Abstract
Extracellular vesicles (EVs) can contain DNA, RNA, proteins and metabolic molecules from primary origins; they are coated with a phospholipid bilayer membrane and released by cells into the extracellular matrix. EVs can be obtained from various body liquids, including the blood, saliva, cerebrospinal fluid, and urine. As has been proved, EVs-mediated transfer of biologically active molecules is crucial for various physiological and pathological processes. Extensive investigations have already begun to explore the diagnosis and prognosis potentials for EVs. Furthermore, research has continued to recognize the critical role of nucleic acids and proteins in EVs. However, our understanding of the comprehensive effects of metabolites in these nanoparticles is currently limited and in its infancy. Therefore, we have attempted to summarize the recent research into the metabolomics of EVs in relation to potential clinical applications and discuss the problems and challenges that have occurred, to provide more guidance for the future development in this field.
Keywords: extracellular vesicles, metabolomics, metabolites, clinical application
Introduction
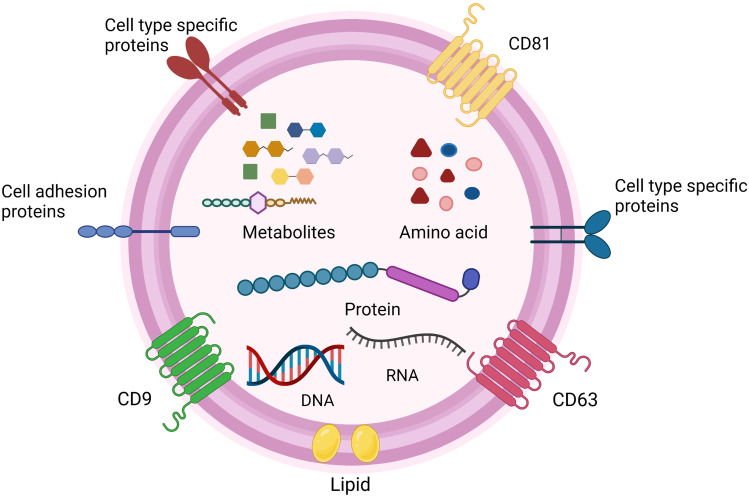
Extracellular vesicles (EVs) are membrane-bound lipid nanovesicles secreted by various cells.1 While the EVs from different cells display similar physical characteristics, they are highly heterogeneous.2 EVs can usually be roughly subdivided into microvesicles (MVs), exosomes, and apoptotic bodies, and they differ from each other in size, shape, formation process, and biological functions.3 Originally, EVs were thought as a trash accumulator for cells, as a way to deal with unneeded components. Up to now, it has been gradually demonstrated that EVs also regulate physiological and pathological responses. Extensive studies have recognized the significance of EVs in information transfer and cell-to-cell communication, such as tumor development,4 immune responses,5 and cell apoptosis.6 EVs are composed of thousands of nucleic acids, proteins, and lipids,7 and their surfaces are composed of a sophisticated lipid bilayer which can protect their inner biomolecules.8 They can also pass through the blood–brain barrier, which characterizes their value in initiation and development of neurological diseases (Figure 1).9
Figure 1.
The structure and content of EVs. EVs have a complex composition of nucleic acids (DNA, RNA), protein, metabolites and amino acid.7 Some proteins are on the surface of the particles and used as markers for EVs, such as CD9, CD63, CD81.1–3 Additionally, EVs surface also include cell adhesion proteins, cell‐type‐specific proteins, and lipid.4–9 Created with BioRender.com.
The role of nucleic acids and proteins in EV-mediated communication has been widely reported.10–12 Considering the similarity of the cargoes in EVs and primary cells, EVs can function as biomarkers for the diagnosis of disease and disease stages.13 In the era of tissue biopsy as the gold standard for cancer diagnosis, EVs can be utilized as novel and non-invasive tools to enable early diagnosis and timely monitoring.14 Increasingly, researchers have designed EVs as targeted carriers to package drugs, miRNAs, and proteins, and thus facilitate the remarkable and precise treatment of diseases.15
In laboratory testing, metabolomics focuses on small-molecule metabolites that are less than or equal to 1500 Da in biological specimens, including carbohydrates, organic acids, lipids, amino acids and aromatic hydrocarbons.16,17 Genomics and transcriptomics have been widely used and shown great potential in various studies. Genomics reveals what a cell is capable of, while transcriptomics elucidates what the cells plan. Specifically, proteomics and metabolomics can elucidate what the cell is doing and reflect the direct physical changes.2,18 By analyzing the constituents of the metabolic compounds, we can identify the chemical processes that having occurred and the reactions currently existing in the body, and this can enhance the rapid assessment of phenotypes for different diseases. Interpretations combined with relevant knowledge can provide comprehensive information on the changes in the upstream molecules, including DNA, RNA, and proteins.1,19 While there may only be a minor difference in the upstream protein structure or composition, this can lead to an obvious transformation in metabolomics.20 Therefore, it is necessary to highlight that metabolomics is the ultimate “omics” which can identify sensitive and specific biomarkers to clarify slight pathological changes in donor cells.21 In turn, metabolites also have a reflected effect on gene replication, transcription, and translation, which ultimately influences the initiation and progression of diseases.22 As it aids in our understanding of molecular mechanism diversity, metabolomics can also be applied to precision medicine to improve human health, such as for metabolic and cardiac diseases, cancer, longevity, and nervous system diseases.23,24 Additionally, due to the interference of environmental enzymes, initial genomics and transcriptomics are not completely linear with the downstream metabolites, as side reactions may simultaneously happen, resulting in unexpected products.25 It is thus not enough to concentrate solely on the genomics and transcriptomics, as the whole molecular landscape should be understood. Accordingly, the two main analytical techniques that have recently been applied to EV metabolomics are nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (MS), of which MS has a higher sensitivity and specificity. To date, however, detection platforms are generally unable to detect all compounds in the sample at the same time, and this can only be achieved by selective extraction combined with parallel detection in each platform.26–28 We must comprehensively consider sample situations and individual needs to select more suitable analytical techniques. When comparing their information processing abilities, both genomics and metabolomics can produce massive databases. Considering the costs associated with using these technologies, mass spectrometry-based metabolomics is cheaper and faster than genomics.29
Ultimately, the cargo within EVs depends not only on the producing cells but also on cell growth conditions.30 A previous study compared EVs growing in conventional culturing conditions with in vitro cell models. It surprisingly concluded that there are few variations regarding their size distribution and surface biomarkers. However, the metabolic composition represents large-scale statistical diversity, revealing the inconsistency of various culture conditions.31 With the emergence of high EVs-output bioreactors, an increasing number of clinical trials have focused on the development of EVs as drug delivery vectors or therapeutic agents.32–34 Nevertheless, it is still unknown whether the current knowledge of EVs in vivo studies can be fully applied to bioreactor-derived vesicles, especially the effects on the metabolic processing of recipient cells.
To summarize the current research and progress related to EV metabolomics in clinical applications, we have used “Extracellular vesicles” and “Metabolomics” as the main search keywords. It should be noted, however, that lipids in organisms are classified as metabolites,35 and there is crosstalk between EV metabolomics and lipidomics,36 but the study of lipids in EVs has progressed more.37,38 Accordingly, we have combined the clinical progress both in EV metabolomics and lipidomics to enhance the content and integrity of this review.
However, despite a promising start into EV metabolomics, most studies to date have been preclinical tests. More clinical tests will be required in the future to provide, more extensive and verified knowledge. We intend to summarize the efforts made to investigate metabolites in EVs as biomarkers for purposes such as diagnosis and prognosis, to help promote EV metabolomics to have a more practical role in large-scale clinical environments.
In the following review, we begin by introducing the relationship between metabolites in EVs with diseases. We then generalize the existing programs related to EV metabolomics. The main applications addressed in this paper are as follows: a) diagnosis, b) therapy, c) detection of disease progression, d) prognosis and e) multi-omics research. We focus on understanding how the metabolomics of EVs improves clinical practice performance, to provide improved guidelines and reference data to improve the use of these techniques and contribute to a deeper understanding of the metabolomics in EVs.
How Do Extracellular Vesicles Play a Key Role in Multiple Diseases?
