Abstract
Dysmenorrhea with high prevalence has been categorized as primary dysmenorrhea (PD) and secondary dysmenorrhea due to differences in pathogenesis. A significant number of reproductive females suffering from monthly menstruation have to deal with negative impacts on their quality of life, work/study productivity, activities, and social relationships. In addition to medical treatment, exercise has been recognized as a complementary and alternative strategy for disease prevention, alleviation, and rehabilitation. This study aimed to investigate the potential effects of exercise on the severity of primary dysmenorrhea, physiological modulation, and physical fitness. Participants consisted of university students who were enrolled in the study and divided into a non-PD (Control) and a PD group based on recruiting criteria, the latter being randomly assigned to either an untreated dysmenorrhea group or a dysmenorrhea group that underwent 10 weeks of high intensity interval training (HIIT) exercise (Dysmen and DysmenHIIT, respectively). The DysmenHIIT group used spinning bikes and the training intensity was validated by heart rate monitors and BORG rating of perceived exertion. Forms containing participant information (premenstrual symptoms, menstrual distress, and a Short Form McGill Pain Questionnaire) as well as physical fitness, biochemical variables, hormone and prostaglandin (PGE2 and PGF2α) levels were assessed before and after the exercise intervention. After intervention, premenstrual symptoms (anger, anxiety, depression, activity level, fatigue, etc.), menstrual distress symptoms (cramps, aches, swelling, etc.), and pain severity were shown to be significantly mitigated, possibly through hormone (estradiol, prolactin, progesterone, and cortisol) modulation. Furthermore, high-sensitivity C-reactive protein (HsCRP), PGE2 and PGF2α levels were also down-regulated, resulting in the amelioration of uterine contraction and inflammation. Participants’ physical fitness, including cardiovascular endurance and explosive force, was significantly improved after HIIT. The 10-week HIIT spinning bike exercise used in this study could be employed as a potential and complementary treatment for PD symptoms alleviation and considered as part of an educational health plan for promoting women’s health. However, the effects of HIIT utilizing different exercise methods and accounting for different age populations and secondary PD warrant further investigation.
Key points.
The 10-week high intensity interval training using a spinning bike could ameliorate the premenstrual symptoms, menstrual distress, and pain severity with primary dysmenorrhea.
The 10-week spinning bike HIIT exercise could modulate the dysmenorrhea-related inflammation and hormones for amelioration of dysmenorrhea.
The physical fitness, including cardiovascular fitness and muscular power of lower extremity, could be improved by implementation of 10-week spinning bike HIIT exercise.
The intensity monitoring could be critical factors for physiological modulation during exercise intervention.
Key words: High intensity interval training, inflammation, menstrual distress, primary dysmenorrhea, premenstrual symptoms, hormones
Introduction
Menstruation is regulated by hormonal modulation mediated by the Hypothalamic-Pituitary-Gonadal (HPG) axis through the gonadotropin-releasing hormone (GnRH), follicular-stimulating hormone (FSH), and luteinizing hormone (LH). The GnRH release from the hypothalamus to the pituitary gland, and the gonadotropins (FSH and LH) primarily act on granulosa and theca cells of the ovaries to initiate the production of steroid hormone such as androgen, estrogen, and inhibin, regulated by positive and negative feedback signaling during the menstrual cycle (Howard, 2021). This menstrual period may vary across individuals, with an average length of 29.3 days, a mean of 16.9 days in the follicular phase and a mean of 12.4 days in the luteal phase (Bull et al., 2019). Dysmenorrhea is defined as the severe and frequent cramps, pain, and discomfort a person may suffer during their menstrual period and is considered a common gynecological condition that most women experience, regardless of age and race (Proctor and Farquhar, 2006). Dysmenorrhea can be classified into two categories, primary and secondary dysmenorrhea, with the pathological syndromes being the main factor differentiating the two (Harada, 2013). Primary dysmenorrhea results in uterine contraction and cramping pain caused by the physiological impacts of released prostaglandins and active lipid compounds before or during menstruation. While it is known to have a good prognosis, it remains a factor in causing low quality of life (Bernardi et al., 2017). Secondary dysmenorrhea is associated with organic pathogenesis in the female reproductive tract, such as endometriosis, adenomyosis, and pelvic inflammation disease. Symptoms worsen over time and last longer than those that occur during primary dysmenorrhea.
Dysmenorrhea has a high prevalence among students and may affect their quality of life and learning in several ways. A cross-sectional study revealed that high school student populations (aged 16-21 years) have a prevalence of dysmenorrhea as high as 85.6% (Al-Matouq et al., 2019). University populations showed an even higher prevalence of up to 91.5%. Strategies used to manage symptoms included non-pharmacological methods such as heat application (79%), rest (60.4%), hot showers/baths (40.9%), and exercise (25.7%), as well as pharmacological treatments, particularly the use of paracetamol (Durand et al., 2021). Reporting of the symptom management strategies used by university students did not provide in-depth information about the application of these strategies and medications, the students’ educational knowledge related to health, or optimization of self-management (Karout et al., 2021). It has been shown that the overall prevalence of dysmenorrhea in women between menarche and menopause varies between 16% and 91%, with 2%-29% suffering severe pain. Improvements in dysmenorrhea and its symptoms are associated with increased age, parity, and the use of oral contraceptives, but the condition is exacerbated by stress and a family history of dysmenorrhea (Ju et al., 2014).
Several related syndromes, including the psychological, physiological, behavioral, and physical impacts, are considered part of premenstrual syndrome (PMS) which may arise during the late luteal phase of the menstrual cycle or before the onset of menstruation. Dietary behaviors (consumption of high fat foods and excessive sugar intake), less physical activity, and poor sleep quality have been significantly associated with PMS (Cheng et al., 2013). It is possible that the estrogen hormone fluctuation during the menstrual cycle may result in norepinephrine secretion from the hypothalamus to trigger a subsequent decline in acetylcholine, dopamine, and serotonin, resulting in the symptoms of PMS. This estrogen-serotonin regulation could indicate a correlation with the causality of hormone, mood, and physiological disorders that are considered a part of the overarching characterization of PMS (Barth et al., 2015). PMS severity was assessed and evaluated through survey questionnaires, such as the Premenstrual Symptoms Screening Tool (PSST) and Premenstrual Symptoms Questionnaire (PSQ), and cases that were determined to constitute moderate-to-severe PMS were associated with lower work productivity and increased absenteeism, and as having potential effects on social economic burden and learning ability (Heinemann et al., 2010; Tadakawa et al., 2016). The Menstrual Distress Questionnaire (MDQ) and the Short Form McGill Pain Questionnaire (SF-MPQ) have also been used to predict related conditions; for example, age, mother’s occupation, menstrual pain, and menstrual attitude were found to be predictive of adolescent menstrual distress (Chen and Chen, 2005).
Insufficient or irregular exercise have also been indicated as being significant predictors and factors regarding the onset of dysmenorrhea and PMS. A meta-analysis study evaluated the effectiveness and safety of exercise for women afflicted with primary dysmenorrhea. There were weak indications that exercise, performed for approximately 45 to 60 minutes each time, three times per week or more, regardless of intensity, may provide a significant reduction in menstrual pain intensity, according to answers obtained using the Visual Analog Scale (VAS) of the SF-MPQ (Armour et al., 2019). Exercise intensity has been shown to be an important treatment factor for physiological adaptation, and vigorous exercise appears to convey greater cardioprotective benefits than exercise regimens of a moderate intensity (Swain and Franklin, 2006). Performing high intensity interval training (HIIT) on a treadmill and analyzing its effects on systemic inflammatory and hormonal markers showed a decrease in levels of pro-inflammatory markers and adipokine modulation, implying beneficial health effects for postmenopausal women with obesity (Steckling, 2019). The mechanism behind dysmenorrhea could be associated with dysregulation of inflammation, lipid metabolism, and hormones, resulting in the menstrual distress and cramp, during menstruation (Barcikowska et al., 2020). HIIT is a popular exercise model in a worldwide survey on fitness trends in 2022 by ACSM’s Health & Fitness, but studies focusing on the exercise model’s potential effects on dysmenorrhea symptom amelioration are limited in number.
In the present study, based on a randomized controlled trial design, female university students with primary dysmenorrhea performed HIIT exercises using a spinning bike while under intensity surveillance. This was used for exercise intensity verification and comprehensive primary outcome evaluations (PMS questionnaire, MDQ, SF-MPQ, VAS, biochemical variables, sex hormones, and prostaglandins). The aim of the present study was to investigate the effects of HIIT intervention on the amelioration of discomfort and distress symptoms characteristic of primary dysmenorrhea and its effects on inflammation and hormone modulation. This exercise treatment displayed promise for the possible amelioration of primary dysmenorrhea symptoms from the physiological perspective and could potentially be used in the future by health care providers as a part of female health management.
Methods
Experimental design
The experimental design was a randomized controlled trial, designed to investigate any effects that HIIT exercise may have on the amelioration of symptoms characteristic of primary dysmenorrhea as well as its effects on inflammation and hormone modulation. The participants in the control (Control; n = 15) and dysmenorrhea (n = 30) groups were enrolled by the research team and selected based on specific criteria. The dysmenorrhea group was further split into the more distinct dysmenorrhea (Dysmen; n = 15) and dysmenorrhea + HIIT (DysmenHIIT; n = 15) groups using a random-numbers table. The blood sampling, questionnaires (PMS, MDQ, and SF-MPQ), and physical fitness of participants were evaluated before and after the experimental intervention. Individuals’ blood was also sampled about 3 days before the start of their respective upcoming menstruation cycles for in-depth assessment of biochemical variables and hormone levels. The study was approved by the Fu Jen Catholic University Institutional Review Board (New Taipei City, Taiwan; FUJ-IRB NO: C110039) and publicly registered at clinicaltrials.gov (NCT Number: NCT05326217). The study experimental scheme and study flow diagram is illustrated in Figure 1 and Figure 2.
