Abstract
The destructive effects of coronavirus disease 2019 (COVID-19) on the elderly and people with cardiovascular disease have been proven. New findings shed light on the role of aging pathways on life span and health age. New therapies that focus on aging-related pathways may positively impact the treatment of this acute respiratory infection. Using new therapies that boost the level of the immune system can support the elderly with co-morbidities against the acute form of COVID-19. This article discusses the effect of the aging immune system against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the pathways affecting this severity of infection.
Keywords: Immunosenescence, Inflamm-ageing, SARS-CoV-2, COVID-19
1. Introduction
As people get older, their immunity to pathogens decreases, and this lack has been proven in experiments. Studies show that aging reduces the effectiveness of the immune system against pathogens. In older people, the body shows reduce tolerance to the pathogen, and the effectiveness of related vaccines decreases (Goodwin et al., 2006, Hainz et al., 2005, Kaml et al., 2006, Melegaro and Edmunds, 2004, Wolters et al., 2003, Yoshikawa, 2000, Zinatizadeh et al., 2022). So it is clear that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected the most vulnerable and older people. Since the coronavirus disease 2019 (COVID-19) outbreak, age has been proposed and proven to be the most critical factor influencing the severity of this disease (Wu et al., 2020). The death toll from the infection of SARS-CoV-2 is high in the elderly (Bajgain et al., 2021, Santesmasses et al., 2020, Zheng et al., 2020c). The incidence of SARS-CoV-2 infection and mortality in the elderly is high worldwide. In the United States alone, the incidence of hospitalization and death from COVID-19 is about 1300 folds larger in the 65–74 age group and 8700 folds greater in the 85-year-old than in the 5–17 age group (Gold et al., 2020). Also, a study in Iran showed that most cases of acute infections were in the age group of 50–60 years. And mortality is significantly higher in the elderly (Nikpouraghdam et al., 2020). In addition, studies in Saudi Arabia (Ibrahim et al., 2021), Iraq (Al-Mosawi, 2021), China (Leung, 2020), and Brazil (Machado et al., 2020) have also shown that age has a special role in mortality. Other diseases of the elderly, such as cardiovascular problems and diabetes, also play a role in the increasing vulnerability of this group to COVID-19. However, it should be noted that age quantity is still the most critical factor influencing the severity of this disease (Bajgain et al., 2021, Ho et al., 2020).
This article has tried to investigate the role of the age factor on the severity of COVID-19 disease, its host action and responses during infection, and physiological and immunological mechanisms against the pathogen.
2. Dysregulation of immune system on ageing
The main functions of the immune system in responding to a viral infection include the following: A) Initiation of a local inflammatory response to activate immune cells; B) Destroying the cells involved in the virus; C) Activation of the adaptive immune response. As they grow older, the immune system will not be able to process these functions properly. The phenomenon of inflamm-ageing, which represents the attendance of systemic inflammatory mediators in the body of the elderly, causes more turbulences to the immune system and strengthens many persistent diseases of them. This phenomenon further causes the weakening of the immune system in people with increasing age, also known as immunosenescence (Ferrucci and Fabbri, 2018). In response to internal and external physiological stresses, inflammation occurs in the body and is usually caused by aging and senescence of immune cells. These stressors can explain the difference in inflammatory biological ages (Alpert et al., 2019, Furman et al., 2019).
Many inflammatory intermediates are generated by immune cells and can affect their function. Excessive release of proinflammatory cytokines can make it difficult for immune cells to signal. Such problems can be noted in the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, myeloid differentiation factor 88 (MyD88), nuclear factor kappa B (NF-κB), and inflammasomes, resulting in high basal activation but impaired immune cell response to more cytokine and pattern recognition receptors (PRRs) excitation (Rea et al., 2018, Shen-Orr et al., 2016). It can be found that many immune cells respond less to external and internal stresses as we age (Chougnet et al., 2015, Cumberbatch et al., 2002, Manser and Uhrberg, 2016, Metcalf et al., 2015, Metcalf et al., 2017, Rea et al., 2018, Van Duin et al., 2007, Zacca et al., 2015). This may explain why mediated chronic systemic inflammation is associated with optimal vaccine responses in younger and older (Fourati et al., 2016, McDade et al., 2011, Verschoor et al., 2017). Immunosenescence with symptoms such as impaired antigen exposure and naive priming of T cells, tendency to segregation myeloid lineage in the bone marrow, delayed type I interferon (IFN) response, decreased cytotoxic function of CD8+ T cells, reduced phagocytic function for many types of the innate immune cell, a confined naive T cell, and B cell repertoire and ruinous generation of highly agitated antibodies ( Table 1) (Derhovanessian et al., 2008, Weiskopf et al., 2009). This incapability in the immune system makes older people more defenseless to viral diseases such as SARS-CoV and SARS-CoV-2. The effect of aging on immune system strength has been widely investigated (Akha, 2018, Boraschi and Italiani, 2014, Nikolich-Žugich, 2018, Nikolich-Zugich et al., 2020, Weiskopf et al., 2009). This paper focuses on the effects of age on immune system response to COVID-19.
Table 1.
Immunosenescence and its negative consequences in elderly COVID-19 patients.
| Immunosenescence characteristics | Immune response | consequences | References |
|---|---|---|---|
| Monocytes/macrophages | Innate | Altered TLR expressionDecreased phagocytic abilityDecreased antigen presentationIncreased cytokine production | (Zheng et al., 2020b) |
| Dendritic cells | Innate | Reduced of pDCs | (Márquez et al., 2020, Zingaropoli et al., 2021) |
| Neutrophils | Innate | Altered TLR expressionVariations in oxidative stress pathwaysInflammatory activityRaised cellular module and SASPDecreased autophagy and DNA damage | (Bajaj et al., 2021b) |
| NK cells | Innate | Prevention of upregulation HLA Class IAltered NKG2A expression | (Wen et al., 2020, Wilk et al., 2020, Witkowski et al., 2022, Yoo et al., 2021) |
| B cells | Adaptive | Decreased antibody titers | (Singh et al., 2021) |
| T cells | Adaptive | Decreased numbers of T cellDecreased number of regulatory T cellsScarcity of naive T cellsDisrupted of antigen-specific responsesUncoordinated adaptive immune responses | (Cunha et al., 2020, Diao et al., 2020, Moderbacher et al., 2020) |
3. How immunosenescence and inflamm-ageing may contribute to severity of COVID-19
3.1. Age-related alterations in ACE2 receptor
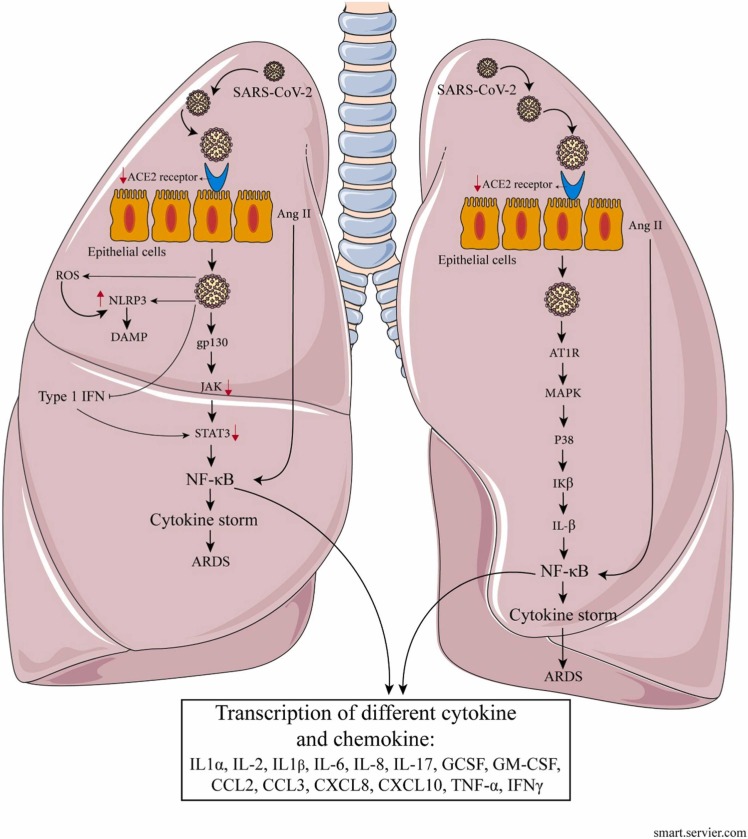
The angiotensin-converting enzyme 2 (ACE2) cellular receptor plays an essential role in primary inflammatory procedures through the renin-angiotensin-aldosterone signaling pathway. It is involved in processing the functions of the innate immune system. As mentioned earlier, the onset of an inflammatory response leads to the activation of immune cells and the recruitment of these cells. Angiotensin II is converted by ACE2 to angiotensin 1–7. Signaling of the angiotensin II in vascular cells causes a proinflammatory condition (Wang et al., 2014, Zarandi et al., 2021). Animal examines performed on mice suffering from acute respiratory distress syndrome (ARDS) have shown that ACE2 has an anti-inflammatory effect and prevents critical lung damage (Imai et al., 2005). On the other hand, angiotensin 1–7 has been shown to reduce the generation of proinflammatory cytokines such as interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and IL-8 by inhibiting the signaling pathway of P38 mitogen-activated protein kinase (MAPK)-NF-kB. In addition, it upregulates the expression of the anti-inflammatory cytokine IL-10 (Yu et al., 2018). During the initial response to SARS-CoV-2, the raised presence of angiotensin II, possibly due to overproduction of TNF-α and activation of local macrophages, has a significant effect on the promotion of severe COVID-19 related cytokine release (Banu et al., 2020). Animal studies have shown a decrease in ACE2 expression in the lungs of older mice contrasted to younger mice (Xudong et al., 2006). In addition, a study found a reduction in ACE2 mRNA in several tissues in older humans (Chen et al., 2020a). Downregulation of ACE2 levels has been observed in people with cardiovascular diseases and diabetics, which is also associated with the severity of COVID-19 (Tikellis and Thomas, 2012). Indeed, ACE2 expression is inversely correlated with the severity of COVID-19 (Chen et al., 2020a), which can be attributed to reduced disease tolerance (Wanhella and Fernandez-Patron, 2022). Disease tolerance declines with increased age and as a result of deteriorated immunity and may affect older individuals infected by SARS-CoV-2. Tolerance may become impaired with age due to declining tissue maintenance and capacity to repair damaged cells (Medzhitov et al., 2012). Although decreased ACE2 levels may be due to minor SARS-CoV-2 attack on host cells, excessive reduced levels of ACE2 may intensify proinflammatory response that leads to cytokine storm, severe lung damage, and ARDS ( Fig. 1). The high levels of angiotensin II shown in the plasma of patients with severe COVID-19 also support this hypothesis (Liu et al., 2020b). It should be noted that in studies on old and young rhesus macaques with SARS-CoV-2 infection, proinflammatory cytokines levels were higher in older macaques and emphasized the hypothesis that disease tolerance in older individuals is reduced (Rosa et al., 2021).
