Abstract
BACKGROUND
The benefits of removing small (≤6 mm), asymptomatic kidney stones endoscopically is unknown. Current guidelines leave such decisions to the urologist and the patient. A prospective study involving older, nonendoscopic technology and some retrospective studies favor observation. However, published data indicate that about half of small renal stones left in place at the time that larger stones were removed caused other symptomatic events within 5 years after surgery.
METHODS
We conducted a multicenter, randomized, controlled trial in which, during the endoscopic removal of ureteral or contralateral kidney stones, remaining small, asymptomatic stones were removed in 38 patients (treatment group) and were not removed in 35 patients (control group). The primary outcome was relapse as measured by future emergency department visits, surgeries, or growth of secondary stones.
RESULTS
After a mean follow-up of 4.2 years, the treatment group had a longer time to relapse than the control group (P<0.001 by log-rank test). The restricted mean (±SE) time to relapse was 75% longer in the treatment group than in the control group (1631.6±72.8 days vs. 934.2±121.8 days). The risk of relapse was 82% lower in the treatment group than the control group (hazard ratio, 0.18; 95% confidence interval, 0.07 to 0.44), with 16% of patients in the treatment group having a relapse as compared with 63% of those in the control group. Treatment added a median of 25.6 minutes (interquartile range, 18.5 to 35.2) to the surgery time. Five patients in the treatment group and four in the control group had emergency department visits within 2 weeks after surgery. Eight patients in the treatment group and 10 in the control group reported passing kidney stones.
CONCLUSIONS
The removal of small, asymptomatic kidney stones during surgery to remove ureteral or contralateral kidney stones resulted in a lower incidence of relapse than nonremoval and in a similar number of emergency department visits related to the surgery. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Veterans Affairs Puget Sound Health Care System; ClinicalTrials.gov number, NCT02210650.)
During the removal of symptomatic stones from the kidneys or ureters, other small, asymptomatic renal stones are often noted on imaging studies.1 Current U.S. and European guidelines are equivocal; the U.S. guidelines suggest that asymptomatic renal stones may be actively monitored but that patients who have small, residual renal-stone fragments after surgery should be offered endoscopic surgery to remove them.2,3 U.S. and European guidelines cite several studies showing that patients with asymptomatic renal stones and fragments had approximately a 50% chance of relapse within 5 years after surgery.2,3 The only prospective study that was referenced in either the U.S. or European guidelines did not test the effectiveness of endoscopic surgery but instead tested shock-wave lithotripsy. That study favored observation of asymptomatic renal stones over surgery, given no significant differences between the two approaches in the number of patients who were free of kidney stones or in the number of additional treatments after 1 year.4 Two of three retrospective studies conducted since either of the guidelines was published supported observation. One study showed that concurrent treatment of asymptomatic contralateral stones was not associated with fewer surgical interventions after 2 years.5 In another study, decision analysis was used to assess the quality-of-life effects of surgery to remove a 10-mm, asymptomatic renal stone, and the authors concluded that active surveillance was the preferred management decision.6 The third study showed that patients with symptomatic ureteral calculi and asymptomatic renal calculi smaller than 15 mm who underwent ureteroscopy with active treatment for renal calculi had fewer future ipsilateral surgical interventions and stone-related events within 2 years than patients who received shock-wave lithotripsy for ureteral calculi.7 The debate regarding the removal of asymptomatic stones has even extended to dueling editorials by experts in the field.8,9
Therefore, surgeons may or may not elect to increase the surgical time and possibly increase patient risk to remove these asymptomatic, or secondary, stones during surgery for primary stones. We defined primary stones as those that were located within the ureter or a kidney and that produced symptoms or were considered at high risk of causing an adverse clinical event. Secondary stones were defined as small (≤6 mm), asymptomatic renal stones that were located in the contralateral kidney (in the case of a primary renal stone) or in either kidney (in the case of a primary ureteral stone, with the specific kidney identified before randomization). Before the advent of modern endoscopes and lasers, concomitant removal of primary and secondary stones was problematic4; however, the procedure is currently quite feasible.5,7 Given the lack of guidance for the removal of small, asymptomatic renal stones, we randomly assigned patients in this trial to undergo endoscopic removal of either their primary and secondary stones (treatment group) or their primary stones alone (control group). Relapse, added surgical time, and clinically significant adverse surgical events were measured to determine whether the removal of small stones would result in fewer relapses among patients in the treatment group than in the control group.
Methods
TRIAL OVERSIGHT
This multicenter, prospective, investigator-initiated, unblinded, randomized, controlled trial was designed and conducted by the authors, who obtained a National Institutes of Health certificate of confidentiality. The trial was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with support from the Veterans Affairs Puget Sound Health Care System. The trial was approved by the institutional review boards at the participating institutions.