Figure 1.
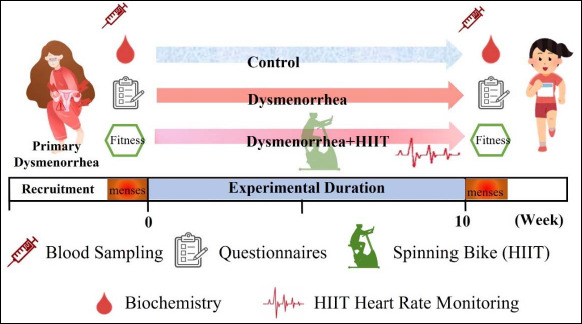
Experimental scheme. The experiment was based on a single-blind and randomized controlled trial design. The primary dysmenorrhea participants who met the inclusion criteria were randomly assigned to the Dysmenorrhea (Dysmen) and Dysmenorrhea + HIIT (DysmenHIIT) groups, and the HIIT treatment was implemented for a 10-week period. During the HIIT exercise, participants’ heart rates were required to reach at least 85% of their maximum heart rate, as indicated by a heart rate belt. Individual blood samples were obtained approximately three days before participants’ menstruation cycles were due to begin to analyze biochemical variables, hormone, and prostaglandin levels. The questionnaires (PMS, MDQ, and SF-MPQ), physical fitness, and blood sampling were conducted before and after HIIT intervention. HIIT: high intensity interval training.
Figure 2.
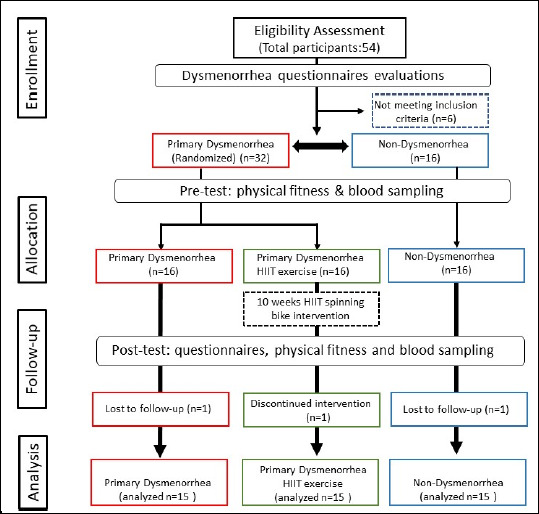
Study flow diagram. This study is a randomized, parallel-controlled clinical trial. There were 48 eligible participants, who were randomly assigned into three groups, allocated as Non-Dysmenorrhea (Control), Primary Dysmenorrhea (Dysmen), and Primary Dysmenorrhea undergoing HIIT exercise (DysmenHIIT). Dysmenorrhea was defined by menstrual pain intensity, measured using the VAS method in the SF-MPQ. The questionnaires and physical fitness were assessed before and after the experimental intervention. Blood samples were taken three days before menstruation and biochemical variables and hormones associated with menstrual pain were analyzed before and after the intervention.
Sample size calculation
The repeated measures design equation implemented in the G*power software was used for calculating the required sample size. The parameters (the pain severity, plasma prostaglandin, and self-rating menstrual symptoms) were set as an effect size of 0.25, a power of 80%, three groups, and measurements with two replications, as used in a previous study (Xing et al., 2021). A total of 42 participants was calculated as an adequate sample size to proceed with the exercise intervention. The dropout rate of participants was estimated to be approximately 10%, so a total of 50 participants had to be recruited as a minimum requirement.
Subjects
The participants had to meet inclusion criteria that included the age range under investigation (18-40 years old), being afflicted with primary dysmenorrhea, and its degree of severity based on the visual analog score of the McGill Pain Questionnaire (0-2 for non-dysmenorrhea control; > 5 for dysmenorrhea). Variables including pregnancy, menstrual disorders, obesity (BMI > 30), smoking, alcohol or drug addiction, and certain health conditions (cardiovascular disease, hypertension, diabetes, asthma, chronic pulmonary obstruction, and mental illness) were excluded in the current study. The participants were female students recruited from the National Taipei University of Nursing and Health Sciences. Three subjects were dropped from the study due to lower participation rate (< 80%), graduation, or work reasons, resulting in a final total of 45 participants who fulfilled the eligible criteria, and who completed all experimental steps for complete data analysis. The participants were also requested to avoid nutrient supplements (probiotic or fermented products) and to maintain their regular lifestyle for the duration of the experiment. All participants provided their informed consent before participating in the exercise program and its assessments. The anthropometric and menstrual data of the participants’ characteristics are presented in Table 1. All assessments, blood sampling, and exercise intervention were performed in professional classrooms at the National Taipei University of Nursing and Health Sciences.
Table 1.
Basal anthropometric and menstrual characteristics of the participants.
| Characteristic | Control (1) | Dysmen (2) | DysmenHIIT (3) |
|---|---|---|---|
| Age (years) | 23.1 ± 4.6 | 21.5 ± 2.1 | 20.6 ± 2.5 |
| Height (m) | 1.60 ± 0.05 | 1.60 ± 0.06 | 1.61 ± 0.05 |
| Weight (kg) | 57.4 ± 8.9 | 55.4 ± 7.9 | 55.9 ± 9.1 |
| BMI | 22.4 ± 3.0 | 21.5 ± 2.4 | 21.5 ± 2.4 |
| Systolic blood pressure (mmHg) | 113 ± 13.0 | 117 ± 14.0 | 111 ± 8.6 |
| Diastolic blood pressure (mmHg) | 77 ± 11.0 | 78 ± 10.0 | 74 ± 6.2 |
| Resting heart rate (bpm) | 86 ± 13.0 | 84 ± 14.0 | 77 ± 9.3 |
| Menarche age (years) | 11.8 ± 0.9 | 12.3 ± 1.3 | 11.9 ± 1.2 |
| Interval of menstrual cycle (days) | 28.6 ± 1.2 | 29.0 ± 1.7 | 28.9 ± 2.4 |
| Duration of menstrual cycle (days) | 4.6 ± 1.0 3 | 5.3 ± 1.2 | 5.8 ± 1.2 1 |
| Menstrual flow + | 1.8 ± 0.7 | 2.1 ± 0.6 | 2.1 ± 0.5 |
| Dysmenorrhea days (during menses) | 1.0 ± 0.7 2,3 | 3.3 ± 1.2 1 | 3.3 ± 1.3 1 |
| Painkiller medication | |||
| Yes | 0 2,3 | 8 1 | 9 1 |
| No | 15 2,3 | 7 1 | 6 11 |
| Regular exercise | |||
| none | 6 | 8 | 3 |
| 3 or less times / week | 6 | 6 | 8 |
| 4 or more times / week | 3 | 1 | 4 |
‘+’: perceived menstrual flow rated as light, medium, or heavy (score 1 to 3). Control and Dysmenorrhea groups were defined according to the definitions of inclusion criteria from the VAS scores of the Short Form McGill Pain Questionnaire in the current study. Superscripts numbers (1, 2, and 3) indicate significant differences (P < 0.05) between the groups. Dysmen and DysmenHIIT represent the dysmenorrhea groups not undergoing exercise and undergoing exercise treatments, respectively.
High intensity interval training intervention
The HIIT program was implemented as a group class under the supervision of an EOXi® certified cycling coach using spinning bikes, where the participants were instructed on how to safely manipulate the equipment before the initiation of the program. The participants were required to attend two sessions per week for a 10-week duration, with at least one day between sessions to allow for sufficient recovery. Each exercise session was 30-35 min in length, including a warm-up (5 min), the main sprint-exercise course (15-20 min), a cool-down (5 min), and a stretching period (5 min). During the main period, a 20 s all-out sprint-exercise was performed fifteen times, separated by 40 s active recovery periods each visit, and the resistance of the spinning bikes was adjusted for 15 subjective levels of the BORG scale of perceived exertion while maintaining the same pace of biking during the all-out phase. The duration of the all-out phase was increased to 25 s with the same rest time intervals as that of the 7th to the end. Additionally, the participants’ heart rate was continuously monitored using an Obeat1 heart rate belt (Alatech, Taichung, Taiwan) to check for exercise intensity (> 85% maximum heart rate) and the participants were requested to meet the subjective degree (> 15) of perceived exertion BORG scale for indicated intensity during the HIIT program.
Menstrual questionnaire surveys
The participants were required to complete the Premenstrual Syndrome Questionnaire, the Menstrual Distress Questionnaire, and the Short Form McGill Pain Questionnaire for an in-depth evaluation of their respective status regarding physiological, psychological, behavioral, and social activity parameters before and during their menstrual period. During the premenstrual syndrome evaluation, the Premenstrual Symptoms Screening Tool (PSST) was used to quantify symptom severity and its effects on a person’s activities (Steiner et al., 2003). A 4-point Likert Scale, graded 1 to 4 (“not at all”, “mild”, “moderate” or “severe”, respectively), was applied to the PSST, MDQ, and SF-MPQ surveys, with higher scores representing higher distress or degree of perceived feeling before and during the menstruation. The PSST questionnaire was divided into two parts: one for physiological or psychological syndromes and another for activity interferences. A modified 16-item MDQ version, validated for internal consistency (Cronbach α = 0.83) (Yeh et al., 2013), was used to grade pain, water retention, and autonomic reactions during premenstrual and menstrual phases. The SF-MPQ has been widely used to quantify the quality and intensity of pain; it includes 15 descriptions related to sensory (11 items) and affective (4 items) variables. In addition, the visual analogue scale (VAS) was used, represented by a 10 cm rating scale for ranking the overall degree of pain.
