Abstract
This study aimed to investigate the relationship between the muscle shear modulus of the biceps brachii, urinary titin N-terminal fragment (UTF), and other damage markers after eccentric exercise. Seventeen healthy males performed five sets of ten eccentric exercises with dumbbells weighing 50% of the maximum voluntary contraction (MVC) at the elbow joint. Muscle shear modulus with range of interest set to only biceps brachii muscle measured by ultrasound shear wave elastography, UTF, MVC, range of motion (ROM), and soreness (SOR) were recorded before, immediately after, and 1, 24, 48, 72, 96, and 168 h after eccentric exercise. Each marker changed in a time course pattern, as found in previous studies. The peak shear modulus showed a moderate negative correlation with peak MVC (r = –0.531, P < 0.05) and a strong positive correlation with peak UTF (r = 0.707, P < 0.01). Our study results revealed a significant relationship between muscle strength, shear modulus measured by ultrasound SWE, and titin measured by UTF, as a non-invasive damage marker after eccentric exercise to track changes in EIMD.
Key points.
This study was investigated based on the hypothesis that the shear modulus measured by ultrasound shear wave elastography of biceps brachii muscle and urinary titin N-terminal fragment (UTF) after eccentric exercise that occurs during eccentric exercises are involved in damage markers similar to those used in previous studies.
The results revealed that shear modulus showed a significant relationship between muscle strength and titin content.
The relationship between shear modulus as an index of stiffness and titin measured by UTF will be useful for observing changes in skeletal muscle condition that result from high-intensity training and games/practices in the field of sports competition.
Key words: Shear wave elastography, stiffness, sarcomere, non-invasive method, exercise-induced muscle damage
Introduction
Muscle damage often occurs after intense/strenuous exercise, training, or sports. Among them, exercise-induced muscle damage (EIMD), including eccentric contraction, causes stronger stiff muscles (Murayama et al., 2000) and leads to muscle soreness associated with damage to the sarcomeres and muscle fibers (Howell et al., 1993; Yu et al., 2003). Consequently, there is a prolonged reduction in muscle force (Yamaguchi et al., 2020b). To track changes in skeletal muscles due to EIMD, a non-invasive method that allows repeated measurements is required. These methods are also important for exploring the possibility of applying them to field measurements beyond the laboratory measurement framework. EIMD-affected muscle fibers are locally damaged (Jang et al., 2018), and proteins found in the matrix and mitochondria are triggered by this damage and released into the blood (Kanda et al., 2014). The concentration of these proteins in the blood can be used to objectively measure EIMD, and creatine kinase (CK), in particular, has been used in several previous studies as the most common and sensitive marker (Brancaccio et al., 2010; Stožer et al., 2020). However, it has major limitations which are as follows: 1) it may pose a physical and mental barrier for study participants owing to its invasive nature (Yamaguchi et al., 2020b) and 2) blood samples can only be collected by qualified personnel (Kanda et al., 2017; Yamaguchi et al., 2020c). On the other hand, as a non-invasive method to track changes in EIMD, biochemical measurements that can be performed using the urinary titin N-terminal fragment (UTF) have recently been developed (Kanda et al., 2017; Yamaguchi et al., 2020b; Yamaguchi et al., 2020c). UTF after EIMD is correlated with related muscle damage indicators (e.g., maximum voluntary contraction [MVC] (r = -0.596), range of motion [ROM] (r = -0.738), and soreness [SOR] (r = 0.626)) and especially with CK (r = 0.966) (Yamaguchi et al., 2020c). In addition, it sensitively reflects the repeated bout effect (Yamaguchi et al., 2020b) and resolves the considerations and/or limitations mentioned above. Therefore, UTF may also be used as an alternative to plasma biomarkers of EIMD. The application of UTF in the field of sports science has just begun; for example, the relationship between local changes occurring in muscles involved in the production of MVC and muscle mechanical property (e.g., muscle stiffness) changes related to ROM has not yet been understood. The use of UTF, which can directly quantify changes that specifically occur in EIMD, further deepens the understanding of the relationship between related muscle damage markers, such as muscle force, ROM, and SOR, including CK.
The mechanical properties of skeletal muscle are also vital for understanding muscle function, muscle morphology, and ultimately muscle condition (Murayama et al., 2000). Muscle stiffness, one of the mechanical properties of muscles, is affected by conditions, such as spasms, edema, contracture, swelling, and compartment syndrome (Inami and Kawakami, 2016), and changes in muscle stiffness greatly depend on EIMD (Murayama et al., 2000). Although histological examination of muscle biopsy samples by determining the number of myofibrillar disruptions can accurately quantify local muscle damage, including EIMD, (Raastad et al., 2010), it is unlikely to be routinely used as the first choice for muscle stiffness. In addition, the amount of muscle stiffness can be evaluated by measuring the relationship between the joint angle and torque, but this measurement is considered to represent both muscle and non-muscle, such as tendons and ligaments and does not reflect muscle stiffness directly (Inami and Kawakami, 2016). However, the use of ultrasonic shear wave elastography (SWE) to directly measure muscle stiffness has increased in recent years. Muscle shear modulus in vivo measured by SWE measurement is used to noninvasively analyze hardness distribution of the muscle tissue as an index of mechanical property changes in the muscle and can be safely used for repetitive measurements (Inami and Kawakami, 2016).
