Abstract
Loss of imprinting (LOI) is an epigenetic event which relaxes an allele-specific restriction on gene expression. One gene that experiences LOI is the paracrine insulin-like growth factor IGF2, which occurs commonly in human prostate tissues during aging and tumorigenesis. However, the relationship between IGF2 LOI and prostate tumorigenesis has not been established functionally. In this study, we created a mouse model with CTCF binding site mutations at the Igf2-H19 imprint control region that abolishes CTCF insulator activity, resulting in biallelic Igf2 expression that mimics increased levels seen with aging-induced LOI. We found that Igf2 LOI increased the prevalence and severity of prostatic intraepithelial neoplasia (PIN), a pre-malignant lesion. Engineering Nkx3.1 deficiency into our model increased the frequency of PIN lesions in an additive fashion. Prostates harboring LOI displayed increased MAPK signaling and epithelial proliferation. In human prostate tissue arrays, we documented a positive correlation in benign tissues of IGF2 levels with phospho-ERK and phospho-AKT levels. Overall, our results establish that Igf2 LOI is sufficient on its own to increase rates of neoplastic development in the prostate by upregulating critical cancer-associated signaling pathways.
Keywords: Loss of imprinting, prostate cancer, aging, Igf2, epigenetics
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer in the male U.S. population, and the second most common cause of death in aging men (1). Several etiologic factors, including diet and aging, have been strongly implicated in the development of this disease (2). Nearly 80% of men age 80y develop PCa based on autopsy findings (3). In addition, numerous foci of cancer are often found upon histopathological examination confirming the multifocal nature of PCa (3). How these epidemiologic factors contribute to the initiation and progression of PCa at the molecular level remains elusive and is an important key in understanding and preventing this disease.
Accumulating data supports an age-related erosion of the epigenome in the predisposition of the prostate to cancer (2). To date, one of the few alterations implicated in aging non-cancerous peripheral prostate tissue and in associated prostate tumors is the finding of a loss in the typical imprint of the Insulin-like growth factor-II gene (IGF2) (4). Genomic imprinting is an epigenetic modification that leads to the differential expression (i.e. only from one allele) of a gene based on parental origin. IGF2, a paracrine and autocrine regulator of cell proliferation is tightly regulated and maintains a strict imprint pattern in adult tissues. Alterations in IGF2 regulation, via a loss of imprinting (i.e. biallelic expression) have been demonstrated to occur in the human prostate with aging (4,5). Whether IGF2 LOI directly underlies proliferative changes in the aging prostate remains an unanswered question.
IGF2 shares structural similarity to insulin, and possesses mitogenic activity (6). IGF2 signals by binding to either IGF-1 receptor (IGF1R), Insulin receptor isoform A (IR-A), or the IGF1R-IR-A hybrid receptor. The IGF2 receptor can bind IGF2, but lacks intracellular signaling activity. IGF2 signaling is complex, however, mitogenic responses to IGF2 are predominantly achieved via IR or IGF1R induced PI3-K/Akt or MAPK/ERK pathway activation (6). In addition, IGF2 binding proteins (IGFBPs) are reactivated in a variety of malignancies, and have been hypothesized to enhance or alter the response to IGF2 signaling (7). The IGF2 gene is located within the IGF2-H19 imprinted locus, which includes a differentially methylated region (DMR) and shared downstream enhancers. Imprinted expression is achieved via allele-specific methylation at the differentially methylated region (DMR), dictating CTCF insulator activity and ultimately directing enhancer association with either IGF2 or H19 promoter sequences, driving their expression.
The homeobox gene NKX3.1 is continuously expressed in the epithelium during prostate development and in adulthood, but is commonly downregulated early in prostate cancer development (8). Human NKX3.1 exists at chromosome 8p21, a region that frequently undergoes loss of heterozygosity (LOH) early in prostate carcinogenesis (9,10). It is also regulated in part by PTEN, a gene mutated in 30% of primary prostate cancers (11). Nkx3.1 mutant mice develop prostatic hyperplasia and dysplasia yet fail to progress to frank cancers (8,12). Restoration of NKX3.1 expression in PTEN deficient cells reverts their phenotype and the progression of prostate cancer in the Pten null mouse (13), suggesting a key early role for NKX3.1 in prostate cancer.
IGF2 loss of imprinting (LOI) has been implicated in a range of human disease following its original description in Wilm’s Tumors (14). In humans, IGF2 LOI has been demonstrated in prostate (4), ovarian (15), esophageal (16), breast (17), and colon cancers (18). In addition to tumors of the prostate, IGF2 LOI is seen in histologically normal human peripheral prostate tissue and was more extensive in prostates with associated cancer (4,5). Various animal models leading to IGF2 overexpression have demonstrated increased risk of malignancy including mammary, lung, and colon cancers (6). However, these models often alter H19 expression, which may also play a role in tumorigenesis (19,20), or use transgenic overexpression of IGF2 resulting in levels not normally seen with aging (21,22).
A mouse with point mutations within CTCF binding sites at the H19 ICR results in biallelic Igf2 expression without altering H19 expression (23). Using this animal model, the role of biallelic Igf2 expression on neoplastic development was assessed in the prostate. In addition, this animal model was crossed with the Nkx3.1 mutant mouse model. Nkx3.1 mutant mice develop prostatic intraepithelial neoplasia (PIN) lesions without progression to cancer (24). Nkx3.1 is also known to negatively impact AKT signaling (13), a common downstream target of Insulin-like growth factor signaling. Therefore, we tested whether Igf2 LOI in the context of Nkx3.1 loss results in increased rates of PIN or cancer development.
Materials and Methods
Maintenance (MOI) and loss of imprinting (LOI) mice generation and Nkx3.1 crosses
All mouse experiments were performed under a UW-IACUC approved IRB protocol. A mouse model of Igf2 LOI previously generated by Pant et al. contains mutations in three of the four CTCF target sites within the Igf2-H19 imprint control region (ICR) changing the core sequence from GTGG to ATAT (142* mutation) (23,25). This sequence change deletes essential CTCF contact points while preserving the CpGs responsible for the methylation-sensitive CTCF targeting. Maternal transmission of 142* mutation results in offspring exhibiting Igf2 LOI (H19+/−) thereby expressing the normally silenced allele. Mice carrying the 142* mutation were backcrossed on a C57Bl/6J background for 7 generations.
An Nkx3.1 knockout mouse containing a targeted gene disruption of the Nkx3.1 homeodomain and carboxy-terminal protein sequences that generates a null mutation were used (8), available from the NCI mouse repository (Strain Number: 01XB3). These mice have been maintained on a hybrid 129/SvlmJ and C57Bl/6J background. To create experimental mice with their Igf2 imprint maintained on the prostate specific Nkx3.1 knockout background, 142* mice were bred with Nkx3.1 knockout mice to create male breeders (H19−/−;Nkx3.1+/−). These male breeders (H19−/−;Nkx3.1+/−) were bred with female H19+/+,Nkx3.1+/− and the subsequent male offspring carry Nkx3.1 mutation at varying rates (+/+, +/−, −/−) and inherit the 142* mutation paternally. Their maternal imprint is maintained, resulting in normal Igf2 expression levels. To generate Igf2 LOI mice, H19−/−Nkx3.1+/− female breeders were created and bred with H19+/+;Nkx3.1+/− male mice. These offspring inherit characteristic Nkx3.1 mutations, but maternally inherit the mutant H19 ICR allele. However, the male offspring express both Igf2 alleles since Igf2 silencing is lost with the 142* mutation on the maternal allele (Fig. 1A). Mice were aged to 6, 12, and 18 months before tissue collection.
