Abstract
Background:
Oral cancer is often preceded by Potentially Malignant Disorders (PMDs) and important role of biochemical markers for early diagnosis has been well documented; however, there is limited evidence of Serum lactate dehydrogenase (SLDH) as an effective biochemical marker in diagnosis of PMDs. The present meta-analysis was conducted to assess if serum LDH can be a used as standard biomarker for PMDs and consequently aid in diagnosis of oral cancer.
Methods:
A comprehensive search was conducted in Medline, Scopus, Web of Science, EBSCO host, Cochrane databases and Google Scholar for studies evaluating estimation of SLDH in PMDs. Search strategy included all types of studies evaluating level of SLDH in patients with PMDs. PRISMA guidelines were followed for the meta-analysis. Fixed-effects model was used to assess the mean differences in SLDH levels between healthy controls and PMDs.
Results:
A total number of nine studies were included in meta-analysis after screening for inclusion and exclusion criteria. Potentially malignant disorder was significantly associated with increased serum LDH level compared to healthy controls (pooled SMD: 1.83 (95% CI, 1.52, 2.15) (P < 0.00001; Subgroup analysis of OSMF (Oral Submucous Fibrosis) studies showed significant association with increased serum LDH level compared to healthy controls (pooled SMD: 2.57 (95% CI, 2.16, 2.98; P < 0.00001). Sensitivity analysis for the five studies reflected a significant reduction in I2 values to 24 % (P=0.26). Funnel plots were derived for any evidence of publication bias among the studies.
Conclusion:
Meta-analysis suggests that SLDH is increased in potentially malignant disorders compared to healthy controls. The results of this metanalysis should encourage use of SLDH as a biomarker in diagnosis of PMDs.
Key Words: Potentially malignant disorders (PMDs), oral cancer, lactate dehydrogenase enzyme, precancerous lesion
Introduction
Oral cancer is amongst the most prevalent cancers worldwide and one of the major threats to public health with growing number of new cases every year. Oropharyngeal cancer is more common in developing than developed countries. In south-central Asia and also in India, cancer of the oral cavity ranks among the three most common types of cancer. The 58th World Health Assembly Resolution on Cancer Prevention and Control (WHA 25 May 2005) urged Member States to develop and reinforce national cancer control programs, prioritizing preventable tumors and risk factors intervention, with a focus on cancers amenable to early detection and treatment, such as oral cancer (Petersen PE, 2005).
Oral cancer defined as the cancer of lips and oral cavity is often preceded by Potentially Malignant Disorders (PMD’s) which are the precursors of oral cancer. PMD’s consist of both premalignant lesions and conditions which include oral leukoplakia, erythroplakia, oral sub-mucous fibrosis and lichen planus (Sankaranarayanan et al., 2005).Oral cancer mostly has a poor prognosis as most of the cases report at a very late stage due to the lack of availability of early screening or the initial ignorance by the patients. Early diagnosis through oral cancer screening is the key to reduce mortality and morbidity in the society. Use of biochemical markers has been well documented in the literature and plays an important role in early detection of cancer providing a cost effective and reliable technique. Studies indicate that there is increased level of Serum lactate dehydrogenase (SLDH) and its isoenzymes in patients suffering from various cancers including that of breast, lung and oral cancer. Several studies also indicate the use of SLDH as a biochemical marker in diagnosis PMDs (Fritz et al., 2000; Rivera, 2015). However, there is limited evidence for its effective applicability in the early screening of oral malignancies. It consequently becomes imperative to determine whether serum LDH can be effectively used as a biochemical marker in diagnosis of PMDs. Hence, the present meta-analysis was conducted to provide conclusive evidence if serum LDH can be a standard biomarker for PMDs and consequently aid in diagnosis of oral cancer.
Materials and Methods
Search Strategy
A comprehensive search for literature was conducted for original studies on estimation of SLDH in PMDs. The database was searched by two independent investigators in Medline, Embase, Scopus, Web of Science, CINHAL, Google scholar databases for studies through March 2021. The search strategy included the following MeSH terms; ‘serum lactate dehydrogenase’, ‘premalignant disorder’, ‘precancerous lesions’ ‘leukoplakia’, ‘lichen planus’, ‘oral submucous fibrosis’. PRISMA guidelines were followed for the meta-analysis. The articles were searched using English keywords. No restrictions were placed on the language of publications. Grey literature was searched for unpublished articles through manual searching of nonindexed journals, but no relevant articles were found. Database was searched for pertinent publications by two investigators independently (WB and BP) according to pre-defined criteria. Title and abstracts were pre-screened for articles to be retrieved in full and exclude ineligible studies. Duplicates were removed by Zotero 6 online bibliography tool. Differences between the two investigators were resolved by discussion.