According to Lacourpaille et al. (Lacourpaille et al., 2014), on investigating the muscle shear modulus (biceps brachii and brachialis) of three different elbow joint angles (70º, 110º, and 160°) after eccentric exercise, no significant differences were observed after 1 h at 70°, and the shear modulus of biceps brachii muscles was increased by 46 ± 25% at 110° and by 72 ± 50% at 160°. The shear modulus of the biceps brachii at 160° was significantly higher even after 48 h. Hence, the length dependency of changes in the muscle mechanical properties was observed. An increase in muscle shear modulus due to EIMD that occurs after eccentric exercise has also been shown in other studies (Lacourpaille et al., 2017; Xu et al., 2019). Increasing the sensitivity of muscle fibers to Ca2+ is considered as one reason for changes in muscle shear modulus (Stephenson and Wendt, 1984; Balnave and Allen, 1996; Claflin et al., 1998), which may be due to the involvement of calcium-dependent physiological processes, such as cross-bridge number or titin giant protein (Lacourpaille et al., 2014).
We hypothesized that the muscle shear modulus measured by ultrasound SWE and titin measured by UTF after EIMD from eccentric exercises are involved in similar damage markers used in previous studies (Kanda et al., 2017; Yamaguchi et al., 2020b; Yamaguchi et al., 2020c), such as muscle strength, ROM, and SOR. This study aimed to investigate the relationship between the muscle shear modulus of the biceps brachii, titin, and other damage markers.
Methods
Participants
Seventeen healthy male participants (age, 22.0 ± 4.7 years; height, 1.7 ± 0.43 m; weight, 61.6 ± 4.7 kg) participated in this study. The sample size was estimated based on our previous study using G*Power (G*Power 3.1.9.2; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) with an effect size of 0.6, α level of 0.05, and power (1 - β) of 0.80 for a possible correlation between UTF and shear modulus, showing that 17 participants were necessary.
During the month prior to and during the experiment, the patients were requested and cautioned to avoid strenuous or unfamiliar exercise, to maintain normal dietary and sleeping habits, and not to take anti-inflammatory drugs (e.g., non-steroidal anti-inflammatory drugs) or dietary supplements (e.g., vitamins, proteins/amino acids).
They were informed of the nature, aims, and risks associated with the experimental procedures before their written consent was provided. This study was conducted following the principles of the Declaration of Helsinki. The present study was approved by the university ethics committee (21-003).
Experimental design
The study was conducted over nine days. Before the experiment, the participants were familiarized with the method of muscle strength measurement. On the first day, before eccentric exercise, a test session was conducted [PRE], and eccentric exercise was performed on the elbow flexor muscles. After eccentric exercise, same test sessions were repeated immediately after [POST], and 1 hour [POST-1], 24 hours [Day 1], 48 hours [Day 2], 72 hours [Day 3], 96 hours [Day 4], and 168 hours [Day 7] later. All measurements were taken in the same order at each test session [PRE, POST, POST-1, Day1–Day7].
Eccentric exercise
Participants were seated on an arm-curl bench so that the hip was flexed at 85° (0° = full hip extension). As described in a previous study (Nosaka and Newton, 2002), during exercise, participants were tightly secured to the arm-curl bench with nonelastic straps. They completed five sets of ten eccentric exercises with dumbbells weighing 50% of the elbow joint MVC of the left arm measured in the PRE session. The elbow joint was extended from 90° to 180° (180° = full extension) to the 60-bpm rhythm of the metronome (i.e., extended 90° in 5 s). To ensure that only eccentric actions were performed, the examiner supported the participants’ elbow flexion during the concentric phase. All actions were repeated every 3 s, and a recovery period of 2 min was performed between each set. At this time, to prevent active concentric contraction and weakness during eccentric contractions, muscle activity was monitored with an electromyogram (EMG; DataLITE EMG, Biometrics Ltd, Japan) attached to the skin of the biceps brachii. The sampling frequency was set to 1000 Hz, and an offline digital filter was applied with a band-pass filter of 10–450 Hz. Surface EMG signals of the biceps brachii muscles were recorded during eccentric exercise and muscle strength measurements.
Muscle strength [elbow MVC]
Maximal isometric elbow flexion strength was evaluated during 5 s of isometric MVC performed at an elbow angle of 90° using a hand-held dynamometer (Mobie, SAKAI Medical Co., Ltd. Japan). Two trials were performed, and the trial with the highest isometric strength (MVC) was considered for further analysis. If the difference between the two measurements exceeded 10%, a third measurement was taken.
Active ROM
A semi-permanent marker was used to mark the center of the acromion, the lateral epicondyle, and the ulnar styloid. The elbow joint angle was photographed in a relaxed and flexed state to determine the active ROM, and the angle formed between the line connecting the center of the acromion and the lateral epicondyle and that connecting the lateral epicondyle and the ulnar styloid was calculated using ImageJ (version 1.39, NIH, USA). After the relaxed and flexed angles were determined, the re-relaxed angle was subtracted from the flexed angle to determine the ROM of the elbow joint (Yamaguchi et al., 2020b).
Subjective evaluation
We measured muscle soreness (SOR) and muscle fatigue as subjective evaluation of muscle damage (Nakagawa et al., 2018). SOR and muscle fatigue were assessed using a 100-mm visual analogue scale (VAS), with 0 indicating no pain (or no fatigue) and 100 representing extreme pain (or extreme fatigue). SOR was measured when participants actively extended their arms. Participants were instructed to hold their shoulder joints at 90° flexion and elbow joints at 180° active extension and to mark their levels of perceived soreness on the VAS. In muscle fatigue, participants evaluated the perception of “difficulty in an active movement during elbow joint extension/flexion” in the muscles according to the findings of recent studies (Place et al., 2010; Shi et al., 2021).
