Background:
Alzheimer disease and related dementia (ADRD) is one of the most expensive health conditions in the United States. Understanding the potential cost-savings or cost-enhancements of Health Information Technology (HIT) can help policymakers understand the capacity of HIT investment to promote population health and health equity for patients with ADRD.
Objectives:
This study examined access to hospital-based HIT infrastructure and its association with racial and ethnic disparities in Medicare payments for patients with ADRD.
Research Design:
We used the 2017 Medicare Beneficiary Summary File, inpatient claims, and the American Hospital Association Annual Survey. Our study focused on community-dwelling Medicare fee-for-service beneficiaries who were diagnosed with ADRD. Our study focused on hospital-based telehealth-postdischarge (eg, remote patient monitoring) and telehealth-treatment (eg, psychiatric and addiction treatment) services.
Results:
Results showed that hospital-based telehealth postdischarge services were associated with significantly higher total Medicare payment and acute inpatient Medicare payment per person per year among patients with ADRD on average. The associations between hospital-based telehealth-treatment services and payments were not significant. However, the association varied by patient’s race and ethnicity. The reductions of the payments associated with telehealth postdischarge and treatment services were more pronounced among Black patients with ADRD. Telehealth-treatment services were associated with significant payment reductions among Hispanic patients with ADRD.
Conclusion:
Results showed that having hospital-based telehealth services might be cost-enhancing at the population level but cost-saving for Black and Hispanic patients with ADRD. Results suggested that personalized HIT services might be necessary to reduce the cost associated with ADRD treatment for racial and ethnic minority groups.
Key Words: ADRD, medicare payments, health information technology, racial and ethnic disparities
Alzheimer disease and related dementia (ADRD) is considered one of the most expensive health conditions, with total payments estimated at $305 billion in 2020 and projected costs estimated to reach $1.1 trillion in 2050.1 More than 5.5 million Americans suffer from ADRD. This number is expected to reach 14 million Americans by 2060. Black older adults (65 years and older) are about twice as likely, and Hispanic older adults are about 1.5 times as likely to have dementia than White older adults.2–4 Conditions such as cardiovascular disease and diabetes, which increase the risk of developing ADRD, contribute to the high prevalence of ADRD among racial and ethnic minority groups.5,6
Evidence suggests that racial and ethnic minority groups are more likely to have later-stage diagnoses, experience delays in timely primary care, and lack access to coordinated care.7 Among Medicare beneficiaries with ADRD, Black patients had the highest Medicare payments, with a total average annual payment of $29,934 per person; Hispanic patients averaged a total annual payment of $23,725 per person and White patients averaged a total annual payment of $22,135 per person in 2020.8 Understanding these cost disparities is a critical step to achieving the Triple Aim of Health Care (ie, improving care and health while lowering cost), and finding approaches that address these cost disparities are important for equity research.
Care coordination strategies can improve the quality of care and quality of life for patients with ADRD.9–11 Care coordination can be successfully achieved by leveraging health information technology (HIT) systems that encompass efficient electronic data sharing,12–15 health information exchanges (HIEs),16,17 and automatic notifications regarding care transitions.18–20 For example, evidence has suggested that automatic notifications of ED visits, inpatient admissions, and discharges to primary care providers could reduce the risk of readmissions among Medicare fee-for-service (FFS) beneficiaries and encourage timely follow-ups.20 A recent study suggested that patients with ADRD treated in hospitals that utilized HIT care coordination and patient engagement services reported lower rates of preventable ED visits.21
While HIT can improve efficiency, Black and Hispanic patients with ADRD have less access to HIT-integrated health care systems compared with their Non-Hispanic White counterparts.22,23 A recent study delineated a conceptual framework for racial and ethnic disparities in ADRD care and discussed how the HIT-supported care coordination model could reduce structural racism and discrimination (eg, racial residential segregation and structural barriers in health care) for patients with ADRD.24 Building on that model, this present study provided empirical evidence on the association between the HIT infrastructure and racial and ethnic disparities in health care costs among patients with ADRD.
In particular, the study examined the association between hospital-based HIT infrastructure with Medicare payments among patients with ADRD by race and ethnicity. We hypothesized that HIT infrastructures promoting care coordination might reduce Medicare payment, especially for Black and Hispanic patients with ADRD. For instance, improved HIT-supported services can improve care coordination, identify patient needs and cultural preferences, and thus reduce overall costs for racial and ethnic minority groups. We expect that results can be helpful for future evaluations of telehealth capacity-building efforts that aim to improve HIT investment and designs for racial and ethnic minority aging populations.
METHODS
Data
We used the linked data sets of the 2017 Centers for Medicare and Medicaid Services (CMS) Medicare 100% inpatient claims data and Medicare Beneficiary Summary File. These data were further linked with the American Hospital Association (AHA) Annual Survey to obtain the hospital characteristics and hospital-based HIT measures.25 Finally, we linked the American Community Survey and the Area Health Resource File to capture zip-code and county-level measures that were commonly used in the literature.
Our study focused on community-dwelling Medicare FFS beneficiaries with a diagnosis of ADRD aged 65 and older and who had at least 1 hospital visit in 2017. Elderly patients with Medicare Advantage or Medicare and Medicaid dual-eligible patients were not included. We used the definition of ADRD reported by the CMS chronic conditions data warehouse codes.26 We focused on non-Hispanic White (White), Black, and Hispanic patients with ADRD.
Outcome Measures
To be consistent with the literature, we employed the Medicare payment measures used in the 2021 Medicare payment Alzheimer disease facts and figures special report, Race, Ethnicity, and Alzheimer in America.8 Outcome measures were obtained from the Medicare Beneficiary Summary Cost and Use file. We used the “acute inpatient Medicare payments” to estimate its variation and association with hospital-based HIT. We created a “total Medicare payments per person per year” variable as the summation of Medicare payments on major services, including acute inpatient, other inpatient hospital, skilled nursing facility, hospice, home health, hospital outpatient, ambulatory surgery center, anesthesia, Part B drug, evaluation and management, Part B physician, other procedure, imaging, test, other Part B carrier, and Part D Medicare payments.
