Abstract
Background:
Cholangiocarcinoma (CCA) is a highly aggressive tumor with a greater risk of distant metastasis. A drug that prevents CCA development and spread is urgently needed. In this research, we investigated the effect of β-eudesmol on the migration and invasion and epithelial-mesenchymal transformation (EMT) of the CCA cell line.
Materials and Methods:
MTT and transwell assays were used to investigate the antiproliferative activity, as well as activity on cell migration and cell invasion. Real-time PCR and western blot analysis were used to investigate the expression of EMT marker genes and proteins.
Results:
β-eudesmol was shown to exhibit potent antiproliferative activity (IC50 92.25-185.67 µM) and to significantly reduce CCA cell migration and invasion (27.3-62.7%). At both mRNA and protein levels, it significantly up-regulated the expression of epithelial marker E-cadherin (3-3.4-fold), while down-regulated the expression of mesenchymal markers-vimentin (0.6-0.8-fold) and snail-1 (0.4-0.6-fold). Furthermore, β-eudesmol inhibited PI3K and AKT phosphorylation (0.5-0.8-fold), while activating p38MAPK activity (1.2-3.6-fold).
Conclusion:
Altogether, the anti-metastatic activity of β-eudesmol might be due to its suppressive effect on EMT via modulating the PI3K/AKT and p38MAPK signaling cascades.
Key Words: Atractylodes lancea, EMT, migration and invasion, cell proliferation
Introduction
Cholangiocarcinoma (CCA), a malignant transformation of epithelial cells lining the bile duct, is a serious public health issue in several parts of the world, especially in Asia. Thailand is the country where the incidence of CCA is highest in the world (Goral, 2017). The ingestion of improperly cooked, fermented, or preserved cyprinid fish species food containing the fluke, Opisthorchis viverrini, is linked to the highest prevalence of CCA in Northeast Thailand (Aukkanimart et al., 2017; Kamsa-ard et al., 2021). Besides, genetic and epigenetic changes in regulatory genes in cholangiocytes have been associated with activation of oncogenes and deregulation of tumor suppressor genes in CCA (Fava and Lorenzini, 2012).
Metastasis is one of the primary cause of CCA related death (Hahn et al., 2020). The increased motility and invasiveness of migrating cells are needed for the first few steps of metastasis, and they have been connected to the epithelial-mesenchymal transition (EMT). EMT is a reversible transformation of epithelial cells into mesenchymal cells with the capacity to migrate and invade. Reduced expression of epithelial markers like E-cadherin and β-catenin and increased expression of mesenchymal markers like N-cadherin, Vimentin, and Snail-1 have been reported in both intrahepatic and extrahepatic CCA (Settakorn t al., 2005; Sat et al., 2010; Ryu et al., 2012; Huang et al., 2014; Vaquero et al., 2017). The loss of E-cadherin, a primary epithelial marker, is a necessary condition for EMT and metastasis stimulation (Heerboth et al., 2015). The main obstacles in CCA management are the lack of early screening tools and poor clinical results. Metastasis emerges at the time of diagnosis is due to the late clinical presentation and the lack of effective non-surgical therapies. So, a drug that stops CCA from progressing and spreading is urgently needed.
The dried rhizome of the Atractylodes lancea (AL) has been used in Japanese Kampo medicine (“So-jutsu”), Thai traditional medicine (“Khod-Kha-Mao”), and Chinese traditional medicine (“Cang Zhu”) for treatment of various illnesses, including gastrointestinal problems, rheumatic diseases, influenza, and night blindness (Koonrungsesomboon et al., 2014; Na-Bangchang et al., 2017). The β-eudesmol, a sesquiterpenoid alcohol, is a major component of the AL rhizome extract. It exhibits diverse biological and therapeutic activities (Achrya et al., 2021). It shows potent antiproliferative activity against CCA, liver cancer, leukemia, and melanoma cells (Bomfim et al., 2013; Li et al., 2013). Previous research on CCA cells in vitro and in animal models has shown promising anti-CCA activity of β-eudesmol (Plengsuriyakarn et al., 2015; Mathema et al., 2017; Kotawong et al., 2018). It induced apoptosis in CCA cell lines through the activation of caspase-3 and 7 (Kotawong et al., 2018). The expression of detoxifying enzyme heme oxygenase (HO)-1 and NAD(P)H quinone dehydrogenase (NOQ)-1 in CCA cells is also suppressed by β-eudesmol (Mathema et al., 2017; Srijiwangsa et al., 2018). The antiproliferative activity of β-eudesmol against CCA cells is attributed to its inhibitory activity on STAT1/3 phosphorylation and NF-κB expression (Mathema et al., 2017). In the xenografted nude mouse model of CCA, a high dose of β-eudesmol (100 mg/kg body weight for 30 days) prevented tumor volume and lung metastasis (Plengsuriyakarn et al., 2015). However, the influence of β-eudesmol on CCA cell migration in vitro and its possible mechanism have yet to be investigated. The present study reported significant inhibitory activity of β-eudesmol on the migration and invasion of intrahepatic CCA cell line (HuCCT1). The anti-migration activity of β-eudesmol might be due to its suppressive action on EMT via PI3K/AKT and p38MAPK modulation.
