Abstract
Introduction:
Infants born to women living with HIV initiating combination antiretroviral therapy (cART) late in pregnancy are at high risk of intrapartum infection. Mother/infant perinatal antiretroviral intensification may substantially reduce this risk.
Methods:
In this single-arm Bayesian trial, pregnant women with HIV receiving standard of care antiretroviral prophylaxis in Thailand (maternal antenatal lopinavir-based cART; nonbreastfed infants 4 weeks’ postnatal zidovudine) were offered “antiretroviral intensification” (labor single-dose nevirapine plus infant zidovudine-lamivudine-nevirapine for 2 weeks followed by zidovudine-lamivudine for 2 weeks) if their antenatal cART was initiated ≤8 weeks before delivery. A negative birth HIV-DNA polymerase chain reaction (PCR) followed by a confirmed positive PCR defined intrapartum transmission. Before study initiation, we modeled intrapartum transmission probabilities using data from 3738 mother/infant pairs enrolled in our previous trials in Thailand using a logistic model, with perinatal maternal/infant antiretroviral regimen and predicted viral load at delivery as main covariates. Using the characteristics of the women enrolled who received intensification, prior intrapartum transmission probabilities (credibility intervals) with/without intensification were estimated. After including the transmission data observed in the current study, the corresponding Bayesian posterior transmission probability was derived.
Results:
No intrapartum transmission of HIV was observed among the 88 mother/infant pairs receiving intensification. The estimated intrapartum transmission probability was 22% (95% credibility interval 0.5–6.1) without intensification versus 0.3% (0.0–1.6) with intensification. The probability of superiority of intensification over standard of care was 94.4%. Antiretroviral intensification appeared safe.
Conclusion:
Mother/infant antiretroviral intensification was effective in preventing intrapartum transmission of HIV in pregnant women receiving ≤8 weeks antepartum cART.
Keywords: HIV, prevention of mother-to-child transmission, Bayesian design, antiretroviral therapy, clinical trial, historical control, meta-analysis, Thailand
INTRODUCTION
Perinatal transmission of HIV is dramatically reduced with antiretroviral use during pregnancy, at delivery and the postnatal period.1 Since 2013, World Health Organization (WHO) guidelines recommend lifelong combination antiretroviral therapy (cART) for all pregnant and breastfeeding women living with HIV regardless of CD4 count or WHO clinical stage.2
Despite worldwide efforts to expand access to early antenatal HIV care, some women are diagnosed late or initiate cART late in pregnancy and deliver after no or only a few weeks of cART. In such situations, their infants are at high risk of perinatal HIV infection.3–5
The original PHPT-5 trial comparing 3 maternal and infant prophylactic regimens was stopped early due to the adoption of cART prophylaxis for all HIV pregnant women in the Thai guidelines6; however, a major risk factor associated with transmission, regardless of randomized regimen, was a duration of ART less than 8 weeks before delivery.4 In this trial, among women randomized to lopinavir-based ART, those who initiated prophylaxis late in pregnancy had unsuppressed RNA viral load at the time of delivery, thereby increasing their risk of intrapartum transmission. Because maternal single-dose nevirapine during labor alone7 or infant postnatal prophylaxis4,8 significantly decreases intrapartum transmission, we hypothesized that in such a situation, nevirapine-based antiretroviral intensification both during labor in women and immediately after birth in their child would reduce intrapartum transmission.
To demonstrate the efficacy of antiretroviral intensification in high-risk pregnant women, a head-to-head comparison trial with/without intensification would have been ethically questionable and required a very large sample size, making this study unfeasible in the Thai context where most pregnant women present early for antenatal care, are systematically tested for HIV and, if found HIV-positive, initiate cART immediately. Considering the substantial historical data available through our previous prevention of mother-to-child HIV transmission (PMTCT) trials,4,9,10 we designed a single-arm Bayesian clinical trial to evaluate the efficacy of perinatal antiretroviral intensification in protecting infants at high risk of acquiring HIV at the time of delivery.
METHODS
Study Design and Setting
We performed an adaptive, single-arm, multicenter, phase III, clinical trial with a Bayesian design to evaluate the efficacy of ART intensification in reducing the risk of HIV intrapartum transmission in women who initiated antepartum cART ≤8 weeks before delivery (NCT01511237; PHPT-5 second phase).
Participants
Pregnant women with confirmed HIV infection participating in the Thai national PMTCT program could enroll if they agreed not to breastfeed per Thai guidelines, were ≥18 years of age, intended to receive care at 1 of 41 study sites, and provided written informed consent.
All women and their infants received the standard of care for PMTCT at the time,6 that is, maternal cART during pregnancy regardless of CD4 count: zidovudine (300 mg), lamivudine (150 mg) plus lopinavir/ritonavir (400/100 mg) twice a day, followed by zidovudine (300 mg) every 3 hours during labor; newborn zidovudine (4 mg/kg) twice a day for 4 weeks. In addition, women who initiated cART ≤8 weeks before delivery, received, together with their infants, “antiretroviral intensification”: single-dose nevirapine (200 mg tablet) at onset of labor and their newborn nevirapine syrup (2 mg/kg once a day for the first week of life, then 4 mg/kg once a day for the next week) plus lamivudine syrup (2 mg/kg twice a day for 1 month), in addition to standard zidovudine syrup (intensification group).
At the time of the study, Thai 2010 guidelines did not recommend continuing ART for life for nonimmunocompromised women.6 Thus, women who had received single-dose nevirapine continued cART for at least 1 month after delivery to prevent selection of non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance mutations.11,12
Women who had received cART for >8 weeks before delivery were not given ART intensification and were followed concurrently with their infants to provide comparative safety data (observational group).
Follow-up
Women had an obstetrical, hematologic, and biochemical evaluation at enrollment. HIV-RNA VL (Abbott m2000 RealTime© HIV-1 assay; Abbott Molecular Inc., Des Plaines, IL; limit of quantification 40 copies/mL) and CD4 cell count were measured at baseline and delivery.
Infants were examined at birth and 2 weeks, 1, 2, 4, and 6 months of life. The child’s interval history was recorded, a physical examination performed, and blood obtained for HIV-DNA testing by real-time polymerase chain reaction (PCR) assay on peripheral blood spotted onto filter papers, dried, and stored at −20°C.13 Hematology and chemistry tests were performed soon after birth, at 2 and 4 weeks.