POPULATION
We screened patients who were 21 years of age or older and were scheduled to undergo endoscopic surgical treatment of a primary stone at the urology clinics of the participating large, urban, tertiary-care centers. The type of surgery for the primary stone — ureteroscopy or percutaneous nephrolithotomy — was a clinical decision made on the basis of each surgeon’s practice preference and was not altered for the trial. Patients who were able to provide informed consent and who had one or more secondary stones on computed tomography (CT) within 90 days before randomization were included. Patients with known systemic disease or anatomical disorders such as medullary sponge kidney, primary hyperparathyroidism, renal tubular acidosis, sarcoidosis, and horseshoe kidney were excluded.
TRIAL PROCEDURES
Patients were randomly assigned, in a 1:1 ratio, to either the treatment group or the control group. Randomization was performed in blocks of 10 according to one central schedule that was designed to minimize the possible effects of recruitment over time. Secondary stones in patients in the treatment group were removed by ureteroscopy. The surgical durations for the removal of both the primary and secondary stones were recorded.
Postoperative imaging with low-dose CT was obtained approximately 90 days after surgery and then approximately once a year thereafter. The first postsurgical scan served to establish a new baseline and allowed the determination of residual fragments; the annual scans thereafter were used to identify stone growth and formation of new stones in the trial kidney. Patients were contacted by telephone or at clinic visits every 3 months to record any interval stone events, emergency department visits, or stone-related surgeries. Patients were followed for up to 5 years. All CTs were reviewed by a single radiologist who was unaware of the group assignments.
All the patients were offered standard metabolic evaluation and education regarding appropriate dietary and fluid-intake protocols for stone prevention. Stones retrieved were analyzed by microCT (SkyScan 1172, Bruker) and Fourier-transform infrared spectroscopy.10
OUTCOMES
The primary outcome was relapse in each group, which was defined according to any of three measures — an emergency department visit owing to stones on the same side where the original asymptomatic stone had occurred (trial side) during the follow-up period (2 weeks to 5 years after surgery); subsequent surgery to remove stones on the trial side in the follow-up period; or growth of an original secondary stone, as measured with the use of CT. A single patient could only be counted as having relapsed once despite possibly having multiple relapse events. Secondary outcomes were the measured surgical time needed to treat the secondary stones in the treatment group; emergency department visits within 2 weeks after surgery that were associated with the surgery, a stent, or stones; and patient-reported stone passage and new stone growth. Stone growth was defined as an increase in stone size of more than 1 mm in one dimension or 1 mm or more in two dimensions, as measured by subsequent CT scans.
STATISTICAL ANALYSIS
Power calculations and statistical simulations showed that 35 patients per group would provide the trial with a statistical power of 80% with the type I error rate kept at the 0.05 level. Details regarding the protocol and statistical analysis plan are available with the full text of this article at NEJM.org. We assumed an incidence of relapse of 50% or more in the control group and 15% in the treatment group after 2 years. The sample size was calculated with the use of Weibull regression to potentially handle a mixture of interval and right censoring. Previous studies that assessed the effects of medical interventions on stone relapse have shown significant effects with sample sizes of 19 to 25 patients per group11-13 and a similar primary outcome of symptomatic events to mark the occurrence of relapse. In addition, our trial also used radiologic evidence of stone growth, which was probably a more sensitive measure of relapse than that used in previous studies. Thus, we expected that we could detect relapse earlier after surgery than in previous studies.
We planned that the overall type I error rate would be controlled with the use of a single primary hypothesis testing, so no adjustment procedures for multiple comparisons were needed. For the secondary end points, summary statistics and 95% confidence intervals were calculated without P values.
Demographic and clinical variables that were collected from patients’ medical records were summarized by count and percentage for categorical variables and by median and interquartile range for continuous variables. The cumulative incidence of stone relapse was estimated with the use of the Kaplan–Meier method, and a log-rank test was used to compare the treatment and control groups. We tested the proportional-hazards assumption by plotting the log–minus–log survival function against the log(time) and by conducting a resample-based Kolmogorov-type supremum test, neither of which indicated violation of the Cox proportional-hazards assumption. Because of the limited time period, we could not estimate the median time to relapse, so we report the data as the restricted mean time to relapse (the average duration of event-free survival as the area under the survival curve) and standard error. The restricted mean time to relapse is smaller than the true (unrestricted) mean time to relapse because of the limited trial period. The difference in the restricted mean time to relapse between the treatment and control groups can be viewed as the mean delay of an event over the trial period. Given that this was a randomized clinical trial and no imbalance of demographic or clinical variables was observed between the treatment and control groups, no multivariable survival models were fitted. The various event categories that were included in the efficacy results were also summarized by counts and percentages without comparisons between groups because all the patients were not followed for the same duration. A two-sided P value of less than 0.05 was considered to indicate statistical significance. All analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
POPULATION CHARACTERISTICS
The trial was conducted from May 2015 through September 2021, and the last patient was enrolled in May 2020. A total of 75 patients were enrolled (Fig. 1). One patient in each group did not have surgery and was not included in the trial. One patient in each group died from causes unrelated to urologic issues during the follow-up period. Three patients in each group voluntarily withdrew from the trial owing to the reasons listed in Figure 1. One patient in the control group was lost to follow-up. All data were included up to the point of death, withdrawal, or loss of contact with the patient. The groups were balanced with respect to baseline characteristics (Table 1) and were representative of the population that typically undergoes surgery to remove stones (Table S1 in the Supplementary Appendix, available at NEJM.org). No patients with a single kidney and no Hispanic patients were enrolled. No mechanism was in place to tabulate the patients who declined to participate, but most patients who declined to participate expressed a desire to avoid the possibility of being randomly assigned to the control group because they wanted to ensure that the asymptomatic stones would be removed.