Materials and Methods
Chemical and Reagents
HuCCT1 cells were purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB), Japan. Roswell park memorial institute-1640 (RPMI-1640), fetal bovine serum (FBS), trypsin-EDTA (0.25 %), and antibiotic-antimycotic (100x) containing 10,000 U/ml of penicillin, and 10,000 U/ml streptomycins were purchased from Gibco BRL life technologies (Grand Island, NY, USA). The pure β-eudesmol was purchased from the Wako Pure Chemical Industry (Osaka, Japan). The 3-(4, 5-dimethyl-2-thiazoyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) was purchased from Sigma Aldrich (St. Louis, MO, USA). Matrigel-coated transwell membrane was purchased from BD Biosciences (San Jose, CA, USA). The TRIzol reagent was purchased from Sigma Aldrich (St. Louis, MO, USA). RQ1 RNase-free DNase kit was purchased from Promega (Madison, WI, USA). SuperScriptTM III first-strand synthesis cDNA synthesis kit, PierceTM BCA protein assay kit, and alkaline phosphatase (AP)-labeled anti-rabbit secondary antibody were obtained from Thermo Fischer Scientific (Waltham, MA, USA). The iTaqTM universal SYBR green supermix was purchased from Bio-Rad (Hercules, CA, USA). All the rabbit primary antibodies (β-actin, PI3K, p-PI3K, AKT, p-AKT, p38MAPK, p-p38MAPK, E-cadherin, Vimentin, and Snail-1), protease inhibitor cocktail, and RIPA lysis buffer were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). The AP-chromogen BCIP/NBT was purchased from Amresco (Solon, OH, USA).
Cell Culture
HuCCT1 cells were grown in RPMI medium supplemented with 10% FBS and 1% antibiotic-antimycotic solution. Cells were maintained in a 5% CO2 environment at 37 oC. Cell subcultures were performed every 3-4 days using 0.25% trypsin-EDTA.
Antiproliferative Assay
The standard colorimetric MTT assay was used to assess cell viability. HuCCT1 cells were seeded (10,000 cells/well) onto a 96-well microtiter plate and incubated at 37°C for 24 hours. The cells were exposed to different concentrations of β-eudesmol (17.5, 35, 70, 140, 280, 560, and 1,120 µM) and further incubated for additional 24 hours, 48 hours, and 72 hours, respectively. The culture medium was discarded, and 20 µL of 5 mg/mL MTT reagent was added to each well. The plate was incubated at 37 °C in the dark for 4 hours. To solubilize the formazan crystals, 100 µL DMSO was applied to each well after withdrawal of the supernatant without disturbing the bottom layer. The plate was incubated at room temperature (25 oC) for 30 minutes (with gentle shaking), and the absorbance was measured at 570 nm using 96-well VarioskanTM Microplate Reader (Thermo Scientific, Rockford, USA). CalcuSyn version 2.11 software (Biosoft, Cambridge, UK) was used to determine the cell viability (%) and the corresponding IC50 (half-maximal inhibitory concentration).
Transwell Migration and Invasion Assay
Transwell assay (24 well, 8 µm pore size membrane, Corning Inc., NY, USA) was utilized to evaluate the migration of HuCCT1 cells (both treated and control) following the previously described protocol with modifications (Trnh et al., 2017). The HuCCT1 cells were collected and resuspended in a serum-free RPMI medium after being pretreated with β-eudesmol for 24 hours. The transwell was loaded with 200 µL of cell suspension (1x105 cells) in the upper chamber and 600 µL of 20 % FBS-containing RPMI media in the lower chamber. The cells that did not migrate and remained inside the cup were removed with a cotton swab after 24 hours of incubation. The cells that migrated through the membrane to the bottom surface of the cup were fixed in methanol, stained with Giemsa, and counted under a light microscope. A similar protocol was used for the invasion assay, with the exception that the transwell chambers were pre-coated with 100 µl of matrigel (diluted in serum-free RPMI media) and incubated at 37 oC for 1 hour. The cells were fixed, stained, and counted. Each experiment was repeated three times.