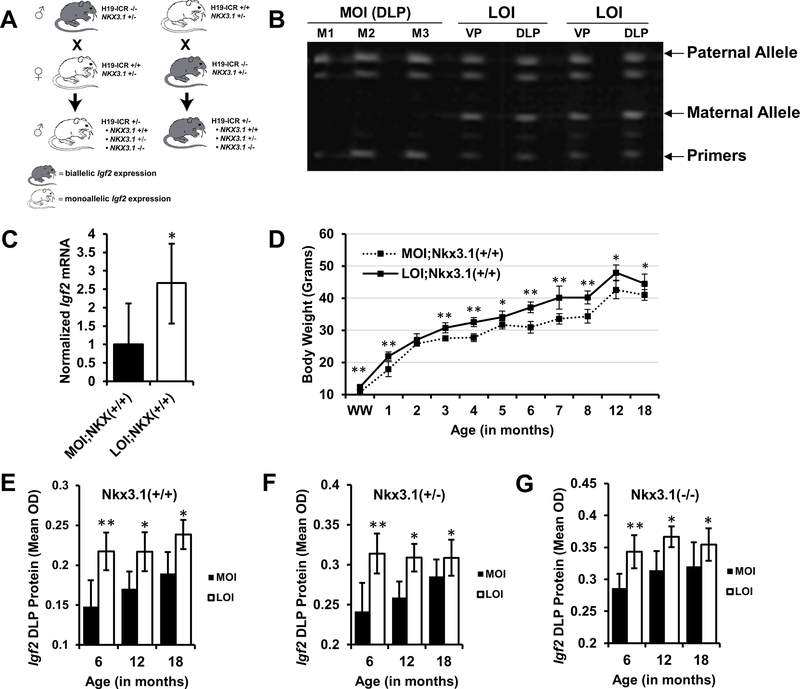
Figure 1. Biallelic expression of Igf2 in 142* mice.
A, Breeding schemes for generation of maintenance (monoallelic Igf2) and loss of imprinting (biallelic Igf2) mice. Maternal transmission of 142* mutation (H19-ICR +/−) results in biallelic expression in offspring by abolishing CTCF insulator activity at the normally silenced maternal allele. Paternal transmission leads to the typical monoallelic expression. B, Ventral prostate (VP) and Dorsolateral prostate (DLP) tissues were analyzed for maintenance of imprinting (MOI) and loss of imprinting (LOI) in mice and maternal and paternal allelic expression was quantitated using FluPE as described in methods. MOI mice maintain expression from a single allele, while LOI mice exhibit biallelic expression. C, Igf2 mRNA expression measured by qPCR in mouse prostates (*P<0.05, mean+SD; n = 6 each group). D, Body weights of experimental animals were recorded throughout their lifetime. LOI animals expressing biallelic Igf2 were persistently heavier throughout their life (mean+95% CI; **P<0.01, *P<0.05). E, F, G, Igf2 protein expression was quantitatively analyzed on tissue microarrays constructed from mouse prostate tissues. Igf2 protein expression was significantly elevated in LOI animals with (E) Nkx3.1+/+ (P=0.001, P=0.015, P=0.012; 6, 12, 18 months), (F) Nkx3.1+/− (P=0.002, P=0.02, P=0.04; 6, 12, 18 months) and (G) Nkx3.1−/− (P<0.001, P=0.029, P=0.043; 6, 12, 18 months), backgrounds across all ages (*P<0.05, columns represent Mean+SD; n > 8 for each group).
Analysis of Igf2 LOI via FluPE (Fluorescent Primer Extension Assay)
C57Bl/6(cast H19-p57), a C57Bl/6 congenic strain heterozygous for distal chromosome 7 sequences from Mus castaneus were used to analyze Igf2 LOI status in 142* mice prostates. Male mice homozygous for M. castaneus alleles (H19-p57) were bred with female 142* mutant heterozygous mice (H19+/−). Igf2 LOI in male mice aged 2 months were analyzed using FluPE assay as previously described (4).
Analysis of Prostate Tissue Pathology
Prostate tissues were scored for Prostatic Intraepithelial Neoplasia (PIN based on criteria as described by Park et al. (26). Low grade PIN lesions (PIN 1/2) consist of foci with few layers of atypical cells, pale cytoplasm and abundant heterochromatic nuclei, but containing minimal pleomorphism. Intermediate grade PIN lesions (PIN 3) consist of larger foci that do not fill the lumen of the ducts but exhibit cribiform, papillary, or tufting patterns. Glandular structure is compromised with poor orientation of atypical cells. Nuclei exhibit pleomorphism and heterochromasia with abundant pale cytoplasmic staining. High grade PIN lesions (PIN 4) consist of atypical cells filling the lumen of the ducts, nuclear and cytoplasmic patterns are similar to intermediate grade PIN, however increasingly severe pleomorphism and hyperchromasia are seen. Inflammatory responses are marked by lymphocyte invasion.
Histologic analyses of mouse prostate tissues were performed on male mice at 6, 12, and 18 months of age. Upon tissue collection ventral (VP) and dorsolateral prostate (DLP) tissues were formalin-fixed and paraffin embedded. H&E stained slides were analyzed on a blinded basis independently by W.Z. and M.L.B. Prostate tissues of experimental animals were first analyzed for the highest scoring PIN lesion seen in serial FFPE H&E stained tissue sections (Table 1). H&E slides were then scanned and, in a blinded fashion, the total number of normal and PIN (of any grade) affected glands were scored for each prostate. Sections from the same animal were averaged to obtain a percentage of PIN affected glands.
Table 1:
Prostatic Intraepithelial Neoplasia (PIN) Occurrence in Experimental Animals
| Maintenance of Imprinting | Loss of Imprinting | |||||||
|---|---|---|---|---|---|---|---|---|
| Nkx3.1(+/+) | Total | NL | LG | IG/HG | Total | NL | LG | IG/HG |
| 6 Months | 16 | 4 | 10 | 2 | 13 | 1 | 5 | 7 |
| 12 Months | 15 | 4 | 6 | 5 | 12 | 3 | 4 | 5 |
| 18 Months | 11 | 2 | 5 | 4 | 16 | 0 | 6 | 10 |
| 42 | 10 (24%) | 21 (50%) | 11 (26%) | 41 | 4 (10%) | 15 (37%) | 22 (54%) | |
| Nkx3.1(+/−) | ||||||||
| 6 Months | 11 | 1 | 7 | 3 | 12 | 2 | 3 | 7 |
| 12 Months | 11 | 2 | 4 | 5 | 13 | 1 | 5 | 7 |
| 18 Months | 10 | 3 | 3 | 4 | 16 | 2 | 4 | 10 |
| 32 | 6 (19%) | 14 (44%) | 12 (38%) | 41 | 5 (12%) | 12 (29%) | 24 (59%) | |
| Nkx3.1(+/−) | ||||||||
| 6 Months | 11 | 0 | 4 | 7 | 10 | 0 | 2 | 8 |
| 12 Months | 10 | 0 | 3 | 7 | 15 | 0 | 3 | 12 |
| 18 Months | 11 | 0 | 3 | 8 | 14 | 0 | 5 | 9 |
| 32 | 0 (0%) | 10 (31%) | 22 (69%) | 39 | 0 (0%) | 10 (26%) | 29 (74%) | |
*NL (normal), LG (Low grade PIN), IG (Intermediate grade), HG (High grade). The highest scoring lesion in each mouse prostate was determined.
cDNA preparation and quantitative PCR
Total RNA was extracted from OCT embedded or flash frozen tissues using the PerfectPure RNA Tissue Kit (5prime) as described by the manufacturer. RNA was DnaseI treated during extraction, cDNA was synthesized from 500 ng of total RNA using Omniscript RT reagents (Qiagen) and both oligo(dT) and random hexamers. Samples without reverse transcriptase served as negative control to detect gDNA contamination. Quantitative PCR (qPCR) was performed with PerfeCta SYBR Green FastMix (Quanta Biosciences) on a CFX96 (Bio-Rad). A geometric mean using Gapdh, 18s, and Actb was used for reference. Primer sequences are provided in Supplementary Table 1.
