Abstract
Background:
Metastatic adenocarcinoma (MAC) accounts for most cases of malignant effusions. Sometimes, it can be difficult to distinguish MAC from reactive mesothelial cells (RMC) in cytologic specimens. Our aim was to assess the diagnostic performance of a novel immunohistochemical panel composed of claudin-4 and EZH2 in differentiating MAC from RMC in effusion cytology.
Methods:
A total of 80 cases of serous effusions (48 MAC and 32 RMC) were included. Immunohistochemistry using claudin-4 and EZH2 was performed on cell block sections of these cases. Assessment of staining patterns, intensity and percentage of target cells stained was done.
Results:
Claudin-4 showed membranous staining in 46/48 of MAC and 1/32 of RMC. High EZH2 (≥ 50% of target cells) was detected in 42/48 MAC and 2/32 RMC. For the discrimination between MAC and RMC, claudin-4 exhibited 95.8% sensitivity and 96.9% specificity, high-EZH2 exhibited 87.5% sensitivity and 93.8% specificity, while the combination of both claudin-4 and high EZH2 showed 100% sensitivity and 90.6% specificity.
Conclusion:
Claudin-4 shows high sensitivity and specificity in differentiation between MAC and RMC in effusion cytology, and might be useful as a solitary marker for MAC. Adding EZH2 to claudin-4 increases the sensitivity to 100%. However, the interpretation of EZH2 results can be challenging due to its focal expression in RMC and inflammatory cells.
Key Words: Claudin-4- EZH2, immunohistochemistry, effusion cytology, adenocarcinoma
Introduction
Effusions of serous membranes may complicate a variety of pathologic conditions, both neoplastic and non-neoplastic. Cytologic examination of this fluid is indispensable for evaluation of etiology, and thus for guidance of the management plan (Cibas, 2019). Approximately 20% of effusions are malignant, with the majority caused by metastatic adenocarcinoma (Shidham and Layfield, 2021). In case of pleural and pericardial effusion, the adenocarcinoma most commonly originates from lung or breast, while in ascitic fluid, the most common primary sites are gastrointestinal and female genital tracts (Lew et al., 2021).
Diagnosis of metastatic adenocarcinoma (MAC) in effusion samples is not always straightforward. In some cases, morphologic distinction between MAC and reactive mesothelial cells (RMC) is challenging, as both may show wide range of morphologic variations, or only few suspicious cells might be seen (Ikeda et al., 2011). In such doubtful cases, application of immunohistochemical markers to cell blocks allows more precise diagnosis of malignancy (Subbarayan et al., 2019).
Currently, several markers: mesothelial (e.g. calretinin, CK5/6, mesothelin, WT-1, D2-40) and epithelial (e.g. CEA, Ber-Ep4, B72.3, and MOC-31) are used. Owing to the unsatisfactory sensitivity and specificity of any one marker, the diagnostic workup often requires immunohistochemical panels, that usually involve multiple markers (Cibas, 2019). These panels, however, are not cost-effective, so they are not suitable for routine use especially in resource-limited and work-loaded centers. The search continues for new markers with optimal accuracy and reasonable cost (Li et al., 2021).
Claudin-4 and Enhancer of zeste homologue 2 (EZH2) are promising carcinoma markers recently proposed in literature. Both recognize carcinoma cells in different ways; claudin-4 recognizes their epithelial origin, while EZH2 identifies their malignant phenotype mediated by aberrant protein expression (Vojtek et al., 2019; Ang et al., 2020).
Claudin-4, a member of the claudin family, is a tight junction protein normally expressed in the epithelium of several organs e.g. lung, colon and endometrium, but rarely encountered in mesothelial cells (Liu and Li, 2020). Recent studies suggest that it might be used to differentiate MAC from RMC in cytology specimens (Oda et al., 2016; Vojtek et al., 2019).
EZH2 is a core subunit of the polycomb repressive complex 2 (PRC2), a histone methyltransferase enzyme that mediates epigenetic silencing of target genes through induction of chromatin compaction (Kang and Chun, 2020). Hyperactivation of EZH2 promotes carcinogenesis by altering the expression of many genes essential for cell cycle regulation, lineage specification, and DNA repair (Duan et al., 2020). Over-expression of EZH2 has been demonstrated in many tumors including mesothelioma, melanoma, and carcinomas of lung, breast, ovary, colon, and liver (Chang and Hung, 2012). Recently, few studies examined the expression of EZH2 by immunohistochemistry on cytology material, and found it to be highly sensitive and specific in detection of malignant cells (Sadullahoglu et al., 2017; Ang et al., 2020).