Biochemical, hormone and prostaglandin analysis
Participants were requested to record their menstrual period in order to obtain blood samples approximately 3 days before menstruation. 10 mL of whole blood was collected from the median cubital vein on the forearm into collection tubes by a clinical research nurse. The serum and plasma were collected after centrifugation at 1000 × g for 10 min and immediately stored at -20°C for further biochemical and hormone analysis. For biochemical variable analysis, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK), lactate dehydrogenase (LDH), high-sensitivity C-reactive protein (HsCRP), blood urea nitrogen (BUN), creatinine (CREA), triglycerides (TG), and total cholesterol (CHOL) were assessed using AU5800 Clinical Chemistry Analyzers (Beckman Coulter, Tokyo, Japan). The hormones FSH, LH, E2-estradiol, prolactin, progesterone, and cortisol, were assessed with a UniCel DxI 800 Access Immunoassay System (Beckman Coulter, California, USA). The prostaglandins E2 (PGE2) and F2α (PGF2α) were also measured using enzyme-linked immunosorbent assays for prostaglandin metabolite analysis (for E2: R&D Systems, MN, USA; for F2α: Cayman Chemical, MI, USA).
Functional Fitness: Cardiovascular fitness
The purpose of the assessment was to measure heart rate recovery throughout a 3 min period, following 3 min of stepping exercises. Participants were required to stand on a wooden crate 35 cm high and perform 24 sets per minute of consistent, repeated up and down stepping motions, for three minutes. A single set comprised two stepping up and two stepping down motions, performed within four beats. The preset rhythm for the test was 96 beats per minute, measured using an electronic metronome. The participants’ actual exercise time was recorded using a stopwatch. Recovery pulse rate during the 30 s intervals that took place at the 1 min, 2 min, and 3 min time points was measured while participants were in a sitting posture, regardless of whether they completed the full 3 min of the exercise or stopped earlier. The three pulse rates were used in the following formula to calculate the stepping cardiovascular function index: (exercise persistence duration × 100) / (2 × the sum of the three pulse rates). The 3-minute step test was also validated for significantly predicting VO2max in previous study (Kieu et al., 2020).
Hand grip strength
The participants’ grip strength (kg) was measured using a hand dynamometer (FBA_EH101, Camry, CA, USA). The towing capacity of the dominant hand was measured with two times tests, allowing for rest intervals of at least 2 minutes, and the maximum value from each individual measurement recorded for analysis.
Horizontal jump assessments
The horizontal jump assessments were composed of standing long jump and triple jump tests, a common and easy way to evaluate the explosive power in the legs. The participant stood, with feet slightly apart, behind a marked line in front of a scaled long jump landing mat. Using both feet, they were to jump and land, solely using the momentum from swinging their arms and bending their legs to drive themselves forward. The jump distance was measured from the starting line to where the participant’s heels landed on the mat, rounded down to the nearest centimeter for analysis. While performing the standing triple jump, participants were required to minimize the ground contact time between three jumps. The participant would attempt to jump as far as possible, landing on both feet without falling backwards, and two measurements from individual tests were obtained with a rest interval of at least 1 minute between assessments. The related jump tests demonstrated significant correlation with explosive force performance (Loturco et al., 2015).
Core strength endurance
Muscular endurance was measured using the 1-minute bent-knee sit-up test. Participants were asked to lie on their backs on a mat, their arms crossed over their chests, and palms resting lightly on their shoulders. They were to sit up to the point where both elbows touched their knees, and subsequently return to the starting position for another repetition. The next curl-up could only be initiated once both shoulder blades had touched the mat. These sit-up motions were repeated for 60 s, and the number of sit-ups performed by each participant during this period was recorded as their core strength endurance, appropriate for core muscle endurance measurement for both male and female (Bianco et al., 2015).
Statistical analysis
The participants’ socio-demographic and menstrual characteristics were analyzed using descriptive statistics and a chi-square test. All dependent variables were assessed under the assumption of a normal distribution, using Kolmogorov–Smirnov tests for appropriate parametric (P > 0.05) and nonparametric analyses (P < 0.05). Nonparametric (Wilcoxon signed-rank, Pearson chi-squared, and Kruskal-Wallis tests) and parametric (MANOVA and paired t-tests) methods were used to test for significant differences in dependent variables between and within groups. The correlation between hormones, inflammatory variables, and menstrual distress were further analyzed by Pearson’s correlation coefficient. SPSS v.22 (IBM, Armonk, NY, USA) was used for all statistical analyses, and statistical significance was assumed when the probability of a type I error was < 0.05. Significant differences between treatments are represented by various subscript Roman letters (a, b, and c) with the compact letter display method in figures and tables.
Results
Basal anthropometric and menstrual characteristics of participants
The assessed anthropometric data, consisting of age, height, weight, BMI, heart rate, and systolic and diastolic pressure, demonstrated no significant differences (F(4, 42) = 0.235 to 2.248, p > 0.05) among the three groups. Across the various menstrual factors, there were no significant differences in menarche age, menstrual cycle lengths, and menstrual flow among the study groups, however menstrual duration, days of dysmenorrhea onset, and analgesic use showed significant differences among groups (F(2, 42) = 3.71 to 22.56, p < 0.05; χ2 (2) = 13.8, p = 0.001). The days of dysmenorrhea onset during menses were shown to be significantly longer and there was significantly greater dependency regarding the use of analgesics in dysmenorrhea groups compared to the control group, although only the menstrual duration in the DysmenHIIT group was significantly longer than that of the control group. No significant differences in exercise frequency during physical activities were observed between groups (χ2 (4) = 4.38, p = 0.356).
Effects of HIIT training on premenstrual syndromes
The PSST was used to survey participants regarding symptoms before menstruation. The mean score of each premenstrual symptom (further classified according to severity) is shown in Table 2. In the pre-test survey, physical symptoms, fatigue/lack of energy, hypersomnia, depression, decreased interest in work, home and social activities, decreased work productivity, and social activity interference were ranked by perceived severity and displayed a significant increase in dysmenorrhea groups compared to the control. Additionally, the total scores obtained from the sum of premenstrual symptoms described by participants were significantly higher in dysmenorrhea groups than the control group. After the 10-week exercise training regimen, the post-test survey showed that symptoms such as anger/irritability, anxiety/tension, emotional sensitivity, decreased interest in home and social activities, difficulty concentrating, and fatigue/lack of energy, were significantly alleviated in the DymenHIIT group compared to the Dysmen group. Furthermore, these same symptoms also showed a significant decrease in prevalence within the DysmenHIIT group compared to before this group performed the exercise regimen. Regarding activity interference variables, significant improvements were observed in relationships between colleagues/classmates and family members alongside improved social activities as a result of HIIT exercise intervention, as observed in the DysmenHIIT group compared to the Dysmen group. Overall, the total scores of premenstrual symptoms in PSST demonstrated significant improvement in the DysmenHIIT group as compared to the Dysmen group.
Table 2.
Mean score and intensity of premenstrual syndromes before and after the HIIT spinning exercise intervention.
