Objective:
To investigate the risk factors for cytomegalovirus (CMV) infection within 100 days and the relationship between early CMV infection and 1-year relapse for patients with acute leukemia following allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods:
Three hundred fifty-nine patients with acute leukemia who received allo-HSCT at our center between January 2015 and January 2020 were retrospectively reviewed.
Results:
Of 359 patients, 48.19% (173) patients experienced CMV infection within 100 days posttransplantation. In univariate and multivariate logistic analysis, haploidentical-related donor (HRD) (P < 0.001; odds ratio [OR], 5.542; 95% confidence interval [CI], 3.186–9.639), and ratio of CD3+CD8+ cells in lymphocytes <14.825% (P < 0.001; OR, 3.005; 95% CI, 1.712–5.275) were identified as 2 independent risk factors. One-year relapse rate (RR) between the CMV infection group and the non-CMV infection group was not statistically significant (18.5% vs 19.9%, P = 0.688). When we divided the total cohort into AML, ALL, and MAL subgroups, there were no significant differences as well (P = 0.138; P = 0.588; P = 0.117; respectively).
Conclusion:
In conclusion, donor type (HRD) and the insufficient recovery of CD3+CD8+ cells were independent risk factors for CMV infection within 100 days posttransplantation in patients with acute leukemia. CMV infection within 100 days did not influence the incidence of relapse in 1 year for patients with acute leukemia.
Keywords: Acute leukemia, Allogeneic hematopoietic stem cell transplantation, Cytomegalovirus, Risk factors, Relapse
1. INTRODUCTION
Cytomegalovirus (CMV) is a member of herpes viruses and is ubiquitous worldwide. In industrialized countries, it infects between 60% and 70% of adults, whereas the prevalence is almost 100% in emerging countries.1 CMV infection may be latent in healthy people, but it can reactivate and cause manifestations from asymptomatic DNAemia to life-threatening end-organ diseases when the immune system is impaired. CMV infection is the most common opportunistic infection and significant cause of mortality for patients undergoing hematopoietic stem cell transplantation (HSCT). It occurs in 60% to 70% of CMV-seropositive recipients and 20% to 30% of CMV-seronegative recipients with CMV-seropositive donors.2 Currently, treatment for CMV infection includes universal prophylaxis and the preferred preemptive therapy. Although current therapy has reduced the risk of death from CMV disease to below 10%, early cytomegalovirus reactivation (with 100 days posttransplantation) remains associated with increased transplant-related mortality.3–6 It may be due to the renal toxicity and bone marrow suppression of current antiviral drugs and “indirect effects” of CMV infection, including secondary bacterial and fungal infections, graft failure, graft-versus-host disease (GVHD), and so on.2,7 In this article, we analyze some parameters of immune reconstruction posttransplantation, together with conventional risk factors, intending to identify early indicators for CMV infection in patients with acute leukemia. In addition, some articles8–11 suggested that CMV infection was associated with decreased incidence of relapse in acute leukemia patients, but other studies did not find significance.3 We also discuss the controversial question whether CMV infection could reduce the relapse of acute leukemia in this article.
2. RESULTS
2.1. Incidence and characteristics
A total of 359 patients with acute leukemia who underwent allo-HSCT between January 2015 and January 2020 were enrolled in the incidence cohort, and 48.18% (173/359) patients experienced CMV infection with 100 days posttransplantation. The median time for CMV infection was 39 days after allo-HSCT, ranging from 5 to 83 days. The characteristics of the patients were listed in Table 1.
Table 1.
