNEL positive cell indexes of treated AgNPs were significantly different compared to TUNEL positive cell control indices at all time periods (Figs. 4, 5, 6, 7).
TUNEL analysis for spheroid (3D) cultures
Spheroids treated with AgNPs appear to have more TUNEL positive cells compared to control. These findings obtained in spheroid culture support our results that the spheroids of the AgNPs-treated group in monolayer culture indicated more TUNEL positive MCF-7 and MDA-MB-231 cells (Figs. 10, 11, 12, 13).
Gene expression assay
According to the findings of gene expression determination in two-dimensional culture, no increase in caspase-3 gene expression was observed in both MCF-7 and MDA-MB-231 cells at all time periods. An increase in caspase-8 and caspase-9 gene expressions was observed in MCF-7 cells at all hours, but this increase was not statistically significant. There was a slight increase in caspase-8 gene expressions at 24 and 48 h in MDA-MB-231 cells, which was not statistically significant. There was no increase in caspase-8 gene expression at 72nd hours in MDA-MB-231 cells. A slight increasing was in caspase-9 gene expressions in MDA-MB-231 cells at all time periods, which was not statistically significant. There was no increment in caspase-9 gene expression at the 72nd hours in MDA-MB-231 cells (Fig. 14).
Fig. 14.
Gene expression determination in MCF-7 and MDA-MB-231 cells
Endo G gene expression was increased in MCF-7 cells at all time periods in two-dimensional culture. While this increase was not statistically significant for 24 h compared to the control group, it was significant for 48 and 72nd hours (p < 0.05). A statistically significant increase in Endo G gene expression was observed in MDA-MB-231 cells at all time periods compared to the control group (p < 0.05) (Fig. 14).
Oxidant/antioxidant status with AgNPs therapy
The total oxidant/antioxidant status were measured with spectrophotometrically in accordance with the procedure. To investigate the effect on the oxidant/antioxidant status, cells from both cell lines were treated with 10 μg/mL AgNPs for 24, 48 and 72nd hours. Cells not treated with AgNPs at the same time were taken as controls. TAS levels, compared to its control at the same time frame, were significantly increased by AgNPs treatment applied to MCF-7 and MDA-MB-231 cells (Table 1). Similarly, AgNPs treatment caused a significant increase in TOS activity when compared to own control at the same time frame. It is also seen that AgNPs therapy causes an increase in TOS depending on time (Table 1). OSI production levels were calculated with the ratio of TOS to TAS. The results showed that the OSI were increased in AgNPs therapy groups. Compared to the control group, statistically significant changes were observed in OSI values depending on time, and the highest rising was observed at 72 h in both cell lines (Table 1).
Table 1.
TOS, TAS and OSI values of samples
| GROUPS | TAS (mmol Trolox eq/L) | TOS (μmol H2O2 eq/L) | OSI (AU) |
|---|---|---|---|
| MDA C24 | 83.40 ± 0.83 | 4016.66 ± 76.37 | 4.81 ± 0.13 |
| MDA C48 | 110.33 ± 1.44 | 2200 ± 33.33 | 1.99 ± 0.00 |
| MDA C72 | 117.77 ± 3.42 | 2344.44 ± 38.49 | 1.99 ± 0.08 |
| MDA AgNP24 | 105.91 ± 2.61● | 7486.88 ± 396.35● | 7.06 ± 90.20● |
| MDA AgNP48 | 383.48 ± 7.01● | 23,660.66 ± 0.001● | 6.17 ± 0.11● |
| MDA AgNP72 | 274.03 ± 7.26● | 25,660.66 ± 1732.05● | 9.36 ± 0.54● |
| MCF C24 | 119.01 ± 5.22 | 2530.33 ± 204.59 | 2.12 ± 0.21 |
| MCF C48 | 124.15 ± 1.14 | 2860 ± 103.92 | 2.30 ± 0.09 |
| MCF C72 | 113.97 ± 3.47 | 2522.22 ± 50.91 | 2.21 ± 0.06 |
| MCF AgNP24 | 304.79 ± 3.86● | 8431.33 ± 199.30● | 2.76 ± 0.07● |
| MCF AgNP48 | 493.44 ± 5.20● | 23,883.88 ± 386.63● | 4.84 ± 0.10● |
| MCF AgNP72 | 380.66 ± 0.72● | 25,220.22 ± 190.71● | 6.62 ± 0.06● |
MDA-MB-231 and MCF-7 Controls (MDA C and MCF C) refer to untreated group; MDA-MB-231and MCF-7 AgNP (MDA AgNP and MCF AgNP) refer to 10 µM/mL AgNPs treated group
