Abstract
Staphylococcus aureus killed during imipenem or ceftazidime chemotherapy in mice elicited an early release of tumor necrosis factor alpha (TNF-α) into the systemic circulation. This response was coincident in time with an increase in leukocyte-endothelium adhesive interactions in the microvasculature. Equivalent responses were not observed without the antibiotic treatment (imipenem or ceftazidime). Protective efficacy of the same antibiotic treatment was markedly diminished in d-galactosamine-treated mice compared to controls; e.g., it dropped from 2,000-fold to 70-fold with 4 mg of imipenem per kg given at the time of challenge. Nevertheless, protection was quantitatively restored upon concurrent administration of neutralizing anti-TNF-α antibody or 4 mg of dexamethasone per kg to these TNF-α-hypersensitive mice. Importantly, protection afforded by dexamethasone was not seen when the animals were challenged with viable organisms but without the concurrent administration of antibiotic. An early TNF-α response could also be demonstrated upon challenge with Escherichia coli, but in this instance, neither the timing nor the magnitude of that response was influenced by treatment with these antibiotics. We conclude from these studies that the inflammatory response to viable versus killed bacteria may differ markedly depending on the particular bacterium, host sensitivity to TNF-α, and possibly the Gram stain classification.
A recognition that the killing of bacteria can trigger a potentially serious host inflammatory response dates back at least 100 years to the very early published studies of Jarisch (22) on the clinical consequences of mercury use to treat patients with syphilis. “Frapper fort ou frapper doucement” (to strike hard or to strike softly) was the way in which concern regarding the host inflammatory response to antibiotic treatment of sepsis due to gram-negative bacteria was succinctly stated in a 1978 editorial questioning whether antibiotic treatment had become too aggressive (4). More recently, such concerns have been described in terms of a “therapeutic paradox” (32). However, when increased morbidity and mortality appear to be associated with antibiotic treatment, it cannot be assumed that the latter is necessarily the source of the problem (29). In this respect, there is currently little direct evidence that would link antibiotic chemotherapy and overall efficacy of various antibiotic strategies, while there is a growing body of experimental evidence to support the concept that antibiotic choice can, under carefully controlled conditions, influence both morbidity and mortality.
Antibiotic action on microbes in the host can result in the release of bacterial components that will trigger a host proinflammatory response (20, 32, 44; D. C. Morrison, Editorial, J. Endotoxin Res. 3:171, 1996). Correspondingly, ex vivo studies with whole blood indicate that both gram-positive and gram-negative bacteria killed by antibiotic can lead to increased levels of biologically active cytokines (14, 30, 36). The relative impact of such host inflammatory responses is likely to depend on a number of variables, including the extent of microbe proliferation and the specific proinflammatory and anti-inflammatory responses that would be anticipated to be elicited from the host even in the absence of antibiotic treatment.
There is a general level of recognition that the proinflammatory mediator tumor necrosis factor alpha (TNF-α) is a potentially important mediator of mortality in response to endotoxin and, perhaps more generally, following infection with virulent gram-negative bacteria (2, 43). TNF-α also contributes significantly to multiple-organ failure, shock, and death resulting from challenge with purified gram-positive bacterial components (e.g., peptidoglycan), as well as with live or heat-killed gram-positive organisms (7, 10, 13, 19). Conversely, the possibility exists that under some circumstances TNF-α will be beneficial to recovery from sepsis. In that regard, a recent clinical trial revealed a dose-dependent trend toward increased mortality upon TNF receptor-Fc fusion protein treatment of patients infected with gram-positive organisms. The same trend did not hold for patients infected with gram-negative organisms (12).
Previous experiments from our laboratory have examined the TNF-α response from peritoneal exudate macrophages taken from CF-1 outbred mice and then cultured in vitro and stimulated with either paraformaldehyde-fixed Escherichia coli or paraformaldehyde-fixed Staphylococcus aureus. The results of these studies indicated markedly different levels of TNF-α released in vitro and, in particular, a significantly lower TNF-α response to S. aureus than to E. coli, even though in vivo S. aureus was more lethal than E. coli in terms of the dose of viable organisms required to elicit 50% mortality among normal, i.e., unsensitized, mice (40).
The reversible hepatotoxin d-galactosamine is known to markedly sensitize animals to the adverse pathophysiologic consequences of TNF-α (15, 16). Conversely, in mice genetically transformed so that TNF-α function is lost due to absence of TNF-α itself (25) or of TNF-α receptors (35) (knockout mice), d-galactosamine does not play a critical role in mortality. In our own published studies, d-galactosamine was found to sensitize mice to lethal infection with viable S. aureus by fivefold. By contrast, with live E. coli infection, sensitization was 10,000-fold, comparable in degree to that seen in parallel experiments with E. coli lipopolysaccharide (40). These results would, therefore, appear to be entirely consistent with those for in vitro TNF-α release, as described in the preceding paragraph. Importantly, however, and in seeming contrast to these findings, Freudenberg and Galanos (13) had earlier published studies showing that when dead (heat-killed) bacteria were injected into mice, d-galactosamine sensitized the mice to the S. aureus-mediated lethal response to a much greater extent (approximately 1,000-fold).
Such studies led us to hypothesize that significant differences in host response, as assessed by TNF-α levels, would be present in vivo following live E. coli versus S. aureus challenge of normal mice and that killing of the bacteria in vivo would lead to significant differences in the appearance of TNF-α in the circulation following bacterial challenge. As a correlative index of early host inflammatory responses, parallel studies to examine leukocyte-endothelial cell adhesive interactions would be expected to reveal parallel definable host pathophysiological manifestations in response to viable versus killed S. aureus. In addition to these differential responses that would be anticipated with normal animals, we further hypothesized that these differences might be reflected in differences in the survival of TNF-α-hypersensitive mice (e.g., d-galactosamine-treated mice) in the presence of appropriate antibiotic chemotherapy. The studies reported here are fully supportive of these anticipated findings.
MATERIALS AND METHODS
Animals.