Real-time PCR
The 2 x 105 cells were plated onto a -well plate and incubated at 37 oC overnight. The cells were further incubated for 24 hours after being treated with different concentrations of β-eudesmol. Only the culture media was used to treat the control cells (negative control). Total RNA was extracted from the cells using TRIzol reagent following the manufacturer’s instructions. NanoDrop was used to determine the RNA concentration and purity. The RQ1 DNase kit was used to remove contaminating genomic DNA from the total RNA extracted. Single-stranded cDNA was synthesized from total RNA (1 µg) using Superscript III reverse transcriptase cDNA construction kit. The concentration of the synthesized cDNA product was determined and used as a template for real-time PCR. The CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) was used to determine the mRNA expression level of the targeted genes using iTaq Universal SYBR green supermix and specific primer sequences. The PCR conditions were denaturation at 95oC for 5 min, and 40 cycles of amplification at 95 oC for 15 sec and annealing at 62 oC for 1 min. The delta-delta Ct technique was used to measure gene expression levels in comparison to controls (Livak et al., 2001), and the expression was normalized using the housekeeping gene GAPDH. The list of target genes and primer sequences is shown in Table 1.
Table 1.
List of Genes and Primers Used for PCR
| Genes | Primer |
| E-cadherin | Forward (5’-3’): TTTCTTGGTCTACGCCTGGG |
| Reverse (5’-3’): TCCTTGGCCAGTGATGCTGT | |
| Vimentin | Forward (5’-3’): AGCTACGTGACTACGTCCAC |
| Reverse (5’-3’): AGCTCCACCTTCTCGTTGGT | |
| Snail-1 | Forward (5’-3’): TCGCTGCCAATGCTCATCTG |
| Reverse (5’-3’): AAGCCTGGGAAGGCATA | |
| GAPDH | Forward (5’-3’): ATTTGGTCGTATTGGGCGCCT |
| Reverse (5’-3’): GATGATGACCCTTTTGGCTCC |
Western Blot Analysis
Western blot analysis was performed according to the previously described method with slight modifications (Mathema et al., 2017) to evaluate the expression levels of EMT-related proteins and signaling pathways. Briefly, after exposing to β-eudesmol for 24 hours, the cells were washed with PBS and lysed in RIPA buffer containing a protease and phosphatase inhibitor cocktail. The PierceTM BCA protein assay kit was used to determine the amount of protein in the sample. After heat denaturation, equal amounts of protein samples (30 µg/lane) were separated on a nitrocellulose membrane using 8-12.5 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The membrane was blocked for 30 minutes in TBS with 5% BSA before being incubated overnight at 4°C with appropriate dilutions of the primary antibodies. After washing with TBS containing 0.1% Tween-20, the membrane was incubated with an AP-conjugated secondary antibody for 1 hour. The AP chromogen (BCIP/NBT) system was used to colorimetrically detect the corresponding protein bands. Finally, the protein band was densitometrically analyzed with Image J software (NIH, USA), and the amount was normalized to an β-actin internal control.
Statistical Analysis
IBM SPSS Statistics 21 (Chicago, Illinois, USA) was used to perform the statistical analysis. Data are presented as a median (range). The non-parametric tests Mann-Whitney-U and Kruskal-Wallis tests were used for comparison of two or more than two groups of quantitative variables. The statistical significance level was set at 0.05.
Results
Antiproliferative Activity of β-eudesmol
The antiproliferative activity of β-eudesmol was assessed using the MTT assay after 24, 48, and 72 hours of cells exposure. The compound showed potent antiproliferative activity on HuCCT1 cells in a time- and concentration-dependent manner (Figure 1A). The corresponding IC50 values [median (range)] for the 24, 48 and 72 hours exposure were 180 (185.67-175.63), 157 (149.75-162.35), and 99.90 (92.25-105.65-) µM, respectively. Under light microscopy, the irregular morphology and visible signs of cellular debris and floating dead cells were visible after β-eudesmol treatment at the concentrations of 78.5 µM and 157 µM (Figure 1B).
Figure 1.