●p < 0.005 AgNPs vs Control (C) at the same time
Discussion
Biological synthesis from medicinal plants is accepted as the most suitable method for metal nanoparticle synthesis when compared with physical and chemical means. Due to the toxicity problems of current therapeutic approaches, the synthesis method of AgNPs from medicinal plants by green synthesis is gaining importance (Chandra et al. 2020). The term “green” is used to describe the synthesis of nanoparticles from metal salts using the reducing property of biologically active compounds of plants/microorganisms (Parveen et al. 2016; Hussain et al. 2015). The biomolecules in plants mediate the easy bioreduction of metal ions into nanoparticles (Makarov et al. 2014; Hano and Abbasi 2022). The development of the green synthesis of AgNPs has received worldwide attention in medical science and disease therapy (Mousavi et al. 2018).
In current study, the biosynthesis of AgNPs by means of the green pathway was performed using Phlomis armeniaca extract. Bio-components in the plant extract are in charge for the reduction of Ag+ ions to Ag0 in a single step. The existence of phenolic profiles, the total phenol and flavonoid content, and the free radical scavenging activity of Phlomis armeniaca extract have previously been reported (Saracoglu et al. 1995; Karakas and Turker 2016). Sarikurkcu et al. (2015) reported that investigating three different Phlomis armeniaca extracts (ethyl acetate, methanol, and water) for total phenolic, flavonoid, flavonol and saponin contents. In addition, the chelating effects and reducing powers of the extracts were determined in the same research. The findings of this study indicate the anticancer activities of AgNPs synthesised using Phlomis armeniaca, which represents a green and cost-effective method of synthesis.
The physicochemical characterisation of the NPs was performed prior to the in vitro studies, as physicochemical properties are known have a significant effect on biological properties (Shin et al. 2015). In current study, a colour change from yellow to dark brown was monitored during observation of the reaction after adding Phlomis armeniaca extract to AgNO3 and the application of heat via a microwave (Fig. 1A). The optical properties of AgNPs depend on the size, shape and aggregation of the NPs in the solution, which are controlled by the surface plasmon resonance (SPR), with a colour change from yellow to red being indicative of this relation (Jahed and Hamidi 2020; Nilghaz et al. 2021). The AgNPs were characterised by means of UV–Vis spectroscopy, which has been proven to be a highly useful and valuable technique for the analysis of NPs (Gurunathan et al. 2013a, b). The AgNPs formed via Phlomis armeniaca showed an absorption peak at 420 nm in the UV–Vis spectrum, which corresponded to the plasmon resonance peak of the AgNPs in current study (Fig. 1B). The SEM images of the AgNPs revealed cubic structural features, mostly at a dimension below 100 nm (labelled in the 38.20–62.88 nm range), as shown in Fig. 1C. The results of element mapping showed the presence of silver element predominantly in the relevant sample (Fig. 1D). The EDS spectrum from AgNPs shows a characteristic energy line corresponding to plant-derived oxygen (O) (0.5 keV), indicative of green synthesis of AgNPs, while at the same time multiple energies corresponding to Ag at the largest 3.4 keV confirmed the line. (Fig. 1D). The SEM–EDS images were similar to those obtained in prior biosynthetic AgNP investigations (Kumar et al 2014; Chaudhari and Ingale 2016). In the current study, it is seen that AgNPs were successfully biosynthesized by Phlomis armeniaca extract as a reducing agent without the need for any component other than Phlomis armeniaca, which is an endemic plant in Turkey. As seen in the literature examples above, although many herbal extracts have been used in the biosynthesis of AgNPs, Phlomis armeniaca is not one of those extracts.