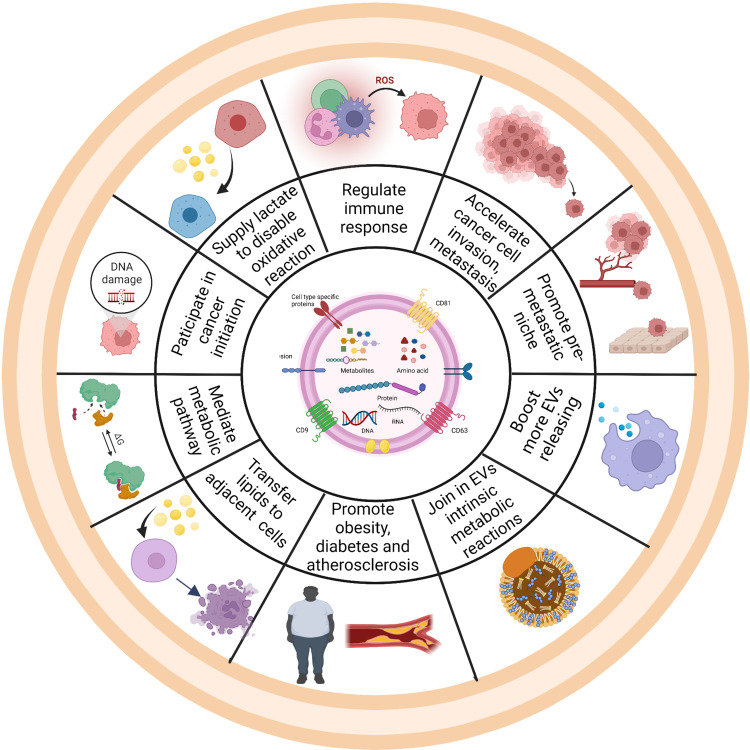
As carriers, EVs play vital roles in intracellular communication, especially through their encapsulated cargos, which includes DNA, various RNA species and proteins.39 In this part, we will focus specifically on the effects of metabolites in EVs on cancer, metabolic diseases, inflammation and neurological disorders (Figure 2).
Figure 2.
Schematic representation of the impact of metabolites in EVs on the hallmarks of different diseases. Ten hallmark capabilities are as follows: accelerating cancer invasion and metastasis,51 supplying lactate and lipids,44 regulating immune response,45 promoting pre-metastatic niche,46–50 mediating metabolic pathway,56 boosting more EVs releasing,40–42 joining in EVs intrinsic metabolic reactions,48 promoting obesity and atherosclerosis,50 transferring lipids to adjacent cells and initiating cancer development.58 Created with BioRender.com.
How Critical are Extracellular Vesicles in Cancerous Growth?
EVs are important mediators of cargo delivery and intracellular communication, which is crucial for the progression of cancer. Furthermore, when normal cells converse into cancer cells, an increased number of EVs are secreted.40–42 Exosomes can deliver multiple signaling molecules to their targets, which leads to reprogramming of cell biological processing. More importantly, it also accelerates the invasion and distant metastasis of tumors.1,43 In tumor microenvironment (TME), cancer-associated fibroblasts (CAFs), macrophages, T cells, dendritic cells (DC), natural kill cells (NKs) and tumor cells can secret EVs to crosstalk with each other. Most metabolites are actually source of nutrients and redistributed through EV-mediated-transport protein expression to increase uptake of cancer-required nutrients, such as the upregulation of glucose and lactate transporter, which includes GLUT1, and MCT1&4.44 For instance, studies of prostate and pancreatic cancers have provided information on CAFs and how they rearrange the metabolic pathways in cancers. The results provide compelling evidence that CAF-derived EVs act as a supply of lactate, amino acids and lipids to disable mitochondrial oxidative processing and promote glycolysis in cancer, thereby maintaining normal tumor proliferation. Furthermore, as tumors are developing, their requirement for energy is increasing, and consequently, they can alter the metabolic patterns to meet the increasing energy demands. The cargos carrying in EVs from surrounding cells and tissues, even if under nutrient deprivation conditions, can enable cancer cells to continue growing and differentiating. This could help to improve our understanding as to why cancer cells maintain rapid proliferation, while the tumor environment maintains an immunosuppressive status with low glucose and low PH.45 Furthermore, EVs participate in the induction of angiogenesis, regulating immune responses and forming a pre-metastatic niche to promote cancer-targeted metastasis.46–50 The vesicles have been confirmed to carry out intrinsic metabolic reactions. Fonseca et al have pointed out that nanoparticles are capable of glycolysis through internal metabolic enzymes to synthesize adenosine triphosphate.48 Thus, after being secreted by cancer cells, EVs can produce special substances that do not exist in primary cells, which may enhance tumor malignancy.
Considering EVs from the same donor cells, their inside cargos can be evidently distinct from each other under different conditions. Kudo et al have further found that before reaching their targets, hydrolysis function of secreted phospholipase A2 (sPLA2) can be used to verify the structures and functional characteristics of EVs. This novel point breaks the dogma that during the transfer from their parental cells to recipient destination, the particles do not exhibit any extracellular modifications and that they only display their functional characteristics after reaching target cells. It has indicated that several biological reactions are simultaneously taking place during the transfer process. The above research is also the first in vivo study to identify sPLA2 as a hydrolytic platform to accelerate tumor progression and has been investigated as a new prognostic biomarker and therapy target for diffuse large B cell lymphoma.51
In short, EVs can modulate the physical reactions of recipient cells and contribute substantially to cancer initiation, progression and invasion, beyond the delivery of various surface and intracellular metabolites of donor cells. EVs also have their own metabolic pathways to alter which they want to contain.
How Important are Extracellular Vesicles in Metabolic Diseases?
An increasing number of studies have declaimed the participation of EVs in metabolic syndromes. For example, flow cytometry has been used to show that plasma-EVs are increased in diabetes and non-alcoholic fatty liver disease (NAFLD), especially in platelet-derived EVs.52–54 Studies have highlighted the lipid droplet protein perilipin A as a promising marker to identify the stress intensity in adipose tissue, which is found to be downregulated 35% in reduced calorie diets.55 This indicates that perilipin A may activate the “find me” signal of macrophages to promote more immune cell migration. Of note, metabolic diseases are often associated with various diseases. EVs may function as mediators to bridge the relationship. Circulating exosomes are found to concurrently facilitate multiple dependent issues. For example, in vitro experiments show that EVs can absorb free fatty acid (FFA) directly from serum and transmit it to cardiac cells, which greatly mediates the relationship between obesity and heart damage. The results were also observed in mouse models, which suggest the involvement of EVs in progression and crosstalk of obesity, diabetes and atherosclerosis.50
How Notable are Extracellular Vesicles in Inflammatory Disease?
As suggested, researches on the relationship between immune and inflammatory diseases and EVs have poorly focused on metabolomics, and the situation is similar for the next part of neurological study. Only a lipidomics analysis found that after applying lipopolysaccharide (LPS) to induce acute lung injury (ALI) mice, more EVs have been observed to release into lung interstitium. These vesicles mainly come from alveolar epithelial cells, macrophages and neutrophils. The characteristic change is the carrying of omega-3 and omega-6 polyunsaturated fatty acids (PUFAs). Furthermore, when alveolar macrophages stay in different inflammatory conditions, the content of eicosanoic acid, arachidonic acid, cytosolic phospholipase A2, and cyclooxygenase in EVs will change diversely.56 The results reveal that inflammation can mediate metabolic pathway changes in EVs. Meanwhile, molecules in EVs can also regulate cellular inflammation. Just take the study of COVID-19 for example, Song et al have suggested that the plasma of COVID-19 patients is enriched in monsialodihexosyl ganglioside (GM3)-exosomes. However, GM3 has been reported to target CD4+T cells. GM3-exosomes may contribute to the immunosuppressive environment. Hence, we can conclude that metabolome in exosomes partakes in pathological processes related to inflammation.57
How Considerable are Extracellular Vesicles in Nervous System Diseases?
As we know, it is not easy to obtain samples from brain when compared with other tissue sources, and this creates difficulties in the diagnosis and development of neurological diseases.14 Due to their smaller size and special surface structures, extracellular vesicles can smoothly transfer across the blood–brain barrier, providing a unique and non-invasive tool to obtain brain tissues.4 Hence, they have been gradually recognized as indispensable source to tell distinct pathological changes for nervous system, as well as in drug delivery system.32 Metabolites from EVs are reported to produce remarkable effects on nervous system disorders. For instance, Scesa et al have discussed that nerve cells promote EV release to restore cellular homeostasis in order to clear accumulated lipids, which is consistent with the initial understanding of EVs. However, they also found that these sphingolipid-loaded EVs actually act as conveyor belts, to transfer lipids to unaffected cells, resulting in a pathological state of non-cell-autonomous defects.58 Ceramide is a rich component on the surface of EV membrane. In Alzheimer’s disease, amyloid beta 1-42 (Ab1-42) can make astrocyte derived-EVs carry more ceramide and promote apoptosis of surrounding cells.59 To the best of our knowledge, most of the current understanding of neurological diseases is based on genome, transcriptome and proteome, such as the data for glioma,60 brain injury,61 Alzheimer’s disease,62,63 and Parkinson’s disease.64 Nevertheless, the analysis of the metabolome in EVs is still in its infancy. Considering evident potential for identifying multiple pathological reactions in the brain, EV metabolomics should be the focus of many future investigations.
How Potential of Metabolomics of Extracellular Vesicles in Clinical Diagnostic Applications?
