Abstract
Toxoplasmosis has been categorized as one of the long-lasting protozoan parasitic infections. It affects almost one-third of the world’s population. In recent years, several documented studies have elucidated that infected individuals have a remarkably higher incidence of distinct health problems and show various adverse effects. In the PCR-positive COVID-19 patients in Gonbad-e-Kavus, Kalaleh, and Minoodasht counties in the northern part of Iran from June 2021 to December 2021, we sought to investigate any potential relationships between the severity of COVID-19 symptoms and acute and latent toxoplasmosis caused by Toxoplasma gondii (T. gondii). Whole blood samples of 161 COVID-19 patients with positive PCR. The samples were centrifuged to separate serum and screened for two important antibodies against T. gondii (IgM and IgG) by using ELISA kits for human anti-T. gondii IgM and IgG. Anti-T. gondii IgM and IgG antibodies were detected in 8/161 (5.0%) and 42/161 (26.1%) COVID-19 patients, respectively. No significant relationships were found between Toxoplasma IgM and IgG results with clinical signs, age, sex, contact with animals, comorbidities, and also the mortality rate of people with COVID-19. These findings showed that acute and latent toxoplasmosis infections are common among patients with COVID-19; however, no significant associations were found between toxoplasma infections and the symptoms of COVID-19. Therefore, toxoplasmosis is not considered a risk factor for COVID-19.
Keywords: Toxoplasmosis; Toxoplasma gondii, COVID-19; Symptoms; Cat; Golestan province; Iran
Introduction
Toxoplasma gondii (T. gondii), an obligate intracellular protozoan parasite that infects a wide range of species including birds and mammals but notably felids (cats), is the cause of the neglected parasitic illness toxoplasmosis (Zarean et al. 2017). Depending on social and cultural habits, geographical factors, climate and transmission routes, the Seroprevalence of toxoplasmosis varies widely between different countries (10–80%) (Zarean et al. 2017). On a global scale, T. gondii infection is thought to affect more than a billion people (Firouzeh et al. 2021). Ingesting cat feces-contaminated food or eating tissue cysts from raw or undercooked meat are the two major ways that people get sick, so that is the second most important food-borne parasitic disease in Europe (Firouzeh et al. 2021). In those with immune deficiencies or immune breakdown, this protozoan is regarded as an opportunistic and potentially fatal parasite (Sina et al. 2021). In addition, in immunocompromised patients it leads to severe clinical signs such as Toxoplasma encephalitis (TE), which can be fatal in severe cases (Sina et al. 2021) and also this disease is associated with several brain related disorders in both mothers and newborn (Fallahi et al. 2018) and furthermore, it should not be neglected as a cause of retinochoroiditis (Norouzi et al. 2016).
Severe acute respiratory syndrome may be caused by SARS-CoV-2. It is mostly spread by droplets and has caused major public health complications (Moriyama et al. 2020). The studies of the early accumulation of virus in the upper respiratory tract indicate that they attack and kill goblet cells and ciliated cells in the lungs. Then, these dead cells enter the lungs, the lungs gradually become blocked, and the person develops pneumonia (Moriyama et al. 2020).
SARS-CoV-2 lifecycle is similar to SARS-CoV as the virus binds to Angiotensin-converting enzyme 2 (ACE2) receptor on the surface of the host cell membrane via spike proteins (S-protein) and begins its infectious lifecycle (Senapati et al. 2021). Some people with coronavirus are asymptomatic, but in others, it can cause mild to severe pneumonia as well as fever, cough, anosmia, and ageusia. COVID-19 disease requires special care in the elderly, heart problems, hyperglycemia, and corticosteroid consumption people (Struyf et al. 2021), and they may have a high risk of death in these situations (Li et al. 2020).
Experimental evidence has shown that some parasitic infections can have immunogenic potential in preventing some diseases. In a retrospective case-control study, participants with a history of previous cutaneous leishmaniasis scars were significantly prevented from COVID-19 complications and mortality (Bamorovat et al. 2021). Coronavirus 2019 also has elucidated a statistically lower incidence in malaria-endemic areas due to the possible interaction via HLA-A ∗ 02: 01 and activation of CD8 + T-cell. The result of the common protection of the immunodominant epitope between SARS-CoV-2 (N and open reading frame (ORF) 1ab) and Plasmodium falciparum thrombospondin-related anonymous protein (TRAP) could provide a low incidence of COVID-19 in these areas (Iesa et al. 2020).