Fig. 1.
The presumptive pattern of immunosenescence and inflamm-ageing in COVID-19.
3.2. Uninhibited inflammatory responses with age
Lung tissue damage results from uninhibited inflammatory responses to SARS-CoV-2 infection (Fig. 1). In patients with severe COVID-19, high levels of pro-inflammatory cytokines and chemokines, including IL-6, IL-1β, IL-2, IL-8, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage (GM)-CSF, C–X–C motif chemokine ligand 10 (CXCL10), CCL2, CCL3, and TNF is observed (Dinnon et al., 2020, Qin et al., 2020, Xu et al., 2020b, Zarandi et al., 2021). Similar cytokines, such as IL-6, IL-1α, IL-1β, TNF-α, and the chemokine CCL2, were also related to the senescent host response to SARS-CoV-2 in experiments on mice (Dinnon et al., 2020, Leist et al., 2020). Many levels of these cytokines have been shown to rise in the elderly due to inflamm-ageing. Research has shown that an increase in IL-6 is a negative consequence of COVID-19, a parameter of aging (Johnson, 2006). The IL-6 transcription factor activates the NF-κB pathway. This transcription factor has a significant role in regulating many pro-inflammatory genes (Brasier, 2010). Aging, NF-κB signaling, and inflammation are closely related (Salminen et al., 2008, Zinatizadeh et al., 2021). On the other hand, an anti-IL-6 receptor antibody has been shown to reduce the severity of COVID-19 in a group of elderly patients and points to the role of some cytokines in disease intensity (Guaraldi et al., 2020). Although IL-6 may play an important role in both local and systemic inflammation, it is unlikely to be the primary cause of inflamm-ageing.
The critical role of cytokines IL-1β and IL-18 in inflamm-ageing has also been proven (Rodrigues et al., 2020). These cytokines, which belong to the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs)-pyrin domain-containing protein 3 (NLRP3), are involved in the pathology of aging-related diseases (Youm et al., 2013). SARS-CoV-2 activates the NLRP3 inflammasome and IL-1β and IL-18 levels in the elderly patient and increases inflammation and the severity of COVID-19 by potentiating pyroptosis and releasing damage-associated molecular patterns (DAMPs) (Junqueira et al., 2021, Rodrigues et al., 2021). Increasing reactive oxygen species (ROS) levels in the aging process leads to the activation of NLRP3 inflammation and intensifies the COVID-19 process in the elderly patient (Mishra et al., 2021, Tay et al., 2020, Zheng et al., 2020b). It should be noted that metformin reduces the severity of the disease by reducing the levels of adenosine triphosphate (ATP) and mitochondrial ROS levels that can stimulate NLRP3-mediated IL-1β generation (Xian et al., 2021). On the other hand, results using anti-IL-1 receptor agents have been shown to reduce COVID-19 fatalities and suggest NLRP3 inflammasome as the target of COVID-19 (Barkas et al., 2021, Shah, 2020).
On the other hand, an increase in NF-κB signaling has been shown in aging. Immune cells derived from an inflamm-ageing conditions have decreased response to severe ex vivo stimuli. However, monocytes emerge to be having an abnormal degree of responsiveness (Sayed et al., 2021). In a clinical study, analysis of monocytes in elderly COVID-19 patients cleared that they were enhanced for the IFN-γ response and TNF-α, IL-1β, and CXCL8 expression (Zheng et al., 2020b). Senescent monocytes were also enhanced for wide pathways related to toll-like receptor (TLR) signaling, oxidative stress, MAPK, and NF-κB, as well as the cyclin-dependent kinase inhibitor (p21), made this hypothesis that the existence of inflammatory aged or senescent cells with a senescence-associated secretory phenotype (SASP) was one activity for severe inflammatory in these cells in COVID-19 (Zheng et al., 2020b). Aging cells remain and cumulate in aging, indicating an extraordinary secretory phenotype defined by the existence of inflammatory cytokines. This SASP is defined by generating cytokines, chemokines, growth factors (GFs), matrix metalloproteinases (MMPs), fibronectin, and ROS (Coppé et al., 2010). A recent study found that exposure of senescent cell lines to pathogen-associated molecular patterns (PAMPs) and the SARS-CoV-2 spike glycoprotein results in considerably higher SASP synthesis and expression of the viral entrance genes ACE2 and transmembrane protease serine 2 (TMPRSS2) (Camell et al., 2021).
Some reduced responses are seen in the neutrophils of older people, including reduced bactericidal activity, reduced respiratory burst, and reduced neutrophil extracellular trap formation (Ortmann and Kolaczkowska, 2018). However, aberrant colonization and increased degranulation are also seen in the neutrophils of these people, which shows the defect of the neutrophils of the elderly in fighting pathogens and producing much inflammatory (Sapey et al., 2014). On the other hand, high levels of IL-6, which is caused by a pathogen or inflamm-ageing, lead to the long-term duration of neutrophils by decreasing apoptosis and indicate poor clinical outputs (Asensi et al., 2004, Liu et al., 2020a).
Therefore, the function of innate immune cells and inflammatory response to SARS-CoV-2 are not regulated in the elderly. Studies have shown a relationship between neutrophils and monocytes in the blood and severe COVID-19 (Kuri-Cervantes et al., 2020, Lucas et al., 2020). Based on the information, it can be concluded that these changes are partly due to the activation of age-related vital TLR, variations in oxidative stress pathways, inflammatory activity, raised cellular module and SASP, as well as other pathways like decreased autophagy and DNA damage with age (Bajaj et al., 2021a). Therefore, it can be concluded that SARS-CoV-2 infection, along with inflammation, worsens the consequences of COVID-19, and further studies are needed to eliminate the relationship between these two options.
3.3. Metabolites reprogramming
Numerous investigations have shown the point of creating between intracellular metabolism and inflammation. The field of immunometabolism concentrates on the shifts in intracellular metabolism that follow immune cell activation and regulate immune cell activities. Immune cells undergo substantial metabolite reprogramming during activation to meet the dramatic demand for energy and sustain immune cell capabilities such as strong cytokine generation, fast proliferation, and migratory processes (O'Neill et al., 2016). The information required to establish the metabolic effects of COVID-19 infection is currently being compiled. A number of studies have investigated the metabolic profiles of COVID-19 patients (Gardinassi et al., 2020).
Based on the most recent studies conducted on the effect of COVID-19 on metabolism, infected patients may have elevated blood glucose and fatty acid levels, as well as aberrations in amino acid (AA) metabolism. The gene expression encoding tryptophan metabolic enzymes, such as kynurenine and indoleamine 2, 3 dioxygenase (IDO), has been shown to have increased. (Blasco et al., 2020, Thomas et al., 2020b). The elevated amount of these metabolites in the circulation of severe COVID-19 patients has been linked to a reduction in tryptophan (Chen and Guillemin, 2009, Guillemin et al., 2003). Due to the decreased supply of this essential AA that activates general control non-derepressible-2 (GCN-2) or eukaryotic initiation factor-2 (eIF-2) stress kinase, tryptophan catabolism inhibits T cell proliferation and stimulates their anergy in severe COVID-19 patients, as illustrated by the reduces in various circulating T cells (Mellor and Munn, 2003, Moffett and Namboodiri, 2003, Munn et al., 2005). The elevated levels of kynurenic acid or kynurenate in the circulating suppress the pro-inflammatory action of monocytes and macrophages (Moroni et al., 2007, Sekkaï et al., 1997). For instance, in patients with severe COVID-19, circulating monocytes and macrophages do not contribute to the formation of the cytokine storm. Furthermore, tryptophan depletion in circulating DCs, monocytes, and macrophages increases GCN-2 activation, which boosts the production of anti-inflammatory cytokines (IL-10 and TGF-β) by phosphorylating eIF-2 at serine 51 and rendering it passive (Munn et al., 2005, Ravishankar et al., 2015, Sorgdrager et al., 2019). As a consequence of modified immunometabolism, circulating tryptophan metabolites inhibit circulating monocytes/macrophages, DCs, and T cells in severe COVID-19 patients.
Further intermediates involved in the metabolism of arginine, aspartate, tyrosine, and lysine may be changed in infected people (Blasco et al., 2020, Moolamalla et al., 2021, Thomas et al., 2020b). Serum from severe COVID-19 individuals demonstrates a reduction in apolipoprotein A1 (APOA1) and apolipoprotein M (APOM) (Shen et al., 2020). Patients with COVID-19 experience downregulation of sphingolipids as well. Glycerophospholipid levels in the serum are continuously reduced by SARS-CoV-2 infection and reach dangerously low levels in COVID-19 patients. In individuals with severe COVID-19, choline and its derivatives declined as well, although phosphocholine levels increased (Shen et al., 2020). These case studies demonstrate that many aspects of healthy metabolism can be disturbed as a result of sickness, making it difficult for any therapy used to restore metabolic equilibrium.
Sphingosine-1-phosphate (S1P) levels in plasma drop in COVID-19 patients but the rise in those who are convalescing (Song et al., 2020). The largest concentrations of S1P are seen in human red blood cells and platelets because these cells lack the pyridoxal phosphate-dependent S1P lyase (S1PL) needed to break down S1P into 2 hexadecenal and ethanolamine phosphate (Ito et al., 2007, Saba and Hla, 2004). So, under typical circumstances, hematopoietic and vascular endothelial cells expressing the ABC and spinster homolog 2 (Spns2) transporters carry S1P out of these cells and into the circulation (Fukuhara et al., 2012, Kim et al., 2009, Venkataraman et al., 2008). Under homeostasis, the mature T cell and B cell egress from the thymus and bone marrow to the circulation and the secondary lymphoid organs (SLOs) is promoted by the circulating S1P produced from vascular endothelial cells (Fukuhara et al., 2012, Nijnik et al., 2012). Although animals missing the Spns2 transporter exhibit a significant reduction in S1P in the lymph, the loss of circulating lymphocytes is caused by a slight reduction in S1P in the plasma (Mendoza et al., 2012). Therefore, a decline in the level of circulating S1P in COVID-19 patients reflects a reduction in the number of T cells in the blood, but an increase in the inflamed organ, which is more pronounced in patients with severe COVID-19. SARS-CoV2 infection reduces the level of circulating S1P in COVID-19 patients by infecting RBCs, platelets (thrombocytopenia), and vascular endothelial cells (Cavezzi et al., 2020, Lippi et al., 2020, Thomas et al., 2020a, Xu et al., 2020a).
In COVID-19 patients, levels of several acylcarnitines, including palmitoylcarnitine, stearoylcarnitine, and oleoylcarnitine, fell. Reduced levels of acylcarnitines in the blood may signify that the entry of fatty acids into the mitochondria for β-oxidation or fatty acid oxidation (FAO) has been reduced (Song et al., 2020). Citrate, succinate, and other tricarboxylic acids (TCAs) cycle metabolites typically decline in COVID-19 patients, and this decline increases with infection severity. Therefore, a diminished metabolic response to the decreased lung functions and blood oxygen to a lower dependency on oxygen for cellular energy production may be indicated by decreased TCA cycle metabolites in the circulation in severe COVID-19 patients (Song et al., 2020).