The aim of this study was to examine the immunohistochemical expression of claudin-4 and EZH2 in cell block sections, in order to assess their diagnostic role in differentiating between MAC and RMC in effusion samples.
Materials and Methods
Case selection
This is a retrospective study that included 80 cases of serous effusions (45 pleural, 32 peritoneal and 3 pericardial) diagnosed between November 2019 and October 2021 at the Pathology Department of Assiut University Hospital. The study was approved by The Institutional Review Board (IRB) of Faculty of Medicine, Assiut University (Approval Number: 17200276). The sample size was calculated using Open EPI with 95% two-sided confidence level and 90% power according to results of Ang et al., (2020).
Among the included cases, there were 48 metastatic adenocarcinomas (MAC) and 32 benign effusions with reactive mesothelial cells (RMC). For all cases, diagnosis was established by clinical and radiological correlation, histologic specimens of primary tumors (available for 30 cases of MAC), and/or confirmatory immunohistochemical studies. All cases had available cell block material with adequate cells of interest (at least 50) on cell block slide. Cell blocks were prepared using the alcohol-formalin method described by Nathan et al., (2000). Cases with inconsistent clinicopathologic data or inadequate cellularity were excluded from the study.
Immunohistochemistry
For immunohistochemistry, 4 μm-thick sections of paraffin-embedded cell blocks were mounted on positively charged slides. The slides were deparaffinized, and endogenous peroxidase activity was blocked by treating with hydrogen peroxide for 5 minutes. For antigen retrieval, slides were microwaved for 15 min in 10mM sodium citrate buffer (pH 6.0). Sections were incubated with Claudin-4 mouse monoclonal antibody (clone 3E2C1, Invitrogen) diluted at 1:150 overnight at 4oC, and with EZH2 mouse monoclonal antibody (Clone 144CT2.1.1.5, Invitrogen) diluted at 1:50, for 30 minutes at room temperature. Detection was performed by EconoTek Biotinylated Anti-polyvalent antibody for 30 minutes, and EconoTek Horseradish peroxidase for 30 minutes (ScyTek Laboratories, USA). The reaction was developed using DAB chromogen, then sections were counterstained with hematoxylin. The negative controls were processed similarly with omission of the primary antibody. Sections of normal colon and tonsil were used as positive control for claudin-4 and EZH2 respectively.
Blinded to any diagnostic information, the immunostained slides were separately examined by two authors and scored for intensity of staining (absent, weak, moderate or strong) and percentage of target cells stained (score 0 for <5%, 1 for 5-49%, 2 for 50-89% and 3 for ≥90%). A positive result was defined as moderate to strong membranous staining for claudin-4, and moderate to strong nuclear staining for EZH2, encountered in ≥ 5% of the target cells (Jiang et al., 2014; Patel et al., 2020).
Furthermore, EZH2 positive specimens were divided into two groups, EZH2-low (score 1) and EZH2-high (score 2–3) following previous reports of Shinozaki-Ushiku et al., (2017) and Yoshimura et al., (2019) on biopsy material.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics version 21. Analyses of the differences between categorical variables were performed using Chi-square test. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated for each marker. P-value <0.05 was considered statistically significant.
Results
The study included 80 cases of serous effusions, including 36 males (45%) and 44 females (55%). The mean age was 55 years (range 26-85) for patients with benign effusion, and 60 years (range 23-91) for patients with MAC. The primary tumor sites of the MAC cases included lung (18), breast (6), ovary (6), endometrium (4), colon (4), stomach (2), esophagus (1), bladder (1), pancreas (1), biliary tract (1), cervix (1), and unknown origin (3). The results of immunohistochemistry are summarized in Tables 1 and 2.
Table 1.
Immunohistochemical Results in the Study Groups
| Metastatic adenocarcinoma, n (%) | Reactive mesothelial cells, n (%) | P value* | ||
|---|---|---|---|---|
| Claudin-4 | Positive | 46 (95.8%) | 1 (3.1%) | <0.001 |
| Negative | 2 (4.2%) | 31 (96.9%) | ||
| EZH2 | Positive | 45 (93.8%) | 10 (31.3%) | <0.001 |
| Negative | 3 (6.3%) | 22 (68.8%) | ||
| EZH2 | High | 42 (87.5%) | 2 (6.3%) | <0.001 |
| No/low | 6 (12.5%) | 30 (93.8%) | ||
| Total | 48 (100%) | 32 (100%) | ||
* calculated by Chi Square test.