Primary Endpoint
Infants were confirmed HIV-infected if blood obtained on 2 separate occasions tested positive for HIV-DNA by PCR, unconfirmed infected if only one sample was tested positive. They were confirmed uninfected if samples tested negative twice including at least once after 2 months of age; otherwise, they were unconfirmed negative. Infants with a negative birth test with no further confirmation were to be excluded from the analysis. Infants with confirmed-HIV infection were considered infected intrapartum if their sample obtained within 3 days of birth was negative.14 To take into account a possible delay in detecting HIV infection in infants exposed to postnatal antiretroviral intensification,15,16 the last HIV-PCR testing occurred 5 months after antiretroviral discontinuation thus ruling out misdiagnosis due to viral suppression.
Safety and Adherence
Adverse events were graded using the Division of AIDS, NIAID Table.17 Women’s adherence to cART was evaluated by pill count at each visit while their single-dose nevirapine intake was directly observed. Newborn study drugs intake was directly observed at the hospital, and after discharge, adherence was assessed by evaluating the remaining drug syrups at each study visit.
Bayesian Modeling and Statistical Methods
We used historical data from 3738 mother/infant pairs enrolled in 3 previous randomized controlled trials (PHPT-19, PHPT-210, and PHPT-54) performed by our group in the same Thai setting, to build a predictive transmission model and derive prior probability distributions of intrapartum transmission with/without antiretroviral intensification.18,19 The characteristics of these historical mother/infant pairs are shown in Table 1, Supplemental Digital Content, http://links.lww.com/ QAI/B451, and a summary of the study designs is below:
TABLE 1.
Sample Sizes and Corresponding Decision Rules Depending on the Number of Intrapartum Transmissions Observed at the Time of Interim Analyses
| No. of Intrapartum Transmissions Observed | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Interim Analyses | Sample Size | N = 0 | N = 1 | N = 2 | N = 3 | N = 4 | N >4 |
|
| |||||||
| 1st | 58 | Continue | Continue | Stop for futility | Stop for futility | Stop for futility | Stop for futility |
| 2nd | 118 | Stop for efficacy | Continue | Continue | Stop for futility | Stop for futility | Stop for futility |
| 3rd | 275 | — | Stop for efficacy | Continue | Continue | Stop for futility | Stop for futility |
| Final | 410 | — | — | Final success | Final success | Final success | |
Stopping criteria are met if there is either 80%-probability of futility (italic cells) or 95%-probability of efficacy (bold cells). Interim time-points were determined using Monte Carlo simulations, under a Beta-Binomial model with assumption based on the predictive probabilities to achieve either futility or efficacy at interim analyses.21 With a sample size of 410 mother–infant pairs receiving antiretroviral intensification, there was a 82% probability of obtaining the study results earlier, at one of the interim looks at N = 58, N = 118, or N = 275.
PHPT-1 (NCT00386230, 1996–2000) compared the efficacy of zidovudine starting at 28 weeks’ gestation plus 6 weeks’ zidovudine in infants (“long-long”) versus zidovudine starting at 35 weeks’ gestation, with 3 days in infants (“short-short”) and long-short and short-long regimens.9
PHPT-2 (NCT00398684, 2000–2004) compared the efficacy of single-dose nevirapine in mothers during labor and in neonates or in mothers only, in addition to zidovudine starting at 28 weeks’ gestation and at least 1 week in children.10 Women enrolled in a PHPT-2 pharmacokinetic substudy of nevirapine as well as in an open-label study for those presenting too late to be randomized in the main trial were also included.20
PHPT-5 (NCT00409591, 2008–2010) compared 3 antiretroviral (ARV) prophylaxis regimens initiated at 28 weeks’ gestation (1) maternal zidovudine monotherapy plus single-dose nevirapine at onset of labor and 2 infant nevirapine doses (at birth and 48 hours of life), (2) maternal zidovudine monotherapy and 2 infant nevirapine doses, and (3) maternal 2-drugs zidovudine plus lopinavir/ritonavir therapy, with no maternal or infant nevirapine.4
Briefly, we developed a dose–effect model using VL measurements during pregnancy and at delivery in these historical trials to predict the VL level at delivery (VLd) depending on the antiretroviral regimen used and its duration until delivery.18 The VL model accounted for all the ART regimens (type and duration) as well as subject specific risk factors known at the time, for example, CD4 at baseline, VL at baseline and throughout pregnancy, gestational age (GA) at ARV initiation (not significant). Using the predicted VLd, we built a logistic regression model with random effects to estimate probabilities of intrapartum transmission, with and without antiretroviral intensification. Within this model, maternal/infant perinatal nevirapine was used as a proxy for antiretroviral intensification. Covariates retained in the model included delivery CD4 count and premature labor (GA <37 weeks).19
Three interim analyses were planned with stopping rules for futility or efficacy defined according to the number of transmissions observed (Table 1).
Efficacy and Safety Analyses
After completion of this trial, we updated the dose–effect VLd and intrapartum transmission models by including the data obtained from the observational group where pregnant women had received cART >8 weeks as per standard of care.
Then, using the specific characteristics of the women in the intensification group, we computed the prior probabilities of intrapartum transmission with and without intensification. Accounting for the intrapartum transmissions actually observed in the intensification group, we computed the Bayesian posterior distribution of the risk of intrapartum transmission21 and calculated the probability of superiority of intensification over standard of care for the prevention of intrapartum transmission, as well as that of a 2-fold reduction of intrapartum transmission attributable to intensification.
Although the prepartum maternal data from the observational group were critical to update the VLd model with use of cART, data from this group were also used to assess the safety of intensification in mothers and infants. Safety events were described and comparison between the proportion of safety events in the intensification and observational groups performed using the Fisher exact test.
Ethics
The ethics committees of the Thai Ministry of Public Health, the Faculty of Associated Medical Sciences of Chiang Mai University, the Harvard T.H. Chan School of Public Health, and local hospitals approved the protocol. All study sites complied with research regulations of the US Department of Health and Human Services.