Muscle shear modulus of the biceps brachii muscle
The shear modulus of the biceps brachii muscle was measured at 180° elbow joint, 10° shoulder joint extension, and 30° shoulder joint abduction while lying supine on a bed. The ultrasonographic apparatus used an ultrasound shear wave scanner in “shear wave” mode coupled with a linear array transducer (Aplio 300, Canon Co., Ltd., Japan). The ultrasound transducer was placed over the muscle belly of the long head of the biceps brachii (i.e., the location of the probe was approximately 50% of the upper arm length from the acromial process of the scapula to the lateral epicondyle of the humerus) (Murayama et al., 2022). Using a semi-permanent ink marker, the probe was attached at the same location across sessions and days, and the measurement marks were maintained during the experimental period. The images were acquired thrice (i.e., three images were acquired) after ensuring that the color map and propagation imaging of the shear wave speed was stable for a few seconds in the session. When it was uncomfortable to fully extend the elbows due to pain, the elbow joint was slowly extended and fully extended over time while consulting the participant so that the stretch reflex does not occur (Murayama et al., 2000). In addition, throughout the scanning, care was taken not to press and deform the muscles. The mean shear modulus was calculated over the largest region of interest, from which the aponeurosis and subcutaneous adipose tissues were excluded on reference to B-mode images. The elastographic images were transferred to a computer as bitmap (.bmp) files, calculated and averaged using dedicated software (iElastographic image analyzer, Takei Scientific Instruments Co., Ltd., Japan), and used for further analyses. All measurements and analyses of the ultrasonography data were performed by an experienced examiner (>10 years of practice). The shear modulus in the resting muscle condition had a coefficient of variation of 2.0% ± 1.9% and an intraclass correlation coefficient of 0.965 (P < 0.001).
Titin N-terminal fragment excretion assays
Approximately 3 ml of urine was collected from each participant to measure UTF concentrations via an enzyme-linked immunosorbent assay (ELISA) system using a titin N-terminal fragment assay kit (Immuno-Biological Laboratories Co. Ltd., Japan) as per a previous study (Yamaguchi et al., 2020a). The samples were stored at -20°C for later analyses. Thawed urine samples were diluted 1:5–1:500 so that the diluted samples were within the linear detection range. Diluted samples and standard solutions were added to each antibody-coated well of the 96-well microplates and incubated at 37°C for 60 min. Subsequently, the microplates were washed four times with wash buffer, labeled antibodies were added to each well, and the plates were incubated again at 37°C for 30 min. The tetramethylbenzidine solution was incubated at room temperature for 30 min after washing five times with the wash buffer. Stop solutions were added to each well as the final step of the ELISA procedure. Absorbance was measured using a microplate reader at the main wavelength of 450 nm and a sub-wavelength of 650 nm (Thermo Fisher Scientific, Multiskan FC, USA). UTF concentration was calculated using a linear regression model, and urinary creatinine levels were estimated using an automated analyzer (Bio Majesty JCA-BM8060, JEOL, Japan). UTF values were normalized relative to urinary creatine (Cr) (each raw data point in urine/Cr concentration) (Maruyama et al., 2016). The normal range of UTF concentration in the general population is 0.9–7.6 pmol/mg/dl (Yamaguchi et al., 2020b).
Statistical analysis
Values are expressed as the mean ± SD. The Shapiro–Wilk test was used for normal distribution analysis, and the normal distribution of MVC, ROM, SOR, shear module and UTF, but not muscle fatigue, were confirmed. The change in time course of muscle fatigue in the eccentric condition was compared using the Friedman test, and MVC, ROM, SOR, the shear module, and UTF were analyzed by one-way analysis of variance. Significant differences were found by the Bonferroni post hoc test for comparing values at different time points. This test indicated a non-normal distribution of the UTF data; therefore, a logarithmic transformation (log10) was applied before analysis (Lacourpaille et al., 2014). The statistical significance level was set at p < 0.05. All statistical analyses were performed using the IBM SPSS Statistics 28 software package (SPSS, Inc., Chicago, IL, USA).
Results
Figure 1, Figure 2 and Figure 3 summarize the changes in each muscle damage index at each time point.
Figure 1.

Changes in (a) maximum voluntary contraction [MVC], (b) range of motion [ROM], (c) soreness [SOR], and (d) muscle fatigue. The values for each time course were significantly different from those before the first session [PRE] (***p < 0.001, **p < 0.01, *p < 0.05).
Figure 2.
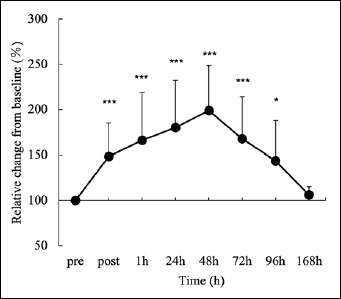
Changes in shear modulus (normalized change from baseline (%)). The values for each time course were significantly different from those before the first session [PRE] (***p < 0.001, *p < 0.05).
Figure 3.
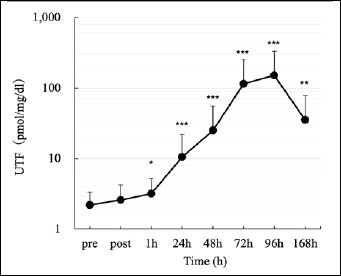
Changes in urinary titin N-terminal fragment [UTF]. The values for each time course were significantly different from those before the first session [PRE] (***p < 0.001, **p < 0.01, *p < 0.05).