Table 2.
Detailed Results of Claudin-4 & EZH2 Immunohistochemistry in Reactive and Malignant Serous Effusions
| Diagnosis | Number | Claudin 4 percentage score | EZH2 percentage score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| Reactive mesothelial cells | 32 (100) | 31 (96.9) | 1 (3.1) | 0 (0) | 0 (0) | 22 (68.8) | 8 (25.0) | 2 (6.3) | 0 (0) | |
| Metastatic adenocarcinoma | 48 (100) | 2 (4.2) | 4 (8.3) | 29 (60.4) | 13 (27.1) | 3 (6.3) | 3 (6.3) | 32 (66.7) | 10 (20.8) | |
| Origin | Lung | 18 | 1 | 1 | 11 | 5 | 0 | 0 | 12 | 6 |
| Breast | 6 | 0 | 0 | 3 | 3 | 1 | 0 | 5 | 0 | |
| Ovary | 6 | 0 | 1 | 3 | 2 | 1 | 0 | 4 | 1 | |
| Endometrium | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 3 | 1 | |
| Esophagus | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Colon | 4 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 1 | |
| Stomach | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | |
| Bladder | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | |
| Pancreas | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | |
| Biliary tract | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Cervix | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Unknown origin | 3 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | |
Figures in parentheses are percentages
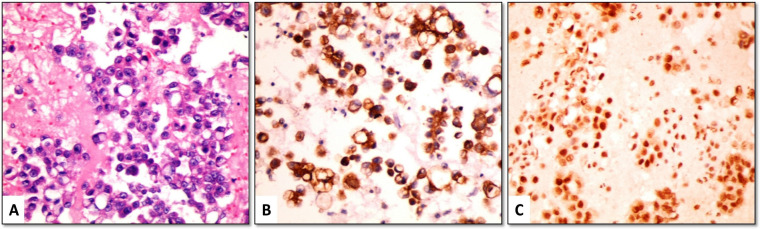
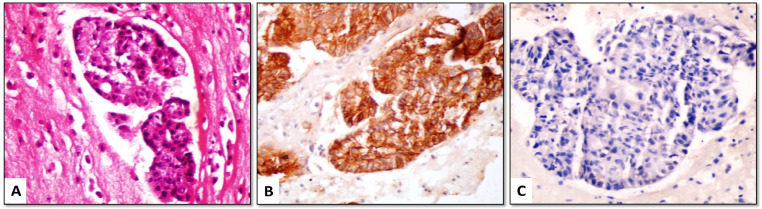
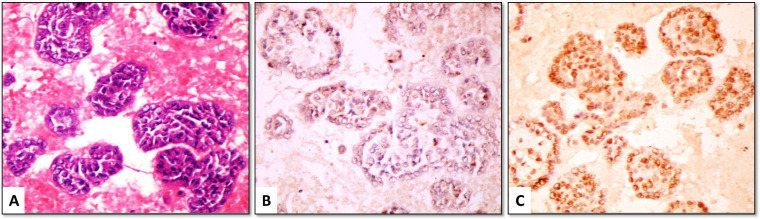
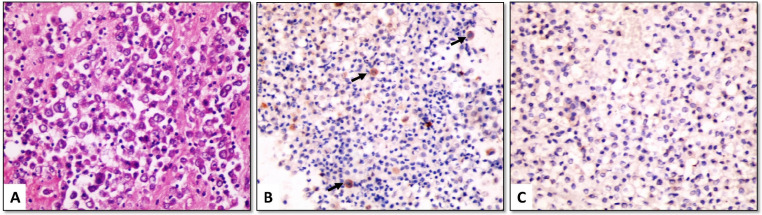
Claudin-4 positive cells showed linear membranous staining with or without cytoplasmic staining. Among the MAC cases, 46/48 (95.8%) were positive for claudin-4, with most of them (42/48 [87.5%]) showed positive staining in ≥ 50% of malignant cells (score 2 or 3) (Figures 1-3). The positive cells occurred as small clusters or separate cells. The two negative cases included one metastatic from lung adenocarcinoma (Figure 4) and one from endometrial carcinoma.
Figure 1.