All animals were housed in the Association for Assessment and Accreditation of Laboratory Animals Care, Inc.-accredited animal care facility at the University of Kansas Medical Center and provided with nonsterile laboratory chow (Harlan Teklab, Madison, Wis.) and water ad libitum. All procedures were performed with the approval of the Institutional Animal Care and Use Committee following guidelines provided by the U.S. Public Health Service. Female CF-1 outbred mice aged 9 to 11 weeks were obtained from Harlan Sprague Dawley, Inc., Indianapolis, Ind., or Charles River Laboratories, Wilmington, Mass. Sprague-Dawley male rats, weighing 250 to 300 g, were purchased from Harlan Sprague Dawley, Inc. All the animals were allowed to acclimate to the laboratory for a minimum of 5 to 7 days prior to initiation of experiments.
Bacteria.
E. coli O111:B4 was the gift of List Biological Laboratories, Campbell, Calif.; S. aureus M was a gift from Chia Y. Lee, Department of Microbiology, Molecular Genetics, and Immunology, Kansas University Medical Center.
Bacterial growth.
Bacterial growth in liquid culture was initiated by picking several colonies from a streaked plate of E. coli grown overnight on MacConkey agar or of S. aureus grown on Trypticase soy agar. Bacteria were inoculated into 1 to 2 ml of Trypticase soy broth in a 10-ml culture tube and aerated by mechanical shaking overnight at 37°C. A 1.0-ml volume of the overnight culture was subcultured in 50 to 100 ml of Trypticase soy broth and grown with aeration until mid-log phase as monitored by light scattering at 660 nm. Final concentrations were then achieved by suitable dilution, depending on the requirements for a particular experiment. Pyrogen-free saline (Baxter Healthcare, Deerfield, Ill.) was used as a diluent in the preparation of all final microbe suspensions used for administration in the in vivo experiments.
Antibiotics.
Imipenem/cilastatin was obtained from Merck & Co. (West Point, Pa.); ceftazidime was obtained from Glaxo (Research Triangle Park, N.C.). Both were prepared fresh in sterile saline just before use.
Monitoring of antimicrobial efficacy. (i) In vitro.
The MICs were determined by the E-test method (AB Biodisk, Solna, Sweden).
(ii) In vivo.
Mice were treated concomitantly with bacteria and either antibiotic or saline vehicle in separate intraperitoneal injections at the beginning of all the experiments. At different times following infection, animals were euthanized by cervical dislocation to assess antibiotic antimicrobial efficacy. A 5-ml volume of normal saline was rapidly injected into the peritoneum followed by immediate lavage. The resulting exudate fluid was harvested and serially diluted into sterile saline in culture tubes, and 10-μl samples were micropipetted into Trypticase soy agar plates, incubated overnight at 37°C, and quantitated for viable CFU.
TNF-α levels in serum.
To assess circulating levels of TNF-α following the initiation of infection with or without antibiotic chemotherapy, trunk blood was collected at various times by decapitation. Whole blood was allowed to clot at 37°C for 15 min, and serum was then separated by centrifugation at 2,000 × g for 10 min at 4°C. Aliquots of serum were stored at −70°C until assays for TNF-α could be performed. For TNF-α determinations, thawed aliquots were diluted in RPMI 1640 medium with 10% fetal calf serum and added to 96-well tissue culture plates (Costar Corp., Cambridge, Mass.) containing 5 × 104 transformed L929 mouse fibroblasts that had been previously treated for 2 h with 5 mg of actinomycin D (Merck & Co.) per ml. The L929 cells were then incubated at 37°C in a 5% CO2 incubator for 24 h and washed twice with RPMI 1640 medium without phenol red, and TNF-α dependent cytotoxicity was assayed with thiazolyl blue (MTT) (Sigma Chemical Co., St. Louis, Mo.) (28). The detection limit of this assay is 1.95 pg/ml using recombinant mouse TNF-α (purchased from R&D Systems, Inc., Minneapolis, Minn.) as a standard. In some experiments, the results of the above cytotoxicity assays were confirmed by quantitative enzyme-linked immunosorbent assay (ELISA) using Duoset kit reagents supplied by R&D Systems, Inc., as recommended by the manufacturer.
Mouse mortality studies.
The experimental conditions for antibiotic or saline vehicle administration following infection were the same as those for determination of the TNF-α levels in serum. The dose of imipenem was 20 mg/kg administered in a volume of 0.2 ml. Injection was intraperitoneal (i.p.) and was performed concurrently with i.p. injection of bacteria. Administration of bacteria was exactly the same as for the mice used for TNF-α determinations in serum, except that 3 to 4 orders of magnitude (logarithmically graded) doses of bacteria were used, in serial dilutions, to assess lethal sensitivity over a period of 72 h postinfection. By 48 to 72 h, no further significant changes in mortality were anticipated or observed. The 50% lethal doses (LD50) were determined by the method described by Reed and Muench (33), with cumulative data from at least two separate experiments, four to six mice per datum point per experiment, used to determine the final values. For some experiments, 800 mg of d-galactosamine (Sigma Chemical Co.) per kg was also administered to mice along with the bacteria in the same injection. In such experiments, the d-galactosamine was first dissolved in phosphate-buffered saline, made fresh immediately before use from the sodium salts. The bacteria at the desired number of CFU were then suspended in the buffered solution containing d-galactosamine just prior to i.p. administration.
Leukocyte-endothelial cell adhesive interactions.
As a second experimental model to investigate the effects of antibiotic chemotherapy on the host inflammatory response in otherwise normal animals challenged with bacteria, leukocyte-endothelial cell interactions in the mesenteric microcirculation of rats were examined using intravital microscopy. The same reagents as those in the mouse studies, prepared in the same way and at comparable doses on a per weight basis, were used.
(i) Surgical preparation for intravital microscopy.
After an overnight fast, rats were anesthetized by intramuscular injection of 1.5 g of urethane per kg. During all procedures, the body temperature was maintained at 36 to 38°C using a homothermic blanket system (Harvard Apparatus, Natick, Mass.) connected to an intrarectal temperature probe. Polyethylene cannulas (PE-50) were inserted into the jugular vein and carotid artery. Blood pressure was continuously monitored using the carotid artery cannula connected to a digital pressure monitor (Micro-Med, Inc., Louisville, Ky.). Bacteria, and imipenem or saline vehicle, were injected via the jugula vein cannula.
An electrocautery was used to make an approximately 1-in. incision along the abdominal midline, and the animal was then positioned on a Plexiglas sheet on top of the stage of a Zeiss Axiovert inverted microscope. A section of the small intestine was carefully removed from the abdomen and positioned over a section of a coverslip in the Plexiglas sheet. The mesentery was bathed (superfused) with bicarbonate-buffered saline at 37°C, at a rate of 0.5 ml per min, and aerated with 95% N2–5% CO2 to prevent tissue drying. Images of mesenteric venules (40× objective, 10× eyepiece) were recorded on a videocassette recorder with a time-date generator and using a color video camera (Panasonic Corp., Cypress, Calif.).