Characteristics of all patients.
| Characteristics | All patients (N = 359) |
|---|---|
| Underlying diseases | |
| AML | 237 |
| ALL | 112 |
| MAL | 10 |
| Gender | |
| Male | 198 |
| Female | 161 |
| Risk stratification | |
| High | 166 |
| Standard | 193 |
| Disease status | |
| Non-CR | 40 |
| CR | 319 |
| Donor type | |
| HRD | 195 |
| MSD | 164 |
| Donor/recipient serostatus | |
| D+/R+ | 311 |
| D−/R+ | 19 |
| D+/R− | 11 |
| D−/R− | 0 |
| Graft | |
| PBSC | 340 |
| BM+PBSC | 19 |
| Age (y) | 34.00 (3–58) |
| MNC (×108/kg) | 10.00 (3.21–25.63) |
| CD34+ cells (×106/kg) | 2.81 (1.3–19.76) |
ALL = acute lymphocytic leukemia, AML = acute myeloid leukemia, BM = bone marrow, CR = complete remission, HRD = haploidentical-related donor, MAL = mixed acute leukemia, MNC = mononuclear cell, MSD = matched sibling donor, PBSC = peripheral blood stem cell.
2.2. Risk factors and relapse
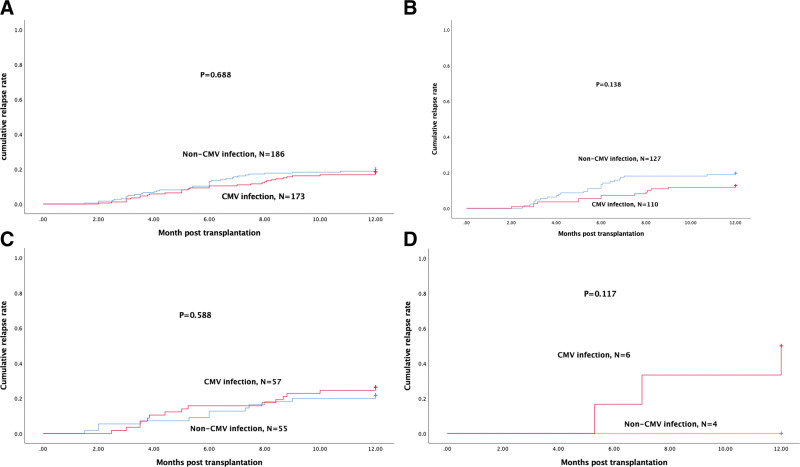
The patients were divided into 2 groups according to whether they were infected by CMV within 100 days following HSCT or not. In univariate analysis, donor type (HRD), ATG, mycophenolate mofetil (MMF), corticosteroid therapy in 30 days, GVHD in 30 days, and the ratio of CD3+CD8+ cells in lymphocytes were identified as potential risk factors (P < 0.05) (Table 2). However, as ATG, MMF, and corticosteroid therapy in 30 days were strongly associated with donor type, they were not taken into multivariate logistic analysis. Two independent risk factors were identified in the multivariate logistic analysis: donor type (HRD) (P < 0.001; odds ratio [OR], 5.542; 95% CI, 3.186–9.639), and the ratio of CD3+CD8+ cells in lymphocytes <14.825% (P < 0.001; OR, 3.005; 95% CI, 1.712–5.275) (Table 3). We also compared the relapse rate (RR) in 1 year posttransplantation between the CMV infection group and the non-CMV infection group, but it was not statistically significant (18.5% vs 19.9%, P = 0.688). Then, we further analyzed the relationship between the RR and CMV infection in 3 subgroups. In the AML, ALL, and MAL subgroups, the RR was also not significantly different (P = 0.138; P = 0.588; P = 0.177; respectively) (Fig. 3).
Table 2.
Univariate analysis of risk factors for CMV infection.