(A) Metastatic lung adenocarcinoma (H&E) showing (B) strong membranous positivity for claudin-4, and (C) strong nuclear positivity for EZH2 (cell-block material from pleural effusion, ×400)
Figure 3.
(A) Metastatic ductal carcinoma of breast (H&E); (B) tumor cells show strong membranous positivity for claudin-4, (C) but are negative for EZH2 (cell-block material from pleural effusion, ×400)
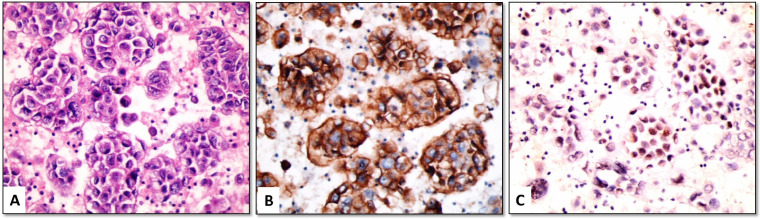
Figure 4.
(A) Metastatic lung adenocarcinoma (H&E); (B) tumor cells are negative for claudin-4, (C) but show strong nuclear positivity for EZH2 (cell-block material from pleural effusion, ×400)
Among the 32 benign effusions with RMC, only one case showed positive staining for claudin-4. This case was a pleural fluid sample from a 67 years old male with para-pneumonic effusion. It showed moderate membranous staining in about 30% of mesothelial cells. The remaining cases did not show membranous staining for claudin-4, however, few scattered cells showed cytoplasmic positivity (Figure 5).
Figure 5.
(A) Sheets of reactive mesothelial cells (RMCs) (H&E). (B) RMCs show negative membranous staining for claudin-4, while few scattered cells (arrows) show cytoplasmic positivity. (C) RMCs are negative for EZH2 (cell-block material from pleural effusion, x400)
For the discrimination between MAC and RMC, claudin-4 showed 95.8% sensitivity, 96.9% specificity, 97.9% positive predictive value and 93.9% negative predictive value (Table 3).
Table 3.
Sensitivity, Specificity, Positive and Negative Predictive Values and Diagnostic Accuracy of Claudin-4 and EZH2
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | DA, % | |
|---|---|---|---|---|---|
| Claudin-4 | 95.83 | 96.88 | 97.87 | 93.94 | 96.25 |
| EZH2* | 93.75 | 68.75 | 81.82 | 88.00 | 83.75 |
| Claudin-4 + EZH2* | 100.00 | 65.62 | 81.36 | 100.00 | 86.25 |
| EZH2-high** | 87.50 | 93.75 | 95.45 | 83.33 | 90.00 |
| Claudin-4 + EZH2-high** | 100.00 | 90.62 | 94.12 | 100.00 | 96.25 |
* At a cut-off value 5%; ** At a cut-off value 50%; Abbreviations: PPV, positive predictive value; NPV, negative predictive value; DA, diagnostic accuracy
Positivity for EZH2 was detected as moderate to strong nuclear staining in mesothelial or malignant cells. The background inflammatory cells also showed focal positivity.
At a 5% cut-off value, EZH2 was positive in 45/48 (93.8%) cases of MAC (Figures 1, 2 and 4) and 10/32 (31.3%) cases of benign cytology. The three cases of MAC that were negative for EZH2 included metastatic breast (Figure 3), ovarian and colorectal carcinomas. As a diagnostic marker for MAC, EZH2 exhibited 93.8% sensitivity, 68.8% specificity, 81.8% positive predictive value and 88% negative predictive value (Table 3).
Figure 2.
(A) Metastatic ovarian serous adenocarcinoma (H&E) showing (B) strong membranous positivity for claudin-4, and (C) moderate nuclear positivity for EZH2 (cell-block material from peritoneal effusion, ×400)
Using 50% as a cut-off value, 42/48 (87.5%) cases of MAC and only 2/32 (6.3%) cases of RMC showed high EZH2 expression (≥ 50% of target cells). High EZH2 exhibited a higher specificity (93.8%) but a lower sensitivity (87.5%) for discriminating MAC from RMC (Table 3).
The combination of positive claudin-4 and high EZH2 expression showed 100% sensitivity, 90.6% specificity, 94.1% positive predictive value and 100% negative predictive value (Table 3).
Discussion
Several immunohistochemical markers have been proposed in literature for the discrimination between MAC and RMC in effusion specimens, however, none has been widely approved for use as a solitary marker or in a limited cost-effective panel (Li et al., 2021).
