Abstract
Klotho long recognized for its role in anti-aging, is potentially implicated in the pathogenesis of rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. Aging of the immune system coincides with the inability of the body to recognize self-antigens, which often leads to autoimmune responses. The role of Klotho in these autoimmune diseases should be of high interest; however, few articles have been published exploring the role of Klotho in the pathogenesis, organ involvement, or clinical manifestation of rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. Herein, we discuss information gathered from peer-reviewed publications to describe the emerging role of Kl in these select rheumatologic autoimmune diseases.
Key terms: Klotho, Rheumatoid arthritis, Systemic lupus erythematosus, Systemic sclerosis
INTRODUCTION
Autoimmune disease occurs when the immune system mediates an attack on the body’s organs, leading to tissue injury. Autoimmune disorders are classified as systemic or organ-specific depending on the degree of their clinical manifestation and pathogenesis. The autoimmune disorders covered in this review, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and systemic sclerosis (SSc) are classified as systemic.1 The prevalence of autoimmune disease increases with age; however, occurrence of RA and SLE is high during the reproductive years as well. Aging is an important risk factor for immune system impairment referred to as immunosenescence and is associated with functional dysregulation and a reduction in immune responsiveness. Specifically, accelerated T cell aging along with telomeric shortening may predispose to autoimmune responses and thus explain the amplified vulnerability for chronic inflammatory diseases in the elderly population.2 Costenbader et al. reported that telomere shortening might present a common biomarker for aging, immunosenescence, and autoimmune disease.3
This review will focus on the protein Klotho (Kl), which is known as an anti-aging and anti-inflammatory factor that may play an important role in the process of autoimmunity. The specific mechanisms through which Kl expression is downregulated and no longer acting in an anti-inflammatory manner are still lacking. Elucidating the cellular and molecular mechanisms of action for increasing Kl at the transcriptional and protein level, along with uncovering the anti-inflammatory potential of Kl may lead to prospective therapies for autoimmune diseases.
Klotho: aging and inflammation
The human Klotho gene encodes the α-Kl protein, along with the β-Kl and γ-Kl isoforms. α-Kl is produced within the kidney and choroid plexus of the brain.4,5 Three α-Kl protein types with different functions have been identified: the full-length transmembrane α-Kl (m-Kl), secreted truncated α-Kl (s-Kl), and proteolyzed shed α-Kl (p-Kl) (Figure 1). m-Kl protein consists of a short intracellular domain and an extracellular domain that contains two internal repeats, Kl1 and Kl2, which have sequence homology to family 1 β-glycosidases.4 m-Kl is an essential component of some fibroblast growth factor (FGF) receptor complexes, being necessary for high affinity binding of FGF23 to its respective receptors.6–8 Osteoblasts and osteocytes are the major sources for circulating FGF23, and the proximal renal tubules, along with the pituitary and parathyroid glands are the main sites for m-Kl/FGF receptor interaction.7,9 s-Kl is generated by the alternative mRNA splicing of Klotho and contains Kl1 with an additional C-terminal sequence.10,11 p-Kl is cleaved from the extracellular domain of m-Kl by the membrane-anchored proteases a disintegrin and metalloprotease 10 (ADAM10), a disintegrin and metalloprotease 17 (ADAM17), and beta-secretase 1 (BACE1).12 Thus, soluble Klotho protein enters the circulation by alternative mRNA splicing (s-Kl) and proteolytic cleavage (p-Kl), and can function as a humoral factor with pleiotropic activities, including inhibiting certain growth factors,13–16 suppressing oxidative stress,17,18 and regulating ion channels and transporters19–24 (Figure 2).
FIGURE 1.