The AgNPs synthesised by means of the green pathway were evaluated in terms of their anti-cancer activity in both cell lines (MCF-7 and MDA-MB-231) using a variety of cytotoxicity, proliferation, gene expression and immunohistochemical assays, which assessed the cytoplasmic membrane, mitochondrial and DNA changes. In vitro studies have shown that AgNPs biosynthesised using medicinal plant extracts exhibit potent cytotoxic and antiproliferative effects against tumour cells (Salman et al. 2021; Yeşilot and Dönmez 2021; Simsek et al. 2021). The cytotoxic and anticancer activities of Phlomis armeniaca extract against various cancer cells have previously been reported in the literature (Karakas and Turker 2016; Turker and Yıldırım 2013). However, no prior study has examined the anticancer and antiproliferative effects of AgNPs synthesised from Phlomis armeniaca extract.
AgNPs are currently being studied as an alternative option for cancer treatments in different cancer models due to their specific biophysicochemical properties. A study conducted on the MCF-7 and MDA-MB-231 cell lines found that biosynthetic AgNPs induced dose-related cytotoxicity after 72 h of exposure, with the IC50 doses being 3,859 μg/mL and 1,128 μg/mL, respectively (Satpathy et al. 2018). Ullah et al. (2020) determined the IC50 values of AgNPs synthesised using Fagonia indica extract to be 12.35 μg/mL in the MCF-7 cell line and 25.09 μg/mL in cells treated with the extract. Fard et al. (2018) found the IC50 values of NPs to be 8.76 and 5.0 μg/mL after 24 and 48 h of treatment in the MCF-7 cell line, respectively. Erdogan et al. (2019) reported that cell growth, cell viability and cell migration via free radical formation in MCF-7 cells were all inhibited by AgNPs with an IC50 dose of approximately10 μg/mL. Similarly, in the current study, the IC50 dose was used as approximately 10 μg/mL.
It is reported that spheroid cultures have in vivo-like tissue culture characteristics and are the best adapted model to evaluate the in vitro resistance specifications of cells. These features of the spheroids are thought to indicate the applied agent effects quite realistically, including restrictions in cell signal feedback mechanisms (Bayram et al. 2019). Given the assumption that substances capable of affecting the cell cycle may have anticancer properties, it is highly important to evaluate the impacts of NPs on the cell cycle (Alimbetov et al. 2018; Taha 2022). In a study in which AgNPs were synthesised from 58 different plants, it was determined that some of the green synthesised AgNPs exhibited an anticancer effect by inducing the arrest of the S1 cycle phase (Ahn and Park 2020). In addition, El-Deeb et al. (2022) reported that the biogenic gold nanoparticles (AuNPs) they synthesized exhibited anticancer effects by arresting MCF-7 cells in the S phase and increasing the cellular population in the sub-G0 (apoptotic) phase. Moreover, a prior study found that biosynthetic AgNPs elicit apoptosis against MCF-7 cancer cells during the S phase of the cell cycle (Khader et al. 2020). In the current study, the BrDU method was used to observe cell proliferation in the monolayer (2D) and spheroid (3D) cultures during the DNA synthesis phase, while the TUNEL method was used to observe possible apoptosis (Figs. 4, 5, 6, 7; 10, 11, 12, 13). The present study is the first to demonstrate the anticancer effect of AgNPs in 2D and 3D cultures using immunohistochemistry and gene expression techniques in breast cancer cell lines.