| PSST component | Control (1) | Dysmen (2) | DysmenHIIT (3) | |||
|---|---|---|---|---|---|---|
| Rank mean | Rank mean | Rank mean | ||||
| Symptoms | Pre | Post | Pre | Post | Pre | Post |
| Anger/irritability | 1.5 ± 0.5 | 1.5 ± 0.5 2 | 2.0 ± 0.5 | 2.3 ± 0.6 1,3 | 2.0 ± 0.8 | 1.6 ± 0.5 2,* |
| Anxiety/tension | 1.4 ± 0.5 | 1.3 ± 0.5 2 | 1.8 ± 0.6 | 2.0 ± 0.6 1,3 | 2.0 ± 0.8 | 1.3 ± 0.5 2,* |
| Tearfulness | 1.3 ± 0.5 | 1.3 ± 0.4 2 | 1.8 ± 0.8 | 2.0 ± 0.7 1,3 | 1.7 ± 0.6 | 1.4 ± 0.6 2 |
| Depressed mood | 1.5 ± 0.5 2,3 | 1.5 ± 0.5 2 | 2.2 ± 0.6 1 | 2.3 ± 0.7 1 | 2.3 ± 0.8 1 | 1.7 ± 0.5 * |
| Decreased interest in work activities | 1.2 ± 0.4 2,3 | 1.3 ± 0.5 2 | 1.9 ± 0.9 1 | 2.0 ± 0.8 1 | 1.9 ± 0.5 1 | 1.4 ± 0.5 * |
| Decreased interest in home activities | 1.3 ± 0.5 2,3 | 1.2 ± 0.4 2 | 1.9 ± 0.6 1 | 1.9 ± 0.7 1,3 | 1.8 ± 0.6 1 | 1.3 ± 0.5 2,* |
| Decreased interest in social activities | 1.1 ± 0.3 2,3 | 1.3 ± 0.5 2 | 1.6 ± 0.5 1 | 1.9 ± 0.6 1,3 | 2.3 ± 0.9 1 | 1.3 ± 0.5 2,* |
| Difficulty concentrating | 1.2 ± 0.4 | 1.3 ± 0.5 | 1.5 ± 0.8 | 1.9 ± 0.8 3 | 1.7 ± 0.8 | 1.2 ± 0.4 2,* |
| Fatigue/lack of energy | 1.4 ± 0.5 2,3 | 1.7 ± 0.5 2 | 2.3 ± 0.7 1 | 2.6 ± 0.6 1,3 | 2.3± 0.8 1 | 1.6 ± 0.3 2,* |
| Overeating/food cravings | 1.7 ± 0.6 | 1.7 ± 0.6 | 2.1 ± 0.5 | 2.2 ± 0.7 | 1.9 ± 0.8 | 1.7 ± 0.6 |
| Insomnia | 1.0 ± 0 | 1.0 ± 0 2 | 1.2 ± 0.4 | 1.5 ± 0.5 1 | 1.2 ± 0.4 | 1.3 ± 0.6 |
| Hypersomnia | 1.3 ± 0.5 2,3 | 1.4 ± 0.5 | 2.3 ± 0.8 1 | 1.9 ± 0.7 | 2.2 ± 0.8 1 | 1.6 ± 0.7 * |
| Feeling overwhelmed/out of control | 1.1 ± 0.3 | 1.1 ± 0.4 | 1.2 ± 0.4 | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.3 ± 0.5 |
| Physical symptoms+ | 1.4 ± 0.5 2,3 | 1.7 ± 0.5 2 | 2.5 ± 0.8 1 | 2.6 ± 0.7 1 | 2.3 ± 0.6 1 | 2.1 ± 0.6 |
| Interference with activities | ||||||
| Work productivity/efficiency | 1.3 ± 0.5 2 | 1.3 ± 0.5 2 | 1.9 ± 0.9 1 | 2.0 ± 0.9 1 | 1.7 ± 0.6 | 1.4 ± 0.5 |
| Colleagues/classmate | 1.0 ± 0 | 1.1 ± 0.3 2 | 1.3 ± 0.5 | 1.5 ± 0.5 1,3 | 1.3 ± 0.6 | 1.1 ± 0.3 2 |
| Family relationships | 1.0 ± 0 | 1.0 ± 0 2 | 1.3 ± 0.5 | 1.5 ± 0.5 1,3 | 1.3 ± 0.5 | 1.1 ± 0.3 2 |
| Social activities | 1.0 ± 0 2,3 | 1.1 ± 0.4 2 | 1.5 ± 0.6 1 | 1.7 ± 0.6 1,3 | 1.5 ± 0.6 1 | 1.1 ± 0.4 2,* |
| Home responsibilities | 1.0 ± 0 | 1.0 ± 0 2 | 1.1 ± 0.3 | 1.3 ± 0.5 1,* | 1.1 ± 0.3 | 1.1 ± 0.4 |
| Total scores | 23.6 ± 2.7 2,3 | 24.8 ± 3.4 2 | 33.1 ± 4.8 1 | 36.7 ± 5.9 1,3 | 33.7 ± 6.1 1 | 26.1 ±4.4 2,* |
The physical symptoms (+) of premenstrual syndromes include breast tenderness, headaches, joint/muscular pain, bloating and weight gain. Control and Dysmenorrhea groups were defined according to the definitions of inclusion criteria from the VAS scores of the Short Form McGill Pain Questionnaire in the current study. Superscripts numbers (1, 2, and 3) indicate significant differences (P < 0.05) between the groups in pre- or post-testing and
‘*’ indicates significant differences between pre- and post-testing.
Effects of HIIT training using the Menstrual Distress and Short Form McGill Pain Questionnaires
The MDQ and SF-MPQ surveys were used to evaluate relevant symptoms and their corresponding pain severity during menstruation. The mean score of each menstrual distress symptom is shown in Table 3. In the pre-test survey, symptoms including cramping, fatigue, breast tenderness, backaches, swelling, general aches and pains, and cold sweats were ranked by perceived severity and displayed a significant increase in dysmenorrhea groups compared to the control group. The total scores from the sum of menstrual distress symptoms described by participants were significantly higher in dysmenorrhea groups than in the control group. The total scores from the sum of the answers obtained from the SF-MPQ and VAS surveys were significantly higher in dysmenorrhea groups (Dysmen and DysmenHIIT) than the control (31.2 ± 11 and 30.1 ± 6.3 versus 17.6 ± 2.5; 7.2 ± 0.5 and 7.1 ± 1.3 versus 1.4 ± 0.7, respectively) in pre-test. After the 10-week exercise intervention, menstrual cramping symptoms were shown to be significantly ameliorated, not only within the DysmenHIIT group but also compared to the Dysmen group. The other symptoms, such as fatigue, backaches, swelling, general aches and pains, cold sweats, muscle stiffness, and skin blemishes or disorders, were also shown to be alleviated within the DysmenHIIT group. In conclusion, the total scores representing the overall severity of dysmenorrhea, in addition to the answers obtained from the VAS surveys, demonstrate that HIIT exercise training may have aided in significantly decreasing pain severity by approximately 29 mm on the VAS scale in the DysmenHIIT group compared to the Dysmen group.
Table 3.
Mean ± SD score and intensity of Menstrual Distress and Short Form McGill Pain Questionnaire scores before and after the HIIT spinning exercise intervention.
| Distress component | Control (1) | Dysmen (2) | DysmenHIIT (3) | |||
|---|---|---|---|---|---|---|
| Rank mean | Rank mean | Rank mean | ||||
| Items | Pre | Post | Pre | Post | Pre | Post |
| Cramp | 1.4 ± 0.5 2,3 | 1.3 ± 0.5 2 | 3.1 ± 0.5 1 | 2.9 ± 0.6 1,3 | 2.7 ± 0.6 1 | 1.9 ± 0.8 2,* |
| Fatigue | 1.7 ± 0.5 2,3 | 1.6 ± 0.6 | 2.5 ± 0.5 1 | 2.1 ± 0.5 * | 2.5 ± 0.5 1 | 1.8 ± 0.7 * |
| Backache | 1.4 ± 0.5 2,3 | 1.5 ± 0.6 2 | 2.4 ± 0.9 1 | 2.3 ± 0.6 1 | 2.8 ± 0.7 1 | 1.9 ± 0.8 * |
| Swelling (chest/abdomen) | 1.5 ± 0.5 2,3 | 1.5 ± 0.5 2 | 2.3 ± 0.8 1 | 2.5 ± 0.8 1 | 2.2 ± 0.6 1 | 1.7 ± 0.7 * |
| Painful or tender breast | 1.5 ± 0.5 2,3 | 1.3 ± 0.5 2 | 2.5 ± 0.5 1 | 2.2 ± 0.5 1 | 2.3 ± 0.5 1 | 1.8 ± 0.7 |
| General ache and pains | 1.3 ± 0.5 2,3 | 1.3 ± 0.5 2 | 2.3 ± 0.6 1 | 2.1 ± 0.6 1 | 2.3 ± 0.6 1 | 1.6 ± 0.6 * |
| Dizziness | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.7 ± 0.7 | 1.6 ± 0.6 | 1.6 ± 0.9 | 1.3 ± 0.6 |
| Cold sweat | 1.0 ± 0.0 2,3 | 1.0 ± 0.0 2 | 1.8 ± 0.8 1 | 1.5 ± 0.6 1 | 1.7 ± 0.6 1 | 1.1 ± 0.4 * |
| Headache | 1.4 ± 0.8 | 1.3 ± 0.6 2 | 1.9 ± 1.0 | 2.0 ± 1.0 1 | 1.4 ± 0.5 | 1.5 ± 0.6 |
| Nausea/vomiting | 1.1 ± 0.4 | 1.0 ± 0 | 1.5 ± 0.9 | 1.5 ± 0.9 | 1.3 ± 0.6 | 1.2 ± 0.4 |
| Hot flashes | 1.0 ± 00 | 1.0 ± 0 2 | 1.3 ± 0.6 | 1.4 ± 0.5 1 | 1.1 ± 0.4 | 1.3 ± 0.5 |
| Muscle stiffness | 1.1 ± 0.5 | 1.1 ± 0.3 | 1.3 ± 0.6 | 1.6 ± 0.5 | 1.3 ± 0.6 | 1.1 ± 0.3 * |
| Swelling legs | 1.4 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.5 | 1.6 ± 0.5 | 1.5 ± 0.6 | 1.5 ± 0.8 |
| Heart pounding | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.3 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.6 | 1.1 ± 0.4 |
| Skin blemish or disorder* | 1.7 ± 0.7 | 1.8 ± 0.6 | 2.1 ± 0.6 | 1.6 ± 0.6 * | 2.4 ± 1.1 | 1.8 ± 0.6 * |
| Numbness | 1.0 ± 0 | 1.0 ± 0 2 | 1.4 ± 0.8 | 1.5 ± 0.6 1,3 | 1.3 ± 0.6 | 1.1 ± 0.3 2 |
| Total scores | 20.8 ± 3.1 2,3 | 20.3 ± 3.3 2 | 31.0 ± 5.9 1 | 29.4 ± 3.6 1,3 | 29.9 ± 4.5 1 | 23.7 ± 3.7 2,* |
| Short-Form McGill Pain Questionnaire (total score) | 17.6 ± 2.5 2,3 | 17.3 ± 1.9 2,3 | 31.2 ± 11.0 1 | 31.1 ± 8.3 1,3 | 30.1 ± 6.3 1 | 21.9 ± 4.5 1,2,* |
| Visual Analog Scale of Pain | 1.4 ± 0.7 2,3 | 1.4 ± 0.7 2,3 | 7.2 ± 0.8 1 | 7.4 ± 0.9 1,3 | 7.1 ± 1.3 1 | 4.2 ± 1.8 1,2,* |
Control and Dysmenorrhea groups were defined according to the definitions of inclusion criteria from the VAS scores and the Short Form McGill Pain Questionnaire in the current study. Superscripts numbers (1, 2, and 3) indicate significant differences (P < 0.05) between the groups in pre- or post-testing and
‘*’ indicates significant differences between pre- and post-testing.