Quantitative Automated Immunohistochemistry
Tissue microarrays were constructed using mouse DLP tissues to quantitatively analyze protein expression in prostate tissues. Preparation of slides and antigen retrieval methods have been previously described (27). Two dual stains were performed on sequential sections, IGF2 (LS-B9544; LSBio, Seattle, WA) versus p-ERK (#4370, Cell Signaling, Danvers, MA) and IGF2 versus p-AKT (#4060, Cell Signaling, Danvers, MA). KI67 (ab15580; Abcam, Cambridge, UK) and TUNEL (Cat#: 12156792910, Roche, Basel, Switzerland) staining was performed on additional sections. Stained slides were scanned as previously described (27). Human tissue microarrays (hTMA) constructed as previously described (27) containing duplicate cores of 48 benign prostate tissues (from radical prostatectomy specimens), 74 PCa tissues, and 22 metastatic samples from PCa patients were used for IHC analysis. Two triple stains were performed on hTMA slides using IGF2/p-ERK/E-cadherin (Ventana #790–4497; Roche, Basel, Switzerland) antibodies and IGF2/p-AKT/E-cadherin antibodies. E-cadherin antibodies were used to segment epithelial and stromal compartments in human prostate tissues. Cellular protein for individual cores was quantitated using the VECTRA™ imaging system according to manufacturer’s protocols (Caliper Life Sciences, Hopkinton, MA). InForm 1.2™ software was used to segment epithelium vs. stroma and nuclear vs. cytoplasmic tissue compartments. Cores from the same patient were averaged to give a more precise estimate. Cores with <5% epithelial component or loss of tissue were excluded from the analysis.
Prostate Cell Lines
LNCaP cell lines were obtained from ATCC in 2006, stocks were generated and frozen at low passage (<25). Mycoplasma testing and cell line authentication by DNA Short Tandem Repeats (STR) analysis was conducted in 04/2016 on cultures from frozen stocks by UW Madison TRIP Laboratory. C4–2B cell lines were were generated by George Thalmann and obtained from the the Lelund Chung Laboratory at low passage (<10) in 2006. Mycoplasma testing and cell line authentication (STR analysis) on cultures from frozen stocks were conducted in 12/2015 by DDC Medical. BHPrE1 and BPH1 cell lines were developed and obtained from the Simon Hayward Laboratory at low passage (<20) in 2010. Mycoplasma testing on cultures from frozen stocks was performed in 03/2016. Since these are not commercially available to compare STRs to known libraries, they were tested for human specificity using species PCR insert SV40Tag for BPH1, and p21/RB high expression and GFP green fluorescence for BHPrE1, which was developed by retroviral infection with a pBird–cytomegalovirus–enhanced green fluorescent protein (EGFP) vector and selected by FACS–green fluorescent protein (GFP) sorting in 11/2014.
All cell line experiments were conducted in 03/2017. Frozen stocks originating from the same freeze dates as those tested here were thawed and allowed at least 2 passages to establish cultures (~ 1 week) before beginning experiments.
Nkx3.1 Knockdown in Prostate Cell Lines and Western Blotting
Immortalized non-tumorigenic human prostate epithelial cell line BHPrE1 and BPH1 cells, dervived from BPH patients, was used to detect Nkx3.1 effects on ERK/AKT phosphorylation. BHPrE1 has been maintained in 50/50 DMEM/F12 medium containing 5% FBS, 1% ITS-X, 0.4% BPE, 10ng/ml EGF and 1% PS. BPH1 cells were cultured in RPMI-1640 supplemented with 5% FBS and 1% PS. LNCaP and C42B were cultured in DMEM media containing 5% FBS and 1% PS.
Human Nkx3.1 shRNA and control shRNA lentiviral particles were purchased from Santa Cruz Biotechnology. Nkx3.1 shRNA lentiviral particles are a pool of concentrated, transduction ready viral particles containing 3 target-specific constructs that encode 19–25 nt (plus hairpin) shRNA designed to knock down gene expression. BHPrE1 cells were plated at 1×105 into a 12-well plate, 24 hours later the cells reached approximately 50% confluent. Cells were transduced with lentiviral particles and 5 μg/ml polybrene, per manufacturer’s protocols. Stable clones were selected using 8ug/ml of puromycin, replaced every 48h. Cells were collected after one week and lysed in 30mM Tris buffer containing 250mM NaCl, 1mM EDTA, 1%NP-40 and protease/phosphatase inhibitors. 40 micrograms of protein lysis were loaded for western blotting and probed for NKX3.1 (Cat#: 820C3a; Santa Cruz Biotechnology, Santa Cruz, CA), p-AKT, p-ERK (using previously referenced antibodies), and α-Tubulin (Cat#: CP06100UG; EMD Millipore, Billerica, MA)
Statistical Analysis
Statistical analyses were performed with the use of GraphPad Prism 5.0 Software and Excel 2016 (Microsoft). Quantitative IHC, mRNA expression, and histologic analyses were compared using the Student’s t-test when assumptions are met (normal distribution and equality of variance), or the Wilcoxon rank sum test where appropriate. Categorical data was compared using Fisher’s Exact. P values of <0.05 were considered as significant.
Results
142* Mutant (LOI) Mice Exhibit Biallelic and Increased Igf2 Expression in the Prostate
Our previous work had demonstrated that biallelic Igf2 expression occurs in the aging mouse and human prostate and this finding in the histologically normal prostate tissue was linked to the presence of human cancer (4). Construction of a mouse model with mutated CTCF binding sites at the H19-Igf2 imprint control region leads to a premature biallelic Igf2 expression (23). Figure 1A demonstrates the breeding schema employed for the current study. Allele-specific expression was initially analyzed in experimental mouse prostates at 2 months of age by Fluorescent Primer Extension Assay (FluPE). Wildtype mice exhibit monoallelic Igf2 expression indicating maintenance of imprinting (MOI) in both the ventral prostate (VP) and dorsolateral prostate (DLP) tissues. In contrast, the normally silenced Igf2 allele is expressed when the 142* mutation is inherited maternally (LOI) resulting in biallelic Igf2 expression (Fig. 1B). In kidney, liver, bladder, and testis, control mice maintain Igf2 imprinting while LOI mice exhibit biallelic expression in these tissues (data not shown).
To determine if LOI translates into changes in Igf2 gene expression, mRNA was analyzed in experimental mice using quantitative real-time PCR. Experiments focused on the mouse DLP due to its anatomic similarities to the human peripheral prostate, the site of PCa development, and the previous association of LOI with aging in this prostatic region (4). At 6 months of age, Igf2 mRNA expression is 2.7 fold higher in LOI mouse prostates when compared to controls (P = 0.02) (Fig. 1C). Expression of H19, a downstream tumor suppressor gene, was not significantly altered in LOI mice (Supplementary Fig. S1A), an advantage in utilizing the 142* mice that specifically target CTCF binding.
In LOI animals biallelic expression of Igf2 occurs in all tissues tested. Body weight measurements taken throughout experimental mice lifetimes (Fig. 1D) demonstrate heavier mice at weaning (21 days of age) compared to MOI (12.3g vs. 10.7g, P=0.016). LOI mice are 14% heavier (on average) throughout their lifetime with the largest differences seen at 6 and 7 months of age (20%, both P<0.001). Similar results were seen in Nkx3.1+/− and Nkx3.1−/− animals containing LOI (Supplementary Fig. S1B-C). Enlarged liver size (8%) is noted in older LOI animals (>18mo), but no other gross abnormalities were seen at necropsy.
NKX3.1 mutant mice, a homeobox 1 family member frequently inactivated early in PCa development (28), was bred onto MOI and LOI Igf2 backgrounds to examine whether a synergistic effect of increased androgen receptor (AR) and AKT signaling on neoplastic potential occurs in these mice prostates (Fig. 1A). Immunohistochemical analysis for IGF2 on tissue microarrays constructed from mouse prostate tissues confirmed the mRNA results. A significant increase in IGF2 protein was observed in all LOI genotypes including LOI;Nkx3.1+/+, LOI;Nkx3.1+/−, and LOI;Nkx3.1−/− mice (Fig. 1E-G) compared to genotype matched MOI animals, although a less extensive change was noted for homozygous Nkx3.1 deletion prostates. MOI mice for all three Nkx3.1 genotypes exhibit increases in Igf2 expression with older age as previously demonstrated (4).