Increased cytosolic phospholipase A2 (cPLA2) activity in severe COVID-19 may worsen lung inflammation (Bhowmick et al., 2017, Song et al., 2020). The increase in PLA2 results in an increase in the levels of circulating glycerophospholipids (Song et al., 2020). In severe COVID-19 patients, there is a strong correlation between the rise in plasma sphingolipid GM3 enriched exosomes and the fall in T cell and CD4+ T cell counts (Song et al., 2020).
Inflammatory cytokines and chemokines are produced when acute macrophage activation occurs under inflammatory conditions (IFN- γ or lipopolysaccharide (LPS) stimulation). This is done by starting glycogenesis (glycogen synthesis), which produces glucose-6-phosphate for glycolysis to aggravate it further (Ma et al., 2020). In macrophages isolated from the lungs of severe COVID-19 patients, high glucose levels promote the multiplication of the SARS-CoV-2 and the production of pro-inflammatory cytokines, leading to cytokine storms (Codo et al., 2020). This happens as a result of the SARS-CoV-2 infection's overexpression of several glycolysis-related genes in these macrophages (Codo et al., 2020). Thus, only SARS-CoV-2 infection causes elevated glycolysis in human macrophages isolated from COVID-19 patients with severe lung disease. Compared to OXPHOS, which provides frequent energy for macrophages during homeostasis to transcribe and translate pro-inflammatory genes, glycolysis is induced in SPP1+ monocyte-derived macrophages that have become infiltrated in the lungs of severe COVID-19 patients. Additionally, these included increased transcriptional activity for enzymes such as lactate dehydrogenase A (LDH-A), pyruvate kinase M2, glucose transporter 1 (GLUT-1), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) (Bost et al., 2020, Codo et al., 2020). The elevation of the pro-inflammatory macrophage transcriptome for different cytokines and chemokines (TNF-, IL-6, IL-12A, IL-23A, and CCL3, CCL4, CCL5, CCL20, CCL23, CXCL2, CXCL11) isolated from sepsis patients is attributed to the HIF-1α overexpression (Kumar, 2018, Shalova et al., 2015). Immune cells' levels of LDH rise as COVID-19 severity increases. Even so, there is no discernible difference between COVID-19 patients and controls in the plasma lactate level (Song et al., 2020). The Krebs or TCA cycle intermediates (succinate and citrate) and the TCA cycle-derived itaconate accumulate as a result of enhanced glycolysis in pro-inflammatory macrophages, which also controls the expression of inflammatory genes (Ryan and O'Neill, 2020, Seim et al., 2019). The direct inhibition of prolyl hydroxylase domain (PHD) activity by succinate transfer to the cytosol stabilizes the HIF-1α and increases glycolysis (Codo et al., 2020, Ryan and O'Neill, 2020, Tannahill et al., 2013). Complex II, also known as succinate dehydrogenase (SDH), oxidizes succinate in the mitochondria, triggering reverse electron transport (RET), which encourages the production of mitochondrial ROS (mtROS), which suppresses PHD and stabilizes HIF-1α and glycolysis (Codo et al., 2020, Ryan and O'Neill, 2020, Tannahill et al., 2013). Thus, SARS-CoV-2 replication in infected macrophages depends critically on succinate oxidation.
3.4. The relationship between aging cells and thrombosis
In addition to the relationship between aging cells and the severity of inflammation in COVID-19, there is another link between them: thrombosis. Aging cells have a paracrine pro-coagulation effect (Wiley et al., 2019). In experiments performed on mice, infusion of a cellular aging reporter by doxorubicin remedy, p16–3MR, was related to notably minor bleeding time, higher platelet numbers, more activated platelets, and higher serum thrombopoietin (Wiley et al., 2019). The mission of aging cells in vivo turned these pro-thrombotic phenotypes. Stable isotope labeling by amino acids in cell culture analysis of aging cells displayed the further activity of human platelets stimulate by aging cell supernatants (Wiley et al., 2019).
Severe COVID-19 cases are related to agents such as venous thromboembolism and arterial thrombosis (Chen et al., 2020b, Cui et al., 2020, Fox et al., 2020, Guan et al., 2020, Klok et al., 2020, Lodigiani et al., 2020, Tang et al., 2020, Wu et al., 2020, Xu et al., 2020b, Zhou et al., 2020a). More precisely, factor CD142 (a blood coagulation stimulant) is expressed by proinflammatory cytokines on some of the immune cells. Supplement activity, neutrophil extracellular traps, and lung hypoxia further boost pro-thrombotic conditions, linking thrombosis and the innate immune system (Price et al., 2020). Generation of viral-induced pro-thrombotic auto-antibodies versus phospholipids and phospholipid-binding proteins is also seen within severe SARS-CoV-2 infection (Zuo et al., 2020). Recently, a study has shown that patients with critical COVID-19 pneumonia produce autoantibodies against type I INF. The response of extrafollicular B cell causes the production of these autoantibodies, which in turn cause thrombosis (Knight et al., 2021). Therefore, it can be concluded that the uncontrolled inflammatory response to SARS-CoV-2 in thrombotic-prone elderly can intensify the coagulation resulting from COVID-19.
3.5. Delayed response of type I IFN with ageing
IFN deficiency has been seen in a patient's serum with acute COVID-19 (Hadjadj et al., 2020). In addition, experiments on elderly macaques with SARS-CoV-2 infection also showed fewer type I IFN and Notch signaling pathways in the lung contrasted to young macaques (Rosa et al., 2021). The reduction in these responses includes a decrease in the number of plasmacytoid dendritic cells (pDCs), potent producer of IFN-α, that have also been seen in a patient with acute COVID-19 (Zhou et al., 2020b). It is noteworthy that only ten percent of patients with acute COVID-19 have type I anti-IFNs antibodies (Bastard et al., 2020). Dysfunction of genes associated with the TLR3 and interferon regulatory factor 7 (IRF7) pathways involved in inducing and amplifying type I IFNs response has also been observed in patients with acute infection (Zhang et al., 2020b). They can effectively prevent the spread of viral infection. They play a vital role in the optimal activity of macrophages, the presentation of antigens by DCs, and the growth of effective antiviral T cell responses (McNab et al., 2015).
Experiments have shown the critical effect of type I IFN responses on the adjustment of monocytes and neutrophils following SARS-CoV-2 infection. In experiments on patients with mild COVID-19, more classical monocytes were observed in the blood, indicating the early and passing effect of a type I IFN. Nevertheless, on the other hand, monocytes and neutrophils in the patient with acute COVID-19 showed more genes involved in NF-κB signaling and the generation of ROS or nitric oxide synthase during the disease. During viral infections, non-classical and intermediate proinflammatory subsets of monocytes develop (Wong et al., 2012). Therefore, they also spread during SARS-CoV-2 infection (Zhang et al., 2020a, Zhou et al., 2020c). Of course, the dysregulated strong response of these cells also helps the inflammatory conditions seen in the severe pathophysiology of COVID-19. In addition, non-classical monocytes also generate type I IFN (IFN-α) in response to TLR3 (Boyette et al., 2017). Therefore, the kinetics of non-classical monocyte dysregulation should be noted as severe consequences. Early monocyte deficiency decreases type I IFN response in patients with acute COVID-19 (Schulte-Schrepping et al., 2020, Silvin et al., 2020).
The aging process delays type I IFN responses, which has also been seen in the SARS-CoV infection. Although the effect of aging on susceptibility to viral infection is not well understood, it causes damage to the early and peripheral retinoic acid-inducible gene I (RIG-I) signaling pathways that rein the expression of many types of I IFN genes. This reduces the generation of I IFNs in people over 65 years of age and thus disrupts their body's antiviral responses (Molony et al., 2017). During SARS-CoV infection, the type I IFN signaling proteins downstream of RIG-I more decreases because of mitochondrial dysfunction associated with the mitochondrial antiviral signaling (MAVS) (Shi et al., 2014). In the elderly, factors such as basal mitochondrial dysfunction decreased TNF receptor-related agent adapter protein, and phosphorylated IRF3 amount associated with RIG-I signaling increases vulnerability to RIG-I deficiency (Feng et al., 2021, Molony et al., 2017). It should be noted that RIG-I can inhibit the proliferation of SARS-CoV-2 in human lung cells, although this function does not need I IFN signaling strength (Yamada et al., 2021). On the other hand, a decrease in the sum number of pDCs has been seen in the elderly. In addition, a decrease in TLR7 and I IFNs production ability has been observed in these elderlies (Feng et al., 2021, Jing et al., 2009, Pérez-Cabezas et al., 2007, Shodell and Siegal, 2002). Although there are many similarities between reduced IFN responses in patients with acute COVID-19 and the elderly, more research is needed to understand their relationship clearly.
3.6. Age-associated modifications to antigen presentation
With proper activation of T lymphocytes, innate immunity plays an essential role in generating adaptive immune responses. In order to efficiently prim T cells, innate immune cells have two functions: (1) to present antigen through the molecules of major histocompatibility complex (MHC) co-stimulatory receptors through a cell-cell interplay between T cells and antigen-presenting cells (APCs) and (2) generate appropriate cytokines to slant the separation of CD4+ T cells into a particular responsibility for the invasive pathogen. Disruption of each APC function has adverse effects on the adaptive immune responses. Like DCs and monocytes, APCs showed a shortage of antigen presentation in people with severe COVID-19. COVID-19-induced DC experiments showed the lowest expression of the chemokine receptors CD80, CD86, C-C chemokine receptor 7 (CCR7), and human leukocyte antigen (HLA)-DR (Giamarellos-Bourboulis et al., 2020, Schulte-Schrepping et al., 2020, Silvin et al., 2020, Zhou et al., 2020c). Disorders in antigen presentation occur with getting old. The number of monocytes also changes during this process, and the accumulation of non-classical monocytes during this period causes a downregulating of HLA-DR (Seidler et al., 2010). In mice, one study revealed a decrease in MHC class II, CD40, and CD86 levels in old DC subsets following activity by TLR agonists. Of course, there are contradictory observations, and further studies are needed (Wong and Goldstein, 2013). In infection of SARS-CoV, older mice lung DCs showed a ruined strength to immigrate to the draining lymph node, which hurt following T cell priming (Zhao et al., 2011). Raised amount of prostaglandin D2 in the lungs of older mice causes this immigration problem, which in turn leads to a decrease in CCR7 surface expression in DCs (Zhao et al., 2011). In turn, the aging factor disrupts T-cell antigen and antigen presentation, so it can be concluded that with viral infections that impair these functions, older people are more prone to dysfunctional adaptive responses of the immune system.