Key Independent Variables
Hospital-based HIT measures were obtained in the Facilities and Services section of the AHA Annual Survey. Seven measures were included: telehealth consultation and office visits; eICU; telehealth stroke care; telehealth psychiatric and addiction treatment; telehealth remote patient monitoring postdischarge, and telehealth remote patient monitoring ongoing chronic care management. Hospital-based HIT was grouped into 2 categories: (1) the telehealth-postdischarge group, which included the services of the remote patient monitoring postdischarge, or the remote patient monitoring ongoing chronic care management; (2) the telehealth-treatment group, which included services that supported telehealth consultation and office visits, eICU, stroke care, or psychiatric and addiction treatment.
In the sensitivity analysis, we tested different measures of telehealth indices. In the appendix, we presented results using: (1) the total number of telehealth services adopted by the hospital, (2) any telehealth services adopted, and (3) the cross-tabulation of telehealth-postdischarge and telehealth-treatment services. Results were similar (Appendix 1, Supplemental Digital Content 1, http://links.lww.com/MLR/C547).
Other Independent Variables
Other independent variables at the beneficiary level included race/ethnicity, age, sex, and health. We used the Research Triangle Institute Race Code for Non-Hispanic White, Non-Hispanic Black, and Hispanic. Health indicators included were those considered as common co-existing chronic conditions of ADRD8: acute myocardial infarction; asthma; atrial fibrillation; heart failure; chronic obstructive pulmonary disease (COPD); depression; diabetes; hyperlipidemia; and hypertension. Diagnoses of these diseases were also obtained from the CMS chronic conditions data warehouse codes.26 Covariates at the hospital level included teaching status, type of controls, and bed size. Area-level variables included rurality indices indicating 3 categories: rural, micropolitan, and metropolitan area. In addition, we included high school education; poverty as the percentage of people at the poverty level by the zip code; the proportion of Black people (divided by the total population) in that zip code using the ACS 5-year (2011–2015) average data; and rates of physicians per 1,000 residents at the county-level.
In the sensitivity analysis, we tested the model with different geographic specifications. We used the Area Deprivation Index (ADI), which allowed for “rankings of neighborhoods by socioeconomic disadvantage in a region of interest” (eg, at the state or national level). ADI is a composite measure that includes population-level factors across the domains of income, education, employment, and housing quality which can be used to inform health delivery and policy, especially for the most “disadvantaged neighborhoods.”27 We categorized beneficiaries’ resident areas into 3 groups: ADI below the 25th percentile (the best), the 25th–75th percentile, and the 75th percentile (the most disadvantaged areas). Results were similar (see Appendix 2, Supplemental Digital Content 1, http://links.lww.com/MLR/C547).
Analysis
Our unit of analysis was at the beneficiary-hospital level. Approximately 70% of beneficiaries visited the same hospital, 22% were admitted in 2 hospitals, 6% were admitted in 3 hospitals, and 2% were admitted in more than 3 hospitals. We created beneficiary-hospital level data to account for situations when beneficiaries visited multiple hospitals, and we adjusted for the number of visits in the regression analyses.
We first presented the likelihood of being treated in hospitals with telehealth-postdischarge and telehealth-treatment services for patients with ADRD by race and ethnicity. We then presented and compared beneficiary, hospital, and geographic characteristics. Given the skewed distributions of the payments, generalized linear models with log link and gamma distributions were used to estimate the association between telehealth measures and Medicare payments.28–30 Interaction terms between race and ethnicity (Blacks and Hispanics) and telehealth-postdischarge and telehealth-treatment services were included in the regressions to test the different associations by race and ethnicity. We then reported the marginal effects of telehealth-postdischarge and telehealth-treatment services for each race and ethnicity group. Medicare reimbursement models vary by state policies (eg, the State of Maryland uses the global budget review model, now called the total cost of care model, for hospital reimbursement). Hence, state-fixed effects were applied to all the regressions. In the sensitivity analysis, we also presented the results of clustered standard errors to help account for heterogeneity. Results were similar (see Appendix 3, Supplemental Digital Content 1, http://links.lww.com/MLR/C547).
RESULTS
Compared with non-Hispanic White patients, Black patients with ADRD were less likely to be treated in hospitals with telehealth-postdischarge services but were more likely to be treated in hospitals with telehealth-treatment services (Fig. 1). Hispanic patients with ADRD were more likely to be treated in hospitals with telehealth-treatment services, and the likelihood of being treated in hospitals with telehealth-postdischarge services was similar to that of White patients.
FIGURE 1.
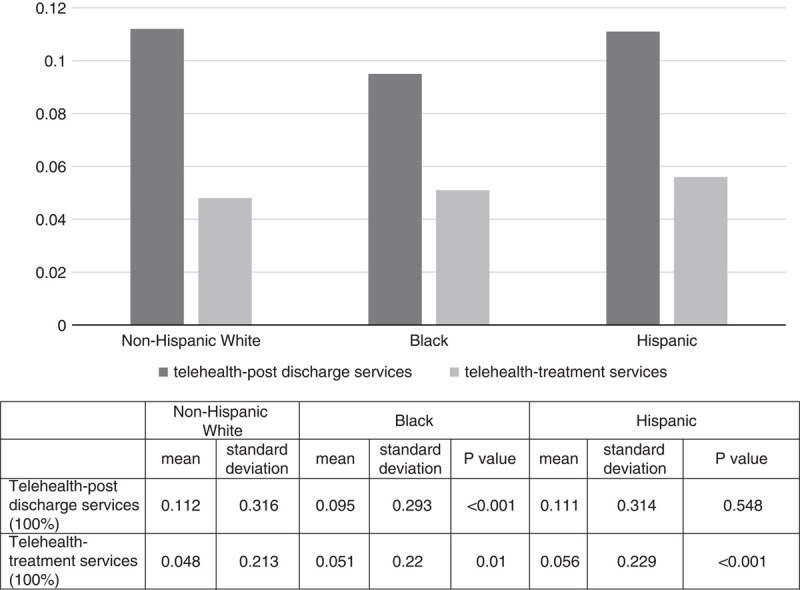
Probability of being served in hospitals with telehealth infrastructure among Medicare fee-for-service beneficiaries with Alzheimer disease and related dementia by race and ethnicity. This study focused on community-dwelling Medicare fee-for-service beneficiaries who had a diagnosis of Alzheimer disease and related dementias (ADRD) that were aged 65 and older and had at least one hospital visit in 2017. Elderly with Medicare Advantage or dual eligibilities were not included. ADRD was defined using the Centers for Medicare and Medicaid Services (CMS) chronic conditions data warehouse codes. Non-Hispanic White (White), Black, and Hispanic patients with ADRD are reported where non-Hispanic Whites were used as the reference group.
