Abstract
Objectives
To longitudinally compare SARS-CoV-2-specific T cell and humoral immune responses between convalescent individuals who are HIV-positive (HIV+) and HIV-negative (HIV-).
Methods
We conducted enzyme-linked immunospots to determine the SARS-CoV-2-specific T cell responses to spike and nucleocapsid, membrane protein, and other open reading frame proteins (NMO), whereas an immunofluorescence assay was used to determine the humoral responses. Participants were sampled at baseline and after 8 weeks of follow-up.
Results
Individuals who are HIV- had significantly more T cell responses to NMO and spike than individuals who are HIV+ at baseline, P-value = 0.026 and P-value = 0.029, respectively. At follow-up, T cell responses to NMO and spike in individuals who are HIV+ increased to levels comparable with individuals who are HIV-. T cell responses in the HIV- group significantly decreased from baseline levels at the time of follow-up (spike [P-value = 0.011] and NMO [P-value = 0.014]). A significantly higher number of individuals in the HIV+ group had an increase in T cell responses to spike (P-value = 0.01) and NMO (P-value = 0.026) during the follow-up period than the HIV- group. Antispike and antinucleocapsid antibody titers were high (1: 1280) and not significantly different between individuals who were HIV- and HIV+ at baseline. A significant decrease in antinucleocapsid titer was observed in the HIV- (P-value = 0.0001) and the HIV+ (P-value = 0.001) groups at follow-up. SARS-CoV-2 vaccination was more effective in boosting the T cell than antibody responses shortly after infection.
Conclusion
There is an impairment of SARS-CoV-2-specific T cell immunity in individuals who are HIV+ with advanced immunosuppression. SARS-CoV-2-specific T cell immune responses may be delayed in individuals who are HIV+, even in those on antiretroviral therapy. There is no difference in SARS-CoV-2-specific humoral immunity between individuals who are HIV- and HIV+.
Keywords: SARS-CoV-2, T cell responses, Humoral immunity, HIV, Vaccination
Introduction
The SARS-CoV-2 is a single-stranded, encapsulated RNA virus that causes COVID-19 [1]. SARS-CoV-2 has so far caused millions of infections and deaths since its identification in 2019 [2]. There have been several waves of infections, with some countries experiencing up to four waves and increased transmissibility of the mutated virus with each successive wave [3,4].
Several populations are at a higher risk of severe COVID-19 disease and death due to many comorbidities that may compromise their ability to fight the infection. Among these vulnerable populations are the elderly; individuals with HIV/AIDS who have high HIV viral loads and low clusters of differentiation (CD4) counts; individuals with cancer; and individuals with other comorbidities, including diabetes, hypertension, and obesity [5], [6], [7], [8]. It has been reported by a study conducted in Spain that SARS-CoV-2 seroprevalence was much higher among people living with HIV than the general population [9]. Also, other studies have reported that individuals with HIV who are not on antiretroviral therapy (ART) are at a higher risk of COVID-19 in-hospital mortality than individuals without HIV and individuals with HIV who are on ART [10], [11], [12].
HIV infects CD4+ T lymphocytes and results in a progressive depletion of this cell population and ultimately leads to an impairment of cell-mediated immunity [13]. This then increases the risk of opportunistic infections and cancer [14]. There are several subsets of CD4+ T cells, some of which are helper T cells that are required for the survival of memory CD8+ T cells during viral infections [15]. CD8+ T cells, also known as cytotoxic T lymphocytes, mediate adaptive immunity and are important for killing cancerous cells and virus-infected cells [16]. The importance of T cell immunity in SARS-CoV-2 infection has been reported, where elderly patients with COVID-19 aged >80 years old have been observed to have diminished CD8 T cell responses, which could explain the more frequent severity of COVID-19 in the elderly [17]. Therefore, it is apparent that both CD4+ and CD8+ T cell-mediated immunity, in addition to humoral and innate immunity, are a critical part of the host responses against SARS-CoV-2 infection.
Most immunological studies during the COVID-19 pandemic have focused on the humoral immunity against SARS-CoV-2 [18,19] because antibodies are especially important in neutralizing viruses and preventing infection, especially after vaccination [20,21]. However, when the prevention of infection fails, as in breakthrough infections, T cell immunity will be the key to recognizing and killing infected cells [22]. Studies on SARS-CoV-2-specific T cell immunity have been reported, mostly in developed countries, and those conducted in Africa have not focused on the effect of HIV infection [23], [24], [25], [26]. In a study conducted in South Africa, it was observed that the depletion of CD4+ T cells by HIV infection was associated with suboptimal SARS-CoV-2-specific T cell and humoral responses, including a decrease in the polyfunctionality of SARS-CoV-2-specific T cells [27]. A cross-sectional study on SARS-CoV-2-specific humoral and T cell immune responses done in the United Kingdom reported that at 5-7 months after infection with SARS-CoV-2, the immune responses were comparable between individuals who are HIV-positive (HIV+) and HIV-negative (HIV-), and that the T cell responses were dominated by CD4+ T cells [28]. In this study, we longitudinally investigated SARS-CoV-2-specific T cell and humoral immunity in individuals who are HIV+ who had recovered from COVID-19, and compared their immune response to individuals who are HIV- who had also recovered from COVID-19.
Methods
Study design and participants
We conducted a prospective cohort study of individuals who had been previously infected with SARS-CoV-2. Study participants were adult males and females who are HIV+ and HIV- who had been diagnosed with SARS-CoV-2 infection by reverse transcriptase-polymerase chain reaction in the past 12 weeks. We obtained written informed consent from the study participants, then collected clinical, sociodemographic, and HIV status information using the study questionnaire, followed by collection of venous whole blood for subsequent laboratory assays. At the time of recruitment, the participants had recovered from COVID-19. Participants were mainly recruited at the outpatient clinics within the University Teaching Hospital in Lusaka (Zambia) during their follow-up visits after the hospitalization for COVID-19 or after infection with SARS-CoV-2. The follow-up of the study participants was done approximately 8 weeks after recruitment. The ethical approval to conduct this study was obtained from the University of Zambia Biomedical Ethics Research Committee (Ref. No. 019-017-18) and from the National Health Research Committee (Ref. No. NHRA00001/17/09/2021).
Sampling of study participants
We collected 16 ml of venous whole blood at both baseline and follow-up visits. About 0.1 ml of the blood was used for CD4 counting, whereas 0.3 ml was used for T cell immunophenotyping by flow cytometry, and then the rest of the blood was subjected to centrifugation to separate plasma. About 1 ml of the plasma was used for determining HIV viral load in participants with HIV, whereas the rest was stored at -80°C for subsequent immunofluorescence assay to detect and titer the anti-SARS-CoV-2 antibodies. After plasma separation, the remaining cellular component was then subjected to density gradient centrifugation to isolate the peripheral blood mononuclear cells, which were used for the interferon-γ (IFN-γ) enzyme-linked immunospot assays on the same day of the collection.
