Abstract
Background:
Antigen-specific immunoglobulin responses have yet to be studied at the oral mucosal surface during peanut oral immunotherapy (PnOIT).
Objective:
We aimed to quantify salivary peanut-specific and total IgG4 and IgA in participants from the Immune Tolerance Network’s IMPACT study, a phase 2 PnOIT trial.
Methods:
Peanut allergic children, aged 12 to <48 months at screening, were enrolled and randomized to peanut or placebo OIT for 134 weeks. Per protocol analysis included 69 PnOIT and 23 placebo participants. Double-blind, placebo-controlled food challenges were conducted at weeks 134 and 160 to assess desensitization and remission, respectively. Saliva samples were collected at baseline, 30, 82, 134, and 160 weeks to quantify peanut-specific and total IgG4 and IgA.
Results:
Participants who received PnOIT experienced significant increases in peanut-specific IgG4 in saliva, whereas participants on placebo did not (p<0.01 at all time points). Peanut-specific IgA/total IgA ratio was also significantly increased in participants treated with PnOIT when compared to those receiving placebo at 30 and 82 weeks (p<0.05). During PnOIT, desensitized participants had increased peanut-specific IgA that plateaued, whereas the not desensitized/no remission group did not change over time. Interestingly, when the PnOIT group was evaluated by clinical outcome, peanut-specific IgA was higher at baseline in the not desensitized/no remission group compared to the desensitized/remission group (p<0.05).
Conclusions:
PnOIT induces substantial increases in allergen-specific IgG4 and IgA in saliva. These data provide insight into OIT-induced mucosal responses and suggest the utility of these easily obtained samples for biomarker development.
Keywords: peanut allergy, oral immunotherapy, IgA, IgG4, saliva
Introduction
Peanut allergy is a growing public health concern affecting 2% of the US population.(1) The current standard of care is avoidance and ready access to epinephrine, although food specific immunotherapies are actively being investigated. Oral immunotherapy (OIT) has been studied for the last 15 years,(2, 3) and is an effective treatment option for peanut allergy. Indeed, one PnOIT product, Palforzia, recently gained FDA approval after a successful phase 3 study.(4) The biological mechanisms of PnOIT remain unclear, but consistent changes in systemic immunoglobulin levels have been observed.(5) Notably, peanut-specific IgG4 in plasma increases sharply within the first few months with some studies also showing increases in peanut-specific IgA throughout therapy.(6, 7) Peanut-specific IgE increases for the first 6 months on OIT, and then decreases below baseline levels after ~12 months.(8, 9) While these changes are consistently observed with treatment, they have had little success when used as biomarkers to predict OIT outcomes.(10) However, since OIT is administered at the oral and gastrointestinal mucosal surfaces, antigen-specific immunoglobulin responses at these sites may be more informative.
Oral tolerance is a naturally occurring process in non-allergic individuals that typically results in elevated levels of serum antigen-specific IgG4 and IgA. (11) For example, in the LEAP trial, early introduction of peanut in infants that were protected from peanut allergy resulted in substantial increases in peanut-specific IgG4, not seen in the infants avoiding peanut. (12) In a separate study, serum antigen-specific IgA2 was shown to play a role in tolerance in subjects that outgrew their egg allergy. (13) Collectively, these studies suggest antigen-specific IgG4 and IgA may dampen IgE responses. Food allergen-specific immunotherapies are employed to induce increased production of antigen-specific IgG4 and IgA, which have been demonstrated to block effector cell degranulation. (7, 14, 15) While these effects have been demonstrated with serum antigen-specific immunoglobulins, the effects at mucosal surfaces are not well-defined.
To evaluate local immune responses, we utilized samples from the Immune Tolerance Network’s IMPACT study, a phase 2 randomized, placebo-controlled trial of PnOIT.(16) Briefly, the IMPACT trial was designed to study desensitization and remission after PnOIT. Specifically, children aged 12 to <48 months with double-blind, placebo-controlled food challenge (DBPCFC)-confirmed peanut allergy were assigned to PnOIT (n=96) or placebo OIT (n=50) for 134 weeks. Participants were assessed for desensitization and remission by DBPCFCs at week 134 (end of treatment) and 160 (after 6 months of avoidance), respectively. The 69 participants that completed the protocol (i.e. the per protocol population) and passed the DBPCFC at weeks 134 and 160 were categorized as desensitized/remission (n=19), participants that passed the DBPCFC at week 134 but failed at week 160 were categorized as desensitized/no remission (n=40), and participants that did not pass the DBPCFC at week 134 were categorized as not desensitized/no remission (n=10) (Table 1). To assess the immunologic response to PnOIT at the oral mucosal surface and its potential to predict treatment outcomes, we aimed to quantify salivary peanut-specific and total IgG4 and IgA in the IMPACT trial and compare these to their sera counterparts.
