Abstract
Animals and viruses have constantly been co-evolving under natural circumstances and pandemic like situations. They harbour harmful viruses which can spread easily. In the recent times we have seen pandemic like situations being created as a result of the spread of deadly and fatal viruses. Coronaviruses (CoVs) are one of the wellrecognized groups of viruses. There are four known genera of Coronavirus family namely, alpha (α), beta (β), gamma (γ), and delta (δ). Animals have been infected with CoVs belonging to all four genera. In the last few decades the world has witnessed an emergence of severe acute respiratory syndromes which had created a pandemic like situation such as SARS CoV, MERS-CoV. We are currently in another pandemic like situation created due to the uncontrolled spread of a similar coronavirus namely SARSCoV-2. These findings are based on a small number of animals and do not indicate whether animals can transmit disease to humans. Several mammals, including cats, dogs, bank voles, ferrets, fruit bats, hamsters, mink, pigs, rabbits, racoon dogs, and white-tailed deer, have been found to be infected naturally by the virus. Certain laboratory discoveries revealed that animals such as cats, ferrets, fruit bats, hamsters, racoon dogs, and white-tailed deer can spread the illness to other animals of the same species. This review article gives insights on the current knowledge about SARS-CoV-2 infection and development in animals on the farm and in domestic community and their impact on society.
Keywords: Coronaviruses, MERS, SARS CoV-2, Pandemic, Transmission, Animal models
Introduction
The Coronaviridae family of viruses have a single-stranded, positive-sense RNA genome. They have been dormant in the environment for a long time and are popular and extensively researched family of viral pathogens in veterinary science. Every pet breed, companion animal, and wild animal has certainly experienced at least one viral infection from this family at a certain point in its life. Coronaviruses (CoVs) are majorly classified into four genera such as alpha, beta, gamma, and delta coronavirus (Perlman 2018). There was a severe outbreak of SARS two decades ago in the major parts of China (Nova 2021), which proved to be the discovery of the natural reservoir of bats family. Since then, there has been a close association as there was high similarity between the bat coronavirus with SARS-CoV, and now, the ongoing SARS-CoV-2 (Li et al. 2005), Zhou et al. (2020) reported a close association of the bat origin of SARS-COV-2 as there was 96% whole-genome sequence identity between the RaTG13 (SARS r-CoV) and SARS-CoV-2. The 800 amino acids containing the ACE-2 receptor’s first description appeared in 2000.The ACE-2 receptor is present in a wide range of animal species and tissues, such as the heart, kidneys, testes, large intestines, and kidneys. The viral spike’s receptor-binding domain RBD interacts with the ACE-2 receptor (Saravanan et al. 2022). Protein sequences from several species were analyzed to find out the similarity in the ACE-2 receptor region which plays a pivotal role in binding to the spike protein thus giving the virus an entry into the human host. There is evidence about 5 critical hotspots of ACE-2 (Position 31, 53, 38, 82, and 353) to the SARS-CoV-2 and for cross-species transmission. Sequence analysis of ACE-2 shows that the crab-eating macaque and chimpanzee share identical amino acids with humans in all the five hotspot areas proving to be ideal candidates for being animal models. Other animals like cattle and pig show four sites which are identical with a single variation sites. Ferrets and dogs possess three identical sites with two different sites. Bats and mice have only two similar amino acid with humans (Sharun et al. 2020).
The most variable region of the coronavirus genome is the receptor-binding domain (RBD) found in the spike protein (Zhou et al. 2020). It has been demonstrated that six RBD amino acids are essential for SARS-CoV-like viral host range determination and binding to ACE2 receptors (Wan et al. 2020). When compared to SARS-CoV, the main mutations include L455, F486, Q493, S494, N501, and Y505 which were found on SARS-CoV-2 (Wan et al. 2020). The crown-shaped spikes on the surfaces of coronaviruses give it the name. They are divided into the alpha, beta, gamma, and delta subgroups which are commonly found coronaviruses such as 229E (alpha coronavirus), NL63 (alpha coronavirus), OC43 (beta coronavirus), HKU1 (beta coronavirus). The viruses known to affect humans are SARS-CoV, MERS-CoV, and SARS-CoV-2 (Lim et al. 2016).
Middle East Respiratory Syndrome (MERS-CoV) was a highly contagious viral disease. Bats are thought to be the natural hosts for coronaviruses. In numerous Middle-eastern nations, camels have been shown to carry different strains of this virus. Nearly, 90% of camels and calves tested positive for MERS-CoV antibodies in Saudi Arabia, according to serological tests. According to a report, human MERS-CoV sequences and camel MERS-CoV sequences are closely linked (Alnuqaydan et al. 2021). The dipeptidyl peptidase DPP4 (also known as CD 26), which was discovered to be the host cell receptor for MERS-CoV, was one of two essential components needed for MERS-CoV to adhere to or fuse with host cells in order to begin infection. DPP4 is only expressed in specific animal species, which accounts for the limitation of the MERS-species CoV’s tropism (Alnuqaydan et al. 2021).
The H5N1 strain of the avian flu first appeared in farm-grown geese which then went on to spread in chickens. The most dangerous types of bird flu that can be spread from birds to people include H5N1 and H7N9, which have killed thousands of people in China and across the globe. People who come into contact with the feathers, flesh, or droppings of infected birds were at high risk of contracting the virus (PETA U. Coronavirus, Swine Flu, n.d. XXXX). The current pandemic, driven by the severe acute respiratory syndrome (SARS) coronavirus-2, is having a devastating effect on society and the planetary health and also serves as an indicator of multiple dangers that may rapidly evolve to cause decline of human and animal populations.
We are in a fierce battle to lessen the effects of the pandemic on human health and wellbeing. In order to survive this socioeconomic disaster, required steps must be taken everywhere to flatten the pandemic curve that SARS-CoV-2 has caused. Additionally, it has the capacity to infect most mammalian species and induce serious respiratory distress. It has been documented that humans can transmit the disease to canines and minks. In numerous nations, there has been intraspecific transmission between minks that has been closely observed and documented (WHO-FAO-WOAH 2021).
Potential source of transmission
Researchers have traced the SARS-CoV-2 outbreak back to a first identified case in massive market at Wuhan that retailed live wild animals among other goods and put forward the hypothesis that most possible reason is spillover of SARS-CoV-2 virus to humans from animals sold at the market (Maxmen 2022; Gao et al. 2022). The SARS-CoV-2 samples collected from the Wuhan market environment and infected people during December 2019 and January 2020 for genetic analysis showed many of the positive cases were found in the samples obtained from regions where live animals were sold at the wet market. The SARS-CoV-2-specific antibodies was found in all blood samples collected from the infected people in the market and from the animals for sale in the market (Gao et al. 2022). Researchers believed that the reservoir may very well be squat dog-like species, particularly raccoon dogs that were sold in the live market and had positive cases. The animals harboring the virus before transmitting it to humans were still stable(Worobey et al. 2022). Five samples tested positive, which were from single live animal shop where animals were housed and moved in metal cages, carts, and a machine used for removing bird feathers. It is believed they could have contracted the virus when they were stored in the caged containers in the same place.
The speed at which the SARS-CoV-2 is creating a pandemic is alarming. Most of the countries are in the fourth wave of the pandemic. There are high chances of SARS-CoV-2 re-infection. The infected population often produces a very high viral load; this increases the chances of spillover to other animal species such as pet animals, farm animals, and wild animals which inhabit regions close to human settlements. It is reported that the likelihood of the viral amplification in pigs does not have high occurrences, and similar instances must be monitored very cautiously in order to prevent any spillovers (Opriessnig and Huang 2020). The Centre for Disease Control and Prevention (CDC), USA, has strongly advised that all laboratory-confirmed COVID-19 cases (RT-PCR positive) limit their interaction with their companion pet animals in light of the potential for SARS-CoV-2 transmission between humans and animals (Mallapaty 2020). The Coronaviruses infection initially has cold-like symptoms which further worsens into respiratory difficulties, chest congestion in humans, while other viruses can cause illness in certain types of animals such as bats, camel, and cattle.