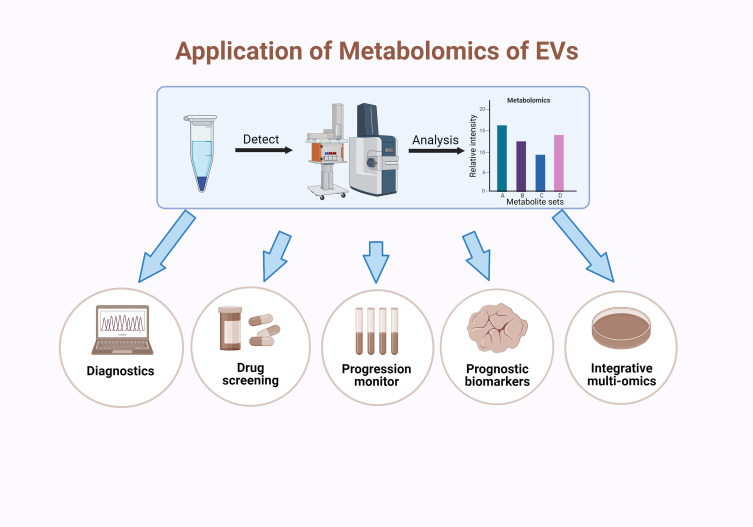
There have been several preclinical researches, which are basic or proof-of-concept studies, that have focused on the clinical potential of EV metabolomics.27,30 Most researches on metabolomics of EVs have attempted to investigate their promising roles as diagnostic biomarkers because of their heterogeneous profiles, which is of great importance for the definition of multiple diseases and identification of the specific illness stages. The detection of EVs is a non-invasive liquid biopsy, so it has consequently attracted a lot of interest. Moreover, EV-harbored cargoes do not come from debris of cellular compositions randomly, but are sorted by a strict separating system, making EVs more informative (Figure 3).65
Figure 3.
Application of EV metabolomics. The analysis of EVs metabolomics usually requires for several steps, including isolation of EVs, mass spectrometry analysis of inner metabolites and analysis of the metabolic maps. Metabolite analysis mainly depends on the distribution of species and the relative intensity of their content.26–28 After these steps, EVs metabolomics can apply to diagnosis,89–98 drug efficacy screening,99–104 disease progression monitoring,51,105–108 identifying prognostic biomarkers43,47,109,110 and cooperating with other omics.83,111,112 Created with BioRender.com.
Where Do the Sources of Extracellular Vesicles Often Used in Metabolomics Come from?
Metabolomics of EVs can be conducted on a diverse range of biomaterials, such as newly acquired or properly preserved clinical or experimental animal samples, and cells or cell lines cultured in vitro. Different sample sources have their various EV separation methods and corresponding metabolite analysis techniques, which have previously been summarized in the literature.30
As for the clinical specimens, blood should be specifically divided into plasma, serum and blood sources. As plasma is often collected in clinical researches, most detected EVs come from plasma when compared with serum and whole blood. In 2016, Altadill et al have detected the metabolites in EVs from plasma. According to the study, they have been the first group to come up with a critical protocol that can be generally used in EV metabolomics through high-resolution mass spectrometry.66 Plasma-derived EVs have also been detected to enable quantitative metabolic profiling for the diagnosis of acute pancreatitis and differentiate EV subtypes by proteomics and metabolomics.67,68 In addition, for patients with ST short elevation myocardial infarction components, plasma EVs were assessed to identify biomarkers and signal transduction mechanisms related to myocardial ischemia.69 Meanwhile, Zhao et al focused on pregnant women and implemented lipidomics to plasma EVs in order to build a preterm birth prediction model.70 Likewise, mouse plasma EVs can also be detected after exposure to ionizing radiation in the brain for metabolomics.71 Recently, an effective EV capture system using Titanium Dioxide microparticles has been designed to facilitate plasma-based liquid biopsies to understand the metabolic signatures of diabetic retinopathy.72
Serum-derived EVs are essential not only for bipolar disorder research,73 they also provide anti-inflammatory substances and antibiotics to COVID-19 patients,74 as well as in Alzheimer’s disease.50,75 Due to the complexity of whole blood substances, blood-derived EVs are rarely used. There is just one study performed on the identification, diagnosis, and prognosis of breast cancer.47
Urine-derived EVs have primarily been studied in relation to the kidney and prostate.76,77 For example, after applying antioxidant Tempol to mice with salt-sensitive hypertension, EVs were used to clarify lipid changes in urine,78 and they were also used intensively as a diagnostic biomarker for prostate cancer (PCa).79 As for the changes in prostate cancer patients before and after prostatectomy, Puhka et al have compared and profiled urinary EVs from the patients and healthy controls.80 Urine-derived EVs have also been applied to investigate the variation in cardiovascular disease, which identifies novel cardiovascular risk signatures.81
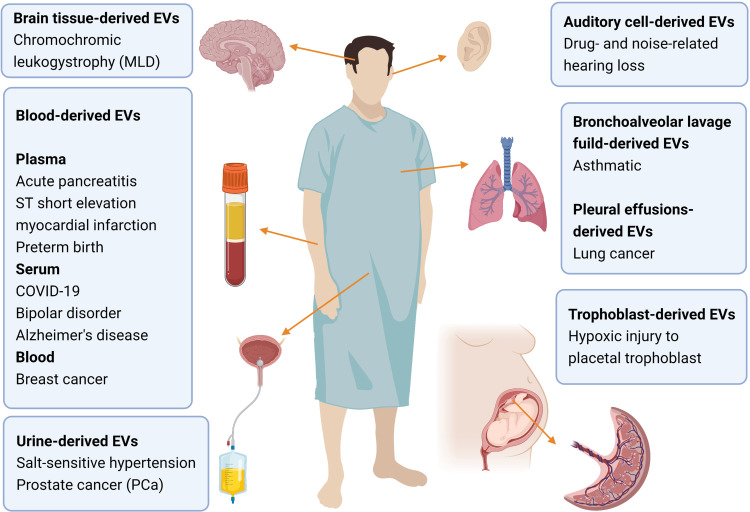
For respiratory diseases, EVs from bronchoalveolar lavage fluid are widely used for purposes, such as comparing lipid between asthmatic patients and healthy people.49 Brain tissue-derived EVs include samples collected in chromochromic leukodystrophy (MLD) and control mice.82 Cohn et al isolated microglia CD11b-positive small EVs from cryopreserved human brain tissue.83 Hypoxic injury to placental trophoblasts has also been assessed by measuring the membrane viscosity of trophoblast microvesicles.84 A study has used T cell surface-specific CD3 to isolate T cell derived-EVs. Through proteomics and lipidomics analysis, the study has verified some known characteristics of lymphocytes and revealed possible markers indicating the activation of plasma T cells.85 Some researches also highlight the use of EVs from auditory cells (Figure 4).86
Figure 4.
Schematic of the diverse sources involved in the research of metabolomics of EVs and the associated diseases. The source types include brain tissue, blood, bronchoalveolar lavage fluid, pleural effusion, urine and trophoblast. Brain tissue has been researched on chromochromic leukodystrophy (MLD).82 Blood can be specifically divided into plasma, serum and blood. Plasma has been applied to acute pancreatitis,67,68 ST short elevation myocardial infarction,69 preterm birth.70 For serum-derived EVs are notable in bipolar disorder,73 COVID-19,74 Alzheimer’s disease,50,75 The whole blood has been displayed on breast cancer.47 Urine tissue are always applied to research on kidney and prostate, such as salt-sensitive hypertension,78 and prostate cancer (PCa).79 Auditory cell-derived EVs has been utilized in drug- and noise-related hearing loss.86 For respiratory system, bronchoalveolar lavage fluid-derived EVs are conducted on asthmatic.49 In addition, lung cancer can be studied based on pleural effusions-derived EVs.90 Moreover, trophoblast-derived EVs can be used to evaluate hypoxic injury to placental trophoblast.84 Created with BioRender.com.
It is not difficult to conclude that samples come from a wide-range system. Blood-derived EVs are the most widely used. Special sources of EVs, such as urine and bronchoalveolar lavage fluid, are mainly utilized in their specific systems. Of note, for patients with neurological disease and pregnant women, invasive tissue biopsies are not suitable for them, which provide a new function and expand broad prospects for EVs.
How Metabolomics of Extracellular Vesicles Used in Diagnosis of Metabolic and Cardiovascular Diseases?
It has been strongly suggested that the EVs released by cardiac cells and tissues contribute to angiogenesis, cellular proliferation, and maturation. Different pathological states of the cardiac cells result in various nanoparticle compositions. Take adipose cells, for example, Shalaby et al elucidated C16:0 ceramide and their derivative lipids in vesicles were the most secreted metabolites from adipose tissue, suggesting that this molecule displays a major role in cardiovascular redox status in obese patients. At the same time, C16:0 ceramide is directly related to the late myocardial necrosis of atherosclerotic individuals,87 which suggests novel drug targets and new insights into the pathogenesis. Except for the implementation of metabolomics in chronic diseases mentioned above, it reportedly benefits the diagnosis and treatment of other acute ones, such as acute pancreatitis (AP). The recent research has compared metabolic profiling of plasma EVs from patients with different severities of AP, including healthy controls, people with severe AP and those with mild AP. Consequently, four potential biomarkers have been determined to serve as candidates for early diagnosis and severity differentiation of AP, including eicosatrienoic acid (C20:3), thiamine triphosphate, 2-Acetylfuran, and cis-Citral. Furthermore, it has given new thought into the possible treatment ideas for AP.68 Furthermore, Weingrill et al found that plasma EVs could also be used to provide an initial assessment of placental function and to develop diagnosis, treatment, and prognosis of gestational hypertension.88 Notably, for ST elevation myocardial infarction, evidence has demonstrated that plasma EVs in patients contain more lipid molecules, revealing possible biomarkers and signal transduction mechanisms.69
How Metabolomics of Extracellular Vesicles Used in Diagnosis of Cancers?