CD4 counts and HIV viral loads
CD4 counts were determined using the BD TriTest kit (BD Biosciences), according to the manufacturer's protocol, on a BD FACSCalibur (BD Biosciences). The HIV viral loads were measured using the Aptima HIV-1 Quant Dx Assay kit on a Hologic Panther (Hologic), according to the manufacturer's protocol.
Enzyme-linked immunospot peptide pools and assay
The peptides, including SARS-CoV-2 S1 scanning pool and SARS-CoV-2 nucleocapsid, membrane protein, and other open reading frame (ORF) protein (NMO)-defined peptide pool, were obtained from MABTECH AB (Sweden). The SARS-CoV-2 S1 scanning pool contains 166.15-mer peptides, which overlap with 11 amino acids and covers the S1 subunit of the spike protein. The SARS-CoV-2 NMO-defined peptide pool contains 101 peptides derived from the NMO, including ORF1, nonstructural protein 3, ORF-3a, ORF-7a, and ORF8. These peptides are specific for the different human leukocyte antigens under both classes 1 and 2.
All the SARS-CoV-2 proteins whose peptides were used in our study have many important functions. The S1 subunit of the spike protein contains the receptor binding domain and is important for the viral entry into the host cells by binding to the host's angiotensin-converting enzyme 2 receptor [29]. The nucleocapsid is an RNA-binding protein that is important for viral packaging and has been observed to be a strong enhancer of virion quality and infectivity [30]. The membrane protein also plays an important role in the viral assembly by interacting with all the other structural proteins [31]. The nonstructural protein 3 is the largest SARS-CoV-2 protein that contains a macrodomain that suppresses the host's IFN response [32]. ORF-3a is a protein involved in viral replication, assembly, and release [33]. ORF-7a and ORF8 are accessory proteins that have been shown to antagonize IFN-1 signaling and therefore impair the host immune response [34,35]. Refer to Supplementary Information for the enzyme-linked immunospot assay.
Immunofluorescence assay
We performed an immunofluorescence assay to detect the presence and titer of antibodies against SARS-CoV-2 spike and nucleocapsid proteins, as described previously [36]. The transfected HEK-293T cells (ATCC, USA) that expressed either the spike (Addgene, USA) or nucleocapsid (Sini Biological, USA) proteins of SARS-CoV-2 were fixed and seeded onto 12-well polytetrafluoroethylene printed slides (Electron Microscopy Sciences, USA). After a 1: 20 dilution in 1X PBS with 0.1% Tween-20, the plasma was added to the corresponding wells on the slide and incubated for 1 hour at 37°C. The slides were then washed and incubated with secondary mouse monoclonal antihuman immunoglobulin G antibody (ATCC, USA), with subsequent removal of unbound antibodies and incubation with tertiary CY2-conjugated donkey antimouse immunoglobulin G (Jackson Immuno Research Laboratories, USA). This was then followed by counterstaining the cells with Evans blue dye. For the titration, the serial dilutions on positive samples were done beginning from 1: 20 until a negative reading. The reading of the slides was done by three independent readers on a Nikon E400 fluorescence microscope to determine positive or negative signals, and only harmonious results from at least two independent readers were reported as the outcome.
Flow cytometry
Whole blood was stained for 30 minutes with CD3-APC-Vio770, CD4-PE, CD8-PE-Vio770, CD45RO-APC, and CD197-CCR7-FITC, and the flow cytometry was carried out using a 6-color BD FACSVerse instrument. The antibodies were obtained from Miltenyi Biotec (Germany) and BD Biosciences (Belgium). Fluorescence-minus-one controls were used to identify and gate the cell populations. The gating strategies used are shown in Supplementary Figure 2. The analysis of the flow data was done using Flow Jo version 10 (TreeStar, Ashland, OR, USA).
Statistical analysis
Descriptive statistics were used to analyze the baseline characteristics. Continuous variables are presented as median and interquartile range for skewed data or mean and standard deviation for normally distributed data, whereas categorical or binary variables are presented as percentages. The comparison of continuous variables between the two groups was done using the Wilcoxon rank-sum test for skewed data, and Student's t-test for normally distributed data. The Shapiro-Wilk test was used to test for normality. The within-group comparisons of baseline and follow-up values were done using the Wilcoxon matched-pairs signed rank test. The determination of the correlation between the continuous variables was done using the Spearman rank correlation. We also used the Fisher's exact test to determine whether there was any significant difference in binary variables between the two groups. A P-value of less than 0.05 was considered statistically significant. Stata version 17 (StataCorp LLC, USA) was used for all statistical analyses. Prism 9 (GraphPad Software) was used to generate the figures.
Results
Baseline characteristics
We recruited 85 study participants who previously had polymerase chain reaction-confirmed SARS-CoV-2 infection. A total of 46 (54.1%) of the participants were HIV- and 39 (45.9%) were HIV+. The median time since SARS-CoV-2 diagnosis for the HIV- group was 10.5 weeks in the HIV- group, whereas it was 10 weeks in the HIV+ group. The proportion of hospitalizations was higher among individuals who are HIV+ than individuals who are HIV- (P-value = 0.012; Table 1 ). The proportions of fully vaccinated individuals were not significantly different between the two groups at baseline (P-value = 0.38). The median HIV viral load was undetectable in individuals who are HIV+, and most (94.3%) but not all were on ART at baseline. The median CD4 T cell counts were >450 cells/µl in both groups, but the counts were much higher in individuals without HIV (705 [556-905] vs 475 [359-738]; P-value = 0.001) as expected (Table 1). The rest of the baseline characteristics, including age, gender, body mass index, and presence of comorbid conditions, were not significantly different between the two groups (Table 1).
Table 1.
Baseline characteristics of study participants.
| HIV-(N = 46) | HIV+(N = 39) | P-value | |
|---|---|---|---|
| Age (years) | 48.7 (±14.7) | 53 (±10.3) | 0.13 |
| Males | 18 (39.1%) | 15 (38.5%) | 1.00 |
| Body mass index (kg/m2) | 28.9 (25-32.7) | 27.3 (26.2-30.5) | 0.91 |
| Clusters of differentiation 4 count (cells/µl) | 705 (556-905) | 475 (359-738) | 0.001 |
| HIV viral load (copies/ml) | N/A | 0 (0-0) | N/A |
| On antiretroviral therapy | N/A | 33 (94.2%) | N/A |
| Fully vaccinated | 23 (50%) | 15 (38.5%) | 0.38 |
| Days since COVID-19 diagnosis | 74 (56-77) | 70 (56-91) | 0.49 |
| Hospitalized for COVID-19 | 24 (52.2%) | 31 (79.5%) | 0.012 |
| Comorbidities (diabetes, hypertension, and obesity) | 35 (76.1%) | 22 (56.4%) | 0.07 |
N/A, not applicable.
Among HIV-infected individuals, the total number with antiretroviral therapy status information was 35 out of 39. All continuous variables are presented as median and interquartile ranges.