Antiproliferative Activity of β-eudesmol on HuCCT1 Cells. Figure (A) shows the viability % of HuCCT1 cells after exposure to different concentrations of β-eudesmol for 24 hours, 48 hours, and 72 hours. The cell viability was determined by MTT assay. Data are expressed as the median (range) from the three independent experiments. Figure (B) shows the morphological evidence of β-eudesmol-induced cell death. The black arrow in the picture indicates normal cells (in control) and dead cells (in treated), respectively
Inhibitory Effects on Migration and Invasion
The number of cells that migrated through the transwell membrane was stained and counted. β-Eudesmol inhibited HuCCT1 cell migration in a concentration-dependent manner. After 24 hours of exposure to 78.5, 157, and 240 µM of β-eudesmol, the number of migrating cells was significantly decreased compared to untreated control cells (Figure 2A). At 78.5, 157, and 240 µM of β-eudesmol exposure, the percents [median (range)] of cells migrating were 59.4 (56.1-62.7) %, 29.7 (27.3-32.1) %, and 13.26 (10.26- 16.26) %, respectively.
Figure 2.
Effects of β-eudesmol on Migration and Invasion of HuCCT1 Cells. Figure (A) shows the representative number of cells migrated after exposure to 0, 78.5, 157, 240 μM ATD for 24 hours. Figure (B) shows the representative number of cells invaded after exposure to 0, 78.5, 157, 240 μM ATD for 24 hours. The cells were fixed, stained, and ten representative fields were counted under the light microscope. The bar graph data represents the median (range) from three independent experiments. *p=0.05, **p=0.04 vs untreated control cells
The invasion assay was performed with a matrigel-coated transwell membrane, and the number of cells passing through the coated membrane after β-eudesmol exposure was stained and counted. β-Eudesmol at 78.5, 157, and 240 µM substantially reduced cell invasion to 62.98 (56.18-69.78) %, 41.09 (38.29-43.89) %, and 29.52 (27.72-31.32) %, respectively, compared with the untreated control cells (Figure 2B).
Modulatory Effects on Genes Associated with EMT
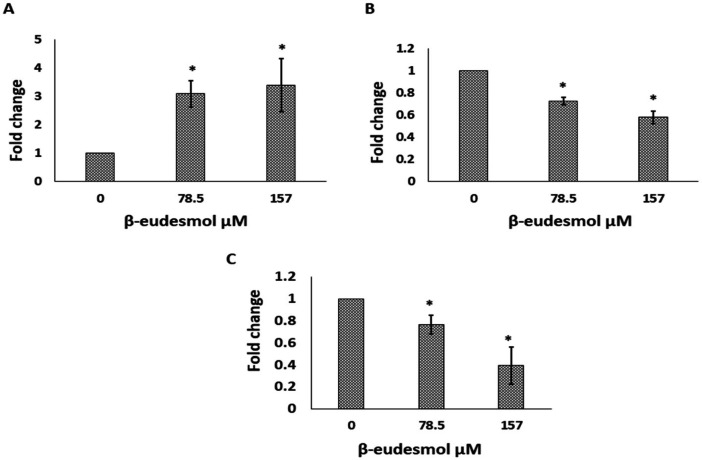
mRNA expression levels of the three EMT-associated genes, i.e., E-cadherin, vimentin, and snail-1 were examined and compared to the untreated control after a 24-hour β-eudesmol exposure (Figures 3 A, B, C). Results showed that exposure to 78.5 µM and 157 µM β-eudesmol enhanced E-cadherin gene expression by 3-fold (p=0.037) and 3.4-fold (p=0.037), respectively. Similarly, 78.5 µM reduced vimentin gene expression by 0.7-fold (p=0.037), while 157 µM reduced gene expression by 0.5-fold (p=0.037). Likewise, β-eudesmol at 78.5 µM and 195 µM reduced the expression of the snail-1 gene by 0.8-fold (p=0.03) and 0.4-fold (p=0.037), respectively.
Figure 3.