Some studies have shown that T. gondii demonstrates antiviral effects both directly and indirectly by causing the secretion of Dense Granule Protein-7 (GRA-7) in its host cells (Fekadu et al. 2010; Flegr 2013; Weeratunga et al. 2017). According to different studies, this intracellular parasite inhibited virus replication (Fekadu et al. 2010; Flegr 2013; Jankowiak et al. 2020a; Weeratunga et al. 2017). Both in vivo and in vitro studies have confirmed this evidence has been proven both in in vitro and in vivo experiments against herpes simplex virus, influenza A virus, coxsackie virus, and Indiana vesiculovirus (Jankowiak et al. 2020a) . Generally, GRA–7 has shown immune-stimulant effects and a wide range of antiviral effects through interferons signaling type I (Weeratunga et al. 2017). Moreover, apicoplast proteins are known to have immunogenic activities (Can et al. 2020). The immune response to Toxoplasma is a combination of TH1 cellular response and humoral immune response or specific anti-Toxoplasma antibodies (Munoz et al. 2011).
To date, an experiment has demonstrated a negative relationship between the incidence of coronavirus disease and distinct infections caused by parasites (Jankowiak et al. 2020a). It is noteworthy that COVID-19 and T. gondii infection can activate host innate immune responses via the same pathway. Indeed, in both intracellular pathogens, infected host toll-like receptors (TLR), such as TLR 2, TLR4, and TLR7, are strongly activated through the canonical or signaling pathway. Accordingly, some stimulated cytokines in toxoplasmosis patients may worsen the severity of coronavirus disease (Jankowiak et al. 2020a; Flegr 2013). Therefore, we hypothesized that Toxoplasma infection maybe has a possible relationship with COVID-19 in hospitalized patients. In this regard, the current experiment was mainly aimed to determine whether Toxoplasma infection has any positive/negative effects on the severity of COVID-19 symptoms or not. Hence, serological tests were used to detect T. gondii infection in 161 PCR-positive COVID-19 hospitalized individuals in the Gonbad-e-Kavus, Kalaleh, and Minoodasht counties in the northern part of Iran from Jun 2021 to December 2021.
Materials and methods
Ethics approval
The present study was conducted in accordance with the principles of the Helsinki Declaration. The ethical approval of this cross-sectional study was obtained from the Research Ethics Committees of Isfahan University of Medical Sciences, School of Medicine, Isfahan, Iran (IR.MUI.MED.REC.1400.049 to Dr. Seyed Hossein Hejazi). Before starting patient recruitment, the Institutional Review Board (IRB) of Isfahan University of Medical Sciences, Isfahan, Iran reviewed and approved our application for the current research projects. In the present study, adult participants and at least one parent or guardian of the child participants signed the written informed consent.
Study area and participants
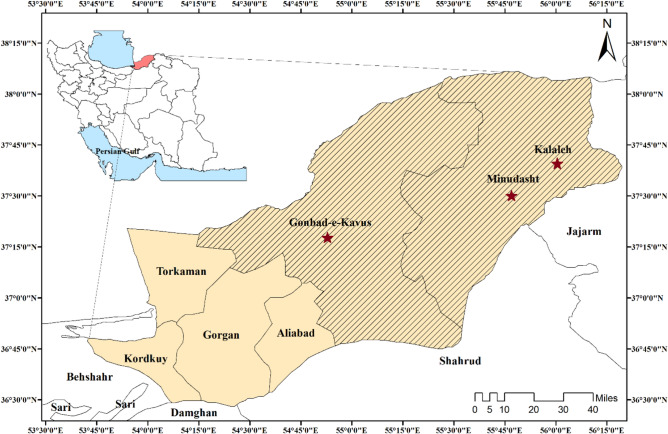
From Jun 2021 to December 2021, we examined 161 PCR-positive COVID-19 individuals at referral hospitals in three countries, including Payambar-E Azam Hospital in Gonbad-e-Kavous county, Rasool Akram Hospital in Kalaleh county, and Fatemeh Zahra Hospital in Minoodasht county Hospitals, which located in the Golestan province in the northern part of Iran (Fig. 1).