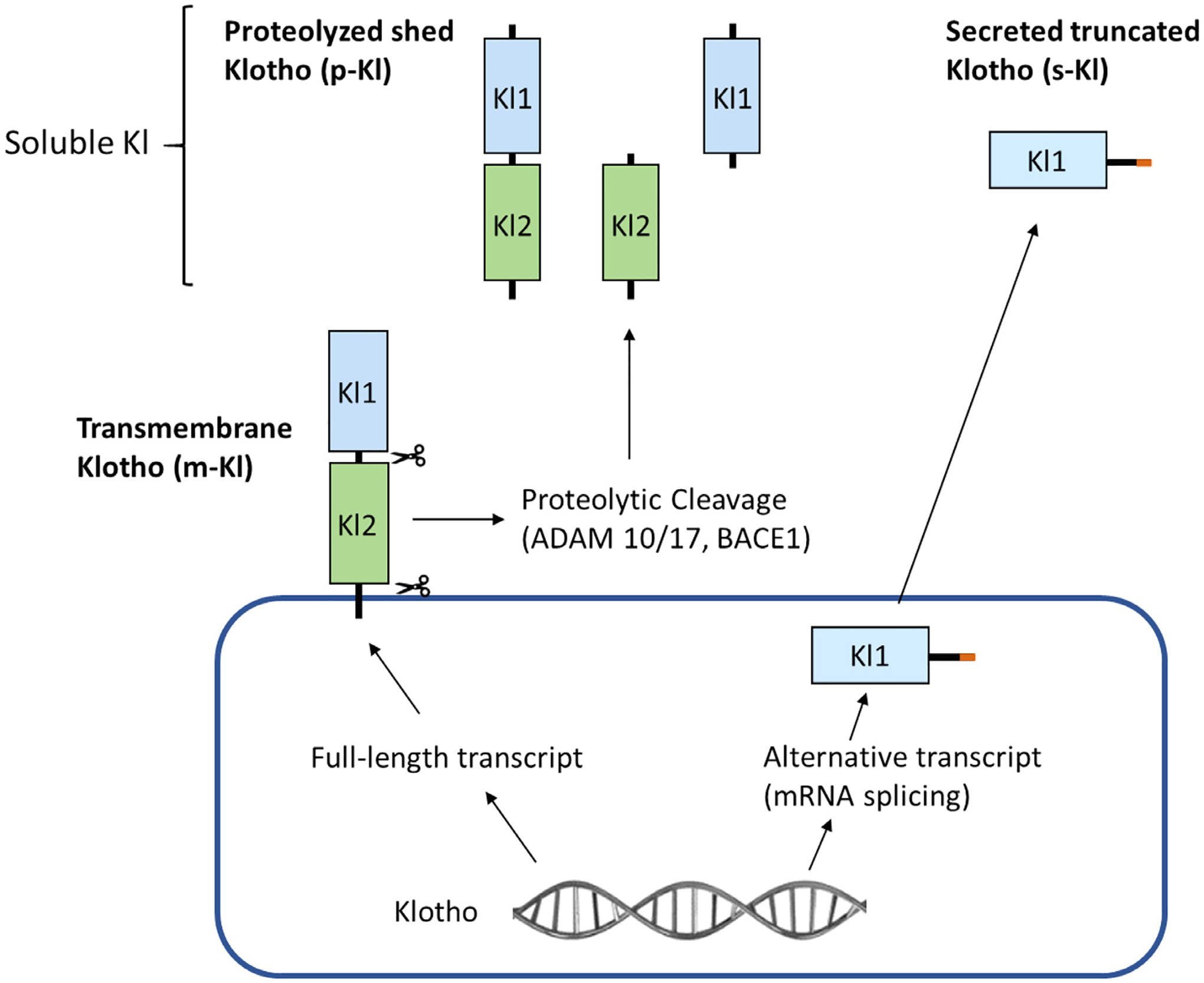
Forms of α-Klotho (KI).
Three α-Kl protein types have been identified: the full-length transmembrane α-Kl (m-Kl), secreted truncated α-Kl (s-Kl), and proteolyzed shed α-Kl (p-Kl). m-Kl protein consists of a short intracellular domain and an extracellular domain that contains two internal repeats, Kl1 and Kl2. s-Kl is generated by the alternative mRNA splicing of Klotho and contains Kl1 with an additional C-terminal sequence (orange segment). p-Kl is cleaved from the extracellular domain of m-Kl by the membrane-anchored proteases a disintegrin and metalloprotease 10 (ADAM10), a disintegrin and metalloprotease 17 (ADAM17), and beta-secretase beta-APP cleaving enzyme 1 (BACE1), resulting in three different forms of p-Kl depending up where the proteases cleave.
FIGURE 2.
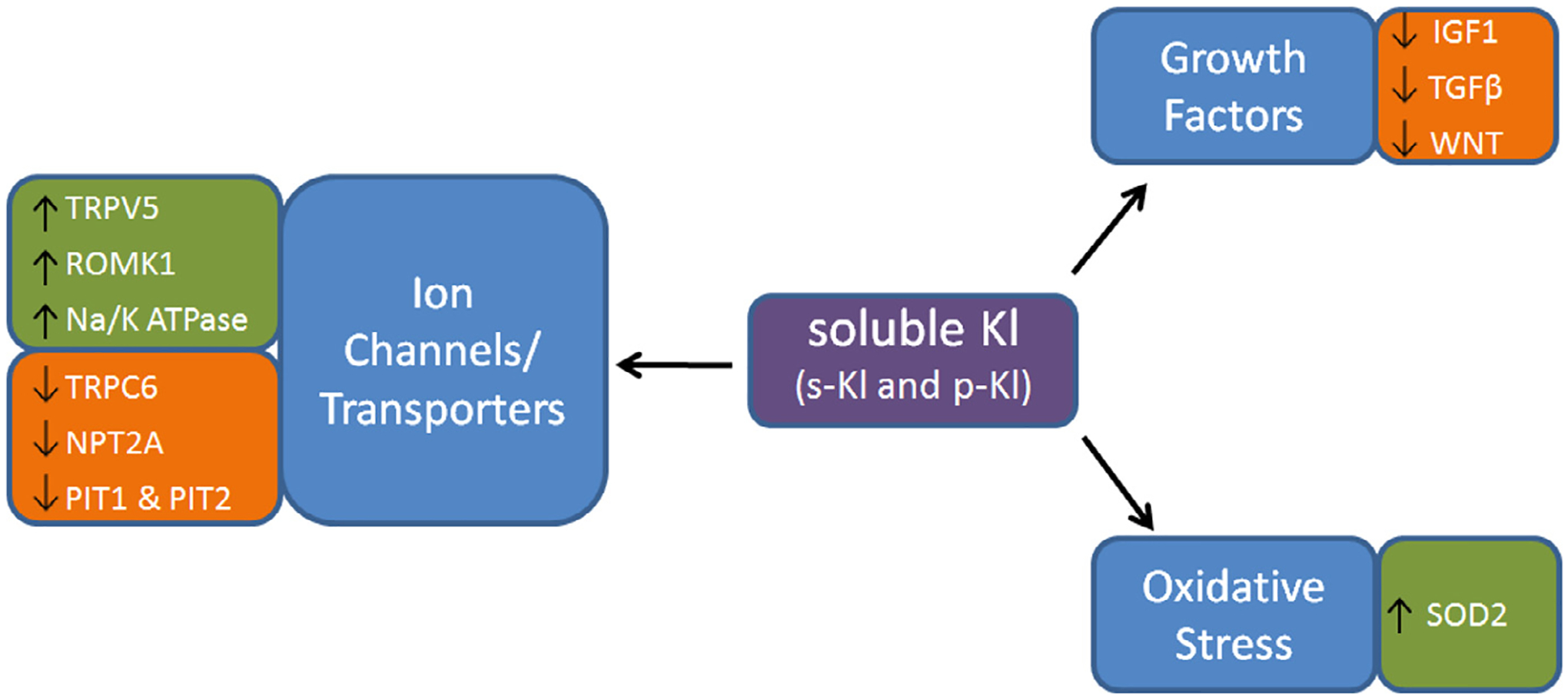
Functions of Soluble Klotho (KI).
Soluble Klotho affects ion channels and transporters, including Transient Receptor Potential Cation Channel Subfamily V Member 5 (TRPV5), Renal Outer Medullary Potassium Channel 1 (ROMK1), Sodium/Potassium ATPase (Na/K ATPase), Transient Receptor Potential Cation Channel Subfamily C Member 6 (TRPC6), Sodium-Dependent Phosphate Transport Protein 2A (NPT2A), Pituitary-specific positive transcription factor 1 (PIT1), Pituitary-specific positive transcription factor 2 (PIT2). Soluble Klotho is also known to inhibit growth factors, including Insulin-like Growth Factor 1 (IGF1), Transforming Growth Factor Beta (TGFβ), and Wingless-Type MMTV Integration Site Family Member 1 (WNT). Soluble Klotho further functions to inhibit oxidative stress via increasing Superoxide Dismutase 2 (SOD2).
Kl is an important anti-aging factor known to suppress aging through multiple mechanisms, including anti-oxidation, vasculoprotective effects, stem cell preservation, and inhibited inflammatory responses through modulation of signaling pathways. Aging is characterized by the progressive loss of tissue and organ function. The oxidative stress theory of aging emphasizes that this loss of function is due to the accumulation of reactive oxygen and nitrogen species, which correspondingly leads to decreases in nitric oxide (NO) bioavailability. One means by which Kl reduces oxidative stress is through increased expression of the mitochondrial anti-oxidant, superoxide dismutase 2 (SOD2).17 Kl protein first inhibits Forkhead box O (FOXO) phosphorylation by inhibiting the insulin/insulin-like growth factor 1 pathway. FOXO can then translocate to the nucleus where it binds the SOD2 promoter, which upregulates SOD2 expression, thereby facilitating the catalysis of superoxide generated by mitochondrial respiration into hydrogen peroxide.17 This leads to a decrease in reactive oxygen species, implicating Kl as an important factor in resistance to oxidative stress.