(ii) Venular wall shear rate.
Venular diameter was measured using a video caliper (Microcirculation Research Institute, College Station, Tex.) either directly on-line or off-line during playback of videotapes. An optical Doppler velocimeter, also obtained from Microcirculation Research Institute, was used to measure the centerline red blood cell velocity in venules. The average red blood cell velocity was calculated as centerline velocity ÷ 1.6 (9). The wall shear rate, which represents the physical force generated at the vessel wall due to movement of blood, was calculated as 8 × (average red blood cell velocity ÷ venular diameter) (21).
(iii) Leukocyte rolling, adherence, and emigration.
After a 30-min initial stabilization period, mesenteric venules were selected for experiments based on the following important criteria: unbranched vessels of at least 100 μm, a diameter of approximately 25 to 40 μm, and fewer than three adherent leukocytes within a 100-μm segment of the venule during the control period of evaluation. These venules were observed for two fixed control periods, with fixed intervening periods. The maximum time for this phase of the experiment was 35 min (10, 15, and 10 min each). Adhesive interactions of leukocytes with mesenteric venules were subsequently measured during 10-min periods at various times throughout each experiment. The average rolling velocity of leukocytes along the venular endothelium was calculated by measuring the time required for a given leukocyte to move along the vessel wall between two points 50 μm apart. This distance was accurately determined using a stage micrometer (Fine Science Tools, Inc., Foster City, Calif.). Rolling velocities were measured for one leukocyte during each 1 min of the 10-min observation periods, and average values were then calculated. The total number of adherent leukocytes was also determined during off-line analysis, by quantitating the leukocytes remaining stationary for periods longer than 30 s (24, 45). Leukocyte emigration was expressed as the number of leukocytes surrounding the venule after correcting for any leukocytes within the examination region at the start of an experiment (47).
Statistics.
The TNF-α level in serum, venular diameter, leukocyte adherence, rolling velocity, emigration, and venular shear rate data are presented as means ± standard errors of the means (SEM), with differences between groups assessed for significance by the Student paired t test method (6). Differences in mortality were compared using the Fisher exact probability test (37). With each statistical method, a calculated P value of <0.05 was considered significant.
RESULTS
Efficacy of imipenem killing of S. aureus and of E. coli following experimental mouse peritoneal infection.
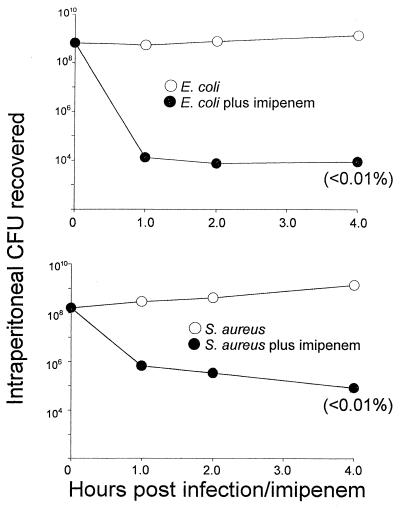
We first undertook control studies to establish that the antibiotic imipenem was effective in reducing the levels of viable bacteria in vivo following administration to CF-1 mice. For these experiments, mice were given either E. coli or S. aureus, and CFU were recovered from peritoneal lavage fluids and quantitated over the 4 h following each bacterial challenge with imipenem or vehicle control. Preliminary determinations of the susceptibility of S. aureus and E. coli to imipenem in vitro confirmed that this antibiotic's broad-spectrum efficacy extends to these two bacterial strains. Its MICs, using the E-test method, were established to be 0.032 and 0.125 μg/ml, respectively. The results of one representative experiment of the subsequent in vivo studies are shown in Fig. 1. As evident from the CFU data presented, imipenem at 20 mg/kg dramatically reduced the level of viable bacteria in the peritoneal cavity within 1 h. With E. coli, the reduction was to 0.002% viable bacteria, from 6.5 × 108 to 1.3 × 104 CFU; with S. aureus, the corresponding reduction was to 0.4%, from 1.6 × 108 to 6.7 × 105 CFU. Further reductions to <0.01% of controls were routinely observed over the 4 h of this study. Control (no antibiotic) data showed, if anything, a modest increase in CFU. At 18 to 24 h, microbes continued to proliferate in saline vehicle-treated mice whereas the corresponding levels of bacteria recovered in peritoneum lavage of experimental animals treated with imipenem were essentially undetectable (data not shown).
FIG. 1.
In situ killing of 4 × 109 CFU of bacteria per kg by 20 mg of imipenem per kg (●), each administered concomitantly and by separate i.p. injections to CF-1 mice. The bacterial count was determined following peritoneal lavage. Data obtained when saline vehicle was injected in place of antibiotic are also shown (○). (Top) E. coli O111:B4; (bottom) S. aureus M. Three mice were used per datum point. Conditions of bacterial growth are as described in Materials and Methods.
Profiles of TNF-α in serum following challenge with live S. aureus or live E. coli.
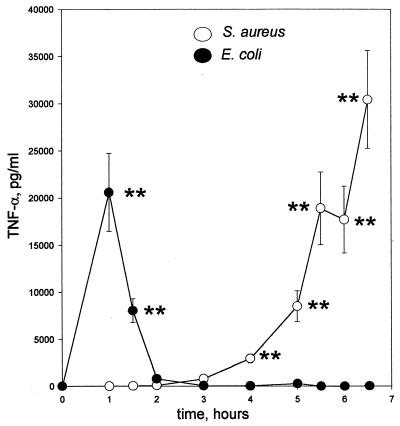
There have been strong suggestions from a number of experimental models of lipopolysaccharide and bacterial lethality that the proinflammatory cytokine TNF-α is a key mediator in the host response. To assess the relative levels of circulating TNF-α in CF-1 mice infected with either E. coli or S. aureus, the appearance of TNF-α in the circulation was monitored over time following live bacterial challenge. TNF-α profiles in serum for mice challenged with these bacteria showed a fundamental difference in response kinetics (Fig. 2). With S. aureus challenge, the rise in the TNF-α level in serum occurred later, at times when the TNF-α levels in serum following E. coli challenge not only had peaked but also had returned to levels indistinguishable from those in controls.