MVC decreased significantly from an initial value of 18.6 ± 2.9 kgf to 9.6 ± 3.3 kgf (51.4 % from baseline) immediately after exercise and remained significantly lower than baseline until 168 hours after exercise (Figure 1a). ROM significantly decreased from an initial value of 163.4 ± 5.2° to 155.4 ± 8.5° (77.4 % from baseline) immediately after exercise and remained significantly lower than baseline until 168 h after exercise (Figure 1b). Pain on elbow extension (SOR) was significantly higher immediately after and 24-96 h after exercise than at baseline (Figure 1c). Shear modulus significantly increased from an initial value of 46.6 ± 8.8 kPa to 92.3 ± 27.3 kPa (198.9 % from baseline) 48 h after exercise and decreased until full recovery at 168 h after exercise (Figure 2). UTF increased significantly from an initial value of 2.2 ± 1.2 pmol/mg/dl to 151.3 ± 178.7 pmol/mg/dl 96 h after exercise and remained significantly higher than baseline until 168 hours after exercise (Figure 3).
Figure 4 and Table 1 presented the correlation coefficients between the peaks of the muscle damage indices after eccentric exercise. Peak shear modulus showed a moderate negative correlation with peak MVC (ρ = -0.531; P < 0.05) and a strong positive correlation with peak UTF (ρ = 0.648; P < 0.01). Peak UTF showed a moderate negative correlation with peak ROM (ρ = -0.504; P < 0.05) and a strong negative correlation with peak MVC (ρ = -0.751; P < 0.01).
Figure 4.

Correlation coefficients between damage markers. The relationship between (a) peak maximum voluntary contraction (MVC) and peak shear modulus, (b) peak MVC and peak logarithmically transformed urinary titin N-terminal fragment (UTF log), (c) peak range of motion (ROM) and peak UTF log, and (d) peak shear modulus and peak UTF log. **p < 0.01, *p < 0.05.
Table 1.
Correlation coefficients between peak value of damage markers.
| ROM | MVC | Soreness | Shear modulus | Urinary Titin | |
|---|---|---|---|---|---|
| ROM | 1.000 | ||||
| MVC | 0.686** | 1.000 | |||
| Soreness | 0.019 | -0.159 | 1.000 | ||
| Shear modulus | -0.417 | -0.531* | 0.314 | 1.000 | |
| Urinary Titin | -0.504* | -0.750** | 0.490 | 0.707** | 1.000 |
**p < 0.01,
*p < 0.05.
As shown in Table 2, after eccentric exercise, there was a negative correlation between UTF and ROM at 24 (r = -0.516, P < 0.05), 48 (r = -0.493, P < 0.05), 72 (r = -0.581, P < 0.05), and 96 hours (r = -0.610, P < 0.01); between UTF and MVC at 24 (r = -0.745, P < 0.01), 48 (r = -0.757, P < 0.01), 72 (r = -0.831, P < 0.01), 96 (r = -0.824, P < 0.01), and 168 hours (r = -0.772, P < 0.01). There was a positive correlation between UTF and SOR at 72 (r = 0.642, P < 0.01), 96 (r = 0.713, P < 0.01), and 168 hours (r = 0.622, P < 0.01); between UTF and muscle fatigue at 48 (ρ = 0.571, P < 0.05), 72 (ρ = 0.715, P < 0.01), 96 (ρ = 0.756, P < 0.01), and 168 hours (r = 0.490, P < 0.05); and between UTF and shear modulus at 24 (r = 0.495, P < 0.05), 48 (r = 0.525, P < 0.05), 72 (r = 0.686, P < 0.01), and 96 hours (r = 0.602, P < 0.05). As shown in Table 2, after eccentric exercise, there was a negative correlation between shear modulus and ROM at 72 (r = -0.672, P < 0.01) and 96 hours (r = -0.552, P < 0.05); and between shear modulus and MVC at 24 (r = -0.642, P < 0.01), 48 (r = -0.640, P < 0.01) 72 (r = -0.716, P < 0.01), and 96 hours (r = -0.645, P < 0.01). There was a positive correlation between shear modulus and SOR at 72 hours (r = 0.605, P < 0.01).
Table 2.
Correlation coefficients between each time point of damage markers.
| Urinary titin | ||||||||
|---|---|---|---|---|---|---|---|---|
| pre | post | 1h | 24h | 48h | 72h | 96h | 168h | |
| ROM | 0.186 | -0.348 | -0.220 | -0.516* | -0.493* | -0.581* | -0.610** | -0.113 |
| MVC | -0.452 | -0.410 | -0.465 | -0.745** | -0.757** | -0.831** | -0.824** | -0.772** |
| Soreness | 0.000 | 0.135 | -0.127 | 0.299 | 0.424 | 0.642** | 0.713** | 0.622** |
| Shear modulus | -0.292 | -0.118 | -0.029 | 0.495* | 0.525* | 0.686** | 0.602* | 0.056 |
| Shear modulus | ||||||||
| ROM | -0.042 | -0.373 | -0.368 | -0.312 | -0.387 | -0.672** | -0.552* | -0.140 |
| MVC | 0.101 | -0.379 | -0.346 | -0.642** | -0.640** | -0.716** | -0.645** | -0.091 |
| Soreness | 0.000 | 0.396 | 0.097 | -0.012 | 0.230 | 0.605* | 0.472 | 0.070 |
| Urinary titin | -0.292 | -0.118 | -0.029 | 0.495* | 0.525* | 0.686** | 0.602* | 0.056 |
**p < 0.01,
*p < 0.05
Discussion
This study was conducted based on the hypothesis that the muscle shear modulus measured by ultrasound SWE and titin measured by UTF after EIMD that occurs during eccentric exercises are involved in damage markers similar to those used in previous studies (Kanda et al., 2017; Yamaguchi et al., 2020b; Yamaguchi et al., 2020c). The results revealed that shear modulus showed a significant relationship between muscle strength and titin content, thereby supporting our hypothesis.