Loss of Igf2 Imprinting Increases Rates of High Grade Prostatic Intraepithelial Neoplasia in Mouse Prostate Tissues
Prostatic intraepithelial neoplasia (PIN) is a marker of early neoplastic disease in humans and is used as a histologic surrogate in rodent models for neoplasia (26). Increased PIN in the context of Nkx3.1 knockout (24) and other genetically engineered mouse models has been described (26). Tissues were analyzed for the presence and prevalence of PIN lesions using validated grading criteria established by Park et al (26) (Fig. 2A-F). The number of mice with low (PIN 1 and 2) versus intermediate/high grade (PIN 3 and 4) lesions were quantified based upon the most severe lesion found within the prostate (Table 1).
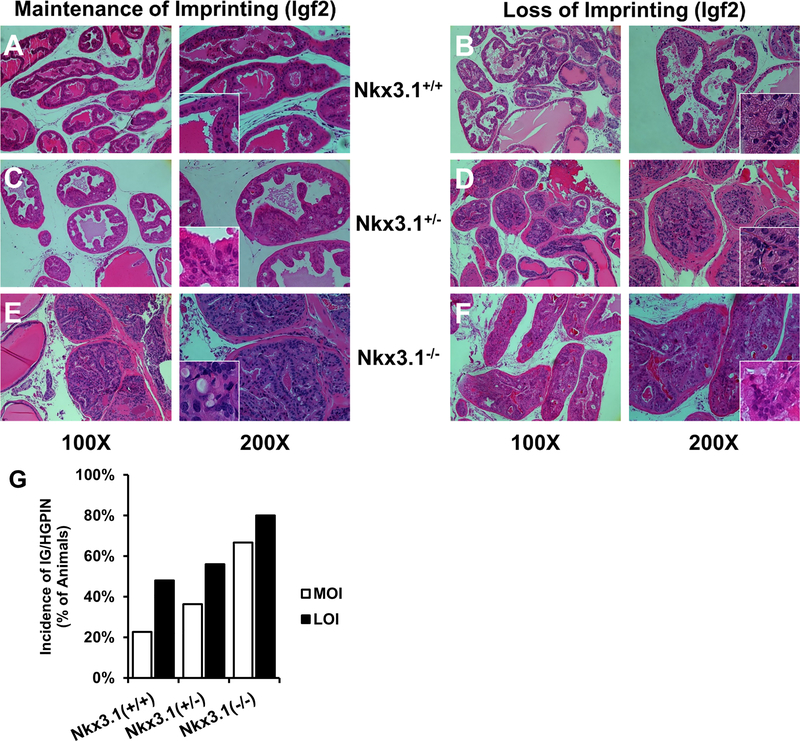
Figure 2. Histopathology of PIN lesions and demonstration of increased prevalence of PIN in LOI mice.
H&E stained FFPE mouse prostate tissues matched by Nkx3.1 status (horizontally) and Igf2 imprint status (vertically) at 12 mo. PIN grading was performed as described by Park et al. (25) and low (PIN 1 and 2) versus intermediate/high grade (PIN 3 and 4) lesions were based on the most severe lesion within the prostate. (A) In normal MOI;Nkx3.1+/+ prostate tissues, glands are lined with columnar epithelial cells and exhibit uniform staining of cytoplasmic and nucleic structures (400X Inset). Low grade (LG) PIN lesions (B,C) were seen in all genotypes surveyed, marked by nuclear pleomorphism and mild disorganization of cell layers projecting into the lumen. More severe PIN lesions (D, E, F) contain predominantly cribriform patterns, with increasing severity seen with Nkx3.1 deletion. G, Incidence of IG/HGPIN in 6 and 12 month prostate tissues. LOI prostates display increased prevalence and severity of PIN lesions. Multiple adjacent glands are affected with similar grade PIN in LOI animals.
When examining all genotypes, the presence of any PIN lesion (1–4) in Igf2 LOI prostates occurred with modestly increased frequency when all genotypes and ages were compared to MOI mice (77% vs 89%; MOI, LOI respectively, P=0.088; Fisher’s Exact). Examining only IG/HGPIN demonstrates higher rates in LOI animals compared to MOI animals (62% vs. 42%; respectively, P=0.004; Fisher’s Exact). As shown in Table 1 and Figure 2G, LOI mice develop increasing rates of IG/HG PIN more commonly than MOI animals across multiple NKX3.1 genetic backgrounds.
Loss of Igf2 Imprinting Increases the Multifocality of Prostatic Intraepithelial Neoplasia
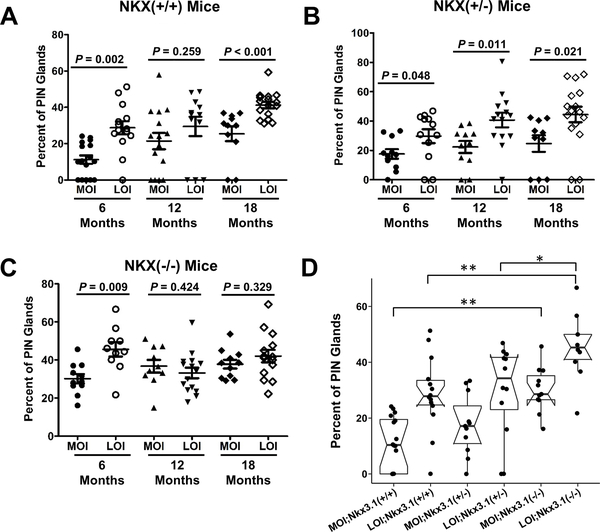
On histologic analysis, LOI prostates displayed the increased presence of multifocal PIN similar to a widespread field effect seen in human disease (2). To quantify this observation, the number of PIN affected glands (any PIN) out of total glands within each prostate was quantified (Fig. 3A-C). LOI mice with a wildtype Nkx3.1 background exhibit an increased proportion of PIN lesions at 6 months compared to MOI;Nkx3.1+/+ (11%+2.3 vs. 29%+3.5; MOI, LOI respectively, P = 0.002) and at later time points (Fig. 3A). LOI in prostate tissues from Nkx3.1+/− mice also demonstrate an increased prevalence of PIN glands at all ages examined compared to MOI (Fig. 3B). In Nkx3.1 homozygous knockout animals (Nkx3.1−/−) (Fig. 3C) significantly higher frequencies of PIN lesions were seen in LOI mice at 6 months (P = 0.009), however the frequency of PIN lesions in this genotype did not significantly differ at later time points.
Figure 3. Igf2 LOI results in an increased multifocality within PIN affected prostate glands.
H&E stained sections of mouse prostate tissue were quantified for the number of normal and PIN appearing glands. Proportions of PIN affected glands were compared in (A) Nkx3.1+/+, (B) Nkx3.1+/−, and (C) Nkx3.1−/− mice. A, LOI;Nkx3.1+/+ animals present significantly greater PIN affected glands at 6 and 18 months, at 12 months similar differences were apparent but non-significant. B, LOI;Nkx3.1+/− animals present significantly greater PIN affected glands at all ages (6, 12, 18 mo). C, Nkx3.1−/− animals exhibit a significantly increased proportion of PIN affected glands at 6 months, but non-significant differences at 12 and 18 months. D, Frequency of PIN lesions increase with Nkx3.1 mutation and in the presence of LOI in an additive fashion in 6mo animals (*P<0.05, **P<0.01).
A comparison across all genotypes at 6 months of age demonstrates an additive effect of Nkx3.1 mutation and Igf2 LOI on PIN formation (Fig. 3D). This effect is clearly demonstrated when comparing Nkx3.1−/− mice to their age and imprint status-matched controls. MOI;Nkx3.1−/− developed a significantly higher proportion of PIN affected glands than MOI;Nkx3.1+/+ (P < 0.001) and MOI;Nkx3.1+/− (P = 0.006) animals. LOI;Nkx3.1−/− animals at 6 months of age also display a significantly higher proportion of PIN than LOI;Nkx3.1+/+ (P = 0.004) and LOI;Nkx3.1+/− (P = 0.016) animals, while also exhibiting higher percentage of PIN than MOI;Nkx3.1−/− (P = 0.009) (Fig. 3D). Thus, the Nkx3.1 background leads to an increased frequency of PIN affected glands when compared to wildtype animals (Fig. 3A-C) while an additive effect on PIN formation is observed in the presence of LOI (Fig. 3D). Nkx3.1 and LOI together did not result in the formation of invasive cancer within the time points examined.