In this study, we proposed that a limited panel composed of claudin-4, an epithelial marker, and EZH2, a marker of aberrant protein expression in malignant cells, would be perfect for this discrimination. We examined the expression of claudin-4 and EZH2 in cell block sections of 48 cases of metastatic adenocarcinoma and 32 cases with benign cytology.
Claudin-4 is a tight junction protein normally expressed in many types of epithelium (Liu and Li, 2020). We found claudin-4 to be positive in 95.8% of adenocarcinomas originating from different sites, including lung, breast, ovary and gastrointestinal tract, which are the most common primary sites of malignant effusion. Our findings are in line with those of Jo et al.,(2014), Kim et al., (2016), Vojtek et al., (2019) and Bernardi et al., (2021) who examined claudin-4 expression in effusion cell blocks, and reported positive staining in 98.8%, 100%, 95.6% and 100% of adenocarcinomas respectively. Lonardi et al., (2011) found claudin-4 immunoreactivity in smears of 99% of adenocarcinomas.
Similar results were also reported in biopsies. Ordóñez et al., (2013) found that, in surgical biopsy specimens, claudin-4 was positive in all adenocarcinomas of the lung, breast, colon, pancreas, and prostate and in 98% of ovarian carcinomas. Naso (2020) assessed claudin-4 immunoreactivity in surgical specimens of lung tumors, and reported positivity in 87% of lung adenocarcinomas. Furthermore, claudin-4 was reported by separate reports in most cases of endometrial, breast, gastric, prostatic and ovarian carcinomas (Pan et al., 2013; Abd-Elazeem and Abd-Elazeem, 2015; Shareef et al., 2015; Radi and Abd-Elazeem, 2016; Martín de la Fuente et al., 2018).
In our study, claudin-4 was positive in only one case of benign effusions with RMC. Our results are comparable to those of Jo (2014), Vojtek (2019) and Bernardi (2021) who did not notice claudin-4 positivity in any of the reactive mesothelial cases. Kim (2016), however, reported positive staining in 27.5% of RMC, despite using the same antibody clone we used. This discrepancy may be explained by different definition of positive staining. We considered the case positive when ≥ 5% of cells are stained, while Kim (2016) defined the case as positive when any cell showed positive staining.
In adenocarcinomas, claudin-4 immunoreactivity was discernible as moderate to strong linear membranous positivity, even in sparsely cellular samples. The results were easy to interpret and differentiate from scattered cytoplasmic positivity detected in reactive mesothelial cells. Similar cytoplasmic reactivity was reported by Lonardi et al., (2011) and Jo et al., (2014) in mesothelial and inflammatory cells, and was considered as a non-specific finding attributed to antigen retrieval conditions. Vojtek (2019) also described distinct dot-like pattern of cytoplasmic positivity limited to mesothelial cells.
Claudin-4 exhibited high sensitivity and specificity for differentiation between MAC and RMC (95.8% and 96.9% respectively). Close figures were previously reported by Jo et al., (2014), Oda et al., (2016), Vojtek et al., (2019) and Patel et al., (2020). The sensitivity of claudin-4 was 100%, 96.4%, 95.6% and 100%, while specificity was 99%, 100%, 99.1% and 100% respectively.
To our knowledge, none of the other commonly used epithelial markers have shown such high sensitivity and specificity in this context. According to meta-analyses, MOC-31 showed 85% sensitivity and 97% specificity, while Ber-EP4 showed 80% sensitivity and 94% specificity (Li et al., 2014; Wang et al., 2014). CD15 (LeuM1) and B72.3 exhibited variable rates of sensitivity, ranging from 24% to 73% for the former (Shield et al., 1994; Bailey et al., 1996), and 62% to 88% for the latter (Afify et al., 2005; Patel et al., 2020). Furthermore, some markers show variable sensitivity according to the origin of carcinoma. For example, CEA is commonly expressed in adenocarcinomas of lung, breast and GIT, but rarely expressed in ovarian carcinomas (Ordóñez, 2006).
EZH2 is a chromatin-modifying enzyme with a well-established role in the pathogenesis of several types of cancer e.g. breast and lung carcinomas (Duan et al., 2020). Recent studies suggested a promising role of EZH2 in diagnosis of malignancy in effusions (Jiang et al., 2014; Sadullahoglu et al., 2017; Ang et al., 2020). Our study found a modest diagnostic accuracy of EZH2 in effusion cytology.