Table 1 presents the total Medicare and acute inpatient Medicare payment per person per year by race and ethnicity according to whether hospitals had HIT infrastructures. Results showed that the payments were significantly higher among White patients with ADRD who were treated in hospitals with telehealth-postdischarge or telehealth-treatment services. However, the total Medicare payment was significantly lower among Black patients with ADRD who were treated in hospitals with telehealth postdischarge or telehealth-treatment services. Acute inpatient Medicare payments were slightly higher among Black patients with ADRD who were treated in hospitals with telehealth-treatment services and Hispanic patients with ADRD who were treated in hospitals with telehealth postdischarge services.
TABLE 1.
Medicare Payments by Race and Ethnicity and Hospital Provision of Telehealth-Postdischarge and Telehealth-Treatment Services
| Telehealth-Postdischarge Services | |||||||
|---|---|---|---|---|---|---|---|
| YES | NO | ||||||
| WHITE | N=33,507 | N=263,434 | |||||
| Mean | 95% CI | Mean | 95% CI | P | |||
| Total Medicare payment per person per year | $38,417.06 | $37,978.86 | $38,855.26 | $36,416.49 | $36,276.78 | $36,556.19 | <0.001 |
| Acute inpatient Medicare payment | $18,231.74 | $17,950.77 | $18,512.70 | $15,552.21 | $15,469.62 | $15,634.81 | <0.001 |
| BLACK | N=2,642 | N=25,034 | |||||
| Mean | 95% CI | Mean | 95% CI | P | |||
| Total Medicare payment per person per year | $38,321.18 | $36,610.24 | $40,032.12 | $40,944.06 | $40,395.67 | $41,492.46 | <0.001 |
| Acute inpatient Medicare payment | $18,700.76 | $17,524.88 | $19,876.63 | $18,030.08 | $17,696.51 | $18,363.65 | 0.23 |
| HISPANIC | N=1,308 | N= 10,470 | |||||
| Mean | 95% CI | Mean | 95% CI | P | |||
| Total Medicare payment per person per year | $41,570.97 | $39,072.50 | $44,069.44 | $42,696.93 | $41,857.53 | $43,536.33 | 0.38 |
| Acute inpatient Medicare payment | $20,555.89 | $18,828.87 | $22,282.91 | $18,413.68 | $17,932.72 | $18,894.65 | <0.001 |
| Telehealth-Treatment Services | |||||||
| YES | NO | ||||||
| WHITE | N=14,109 | N=282,832 | |||||
| Mean | 95% CI | Mean | 95% CI | P | |||
| Total Medicare payment per person per year | $38,752.57 | $38,061.25 | $39,443.90 | $36,536.96 | $36,401.16 | $36,672.75 | <0.001 |
| Acute inpatient Medicare payment | $18,609.10 | $18,153.64 | $19,064.57 | $15,717.17 | $15,636.46 | $15,797.89 | <0.001 |
| BLACK | N=1,411 | N=26,265 | |||||
| Mean | 95% CI | Mean | 95% CI | P | |||
| Total Medicare payment per person per year | $37,735.00 | $35,193.67 | $40,276.33 | $40,852.62 | $40,319.51 | $41,385.74 | <0.001 |
| Acute inpatient Medicare payment | $19,485.57 | $17,519.80 | $21,451.34 | $18,019.35 | $17,696.99 | $18,341.72 | 0.05 |
| HISPANIC | N=658 | N=11,120 | |||||
| Mean | 95% CI | Mean | 95% CI | P | |||
| Total Medicare payment per person per year | $38,708.45 | $35,481.88 | $41,935.02 | $42,800.50 | $41,979.38 | $43,621.62 | 0.38 |
| Acute inpatient Medicare payment | $19,029.72 | $16,611.01 | $21,448.43 | $18,629.25 | $18,153.83 | $19,104.67 | 0.70 |
Our study focused on community-dwelling Medicare fee-for-service beneficiaries who had a diagnosis of Alzheimer disease and related dementias (ADRD) aged 65 and older and who had at least 1 hospital visit in 2017. Elderly with Medicare Advantage or dual eligibilities were not included. We used the definition of ADRD reported by the Centers for Medicare and Medicaid Services (CMS) chronic conditions data warehouse codes. We focused on Non-Hispanic White (White), Black, and Hispanic patients with ADRD. Whites were used as the reference group.
Summaries of beneficiary, hospital, and community-level characteristics are presented in Table 2. Compared with White patients, Black and Hispanic patients with ADRD had significantly higher acute inpatient Medicare payments and total Medicare payments per person per year. Black and Hispanic patients, on average, were younger. Rates of co-existing ADRD comorbidities varied by race and ethnicity. Compared with White patients, Black patients were more likely to have asthma, heart failure, diabetes, hyperlipidemia, and hypertension, and less likely to have depression and COPD; Hispanic patients were also more likely to have diabetes and hypertension. Compared with White patients, Black and Hispanic patients with ADRD were more likely to live in areas with high poverty rates and low education levels.