| CMV infection (N = 173) | Non-CMV infection (N = 186) | P value | ||
|---|---|---|---|---|
| Underlying diseases | AML | 110 | 127 | 0.553 |
| ALL | 57 | 55 | ||
| MAL | 6 | 4 | ||
| Gender | Male | 96 | 102 | 0.901 |
| Female | 77 | 84 | ||
| Risk stratification | High | 78 | 88 | 0.673 |
| Standard | 95 | 98 | ||
| Disease status | Non-CR | 15 | 25 | 0.151 |
| CR | 158 | 161 | ||
| Donor type | HRD | 139 | 56 | <0.001 |
| MSD | 34 | 130 | ||
| Donor/recipient serostatus | D+/R+ | 153 | 158 | 0.166 |
| D−/R+ | 12 | 7 | ||
| D+/R− | 3 | 8 | ||
| TBI in conditioning regimen | Yes | 42 | 42 | 0.704 |
| No | 131 | 144 | ||
| ATG | Yes | 157 | 82 | <0.001 |
| No | 16 | 104 | ||
| MMF | Yes | 109 | 53 | <0.001 |
| No | 64 | 133 | ||
| MRD | Positive | 47 | 60 | 0.292 |
| Negative | 126 | 126 | ||
| Corticosteroid therapy in 30 d | Yes | 145 | 83 | <0.001 |
| No | 28 | 103 | ||
| GVHD in 30 d | Yes | 36 | 24 | 0.045 |
| No | 137 | 162 | ||
| Age (y) | Mean ± SD | 33.93 ± 13.30 | 33.72 ± 13.33 | 0.878 |
| Donor age (y) | Mean ± SD | 37.02 ± 13.43 | 35.08 ± 12.55 | 0.157 |
| MNC (×108/kg) | Mean ± SD | 10.87 ± 3.50 | 10.27 ± 3.30 | 0.097 |
| CD34+ cells (×106/kg) | Mean ± SD | 3.20 ± 1.11 | 3.14 ± 1.68 | 0.691 |
| Tregs in lymphocytes (%) | Mean ± SD | 1.53 ± 2.62 | 2.11 ± 4.81 | 0.168 |
| CD3+CD8+cells in lymphocytes (%) | Mean ± SD | 15.25 ± 15.38 | 25.84 ± 14.60 | <0.001 |
ALL = acute lymphocytic leukemia, AML = acute myeloid leukemia, ATG = antithymocyte globulin, BM = bone marrow, CMV = cytomegalovirus, CR = complete remission, GVHD = graft-versus-host disease, HRD = haploidentical-related donor, MAL = mixed acute leukemia, MMF = mycophenolate mofetil, MNC = mononuclear cell, MRD = minimal residual disease, MSD = matched sibling donor, PBSC = peripheral blood stem cell, TBI = total body irradiation, Tregs = regulatory T cells.
Table 3.
Multivariate logistic analysis of risk factors for CMV infection.
| Potential risk factors | P value | OR | 95% CI | |
|---|---|---|---|---|
| Donor type | <0.001 | 5.542 | 3.186 | 9.639 |
| HRD | ||||
| MSD | ||||
| CD3+CD8+ cells in lymphocytes (%) | <0.001 | 3.005 | 1.712 | 5.275 |
| <14.825 | ||||
| ≥14.825 | ||||
CMV = cytomegalovirus, MSD = matched sibling donor.
Figure 3.
One-year cumulative relapse rate of patients in the CMV infection group and non-CMV infection group. (A) Cumulative relapse rate for all the patients. (B) Cumulative relapse rate for AML patients. (C) Cumulative relapse rate for ALL patients. (D) Cumulative relapse rate for MAL patients. ALL = acute lymphocytic leukemia, AML = acute myeloid leukemia, CMV = cytomegalovirus, MAL = mixed acute leukemia.
3. DISCUSSION
CMV infection is the most common viral infection and an important cause of mortality for patients undergoing allo-HSCT. In this study, we wanted to identify risk factors for CMV infection in patients with acute leukemia within 100 days posttransplantation as the majority of CMV infection occurred in the first 100 days.3 According to previous studies, donor/recipient serostatus (IgG), unrelated donor, GVHD, myeloablative conditioning regimen, total body irradiation (TBI), antithymocyte globulin, mycophenolate mofetil, and corticosteroid therapy were demonstrated as risk factors for CMV infection.5,7,12 Donor/recipient serostatus (IgG) before transplantation was considered to be a major risk factor for CMV infection.7,13–15 Seronegative donor/seropositive recipient was at the highest risk, while when a seronegative recipient received graft from a seronegative donor, the risk of developing CMV infection is the lowest. Since CMV is very prevalent in emerging countries, the majority of our patients were seropositive 96.94% (348/359) and so were the donors, which was different from previous studies. A total of 341 patients had the corresponding serostatus information of their donors, and of which, 311 were D+/R+ (donor-seropositive/recipient-seropositive), 19 were D−/R+ (donor-seronegative/ recipient-seropositive), and 11 were D+/R− (donor-seropositive/recipient-seronegative) (Table 1). We incorporated the risk factors reported previously in our study, intending to find out risk factors and early predictors for CMV infection. In the univariate analysis, donor type (HRD), corticosteroid therapy in 30 days, ATG, MMF, GVHD in 30 days, and the ratio of CD3+CD8+ cells in lymphocytes were identified as potential risk factors (Table 2). As the interaction between ATG, MMF, and corticosteroid therapy in 30 days with donor type (HRD) was statistically significant (P < 0.05), they were not incorporated into multivariate logistic analysis. On the other hand, as serostatus has been suggested as a main risk factor for CMV infection, we also took it into the multivariate logistic analysis although it did not show significance in univariate analysis. Finally, 2 independent risk factors were identified: donor type (HRD) and ratio of CD3+CD8+ cells in lymphocytes <14.825%.