Some coronaviruses, which belong to canine and feline coronaviruses family, infect only animals and not humans. In an extensive study, 1914 serum samples were collected from 35 animal species with symptoms and suspected of SARS-CoV-2 infection and were subjected to ELISA with double antigen sandwich that detects SARS-CoV-2-specific antibodies. The findings indicated the absence of SARS-CoV-2-specific antibodies, which ruled out the notion that an animal species could serve as an intermediate transmission host for SARS-CoV-2 infection (Deng et al. 2020). The spread of the virus raised many concerns by the pet owners, which led them to a state of anxiety and fear which became one of the major reason for pets being abandoned and rendered homeless in many cities. This has created a huge impact on the welfare of the animals.
Genetic diversity of SARS-CoV-2
Coronaviruses are enclosed as positive-sense single-stranded RNA viruses that are members of the family Coronaviridae, sub-class Orthocoronavirinae, and are named after their surface proteins with a crown-like structure. They are divided into four genera such as alpha coronavirus, beta coronavirus, delta coronavirus, and gamma coronavirus. The first two class of the virus majorly cause infection in mammals where as in birds the source of infection is gamma coronaviruses. Delta coronaviruses infect both mammals and birds.
SARS-CoV-2 is an enveloped single-stranded RNA virus which has a genomic size of 30,000 nucleotides and eleven open reading frames (ORFs) that encode for 29 proteins. 16 non-structural proteins are encoded by the first 21,552 nucleotides of the genome, which make up ORF1ab (nsp1–nsp16). The structural proteins are spike (S), envelope (E), matrix (M), and nucleocapsid (N), and nine auxiliary proteins are encoded in the final part of the genome ORF3a, ORF3b, ORF6, ORF7a, ORF7b, ORF8b, ORF9b, ORF9c, and ORF10 (Helmy et al. 2020; Bai et al. 2022).
The SARS-CoV-2 has evolved over the period of time in multiple locations and has been classified by the World Health Organization under specific categories such as variant of interest, variant of concern, and variant under monitoring. This classification is based on the severity and progression of the SARS-CoV-2 variants. There have been several variants that had been circulating around the globe and causing loss of human and animals. There are five major variants of concern such as alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and Omicron (BA.1). The Omicron variant shows diversity as it has been found in multiple locations in the world; they have been sub-classified as Omicron (BA.2), Omicron (BA.4), Omicron (BA.5), Omicron (BA.2.12.1), Omicron (BA.2.75). Multiple SARS-CoV-2 variations have been identified, with a few classified as variants of concern (VOCs) owing to their public health implications. VOCs have been linked to increased transmissibility or virulence, decreased neutralization by antibodies that are produced by natural infection or vaccination, the capacity to elude detection, and a reduction in therapeutic or vaccine efficiency (Aleem et al. 2021).
All five of the reported VOCs such as have multiple mutations in the receptor-binding domain and the N-terminal domain of the spike protein, with the exception of the delta variant, which has a N501Y mutation on the RBD which has resulted in the increased affinity of the interaction between the spike protein and ACE 2 receptors, enhancing viral attachment and subsequent entry into host cells. All the major mutations encoding the structural proteins of SARS -CoV-2 and its variants are given in the following Table 1. The WHO has presently identified eight variants of interest (VOIs) since the beginning of the pandemic, which include epsilon (B.1.427 and B.1.429), zeta (P.2), eta (B.1.525), theta (P.3), iota (B.1.526), kappa (B.1.617.1), lambda (C.37), and mu (B.1.621). VOIs are identified as genetic variants with particular genetic markers that have been linked to changes that may result in increased transmissibility or virulence and decrease in the neutralization capability of antibodies generated through natural infection or due to vaccination.
Table 1.
Structural protein mutations (Spike (S), envelope (E), membrane, (M), and nucleocapsid (N)) of different variants of SARS-CoV-2 isolated from humans
| Name of the variant | Pango Lineage | Origin | Mutations on the Structural protein | References | |||
|---|---|---|---|---|---|---|---|
| Spike | Nucleocapsid | Membrane | Envelope | ||||
| Alpha | B.1.1.7 |
Dec 2020 England |
N501Y, A570D, P681H, T716I, S928A, D1118H | D3L, S235F | – | – | Aleem et al. (2021) |
| Beta | B.1.351 |
Dec 2020 South Africa |
N501Y, E484K, L18F, K417N D80A |
T205I | – | P71L | Tegally et al. (2021) |
| Gamma | P.1 |
Jan 2021 Brazil |
N501Y, E484K, D614G, H655Y |
P80R, R203K G204R |
– | – | Amanat et al. (2021) |
| Delta | B.1.617.2 |
2021 India |
T19R, G142D, R158G, L452R, T478K, D614G, P681R, D950N |
D63G, R203M D377Y |
I82T | – | Kannan et al. (2021) |
| Omicron | BA.1 |
Nov 2021 South Africa |
A67V, T95I, Y145D, L212I, G339D, S371L, S373P,S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K | P13L, R203K, G204R | D3G, Q19E, A63T | T91 | Zhang et al. (2022) |
Animal-to-human transmission
A lethal zoonotic virus known as SARS-CoV-2 is thought to have spread from one of the animal species to humans after infecting and spreading among other animals. A team of researchers used whole-genome sequencing to demonstrate that in the early stages of the 2021 (March) pandemic, SARS-CoV-2 infections were widespread among mink farms in the southeast of the Netherlands. 68% of mink farm employees screened positive for the infection or have had antibodies against SARS-CoV-2. The human COVID-19 patients that had viruses with the D614G mutation were what started these sizable clusters of infection. Sequencing has since demonstrated that mink-to-human transfer also took place (Munnink et al. 2021).
Another incident of animal-to-human transfer being suspected included hamsters at a pet store. A team of researchers published the results of their epidemiological and viral analyses of a COVID-19 epidemic linked to a pet shop that may have involved interspecies transmission. The 3 vaccine recipients in this cluster were found to have contracted the most closely related virus strain of the SARS-CoV-2, the delta variant AY.127, according to the epidemiological research. The hamsters at the warehouse that were harboring SARS-CoV-2 were not visited by the afflicted patient. Therefore, it seemed most likely that the patient contracted the virus at the pet store, either by close contact with sick animals or by being exposed to contaminated surroundings (Chan et al. 2020).
A case was reported in Thailand, where there was a spread of the virus from a cat to human as the lady veterinarian who had handled the cat showed severe symptoms. The cat's nasal swab’s comparatively low RT-PCR cycle thresholds indicate that the virus load was high and contagious (Sila 2022).
The Ohio area underwent a thorough research in the winter of 2021, testing white-tailed deer (O. virginianus) at random locations in nine distinct locations for the presence of SARS-CoV-2 and its variations. Out of these, four areas where further sampling was conducted to indicate it as a significant deer cluster were where the highest prevalence estimates of SARS-CoV-2 (B.1.596 viruses) were discovered. The sequencing unmistakably demonstrated that a transfer from deer to deer had occurred. A variety of mutations, including one in the receptor-binding motif, were found in white-tailed deer but only at extremely low frequency in humans. Such alterations could be enhanced in a fresh reservoirs host with significant infection rates and other evolutionary constraints (Hale et al. 2022). A schematic representation of the possible route of transmission of SARS-CoV-2 starting from animal as a primary host to the intermediate and then jumping to humans is illustrated in Fig 1. The animals infected with the virus were studied; their samples were sequenced, and the data are submitted to GISAID databank. The following table gives an overview about the mutations found in SARS-CoV-2 isolated from animal sources across the globe. This data have been retrieved and analyzed for mutations from the GISAID databank.
Fig. 1.
Above image shows the possible route of transmission of SARS-CoV-2 from animals starting from primary host, then to intermediate and finally to humans and animals. The image was produced using BioRender.com
Structural protein mutations in SARS-CoV-2 spike, envelope, membrane, and nucleocapsid isolated from humans for different variants at multiple time points as given in Table1. The most frequently occurring mutations were selected, compared, and analyzed with the SARS-CoV-2 viral isolates found in domestic and wild animals. Based on the analysis, we have summarized the presence and absence of the mutations in animals in Table 2.
Table 2.