TABLE 2.
Characteristics of Medicare Fee-For-Service Beneficiaries With Alzheimer Disease and Related Dementias Aged 65 and Older
| White | Black | Hispanic | ||||||
|---|---|---|---|---|---|---|---|---|
| N=296,941 | N=27,676 | N=11,778 | ||||||
| Mean | SD | Mean | SD | P | Mean | SD | P | |
| Total Medicare payment per person per year ($) | 36,642.23 | 37,105.35 | 40,693.68 | 44,329.79 | <0.001 | 42,571.89 | 44,071.37 | <0.001 |
| Acute inpatient Medicare payment ($) | 15,854.6 | 22,212.38 | 18,094.12 | 27,319.92 | <0.001 | 18,651.63 | 25,945.82 | <0.001 |
| Age (100%) | ||||||||
| Age 65–74 year | 0.22 | 0.41 | 0.31 | 0.46 | <0.001 | 0.28 | 0.45 | <0.001 |
| Age 75–84 year | 0.39 | 0.49 | 0.39 | 0.49 | 0.71 | 0.39 | 0.49 | 0.78 |
| Age 85 y and over | 0.40 | 0.49 | 0.31 | 0.46 | <0.001 | 0.33 | 0.47 | <0.001 |
| Female (100%) | 0.56 | 0.50 | 0.56 | 0.50 | 0.18 | 0.53 | 0.50 | <0.001 |
| Co-comorbidities (100%) | ||||||||
| Depression | 0.42 | 0.49 | 0.29 | 0.45 | <0.001 | 0.40 | 0.49 | <0.001 |
| Acute myocardial infarction | 0.05 | 0.22 | 0.05 | 0.22 | 0.82 | 0.05 | 0.23 | 0.44 |
| Asthma | 0.09 | 0.29 | 0.11 | 0.31 | <0.001 | 0.11 | 0.31 | <0.001 |
| Heart failure | 0.44 | 0.50 | 0.48 | 0.50 | <0.001 | 0.44 | 0.50 | 0.81 |
| Chronic obstructive pulmonary disease | 0.31 | 0.46 | 0.27 | 0.44 | <0.001 | 0.25 | 0.43 | <0.001 |
| Diabetes | 0.38 | 0.48 | 0.57 | 0.50 | <0.001 | 0.57 | 0.49 | <0.001 |
| Hyperlipidemia | 0.74 | 0.44 | 0.73 | 0.44 | 0.013 | 074 | 0.44 | 0.31 |
| Hypertension | 0.91 | 0.29 | 0.96 | 0.19 | <0.001 | 0.93 | 0.26 | <0.001 |
| Teaching hospital | 0.18 | 0.38 | 0.28 | 0.45 | <0.001 | 0.19 | 0.39 | 0.05 |
| Hospital control (100%) | ||||||||
| For-profit hospitals | 0.12 | 0.33 | 0.13 | 0.34 | <0.001 | 0.25 | 0.43 | <0.001 |
| Non-profit hospitals | 0.77 | 0.42 | 0.75 | 0.43 | <0.001 | 0.65 | 0.48 | <0.001 |
| Government hospitals | 0.10 | 0.30 | 0.11 | 0.32 | <0.001 | 0.11 | 0.31 | 0.37 |
| Bed size (100%) | ||||||||
| Bed size<200 | 0.31 | 0.46 | 0.21 | 0.41 | <0.001 | 0.25 | 0.57 | <0.001 |
| Bed size ≥200 | 0.69 | 0.46 | 0.79 | 0.41 | <0.001 | 0.75 | 0.43 | <0.001 |
| Urban/rural location (100%) | ||||||||
| Rural area | 0.03 | 0.18 | 0.02 | 0.13 | <0.001 | 0.01 | 0.10 | <0.001 |
| Micropolitan area | 0.17 | 0.38 | 0.08 | 0.28 | <0.001 | 0.09 | 0.29 | <0.001 |
| Metropolitan area | 0.79 | 0.40 | 0.90 | 0.30 | <0.001 | 0.90 | 0.30 | <0.001 |
| 100% Black population in the community above 50% of the population median | 0.46 | 0.50 | 0.94 | 0.25 | <0.001 | 0.49 | 0.50 | <0.001 |
| 100% Population with high school degrees in the community above 50% of the population median | 0.53 | 0.50 | 0.26 | 0.44 | <0.001 | 0.29 | 0.45 | <0.001 |
| 100% Population living in poverty in the community above 50% of the population median | 0.48 | 0.50 | 0.76 | 0.43 | <0.001 | 0.67 | 0.47 | <0.001 |
| 100% Physicians per 1,000 residents in the community above 50% of the population median | 0.49 | 0.50 | 0.54 | 0.50 | <0.001 | 0.50 | 0.50 | 0.25 |
Data source: the linked data sets of 2017 Centers for Medicare and Medicaid Services (CMS) Medicare 100% inpatient claims data, Medicare Beneficiary Summary File, American Hospital Annual Survey, American Community Survey, and the Area Health Resource File.
This study focused on community-dwelling Medicare fee-for-service beneficiaries who had a diagnosis of Alzheimer disease and related dementias (ADRD) that were aged 65 and older and had at least one hospital visit in 2017. Elderly with Medicare Advantage or dual eligibilities were not included. ADRD was defined using the CMS chronic conditions data warehouse codes. Non-Hispanic White (White), Black, and Hispanic patients with ADRD are reported where non-Hispanic Whites were used as the reference group.
Percentages are commonly rounded; as a result, the sum of the values may not always add to 100%.
After controlling for beneficiary, hospital, and area-level characteristics, results showed that telehealth postdischarge services were significantly associated with higher total Medicare payment (coefficient=0.034, P value<0.001) and acute inpatient Medicare payment (coefficient=0.048, P value<0.001) (Table 3). Telehealth-treatment services were associated with higher total Medicare payments (coefficient=0.007, P value=0.34) and acute inpatient Medicare payments (coefficient=0.01, P value=0.24), but these findings were not statistically significant. Black and Hispanic patients with ADRD had higher total Medicare payments and acute inpatient Medicare payments compared with Whites.