In this study, the choice of a cut-off value for EZH2 immunoreactivity was problematic due to the wide discrepancy of values reported in literature. While most reports on cytology material adopted 5% as a cut-off value for EZH2 positivity (Jiang et al., 2014; Sadullahoglu et al., 2017), many reports on biopsy specimens divided cases into EZH2-low or high according to a 50% cut-off value (Shinozaki-Ushiku et al., 2017; Hakim and Abou Gabal, 2021). To solve this problem, we assessed the diagnostic utility of EZH2 at both cut-off values, 5% and 50%.
At 5% cut-off value, EZH2 was positive in 93.8% of MAC cases and 31.2% of benign cases with RMC. When 50% was used as a cut-off value, most of the MAC cases (87.3%) showed high expression (i.e. in ≥ 50% of target cells), while the majority of benign cases (93.8%) showed no/low expression. For the identification of MAC in effusions, positive EZH2 (≥ 5%) exhibited high sensitivity (93.8%) but low specificity (68.8%), while high EZH2 (≥ 50%) showed improved specificity (93.8%) but lower sensitivity (87.5%).
Previous studies by Jiang et al., (2014), Sadullahoglu et al., (2017) and Ang et al., (2020) reported similarly high sensitivity of EZH2 in detection of MAC in cell block sections of effusion samples (90%, 93% and 95.5% respectively). However, they reported 100% specificity, which is different from the results of this study. Our results are supported by Shinozaki-Ushiku et al., (2017) and Hakim and Abou Gabal (2021) who detected EZH2 expression in histologic specimens of reactive mesothelial hyperplasia.
In our work, EZH2 exhibited discernible nuclear staining in target cells, with minimal staining of the background proteinaceous material. However, focal positivity of EZH2 was detected in inflammatory cells as described by others (Jiang et al., 2014; Sadullahoglu et al., 2017; Ang et al., 2020). This might cause diagnostic problems, especially in specimens with dyscohesive malignant cells dispersed in background rich in inflammatory cells. Based on our observations, it is not advised to use EZH2 as a solitary marker for adenocarcinoma in effusion samples, and it is better used in combination with other markers.
In our study, combining high EZH2 with positive claudin-4 showed the highest sensitivity (100%) with a good specificity (90.6%). The cut-off value for high EZH2 is fairly easy to follow, and its nuclear staining pattern is a good addition to the membranous pattern of claudin-4. Furthermore, high EZH2 identifies adenocarcinoma cells based on their aberrant protein expression and not their primary origin, thus, it is supposed to complement the role of claudin-4 which recognizes their epithelial phenotype (Vojtek et al., 2019; Ang et al., 2020).
In conclusion, claudin-4 shows high sensitivity and specificity in differentiation between MAC and RMC in effusion cytology, and might be useful as a solitary marker for MAC in selected cases. Adding EZH2 to claudin-4 would increase the sensitivity. However, the interpretation of EZH2 results can be challenging due to its focal expression in RMC and inflammatory cells.
Author Contribution Statement
Aliaa Elhosainy: Conception and design of the work; acquisition, analysis and interpretation of data; drafting of the work; statistical analysis; obtaining funding; and administrative, technical, and material support. Momen M. A. Hafez: Conception and design; critical revision of the of the work; administrative, technical, and material support; and supervision. Etemad H. Yassin: Conception and design; critical revision of the of the work; and supervision. Mohamed Adam: Acquisition of data; critical revision of the of the work; and supervision. Maha S. Elnaggar: Acquisition of data; and critical revision of the of the work. Noha A. Aboulhagag: Conception and design; critical revision of the of the work; and administrative, technical, and material support. All authors have approved the final version of the paper and accept responsibility for all aspects of the work.
Acknowledgements
Funding
This research received 25,000 LE grant from the Funding Unit in Faculty of Medicine, Assiut University. The Funding Unit had no involvement in study design, collection, analysis and interpretation of data, or writing of the report.
Protocol approval
This study is a part of the MD thesis of the corresponding author. The protocol of this thesis was approved by Faculty of Medicine, Assiut University in November 2018. The protocol was registered in ClinicalTrials.gov (ID: NCT04279327).
Ethical approval
The study was approved by The Institutional Review Board (IRB) of Faculty of Medicine, Assiut University (Approval Number: 17200276).
Conflict of interest
We have no conflict of interest to declare.