3.7. Dysregulation of B and T lymphocytes during ageing
3.7.1. B lymphocytes
Following the SARS-CoV-2 infection, B cells generate the amount of immunoglobulin M (IgM), IgG, and IgA antibodies for SARS-CoV-2 up to 1 week after symptoms and up to 2 weeks, most patients seroconverted to IgG and IgM. By identifying SARS-CoV-2-particular follicular T helper cells in the circulation, this response begins and plays a role in generating T cell-dependent antibodies. Counteracting antibodies of the SARS-CoV-2 receptor-binding domain (RBD) have been demonstrated in mice and humans, and translocation of these antibodies in experiments has decreased the severity of SARS-CoV-2 disease (Alsoussi et al., 2020, Zost et al., 2020). However, serum samples of a patient showing improvement have shown positive effects (Devarasetti et al., 2021). Determining the innate role of antibodies during COVID-19 requires further investigation.
The results of many experiments have shown a relationship between greater IgG antibody titers and disease severity (Moderbacher et al., 2020). The ambivalent role of antibodies within viral infections can clear the different results: Although counteracting antibodies are generally effective in combating and eliminating the viral agent, the initial production or pre-existence of pre-circulating non-counteracting antibodies can conduce to the antibody-mediated enhancement of viral entry and infuses severe inflammatory response. Here, an antibody-related issue exacerbates the disease. However, the exact role of antibodies in exacerbating SARS-CoV-2 infection has not yet been established. In addition, antibodies such as IgG with afucosylated IgG Fc series may exacerbate SARS-CoV-2 and further damage due to pro-inflammatory activity via FcγRIIIa (Larsen et al., 2021).
The effectiveness of the humoral immune response also decreases with aging, and older B cells show less power to sustain with physical mutation, which reduces the generation of counteracting antibody titers in the elderly (Frasca et al., 2017). Experiments on animals have shown that the IgG amount of viral S, protein-particular at the onset of acute SARS-CoV-2 infection, is less in older macaques than in young macaques; therefore, the establishment of antibody titers against viral infection decreases with aging (Singh et al., 2021).
On the other hand, B cell accumulation acquires unique attributes with aging. Another study in mice has shown that TLR7 responses elevate these cells (Cancro, 2020). These cells reside in the late human memory segment and release inflammatory mediators such as TNF-α, IL-6, and IL-8, thus having a role in autoimmune disease and recently in COVID-19 (Cancro, 2020, Frasca, 2018, Woodruff et al., 2020). In severe COVID-19 patients, the development of such cells has been observed to induce CD11c and T-bet expression in a skewed extra-follicular B cell response (Tay et al., 2020).
Favorable to the predominant extra-follicular B cell response, experiments have confirmed defective germinal center (GC) responses in patients with acute SARS-CoV-2 infection in the secondary lymphatic organs. Decreased GC establishment has also been reported in the results of one of these experiments and occurs when there are many amounts of TNF-α in lymph node follicles. TNF-α production also raises during aging and may play a role in phenotype (Bruunsgaard et al., 2003, Fagiolo et al., 1993, Penninx et al., 2004). Other aging problems also affect GC establishment. The expression of CD40 ligand, a co-stimulatory molecule essential for well T-cell and B-cell interplay, reduces with the increasing age of CD4+ T cells. In addition, lymphopenia in the elderly and patients with acute COVID-19 could mention by reducing access to CD4+ T cells to employ with B cells. Since the likelihood of T-cell and B-cell interaction is considerably decreased, the loss of a clonal diversity of TCR and B-cell receptor (BCR) in the elderly exacerbates this problem. Lack of longanimity and the appearance of auto-antibodies are other difficulties associated with aging. According to research on SARS-CoV-2, the lethality of this agent, especially in men, has been linked to the existence of auto-antibodies particular to the type I IFNs (Bastard et al., 2020).
Although these auto-antibodies have been found at different ages, their existence has been seen more in those patients over 65 years old. Based on these findings, it can be concluded that the effect of aging on the production of these antibodies and other auto-antibodies that are effective in exacerbating COVID-19 should be carefully investigated. On the other hand, the production of auto-antibodies, especially in men, requires more research.
3.7.2. T lymphocytes
One week after SARS-CoV-2 infection, adaptive responses are activated, and CD4+ and CD8+ T cells appear (Moderbacher et al., 2020). T helper 1 (TH1) cells respond to SARS-CoV-2 by producing IFN-γ and IL-2, among other cytokines, including TNF-α (Braun et al., 2020, Grifoni et al., 2020, Kaneko et al., 2020, Moderbacher et al., 2020, Zhou et al., 2020c). It should be noted that raised chemokines like CXCL9 and CXCL10 in the blood of COVID-19 patients have a role in the recruiting and/or differentiating naive T cells into TH1 cells. Experiments have shown that CD4+ and CD8+ T cells have protective effects on former coronaviruses (CoVs) (Zhao et al., 2010). To date, the role of T cells' response to SARS-CoV-2 infection has been demonstrated, although cytokine storms in these cells lead to the dysfunctional activity of monocytes (Moderbacher et al., 2020, Nelde et al., 2021, Peng et al., 2020). Considering the role of the T cell in fighting SARS-CoV-2 infection and its changes in aging, it is necessary to investigate the effect of biological changes of this cell to fight against this infection.
3.7.2.1. Decrease in clonal lymphocyte
To have a strong immune system, it is necessary to have a varied set of T-cell receptors (TCRs), but with increasing age, the diversity of CD4+ and CD8+ naive TCR species decreases (Britanova et al., 2014). A detailed study of the factors affecting TCR variability in adults is still ongoing. The process of thymic shrinkage in old age prevents the expansion of new T cells in the elderly and reduces the TCR set versatility. Experimental evidence has shown that with the introduction of naive, newly produced T cells into the old T cell set, the effectiveness of these cells decreases, and they produce fewer efficient memory cells. Of course, the problem of these young T cells, particularly CD8+ T cells, is not the cause of all the problems. Modified homeostatic proliferation in humans and the silencing preservation of T cell inactivity stillness are essential for a stable, naive peripheral T cell compartment. These two agents play an essential role in maintaining the diversity of naive T cells during aging (Goronzy and Weyand, 2019).
On the other hand, chronic antigen motivation may lead to developing a polyclonal T cell memory repository that reduces the existence of naive polyclonal T cells. In severe COVID-19, less TCR variability was observed against SARS-CoV-2 epitopes (Nelde et al., 2021, Peng et al., 2020). Compared with patients with moderate COVID-19, patients with severe the disease have a weaker T cell response to the N-terminal segment of the SARS-CoV-2 S protein (Braun et al., 2020). Low T cell frequency is also directly related to the effects of severe COVID-19 (Moderbacher et al., 2020). Therefore, the relationship between reduced TCR in the elderly and worsening consequences of SARS-CoV-2 infection can be understood.
3.7.2.2. Lymphopenia
Lymphopenia is one of the factors influencing the severity of COVID-19 and a decrease in the total number of peripheral T cells in the blood (Huang and Pranata, 2020). Experiments have shown the role of blood lymphocyte percentage as a criterion for diagnosing mild, severe disease and choosing the appropriate treatment process for the condition (Tan et al., 2020). Lymphopenia during COVID-19 can have a more adverse effect on the elderly and exacerbate poor T-cell responses by reducing the number of T cells. COVID-19-related lymphopenia resulted from increased T cell migration to infection sites. However, experiments have shown that lymphocyte migration is not the only factor affecting the lymphopenia related to COVID-19. In addition, a study has shown that the whole number of CD8+ T cells in the tissue of patients with mild COVID-19 is higher than in patients with acute symptoms (Liao et al., 2020).
Experimental findings confirming that T-cell immigration to the lungs alone does not cause blood lymphopenia in patients with severe COVID-19, as showed that lung macrophages in patients with severe COVID-19 mainly use chemokines to use inflammatory monocytes and neutrophils express, while lung macrophages in patient of mild COVID-19 express higher amount T cell-activating chemokines. Other factors that may reduce T cells in SARS-CoV-2 infection include the direct involvement of these cells in the virus or the cell death increase in response to antigen and the release of cytokines (Merad and Martin, 2020). In these experiments, dependence on the amount of cytokines IL-6, IL-10, or TNF-α and blood lymphopenia was seen in disease, and IL-6 receptor antagonist tocilizumab raised the issue of lymphocytes in the blood of COVID-19 patients (Diao et al., 2020, Giamarellos-Bourboulis et al., 2020, Wan et al., 2020). In addition, there is a direct relationship between lymphopenia and the age of the COVID-19 patient. Notably, in patients over 60, the whole number of blood T cells reaches its lowest point (Diao et al., 2020). Findings on the influenza virus have shown a decrease in the multiplication of old CD8 + T cells and its negative effect on the fight against the viral agent in older patients (Gruver et al., 2007).
3.7.2.3. T cell exhaustion
The effectiveness of T cell activity reduces with age, and on the other hand, the exhaustion of these cells also raises. So far, conflicting observations about the activity of these cells after SARS-CoV-2 infection have been seen. Weakness of essential activities of CD4+ T cells, such as production of IFN-γ, IL-2, and/or TNF-α, has been observed in patients with acute conditions. However, other experiments have not observed this weakness (Mazzoni et al., 2020, Zheng et al., 2020a). Decreased cytotoxicity of CD8+ and production of cytokines at CD8+ T cells activity in acute conditions have been observed, while the inverse of that has been observed in other experiments; it should be noted that in some experiments, no variation was seen in the function of these cells (Moderbacher et al., 2020, Peng et al., 2020, Zheng et al., 2020a). The variation in cytokine sampling time seems to be the reason for these different results. Due to the negative role of aging in the adaptive immune process, in our view, in the elderly, the effectiveness of T cell activity decreases and leads to a decrease in cytokine production and/or cytotoxicity, resulting in a weak fight against the virus disease. Experiments have identified the existence of IFN-γ-producing CD8+ T cells in the acute stages of the disease as a sign of the moderate COVID-19 effects (Moderbacher et al., 2020). In addition, in acute cases of COVID-19, higher expression of exhaustion indicators by CD4+ and CD8+ T cells has been observed (Diao et al., 2020). Recent findings have indicated the upregulation of exhaustion indicators such as programmed cell death protein-1 (PD-1) and T-cell immunoglobulin mucin 3 (Tim-3) by SARS-CoV-2 infection (Diao et al., 2020, Wu et al., 2021). However, these indicators may be signs of activation, not function exhaustion (Rha et al., 2021).
3.7.2.4. Pre-existing SARS-CoV-2 epitopes in unexposed individuals
CD4+ T cells that fight SARS-CoV-2 infection have already been seen in people who did not expose to the disease (Grifoni et al., 2020). In vitro studies have shown that T cells that fight SARS-CoV-2 infection, which already exists in the body, are mainly from the memory T cells (Mateus et al., 2020). Thus, many people have memory T cells that can cross-recognize SARS-CoV-2 epitopes (Braun et al., 2020). Other experiments have suggested a role for the absence of these cells in the TCR set in the exacerbation of COVID-19 (Nelde et al., 2021). The results of these experiments still need further investigation. For example, it should be investigated to what extent older adults have been at risk for CoV infections and whether this led to memory T cell responses. The absence of TCR diversity as we age can prevent memory T cells from proliferating in the previous face of CoVs. It may be due to inflating memory that memory T cells of the elderly may not be well stored in the peripheral repertoire (Klenerman and Oxenius, 2016).