Previous studies have evaluated various cellular parameters related to the cellular uptake of AgNPs, with cell membrane damage, DNA damage, mitochondrial damage and apoptosis being considered to be among the possible cytotoxic mechanisms of AgNPs (Morais et al. 2020). Among these proposed toxicity mechanisms, an increase in ROS production and the induction of apoptosis appear to be the most common. The toxicity caused within the mitochondria by AgNPs mainly stems from the increase in ROS production (Nayak et al. 2016; Cameron et al. 2018). The production of ROS, which forms part of the cytotoxic mechanism of metallic NPs, causes DNA damage, cell cycle arrest, apoptosis/necrosis and the inhibition of cell proliferation (De Matteis et al. 2018). Mitochondrial phosphorylation-associated ROS production is also involved in pro-apoptotic mitochondrial events during tumour cell processes, whereby the cells tend toward apoptosis (Ferrín et al. 2011). In our study, it was observed that, as the parameter of the oxidative stress level, OSI increased significantly especially at the 72nd hour in the samples taken from the cell culture medium (Table 1). These results suggest that the production of ROS was released from cells treated with AgNPs.
Most prior studies have shown that the cell damage caused by AgNPs guides to cell death and, therefore, apoptosis. The pathways regulated by caspase-9 (intrinsic pathway) and caspase-8 (extrinsic pathway) are the two main signalling pathways that typically induce apoptosis (Ullah et al. 2020; Al-kawmani et al. 2020). AgNPs mainly cause damage to a cell’s nucleus, cytoplasmic membrane and mitochondria. Such damage leads to cell cycle arrest and, ultimately, apoptosis (Saravanakumar et al. 2018). In the literature, it has been reported that the treatment of green synthesised AgNPs changed the expression of apoptotic proteins and inflammatory markers, such as cyclooxygenase-2 in MCF-7 cells (Rohini et al. 2019; Shariq Ahmed et al. 2019). Furthermore, Gurunathan et al.’s studies (2013a, 2015; b) involving biosynthetic AgNPs in MDA-MB-231 breast cancer cells revealed that the lactate dehydrogenase leakage, ROS activation and dose-dependent toxic effect of AgNPs confirmed by the TUNEL-positive cells served to induce cellular apoptosis via various signalling pathways. The specific type of caspase-independent programmed cell death may contain specific mitochondrial ingredients. It has been indicated that oxidative stress can spark neuronal caspase-independent cell death mediated by Endo G (Higgins et al. 2009). Endo G, a mitochondrial nuclease that acts as an apoptotic endonuclease, is released from mitochondria when sparked by cell death stimuli. In addition, it is claimed to have a role in apoptotic process and nuclear DNA fragmentation (Li et al. 2001). Lemarié et al. (2004) showed that cadmium induces apoptosis in Hep3B cells by the disruption of mitochondria due to calcium and oxidative stress, which promotes Endo G and the release of apoptosis-inducing factor. Significantly increased Endo G gene expression in both cell lines is also in line with our other findings related to oxidative stress promoting caspase-independent apoptosis. The results of the current study also indicate caspase-independent endo-G-mediated cell death due to mitochondrial damage, which supports the immunohistochemical data (Fig. 14).
From the findings, it was concluded that AgNPs synthesized via Phlomis armeniaca exhibited multifunctional properties on breast cancer cell lines. We have demonstrated AgNPs therapy activates scavenging of reactive oxygen species and modulation of mitochondria-mediated apoptotic pathway in MCF-7 and MDA-MB-231 breast cancer cells.
Conclusions
AgNPs mediated by Phlomis armeniaca, synthesized by a green method, successfully induced damage to mitochondria, resulting in cell cycle arrest and consequent cell proliferation blockade and death in both MCF-7 and MDA-MB-231 cells. This is the first study to evaluate the in vitro anticancer effects of AgNPs for their apoptotic effects in human breast cancer lines using immunohistochemical and gene expression techniques via monolayer (2D) and spheroid cultures (3D). In the future, the cytotoxic potential of these AgNPs may be promising for theranostic applications in breast cancer patients. Therefore, these data may provide guidance for evaluating the potential of these AgNPs as anticancer agents for different stages of breast cancer disease.