Effects of HIIT training on physical fitness
The participants’ upper limb strength, explosive force, cardiovascular fitness, and core strength were evaluated before and after HIIT intervention (Table 4). There were no significant differences in the grip strength, standing long jump distance, standing triple jumps distance, 3-min step workout, and bent-knee sit-up test measurements (F(2, 42) = 0.922 to 2.994, p > 0.05) among the three groups in pre-test assessments. After the 10-week duration of exercise training, the grip strength, core strength (30 seconds), and endurance (60 seconds) demonstrated by participants revealed no significant differences among or within groups, however the significant differences were observed with regards to the explosive force and cardiovascular fitness (F(2, 42) = 4.862 to 10.392, p < 0.05) among groups. The 10-week duration of exercise training resulted in significant improvements in explosive force and cardiovascular fitness in the DysmenHIIT group compared to the Control and Dysmen groups. Furthermore, the DysmenHIIT group was shown to have significantly improved in explosive force and cardiovascular fitness when comparing post-exercise test to pre-exercise test measurements.
Table 4.
Effects of HIIT spinning exercise intervention on physical fitness.
| Control (1) | Dysmen (2) | DysmenHIIT (3) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Grip strength (kg) | 27.0 ± 3.8 | 26.9 ± 3.4 | 26.6 ± 4.1 | 26.8 ± 3.9 | 28.9 ± 6.2 | 28.6 ± 5.4 |
| Standing long jump (cm) | 150 ± 26 | 150 ± 28 3 | 147 ± 23 | 146 ± 21 3 | 160 ± 33 | 172 ± 23 1,2,* |
| Standing triple jumps (cm) | 438 ± 62 | 437 ± 58 3 | 417 ± 65 | 431 ± 50 3 | 433 ± 64 | 496 ± 60 1,2,* |
| 3-min step test | 52 ± 6.3 | 52 ± 5.5 3 | 52 ± 7.3 | 52 ± 6.2 3 | 57 ± 7.9 | 63 ± 9.4 1,2,* |
| Bent-knee sit-up | ||||||
| (30 seconds) | 15.8 ± 2.4 | 15.8 ± 2.9 | 17.0 ± 4.0 | 16.9 ± 4.4 | 17.5 ± 1.8 | 18.1 ± 2.3 |
| (60 seconds) | 27.6 ± 5.9 | 27.7 ± 5.7 | 29.4 ± 9.2 | 28.9 ± 9.2 | 32.5 ± 4.9 | 32.1 ± 5.9 |
Control and Dysmenorrhea groups were defined according to the definitions of inclusion criteria from the VAS scores of the Short Form McGill Pain Questionnaire in the current study. Superscripts numbers (1, 2, and 3) indicate significant differences (P < 0.05) between the groups in pre- or post-testing and
‘*’ indicates significant differences between pre- and post-testing.
Effects of HIIT training on biochemical variables and hormones
Blood samples were obtained from participants three days before menstruation was expected to commence. In the pre-test analysis (Table 5), indices representing biochemical markers for the liver (AST and ALT), kidney (BUN and CREA), lipid metabolism (TG and CHOL), and tissue injury (LDH and CK) did not display any significant differences among groups (F (2, 42) = 0.048 to 2.401, p > 0.05). However, significant differences were observed for HsCRP (F (2, 42) = 4.529, p = 0.017), where the dysmenorrhea groups displayed significantly higher values than the control group. HsCRP is produced when inflammation occurs and is considered to be an indicator of systemic inflammation. The hormones (estradiol, PRL, progesterone, and cortisol) also showed significant differences among groups (F (2, 42) = 4.202 to 9.972, p < 0.05). The values represented by the dysmenorrhea groups were significantly higher in estradiol and PRL and lower in progesterone and cortisol compared to those of the control group. There were no significant differences in LH and FSH hormone levels among groups (F (2, 42) = 0.126, p = 0.882 and F (2, 42) = 0.595, p = 0.556, respectively). After 10 weeks of HIIT training, the AST, ALT, BUN, CREA, TG, CHOL, LDH, CK, LH, and FSH levels from post-test assessments showed no significant differences among groups (F (2, 42) = 0.136 to 2.849, p > 0.05). There were, however, significant differences among groups observed in levels of HsCRP (F (2, 42) = 5.81, p < 0.05), estradiol (F (2, 42) = 8.72, p < 0.05), progesterone (F (2, 42) = 6.55, p < 0.05), and cortisol (F (2, 42) = 4.41, p < 0.05) (Table 5). More specifically, the study group undergoing HIIT intervention (DysmenHIIT) showed significant increases in progesterone and cortisol levels, and decreases in HsCRP and estradiol compared to the Dysmen group. In comparing the pre- and post-test assessments, significant decreases in LDH, HsCRP, estradiol, and PRL, in addition to increases in progesterone and cortisol were observed within the DysmenHIIT group as a result of HIIT intervention.
Table 5.
Effects of HIIT spinning exercise intervention on biochemical variables and hormones.
| Control (1) | Dysmen (2) | DysmenHIIT (3) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| AST (U/L) | 16.1 ± 6.5 | 13.2 ± 3.0 | 15.3 ± 2.3 | 14.3 ± 3.8 | 15.0 ± 2.2 | 13.8 ± 2.3 |
| ALT (U/L) | 09.5 ± 3.7 | 09.4 ± 3.4 | 09.0 ± 2.3 | 09.2 ± 4.6 | 07.1 ± 2.2 | 07.4 ± 2.8 |
| BUN (mg/dL) | 11.5 ± 3.6 | 12.3 ± 3.8 | 11.4 ± 2.1 | 12.3 ± 3.1 | 13.7 ± 3.6 | 13.4 ± 3.6 |
| CREA (mg/dL) | 0.66 ± 1.14 | 0.63 ± 0.08 | 0.66 ± 0.64 | 0.66 ± 0.05 | 0.72 ± 0.08 | 0.70 ± 0.09 |
| TG (mg/dL) | 64.7 ± 23 | 59.2 ± 12 | 69.7 ± 15 | 70.4 ± 19 | 67.1 ± 26 | 67.3 ± 21 |
| CHOL (mg/dL) | 161. ± 37 | 154. ± 29 | 158. ± 22 | 155. ± 26 | 160. ± 20 | 158. ± 19 |
| LDH (U/L) | 112. ± 18 | 103. ± 12 | 113. ± 15 | 108. ± 18 | 125. ± 20 | 116. ± 18 * |
| CPK (U/L) | 101. ± 86 | 083. ± 69 | 103. ± 48 | 071. ± 49 | 109. ± 58 | 104. ± 51 |
| HsCRP (mg/dL) | 0.031 ± 0.02 2,3 | 0.037 ± 0.02 2 | 0.136 ± 0.02 1 | 0.139 ± 0.11 1,3 | 0.134 ± 0.06 1 | 0.043 ± 0.03 2,* |
| Estradiol (pg/mL) | 188 ± 51 2,3 | 177 ± 41 2 | 233 ± 35 1 | 228 ± 40 1,3 | 221 ± 34 1 | 174 ± 37 2,* |
| FSH (mIU/mL) | 03.8 ± 1.7 | 4.0 ± 2.5 | 04.1 ± 2.3 | 4.5 ± 2.5 | 04.2 ± 2.4 | 3.5 ± 1.7 |
| LH (mIU/mL) | 08.1 ± 5.2 | 5.7 ± 3.1 | 07.4 ± 6.3 | 6.8 ± 5.0 | 06.1 ± 3.9 | 6.1 ± 4.2 |
| PRL (ng/mL) | 24.3 ± 9.0 3 | 25.9 ± 8.3 2 | 32.8 ± 9.0 | 33.7 ± 7.8 1 | 35.7 ± 15 1 | 28.0 ± 12 * |
| Progesterone (ng/mL) | 11.7 ± 4.6 2,3 | 11.2 ± 4.1 2 | 05.8 ± 3.7 1 | 06.0 ± 5.3 1,3 | 05.4 ± 4.2 1 | 9.7 ± 2.2 2,* |
| Cortisol (μg/dL) | 9.6 ± 3.3 3 | 11.6 ± 2.8 2 | 7.9 ± 2.6 | 8.7 ± 3.1 1,3 | 6.7 ± 2.3 1 | 11.6 ± 3.6 2,* |
Control and Dysmenorrhea groups were defined according to the definitions of inclusion criteria from the VAS scores of the Short Form McGill Pain Questionnaire in the current study. Superscripts numbers (1, 2, and 3) indicate significant differences (P < 0.05) between the groups
‘*’ indicates significant differences between pre- or post-testing.
Effects of HIIT training on prostaglandin levels
Arachidonic acid, a fatty acid, accumulates in the phospholipid bilayer of the cell membrane after ovulation alongside a decrease in progesterone levels, and plays a role in the metabolism of prostaglandins (PGF2α and PGE2), prostacyclins and thromboxane for inflammation vasoconstriction and uterine hypercontraction. In pre-test analyses (Figure 3), significant differences in PGF2α and PGE2 levels were observed (F (2, 42) = 24.108, p < 0.0001 and F (2, 42) = 16.439, p < 0.0001, respectively) and the dysmenorrhea groups displayed significantly higher PGF2α and PGE2 levels compared to the control group. After the 10-week HIIT training, significant differences in PGF2α and PGE2 levels between the study groups remained (F (2, 42) = 15.61, p<0.0001 and F (2, 42) = 17.4, p<0.0001, respectively). This was most notable in the significant decreases displayed by the DysmenHIIT group compared to those of the Dysmen group, however the DysmenHIIT group hormone levels were still significantly higher than those in the control. Additionally, both PGF2α and PGE2 hormone levels were shown to be significantly decreased within the DysmenHIIT group, possibly as a result of the HIIT intervention.