RESULTS
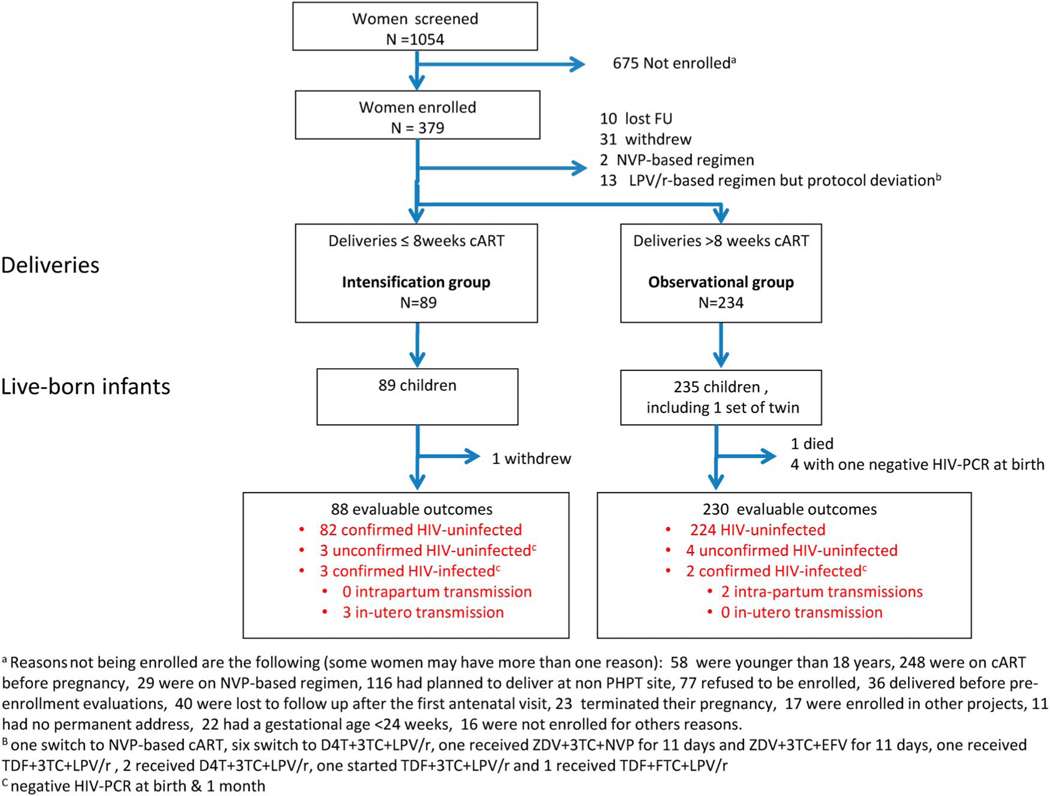
A total of 1054 pregnant women living with HIV were screened for eligibility and 379 enrolled from November 2011 to May 2014 (Fig. 1). At its second meeting, the Data Safety and Monitoring Board recommended to stop enrollment and proceed to the final analysis before reaching the second interim analysis time-point as enrollment was slower than expected due to the success of the Thai PMTCT program and primary results were urgently needed given their public health relevance.
FIGURE 1.
Population disposition for the efficacy analysis.
Among the 379 women enrolled, 10 were lost to follow-up, 31 withdrew before delivery, and 15 were excluded because they received nonprotocol antiretroviral regimens during pregnancy. Of the remaining 323 women, 89 had initiated cART ≤8 weeks before delivery (intensification group) while 234 had initiated cART >8 weeks before delivery (observational group). These women gave birth to 89 and 235 liveborn infants, respectively. Transmission outcomes were evaluable for 88 women in the intensification group and 230 in the observational group. Enrollment, loss to follow-up, pregnancy outcomes, and available endpoints are summarized in Figure 1.
Characteristics of the Women, Deliveries, and Infants
Table 2 summarizes the characteristics of the mothers and infants in the intensification and observational groups. At cART initiation, median (interquartile range) GA was 34$0 weeks (32.4–36.3) and 19.0 weeks (15.1–24.0) in the intensification and observational groups, respectively, with similar median VL in the 2 groups 4.3 log10 copies/mL (3.7–4.7). Enrollment median age was 26 years (22–33) and 28 years (23–32) in the intensification and observational groups, respectively, with median CD4 cell counts of 372 cells/mm3 (256–500) and 360 cells/mm3 (250–485), respectively. At delivery, median GA was 38.6 weeks in both groups. Duration of cART was shorter in the intensification compared with the observational group [4.2 weeks (2.6–6.3) versus 19.4 weeks (14.1–23.1), P < 0.001], and median VLd was higher in the intensification group than in the observational group [2.3 log10 copies/mL (1.8–2.9) versus 1.3 (1.3–1.7), P < 0.001]. The percentages of women undergoing caesarean section were similar, 36% and 42% in the intensification and observational groups, respectively. Median birth weight was similar in the 2 groups (2.8 Kg).
TABLE 2.
Characteristics of Mothers and Infants Enrolled in the Intensification and Observational Groups*
| Characteristics of Women | Intensification Group, N = 88 | Observational Group, N = 229 | P † |
|---|---|---|---|
|
| |||
| At cART initiation | |||
| Median gestational age (IQR) (wk) | 34.0 (32.4–36.4) | 19.0 (15.1–24.0) | <0.001 |
| Median VL (IQR) (log10 copies/mL)‡ | 4.3 (3.7–4.7) | 4.3 (3.7–4.7) | 0.947 |
| At enrolment | |||
| Median age (IQR) (yr) | 26 (22–33) | 28 (23–32) | 0.988 |
| Median VL (IQR) (log10 copies/mL) | 4.0 (3.2–4.6) | 3.7 (2.4–4.4) | 0.006 |
| Median CD4 (IQR) (cells/mm3) | 372 (256–500) | 360 (250–485) | 0.914 |
| At delivery | |||
| Median gestational age (IQR) (wk) | 38.6 (38.0–39.3) | 38.6 (37.6–39.4) | 0.531 |
| Median cART duration (IQR) (wk) | 4.2 (2.6–6.3) | 19.4 (14.1–23.1) | <0.001 |
| Median VLd (IQR) (log10 copies/mL) | 2.3 (1.8–2.9) | 1.3 (1.3–1.7) | <0.001 |
| VLd <50 copies/mL, n (%) | 17 (19%) | 165 (72%) | <0.001 |
| Median CD4 (IQR) (cells/mm3) | 431 (332–623) | 520 (349–652) | 0.247 |
| C/section, n (%) | 32 (36%) | 97 (42%) | 0.372 |
|
| |||
| Characteristics of Neonates | Intensification Group, N = 88 | Observational Group, N = 230§ | P† |
|
| |||
| Median birth weight (IQR) (Kg) | 2.8 (2.6–3.1) | 2.8 (2.5–3.1) | 0.128 |
| Intrapartum transmissions, n (%) | 0 (0%) | 2 (0.87%) | 0.521 |
The total number shown for each group is the number of mother–infant pairs included in the analysis. Only women whose infant had an evaluable outcome were included.
Comparison between intensification and observational groups. The Fisher exact test was used to compare proportions, and the Wilcoxon rank-sum test to compare distributions of continuous data.
Women could enroll in the study after cART initiation. VL before cART was available for 67 women in the intervention group and 143 in the observational group.
One woman had twins.