Patient receiving graft from an HRD had a higher risk of developing CMV infection (P < 0.001; OR: 5.542; 95% CI, 3.186–9.639). An SFGM-TC (Francophone Society of Bone Marrow Transplantation and Cellular Therapy) study12 focusing on HLA-matched donor showed that unrelated donor is an independent risk factor for CMV infection. This article actually proved the same thing from different points of view that receiving graft from a HLA-matched sibling donor was a protective factor for prevention of CMV infection after transplantation. Graft from either HLA-matched unrelated donor or HRD increased the hazard of developing CMV infection. This may due to more intensive preconditioning regimen, such as the use of ATG and TBI, which resulted in deeply impaired immune system and susceptibility to infection.
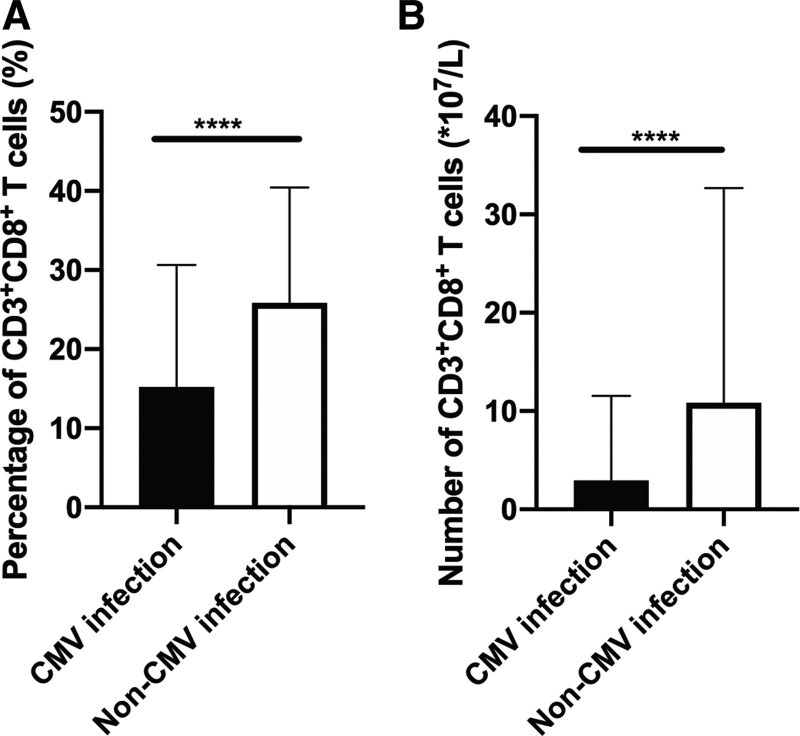
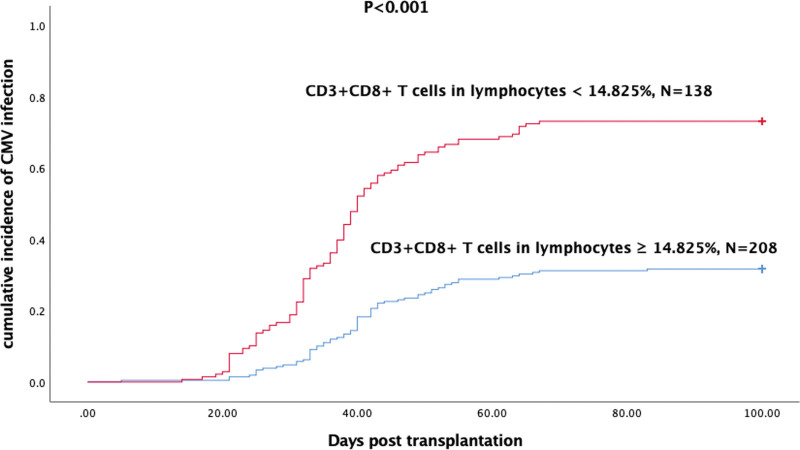
Another independent risk factor was the ratio of CD3+CD8+ cells in lymphocytes <14.825% (P < 0.001; OR, 3.005; 95% CI, 1.712–5.275). In recent years, immune reconstruction posttransplantation has drawn more and more attention, and monitoring of immune construction has showed to be a predictor of CMV infection.16–22 Liu et al18 demonstrated that patients with lower level of CMV-specific CD8+ TCM (central memory T cells) at day 30 post-HSCT had increased risk of refractory and recurrent CMV comparing with the higher one (P < 0.001). A more recent study19 suggested that 2 CMV-specific CD8+ T-cell functional subsets were strongly associated with risk of CMV: the nonprotective signature (NPS; IL-2− IFN-γ+ TNF-α− MIP-1β+) and the PS (IL-2+ IFN-γ+ TNF-α+ MIP-1β+) after the stimulation of CMV-pp65 peptides. High levels of the NPS and low levels of PS increased 100-day cumulative incidence of clinically significant CMV infection (35% vs 5%; P = 0.02; and 40% vs 12%; P = 0.05, respectively). Although CMV-specific immunity is of good predictive value of CMV infection, it needs specific detection and the parameters varied among institutions. We seek to find a common and universal parameter to assess the immune reconstruction and predict CMV infection. Then, we analyzed the immune cell subsets and focused on the ratio of CD4+CD25+ Tregs (regulatory T cells) and CD3+CD8+ T (cytotoxic T cells) cells in lymphocytes. In the univariate