Author contributions
SY: designed the study, performed the synthesis and characterization of nanoparticles, investigated biochemical experiments, involved in statistical analysis and drafted the paper. DB: designed the study and methodology, performed cell culture experiments, investigated histological and immunohistochemical assays, involved in statistical analysis. MO: performed cell culture experiments, investigated histological and immunohistochemical assays, visualizationed the results. VAT: performed molecular experiments, visualizationed the results, involved in statistical analysis. All authors have read and approved the final manuscript.
Funding
No funds, grants, or other support was received.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Abdal Dayem A, Hossain MK, Lee SB, Kim K, Saha SK, Yang GM, Choi HY, Cho SG. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci. 2017;18(1):120. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn EY, Park Y. Anticancer prospects of silver nanoparticles green-synthesized by plant extracts. Mater Sci Eng C Mater Biol Appl. 2020;116:111253. doi: 10.1016/j.msec.2020.111253. [DOI] [PubMed] [Google Scholar]
- Alimbetov D, Askarova S, Umbayev B, Davis T, Kipling D. Pharmacological targeting of cell cycle, apoptotic and cell adhesion signaling pathways implicated in chemoresistance of cancer cells. Int J Mol Sci. 2018;19(6):1690. doi: 10.3390/ijms19061690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-kawmani AA, Alanazi KM, Farah MA, Ali MA, Hailan WAQ, Al-Hemaid FMA. Apoptosis-inducing potential of biosynthesized silver nanoparticles in breast cancer cells. J King Saud Univ Sci. 2020;32:2480–2488. doi: 10.1016/j.jksus.2020.04.002. [DOI] [Google Scholar]
- Bagur H, Medidi RS, Somu P, Choudhury PWJ, Karua CS, Guttula PK, Melappa G, Poojari CC. Endophyte fungal isolate mediated biogenic synthesis and evaluation of biomedical applications of silver nanoparticles. Mater Technol. 2020;37(3):167–178. doi: 10.1080/10667857.2020.1819089. [DOI] [Google Scholar]
- Bayram D, Çetin E, Kara M, Özgöçmen M, Candan I. The apoptotic effects of silibinin on MDA-MB-231 and MCF-7 human breast carcinoma cells. Hum Exp Toxicol. 2017;36(6):573–586. doi: 10.1177/0960327116658105. [DOI] [PubMed] [Google Scholar]
- Bayram D, Armagan İ, Özgöcmen M, Senol N, Calapoglu M. Determination of apoptotic effect of juglone on human bladder cancer TCC-SUP and RT-4 cells: an in vitro study. J Environ Pathol Toxicol Oncol. 2018;37(2):173–181. doi: 10.1615/JEnvironPatholToxicolOn. [DOI] [PubMed] [Google Scholar]
- Bayram D, Özgöçmen M, Armagan I, Sevimli M, Türel GY, Şenol N. Investigation of apoptotic effect of juglone on CCL-228-SW 480 colon cancer cell line. J Can Res Ther. 2019;15:68–74. doi: 10.4103/jcrt.JCRT_880_17. [DOI] [PubMed] [Google Scholar]
- Berumen A, Jimenez Moyao G, Rodriguez NM, Ilbawi AM, Migliore A, Shulman LN. Defining priority medical devices for cancer management: a WHO initiative. Lancet Oncol. 2018;19(12):e709–e719. doi: 10.1016/S1470-2045(18)30658-2. [DOI] [PubMed] [Google Scholar]
- Cameron SJ, Hosseinian F, Willmore WGA. Current overview of the biological and cellular effects of nanosilver. Int J Mol Sci. 2018;19:2030. doi: 10.3390/ijms19072030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelik DA, Gurbuz N, Toğay VA, Özçelik N. Ochratoxin A causes cell cycle arrest in G1 and G1/S phases through p53 in HK-2 cells. Toxicon. 2020;180:11–17. doi: 10.1016/j.toxicon.2020.03.012. [DOI] [PubMed] [Google Scholar]
- Chandra H, Kumari P, Bontempi E, Yadav S. Medicinal plants: treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal Agric Biotechnol. 2020;24:101518. doi: 10.1016/j.bcab.2020.101518. [DOI] [Google Scholar]