Structural protein mutations (Spike (S), envelope (E), membrane, (M) and nucleocapsid (N)) of different variants of SARS-CoV-2 isolated from wild and domestic animals
Natural infection of SARS-CoV-2 in animals
The SARS-CoV-2 has four structural proteins, namely spike (S), membrane (M), envelope (E), and nucleocapsid (N) which collectively constitute the structure of the virion. The virion binds to the host cell receptor with the help of S1 subunit of spike protein. This interaction between the spike protein of SARS-CoV-2 and the host receptor determines the host species range and also the tissue tropism of the virus. The alpha coronavirus uses the aminopeptidase N as its receptor for interaction, whereas ACE-2 is used as a host receptor by SARS-CoV and HCoV NL63. Another group of viruses such as MHV utilizes the CEACAM1 to enter the human cells. MERS-CoV majorly binds to the dipeptidyl peptidase4 (DPP4) to enter the human cells (Hamming et al. 2004). The angiotensin-converting enzyme 2 (ACE2) in human binds to the spike protein of the SARS-CoV-2 virus (Luan et al. 2020). There have been reported evidences of transmission of SARS-CoV-2 between other domestic animals such as cats, dogs, minks, hamsters, and ferrets and some wild animals such as lions and tigers. The key ACE-2 residue plays a very important role in recognizing the spike/S1 subunit protein; it was analyzed in order to identify all the possible potential host ranges of SARS-CoV-2. The SARS-CoV-2 virus was inoculated in numerous animal model species for an experimental examination, and the results revealed that ferrets and cats are extremely vulnerable to the virus whereas dogs are relatively less susceptible. The researchers discovered that pigs, chickens, and ducks are either not at all or only marginally infected with SARS-CoV-2 (Shi et al. 2020a, b).
SARS-CoV-2 in apes
The studies conclude that, the Hominidae family of wild great apes is extremely vulnerable to a variety of viral infections that affect humans. The diseases that constantly endanger humans also represent a serious threat to global efforts to save wild animal populations. The traits of ACE2 indicate that primates, especially Old-World species, will be very susceptible to SARS-CoV-2. A study proves that there is a naturally acquired infection in captive gorillas. Direct and indirect contact of humans to primates leads to the covid infection. The interaction with humans is rising constantly due to deforestation, rehabilitation, research, and tourism. Most of the primates’ conservation habitat is geographically placed near highly populated urban areas; hence, there is a sharp rise in the susceptibility toward SARS-CoV-2 in primates. Great apes Western low land gorillas are infected with SARS-CoV-2. Infected animals had visible symptoms of coughing, nasal discharge, and some old apes’ showed pneumonia like symptoms, which reduced after two or more days. The severity of infection in wild gorillas was very high and unpredictable. Strict precautions must be taken by the all people who come in close contact with primates such as tourists, researchers, and conservation workers. Other apes belonging to different families such as captive bonobos (Pan paniscus) and orangutans (Pongo sp.) are also vulnerable to such viral infections; therefore, administration of vaccines is of prime importance (Delahay et al. 2021).
SARS-CoV-2 in dogs
In the past, when the pandemic had hit in 2003 due to the emergence of SARS-CoV, it was detected in domestic dogs (Group 2003). In the current pandemic, there has been multiple cases about the susceptibility of domestic pet mammals to SARS-CoV-2. The testing is done using RT PCR method. In a research study from Hong Kong, it was discovered that 2 out of 15 dogs from residents that had confirmed human cases of COVID-19 were found to be infected with SARS-CoV-2. In a second investigation, the virus was isolated from nasal and oral swabs, and the 2.5 year-old male German shepherd was confirmed to be positive for SARS-CoV-2 RNA on two separate occasions. Plaque-reduction-neutralization assays were used to evaluate the dog’s antibody responses. The genetic material of the viruses obtained from the two dogs matched those of the corresponding human cases. These indications reflect the instances of SARS-CoV-2 transmission from humans to animals (Goumenou et al. 2020).
Dogs may have served as intermediary hosts in the spread of SARS-CoV-2 among people in Italy, according to a group of researchers. This assumption was supported by the finding that, despite the implementation of stringent laws and regulations in Italy, the number of cases increased rapidly. There is one dog for every six people living in Italy, the likelihood of both human-to-human and animal-to-human transmission. A case was studied in the United States Department of Agriculture’s (USDA) National Veterinary Services Laboratories (NVSL), where a pet dog (German shepherd) had showed signs of respiratory distress. Swab samples from two dogs were taken after its owner tested positive. One of the dogs showed symptoms whereas the other did not have symptoms. Antibodies were also detected in both samples. This showed transmission from human to animals. SARS-CoV-2 infections have been reported in a small number of animals across the world, mostly in animals that are in close contact with an infected person.
The RBD of dog ACE-2 complex and the RBD of human ACE-2 complex have several structural similarities. The two complexes’ interaction interfaces, however, are marginally dissimilar. The RBD/dACE-2 interface has significantly fewer contact atoms, residues, and hydrogen bonds than the RBD/hACE-2 interface, which accounts for the 6.65 times lower binding efficiency of dACE-2 for RBD than that of hACE-2.
SARS-CoV-2 in cats
Considering that cats were previously known to have been infected with severe acute respiratory syndrome coronavirus SARS-CoV during the 2003 outbreak; their sensitivity to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was predicted to be more transmissible. SARS-CoV-2 has been discovered to replicate in domestic cats, and experimental cat-to-cat viral transmission has been noted. Infection from COVID-19-positive pet owners via human-to-cat SARS-CoV-2 transmission has been confirmed in reports from various countries. The disease manifested in varying degrees of severity in the affected cats (Halfmann et al. 2020). In New York, two pet cats tested positive. One of the pet owners was a symptomatic virus carrier. Both animals had a slight respiratory infection that caused breathing difficulties. There may have been a human-to-animal transfer or an animal-to-human transmission, according to reports. A study was conducted for the alleged infection that 22 pet cats may have had. The owners themselves were thought to have contracted the disease or to be the source of it. Rectal, nasopharyngeal, and biological fluids were used to collect the swabs for the RT-qPCR test, which looked for 2 SARS-CoV-2 genes. Antibodies to the SARS-CoV-2 were found, according to a serological investigation. Additionally, it was revealed that the SARS-CoV-2 genome sequence analysis belonged to clade a2a, which shared similarities with the human SARS-CoV-2 samples isolated from France (Sailleau et al. 2020).
SARS-CoV-2 in minks
First confirmed case of SARS-CoV-2 was reported in Netherlands inside a mink farm in the first wave of the SARS-COV-2 outbreak. The neighboring farm had a probable suspected case; both farms exhibited respiratory difficulties, which led to a greater death rate. The farm mink may have been infected after being exposed to a virus-infested droplet (Oreshkova 2020). A farm worker was suspected to have contracted SARS-CoV-2 from mink raised for food. Four distinct mutations were found in the S gene that gave birth to the mink adapted variant (G75V, M177T, Y453F, and C1247F) in an isolate taken from a farm worker who tested positive for SARS-CoV-2. The additional alterations also included the Y453F mutation, which had been previously noted to have emerged in mink during sequential passages, and a unique mutation that truncates ORF 7b at location L22 and is absent in any other global SARS-CoV-2 isolate.
The virus’s apparent adaptation to the mink host led to the emerging variation, with some point mutations being repaired early during the mink’s dissemination and further modifications accumulating over time (Bayarri-Olmos et al. 2021; Rabalski et al. 2022). Scientists examined over 91 mink samples for SARS-CoV-2 infection. It was validated that 15 animals screened tested positive for SARS-CoV-2 combining detection technique such as RT- PCR, antigen-based detection, and NGS technique. On sequencing the whole viral genomes, researchers validated this discovery that there was presence of virus variation with sporadic mutations on the protein sequence such as G75V and C1247F which coded for spike protein (Rabalski et al. 2021).
SARS-CoV-2 in Asiatic lion
In one of the biggest zoos in Asia, the Nehru zoological park in Hyderabad, eight of the Asiatic lions were found to be positive against SARS-CoV-2 during the second wave of covid-19 which was at its peak during April 2021–June 2021, and more than two dozen of the zoo staffers had been tested positive at the time. This reported case was one of a kind to prove that there is human-to-animal transmission. There was an initial suspect when one of the caretakers found that these big cats developed symptoms such as dry cough, nasal discharge, and loss of appetite. These alarming symptoms were soon addressed by the in-house veterinary officials who took the oropharyngeal swab samples for further testing to test and find out that they were positive for SARS-CoV-2.