The importance of Kl in protecting the vasculature was elucidated when Kl-deficient mice showed decreased endothelium-dependent vasodilation after treatment with acetylcholine compared to their wild-type counterparts, indicating that NO had been reduced in the vascular endothelium.25 They also found that NO metabolites in the urine were decreased in Kl heterozygous mice compared to wild type. An additional study showed that Kl may play a role in regulating endothelial Nitric Oxide Synthase (eNOS), the enzyme that controls NO production in the vascular endothelium. Delivery of mesenchymal stromal cells overexpressing secreted Kl abolished endothelial dysfunction caused by monocrotaline, and an increase in secreted Kl levels abolished the monocrotaline-induced down regulation of eNOS.26 In patients with chronic kidney disease, decreases in Kl serum levels were found to correlate with signs of vascular dysfunction, including arterial stiffness, atherosclerosis, and vascular calcification.27 Another mechanism by which Kl is capable of asserting vasculoprotective effects is through the inhibition of autophagy, which is critical for cell survival.28
One of the most important mechanisms by which Kl functions as an anti-aging factor is by impeding inflammation. In renal cells and human umbilical vein endothelial cells (HUVECs), Kl was found to inhibit TNF-α-induced pro-inflammatory cytokine (IL-6 and IL-8) production and expression of adhesion molecules, while attenuating NF-κB promoter activity, a critical transcription factor regulating inflammatory responses.29,30 It was also discovered that Kl deficiency leads to increased airway inflammation, and soluble Kl was protective against the pro-inflammatory effects of FGF23 and smoke.31 Furthermore, there was an inverse correlation between inflammatory TNF-α and Kl as was found when Kl protein inhibited TNFα-induced expression of adhesion molecules in HUVECs.32 There was also found to be a significant positive correlation between anti-inflammatory IL-10 and Kl gene expression in the vascular beds of patients with clinical atherosclerotic disease.32 In human kidney proximal tubular (HK-2) cells, Kl protected against lipopolysaccharide-induced inflammation injury via decreased reactive oxygen species production and lower mRNA levels of TNF-α and IL-6, along with inhibition of Wnt and NF-κB pathways.33 Kl further functions as an anti-aging factor by suppressing RIG-I-mediated senescence-associated inflammation. Intracellular Kl binds RIG-I and blocks its multimerization, which inhibits RIG-I-induced expression of the inflammatory factors IL-6 and IL-8.34 Kl also acts to inhibit the aging process through stem cell preservation. Kl deficient mice had a decrease in stem cell number and increase in progenitor cell senescence. Wnt exposure triggered accelerated cellular senescence, and Kl was found to bind to multiple Wnt family members, with Wnt-Kl interaction suppressing Wnt biological activity.35 The role of Kl in suppressing inflammation is evident and is an essential element of Kl’s potent anti-aging effects.
