Abstract
Plane-wave imaging (PWI) with angular compounding has gained in popularity over recent years, because it provides high frame rates and good image properties. However, most linear arrays used in clinical practice have a pitch that is equal to than the wavelength of ultrasound. Hence, the presence of grating lobes is a concern for PWI using multiple transmit angles. The presence of grating lobes produces clutter in images and reduces the ability to observe tissue contrast. Techniques to reduce or eliminate the presence of grating lobes for PWI using multiple angles will result in improved image quality. Null subtraction imaging (NSI) is a nonlinear beamforming technique that has been explored for improving the lateral resolution of ultrasonic imaging. However, the apodization scheme used in NSI also eliminates or greatly reduces the presence of grating lobes. Imaging tasks using NSI were evaluated in simulations and physical experiments involving tissue-mimicking phantoms and rat tumors in vivo. Images created with NSI were compared with images created using traditional delay and sum (DAS) with Hann apodization and images created using a generalized coherence factor (GCF). NSI was observed to greatly reduce the presence of grating lobes in ultrasonic images, compared to DAS with Hann and GCF, while maintaining spatial resolution and contrast in the images. Therefore, NSI can provide a novel means of creating images using PWI with multiple steering angles on clinically available linear arrays while reducing the adverse effects associated with grating lobes.
Index Terms—: Apodization, beamforming, null subtraction imaging (NSI), plane-wave imaging (PWI), ultrasonic imaging
I. Introduction
Improving the quality of ultrasonic B-mode imaging remains an important area of research, because B-mode imaging is ubiquitous in the clinical setting. In general, image quality is a function of contrast, spatial resolution, and the signal-to-noise ratio (SNR). Different approaches have been explored to improve each of the image quality features often by trading off one quality to improve another. For example, approaches have been applied to improve the SNR of B-mode imaging using techniques, such as coded excitation and pulse compression [1], [2] or compounding [3]. Techniques to improve spatial resolution in B-mode ultrasound have been attempted using a variety of methods, including beamforming techniques [4], coded excitation [5], and signal processing [6]. Finally, contrast-to-noise ratio (CNR) is often considered the most important image quality metric. Because the contrast of ultrasound targets is often inherently low, it is important to remove artifacts that can reduce the ability to observe contrast in B-mode imaging. Reverberation and clutter due to things, such as sidelobes or grating lobes, can result in a reduction in the observable contrast of targets in B-mode ultrasound. Therefore, a large number of studies have occurred, and papers have been written about reducing the impact of reverberation and clutter on B-mode imaging.
The list of works pertaining to the goal of improving contrast in B-mode ultrasound is very long, and calling attention to all of the works in the field is the role of a review paper. However, in this work, we focus on techniques to reduce sidelobe and grating lobe artifacts in B-mode imaging when applying plane-wave imaging (PWI) compounding. The idea of PWI coherent compounding was first introduced in a series of papers by Lu and Greenleaf [7], [8], [9], Lu [10], [11], and Cheng and Lu [12] and then popularized by Montaldo et al. [13]. In PWI in 2-D, a linear array sends out “plane”-wave transmissions at multiple angles by firing off each element of the array simultaneously for zero-degree (or broadside) plane-waves or with sequentially increasing or decreasing time delays to elements resulting in planes-wave transmissions at different angles relative to broadside. Image quality is improved by coherently or incoherently compounding received signals from plane-wave transmissions from multiple angles resulting in the ability to focus throughout the field.
One of the main attractive features of PWI is that it facilitates ultrafast B-mode imaging with frame rates on the order of 103 per second or higher [14]. PWI and ultrafast imaging have been instrumental in improving vasculature imaging [15], [16], [17]. Furthermore, PWI and ultrafast imaging have allowed the development of super-resolution imaging for visualizing the vasculature for cardiac applications [18], [19], tumor imaging [20], [21], and functional imaging of the brain [22], [23]. Therefore, the adoption of PWI has had a significant impact on ultrasonic imaging with many of these techniques making their way into the clinic.
One issue associated with using PWI on most clinically available linear arrays is that these linear arrays were not constructed with the steering of beams at moderate-to-high angles in mind. Most linear arrays have a pitch that is close to the wavelength of the center frequency of the probe, and a few arrays exist where the pitch is larger than a wavelength. For broadside, i.e., zero-degree angle plane-wave, beam formation, such a pitch would result in appreciable grating lobes in the field of view [24]. As the plane-wave is steered away from broadside, grating lobes become more obvious in the field of view. For broadband probes, these grating lobes are reduced in magnitude compared with the main lobe, but their energy is smeared out spatially. The high amplitudes of these grating lobes result in image artifacts and can greatly reduce the observable contrast of targets in the imaging field and potentially obscure important image details.
To address grating lobes in PWI, several approaches have been explored. In one approach, nonuniform plane-wave angles were used to help suppress grating lobes [25]. In simulation, this technique reduced the amplitude of some grating lobes but did not completely mitigate their presence. In another approach, physically shifting of the transducer laterally between successive frame acquisitions was used to decrease the effective pitch between elements and mitigate grating lobes [26].
Similar to grating lobes, sidelobes also produce clutter in ultrasound B-mode images. Reducing sidelobe levels of ultrasonic beams has been an area of research since the foundations of diagnostic ultrasound. The simplest method for reducing sidelobe levels in array imaging is to implement apodization using tapered windows. By applying a tapered apodization, such as a Hann apodization, the sidelobe levels can be reduced but at the expense of a broadening main lobe [27]. Many other approaches to reducing sidelobe levels have been investigated over the years with different levels of success [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. However, for many of these techniques, success in sidelobe reduction comes with a trade-off of higher computational cost.