Figure 3.
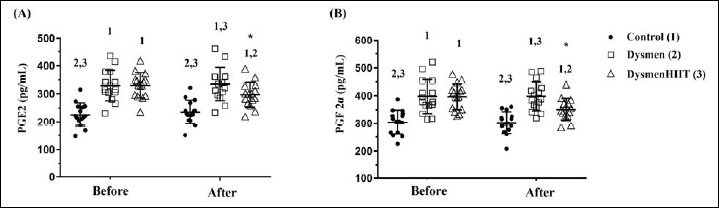
The effect of HIIT spinning exercise on prostaglandin metabolite levels. Blood samples were obtained from the Control, Dysmen, and DysmenHIIT groups approximately three days before menstruation. Levels of the prostaglandins PGE2 (A) and PGF2α (B) were assessed before and after the 10-week HIIT exercise training program. The data are presented as means ± SD. Superscripts numbers (1, 2, and 3) indicate significant differences (P < 0.05) between the groups. as evaluated by a one-way analysis of variance (ANOVA). ‘*’ represents significant differences within groups.
Correlation between hormones, inflammatory variables, and menstrual distress
Independent correlation analyses between MDQ, SF-MPQ, prostaglandins, cortisol, progesterone, estradiol and HsCRP are presented in Table 6. Significant negative correlations between progesterone and HsCRP as well as progesterone and PGF2α were detected (HsCRP, r = -0.395, p = 0.007; PGF2α, r = -0.312, p = 0.037), whereas a significant positive correlation was observed between cortisol and progesterone levels (r = 0.362, p = 0.029). Additionally, the levels of PGE2 and PGF2α were shown to have significantly positive correlations with HsCRP (r = 0.408, p = 0.005 and r = 0.297, p = 0.048, respectively). For the dysmenorrhea symptoms correlations, the MDQ showed significant correlations with SF-MPQ (r = 0.788, p < 0.01), PGE2 (r = 0.515, p < 0.01), PGF2α (r = 0.576, p < 0.01), progesterone (r = -0.377, p = 0.011), estradiol (r = 0.409, p = 0.005), and HsCRP (r = 0.312, p = 0.037).
Table 6.
The correlation between hormones, inflammatory variables, and menstrual distress with sprint-exercise intervention.
| MDQ | SF-MPQ | PGE2 | PGF2α | Cortisol | Progesterone | estradiol | HsCRP | |
|---|---|---|---|---|---|---|---|---|
| MDQ | 1 | 0.788 ** | 0.515 ** | 0.576 ** | -0.284 | -0.377 * | 0.409 ** | 0.312 * |
| SF-MPQ | 0.788 ** | 1 | 0.367 * | 0.493 ** | -0.295 * | -0.394 ** | 0.427 ** | 0.361 * |
| PGE2 | 0.515 ** | 0.367 * | 1 | 0.690 ** | -0.155 | -0.202 | 0.107 | 0.408 ** |
| PGF2α | 0.576 ** | 0.493 ** | 0.690 ** | 1 | -0.164 | -0.312 * | 0.166 | 0.297 * |
| Cortisol | -0.284 | -0.295 * | -0.155 | -0.164 | 1 | 0.326 * | -0.261 | -0.215 |
| Progesterone | -0.377 * | -0.394 ** | -0.202 | -0.312 * | 0.326 * | 1 | -0.129 | -0.395 ** |
| estradiol | 0.409 ** | 0.427 ** | 0.107 | 0.166 | -0.261 | -0.129 | 1 | 0.121 |
| HsCRP | 0.312 * | 0.361 * | 0.408 ** | 0.297 * | -0.215 | -0.395 ** | 0.121 | 1 |
The MDQ and SF-MPQ represent the menstrual distress and the short form McGill pain questionnaires, respectively.
‘*’ and
‘**’ indicates significant correlations between the variables (P < 0.05 and P < 0.01, respectively).
Besides, the significant differences also were observed between SF-MPQ and PGE2 (r = 0.367, p = 0.013), PGF2α (r = 0.493, p < 0.01), cortisol (r = -0.295, p = 0.049), progesterone (r = -0.394, p = 0.007), estradiol (r = 0.427, p = 0.003), and HsCRP (r = 0.361, p = 0.015).
Discussion
Different types of aerobic and resistance exercise have been considered as alternative medical avenues for the alleviation of primary dysmenorrhea symptoms, due to the hormonal changes the uterine lining undergoes as a consequence of performing such exercises. However, the various hormonal changes and interactions within the endocrine system that play a role in exercise-induced pain alleviation are not clearly understood (Jaleel et al., 2022). In the present study, spinning bike exercises were performed as a form of HIIT intervention, and were implemented while participants were monitored for exercise intensity. The results suggest that such an exercise model could improve a person’s physical attributes such as cardiovascular fitness and explosive force. Additionally, premenstrual symptoms, menstrual distress, and menstrual pain were also significantly ameliorated on implementation of the 10-week HIIT spinning bike exercise, possibly through the modulation of hormones, prostaglandins, and decreased inflammation.
In a previous meta-analysis, both low- and high-intensity exercises were demonstrated to have a large effect on reducing menstrual pain intensity, resulting in a 25 mm reduction on a 100 mm visual analogue scale (VAS) (Armour et al., 2019). Previous studies have also shown that both aerobic and stretching exercises are effective in reducing the severity of dysmenorrhea in female students (Vaziri et al., 2015). However, studies related to the effects of HIIT on dysmenorrhea symptom amelioration are limited. High intensity interval training (HIIT) as a whole encompasses a variety of workout methods involving short periods of intense exercise interspersed with recovery periods. HIIT, intensity maintained at 85% - 95% of an individual maximum heart rate, has been recognized as an efficient exercise protocol, and the short exercise sessions characteristic of this regimen have been shown to have an effect in improving cardiovascular and metabolic capacity in different populations (Ito, 2019) and the HIIT in 15 repetitions × 30-seconds cycling at ~85-90% maximum heart rate with 30-seconds of active recovery could also apply to cardiac rehabilitation on patients with coronary artery disease for improvement of aerobic fitness, blood pressure and body fat levels (Keech et al., 2020). The VAS used by the DysmenHIIT group to measure pain severity displayed a significant decrease in pain intensity (by approximately 29 mm) within the group, consistent with previous meta-analysis results. However, a previous study reported that an 8-week long HIIT regimen did not result in any significant differences in dysmenorrhea pain intensity between the control or HIIT groups, both of whom also underwent nutrition supplementation (Atashak and Rashidi, 2018). Furthermore, dysmenorrhea symptom alleviation is likely affected by many factors, such as types of exercise, exercise frequency, HIIT conditions, evaluation tools, and intensity monitoring approach. Prescribing HIIT exercise as a pain-relieving method while monitoring the exercise intensity could be an important avenue and warrants further validation.
A previous meta-analysis indicated that HIIT had a moderately positive effect on improving cardiorespiratory fitness (Martin-Smith et al., 2020). A 4-week cycle-based HIIT regimen, performed by an adolescent population, was also shown to significantly improve anaerobic threshold, power, and heart rate recovery compared to continuous training of moderate intensity (Fang et al., 2021). The results of the present study show significant improvements in cardiovascular fitness and power indexes within and among the study groups, and these improvements were positively correlated with HIIT intervention. The effects of resistance training in the lower body have also been shown to improve upper-body adaptations, as mentioned in a study by Bartolomei et al. (2018). Hypertrophy training programs (10-12 reps at 65% - 70% of 1-RM) designed for the lower body and geared towards improving upper-body maximal strength were shown to stimulate greater strength and power gains in the upper body compared to high intensity resistance training programs designed for both the upper and lower body (Bartolomei et al., 2018). However, in the present study, there was no significant improvement in grip strength within and among treatment groups. Resistance intensity and type, implemented by a spinning bike, seemed to present insufficient stimulation for upper-body adaptation, and additionally the assessments used for physical fitness were performed using grip strength as the measured variable, rather than the bench press used in the Bartolomei et al. (2018) study. The core strength and muscular endurance assessment results from the current study didn’t show significant differences, possibly due to differences in which muscle groups were tested, the type of exertion spinning bikes demand of the body, and the physical fitness test parameters.
Pharmaceuticals, physiotherapy, nutrition, and exercise strategies have been applied and have shown promise for dysmenorrhea symptom amelioration. Non-steroid anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, diclofenac potassium, and meclofenamate, alongside hormonal contraceptives, were the first and second modes of medical intervention against dysmenorrhea through their modulation of prostaglandins, leukotrienes, and progesterone and the resulting inhibition of cyclooxygenase and limitation of endometrial growth. The mechanisms behind menstrual distress and pain are well known, and are a result of inflammation and imbalanced hormone regulation. When fertilization fails to occur in the late luteal phase, progesterone levels rapidly decline and inflammation-associated cytokines (TNF-α) and prostaglandins (PGE2 and PGF2α) are secreted from endometrial stromal cells and arachidonic acid is metabolized by cyclooxygenase for prostaglandin, prostacyclin, and thromboxane. Consequently, the pain threshold is decreased due to ischemia caused by hypercontractility of the myometrium (Barcikowska et al., 2020). In a study involving adolescents, the stress and prostaglandin levels also significantly contributed to the occurrence of dysmenorrhea (Tahir et al., 2021). Another study also suggested that the occurrence of both premenstrual syndrome and primary dysmenorrhea were related to higher levels of HsCRP, prooxidant-antioxidant balance, and the neutrophil-to-lymphocyte ratio (Bahrami et al., 2020). In the present study, in samples taken during the late luteal phase, prostaglandin and HsCRP levels were shown to be significantly higher in dysmenorrhea groups than the control group, and the HIIT intervention was shown to significantly decrease prostaglandin and HsCRP levels both within and among groups. Additionally, an increase in concentration of prolactin has been indicated as a potential risk factor for the pathogenesis of adenomyosis, and a prolactin inhibitor may significantly improve menstrual bleeding, pain, and quality of life (Andersson et al., 2019). In the present study, the modulation of prolactin and progesterone levels in patients suffering from dysmenorrhea was significantly improved on completion of the 10-week HIIT exercise program. In previous study related hormones correlation of dysmenorrhea, PGF2α levels in the uterine muscle layer were also shown as having a positive correlation with estradiol levels and a negative correlation with progesterone levels in the uterine vein (Xue et al., 2014). Cortisol levels demonstrated a decreasing trend from the follicular to the luteal phase of the menstrual cycle, and the cortisol levels in patients with dysmenorrhea were significantly lower than the health control group (Vincent et al., 2011). Thus, dysmenorrhea symptom amelioration could be modulated through the homeostasis of prostaglandins and hormones (estradiol, progesterone, cortisol, and prolactin) using the 10-week HIIT exercise intervention strategy that was the focus of the current study.