Figure 1.
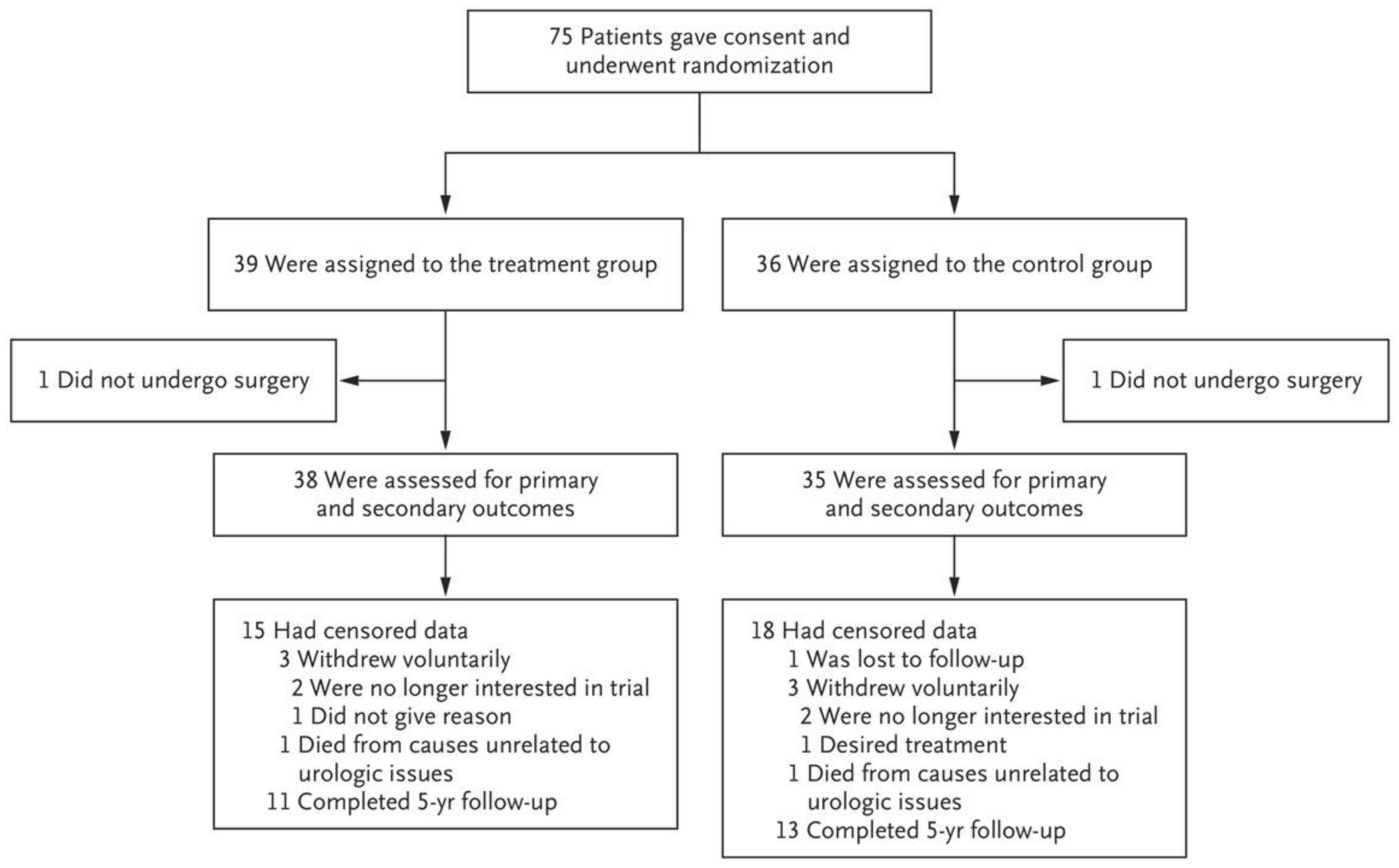
Enrollment and Randomization.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline and Follow-up Data.*
| Characteristic | Treatment (N = 38) | Control (N = 35) |
|---|---|---|
| Median age (IQR) — yr | 64 (54–69) | 60 (49–67) |
| Sex — no. (%) | ||
| Female | 6 (16) | 9 (26) |
| Male | 32 (84) | 26 (74) |
| Race — no. (%)† | ||
| White | 35 (92.1) | 29 (82.9) |
| Black | 2 (5.3) | 3 (8.6) |
| Asian | 1 (2.6) | 2 (5.7) |
| Unknown or not reported | 0 | 1 (29) |
| Median body-mass index (IQR)‡ | 29.6 (25.4–33.6) | 30.7 (26.4–34.6) |
| Primary stone — no. (%) | ||
| Location | ||
| Kidney | 20 (53) | 18 (51) |
| Ureter | 18 (47) | 17 (49) |
| Left side | 20 (53) | 21 (60) |
| Right side | 18 (47) | 14 (40) |
| Removal method | ||
| Ureteroscopy | 33 (87) | 31 (89) |
| Percutaneous nephrolithotomy | 5 (13) | 4 (11) |
| Secondary stone — no. (%) | ||
| Location | ||
| Contralateral | 34 (90) | 32 (91) |
| Ipsilateral | 4 (10) | 3 (9) |
| Median size (IQR) — mm | 3 (3–4) | 4 (2–4) |
| Median no. (IQR) | 1 (1–2) | 1 (1–3) |
| Stone composition — no. (%) | ||
| Calcium oxalate | 33 (87) | 30 (86) |
| Calcium phosphate | 1 (11) | 3 (9) |
| Uric acid | 4 (3) | 0 |
| Other or unknown | 0 | 2 (6) |
| Median follow-up (IQR) — yr | 4.1 (2.9–5.0) | 4.2 (3.5–5.0) |
| Receipt of prescribed medication for stone prevention — no. (%) | 10 (26) | 8 (23) |
IQR denotes interquartile range.
Race was determined by a review of medical records or was reported by the patient.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
FOLLOW-UP AND OUTCOME