Recently, our group introduced a nonlinear beamforming technique, null subtraction imaging (NSI), that utilized three apodization schemes on receive only [4]. In this technique, the first apodization consisted of a subaperture of N elements where the first N/2 elements had a +1 weight, and the second N/2 elements had a −1 weight resulting in a zero-mean apodization across the receive subaperture. The resulting receive beam pattern had a null at zero degrees. The second apodization was equal to the first apodization but with a small dc offset value added to the apodization resulting in a nonzero-mean apodization across the receive subaperture. The third apodization was a flipped version of the second aperture. The envelopes of the received and beamformed signals for the second and third apodizations are then summed, and the envelope for the first apodization is subtracted from the sum. This results in the zero-degree null becoming the beam and accompanied by low sidelobe levels.
In this study, we examine the use of NSI to dramatically reduce grating lobes and sidelobes when using PWI and both coherent and incoherent compounding techniques. To test the approach, we constructed images of wire targets and acquired images from tissue-mimicking phantoms and from rat tumors in vivo. We compared the NSI approach to Hann apodization and to a generalized coherence factor (GCF) approach [31]. Grating lobe levels were assessed relative to the main lobe energy, and image quality was assessed through reduction in clutter energy in the in vivo images.
II. Methods
A. NSI
NSI makes the use of multiple apodizations on receive on a linear array to create multiple images and then subtracts combinations of the images from each other to obtain a composite image with improved properties in terms of sidelobe levels, grating lobe levels, and main lobe width. Specifically, transmission of ultrasound can occur through multiple means, e.g., linear sequential scanning with focusing or plane-wave coherent compounding. However, linear sequential receive beamforming on receive is required for NSI implementation. In linear sequential scanning, subapertures are used to beam-form and create individual scan lines; then, the subapertures are electronically translated to create the next scan lines. An image is formed by combining these scan lines and possibly interpolating lines in between the actual measured scan lines.
With NSI, three images are created from the scan lines by applying three different apodizations on each subaperture [4]. The first apodization consists of a zero-mean window with the first half given the value of +1 and the second half given the apodization value of −1
where AZM,i is the zero-mean apodization, and N is the number of elements in the subaperture. The second apodization is the same as the zero-mean apodization except for a dc offset
and the third apodization is a flipped version of the second. An NSI image is created by subtracting the zero-mean image from the sum of the dc images
where EZM is the zero-mean envelope image, and Edc1 and Edc2 are the two dc offset envelope images. The resulting B-mode image is constructed by converting to a decibel scale and scaling the image to the maximum value (i.e., 0 dB) and displayed in gray scale
| (1) |
It has been shown that NSI could effectively improve the resolution by reducing main lobe width [4].
However, NSI also reduces the grating lobe levels. The reason for this reduction is based on the broadband nature of ultrasonic imaging. For the zero-mean apodization, a null exists at 0°. On the other hand, grating lobes will not have a null, because, due to the broadband nature of ultrasonic imaging, only a few sequential elements in a subaperture will contribute to the construction of the grating lobe at a single point in the field. Hence, the grating lobes will not see a zero-mean aperture. Similar grating lobe profiles will be produced in the second and third apodizations. Therefore, similar to the sidelobes, the grating lobes are canceled when subtracting the zero-mean apodization image from the dc offset images.
B. Filtered IC-NSI
In this work, we also propose a filtered incoherent NSI (IC-NSI) as an imaging scheme that is designed to maintain the advantages of both the higher lateral resolution of NSI and the better CNR of conventional methods for forming B-mode images. The IC-NSI process is illustrated in Fig. 1. The raw RF data are first beamformed with three NSI apodizations individually for each angle. Then, the beamformed RF data are filtered. An Equiripple finite impulse response (FIR) low pass filter (LPF) with a normalized passband frequency at 0.3, a stopband frequency at 0.8, and a stopband attenuation at 60 dB was generated by MATLAB Filter Designer and applied to the angular dimension of the beamformed data individually for each NSI apodization. The aim of the LPF is to filter out signals that vary rapidly over different steering angles, which improves the lateral resolution. After filtering, the envelopes were detected for each angle and each apodization. Then, the ZM image was subtracted from the sum of dc images individually for each angle. The resulting NSI images for each angle are summed together to generate the final image. In this way, the lateral resolution of NSI is maintained by the LPF, which filters out the signals that vary rapidly over different steering angles. At the same time, a smoother speckle pattern is preserved for incoherent compounding in comparison with coherently summed NSI (C-NSI) [4]. Application of the LPF to the C-NSI did not result in any appreciable differences.
Fig. 1.

Block diagram of IC-NSI. The raw channel RF data is first beamformed with three different apodizations for each angle. An LPF is applied to each apodization result in the angular dimension. The envelopes of the filtered ZM signal are subtracted from the sum of the filtered dc signal. Finally, incoherent compounding is performed on all the angular data to generate the final image.
III. Experimental Setup
A. Simulation
To evaluate the grating lobe reduction on imaging performance, we first simulated imaging of single targets using the Field-II [43], [44] simulator. In the simulation, we placed a single scatterer in the field of view. The simulation parameters were set according to an L14-5/38 array transducer (Ultrasonix, BC, Canada). This particular array was used in the animal studies because of its broad bandwidth (nominal 5–14 MHz) and was, therefore, used in simulations to match the physical experiments. The array had a nominal center frequency of 7.82 MHz (λ ≈ 197 μm), 128 elements, a pitch of 0.3048 mm, a total length of 38 mm, and an elevational focus of 16 mm. A fixed F-number of 1.5 was used in the beamforming process. The Field-II simulator represents an ideal case where all elements on the array are assumed to be perfectly matched in their element factors and sensitivities, and the SNR can be controlled. A wire target at a depth of 5 mm was imaged using PWI with a total of 33 plane-waves transmitted with steering angles spanning from −16° to +16° in 1° increments. With this pitch, grating lobes are predicted to occur at angles close to ±40°.
B. Physical Samples
In physical experiments, grating lobe reduction was assessed using NSI for different imaging tasks from three different types of samples: wire targets in water, a tissue-mimicking phantom, and rat tumors in vivo.