Comparison of T cell responses between individuals who are HIV+ and HIV-
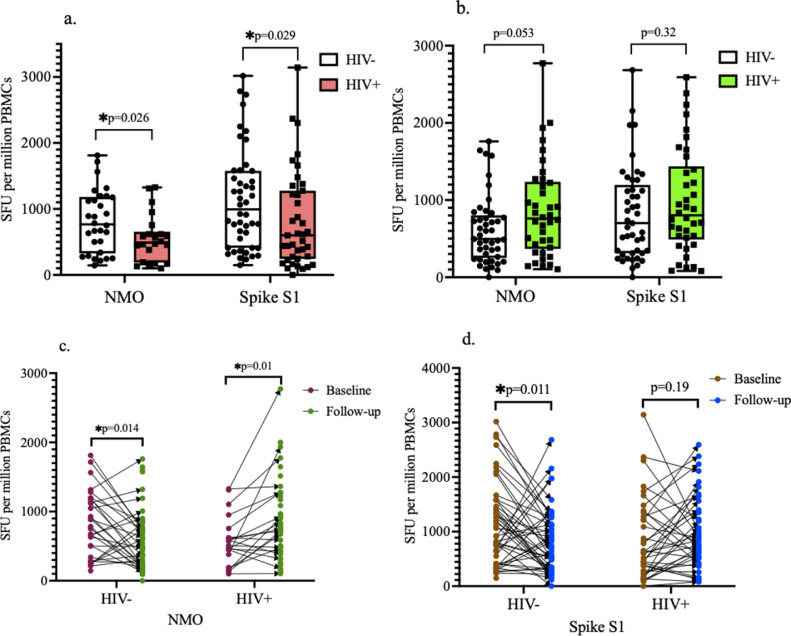
At both baseline and follow-up, 97-100% of individuals in both groups had detectable responses (>55 spot-forming units [SFU]/million cells) to spike and/or NMO peptide pools. At baseline, individuals who are HIV- had a significantly higher number of SFU toward the S1 subunit of the spike and NMO peptide pools than individuals who are HIV+ (Figure 1 a). At follow-up, there was no significant difference in the T cell responses toward both spike and NMO peptide pools between the two groups (Figure 1b). After 8 weeks of follow-up, there was a significant decrease in the T cell responses toward the NMO peptide pool in the HIV- group, whereas a significant increase toward the NMO peptide pool was observed in the HIV+ group (Figure 1c). Also, a statistically significant decrease in the T cell responses toward the spike peptide pool was observed after 8 weeks of follow-up in the HIV- group, whereas the T cell responses in the HIV+ group increased, but this was not statistically significant (Figure 1d).
Figure 1.
SARS-CoV-2-specific T cell responses. (a) At baseline, HIV- individuals had significantly higher number of T cell responses toward both spike S1 and NMO peptide pools compared to HIV+ individuals as indicated by the higher number of SFU per million cells. (b) At follow-up, there was an increase in T cell responses among HIV+ individuals to levels that were not significantly different from that of the HIV- group. (c) HIV- individuals had a significant decrease in number of T cell responses toward the NMO peptide pool after 8 weeks of follow-up while HIV+ individuals had a significant increase in number of T cell responses. (d) HIV- individuals had a significant decrease in number of T cell responses toward the S1 subunit of spike after 8 weeks of follow-up, while no significant difference between baseline and follow-up was observed in the HIV+ group.
NMO, nucleocapsid, membrane protein, and other open reading frame proteins; PBMC, peripheral blood mononuclear cells; SFU, spot-forming units.
A total of 34% (10/29) of individuals who are HIV- had an increase in the T cell responses against NMO, whereas 71.4% (15/21) of individuals who are HIV+ had an increase in the T cell responses against NMO during the follow-up period. Among the individuals who are HIV-, 31% (14/45) had an increase in the antispike T cell responses, whereas 55.3% (21/38) of individuals who are HIV+ had an increase in the spike T cell responses during the follow-up period. Individuals who are HIV+ had a statistically higher proportion of individuals that experienced an increase in the T cell responses against NMO (P-value = 0.026) and spike (P-value = 0.01) during the follow-up period. The comparison of clinical and demographic characteristics of individuals who are HIV+ who had an increase in the spike and NMO T cell responses to those who had a decrease or no increase in the T cell responses showed that higher CD4 counts were associated with an increase in T cell counts during the follow-up period (Supplementary Table 1).
Comparison of humoral responses between individuals who are HIV+ and HIV-
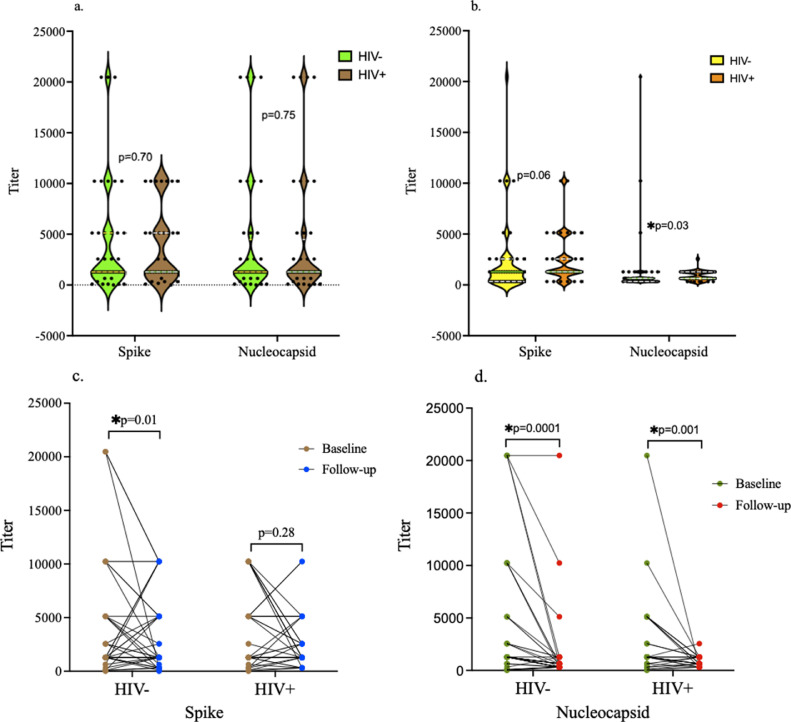
Both groups had 97.7% of individuals with detectable antibody responses at baseline, whereas 100% of the participants in both groups had detectable antibody responses at follow-up. At baseline, there was no significant difference in the antispike and antinucleocapsid antibody titers between the HIV- and HIV+ groups (Figure 2 a). At follow-up, the antispike antibody titers were not significantly different between the two groups, whereas the antinucleocapsid antibody titers were significantly higher in the HIV+ group than in the HIV- group (Figure 2b). There was a significant decrease in the antispike antibody titers in the HIV- group after 8 weeks of follow-up, whereas no significant difference between the baseline and follow-up antispike antibody titers was observed in the HIV+ group (Figure 2c). There was a significant decrease in the antinucleocapsid antibody titers in both groups after 8 weeks of follow-up (Figure 2d).
Figure 2.