TABLE 3.
Associations Between Telehealth-Postdischarge and Telehealth-Treatment Services With Total Medicare Payments and Acute Inpatient Medicare Payments
| Total Medicare Payment | Acute Inpatient Medicare Payment | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | |||
| Telehealth-postdischarge services | 0.034 | 0.024 | 0.045 | <0.001 | 0.048 | 0.035 | 0.061 | <0.001 |
| Telehealth-treatment services | 0.007 | −0.008 | 0.023 | 0.343 | 0.011 | −0.007 | 0.029 | 0.242 |
| Black | 0.036 | 0.024 | 0.048 | <0.001 | 0.030 | 0.015 | 0.044 | <0.001 |
| Hispanic | 0.012 | −0.006 | 0.029 | 0.197 | 0.063 | 0.042 | 0.084 | <0.001 |
| Telehealth-postdischarge services * Black | −0.098 | −0.134 | −0.062 | <0.001 | −0.108 | −0.150 | −0.067 | <0.001 |
| Telehealth-treatment services * Black | −0.098 | −0.149 | −0.048 | <0.001 | −0.068 | −0.128 | −0.008 | 0.026 |
| Telehealth-postdischarge services* Hispanic | 0.012 | −0.038 | 0.063 | 0.631 | 0.032 | −0.034 | 0.098 | 0.341 |
| Telehealth-treatment services* Hispanic | −0.121 | −0.193 | −0.049 | 0.001 | −0.095 | −0.190 | −0.001 | 0.048 |
| Age 65–74 y | Reference | |||||||
| Age 75–84 y | −0.170 | −0.179 | −0.162 | <0.001 | −0.246 | −0.256 | −0.236 | <0.001 |
| Age 85 y and older | −0.317 | −0.325 | −0.309 | <0.001 | −0.460 | −0.470 | −0.450 | <0.001 |
| Female | −0.085 | −0.091 | −0.080 | <0.001 | −0.131 | −0.138 | −0.124 | <0.001 |
| Depression | 0.142 | 0.136 | 0.147 | <0.001 | 0.029 | 0.022 | 0.036 | <0.001 |
| Acute myocardial infarction | 0.223 | 0.211 | 0.234 | <0.001 | 0.448 | 0.434 | 0.462 | <0.001 |
| Asthma | 0.074 | 0.065 | 0.083 | <0.001 | 0.059 | 0.047 | 0.070 | <0.001 |
| Heart Failure | 0.305 | 0.299 | 0.311 | <0.001 | 0.364 | 0.357 | 0.371 | <0.001 |
| Chronic obstructive pulmonary disease | 0.138 | 0.132 | 0.144 | <0.001 | 0.153 | 0.145 | 0.161 | <0.001 |
| Diabetes | 0.092 | 0.086 | 0.098 | <0.001 | 0.034 | 0.027 | 0.042 | <0.001 |
| Hyperlipidemia | 0.053 | 0.046 | 0.060 | <0.001 | 0.081 | 0.073 | 0.089 | <0.001 |
| Hypertension | 0.102 | 0.090 | 0.113 | <0.001 | 0.088 | 0.076 | 0.101 | <0.001 |
| Teaching hospitals | Reference | |||||||
| Non-teaching hospitals | −0.249 | −0.258 | −0.241 | <0.001 | −0.398 | −0.408 | −0.387 | <0.001 |
| For-profit hospitals | Reference | |||||||
| Non-profit hospitals | −0.118 | −0.127 | −0.109 | <0.001 | −0.049 | −0.060 | −0.038 | <0.001 |
| Government hospitals | −0.101 | −0.114 | −0.089 | <0.001 | −0.012 | −0.027 | 0.003 | 0.111 |
| Bed size<200 | Reference | |||||||
| Bed size (≥200) | 0.033 | 0.027 | 0.040 | <0.001 | 0.086 | 0.078 | 0.094 | <0.001 |
| Metropolitan area | Reference | |||||||
| Rural area | −0.008 | −0.025 | 0.009 | 0.361 | 0.024 | 0.004 | 0.044 | 0.020 |
| Micropolitan area | −0.004 | −0.012 | 0.005 | 0.396 | 0.042 | 0.032 | 0.053 | <0.001 |
| % Black population in the county above 50% of the population median | 0.016 | 0.009 | 0.023 | <0.001 | −0.010 | −0.019 | −0.002 | 0.021 |
| % Population with high school degrees in the county above 50% of the population median | −0.027 | −0.034 | −0.020 | <0.001 | 0.003 | −0.006 | 0.011 | 0.553 |
| % Population living in poverty in the county above 50% of the population median | 0.007 | 0.000 | 0.014 | 0.038 | 0.000 | −0.009 | 0.008 | 0.914 |
| % Physicians per 1,000 residents in the county above 50% of the population median | 0.008 | 0.002 | 0.015 | 0.012 | −0.011 | −0.018 | −0.003 | 0.006 |
| Constant | 10.292 | 10.263 | 10.322 | <0.001 | 9.467 | 9.432 | 9.502 | <0.001 |
| State-fixed effect | Controlled | |||||||
This study focused on community-dwelling Medicare fee-for-service beneficiaries who had a diagnosis of Alzheimer disease and related dementias (ADRD) that were aged 65 and older and had at least one hospital visit in 2017. The elderly with Medicare Advantage or dual eligibilities were not included. ADRD was defined using the Centers for Medicare and Medicaid Services (CMS) chronic conditions data warehouse codes. Non-Hispanic White (White), Black, and Hispanic patients with ADRD are reported. Whites were used as the reference group.
HIT indicates health information technology.