Study Drug Administration and Adherence
During pregnancy, among the 89 women who had received ≤8 weeks ART at delivery, 87 (99%) received zidovudine, lamivudine plus lopinavir/ritonavir as per the Thai recommendations, and 2 women had no antepartum cART. Adherence to antenatal cART was >90% in 92% of women at 36 weeks GA and 93% at delivery. Women received single-dose nevirapine a median of 3.4 hours (1.3–6.4) before delivery, and 12 (13%) did not receive their dose. Infant intensification started a median of 0.7 hours (0.5–1.5) after birth. Adherence to infant intensification as assessed by the pediatrician was >90% in 97% of infants at 2 and 95% at 4 weeks.
In the observational group, >90% adherence to cART during pregnancy was observed in 91% of the women at 36 weeks’ GA and 95% at delivery. Adherence to infant standard of care as assessed by the pediatrician was >90% in 100% of the infants at 2 weeks and in 99% at 4 weeks.
Efficacy Analysis
In the intensification group, endpoints were available for 88 (99%) of the 89 live-born infants. Eighty-two infants were confirmed uninfected, 3 unconfirmed uninfected (negative PCR at birth and 1 month but no confirmation on a later sample), and 3 confirmed HIV-infected, all in utero. In the observational group, endpoints were available for 230 (98%) of the 235 live-born infants. 224 infants were confirmed uninfected, 4 unconfirmed uninfected, and 2 confirmed HIV-infected, both intrapartum (Fig. 1).
Using our model, intrapartum transmission probabilities (priors) based on the characteristics of the 88 women with ≤8 weeks’ antenatal cART were predicted to be 0.5% (95% credibility intervals: 0.0%–2.5%) with antiretroviral intensification and 2.2% (95% Crl: 0.5%–6.1%) without. After observing no intrapartum transmissions of HIV in the 88 women enrolled in the intensification group, the posterior probability of intrapartum transmission was estimated at 0.3% (credibility intervals:0.0%–1.6%) with intensification (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B451). The probability of superiority of intensification over standard of care (risk ratio < 1) was 94.4%, and that of at least a 2-fold reduction of risk (risk ratio < 0.5) was 83.5% (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B451). Sensitivity analyses where unconfirmed intrapartum infection status was excluded gave similar results (data not shown).
Maternal and Infant Safety
Table 3 details the maternal safety events in the intensification and observational groups. A total of 25 women (8%) experienced 27 serious adverse events (SAE) during pregnancy up to 1-month postpartum, and rates were not significantly different in the intensification compared with observational group (4% vs. 9%, P = 0.245). Eight SAEs were related to pregnancy, 6 to delivery complication, 6 to infection, 2 to HIV, and 5 to other causes. There was no significant difference between groups for the frequency of metabolic abnormalities.
TABLE 3.
Safety in Women During Pregnancy and Up to 1 Month After Delivery*
| Intensification Group, (N = 89) | Observational Group, (N = 234) | P † | All Women (N = 323) | |
|---|---|---|---|---|
|
| ||||
| At least one SAE, n (%) | 4 (4%) | 21 (9%) | 0.245 | 25 (8%) |
| SAEs, n | 4 | 23 | 27 | |
| HIV related, n | 1 | 1 | 2 | |
| Pregnancy related, n | 0 | 8 | 8 | |
| Delivery related, n | 1 | 5 | 6 | |
| Possibly ART related, n | 0 | 1 | 1 | |
| Infections, n | 1 | 5 | 6 | |
| Others, n | 1 | 3 | 4 | |
| Metabolic toxicity at least once at any visit | 19 (21%) | 68 (29%) | 0.206 | 87 (27%) |
| Anemia grade ≥2, n | 1 | 12 | 0.123 | 13 |
| Fasting glucose grade ≥2, n | 0 | 6 | 0.193 | 6 |
| Fasting cholesterol grade ≥3, n | 12 | 35 | 0.860 | 47 |
| Fasting triglycerides grade ≥2, n | 1 | 6 | 0.678 | 7 |
| Total bilirubin grade ≥1, n | 3 | 13 | 0.571 | 16 |
| Alanine aminotransferase grade ≥2, n | 1 | 4 | >0.99 | 5 |
| Rashes | ||||
| At delivery, n | 1 | 0 | 0.276 | 1 |
| 7–10 days postpartum, n | 0 | 0 | — | 0 |
| Delivery outcomes | ||||
| Preterm (<37 weeks’ GA), n (%) | 8 (9%) | 24 (10%) | 0.837 | 32 (10%) |
| Very preterm (<34 weeks’ GA), n (%) | 0 (0%) | 4 (2%) | 0.579 | 4 (1%) |
| Stillborn, n (%) | 0 (0%) | 0 (0%) | — | 0 (0%) |
| Low birth weight (<2500 g), n (%) | 8 (9%) | 58 (25%) | 0.001 | 66 (20%) |
| Very low birth weight (<2000 g), n (%) | 1 (1%) | 9 (4%) | 0.295 | 10 (3%) |
| Small for GA (<10th percentile), n (%) | 1 (1%) | 9 (4%) | 0.295 | 10 (3%) |
The total number shown for each group is the number of women who delivered in the study.
The Fisher exact test was used to compare proportions, and the Wilcoxon rank-sum test was used to compare distributions of continuous data.
Rates of preterm and very preterm deliveries were similar in the 2 groups. However, the rate of low birth weight (<2500 g) was significantly lower in the intensification group compared with the observational group (9% vs. 25%, P < 0.001).
Among newborns, 61 SAEs in 60 infants (19%) were reported during the first 6 months of life with no difference between groups (Table 4). Twenty-six events were related to infections, 5 possibly related to antiretrovirals (anemia and/or neutropenia), and none to HIV. There was significantly less frequent anemia grade ≥2 in the intensification group (30%) than in the observational group (48%) (P = 0.008). There were 4 deaths, all in HIV-negative or unconfirmed-negative children: one in the intensification group at 35 days of life from sudden death, and 3 in the observational group: one at birth from severe prematurity, one at 15 days from Down syndrome and sepsis, and one at 5 months from fever and seizures.
Table 4.