SARS-CoV-2-specific antibody responses. (a) No significant difference in antispike and antinucleocapsid antibody titers between HIV- and HIV+ groups at baseline. The antibody titers were high in both groups. The median dilutions for both antispike and antinucleocapsid antibodies in both groups was 1: 1280. (b) At follow-up, the median antibody titer against spike (1: 1280) was higher than that against nucleocapsid protein (1: 640) in both groups. There were no significant differences in antispike antibody titers between the two groups at follow-up, while the antinucleocapsid antibody titer was significantly higher in the HIV+ group as indicated by the distribution of more individuals with higher titers compared to the HIV- group. (c) There was a significant decrease in antispike antibody titers in the HIV- group at follow-up, while no significant change between baseline and follow-up titers was observed in the HIV+ group. (d) There was a statistically significant decrease in antinucleocapsid antibody titers after 8 weeks of follow-up in both the HIV- and HIV+ groups. The titer on Y-axis indicate the number of dilutions.
Correlation of T cell and humoral responses with CD4 T cell counts in the HIV+ group
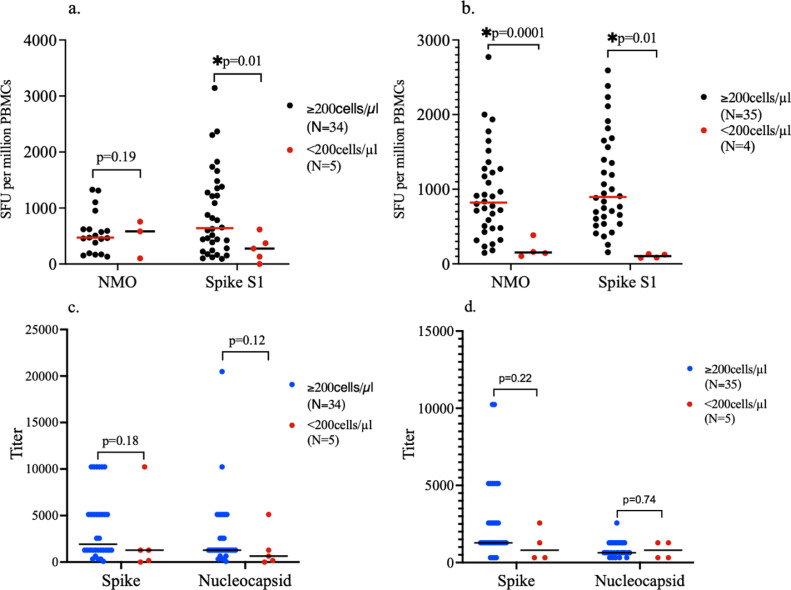
The individuals who are HIV+ were further categorized into those with CD4 counts <200 cells/µl and those with CD4 counts ≥200 cells/µl. Individuals with CD4 counts <200 cells/µl had significantly lower T cell responses toward the S1 subunit of spike protein than those with counts ≥200 cells/µl at baseline (Figure 3 a). Also, at the time of follow-up, the T cell responses toward both peptide pools were significantly higher among individuals with higher CD4 T cell counts than individuals with low CD4 T cell counts (Figure 3b). There was no significant correlation between the CD4 counts and T cell responses to spike (rho = 0.05; P-value = 0.75) and NMO (rho = 0.20; 0.38) at baseline. However, there were statistically significant positive correlations between the CD4 counts and T cell responses to spike (rho = 0.59; P-value = 0.0001) and NMO (rho = 0.51; P-value = 0.001) at follow-up. The antispike and antinucleocapsid antibody titers were not significantly different between the two groups at both baseline and follow-up (Figures 3c and 3d). There were no significant correlations between the CD4 counts and antispike or antinucleocapsid titers at both baseline and follow-up (rho = 0.12; P-value = 0.46, or rho = 0.19; P-value = 0.27 and rho = 0.31; P-value = 0.08, or rho = 0.03; P-value = 0.88, respectively).
Figure 3.
SARS-CoV-2-specific T cell and antibody responses by CD4 counts in HIV+ Group. (a) A subanalysis by CD4 counts was done in the HIV+ group. There was no significant difference in T cell responses toward the NMO peptide pool at baseline between individuals with low CD4 counts (<200 cells/µl) and those with high CD4 counts (≥200 cells/µl). T cell responses toward spike S1 subunit were significantly higher at baseline in individuals with higher CD4 counts (≥200 cells/µl) compared to those with low CD4 counts (<200 cells/µl). (b) At follow-up, T cell responses toward the NMO and spike peptide pools were significantly more in individuals with CD4 counts ≥200 cells/µl than those with lower CD4 counts. (c) No significant difference in antispike and antinucleocapsid antibody titers at baseline between HIV+ individuals with high and low CD4 counts. (d) No significant difference in antispike and antinucleocapsid antibody titers at follow-up between HIV+ individuals with high and low CD4 counts. At baseline, a few participants did not have their cells stimulated with NMO due to unavailability of the peptide pool at the beginning of the study. Therefore, the number of participants analyzed for the NMO peptide pool is slightly lower than those analyzed for the spike peptide pool at baseline (Figure 3a). Figure 3b shows a slight increase in the total number of individuals with higher CD4 counts and a slight decrease in those with lower CD4 counts at follow-up because of an individual who had an improvement in the CD4 count during the follow-up period.
CD, clusters of differentiation; NMO, nucleocapsid, membrane protein, and other open reading frame proteins; PBMC, peripheral blood mononuclear cells; SFU, spot-forming units.
Among individuals with low CD4 counts <200 cells/µl, the majority (83.3%) had been hospitalized at the time of diagnosis, 16.7% of individuals had breakthrough infections after vaccination, and 33.3% had other comorbid conditions. Only 16.7% of the individuals were vaccinated at baseline, whereas 66.7% of the individuals were vaccinated at follow-up. However, a comparison of spike T cell responses between baseline and follow-up was not statistically significant (324 SFU [132-408] vs 270 SFU [84-580]; P-value = 0.46).
Effect of vaccination on T cell and humoral responses to spike
At baseline, there was no significant difference in the proportion of individuals between the two groups who were vaccinated (52.2% in HIV- and 56.4% in HIV+; P-value = 0.88) or fully vaccinated against SARS-CoV-2 (Table 1 and Supplementary Table 2). Also, some of the participants were vaccinated during the follow-up period. However, there were no significant differences in the SARS-CoV-2 vaccination status at baseline and during follow-up between the participants in the HIV- and HIV+ groups. The participants all received either of two viral vector COVID-19 vaccines (Johnson & Johnson or Oxford AstraZeneca), which were the only vaccines available in the country at the time of recruitment and follow-up. There was no significant difference in the type of vaccine received between the two groups. However, the participants who are HIV- had a significantly shorter time from the last SARS-CoV-2 vaccine dose to recruitment into the study than the participants who are HIV+ (P-value = 0.013; Supplementary Table 2). Because the study participants were previously infected with SARS-CoV-2, full vaccination was considered approximately 2 weeks after the single dose of the Johnson & Johnson vaccine and at least 3 weeks after the first dose of the Oxford AstraZeneca vaccine [37], [38], [39]. Based on vaccination history, only 2/46 (4.35%) of the individuals in the HIV- group and 3/39 (7.7%) of the individuals in the HIV+ group had breakthrough infections, which was not significantly different between the two groups (P-value = 0.66). The majority of the participants had no reported or confirmed reinfection during the follow-up period.