Fig. 1.
Map of Gonbad-e-Kavus, Kalaleh, and Minoodasht counties which are located in the Golestan Province in Northern Iran, showing the study area and sampling site from PCR-positive COVID-19 individuals in the referral centers (Created by Arc GIS version 10.2)
Sample collection
Having positive COVID19 PCR was the inclusion criteria in the current study. Blood specimens were taken from COVID-19 individual with positive PCR in Eppendorf tubes and, after 20 min, centrifuged for 4 min at 3000 rpm. The serum was then separated from the plasma and kept in the freezer at − 20 °C. Finally, all taken sera were submitted to the Department of Parasitology and Mycology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran, for further examination.
Sociodemographic and clinical evaluation
By using a questionnaire checklist, sociodemographic data such as age, gender, clinical data including hospitalization or non-hospitalization due to COVID19, duration of symptoms, length of hospital stay, place of residence, history, different signs and symptoms, occupation, use of a ventilator, and type of treatment were documented. All extra data was taken from the patient’s physicians and documented records of medical examination.
Serological analysis
The serum samples were screened for two main anti-T. gondii antibodies, including IgM and IgG, by using a human Anti-T. gondii IgM and IgG by ELISA kit (Vircell, Granada, Spain), following the manufacturer’s protocol. For all samples, the optical density was determined at the wavelength of 450 nm, and both antibodies’ titers equal to or above 1.1 were considered positive. High IgM titer is an indication of active or acute toxoplasmosis, but increased IgG is used for showing latent toxoplasmosis. The tests had a sensitivity of 99.9% and specificity of 100%.
Statistical analysis
SPSS software (version 19; SPSS Inc., Chicago, IL, USA) was applied to the statistical analysis of the data. Kolmogorov–Smirnov (KS) test was used to examine the normal distribution of the quantitative variables. Frequency, median and interquartile ranges (IQR) were used to describe quantitative variables. The Chi-square and student’s t-tests were utilized to investigate the relationship between qualitative variables. Using Kruskal-Wallis test, the age variable was compared in all tests. P-values below 0.05 were considered statistically significant.
Results
In the present study, 161 PCR-positive COVID-19 patients were studied in the referral centers of Gonbad-e-Kavus, Kalaleh, and Minoodasht counties in the northern part of Iran (Fig. 1). Among the participants, 81 (50.3%) were female, and 80 (49.7%) were male. The mean age of participants was 50.0 (23%). Table 1 presents the rate of infection with T. gondii in COVID-19 patients. Complete sociodemographic, clinical data and serological analysis are shown in Tables 2 and 3. The analyzed data of the independent t-test elucidated that the mean age of participants was not significantly different among both men and women (P > 0.05). Among 161 PCR-positive COVID-19 individuals, 39 (24.2%), 41 (25.5%), and 60 (37.3%) had a fever above 38 °C, cough, and dyspnea, respectively, which showed the severe form of COVID-19. Our finding has shown no significant correlation between both gender and the severity of COVID-19 symptoms (P > 0.05) (Tables 2 and 3).
Table 1.
The prevalence of Toxoplasma gondii infection in COVID-19 patients
| Toxoplasma gondii IgM | Toxoplasma gondii IgG | |||||
|---|---|---|---|---|---|---|
| Positive | Borderline | Negative | Positive | Borderline | Negative | |
| N (%) | 8 (5.0) | 6 (3.7) | 147 (91.3) | 42 (26.1) | 5 (3.1) | 114 (70.8) |
Table 2.