Klotho and autoimmunity
Kl is known to play an important role in aging and one of the most apparent signs of aging is a decline in the integrity of the immune system; however, data implicating Kl in immune system function is limited and mostly pertains to murine models. For example, Kl knockout mice have underdeveloped immune organs and impaired B cell differentiation and development.4,36 Aging of the immune system accompanies the inability of the body to recognize self-antigens, which often leads to autoimmune responses. The role of Kl in autoimmune response is recognized in diseases such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by joint swelling, pain, and deformity. Systemic manifestations of the disease include osteoporosis, pleuritis, and pericarditis, as well as inflammation of heart and blood vessels. Pásztói et al.37 demonstrated a lower expression level of Kl in synovial fibroblasts than in synovial membrane samples isolated from the knee joints of RA patients. Additionally, Kl is an anti-inflammatory modulator negatively regulating NFκ-B, decreasing pro-inflammatory gene transduction. Excessive stimulation of NFκ-B activates the immune response, which is linked to the pathogenesis of RA.38 In RA, the impaired function of peripheral blood CD4+ lymphocytes is thought to affect the function of the synovium and induce inflammation. These CD4+ cells are characterized by decreased proliferation upon stimulation, which is associated with shorter telomeres.39 The peripheral blood CD4+ lymphocytes of patients with RA are similar to lymphocytes during physiological aging, which may suggest that RA causes accelerated aging of the T cell population.39 Additionally, the peripheral blood of RA patients contains enlargement of both memory (CD4+ CD45RO+) and CD4+ CD28− T cell populations, with CD28 being required for activation of these lymphocytes upon antigen challenge.40,41 The down-regulation of CD28 expression on the surface of CD4+ cells of both healthy elderly and patients with RA is dependent on increased levels of TNF-α.42,43 Witkowski et al.44 showed that Kl was down-regulated at the mRNA and protein levels in CD4+ lymphocytes from healthy elderly and RA subjects. They also showed that reduced Kl mRNA levels occurred in concert with reduced expression levels of CD28 for RA patients, but there was only a marginal correlation for healthy individuals. The authors state that although the exact mechanism of Kl activity for CD4+ cell function is unknown, it may be involved in the anti-inflammatory processes occurring in young healthy individuals, while being reduced in healthy elderly and RA patients. Thus, this mechanism of Kl down-regulation may underlie a physiological disease process for T cell aging that occurs in RA patients. Soroczyńska-Cybula et al.45 showed impaired protein binding activity of the ‘α’ sequence in the promoter that controls expression of CD28; the corresponding region near the promoter of Kl reaches 80% homology and appears to be binding nuclear proteins similar to the ‘α’ sequence, which makes them more susceptible to the factors controlling the ‘α’ itself, especially TNF. This data may suggest the common mechanism decreasing the expression of CD28 is Kl and the decreased expression of Kl in the resting peripheral blood CD4+ cells of patients with RA would be a likely indication of their premature aging. Alvarez-Cienfuegos et al.46 analyzed soluble Kl in a cohort of RA patients and assessed possible links between Kl and different characteristics of the disease. Serum Kl concentrations were significantly increased in RA patients as compared to the control group. Higher plasma concentrations of Kl were associated with higher levels of autoantibodies: rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPAs), and DAS-28-ESR (an activity score calculated using the number of swollen and tender joints and the erythrocyte sedimentation rate). The level of Kl was increased in RA patients undergoing treatment with biological agents. No correlation was found between Kl and age or hypertension. However, the authors expected diminished levels of Kl in RA patients because serum Kl has been reported to act as an anti-inflammatory molecule.29,47 They concluded the positive correlation between elevated levels of Kl and disease activity may be a compensatory response to inflammation. This study was a cross-sectional analysis and expression of Kl in RA tissues was not performed. From the combined results, there are strong associations between Kl and inflammation in RA. However, further studies should be performed to elucidate the mechanisms by which Kl impacts inflammation in RA in order to support translation of these findings into new therapies.