Effect of β-eudesmol Treatment on the Expression of EMT Associated Genes. Bar graph (A) shows the fold change in expression of E-cadherin mRNA. Bar graph (B) shows the fold change in expression of Vimentin mRNA. Bar graph (C) shows the fold change in expression of the Snail-1 mRNA. The data represents the median (range) of the three independent experiments. * p=0.037 vs untreated control
Suppression of EMT via PI3K/AKT and p38MAPK Modulation
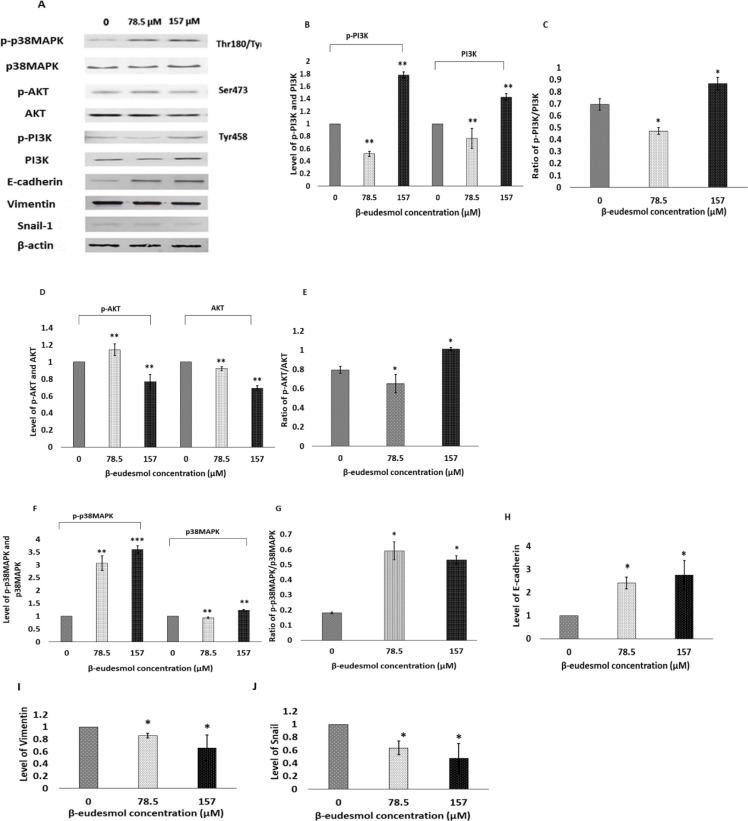
HuCCT1 cells were exposed to β-eudesmol for 24 hours and the expression of EMT marker proteins (E-cadherin, vimentin, and snail) in the extracted lysate was investigated using Western blot analysis. The results showed that β-eudesmol increased E-cadherin expression by 2.4-fold (p=0.037) and 2.7-fold (p=0.037) at 78.5 µM and 157 µM, respectively (Figures 4 A, H). Vimentin expression was decreased by 0.8-fold (p=0.037) and 0.6-fold (p=0.037) at both concentrations (Figsures 4 A, I). In addition, snail-1 expression was decreased by 0.6-fold (p=0.037) and 0.4-fold (p=0.037), respectively, at both concentrations (Figures A, J). Altogether, it was concluded that β-eudesmol down-regulated EMT by modulating the expression E-cadherin, vimentin, and the transcription factor snail-1.
Figure 4.
Western Blot Results Showing the Effects of β-eudesmol on EMT Markers and PI3K/AKT and MAPK Expression. The protein was extracted by using the RIPA buffer and 30 µg whole protein per lane was separated by SDS-PAGE (8-12.5% gel concentration). Figure A shows the representative pictures of the protein bands showing the expression levels of p-p38MAPK, p38MAPK, p-AKT, AKT, p-PI3K, PI3K, E-cadherin, Vimentin, and Snail-1 in HuCCT1 cells treated with β-eudesmol at 0, 78.5 µM and 157 µM for 24 hours. Bar graph B shows the fold change in expression of p-PI3K and PI3K, and bar graph C shows their ratios. Bar graph D shows the fold change in expression of p-AKT and AKT, and bar graph E shows their ratios. Bar graph F shows the fold change in expression of p-p38MAPK and p38MAPK, and bar graph G shows their ratios. Bar graph H, I, and J show the fold change in expression of E-cadherin, vimentin, and snail-1, respectively. β-actin was taken as an internal loading control. *p=0.05, **p=0.037, ***p=0.034 vs untreated control cells. Abbreviations: PI3K, Phosphatidylinositide-3 kinase; AKT, Protein kinase B; MAPK, Mitogen-activated protein kinase; EMT, epithelial-mesenchymal transition
PI3K/AKT and p38MAPK are among the upstream regulator of the EMT process. The expression levels of these proteins in β-eudesmol treated cells were determined. The expression levels of total PI3K, phospho-PI3K at Tyr 458, and their ratios were initially investigated. The results revealed that exposing HuCCT1 cells to 78.5 µM β-eudesmol reduced total PI3K and p-PI3K expression by 0.8-fold (p=0.03) and 0.5-fold (p=0.03), respectively. On the other hand, β-eudesmol at 157 µM slightly increased the expression of total PI3K and p-PI3K by 1.4-fold (p=0.03) and 1.8-fold (p=0.03), respectively. On a basal level, 78.5 M, and 157 M, the ratios of p-PI3K to PI3K were 0.7, 0.4, and 0.9, respectively (Figures A, B, C). Similarly, total AKT, phospho-AKT at Ser 473, and their ratios were also affected by the β-eudesmol treatment. At 78.5 µM, the expression of p-AKT was increased by 1.2-fold (p=0.037), while total AKT expression was decreased by 0.9-fold (p=0.037). At 157 µM, however, the expression of total and p-AKT nearly was reduced by 0.8-fold (p=0.037) and 0.7-fold (p=0.037), respectively. At the basal level, the ratio of p-AKT to AKT was 0.8, but treatment with 78.5 µM and 157 µM β-eudesmol reduced the ratio to 0.6 (p=0.05) and 1 (p=0.05), respectively (Figures 4 A, D, E). These results suggested that β-eudesmol at low concentration (78.5 µM) inhibited the phosphorylation of PI3K and AKT proteins.