Proliferation and p-ERK is Significantly Elevated in Mice Exhibiting Increased PIN
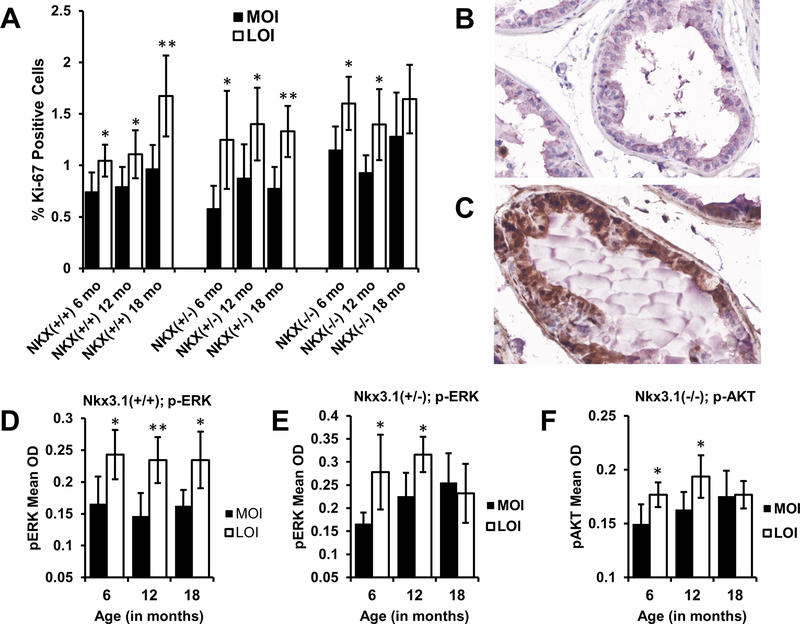
IGF2 signaling occurs by binding to the Insulin-like growth factor 1 (IGF1R), Insulin Receptor isoform A (IR-A), or the IGF1R-IR-A hybrid receptors. Signaling via these receptors activates unique and shared downstream pathways including PI3K/AKT and MAPK/ERK leading to cell growth and proliferation (29). Using tissue microarrays constructed from mouse prostates, VECTRA was used to independently quantitate proliferation rates by measuring percent KI67 immunohistochemical staining. Biallelic Igf2 expression on any Nkx3.1 genotype results in a significant increase in the percentage of KI67 positive cells at 6, 12 and 18 months of age (P = 0.027, P = 0.043, P = 0.019 respectively) compared to MOI. (Fig. 4A). No synergistic increase in proliferation was found when both phenotypes, the LOI;Nkx3.1−/− mice, were compared to Nkx3.1+/+. Furthermore, no significant differences in apoptosis were noted between MOI and LOI animals as identified by TUNEL assay (Supplementary Fig. 2A).
Figure 4. Igf2 LOI animals display increased proliferation and phosphorylated ERK expression.
Tissue microarrays constructed from mouse prostate tissues were IHC stained for Ki-67 and Igf2 downstream signaling markers. A, Igf2 LOI resulted in significantly higher rates of prostate epithelial cell proliferation (Ki-67) ((**P<0.01, *P<0.05; columns represent mean+95% CI). Protein expression levels were quantified by Vectra analysis and average levels of p-ERK staining analyzed by age and genotype. Representative p-ERK staining (brown) in (B) MOI and (C) LOI prostate tissues from 12 mo Nkx3.1+/+ mice. D, LOI;Nkx3.1+/+ prostate tissues showed significantly elevated levels of p-ERK staining at 6, 12, and 18 months (P=0.013, P=0.004, P=0.012 respectively). E, Levels of p-ERK were increased at 6 (P=0.028) and 12 months (P=0.016) in LOI;Nkx3.1+/− mice compared to their age matched counterparts. F, Expression of p-AKT significantly increased in LOI;Nkx3.1−/− prostate tissues at 6 (P=0.038) and 12 (P=0.02) months. Figures 4D, 4E, 4F, columns represent mean+95% CI; **P<0.01; *P<0.05.
To uncover the activated signaling pathways boosting proliferation and PIN formation in Igf2 LOI animals we assessed p-AKT, and p-ERK by quantitative IHC using VECTRA image analysis. IHC demonstrates p-ERK staining occurs primarily in the glandular epithelium, therefore analysis focused on the epithelial compartment (Fig. 4B-C). Igf2 LOI was associated with elevated p-ERK in both Nkx3.1+/+ (Fig. 4D) and Nkx3.1+/− mice (Fig. 4E). No significant change in p-ERK staining was detected in LOI;Nkx3.1−/− mice despite a significant increase in proliferation (Supplementary Fig. S2B). To further investigate this, an analysis of p-AKT was performed that revealed significantly elevated p-AKT levels at 6 and 12 months in LOI;Nkx3.1−/− mice vs. MOI;Nkx3.1−/− (Fig. 4F) but not in Nkx3.1+/+ and Nkx3.1+/− (p-AKT staining, Supplementary Fig. S2C-D). In sum, Igf2 LOI induces ERK signaling primarily, but with loss of Nkx3.1 function, p-AKT activation becomes prominent.
To identify whether IGF2 LOI resulted in altered expression of the receptors potentially affecting these signaling pathways, we quantified Insulin receptor isoform and Igf1r expression in mouse prostate tissues. We note nonsignificant increases in Ir-A isoform expression in LOI;Nkx3.1+/+ compared to MOI;Nkx3.1+/+ prostates (Supplementary Fig. S3A-B). In addition, these tissues displayed increasing Igf1r approaching significance (Supp Fig. S3C), however no difference in Igf1r expression was apparent in Nkx3.1−/− prostates (Supp Fig. S3D).
Igf2 LOI Leads to Increased Transcription of MAPK/ERK Effector and Proliferation Related Genes; Nkx3.1 Status is Associated with AKT/ERK Activation
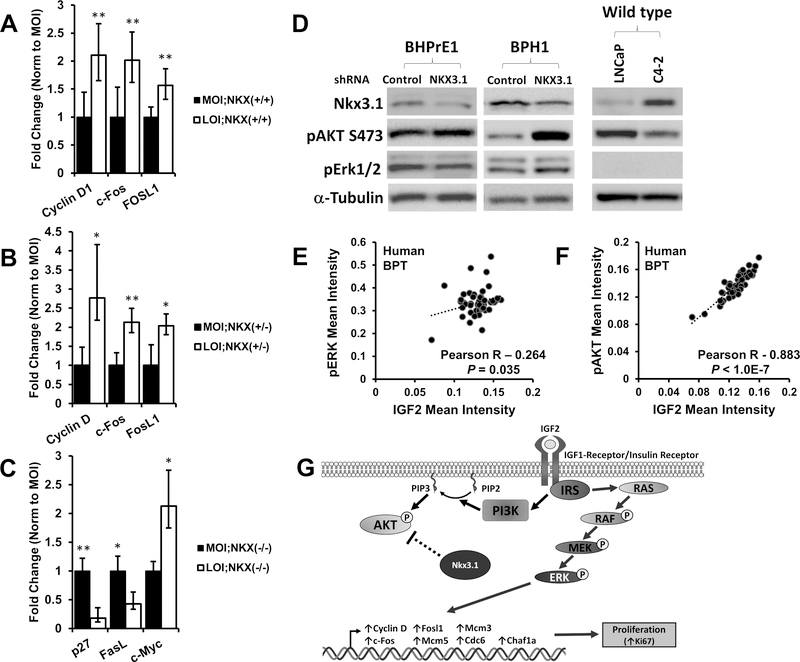
IGF2 exhibits structural similarity to insulin, with the ability to elicit signaling through both the insulin–like growth factor 1 receptor (IGF1R) and insulin receptor (IR). Ligand binding to these growth receptors results in context dependent signaling through PI3K/AKT and MAPK pathways (6). As demonstrated (Fig. 3), upregulation of p-ERK levels occurred predominantly in LOI;Nkx3.1+/+ mice, but in LOI;Nkx3.1−/− prostates AKT signaling significantly increases. We performed further transcriptional analyses to determine potential MAPK/ERK effector genes associated with the increased proliferation in LOI mouse prostates. Using 12mo Nkx3.1+/+ and Nkx3.1+/− prostate specimens, the effect of biallelic expression was compared to MOI by qPCR for immediate early genes (IEGs) which are activated in response to growth factor stimuli (30). C-Fos transcription is significantly elevated in LOI prostates (2-fold, P = 0.003) indicating increased activated MAPK signaling (Fig. 5A and 5B). Phosphorylation of c-FOS and c-JUN by MAPK results in the formation of the AP-1 transcription factor complex. Increased FosL1 (Fra-1) mRNA in LOI prostates (1.5-fold, P = 0.009) confirms active AP-1 which is required for FosL1 transcription (31). MAPK signaling results in transcription of cell cycle-related genes including Cyclin D which is also increased in LOI mouse prostates compared to MOI (2.1 fold, P = 0.003). Analysis of downstream transcription of p-AKT signaling was performed in a similar fashion using Nkx3.1−/− prostate tissues. We observed decreased transcription of p27 and FasL and increased transcription of c-Myc in LOI;Nkx3.1−/− compared to MOI;Nkx3.1−/− prostate tissues (Fig. 5C) consistent with decreased p-AKT signaling noted in previous reports (32) (33) (34).