Safety in Children Up to 6 Months After Birth
| Intensification Group (N = 89) | Observational Group (N = 235) | P * | All Women (N = 324) | |
|---|---|---|---|---|
|
| ||||
| At least one SAE, n (%) | 11 (12%) | 49 (21%) | 0.108 | 60 (19%) |
| SAE, n | 11 | 50 | 61 | |
| Infections, n | 4 | 22 | 26 | |
| Birth related, n | 3 | 13 | 16 | |
| HIV related, n | 0 | 0 | 0 | |
| Anemia/neutropenia, n | 0 | 5 | 5 | |
| Congenital anomalies, n | 1 | 4 | 5† | |
| Others, n | 3 | 6 | 9 | |
| Deaths | 1‡ (1%) | 3§ (1%) | >0.999 | 4 (1%) |
| Neonatal death (within 28 days after birth) | 0 (0%) | 1 (0.48%) | >0.999 | 1 (0.31%) |
| Metabolic toxicity at least once at any visit, n (%) | 25 (28%) | 97 (41%) | 0.030 | 122 (38%) |
| Anemia grade ≥2 at 1 mo, n (%) | 25 (30%) | 97 (48%) | 0.008 | 122 (42%) |
| Alanine aminotransferase grade ≥2 at 7–10 days, n (%) | 0 (0%) | 0 (0%) | — | 0 (0%) |
| Hemoglobin grade ≥2, n (%) | 25 (30%) | 97 (48%) | 0.008 | 122 (42%) |
| Abnormal hematocrit (<26%), n (%) | 20 (23%) | 66 (29%) | 0.325 | 86 (27%) |
| White blood cells grade ≥2, n (%) | 0 (0%) | 0 (0%) | — | 0 (0%) |
| Absolute neutrophils grade ≥2, n (%) | 5 (6%) | 13 (6%) | >0.999 | 18 (6%) |
| Abnormal absolute lymphocytes (<6000 cells/mm3), n (%) | 36 (43%) | 93 (46%) | 0.697 | 129 (45%) |
| Platelets grade ≥2, n (%) | 1 (1%) | 0 (0%) | 0.296 | 1 (0.3%) |
| Creatinine grade ≥2, n (%) | 0 (0%) | 0 (0%) | — | 0 (0%) |
| SGPT grade ≥2, n (%) | 3 (4%) | — | — | 3 (4%) |
| Total bilirubin grade ≥2, n (%) | 1 (1%) | — | — | 1 (1%) |
The Fisher exact test was used to compare the proportions, and the Wilcoxon rank-sum test was used to compare median.
Congenital anomalies: one polydactyly of right thumb, one Down syndrome, one 4–5 toe syndactyly of left foot, one ankyloglossia, and one deformity of the fourth right toe.
Sudden death at 35 days (unconfirmed uninfected).
One death at birth from severe prematurity (indeterminate HIV status); one death at 15 days from Down syndrome and sepsis (unconfirmed uninfected); and one death at 156 days from fever and seizures (confirmed uninfected).
DISCUSSION
This study demonstrates that perinatal antiretroviral intensification—in this case, maternal intrapartum single-dose nevirapine, and infant triple combination of zidovudine, lamivudine plus nevirapine for 2 weeks followed by zidovudine plus lamivudine for 2 weeks—significantly reduces intrapartum HIV transmission for women who received too short a cART duration to suppress viral load by the time of delivery. Antiretroviral intensification was found to be safe and well tolerated and is recommended in the most recent Thai National guidelines.22
In the Thai context, where transmission rates of HIV are low, a comparative study to show superiority of intensification over standard of care or to compare different intensification schemes would have required a sample size that would made it unfeasible. More importantly, there was no equipoise since there was clear indication from previous PMTCT studies that various forms of maternal/infant perinatal intensification could help prevent intrapartum transmission.23 In our PHPT-2 study, we observed that women on zidovudine monotherapy, most of whom not virologically suppressed at delivery, had intrapartum transmission reduced by approximately 75% when maternal/infant single-dose nevirapine was added.10 Although its efficacy was not formally demonstrated, intensification was already used in clinical practice and recommended for high-risk women (ie, no maternal cART or detectable viral load at delivery) in several guidelines.22,24,25
Thus, we opted for a Bayesian approach with a single intervention arm in high-risk women. With the PHPT-1, −2, and −5 trials data,4,9,10 there was sufficient historical information to model intrapartum transmission accurately and estimate the prior distributions of intrapartum transmission probabilities in women who would have received a short antenatal cART course with/without peripartum intensification. Borrowing historical information requires careful judgment about the relevance of the data to be used.26 In our case, the data were collected by the same team, in the same network of Thai hospitals, with the same virological evaluations and data management quality standards. It was critical to use the large number of women on zidovudine monotherapy in our historical data to develop our intrapartum HIV transmission model as it is a direct result of unsuppressed VLd. The use of maternal/infant perinatal single-dose nevirapine as a proxy for antiretroviral intensification to compute the prior distribution of the risk of intrapartum transmission was very conservative and could only underestimate the magnitude of the effect of the more potent intensification regimen used in this study.
The Data Safety and Monitoring Board recommended to stop enrollment just before the planned second interim analysis and to proceed to final analysis as the target sample size could not be reached in a reasonable time frame and results needed to be made public. In this context, an advantage of using a Bayesian framework is that the posterior probability of intrapartum transmission with antiretroviral intensification was directly interpretable27 and the computed probability that antiretroviral intensification was of superior efficacy than standard of care precisely reflected the information gathered at study end (see Figure A, Supplemental Digital Content, http://links.lww.com/QAI/B451).
Several studies have investigated perinatal interventions in children at high risk of HIV infection.28 HPTN040/PACTG 1043 compared the efficacy and safety of 3 postnatal ART regimens in 1684 formula-fed infants whose mothers had received no antepartum prophylaxis29: 6-week zidovudine, 6-week zidovudine plus 3 doses of nevirapine during the first 8 days of life, and 6-week zidovudine, plus nelfinavir and lamivudine for 2 weeks. Intrapartum transmission rates were 4.8%, 2.2%, and 2.4%, respectively. In these women with high viral load at delivery, the 2- or 3-drug ART regimens were of superior efficacy than zidovudine alone, but the 3-drug regimen had significant toxicity, in particular neutropenia.29 The relative efficacy of 2 (ie, zidovudine-nevirapine) vs. 3-drug (ie, zidovudine-lamivudine-nevirapine) intensification in high-risk infants remains unclear, and a 2-drug regimen may have sufficed.29 Observational studies support the efficacy and safety of antiretroviral intensification with 3 drugs for infants at high risk of intrapartum transmission.23,30,31 Zidovudine-lamivudine-nevirapine for the first 2 weeks followed by zidovudine-lamivudine for 2 more weeks was chosen to cover the nevirapine “tail” which occurs after stopping due to its long half-life and to prevent the selection of NNRTI-resistant viruses.32 It also reflected drug options for neonates at the time and appeared easy to implement.