The primary outcome is shown in Figure 2A as the cumulative incidence of relapse; by that measure, the control group had more relapses than the treatment group (P<0.001 by log-rank test). The restricted mean time to relapse was 1631.6±72.8 days for the treatment group and 934.2±121.8 days for the control group. The hazard ratio for relapse in the treatment group as compared with the control group was 0.18 (95% confidence interval [CI], 0.07 to 0.44). Relapse events are summarized in Table 2. Relapse occurred in 6 of 38 patients (16%) in the treatment group and 22 of 35 patients (63%) in the control group. This difference of 47 percentage points was larger than the difference of 35 percentage points used in the power calculations.
Figure 2. Cumulative Incidence of Relapse.
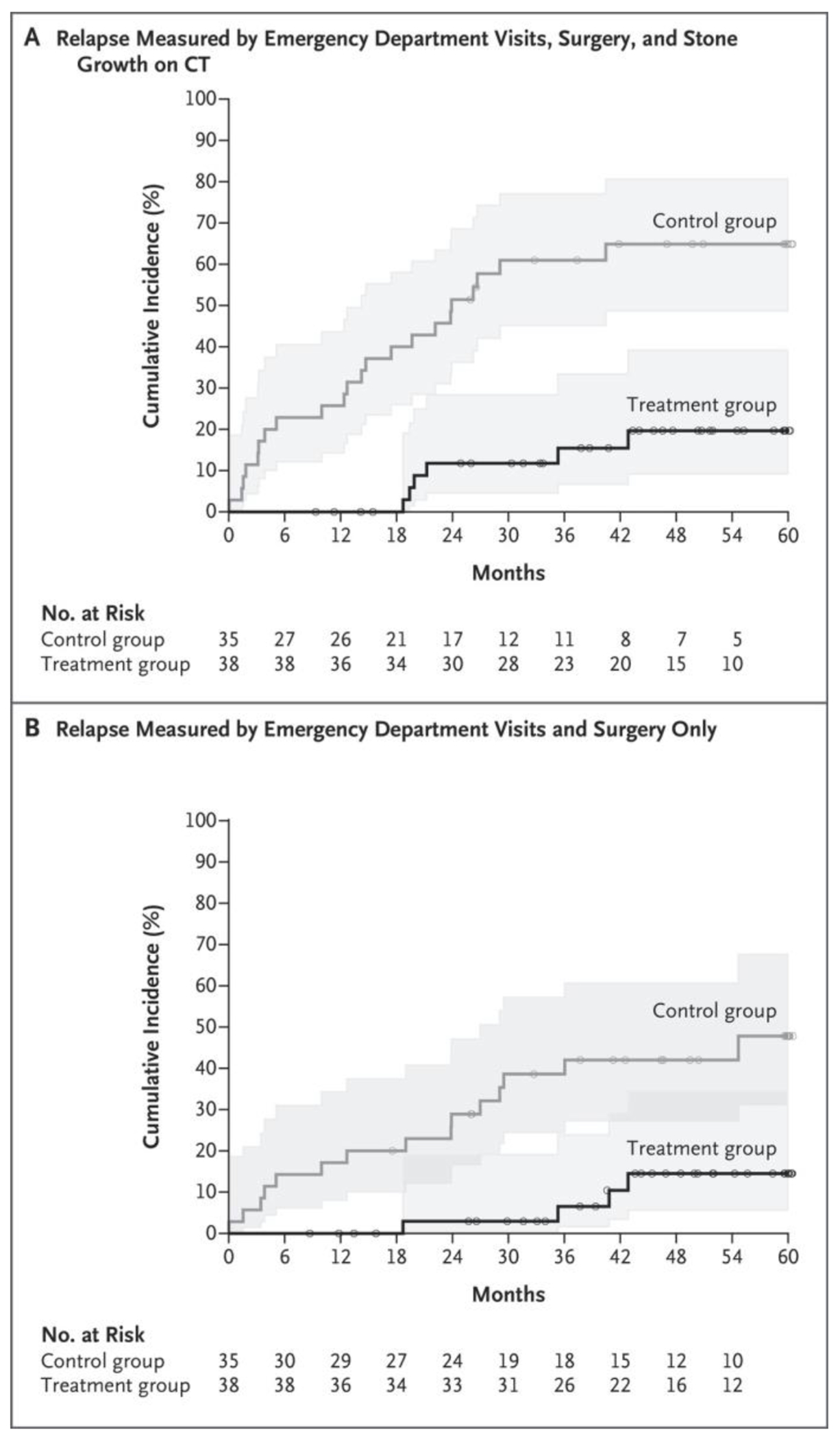
Shaded areas represent 95% confidence intervals. Small circles represent censored data. Patient relapse data were censored when the patient did not have a relapse before being lost to follow-up or by the end of follow-up. CT denotes computed tomography.
Table 2.
Primary Effectiveness Outcome (Relapse).
| Measure | Treatment (N = 38) | Control (N = 35) |
|---|---|---|
| number of patients (percent) | ||
| Surgery | 2 (5) | 9 (26) |
|
| ||
| Emergency department visit | 2 (5) | 10 (29) |
|
| ||
| Total surgeries, emergency department visits, or both | 4 (11) | 15 (43) |
|
| ||
| Growth of secondary stones or fragments* | 3 (8) | 13 (37) |
|
| ||
| Total surgeries, emergency department visits, or growth of stones | 6 (16) | 22 (63) |
Seven patients in the treatment group had residual fragments after ureteros-copy. Of the 13 patients in the control group who did not have a relapse, 2 were free of kidney stones after ureteroscopy.