Study selection criteria
The inclusion and exclusion criteria for study selection was determined prior to literature search. A randomized or nonrandomized controlled trials, retrospective or prospective cohort studies, case-control, cross sectional studies comparing the level of serum lactate dehydrogenase (SLDH) in patients with PMDs and healthy controls and providing sufficient information to estimate the level of SLDH were included. Studies including in vitro studies, animal studies, papers with abstract only, literature reviews, case report or case series were excluded from analysis. Studies without a control group were also excluded. All the studies extracted for systematic review was available in English literature. Studies comparing SLDH among subclassifications of PMDs such as OSMF, leukoplakia and lichen planus were also extracted for meta-analysis; however, the estimates were considered separately. The Population Exposure Comparison Outcome (PECO) used to define the research question was Population: Patients diagnosed with PMDs; Intervention: Premalignant disorders; Comparator: healthy controls without PMDs; Outcome variable: SLDH levels
Data extraction and quality assessment
The data extraction protocol included the relevant data like author information, publication year, country, study location, sample size and study design from the selected studies (Figure 1). A total number of nine studies were included in meta-analysis after screening the studies for inclusion and exclusion criteria. There were no RCT or Cohort study reported on this topic. All the studies done were cross sectional in nature with comparison group. Baseline characteristics of the studies including participants age and gender, confirmed diagnosis of premalignant disorder, outcome measures (serum LDH level) were extracted and presented in Table 1. Outcome data were extracted by the two investigators using guidelines published by the Cochrane Collaboration. Mean and standard deviations (SD) were obtained from all the studies selected for the estimation of effect.
Figure 1.
Flow Diagram to Illustrate the Study Selection Procedure
Table 1.
Characteristics of included studies: Serum LDH in PMD versus Healthy individuals
| Study Reference (Author, Year) |
Study design and setting | Diagnosis | Age, Gender Distribution | Sample Size (PMD: Healthy Controls) |
Outcome variable (Serum LDH) PMD: Healthy Controls |
Outcome difference (P value) |
|---|---|---|---|---|---|---|
| Sharma G et al. 2016 |
India, comparative study | Clinical and histopathological diagnosis of PMD including leukoplakia, erythroplakia, and oral submucous fibrosis | 21 to 35 years (PMD) 38 to 70 years (Healthy Controls) |
11:15 | 485.66±123.98 338.82±75.24 |
<0.005 |
| Swamy KM et al. 2016 |
India, comparative study | Clinical & histopathological diagnosis of OSMF |
49.5 ± 11.7 (OSMF)& 46. 2 ± 10.3 (Healthy controls) |
30:30:00 | 492.20 ± 16.41 117.23 ± 19.47 |
p <0.0001 |
| Chari A et al. 2107 |
India, comparative study | Clinical diagnosis of PMD | Patients aged between 18 and 65 years |
35:20:00 | 446.74±136.65 345.3±90.23 |
P=0.005 |
| Rathore A et al. 2015 |
India, comparative study | Clinical and histopathological diagnosis of leukoplakia& OSMF | 20-60 years irrespective of gender |
30:30:30 | 277.91±33.34 249.68±44.65 161.39±36.14 |
P<0.0001 |
| Mishra et al. 2018 |
India, comparative study | Clinically diagnosed OSMF | Above 18 years | 20:20 | 408.35±158.35 313.05±82.69 |
P=0.02 |
| Nandita A et al. 2017 |
India, comparative study | Clinically diagnosed OSMF, leukoplakia | 20-60 years irrespective of gender |
10:10 | 512.7±46.7 471.6±72.3 251.5±48.3 |
P<0.001 |
| Nalin AS et al. 2018 |
India, comparative study | Clinical & histopathological diagnosis according to the modified WHO diagnostic criteria for OLP | 20 to 50 years irrespective of gender | 10:10 | 181.50 ±56.139 144.50 ±18.441 |
p=0.427 |
| Mohan M et al. 2017 |
India, comparative study | Clinically diagnosed and Histopathologically confirmed cases of PMD | 30-60 yrs | 10:10 | 422.20± 92.53 3,516.80±1,297.30 |
Not provided |
| Pereira T et al. 2015 |
India, comparative study | Clinically diagnosed and Histopathologically confirmed cases OF osmf & leukoplakia |
age and gender matched participants | 30:25:20 | 488.67 ±23.18 339.90± 61.54 |
p<0.001 |
The Newcastle–Ottawa tool adapted for case control studies (Modesti et al., 2016) was used to assess study quality on three domains (participant selection, comparability, and outcome). A score of ‘0’ was awarded when criteria were not satisfied, ‘1’ was awarded when the criteria were satisfied and ‘2’ was awarded when the study analysis was controlled for any second important factor. The sum of scores for all subscale items categorised overall study quality as high (> 7), moderate (5–7), or low (< 5).