How Metabolomics of Extracellular Vesicles Used in Diagnosis of Lung Cancer?
Up to now, in the world ranking of cancer-related deaths, lung cancer still remains first. Implementing more sensitive screening programs to improve the diagnosis of lung cancer is one of the major steps to reduce mortality.89 Despite the use of bacteriological and histopathological methods, the delayed or missed diagnosis cannot yet be completely avoided, especially in patients loaded with lower dose of M. tuberculosis and fewer tumor cells. Thus, more sensitive approaches need to be developed. Our previous study has attempted to use large and small EVs from pleural effusions of patients diagnosed with tuberculosis or lung cancer to clarify the metabolic profiling and reprogrammed reactions in these two diseases, with a total of 579 metabolites analyzed. We have discovered that the metabolite profiling of vesicles and pleural effusions shows extremely distinct distribution. Through comprehensive detection and validation workflow, phenylalanine, leucine, phosphatidylcholine 35:0 and sphingomyelin 44:3 have been determined as novel marker candidates for distinguishing tuberculosis from lung cancer.90 We have provided a new metabolic perspective to discriminate lung cancer from benign nodules. In another similar study which gathered serum EVs from patients with lung cancer and pulmonary nodules, a high degree of heterogeneity in lipid profiles was explored. The study focused on hundreds of patients including patients free of nodules or diagnosed with benign nodules and those with lung cancer. Then, a targeted mass spectrometry was then utilized to detect 352 lipids. Ceramides have exhibited up-regulated expression in cancer cell vesicles, with a concomitant decrease in phosphatidylcholine and polyunsaturated acyl chains. Significant differences in EVs and serum lipid profiles were also observed.91 Accordingly, these researches have demonstrated the sensitivity of metabolic markers to clarify malignant cancer, especially for highly heterogeneous nodules when using low-dose computed tomography (CT). However, more information and tests are needed to promote the availability of the technology.92 It seems a common problem that these studies both lack large populations to externally validate the results. This is a limitation for EV metabolomics to apply for clinical testing program.
How Metabolomics of Extracellular Vesicles Used in Diagnosis of Prostate Cancer?
As is well known, testing for prostate-specific antigen (PSA) in blood is often conducted as a preliminary screening experiment for potential PCa patients.93 Nowadays, a growing number of urine specimens have been investigated to determine new markers for their availability to obtain.94 However, due to the dilution of renal tubules, not all the biomarkers can be detected by current technologies. Thus, it requires more concentrated samples. Given the generative process of EVs and presentation in all body fluids, EVs can provide more concentrated molecules to improve the detection of PCa biomarkers.95 For example, Skotland et al compared the urinary EVs from healthy individuals with those from PCa samples. They found nine distinctly expressed lipids that could be potential markers for PCa.79 Likewise, a recent study has analyzed the metabolite difference between PCa and benign prostate hyperplasia (BPH) patients. Seventy-six of the detected metabolites exhibited significant differential abundance, supporting the use of urinary EVs as a method to clarify the metabolic profile of PCa.31
Some studies also compared EVs from lymphoma and colorectal cancer cell lines. They found that cancer-derived EVs have higher levels of amino acids and B vitamins, which can be called “metabolic fingerprints”. In the future, how to deeply understand the role of “metabolic fingerprints” and pursue more applications of EV metabolomics in cancer deserves further research.96
How Metabolomics of Extracellular Vesicles Used in Diagnosis of Nervous System Diseases?
Increasing studies have focused on how the metabolites in EVs perform in nervous system disorders. In a related study, Du et al conducted a large multicenter project to identify 15 exosome-derived substances in schizophrenia. These markers were determined to have excellent performance in differentiating bipolar disorder (BD), major depressive disorder and schizophrenia. In early stages, the symptoms of these diseases are mild and similar to each other, resulting in difficulty in classification. To deal with this problem, EVs can be developed as possible diagnostic biomarkers. Several bioreactions have been revealed to exhibit great importance during the pathogenesis, including galactose biochemical processing, amino sugar and nucleotide sugar reactions, and the mutual interconversion between pentose and glucuronic acid.73 This is consistent with a previous study of multi-pathway alterations in BD plasma metabolomics.97 This has strongly suggested that the dysfunction of glucose metabolism is closely related to the occurrence and deterioration of BD.
In another study, untargeted lipidomic analysis has been utilized to identify EVs in brain tissue of MLD mice. This work specifically noted that when compared with control mice, EVs’ size and protein concentration were not significantly different in MLD mice. However, the sulfides of short fatty acyl chains were significantly increased.82 Therefore, we can identify that lipidomics may present unique advantages in diagnosing neurological diseases and following pathological change. However, human samples are still required to verify and supplement this assumption. In 2016, Moyano et al identified EV changes in plasma of patients with multiple sclerosis, characterizing the increase of C16:0 sulfatide in small vesicles.98
Overall, these studies have fully indicated that implementing metabolomics of EVs to neurological disorders is a feasible approach to improve early diagnosis and treatment for these diseases.
How Potential of Metabolomics of Extracellular Vesicles for Disease Treatment?
As has been suggested, many endogenous small molecules can directly participate in various forms of metabolism. To a certain extent, their levels can reflect the function and state of the biochemical metabolism in the body, thus helping to discover new drug targets. For example, the data of auditory cells suggest that large and small vesicles contain diverse concentrations of eicosanoids. PUFAs are more efficiently loaded into small vesicles. PUFAs promote the production of pro-inflammatory agents in vesicles, alter the content composition, and influence the effect of drugs.86 EV metabolomics has also been applied to study human pharmacokinetics. After taking resveratrol, healthy subjects have been found to secrete more EVs in plasma. Furthermore, the metabolites encapsulated in EVs are concentrated and consist of only nine resveratrol derivatives, not all the seventeen kinds of resveratrol metabolites. Moreover, the metabolites from the intestinal microbiota derived EVs were higher than those of the plasma, suggesting a higher intestinal entrapment rate for resveratrol. This provides a new direction and pathway to clarify the internal transport of drugs.99 Many studies have been devoted to the use of EVs for the encapsulation of drugs.100 However, it is not yet clear whether the metabolites primarily in EVs will affect the drug encapsulation and therapy efficacy. With the increasing number of tumor metabolism inhibitors and drugs for metabolic diseases receiving a larger amount of attention and applying to clinical trials,101,102 here comes more demand for real-time monitoring of drug efficacy to acquire large-scale metabolomic characterization. Exosomes are widely used for drug delivery because of their excellent loading capacity and natural targeting potential. However, their targeting effect is not as satisfactory as previously predicted. To optimize targeting advantages, metabolomics analysis was applied to know the specific lipid components on the surface of EVs. According to the structure, proteins can also be attached to lipid molecules on EVs to increase cell uptake and drug delivery efficiency (Table 1).103
Table 1.
Clinical Use of Metabolomics of EVs in Diagnosis and Therapy
| Scheme | Diseases | Source of EVs | Metabolites Studied | Ref. |
|---|---|---|---|---|
| Diagnosis | Late myocardial necrosis of atherosclerotic | Adipose tissue | C16:0 ceramide and their derivatives | [87] |
| Acute pancreatitis | Plasma | Eicosatrienoic acid (C20:3), thiamine triphosphate. 2-Acetylfuran, cis-Citral | [68] | |
| Gestational hypertension | Plasma | SM, acetyl CoA, methionine, phenylalanine, tryptophan, carotenoids, tyrosine, arginine, leucine | [88] | |
| ST elevation myocardial infarction | Plasma | Ceramides, dihydroceramides, and sphingomyelins | [69] | |
| Lung cancer | Pleural effusions | Phenylalanine, leucine, PC 35:0 and SM 44:3 | [91] | |
| Serum | Ceramides, PC and polyunsaturated acyl chains | [92] | ||
| Prostate cancer | Urinary | PS 18:1/18:1 and lactosylceramide (d18:1/16:0) | [93] | |
| Benign prostate hyperplasia | Urinary | PC, PE, SM | [31] | |
| Bipolar disorder | Serum | lysoPE 18: 0, lysoPE 14: 0, lysoPC 18: 0, and lysoPC 20: 1 | [73] | |
| Schizophrenia | ||||
| Depressive disorder | ||||
| Chromochromic leukogystrophy | Brain tissue | sulfides of short fatty acyl chains | [82] | |
| Multiple sclerosis | Plasma | C16:0 sulfatide | [98] | |
| Therapy | Drug- and noise-related hearing loss | Auditory cells | Eicosanoids | [104] |
| Advanced Alzheimer’s disease | Microglia | Cholesterol, docosahexaenoic acid (DHA) | [83] |
Abbreviations: PE, phosphatidylethanolamine; PI, phosphatidylinositol; PC, phosphatidylcholine; PS, phosphatidylserine; PA, phosphatidic acid; SM, sphingomyelins.