Because of concerns that stone growth could be driving relapse disproportionately, sensitivity analyses were conducted. When stone growth was excluded as a marker of relapse (Fig. 2B), relapse remained greater in the control group (P = 0.002 by log-rank test). The time to relapse was 36% longer in the treatment group than in the control group, with a restricted mean time to relapse of 1717.1±51.7 days in the treatment group and 1262.8±117.7 days in the control group. An emergency department visit or surgery — two of the measures of relapse — occurred in 4 patients (11%) in the treatment group and 15 patients (43%) in the control group. The total number of emergency department visits and surgical procedures (including stent placement), not counting any visits or procedures that occurred after surgery at the time of relapse to remove the stone, was 4 (including 2 surgeries, both bilateral) in the treatment group and 23 (including 10 surgeries) in the control group.
For the secondary outcome of measured surgical duration for treatment of secondary stones, additional surgery time was longer in the treatment group. The median time added in all operations performed in patients in the treatment group was 25.6 minutes (interquartile range, 18.5 to 35.2), accounting for 27% of the total surgery time. The median total surgery time was 93.6 minutes (interquartile range, 79.5 to 107.5) for the treatment group and 59.8 minutes (interquartile range, 46.2 to 78.5) for the control group. The median time added was 25.0 minutes (interquartile range, 20.8 to 29.2) for ipsilateral ureteroscopy and 25.1 minutes (interquartile range, 17.5 to 36.1) for contralateral ureteroscopy. The median time added in the five percutaneous nephrolithotomies was 30.0 minutes (interquartile range, 26.0 to 38.5).
The secondary outcome, emergency department visits within 2 weeks after surgery, was observed in five patients (13%) in the treatment group and four (11%) in the control group (odds ratio, 1.17; 95% CI, 0.29 to 4.78). All visits were for stent pain.