In Nahargarh Biological Park, India, one of the lions was displaying mild clinical symptoms, leading to discovery of an additional case of the feline SARS-CoV-2 infection. Testing revealed a positive result for SARS-CoV-2 employing primers and probes targeting RdRP, the E gene, and RT-PCR results were verified by Sanger sequencing of the S, N, and E genes. A further 14 days of isolation and quarantine followed. Testing of the entire zoo crew indicated that the veterinarian who was caring for the sick lions also experienced clinical symptoms (Karikalan et al. 2021). A similar case of feline infection was reported in Singapore zoo, where four endangered Asiatic lions had started to show symptoms such as coughing and sneezing after they had contact with one of the zoo keeper who was found to be infected. They were immediately isolated and quarantined in a separate den. They remained bright and active all the while and showed signs of improvement.
Two Asiatic lions from Vandalur Zoo in Chennai died due to severe infection of SARS-CoV-2. Nine of the lions were tested during the time. Of the two, one was 9 year-old lioness, and the other was 12 year-old male. This test report was issued by NISHAD in Bhopal. The lion had been kept in the intensive care unit and was receiving treatment since a long time. These two cases were reported of feline deaths in India (Mishra et al. 2021).
SARS-CoV-2 in white tailed deer
The ACE2 receptor, which is the primary entry point for the viral invasion, is shared by humans, reindeer, and Pere David’s deer (Damas et al. 2020).
SARS-CoV-2 was also reported in several deer spp. The contagious virus was discovered in deer excrement. RT-PCR was used to analyze the samples, which were tested from nasal and rectal swabs. In several cases, the body temperature was temporarily increased, but there are no evident lesions that were visibly seen. Acute alveolar damage-related lesions were seen histologically, that resembled infections in humans, but no viral RNA was discovered, suggesting the pathogen had already been wiped off (Michelitsch et al. 2021).
The main risk that is associated with the ongoing pandemic caused by SARS-CoV-2 outbreak is the transmission of the virus from human to human. It is true to believe that the persisting evidences show and support that the plausibility of the deadly SARS-CoV-2 spilling over to the undiscovered new hosts through medium involving fecal shedding by infected humans which reaches the natural aquatic environment through the waste water treatment system (Franklin and Bevins 2020).
SARS-CoV-2 in avian species
A group of researchers studied 5 different species of poultry such as chicken (Gallus gallus domesticus), turkey (Meleagris gallopavo), quail (Coturnix japonica), Pekin ducks (Anas platyrhinchos domesticus), and white Chinese geese (Anser cygnoides). They challenged 10 birds from each species with SARS-CoV-2 virus obtained from the Biodefense and Emerging Infectious Resources Repository, USA. The swab samples particularly the oropharyngeal and cloacal were collected and analyzed for the presence of the virus at different time intervals. Their serum was also analyzed for antibody levels and the virus neutralization. The results revealed absence of viral antibodies even at a window period of 14 days post the challenge. These experiments clearly state that the virus did not replicate in any of the avian species (Suarez et al. 2020).
Challenges in animal model
The search for a preclinical animal model that accurately replicates the severe and fatal type of human COVID-19 is one of the major limitations in the advancement of SARS-CoV-2 infection models. It will be an advantage for several groups of researchers working on animal models. Furthermore, it would offer a method for evaluating the transition from a moderate to a severe illness, which may help to identify disease and its biomarkers. Additionally, it would broaden the scope of currently existing animal models for testing vaccinations and treatments, which may lead to the development of critically required medical countermeasures during rescue situations.
There are still a number of unsolved concerns about how SARS-CoV-2 is spread. Are there any other possible transmission routes, such as mother-to-child or fecal contamination. Due to the disease’s unexpected outcomes, it has been challenging to comprehend the pathophysiology of the condition. The disease is more infectious than MERS and SARS for what reasons? Why are older people and others who have underlying medical conditions more vulnerable (Rothan 2020). Does the host’s sex or genetic composition also affects pathogenicity? More study is required, and these issues should be examined using physiologically plausible animal models. Most of the SARS-CoV-2 comorbidities and co-infections remain unknown. Concerns exist regarding why some drugs that have been repurposed for COVID-19 therapy are effective for some infected people. As a result, in vivo research using the most effective animal models is necessary to gather preliminary information. The wide variation in human genetic make-up makes it difficult to understand the workings of processes.
Animal models such ferrets, mice, and hamsters can be used to study a wide range of experiments including the mechanistic of action of antivirals, the efficacy and safety of vaccines, and the impact of comorbidities on the development of COVID-19 (Olwenyi et al. 2020). As a result, in vivo research using the most effective animal models is necessary to gather first information. The wide variation in human genetic make-up makes it difficult to understand the workings of processes. Animal models such ferrets, mice, and hamsters can be used to study a variety of topics, including the mechanism of action of antivirals, the efficacy and safety of vaccines, and the impact of comorbidities on the development of COVID-19. No one animal model is likely to be able to handle all the issues associated to translating from humans because of inherent differences in the development and physiology of the organism (Pandey et al. 2021). Furthermore, there is a great deal of heterogeneity among the animals, including variances in their biology, genetics, and level of ACE2 receptor expression, all of which might impact the infection rate. Understanding SARS-CoV-2 pathophysiology requires the use of primary cell lines, organoids, and in vitro models (Leist et al. 2020). The creation of humanized mouse models with tissues similar to those of humans may also pave the door for fast testing of antivirals and vaccines.
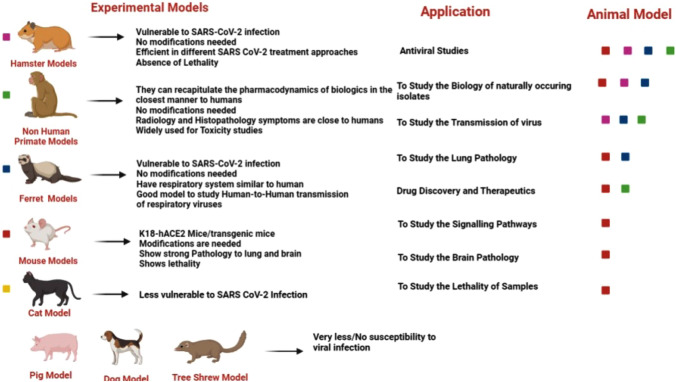
To further prove the safety and effectiveness of potential therapies and vaccines, researchers can use a number of small and large animal models to study critical aspects of SARS-CoV-2 including pathogenesis, transmission, and host reactions to SARS-CoV-2. In order to measure the pathogenic pathways and prospective treatments, it is essential for this effort to advance investigations on several animal models of COVID-19. To this purpose, research for SARS-CoV-2 has made good use of a variety of animal species, such as mouse, great apes, hamsters, ferrets, and cats. A wide variety of experimental animal model for SARS-CoV-2 infection and their application is widely discussed in Fig. 2
Fig. 2.
Schematic representation of experimental animal model for SARS-CoV-2 with their application in research and development
Mouse models
For several viral experiments, the mouse model has indeed been widely employed. The hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), Zika virus, and other viruses have all been studied using it as the best small animal model. The mouse model is appropriate for extensive research on viruses, both for pathogenesis and antiviral treatments, due to its economic cost, compact size, simple operation, and excellent repeatability. Mice are easier to handle as compared to non-human primates in a laboratory with a higher level of biosafety because of their comparatively small size and lack of operational challenges. Importantly, mice may be easily genetically altered for accurate study. For investigations on viral infection and transmission limitation, viral pathogenesis, and antiviral immunity, a large number of genetically altered mice are available. Furthermore, it is possible to research viral pathogenesis and host immune responses using modern mouse immunological tools.
Several viral investigations, such as those involving the Middle East respiratory syndrome coronavirus and SARS-CoVs, have made good use of the mouse (Mus musculus) model (Bi et al. 2021). Since SARS-CoV-2 penetrates host cells primarily through the human angiotensin-converting enzyme 2 (ACE2) receptor rather than the mouse ACE2 receptor, the main challenge to SARS-CoV-2 infection in mice is a lack of appropriate receptors (Bi et al. 2021). The strategies implemented for using mouse as an animal model will involve alterations in the animals to express human ACE-2. To introduce hACE-2 in mice, CRISPR/Cas9 knock-in technology or recombinant plasmids are used to construct permanent genetic alterations. The human lung ciliated epithelial cell-specific HFH4/FOXJ1 promoter, the human epithelial cell cytokeratin-18 (K18) promoter, and the mouse ACE2 (mACE2) promoter all regulate the production of hACE-2 (Zhao et al. 2020a, b). By employing reverse genetics or serial passaging, viruses can be modified to directly infect wide-type mice. The SCID mice were surgically implanted with human fetal lung tissue under their dorsal skin to create a SARS-CoV-2 xenograft model. When infected with SARS-CoV-2, the human lung xenografts exhibited high viral replication with spreading to the entire lung tissue and generated mature structures that nearly resemble the normal human lung (Fu et al. 2021).