- Chaudhari AN, Ingale AG. Syzygium aromaticum extract mediated, rapid and facile biogenic synthesis of shape-controlled (3D) silver nanocubes. Bioprocess Biosyst Eng. 2016;39:883–891. doi: 10.1007/s00449-016-1567-z. [DOI] [PubMed] [Google Scholar]
- Dalar A, Uzun Y, Mukemre M, Turker M, Konczak I. Centaurea karduchorum Boiss. from Eastern Anatolia: phenolic composition, antioxidant and enzyme inhibitory activities. J Herb Med. 2015;5:211–216. doi: 10.1016/j.hermed.2015.09.006. [DOI] [Google Scholar]
- De Matteis V, Cascione M, Toma CC, Leporatti S. Silver nanoparticles: synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials. 2018;8(5):319. doi: 10.3390/nano8050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts. 2021;11(8):902. doi: 10.3390/catal11080902. [DOI] [Google Scholar]
- El-Deeb NM, Khattab SM, Abu-Youssef MA, Badr AMA. Green synthesis of novel stable biogenic gold nanoparticles for breast cancer therapeutics via the induction of extrinsic and intrinsic pathways. Sci Rep. 2022;12(1):11518. doi: 10.1038/s41598-022-15648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan O, Abbak M, Demirbolat GM, Birtekocak F, Aksel M, Pasa S, et al. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: the characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS One. 2019;14(6):e0216496. doi: 10.1371/journal.pone.0216496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Fard SE, Tafvizi F, Torbati MB. Silvernanoparticles biosynthesisedusing Centella asiatica leaf extract: apoptosis induction in MCF-7 breast cancer cell line. IET Nanobiotechnol. 2018;12(7):994–1002. doi: 10.1049/iet-nbt.2018.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrín G, Linares CI, Muntané J. Mitochondrial drug targets in cell death and cancer. Curr Pharm Des. 2011;17(20):2002–2016. doi: 10.2174/138161211796904803. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Raman J, Abd Malek SN, John PA, Vikineswary S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells. Int J Nanomed. 2013;8:4399–4413. doi: 10.2147/IJN.S51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, Han JW, Eppakayala V, Jeyaraj M, Kim J-H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Res Int. 2013;2013:1–10. doi: 10.1155/2013/535796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, Park JH, Han JW, Kim JH. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy. Int J Nanomed. 2015;29(10):4203–4222. doi: 10.2147/IJN.S83953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hano C, Abbasi BH. Plant-based green synthesis of nanoparticles: production, characterization and applications. Biomolecules. 2022;12:31. doi: 10.3390/biom12010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GC, Beart PM, Nagley P. Oxidative stress triggers neuronal caspase-independent death: endonuclease G involvement in programmed cell death-type III Cell. Mol Life Sci. 2009;66:2773–2787. doi: 10.1007/s00018-009-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Singh NB, Singh A, Singh H, Singh S. Green synthesis of nanoparticles and its potential application. Biotechnol Lett. 2015;38:545–560. doi: 10.1007/s10529-015-2026-7. [DOI] [PubMed] [Google Scholar]
- Jahed FS, Hamidi S. Applications of surface plasmon resonance in human health care. Nanomedicine. 2020;15(19):1823–1827. doi: 10.2217/nnm-2020-0170. [DOI] [PubMed] [Google Scholar]
- Javed R, Zia M, Naz S, et al. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnol. 2020;18:172. doi: 10.1186/s12951-020-00704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas FP, Turker AU. Improvement of shoot proliferation and comparison of secondary metabolites in shoot and callus cultures of Phlomis armeniaca by LC-ESI-MS/MS analysis. In Vitro Cell Dev Biol Plant. 2016;52:608–618. doi: 10.1007/s11627-016-9792-3. [DOI] [Google Scholar]