The secondary outcome of patient-reported stone passage was observed in 8 patients (21%) in the treatment group and 10 patients (29%) in the control group (odds ratio, 0.67; 95% CI, 0.23 to 1.94). These data include 4 patients in each group in whom neither the patient nor the trial staff could determine the affected side. Seven patients in the treatment group and 6 in the control group reported asymptomatic stone or fragment passage. In addition, new stone formation that was detected by imaging (Fig. 3) and restricted mean time to relapse (time to new stone formation) were similar — 1338.8±104.0 days in the treatment group and 1381.1±100.5 days in the control group. New stones formed in 14 patients (37%) in the treatment group and 13 patients (37%) in the control group (odds ratio, 0.99; 95% CI, 0.38 to 2.56).
Figure 3. Cumulative Incidence of New Stones in the Trial Kidney.
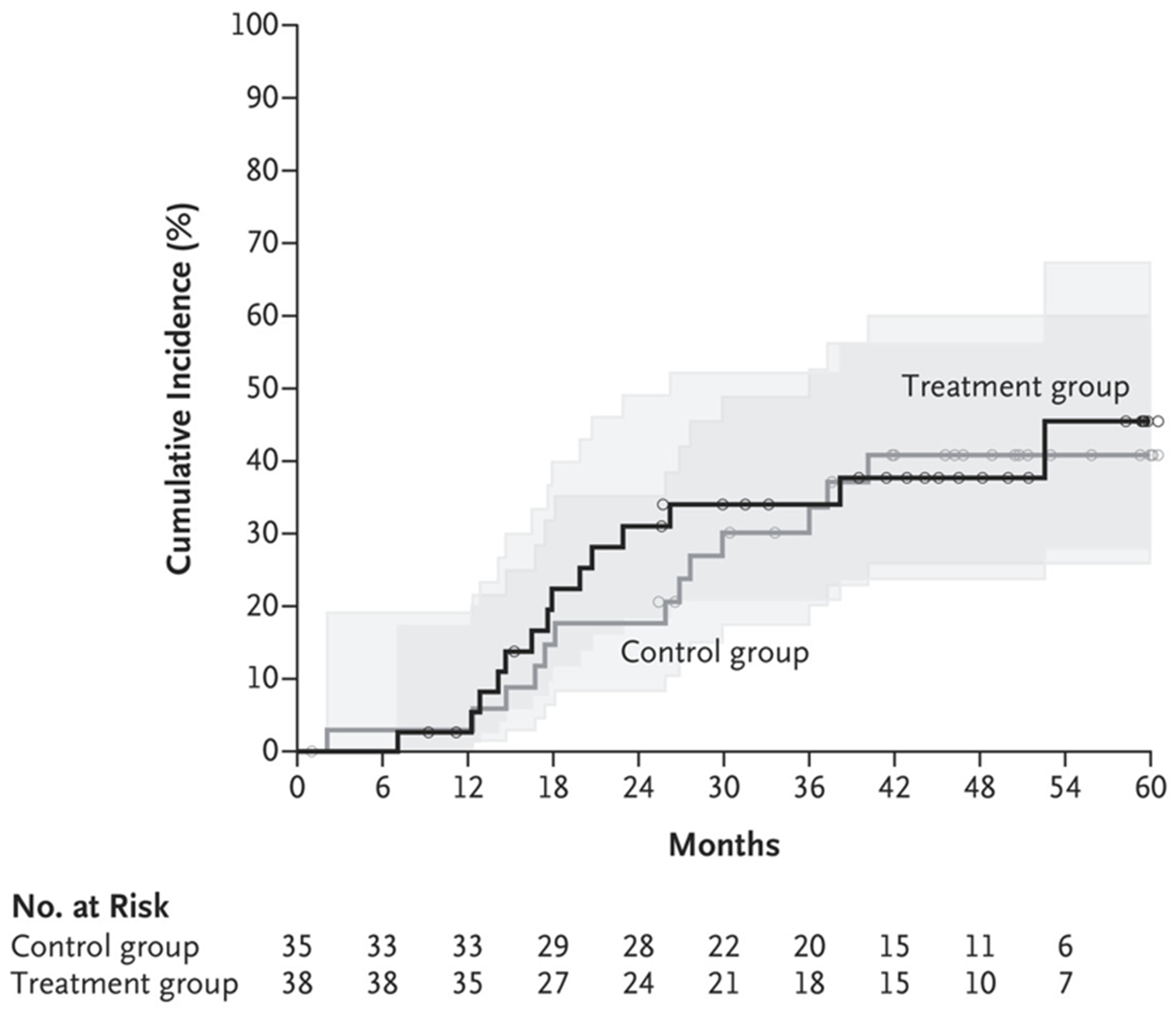
New stones were detected on CT. Small circles represent censored data. Shaded areas represent 95% confidence intervals.