FIG. 2.
Kinetic TNF-α profiles in serum for CF-1 mice challenged i.p. with 4 × 109 CFU of bacteria per ml. ○, S. aureus M; ●, E. coli O111:B4. Two experiments were performed per profile. The conditions of bacterial growth are as described in Materials and Methods. ∗∗, P < 0.005.
Effect of imipenem or ceftazidime on TNF-α profiles with S. aureus or with E. coli.
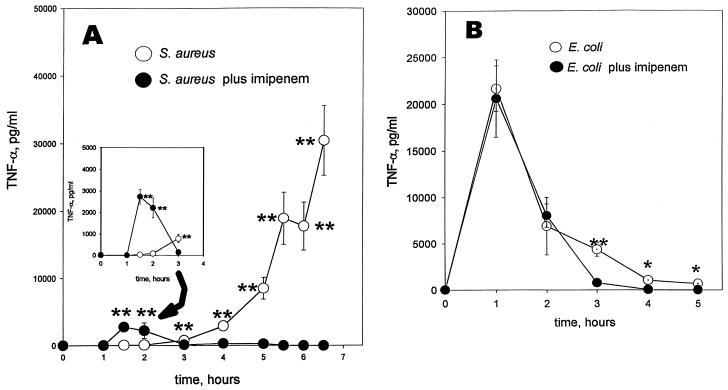
Differences in host inflammatory response to antibiotic chemotherapy of bacterial infection continue to be the subject of investigation. The effects of 20 mg of imipenem per kg, added at the time of challenge with S. aureus or with E. coli, reveal additional distinctive differences in circulating TNF-α levels (Fig. 3). Against S. aureus, imipenem had the effect of altering the host TNF-α response quantitatively and temporally (Fig. 3A). In contrast, it did neither with regard to the host response to E. coli (Fig. 3B) despite the effectiveness of this broad-spectrum antibiotic against both bacteria. The conditions of challenge with each of the bacteria are the same as described for Fig. 1. With imipenem chemotherapy for S. aureus infection, a new and significant TNF-α peak, at least 2 ng/ml in magnitude, appeared in CF-1 mice between 1 and 2 h after challenge. In addition, the late rise in the TNF-α level that would otherwise have reached at least 30 ng/ml was no longer apparent. Similar profiles to those seen with imipenem were also apparent upon comparable therapeutic treatment with ceftazidime and also when the mice were made sensitive to TNF-α by treatment with d-galactosamine (data not shown). In the absence of bacteria, it was confirmed that there was no detectable TNF-α response in serum from each of these antibiotics alone.
FIG. 3.
TNF-α profiles in serum with time for CF-1 mice challenged i.p. with 4 × 109 CFU of bacteria per kg plus saline vehicle (○) or plus 20 mg of imipenem per kg (●). Imipenem or saline vehicle was given by separate i.p. injection at the time of challenge. Blood was collected from the trunk following decapitation. Five mice were used per datum point per experiment. Two experiments were performed per profile. Data are means ± SEM. ∗∗, P < 0.005. (A) S. aureus M. (B) E. coli O111:B4. In the absence of bacterial treatment, TNF-α was undetectable in serum, with or without the imipenem treatment. Conditions of bacterial growth are as described in Materials and Methods. The inset includes the same data from 0 to 3 h but with the scale of the ordinate axis expanded threefold.
Mortality of normal mice as a result of S. aureus or E. coli challenge.
The data summarized in the previous sections have established that significant temporal differences in circulating levels of TNF-α exist following challenge by E. coli versus S. aureus. Further, the TNF-α profile associated with the S. aureus but not with the E. coli challenge is markedly influenced by treatment with the antibiotic imipenem or ceftazidime. Inasmuch as TNF-α has been strongly implicated as an important proinflammatory cytokine in the pathogenesis of sepsis, we therefore tested for correlations between these differences in TNF-α response in serum and differences in lethal outcome. For such studies, the same conditions of bacterial challenge and antibiotic treatment were maintained as described above and mortality was assessed at 72 h. The results of these studies are summarized in Table 1. They indicate, as expected, that the antibiotic chemotherapy confers marked protection against the lethal effects of S. aureus infection. S. aureus at a dose of 106 CFU, plus saline vehicle in place of antibiotic, was lethal to 8 of 11 infected mice. In contrast, infection of mice with S. aureus plus imipenem resulted in 100% survival at 108 CFU and in only 5 deaths in 11 mice at 109 CFU, reflecting an approximate 2,000-fold level of protection.
TABLE 1.
Effect of imipenem, with or without dexamethasone, on survival from S. aureus M versus E. coli O111:B4 challenge of normal and TNF-α-sensitized (d-galactosamine-treated) CF-1 micea
| Sensitization status and challenge | Treatment | LD50 (CFU) | Protection (fold) |
|---|---|---|---|
| Nonsensitized (normal) mice | |||
| S. aureus | Saline | 5 × 105 | |
| Imipenem | 1 × 109 | 2,000 | |
| S. aureus plus dexamethasone | Saline | 2.5 × 105 | |
| Imipenem | 7 × 108 | 1,400 | |
| E. colib | Saline | 7 × 106 | |
| Imipenem | 6 × 108 | 90 | |
| TNF-α-sensitized (d-galactosamine-treated) mice | |||
| S. aureus | Saline | 1 × 105 | |
| Imipenem | 7 × 106 | 70 | |
| S. aureus plus dexamethasone | Saline | 7 × 104 | |
| Imipenem | 1 × 109 | 14,000 | |
| E. coli | Saline | 1 × 103 | |
| Imipenem | 4 × 104 | 40 | |
| E. coli plus dexamethasone | Saline | 1.5 × 105 | |
| Imipenem | 1.8 × 106 | 12 |
Mice were treated with bacteria immediately preceded by a separate i.p. injection of 20 mg of imipenem per kg saline vehicle (0.2 ml). In some experiments, as shown, mice were also subjected at the time of challenge to i.p. injection of 4 mg of dexamethasone per kg. Mortality was scored at 72 h.
With E. coli plus dexamethasone plus imipenem, the effect of dexamethasone was to decrease protection by threefold (data not shown).