- Khader SZA, Syed Zameer Ahmed S, Ganesan GM, et al. Rhynchosia rufescens AgNPs enhance cytotoxicity by ROS-mediated apoptosis in MCF-7 cell lines. Environ Sci Pollut Res. 2020;27:2155–2164. doi: 10.1007/s11356-019-06479-y. [DOI] [PubMed] [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Konczak I, Dalar A, Konczak-Islam KA. Health attributes, antioxidant properties and phytochemical composition of traditional medicinal plants from Eastern Anatolia (chapter 7) In: Pereira DAM, editor. Medicinal plants. Hauppauge: Nova Science Publishers Inc; 2014. pp. 183–227. [Google Scholar]
- Kumar B, Smita K, Cumbal L, Debut A. Sacha inchi (Plukenetia volubilis L.) oil for one pot synthesis of silver nanocatalyst: an ecofriendly approach. Ind Crop Prod. 2014;58:238–243. doi: 10.1016/j.indcrop.2014.04.021. [DOI] [Google Scholar]
- Lemarié A, Lagadic-Gossmann D, Morzadec C, et al. Cadmium induces caspase-independent apoptosis in liver Hep3B cells: role for calcium in signaling oxidative stress-related impairment of mitochondria and relocation of endonuclease G and apoptosis-inducing factor. Free Radic Biol Med. 2004;36:1517–1531. doi: 10.1016/j.freeradbiomed.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO. "Green" nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae. 2014;6(1):35–44. doi: 10.32607/20758251-2014-6-1-35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cabanas M, López-García M, Rodríguez-Barro P, Vilariño T, Lodeiro P, Herrero R, Barriada JL, Sastre de Vicente ME. Antioxidant capacity assessment of plant extracts for green synthesis of nanoparticles. Nanomaterials (basel) 2021;11(7):1679. doi: 10.3390/nano11071679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais M, Teixeira AL, Dias F, Machado V, Medeiros R, Prior JAV. Cytotoxic effect of silver nanoparticles synthesized by green methods in cancer. J Med Chem. 2020;63(23):14308–14335. doi: 10.1021/acs.jmedchem.0c01055. [DOI] [PubMed] [Google Scholar]
- Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Dashtaki AS, Babapoor A, Arjmand O. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol. 2018;46(sup3):855–872. doi: 10.1080/21691401.2018.1517769. [DOI] [PubMed] [Google Scholar]
- Nayak D, Kumari M, Rajachandar S, Ashe S, Thathapudi NC, Nayak B. Biofilm impeding agnps target skin carcinoma by inducing mitochondrial membrane depolarization mediated through ros production. ACS Appl Mater Interfaces. 2016;8:28538–28553. doi: 10.1021/acsami.6b11391. [DOI] [PubMed] [Google Scholar]
- Nilghaz A, Mousavi SM, Tian J, Cao R, Guijt RM, Wang X. Noble-metal nanoparticle-based colorimetric diagnostic assays for point-of-need applications. ACS Appl Nano Mater. 2021;4(12):12808–12824. doi: 10.1021/acsanm.1c01545. [DOI] [Google Scholar]
- Pandit C, Roy A, Ghotekar S, Khusro A, Islam MN, Emran TB, Lam SE, Khandaker MU, Bradley DA. Biological agents for synthesis of nanoparticles and their applications. J King Saud Univ Sci. 2022;34:101869. doi: 10.1016/j.jksus.2022.101869. [DOI] [Google Scholar]
- Parveen K, Banse V, Ledwani L. Green synthesis of nanoparticles: their advantages and disadvantages. AIP Conf Proc. 2016;1724:020048. doi: 10.1063/1.4945168. [DOI] [Google Scholar]
- Rohini B, Akther T, Waseem M, Khan J, Kashif M, Hemalatha S. AgNPs from Nigella sativa control breast cancer: an in vitro study. J Environ Pathol Toxicol Oncol. 2019;38:185–194. doi: 10.1615/JEnvironPatholToxicolOncol.2019027318. [DOI] [PubMed] [Google Scholar]