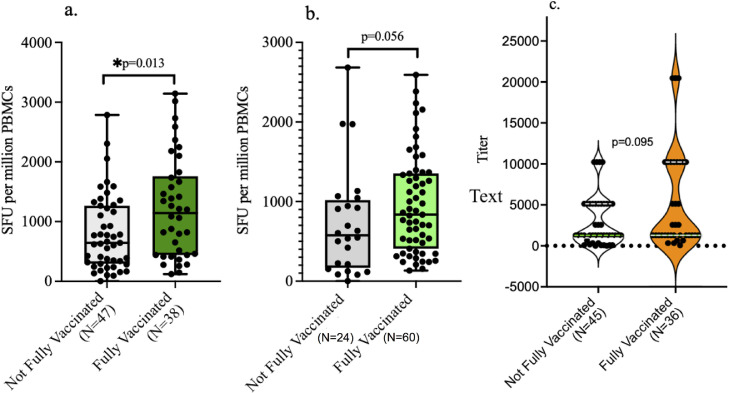
At baseline, about 47% of the participants were not vaccinated and approximately 8.2% were partially vaccinated, whereas 44.7% were fully vaccinated. At follow-up, about 23.5% of the participants were not vaccinated, about 5.8% were partially vaccinated, and about 70.6% were fully vaccinated. Because there were very few partially vaccinated individuals, they were combined with unvaccinated individuals for statistical analysis purposes. Overall, fully vaccinated individuals had significantly higher number of T cell responses to the spike S1 subunit than unvaccinated/partially vaccinated individuals at baseline (Figure 4 a). At follow-up, there was a trend toward a higher number of T cell responses in fully vaccinated than in unvaccinated/partially vaccinated individuals, but this was not statistically significantly different possibly due to insufficient number of individuals in the unvaccinated/partially vaccinated group to make statistical comparisons (Figure 4b). There were high numbers of T cell responses to spike in fully vaccinated individuals than in unvaccinated/partially vaccinated individuals at baseline and follow-up in the HIV- group; although, this was not statistically significant, possibly because the study was not powered enough to address this subgroup analysis (Table 2 ). Although T cell responses in fully vaccinated individuals who are HIV+ at baseline were not significantly different from those in unvaccinated/partially vaccinated individuals who are HIV+, these responses were significantly higher in fully vaccinated individuals at the time of follow-up (Table 2).
Figure 4.
Effect of vaccination on T cell and antibody responses. (a) Fully vaccinated individuals had significantly higher number of spike-specific T cell responses than unvaccinated/partially vaccinated individuals at baseline. (b) Fully vaccinated individuals had marginally significant higher number of spike-specific T cell responses that unvaccinated/partially vaccinated individuals at follow-up. (c) No significant difference in antispike antibody titers between fully vaccinated and unvaccinated/partially vaccinated individuals at baseline. (d) No significant difference in antispike antibody titers between fully vaccinated and unvaccinated/partially vaccinated individuals at follow-up.
Table 2.
SARS-CoV-2-specific T cell and antibody responses to spike in HIV- and HIV+ individuals by vaccination status
| HIV- |
HIV+ |
|||||
|---|---|---|---|---|---|---|
| Fully vaccinated | Unvaccinated/ partially vaccinated | P-value | Fully vaccinated | Unvaccinated/ partially vaccinated | P-value | |
| T cell responses | ||||||
| Baseline | 1344 SFU (412-2100) | 772 SFU (408-1264) | 0.084 | 820 SFU (440-1736) | 406 SFU (170-1248) | 0.075 |
| Follow-up | 718 SFU (344-1190) | 548 SFU (212-1132) | 0.58 | 1000 SFU (536-1684) | 604 SFU (124-908) | 0.015 |
| Antibody responses (titers) | ||||||
| Baseline | 1:2560 (1:1280-1:10240) | 1:1280 (1:320-1:2560) | 0.01 | 1:1280 (1:1280-1:5120) | 1:3840 (1:1280-1:5120) | 0.87 |
| Follow-up | 1:1280 (1:320-1:2560) | 1:1280 (1:320-1:1280) | 0.69 | 1:1280 (1:1280-1:2560) | 1:1280 (1:1280-1:5120) | 0.84 |
SFU, spot-forming units.
There were no statistically significant differences in the median antispike antibody titers at both baseline and follow-up between the fully vaccinated and unvaccinated/partially vaccinated individuals (Figures 4c and 4d). However, when a subgroup analysis was done, fully vaccinated individuals who are HIV- had higher antispike antibody titers than unvaccinated/partially vaccinated individuals who are HIV- at baseline, whereas no significant difference in the antispike antibody titers was observed between the fully vaccinated and unvaccinated/partially vaccinated individuals who are HIV+ (Table 2). At follow-up, there were no significant differences in the antispike antibody titers between the fully vaccinated and unvaccinated/partially vaccinated individuals in both the HIV- and HIV+ groups (Table 2).
Changes in CD4 count, HIV viral load, and COVID-19 vaccination status during follow-up
There were no statistically significant differences between the baseline and follow-up CD4 counts and HIV viral loads among the individuals who are HIV+ (475 cells/µl [359-738] vs 550 cells/µl [346-762]; P-value = 0.20 and 0 copies/ml [0-0] vs 0 copies/ml [0-0]; P-value = 0.77, respectively). The full vaccination status in the HIV- group increased from 50% at baseline to 71.7% at follow-up, whereas the full vaccination status in the HIV+ group increased from 38.5% at baseline to 69.2% at follow-up. There were no significant differences in vaccination status between the HIV+ and HIV- groups at both baseline (P-value = 0.38) and at follow-up (P-value = 0.82).