Following infection with E. coli, there were no deaths (0 of 12) at 106 CFU, 7 deaths in 12 mice at 107 CFU, and 12 deaths in 12 mice at 108 CFU. With E. coli plus imipenem, there were no deaths in 16 mice at 108 CFU and 7 deaths in 8 mice at 109 CFU, corresponding to 90-fold protection resulting from the antibiotic treatment (Table 1).
Mortality from S. aureus in TNF-α sensitized mice.
To test whether a correlation with mortality could also be discerned in association with the smaller and earlier TNF-α response (Fig. 3A, inset) a model of acute and early TNF-α lethal hypersensitivity (mediated by d-galactosamine) was used. For these studies the d-galactosamine was added with the bacteria and at a dose that our studies (1, 18, 38, 40, 41) and studies from other laboratories (5, 11, 13, 15, 23, 25, 26, 35, 42) have shown to markedly sensitize mice toward the harmful effects of TNF-α, if appearing in the circulation within 2 to 3 h after d-galactosamine administration. Earlier studies from our laboratory had shown only a 5-fold difference in mortality from S. aureus infection (compared to 10,000-fold from E. coli) with and without d-galactosamine (40). Using the same model as previously (40) but with the added feature of imipenem treatment, 6 of 10 and 11 of 12 mice died after infection with 107 and 108 CFU of S. aureus, respectively. This corresponds to 70-fold protection by imipenem (Table 1), much less than the 2,000-fold seen for normal mice (Table 1). These findings suggest that the appearance of the earlier TNF-α peak in serum elicited by the imipenem treatment may, in fact, have proven particularly detrimental to the TNF-α-sensitized mice. Such a possibility was tested directly with neutralizing anti-TNF-α antibody raised in rabbits. With control rabbit serum, 2 of 5 and 5 of 5 mice died after infection with 107 and 108 CFU of S. aureus, respectively, in this TNF-α sensitivity model. Replacement of control serum with corresponding anti-TNF-α antiserum resulted in no deaths among the five animals challenged with each of the same bacterial doses. Therefore, we conclude that the early and singular TNF-α peak associated with the imipenem chemotherapy in S. aureus-infected mice had contributed to mortality in this model.
E. coli lethality in normal versus TNF-α sensitized mice.
With E. coli, and in the absence of antibiotic, d-galactosamine sensitized by approximately 4 orders of magnitude, reducing the LD50 from 7 × 106 to 1 × 103 CFU. When imipenem treatment was added, a similar increase in the magnitude of sensitization due to d-galactosamine was again evident, with a reduction in LD50 from 6 × 108 to 4 × 104 CFU (Table 1). This marked but parallel degree of sensitization to d-galactosamine remained consistent not only with an important role for TNF-α in mediating E. coli lethality but also with the inability of the imipenem treatment to alter circulating TNF-α levels. The imipenem protective efficacy of 90- and 40-fold with and without the d-galactosamine treatment, respectively (Table 1), is based on its antibacterial effect and/or, possibly, on modulation of other potential mediators.
Effects of dexamethasone on mortality in TNF-α sensitized mice.
As an alternative experimental approach to further explore the concept that the TNF-α peak level elicited by S. aureus plus imipenem may have proven detrimental to survival in d-galactosamine-treated mice, 4 mg of dexamethasone was administered at the time of challenge. In that regard, we had earlier provided data to show that, importantly, in the absence of antibiotic treatment, dexamethasone was ineffective at protecting either normal or d-galactosamine-treated mice against lethal S. aureus infection (40), with even a hint of a slightly increased mortality with dexamethasone (Table 1). With the addition of imipenem treatment, dexamethasone was now found to be dramatically beneficial for survival in the d-galactosamine model, raising the total level of protection from 70-fold to almost 14,000-fold (Table 1). Thus, there were only 4 deaths in 16 mice (25%) at 108 CFU and only 4 deaths in 10 mice (40%) at 109 CFU of S. aureus. We conclude that (i) the marked protection by dexamethasone is directly linked to the effects of the imipenem and (ii) such protection, as well as that provided by anti-TNF-α antibody, may have more immediately resulted from the ability to eliminate or neutralize the TNF-α peak in serum that would otherwise have become manifest 1 to 3 h following S. aureus challenge plus imipenem (or ceftazidime [data not shown]). In accord with this view, we were able to show that dexamethasone completely abrogated the appearance of this early TNF-α peak. With the addition of the dexamethasone treatment, the TNF-α level in serum elicited from S. aureus plus imipenem at 2 h, for example, was reduced in normal mice from 2,410 ± 251 to 9 ± 2 pg/ml (P < 0.005). In contrast, the fact that imipenem does not alter the TNF-α response to E. coli (Fig. 3B) is entirely consistent with the finding that dexamethasone protection against E. coli, even in the TNF-α-sensitized mouse model and unlike that against S. aureus, was not enhanced by the antibiotic treatment. In fact, it appears to have been slightly reduced, from 40- to 12-fold, in contrast to the increase in protection from 70- to 14,000-fold against S. aureus (Table 1).
Leukocyte-endothelial cell adhesive interactions.
Thus far, we have focused on differences in TNF-α profiles in serum and corresponding changes in lethal outcomes. In this regard, S. aureus-infected mice treated with antibiotic were found to elicit an early TNF-α response that was not seen in the absence of the antibiotic treatment. Collectively, several pieces of independent evidence demonstrate that this early TNF-α peak is associated with decreased survival in TNF-α sensitized animals. To test whether the same antibiotic treatment may also lead to pathophysiological consequences even in normal animals, we used rat mesenteric intravital microscopy to probe for changes in leukocyte adhesion in the circulation as an index of inflammation.