Systemic lupus erythematosus
Systemic Lupus Erythematosus (SLE) is a heterogeneous autoimmune disease involving multiple organs, including the skin, joints, kidneys, lungs, heart, and nervous system. SLE is characterized by autoantibody production, such as anti-double-stranded DNA antibodies (anti-dsDNA), anti-nuclear antibodies (ANA), and anti-Smith antibodies, followed by formation of immune complexes deposited in the kidneys and other organs, causing cellular injury.48 Previous studies demonstrated higher levels of pro-inflammatory cytokines and chemokines, including IL-6, IL-8, IL-10, TNF-α, IFN-γ, CCL2/MCP-1, CXCL10/IP-10 in the cerebrospinal fluid (CSF) of patients with neuropsychiatric SLE (NPSLE).49,50 Ushigusa et al.51 demonstrated that lower levels of soluble Kl in CSF, lower serum levels of anti-Smith antibodies, and increased serum levels of C3 were important factors for predicting NPSLE compared to SLE in multivariable analyses. Additionally, CSF Kl and CSF granulocyte-macrophage colony stimulating factor were inversely correlated in NPSLE patients. One of the main causes of morbidity in patients with SLE is end stage renal disease. Previous studies have shown that Kl protein is highly expressed in the kidneys52 and has a renal protective effect in glomerulonephritis and acute kidney injury of mice.53–55 Furthermore, Kl suppresses pro-inflammatory cytokines such as MCP-1and IL-8 in a human kidney proximal tubular cell line (HK-2); and RANTES and IL-6 in the human embryonic kidney 293 cells (HEK293).30,33 Kl down-regulates NF-κB, known to be activated and constitutively maintained in B lymphocytes from active stage SLE patients, and the activation of NF-κB is associated with the initiation and progression of lupus nephritis.56–58 Furthermore, Ye et al.55 demonstrated that down-regulation of Kl by miR-199a lead to NF-κB activation and inflammatory cytokine secretion in response to lipopolysaccharide stimulation in human embryonic kidney 293T cells. Whether this mechanism is involved in immune cells and kidney cells during the pathological process of lupus nephritis has yet to be determined.
Systemic sclerosis (Scleroderma)
Systemic Sclerosis (SSc) is an autoimmune disease of the connective tissue characterized by microangiopathy, early inflammation, and subsequent fibrosis of internal organs and thickening of the skin. Talotta et al.59 evaluated the serum concentration of Kl in patients with SSc and healthy controls. Their data demonstrated a lower concentration of Kl in the serum of SSc patients (age 63.9 ± 13.1) compared to that of healthy controls (age 50.5 ± 10.7), without any significant association with the clinical manifestations described above or with autoantibody levels. Kl levels in SSc patient’s serum were not associated with age; however, Kl was directly related to the age in matched controls. This may suggest that diminished levels of Kl are connected to premature aging phenotype in SSc rather than to the age of the patient.
Previous studies showed that Kl is a known co-receptor necessary for FGF23 activity.6,7 FGF23/Kl signaling inhibits renal phosphate reabsorption, activation of Vitamin D as well as reduced secretion of parathyroid hormone.60–62 Kotyla et al.63 demonstrated that the ratio of FGF23 to α-Kl was both significantly reduced in SSc patients with the diffuse form of the disease and significantly correlated with disease activity score according to European Scleroderma Trials and Research Group (Eustar) 2017 guidelines.64 The authors proposed this ratio as a potential novel marker of SSc activity.
One manifestation of SSc is Raynaud’s phenomenon or a reduced blood flow to the fingers resulting from cold exposure. In SSc, this reduction in blood circulation to the fingers and toes can lead to microangiopathy and digital ulcers. Deficiency of Kl can impair the healing of digital ulcers related to microvessel damage. The retrospective studies performed by Tallota et al.65 showed an inverse correlation between serum Kl concentration and the severity of the nailfold capillaroscopic pattern, a clinical indicator of microangiopathy. There was no significant correlation between the presence of capillary avascular areas, megacapillaries, or neoangiogenesis. The main limitations of the study were that no healthy subjects were enrolled as a control group, only total concentration of Kl was evaluated, and the nailfold capillaroscopic images were assessed qualitatively rather than quantitatively. A study published by Mazzotta et al.66 demonstrated decreased expression of α-Kl in skin biopsies from patients with SSc at microvascular level. Additionally, α-Kl was significantly diminished in cultured SSc microvascular endothelial cells (MVECs) as compared to healthy MVECs, and the administration of soluble Kl improved SSc MVEC functions in vitro by acting as a pro-angiogenic factor. Mechanisms through which Kl expression may be increased in SSc have yet to be uncovered.