analysis, the ratio of CD4+CD25+ Tregs in lymphocytes showed no difference between the CMV infection group and the noninfection group, whereas the ratio of CD3+CD8+ T cells in lymphocytes was significantly lower in the CMV infection group (Fig. 1A). Furthermore, we compared the absolute number of CD3+CD8+ T cells in the 2 groups, and it was also significantly lower in the CMV infection group (Fig. 1B). As ratio of CD3+CD8+ T cells was continuous variables, we used statistics method (Youden index) to determine a cutoff (14.825%) for this parameter. Then, we divided patients into 2 groups according to the ratio of CD3+CD8+ T cells and the cumulative incidence of CMV infection within 100 days was significantly higher in the group below the cutoff (Fig. 2). In multivariate logistic analysis, the ratio of CD3+CD8+ T cells was an independent risk factor for CMV infection, suggesting insufficient recovery of CD3+CD8+ T cells was associated with CMV infection.
Figure 1.
Patients with CMV infection had lower ratio. (A) and absolute number (B) of CD3+CD8+ T cells. CMV = cytomegalovirus, **** indicates P < 0.0001.
Figure 2.
Patients with lower ratio of CD3+CD8+ T cells had higher incidence of CMV infection within 100 days posttransplantation. CMV = cytomegalovirus.
Some articles suggested that early CMV infection 8−11 was associated with reduced risk of relapse in patients with AML. However, the mechanisms of how CMV reactivation protect against AML relapse remain unclear. The possible mechanisms included: CMV infection promoted expansion in educated NKG2C+ natural killer with enhanced interferon γ production23; γδT cells elicited by CMV reactivation recognized CMV peptides which were cross-reactive against leukemia cells.24 However, in contrast to adult AML, in pediatric patients, CMV reactivation was associated with increased RR.25 A CIBMTR (Center for International Blood and Marrow Transplant Research) study3 including 9469 patients demonstrated that no protective effect of CMV reactivation in preventing leukemia relapse was observed. In our study, there was no significant difference between the CMV infection group and the non-CMV infection group (Fig. 3A). When we divided all the patients into subgroups according to the underlying diseases, the difference was not significant as well (Fig. 3B–D).