Adverse side effects of pharmaceuticals used for the treatment of dysmenorrhea, including nausea, dyspepsia, headaches, dizziness, drowsiness, dry mouth, organs injury, and risk of thromboembolic complications, have been documented (Zahradnik et al., 2010). In a previous meta-analysis, evidence suggested that therapeutic exercise had a moderate effect on reducing pain intensity in patients diagnosed with primary dysmenorrhea, but little evidence was obtained regarding its effects on the overall duration of pain or the quality of life of the patient (Carroquino-Garcia et al., 2019). The goal of the present study was therefore to establish an optimized exercise treatment for the amelioration of primary dysmenorrhea symptoms and pain. Unfortunately, while the HIIT model designed for cycling showed better local tissue oxygenation, its perceived exertion (as rated by participants) was also higher when compared to running exercises, and it was considered a less enjoyable physical activity (Kriel et al., 2018). The adherence to exercise on part of participants who are afflicted with dysmenorrhea should also be considered as a factor for promoting exercise management. Additionally, a previous study on appetite-regulating hormones also demonstrated that the increasing levels of intensity-dependent signals lead to greater orexigenic suppression and greater anorexigenic stimulation (Hazell et al., 2016). Participants were encouraged to meet the required exercise intensity by monitoring their heart rate, which is a critical aspect in exercise interventions for reaching optimal physiological adaptation parameters related to hormone and inflammation homeostasis. On the intensity implementation of HIIT, the different intensity validation and exercise performance assessment could be also precisely adjusted and evaluated by cardiopulmonary exercise test (CPX test), which could be considered as limitation on intensity validation and physiological adaptation in present study.
Conclusion
Various treatment strategies, including pharmaceuticals, alternative medicine, nutrition, the use of hot compresses, and physiotherapy, have been widely used for alleviating dysmenorrhea symptoms, and exercise may now also be considered as a potential treatment method for this condition. The 10-week HIIT spinning bike training was shown to improve the physical fitness (cardiovascular fitness and power) of participants, and the symptoms and pain usually felt for the duration of the premenstrual and menstrual cycles were also alleviated through the modulation of hormones, inflammation, and prostaglandins. While these results are encouraging, the alleviation of dysmenorrhea (primary and secondary) symptoms through the use of different types of exercise adapted for HIIT models as well as more varied study parameters, such as age population and complementary nutrition, warrants further investigation. Furthermore, exercise intensity has been shown to play an important role in reaching optimal physiological adaptation parameters as well as encouraging participant adherence to the practical application of the prescribed exercise intervention. Thus, the exercise intervention described in the present study may be considered as an avenue for promoting women’s health and as an adequate way for improving quality of life, study/work productivity, and as a possible prevention method against gynecological disorders in the adolescent population.
Acknowledgements
We would like to state our appreciation for the students from NTUNHS for their assistance with the physical fitness tests and thank Uni-edit (www.uni-edit.net) for editing and proofreading this manuscript. This work was financially supported by Taiwan’s Ministry of Science and Technology (MOST111-2628-H-227-001-MY2). The experiments complied with the current laws of the country in which they were performed. The authors have no conflicts of interest to declare. The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author who was an organizer of the study.
Biographies

Wen Ching HUANG
Employment
Depart. of Exercise and Health Science, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
Degree
PhD
Research interests
Sports nutrition, Exercise Performance, Exercise physiology, Health Food Development
E-mail: wenching@ntunhs.edu.tw

Pei Chi CHIU
Employment
Depart. of Exercise and Health Science, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
Degree
BSc.
Research interests
Exercise Fitness Assessment, Exercise Instruction, Weight management
E-mail: nikaaaa8726@gmail.com

Chi Hong HO
Employment
Depart. of Obstetrics and Gynecology, Taipei Veterans General Hospital, Taipei, Taiwan
Degree
Ph.D.
Research interests
Polycystic Ovary Syndrome, Endometriosis, Dysmenorrhea, Laparoscopic and Hysteroscopic Surgery, Obstetric Examination and Delivery
E-mail: hochwater@gmail.com
References
- Al-Matouq S., Al-Mutairi H., Al-Mutairi O., Abdulaziz F., Al-Basri D., Al-Enzi M., Al-Taiar A. (2019) Dysmenorrhea among high-school students and its associated factors in Kuwait. BMC Pediatrics 19, 80. https://doi.org/10.1186/s12887-019-1442-6. 10.1186/s12887-019-1442-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.K., Khan Z., Weaver A.L., Vaughan L.E., Gemzell-Danielsson K., Stewart E.A. (2019) Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: A pilot study. Acta Obstetricia et Gynecologica Scandinavica 98, 1341-1350. https://doi.org/10.1111/aogs.13632. 10.1111/aogs.13632 [DOI] [PubMed] [Google Scholar]
- Armour M., Ee C.C., Naidoo D., Ayati Z., Chalmers K.J., Steel K.A., de Manincor M.J., Delshad E. (2019) Exercise for dysmenorrhoea. Cochrane Database of Systematic Reviews 9, CD004142. https://doi.org/10.1002/14651858.CD004142.pub4. 10.1002/14651858.CD004142.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atashak S., Rashidi S. (2018) Effect of eight-week high-intensity interval training and ginger supplementation on primary dysmenorrhea in nonathletic female students. Iranian Journal of Obstetrics, Gynecology and Infertility 20, 23-31. https://doi.org/10.22038/ijogi.2017.10425 10.22038/ijogi.2017.10425 [DOI] [Google Scholar]
- Bahrami A., Avan A., Sadeghnia H.R., Esmaeili H., Tayefi M., Ghasemi F., Nejati Salehkhani F., Arabpour-Dahoue M., Rastgar-Moghadam A., Ferns G.A., Bahrami-Taghanaki H., Ghayour-Mobarhan M. (2018) High dose vitamin D supplementation can improve menstrual problems, dysmenorrhea, and premenstrual syndrome in adolescents. Gynecological Endocrinology 34, 659-663. https://doi.org/10.1080/09513590.2017.1423466. 10.1080/09513590.2017.1423466 [DOI] [PubMed] [Google Scholar]
- Bahrami A., Bahrami-Taghanaki H., Khorasanchi Z., Timar A., Jaberi N., Azaryan E, Tayefi M., Ferns G.A., Sadeghnia H.R., Ghayour-Mobarhan M. (2020) Menstrual problems in adolescence: relationship to serum vitamins A and E, and systemic inflammation. Archives of Gynecology and Obstetrics 301, 189-197. https://doi.org/10.1007/s00404-019-05343-1. 10.1007/s00404-019-05343-1 [DOI] [PubMed] [Google Scholar]
- Barcikowska Z., Rajkowska-Labon E., Grzybowska M.E., Hansdorfer-Korzon R., Zorena K. (2020) Inflammatory Markers in Dysmenorrhea and Therapeutic Options. International Journal of Environmental Research and Public Health 17, 1191. https://doi.org/10.3390/ijerph17041191 10.3390/ijerph17041191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C., Villringer A., Sacher J. (2015) Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience 9, 37. https://doi.org/10.3389/fnins.2015.00037 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei S., Hoffman J.R., Stout J.R., Merni F. (2018) Effect of Lower-Body Resistance Training on Upper-Body Strength Adaptation in Trained Men. Journal of Strength and Conditioning Research 32, 13-18. https://doi.org/10.1519/JSC.0000000000001639. 10.1519/JSC.0000000000001639 [DOI] [PubMed] [Google Scholar]
- Bernardi M., Lazzeri L., Perelli F., Reis F.M., Petraglia F. (2017) Dysmenorrhea and related disorders. F1000Research 6, 1645. https://doi.org/10.12688/f1000research.11682.1 10.12688/f1000research.11682.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A., Lupo C., Alesi M., Spina S., Raccuglia M., Thomas E., Paoli A., Palma A. (2015) The sit up test to exhaustion as a test for muscular endurance evaluation. Springerplus 4, 309. https://doi.org/10.1186/s40064-015-1023-6. 10.1186/s40064-015-1023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J.R, Rowland S.P., Scherwitzl E.B., Scherwitzl R., Danielsson K.G., Harper J. (2019) Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. Nature Partner Digital Medicine 2, 83. https://doi.org/10.1038/s41746-019-0152-7 10.1038/s41746-019-0152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroquino-Garcia P., Jiménez-Rejano J.J., Medrano-Sanchez E., de la Casa-Almeida M., Diaz-Mohedo E., Suarez-Serrano C. (2019) Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Physical Therapy 99, 1371-1380. https://doi.org/10.1093/ptj/pzz101. 10.1093/ptj/pzz101 [DOI] [PubMed] [Google Scholar]