SUMMARY AND CONCLUSION
Kl plays an important role in the process of aging, inflammation, and autoimmunity (Table 1). A decline in immunocompetence with aging leads to the inability of the body to recognize self-antigens, which often leads to autoimmune responses. Several clinical studies demonstrate that Kl may function through multiple mechanisms associated with the pathogenesis and progression of certain autoimmune diseases, including RA, SLE, and SSc. Accelerated aging of T cell population together with diminished expression of Kl in CD4+ cells and reduced expression levels of CD28 for RA patients may suggest an interplay between immunosenescence processes and the anti-aging gene Kl. Although the exact mechanism of Kl in CD4+ cell function is unknown, it may be involved in the anti-inflammatory processes occurring in young healthy individuals, while being reduced in healthy elderly and RA patients. Elderly patients with SLE have different clinical and serological manifestations and poorer prognosis compared with young SLE patients.67 In SSc, processes such as fibrosis, inflammation, and vasculopathy may be accelerated by aging-associated phenotypes including immune dysregulation, cellular senescence, or epigenetic modifications. Because patients with active, diffuse SSc have a reduced ratio of FGF23 to α-Kl, future studies could validate this as a novel marker of disease activity. Decreased levels of Kl in serum from patients with SLE and SSc, but increased levels of Kl in serum of RA patients compared to healthy controls reveal there may be differences in the role of Kl in these autoimmune diseases. However, these discrepancies could also be due to the methods used for measuring α-Kl. The positive correlation between elevated levels of Kl and disease activity may be a compensatory response to inflammation.46 Whether the interference from rheumatoid factor was considered when ELISAs were performed on serum taken from RA patients is also not known. As shown in Figure 1, there are multiple forms of α-Kl, which adds complexity to measuring Kl levels as each form may play a different role in varying diseases. Olejnik et al.68 noted the difficulty of measuring soluble Klotho in serum, in particular, differentiating between s-Kl and p-Kl, suggesting that an antibody specific for the C-terminal sequence of s-Kl may resolve this issue. It is also pertinent to note that examining the function and activity of Kl instead of solely measuring levels of Kl may help further differentiate its role in these autoimmune diseases. Taken together, the association between Kl gene expression or serum levels (Table 1) and changes in immune system function suggest a role for Kl in RA, SLE, and SSc; however, further investigation into the cellular and molecular mechanisms of these associations is necessary to support translation of these findings into novel treatments. More precise methods for measurement of the different forms of Kl will allow more rapid progress in determining these mechanisms.
Table 1.
Klotho (KI) involvement in RA, SLE, SSc.
| Autoimmune Disease | Klotho Level/Correlation | References |
|---|---|---|
| Rheumatoid Arthritis | Low Kl expression in synovial membrane, synovial fibroblasts | 37 |
| Kl is downregulated in CD4+ cells, compared to healthy controls | 44 | |
| Kl positively correlates with CD28 on CD4+ cells | 45 | |
| Increased Kl in serum, compared to healthy controls | 46 | |
| Kl positively correlates with anti-cyclic citrullinated peptide antibody and rheumatic factor | 46 | |
| Systemic Lupus Erythematosus | Low Kl in cerebrospinal fluid (CSF) is associated with endothelial dysfunction and neuronal damage | 51 |
| Lower CSF Kl, lower serum anti-Smith antibodies, and higher serum C3 were significant factors for predicting neuropsychiatric SLE | 51 | |
| Kl in CSF inversely correlates with granulocyte/macrophage-colony stimulating factor in CSF | 51 | |
| Systemic Sclerosis | Decreased Kl in serum, compared to healthy controls | 59 |
| Lower Kl in microvascular endothelial cells, compared to healthy controls | 66 |
Funding:
This work was supported by funding from the Department of Veterans Affairs (101 CXC001248–01A2).
Footnotes
Conflict of Interest: The authors have no financial or other conflicting interests to disclose.