Clinical features in COVID-19 positives and results of Toxoplasma IgM test
| Variable | All patients N (%) | Toxoplasma IgM (Positive) N (%) | Toxoplasma IgM (Borderline) N (%) | Toxoplasma IgM (Negative) N (%) | P value | |
|---|---|---|---|---|---|---|
| Sociodemographic features | Female | 81 (50.3) | 5 (62.5) | 5 (83.3) | 71 (48.3) | 0.189 |
| Male | 80 (49.7) | 3 (37.5) | 1 (16.7) | 76 (51.7) | ||
| Age [median (IQR)] | 50.0 (23) | 44.5 (26) | 49.5 (24) | 51.0 (23) | 0.464 | |
| Contact with animals | 44 (27.3) | 44 (50.0) | 3 (50.0) | 37 (25.2) | 0.138 | |
| No contact with animals | 117 (72.7) | 44 (50.0) | 3 (50.0) | 110 (74.8) | ||
| Clinical symptoms and signs | Fever > 38 °C | 39 (24.2) | 1 (12.5) | 2 (33.3) | 36 (24.5) | 0.645 |
| Cough | 41 (25.5) | 3 (37.5) | 3 (50.0) | 35 (23.8) | 0.256 | |
| Other | 17 (10.6) | 0 (0.0) | 0 (0.0) | 17 (11.6) | 0.405 | |
| Shortness of breath | 60 (37.3) | 4 (50.0) | 1 (16.7) | 55 (37.4) | 0.439 | |
| Non-specific symptoms | Body pain | 21 (13.0) | 0 (0.0) | 3 (50.0) | 18 (12.2) | 0.014 |
| Headache | 18 (11.2) | 1 (12.5) | 0 (0.0) | 17 (11.6) | 0.673 | |
| Trembling | 13 (8.1) | 0 (0.0) | 0 (0.0) | 13 (8.8) | 0.510 | |
| Diarrhea | 5 (3.1) | 0 (0.0) | 0 (0.0) | 5 (3.4) | 0.782 | |
| Comorbidities | Hypertension | 20 (12.4) | 0 (0.0) | 1 (16.7) | 19 (12.9) | 0.530 |
| Chronic heart disease | 25 (15.5) | 0 (0.0) | 1 (16.7) | 24 (16.3) | 0.641 | |
| Chronic pulmonary disease | 13 (8.1) | 1 (12.5) | 1 (16.7) | 11 (7.5) | 0.645 | |
| No spleen and chronic hepatic disease | 6 (3.7) | 0 (0.0) | 0 (0.0) | 6 (4.1) | 0.743 | |
| Diabetes | 12 (7.5) | 2 (25.0) | 1 (16.7) | 9 (6.1) | 0.096 | |
| Patient condition | Death | 113 (70.6) | 3 (37.5) | 3 (50.0) | 107 (73.3) | 0.051 |
| Recovery (alive) | 47 (29.4) | 5 (62.5) | 3 (50.0) | 39 (26.7) |
Table 3.
Clinical features in COVID-19 positives and results of Toxoplasma IgG test
| Characteristic | All patients N (%) |
Toxoplasma IgG (Positive) N (%) | Toxoplasma IgG (Borderline) N (%) | Toxoplasma IgG (Negative) N (%) | P value | |
|---|---|---|---|---|---|---|
| Sociodemographic features | Female | 81 (50.3) | 22 (52.4) | 0 (0.0) | 59 (51.8) | 0.073 |
| Male | 80 (49.7) | 20 (47.6) | 5 (100.0) | 55 (48.2) | ||
| Age [median (IQR)] | 50.0 (23) | 51.5 (22) | 53.0 (19) | 48.5 (24) | 0.639 | |
| Contact with animals | 44 (27.3) | 40 (95.2) | 1 (20.0) | 3 (2.6) | < 0.001 | |
| No contact with animals | 117 (72.7) | 2 (4.8) | 4 (80.0) | 111 (97.4) | ||
| Clinical symptoms and signs | Fever > 38 °C | 39 (24.2) | 9 (21.4) | 0 (0.0) | 30 (26.3) | 0.359 |
| Cough | 41 (25.5) | 10 (23.8) | 0 (0.0) | 31 (27.2) | 0.378 | |
| Other | 17 (10.6) | 3 (7.1) | 2 (40.0) | 12 (10.5) | 0.078 | |
| Shortness of breath | 60 (37.3) | 20 (47.6) | 3 (60.0) | 37 (32.5) | 0.125 | |
| Non-specific symptoms | Body pain | 21 (13.0) | 7 (16.7) | 0 (0.0) | 14 (12.3) | 0.523 |
| Headache | 18 (11.2) | 3 (7.1) | 0 (0.0) | 15 (13.2) | 0.413 | |
| Trembling | 13 (8.1) | 5 (11.9) | 0 (0.0) | 8 (7.0) | 0.487 | |
| Diarrhea | 5 (3.1) | 1 (2.4) | 0 (0.0) | 4 (3.5) | 0.863 | |
| Comorbidities | Hypertension | 20 (12.4) | 6 (14.3) | 0 (0.0) | 14 (12.3) | 0.655 |
| Chronic heart disease | 25 (15.5) | 4 (9.5) | 2 (40.0) | 19 (16.7) | 0.169 | |
| Chronic pulmonary disease | 13 (8.1) | 2 (4.8) | 0 (0.0) | 11 (9.6) | 0.487 | |
| No spleen and chronic hepatic disease | 6 (3.7) | 1 (2.4) | 0 (0.0) | 5 (4.4) | 0.762 | |