Coefficients of interaction terms between telehealth-postdischarge services and Black patients with ADRD were significantly negative in the regressions of total Medicare payments (coefficient=−0.10, P value<0.001) and acute inpatient Medicare payments (coefficient=−0.11, P value <0.001). Coefficients of interaction terms of telehealth-treatment services and Black were also significantly negative in both payment regressions (coefficient=−0.1, P value<0.001 and coefficient=−0.07, P value=0.03). The coefficients of the interaction terms of telehealth-treatment services and Hispanic were significantly negative (coefficient=−0.12, P value <0.001 and coefficient=−0.1, P value=0.05).
Table 3 suggested that being treated in hospitals that offered telehealth postdischarge services was associated with reduced costs for Black patients with ADRD. Hence, we calculated the marginal effects of telehealth postdischarge services (Table 4). Results showed a decrease of $1,104 ($32,788 vs. $33,892) in the total Medicare payment and a decrease of $569 ($14,194 vs. 14,763) in the acute inpatient Medicare payment per person per year when Black patients with ADRD were treated in hospitals with telehealth postdischarge infrastructure.
TABLE 4.
Marginal Effects of Telehealth-Postdischarge and Telehealth-Treatment Services
| Telehealth-Postdischarge Services | |||||||
|---|---|---|---|---|---|---|---|
| YES | NO | ||||||
| ME | 95% CI | ME | 95% CI | Difference | |||
| Black patients with ADRD | |||||||
| Total Medicare payment | $32,787.87 | $31,644.04 | $33,931.70 | $33,892.29 | $33,484.21 | $34,300.37 | $ (1,104.42) |
| Acute inpatient Medicare payment | $14,193.87 | $13,629.33 | $14,758.42 | $14,763.09 | $14,536.57 | $14,989.60 | $ (569.22) |
| Telehealth-Treatment Services | |||||||
| YES | NO | ||||||
| ME | 95% CI | ME | 95% CI | Difference | |||
| Black patients with ADRD | |||||||
| Total Medicare payment | $32,317.64 | $30,719.59 | $33,915.69 | $33,860.62 | $33,466.13 | $34,255.12 | $ (1,542.98) |
| Acute inpatient Medicare payment | $14,432.64 | $13,590.42 | $15,274.87 | $14,714.42 | $14,496.79 | $14,932.05 | $ (281.78) |
| Hispanic patients with ADRD | |||||||
| Total Medicare payment | $33,309.41 | $30,846.03 | $35,772.80 | $35,388.49 | $34,768.11 | $36,008.87 | $ (2,079.08) |
| Acute inpatient Medicare payment | $14,872.03 | $13,438.72 | $16,305.33 | $15,499.53 | $15,137.49 | $15,861.57 | $ (627.50) |
This study focused on community-dwelling Medicare fee-for-service beneficiaries who had a diagnosis of ADRD that were aged 65 and older and had at least 1 hospital visit in 2017. Elderly with Medicare Advantage or dual eligibilities were not included. ADRD was defined using the CMS chronic conditions data warehouse codes. Non-Hispanic White (White), Black, and Hispanic patients with ADRD are reported where non-Hispanic Whites were used as the reference group.
ADRD indicates Alzheimer disease and related dementias; ME, marginal effect.
Table 3 suggested that telehealth treatment services were associated with reduced Medicare payments for Black and Hispanic patients with ADRD. Similarly, we calculated the marginal effects of telehealth-treatment services on cost (Table 4). Results showed a reduction of $1,543 ($32,318 vs. $33,860) in the total Medicare payment and a reduction of $281 ($14,432 vs. $14,714) in the acute inpatient Medicare payment when Black patients with ADRD were treated in hospitals with telehealth postdischarge infrastructure. Reductions were also found among Hispanic patients with ADRD (ie, a reduction of $2,079 ($33,309 vs. $35,388) in the total Medicare payment and a reduction of $628 ($14,872 vs. $15,500) in the acute inpatient Medicare payment when Hispanic patients with ADRD were treated in hospitals with the telehealth treatment infrastructure.
DISCUSSION
Building healthy and sustainable aging-friendly health care structures requires population-based integrated ADRD care initiatives at the health care system level. The 2020 National Health IT Priorities for Research report by the National Coordinator for Health Information Technology has acknowledged how HIT can bridge gaps in the continuity of care for underserved patients with complex health needs by improving provider access to health information.31 Understanding the potential cost-saving or cost-enhancing of HIT adoption can help policymakers understand the capacity of HIT investment for population health and health equity.
The results of our study showed that hospital-based telehealth-postdischarge services were associated with significantly higher total Medicare payment and acute inpatient Medicare payment per person per year among patients with ADRD. The associations between hospital-based telehealth-treatment services and payments were not significant. In addition, results showed that these associations varied by patients’ races and ethnicities. The reductions of the payments associated with telehealth postdischarge and treatment services were more pronounced among Black patients with ADRD. Telehealth-treatment services were associated with significant payment reductions among Hispanic patients with ADRD. Results of the study suggested that adopting HIT infrastructure might be cost-enhancing overall, but HIT can be cost-saving among racial and ethnic minority groups who could benefit from telehealth services.
Racial and ethnic group members with ADRD are at high risk for uncoordinated and low-quality care, including higher rates of hospitalizations, higher levels of impairment at the time of referral to ADRD services, and lower rates of being prescribed anti-dementia medications. By incorporating HIT in care coordination and treatment, racial and ethnic minority individuals, especially those who live in underrepresented communities dealing with complex health issues,22,23,32,33 may receive more informed and timely follow-up health care. Telehealth services include remote patient monitoring and ongoing chronic care management, two substantial components of care coordination. Having these functions can be particularly helpful for ADRD patients with complex medical needs who require frequent medical care. Studies have shown that HIT services focused on improved patient monitoring and care management contribute to better health outcomes for chronically-ill patients who often face difficulties managing self-care regimens.8 This may be particularly beneficial for Black and Hispanic patients with a disproportionately high prevalence of ADRD who might encounter unique barriers to access care.34 This study supports the notion that HIT services may potentially assist clinicians with ADRD patient monitoring, providing early diagnosis and treatment for ADRD patients, improving the management of complex and co-existing conditions, and working towards improved communication between providers and caregivers.