Accordingly, a comprehensive understanding of the composition of EVs would be indispensable, as it would help to carefully specify the type and quantity of encapsulated drugs and to ensure they can successfully exert their desired therapeutic effects.104 The role of EV metabolomics in reflecting the efficacy of novel metabolic treatments deserves further attention.
What is the Potential of Metabolomics of Extracellular Vesicles in Monitoring Disease Progression?
Although there is overwhelming evidence indicating the subtype and arrangement of EVs’ molecules and structuresto date, only a few metabolites related biomarkers have been studied to monitor disease progression.
For instance, metabolomic testing has been performed to screen for biomarkers in rectal cancer patients, aiming to assess the neoadjuvant chemotherapy response.105 What is more, this research also compared the sensitivity of proteins and lipids as biomarkers. At present, there are numerous clinical studies screening differential markers, while only a few studies have been conducted to compare the effectiveness between a single omics and a combination of multiple omics. It is hoped that future research will focus on this problem to help promote the clinical translation of EVs as a pathway for disease diagnosis. As for abdominal aortic aneurysm (AAA), Dang et al have demonstrated that activated T cell derived-EVs may deliver more PUFA phospholipids to macrophages. PUFAs can provide more substrate for redox imbalance and migration of macrophages, thus accelerating the progression of AAA. This result has revealed how T cells interact with macrophages and suggested a potential targeted therapeutic strategy for AAA.106 In addition, anti-inflammatory molecules (LysoPS, 7-α, 25-dihydroxycholesterol and 15-d-PGJ2) and coagulation (thromboxane and ellagic acid) related metabolites in EVs may play critical roles in reducing inflammation of COVID-19.74 EVs metabolomic analysis was also applied to monitor glioblastoma.107 Surprisingly, the technique has been practiced to measure the surface lipid composition and viscosity of the nanoparticles, and evaluate intrauterine hypoxic injury of placental trophoblasts. In short, the results suggest a potential strategy to characterize the health of fetal membranes during pregnancy, as well as to achieve real-time monitoring.84 In addition, five lipid markers can construct a predictive model to assess how high the risk of premature delivery is for pregnant women in the second trimester (12–24 weeks), facilitating early clinical intervention.70
As is known, ionizing radiation exposure induces structural and functional changes in the brain, associated with spinal density, inflammation, and cognitive decline. However, many of these pathological changes cannot be observed in the early stage of irradiation. Using metabolomic analysis of plasma EVs from short-term radiation-exposed mice can help identify minimally invasive biomarkers of ionizing radiation injury in the central nervous system. Together, all these results have stated the utility of EVs in the blood for early disease progression after ionizing radiation.71
In addition, EVs from paroxysmal nocturnal hemoglobinuria patients have been observed to contain pro-inflammatory metabolic substances such as prostaglandin F2-α (PGF2α). The molecules were found to increase thrombotic events in patients.108 In a recently published study on B lymphomas, Kudo et al showed that lymphoma-derived EVs were driven by sPLA2 to enhance lipid metabolism. sPLA2 hydrolyzed the membranes of EVs phospholipid bilayers, increasing the output of fatty acids, lysophospholipids and their derivatives in the blood. sPLA allows vesicles to change structural and functional properties before reaching target cells. These findings provide new avenues for cancer invasion and metastasis. Furthermore, lipids are more concentrated in EVs when compared with the parent origins.51 This has aroused the speculation that in future studies we can not only focus on changes in vesicle components but also compare differences between the primary cells and EVs, as this could help elucidate new mechanisms (Table 2).
Table 2.
Clinical Use of Metabolomics of EVs in Progression Monitor, Prognosis and Multi-Omics
| Scheme | Diseases | Source of EVs | Metabolites Studied | Ref. |
|---|---|---|---|---|
| Progression monitor | Rectal cancer | Serum | 129 metabolites | [86] |
| Abdominal aortic aneurysm | Activated T cell | PUFAs phospholipids | [106] | |
| COVID-19 | Serum | Anti-inflammatory molecules (LysoPS, 7-α, 25-dihydroxycholesterol and 15-d-PGJ2) and coagulation (thromboxane and ellagic acid) | [74] | |
| Glioblastoma | Glioblastoma Cell | Glycerol, tryptophan, carnitine and oxidized glutathione | [107] | |
| Intrauterine hypoxic injury of placental trophoblasts | Trophoblast | PE, PI, PC, PS, PA | [84] | |
| Preterm birth | Plasma | PS (34:0) | [70] | |
| Radiation injury in the central system | Plasma | Triglycerides, platelet activating factor, carnitine and C-16 sphinganine | [71] | |
| Paroxysmal nocturnal hemoglobinuria | Plasma | Prostaglandin F2-α (PGF2α) | [108] | |
| B lymphomas | Lymphoma cell | Secreted phospholipase A2 | [51] | |
| Prognosis | Breast cancer | Plasma | (lyso)-PCs and SM | [43,47] |
| Pancreatic cancer | Serum | LysoPC 22:0, PC (P-14:0/22:2) and PE (16:0/18:1) | [109] | |
| Non-small cell lung cancer | Neural stem/progenitor cell | SM | [110] | |
| Multi-omics | Neoadjuvant radiotherapy in patients | Serum | 129 metabolites | [105] |
| Advanced Alzheimer’s disease | Microglia | Cholesterol, docosahexaenoic acid (DHA) | [83] |
Abbreviations: PE, phosphatidylethanolamine; PI, phosphatidylinositol; PC, phosphatidylcholine; PS, phosphatidylserine; PA, phosphatidic acid; SM, sphingomyelins.
What is the Potential of Metabolomics of Extracellular Vesicles in Prognostic Evaluation?
The application of extracellular metabolomics for prognosis assessment is in its initial stage of development. There are only a few studies focusing on cancer prognosis and drug resistance. Rather than soluble plasma compounds, vesicles have been shown to mediate breast cancer invasiveness. The lipid molecules not only differentiate breast cancer patients from controls but also evaluate the future prognosis.43,47 Pancreatic cancer is a highly malignant tumor with poor prognosis due to the difficulties in achieving early diagnosis. EVs monitoring 61 clinical serum samples by LC-MS untargeted metabolomics have identified Lyso phosphatidylcholine (PC) 22:0, PC (P-14:0/22:2) and phosphatidylethanolamine (PE) (16:0/18:1). These bioactive molecules correlated with tumor stage, CA199, CA242 and tumor diameter. Furthermore, overall survival was significantly associated with the PE levels in vesicles (16:0/18:1).109
As for drug resistance, take non-small-cell lung cancers (NSCLCs), for example. EVs phospholipid composition was found to be significantly different in gefitinib-resistant cells and gefitinib-sensitive cells. The transition to an acquired resistance phenotype is accompanied by alterations in phospholipids that may be associated with gefitinib resistance. Accordingly, lipid profiles in EVs may associate with clinicopathological features of breast cancers, which can provide new ideas for predicting drug resistance to gefitinib in breast cancers (Table 2).110
How Does Metabolomics of Extracellular Vesicles Cooperate with Other Omics?
Recently, a considerable amount of literature has developed around the theme of multi-omics studies. Take combination of proteomics and metabolomics, for example, they have been applied to evaluate the effect of neoadjuvant radiotherapy in patients with advanced rectal cancer. Several proteins and metabolites relevant to the unexpected results have consequently been revealed, as well as the involved signaling pathway, such as immune response, complement activation cascade, and platelet functions.105 Studies based on radiation therapy have been described to alter exosome content, thus affecting unirradiated cells and mediating radiation tolerance.111 At the same time, through fecal microbial EVs, genomics and metabolomics were used to construct a colorectal cancer diagnostic model.112 As expected, a multicomponent approach has demonstrated that using different types of exosome production may potentially improve the specificity and sensitivity of exosome-based diagnostics.1 Likewise, Cohn et al used a combination of proteomics, targeted lipidomics, and NanoString nCounter technology to detect microglial EVs from advanced AD patients and normal/low pathology cases. The results have revealed 1000 proteins, 594 lipids, and 105 miRNAs, suggesting that new AD biomarkers may be derived from the above biomolecules.83
Of note, there exists a multi-omics research conducted on COVID-19, which concentrated on proteomics and lipidomics to clarify the differences among various stages of COVID-19. Their important results provide a deeper understanding and insights into metabolic dysregulation of COVID-19 patients.113 The encouraging results also indicate the promising role of EV metabolomics in clinical applications.