Figure 5. Transcription of p-ERK and p-AKT effector genes are altered in Igf2 LOI prostates, IGF2 correlates with p-ERK and p-AKT in human prostate tissues.
Gene transcription was analyzed in MOI and LOI mouse prostate tissues (Minimum n=5 each group). A, Increased mRNA expression of p-ERK target genes Cyclin D1 (P=0.003), c-Fos (P=0.003), and Fosl1 (P=0.009) was demonstrated in LOI;Nkx3.1+/+ prostates. B, CyclinD1 (P=0.029), c-Fos (P=0.004), and Fosl1 (P=0.017) were significantly increased in LOI;Nkx3.1+/− prostates. C, Altered mRNA expression of p-AKT target genes p27 (P=0.0081), Fasl (P=0.033), and c-Myc (P=0.044) in LOI;Nkx3.1−/− prostate tissues D, Western blot of NKX3.1 knockdown. An siRNA for NKX3.1 (and non-silencing control) was transfected into the BHPrE1, a nontumorigenic human epithelial cell line, and BPH1 cells and protein lysates analyzed. Knockdown of NXX3.1 results in a pAKT increase in BHPrE1 and BPH1 cells and pERK downregulation in BHPrE1. An inverse correlation between NKX3.1 expression and pAKT is noted in LNCaP and its derivative C4–2. E, Quantitative IHC in human prostate tissues demonstrates a significant correlation between IGF2 and p-ERK expression in benign prostate tissues (BPT). F, IGF2 and p-AKT protein expression strongly correlate in human benign prostate tissues. Correlation coefficient and P-value calculated using Pearson’s product moment correlation (BPT, n=48). G, In LOI affected prostates containing at least one Nkx3.1 allele, proliferation is predominantly mediated via MAPK/ERK signaling (right). In contrast, Nkx3.1 negatively regulates AKT signaling in Nkx3.1+/n animals, and Nkx3.1−/− prostates exhibit increased p-AKT.
In addition to MAPK/ERK and p-AKT related gene transcription, we analyzed a DNA replication gene set previously shown to be induced with Igf2 LOI in mouse colon tissue (Supp Fig. 3E) (19). Of these genes, G0/G1 associated genes Mcm5 (1.48 fold, P = 0.009), Mcm3 (1.56 fold, P = 0.012), Cdc6 (2.25-fold, P = 0.001), and Chaf1a (1.73-fold, P = 0.005) were significantly upregulated in LOI prostates (non-significant difference in Lig1, Tiam2, Axin1, and NF-κB subunits p50 and p65), supporting the enrichment of proliferating cells as identified by KI67 staining (Fig. 4A).
Our mouse model data suggests Nkx3.1 status impacts the status of MAPK/ERK or AKT activation. To recapitulate these findings in vitro, Nkx3.1 knockdown was performed in the immortalized non-tumorigenic human prostate epithelial cell line BHPrE1 and BPH-1 cells. Following Nkx3.1 knockdown, a modest increase in p-AKT and decrease in p-ERK were noted in BHPrE1 cells, along with a strong increase in p-AKT following Nkx3.1 knockdown in BPH-1 cells (Fig. 5D). These results agree with animal experiments where homozygous mutant (Nkx3.1−/−) animals experienced preferentially increased p-AKT. Similarly, the isogenic LNCaP and C4–2 prostate cancer cell lines demonstrate an inverse correlation between Nkx3.1 and p-AKT levels (Fig. 5D).
IGF2 Protein Expression Correlates with p-AKT and p-ERK in Nontumor Human Prostate Tissue from Radical Prostatectomy Specimens
To determine the relevance of these findings to human prostate disease we examined IGF2, p-AKT, and p-ERK protein levels using a human tissue microarray (hTMA) containing benign prostate tissues (BPT) and cancer. Our animal studies revealed increased IGF2 expression results in induction of both p-ERK and p-AKT signaling. Therefore, we performed a correlation analysis between IGF2 and p-ERK and p-AKT to establish a potential link between these proteins (Fig. 5E-F). A significant positive correlation exists between increased IGF2 and p-ERK in BPT (Pearson R = 0.26, P = 0.035) (Fig. 5E). IGF2 and p-AKT exhibited an even more marked positive correlation (Pearson R = 0.88; P < 1.0E-7) (Fig. 5F). Notably, these correlations were less pronounced in primary PCa tissues (Supplementary Fig. S4A-B), although higher IGF2 and p-AKT protein expression was noted (Supp Fig. S4C-D), but not p-ERK (Supp Fig. S4E). Loss of IGF2 imprinting is a common finding in benign human tissues removed at cancer surgery (4,35). While this approach did not examine IGF2 imprint status, the underlying increased IGF2 expression noted results supports our animal model findings that higher Igf2 expression is associated with increased p-AKT and p-ERK signaling.
Discussion
The human prostate develops cancer remarkably frequently with aging (3) yet a clear genetic etiology has not been identified. In parallel, Igf2 LOI is more extensive in the normal peripheral prostate tissue of patients that develop prostate cancer compared to age-matched controls, and furthermore an erosion of this allelic imprinting occurs with aging in mouse and human prostates (4). In the current study, we show Igf2 loss of imprinting alone plays a role in the development of prostatic disease using a mouse model of Igf2 LOI that mimics the biallelic, increased Igf2 levels seen in the peripheral prostate during aging (4,36). Biallelic expression of Igf2, induced by mutation of CTCF binding sites within the intergenic imprint control region, resulted in widespread PIN in the mouse prostate. Significant upregulation of p-ERK signaling associates with increased epithelial cell proliferation. We concurrently test Nkx3.1 loss, a common early molecular alteration in established prostate cancer (24), and find LOI;Nkx3.1+/− animals developed significantly more extensive PIN in an additive fashion, but not frank cancer. These novel epigenetic results support a model in which the widespread multifocal development of neoplasia in the peripheral human prostate is dependent on the presence of Igf2 LOI. This observation explains, in part, the remarkable predisposition of the human prostate for the development of cancer with aging.