1). Wire Target Experiments:
In the wire target experiments, a 100-μm-diameter tungsten wire was placed in a tank of degassed water, which was then scanned by the linear array. A total of 33 plane-waves were transmitted with steering angles spanning from −16° to +16° in 1° increments. Received signals were recorded by the Verasonics system on all 128 channels. On receive, a fixed F-number subaperture was used to create each scan line using delay and sum (DAS). The usable subaperture size is limited by the total number of elements (channels) when the depth is too large. When the subaperture moved beyond the edges of the array, we padded zeros to span the expected aperture. Images were created using three approaches: simple DAS beamforming with a Hann apodization, the same but with the GCF applied and using NSI. For NSI, different values of the dc offset were chosen (0.1 and 1.0) to quantify grating lobe level reduction versus the spatial resolution gain provided by NSI, i.e., a smaller dc offset results in better lateral resolution and lower grating lobe levels. In addition, NSI was assessed when using either coherent summing of the plane-wave (C-NSI) or incoherent summation (IC-NSI). For GCF, the cutoff frequency M0 [31] was empirically chosen to be 2, because it provided the best CNR in the phantom contrast targets.
2). CIRS Phantom Experiments:
In the phantom experiments, we scanned the CIRS 040GSE phantom (Computerized Imaging Reference Systems, Norfolk, VA, USA). As before, 33 plane-waves were fired with steering angles equally spanning from −16° to +16°. Received signals were recorded by the Verasonics system on all 128 channels. Images were constructed using the same beamforming approaches that were performed in the wire target experiments.
3). In Vivo Experiments:
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana–Champaign. Tumors were induced in the mammary fat pad of the female F344 rats by injecting MAT tumor cells (5 × 102 cells in 100 μL) on each side of the abdomen. Once the tumors grew to 5–15 mm in diameter, the animals were anesthetized using isoflurane and imaged. The skin above a tumor was shaved, and tumors were coupled to the transducer array using ultrasound gel. Seven plane-waves were transmitted with steering angles equally spanning from −12° to +12°. All signals were received using the Verasonics system, and the same beamforming approaches used in the wire target experiments were also used in the tumor imaging.
C. Image Quality Metrics
To evaluate the grating lobe reduction performance, we compared the lateral profile of axially integrated power estimated from the wire targets using Hann apodization and NSI, because the energy in the grating lobes spreads axially. We also estimated the amplitude differences between the grating lobes and the main lobe for Hann apodization and NSI. CNR was measured for the CIRS phantom scan data. However, for the tumor images, the improvement in image quality was estimated by quantifying the reduction in the image intensity artifact due to grating lobes. Specifically, the intensity in regions observed to be artifacts from the grating lobes was compared between images created using the different beamforming approaches.
IV. Results
A. Simulation
Images from a single scatterer using Hann apodization, the three NSI’s apodization (dc offset of 1.0), and the combined NSI image based on single zero-degree plane-wave simulations are shown in Fig. 2. The grating lobes appear as smeared out intensity blobs between 5 and 7 mm axially and about ±4 mm laterally from the scatterer (≈40°). The Hann image shows that the grating lobes are symmetric about the scatterer. The dc offset images show grating lobes only on the left or right depending on which apodization is displayed. The zero-mean apodization is similar to the Hann apodization except for the null at broadside, i.e., zero degrees. Note that the grating lobes produced by the zero-mean apodization do not have a null at the center. In the NSI image, the grating lobes are subtracted out.
Fig. 2.

Top to bottom: single scatterer image using Hann apodization, using the zero-mean apodization, the zero mean plus dc offset of 1.0, the flipped version of the previous apodization, and the NSI image. Dynamic range is 60 dB for all five images.