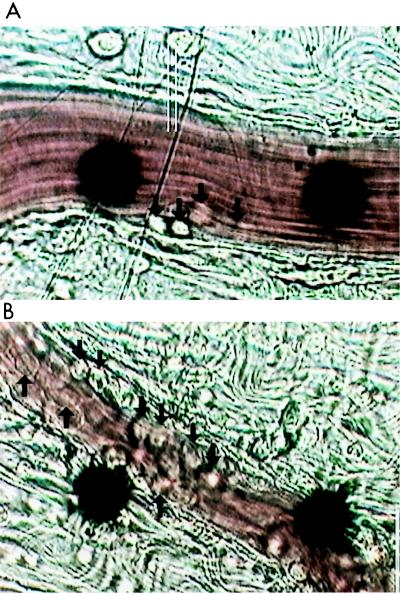
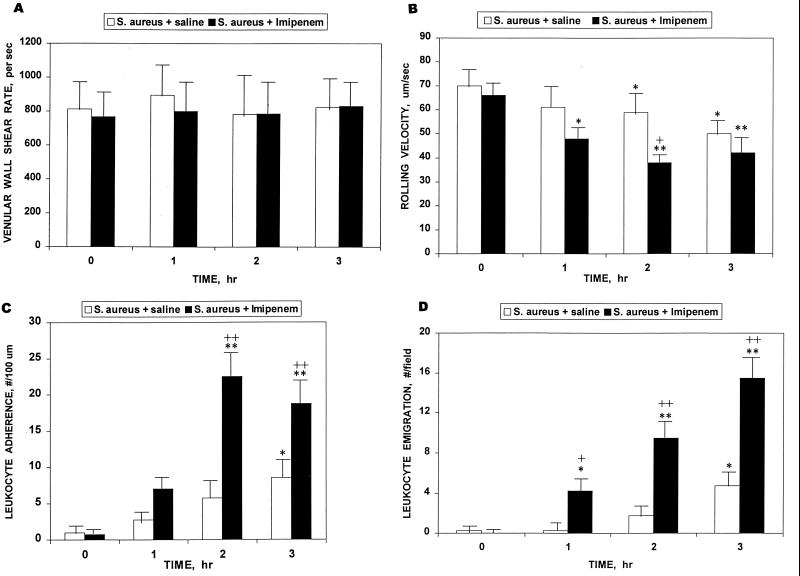
Leukocyte adhesion can be dissected into at least three phases that may be quantitated and statistically evaluated: decreased rolling velocity, increased adherence, and emigration. Figure 4 illustrates photographically the profound differences in the microvasculature 3 h after infection with S. aureus with and without concurrent imipenem chemotherapy. It is evident that by that time the imipenem treatment had resulted in increased leukocyte-endothelial cell adhesive interactions, as shown in the representative photographs. Each of the panels of Fig. 5 dissects and quantitates individual underlying features as a function of time. The venular wall shear rate (Fig. 5A) was monitored as an internal control. Imipenem, of itself, elicited no evidence of leukocyte-endothelial cell adhesive interactions. There was no statistically significant difference in venular diameter between the two groups of rats: S. aureus plus saline, 30.4 ± 2.5 μm; S. aureus plus imipenem, 27.4 ± 2.6 μm (P = 0.42; n = 5 per group; data are means ± SEM; Student's paired t test method) (6).
FIG. 4.
Photomicrographs of inflammation within the microvasculature following 3 h of treatment of rats with 4 × 109 CFU of S. aureus M per kg. (A) S. aureus plus saline vehicle. (B) S. aureus plus 20 mg of imipenem per kg. Arrows point to leukocytes that have adhered to the endothelium. The black dots are part of an optical Doppler velocimeter used to measure centerline red blood cell velocity within the venule. Conditions of bacterial growth are as described in Materials and Methods.
FIG. 5.
Kinetics of leukocyte-endothelial cell adhesive interactions following treatment of rats with 4 × 109 CFU of S. aureus M per kg plus, in a separate i.p. injection, either saline vehicle or 20 mg of imipenem per kg. (A) Venular wall shear rate. (B) Rolling velocity. (C) Adherence. (D) Emigration. Five rats were used per datum point. Data are means and SEM. Paired t test: +, P < 0.05; ++, P < 0.005; comparison with t = 0: ∗, P < 0.05; ∗∗, P < 0.005.
As shown by the data in Fig. 5A, the average venular shear rate did not change throughout the 3-h monitoring period, nor was it significantly altered by the imipenem treatment. Shear rate allows an estimation of the overall physical forces that are generated at the vessel wall due to the movement of blood and that counteract leukocyte adhesion. The fact that there were no differences in shear rate between groups is consistent with the conclusion that this factor could not account for any observed differences in leukocyte-endothelial cell adhesive interactions. The microvascular inflammatory changes illustrated in Fig. 5B to D show the results of further evaluation of changes in leukocyte circulatory velocity with concomitant interaction with the microvascular wall, i.e., leukocyte rolling velocity (Fig. 5B), leukocyte adherence to the vascular wall (Fig. 5C), and leukocyte emigration across the wall (Fig. 5D). The data shown in Fig. 5B demonstrate that when the microvasculature of imipenem- or saline vehicle-treated rats challenged with S. aureus is assessed for variations in rolling velocity, decreases are readily seen throughout the 3-h period compared to initial values. By 2 h, the differences between the two groups became statistically significant. Differences in the extent of leukocyte adherence also became statistically significant with and without the antibiotic chemotherapy by 2 h (Fig. 5C). The differences with respect to leukocyte emigration were the most pronounced, since even by 1 h there was significant emigration through the endothelium in the imipenem-treated, S. aureus-challenged animals both in comparison to initial time and with respect to the control group. Such differences become even more pronounced by 2 and 3 h (Fig. 5D), as is also seen dramatically in Fig. 4. Leukocyte emigration in the control group, by contrast, did not become significant until the 3-h time point, reaching approximately the same magnitude by that time as was seen in the S. aureus-plus-imipenem treated group at 1 h (approximately four per field). These various changes in leukocyte adhesion, taken individually or collectively, provide a second and independent indication that imipenem treatment for S. aureus infection can lead to a distinctive, early, and potentially detrimental inflammatory response that is far more pronounced than that seen during the same time frame in the absence of the antibiotic treatment.
DISCUSSION
The data presented in this paper document that the production of the proinflammatory mediator TNF-α is critically dependent not only upon the microbe used to establish the experimental infection but also upon the therapeutic intervention used to treat the infection. Imipenem or ceftazidime chemotherapy of S. aureus had the effect of altering the host TNF-α response quantitatively and temporally. These antibiotics did neither, in contrast to the host TNF-α response to E. coli. The appearance of an early TNF-α peak associated with imipenem or ceftazidime treatment of S. aureus had a detrimental effect on survival in d-galactosamine-treated mice. This was evident not only from a reduction in protective efficacy of each of these antibiotics in this model but also from the concomitant protective efficacy of either anti-TNF-α neutralizing antibody or dexamethasone therapy. In normal mice, however, the efficacy of these antibiotics against S. aureus was much greater, even without the dexamethasone treatment. Moreover, dexamethasone not only did not protect but also appeared to have a slightly adverse effect on survival, suggesting a possibly beneficial aspect to the early and relatively small TNF-α peak that had been abrogated by the dexamethasone treatment.