| Diabetes | 12 (7.5) | 4 (9.5) | 1 (20.0) | 7 (6.1) | 0.430 | |
| Patient condition | Death | 113 (70.6) | 25 (59.5) | 4 (80.0) | 84 (74.3) | 0.178 |
| Recovery (alive) | 47 (29.4) | 17 (40.5) | 1 (20.0) | 29 (25.4) |
Anti-T. gondii IgM and IgG antibodies were identified in 8/161 (5.0%) and 42/161 (26.1%) COVID-19 patients, respectively. No significant relationships were found between Toxoplasma IgM and IgG results with clinical signs, age, sex, and comorbidities and also the mortality rate of due to COVID-19. However, in non-specific symptoms, the results showed that body pain was significantly different in individuals of different Toxoplasma IgM groups (P > 0.05) (Table 2). Accordingly, out of 147 people with Toxoplasma IgM negative test, 18 patients (12.2%) reported physical pain, which was 0% and 50% for those with a positive test and borderline test, respectively. The level of T. gondii IgG antibody was higher in COVID-19 patients in comparison with IgM antibody (Table 1). The results of the Toxoplasma IgG test revealed a significant relationship between animal contacts and Toxoplasma IgG test results (P > 0.05). Accordingly, 95.2% of those with a positive IgG test had a history of contact with animals, which was 20% and 2.6% in borderline individuals and those with a positive test, respectively (Table 3).
Discussion
Patients with COVID-19 who suffer from underlying diseases or receiving immunosuppressive therapy are at increased risk for opportunistic infections, in which prevention, screening, early diagnosis, and treatment is recommended (Abdoli et al. 2021; Mewara et al. 2021). According to the literature review, the association between toxoplasmosis and the severity of COVID-19 is not well-known. A study demonstrated a significant negative correlated variation between toxoplasmosis and COVID-19 (Jankowiak et al. 2020a).
We evaluated 161 PCR-positive COVID-19 individuals for acute and latent (chronic) toxoplasmosis by using anti-T. gondii antibodies (IgM and IgG). The current study demonstrated that 8/161 (5.0%) and 42/161 (26.1%) of the patients with COVID-19 were positive for anti-T. gondii antibodies (IgM and IgG). The study revealed a relatively high prevalence of latent toxoplasmosis in these patients; however, this high prevalence was not statistically significant Although in areas with high temperature, humidity and precipitation, the prevalence rates of toxoplasmosis are higher (Rostami et al. 2021). Several reports in the other parts of Iran showed reduced levels of T. gondii seroprevalence in different diseases (Ghaffari et al. 2021; Kalantari et al. 2018), which is in agreement with the current experiment.
In a study in Iran, the rate of latent IgG positive toxoplasmosis was reported at about 39% (Daryani et al. 2014). In another study in Golestan province, the rate of chronic Toxoplasmosis during pregnancy was reported to be 39.8% (Sharbatkhori et al. 2014). Nairi et al. in 2020 reported a global rate of antibodies against Toxoplasma of 33% and 43% in women who had recently had an abortion and those with previous abortion, respectively (Nayeri et al. 2020). Najm et al. in 2020 reported positive abortion between rubella virus infection and T. gondii (Najm et al. 2020). In Egypt, the rate of parasitic infections was 68.8%, in patients with COVID-19 and the most common parasite was T. gondii, with a prevalence of 22.4% (Abdel-Hamed et al. 2021).