References
- Abd-Elazeem MA, Abd-Elazeem MA. Claudin 4 expression in triple-negative breast cancer: correlation with androgen receptors and Ki-67 expression. Ann Diagn Pathol. 2015;19:37–42. doi: 10.1016/j.anndiagpath.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Afify A, Zhou H, Howell L, Paulino AF. Diagnostic utility of GLUT-1 expression in the cytologic evaluation of serous fluids. Acta Cytol. 2005;49:621–6. doi: 10.1159/000326249. [DOI] [PubMed] [Google Scholar]
- Ang PP, Tan GC, Karim N, Wong YP. Diagnostic value of the EZH2 immunomarker in malignant effusion cytology. Acta Cytol. 2020;64:248–55. doi: 10.1159/000501406. [DOI] [PubMed] [Google Scholar]
- Bailey ME, Brown RW, Mody DR, Cagle P, Ramzy I. Ber-EP4 for differentiating adenocarcinoma from reactive and neoplastic mesothelial cells in serous effusions Comparison with carcinoembryonic antigen B72 3 and Leu-M1. Acta Cytol. 1996;40:1212–6. doi: 10.1159/000333982. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Bizzarro T, Pironi F, et al. The “Brescia panel” (Claudin-4 and BRCA-associated protein 1) in the differential diagnosis of mesotheliomas with epithelioid features versus metastatic carcinomas. Cancer Cytopathol. 2021;129:275–82. doi: 10.1002/cncy.22368. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012;106:243–7. doi: 10.1038/bjc.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibas ES. In: Cytology: Diagnostic Principles and Clinical Correlates. Eds Cibas ES, Ducatman BS., editors. Elsevier; 2019. [Pleural, Pericardial, and Peritoneal Fluids]. pp. 141–70. [Google Scholar]
- Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:1–12. doi: 10.1186/s13045-020-00937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim SA, Abou Gabal HH. Diagnostic utility of BAP1, EZH2 and Survivin in differentiating pleural epithelioid mesothelioma and reactive mesothelial hyperplasia: Immunohistochemical study. Pathol Oncol Res. 2021;27:600073. doi: 10.3389/pore.2021.600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Tate G, Suzuki T, Kitamura T, Mitsuya T. Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using cytospin preparations. Diagn Cytopathol. 2011;39:395–401. doi: 10.1002/dc.21398. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gupta R, Somma J. EZH2, a unique marker of malignancy in effusion cytology. Diagn Cytopathol. 2014;42:111–6. doi: 10.1002/dc.22999. [DOI] [PubMed] [Google Scholar]
- Jo VY, Cibas ES, Pinkus GS. Claudin-4 immunohistochemistry is highly effective in distinguishing adenocarcinoma from malignant mesothelioma in effusion cytology. Cancer Cytopathol. 2014;122:299–306. doi: 10.1002/cncy.21392. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Chun T. Structural heterogeneity of the mammalian polycomb repressor complex in immune regulation. Exp Mol Med. 2020;52:1004–15. doi: 10.1038/s12276-020-0462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NI, Kim GE, Lee JS. Diagnostic usefulness of Claudin-3 and Claudin-4 for immunocytochemical differentiation between metastatic adenocarcinoma cells and reactive mesothelial cells in effusion cell blocks. Acta Cytol. 2016;60:232–9. doi: 10.1159/000447008. [DOI] [PubMed] [Google Scholar]
- Lew M, Cantley R, Heider A, Jing X. Diagnosis and categorization of malignant effusions: A 6-year review from a single academic institution. Diagn Cytopathol. 2021;49:615–21. doi: 10.1002/dc.24433. [DOI] [PubMed] [Google Scholar]
- Li D, Wang B, Hu Q, et al. Diagnostic accuracy of MOC-31 for malignant effusions: a meta-analysis. Tumour Biol. 2014;35:6003–9. doi: 10.1007/s13277-014-1795-2. [DOI] [PubMed] [Google Scholar]
- Li M, Zhao L, Zhou X, et al. Detection of carcinoma in serous effusions: a review. Am J Cancer Res. 2021;11:43–60. [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li M. The role of claudin-4 in the development of gastric cancer. Scand J Gastroenterol. 2020;55:1072–8. doi: 10.1080/00365521.2020.1795923. [DOI] [PubMed] [Google Scholar]