CD4 and CD8 T cell immunophenotypes
To determine whether HIV infection affects the proportions of the circulating T cell subsets in convalescent individuals with COVID-19, we compared proportions of the naïve, central memory, effector memory, and effector CD4+ and CD8+ T cells between the individuals who are HIV+ and HIV- and the changes between the groups after 8 weeks of follow-up. We observed that the proportions of the T cell subsets were not significantly different at baseline and at follow-up between the HIV+ and HIV- groups. However, there was a significant change in the proportion of the subsets within the groups at follow-up. For CD4+ T cells, the proportion of naïve cells in the whole blood of both HIV+ and HIV- groups significantly increased from baseline levels after 8 weeks of follow-up (26.9% [16.8-32.4] vs 29.8% [18.3-41.2]; P-value = 0.0001 and 24.4% [14.8-33.9] vs 28.7% [21.6-38.1]; P-value = 0.0001, respectively; Supplementary Table 3). On the other hand, the proportion of the CD4 effector memory cells significantly reduced from baseline levels after 8 weeks of follow-up in both the HIV+ and HIV- groups (24.2% [11.8-34.9] vs 15% [10.5-22.6]; P-value = 0.001 and 17.8% [12.3-38.8] vs 15.9% [10.8-20.9]; P-value = 0.002, respectively). In addition, there was a significant increase in the proportion of the CD4 effector cells in the HIV+ group at follow-up (0.5% [0.1-1.3] vs 0.7% [0.5-1.9]; P-value = 0.015) but not in the HIV- group. For CD8+ T cells, the proportion of central memory cells significantly increased from baseline levels in both the HIV+ and HIV- groups (2% [1.5-4.6] vs 5.3% [2.8-79.5]; P-value = 0.0001 and 2.7% [1.2-4.5] vs 5.6% [2.9-13.5]; P-value = 0.0001, respectively; Supplementary Table 4). The proportion of the effector cells significantly reduced in both the HIV+ and HIV- groups (37.8% [17.8-55.9] vs 30.5% [2.3-50.1]; P-value = 0.003 and 38.3% [27.3-58] vs 31.3% [13.1-43.5]; P-value = 0.001, respectively), whereas the proportion of the effector memory cells significantly reduced in the HIV+ group (11.5% [6.9-18.4] vs 7.4% [4.3-13.4]; P-value = 0.01), with a statistically insignificant reduction in the HIV- group. We also compared the proportion of CD4 (25.1% [12.7-33.9] vs 24.2% [9.5-54.8]; P-value = 0.76) and CD8 (12.5% [6.9-18.2] vs 8% [7.7-28]; P-value = 0.96) effector memory T cells at baseline between the individuals who are HIV+ with high CD4 counts and those with lower CD4 counts and observed no significant difference in the proportions, which could be due to the low sample size for the subanalysis.
Discussion
Cell-mediated and humoral immunity are the two major aspects of adaptive immunity that play a critical role against SARS-CoV-2 infection. Hence, our study focused on SARS-CoV-2-specific T cell and antibody responses in individuals who are HIV+ versus HIV- in Africa, who were previously infected with SARS-CoV-2 and had recovered from COVID-19. We also looked at how this immunity changed over an 8-week follow-up period.
We observed that at 10 weeks post-infection with SARS-CoV-2, SARS-CoV-2-specific T cell responses are present in individuals who are HIV+ and HIV-. However, they are significantly greater in the individuals who are HIV- than the individuals who are HIV+. There was a significantly shorter time from the last SARS-CoV-2 vaccine dose to recruitment in participants who are HIV- than HIV+, possibly explaining the observed differential T cell responses to spike. Nevertheless, the T cell responses to NMO were also significantly higher in the HIV- group at baseline, and only about half of the entire study population was vaccinated at baseline. This observation changed after an 8-week follow-up period, where the SARS-CoV-2-specific T cell responses in the HIV+ group significantly improved to match the levels observed in the HIV- group. It is possible that this improved response in the HIV+ group over the follow-up period could be due to ongoing/prolonged asymptomatic infection and/or improved vaccination status over time. Our findings are similar to a previous study, where an observation was made that both humoral and T cell responses are comparable in individuals without HIV and individuals with HIV who are on ART and have suppressed HIV viral loads [28]. However, our data suggest that individuals who are HIV+ mount a less robust SARS-CoV-2-specific T cell response both at baseline and follow-up, but that these can be boosted by vaccination.
It is interesting to note that the T cell responses in our cohort of individuals who are HIV+ and HIV- were still present at almost 4.5 months after the initial infection because our baseline analysis was at about 10 weeks after the infection. These responses increased in the HIV+ group to levels comparable with that of the HIV- group during the 8-week follow-up period. This is similar to a previous study by Zuo et al. [40], where it was observed that functional SARS-CoV-2-specific T cell responses were maintained at 6 months after the primary infection. In another study, it was observed that adaptive cellular immunity was durable at 7 months after the primary infection [41]. A study by Lu et al. [42] observed that the T cell responses were still detectable after a longer period of 12 months after SARS-CoV-2 infection, and that individuals with severe illness had a higher frequency of SARS-CoV-2-specific T cells and antibodies. In our study, attempts to categorize the immune responses based on the initial COVID-19 severity were not done because some participants had missing clinical information for the time when they were acutely infected because the study recruited participants after hospital discharge or recovery from acute infection. On the other hand, hospital admission, which may be associated with disease severity, was not associated with the differences in the immune responses between the two groups. The slow development of T cell responses in individuals who are HIV+ over time could also be due to more prolonged asymptomatic infection or reinfection during the follow-up period. Nevertheless, based on our studies and that of others, T cell and humoral immunity appears to be present for at least several months after the primary infection in both the individuals who are HIV+ and HIV-. However, at least in the HIV- population, these responses seem to weaken over time, necessitating the need for SARS-CoV-2 vaccination and/or boosters to prevent reinfections and COVID-19 disease. Our study is unique from the other studies in that we investigated the T cell and humoral responses in people living with HIV and how these responses change over time in comparison to individuals who are HIV-.
We observed that humoral immune responses against the two SARS-CoV-2 proteins (spike and nucleocapsid) were detectable in most of the participants in both the HIV- and HIV+ groups at baseline and follow-up. The antibody titers were high and not significantly different between the two groups at baseline. However, at follow-up, the antinucleocapsid antibody titers were significantly higher in the HIV+ group than in the HIV- group. Our observation is similar to other studies that have reported that SARS-CoV-2 seroconversion is similar between people living with HIV who are on ART and individuals without HIV [28,43]. After 8 weeks of follow-up, the antibody responses were still detectable in all the participants, including those that were undetectable at baseline. The one individual who had undetectable levels of anti-SARS-CoV-2 antibody at baseline and detectable titers at follow-up may represent a case of some individuals with weak/delayed initial responses, vaccine response, or reinfection during the study period. However, there was a significant decrease in the titers against both the spike and nucleocapsid proteins in both groups, except against spike in the HIV+ group. Other studies have also reported that the antibody titers against SARS-CoV-2 decrease after a few months but are still detectable several months after the infection [44]. Our findings and that of others indicate that SARS-CoV-2 vaccination or boosters may be very important to maintain the immunity and prevent reinfections.
Most of our study participants who are HIV+ had suppressed and/or undetectable HIV viral loads at baseline, and 33 (94.2%) were on ART. Despite this, we observed that individuals who are HIV+, with CD4 counts <200 cells/µl, had significantly weaker T cell responses than those with higher CD4 counts at both baselines and after 8 weeks of follow-up. This is in line with other studies that have reported that severe immunosuppression, with CD4 counts <200 cells/µl, is associated with severe COVID-19 disease [45]. On the other hand, no significant difference was observed in the antibody titers against spike and nucleocapsid between individuals with HIV with higher CD4 and low CD4 counts. Our observation may be due to insufficient numbers to make comparisons for this subgroup analysis or may be due to a better humoral than T cell immunity against SARS-CoV-2 in severely immunosuppressed individuals with HIV. Furthermore, the higher proportion of hospitalizations in the HIV+ than the HIV- populations may be due to our observed less robust initial T cell responses to SARS-CoV-2 in the HIV+ population.