In conclusion, HRD and insufficient recovery of CD3+CD8+ T cells were associated with CMV infection within 100 days after allo-HSCT for patients with acute leukemia. The ratio of CD3+CD8+ T cells in lymphocytes <14.825% could be an early predictor for CMV infection and clinicians must be cautious of these patients. In addition, early CMV reactivation showed no protective effect in preventing 1-year leukemia relapse in patients with acute leukemia.
4. MATERIAL AND METHODS
4.1. Patients and definitions
Three hundred fifty-nine acute leukemia patients receiving allo-HSCT at the Hematopoietic Stem Cell Transplantation Center of Blood Diseases Hospital, Chinese Academy of Medical Sciences between January 2015 and January 2020 were retrospectively reviewed. CMV infection is defined as nucleic acid or virus isolation or detection of viral antigens in any body fluid or tissue specimen.26 In the current study, CMV DNA was detected by plasma sample using real-time PCR and CMV infection was defined as >1000 copies/mL. The detection was regularly performed at least twice a week when patients were in the hospital (the first 30 days posttransplantation) and once a week when they were out of the hospital within 100 days following HSCT. Once the patient was infected, the detection would be more frequent. The first time of immune cell subsets assay following HSCT was analyzed in the study. It was regularly performed two weeks posttransplantation, but it slightly varied among patients, and the median time was 18 days (11–36 days). All patients undergoing allo-HSCT received a myeloablative preconditioning regimen. The main regimen for acute myeloid leukemia (AML) was busulfan and cyclophosphamide (Bu+Cy), in addition of fludarabine (Flu), cytarabine (Ara-c), antithymocyte globulin (ATG), or not. For patients with acute lymphocytic leukemia (ALL), the regimen was TBI/melphalan (Mel) + cyclophosphamide (CTX) regimen in combination with Flu, Ara-c, ATG, or not.
For GVHD prophylaxis, all transplant recipients received FK506 or cyclosporine A, short-term methotrexate, in addition to MMF or not. All patients or their legal representatives provided written informed consent before transplantation. This study was approved by the Ethics Review Committee of our center and was in compliance with the Declaration of Helsinki.
4.2. Statistical Analysis
The clinical data were analyzed by the software GraphPad Prism 8 and IBM SPSS statistics 25. The descriptive statistics for continuous variables and chi-square test and Fisher exact test for categorical variables were used to compare incidence in univariate analysis. P <0.05 was regarded as potential risk factors in univariate analysis and further analyzed by multivariate logistic regression. The Kaplan–Meier method was used to estimate the cumulative incidence/relapse and differences were compared by the log-rank test. A two-sided P < 0.05 was considered as statistically significant.
ACKNOWLEDGMENTS
This work was funded by grants from the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant numbers 2021-1-I2M-017 and 2021-I2M-C&T-B-080). We are grateful to all the patients, doctors, and nurses participating in the study.
Footnotes
Author Contributions: S.F. contributed to study design and manuscript reviewing. J.C. contributed to the data collection, analysis, and manuscript composition. A.P., Y.Z., L.L., J.Z., and R.M. contributed to the data collection and interpretation. J.W., X.C., Y.H., D.Y., R.Z., W.Z., Q.M., E.J., and M.H. contributed to the treatment of the disease and data collection. All authors contributed to the article and approved the submitted version.
The authors declare that they have no conflict of interest.
This work was funded by grants from the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant numbers 2021-1-I2M-017 and 2021-I2M-C&T-B-080).