In the future, how to make metabolomics directly promote precise treatment is a problem worthy to be further discussed. Take cancer, for example, except for identifying risk factors, metabolomics of EVs can also realize screening, diagnosis and monitoring of cancers. At the same time, to regulate abnormal proliferation environment and interfere with the metabolism of cancer cells, several inhibitors have been used to target metabolic molecules or pathways. To date, there have only been a few clinical trials of metabolic enzyme inhibitors. In addition, metabolomics is expected to be used with other omics. We believe that, as more standardized schemes, relevant instruments and user-friendly analysis platforms develop and become more easily accessible, EV-based metabolomics will also develop and result in beneficial clinical applications.
What are the Problems and Challenges in Metabolomics of Extracellular Vesicles?
However, there still exist some limitations in our attempts to fully harness the potential of EV metabolomics. First, the lack of global consensus on standard protocols to extract and analyze EVs consistently remains an obstacle to comparing different results based on similar researches and data reproducibility. Although the International Society for Extracellular Vesicles (ISEV) in 2018 has described the protocols of EV isolation and characterization, there still lacks a consensus on how to choose appropriate isolation and characterization methods for various sources of EVs.2,18 Normalization methods obviously vary from different institutions. In addition, low recovery efficiency and EV contraction indicate that the isolation and detection methods require further development.114 Hence, we urgently need to build a unified and effective method to guarantee the reliability and efficiency of these studies.
In addition, the complexity of inner components impacts on the ability to identify reliable EV-metabolite biomarkers. Some studies found that regardless of the source of samples, the extraction and storage conditions of metabolites are pre-variables that will significantly affect the results.115–117 It also appeals for more details and standards on associated factors of metabolites treatment. More importantly, metabolites are highly diverse in chemical structure and composition. For instance, the main lipids on the membrane include glycerophosphingolipids, sphingolipids, and sterols (mainly cholesterol), which indicates great variation.118
Moreover, the identification and quantitative methods for metabolites are very limited. MS has become one of the most effective methods to study EV metabolomics,30 while there also exist some challenges for MS. The most challenging limitation of MS-based metabolomics is the lack of standardized analyzing protocols and consensus on spectrogram database, which can be utilized to compare differential metabolites.119 Specifically, the ability of MS to distinguish isomers has not yet reached a high level of accuracy, resulting in a low resolution of metabolites.120 In addition, due to the limitations of ion utilization and transmission efficiency, the detection sensitivity of MS is not sufficient to achieve a larger scale of metabolic profiling.121 Consequently, more effective methods need to be further developed to facilitate the application of EV metabolomics.
We should have to acknowledge that research on the metabolic profiling of vesicles is in the earliest stage of development. Much less information is available in clinic. With regard to the metabolites of EVs reported in studies, they are still in infancy. Although there is currently a prospective exosome‐focused translational research for afatinib with NSCLC patients (EXTRA study), that aims to identify predictive biomarkers of these treatment efficacy.122 That may not be enough. More clinical tests with large independent cohorts of patients need to be explored in the future, giving more extended and validated knowledge to this field, and to fully ascertain their sensitivity, specificity and accuracy.
The Future Prospectives
In summary, this review provides a comprehensive view of our current knowledge of metabolomics in EVs. We have focused on the relationship between diseases and metabolites via the nanovesicles, especially the various clinical practice of EV metabolomics. The main clinical applications include diagnosis, therapy, disease progression detection, prognosis, and multi-omics research. Understanding the throughout practice of metabolomics and previously established research will help us realize the current landscape and existing limitations of this technique. Although EV metabolomics appears to be promising for the future of disease diagnosis, therapy, and prognosis, to enable further clinical utilization, considerable effort will be required to address the challenges and problems that have occurred so far. Due to the vast heterogeneity and complexity of EVs, more standardized isolation and analytical guidelines should be constructed to guarantee the reliability of EVs-based research. As for metabolomics analysis, more sensitive and high-resolution methods should be provided to bring larger perspectives to decipher the inner metabolites of EVs. However, it is accompanied by the extreme lack of the clinical research. Hence, more clinical tests should be explored to provide meaningful and multidimensional information, to help cover the numerous limitations of EV-metabolomics research.
We believe that after properly addressing the current limitations and deficiency, EV metabolomics will achieve more breakthroughs for human health and disease management, as well as embrace more possibilities to acquire clinical transformation.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China [No.: 82070099, 81900095, 82001988, 82100112, 82102496], General Program and Health Commission of Hubei Province [No.: WJ2021M234, WJ2021M226]. We thank the National Natural Science Foundation of China, General Program and Health Commission of Hubei Province. We appreciate the contribution of the online tool BioRender (https://biorender.com/) to help us create excellent drawings.
Abbreviations
EVs, extracellular vesicles; CAFs, cancer-associated fibroblasts; sPLA2, secreted phospholipase A2; NAFLD, non-alcoholic fatty liver disease; FFA, free fatty acid; LPS, lipopolysaccharide; ALI, acute lung injury; PUFAs, polyunsaturated fatty acids; GM3, monsialodihexosyl ganglioside; MLD, chromochromic leukodystrophy; PCa, prostate cancer; AP, acute pancreatitis; CT, computed tomography; PSA, prostate-specific antigen; BD, bipolar disorder; AAA, abdominal aortic aneurysm; PGF2α, prostaglandin F2-α; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PC, phosphatidylcholine; PS, phosphatidylserine; PA, phosphatidic acid; SM, sphingomyelins; NSCLCs, non-small-cell lung cancers; AD, Alzheimer’s diseases; TME, tumor microenvironment; DC, dendritic cells; NKs, natural kill cells.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interests in this work.
References
- 1.Kalluri R, LeBleu V. The biology function and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nam G, Choi Y, Kim G, Kim S, Kim S, Kim I. Emerging Prospects of Exosomes for Cancer Treatment: from Conventional Therapy to Immunotherapy. Adv Mater. 2020;32(51):e2002440. [DOI] [PubMed] [Google Scholar]
- 6.Thietart S, Rautou P. Extracellular vesicles as biomarkers in liver diseases: a clinician’s point of view. J Hepatol. 2020;73(6):1507–1525. [DOI] [PubMed] [Google Scholar]
- 7.Bebelman M, Smit M, Pegtel D, Baglio S. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. [DOI] [PubMed] [Google Scholar]
- 9.Gratpain V, Mwema A, Labrak Y, Muccioli G, van Pesch V. Extracellular vesicles for the treatment of central nervous system diseases. Adv Drug Deliv Rev. 2021;174:535–552. [DOI] [PubMed] [Google Scholar]
- 10.Channon L, Tyma V, Xu Z, et al. Small extracellular vesicles (exosomes) and their cargo in pancreatic cancer: key roles in the hallmarks of cancer. Biochim Biophys Acta Rev Cancer. 2022;1877(3):188728. [DOI] [PubMed] [Google Scholar]
- 11.Han C, Yang J, Sun J, Qin G. Extracellular vesicles in cardiovascular disease: biological functions and therapeutic implications. Pharmacol Ther. 2022;233:108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sáez T, Toledo F, Sobrevia L. Impaired signalling pathways mediated by extracellular vesicles in diabesity. Mol Aspects Med. 2019;66:13–20. [DOI] [PubMed] [Google Scholar]
- 13.Shehzad A, Islam S, Shahzad R, Khan S, Lee Y. Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol Ther. 2021;223:107806. [DOI] [PubMed] [Google Scholar]
- 14.Arifin DR, Witwer KW, Bulte JWM. Non-Invasive imaging of extracellular vesicles: quo vaditis in vivo? J Extracell Vesicles. 2022;11(7):e12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullah M, Kodam SP, Mu Q, Akbar A. Microbubbles Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano. 2021;15(3):3612–3620. [DOI] [PubMed] [Google Scholar]
- 16.Yuana Y, Koning RI, Kuil ME, et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles. 2013;2:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019;20(6):353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng JY, Xiang XQ, Ding LW, et al. Extracellular vesicles, the cornerstone of next-generation cancer diagnosis? Semin Cancer Biol. 2021;74:105–120. [DOI] [PubMed] [Google Scholar]
- 19.Gezsi A, Kovacs A, Visnovitz T, Buzas EI. Systems biology approaches to investigating the roles of extracellular vesicles in human diseases. Exp Mol Med. 2019;51(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int J Mol Sci. 2017;18(6):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernie A, Trethewey R, Krotzky A, Willmitzer L. Metabolite profiling: from diagnostics to systems biology. Nat Rev Mol Cell Biol. 2004;5(9):763–769. [DOI] [PubMed] [Google Scholar]