Igf2 exerts its mitogenic effects by signaling through the IGF1 receptor, insulin receptor isoform A, or heterodimeric hybrid receptors, which bind Igf2 with differing affinity (37). The MAPK/ERK and PI3K/AKT pathways are the predominant intracellular signaling pathways affected by IGF2 stimulation (Fig. 5G). Interrogation of these pathways by quantitative IHC revealed strong upregulation of p-ERK signaling with Igf2 LOI. Conversely, in mice harboring a complete loss of Nkx3.1, the alternate and competing pathway, p-AKT is increased. This is consistent with in vitro studies showing that in NKX3.1 deficiency, the repressive effect on AR signaling is lost and p-AKT upregulated (13). These results may be explained by the differential dosage sensitivity to NKX3.1 loss seen in certain genes, where Nkx3.1−/− prostates exhibit a drastically altered transcriptional profile compared to less significant differences between Nkx3.1+/+ and Nkx3.1+/− animals (38). Other studies show homozygous inactivation of Nkx3.1 results in increased AKT signaling (13) and activated AKT signaling is implicated in the negative regulation of MAPK/ERK signaling (39).
Previous research demonstrated Igf1r conditional deletion is negatively correlated with ERK activation in mouse prostate tissues (40). This suggests the upregulation of p-ERK in our study could potentially be attributed to IR-A isoform mediated IGF2 signaling. Although we did not note a significant strong upregulation of IR-A in experimental prostate tissues (Supp. Fig 3), receptor balance may be further disturbed specifically in PIN tissues and warrants future study.
We examined the relationship of IGF2 and these downstream signaling markers, p-ERK and p-AKT, in human prostate samples using automated quantitative image analysis. Interestingly, p-AKT exhibited a very strong correlation with IGF2 in benign prostate tissues with a less pronounced, but significant p-ERK correlation (Fig. 5E-F). This stronger relationship between IGF2 and p-AKT signaling in humans might be due to species-specific differences. A recent report demonstrated IGF2 LOI correlates with increased p-AKT in human colorectal cancer tissues (41). Alternatively, the histologically normal prostate specimens analyzed here originated from prostates containing cancer, and potentially harbor epigenetic alterations as part of a well-described field effect (42,43) encouraging signaling through p-AKT. The positive correlations between IGF2 and p-AKT or p-ERK were less pronounced in prostate cancer tissues. This lessened dependence on IGF2 is likely explained by additional genetic alterations acquired in cancerous lesions affecting ERK and AKT signaling, confounding the observed relationship between IGF2 and these signaling pathways. In sum, these data support our animal model findings in which animals expressing greater levels of IGF2 through LOI lead to increases in p-ERK or p-AKT signaling.
Using KI67 immunohistochemistry, the current work demonstrates significantly increased levels of epithelial-specific proliferation in mouse prostates containing Igf2 LOI (Fig. 4A). Kaneda et al. previously found Igf2 LOI in the murine colon induces increased expression of proliferation related-genes (19). This mouse model achieved Igf2 LOI in the colon through genomic deletion of the ICR and H19 gene sequences, a potentially confounding factor given the emerging role of H19 long noncoding sequences in cancer development and progression (44). The animal model used in the current study has clear advantages namely achieving Igf2 LOI while leaving H19 sequences and expression intact (Supplementary Fig. S1A) (23). Despite these model differences we find, by RT-qPCR, a similar set of cell-cycle related genes to be upregulated with Igf2 LOI alone. In addition, we demonstrate upregulation of transcriptional markers of activated p-ERK signaling (Fig. 5A) emphasizing the role of this epigenetic alteration in driving proliferation. A recent report analyzing the field effect in prostate cancer demonstrated a number of genes induced in normal appearing, cancer-adjacent tissues that are predictive of worse clinical outcomes (42). Among these prognostic genes were KI67, FOS, and CHAF1A, all significantly altered with Igf2 LOI in this study. This suggests that the IGF2 LOI found in the field effect, commonly seen in human cancer, may hold prognostic value in normal prostate tissues, such as negative biopsy specimens.
One potential issue with the 142* mouse model of Igf2 LOI in the study of prostatic disease is the Igf2 LOI experienced in all tissues of these animals. The effects of systemic LOI were indeed apparent in these animals, including increased bodyweight (Figure 1D; Supplementary Fig. S1B and S1C) at all ages and liver enlargement in older animals. However, this model has specific advantages over studies previously conducted in other organ systems. Compared to transgenic models that experience 20- to 30-fold increases in serum Igf2 (45) and large increases in tissue levels (21,46), our animals experience ~2 fold increase in Igf2 expression, physiologically similar to the levels seen with aging (4) and in tumors (47). Other animal models have achieved physiologic levels of Igf2 LOI, but these require deletion of the Igf2-H19 ICR and/or the H19 gene (19). Of note, in older animals (18mo) the additive effects of NKX3.1 and LOI are less marked in part due to a physiologic LOI in mice prostates that we have previously demonstrated occurs with aging (4). Our LOI animals also do not develop invasive cancer. We tested whether Nkx3.1 loss acts in a synergistic fashion to generate cancer, but this was not found to occur. Igf2 LOI has been studied for its contribution to cancer development but typically requires other strong genetic alterations to form tumors, such as ApcMin/+ in the colon (48), SV-40 large T-antigen in the liver (49), and Wilm’s Tumor 1 (Wt1) in Wilm’s tumor models (50).
Ultimately, PCa is an age-related disease that is marked by multiple foci of cancer. Mirroring this observation, IGF2 loss of imprinting progresses in an age-related manor, and can be found throughout the peripheral prostate (4,35). Herein, we provide evidence for the first time that Igf2 LOI encourages early prostatic neoplasia. Furthermore, underlying increased disease was an induction of proliferation mediated by increased MAPK/ERK signaling. Taken together, Igf2 LOI acts as a promoter of disease formation by increasing the fitness of affected cells, subsequently raising the possibility of further adverse genomic events. Ultimately, Igf2 LOI may be an important biomarker for future disease risk and the development of this epigenetic alteration may provide a reversible target for chemoprevention. Recent research suggests metformin may dampen IGF axis signaling through negative regulation of p-ERK (51,52) and is currently being investigated as a prevention agent for early prostate cancer (53). Screening for Igf2 LOI, or potentially p-ERK or p-AKT signaling induction in patients with negative biopsies may provide important future opportunities for identifying these chemoprevention targets.
Supplementary Material
Acknowledgements
The authors thank the University of Wisconsin Translational Research Initiatives in Pathology laboratory, in part supported by the UW Department of Pathology and Laboratory Medicine and UWCCC grant P30 CA014520, for use of its facilities and services. We would like to thank Dr. William William Ricke for providing the BPH1 and BHPrE1 cell lines and Dr. Simon Hayward for making these cell lines.