DISCUSSION
In this multicenter, randomized, controlled trial, patients undergoing endoscopic treatment of a primary ureteral or kidney stone were assigned to the treatment group, in which they had small, asymptomatic (secondary) renal stones removed, or to the control group, in which those stones were not removed. We found that removal of secondary stones in the treatment group resulted in a 75% lower incidence of relapse — 6 of 38 patients (16%) in the treatment group as compared with 22 of 35 patients (63%) in the control group. Time to relapse was longer in the treatment group by 697 days (75%), but treatment added a median of 25.6 minutes to surgery times. Treatment of secondary stones did not increase emergency department visits that occurred within 2 weeks after surgery (13% in the treatment group vs. 11% in the control group) and did not affect new stone formation (37% in both groups) or self-reported stone passage (21% vs. 29%). Published studies have similarly reported an additional 16.7 minutes of surgical time to treat asymptomatic kidney stones during treatment of a primary ipsilateral ureteral stone as well as emergency department visits within 30 days after 8% of ureteroscopy surgeries.14,15
Results of our prospective, randomized trial support removal of small, asymptomatic renal stones at the time of surgery to remove a symptomatic stone. Whether to remove small, asymptomatic kidney stones is a common surgical decision that currently lacks specific guidelines2,3 and may involve hundreds of thousands of surgeries annually in the U.S. alone.16 The additional 25 minutes needed to remove small, asymptomatic renal stones at the time of surgery for a primary stone (extending the procedure by 38%) should be weighed against the potential need for repeat surgery in the 63% of patients who had a relapse. One comparison is financial cost: 100 surgical procedures with 25 additional minutes at $36 per minute17 would add $90,000 to the cost of the surgeries, whereas 63 emergency department visits at an average cost of $3,437 per visit would cost an estimated $217,000 — 2.4 times as much.18
The present trial showed more adverse outcomes within 5 years when small, asymptomatic kidney stones were left in place than when they were surgically removed. However, our trial has certain limitations. It was relatively small, and few of the patients were non-White, which attenuates generalization to other groups. Surgeons were aware of the patients’ group assignments during surgery and continued care; however, the shared patient-care model and the high levels of preventive evaluation in the participating centers probably counterbalanced that limitation. Indeed, the percentage of patients in each group who were prescribed medications for prevention were similar (roughly 25% of patients in each group). Taken together, our data add to a growing body of evidence that supports the efficacy and safety of single-setting treatment of ureteral and kidney stones, the combination of ureteroscopy and percutaneous nephrolithotomy, and bilateral endoscopic procedures.19-22
Some authors and guidance documents have proposed that the benefit of preemptive endoscopic surgery to remove small, asymptomatic renal stones is unclear.2 However, we would argue that a rate of one relapse event per patient might well justify prophylactic surgery on all patients. In the present trial, the control group of 35 patients subsequently had 36 relapse events (i.e., emergency department visit, surgery, or stone growth) — roughly one event per patient — whereas the treatment group had 0.18 events per patient during the follow-up period. Guidelines from the American Urological Association have cited a retrospective study that reported 43% of patients had a relapse within 32 months after surgery, a result that is similar over time to that of the control group in the present trial, in which 63% of patients had a relapse within 52 months.2,23 Residual stone fragments have been reported to remain after 30 to 65% of procedures and were present in 7 of 38 patients (18%) in the treatment group in our trial, including in all 6 patients who had relapse.16,24
Although the present trial was enabled by advancements in surgical technique and technology, the levels of surgical skill and experience of the individual surgeons were not tested here. Indeed, the trial size precluded subanalysis of surgical techniques and technologies. Future advances, such as transcutaneous ultrasonography to break and expel stones without the need for surgery or anesthesia,25,26 and data from validated quality-of-life measurement tools27 may further tilt the balance in favor of early intervention.
Our trial showed that removing small, asymptomatic renal stones during surgery for a ureteral or contralateral stone resulted in fewer subsequent emergency department visits and surgeries and less stone growth than leaving the secondary stones in place.
Supplementary Material
Acknowledgments
Supported by a grant (P01 DK043881) from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health and by the Veterans Affairs Puget Sound Health Care System.