The lateral profile of axially integrated power for the different approaches basing on a single zero-degree plane-wave simulation is shown in Fig. 3. The beamwidths from the NSI are narrower, as the dc offset is decreased. Sidelobe levels are also greatly reduced when using NSI compared with Hann apodization. Finally, grating lobe levels for the Hann apodization are at about −20 dB down from the main lobe. However, for NSI, the grating lobes are below −60 dB from the main lobe level.
Fig. 3.
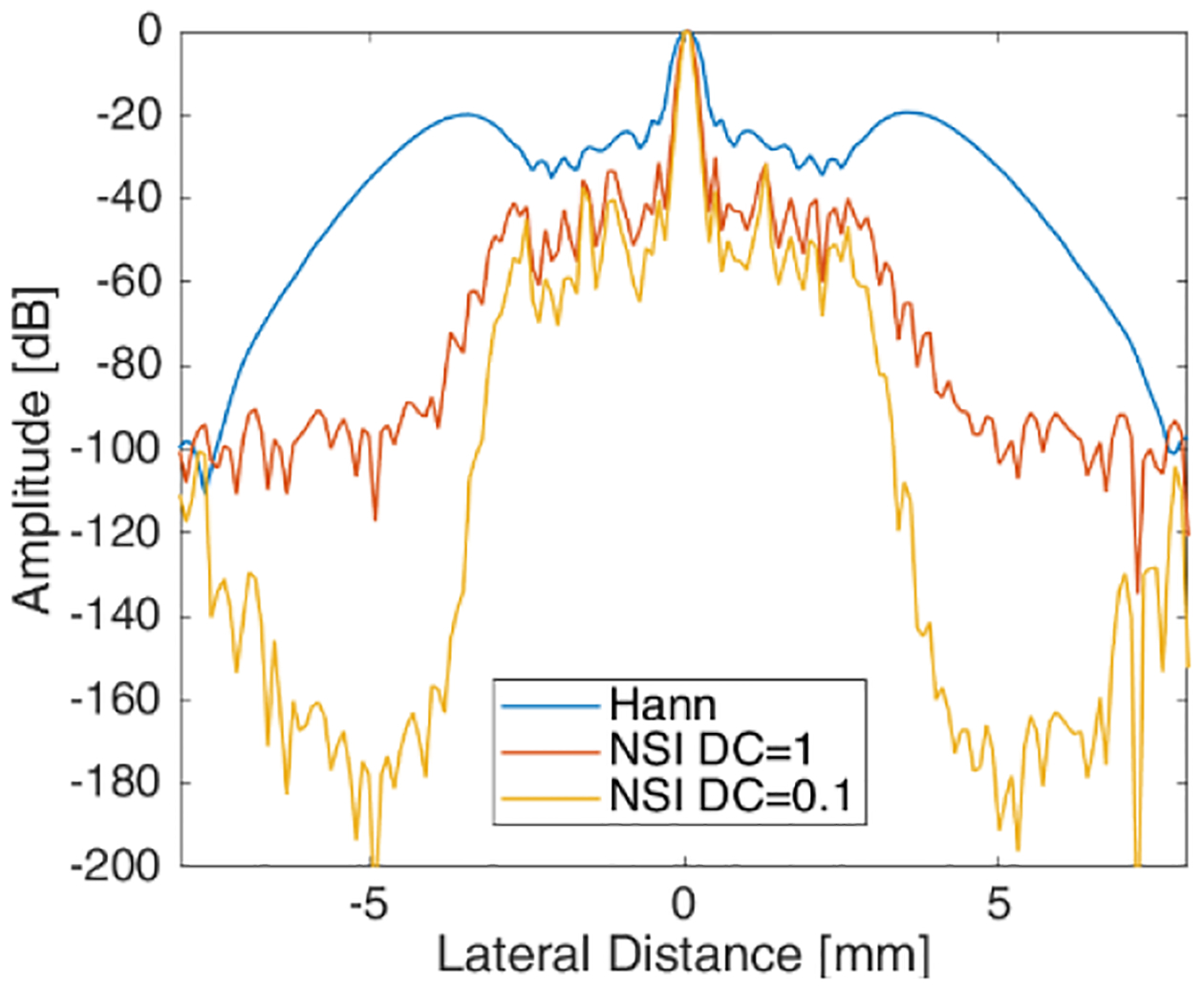
Lateral profile of axially integrated power from Hann and NSI with different dc offsets resulting based on Field-II single scatterer simulation.
Using the Field-II simulator and a single scatterer, the reduction in the grating lobes was quantified for different dc offset values. The reductions in grating lobe levels for C-NSI (coherently summed) and IC-NSI (incoherently summed) with different dc offset and different number of angles used for compounding are shown in Fig. 4 with respect to Hann apodization with the corresponding angular compounding setting. The grating lobe reduction was calculated by GLHann − GLNSI. As the dc offset decreased, the grating lobe reduction increased for NSI. Furthermore, the grating lobes were reduced more when using incoherent summation of plane-waves compared with coherent summation. The larger the number of angles used for PWI, the less the reduction in grating lobe level. These results represent a best-case scenario for high SNR. The SNR in physical experiments will be much lower than achieved in the ideal simulations. To test the effects of SNR on the grating lobe levels using NSI, noise was added to the simulation data. Fig. 5 shows the resulting grating lobe reduction with a dc offset at 1 when noise was added to the simulated raw RF channel data and the SNR calculated from the raw unbeamformed data. The beamforming process will increase the SNR compared with the unbeamformed data, and therefore, the grating lobe reduction is still higher than 0 dB in such cases. As the SNR increases, the grating lobe reduction performance matches the noise free simulation results in Fig. 4.
Fig. 4.

Grating lobe reduction of C-NSI and IC-NSI compared with Hann apodization estimated from axially integrated power of simulations with a single scatterer when using different numbers of angles for compounding.
Fig. 5.

Grating lobe reduction of NSI compared with Hann apodization estimated from axially integrated power of simulations with a single scatterer under different input raw RF channel data SNR.
B. Physical Experiments
The underwater wire target images for Hann, C-NSI, IC-NSI, and GCF are shown in Fig. 6. GCF is implemented with fixed M equal to 2 empirically. In this figure, the dc offset was set to 1.0, which produces only moderate lateral resolution improvements compared with Hann apodization. Lateral cross sections of the wire targets are shown in Fig. 7. The grating lobe reduction for C-NSI, IC-NSI, and GCF with respect to Hann apodization was 20.21, 23.55, and 11.94 dB. These results are comparable to results from the Verasonics simulation when noise was added.
Fig. 6.

Underwater wire target images. Top left: Hann. Bottom left: C-NSI. Top right: IC-NSI. Bottom right: GCF. The dc offset values were 1.0. Dynamic range is 60 dB for all four images.
Fig. 7.

Lateral profile of axially integrated power of underwater target images. The dc offset values were 1.0.
Wire targets imaged from the CIRS phantom are shown in Fig. 8. The wire targets have a speckle background, which makes it difficult to observe the grating lobe artifacts. GCF removed the speckle immediately surrounding the bright wire targets in the phantom, which results in dark spots surrounding the wire targets. This is an image artifact associated with GCF and may be undesirable for certain imaging tasks.
Fig. 8.

CIRS phantom wire target images. Top left: Hann. Bottom left: C-NSI. Top right: IC-NSI. Bottom right: GCF. The dc offset values were 1.0. Dynamic range is 60 dB for all four images.
Contrast targets from the CIRS phantom imaged using the different techniques are shown in Fig. 9 with the NSI using a dc offset of 1.0. For this specific imaging task, GCF provided the best visual visibility with the targets and the highest contrast. The CNR values for Hann apodization, C-NSI, IC-NSI, and GCF with different numbers of compounded angles measured from the marked region of CIRS phantom in Fig. 9 are shown in Fig. 10. Based on the metrics, IC-NSI provided better contrast than C-NSI, because the speckle for C-NSI has larger variance, i.e., lower speckle SNR.
Fig. 9.