A correlation has been found between the severity of COVID-19 and the occurrence of cytomegalovirus (CMV), an early pathogen related to memory inflation. However, more research is needed on the impact of formation, function, and longevity of new T cells and the CMV and other latent viruses on each other (Shrock et al., 2020). CD8+ T cells may also become active as aging and may be isolated without the presence of their homogeneous antigen and in reacting to IL-15 signaling. Although the function of CD8+ T cells has been extensively investigated in mice, it has been proven to be directly related to the age of humans (White et al., 2016). Cytokine-activated CD8+ T cells have an instinctive function and can induce cytotoxic effects without the presence of homogeneous antigen within viral infections. Aside from the antiviral benefits of this, an unrestrained function can cause harm to the host (Kim and Shin, 2019). The exact role of these cells in the effects of COVID-19 requires investigation.
4. Conclusions
Abnormal pathological inflammatory responses cause devastating consequences of SARS-CoV-2 infection and cause uncontrolled local tissue damage, vascular leakage, systemic cytokine storm, and thrombosis. NF-κB signaling causes inflammation and thus leads to an inadequate response to immune cells to severe activation, although monocyte subsets are an exception to this rule. Loss of early type I IFN responses during acute COVID-19, together with JAK-STAT hypo-responsiveness of old immune cells, leads to rapid viral duplication and exacerbates vulnerability to SARS-CoV-2 attack in the elderly. When the aging immune system overcomes the initial signaling and peak viral attack, many pro-inflammatory cytokines are released, which may cause tissue damage and vascular permeability, leading to the continued release of innate inflammatory pathological responses. Excess priming of TLR4 due to secondary bacterial infections after the viral attack is also involved in creating this peak. It should be noted that weak immune responses of the elderly to SARS-CoV-2 can be due to ineffective T cell priming, loss of naive T cell diversity, decreased antibody maturity, and/or impaired memory in these individuals. With its unregulated activity and without the aid of an antigen-specific immune response, the innate immune system exacerbates the complications of COVID-19.
The role of aging in the worsening of SARS-CoV-2 symptoms is one of the hottest topics. Most experiments have investigated the role of aging in people's blood factors, and there is still no substantiated research on tissue information. In the information obtained from the study of the immune system of mice and the role of aging in it, differences have been observed from the information obtained from human experiments. In addition, immune cell subsets are also not always fully maintained between these two experimental species. These contradictions necessitate careful consideration from one model to another. The investigations performed should integrate all the information obtained and provide comprehensive results.
Platforms of mRNA COVID-19 vaccines have proven highly effective in the elderly. The Pfizer vaccine has a success rate of 95% in people over 65 years old, and the Moderna vaccine has a success rate of 86% in this age group. Recently, 17 days after the second dose injection, an experiment showed fewer anti-SARS-CoV-2 antibodies in people over 80 than in those under 60. Determining the lasting efficacy of vaccines provided to the elderly requires further investigation. The long-term effects of SARS-CoV-2 infection have not been studied. The development of a strong immune system against COVID-19 is the main focus of today's research, which continues in the hope of increasing the health age of individuals.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Mohammad Reza Zinatizadeh: Conceptualization, Writing – original draft preparation, Data curation. Peyman Kheirandish Zarandi: Writing – original draft preparation, Data curation. Mohsen Ghiasi: Data curation and figure. Hamid Kooshki: Writing – review & editing. Mozafar Mohammadi: Writing – review & editing. Jafar Amani: Writing – review & editing. Nima Rezaei: Project administration, Supervision, Conceptualization, Writing – review & editing. All authors contributed to the paper and approved the submitted version.
Conflict of interest
The authors report no conflicts of interest in this work.
Acknowledgments
None declared.
References
- Akha A.A.S. Aging and the immune system: an overview. J. Immunol. Methods. 2018;463:21–26. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Al-Mosawi A.J. The pattern of covid-19 disease in Iraq during the year 2020. Sch. Int J. Anat. Physiol. 2021;4:127–134. [Google Scholar]
- Alpert A., Pickman Y., Leipold M., Rosenberg-Hasson Y., Ji X., Gaujoux R., Rabani H., Starosvetsky E., Kveler K., Schaffert S. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019;25:487–495. doi: 10.1038/s41591-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsoussi W.B., Turner J.S., Case J.B., Zhao H., Schmitz A.J., Zhou J.Q., Chen R.E., Lei T., Rizk A.A., McIntire K.M. A potently neutralizing antibody protects mice against SARS-CoV-2 infection. J. Immunol. 2020;205:915–922. doi: 10.4049/jimmunol.2000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensi V., Valle E., Meana A., Fierer J., Celada A., Alvarez V., Paz J., Coto E., Carton J.A., Maradona J.A. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect. Immun. 2004;72:3823–3828. doi: 10.1128/IAI.72.7.3823-3828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front. Physiol. 2021:1793. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front. Physiol. 2021;11 doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am. J. Infect. Control. 2021;49:238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu N., Panikar S.S., Leal L.R., Leal A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to macrophage activation syndrome: therapeutic implications. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas F., Filippas-Ntekouan S., Kosmidou M., Liberopoulos E., Liontos A., Milionis H. Anakinra in hospitalized non-intubated patients with coronavirus disease 2019: a systematic review and meta-analysis. Rheumatology. 2021;60:5527–5537. doi: 10.1093/rheumatology/keab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick R., Clark S., Bonventre J.V., Leong J.M., McCormick B.A. Cytosolic phospholipase A2α promotes pulmonary inflammation and systemic disease during Streptococcus pneumoniae infection. Infect. Immun. 2017;85:e00280–00217. doi: 10.1128/IAI.00280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco H., Bessy C., Plantier L., Lefevre A., Piver E., Bernard L., Marlet J., Stefic K., Benz-de Bretagne I., Cannet P. The specific metabolome profiling of patients infected by SARS-COV-2 supports the key role of tryptophan-nicotinamide pathway and cytosine metabolism. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-73966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol. Lett. 2014;162:346–353. doi: 10.1016/j.imlet.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., Blecher-Gonen R., Cohen M., Medaglia C., Li H. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;181:1475–1488. doi: 10.1016/j.cell.2020.05.006. e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette L.B., Macedo C., Hadi K., Elinoff B.D., Walters J.T., Ramaswami B., Chalasani G., Taboas J.M., Lakkis F.G., Metes D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A.R. The nuclear factor-κB–interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Britanova O.V., Putintseva E.V., Shugay M., Merzlyak E.M., Turchaninova M.A., Staroverov D.B., Bolotin D.A., Lukyanov S., Bogdanova E.A., Mamedov I.Z. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J. Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H., Andersen-Ranberg K., vB Hjelmborg J., Pedersen B.K., Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- Camell C.D., Yousefzadeh M.J., Zhu Y., Prata L.G.L., Huggins M.A., Pierson M., Zhang L., O’Kelly R.D., Pirtskhalava T., Xun P. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021;373 doi: 10.1126/science.abe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancro M.P. Age-associated B cells. Annu. Rev. Immunol. 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020;10:1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., Gong W., Han J.D.J. Individual variation of the SARS‐CoV‐2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19 doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guillemin G.J. Kynurenine pathway metabolites in humans: disease and healthy states. International journal of tryptophan research 2. IJTR. 2009:S2097. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougnet C.A., Thacker R.I., Shehata H.M., Hennies C.M., Lehn M.A., Lages C.S., Janssen E.M. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol. 2015;195:2624–2632. doi: 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A.C., Davanzo G.G., de Brito Monteiro L., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol.: Mech. Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M., Dearman R.J., Kimber I. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin‐1β. Immunology. 2002;105:466–477. doi: 10.1046/j.1365-2567.2002.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha L.L., Perazzio S.F., Azzi J., Cravedi P., Riella L.V. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front. Immunol. 2020:1748. doi: 10.3389/fimmu.2020.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E., Solana R., Larbi A., Pawelec G. Immunity, ageing and cancer. Immun. Ageing. 2008;5:1–16. doi: 10.1186/1742-4933-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarasetti P.K., Rajasekhar L., Baisya R., Sreejitha K., Vardhan Y.K. A review of COVID-19 convalescent plasma use in COVID-19 with focus on proof of efficacy. Immunol. Res. 2021;69:18–25. doi: 10.1007/s12026-020-09169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon K.H., Leist S.R., Schäfer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Hou Y.J., Adams L.E. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolo U., Cossarizza A., Scala E., Fanales‐Belasio E., Ortolani C., Cozzi E., Monti D., Franceschi C., Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Feng E., Balint E., Poznanski S.M., Ashkar A.A., Loeb M. Aging and interferons: impacts on inflammation and viral disease outcomes. Cells. 2021;10:708. doi: 10.3390/cells10030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourati S., Cristescu R., Loboda A., Talla A., Filali A., Railkar R., Schaeffer A.K., Favre D., Gagnon D., Peretz Y. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat. Commun. 2016;7:1–12. doi: 10.1038/ncomms10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D. Senescent B cells in aging and age-related diseases: their role in the regulation of antibody responses. Exp. Gerontol. 2018;107:55–58. doi: 10.1016/j.exger.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D., Diaz A., Romero M., Blomberg B.B. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp. Gerontol. 2017;87:113–120. doi: 10.1016/j.exger.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Investig. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinassi L.G., Souza C.O., Sales-Campos H., Fonseca S.G. Immune and metabolic signatures of COVID-19 revealed by transcriptomics data reuse. Front. Immunol. 2020;11:1636. doi: 10.3389/fimmu.2020.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.A., Rossen L.M., Ahmad F.B., Sutton P., Li Z., Salvatore P.P., Coyle J.P., DeCuir J., Baack B.N., Durant T.M. Race, ethnicity, and age trends in persons who died from COVID-19—United States, May–August 2020. Morb. Mortal. Wkly. Rep. 2020:1517. doi: 10.15585/mmwr.mm6942e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin K., Viboud C., Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019;19:573–583. doi: 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(1489–1501) doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver A., Hudson L., Sempowski G. Immunosenescence of ageing. J. Pathol.: A J. Pathol. Soc. Gt. Br. Irel. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-j, Ni Z.-y, Hu Y., Liang W.-h, Ou C.-q, He J.-x, Liu L., Shan H., Lei C.-l, Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. New Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin G.J., Smith D.G., Smythe G.A., Armati P.J., Brew G.J. Developments in Tryptophan and Serotonin Metabolism. Springer; 2003. Expression of the kynurenine pathway enzymes in human microglia and macrophages; pp. 105–112. [DOI] [PubMed] [Google Scholar]
- Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainz U., Jenewein B., Asch E., Pfeiffer K.-P., Berger P., Grubeck-Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23:3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- Ho F.K., Petermann-Rocha F., Gray S.R., Jani B.D., Katikireddi S.V., Niedzwiedz C.L., Foster H., Hastie C.E., Mackay D.F., Gill J.M. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care. 2020;8:1–10. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M.E., Al-Aklobi O.S., Abomughaid M.M., Al-Ghamdi M.A. Epidemiological, clinical, and laboratory findings for patients of different age groups with confirmed coronavirus disease 2019 (COVID-19) in a hospital in Saudi Arabia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Anada Y., Tani M., Ikeda M., Sano T., Kihara A., Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- Jing Y., Shaheen E., Drake R.R., Chen N., Gravenstein S., Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009;70:777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.E. Recent results: biomarkers of aging. Exp. Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Junqueira C., Crespo Â., Ranjbar S., Lewandrowski M., Ingber J., de Lacerda L.B., Parry B., Ravid S., Clark S., Ho F. Research Square; 2021. SARS-CoV-2 Infects Blood Monocytes to Activate NLRP3 and AIM2 Inflammasomes, Pyroptosis and Cytokine Release. [Google Scholar]
- Kaml M., Weiskirchner I., Keller M., Luft T., Hoster E., Hasford J., Young L., Bartlett B., Neuner C., Fischer K.-H. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine. 2006;24:6808–6811. doi: 10.1016/j.vaccine.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Kaneko N., Kuo H.-H., Boucau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., Piechocka-Trocha A., Lefteri K., Osborn M., Bals J. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157. doi: 10.1016/j.cell.2020.08.025. e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R.H., Takabe K., Milstien S., Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-S., Shin E.-C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 2019;51(1–9) doi: 10.1038/s12276-019-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P., Oxenius A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016;16:367–377. doi: 10.1038/nri.2016.38. [DOI] [PubMed] [Google Scholar]
- Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., Kaptein F., van Paassen J., Stals M., Huisman M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.S., Caricchio R., Casanova J.-L., Combes A.J., Diamond B., Fox S.E., Hanauer D.A., James J.A., Kanthi Y., Ladd V. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021:131. doi: 10.1172/JCI154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. Int. Immunopharmacol. 2018;58:173–185. doi: 10.1016/j.intimp.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M.D., de Graaf E.L., Sonneveld M.E., Plomp H.R., Nouta J., Hoepel W., Chen H.-J., Linty F., Visser R., Brinkhaus M. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. 2021;371 doi: 10.1126/science.abc8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist S.R., Dinnon K.H., III, Schäfer A., Longping V.T., Okuda K., Hou Y.J., West A., Edwards C.E., Sanders W., Fritch E.J. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;183:1070–1085. doi: 10.1016/j.cell.2020.09.050. e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: a review of clinical data in China. Mech. Ageing Dev. 2020;188 doi: 10.1016/j.mad.2020.111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H., Luo M., Chen L., Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.-D., Sacco C., Bertuzzi A. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein J., Castro T.B., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Wei K., Liu J., Tang K., Zhang H., Zhu L., Chen J., Li F., Xu P., Liu J. Glycogen metabolism regulates macrophage-mediated acute inflammatory responses. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-15636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C.J., Pereira C.Cd.A., Viana B.D.M., Oliveira G.L., Melo D.C., Carvalho J.F.M.G.D., Moraes F.L.D., Moraes E.N.D. Estimates of the impact of COVID-19 on mortality of institutionalized elderly in Brazil. Cienc. Saude Coletiva. 2020;25:3437–3444. doi: 10.1590/1413-81232020259.14552020. [DOI] [PubMed] [Google Scholar]
- Manser A.R., Uhrberg M. Age-related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol. Immunother. 2016;65:417–426. doi: 10.1007/s00262-015-1750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez E.J., Trowbridge J., Kuchel G.A., Banchereau J., Ucar D. The lethal sex gap: COVID-19. Immun. Ageing. 2020;17:1–8. doi: 10.1186/s12979-020-00183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., Burger Z.C., Rawlings S.A., Smith D.M., Phillips E. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., Mencarini J., Caporale R., Peruzzi B., Antonelli A. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020;130:4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade T.W., Adair L., Feranil A.B., Kuzawa C. Positive antibody response to vaccination in adolescence predicts lower C‐reactive protein concentration in young adulthood in the Philippines. Am. J. Hum. Biol. 2011;23:313–318. doi: 10.1002/ajhb.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F., Mayer-Barber K., Sher A., Wack A., O'garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegaro A., Edmunds W.J. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur. J. Epidemiol. 2004;19:353–363. doi: 10.1023/b:ejep.0000024701.94769.98. [DOI] [PubMed] [Google Scholar]
- Mellor A.L., Munn D.H. Tryptophan catabolism and regulation of adaptive immunity. J. Immunol. 2003;170:5809–5813. doi: 10.4049/jimmunol.170.12.5809. [DOI] [PubMed] [Google Scholar]
- Mendoza A., Bréart B., Ramos-Perez W.D., Pitt L.A., Gobert M., Sunkara M., Lafaille J.J., Morris A.J., Schwab S.R. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2:1104–1110. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T.U., Cubas R.A., Ghneim K., Cartwright M.J., Grevenynghe J.V., Richner J.M., Olagnier D.P., Wilkinson P.A., Cameron M.J., Park B.S. Global analyses revealed age‐related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell. 2015;14:421–432. doi: 10.1111/acel.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T.U., Wilkinson P.A., Cameron M.J., Ghneim K., Chiang C., Wertheimer A.M., Hiscott J.B., Nikolich-Zugich J., Haddad E.K. Human monocyte subsets are transcriptionally and functionally altered in aging in response to pattern recognition receptor agonists. J. Immunol. 2017;199:1405–1417. doi: 10.4049/jimmunol.1700148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S.R., Mahapatra K.K., Behera B.P., Patra S., Bhol C.S., Panigrahi D.P., Praharaj P.P., Singh A., Patil S., Dhiman R. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int. J. Biochem. Cell Biol. 2021;136 doi: 10.1016/j.biocel.2021.106013. [DOI] [PubMed] [Google Scholar]
- Moderbacher C.R., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J.R., Namboodiri M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- Molony R.D., Nguyen J.T., Kong Y., Montgomery R.R., Shaw A.C., Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolamalla S., Balasubramanian R., Chauhan R., Priyakumar U.D., Vinod P. Host metabolic reprogramming in response to SARS-CoV-2 infection: a systems biology approach. Microb. Pathog. 2021;158 doi: 10.1016/j.micpath.2021.105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F., Fossati S., Chiarugi A., Cozzi A. International Congress Series. Elsevier; 2007. Kynurenic acid actions in brain and periphery; pp. 305–313. [Google Scholar]
- Munn D.H., Sharma M.D., Baban B., Harding H.P., Zhang Y., Ron D., Mellor A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2, 3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Nelde A., Bilich T., Heitmann J.S., Maringer Y., Salih H.R., Roerden M., Lübke M., Bauer J., Rieth J., Wacker M. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021;22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- Nijnik A., Clare S., Hale C., Chen J., Raisen C., Mottram L., Lucas M., Estabel J., Ryder E., Adissu H. The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J. Immunol. 2012;189:102–111. doi: 10.4049/jimmunol.1200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- Nikpouraghdam M., Farahani A.J., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H., Sepandi M., Jafari N.J., Izadi M., Qazvini A. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann W., Kolaczkowska E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018;371:473–488. doi: 10.1007/s00441-017-2751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx B.W., Kritchevsky S.B., Newman A.B., Nicklas B.J., Simonsick E.M., Rubin S., Nevitt M., Visser M., Harris T., Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J. Am. Geriatr. Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Cabezas B., Naranjo-Gómez M., Fernández M.A., Grífols J.R., Pujol-Borrell R., Borràs F.E. Reduced numbers of plasmacytoid dendritic cells in aged blood donors. Exp. Gerontol. 2007;42:1033–1038. doi: 10.1016/j.exger.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Price L.C., McCabe C., Garfield B., Wort S.J. Eur Respiratory Soc.; 2020. Thrombosis and COVID-19 Pneumonia: The Clot Thickens! [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar B., Liu H., Shinde R., Chaudhary K., Xiao W., Bradley J., Koritzinsky M., Madaio M.P., McGaha T.L. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc. Natl. Acad. Sci. U.S.A. 2015;112:10774–10779. doi: 10.1073/pnas.1504276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Alexander H.D., Ross O.A. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 2018:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha M.-S., Jeong H.W., Ko J.-H., Choi S.J., Seo I.-H., Lee J.S., Sa M., Kim A.R., Joo E.-J., Ahn J.Y. PD-1-expressing SARS-CoV-2-specific CD8+ T cells are not exhausted, but functional in patients with COVID-19. Immunity. 2021;54:44–52. doi: 10.1016/j.immuni.2020.12.002. e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues T.S., de Sá K.S., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R. Inflammasome activation in COVID-19 patients. medRxiv. 2020 [Google Scholar]