We found that microvascular inflammatory responses to S. aureus developed more rapidly in imipenem-treated than in saline-treated animals. These responses included a larger reduction in leukocyte rolling velocity and increased numbers of adherent and emigrated leukocytes. An important point is that the antibiotic treatment increased leukocyte-endothelial cell adhesive interactions without a decrease in shear rate. Since this physical force opposes leukocyte-endothelial cell adhesion, our results suggest that the action of imipenem against S. aureus enhances proadhesive responses on either endothelial cells, circulating leukocytes, or both. A potential mechanism is suggested by our observation of an earlier and larger increase in the level of circulating TNF-α in animals given both agents. TNF-α is known to increase selectin expression on endothelial cells (34), which could account for a reduction in leukocyte rolling velocity. Furthermore, TNF-α promotes leukocyte adherence within the mesenteric circulation (8). Future studies are needed to establish whether the early increase in TNF-α levels in serum brought about by the antibiotic treatment and the microvascular responses are causally interrelated.
While the differences in the profiles of TNF-α in serum between E. coli- and S. aureus-infected animals correlate with the observed differences in mortality in the d-galactosamine model (40) (Table 1), they also raise the question of whether the findings with these particular strains of S. aureus and E. coli can be extended to other bacterial organisms. Studies with capsular and acapsular S. aureus, including types 5 and 8, and with two streptococcal strains (S. mitis and S. pneumoniae), along with gram-negative strains including E. coli O18:K1 (a gift of A. Cross), K. pneumoniae, C. diversus, P. aeruginosa, and P. mirabilis, suggest the applicability of our findings to other strains of S. aureus and E. coli and, even more generally, to gram-positive and gram-negative bacterial infection (R. Silverstein, Q. Xue, R. T. Horvat, C. Y. Lee, M. J. Luchi, S. Sau, P. A. Worley, and D. C. Morrison, Prog. Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother. 1997, abstr. B-50, p. 35, 1997).
Finally, there is increasing concern about possibly important differences in host inflammatory responses to sepsis due to gram-positive versus gram-negative bacteria (11, 17, 32), including recent reexamination of the scope and limitation of glucocorticoids to address such concerns (3, 27, 39, 40, 46; D. G. Remick, Editorial, Shock 8:146, 1997). The present findings contribute to our growing knowledge base of differences in host responses to different classes of bacteria and the still further differences resulting from specific antibiotic chemotherapy and, ultimately, should lead to greater harmony between complementary modes of anti-inflammatory and antibiotic intervention strategies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R37-AI23447 from the National Institute of Allergy and Infectious Diseases (D.C.M.) and by grants from the Ernest F. Lied Foundation (R.S.) and Merck & Co., Inc., unrestricted (D.C.M.).
We are grateful to Donald C. Johnson for critical reading of the manuscript and to Steven M. Opal for additional suggestions. We are also grateful to Richard A. Grabbe for assistance with the graphical representations.
REFERENCES
- 1.Amura C R, Silverstein R, Morrison D C. Mechanisms involved in the pathogenesis of sepsis are not necessarily reflected by in vitro cell activation studies. Infect Immun. 1998;66:5372–5378. doi: 10.1128/iai.66.11.5372-5378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B, Krochin N, Milsark I W, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- 3.Bollaert P-E, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–650. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Buxton Hopkin D A. Frapper fort ou frapper doucement: a gram-negative dilemma. Lancet. 1978;ii:1193–1194. [PubMed] [Google Scholar]
- 5.Chapes S K, Beharka A A. Lipopolysaccharide is required for the lethal effects of enterotoxin B after d-galactosamine sensitization. J Endotoxin Res. 1995;2:263–271. [Google Scholar]
- 6.Croxton F E. Elementary statistics with applications in medicine. 1953. pp. 235–239. and 326–327. Prentice-Hall, Inc., Englewood Cliffs, N.J. [Google Scholar]
- 7.Cusumano V, Mancuso G, Genovese F, Cuzzola M, Cook J A, Cochran J B, Teti G. Neonatal hypersusceptibility to endotoxin correlates with increased tumor necrosis factor production in mice. J Infect Dis. 1997;176:168–176. doi: 10.1086/514019. [DOI] [PubMed] [Google Scholar]
- 8.Davenpeck K L, Zagorski J, Schleimer R P, Bochner B S. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: regulation by glucocorticoids and role of cytokines. J Immunol. 1998;161:6861–6870. [PubMed] [Google Scholar]
- 9.Davis M J. Determination of volumetric flow in capillary tubes using an optical Doppler velocimeter. Microvasc Res. 1987;34:223–230. doi: 10.1016/0026-2862(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 10.De Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiedler V B, Loof I, Voehringer V, Galanos C, Fournel M A. Monoclonal antibody to tumor necrosis factor-alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. J Lab Clin Med. 1992;120:574–588. [PubMed] [Google Scholar]
- 12.Fischer C J, Jr, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M H, Benjamin E for the Soluble TNF Receptor Sepsis Study Group. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 13.Freudenberg M A, Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frieling J T M, Mulder J A, Hendriks T, Curfs J H A J, van der Linden C J, Sauerwein R W. Differential induction of pro- and anti-inflammatory cytokines in whole blood by bacteria: effects of antibiotic treatment. Antimicrob Agents Chemother. 1997;41:1439–1443. doi: 10.1128/aac.41.7.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanos C, Freudenberg M A, Katschinski T, Salamao R, Mossmann H, Kumazawa Y. Tumor necrosis factor and host response to endotoxin. In: Ryan J L, Morrison D C, editors. Bacterial endotoxic lipopolysaccharides. II. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 75–104. [Google Scholar]