It should be noted that in this study, we provided maternal single-dose nevirapine at onset of labor because we wanted to ensure the earliest possible fetal prophylaxis and to prevent selection of NNRTI resistance mutations, these women received cART for 1-month post delivery.
Toxicities in infants were limited and similar in the antiretroviral intensification and the observational group. Although infants in both groups received 1-month zidovudine, anemia was less prevalent in the intensification group, reflecting a shorter fetal exposure to zidovudine, which readily crosses the placenta. Zidovudine, lamivudine, and nevirapine for 6 weeks in high-risk HIV-exposed infants is part of the current Thailand national recommendations.22 The higher rate of low birth weight in the observational group may also reflect a longer antepartum exposure to lopinavir-based cART, an observation consistent with the fetal impact of antenatal cART reported in the randomized PROMISE trial.29
Defining mothers at high risk of intrapartum transmission is complex. Depending on guidelines, risk criteria include seroconversion during pregnancy, detectable maternal HIV viral load close to delivery, late presentation for antenatal care, short prenatal cART duration, poor maternal adherence to cART, premature rupture of membranes with detectable HIV, and maternal HIV diagnosis at or after delivery. Our single and simple condition of initiating cART ≤8 weeks before delivery was easy to implement but did not cover all the situation cited above and was determined within the context of lopinavir/ritonavir-based cART during pregnancy. More recently, once-daily efavirenz-based fixed-dose combination has been widely used in pregnancy. It has been shown to be marginally superior to a lopinavir/ritonavir-based treatment in suppressing VLd and time to VL suppression may be somewhat shorter.33 However, the upcoming era of cART may change the profile of women at high risk of intrapartum transmission of HIV. Dolutegravir-based regimens are now recommended by WHO for pregnant women with HIV while safety continues to be monitored and efavirenz vs. dolutegravir clinical trials are ongoing (VESTED NCT03048422; DOLPHIN2 NCT03249181).34 With this potent regimen, VL is reduced within days of initiation, and our definition of high risk with ≤8 weeks’ cART before delivery may need to be re-evaluated. Also, the nature of antiretroviral intensification for infants at high risk will evolve with the introduction of infant formulations of integrase inhibitors. Today, raltegravir, the first integrase inhibitor approved for neonatal use, is the only option to replace nevirapine. Raltegravir also has some limitations, such as a low barrier to resistance and complex use for caregivers. Current WHO guidelines still recommend zidovudine plus nevirapine for 6 weeks in high-risk infants,35 but given the increasing availability of ARVs for neonates, there are discussions at the WHO36 about the possible recommendation of presumptive treatment in high-risk infants, a recommendation already adopted in the US guidelines.24
Supplementary Material
ACKNOWLEDGMENTS
All the women who participated in the trial; The PHPT-5 study team: administrative support: A. Lautissier, L. Barra, N. Chaiboonruang, T. Sriwised, T. Tritungtrakul (Intaboonmar), D. Punyatiam, P. Pirom, S. Jitharidkul (Phromsongsil), P. Palidta, S. Vorayutthanakarn, N. Rawanchaikul, S. Nupradit, T. Tankool, and W. Champa; tracking and supplies: K. Than-in-at, R. Wongsang, M. Inta, N. Mungkhala, P. Saenchitta, K. Oopin, and P. Wimolwattanasarn; safety monitoring: S. S. Chalermpantmetagul, R. Peongjakta, C. Kanabkaew, and J. Chaiwan; site monitoring: P. Sukrakanchana, B. Ratchanee, J. Thonglo, J. Khanmali, N. Kruenual, N. Krapunpongsakul, N. Krueduangkam, R. Kaewsai (Wongsrisai), R. Wongchai, S. Jinasa, T. Thimakam, W. Pongchaisit, W. Khamjakkaew, S. Thammajitsagul, J. Wallapachai, J. Chalasin, P. Kulchatchai, N. Thuenyeanyong, P. Thuraset, P. Chart, and S. Thongsuwan; data management: S. Barbier, R. Seubmongkolchai, K. Yoddee, S. Tanasri, S. Chailert, N. Naratee, R. Suaysod, K. Chaokasem, R. Jitharidkul, N. Jaisieng, P. Chusut, W. Wongwai, B. Tongpanchang, J. Inkom, A. Lueanyod, T. Chitkawin, W. Chanthaweethip, A. Seubmongkolchai, K. Seubmongkolchai, K. Saopang, R. Malasam, S. Kreawsa, T. Yaowarat (Chattaviriya), A. Wongja, D. Jianphinitnan, K. Ruangwut,S. Suekrasae (Onpha), T. Thanyaveeratham (Chimplee), P. Chailert (Supinya), N. Homkham, and P. Pongwaret; statistics: Nicolas Salvadori and Yvonne Pittelkow; laboratory: W. Pilonpongsathorn (Boonprasit), J. Kamkon, P. Moolnoi (Tungyai), P. Pongpunyayuen, Y. Tawon, D. Saeng-ai, L. Laomanit, N. Wangsaeng, P. Khantarag, R. Dusadeepong, S. Surajinda, A. Kaewbundit, P. Punyati, A. Khanpanya, U. Tungchitrapituk, N. Boonpluem, T. Thaiyanant, C. Kasemrat, W. Thimayom, W. Sripaoraya, S. Putthasiraapakorn, W. Danpaiboon, P. Mongkolwat, T. Donchai, and P. Sothanapaisan; The DSMB members: Prof. Suwachai Intaraprasert, Assoc. Prof. Rudiwilai Samakoses, and Dr. Wiput Phoolcharoen.
The authors are also grateful for the advice and assistance from the Thai Ministry of Public Health: Office of the Permanent Secretary, Departments of Health, Department of Diseases Control, especially, S. Thanprasertsuk, P. Sirinirund, N. Premsri, N. Voramongkol, S. Kanshana, N. Voramongkol, S. Pattarakulwanich, and from the National Institute of Child Health and Human Development: L. Mofenson; as well as the following colleagues who contributed to this project in many critical ways: M. Essex, D. Wirth, and E. Kiley.
PHPT-5 Site investigators and number of enrollment per sites.