CIRS phantom hyperechoic and anechoic cyst images. Top left: Hann. Bottom left: C-NSI. Top right: ICNSI. Bottom right: GCF. The dc offset values were 1.0. Dynamic range is 60 dB for all four images.
Fig. 10.
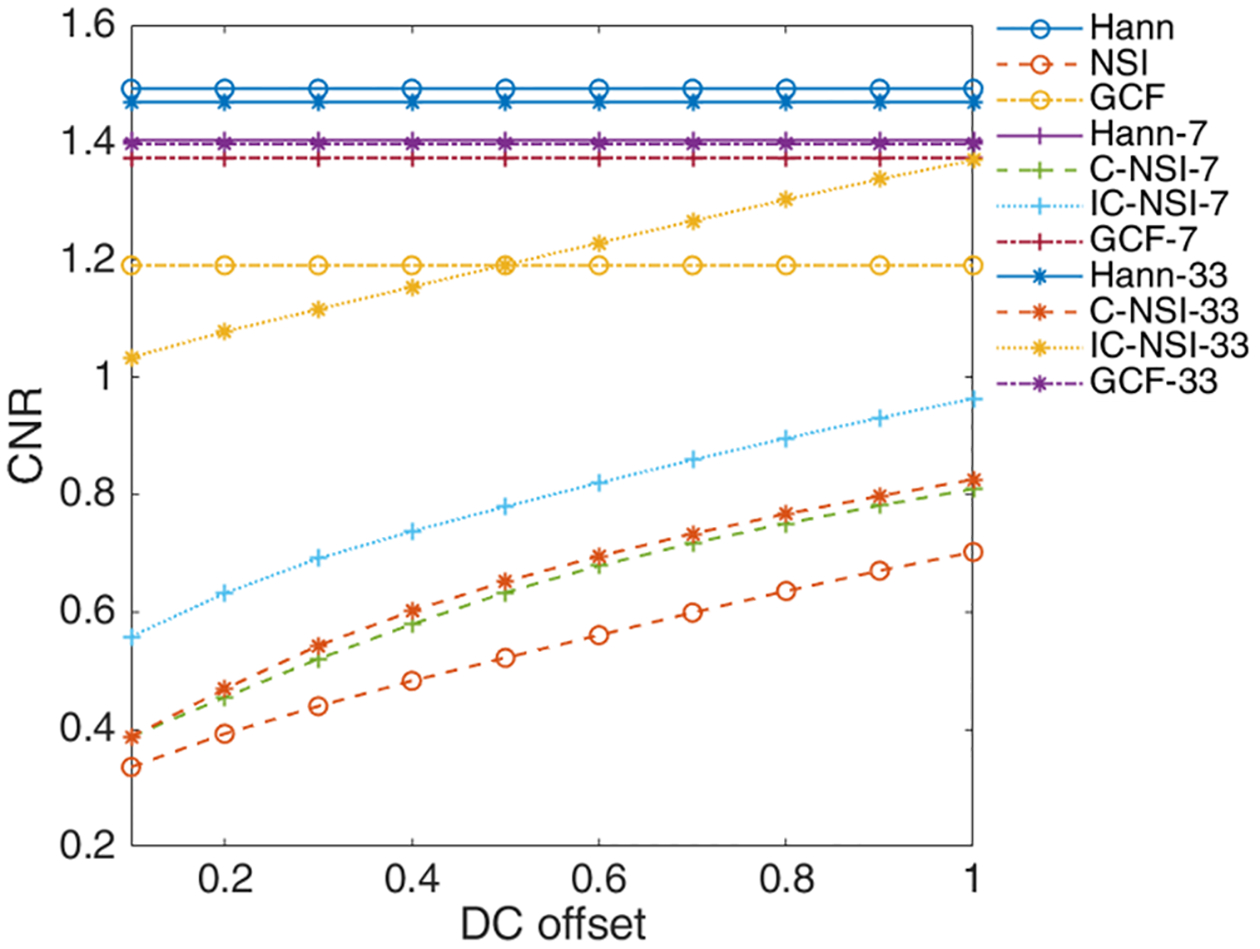
CNR of Hann, C-NSI, IC-NSI, and GCF with different numbers of angle compounding from CIRS phantom scan.
Tumor images from three separate rats are shown in Figs. 11–13 for the different imaging techniques. Arrows point out the suspected grating lobe artifacts in the tumors (see tumor images created using Hann apodization). Each suspected grating lobe arose from a bright interface scatterer and was estimated to be between 36° and 41° off from the scatterer, which was the predicted location of the grating lobes based on the center frequency and pitch of the array. This supports the presence of grating lobes in the tumor images and their mitigation.
Fig. 11.

Rat tumor 1 images. Top left: Hann. Bottom left: C-NSI. Top right: IC-NSI. Bottom right: GCF. A dc offset of 1.0 was used for these images. Dynamic range is 60 dB for all four images.
Fig. 13.

Rat tumor 3 images. Top left: Hann. Bottom left: C-NSI. Top right: IC-NSI. Bottom right: GCF. A dc offset of 1.0 was used for these images. Dynamic range is 60 dB for all four images.
In the first rat tumor image (Fig. 11), a small bubble is present on the tumor surface laterally at about +7 mm. A grating lobe artifact (marked with an arrow) is observed from the bright bubble in the Hann apodization image inside the tumor obscuring the tissue signal underneath. The NSI images appeared to remove the grating lobe artifact completely. Other grating lobe artifacts (marked with an arrow) are present in the Hann apodization image due to the bright layer at a depth of about 14 mm near the center laterally and from a bright interface located at about 11.5 mm axially and +10 mm laterally. The grating lobe artifacts associated with these bright interfaces are removed in the NSI images. The GCF image also appears to remove some of the grating lobe artifacts but is dark, and much of the tissue signal is lost degrading the image quality.