- 16.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J J, Zuvanich E G, Xue Q, Horn D L, Silverstein R, Morrison D C. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J Immunol. 1999;163:4095–4099. [PubMed] [Google Scholar]
- 18.Gonzalez J C, Johnson D C, Morrison D C, Freudenberg M A, Galanos C, Silverstein R. Endogenous and exogenous glucocorticoids have different roles in modulating endotoxin lethality in d-galactosamine-sensitized mice. Infect Immun. 1993;61:970–974. doi: 10.1128/iai.61.3.970-974.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinshaw L B, Emerson T E, Jr, Taylor F B, Jr, Chang A C K, Duerr M, Peer G T, Flournoy D J, White G L, Kosanke S D, Murray C K, Xu R, Passey R B, Fournel M A. Lethal Staphylococcus aureus-induced shock in primates: prevention of death with anti-TNF antibody. J Trauma. 1992;33:568–573. [PubMed] [Google Scholar]
- 20.Horn D L, Opal S M, Lomastro E. Antibiotics, cytokines, and endotoxin: a complex and evolving relationship in gram-negative sepsis. Scand J Infect Dis Suppl. 1996;101:9–13. [PubMed] [Google Scholar]
- 21.House S D, Lipowsky H H. Leukocyte-endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- 22.Jarisch A. Therapeutiche versuche bei syphilis. Wien Med Wochenschr. 1895;45:721–724. [Google Scholar]
- 23.Kirikae T, Hirata M, Yamasu H, Kirikae F, Tamura H, Kayama F, Nakatsuka K, Yokochi T, Nakano M. Protective effects of a human 18-kilodalton cationic antimicrobial protein (CAP18)-derived peptide against endotoxemia. Infect Immun. 1998;66:1861–1868. doi: 10.1128/iai.66.5.1861-1868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurose I, Wolf R, Grisham M B, Aw T Y, Specian R D, Granger D N. Microvascular responses to inhibition of nitric oxide production: role of active oxidants. Circ Res. 1995;76:30–39. doi: 10.1161/01.res.76.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall A J, Denkers E Y. Toxoplasma gondii triggers granulocyte-dependent cytokine-mediated lethal shock in d-galactosamine-sensitized mice. Infect Immun. 1998;66:1325–1333. doi: 10.1128/iai.66.4.1325-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meduri G U, Headley A S, Golden E, Carson S J, Umberger R A, Kelso T, Tolley E A. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 28.Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb D, Bolgos G, Green L, Remick D G. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Norimatsu M, Morrison D C. Correlation of antibiotic-induced endotoxin release and cytokine production in Escherichia coli-inoculated mouse whole blood ex vivo. J Infect Dis. 1998;177:1302–1307. doi: 10.1086/515291. [DOI] [PubMed] [Google Scholar]
- 31.Opal S M, Cohen J. Clinical gram-positive sepsis: does it fundamentally differ from gram-negative sepsis? Clin Care Med. 1999;27:1608–1616. doi: 10.1097/00003246-199908000-00039. [DOI] [PubMed] [Google Scholar]
- 32.Periti P, Mazzei T. Antibiotic-induced release of bacterial cell wall components in the pathogenesis of sepsis and septic shock: a review. J Chemother. 1998;10:427–448. doi: 10.1179/joc.1998.10.6.427. [DOI] [PubMed] [Google Scholar]
- 33.Reed L J, Muench H A. A simple method for estimating fifty percent endpoints. J Hyg. 1938;27:493–497. [Google Scholar]
- 34.Robinson L A, Tu L, Steeber D A, Preis O, Platt J L, Tedder T F. The role of adhesion molecules in human leukocyte attachment to porcine vascular endothelium: implications for xenotransplantation. J Immunol. 1998;12:6931–6938. [PubMed] [Google Scholar]
- 35.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 36.Schneider C M, Huzly D, Vetter C, von Specht B U, Daschner F D. Tumor necrosis factor alpha and interleukin 6 release induced by antibiotic killing of Pseudomonas aeruginosa and Staphylococcus aureus. Eur J Microbiol Infect Dis. 1997;16:467–471. doi: 10.1007/BF02471914. [DOI] [PubMed] [Google Scholar]
- 37.Siegel S. Nonparametric statistics. New York, N.Y: McGraw-Hill Book Co.; 1956. pp. 96–100. [Google Scholar]
- 38.Silverstein R, Johnson W M, Bucklin S E, Johnson D C. The protein kinase C activator PMA modulates LPS lethality in normal mice and protects against lethality in d-galactosamine-sensitized mice. J Endotoxin Res. 1996;3:29–37. [Google Scholar]
- 39.Silverstein R, Johnson D C, Norimatsu M. Glucocorticoid control of endotoxin responses. In: Brade H, Opal S M, Vogel S N, Morrison D C, editors. Endotoxin in health and disease. New York, N.Y: Marcel Dekker, Inc.; 1999. p. 769. [Google Scholar]
- 40.Silverstein R, Norimatsu M, Morrison D C. Fundamental differences during gram-positive versus gram-negative sepsis become apparent during bacterial challenge of d-galactosamine-treated mice. J Endotoxin Res. 1997;4:173–181. [Google Scholar]
- 41.Silverstein R, Turley B R, Christofferesen C A, Johnson D C, Morrison D C. Hydrazine sulfate protects d-galactosamine-sensitized mice against endotoxin and tumor necrosis factor/cachectin lethality: evidence of a role for the pituitary. J Exp Med. 1991;173:357–365. doi: 10.1084/jem.173.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiegs G, Freudenberg M A, Galanos C, Wendel A. Colchicine prevents tumor necrosis factor-induced toxicity in vivo. Infect Immun. 1992;60:1941–1945. doi: 10.1128/iai.60.5.1941-1945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 44.van Langevelde P, van Dissel J T, Ravensbergen E, Appelmilk B J, Schrijver I A, Groenveld P H P. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood J G, Mattioli L F, Gonzalez N C. Hypoxia causes leukocyte adherence to mesenteric venules in nonacclimatized, but not in acclimatized, rats. J Appl Physiol. 1999;87:873–881. doi: 10.1152/jappl.1999.87.3.873. [DOI] [PubMed] [Google Scholar]
- 46.Zawacki J K, Hunt J L, Gamelli R L, Filkins J P. Glucocorticoid regulation of hepatic TNF production following cecal ligation and puncture sepsis. Shock. 1997;8:141–145. doi: 10.1097/00024382-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman B J, Holt J W, Paulson J C, Anderson D C, Miyasaka M, Tamatani T, Todd R F, Rusche J R, Granger D N. Molecular determinants of lipid mediator-induced leukocyte adherence and emigration in rat mesenteric venules. Am J Physiol. 1994;266:H847–H853. doi: 10.1152/ajpheart.1994.266.3.H847. [DOI] [PubMed] [Google Scholar]