- 22.Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME. Extracellular Vesicle Quantification and Characterization: common Methods and Emerging Approaches. Bioengineering. 2019;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Covarrubias V, Martinez-Martinez E, Del Bosque-Plata L. The Potential of Metabolomics in Biomedical Applications. Metabolites. 2022;12(2):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amatya SB, Salmi S, Kainulainen V, Karihtala P, Reunanen J. Bacterial Extracellular Vesicles in Gastrointestinal Tract Cancer: an Unexplored Territory. Cancers. 2021;13(21):5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexandrov T, Ovchinnikova K, Palmer A, et al. METASPACE: a community-populated knowledge base of spatial metabolomes in health and disease. bioRxiv. 2019;1;539478. [Google Scholar]
- 26.Zhu QF, Huang YJ, Yang QS, Liu F. Recent technical advances to study metabolomics of extracellular vesicles. Microchem J. 2021;171:106816. [Google Scholar]
- 27.Zebrowska A, Skowronek A, Wojakowska A, Widlak P, Pietrowska M. Metabolome of Exosomes: focus on Vesicles Released by Cancer Cells and Present in Human Body Fluids. Int J Mol Sci. 2019;20(14):3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alseekh S, Aharoni A, Brotman Y, et al. Mass spectrometry-based metabolomics: a guide for annotation, quantification and best reporting practices. Nat Methods. 2021;18(7):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seydel C. Single-cell metabolomics hits its stride. Nat Methods. 2021;18(12):1452–1456. [DOI] [PubMed] [Google Scholar]
- 30.Williams C, Palviainen M, Reichardt NC, Siljander PRM, Falcon-Perez JM. Metabolomics Applied to the Study of Extracellular Vesicles. Metabolites. 2019;9(11):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palviainen M, Saari H, Kärkkäinen O, et al. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J Extracell Vesicles. 2019;8(1):1596669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pirisinu M, Pham T, Zhang D, Hong T, Nguyen L, Le M. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: recent advances, current obstacles, and challenges for clinical translation. Semin Cancer Biol. 2022;80:340–355. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9(4):1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Tan Q, Yang Z, Jin Y. Engineered extracellular vesicles: potentials in cancer combination therapy. J Nanobiotechnology. 2022;20(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skotland T, Sagini K, Sandvig K, Llorente A. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308–321. [DOI] [PubMed] [Google Scholar]
- 36.Masoodi M, Gastaldelli A, Hyotylainen T, et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835–856. [DOI] [PubMed] [Google Scholar]
- 37.Wu ZJ, Bagarolo GI, Thoroe-Boveleth S, Jankowski J. ”Lipidomics”: mass spectrometric and chemometric analyses of lipids. Adv Drug Deliv Rev. 2020;159:294–307. [DOI] [PubMed] [Google Scholar]
- 38.Baek J, He CC, Afshinnia F, Michailidis G, Pennathur S. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol. 2022;18(1):38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaac R, Reis F, Ying W, Olefsky J. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Achreja A, Zhao H, Yang L, Yun TH, Marini J, Nagrath D. Exo-MFA - A 13C metabolic flux analysis framework to dissect tumor microenvironment-secreted exosome contributions towards cancer cell metabolism. Metab Eng. 2017;43(Pt B):156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma R, Huang X, Brekken RA, Schroit AJ. Detection of phosphatidylserine-positive exosomes for the diagnosis of early-stage malignancies. Br J Cancer. 2017;117(4):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - A new regulatory pathway. Eur J Cancer. 2014;50(5):1025–1034. [DOI] [PubMed] [Google Scholar]
- 43.Menck K, Bleckmann A, Wachter A, et al. Characterisation of tumour-derived microvesicles in cancer patients’ blood and correlation with clinical outcome. J Extracell Vesicles. 2017;6(1):1340745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Niel G, Carter D, Clayton A, Lambert D, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369–382. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016;5:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iraci N, Gaude E, Leonardi T, et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13(9):951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buentzel J, Klemp HG, Kraetzner R, et al. Metabolomic Profiling of Blood-Derived Microvesicles in Breast Cancer Patients. Int J Mol Sci. 2021;22(24):13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonseca P, Vardaki I, Occhionero A, Panaretakis T. Metabolic and Signaling Functions of Cancer Cell-Derived Extracellular Vesicles. Int Rev Cell Mol Biol. 2016;326:175–199. [DOI] [PubMed] [Google Scholar]
- 49.Hough KP, Wilson LS, Trevor JL, et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci Rep. 2018;8(1):10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia NA, González-King H, Grueso E, et al. Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: possible role of CD36. PLoS One. 2019;14(5):e0217546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudo K, Miki Y, Carreras J, et al. Secreted phospholipase A modifies extracellular vesicles and accelerates B cell lymphoma. Cell Metab. 2022;34(4):615–633.e8. [DOI] [PubMed] [Google Scholar]
- 52.Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192(2):268–275. [DOI] [PubMed] [Google Scholar]
- 53.Crewe C, Joffin N, Rutkowski JM, et al. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell. 2018;175(3):695–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eguchi A, Lazic M, Armando AM, et al. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl). 2016;94(11):1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nirujogi TS, Kotha SR, Chung S, et al. Lipidomic Profiling of Bronchoalveolar Lavage Fluid Extracellular Vesicles Indicates Their Involvement in Lipopolysaccharide-Induced Acute Lung Injury. J Innate Immun. 2022;14(5):555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song J, Lam S, Fan X, et al. Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metab. 2020;32(2):188–202.e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scesa G, Moyano AL, Bongarzone ER, Givogri MI. Port-to-port delivery: mobilization of toxic sphingolipids via extracellular vesicles. J Neurosci Res. 2016;94(11):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gratpain V, Mwema A, Labrak Y, Muccioli GG, van Pesch V. Extracellular vesicles for the treatment of central nervous system diseases. Adv Drug Deliv Rev. 2021;174:535–552. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Liu J, Sun G, et al. Glioblastoma extracellular vesicles induce the tumour-promoting transformation of neural stem cells. Cancer Lett. 2019;466:1–12. [DOI] [PubMed] [Google Scholar]
- 61.Goetzl EJ, Yaffe K, Peltz CB, et al. Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. FASEB J. 2020;34(2):3359–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. 2016;3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polanco JC, Li C, Durisic N, Sullivan R, Götz J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol Commun. 2018;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaudhuri AD, Dastgheyb RM, Yoo S-W, et al. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018;9(3):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang D, Zhang W, Zhang H, et al. Progress, opportunity, and perspective on exosome isolation efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altadill T, Campoy I, Lanau L, et al. Enabling Metabolomics Based Biomarker Discovery Studies Using Molecular Phenotyping of Exosome-Like Vesicles. PLoS One. 2016;11(3):e0151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan F, Xiang X, Xie Y, et al. Simultaneous metabolomics and proteomics analysis of plasma-derived extracellular vesicles. Anal Methods. 2021;13(16):1930–1938. [DOI] [PubMed] [Google Scholar]
- 68.Lou D, Shi K, Li H-P, et al. Quantitative metabolic analysis of plasma extracellular vesicles for the diagnosis of severe acute pancreatitis. J Nanobiotechnology. 2022;20(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burrello J, Biemmi V, Dei Cas M, et al. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci Rep. 2020;10(1):16182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Q, Ma Z, Wang X, et al. Lipidomic Biomarkers of Extracellular Vesicles for the Prediction of Preterm Birth in the Early Second Trimester. J Proteome Res. 2020;19(10):4104–4113. [DOI] [PubMed] [Google Scholar]
- 71.Hinzman CP, Baulch JE, Mehta KY, et al. Plasma-derived extracellular vesicles yield predictive markers of cranial irradiation exposure in mice. Sci Rep. 2019;9(1):9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan YJ, Chen TC, Zhang QW, et al. Highly Selective Purification of Plasma Extracellular Vesicles Using Titanium Dioxide Microparticles for Depicting the Metabolic Signatures of Diabetic Retinopathy. Anal Chem. 2022;94(41):14099–14108. [DOI] [PubMed] [Google Scholar]
- 73.Du Y, Dong J-H, Chen L, et al. Metabolomic Identification of Serum Exosome-Derived Biomarkers for Bipolar Disorder. Oxid Med Cell Longev. 2022;2022:5717445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alzahrani FA, Shait Mohammed MR, Alkarim S, et al. Untargeted Metabolic Profiling of Extracellular Vesicles of SARS-CoV-2-Infected Patients Shows Presence of Potent Anti-Inflammatory Metabolites. Int J Mol Sci. 2021;22(19):10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nielsen JE, Maltesen RG, Havelund JF, et al. Characterising Alzheimer’s disease through integrative NMR- and LC-MS-based metabolomics. Metabol Open. 2021;12:100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saito M, Horie S, Yasuhara H, et al. Metabolomic pro fi ling of urine-derived extracellular vesicles from rat model of drug-induced acute kidney injury. Biochem Biophys Res Commun. 2021;546:103–110. [DOI] [PubMed] [Google Scholar]