In the second rat tumor image (Fig. 12), there is a bright interface at about 7 mm axially and +7 mm laterally, which produces a grating lobe artifact on both sides of the interface in the Hann apodization image. The grating lobe artifact (marked with an arrow) is entirely removed in the NSI images. The GCF image does not entirely remove the grating lobes, and they appear on both sides of the bright interface. Furthermore, the darkening of the tissue signals around bright targets is observed in the GCF images reducing their image quality.
Fig. 12.

Rat tumor 2 images. Top left: Hann. Bottom left: C-NSI. Top right: IC-NSI. Bottom right: GCF. A dc offset of 1.0 was used for these images. Dynamic range is 60 dB for all four images.
The third rat tumor image (Fig. 13) shows two prevalent grating lobe artifacts from each side of the tumor generated from bright interfaces. One grating lobe artifact was generated from the interface located at 7-mm depth and −12 mm laterally. The other grating lobe artifact was generated from the interface at 11-mm depth and +7.5 mm laterally. As with the previous images, NSI appears to mitigate the grating lobe artifacts. The GCF image also eliminates the grating lobe artifact, but the image quality is degraded.
To quantify the reduction in the grating lobe levels inside the tumors, we averaged the power of the signal location at the location of the grating lobe artifacts in select regions for each tumor. The grating lobe levels were then reported in dB using the four beamforming schemes in rat tumors and are listed in Table I. For the first tumor image, the signal was averaged for the region marked with red rectangle in the image. For the second tumor image, the signal was averaged for the region marked with red rectangle in the image. For the third tumor image, the signal was averaged for the region marked with red rectangle in the image. From Table I, C-NSI resulted in a 15–18-dB grating lobe reduction, IC-NSI resulted in a 10–14-dB grating lobe reduction, and GCF provided 7–8-dB grating lobe reduction compared with Hann apodization.
TABLE I.
Integrated Signal Energy at the Grating Lobe Locations for Each Tumor Based on Each Beamforming Approach. All Values Are Reported in dB
| GCF | ||||
|---|---|---|---|---|
| Tumor 1 | −39.00 | 57.71 | −53.65 | −46.05 |
| Tumor 2 | −43.42 | −61.78 | −53.34 | −52.25 |
| Tumor 3 | −46.37 | −61.77 | −57.68 | −53.41 |
V. Discussion
In this study, NSI was quantified for its ability to reduce or mitigate grating lobe artifacts for ultrasonic imaging tasks. Reducing grating lobes has increased in its importance with the advent of PWI on clinically available linear arrays that have pitches that are equal to or greater than a wavelength. Simulations and physical experiments on wire targets both indicated that the NSI approach can reduce the presence of sidelobes and grating lobes. Previous studies have also revealed that NSI can reduce sidelobes [4], but this study provided evidence that NSI also reduced or eliminated grating lobes and their resulting artifacts from images.
Simulations on point scatterers indicated that NSI could reduce grating lobes by 60–100 dB, and this reduction increased, as the dc offset decreased. In NSI, the dc offset provides a trade-off between lateral resolution and sidelobe/grating lobe levels and the CNR of an image. Decreasing the dc offset results in better matching of sidelobes and grating lobes between the zero-mean apodization image and the dc offset images. In addition, a lower dc offset results in a narrower NSI main beam. For an imaging task to resolve singular targets, small dc offset values are appropriate (dc ≤0.1). But, for imaging tasks where contrast depends on low variance in the speckle within both a target and background, a low dc offset results in a high variance in the speckle leading to poor speckle SNR and CNR. Therefore, a low dc offset with NSI may not be a good option for observing contrast in ultrasound images. However, the use of a larger dc offset, i.e., a dc offset of 1.0, produces speckle that is more in line with DAS and Hann apodization while still reducing sidelobes and grating lobes. Therefore, NSI imaging of tissue-mimicking phantoms and rat tumors were reported for the dc offsets of 1.0 only. Images of contrast targets using NSI with small dc offsets can be found in [4].
Imaging of physical wire targets in water, i.e., a speckle-free background, revealed higher grating lobe levels using NSI than was predicted by noise-free simulations produced with the Field II. Tests of the simulations after adding noise, i.e., to more closely match the physical measurements, resulted in an increase in estimated grating lobe levels. As the SNR decreased, grating lobes levels increased, because the grating lobes were not perfectly subtracted using NSI.
For the CIRS phantom images, GCF provided the best images in terms of contrast. Grating lobe artifacts were not observed in the CIRS phantom images for any of the approaches. Therefore, in a highly idealized imaging task, such as imaging a test phantom, GCF produced superior results compared with NSI or Hann apodization.
However, while GCF may result in more reduction in sidelobes and grating lobes compared with NSI, there are other trade-offs associated with GCF. For example, GCF produced shading artifacts surrounding bright scatterers in the phantom and in vivo images. Much of the internal structure of the rat tumors was not visible in the GCF tumor images, which created large anechoic-like regions. With additional tuning of the GCF, it is possible that the rat images could improve, but the NSI does not require tuning. The speckle of the IC-NSI in the in vivo rat tumor images was closest to the speckle profile of the Hann apodization images. Furthermore, the computational load for producing GCF images is much higher compared with NSI. Therefore, these results suggest that NSI was superior at the task of imaging these rat tumors.
In comparing C-NSI and IC-NSI, two observations stand out. First, IC-NSI provided better reduction in grating lobe levels compared with C-NSI. However, these differences are hard to observe in the in vivo rat tumor images for C-NSI and IC-NSI. Second, higher CNR values were obtained when using IC-NSI compared with C-NSI. Coherent compounding provides better lateral resolution compared with incoherent compounding; however, incoherent compounding has been used for decades to smooth speckle and increase CNR [45]. This reduction in CNR is related to the decreased speckle SNR produced by the nonlinear processing used in NSI. The speckle SNR was calculated for a homogeneous region in the CIRS phantom for the Hann, C-NSI, and IC-NSI. The speckle SNR for the Hann images, C-NSI, and IC-NSI was 1.69, 0.97, and 1.42, respectively. This indicates that C-NSI was far from producing fully developed speckle (value of 1.91). With the large reduction in grating lobe levels afforded by NSI using either C-NSI or IC-NSI, the superior CNR provided by IC-NSI suggests that IC-NSI is the better choice for general ultrasonic imaging tasks compared with C-NSI when using PWI with multiple transmission angles. However, there are more difficult imaging situations, such as multipath, reverberation, and large sound speed inhomogeneities in real-world imaging tasks where the performance of NSI should be evaluated.