More research is needed to understand how to design telehealth systems to best engage Hispanic patients with ADRD and their caregivers proactively. We speculated that HIT might be able to improve timely patient-physician communications and encourage patient empowerment. Recent surveys of patients with ADRD and their caregivers identified that Hispanic patients with ADRD and their caregivers were more likely to report having language barriers or a lack of health literacy and facing substantial social stigma compared with their White counterparts. They often perceive and experience discrimination, distrust health care providers, and have low confidence in communication.8,35 We also acknowledge that Hispanics are not one monolithic group. Patterns of health care utilization and expenditures, cultural background, immigrant and citizenship status, and patient preferences vary substantially across Hispanic subgroups (eg, Mexican, Puerto Rican, Cuban, etc.).36,37
Due to the cognitive decline associated with ADRD that often impacts patients’ ability to function independently, many patients must rely on families to play a caregiver role or hire caregivers. Although we were not able to incorporate caregivers’ characteristics and preferences in the analysis due to data limitations, we acknowledge that caregivers of patients with ADRD have an essential role in liaising between providers and patients. Providers using telehealth/medicine services for this patient population need to consider potential technological barriers for caregivers in terms of access to technology and their ability to utilize the software associated with the technology.38 Engaging caregivers of patients with ADRD for home-based telemedicine/health services can help patients adhere to care visits and medication management.39,40
Our study has several limitations. First, we examined broad associations between hospital-based HIT services and cost. Future studies may further investigate the impact of HIT on health care access and quality and consider patient disease severity, stages of ADRD development, and ADRD caregivers’ access to HIT when data are available. Second, our study focused on community-dwelling residents. Over 58% of older adults with ADRD are community-dwelling (ie, remain in their residences vs. living in custodial nursing facilities).41 However, individuals with ADRD often move between nursing facilities, hospitals, and homes rather than remaining solely in their residences or nursing facilities.42 Future studies on ADRD patients should examine the role of HIT in postacute care settings (eg, long-term care hospitals, inpatient rehabilitation facilities, skilled nursing facilities, and home health agencies). Finally, our study focused on Medicare FFS beneficiaries with ADRD diagnoses. We were not able to observe the health care patterns of ADRD patients who were dual eligible, enrolled in Medicare Advantage, or other private health insurance. Due to data limitations, our measures of Medicare payments did not cover patients’ out-of-pocket payments.
CONCLUSION
It is an opportune time to understand the barriers to implementing robust HIT systems.43 The literature suggested HIT-supported care coordination has the potential to break down longstanding silos between health care providers, community-based organizations, and social service agencies serving people with ADRD, particularly for racial and ethnic minority populations. The results of this study offer evidence of effective HIT services associated with reduced Medicare payments. Evidence suggests that HIT can be designed to make care integration and HIT implementation sustainable for underserved populations with ADRD. Results of our study also suggested that personalized HIT design (eg, postdischarge services for Black patients and treatment services for Hispanic patients with ADRD) is critical for racial and ethnic minority populations to address heterogeneous barriers and preferences in treatment.
Supplementary Material
Footnotes
This study is supported by the National Institute on Aging (R01AG62315) and the National Institute on Minority Health and Health Disparities (R01MD011523S1).
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.
Contributor Information
Jie Chen, Email: jichen@umd.edu.
Merianne Rose T. Spencer, Email: merianne@umd.edu.
Portia Buchongo, Email: pbuchong@umd.edu.
Min Qi Wang, Email: mqw@umd.edu.
REFERENCES
- 1. Kit M. Primary care physicians on the front lines of diagnosing and providing Alzheimer’s and dementia care: half say medical profession not prepared to meet expected increase in demands. Alzheimer’s Association. 2021. Available at: https://www.alz.org/news/2020/primary-care-physicians-on-the-front-lines-of-diag. Accessed November 16, 2021. [Google Scholar]
- 2. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayeda ER, Glymour MM, Quesenberry CP, et al. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institutes of Health. NIH Professional Judgment Budget for Alzheimer’s disease and related dementias for fiscal year 2023. 2021. Available at: https://www.nia.nih.gov/sites/default/files/2021-07/bypass-budget-report-fy23.pdf?utm_source=NIA+Main&utm_campaign=20b3074da4-20210721_blog&utm_medium=email&utm_term=0_ffe42fdac3-20b3074da4-7505237. Accessed November 16, 2021.
- 5. Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y). 2018;4:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: The impact of ethnoracial differences in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2011;25:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilligan AM, Malone DC, Warholak TL, et al. Health disparities in cost of care in patients with Alzheimer’s disease: an analysis across 4 state Medicaid populations. Am J Alzheimers Dis Other Demen. 2013;28:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alzheimer’s Association. Race, ethnicity and Alzheimer’s in America. 2021. Available at: https://www.alz.org/media/Documents/alzheimers-facts-and-figures-special-report-2021.pdf. Accessed November 16, 2021.
- 9. Hughes S, Lepore MPPM, Wiener JM. Research on care coordination for people with dementia and family caregivers. National Research Summit on Care, Services, and Supports for Persons with Dementia and Their Caregivers; 2017; Bethesda, MD.
- 10. Lines LM, Ahaghotu C, Tilly J, et al. Care coordination for people with Alzheimer’s disease and related dementias: Literature review. Report prepared for the Office of the Assistant Secretary for Planning and Evaluation. Washington, DC: RTI International. 2013. Available at: https://aspe.hhs.gov/system/files/pdf/76771/AlzCC.pdf. Accessed November 15, 2021. [Google Scholar]
- 11. Vertinsky L. Combating Alzheimer’s disease through effective public-private partnerships. Health Affairs Blog. 2021. Available at: 10.1377/hblog20210810.999825. Accessed November 16, 2021. [DOI] [Google Scholar]
- 12. The Office of the National Coordinator for Health Information Technology (ONC). What is a patient portal? 2017. Available at: https://www.healthit.gov/faq/what-patient-portal. Accessed November 16, 2021.