Financial Support: This study was supported by: NIH/NCI 5R01CA097131 (D.F. Jarrard), NIH 2RO1CA097131–06A2 (D.F. Jarrard), The John Livesey Endowment Fund (D.F. Jarrard), The Nast Family Foundation (D.F. Jarrard), and NCI T32 CA009135 (N.A. Damaschke)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29 [DOI] [PubMed] [Google Scholar]
- 2.Damaschke NA, Yang B, Bhusari S, Svaren JP, Jarrard DF. Epigenetic susceptibility factors for prostate cancer with aging. Prostate 2013;73:1721–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guileyardo JM, Johnson WD, Welsh RA, Akazaki K, Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. J Natl Cancer Inst 1980;65:311–6 [PubMed] [Google Scholar]
- 4.Fu VX, Dobosy JR, Desotelle JA, Almassi N, Ewald JA, Srinivasan R, et al. Aging and Cancer-Related Loss of Insulin-like Growth Factor 2 Imprinting in the Mouse and Human Prostate. Cancer Res 2008;68:6797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarrard DF, Bussemakers MJ, Bova GS, Isaacs WB. Regional loss of imprinting of the insulin-like growth factor II gene occurs in human prostate tissues. Clin Cancer Res 1995;1:1471–8 [PubMed] [Google Scholar]
- 6.Livingstone C IGF2 and cancer. Endocr Relat Cancer 2013;20:R321–39 [DOI] [PubMed] [Google Scholar]
- 7.Bell JL, Wächter K, Mühleck B, Pazaitis N, Köhn M, Lederer M, et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci 2013;70:2657–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev 1999;13:966–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, et al. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics 1997;43:69–77 [DOI] [PubMed] [Google Scholar]
- 10.Voeller HJ, Augustus M, Madike V, Bova GS, Carter KC, Gelmann EP. Coding region of NKX3.1, a prostate-specific homeobox gene on 8p21, is not mutated in human prostate cancers. Cancer Res 1997;57:4455–9 [PubMed] [Google Scholar]
- 11.Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer 2000;7:115–29 [DOI] [PubMed] [Google Scholar]
- 12.Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA, et al. Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 2002;22:1495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J, et al. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell 2006;9:367–78 [DOI] [PubMed] [Google Scholar]
- 14.Ogawa O, Eccles MR, Szeto J, McNoe LA, Yun K, Maw MA, et al. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms’ tumour. Nature 1993;362:749–51 [DOI] [PubMed] [Google Scholar]
- 15.Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, et al. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res 2006;4:283–92 [DOI] [PubMed] [Google Scholar]
- 16.Zhao R, DeCoteau JF, Geyer CR, Gao M, Cui H, Casson AG. Loss of imprinting of the insulin-like growth factor II (IGF2) gene in esophageal normal and adenocarcinoma tissues. Carcinogenesis 2009;30:2117–22 [DOI] [PubMed] [Google Scholar]
- 17.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005;353:229–37 [DOI] [PubMed] [Google Scholar]
- 18.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science 2003;299:1753–5 [DOI] [PubMed] [Google Scholar]
- 19.Kaneda A, Wang CJ, Cheong R, Timp W, Onyango P, Wen B, et al. Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proc Natl Acad Sci U S A 2007;104:20926–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haley VL, Barnes DJ, Sandovici I, Constancia M, Graham CF, Pezzella F, et al. Igf2 pathway dependency of the Trp53 developmental and tumour phenotypes. EMBO Mol Med 2012;4:705–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates P, Fisher R, Ward A, Richardson L, Hill DJ, Graham CF. Mammary cancer in transgenic mice expressing insulin-like growth factor II (IGF-II). Br J Cancer 1995;72:1189–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorehead RA, Sanchez OH, Baldwin RM, Khokha R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene 2003;22:853–7 [DOI] [PubMed] [Google Scholar]
- 23.Pant V, Mariano P, Kanduri C, Mattsson A, Lobanenkov V, Heuchel R, et al. The nucleotides responsible for the direct physical contact between the chromatin insulator protein CTCF and the H19 imprinting control region manifest parent of origin-specific long-distance insulation and methylation-free domains. Genes Dev 2003;17:586–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, et al. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res 2002;62:2999–3004 [PubMed] [Google Scholar]
- 25.Kanduri C, Pant V, Loukinov D, Pugacheva E, Qi CF, Wolffe A, et al. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol 2000;10:853–6 [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, et al. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol 2002;161:727–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang W, Hennrick K, Drew S. A colorful future of quantitative pathology: validation of Vectra technology using chromogenic multiplexed immunohistochemistry and prostate tissue microarrays. Hum Pathol 2013;44:29–38 [DOI] [PubMed] [Google Scholar]
- 28.Vocke CD, Pozzatti RO, Bostwick DG, Florence CD, Jennings SB, Strup SE, et al. Analysis of 99 microdissected prostate carcinomas reveals a high frequency of allelic loss on chromosome 8p12–21. Cancer Res 1996;56:2411–6 [PubMed] [Google Scholar]
- 29.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915–28 [DOI] [PubMed] [Google Scholar]
- 30.Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta 2007;1773:1285–98 [DOI] [PubMed] [Google Scholar]
- 31.Bergers G, Graninger P, Braselmann S, Wrighton C, Busslinger M. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol Cell Biol 1995;15:3748–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 2000;20:9138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavurma MM, Khachigian LM. Signaling and transcriptional control of Fas ligand gene expression. Cell Death Differ 2003;10:36–44 [DOI] [PubMed] [Google Scholar]
- 34.Vartanian R, Masri J, Martin J, Cloninger C, Holmes B, Artinian N, et al. AP-1 regulates cyclin D1 and c-MYC transcription in an AKT-dependent manner in response to mTOR inhibition: role of AIP4/Itch-mediated JUNB degradation. Mol Cancer Res 2011;9:115–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhusari S, Yang B, Kueck J, Huang W, Jarrard DF. Insulin-like growth factor-2 (IGF2) loss of imprinting marks a field defect within human prostates containing cancer. Prostate 2011;71:1621–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet P, Reiter E, Bruyninx M, Sente B, Dombrowicz D, de Leval J, et al. Benign prostatic hyperplasia and normal prostate aging: differences in types I and II 5 alpha-reductase and steroid hormone receptor messenger ribonucleic acid (mRNA) levels, but not in insulin-like growth factor mRNA levels. J Clin Endocrinol Metab 1993;77:1203–8 [DOI] [PubMed] [Google Scholar]
- 37.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 2007;28:20–47 [DOI] [PubMed] [Google Scholar]
- 38.Magee JA, Abdulkadir SA, Milbrandt J. Haploinsufficiency at the Nkx3.1 locus. A paradigm for stochastic, dosage-sensitive gene regulation during tumor initiation. Cancer Cell 2003;3:273–83 [DOI] [PubMed] [Google Scholar]
- 39.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 2011;36:320–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland BW, Knoblaugh SE, Kaplan-Lefko PJ, Wang F, Holzenberger M, Greenberg NM. Conditional deletion of insulin-like growth factor-I receptor in prostate epithelium. Cancer Res 2008;68:3495–504 [DOI] [PubMed] [Google Scholar]
- 41.Belharazem D, Magdeburg J, Berton AK, Beissbarth L, Sauer C, Sticht C, et al. Carcinoma of the colon and rectum with deregulation of insulin-like growth factor 2 signaling: clinical and molecular implications. J Gastroenterol 2016;51:971–84 [DOI] [PubMed] [Google Scholar]
- 42.Magi-Galluzzi C, Maddala T, Falzarano SM, Cherbavaz DB, Zhang N, Knezevic D, et al. Gene expression in normal-appearing tissue adjacent to prostate cancers are predictive of clinical outcome: evidence for a biologically meaningful field effect. Oncotarget 2016;7:33855–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang B, Bhusari S, Kueck J, Weeratunga P, Wagner J, Leverson G, et al. Methylation profiling defines an extensive field defect in histologically normal prostate tissues associated with prostate cancer. Neoplasia 2013;15:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raveh E, Matouk IJ, Gilon M, Hochberg A. The H19 Long non-coding RNA in cancer initiation, progression and metastasis - a proposed unifying theory. Mol Cancer 2015;14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, et al. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. Journal of Biological Chemistry 1994;269:13779–84 [PubMed] [Google Scholar]
- 46.Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene 2004;23:6156–62 [DOI] [PubMed] [Google Scholar]
- 47.Ravenel JD, Broman KW, Perlman EJ, Niemitz EL, Jayawardena TM, Bell DW, et al. Loss of imprinting of insulin-like growth factor-II (IGF2) gene in distinguishing specific biologic subtypes of Wilms tumor. J Natl Cancer Inst 2001;93:1698–703 [DOI] [PubMed] [Google Scholar]
- 48.Hassan AB, Howell JA. Insulin-like growth factor II supply modifies growth of intestinal adenoma in Apc(Min/+) mice. Cancer Res 2000;60:1070–6 [PubMed] [Google Scholar]
- 49.Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature 1994;369:414–8 [DOI] [PubMed] [Google Scholar]
- 50.Hu Q, Gao F, Tian W, Ruteshouser EC, Wang Y, Lazar A, et al. Wt1 ablation and Igf2 upregulation in mice result in Wilms tumors with elevated ERK1/2 phosphorylation. J Clin Invest 2011;121:174–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, et al. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer 2012;19:447–56 [DOI] [PubMed] [Google Scholar]
- 52.Cao H, Dong W, Qu X, Shen H, Xu J, Zhu L, et al. Metformin Enhances the Therapy Effects of Anti-IGF-1R mAb Figitumumab to NSCLC. Sci Rep 2016;6:31072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med 2015;66:17–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.