The certainty of evidence was determined using the GRADE assessment (Schünemann et al., 2013), considering the study design, risk of bias, consistency, directness of evidence, precision of results, risk of publication bias, magnitude of the effect, and influence of confounding factors. GRADE assesses the quality of a body of evidence as high, moderate, low, or very low.
Statistical Analysis
Meta-analysis was performed using version 5.0.17 of the Review Manager software (Nordic Cochrane Centre, Copenhagen, Denmark) to generate the forest plots and to assess heterogeneity of the included studies. Heterogeneity was quantified using the I2 statistics and the heterogeneity was defined as low, moderate, and high based on I2 values of 25%, 50% and 75% respectively (Higgins and Thompson, 2002; Higgins et al., 2003). A fixed effects model was used to assess the mean differences with 95% confidence intervals in SLDH levels between PMD patients and healthy controls. Sensitivity analysis was conducted as the results indicated high level of heterogeneity. The data of Oral Submucous fibrosis patients were pooled for the subgroup analysis. Statistical significance was set at p <0.05.
Results
The results of the literature search and characteristics of the studies included in meta-analysis is given in Figure 1 and Table 1 respectively. A total number of nine studies were included in meta-analysis after screening the studies for inclusion and exclusion criteria.
Quality of trials included in the analysis is presented in Table 2. The overall scores indicated moderate quality. All included studies addressed the confounding factor of age by matching among patients with and without PMDs.
Table 2.
Quality: assessment according to the Newcastle-Ottawa Scale
| Sl. No | Studies | Selection (Sample) | Comparability of groups | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Is the case definition adequate | Representativeness of the cases | Selection of Controls | Definition of Controls | Comparability of cases and controls based on the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non- Response rate |
||
| 1. | Sharma G et al. 2016 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| 2. | Swamy KM et al. 2016 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| 3. | Chari A et al. 2107 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| 4. | Rathore A et al. 2015 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 |
| 5. | Mishra et al. 2018 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 |
| 6. | Nandita A et al. 2017 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 |
| 7. | Nalin AS et al. 2018 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| 8. | Mohan M et al. 2017 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| 9. | Pereira T et al. 2015 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
A moderate certainty of evidence was achieved for association between SLDH levels among patients with and without PMDs (Table 3). Similarly, the level of evidence obtained for the association of SLDH levels among OSMF patients and healthy controls (OR: 2.57; 95% CI 2.16-2.98) was moderate.
Table 3.
Grade Analysis for Certainty of Evidence for association between SLDH and PMDs
| No of studies | Design | Certainty of evidence | No of participants | Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SLDH among PMDs and Healthy controls | ||||||||||
| Risk of Bias | Inconsistency | Indirectness | Imprecision | Other considerations | PMDs | Healthy controls | Mean Difference (Relative 95% CI) | Certainty | ||
| 12 | Observational studies (Case control) | Not serious | Not serious | Not serious | Not serious | Strong association | 281 | 255 | 199.57 (95.84-30.30) | |
| MODERATE | ||||||||||
| SLDH among OSMF and Healthy controls | ||||||||||
| 5 | Observational studies (Case control) | Not serious | Not serious | Not serious | Not serious | Strong association | 145 | 140 | 2.57 (2.16-2.98) | |
| MODERATE | ||||||||||
CI, Confidence interval; OR, Odds ratio
The studies by Nandita et al., 2017; Rathore et al., 2015; Pereira et al., 2015 analysed the data of premalignant lesions separately by categorising the study groups into OSMF group, Leukoplakia group and lichen planus group. Therefore, the study parameters were extracted separately for the data analysis under different PMDs of interest. The data of a total 281 PMD patients and 255 healthy controls was available for analysis.