VI. Conclusion
In this work, the NSI beamforming technique was evaluated for reducing the grating lobes associated with PWI using clinically available clinical linear arrays. The presence of grating lobes produces increased clutter in ultrasonic images, which, in turn, reduces image quality. Furthermore, clutter may obscure important information in images. Two NSI approaches, C-NSI and IC-NSI, were evaluated for specific imaging tasks. For general imaging in vivo, the results suggest that IC-NSI (dc offset of 1.0) provided the best performance in terms of spatial resolution, contrast, and clutter reduction from grating lobes.
Acknowledgment
The authors would like to thank Kendall Junger, Divya Nambiar, and Erin Kinaci for the assistance with cell culture.
This work was supported by the National Institutes of Health under Grant R21EB024133 and Grant R01CA251939.
Footnotes
This work involved human subjects or animals in its research. Approval of all ethical and experimental procedures and protocols was granted by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana–Champaign.
References
- [1].Chiao RY and Hao X, “Coded excitation for diagnostic ultrasound: A system developer’s perspective,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 52, no. 2, pp. 160–170, Feb. 2005. [DOI] [PubMed] [Google Scholar]
- [2].Pedersen MH, Misaridis TX, and Jensen JA, “Clinical evaluation of chirp-coded excitation in medical ultrasound,” Ultrasound Med. Biol, vol. 29, no. 6, pp. 895–905, 2003. [DOI] [PubMed] [Google Scholar]
- [3].Adam D, Beilin-Nissan S, Friedman Z, and Behar V, “The combined effect of spatial compounding and nonlinear filtering on the speckle reduction in ultrasound images,” Ultrasonics, vol. 44, no. 2, pp. 166–181, Feb. 2006. [DOI] [PubMed] [Google Scholar]
- [4].Agarwal A, Reeg J, Podkowa AS, and Oelze ML, “Improving spatial resolution using incoherent subtraction of receive beams having different apodizations,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 66, no. 1, pp. 5–17, Jan. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oelze ML, “Bandwidth and resolution enhancement through pulse compression,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 54, no. 4, pp. 768–781, Apr. 2007. [DOI] [PubMed] [Google Scholar]
- [6].Hasegawa H, “Improvement of range spatial resolution of medical ultrasound imaging by element-domain signal processing,” Jpn. J. Appl. Phys, vol. 56, no. 7S1, Jul. 2017, Art. no. 07JF02. [Google Scholar]
- [7].Lu J-Y and Greenleaf JF, “Pulse-echo imaging using a nondiffracting beam transducer,” Ultrasound Med. Biol, vol. 17, no. 3, pp. 265–281, 1991. [DOI] [PubMed] [Google Scholar]
- [8].Lu J-Y and Greenleaf JF, “Ultrasonic nondiffracting transducer for medical imaging,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 37, no. 5, pp. 438–447, Sep. 1990. [DOI] [PubMed] [Google Scholar]
- [9].Lu J-Y and Greenleaf JF, “Experimental verification of nondiffracting X waves,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 39, no. 3, pp. 441–446, May 1992. [DOI] [PubMed] [Google Scholar]
- [10].Lu J-Y, “2-D and 3-D high frame rate imaging with limited diffraction beams,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 44, no. 4, pp. 839–856, Jul. 1997. [DOI] [PubMed] [Google Scholar]
- [11].Lu J-Y, “Experimental study of high frame rate imaging with limited diffraction beams,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 45, no. 1, pp. 84–97, Jan. 1998. [DOI] [PubMed] [Google Scholar]
- [12].Cheng J and Lu J-Y, “Extended high-frame rate imaging method with limited-diffraction beams,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 53, no. 5, pp. 880–899, May 2006. [DOI] [PubMed] [Google Scholar]
- [13].Montaldo G, Tanter M, Bercoff J, Benech N, and Fink M, “Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 56, no. 3, pp. 489–506, Mar. 2009. [DOI] [PubMed] [Google Scholar]
- [14].Tanter M and Fink M, “Ultrafast imaging in biomedical ultrasound,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 61, no. 1, pp. 102–119, Jan. 2014. [DOI] [PubMed] [Google Scholar]
- [15].Villemain O et al. , “Ultrafast ultrasound imaging in pediatric and adult cardiology: Techniques, applications, and perspectives,” JACC, Cardiovascular Imag, vol. 13, pp. 1771–1791, Aug. 2020. [DOI] [PubMed] [Google Scholar]
- [16].Urs R, Silverman RH, and Ketterling JA, “Ultrafast ultrasound imaging of ocular anatomy and blood flow,” Invest. Ophthalmol. Vis. Sci, vol. 57, no. 8, pp. 3810–3816, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cikes M, Tong L, Sutherland GR, and D’Hooge J, “Ultrafast cardiac ultrasound imaging: Technical principles, applications, and clinical benefits,” JACC Cardiovascular Imag, vol. 7, no. 8, pp. 812–823, Aug. 2014. [DOI] [PubMed] [Google Scholar]
- [18].Maresca D et al. , “Noninvasive imaging of the coronary vasculature using ultrafast ultrasound,” JACC, Cardiovascular Imag, vol. 11, pp. 798–808, Jun. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Demené C et al. , “Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity,” IEEE Trans. Med. Imag, vol. 34, no. 11, pp. 2271–2285, Apr. 2015. [DOI] [PubMed] [Google Scholar]