Additionally, the effects of β-eudesmol treatment on total p38MAPK expression, p-p38MAPK (at Thr180/Tyr182) expression, and their ratios were investigated. β-Eudesmol increased the expression of p-p38MAPK by 3-fold (p=0.037) and 3.6-fold (p=0.034) at 78.5 µM and 157 µM. At 157 µM, it slightly increased the expression of total p38MAPK by 1.2-fold (p=0.037). The ratio of p-p38MAK to p38MAPK at basal level was 0.18, and was increased to 0.6-fold (p=0.05) and 0.5-fold (p=0.05) when exposed to 78.5 μM and 157 μM β-eudesmol, respectively (Figures 4 A, F, G). These results suggested that β-eudesmol activated the p38MAPK signaling pathway.
Discussion
β-Eudesmol exhibited potent antiproliferative activity that is time- and concentration-dependent (Mathema et al., 2017; Kotawng et al., 2018). The antiproliferative activity of β-eudesmol on HuCCT1 is qualitatively consistent with the findings from other studies in other types of human cancers, including lung cancer, liver cancer, and prostate cancer (Archarya et al., 2021). It significantly decreased HuCCT1 cell migration and invasion in an in vitro transwell assay in a concentration-dependent manner.
Most CCA-related deaths occur because of metastasis (Hahn et al., 2020). The increased motility and invasive nature of migrating cells are required for the first few steps of metastasis, and it has been suggested that they are linked to the epithelial-mesenchymal transition (EMT) phase. EMT is a reversible mechanism in which epithelial cells transform into mesenchymal cells and develop the ability to migrate and invade (Heerboth et al., 2015). During EMT, epithelial markers such as E-cadherin, zonula occludens (ZO-1) and occludin are down-regulated, while mesenchymal markers such as N-cadherin, vimentin, and fibronectin are up-regulated. Previous studies have shown that CCA patients have lower levels of the epithelial marker E-cadherin and higher levels of mesenchymal markers, including vimentin and snail-1 (Settkorn et al., 2005; Sato et al., 2010; Ryu et al., 2012). In the present study however, β-eudesmol treatment up-regulated the expression of epithelial marker E-cadherin in HuCCT1 cells, while down-regulated the expression of mesenchymal markers (vimentin and snail-1) at both the mRNA and protein levels. Up-regulation of snail-1 expression has been shown to inhibit E-cadherin expression (Cano et al., 2000). It is possible that the low level of snail-1 expression is responsible for the enhancement of E-cadherin expression in β-eudesmol treated HuCCT1 cells. This finding suggested that β-eudesmol inhibited HuCCT1 cell migration and invasion by suppressing the formation of mesenchymal phenotypes.
EMT is a complicated mechanism controlled by the interactions of several signaling pathways. Some of the molecular signaling pathways linked to EMT induction include transforming growth factor (TGF)-β, fibroblast growth factor, epidermal growth factor, Ras, Src, integrin, Wnt/β-catenin, Notch, and PI3K/AKT. The PI3K/AKT signaling pathway can cooperate with other signaling pathways such as TGF-, NF-B, and Wnt/-catenin to induce the EMT process (Xu et al., 2015). Besides, increased PI3K/AKT activation was also linked to increased metastasis in CCA (Yothaisong t al., 2013). The compound is gaining interest as a potential target for the prevention and treatment of metastatic tumors like CCA. The phosphorylation of both PI3K and AKT in HuCCT1 cells was significantly reduced by β-eudesmol at a low concentration (78.5 µM). This suggested that β-eudesmol could be utilized as a PI3K/AKT inhibitor at low concentration. In previous research, natural compound rotenone showed anticancer activity in colon cancer by inhibiting migration and EMT via regulating the activity of the PI3K/AKT signaling pathway (Xue et al., 2020). It is possible that the modulatory effect of β-eudesmol on the PI3K/AKT signaling pathway might be responsible for the up-regulated expression of E-cadherin and down-regulated expression of vimentin and snail-1.