- 77.Kim K, Lee S, Park SC, et al. Role of an unclassified Lachnospiraceae in the pathogenesis of type 2 diabetes: a longitudinal study of the urine microbiome and metabolites. Exp Mol Med. 2022;54(8):1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chacko KM, Nouri M-Z, Schramm WC, et al. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules. 2021;11(12):1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skotland T, Ekroos K, Kauhanen D, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–132. [DOI] [PubMed] [Google Scholar]
- 80.Puhka M, Takatalo M, Nordberg ME, et al. Metabolomic Profiling of Extracellular Vesicles and Alternative Normalization Methods Reveal Enriched Metabolites and Strategies to Study Prostate Cancer-Related Changes. Theranostics. 2017;7(16):3824–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agudiez M, Martinez PJ, Martin-Lorenzo M, et al. Analysis of urinary exosomal metabolites identifies cardiovascular risk signatures with added value to urine analysis. BMC Biol. 2020;18(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pergande MR, Kang C, George D, et al. Lipidomic analysis identifies age-disease-related changes and potential new biomarkers in brain-derived extracellular vesicles from metachromatic leukodystrophy mice. Lipids Health Dis. 2022;21(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohn W, Melnik M, Huang C, et al. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front Pharmacol. 2021;12:766082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang C, Li H, Powell JS, et al. Assessing hypoxic damage to placental trophoblasts by measuring membrane viscosity of extracellular vesicles. Placenta. 2022;121:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zebrowska A, Jelonek K, Mondal S, et al. Proteomic and Metabolomic Profiles of T Cell-Derived Exosomes Isolated from Human Plasma. Cells. 2022;11(12):1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalinec G, Gao L, Cohn W, Whitelegge J, Faull K, Kalinec F. Extracellular Vesicles From Auditory Cells as Nanocarriers for Anti-inflammatory Drugs and Pro-resolving Mediators. Front Cell Neurosci. 2019;13:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akawi N, Checa A, Antonopoulos AS, et al. Fat-Secreted Ceramides Regulate Vascular Redox State and Influence Outcomes in Patients With Cardiovascular Disease. J Am Coll Cardiol. 2021;77(20):2494–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weingrill RB, Paladino SL, Souza MLR, et al. Exosome-Enriched Plasma Analysis as a Tool for the Early Detection of Hypertensive Gestations. Front Physiol. 2021;12:767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thai A, Solomon B, Sequist L, Gainor J, Heist R. Lung cancer. Lancet. 2021;398(10299):535–554. [DOI] [PubMed] [Google Scholar]
- 90.Luo P, Mao K, Xu J, et al. Metabolic characteristics of large and small extracellular vesicles from pleural effusion reveal biomarker candidates for the diagnosis of tuberculosis and malignancy. J Extracell Vesicles. 2020;9(1):1790158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smolarz M, Kurczyk A, Jelonek K, et al. The Lipid Composition of Serum-Derived Small Extracellular Vesicles in Participants of a Lung Cancer Screening Study. Cancers. 2021;13(14):3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Qin H, Man Cheung F, et al. Plasma extracellular vesicle microRNAs for pulmonary ground-glass nodules. J Extracell Vesicles. 2019;8(1):1663666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freedland S. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2011;117(6):1123–1135. [DOI] [PubMed] [Google Scholar]
- 94.Di Meo A, Bartlett J, Cheng Y, Pasic M, Yousef G. Liquid biopsy: a step forward towards precision medicine in urologic malignancies. Mol Cancer. 2017;16(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yáñez-Mó M, Siljander P, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palviainen M, Laukkanen K, Tavukcuoglu Z, et al. Cancer Alters the Metabolic Fingerprint of Extracellular Vesicles. Cancers. 2020;12(11):3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei J, Zhao L, Du Y, et al. A plasma metabolomics study suggests alteration of multiple metabolic pathways in patients with bipolar disorder. Psychiatry Res. 2021;299:113880. [DOI] [PubMed] [Google Scholar]
- 98.Moyano AL, Li G, Boullerne AI, et al. Sulfatides in extracellular vesicles isolated from plasma of multiple sclerosis patients. J Neurosci Res. 2016;94(12):1579–1587. [DOI] [PubMed] [Google Scholar]
- 99.Iglesias-Aguirre CE, Avila-Galvez MA. Exosome-Containing Extracellular Vesicles Contribute to the Transport of Resveratrol Metabolites in the Bloodstream: a Human Pharmacokinetic Study. Nutrients. 2022;14(17):3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma S, Masud M, Kaneti Y, et al. Extracellular Vesicle Nanoarchitectonics for Novel Drug Delivery Applications. Smal. 2021;17(42):e2102220. [DOI] [PubMed] [Google Scholar]
- 101.Lemberg K, Gori S, Tsukamoto T, Rais R, Slusher B. Clinical development of metabolic inhibitors for oncology. J Clin Invest. 2022;132(1):e148550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cully M. Metabolic disorders: IDO inhibitors could change tack to treat metabolic disorders. Nat Rev Drug Discov. 2018;17(8):544. [DOI] [PubMed] [Google Scholar]
- 103.Jiang X, Zhang T, Gao J. The in vivo fate and targeting engineering of crossover vesicle-based gene delivery system. Adv Drug Deliv Rev. 2022;187:114324. [DOI] [PubMed] [Google Scholar]
- 104.Kalinec GM, Gao L, Cohn W, Whitelegge JP, Faull KF, Kalinec F. Extracellular Vesicles From Auditory Cells as Nanocarriers for Anti-inflammatory Drugs and Pro-resolving Mediators. Front Cell Neurosci. 2019;13:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Strybel U, Marczak L, Zeman M, et al. Molecular Composition of Serum Exosomes Could Discriminate Rectal Cancer Patients with Different Responses to Neoadjuvant Radiotherapy. Cancers. 2022;14(4):993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dang G, Li T, Yang D, et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox Biol. 2022;50:102257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Čuperlović-culf M, Khieu NH, Surendra A, Hewitt M, Charlebois C, Sandhu JK. Analysis and Simulation of Glioblastoma Cell Lines-Derived Extracellular Vesicles Metabolome. Metabolites. 2020;10(3):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vallejo F, Yuste JE, Teruel-Montoya R, et al. First exploratory study on the metabolome from plasma exosomes in patients with paroxysmal nocturnal hemoglobinuria. Thromb Res. 2019;183:80–85. [DOI] [PubMed] [Google Scholar]
- 109.Tao L, Zhou J, Yuan C, et al. Metabolomics identifies serum and exosomes metabolite markers of pancreatic cancer. Metabolomics. 2019;15(6):86. [DOI] [PubMed] [Google Scholar]
- 110.Jung JH, Lee MY, Choi D-Y, et al. Phospholipids of tumor extracellular vesicles stratify gefitinib-resistant nonsmall cell lung cancer cells from gefitinib-sensitive cells. Proteomics. 2015;15(4):824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova -I-I. Therapy resistance mediated by exosomes. Mol Cancer. 2019;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim DJ, Yang J, Seo H, et al. Colorectal cancer diagnostic model utilizing metagenomic and metabolomic data of stool microbial extracellular vesicles. Sci Rep. 2020;10(1):2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lam SM, Zhang C, Wang ZH, et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat Metab. 2021;3(7):909–922. [DOI] [PubMed] [Google Scholar]
- 114.Liang YX, Lehrich BM, Zheng SY, Lu MR. Emerging methods in biomarker identification for extracellular vesicle-based liquid biopsy. J Extracell Vesicles. 2021;10(7):e12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guijas C, Montenegro-Burke JR, Warth B, Spilker ME, Siuzdak G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat Biotechnol. 2018;36(4):316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang QJ, Zhao JR, Hao J, et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9(1):71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tian H, Ni Z, Lam SM, et al. Precise Metabolomics Reveals a Diversity of Aging-Associated Metabolic Features. Small Methods. 2022;6(7):e2200130. [DOI] [PubMed] [Google Scholar]
- 118.Royo F, Gil-Carton D, Gonzalez E, et al. Differences in the metabolite composition and mechanical properties of extracellular vesicles secreted by hepatic cellular models. J Extracell Vesicles. 2019;8(1):1575678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu LL, Liu JJ, Zhang WH, et al. FUNCTIONAL METABOLOMICS DECIPHER BIOCHEMICAL FUNCTIONS AND ASSOCIATED MECHANISMS UNDERLIE SMALL-MOLECULE METABOLISM. Mass Spectrom Rev. 2020;39(5–6):417–433. [DOI] [PubMed] [Google Scholar]
- 120.Paglia G, Smith AJ, Astarita G. Ion mobility mass spectrometry in the omics era: challenges and opportunities for metabolomics and lipidomics. Mass Spectrom Rev. 2022;41(5):722–765. [DOI] [PubMed] [Google Scholar]
- 121.Hollerbach AL, Li AL, Prabhakaran A, et al. Ultra-High-Resolution Ion Mobility Separations Over Extended Path Lengths and Mobility Ranges Achieved using a Multilevel Structures for Lossless Ion Manipulations Module. Anal Chem. 2020;92(11):7972–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okuma Y, Morikawa K, Tanaka H, et al. Prospective exosome-focused translational research for Afatinib study of non-small cell lung cancer patients expressing EGFR (EXTRA study). Thorac Cancer. 2019;10(2):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]