An essential covariant factor in the toxoplasmosis epidemiology was recorded as age. Various experiments have revealed the increased rate of seroprevalence for T. gondii infection with aging, and the highest levels were reported above the age of 50 (Kalantari et al. 2018). In our study, the average age of COVID-19 patients was 50 years which is similar to the mentioned experiments. However, our study elucidated that the rate of seropositivity for both acute and latent Toxoplasma infection did not remarkably increase by age. Furthermore, there were no significant associations between the participants’ gender and the rate of seroprevalence regarding acute and latent Toxoplasma infection. These findings are not consistent with other documented studies that report a significant relationship between the seropositivity for T. gondii, gender, and age (Achaw et al. 2019, Ghaffari et al. 2021). This is possibly due to variations in the type and nature of the study population and its related regions (Ghaffari et al. 2021).
In the current study, we have confirmed no statistical relationship between COVID-19 outcomes and/or severity and acute/latent Toxoplasma infection, which has been demonstrated in preceding studies (Ghaffari et al. 2021; Montazeri et al. 2022). However, our results do not support those of Jankowiak et al. (2020a) which they found a significant negative correlated variation between toxoplasmosis and COVID-19.
In some viral infections such as AIDS, Toxoplasma latent tissue cysts containing bradyzoites show a higher risk of being reactivated by a decrease in CD4 + T cells and often present as Toxoplasma encephalitis. In these cases, reactivation is a possible scenario in patients with COVID-19 experiencing progressive lymphopenia but may not be considered due to the characteristics of Toxoplasma encephalitis, including neuropsychiatric symptoms including seizure, and altered sensorium, are also seen patients with COVID-19 (Roe 2021). In several studies, researchers discussed that various parasitic co-infections might be indicate the severity of COVID-19 (Ghaffari et al. 2021; Montazeri et al. 2022; Wolday et al. 2021). This may be due to host immune responses regarding T-helper 1 (TH1), which induce higher levels of IFN-ɣ and causes intense host tissue damage (Montazeri et al. 2022). Several animal co-infection in vivo experiments elucidated positive/negative impacts on the immunity against viral infections. In this regard, further studies should be conducted on COVID-19 severity and various parasitic co-infection is needed.
The main limitation of the present study could be related to the kind of study in which, due to the COVID-19 pandemic, we could not take samples from healthy individuals in the same period for a case-control study. It is recommended that ongoing experiments be carried out as multicenter studies in distinct geographic regions of the country to make more detailed health decisions about the relationship between acute and latent toxoplasmosis and COVID-19 severity. In addition, to precisely analyze the relationship between toxoplasmosis and the severity of COVID-19 symptoms, more comprehensive studies with higher sample sizes are required.
Conclusion
To our knowledge, the current study is a fundamental experiment that scrutinized the seroprevalence of active and latent toxoplasmosis in patients with COVID-19 and investigated their relationship with the severity of COVID-19. These findings showed that acute and latent toxoplasmosis infections are prevalent amongst COVID-19 patients; however, no significant and direct association was seen between toxoplasma infections and COVID-19 severity. Therefore, toxoplasmosis is not considered a risk factor for COVID-19.
Acknowledgements
We thank Dr. Zahra Asadgol (Health Deputy of Iran University of Medical Sciences, Tehran, Iran) for technical assistance in GIS map design.
Authors’ contribution
AG, AB: Formal analysis, investigation, conceptualization, methodology, validation, software. MS, SMM, PM: Investigation, methodology, software, data curation, writing- original draft preparation. ZS, SMH: Visualization, validation, writing - review and editing. RRS, SHH: Formal analysis, investigation, conceptualization, methodology, validation, software, resources, supervision, writing- original draft preparation, writing - review and editing.
Funding
Isfahan University of Medical Sciences funded the present study, with grant number 53462 to Dr. Seyed Hossein Hejazi. The funders were not involved in any steps of designing the study, collecting and analyzing data, publication decision, or manuscript preparation.
Data Availability
The corresponding author can provide the datasets collected during this study upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Seyed Hossein Hejazi and Raheleh Rafiei-Sefiddashti have contributed equally to this work.
Contributor Information
Seyed Hossein Hejazi, Email: hejazi@med.mui.ac.ir.
Raheleh Rafiei-Sefiddashti, Email: rafiei.r@iums.ac.ir.