β-eudesmol increased p38MAPK activity in HuCCT1 cells. Various cellular stresses, including chemotherapeutic agents, stimulate this stress-response pathway. In addition to its function in stress responses, studies have shown that p38 MAPK mediates pathways that lead to cell apoptosis and growth inhibitory signals (Olson et al., 2004). It has been documented that cisplatin-induced cancer cell death needs p38 MAPK activation (Olson et al., 2004). On the other hand, p38MAPK activation has been shown to have both anti-apoptotic and proliferative effects (Wada and Penninger, 2004). TGF-β-mediated EMT and cell migration have been shown to be involved in the activation of p38MAPK (Bakin et al., 2002). However, the relationship between β-eudesmol-induced p38MAPK activation and its role in EMT and metastasis of CCA cells needs further research.
In conclusion, β-eudesmol exhibited potent antiproliferative activity against HuCCT1 cells. It inhibited HuCCT1 cell migration and invasion in vitro. The anti-migration action of β-eudesmol might be due to its suppressive effect on EMT, as evidenced by the changes in expression of EMT markers. The compound also inhibited phosphorylation of PI3K and AKT, while activating phosphorylation of p38MAPK, which could attribute to its anti-migration and anti-EMT effect. The findings from the current study suggested that β-eudesmol can act as a suppressor of EMT in CCA, but further research with additional CCA cell lines and animal models is required.
Author Contribution Statement
BA, WC, and KN were involved in the design of the experimental study. BA performed the experiments. BA and WC performed data analysis. BA drafted the manuscript. KN revised the manuscript. All authors reviewed and approved the final manuscript for submission. All meet the ICMJE criteria for authorship.
Acknowledgements
The authors would like to thank the staff of Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Thammasat University, for the technical supports.
Funding Statement
This study was financially supported by the Ministry of Higher Education, Science, Research and Innovation of Thailand (Frontier Research Seed Fund grant number TUFF20/2564), the Research Team Promotion Grant, National Research Council of Thailand (Kesara Na-Bangchang: Grant No. 020/2563), and Thammasat University (as a part of Mr. Bishwanath Acharya Ph.D. thesis).
Confliect of Interest
The authors declare no competing interests.
References
- Acharya B, Chaijaroenkul W, Na-Bangchang K. Therapeutic potential and pharmacological activities of β-eudesmol. Chem Biol Drug Design. 2021;97:984–96. doi: 10.1111/cbdd.13823. [DOI] [PubMed] [Google Scholar]
- Aukkanimart R, Boonmars T, Sriraj P, et al. Carcinogenic Liver Fluke and Others Contaminated in Pickled Fish of Northeastern Thailand. Asian Pac J Cancer Prev. 2017;18:529–33. doi: 10.22034/APJCP.2017.18.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- Bomfim DS, Ferraz RP, Carvalho NC, et al. Eudesmol isomers induce caspase-mediated apoptosis in human hepatocellular carcinoma HepG2 cells. Basic Clin Pharmacol Toxicol. 2013;113:300–6. doi: 10.1111/bcpt.12097. [DOI] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Fava G, Lorenzini I. Molecular Pathogenesis of Cholangiocarcinoma. Int J Hepatol. 2012;2012:630543. doi: 10.1155/2012/630543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral V. Cholangiocarcinoma: New Insights. Asian Pac J Cancer Prev. 2017;18:1469–73. doi: 10.22034/APJCP.2017.18.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn F, Müller L, Mähringer-Kunz A, et al. Distant Metastases in Patients with Intrahepatic Cholangiocarcinoma: Does Location Matter? A Retrospective Analysis of 370 Patients. J Oncol. 2020;2202:7195373. doi: 10.1155/2020/7195373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X-Y, Zhang C, Cai J-B, et al. Comprehensive Multiple Molecular Profile of Epithelial Mesenchymal Transition in Intrahepatic Cholangiocarcinoma Patients. PLoS One. 2014;9:e96860. doi: 10.1371/journal.pone.0096860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsa-ard S, Santong C, Kamsa-ard S, et al. Decreasing trends in cholangiocarcinoma incidence and relative survival in Khon Kaen, Thailand: An updated, inclusive, population-based cancer registry analysis for 1989–2018. PLoS One. 2021;16:e0246490. doi: 10.1371/journal.pone.0246490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonrungsesomboon N, Na-Bangchang K, Karbwang J. Therapeutic potential and pharmacological activities of Atractylodes lancea (Thunb. ) DC. Asian Pac J Trop Med. 2014;7:421–8. doi: 10.1016/S1995-7645(14)60069-9. [DOI] [PubMed] [Google Scholar]