Thitiporn Siriwachirachai, Ussanee Srirompotong, KhonKaen H. (50); Boonsong Rawangban, Sadhit Santadusit, Anita Luvira, Nopparat Rajathanee H., Bangkok (42); Prapan Sabsanong, Achara Puangsombat, Samutprakarn, (35); Kamol Boonrod, Prateep Kanjanavikai, Siriluk Phanomcheong, Banglamung H., Chonburi (24); Jullapong Achalapong, Kannikar Saisawat, Chulapong Chanta, Kanchana Preedisripipat, Chiangrai Prachanukroh H. (23); Tapnarong Jarupanich, Boonyarat Warachit, Hat Yai H., Songkhla (20); Jantana Jungpipun, Ratikorn Petprakorp, Fang H., Chiang Mai (14); Phaiboon Wanasiri, Sakulrat Srirojana, Kalasin H., Kalasin (13); Supha-arth Phon-in, Wannee Limpitikul, Songkhla H. (13); Jittapol Hemvuttiphan, Pornchai Techakunakorn, Phayao Provincial H. (11); Chaiwat Putiyanun, Vanichaya Wanchaitanawong, Chiang Kham H., Phayao (10); Sookchai Theansavettrakul, Phan H., Chiangrai (10); Suraphan Sangsawang, Kanokwan Jittayanun, Health Promotion Center Region 10, Chiang Mai (9); Prapap Yuthavisuthi, Chaiwat Ngampiyaskul, Prapokklao H., Chantaburi (9); Weerapong Suwankornsakul, Phantip Sreshthatat, Rayong, Rayong (9); Prateung Liampongsabuddhi, Kultida Pongdetudom, Lampang H., Lampang (9); Nantasak Chotivanich, Suchat Hongsiriwon, Chonburi H., Chonburi (8); Supang Varadisai, Sawitree Krikajornkitti, Samutsakhon H. (8); Ruaengkitti Sirikanchanakul, Kraisorn Vivatpatanakul, Sansanee Hanpinitsak, Regional Health Promotion Centre 6, Khon Kaen (7); Aram Limtrakul, Suparat Kanjanavanit, Nakornping H., Chiang Mai (5); Kanchapan Sukonpan, Narong Lertpienthum, Buddhachinaraj H., Pitsa-nuloke (5); Sunida Panna, Nusra Puarattana.aroonkorn, Naruepon Yutthakasemsunt, Nong Khai H. (5); Worapong Worachet, Sakchai Tonmat, Sathaporn Na-Rajsima, Mahasarakam H., Mahasarakam (5); Toranong Pilalai, Wiangpapao H., Chiang Rai (5); Wanmanee Matanasarawut, Pornpun Wannarit, Lamphun H. (4); Sinart Prommas, Prapaisri Layangool, Bhumibol Adulyadej H., Bangkok (4); Prayoon Khamja, Noppadon Akarathum, Sanpatong H., Chiang Mai (4); Annop Kanjanasing, Ratchanee Kwanchaipanich, Bhuddasothorn H., Chachoengsao (3); Arunsri Iamthongin, Apichai Phiyarom, Chomthong H., Chiang Mai (3); Manoch Chakorngowit, Wisith Pholsawat, Panasnikom H., Chonburi (3); Sudanee Buranabanjasatean, Surachai Piyaworawong, Mae Chan H. (2); Rucha Kongpanichkul, Suthunya Bunjongpak, Nakhonpathom H., (2); Sukit Mahattanan, Somsri Kotchawet, Maharaj Nakhon Si Thammarat H. (2); Surachai Pipatnakulchai, Sinchai Wanwaisart, Pranangklao H., Nonthaburi (1); Sompong Wannun, Weerasak Lawtongkum, Vachira Phuket H. (1); Ittipol Chaitha, Chiang Saen H., Chiang Rai (1); Premjit Charoenweerakul, Jariyarat Nitipipatkosol, Mae Sai H., Chiang Rai (0); Somsak Wachirachaikan, Surat Sirinontakan, Health Promotion H. Regional Center I, Bangkok (0).
Study site coinvestigators: Achara Puangsombat, Annop Kanjanasing, Aram Limtrakul, Apichai Phiyarom, Arunsri Lamthongin, Boonsong Rawangban, Boonyarat Warachit, Chaiwat Ngampiyaskul, Chaiwat Putiyanun, Chulapong Chanta, Ittipol Chaitha; Jantana Jungpipun, Jariyarat Nitipipatkosol, Jittapol Hemvuttiphan, Jullapong Achalapong, Kamol Boonrod, Kanchana Preedisripipat, Kanchapan Sukonpan, Kannikar Saisawat, Kanokwan Jittayanun, Kraisorn Vivatpatanakul, Kultida Pongdetudom, Manoch Chakorngowit, Nantasak Chotivanich, Naruepon Yutthakasemsunt, Narong Lertpienthum, Noppadon Akarathum, Nusra Puarattana.aroonkorn, Phaiboon Wanasiri, Phantip Sreshthatat, Pornchai Techakunakorn, Pornpun Wannarit, Prapan Sabsanong, Prapaisri Layangool, Prapap Yuthavisuthi, Prateep Kanjanavikai, Prateung Liampongsabuddhi, Prayoon Khamja, Premjit Charoenweerakul, Ratchanee Kwanchaipanich, Ratikorn Petprakorp, Ruaengkitti Sirikanchanakul, Rucha Kongpanichkul, Sadhit Santadusit, Anita Luvira, Sakchai Tonmat, Sakulrat Srirojana, Sansanee Hanpinitsak, Sathaporn Na-Rajsima, Sawitree Krikajornkitti, Sinart Prommas, Sinchai Wanwaisart, Siriluk Phanomcheong, Sompong Wannun, Somsak Wachirachaikan, Somsri Kotchawet, Sookchai Theansavettrakul, Sudanee Buranabanjasatean, Suchat Hongsiriwon, Sukit Mahattanan, Sunida Panna, Supang Varadisai, Suparat Kanjanavanit, Supha-arth Phon-in, Surachai Pipatnakulchai, Surachai Piyaworawong, Suraphan Sangsawang, Surat Sirinontakan, Suthunya Bunjongpak, Tapnarong Jarupanich, Thitiporn Siriwachirachai, Toranong Pilalai, Ussanee Srirompotong, Vanichaya Wanchaitanawong, Wanmanee Matanasarawut, Wannee Limpitikul, Weerapong Suwankornsakul, Weerasak Lawtongkum, Wisith Pholsawat, and Worapong Worachet.
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), USA (Grant number R01 HD052461 and R01 HD056953); the Ministry of Public Health, Thailand; the Institut de Recherche pour le Développement, France; the Institut National d’Etudes Démographiques, France; and the Thailand International Development Cooperation Agency (TI-CA). GlaxoSmithKline and Boehringer Ingelheim provided study drugs for the historical clinical trials that were meta-analyzed and this study.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
PHPT-5 site investigators are listed in the Acknowledgment section.