REFERENCES
- [1].Gupta M, Shorman M. Cytomegalovirus. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- [2].Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020;135:1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016;127:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016;128:2624–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Camargo JF, Komanduri KV. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther 2017;10:233–238. [DOI] [PubMed] [Google Scholar]
- [6].Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019;19:e260–e272. [DOI] [PubMed] [Google Scholar]
- [7].Melendez-Munoz R, Marchalik R, Jerussi T, et al. Cytomegalovirus infection incidence and risk factors across diverse hematopoietic cell transplantation platforms using a standardized monitoring and treatment approach: a comprehensive evaluation from a single institution. Biol Blood Marrow Transplant 2019;25:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011;118:1402–1412. [DOI] [PubMed] [Google Scholar]
- [9].Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 2013;122:1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jang JE, Kim SJ, Cheong JW, et al. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann Hematol 2015;94:275–282. [DOI] [PubMed] [Google Scholar]
- [11].Litjens NHR, van der Wagen L, Kuball J, et al. Potential beneficial effects of cytomegalovirus infection after transplantation. Front Immunol 2018;9:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beauvais D, Drumez E, Blaise D, et al. Scoring system for clinically significant CMV infection in seropositive recipients following allogenic hematopoietic cell transplant: an SFGM-TC study. Bone Marrow Transplant 2020;56:1305–1315 [DOI] [PubMed] [Google Scholar]
- [13].Stern L, Withers B, Avdic S, et al. Human cytomegalovirus latency and reactivation in allogeneic hematopoietic stem cell transplant recipients. Front Microbiol 2019;10:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yong MK, Cameron PU, Slavin M, et al. Identifying cytomegalovirus complications using the quantiferon-CMV assay after allogeneic hematopoietic stem cell transplantation. J Infect Dis 2017;215:1684–1694. [DOI] [PubMed] [Google Scholar]
- [15].Kalra A, Williamson T, Daly A, et al. Impact of donor and recipient cytomegalovirus serostatus on outcomes of antithymocyte globulin–conditioned hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:1654–1663. [DOI] [PubMed] [Google Scholar]
- [16].Yong MK, Gottlieb D, Lindsay J, et al. New advances in the management of cytomegalovirus in allogeneic haemopoietic stem cell transplantation. Intern Med J 2020;50:277–284. [DOI] [PubMed] [Google Scholar]
- [17].Krawczyk A, Ackermann J, Goitowski B, et al. Assessing the risk of CMV reactivation and reconstitution of antiviral immune response post bone marrow transplantation by the QuantiFERON-CMV-assay and real time PCR. J Clin Virol 2018;99–100:61–66. [DOI] [PubMed] [Google Scholar]
- [18].Liu J, Chang YJ, Yan CH, et al. Poor CMV-specific CD8+ T central memory subset recovery at early stage post-HSCT associates with refractory and recurrent CMV reactivation. J Infect 2016;73:261–270. [DOI] [PubMed] [Google Scholar]
- [19].Camargo JF, Wieder ED, Kimble E, et al. Deep functional immunophenotyping predicts risk of cytomegalovirus reactivation after hematopoietic cell transplantation. Blood 2019;133:867–877. [DOI] [PubMed] [Google Scholar]
- [20].Watanabe M, Kanda J, Hishizawa M, et al. Lymphocyte area under the curve as a predictive factor for viral infection after allogenic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2019;25:587–593. [DOI] [PubMed] [Google Scholar]
- [21].Navarro D, Amat P, de la Cámara R, et al. efficacy and safety of a preemptive antiviral therapy strategy based on combined virological and immunological monitoring for active cytomegalovirus infection in allogeneic stem cell transplant recipients. Open Forum Infect Dis 2016;3:ofw107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ariza-Heredia E, Jiang Y, Shah DP, et al. Cytomegalovirus (CMV) cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: The REACT Study. Clin Infect Dis 2020;71:2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012;119:2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scheper W, van Dorp S, Kersting S, et al. GammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013;27:1328–1338. [DOI] [PubMed] [Google Scholar]
- [25].Jeljeli M, Guérin-El Khourouj V, Porcher R, et al. Relationship between cytomegalovirus (CMV) reactivation, CMV-driven immunity, overall immune recovery and graft-versus-leukaemia effect in children. Br J Haematol 2014;166:229–239. [DOI] [PubMed] [Google Scholar]
- [26].Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017;64:87–91. [DOI] [PubMed] [Google Scholar]