Footnotes
REFERENCES
- 1.Boyce CJ, Pickhardt PJ, Lawrence EM, Kim DH, Bruce RJ. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol 2010;183:1017–21. [DOI] [PubMed] [Google Scholar]
- 2.Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American Urological Association/Endourological Society guideline. J Urol 2016;196:1153–69. [DOI] [PubMed] [Google Scholar]
- 3.EAU guidelines. Presented at the EAU20 Virtual Congress, July 17–19, 2020. [Google Scholar]
- 4.Keeley FX Jr, Tilling K, Elves A, et al. Preliminary results of a randomized controlled trial of prophylactic shock wave lithotripsy for small asymptomatic renal calyceal stones. BJU Int 2001;87:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Quarrier S, Serrell EC, Penniston KL, Nakada SY. Should we treat asymptomatic concurrent contralateral renal stones? A longitudinal analysis. Urolithiasis 2022;50:71–7. [DOI] [PubMed] [Google Scholar]
- 6.Djang R, Stahl JE, Pais VM Jr. Informing the management of symptomatic nephrolithiasis: Markov decision analysis for the 1 cm renal stone. Urol Pract 2021;8:495–502. [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Takahashi T, Kanno T, Okada T, Higashi Y, Yamada H. Decreased recurrence of urolithiasis after simultaneous ureteroscopic surgery for ureter and ipsilateral renal calculi: comparison to shockwave lithotripsy for ureter calculi alone. Urology 2021;147:74–80. [DOI] [PubMed] [Google Scholar]
- 8.Bhalla RG, Hsi RS. Should asymptomatic renal stones be surgically treated? Pro treatment. J Endourol 2021;35:567–9. [DOI] [PubMed] [Google Scholar]
- 9.Streeper NM. Should asymptomatic renal stones be surgically treated? Proobservation. J Endourol 2021;35:570–2. [DOI] [PubMed] [Google Scholar]
- 10.Williams JC Jr, Lingeman JE, Daudon M, Bazin D. Using micro computed tomographic imaging for analyzing kidney stones. C R Chim 2021;24:Suppl 2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghi L, Meschi T, Guerra A, Novarini A. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol 1993;22:Suppl 6:S78–S86. [PubMed] [Google Scholar]
- 12.Ettinger B, Citron JT, Livermore B, Dolman LI. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol 1988;139:679–84. [DOI] [PubMed] [Google Scholar]
- 13.Laerum E Metabolic effects of thiazide versus placebo in patients under longterm treatment for recurrent urolithiasis. Scand J Urol Nephrol 1984;18:143–9. [DOI] [PubMed] [Google Scholar]
- 14.Bilgasem S, Pace KT, Dyer S, Honey RJ. Removal of asymptomatic ipsilateral renal stones following rigid ureteroscopy for ureteral stones. J Endourol 2003;17:397–400. [DOI] [PubMed] [Google Scholar]
- 15.Hiller SC, Daignault-Newton S, Pimentel H, et al. Ureteral stent placement following ureteroscopy increases emergency department visits in a statewide surgical collaborative. J Urol 2021;205:1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinstein L, Matlaga B. Urologic diseases in America. Washington, DC: Government Printing Office, 2018:12–3 (https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/urologic-diseases-in-america). [Google Scholar]
- 17.Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg 2018;153(4):e176233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldwell N, Srebotnjak T, Wang T, Hsia R. “How much will I get charged for this?” Patient charges for top ten diagnoses in the emergency department. PLoS One 2013;8(2):e55491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocuzza M, Colombo JR Jr, Ganpule A, et al. Combined retrograde flexible ureteroscopic lithotripsy with holmium YAG laser for renal calculi associated with ipsilateral ureteral stones. J Endourol 2009;23:253–7. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg H, Holland R, Tal R, Lask DM, Livne PM, Lifshitz DA. The impact of retrograde intrarenal surgery for asymptomatic renal stones in patients undergoing ureteroscopy for a symptomatic ureteral stone. J Endourol 2013;27:970–3. [DOI] [PubMed] [Google Scholar]
- 21.Marguet CG, Springhart WP, Tan YH, et al. Simultaneous combined use of flexible ureteroscopy and percutaneous nephrolithotomy to reduce the number of access tracts in the management of complex renal calculi. BJU Int 2005;96:1097–100. [DOI] [PubMed] [Google Scholar]
- 22.Fiscus G, Marien T, Tangpaitoon T, Kuebker J, Herrell SD, Miller NL. Single session bilateral vs staged bilateral ureteroscopy for nephrolithiasis: an assessment of safety and efficacy. Urology 2019;123:64–9. [DOI] [PubMed] [Google Scholar]
- 23.Raman JD, Bagrodia A, Gupta A, et al. Natural history of residual fragments following percutaneous nephrostolithotomy. J Urol 2009;181:1163–8. [DOI] [PubMed] [Google Scholar]
- 24.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol 2008;179:Suppl:S69–S73. [DOI] [PubMed] [Google Scholar]
- 25.Harper JD, Lingeman JE, Sweet RM, et al. Fragmentation of stones by burst wave lithotripsy in the first 19 humans. J Urol 2022;207:1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MK, Thiel J, Dunmire B, et al. Feasibility study of using point of care ultrasonic propulsion and burst wave lithotripsy (BWL) to noninvasively treat symptomatic ureteral stones. J Urol 2022;208 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penniston KL, Antonelli JA, Viprakasit DP, et al. Validation and reliability of the Wisconsin Stone Quality of Life Questionnaire. J Urol 2017;197:1280–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