Systems based on adenovirus vectors are advantageous for usage with transgenic or factor-deficient mice because they may be utilized to incorporate human receptors into mouse genomes. hDDP4 has been inserted into WT mice using replication-defective adenovirus vectors, making them vulnerable to MERS-CoV infection. Mice that were infected developed pneumonia with significant pulmonary immune cell infiltration and viral clearance by 6 days post-infection (dpi) although they suffered less damage than mice that had completely hDPP4 transgenic bodies (Zhao et al. 2014).
Syrian hamster models
In order to study respiratory viruses, hamsters are extensively employed. The SARS-CoV-2 spike protein is strongly bound by the hamster ACE2 protein, which facilitates entrance. In order to explore the pathophysiology of SARS-CoV-2 infections, hamsters are a prospective infection model (Shou et al. 2021). This model is effective for researching infectious biology, including post-bacterial, viral, and parasitic diseases, because the immunological reactions of Syrian hamsters to pathogenic organisms are comparable to those of humans. It also helps in evaluating the effectiveness and inter-connections of prescribed drugs and vaccines for those pathogens (Miao et al. 2019). The ACE 2 receptor is reported to interact with the most epitopic area of SARS-CoV-2 structural spike glycoprotein. In a study, scientists found that Syrian hamsters are susceptible to SARS-CoV-2 infection (Chan et al. 2020). Syrian hamsters had pathological symptoms after infections that were comparable to those in humans. Focal, diffuse alveolar damage, hyaline membrane development, and mononuclear cell infiltration were all visible in the lung tissue. The Syrian hamster may develop lesions in the kidney, adrenal gland, ovary, spleen, lymph nodes, and other numerous organs depending on the severity of the coronavirus-2 infections. One of these, focal to multifocal inflammation, was seen in the adrenal gland (Song et al. 2021). Hamsters were immune from secondary infection by neutralizing antibodies produced due to the initial SARS-CoV-2 infection. Likewise, passive serum transfer protected the naive hamsters from viral lung replication (Imai et al. 2020).
Ferret models
Numerous human respiratory viruses, including the influenza virus, syncytial virus, para influenza virus, and coronavirus, can infect ferrets. Due to the existence of viral receptors and the similarities in their respiratory tract structure to that of humans, ferrets can mimic the clinical signs of viral infections. In ferrets’ tracheobronchial sub-mucosal glands, type-II pneumocytes and serous epithelial cells primarily express ACE-2. Only, two amino acids differentiate the ferret ACE-2 domain from its human counterpart domain when it comes to binding the spike protein of the SARS-CoV-2 virus.
Ferrets (Mustela putorius furo) have indeed been found to be an extremely useful model for evaluating the virulence and propagation of human respiratory viruses such as influenza and respiratory syncytial virus. It is not unusual, then, that the ferret model has been examined for research into the pathophysiology of SARS-CoV-2 spread. Despite the use of various SARS-CoV-2 isolates, the results were remarkably comparable across all laboratories (Shi et al. 2020a, b). In laboratory conditions, ferrets can easily spread viruses to uninfected ferrets. Transfer between experimentally infected ferrets to naive cage mates happened efficiently, while transmission between ferrets exposed to companion ferrets separated by steel grids did not. These investigations demonstrated the possibility of SARS-CoV-2 airborne transmission and recommended that future research on transmission may benefit from using the ferret model (Richard et al. 2020).
Tree shrew models
An animal resembling rat and squirrel-like is known to be Chinese tree shrew (Tupaia belangeri chinensis) which shares the genetic affinity with primates. Tree shrews have been a new experimental animal that has been successfully tested as a substitute to primates in several researches and drug safety trials which majorly focused on research related to hepatitis C and B viruses. Tree shrews have the benefits of being small in size, low feeding costs, and short reproductive cycles (Fan et al. 2013).
In an experimental context, SARS-CoV-2 was delivered to males as well as female tree shrews of different ages, ranging from 6 months to 7 years. After inoculation, the majority of animals, particularly females, showed an elevation in body temperature but no clinical signs or obvious lesions. Particularly, in younger animals, viral RNA has been discovered in blood samples, nasal samples, and also in throat swabs for up to 12 days. Compared to the existing animal models, the tree shrew is a little less vulnerable to SARS-CoV-2 infection and might not be an appropriate animal for COVID-19-related research. However, as an asymptomatic carrier, the tree shrew may serve as a significant intermediate host for the SARS-CoV-2 virus (Zhao et al. 2020a, b).
Pig models
Pigs are frequently employed in research due to their anatomical, genetic, physiologic, and immunological parallels to humans. Indeed, pig experiments are more reliable than rodent experiments to indicate therapeutic and preventive treatments for humans (Meurens et al. 2012). Piglets can be used as model animals as they were injected with the severe acute respiratory syndrome coronavirus-2 by a variety of methods, including intranasal, intratracheal, intramuscular, and intravenous inoculation. Seroconversion was reported in pigs inoculated parenterally, despite the fact that piglets were not sensitive to SARS-CoV-2 and lacked lesions or viral RNA in tissues/swabs intramuscularly or intravenously (Vergara-Alert et al. 2021). Another group of scientists challenged chickens, turkeys, ducks, quail, and geese with severe acute respiratory syndrome coronavirus-2 or Middle East respiratory syndrome coronavirus, observed no disease and detected no virus replication and no serum antibodies. It was concluded that poultry is unlikely to serve a role in maintenance of either virus.
Dog model
A group of scientists examined at SARS-CoV-2 replication and transmission in canines. Five three-month-old beagles were intra-nasally immunized and kept in a room with two other beagles that were not immunized. Each beagle’s oropharyngeal and rectal swabs were obtained every other day for virus quantification in Vero E6 cells and viral RNA detection. On days 2 and 6, viral RNA was found in the rectal swabs of two virus-inoculated dogs, but no viral RNA was found in any organs or tissues removed from this animal. On the 14th day following infection, serum from all dogs was taken for ELISA antibody detection. These findings suggest that dogs are not very susceptible to SARS-CoV-2 (Shi et al. 2020a, b).
Cat model
Wild cats have been shown to carry the SARS-CoV-2 infection and show detectable symptoms (Mallapaty 2020). Cats are sensitive to experimental infection and have found to shed the viral particle through the nasal turbinates, soft palates, tonsils, tracheas, lungs, and small intestines. Limited shedding of virus via that route was found to be in the tissues except intestine or feces (Rudd et al. 2021; Johansen et al. 2020). Viral RNA had been eliminated from the lungs but managed to remain in other tissues after 6 days post-infection. Although juvenile cats had longer viral RNA shed in lung tissue, their upper respiratory tract had lower levels of viral. In line with this, infected wild cats displayed varying degrees of respiratory problems.
Non-human primates model
Rhesus macaques, cynomolgus monkeys, common marmosets, and African green monkeys, sometimes known as Chlorocebus aethiops, are non-human primates that have been used to study the pathogenesis of SARS-CoV-2 and test therapeutic strategies (Zhao et al. 2022). A study was conducted in which young and old cynomolgus macaques were exposed to the SARS-CoV-2 strain using a combination of routes of administration such as intratracheal and intranasal routes. Except for one old macaque that experienced nasal discharge after the 14th day following vaccination, no other macaques specifically showed any overt clinical signs or weight loss. Early in the infection, the virus was mostly discharged from the throat and nose. It is noteworthy that on the fourteenth day, a macaque shed virus RNA in rectal swabs. During the autopsies of four macaques, SARS-CoV-2 RNA was discovered in several tissues from the tracheobronchial lymph nodes, ileum, and respiratory tract (Rockx et al. 2020). When the SARS-CoV-2 virus was delivered to rhesus macaques in a study, it was shown that the animals exhibited high viral loads both in the upper and lower tracts and showed humoral and cellular immune responses. Most of the symptoms showed signs of viral pneumonia. A set of animals were re-exposed to SARS-CoV-2 after the initial viral clearance, and they had lower median viral loads in both bronchoalveolar lavage and nasal mucosa compared to before the initial infection (Chandrashekar et al. 2020).