References
- Abdel-Hamed EF, Ibrahim MN, Mostafa NE, Moawad HS, Elgammal NE, Darwiesh EM, El-Rafey DS, ElBadawy NE, Al-Khoufi EA, Hindawi SI. Role of interferon gamma in SARS-CoV-2-positive patients with parasitic infections. Gut Pathogens. 2021;13(1):1–7. doi: 10.1186/s13099-021-00427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdoli A, Falahi S, Kenarkoohi A (2021) COVID-19-associated opportunistic infections: a snapshot on the current reports. Clin Exp Med, pp 1–20 [DOI] [PMC free article] [PubMed]
- Achaw B, Tesfa H, Zeleke AJ, Worku L, Addisu A, Yigzaw N, Tegegne Y. Sero-prevalence of Toxoplasma gondii and associated risk factors among psychiatric outpatients attending University of Gondar Hospital, Northwest Ethiopia. BMC Infect Dis. 2019;19(1):1–8. doi: 10.1186/s12879-019-4234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamorovat M, Sharifi I, Aflatoonian MR, Karamoozian A, Tahmouresi A, Jafarzadeh A, Heshmatkhah A, Sharifi F, Salarkia E, Khaleghi T (2021) Prophylactic effect of cutaneous leishmaniasis against COVID-19: a case-control field assessment.International J Infect Dis [DOI] [PMC free article] [PubMed]
- Can H, Alak SE, Köseoğlu AE, Döşkaya M, Ün C. Do Toxoplasma gondii apicoplast proteins have antigenic potential? An in silico study. Comput Biol Chem. 2020;84:107158. doi: 10.1016/j.compbiolchem.2019.107158. [DOI] [PubMed] [Google Scholar]
- Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, Rahimi M-T, Sharif M. Seroprevalence of Toxoplasma gondii in the iranian general population: a systematic review and meta-analysis. Acta Trop. 2014;137:185–194. doi: 10.1016/j.actatropica.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Fallahi S, Rostami A, Shiadeh MN, Behniafar H, Paktinat S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J Gynecol Obstet Hum Reprod. 2018;47(3):133–140. doi: 10.1016/j.jogoh.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Shibre T, Cleare AJ. Toxoplasmosis as a cause for behaviour disorders-overview of evidence and mechanisms. Folia Parasitol. 2010;57(2):105. doi: 10.14411/fp.2010.013. [DOI] [PubMed] [Google Scholar]
- Firouzeh N, Ziaali N, Sheibani V, Doustimotlagh AH, Afgar A, Zamanpour M, Keshavarz H, Shojaee S, Shafiei R, Esmaeilpour K. Chronic Toxoplasma gondii infection potentiates Parkinson’s disease course in mice model. Iran J Parasitol. 2021;16(4):527. doi: 10.18502/ijpa.v16i4.7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013;29(4):156–163. doi: 10.1016/j.pt.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Ghaffari S, Kalantari N, Gorgani-Firouzjaee T, Bayani M, Jalali F, Daroonkola MA (2021) Is COVID-19 associated with latent toxoplasmosis? Environ Sci Pollut Res, pp 1–5 [DOI] [PMC free article] [PubMed]
- Iesa MA, Osman ME, Hassan MA, Dirar AI, Abuzeid N, Mancuso JJ, Pandey R, Mohammed AA, Borad MJ, Babiker HM. SARS-CoV-2 and Plasmodium falciparum common immunodominant regions may explain low COVID-19 incidence in the malaria-endemic belt. New Microb New Infect. 2020;38:100817. doi: 10.1016/j.nmni.2020.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowiak Ł, Rozsa L, Tryjanowski P, Møller AP. A negative covariation between toxoplasmosis and CoVID-19 with alternative interpretations. Sci Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-69351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantari N, Sheikhansari M, Ghaffari S, Alipour J, Gorgani-Firouzjaee T, Tamadoni A, Bayani M. Seroprevalence and molecular detection of Toxoplasma gondii in young healthy blood donors in Northern Iran. Trop Biomed. 2018;35:1017–1027. [PubMed] [Google Scholar]
- Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewara A, Sahni N, Jain A. Considering opportunistic parasitic infections in COVID-19 policies and recommendations. Trans R Soc Trop Med Hyg. 2021;115(11):1345–1347. doi: 10.1093/trstmh/trab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri M, Nakhaei M, Fakhar M, Pazoki H, Pagheh AS, Nazar E, Zakariaei Z, Mirzaeian H, Sharifpour A, Banimostafavi ES (2022) Exploring the association between latent Toxoplasma gondii infection and COVID-19 in hospitalized patients: first registry-based study. Acta Parasitol, pp 1–8 [DOI] [PMC free article] [PubMed]