- Chen H.M., Chen C.H. (2005) Related factors and consequences of menstrual distress in adolescent girls with dysmenorrhea. Kaohsiung Journal of Medical Sciences 21, 121-127. https://doi.org/10.1016/S1607-551X(09)70288-8. 10.1016/S1607-551X(09)70288-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.H., Shih C.C., Yang Y.K., Chen K.T., Chang Y.H., Yang Y.C. (2013) Factors associated with premenstrual syndrome - a survey of new female university students. Kaohsiung Journal of Medical Sciences 29, 100-105. https://doi.org/10.1016/j.kjms.2012.08.017. 10.1016/j.kjms.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand H., Monahan K., McGuire B.E. (2021) Prevalence and Impact of Dysmenorrhea Among University Students in Ireland. Pain Medicine 22, 2835-2845. https://doi.org/10.1093/pm/pnab122. 10.1093/pm/pnab122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B., Kim Y., Choi M. (2021) Effect of Cycle-Based High-Intensity Interval Training and Moderate to Moderate-Intensity Continuous Training in Adolescent Soccer Players. Healthcare (Basel) 9, 1628. https://doi.org/10.3390/healthcare9121628. 10.3390/healthcare9121628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T. (2013) Dysmenorrhea and endometriosis in young women. Yonago Acta Medica 56, 81-84. [PMC free article] [PubMed] [Google Scholar]
- Hazell T.J., Islam H., Townsend L.K., Schmale M.S., Copeland J.L. (2016) Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 98, 80-88. https://doi.org/10.1016/j.appet.2015.12.016. 10.1016/j.appet.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Heinemann L.A., Minh T.D., Filonenko A., Uhl-Hochgräber K. (2010) Explorative evaluation of the impact of severe premenstrual disorders on work absenteeism and productivity. Women's Health Issues 20, 58-65. https://doi.org/10.1016/j.whi.2009.09.005. 10.1016/j.whi.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Howard S.R. (2021) Interpretation of reproductive hormones before, during and after the pubertal transition-Identifying health and disordered puberty. Clinical Endocrinology 95, 702-715. https://doi.org/10.1111/cen.14578. 10.1111/cen.14578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. (2019) High-intensity interval training for health benefits and care of cardiac diseases - The key to an efficient exercise protocol. World Journal of Cardiology 11, 171-188. https://doi.org/10.4330/wjc.v11.i7.171. 10.4330/wjc.v11.i7.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel G., Shaphe M.A., Khan A.R., Malhotra D., Khan H., Parveen S., Qasheesh M., Beg R.A., Chahal A., Ahmad F., Ahmad M.F. (2022) Effect of Exercises on Central and Endocrine System for Pain Modulation in Primary Dysmenorrhea. Journal of Lifestyle Medicine 12, 15-25. https://doi.org/10.15280/jlm.2022.12.1.15. 10.15280/jlm.2022.12.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H., Jones M., Mishra G. (2014) The prevalence and risk factors of dysmenorrhea. Epidemiologic Reviews 36, 104-113. https://doi.org/10.1093/epirev/mxt009. 10.1093/epirev/mxt009 [DOI] [PubMed] [Google Scholar]
- Karout S., Soubra L., Rahme D., Karout L., Khojah H.M.J., Itani R. (2021) Prevalence, risk factors, and management practices of primary dysmenorrhea among young females. BMC Womens Health 21, 392. https://doi.org/10.1186/s12905-021-01532-w. 10.1186/s12905-021-01532-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech A., Holgate K., Fildes J., Indraratna P., Cummins L., Lewis C., Yu J. (2020) High-intensity interval training for patients with coronary artery disease: Finding the optimal balance. International Journal of Cardiology 298, 8-14. https://doi.org/10.1016/j.ijcard.2019.09.060. 10.1016/j.ijcard.2019.09.060 [DOI] [PubMed] [Google Scholar]
- Kieu N.T.V., Jung S.J., Shin S.W., Jung H.W., Jung E.S., Won Y.H., Kim Y.G., Chae S.W. (2020) The Validity of the YMCA 3-Minute Step Test for Estimating Maximal Oxygen Uptake in Healthy Korean and Vietnamese Adults. Journal of Lifestyle Medicine 10, 21-29. https://doi.org/10.15280/jlm.2020.10.1.21. 10.15280/jlm.2020.10.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriel Y., Askew C.D., Solomon C. (2018) The effect of running versus cycling high-intensity intermittent exercise on local tissue oxygenation and perceived enjoyment in 18-30-year-old sedentary men. PeerJ 6, e5026. https://doi.org/10.7717/peerj.5026. 10.7717/peerj.5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loturco I., Pereira L.A., Cal Abad C.C., D’Angelo R.A., Fernandes V., Kitamura K., Kobal R., Nakamura F.Y. (2015) Vertical and Horizontal Jump Tests Are Strongly Associated With Competitive Performance in 100-m Dash Events. Journal of Strength & Conditioning Research 29,1966-1971. https://doi.org/10.1519/JSC.0000000000000849. 10.1519/JSC.0000000000000849 [DOI] [PubMed] [Google Scholar]
- Martin-Smith R., Cox A., Buchan D.S., Baker J.S., Grace F., Sculthorpe N. (2020) High Intensity Interval Training (HIIT) Improves Cardiorespiratory Fitness (CRF) in Healthy, Overweight and Obese Adolescents: A Systematic Review and Meta-Analysis of Controlled Studies. International Journal of Environmental Research and Public Health 17, 2955. https://doi.org/10.3390/ijerph17082955. 10.3390/ijerph17082955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor M., Farquhar C. (2006) Diagnosis and management of dysmenorrhoea. British Medical Journal 332, 1134-1138. https://doi.org/10.1136/bmj.332.7550.1134 10.1136/bmj.332.7550.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckling F.M., Farinha J.B., Figueiredo F.D.C., Santos D.L.D., Bresciani G., Kretzmann N.A., Stefanello S.T., Courtes A.A., Beck M.O., Sangoi Cardoso M., Duarte M.M.M.F., Moresco R.N., Soares F.A.A. (2019) High-intensity interval training improves inflammatory and adipokine profiles in postmenopausal women with metabolic syndrome. Archives of Physiology and Biochemistry 125, 85-91. https://doi.org/10.1080/13813455.2018.1437750. 10.1080/13813455.2018.1437750 [DOI] [PubMed] [Google Scholar]
- Steiner M., Macdougall M., Brown E. (2003) The premenstrual symptoms screening tool (PSST) for clinicians. Archives of Women's Mental Health 6, 203-209. https://doi.org/10.1007/s00737-003-0018-4. 10.1007/s00737-003-0018-4 [DOI] [PubMed] [Google Scholar]
- Swain D.P., Franklin B.A. (2006) Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. American Journal of Cardiology 9, 141-147. https://doi.org/10.1016/j.amjcard.2005.07.130. 10.1016/j.amjcard.2005.07.130 [DOI] [PubMed] [Google Scholar]
- Tadakawa M., Takeda T., Monma Y., Koga S., Yaegashi N. (2016) The prevalence and risk factors of school absenteeism due to premenstrual disorders in Japanese high school students-a school-based cross-sectional study. BioPsychoSocial Medicine 10, 13. https://doi.org/10.1186/s13030-016-0067-3 10.1186/s13030-016-0067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir A., Sinrang A.W., Jusuf E.C., Syamsuddin S., Arsyad A. (2021) The influence of macronutrient intake, stress and prostaglandin levels (pgf2α) of urine with the incidence of dysmenorrhea in adolescents. Gaceta Sanitaria 35, 298-301. https://doi.org/10.1016/j.gaceta.2021.10.039. 10.1016/j.gaceta.2021.10.039 [DOI] [PubMed] [Google Scholar]
- Vaziri F., Hoseini A., Kamali F., Abdali K., Hadianfard M., Sayadi M. (2015) Comparing the effects of aerobic and stretching exercises on the intensity of primary dysmenorrhea in the students of universities of bushehr. Journal of Family and Reproductive Health 9, 23-28. [PMC free article] [PubMed] [Google Scholar]
- Vincent K., Warnaby C., Stagg C.J., Moore J., Kennedy S., Tracey I. (2011) Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 152, 1966-1975. https://doi.org/10.1016/j.pain.2011.03.029. 10.1016/j.pain.2011.03.029 [DOI] [PubMed] [Google Scholar]
- Xing R., Yang J., Wang R., Wang Y. (2021) Extracorporeal shock wave therapy for treating primary dysmenorrhea: A randomized controlled trial. Medicine (Baltimore) 100, e23798. https://doi.org/10.1097/MD.0000000000023798. 10.1097/MD.0000000000023798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Liu C.Z., Gao S.Z., Ma Y.X. (2014) The herbal-partitioned moxibustion for primary dysmenorrhea and it's impact on reproductive endocrinal function of patients. Zhongguo Zhen Jiu 34 209-212. [PubMed] [Google Scholar]
- Yeh M.L., Hung Y.L., Chen H.H., Wang Y.J. (2013) Auricular acupressure for pain relief in adolescents with dysmenorrhea: a placebo-controlled study. Journal of Alternative and Complementary Medicine 19, 313-318. https://doi.org/10.1089/acm.2011.0665. 10.1089/acm.2011.0665 [DOI] [PubMed] [Google Scholar]
- Zahradnik H.P., Hanjalic-Beck A., Growth K. (2010) Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: A Review. Contraception 81, 185-196. https://doi.org/10.1016/j.contraception.2009.09.014. 10.1016/j.contraception.2009.09.014 [DOI] [PubMed] [Google Scholar]