- Salman G, Pehlivanoglu S, Aydin Acar C, et al. Anticancer effects of Vitis vinifera L. mediated biosynthesized silver nanoparticles and cotreatment with 5 fluorouracil on HT-29 cell line. Biol Trace Elem Res. 2021 doi: 10.1007/s12011-021-02923-8. [DOI] [PubMed] [Google Scholar]
- Saracoglu I, Inome M, Calis I, Ogihara Y. Studies on constituents with cytotoxic and cytostatic activity of 2 Turkish medicinal-plants Phlomis armeniaca and Scutellaria salviifolia. Biol Pharm Bull. 1995;18:1396–1400. doi: 10.1248/bpb.18.1396. [DOI] [PubMed] [Google Scholar]
- Saravanakumar K, Chelliah R, Shanmugam S, Varukattu NB, Oh DH, Kathiresan K, Wang MH. Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. J Photochem Photobiol. 2018;185:126–135. doi: 10.1016/j.jphotobiol.2018.05.032. [DOI] [PubMed] [Google Scholar]
- Sarikurkcu C, Uren MC, Tepe B, Cengiz M, Kocak MS. Phlomis armeniaca: phenolic compounds, enzyme inhibitory and antioxidant activities. Ind Crops Prod. 2015;78:95–101. doi: 10.1016/j.indcrop.2015.10.016. [DOI] [Google Scholar]
- Satpathy S, Patra A, Ahirwar B, Delwar Hussain M. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artif Cells Nanomed Biotechnol. 2018;46:71–85. doi: 10.1080/21691401.2018.1489265. [DOI] [PubMed] [Google Scholar]
- Saxena SK, Nyodu R, Kumar S, Maurya VK. Current advances in nanotechnology and medicine. In: Saxena S, Khurana S, editors. NanoBioMedicine. Singapore: Springer; 2020. [Google Scholar]
- Shariq Ahmed M, Soundhararajan R, Akther T, et al. Biogenic AgNPs synthesized via endophytic bacteria and its biological applications. Environ Sci Pollut Res. 2019;26:26939–26946. doi: 10.1007/s11356-019-05869-6. [DOI] [PubMed] [Google Scholar]
- Shin SW, Song IH, Um SH. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials. 2015;5:1351–1365. doi: 10.3390/nano5031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek A, Pehlivanoglu S, Aydin Acar C. Anti-proliferative and apoptotic effects of green synthesized silver nanoparticles using Lavandula angustifolia on human glioblastoma cells. 3 Biotech. 2021;11:374. doi: 10.1007/s13205-021-02923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha RH. Green synthesis of silver and gold nanoparticles and their potential applications as therapeutics in cancer therapy; a review. Inorg Chem Commun. 2022;143:109610. doi: 10.1016/j.inoche.2022.109610. [DOI] [Google Scholar]
- Turker AU, Yıldırım AB. Evaluation of antibacterial and antitumor activities of some Turkish endemic plants. Trop J Pharm Res. 2013;12(6):1003–1010. doi: 10.4314/tjpr.v12i6.20. [DOI] [Google Scholar]
- Ullah I, Khalil AT, Ali M, Iqbal J, Ali W, Alarifi S, Shinwari ZK. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid Med Cell Longev. 2020;2020:1–14. doi: 10.1155/2020/1215395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal K, Rather HA, Rajagopal K, Shanthi MP, Sheriff K, Illiyas M, Rather RA, Manikandan E, Uvarajan S, Bhaskar M, et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J Photochem Photobiol B. 2017;167:282–289. doi: 10.1016/j.jphotobiol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- Yedjou CG, et al. Health and racial disparity in breast cancer. In: Ahmad A, et al., editors. Breast cancer metastasis and drug resistance advances in experimental medicine and biology. 3. Cham: Springer; 2019. pp. 31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeşilot Ş, Dönmez S. Cytotoxic effect of green synthesized silver nanoparticles with Salvia officinalis on MCF-7 human breast cancer cells. TJHSL. 2021;4(3):133–139. [Google Scholar]
- Yumrutas O, Saygideger SD. Determination of antioxidant and antimutagenic activities of Phlomis armeniaca and Mentha pulegium. J Appl Pharm Sci. 2012;02(2012):36–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.