REFERENCES
- 1.Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, eds. Kelley and Firestein’s Textbook of Rheumatology | Sciencedirect. 10th ed. Elsevier; 2017. https://www.sciencedirect.com/book/9780323316965/kelley-and-firesteins-textbook-of-rheumatology. Accessed April 8, 2020. [Google Scholar]
- 2.Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006;41(3):246–251. 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Costenbader KH, Prescott J, Zee RY, De Vivo I. Immunosenescence and rheumatoid arthritis: does telomere shortening predict impending disease? Autoimmun Rev. 2011;10(9):569–573. 10.1016/j.autrev.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390 (6655):45–51. 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 5.Li S-A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29(4):91–99. 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 6.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by Klotho. J Biol Chem. 2006;281(10):6120–6123. 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444 (7120):770–774. 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 8.Kuro-o M. Overview of the FGF23-Klotho axis. In: Pediatric Nephrology. Vol 25 Pediatr Nephrol. 2010:583–590. 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 9.Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone. 2012;51 (3):621–628. 10.1016/j.bone.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-O M, Nabeshima YI. Identification of the human Klotho gene and its two transcripts encoding membrane and secreted Klotho protein. Biochem Biophys Res Commun. 1998;242(3):626–630. 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 11.Shiraki-Iida T, Aizawa H, Matsumura Y, et al. Structure of the mouse Klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424(1–2):6–10. 10.1016/S0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 12.Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for α-, β- and γ-secretase. FEBS Lett. 2009;583(19):3221–3224. 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utsugi T, Ohno T, Ohyama Y, et al. Decreased insulin production and increased insulin sensitivity in the Klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49(9):1118–1123. 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 14.Kurosu H, Yamamoto M, Clark JD, et al. Physiology: suppression of aging in mice by the hormone Klotho. Science (80-). 2005;309(5742):1829–1833. 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286(10):8655–8665. 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol. 2013;24(5):771–785. 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, Clark JD, Pastor JV. et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J Biol Chem. 2005;280(45):38029–38034. 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuro-o M Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389(3):233–241. 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 19.Chang Q, Hoefs S, Van Der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The β-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science (80-). 2005;310(5747):490–493. 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 20.Cha SK, Hu MC, Kurosu H, Kuro-O M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K + excretion by Klotho. Mol Pharmacol. 2009;76(1):38–46. 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sopjani M, Alesutan I, Dërmaku-Sopjani M, et al. Regulation of the Na+/K+ ATPase by Klotho. FEBS Lett. 2011;585(12):1759–1764. 10.1016/j.febslet.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL. Cardio-protection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3. 10.1038/ncomms2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hum JM, O’Bryan LM, Tatiparthi AK, et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble Klotho. J Am Soc Nephrol. 2017;28(4):1162–1174. 10.1681/ASN.2015111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–136. 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248(2):324–329. 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 26.Varshney R, Ali Q, Wu C, Sun Z. Monocrotaline-induced pulmonary hypertension involves downregulation of antiaging protein Klotho and eNOS activity. Hypertension. 2016;68(5):1255–1263. 10.1161/HYPERTENSIONAHA.116.08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE. 2013;8(2): e56695. 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Sun Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J Mol Med. 2019. 10.1007/s00109-019-01841-6. (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-α-induced expression of adhesion molecules in the endothelium and attenuates NF-κB activation. Endocrine. 2009;35(3):341–346. 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Banerjee S, Dey N, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60(7):1907–1916. 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krick S, Grabner A, Baumlin N, et al. Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J. 2018;52(1). 10.1183/13993003.00236-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martín-Nuñez E, Donate-Correa J, López-Castillo Á, et al. Soluble levels and endogenous vascular gene expression of Klotho are related to inflammation in human atherosclerotic disease. Clin Sci. 2017;131 (21):2601–2609. 10.1042/CS20171242. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Kuang Y, Zhou J. Klotho protects against LPS-induced inflammation injury by inhibiting Wnt and NF-κB pathways in HK-2 cells. Pharmazie. 2017;72(4):227–231. 10.1691/ph.2017.6867. [DOI] [PubMed] [Google Scholar]
- 34.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13(3):254–262. 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science (80-). 2007;317 (5839):803–806. 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 36.Okada S, Yoshida T, Hong Z, et al. Impairment of B lymphopoiesis in precocious aging (Klotho) mice. Int Immunol. 2000;12(6):861–871. 10.1093/intimm/12.6.861. [DOI] [PubMed] [Google Scholar]
- 37.Pásztói M, Nagy G, Géher P, et al. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis Res Ther. 2009;11(3). 10.1186/ar2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Sullivan B, Thompson A, Thomas R. NF-KB as a therapeutic target in autoimmune disease. Expert Opin Ther Targets. 2007;11(2):111–122. 10.1517/14728222.11.2.111. [DOI] [PubMed] [Google Scholar]