In this study, dumbbell exercise was used for eccentric exercise. According to a previous study (Damas et al., 2016), the degree of EIMD associated with eccentric exercise strongly appears in the amount of change in MVC. In the exercise task of this study, as described in a previous study (Nosaka and Newton, 2002), participants were tightly secured to the arm-curl bench with nonelastic straps during exercise, and MVC decreased by 48.6% immediately after the exercise task. This change was similar to that reported in previous studies using dumbbell exercise to induce muscle damage (Chen et al., 2007; Lavender and Nosaka, 2006b), and no participants dropped out of the study or reported adverse events. In addition, ROM was most restricted immediately after eccentric exercise, and SOR peaked after 48 h of eccentric exercise; these were also supported by the results of previous studies (Yamaguchi et al., 2020a; Yamaguchi et al., 2020b; Yamaguchi et al., 2020c). These results suggested that the exercise task used in this study was appropriate.
The shear modulus, as an index of stiffness, increased over time and peaked at 48 h. Although there was a slight difference in the time course change when the peak was shown, such as immediately after eccentric exercise or after 48 hours, depending on the difference in experimental settings (different elbow joint angle and/or eccentric exercise load, etc.), some studies (Lacourpaille et al., 2014; Lacourpaille et al., 2017; Xu et al., 2019) have acknowledged that shear modulus measured by SWE increases after eccentric exercise. In detail, the peak value of the shear modulus in this study was approximately twice that of the resting muscle shear modulus (46.6 kPa to 92.3 kPa), and this change was slightly greater than in the study by Lacourpaille et al. (Lacourpaille et al., 2014), who performed eccentric exercises on the biceps brachii (1.7-fold increase: 16.8 kPa to 29.6 kPa). One of the causes for this is the measured joint angle (i.e., muscle fiber length) associated with the muscle. Chen et al. (Chen et al., 2017) and Eby et al. (Eby et al., 2015) also stated that stiffness is affected by muscle fiber length. Lacourpaille et al. (Lacourpaille et al., 2014) had measured the elbow joints at 70°, 110°, and 160°, and the higher values in this study (elbow joint angle 180° and different positions such as the shoulder joint) were because of muscle fiber length on target muscle.
UTF increased after eccentric exercise and peaked at 96 h. This pattern of time course change, amount of change, and range of values supports our previous results (Yamaguchi et al., 2020c). Intense eccentric contractions strain individual muscle fibers, resulting in the destruction of muscle cell membranes and myofibrils (Yu et al., 2003). This destruction is observed immediately after exercise, and several hours later, myofibrils are further degraded by proteases (Yu et al., 2003). Calpain 3, a protease, is expressed primarily in skeletal muscle and exists in association with titin (Ono et al., 2016). Calpain 3 has been reported to specifically cleave and degrade titin, and this phenomenon has been confirmed to induce muscle damage after eccentric contraction (Raastad et al., 2010) The delayed peak of UTF is considered to be strongly influenced by this degrading enzyme, calpain.
In addition, peak UTF measured in this study showed a high correlation between peak MVC and peak ROM, which is consistent with a previous study (Yamaguchi et al., 2020a). These results showed that EIMD was induced by eccentric contraction (Lavender and Nosaka, 2006a) and that UTF was specifically responsive to EIMD. Regarding the relationship between indicators, peak UTF showed a high correlation with peak shear modulus. Titin is a sarcomere constituent protein that mainly supports the contraction of actin and myosin filaments in the fiber direction (longitudinal direction) by eccentric contraction (exercise). Also, As the structure of skeletal muscle, shear modulus (stiffness) measured by SWE has been defined as stiffness in the fiber direction (longitudinal direction) of the muscle, which is the same direction as muscle contraction including eccentric exercise (Inami and Kawakami, 2016). As one of the factors affecting the change in stiffness after eccentric exercise, increased sensitivity of muscle fibers to Ca2+ may be due to the involvement of calcium-dependent physiological processes, such as cross-bridge number or titin giant protein (Stephenson and Wendt, 1984; Balnave and Allen, 1996; Claflin et al., 1998). It is speculated that titin filaments are damaged by eccentric exercise and that the unstable sarcomere improves stiffness by shortening the cross-bridge between actin and myosin.
Although the present study strongly suggests that titin is involved in the change in stiffness after eccentric exercise and demonstrates the speculation of previous studies (Stephenson and Wendt, 1984; Balnave and Allen, 1996; Claflin et al., 1998; Lacourpaille et al., 2014), there is a lack of microscopic knowledge to understand the detailed physiological relationship between the two. If this is the case, the relationship between the shear modulus and titin should also show a stronger correlation; however, the correlation coefficient is 0.648. The participants in this study performed eccentric exercises on the elbow flexor muscles using a dumbbell; however, only the stiffness of the biceps brachii could be evaluated. This was a limitation of this study. If the stiffness of all muscles that contribute to elbow flexion, including the brachialis and forearm flexion muscles, were evaluated and integrated, a better correlation could have been found. Our study suggested that shear modulus and titin are strongly related, but further studies are required to comprehensively examine the differences in physiological processes of stiffness and giant titin, including the differences in time course change (peak at 48 and 96 hours after eccentric exercise, respectively).