- Lonardi S, Manera C, Marucci R, et al. Usefulness of Claudin 4 in the cytological diagnosis of serosal effusions. Diagn Cytopathol. 2011;39:313–7. doi: 10.1002/dc.21380. [DOI] [PubMed] [Google Scholar]
- Martín de la Fuente L, Malander S, Hartman L, et al. Claudin-4 expression is associated with survival in ovarian cancer but not with chemotherapy response. Int J Gynecol Pathol. 2018;37:101–9. doi: 10.1097/PGP.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- Oda T, Ogata S, Kawaguchi S, et al. Immunocytochemical utility of claudin-4 versus those of Ber-EP4 and MOC-31 in effusion cytology. Diagn Cytopathol. 2016;44:499–504. doi: 10.1002/dc.23476. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG. Value of immunohistochemistry in distinguishing peritoneal mesothelioma from serous carcinoma of the ovary and peritoneum: a review and update. Adv Anat Pathol. 2006;13:16–25. doi: 10.1097/01.pap.0000201832.15591.1d. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG. Value of claudin-4 immunostaining in the diagnosis of mesothelioma. Am J Clin Pathol. 2013;139:611–9. doi: 10.1309/AJCP0B3YJBXWXJII. [DOI] [PubMed] [Google Scholar]
- Pan XY, Li X, Che YC, et al. Overexpression of claudin-4 may be involved in endometrial tumorigenesis. Oncol Lett. 2013;5:1422–6. doi: 10.3892/ol.2013.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Borczuk AC, Siddiqui MT. Utility of Claudin-4 versus BerEP4 and B72 3 in pleural fluids with metastatic lung adenocarcinoma. J Am Soc Cytopathol. 2020;9:146–51. doi: 10.1016/j.jasc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- Radi DA, Abd-Elazeem MA. Prognostic significance of lymphatic vessel density detected by D2-40 and its relation to claudin-4 expression in prostatic adenocarcinoma. Int J Surg Pathol. 2016;24:219–26. doi: 10.1177/1066896915611488. [DOI] [PubMed] [Google Scholar]
- Sadullahoglu C, Nart D, Veral A. The importance of EZH2 and MOC-31 expression in the differential diagnosis of benign and malignant effusions. Diagn Cytopathol. 2017;45:118–24. doi: 10.1002/dc.23653. [DOI] [PubMed] [Google Scholar]
- Shareef MM, Radi DM, Eid AM. Tight junction protein claudin 4 in gastric carcinoma and its relation to lymphangiogenic activity. Arab J Gastroenterol. 2015;16:105–12. doi: 10.1016/j.ajg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Shidham VB, Layfield LJ. Approach to diagnostic cytopathology of serous effusions. Cytojournal. 2021;18:32. doi: 10.25259/CMAS_02_03_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield PW, Callan JJ, Devine PL. Markers for metastatic adenocarcinoma in serous effusion specimens. Diagn Cytopathol. 1994;11:237–45. doi: 10.1002/dc.2840110309. [DOI] [PubMed] [Google Scholar]
- Shinozaki-Ushiku A, Ushiku T, Morita S, et al. Diagnostic utility of BAP 1 and EZH 2 expression in malignant mesothelioma. Histopathology. 2017;70:722–33. doi: 10.1111/his.13123. [DOI] [PubMed] [Google Scholar]
- Subbarayan D, Bhattacharya J, Rani P, Khuraijam B, Jain S. Use of panel of markers in serous effusion to distinguish reactive mesothelial cells from adenocarcinoma. J Cytol. 2019;36:28–31. doi: 10.4103/JOC.JOC_13_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek M, Walsh MD, Papadimos DJ, Shield PW. Claudin-4 immunohistochemistry is a useful pan-carcinoma marker for serous effusion specimens. Cytopathology. 2019;30:614–9. doi: 10.1111/cyt.12765. [DOI] [PubMed] [Google Scholar]
- Wang B, Li D, Ou X, Yi Q, Feng Y. Diagnostic accuracy of Ber-EP4 for metastatic adenocarcinoma in serous effusions: a meta-analysis. PLoS One. 2014;9:e107741. doi: 10.1371/journal.pone.0107741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Kinoshita Y, Hamasaki M, et al. Highly expressed EZH2 in combination with BAP1 and MTAP loss, as detected by immunohistochemistry, is useful for differentiating malignant pleural mesothelioma from reactive mesothelial hyperplasia. Lung Cancer. 2019;130:187–93. doi: 10.1016/j.lungcan.2019.02.004. [DOI] [PubMed] [Google Scholar]