Fully vaccinated individuals in the entire combined cohort compared with unvaccinated and partially vaccinated individuals had significantly higher number of T cell responses to spike at baseline and marginally significant higher number of T cell responses at follow-up as expected due to the vaccine stimulatory effect. The subgroup analyses by HIV status showed that the T cell responses against spike were much higher in the fully vaccinated individuals at both time points, with responses in the fully vaccinated HIV+ group being significantly higher at follow-up as the number of fully vaccinated participants increased over time. There was no significant difference in the antispike antibody titers between the fully vaccinated and unvaccinated/partially vaccinated individuals at both baseline and follow-up. This could be due to the durable responses provided by the SARS-CoV-2 primary infection because all these individuals were previously infected with SARS-CoV-2. However, on the subgroup analysis, fully vaccinated individuals in the HIV- group had significantly higher antispike antibody titers than unvaccinated/partially vaccinated individuals. Again, this could be due to the marginal boosting effect of the SARS-CoV-2 vaccine; although, both groups had high titers at baseline. Our observations are similar to previous studies that have observed that people living with HIV develop high antispike antibody titers, which are similar to individuals without HIV infection [46]. Our findings may suggest that vaccination in individuals recently infected with SARS-CoV-2 may be more beneficial in improving T cell immunity. The antibody titers were very high in both groups after infection with SARS-CoV-2, reaching several thousand-fold dilution, and hence, vaccination did not seem to have a profound effect on the titers, which may have affected our comparisons. However, considering the gradual decrease in the antibody titers, including T cell immunity, over time as observed in our study and by others [38], vaccination to boost immunity is absolutely necessary to prevent reinfections and severe COVID-19 in individuals who are HIV+ and HIV-. Also, as noted from our study, the higher antispike antibody titers at follow-up than antinucleocapsid antibody titers may be explained by the effect of the vaccination because the majority of the participants in both groups were vaccinated at follow-up.
We observed significant changes in circulating proportions of CD4+ and CD8+ T cell phenotypes after 8 weeks of follow-up. During this period, the proportions of naïve CD4+ T cells increased from baseline levels, indicating a normalization of circulating CD4+ T cell subsets after the recovery from COVID-19 disease [47,48]. This increase in naïve CD4+ T cells was accompanied by an observed decrease in effector memory cells over time, reflecting a recovery of CD4+ T cell subsets toward normal after infection [49]. The proportion of CD4+ effector T cells in individuals who are HIV+ was observed to significantly increase at follow-up compared with baseline levels, but the proportions were very low compared with other subsets. This could be associated with the observed delayed SARS-CoV-2-specific T cell responses observed in the HIV+ group.
The proportion of CD8+ central memory T cells significantly increased, whereas the CD8+ effector T cells significantly reduced in individuals who are HIV+ and HIV- after 8 weeks of follow-up. These findings are in agreement with studies that have shown that during acute viral infection, naïve CD8+ T cells differentiate into effector cells, which kill target cells [48]. Upon clearance of the infection, a proportion of these cells differentiate into memory cells that protect the host during reinfection [49]. Hence, both CD4+ and CD8+ T cell subsets may be useful in the monitoring of disease progression and recovery from COVID-19 disease in individuals who are HIV+ and HIV-.
Study limitations
The overall small sample size might have limited our ability to elicit some of the differences that might exist between the subgroups. Because immune responses are impacted by vaccination status, timing, and types of vaccine used, the lack of a standardized cohort with a uniform vaccination profile might have confounded some of our findings. However, we observed no difference in the type of vaccine received by both groups and the proportion of vaccinated individuals. Our assessment of immune responses may also be confounded by the lack of data on infection status of the participants during the follow-up period because some responses could be due to ongoing/prolonged SARS-CoV-2 infection or asymptomatic reinfection during the study period. Although most of the participants received SARS-CoV-2 vaccination during follow-up, we cannot rule out the role of reinfection in the observed responses. Another study limitation is the lack of clinical detailed information on the severity of COVID-19 disease and SARS-CoV-2 viral load, where it could have been useful to compare immune responses in different COVID-19 stages and viral burden. However, our study provides important findings on the presence of SARS-CoV-2-specific T cell and antibody responses in individuals who are HIV+ and in HIV- approximately 4.5 months after recovery from acute infection.
Conclusion
SARS-CoV-2-specific T cell and antibody responses are present in individuals who are HIV+ and HIV- after recovery from acute infection. These responses are generally high and still detectable 4.5 months after the initial infection. Participants who are HIV+ have less robust T cell responses both at baseline and follow-up, but these are boosted by vaccination. Severe immunosuppression among individuals with HIV mostly affects T cells and not antibody responses. SARS-CoV-2-specific T cell responses may be delayed in individuals with HIV, despite them being on ART. T cell responses in the individuals who are HIV- and antibody responses in individuals who are HIV- and HIV+ decrease over time, which could increase the risk of reinfection and severe disease and necessitates vaccine boosters.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
The research reported in this publication was supported by the Fogarty International Center and National Cancer Institute of the National Institutes of Health under Award Numbers K43TW011095 and U54CA221204 to ON and K43TW011418 to SJL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We thank all the participants for consenting to participate in this study.
Author contributions
O.N.: performed and supervised the laboratory experiments on T cell work, drafted the manuscript, performed statistical analyses, wrote, and edited the manuscript. S.L.: performed and supervised the laboratory experiments on humoral responses work, drafted the manuscript, performed statistical analyses, and wrote and edited the manuscript, J.R.N., J.M., and F.Y.T.: performed laboratory experiments on humoral response. M.C.M.: performed laboratory experiments on T cell functional assays. M.K.: performed laboratory experiments on flow cytometry. P.K.: recruited, followed up the study participants, and collected specimens. All authors reviewed and edited the manuscript.
Data availability
All the data generated or analyzed during this study are available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.12.009.
Appendix. Supplementary materials
References
- 1.Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12:e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla S, Bakhshwin D, Shirbeeny W, Bakhshwin A, Bahabri F, Bakhshwin A, et al. Successive waves of COVID 19: confinement effects on virus-prevalence with a mathematical model. Eur J Med Res. 2021;26:128. doi: 10.1186/s40001-021-00596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisayo T, Tsukagoshi S. Three waves of the COVID-19 pandemic. Postgrad Med J. 2021;97:332. doi: 10.1136/postgradmedj-2020-138564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danwang C, Noubiap JJ, Robert A, Yombi JC. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022;19(3) doi: 10.1186/s12981-021-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng WH, Tipih T, Makoah NA, Vermeulen JG, Goedhals D, Sempa JB, et al. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. mBio. 2021;12:e03647. doi: 10.1128/mBio.03647-20. –20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peron JPS, Nakaya H. Susceptibility of the elderly to SARS-CoV-2 infection: ACE-2 overexpression, shedding, and antibody-dependent enhancement (ADE) Clinics (Sao Paulo) 2020;75:e1912. doi: 10.6061/clinics/2020/e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berenguer J, Díez C, Martín-Vicente M, Micán R, Pérez-Elías MJ, García-Fraile LJ, et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV research network cohort. Clin Microbiol Infect. 2021;27:1678–1684. doi: 10.1016/j.cmi.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosioni J, Blanco JL, Reyes-Urueña JM, Davies MA, Sued O, Marcos MA, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8:e294–e305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MA. HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa. medRxiv 03 July 2020. https://www.medrxiv.org/content/10.1101/2020.07.02.20145185v2 accessed 22 August 2022.