As one mechanism, the sarcoplasmic reticulum is connected to the T-tubule system by junctophilin and is damaged by high-intensity eccentric exercise (Allen et al., 2005). Damage to the sarcoplasmic reticulum leads to high concentrations of Ca2+ in myofibers and activation of calpain 3. Calpain 3 is a Ca2+-activated protease found in the Z-region of skeletal muscles that cleaves cytoskeletal proteins, such as titin, desmin, vimentin, and α-actinin (Huang and Zhu, 2016). Therefore, it is likely that UTF in this study was catabolized by calpain and deviated into the urine. In addition, titin, a metabolite excreted due to mechanical stimulation locally generated intramuscularly by eccentric exercise, may be explained by the time difference until it could be excreted into the urine via the lymph and blood vessels. In any case, the hypothesis of this study is supported, and this is the first study to substantiate the speculation stated in the previous studies (Stephenson and Wendt, 1984; Balnave and Allen, 1996; Claflin et al., 1998; Lacourpaille et al., 2014).
A significant positive correlation was observed between UTF and muscle fatigue at 48 to 168 hours. The muscle fatigue in this study evaluated the subjective muscle fatigue that participants evaluated through the perception of dullness in the muscles. The condition of tension reduction induced by movements of the degree of daily activities (low frequency stimulation) and the threshold reduction of subjective fatigue caused by tension reduction is called low frequency fatigue (Place et al., 2010). According to Place et al., (Place et al., 2010), the mechanism of low-frequency fatigue is a decrease in Ca2+ sensitivity of myofibrils or a decrease in Ca2+ release/control function of sarcoplasmic reticulum, and is considered to occur mainly in muscle tissue rather than in the middle nervous system (Balog, 2010). On the other hand, it has been reported that titin is decomposed by calpain as described above. In calpain, sarcoplasmic reticulum is disrupted by secondary inflammation occurring after 24 hours of intense eccentric exercise, and Ca2+ is excessively released into muscle cells. It is activated by being active and becomes a major cause of metabolic degradation of titin (Stožer et al., 2020). Therefore, the reason for a strong positive correlation between muscle fatigue and UTF after 48 hours is suggested as the low-frequency fatigue and titin degradation that may have been triggered by a similar mechanism (sarcoplasmic reticulum breakdown due to secondary inflammation).
Although this study found a strong correlation primarily between SWE and UTF, at least two study groups need to be formed, treated differently, and ultimately compared on the effects of interest to reach clear causal conclusions. In addition, although the present results are limited to a correlation analysis between non-invasive measurements, it is necessary to conduct a detailed analysis using invasive measurements such as muscle biopsy to investigate the relationship between the two with a detailed understanding of the condition of muscle tissue after eccentric exercise in the future. Also, both methods used in this study are non-invasive tools for grasping the condition of skeletal muscle, but the ultrasonic device equipped with the SWE function is still expensive, and its portability is limited. In addition, the analysis of titin is not immediate at present; therefore, further simplification is expected. However, if the development of a highly portable and highly immediacy analysis method using the relationship between the indicators clarified in this study progresses in the future, trainers and therapists can determine the athlete's objective situation, and this may aid in the prevention of sports injuries.
Conclusion
Our study results revealed a significant relationship between muscle strength, shear modulus measured by ultrasound SWE, and titin measured by UTF, as a non-invasive damage marker after eccentric exercise to track changes in EIMD.
Acknowledgements
Taisho Pharmaceutical Co., Ltd. funded and collaborated with this study; however, the company did not influence the scientific interpretation of the collected data or the publication of this article. The authors declare that this study complies with the laws and regulations of the host country.
Biographies

Takayuki INAMI
Employment
Institute of Physical Education, Keio University, Japan
Degree
Ph.D.
Research interests
Muscle Biomechanics, Sports Medicine
E-mail: inamit@keio.jp

Shota YAMAGUCHI
Employment
Institute of Physical Education, Keio University, Japan
Degree
Ph.D.
Research interests
Exercise-induced muscle damage
E-mail: yamaguchi.s@keio.jp

Hiroyuki ISHIDA
Employment
Sports Medicine Research Center, Keio University, Japan
Degree
M.D., Ph.D.
Research interests
Sports medicine
E-mail: RXB03102@nifty.ne.jp

Naohiko KOHTAKE
Employment
Graduate School of System Design and Management, Keio University
Degree
PhD
Research interests
System Design Management, Systems Engineering
E-mail: kohtake@sdm.keio.ac.jp

Akihisa MORITO
Employment
Graduate School of System Design and Management, Keio University
R&D Laboratories, Self-Medication, Taisho Pharmaceutical Co., Ltd.
Degree
MSc
Research interests
Sports Science and Nutrition
E-mail: a-morito@taisho.co.jp

Satoshi YAMADA
Employment
R&D Laboratories, Self-Medication, Taisho Pharmaceutical Co., Ltd.
Degree
MSc
Research interests
Sports Science and Nutrition
E-mail: sat-yamada@taisho.co.jp

Masatsugu SHIMOMASUDA
Employment
Research & Development Headquarters, Self-Medication, Taisho Pharmaceutical Co., Ltd.
Degree
MSc
Research interests
Sports Science and Nutrition
E-mail: m-shimomasuda@taisho.co.jp

Maki HARAMOTO
Employment
Research & Development Headquarters, Self-Medication, Taisho Pharmaceutical Co., Ltd.
Degree
Bachelor
Research interests
Sports Science and Nutrition
E-mail: m-haramoto@taisho.co.jp

Naoya NAGATA
Employment
Institute of Physical Education, Keio University, Japan
Degree
M.P.Ed
Research interests
Sports psychology, Attention, Basketball
E-mail: nnagata@keio.jp

Mitsuyoshi MURAYAMA
Employment
Institute of Physical Education, Keio University, Japan
Degree
Ph.D.