- Rodrigues T.S., de Sá K.S., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021:218. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa B.A., Ahmed M., Singh D.K., Choreño-Parra J.A., Cole J., Jiménez-Álvarez L.A., Rodríguez-Reyna T.S., Singh B., Gonzalez O., Carrion R. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS-CoV-2 infection. Commun. Biol. 2021;4:1–14. doi: 10.1038/s42003-021-01829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D.G., O'Neill L.A. Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol. 2020;38:289–313. doi: 10.1146/annurev-immunol-081619-104850. [DOI] [PubMed] [Google Scholar]
- Saba J.D., Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- Salminen A., Huuskonen J., Ojala J., Kauppinen A., Kaarniranta K., Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Santesmasses D., Castro J.P., Zenin A.A., Shindyapina A.V., Gerashchenko M.V., Zhang B., Kerepesi C., Yim S.H., Fedichev P.O., Gladyshev V.N. COVID‐19 is an emergent disease of aging. Aging Cell. 2020;19 doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapey E., Greenwood H., Walton G., Mann E., Love A., Aaronson N., Insall R.H., Stockley R.A., Lord J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood J. Am. Soc. Hematol. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed N., Huang Y., Nguyen K., Krejciova-Rajaniemi Z., Grawe A.P., Gao T., Tibshirani R., Hastie T., Alpert A., Cui L. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging. 2021;1:598–615. doi: 10.1038/s43587-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., Krämer B., Krammer T., Brumhard S., Bonaguro L. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440. doi: 10.1016/j.cell.2020.08.001. e1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler S., Zimmermann H.W., Bartneck M., Trautwein C., Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:1–11. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim G.L., Britt E.C., John S.V., Yeo F.J., Johnson A.R., Eisenstein R.S., Pagliarini D.J., Fan J. Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation. Nat. Metab. 2019;1:731–742. doi: 10.1038/s42255-019-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekkaï D., Guittet O., Lemaire G., Tenu J.-P., Lepoivre M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch. Biochem. Biophys. 1997;340:117–123. doi: 10.1006/abbi.1997.9913. [DOI] [PubMed] [Google Scholar]
- Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front. Immunol. 2020;11:1021. doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalova I.N., Lim J.Y., Chittezhath M., Zinkernagel A.S., Beasley F., Hernández-Jiménez E., Toledano V., Cubillos-Zapata C., Rapisarda A., Chen J. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity. 2015;42:484–498. doi: 10.1016/j.immuni.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., Quan S., Zhang F., Sun R., Qian L. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72. doi: 10.1016/j.cell.2020.05.032. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Orr S.S., Furman D., Kidd B.A., Hadad F., Lovelace P., Huang Y.-W., Rosenberg-Hasson Y., Mackey S., Grisar F.A.G., Pickman Y. Defective signaling in the JAK-STAT pathway tracks with chronic inflammation and cardiovascular risk in aging humans. Cell Syst. 2016;3:374–384. doi: 10.1016/j.cels.2016.09.009. e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-S., Qi H.-Y., Boularan C., Huang N.-N., Abu-Asab M., Shelhamer J.H., Kehrl J.H. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193:3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shodell M., Siegal F. Circulating, interferon‐producing plasmacytoid dendritic cells decline during human ageing. Scand. J. Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Shrock E., Fujimura E., Kula T., Timms R.T., Lee I.-H., Leng Y., Robinson M.L., Sie B.M., Li M.Z., Chen Y. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020;370 doi: 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin A., Chapuis N., Dunsmore G., Goubet A.-G., Dubuisson A., Derosa L., Almire C., Hénon C., Kosmider O., Droin N. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418. doi: 10.1016/j.cell.2020.08.002. e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D.K., Singh B., Ganatra S.R., Gazi M., Cole J., Thippeshappa R., Alfson K.J., Clemmons E., Gonzalez O., Escobedo R. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat. Microbiol. 2021;6:73–86. doi: 10.1038/s41564-020-00841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-W., Lam S.M., Fan X., Cao W.-J., Wang S.-Y., Tian H., Chua G.H., Zhang C., Meng F.-P., Xu Z. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020;32:188–202. doi: 10.1016/j.cmet.2020.06.016. e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgdrager F.J., Naudé P.J., Kema I.P., Nollen E.A., Deyn P.P.D. Tryptophan metabolism in inflammaging: from biomarker to therapeutic target. Front. Immunol. 2019;10:2565. doi: 10.3389/fimmu.2019.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020;5:1–3. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill G., Curtis A., Adamik J., Palsson-McDermott E., McGettrick A., Goel G., Frezza C., Bernard N., Kelly B., Foley N. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Stefanoni D., Dzieciatkowska M., Issaian A., Nemkov T., Hill R.C., Francis R.O., Hudson K.E., Buehler P.W., Zimring J.C. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J. Proteome Res. 2020;19:4455–4469. doi: 10.1021/acs.jproteome.0c00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., Hudson K.E., Zimring J.C., Hansen K.C., Hod E.A. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020:5. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., Thomas M. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012:2012. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin D., Mohanty S., Thomas V., Ginter S., Montgomery R.R., Fikrig E., Allore H.G., Medzhitov R., Shaw A.C. Age-associated defect in human TLR-1/2 function. J. Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- Venkataraman K., Lee Y.-M., Michaud J., Thangada S., Ai Y., Bonkovsky H.L., Parikh N.S., Habrukowich C., Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor C.P., Lelic A., Parsons R., Evelegh C., Bramson J.L., Johnstone J., Loeb M.B., Bowdish D.M. Serum C-reactive protein and congestive heart failure as significant predictors of herpes zoster vaccine response in elderly nursing home residents. J. Infect. Dis. 2017;216:191–197. doi: 10.1093/infdis/jix257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 [Google Scholar]
- Wang M., Jiang L., Monticone R.E., Lakatta E.G. Proinflammation: the key to arterial aging. Trends Endocrinol. Metab. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanhella K.J., Fernandez-Patron C. Biomarkers of ageing and frailty may predict COVID-19 severity. Ageing Res. Rev. 2022;73 doi: 10.1016/j.arr.2021.101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Weinberger B., Grubeck‐Loebenstein B. The aging of the immune system. Transpl. Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.T., Cross E.W., Burchill M.A., Danhorn T., McCarter M.D., Rosen H.R., O’Connor B., Kedl R.M. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat. Commun. 2016;7:1–13. doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C.D., Liu S., Limbad C., Zawadzka A.M., Beck J., Demaria M., Artwood R., Alimirah F., Lopez-Dominguez J.-A., Kuehnemann C. SILAC analysis reveals increased secretion of hemostasis-related factors by senescent cells. Cell Rep. 2019;28:3329–3337. doi: 10.1016/j.celrep.2019.08.049. e3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski J.M., Fulop T., Bryl E. Immunosenescence and COVID-19. Mech. Ageing Dev. 2022;204 doi: 10.1016/j.mad.2022.111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters B., Junge U., Dziuba S., Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21:3623–3628. doi: 10.1016/s0264-410x(03)00399-2. [DOI] [PubMed] [Google Scholar]
- Wong C., Goldstein D.R. Impact of aging on antigen presentation cell function of dendritic cells. Curr. Opin. Immunol. 2013;25:535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.L., Yeap W.H., Tai J.J.Y., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: implications for health and disease. Immunol. Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- Woodruff M.C., Ramonell R.P., Nguyen D.C., Cashman K.S., Saini A.S., Haddad N.S., Ley A.M., Kyu S., Howell J.C., Ozturk T. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Chen D., Ding W., Wu P., Hou H., Bai Y., Zhou Y., Li K., Xiang S., Liu P. The trans-omics landscape of COVID-19. Nat. Commun. 2021;12:1–16. doi: 10.1038/s41467-021-24482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H., Liu Y., Nilsson A.R., Gatchalian R., Crother T.R., Tourtellotte W.G., Zhang Y., Aleman-Muench G.R., Lewis G., Chen W. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54:1463–1477. doi: 10.1016/j.immuni.2021.05.004. e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xudong X., Junzhu C., Xingxiang W., Furong Z., Yanrong L. Age-and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., Sasaki M., Takaoka A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021;22:820–828. doi: 10.1038/s41590-021-00942-0. [DOI] [PubMed] [Google Scholar]
- Yoo J.-S., Sasaki M., Cho S.X., Kasuga Y., Zhu B., Ouda R., Orba Y., de Figueiredo P., Sawa H., Kobayashi K.S. SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nat. Commun. 2021;12:1–17. doi: 10.1038/s41467-021-26910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T.T. Epidemiology and unique aspects of aging and infectious diseases. Clin. Infect. Dis. 2000;30:931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- Youm Y.-H., Grant R.W., McCabe L.R., Albarado D.C., Nguyen K.Y., Ravussin A., Pistell P., Newman S., Carter R., Laque A. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Cui L., Hou F., Liu X., Wang Y., Wen Y., Chi C., Li C., Liu R., Yin C. Angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis prevents pancreatic acinar cell inflammatory response via inhibition of the p38 mitogen-activated protein kinase/nuclear factor-κB pathway. Int. J. Mol. Med. 2018;41:409–420. doi: 10.3892/ijmm.2017.3252. [DOI] [PubMed] [Google Scholar]