- [20].Lowerison MR, Huang C, Lucien F, Chen S, and Song P, “Ultrasound localization microscopy of renal tumor xenografts in chicken embryo is correlated to hypoxia,” Sci. Rep, vol. 10, no. 1, pp. 1–13, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dencks S et al. , “Clinical pilot application of super-resolution U.S. imaging in breast cancer,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 66, no. 3, pp. 517–526, Sep. 2018. [DOI] [PubMed] [Google Scholar]
- [22].Errico C et al. , “Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging,” Nature, vol. 527, no. 7579, pp. 499–502, 2015. [DOI] [PubMed] [Google Scholar]
- [23].Demené C et al. , “Transcranial ultrafast ultrasound localization microscopy of brain vasculature in patients,” Nature Biomed. Eng, vol. 5, no. 3, pp. 219–228, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Udesen J, Gran F, Hansen KL, Jensen JA, Thomsen C, and Nielsen MB, “High frame-rate blood vector velocity imaging using plane waves: Simulations and preliminary experiments,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 55, no. 8, pp. 1729–1743, Aug. 2008, doi: 10.1109/TUFFC.2008.858. [DOI] [PubMed] [Google Scholar]
- [25].Bae S and Song T-K, “Methods for grating lobe suppression in ultrasound plane wave imaging,” Appl. Sci, vol. 8, no. 10, p. 1881, 2018. [Google Scholar]
- [26].Heller M and Schmitz G, “Reducing grating lobe artifacts by exploiting lateral transducer motion,” in Proc. IEEE Int. Ultrason. Symp. (IUS), Sep. 2020, pp. 1–4. [Google Scholar]
- [27].Harris FJ, “On the use of windows for harmonic analysis with the discrete Fourier transform,” Proc. IEEE, vol. 66, no. 1, pp. 51–83, Jan. 1978. [Google Scholar]
- [28].‘t Hoen PJ, “Aperture apodization to reduce the off-axis intensity of the pulsed-mode directivity function of linear arrays,” Ultrasonics, vol. 20, no. 5, pp. 231–236, Sep. 1982. [Google Scholar]
- [29].Mallart R and Fink M, “Adaptive focusing in scattering media through sound-speed inhomogeneities: The van Cittert Zernike approach and focusing criterion,” J. Acoust. Soc. Amer, vol. 96, no. 6, pp. 3721–3732, Dec. 1994. [Google Scholar]
- [30].Hollman KW, Rigby KW, and O’Donnell M, “Coherence factor of speckle from a multi-row probe,” in Proc. IEEE Ultrason. Symp, vol. 2, Oct. 1999, pp. 1257–1260. [Google Scholar]
- [31].Li P-C and Li M-L, “Adaptive imaging using the generalized coherence factor,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 50, no. 2, pp. 128–141, Feb. 2003. [DOI] [PubMed] [Google Scholar]
- [32].Camacho J, Parrilla M, and Fritsch C, “Phase coherence imaging,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 56, no. 5, pp. 958–974, May 2009. [DOI] [PubMed] [Google Scholar]
- [33].Jeong MK and Kwon SJ, “Estimation of side lobes in ultrasound imaging systems,” Biomed. Eng. Lett, vol. 5, no. 3, pp. 229–239, Sep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Synnevag J, Austeng A, and Holm S, “Adaptive beamforming applied to medical ultrasound imaging,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 54, no. 8, pp. 1606–1613, Aug. 2007. [PubMed] [Google Scholar]
- [35].Asl BM and Mahloojifar A, “Minimum variance beamforming combined with adaptive coherence weighting applied to medical ultrasound imaging,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 56, no. 9, pp. 1923–1931, Sep. 2009. [DOI] [PubMed] [Google Scholar]
- [36].Holfort IK, Gran F, and Jensen JA, “Broadband minimum variance beamforming for ultrasound imaging,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 56, no. 2, pp. 314–325, Feb. 2009. [DOI] [PubMed] [Google Scholar]
- [37].Seo C and Yen J, “Sidelobe suppression in ultrasound imaging using dual apodization with cross-correlation,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 55, no. 10, pp. 2198–2210, Oct. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guenther D and Walker W, “Optimal apodization design for medical ultrasound using constrained least squares Part I: Theory,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 54, no. 2, pp. 332–342, Feb. 2007. [DOI] [PubMed] [Google Scholar]
- [39].Guenther D and Walker W, “Optimal apodization design for medical ultrasound using constrained least squares Part II simulation results,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 54, no. 2, pp. 343–358, Feb. 2007. [DOI] [PubMed] [Google Scholar]
- [40].Byram B, Dei K, Tierney J, and Dumont D, “A model and regularization scheme for ultrasonic beamforming clutter reduction,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 62, no. 11, pp. 1913–1927, Nov. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dei K and Byram B, “The impact of model-based clutter suppression on cluttered, aberrated wavefronts,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 64, no. 10, pp. 1450–1464, Oct. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Byram B, Shu J, and Dei K, “Nonlinear beamforming of aperture domain signals,” in Proc. Int. Ultrason. Symp. (IUS), Oct. 2015, pp. 1–6. [Google Scholar]
- [43].Jensen JA, “Field: A program for simulating ultrasound systems,” in Proc. 10th Nordic-Baltic Conf. Biomed. Imag. Published Med. Biol. Eng. Comput, vol. 34, 1996, pp. 351–353. [Google Scholar]
- [44].Jensen JA and Svendsen NB, “Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers,” IEEE Trans. Ultrason., Ferroelectr., Freq. Control, vol. 39, no. 2, pp. 262–267, Mar. 1992. [DOI] [PubMed] [Google Scholar]
- [45].Burckhardt CB, “Speckle in ultrasound B-mode scans,” IEEE Trans. Sonics Ultrason, vol. SU-25, no. 1, pp. 1–6, Jan. 1978, doi: 10.1109/T-SU.1978.30978. [DOI] [Google Scholar]