Another study was done on rhesus macaques by exposing to SARS-CoV-2 developed a respiratory infection. Radiographs of the lungs of infected animals revealed pulmonary infiltrates, changes in respiratory pattern, alterations in piloerection, lack of appetite, hunchback, pale skin, dehydration, and weight loss. The macaque model essentially recapitulates the pathological hallmarks of COVID-19, as demonstrated by the fact that three out of four animals experienced mild to severe interstitial pneumonia with symptoms such as thickening of the alveolar septa, alveolar edema, and hyaline membranes (Munster et al. 2020). The vaccinations appeared to protect non-human primates because when the vaccinated animals contracted viral SARS-CoV-2, sampling of their bronchoalveolar lavage as well as nasal mucosa showed lower virus levels. Furthermore, the human monoclonal antibody CB6 protected rhesus macaques infected with SARS-CoV-2 by lowering viral titters and preventing pathological lung damage, suggesting that CB6 might be used as a COVID-19 therapy (Shi et al. 2020a, b).
According to recent research, African green monkeys (AGMs), another non-human primate, experienced strong SARS-CoV-2 replication and acquired a severe respiratory illness. SARS-CoV-2 shedding from the pulmonary and gastrointestinal tracts was also observed in AGMs, which may closely resemble the cases in humans. In conclusion, the evaluation of COVID-19 therapeutics and vaccines may have another alternative to using AGMs as animal models (Woolsey et al. 2021) (Hartman et al. 2020).
Apart from ethical implications, the main concern is eventually the choice of animal model to be used in the research for SARS-CoV-2.However, the local infrastructure and resources have a significant influence on this choice. Small animal models like hamsters, ferrets, or mice are the sole practical experimental strategy for many researchers.
Vaccination for animals
SARS-CoV-2 variations are now being studied for their impact on the effectiveness of existing vaccinations, treatments, and diagnostics. The SARS-CoV-2 virus has been associated with cross-species jumping in animals, raising zoonotic concerns about the possibility of reintroduction into human populations by interspecies transmission between people and animals. Domesticated animals such as cats, ferrets, and dogs, captive animals such as lion, tiger, puma, gorilla, and leopard, and wild and farmed minks have already been reported to have SARS-CoV-2 infections. The emergence of SARS-CoV-2 further into feral population and its subsequent transmission to wildlife may be prevented if domestic animals were vaccinated. Although it might appear unreasonable from a public health point of view to vaccinate sensitive and susceptible animal species such as dogs, minks, and cats, the complete eradication of SARS-CoV2 will still demand control over the transmission across all vulnerable animal species. This is needed to avoid SARS-CoV-2 from re-emerging in the future.
Vaccinations of domestic animals such as cats can help reduce the spread of SARS-CoV-2 to feral cats and subsequent transmission to wildlife. A coordinated effort has been displayed between Applied DNA Sciences (United States) and Evvi Vax (Italy) in actively developing a vaccine name Linear DNA™, a potential COVID-19 vaccine candidate to be used for cats. This vaccine has successfully acquired approval from the United States Department of Agriculture (USDA) to proceed to clinical studies in domestic felines and further assess its safety and immunogenicity (Brook 2020). Canines, lions, leopards, mice, and rabbits can all be treated with a new vaccine developed in India called Ancovax. The inactivated vaccine is developed using the highly epitopic component of the delta variant of SARS-CoV-2. Alhydrogel is also used as an adjuvant to strengthen the immunological response (Dutt 2022). A Russian-based vaccine called Carnivac Cov, especially designed for animals such as cats, dogs, minks, and foxes, has proven to be effective, and it is also said to be working against the mutated SARS-CoV-2 and its variants. It is also reported to prevent mutations in animals (Tétrault-Farber and Vasilyeva 2021).
Current challenges
In order to understand the etiology of acute infectious diseases and create vaccines and medications, animal models are vital. Animal Biosafety Level 3 (ABSL-3) facilities must be used for any animal investigations containing risk Grade 3 agents, including such SARS-CoV, HIV, Mtb, and H7N9 (Guo et al. 2019). Animal Biosafety Level 3 is the pre-requisite for working with the viruses and pathogens belonging to Risk group 3 micro-organisms (DBT 2018). The Risk Group 3 viruses involve SARS-CoV-2, MERS-CoV-2, Monkey Pox Virus, HIV, Swine Flu Virus, and West Nile Virus. Higher BSL-3 precautions are necessary in scenarios involving virus growth, zoonosis probable aerosol exposure, handling diverse and highly transmissible variants, involving laboratory animals (Yeh et al. 2021). In Nashik, Maharashtra, India, the nation's first mobile Biosafety Level 3 containment laboratory was unveiled. The purpose of the mobile laboratory is to look into viral illnesses that are very contagious and have the potential to be fatal to people. It is important to set up various BSL labs in order to be prepared for any pandemic like situations.
Discussion
This paper offers a summary of the present understanding of the connection between SARS-CoV-2 infections in animals. Based on a common assumption, SARS-CoV-2 started in bats and spread to humans through an unidentified intermediary host. Although it has not been clear about the primary source contributing to the SARS-CoV-2 pandemic, a number of animal species either naturally contracted the virus after coming into touch with an infected person or as a result of experimental infection. It has been suggested that a number of animal models might be used to evaluate potential SARS-CoV-2 vaccines or assess the effectiveness and safety of antiviral drugs.
The animal model proves to be an excellent idea to test the nature and function of the virus infection. The examples of the animal models include mice, hamster models, cat, ferret, and primate models. A well strategized plan should be emphasized which collaborates the interdisciplinary cooperation among several medical and research fields such as public health, environmental sciences, veterinary medicine, and social sciences which are the prime areas where strict and efficient measures should be followed to mitigate the spread of the virus. Combined approaches of classical epidemiology and contemporary biomedicine have helped us discover a great deal about SARS-CoV-2 in the recent year. It is astonishing how quickly the novel synthetic recombinant vaccines against SARS-CoV-2 are being developed, and recent breakthroughs may open the door for other RNA-based vaccinations in the future.
The new recombinant vaccines include the viral protein's mRNA and trigger a cascade that results in the production of potent, neutralizing antibodies, in contrast to conventional vaccinations, which typically provide inactivated viral proteins to create an immunizations process. An inactivated SARS-CoV-2 delta vaccine for animals is the Ancovax Vaccine for Equines Both the Delta and Omicron Variants of SARS-CoV-2 are neutralized by the immunity brought on by Ancovax. The vaccine includes Alhydrogel as an adjuvant and inactivated SARS-CoV-2 (Delta) antigen. Russian scientists have developed a vaccine to counteract the danger of animal-to-human transfer and the emergence of mutant versions. The Karnivak-Kov vaccination strategy has been successfully launched. The new vaccines appear to have excellent translation effectiveness even in light of the many SARS-CoV-2 mutations that are now evolving. Thus, they may be especially helpful for senior citizens who are maintaining companion pet animals. Despite the minimal risk shown by experimental and epidemiological evidence, a generalized warning against the possible pet-to-human transmission is however required while the probability of SARS-CoV-2 transmission between dogs and people is especially low. The most exposed and vulnerable members of the population in many nations begin to receive protection through vaccination programs that are successful. To prevent interaction between sick humans and animals, it is advised to practice basic hygiene precautions.
It has been suggested that a number of animal models might be used to evaluate potential SARS-CoV-2 vaccines or determine the efficacy and safety of antiviral medications. To mitigate the virus from re-emerging in the future, it is important to vaccinate vulnerable animal species like cats, minks, and great apes. This may appear preposterous, but it is the only way to successfully eradicate SARS-CoV-2 is by limiting the transmissions in all vulnerable animal species. A required vaccination passport should also be implemented in order to transfer domestic animals as well as wild and captive animals across international boundaries.
To mitigate the spread of SARS-CoV-2 across wild and domesticated animal species, a stringent transit restrictions must be implemented across international boundaries, and passive or active surveillance frameworks for domesticated, confined, and wild animals must be developed. An attempt to stop the spread of SARS-CoV-2 to local domestic and wild populations of animals and its ensuing re-emergence will be strengthened by such a move.