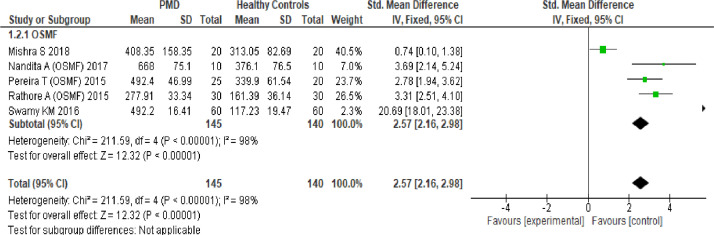
Meta-analysis of the nine studies to assess the level of serum LDH between two groups of participants is presented in Figure 2. Potentially malignant disorder was significantly associated with increased serum LDH level compared to healthy controls (pooled standard mean difference: 1.83 (95% CI, 1.52, 2.15) P < 0.00001) (Figure 2). The 95% confidence intervals of the overall effect estimate did not cross the line of no effect. Heterogeneity between studies was high with I2= 97%, prompting a subgroup and sensitivity analysis. Five of the studies which analysed the data of OSMF patients were pooled for the subgroup analysis. The results showed that OSMF was significantly associated with increased SLDH level compared to healthy controls (pooled standard mean difference: 2.57 (95% CI, 2.16, 2.98; P < 0.00001) (Figure 3). Sensitivity analysis for the five studies reflected a significant reduction in I2 values to 24 % (P=0.26; Figure 4). Funnel plots were derived for any evidence of publication bias among the studies. Visual inspection of the funnel plot did not identify any substantial asymmetry, with the vertical line of funnel plot representing pooled standard mean effect size, and the dotted lines representing the 95% confidence interval (Figure 5).
Figure 2.
Forest Plot Analyses. Forest plots of the meta analysis of the serum LDH in PMD and Healthy Controls
Figure 3.
Sub Group Analyses. Forest plots of the meta analysis of the serum LDH in OSMF and Healthy Controls
Figure 4.
Sensitivity Analyses. Forest plots of the meta analysis of the serum LDH in PMD and Healthy Controls
Figure 5.
Funnel Plot for Publication bias of the Studies Included in Meta Analysis
Discussion
This meta-analysis compared the serum LDH levels in potentially malignant disorders and healthy participants. The metanalysis included nine studies and demonstrated a significant increase in serum LDH level among patients with potentially malignant disorders including OSMF, leukoplakia and lichen planus. All the nine observational studies in the meta-analysis (Chari et al., 2016; Mishra et al., 2018; Mohan et al., 2017; Nalin et al., 2018; Nandita et al., 2017; Pereira et al., 2015; Rathore et al., 2015; Sharma et al., 2016; Swamy and Ganiger et al., 2016) reported a higher level of mean serum LDH for PMDs compared to healthy participants.
Oral cancer often presents a clinical diagnostic challenge to the dental practitioner, particularly in its early stage of development. Typically, they tend to be preceded by a potentially malignant state for a long time (Macdonald et al., 1957; Nair et al., 2012). The increase in LDH levels was consistent in serum of PMD patients and therefore, quantification of SLDH can be used as a biochemical marker, as it is simple and easily accepted by the patient. The present meta-analysis assessed correlation between tumour differentiation and serum Lactate Dehydrogenase levels, which showed a positive correlation. The early diagnosis of cancer and potentially malignant disorder offers the best prognosis with tumour markers playing an important role in the early detection of the lesion (Hong et al., 2010). Due to its extraordinarily widespread distribution in the body, SLDH is abnormal in a host of disorders and could be a valuable predictive biomarker for detection of PMDs (Drent et al., 1996).
Assessment of the consistency of effects across studies is an essential part of meta-analysis. Heterogeneity in meta-analysis is variation in study outcomes between studies. Substantial heterogeneity was observed among the studies of meta-analysis, which can be attributed to the differences in the socio-demographic characteristics of populations, definition of the reference case and the adjustment for confounding factors. The I2 statistic describes the percentage variation across studies that are due to heterogeneity rather than chance. A reduction in I2 value means that the heterogeneity was being contributed by the studies which are not part of this group. A reduction from 97% to 24% was noted for I2 value, probably due to stringent inclusion and exclusion criteria and a standardized technique for estimation of SLDH level across the studies included. A lower heterogeneity is always desirable as it reflects consistent finding across studies.
However, potentially malignant disorders included in the meta-analysis were OSMF, Leukoplakia and lichen planus and not specific to any lesion or condition. Most of the studies in the meta-analysis were confined to a particular geographic area and hence results may be more applicable to Southeast Asian regions where OSMF is more prevalent. Above all, further studies including cohort or long-term follow-up studies and representing wider geographical regions and populations are required to prove the usefulness of SLDH as a biochemical marker for PMDs.