- Zacca E.R., Crespo M.I., Acland R.P., Roselli E., Núñez N.G., Maccioni M., Maletto B.A., Pistoresi-Palencia M.C., Morón G. Aging impairs the ability of conventional dendritic cells to cross-prime CD8+ T cells upon stimulation with a TLR7 ligand. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarandi P.K., Zinatizadeh M.R., Zinatizadeh M., Yousefi M.H., Rezaei N. SARS-CoV-2: from the pathogenesis to potential anti-viral treatments. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., Qian H., Dai T., Zhang T., Lai Y. COVID‐19 infection induces readily detectable morphologic and inflammation‐related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2020 doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Liu Z., Pen J.L., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., Rosain J., Bilguvar K., Ye J., Bolze A., Bigio B., Yang R., Arias A.A., Zhou Q., Zhang Y., Onodi F., Korniotis S., Karpf L., Philippot Q., Chbihi M., Bonnet-Madin L., Dorgham K., Smith N., Schneider W.M., Razooky B.S., Hoffmann H.-H., Michailidis E., Moens L., Han J.E., Lorenzo L., Bizien L., Meade P., Neehus A.-L., Ugurbil A.C., Corneau A., Kerner G., Zhang P., Rapaport F., Seeleuthner Y., Manry J., Masson C., Schmitt Y., Schlüter A., Voyer T.L., Khan T., Li J., Fellay J., Roussel L., Shahrooei M., Alosaimi M.F., Mansouri D., Al-Saud H., Al-Mulla F., Almourfi F., Al-Muhsen S.Z., Alsohime F., Turki S.A., Hasanato R., Beek Dvd, Biondi A., Bettini L.R., D’Angio’ M., Bonfanti P., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Oler A.J., Tompkins M.F., Alba C., Vandernoot I., Goffard J.-C., Smits G., Migeotte I., Haerynck F., Soler-Palacin P., Martin-Nalda A., Colobran R., Morange P.-E., Keles S., Çölkesen F., Ozcelik T., Yasar K.K., Senoglu S., Karabela Ş.N., Rodríguez-Gallego C., Novelli G., Hraiech S., Tandjaoui-Lambiotte Y., Duval X., Laouénan C., Snow A.L., Dalgard C.L., Milner J.D., Vinh D.C., Mogensen T.H., Marr N., Spaan A.N., Boisson B., Boisson-Dupuis S., Bustamante J., Puel A., Ciancanelli M.J., Meyts I., Maniatis T., Soumelis V., Amara A., Nussenzweig M., García-Sastre A., Krammer F., Pujol A., Duffy D., Lifton R.P., Zhang S.-Y., Gorochov G., Béziat V., Jouanguy E., Sancho-Shimizu V., Rice C.M., Abel L., Notarangelo L.D., Cobat A., Su H.C., Casanova J.-L., Foti G., Bellani G., Citerio G., Contro E., Pesci A., Valsecchi M.G., Cazzaniga M., Abad J., Aguilera-Albesa S., Akcan O.M., Darazam I.A., Aldave J.C., Ramos M.A., Nadji S.A., Alkan G., Allardet-Servent J., Allende L.M., Alsina L., Alyanakian M.-A., Amador-Borrero B., Amoura Z., Antolí A., Arslan S., Assant S., Auguet T., Azot A., Bajolle F., Baldolli A., Ballester M., Feldman H.B., Barrou B., Beurton A., Bilbao A., Blanchard-Rohner G., Blanco I., Blandinières A., Blazquez-Gamero D., Bloomfield M., Bolivar-Prados M., Borie R., Bosteels C., Bousfiha A.A., Bouvattier C., Boyarchuk O., Bueno M.R.P., Bustamante J., Agra J.J.C., Calimli S., Capra R., Carrabba M., Casasnovas C., Caseris M., Castelle M., Castelli F., Vera M.Cd, Castro M.V., Catherinot E., Chalumeau M., Charbit B., Cheng M.P., Clavé P., Clotet B., Codina A., Colkesen F., Çölkesen F., Colobran R., Comarmond C., Dalmau D., Darley D.R., Dauby N., Dauger S., Pontual Ld, Dehban A., Delplancq G., Demoule A., Diehl J.-L., Dobbelaere S., Durand S., Eldars W., Elgamal M., Elnagdy M.H., Emiroglu M., Erdeniz E.H., Aytekin S.E., Euvrard R., Evcen R., Fabio G., Faivre L., Falck A., Fartoukh M., Faure M., Arquero M.F., Flores C., Francois B., Fumadó V., Fusco F., Solis B.G., Gaussem P., Gil-Herrera J., Gilardin L., Alarcon M.G., Girona-Alarcón M., Goffard J.-C., Gok F., González-Montelongo R., Guerder A., Gul Y., Guner S.N., Gut M., Hadjadj J., Haerynck F., Halwani R., Hammarström L., Hatipoglu N., Hernandez-Brito E., Heijmans C., Holanda-Peña M.S., Horcajada J.P., Hoste L., Hoste E., Hraiech S., Humbert L., Iglesias A.D., Íñigo-Campos A., Jamme M., Arranz M.J., Jordan I., Jorens P., Kanat F., Kapakli H., Kara I., Karbuz A., Yasar K.K., Keles S., Demirkol Y.K., Klocperk A., Król Z.J., Kuentz P., Kwan Y.W.M., Lagier J.-C., Lambrecht B.N., Lau Y.-L., Bourgeois F.L., Leo Y.-S., Lopez R.L., Leung D., Levin M., Levy M., Lévy R., Li Z., Linglart A., Loeys B., Lorenzo-Salazar J.M., Louapre C., Lubetzki C., Luyt C.-E., Lye D.C., Mansouri D., Marjani M., Pereira J.M., Martin A., Pueyo D.M., Martinez-Picado J., Marzana I., Mathian A., Matos L.R.B., Matthews G.V., Mayaux J., Mège J.-L., Melki I., Meritet J.-F., Metin O., Meyts I., Mezidi M., Migeotte I., Millereux M., Mirault T., Mircher C., Mirsaeidi M., Melián A.M., Martinez A.M., Morange P., Mordacq C., Morelle G., Mouly S., Muñoz-Barrera A., Naesens L., Nafati C., Neves J.F., Ng L.F.P., Medina Y.N., Cuadros E.N., Ocejo-Vinyals J.G., Orbak Z., Oualha M., Özçelik T., Pan-Hammarström Q., Parizot C., Pascreau T., Paz-Artal E., Pellegrini S., Diego R.Pd, Philippe A., Philippot Q., Planas-Serra L., Ploin D., Poissy J., Poncelet G., Pouletty M., Quentric P., Raoult D., Rebillat A.-S., Reisli I., Ricart P., Richard J.-C., Rivet N., Rivière J.G., Blanch G.R., Rodrigo C., Rodriguez-Gallego C., Rodríguez-Palmero A., Romero C.S., Rothenbuhler A., Rozenberg F., Prado M.Y.Rd, Riera J.S., Sanchez O., Sánchez-Ramón S., Schluter A., Schmidt M., Schweitzer C.E., Scolari F., Sediva A., Seijo L.M., Sene D., Senoglu S., Seppänen M.R.J., Ilovich A.S., Shahrooei M., Slabbynck H., Smadja D.M., Sobh A., Moreno X.S., Solé-Violán J., Soler C., Soler-Palacín P., Stepanovskiy Y., Stoclin A., Taccone F., Tandjaoui-Lambiotte Y., Taupin J.-L., Tavernier S.J., Terrier B., Thumerelle C., Tomasoni G., Toubiana J., Alvarez J.T., Trouillet-Assant S., Troya J., Tucci A., Ursini M.V., Uzunhan Y., Vabres P., Valencia-Ramos J., Braeckel E.V., Velde S.Vd, Rym A.M.V.D., Praet J.V., Vandernoot I., Vatansev H., Vélez-Santamaria V., Viel S., Vilain C., Vilaire M.E., Vincent A., Voiriot G., Vuotto F., Yosunkaya A., Young B.E., Yucel F., Zannad F., Zatz M., Belot A., Bole-Feysot C., Lyonnet S., Masson C., Nitschke P., Pouliet A., Schmitt Y., Tores F., Zarhrate M., Abel L., Andrejak C., Angoulvant F., Bachelet D., Basmaci R., Behillil S., Beluze M., Benkerrou D., Bhavsar K., Bompart F., Bouadma L., Bouscambert M., Caralp M., Cervantes-Gonzalez M., Chair A., Coelho A., Couffignal C., Couffin-Cadiergues S., D’Ortenzio E., Silveira C.D., Debray M.-P., Deplanque D., Descamps D., Desvallées M., Diallo A., Diouf A., Dorival C., Dubos F., Duval X., Eloy P., Enouf V.V., Esperou H., Esposito-Farese M., Etienne M., Ettalhaoui N., Gault N., Gaymard A., Ghosn J., Gigante T., Gorenne I., Guedj J., Hoctin A., Hoffmann I., Jaafoura S., Kafif O., Kaguelidou F., Kali S., Khalil A., Khan C., Laouénan C., Laribi S., Le M., Hingrat Q.L., Mestre S.L., Nagard H.L., Lescure F.-X., Lévy Y., Levy-Marchal C., Lina B., Lingas G., Lucet J.C., Malvy D., Mambert M., Mentré F., Mercier N., Meziane A., Mouquet H., Mullaert J., Neant N., Noret M., Pages J., Papadopoulos A., Paul C., Peiffer-Smadja N., Petrov-Sanchez V., Peytavin G., Picone O., Puéchal O., Rosa-Calatrava M., Rossignol B., Rossignol P., Roy C., Schneider M., Semaille C., Mohammed N.S., Tagherset L., Tardivon C., Tellier M.-C., Téoulé F., Terrier O., Timsit J.-F., Trioux T., Tual C., Tubiana S., Werf Svd, Vanel N., Veislinger A., Visseaux B., Wiedemann A., Yazdanpanah Y., Alavoine L., Amat K.K.A., Behillil S., Bielicki J., Bruijning P., Burdet C., Caumes E., Charpentier C., Coignard B., Costa Y., Couffin-Cadiergues S., Damond F., Dechanet A., Delmas C., Descamps D., Duval X., Ecobichon J.-L., Enouf V., Espérou H., Frezouls W., Houhou N., Ilic-Habensus E., Kafif O., Kikoine J., Hingrat Q.L., Lebeaux D., Leclercq A., Lehacaut J., Letrou S., Lina B., Lucet J.-C., Malvy D., Manchon P., Mandic M., Meghadecha M., Motiejunaite J., Nouroudine M., Piquard V., Postolache A., Quintin C., Rexach J., Roufai L., Terzian Z., Thy M., Tubiana S., Werf Svd, Vignali V., Visseaux B., Yazdanpanah Y., Agtmael Mv, Algera A.G., Baarle Fv, Bax D., Beudel M., Bogaard H.J., Bomers M., Bos L., Botta M., Brabander Jd, Bree Gd, Brouwer M.C., Bruin Sd, Bugiani M., Bulle E., Chouchane O., Cloherty A., Elbers P., Fleuren L., Geerlings S., Geerts B., Geijtenbeek T., Girbes A., Goorhuis B., Grobusch M.P., Hafkamp F., Hagens L., Hamann J., Harris V., Hemke R., Hermans S.M., Heunks L., Hollmann M.W., Horn J., Hovius J.W., Jong M.Dd, Koning R., Mourik Nv, Nellen J., Paulus F., Peters E., Poll Tvd, Preckel B., Prins J.M., Raasveld J., Reijnders T., Schinkel M., Schultz M.J., Schuurman A., Sigaloff K., Smit M., Stijnis C.S., Stilma W., Teunissen C., Thoral P., Tsonas A., Valk Mvd, Veelo D., Vlaar A.P.J., Vries Hd, Vugt Mv, Wiersinga W.J., Wouters D., Zwinderman A.H., Beek Dvd, Abel L., Aiuti A., Muhsen S.A., Al-Mulla F., Anderson M.S., Arias A.A., Feldman H.B., Bogunovic D., Bolze A., Bondarenko A., Bousfiha A.A., Brodin P., Bryceson Y., Bustamante C.D., Butte M., Casari G., Chakravorty S., Christodoulou J., Cirulli E., Condino-Neto A., Cooper M.A., Dalgard C.L., David A., DeRisi J.L., Desai M., Drolet B.A., Espinosa S., Fellay J., Flores C., Franco J.L., Gregersen P.K., Haerynck F., Hagin D., Halwani R., Heath J., Henrickson S.E., Hsieh E., Imai K., Itan Y., Karamitros T., Kisand K., Ku C.-L., Lau Y.-L., Ling Y., Lucas C.L., Maniatis T., Mansouri D., Marodi L., Meyts I., Milner J., Mironska K., Mogensen T., Morio T., Ng L.F.P., Notarangelo L.D., Novelli A., Novelli G., O’Farrelly C., Okada S., Ozcelik T., Diego R.Pd, Planas A.M., Prando C., Pujol A., Quintana-Murci L., Renia L., Renieri A., Rodríguez-Gallego C., Sancho-Shimizu V., Sankaran V., Barrett K.S., Shahrooei M., Snow A., Soler-Palacín P., Spaan A.N., Tangye S., Turvey S., Uddin F., Uddin M.J., Beek Dvd, Vazquez S.E., Vinh D.C., Bernuth Hv, Washington N., Zawadzki P., Su H.C., Casanova J.-L., Jing H., Tung W., Luthers C.R., Bauman B.M., Shafer S., Zheng L., Zhang Z., Kubo S., Chauvin S.D., Meguro K., Shaw E., Lenardo M., Lack J., Karlins E., Hupalo D.M., Rosenberger J., Sukumar G., Wilkerson M.D., Zhang X. Vol. 370. 2020. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. (Science). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhao J., Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J. Virol. 2010;84:9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD 2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Investig. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., Dong X.-Q., Zheng Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Liu X., Le W., Xie L., Li H., Wen W., Wang S., Ma S., Huang Z., Ye J. A human circulating immune cell landscape in aging and COVID-19. Protein Cell. 2020;11:740–770. doi: 10.1007/s13238-020-00762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., To K.K.-W., Wong Y.-C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.-Y., Lau T.T.-K. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53:864–877. doi: 10.1016/j.immuni.2020.07.026. e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinatizadeh M.R., Schock B., Chalbatani G.M., Zarandi P.K., Jalali S.A., Miri S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune. Dis. Genes Dis. 2021;8:287–297. doi: 10.1016/j.gendis.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinatizadeh M.R., Zarandi P.K., Zinatizadeh M., Yousefi M.H., Amani J., Rezaei N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingaropoli M.A., Nijhawan P., Carraro A., Pasculli P., Zuccala P., Perri V., Marocco R., Kertusha B., Siccardi G., Del Borgo C. Increased sCD163 and sCD14 plasmatic levels and depletion of peripheral blood pro-inflammatory monocytes, myeloid and plasmacytoid dendritic cells in patients with severe COVID-19 pneumonia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.627548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]