- 13. Dendere R, Slade C, Burton-Jones A, et al. Patient portals facilitating engagement with inpatient electronic medical records: A systematic review. J Med Inter Res. 2019;21:e12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asagbra OE, Burke D, Liang H. The association between patient engagement HIT functionalities and quality of care: Does more mean better? Int J Med Inform. 2019;130:103893. [DOI] [PubMed] [Google Scholar]
- 15. Irizarry T, Dabbs AD, Curran CR. Patient portals and patient engagement: a state of the science review. J Med Inter Res. 2015;17:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Unruh MA, Jung HY, Kaushal R, et al. Hospitalization event notifications and reductions in readmissions of Medicare fee-for-service beneficiaries in the Bronx, New York. J Am Med Inform Assoc. 2017;24(e1):e150–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elysee G, Yu H, Herrin J, et al. Association between 30-day readmission rates and health information technology capabilities in US hospitals. Medicine. 2021;100:e24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orenstein D. EHR integration: a digital health imperative. Health Catalyst 2021. Available at: https://www.healthcatalyst.com/insights/EHR-integration-digital-health-imperative. Accessed February 10, 2021.
- 19. Office of the National Coordinator for Health Information Technology. Improving hospital transitions and care coordination using automated admission, discharge and transfer alerts. 2013. Available at: https://www.healthit.gov/sites/default/files/onc-beacon-lg1-adt-alerts-for-toc-and-care-coord.pdf. Accessed November 16, 2021.
- 20. Abraham J, Meng A, Tripathy S, et al. Effect of health information technology (HIT)-based discharge transition interventions on patient readmissions and emergency room visits: a systematic review. J Am Med Inform Assoc. 2022;29:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang N, Albaroudi A, Benjenk I, et al. Exploring hospital-based health information technology functions for patients with Alzheimer’s Disease and related Dementias. Preventive Medicine Reports. 2021;23:101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lines LM, Wiener JM. Racial and ethnic disparities in Alzheimer’s disease: a literature review. US Department of Health and Human Services, Assistant Secretary for Planning and Evaluation, Office of Disability, Aging and Long-Term Care Policy. 2014. Available at: https://aspe.hhs.gov/reports/racial-ethnic-disparities-alzheimers-disease-literature-review-0. Accessed August 20, 2021.
- 23. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18:223–254. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Buchongo P, Spencer MRT, et al. An HIT-supported care coordination framework for reducing structural racism and discrimination for patients with ADRD. Am J Geriatr Psychiatr. 2022;30:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Hospital Association Annual Survey Database. Available at: https://www.ahadata.com/aha-annual-survey-database. Accessed November 16, 2021.
- 26. CMS chronic conditions data warehouse. Available at: https://www2.ccwdata.org/web/guest/condition-categories. Accessed November 16, 2021.
- 27. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng VKY, Cribbie RA. Using the gamma generalized linear model for modeling continuous, skewed and heteroscedastic outcomes in psychology. Curr Psychol. 2017;36:225–235. [Google Scholar]
- 29. Kirkland EB, Heincelman M, Bishu KG, et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003–2014. J Am Heart Assoc. 2018;7:e008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newton JC, Johnson CE, Hohnen H, et al. Out-of-pocket expenses experienced by rural Western Australians diagnosed with cancer. Support Care Cancer. 2018;26:3543–3552. [DOI] [PubMed] [Google Scholar]
- 31. Office of the National Coordinator for Health Information Technology. National health IT priorities for research: A policy and development agenda. 2020. Available at: https://www.healthit.gov/sites/default/files/page/2020-01/PolicyandDevelopmentAgenda.pdf. Accessed November 16, 2021. [DOI] [PMC free article] [PubMed]
- 32. Weuve J, Barnes LL, de Leon CFM, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (Cambridge, Mass). 2018;29:151; dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z, Hayward MD, Yu YL. Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Social Behavior. 2016;57:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Green-Harris G, Coley A, Koscik R, et al. Addressing disparities in alzheimer’s disease and african-american participation in research: an asset-based community development approach. Front Aging Neurosci. 2019;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borrayo EA, Goldwaser G, Vacha-Haase T, et al. An inquiry into Latino caregivers’ experience caring for older adults with Alzheimer’s disease and related dementias. J Applied Gerontol. 2007;26:486–505. [Google Scholar]
- 36. Vargas-Bustamante A, Chen J. physicians cite hurdles ranging from lack of coverage to poor communication in providing high quality care to Latino patients. Health Affairs. 2011;30:1921–1929. [DOI] [PubMed] [Google Scholar]
- 37. Vargas-Bustamante A, Chen J, Rodriguez H, et al. Use of preventive care services among Latino subgroups: how much are disparities attributable to the health system? Am J Preventive Med. 2010;38:610–619. [DOI] [PubMed] [Google Scholar]
- 38. Yi JS, Pittman CA, Price CL, et al. Telemedicine and Dementia Care: A Systematic Review of Barriers and Facilitators. J Am Med Dir Assoc. 2021;22:1396–1402.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gately ME, Trudeau SA, Moo LR. In-Home Video Telehealth for Dementia Management: Implications for Rehabilitation. Curr Geri Rep. 2019;8:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang W, Homer M, Rossi MI. Use of Clinical Video Telehealth as a Tool for Optimizing Medications for Rural Older Veterans with Dementia. Geriatrics. 2018;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alzheimer’s Association 2017. Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- 42. Callahan CM, Arling G, Tu W, et al. Transitions in care among older adults with and without dementia. J Am Geriatr Soc. 2012;60:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agrawal S, Gandhi T. Telehealth should be expanded—if it can address today’s health care challenges. Health Affairs Blog. 2020. Available at: https://doi.org/. Accessed November 16, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