In conclusion, this meta-analysis provided evidence of strong association between Serum Lactate Dehydrogenase in potentially malignant disorders when compared to healthy controls. The results conclude that SLDH level is increased in potentially malignant lesions. This should encourage judicious application of SLDH as an appropriate, valuable biomarker in screening of PMDs.
Author Contribution Statement
WB conceptualized the topic and undertook literature search and developed the first draft. BMP extracted, analyzed, and contributed for quality assessment of the data and critical review of the final manuscript.
Acknowledgements
We thank All India Institute of Medical Sciences Patna for providing the opportunity to conduct the present meta-analysis and New Delhi.
Ethics approval and consent to participate
Not applicable.
Study registration
The systematic review was conducted according to PRISMA guidelines; however, it was not registered in any data set.
Conflict of interest
None declared.
References
- Chari A, Rajesh P, Prabhu S. Estimation of serum lactate dehydrogenase in smokeless tobacco consumers. Indian J Dent Res. 2016;27:602–8. doi: 10.4103/0970-9290.199594. [DOI] [PubMed] [Google Scholar]
- Drent M, Cobben NA, Henderson RF, Wouters EF, Van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–42. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. p. 240. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH, Roh SY, Ko YH, et al. Prognostic significance of glycolytic metabolic change related to HIF-1alpha in oral squamous cell carcinomas. Korean J Patho. 2010;44:360–9. [Google Scholar]
- Macdonald RP, Simpson JR, Nossal E. Serum lactic dehydrogenase; a diagnostic aid in myocardial infarction. JAMA. 1957;165:35–40. doi: 10.1001/jama.1957.02980190037009. [DOI] [PubMed] [Google Scholar]
- Mishra S, Kritika C, Bajoria AA, et al. Estimation of salivary and serum lactate dehydrogenase in oral submucous fibrosis. J Int Soc Prevent Communit Dent. 2018;8:289–95. doi: 10.4103/jispcd.JISPCD_214_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti A, Reboldi G, Cappuccio P. ESH working group on CV risk in low resource settings Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS One. 2016:11. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N, Krithika S, Mathew S. A study of salivary and serum lactate dehydrogenase levels in tobacco users and potentially malignant disorders. J Med Sci Clin Res. 2017 [Google Scholar]
- Nair DR, Pruthy R, Pawar U, Chaturvedi P. Oral cancer: Premalignant conditions and screening--An update. J Cancer Res Ther. 2012;8:57–66. doi: 10.4103/0973-1482.92217. [DOI] [PubMed] [Google Scholar]
- Nalin AS, Rajeev R, George GB, Padiath S. Level of serum lactate dehydrogenase enzyme in oral lichen planus – A biochemical study. IOSR-JDMS. 2018;17:2026. [Google Scholar]
- Nandita NA, Sowbhagya BS, Balaji P. Lactate Dehydrogenase as a Tumor Marker in Oral Cancer and Oral Potentially Malignant Disorders: A Biochemical study. Int J Prev Clin Dent Res. 2017;4:196–200. [Google Scholar]
- Pereira T, Shetty S, Pereira S. Estimation of serum lactate dehydrogenase level in patients with oral premalignant lesions/conditions and oral squamous cell carcinoma. J Cancer Res Ther. 2015:11. doi: 10.4103/0973-1482.150352. [DOI] [PubMed] [Google Scholar]
- Petersen PE. Strengthening the prevention of oral cancer: the WHO perspective. Community Dent Oral Epidemiol. 2005;33:397–9. doi: 10.1111/j.1600-0528.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- Rathore A, Nagarajappa AK, Sreedevi Evaluation of serum lactate dehydrogenase in oral squamous cell carcinoma, oral leukoplakia and oral submucous fibrosis. J Indian Acad Oral Med Radiol. 2015;27:29–34. [Google Scholar]
- Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884–94. [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–33. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- Sharma G, Sharma P, Kumar P, Kumar R. Study on serum lactate dehydrogenase level in precancerous, cancerous and healthy subjects. Asian J Pharm Clin Res. 2016 [Google Scholar]
- Swamy KM, Ganiger A. Level of serum lactate dehydrogenase in oral submucous fibrosis. Int J Otorhinolaryngol Head Neck Surg. 2016:2. [Google Scholar]