Research interests
Muscle hardness, eccentric exercise
E-mail: murayama@z3.keio.jp
References
- Allen D.G., Whitehead N.P., Yeung E.W. (2005) Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. The Journal of Physiology 567, 723-735. https://doi.org/10.1113/jphysiol.2005.091694 10.1113/jphysiol.2005.091694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave C.D., Allen D.G. (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. The Journal of Physiology 492, 705-713. https://doi.org/10.1113/jphysiol.1996.sp021339 10.1113/jphysiol.1996.sp021339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog E.M. (2010) Excitation-Contraction Coupling and Minor Triadic Proteins in Low-Frequency Fatigue. Exercise and sport sciences reviews 38, 135-142. https://doi.org/10.1097/JES.0b013e3181e3734d 10.1097/JES.0b013e3181e3734d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio P., Lippi G., Maffulli N. (2010) Biochemical markers of muscular damage. Clinical Chemistry and Laboratory Medicine 48, 757-767. https://doi.org/10.1515/CCLM.2010.179 10.1515/CCLM.2010.179 [DOI] [PubMed] [Google Scholar]
- Chen T.C., Nosaka K., Sacco P. (2007) Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. Journal of Applied Physiology 102, 992-999. https://doi.org/10.1152/japplphysiol.00425.2006 10.1152/japplphysiol.00425.2006 [DOI] [PubMed] [Google Scholar]
- Chen J., O’Dell M., He W., Du L.-J., Li P.-C., Gao J. (2017) Ultrasound shear wave elastography in the assessment of passive biceps brachii muscle stiffness: influences of sex and elbow position. Clinical Imaging 45, 26-29. https://doi.org/10.1016/j.clinimag.2017.05.017 10.1016/j.clinimag.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Claflin D.R., Morgan D.L., Julian F.J. (1998) The effect of length on the relationship between tension and intracellular [Ca2+] in intact frog skeletal muscle fibres. The Journal of Physiology 508, 179-186. https://doi.org/10.1111/j.1469-7793.1998.179br.x 10.1111/j.1469-7793.1998.179br.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas F., Nosaka K., Libardi C.A., Chen T.C., Ugrinowitsch C. (2016) Susceptibility to exercise-induced muscle damage: a cluster analysis with a large sample. International Journal of Sports Medicine 37, 633-640. https://doi.org/10.1055/s-0042-100281 10.1055/s-0042-100281 [DOI] [PubMed] [Google Scholar]
- Eby S.F., Cloud B.A., Brandenburg J.E., Giambini H., Song P., Chen S., LeBrasseur N.K., An K. (2015) Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clinical Biomechanics 30, 22-27. https://doi.org/10.1016/j.clinbiomech.2014.11.011 10.1016/j.clinbiomech.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J.N., Chleboun G., Conatser R. (1993) Muscle stiffness, strength loss, swelling and soreness following exercise-induced injury in humans. The Journal of Physiology 464, 183-196. https://doi.org/10.1113/jphysiol.1993.sp019629 10.1113/jphysiol.1993.sp019629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhu X. (2016) The Molecular Mechanisms of Calpains Action on Skeletal Muscle Atrophy. Physiological Research 65, 547-560. https://doi.org/10.33549/physiolres.933087 10.33549/physiolres.933087 [DOI] [PubMed] [Google Scholar]
- Inami T., Kawakami Y. (2016) Assessment of individual muscle hardness and stiffness using ultrasound elastography. The Journal of Physical Fitness and Sports Medicine 5, 313-317. https://doi.org/10.7600/jpfsm.5.313 10.7600/jpfsm.5.313 [DOI] [Google Scholar]
- Jang H.-J., Lee J.D., Jeon H.-S., Kim A.-R., Kim S., Lee H.-S., Kim K.-Y. (2018) Metabolic Profiling of Eccentric Exercise-Induced Muscle Damage in Human Urine. Toxicological Research 34, 199-210. https://doi.org/10.5487/TR.2018.34.3.199 10.5487/TR.2018.34.3.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K., Sakuma J., Akimoto T., Kawakami Y., Suzuki K. (2017) Detection of titin fragments in urine in response to exercise-induced muscle damage. Plos One 12, e0181623. https://doi.org/10.1371/journal.pone.0181623 10.1371/journal.pone.0181623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K., Sugama K., Sakuma J., Kawakami Y., Suzuki K. (2014) Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exercise Immunology Review 20, 39-54. [PubMed] [Google Scholar]
- Lacourpaille L., Nordez A., Hug F., Couturier A., Dibie C., Guilhem G. (2014) Time-course effect of exercise-induced muscle damage on localized muscle mechanical properties assessed using elastography. Acta Physiologica (Oxford, England) 211, 135-146. https://doi.org/10.1111/apha.12272 10.1111/apha.12272 [DOI] [PubMed] [Google Scholar]
- Lacourpaille L., Nordez A., Hug F., Doguet V., Andrade R., Guilhem G. (2017) Early detection of exercise-induced muscle damage using elastography. European Journal of Applied Physiology 117, 2047-2056. https://doi.org/10.1007/s00421-017-3695-9 10.1007/s00421-017-3695-9 [DOI] [PubMed] [Google Scholar]
- Lavender A.P., Nosaka K. (2006a) Comparison between old and young men for changes in makers of muscle damage following voluntary eccentric exercise of the elbow flexors. Applied Physiology, Nutrition, and Metabolism 31(3), 218-225. https://doi.org/10.1139/h05-028 10.1139/h05-028 [DOI] [PubMed] [Google Scholar]