- Moriyama M, Hugentobler WJ, Iwasaki A (2020) Seasonality of respiratory viral infections. Annu Rev Virol 7(1), https://www.annualreviews.org/doi/pdf/10.1146/annurev-virology-012420-022445 [DOI] [PubMed]
- Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240(1):269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- Najm MA, Razaak HAA, Fahad H. Detect the infection with Rubella virus and toxoplasmosis in pregnancy causes suffering from early abortion by using real time PCR. Medico Legal Update. 2020;20(1):397–402. [Google Scholar]
- Nayeri T, Sarvi S, Moosazadeh M, Amouei A, Hosseininejad Z, Daryani A (2020) The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: a systematic review and meta-analysis. PLoS Negl Trop Dis 14(3), e0008103 [DOI] [PMC free article] [PubMed]
- Norouzi M, Tabaei SJS, Niyyati M, Saber V, Behniafar H. Genotyping of Toxoplasma gondii strains isolated from patients with ocular toxoplasmosis in Iran. Iran J Parasitol. 2016;11(3):316. [PMC free article] [PubMed] [Google Scholar]
- Roe K. The symptoms and clinical manifestations observed in COVID-19 Patients/Long COVID-19 symptoms that parallel Toxoplasma gondii infections. J Neuroimmune Pharmacol. 2021;16(3):513–516. doi: 10.1007/s11481-021-09997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A, Riahi SM, Esfandyari S, Habibpour H, Mollalo A, Mirzapour A, Behniafar H, MohammadiMoghadam S, Kyvanani NA, Aghaei S. Geo-climatic factors and prevalence of chronic toxoplasmosis in pregnant women: a meta-analysis and meta-regression. Environ Pollut. 2021;288:117790. doi: 10.1016/j.envpol.2021.117790. [DOI] [PubMed] [Google Scholar]
- Senapati S, Banerjee P, Bhagavatula S, Kushwaha PP, Kumar S. Contributions of human ACE2 and TMPRSS2 in determining host–pathogen interaction of COVID-19. J Genet. 2021;100(1):1–16. doi: 10.1007/s12041-021-01262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharbatkhori M, Moghaddam YD, Pagheh AS, Mohammadi R, Mofidi HH, Shojaee S. Seroprevalence of Toxoplasma gondii infections in pregnant women in Gorgan city, Golestan province, Northern Iran-2012. Iran J Parasitol. 2014;9(2):181. [PMC free article] [PubMed] [Google Scholar]
- Sina S, Mohammad JM, Reza S, Anita M, Soudabeh E, Hadi M. Determination of parasitic burden in the brain tissue of infected mice in acute toxoplasmosis after treatment by fluconazole combined with sulfadiazine and pyrimethamine. Eur J Med Res. 2021;26(1):1–8. doi: 10.1186/s40001-021-00537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, Hooft L, Emperador D, Domen J (2021) Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev; 2(2):CD013665 [DOI] [PMC free article] [PubMed]
- Weeratunga P, Herath TU, Kim T-H, Lee H-C, Kim J-H, Lee B-H, Lee E-S, Chathuranga K, Chathuranga WG, Yang C-S. Dense granule Protein-7 (GRA-7) of Toxoplasma gondii inhibits viral replication in vitro and in vivo. J Microbiol. 2017;55(11):909–917. doi: 10.1007/s12275-017-7392-5. [DOI] [PubMed] [Google Scholar]
- Wolday D, Tasew G, Amogne W, UrbanUrban B, Schallig HD, Harris V, de Wit TFR. Interrogating the impact of intestinal parasite-microbiome on pathogenesis of COVID-19 in Sub-Saharan Africa. Front Microbiol. 2021;12:614522. doi: 10.3389/fmicb.2021.614522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarean M, Shafiei R, Gholami M, Fata A, Rahmati Balaghaleh M, Karimi A, Tehranian F, Hasani A, Akhavan A. Seroprevalence of Anti–Toxoplasma gondii antibodies in healthy voluntary blood donors from Mashhad City, Iran. Arch Iran Med. 2017;20(7):441–445. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author can provide the datasets collected during this study upon reasonable request.