- Kotawong K, Chaijaroenkul W, Muhamad P, Na-Bangchang K. Cytotoxic activities and effects of atractylodin and beta-eudesmol on the cell cycle arrest and apoptosis on cholangiocarcinoma cell line. J Pharmacol Sci. 2018;136:51–6. doi: 10.1016/j.jphs.2017.09.033. [DOI] [PubMed] [Google Scholar]
- Li Y, Li T, Miao C, et al. beta-Eudesmol induces JNK-dependent apoptosis through the mitochondrial pathway in HL60 cells. Phytother Res. 2013;27:338–43. doi: 10.1002/ptr.4727. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mathema VB, Chaijaroenkul W, Karbwang J, Na-Bangchang K. Growth inhibitory effect of beta-eudesmol on cholangiocarcinoma cells and its potential suppressive effect on heme oxygenase-1 production, STAT1/3 activation, and NF-kappaB downregulation. Clin Exp Pharmacol Physiol. 2017;44:1145–54. doi: 10.1111/1440-1681.12818. [DOI] [PubMed] [Google Scholar]
- Na-Bangchang K, Plengsuriyakarn T, Karbwang J. Research and Development of Atractylodes lancea (Thunb ) DC as a Promising Candidate for Cholangiocarcinoma Chemotherapeutics. Evid-Based Compl Alt Med. 2017;2017:16. doi: 10.1155/2017/5929234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125–9. doi: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Plengsuriyakarn T, Karbwang J, Na-Bangchang K. Anticancer activity using positron emission tomography-computed tomography and pharmacokinetics of beta-eudesmol in human cholangiocarcinoma xenografted nude mouse model. Clin Exp Pharmacol Physiol. 2015;42:293–304. doi: 10.1111/1440-1681.12354. [DOI] [PubMed] [Google Scholar]
- Ryu HS, Chung JH, Lee K, et al. Overexpression of epithelial-mesenchymal transition-related markers according to cell dedifferentiation: clinical implications as an independent predictor of poor prognosis in cholangiocarcinoma. Human Pathol. 2012;43:2360–70. doi: 10.1016/j.humpath.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Sato Y, Harada K, Itatsu K, et al. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/Snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177:141–52. doi: 10.2353/ajpath.2010.090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settakorn J, Kaewpila N, Burns GF, Leong ASY. FAT, E-cadherin, beta catenin, HER 2/neu, Ki67 immuno-expression, and histological grade in intrahepatic cholangiocarcinoma. J Clin Pathol. 2005;58:1249–54. doi: 10.1136/jcp.2005.026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srijiwangsa P, Ponnikorn S, Na-Bangchang K. Effect of beta-Eudesmol on NQO1 suppression-enhanced sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. BMC Pharmacol Toxicol. 2018;19:32. doi: 10.1186/s40360-018-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh SX, Nguyen HTB, Saimuang K, Prachayasittikul V, Chan On W. Metformin Inhibits Migration and Invasion of Cholangiocarcinoma Cells. Asian Pac J Cancer Prev. 2017;18:473–7. doi: 10.22034/APJCP.2017.18.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero J, Guedj N, Clapéron A, et al. Epithelial-mesenchymal transition in cholangiocarcinoma: from clinical evidence to regulatory networks. J Hepatol. 2017;66:424–41. doi: 10.1016/j.jhep.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–24. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Men S, Liu R. Rotenone restrains the proliferation, motility and epithelial–mesenchymal transition of colon cancer cells and the tumourigenesis in nude mice via PI3K/AKT pathway. Clin Exp Pharmacol Physiol. 2020;47:1484–94. doi: 10.1111/1440-1681.13320. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yothaisong S, Dokduang H, Techasen A, et al. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34:3637–48. doi: 10.1007/s13277-013-0945-2. [DOI] [PubMed] [Google Scholar]