Conclusion and future prospects
It is very interesting to note that there is a lot of similarity that exists between SARS-CoV-2 and SARS-CoV in several aspects such as the susceptibility of cats and ferrets, transmission of infection to cage mates, and resistance of chickens and pigs to infection. This has given a new path for researchers where one can understand the nature of infection in the animal and wildlife. In order to conserve nature and to protect the wildlife species, it is very important to understand the new emerging viruses and its pathogenesis. It is very important to establish BSL-3 facilities at every regional level which will help in undertaking research related to Risk Group 3 and Risk Group 4 viruses and pathogens in animals. Investigations undertaken on different types of animal model also give us more clarity regarding the same. Large-level vaccination drive should be undertaken against most neglected diseases which can pose a threat in future. It is very important to break the chain of human-to-animal transmission and the animal-to-human transmission; this will only be possible if a large population of mankind and wildlife are vaccinated against a wide range of infectious viruses. Also, the wildlife and zoo authorities must always keep an eye on the infections that the inhabited animals may potentially carry. The petting zoos and animal park that allow the human visitors to interact with animals must be thoroughly screened. This can help in increasing the life expectancy of the wildlife and will also prevent them from becoming endangered or extinct.
Acknowledgements
The authors would like to acknowledge Indian Council of Medical Research (ICMR) (VIR/COVID19/33/2021/ECD-1) for funding.
Data availability
Data of the manuscript will be provided on request.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Aleem A, Bari Akbar Samad A, Slenker AK (2021) Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). StatPearls. http://www.ncbi.nlm.nih.gov/pubmed/34033342 [PubMed]
- Alnuqaydan AM, Almutary AG, Sukamaran A, Yang BTW, Lee XT, Lim WX, Ng YM, et al. Middle east respiratory syndrome (MERS) virus—pathophysiological axis and the current treatment strategies. AAPS PharmSciTech. 2021 doi: 10.1208/s12249-021-02062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F, Thapa M, Lei T, Sayed SM, Ahmed DC, Adelsberg JM, Carreno SS, et al. The plasmablast response to SARS-CoV-2 MRNA vaccination is dominated by non-neutralizing antibodies that target both the NTD and the RBD. MedRxiv. 2021 doi: 10.1101/2021.03.07.21253098. [DOI] [Google Scholar]
- Bai C, Zhong Q, Gao GF. Overview of SARS-CoV-2 genome-encoded proteins. Sci China Life Sci. 2022;65(2):280–294. doi: 10.1007/s11427-021-1964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri-Olmos R, Rosbjerg A, Johnsen LB, Helgstrand C, Bak-Thomsen T, Garred P, Skjoedt MO. The SARS-CoV-2 Y453F mink variant displays a pronounced increase in ACE-2 affinity but does not challenge antibody neutralization. J Biol Chem. 2021;296:100536. doi: 10.1016/j.jbc.2021.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Z, Hong W, Yang J, Shuaiyao Lu, Peng X. Animal models for SARS-CoV-2 infection and pathology. MedComm. 2021;2(4):548–568. doi: 10.1002/mco2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook S (2020) Applied DNA, EvviVax & GVS receive regulatory approval to begin veterinary clinical trial for lead linear DNA COVID-19 vaccine candidate. http://pharmabiz.com/NewsDetails.aspx?aid=133853&sid=2
- Chan JF, Woo AJ, Zhang SY, Poon VKM, Chan CCS, Lee ACY, Chan WM, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19. Clin Infect Dis. 2020;71(9):2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar A, Liu J, Martino AJ, McMahan K, Mercad NB, Peter L, Tostanosk LH, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369(6505):812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, Hiller M, et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci USA. 2020;117(36):22311–22322. doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DBT (2018) Regulations and guidelines on biosafety of recombinant DNA Research & Biocontainment 2017.” http://www.dbtindia.nic.in/wp-content/uploads/Regulations-Guidelines-for-Reocminant-DNA-Research-and-Biocontainment-2017.pdf
- Delahay RJ, de la Fuente J, Smith GC, Sharun K, Snary EL, Flores Girón L, Nziza J, et al. Assessing the risks of SARS-CoV-2 in wildlife. One Health Outlook. 2021 doi: 10.1186/s42522-021-00039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Jin Y, Liu Y, Sun J, Hao L, Bai J, Huang T, Lin D, Jin Y, Tian K. Serological survey of SARS-CoV-2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transbound Emerg Dis. 2020;67(4):1745–1749. doi: 10.1111/tbed.13577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dutt A (2022) India’s first covid-19 vaccine for animals : why the need was felt. The Indian Express June 11, 2.
- Fan Yu, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, He J, et al. Genome of the Chinese tree shrew. Nat Commun. 2013 doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- Franklin AB, Bevins SN. Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen. Sci Total Environ. 2020;733:139358. doi: 10.1016/j.scitotenv.2020.139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Wang W, Yuan L, Lin Y, Huang X, Chen R, Cai M (2021) Theranostics A SCID mouse-human lung xenograft model of SARS-CoV-2 infection. Theranostics 11(13):6607. 10.7150/thno.58321 [DOI] [PMC free article] [PubMed]
- Gao G, Liu W, Wong G, Wang J, Wang F, Li M (2022) Surveillance of SARS-CoV-2 in the environment and animal samples of the Huanan seafood market. Res Square. 10.21203/rs.3.rs-1370392/v1
- Goumenou M, Spandidos DA, Tsatsakis A. Possibility of transmission through dogs being a contributing factor to the extreme Covid-19 outbreak in North Italy. Mol Med Rep. 2020;21(6):2293–2295. doi: 10.3892/mmr.2020.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wang Y, Liu J, Huang Z, Li X. Biosafety and data quality considerations for animal experiments with highly infectious agents at ABSL-3 facilities. J Biosaf Biosecurity. 2019;1(1):50–55. doi: 10.1016/j.jobb.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, Ehrlich M, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–486. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann PJ, Masato Hatta DVM, Chiba S, Tadashi Maemura DVM, Fan S. Transmission of SARS-CoV-2 in domestic cats. New England J Med. 2020;383(6):590–92. doi: 10.1056/nejmc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Nambulli S, McMillen CM, White AG, Tilston-Lunel NL, Albe JR, Cottle E, et al. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog. 2020;16(9):1–24. doi: 10.1371/journal.ppat.1008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020 doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, Watanabe T et al (2020) Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Nat Acad Sci U.S.A. 117(28):16587–95. 10.1073/pnas.2009799117 [DOI] [PMC free article] [PubMed]
- Johansen MD, Irving A, Montagutelli X, Tate MD, Rudloff I, Nold MF, Hansbro NG, et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13(6):877–891. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan SR, Spratt AN, Cohen AR, Hasan Naqvi S, Chand HS, Quinn TP, Lorson CL, Byrareddy SN, Singh K. Evolutionary analysis of the delta and delta plus variants of the SARS-CoV-2 viruses. J Autoimmun. 2021;124:1–5. doi: 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikalan M, Chander V, Mahajan S, Deol P, Agrawal RK, Nandi S, Rai SK, et al. Natural infection of delta mutant of SARS-CoV-2 in Asiatic lions of India. Transbound Emerg Dis. 2021 doi: 10.1111/tbed.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist SR, Schäfer A, Martinez DR. Cell and animal models of SARS-CoV-2 pathogenesis and immunity. DMM Dis Models Mech. 2020 doi: 10.1242/dmm.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Shi Z, Meng Yu, Ren W, Smith C, Epstein JH, Wang H, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lim Y, Ng Y, Tam J, Liu D. Human coronaviruses: a review of virus-host interactions. Diseases. 2016;4(4):26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Yue Lu, Jin X, Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 2020;526(1):165–169. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. Coronavirus can infect cats—dogs, not so much. Nature. 2020 doi: 10.1038/d41586-020-00984-8. [DOI] [PubMed] [Google Scholar]
- Maxmen A. Wuhan market was epicentre of pandemic’s. Nature. 2022;603:15–16. doi: 10.1038/d41586-022-00584-8. [DOI] [PubMed] [Google Scholar]
- Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20(1):50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Chard LS, Wang Z, Wang Y. Syrian hamster as an animal model for the study on infectious diseases. Front Immunol. 2019;10:1–12. doi: 10.3389/fimmu.2019.02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch A, Wernike K, Ulrich L, Mettenleiter TC, Beer M. SARS-CoV-2 in animals: from potential hosts to animal models. Adv Virus Res. 2021;110:59–102. doi: 10.1016/bs.aivir.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Kumar N, Bhatia S, Aasdev A, Kanniappan S, Sekhar AT, Gopinadhan A, et al. Sars-Cov-2 delta variant among Asiatic Lions, India. Emerg Infect Dis. 2021;27(10):2723–2725. doi: 10.3201/eid2710.211500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnink BB, Oude RS, Sikkema DF, Nieuwenhuijse RJ, Molenaar EM, Molenkamp R, Van Der Spek A, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585(7824):268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nova N. Cross-species transmission of coronaviruses in humans and domestic mammals, what are the ecological mechanisms driving transmission, spillover, and disease emergence? Front Public Health. 2021;9:1–11. doi: 10.3389/fpubh.2021.717941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwenyi OA, Ravi Dyavar S, Acharya A, Podany AT, Fletcher CV, Ng CL, Patrick Reid S, Byrareddy SN (2020) Immuno-epidemiology and pathophysiology of coronavirus disease 2019 (COVID-19). J Mol Med 98(10):1369–1383 [DOI] [PMC free article] [PubMed]
- Opriessnig T, Huang YW. Coronavirus disease 2019 (COVID-19) outbreak: could pigs be vectors for human infections? Xenotransplantation. 2020;27(2):1–3. doi: 10.1111/xen.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N. SARS-CoV-2 infection in farmed minks. Euro Surveill. 2020;25(23):1–7. doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K, Acharya A, Mohan M, Ng CL, Reid SP, Byrareddy SN. Animal models for SARS-CoV-2 research: a comprehensive literature review. Transbound Emerg Dis. 2021;68(4):1868–1885. doi: 10.1111/tbed.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETA U. Coronavirus, Swine Flu, SARS: Major Diseases Linked to Eating Animals. n.d. “Swine Flu, SARS : Major Diseases Linked to Eatin Animals
- Perlman S, Fehr AR (2018) Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses: Methods Protoc, Methods Mol Biol, Springer vol.1282, 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed]
- Rabalski L, Kosinski M, Mazur-panasiuk N, Szewczyk B, Bienkowska-szewczyk K, Kant R, Sironen T, Pyrc K, Grzybek M (2022) Zoonotic spill-over of SARS-CoV-2 : mink-adapted virus in humans. Clinical Microbiology an Infectio 1:28(3):451 [DOI] [PMC free article] [PubMed]
- Rabalski L, Kosinski M, Smura T, Aaltonen K, Kant R, Sironen T, Szewczyk B, Grzybek M. Severe acute respiratory syndrome coronavirus 2 in farmed mink (Neovison Vison), Poland. Emerg Infect Dis. 2021;27(9):2333–2339. doi: 10.3201/eid2709.210286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Kok A, de Meulder D, Bestebroer TM, Lamers MM, Okba NMA, Fentener van Vlissingen M, et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun. 2020;11(1):1–6. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, De Meulder D, et al (2020) Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368(6494):1012–15. 10.1126/science.abb7314 [DOI] [PMC free article] [PubMed]
- Rothan HA, Acharya A, Reid SP, Kumar M, Byrareddy SN (2020) Molecular aspects of COVID-19 differential pathogenesis. Pathogens 9(7):538. 10.3390/pathogens9070538 [DOI] [PMC free article] [PubMed]
- Rudd JM, Selvan MT, Cowan S, Kao YF, Midkiff CC, Narayanan S, Ramachandran A, Ritchey JW, Miller CA. Clinical and histopathologic features of a feline SARS-Cov-2 infection model are analogous to acute Covid-19 in humans. Viruses. 2021;13(8):1–16. doi: 10.3390/v13081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailleau C, Dumarest M, Vanhomwegen J, Delaplace M, Caro V, Kwasiborski A, Hourdel V, et al. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound Emerg Dis. 2020;67(6):2324–2328. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan KM, Zhang H, Senthil R, Vijayakumar KK, Sounderrajan V, Wei Y, Shakila H. Structural basis for the inhibition of SARS-CoV2 main protease by indian medicinal plant-derived antiviral compounds. J Biomol Struct Dyn. 2022;40(5):1970–1978. doi: 10.1080/07391102.2020.1834457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severe Acute Respiratory Syndrome (SARS) Epidemiology Working Group (2003) SARS Report WHO. https://apps.who.int/iris/handle/10665/70863
- Sharun K, Tiwari R, Patel SK, Karthik K, Yatoo MI, Malik YS, Singh KP, et al. Coronavirus disease 2019 (COVID-19) in domestic animals and wildlife: advances and prospects in the development of animal models for vaccine and therapeutic research. Hum Vaccin Immunother. 2020;16(12):3043–3054. doi: 10.1080/21645515.2020.1807802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Shou S, Liu M, Yang Y, Kang N, Song Y, Tan D, Liu N, Wang F, Liu J, Xie Y. Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.626553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sila T (2022) Suspected cat-to-human transmission of SARS-CoV-2. Thailand, July–September 2021. Emerg Infect Dis 28(7):1485–1488 [DOI] [PMC free article] [PubMed]
- Song Z, Bao L, Yu P, Qi F, Gong S, Wang J, Zhao B et al (2021) SARS-CoV-2 Causes a systemically multiple organs damages and dissemination in hamsters. Front Microbiol 11:1–15. 10.3389/fmicb.2020.618891 [DOI] [PMC free article] [PubMed]
- Suarez DL, Pantin-jackwood MJ, Swayne DE, Lee SA, Deblois SM (2020) In Poultry 26(12):3074–3076 [DOI] [PMC free article] [PubMed]
- Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- Tétrault-Farber G, Vasilyeva M (2021) Russia registers world’s first COVID-19 vaccine for animals. Reuters [(accessed on 15 May 2021)]. 2021. “Russia Registers ’ World’s First’ COVID Vaccine for Animals,” no. March: 2021–22
- Vergara-Alert J, Rodon J, Carrillo J, Te N, Izquierdo-Useros N, Luisa Rodríguez de la Concepción M, Ávila-Nieto C et al (2021) Pigs are not susceptible to SARS-CoV-2 infection but are a model for viral immunogenicity studies. Transbound Emerg Dis 68 (4): 1721–25. 10.1111/tbed.13861 [DOI] [PMC free article] [PubMed]
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020 doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO-FAO-WOAH (2021) SARS-CoV-2 in animals used in fur farming: GLEWS + Risk Assessment,” no. January.
- Woolsey C, Borisevich V, Prasad AN, Agans KN, Deer DJ, Dobias NS, Heymann JC, et al. Establishment of an African green monkey model for COVID-19 and protection against re-infection. Nat Immunol. 2021;22(1):86–98. doi: 10.1038/s41590-020-00835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobey M, Levy JI, Malpica Serrano LM, Crits-christoph A, Pekar JE, Goldstein SA, Rasmussen AL, Kraemer MUG, Newman C, Koopmans MPG (2022) The Huanan market was the epicenter of SARS-CoV-2 emergence. Zenodo
- Yeh KB, Tabynov K, Parekh FK, Mombo I, Parker K, Tabynov K, Bradrick SS, et al. Significance of high-containment biological laboratories performing work during the COVID-19 pandemic: biosafety level-3 and -4 labs. Front Bioeng Biotechnol. 2021;9:1–9. doi: 10.3389/fbioe.2021.720315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cai Y, Lavine CL, Jianming Lu, Xiao T, Chen B, Zhang J, et al. Article structural and functional impact by SARS-CoV-2 omicron spike mutations Ll Ll structural and functional impact by SARS-CoV-2 omicron spike mutations. Cell Rep. 2022;39(4):110729. doi: 10.1016/j.celrep.2022.110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ, et al. Rapid generation of a mouse model for middle east respiratory syndrome. Proc Natl Acad Sci USA. 2014;111(13):4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Liu P, Zhao L, Ye F, Wang H, Zhou W, Zhu N et al (2020a) The pathogenicity of SARS-CoV-2 in HACE2 transgenic mice. 10.1038/s41586-020-2312-y [DOI] [PubMed]
- Zhao Y, Wang J, Kuang D, Jingwen Xu, Yang M, Ma C, Zhao S, et al. Susceptibility of tree shrew to SARS-CoV-2 infection. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-72563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fan J, Liu E. Animal models for COVID-19 therapeutic development: where we are and where we need to go. Front Microbiol. 2022;13:2–5. doi: 10.3389/fmicb.2022.907406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Ben Hu, Zhang L, Zhang W, Si HR, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of the manuscript will be provided on request.