- Lavender A.P., Nosaka K. (2006b) Changes in fluctuation of isometric force following eccentric and concentric exercise of the elbow flexors. European Journal of Applied Physiology 96, 235-240. https://doi.org/10.1007/s00421-005-0069-5 10.1007/s00421-005-0069-5 [DOI] [PubMed] [Google Scholar]
- Maruyama N., Asai T., Abe C., Inada A., Kawauchi T., Miyashita K., Maeda M., Matsuo M., Nabeshima Y. (2016) Establishment of a highly sensitive sandwich ELISA for the N-terminal fragment of titin in urine. Scientific Reports 6, 39375. https://doi.org/10.1038/srep39375 10.1038/srep39375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M., Inami T., Shim20a N., Yoneda T., Nosaka K. (2022) Changes in biceps brachii muscle hardness assessed by a push-in meter and strain elastography after eccentric versus concentric contractions. Scientific Reports 12, 9214. https://doi.org/10.1038/s41598-022-13184-3 10.1038/s41598-022-13184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M., Nosaka K., Yoneda T., Minamitani K. (2000) Changes in hardness of the human elbow flexor muscles after eccentric exercise. European Journal of Applied Physiology 82, 361-367. https://doi.org/10.1007/s004210000242 10.1007/s004210000242 [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Inami T., Yonezu T., Kenmotsu Y., Narita T., Kawakami Y., Kanosue K. (2018) Unstable rocker shoes promote recovery from marathon-induced muscle damage in novice runners. Scandinavian Journal of Medicine & Science in Sports 28, 621-629. https://doi.org/10.1111/sms.12911 10.1111/sms.12911 [DOI] [PubMed] [Google Scholar]
- Nosaka K., Newton M. (2002) Repeated eccentric exercise bouts do not exacerbate muscle damage and repair. Journal of Strength and Conditioning Research 16, 117-122. https://doi.org/10.1519/00124278-200202000-00018 10.1519/00124278-200202000-00018 [DOI] [PubMed] [Google Scholar]
- Ono Y, Ojima K, Shinkai-Ouchi F, Hata S, Sorimachi H. (2016) An eccentric calpain, CAPN3/p94/calpain-3. Biochimie 122, 169-187. https://doi.org/10.1016/j.biochi.2015.09.010 10.1016/j.biochi.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Place N., Yamada T., Bruton J.D., Westerblad H. (2010) Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. European Journal of Applied Physiology 110, 1-15. https://doi.org/10.1007/s00421-010-1480-0 10.1007/s00421-010-1480-0 [DOI] [PubMed] [Google Scholar]
- Raastad T., Owe S.G., Paulsen G., Enns D., Overgaard K., Crameri R., Steinar K., Angelo B., Linda B., And Jostein H. (2010) Changes in Calpain Activity, Muscle Structure, and Function after Eccentric Exercise. Medicine & Science in Sports & Exercise 42, 86-95. https://doi.org/10.1249/MSS.0b013e3181ac7afa 10.1249/MSS.0b013e3181ac7afa [DOI] [PubMed] [Google Scholar]
- Shi J., Watanabe D., Wada M. (2021) Effects of vigorous isometric muscle contraction on titin stiffness-related contractile properties in rat fast-twitch muscles. American Journal of Physiology. Regulatory, Integrative & Comparative Physiology 321, 858-868. https://doi.org/10.1152/ajpregu.00189.2021 10.1152/ajpregu.00189.2021 [DOI] [PubMed] [Google Scholar]
- Stephenson D.G., Wendt I.R. (1984) Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. Journal of Muscle Research and Cell Motility 5, 243-272. https://doi.org/10.1007/BF00713107 10.1007/BF00713107 [DOI] [PubMed] [Google Scholar]
- Stožer A., Vodopivc P., Križančić Bombek L. (2020) Pathophysiology of exercise-induced muscle damage and its structural, functional, metabolic, and clinical consequences. Physiological Research 69, 565-598. https://doi.org/10.33549/physiolres.934371 10.33549/physiolres.934371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Fu S.N., Zhou D., Huang C., Hug F. (2019) Relationship between pre-exercise muscle stiffness and muscle damage induced by eccentric exercise. European Journal of Sport Science 19, 508-516. https://doi.org/10.1080/17461391.2018.1535625 10.1080/17461391.2018.1535625 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Suzuki K., Inami T., Kanda K., Hanye Z., Okada J. (2020a) Changes in Urinary Titin N-terminal Fragment Concentration after Concentric and Eccentric Exercise. Journal of Sports Science & Medicine 19, 121-129. https://pubmed.ncbi.nlm.nih.gov/32132835/ [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Suzuki K., Kanda K., Inami T., Okada J. (2020b) Changes in urinary titin N-terminal fragments as a biomarker of exercise-induced muscle damage in the repeated bout effect. Journal of Science and Medicine in Sport 23, 536-540. https://doi.org/10.1016/j.jsams.2019.12.023 10.1016/j.jsams.2019.12.023 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Suzuki K., Kanda K., Okada J. (2020c) N-terminal fragments of titin in urine as a biomarker for eccentric exercise-induced muscle damage. The Journal of Physical Fitness and Sports Medicine 9, 21-29. https://doi.org/10.7600/jpfsm.9.21 10.7600/jpfsm.9.21 [DOI] [Google Scholar]
- Yu J.-G., Fürst D.O., Thornell L.-E. (2003) The mode of myofibril remodelling in human skeletal muscle affected by DOMS induced by eccentric contractions. Histochemistry and Cell Biology 119, 383-393. https://doi.org/10.1007/s00418-003-0522-7 10.1007/s00418-003-0522-7 [DOI] [PubMed] [Google Scholar]