REFERENCES
- 1.Fowler MG, Mofenson LM, Taha TE Antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2017;376:699–700. [DOI] [PubMed] [Google Scholar]
- 2.Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection. Recommendations for a Public Health Approach. WHO; 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed July 5, 2013. [PubMed] [Google Scholar]
- 3.Jourdain G, Mary JY, Coeur SL, et al. Risk factors for in utero or intrapartum mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. J Infect Dis. 2007;196:1629–1636. [DOI] [PubMed] [Google Scholar]
- 4.Lallemant M, Le Coeur S, Sirirungsi W, et al. Randomized noninferiority trial of two maternal single-dose nevirapine-sparing regimens to prevent perinatal HIV in Thailand. AIDS. 2015;29:2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warszawski J, Tubiana R, Le Chenadec J, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. AIDS. 2008;22:289–299. [DOI] [PubMed] [Google Scholar]
- 6.Phanuphak N, Lolekha R, Chokephaibulkit K, et al. Thai national guidelines for the prevention of mother to-child transmission of HIV: march 2010. Asian Biomed. 2010;4:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro R, Thior I, Gilbert P, et al. Maternal single-dose Nevirapine may not be needed to reduce mother-to-child HIV transmission in the setting of maternal and infant zidovudine and infant single-dose nevirapine: results of a randomized clinical trial in Bostwana. Paper presented at: 12th Conference on Retroviruses and Opportunistic Infections; February 22–25, 2005; Boston, MA. Abstract 74LB. [Google Scholar]
- 9.Lallemant M, Jourdain G, Le Coeur S, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med. 2000;343:982–991. [DOI] [PubMed] [Google Scholar]
- 10.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–228. [DOI] [PubMed] [Google Scholar]
- 11.Lallemant M, Ngo-Giang-Huong N, Jourdain G, et al. Efficacy and safety of 1-month postpartum zidovudine-didanosine to prevent HIV-resistance mutations after intrapartum single-dose nevirapine. Clin Infect Dis. 2010; 50:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dyke RB, Ngo-Giang-Huong N, Shapiro DE, et al. A comparison of 3 regimens to prevent nevirapine resistance mutations in HIV-infected pregnant women receiving a single intrapartum dose of nevirapine. Clin Infect Dis. 2012;54:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngo-Giang-Huong N, Khamduang W, Leurent B, et al. Early HIV-1 diagnosis using in-house real-time PCR amplification on dried blood spots for infants in remote and resource-limited settings. J Acquir Immune Defic Syndr. 2008;49:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryson YJ, Luzuriaga K, Sullivan JL, et al. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992; 327:1246–1247. [DOI] [PubMed] [Google Scholar]
- 15.King CC, Kourtis AP, Persaud D, et al. Delayed HIV detection among infants exposed to postnatal antiretroviral prophylaxis during breastfeeding. AIDS. 2015;29:1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster C, Pace M, Kaye S, et al. Early antiretroviral therapy reduces HIV DNA following perinatal HIV infection. AIDS. 2017;31:1847–1851. [DOI] [PubMed] [Google Scholar]
- 17.Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events Version 2.0, 2014. Available at: https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. Accessed February 8, 2015.
- 18.Sripan P, Le Coeur S, Ingsrisawang L, et al. Contribution of different antiretroviral regimens containing zidovudine, lamivudine and ritonavir-boosted lopinavir on HIV viral load reduction during pregnancy. Antivir Ther. 2016;21:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sripan P, Le Coeur S, Amzal B, et al. Modeling of in-utero and intrapartum transmissions to evaluate the efficacy of interventions for the prevention of perinatal HIV. PLoS One. 2015;10:e0126647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cressey TR, Jourdain G, Lallemant MJ, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38:283–288. [PubMed] [Google Scholar]
- 21.Spiegelhalter DJ, Thomas A, Best N, et al. WinBUGS User Manual, Version 1.4. Available at: http://www.politicalbubbles.org/bayes_beach/manual14.pdf. 2003. [Google Scholar]
- 22.Lolekha R, Chokephaibulkit K, Phanuphak N, et al. Thai national guidelines for the prevention of moither-to-child transmission of human immunodeficiency virus 2017. Asian Biomed. 2017;11:127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiappini E, Galli L, Giaquinto C, et al. Use of combination neonatal prophylaxis for the prevention of mother-to-child transmission of HIV infection in European high-risk infants. AIDS. 2013;27:991–1000. [DOI] [PubMed] [Google Scholar]
- 24.Panel on treatment of pregnant women with HIV infection and prevention of perinatal transmission. Recommendations for use of anti-retroviral drugs in transmission in the United States. Available at: https//aidsinfo.nih.gov/guidelines/PerinatalGL.pdf. Accessded January 10, 2020.
- 25.de Ruiter A, Taylor G, Clayden P, et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012 (2014 interim review). HIV Med. 2014;15(suppl 4):1–77. [DOI] [PubMed] [Google Scholar]
- 26.Viele K, Berry S, Neuenschwander B, et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat. 2014;13: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry DA. Bayesian clinical trials. Nat Rev Drug Discov. 2006;5:27–36. [DOI] [PubMed] [Google Scholar]
- 28.Beste S, Essajee S, Siberry G, et al. Optimal antiretroviral prophylaxis in infants at high risk of acquiring HIV: a systematic review. Pediatr Infect Dis J. 2018;37:169–175. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen-Saines K, Watts DH, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakkar FW, Samson L, Vaudry W, et al. Safety of combination antiretroviral prophylaxis in high-risk HIV-exposed newborns: a retrospective review of the Canadian experience. J Int AIDS Soc. 2016;19: 20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anugulruengkitt S, Suntarattiwong P, Ounchanum P, et al. Safety of 6-week neonatal triple-combination antiretroviral postexposure prophylaxis in high-risk HIV-exposed infants. Pediatr Infect Dis J. 2019;38: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 32.Cressey TR, Punyawudho B, Le Coeur S, et al. Assessment of nevirapine prophylactic and therapeutic dosing regimens for neonates. J Acquir Immune Defic Syndr. 2017;75:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohan D, Natureeba B, Koss C, et al. Efficacy and safety of lopinavir/ritonavir- versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford M, van Wyk J, Aboud M, et al. Postmarketing surveillance of pregnancy outcomes with dolutegravir use. J Acquir Immune Defic Syndr. 2020:83:e2–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO Technical Brief: Preventig HIV during Pregnancy and Breastfeeding in the Context of Pre-exposure Prophylaxis (PrEP). Geneva, Switzerland: World Health Organization; 2017. Licence:CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 36.Penazzato M, Townsend C, Rakhmanina N, et al. Prioritising the most needed paediatric antiretroviral formulations: the PADO4 list. Lancet HIV. 2019:6:e623–e631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.