- 39.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97(16):9203–9208. 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–7452. 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 41.Pawlik A, Ostanek L, Brzosko I, et al. The expansion of CD4+CD28− T cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5(4): R210. 10.1186/ar766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallejo AN, Bryl E, Klarskov K, Naylor S, Weyand CM, Goronzy JJ. Molecular basis for the loss of CD28 expression in senescent T cells. J Biol Chem. 2002;277(49):46940–46949. 10.1074/jbc.M207352200. [DOI] [PubMed] [Google Scholar]
- 43.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-α. J Immunol. 2001;167(6):3231–3238. 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 44.Witkowski JM, Soroczyńska-Cybula M, Bryl E, Smoleńska Ż, Jóźwik A. Klotho—a common link in physiological and rheumatoid arthritis-related aging of human CD4 + lymphocytes. J Immunol. 2007;178 (2):771–777. 10.4049/jimmunol.178.2.771. [DOI] [PubMed] [Google Scholar]
- 45.Soroczyńska-Cybula M, Bryl E, Smoleńska Z, Witkowski JM. Varying expression of four genes sharing a common regulatory sequence may differentiate rheumatoid arthritis from ageing effects on the CD4+ lymphocytes. Immunology. 2011;132(1):78–86. 10.1111/j.1365-2567.2010.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez-Cienfuegos A, Cantero-Nieto L, Garcia-Gomez JA, Robledo G, González-Gay MA, Ortego-Centeno N. FGF23-Klotho axis in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2020;38 (1):50–57. http://www.ncbi.nlm.nih.gov/pubmed/31025926. [PubMed] [Google Scholar]
- 47.Nakanishi K, Nishida M, Harada M, et al. Klotho-related molecules upregulated by smoking habit in apparently healthy men: a cross-sectional study. Sci Rep. 2015;5. 10.1038/srep14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham M, Marks N, Barnado A, Wirth JR, Gilkeson G, Markiewicz M. Are microparticles the missing link between thrombosis and autoimmune diseases? Involvement in selected rheumatologic diseases. Semin Thromb Hemost. 2014;40(6):675–681. 10.1055/s-0034-1387924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Efthimiou P, Blanco M. Pathogenesis of neuropsychiatric systemic lupus erythematosus and potential biomarkers. Mod Rheumatol. 2009;19 (5):457–468. 10.1007/s10165-009-0198-5. [DOI] [PubMed] [Google Scholar]
- 50.Fragoso-Loyo H, Atisha-Fregoso Y, Llorente L, Sá Nchez-Guerrero J. Inflammatory profile in cerebrospinal fluid of patients with headache as a manifestation of neuropsychiatric systemic lupus erythematosus. Rheumatology. 2013;52(12). 10.1093/rheumatology/ket294. 2218–2212. [DOI] [PubMed] [Google Scholar]
- 51.Ushigusa T, Ichinose K, Sato S, et al. Soluble α-Klotho is a potential biomarker associated with neuropsychiatric systemic lupus erythematosus. Clin Immunol. 2016;165:29–34. 10.1016/j.clim.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. 2017;100:19–35. 10.1016/j.bone.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 53.Haruna Y, Kashihara N, Satoh M, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A. 2007;104(7):2331–2336. 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugiura H, Yoshida T, Mitobe M, et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant. 2010;25(1):60–68. 10.1093/ndt/gfp451. [DOI] [PubMed] [Google Scholar]
- 55.Ye H, Su B, Ni H, et al. microRNA-199a may be involved in the pathogenesis of lupus nephritis via modulating the activation of NF-κB by targeting Klotho. Mol Immunol. 2018;103:235–242. 10.1016/j.molimm.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Mishra RK. Involvement of NF-κB signaling pathway in the pathogenesis of systemic lupus erythematosus. Nephrol Open J. 2016;2(1):9–13. 10.17140/NPOJ-2-112. [DOI] [Google Scholar]
- 57.Buendía P, Ramírez R, Aljama P, Carracedo J. Klotho prevents trans-location of NFkB. Vitamins and Hormones. 101. Academic Press Inc.; 2016:119–150. 10.1016/bs.vh.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Brightbill HD, Suto E, Blaquiere N, et al. NF-κB inducing kinase is a therapeutic target for systemic lupus erythematosus. Nat Commun. 2018;9(1). 10.1038/s41467-017-026720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talotta R, Bongiovanni S, Letizia T, et al. Mesurment of serum Klotho in systemic sclerosis. Dis Markers. 2017;2017: 9545930. 10.1155/2017/9545930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fructuoso AIS, Maestro ML, Pérez-Flores I, et al. Serum level of fibroblast growth factor 23 in maintenance renal transplant patients. Nephrol Dial Transplant. 2012;27(11):4227–4235. 10.1093/ndt/gfs409. [DOI] [PubMed] [Google Scholar]
- 61.Ahmadi R, Hajialilo M, Ghorbanihaghjo A, et al. FGF-23, Klotho and vitamin D levels in scleroderma. Iran J Public Health. 2017;46 (4):530–536. [PMC free article] [PubMed] [Google Scholar]
- 62.De Borst MH, Vervloet MG, Ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-Klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603–1609. 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotyla P, Kruszec-Zytniewska A, Owczarek A, Olszanecka-Glinianowicz M, Chudek J. Fibroblast growth factor 23 to alpha-Klotho index correlates with systemic sclerosis activity: a proposal for novel disease activity marker. J Clin Med. 2018;7(12):558. 10.3390/jcm7120558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valentini G, Iudici M, Walker UA, et al. The European scleroderma trials and research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis. 2017;76(1):270–276. 10.1136/annrheumdis-2016-209768. [DOI] [PubMed] [Google Scholar]
- 65.Talotta R, Rigamonti F, Letizia T, et al. Serum Klotho concentrations inversely correlate with the severity of nailfold capillaroscopic patterns in patients with systemic sclerosis. Reumatismo. 2019;71(1):19–23. 10.4081/reumatismo.2019.1129. [DOI] [PubMed] [Google Scholar]
- 66.Mazzotta C, Manetti M, Rosa I, et al. Proangiogenic effects of soluble α-Klotho on systemic sclerosis dermal microvascular endothelial cells. Arthritis Res Ther. 2017;19(1). 10.1186/s13075-017-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montoya-Ortiz G. Immunosenescence, aging, and systemic lupus erythematous. Autoimmune Dis. 2013;2013. 10.1155/2013/267078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olejnik A, Franczak A, Krzywonos-Zawadzka A, Kabuhna-Oleksy M, Bil-Lula I The biological role of Klotho protein in the development of cardiovascular diseases. 2018. doi: 10.1155/2018/5171945. [DOI] [PMC free article] [PubMed] [Google Scholar]