- 12.Jassat W, Cohen C, Tempia S, Masha M, Goldstein S, Kufa T, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8:e554–e567. doi: 10.1016/S2352-3018(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 16.Chiang SC, Theorell J, Entesarian M, Meeths M, Mastafa M, Al-Herz W, et al. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood. 2013;121:1345–1356. doi: 10.1182/blood-2012-07-442558. [DOI] [PubMed] [Google Scholar]
- 17.Westmeier J, Paniskaki K, Karaköse Z, Werner T, Sutter K, Dolff S, et al. Impaired cytotoxic CD8 + T cell response in elderly COVID-19 patients. mBio. 2020;11:e02243. doi: 10.1128/mBio.02243-20. –20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E, Oh JE. Humoral immunity against SARS-CoV-2 and the impact on COVID-19 pathogenesis. Mol Cells. 2021;44:392–400. doi: 10.14348/molcells.2021.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Deng Y, Zhao Z, Mao B, Lu M, Lin Y, et al. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol Immunol. 2022;19:150–157. doi: 10.1038/s41423-021-00774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 22.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Björkander S, Du L, Zuo F, Ekström S, Wang Y, Wan H, et al. SARS-CoV-2-specific B- and T-cell immunity in a population-based study of young Swedish adults. J Allergy Clin Immunol. 2022;149:65–75. doi: 10.1016/j.jaci.2021.10.014. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima N, Klausner JD. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:12–14. doi: 10.1016/S1473-3099(21)00676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 26.Riou C, Schäfer G, du Bruyn E, Goliath RT, Stek C, Mou H, et al. Rapid, simplified whole blood-based multiparameter assay to quantify and phenotype SARS-CoV-2 specific T-cells. Eur Respir J. 2022;59 doi: 10.1183/13993003.00285-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riou C, du Bruyn E, Stek C, Daroowala R, Goliath RT, Abrahams F, et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest. 2021;131 doi: 10.1172/JCI149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alrubayyi A, Gea-Mallorquí E, Touizer E, Hameiri-Bowen D, Kopycinski J, Charlton B, et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun. 2021;12:5839. doi: 10.1038/s41467-021-26137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia X. Domains and functions of spike protein in Sars-Cov-2 in the context of vaccine design. Viruses. 2021;13:109. doi: 10.3390/v13010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra T, Sreepadmanabh M, Ramdas P, Sahu AK, Kumar A, Chande A. SARS CoV-2 nucleoprotein enhances the infectivity of lentiviral spike particles. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.663688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boson B, Legros V, Zhou B, Siret E, Mathieu C, Cosset FL, et al. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J Biol Chem. 2021;296 doi: 10.1074/jbc.RA120.016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo LC, Tomasin R, Matos IA, Manucci AC, Sowa ST, Dale K, et al. The SARS-CoV-2 Nsp3 macrodomain reverses PARP9/DTX3L-dependent ADP-ribosylation induced by interferon signaling. J Biol Chem. 2021;297 doi: 10.1016/j.jbc.2021.101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azad GK, Khan PK. Variations in Orf3a protein of SARS-CoV-2 alter its structure and function. Biochem Biophys Rep. 2021;26 doi: 10.1016/j.bbrep.2021.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flower TG, Buffalo CZ, Hooy RM, Allaire M, Ren X, Hurley JH. Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2021785118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redondo N, Zaldívar-López S, Garrido JJ, Montoya M. SARS-CoV-2 accessory proteins in viral pathogenesis: knowns and unknowns. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.708264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tso FY, Lidenge SJ, Peña PB, Clegg AA, Ngowi JR, Mwaiselage J, et al. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int J Infect Dis. 2021;102:577–583. doi: 10.1016/j.ijid.2020.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzoni A, Di Lauria N, Maggi L, Salvati L, Vanni A, Capone M, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasikala M, Shashidhar J, Deepika G, Ravikanth V, Krishna VV, Sadhana Y, et al. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int J Infect Dis. 2021;108:183–186. doi: 10.1016/j.ijid.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo J, Dowell AC, Pearce H, Verma K, Long HM, Begum J, et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurevich M, Zilkha-Falb R, Sonis P, Magalashvili D, Menascu S, Flechter S, et al. SARS-COV-2 Memory B and T cells Profile in Mild COVID-19 Convalescents subjects. Int J Infect Dis. 2021;115:208–214. doi: 10.1016/j.ijid.2021.12.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Z, Laing ED, Pena DaMata J, Pohida K, Tso MS, Samuels EC, et al. Durability of SARS-CoV-2-Specific T-cell responses at 12 months postinfection. J Infect Dis. 2021;224:2010–2019. doi: 10.1093/infdis/jiab543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto S, Saito M, Nagai E, Toriuchi K, Nagai H, Yotsuyanagi H, et al. Antibody response to SARS-CoV-2 in people living with HIV. J Microbiol Immunol Infect. 2021;54:144–146. doi: 10.1016/j.jmii.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamayoshi S, Yasuhara A, Ito M, Akasaka O, Nakamura M, Nakachi I, et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalmedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann C, Casado JL, Härter G, Vizcarra P, Moreno A, Cattaneo D, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021;22:372–378. doi: 10.1111/hiv.13037. [DOI] [PubMed] [Google Scholar]
- 46.Ruddy JA, Boyarsky BJ, Bailey JR, Karaba AH, Garonzik-Wang JM, Segev DL, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS. 2021;35:2399–2401. doi: 10.1097/QAD.0000000000003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna SJ, Codd AS, Gea-Mallorqui E, Scourfield DO, Richter FC, Ladell K, et al. T cell phenotypes in COVID-19 - a living review. Oxf Open Immunol. 2021;2 doi: 10.1093/oxfimm/iqaa007. iqaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalpakci Y, Hacibekiroglu T, Trak G, Karacaer C, Demirci T, Kocayigit H, et al. Comparative evaluation of memory T cells in COVID-19 patients and the predictive role of CD4+CD8+ double positive T lymphocytes as a new marker. Rev Assoc Med Bras (1992) 2020;66:1666–1672. doi: 10.1590/1806-9282.66.12.1666. [DOI] [PubMed] [Google Scholar]
- 49.Bordoni V, Brando B, Piselli P, Forini O, Perna FE, Atripaldi U, et al. Naive/Effector CD4 T cell ratio as a useful predictive marker of immune reconstitution in late presenter HIV patients: a multicenter study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analyzed during this study are available from the corresponding author on reasonable request.