Table 1.
Demographics of subjects on PnOIT.
| Desensitized / Remission (n=19) | Desensitized / No Remission (n=40) | Not Desensitized / No Remission (n=10) | |
|---|---|---|---|
| Age (Months) | 30 (24–38.4) | 39.6 (35.7–46.8) | 40.8 (36.9–41.7) |
| Sex | |||
| Female | 7 (37%) | 11 (28%) | 3 (30%) |
| Male | 12 (63%) | 29 (72%) | 7 (70%) |
| Race | |||
| Asian | 3 (16%) | 6 (15%) | 2 (20%) |
| Black | 0 (0%) | 1 (2%) | 0 (0%) |
| Mixed | 2 (11%) | 9 (22%) | 2 (20%) |
| White | 14 (74%) | 24 (60%) | 6 (60%) |
| Other Food Allergy | |||
| at Screening | 12 (63%) | 21 (52%) | 7 (70%) |
| at End of Study | 15 (79%) | 25 (62%) | 8 (80%) |
| Peanut-specific IgE (serum) | 16.5 (10.38–46.5) | 69.5 (38–196.75) | 135.4 (50.5–316.75) |
| Total IgE (serum) | 486 (192.5–703.75) | 400.5 (192.5–617.75) | 452 (247.25–772.5) |
Data are n (%) or median (IQR)
Methods:
Clinical trial description
The IMPACT clinical trial (NCT01867671) information can be found elsewhere. (16)
Saliva samples
Saliva was collected either by suction with a syringe or spitting directly into a cryovial after first rinsing mouth with water or a damp washcloth. Saliva samples were then frozen and stored long term at −80°C until analysis. Samples were deidentified and researchers measured IgA and IgG4 while blinded.
IgG4 and IgA ELISAs
For peanut-specific and total IgG4 ELISAs, 96-well plates were coated with 2 μg/mL of anti-human IgG4 (clone G17–4, BD Biosciences, San Jose, CA) for standard curves or 20 μg/mL peanut protein (extracted as described previously (17)) for saliva samples. Wells were blocked with 2% BSA in PBS with 0.05% Tween 20. Saliva samples were diluted 1:10 for peanut-specific and 1:20 for total IgG4. Standard curves were generated with native human IgG4 protein (Abcam, Cambridge, MA) ranging from 0.24–250 ng/mL. Samples and standards were detected with a 1:1000 dilution of anti-human IgG4 Fc-HRP (clone HP6025, Southern Biotech, Birmingham, AL). Plates were developed using TMB (SeraCare, Milford, MA); the reaction was stopped by using 1% HCl (SeraCare), and the results were read at 450 nm by using a microplate spectrophotometer (BioTek, Winooski, VT).
For peanut-specific and total IgA ELISAs, 96-well plates were coated with 2 μg/mL of anti-human IgA1/IgA2 (clone G18–1, BD Biosciences, San Jose, CA) for standard curves or 20 μg/mL peanut protein for saliva samples. Wells were blocked with 2% BSA in PBS with 0.05% Tween 20. Saliva samples were diluted 1:50 for peanut-specific and 1:20,000 for total IgA. Standard curves were generated with purified human IgA (Bethyl Labs, Montgomery, TX) ranging from 0.6–60 ng/mL. Samples were detected with a 1:1000 dilution of anti-human IgA-HRP (Southern Biotech). Plates were developed using TMB (SeraCare, Milford, MA); the reaction was stopped by using 1% HCl (SeraCare), and the results were read at 450 nm by using a microplate spectrophotometer (BioTek, Winooski, VT).
Serum IgE, IgA, and IgG4 quantification
Serum immunoglobulins were quantified by the ImmunoCAP 1000 System (Phadia-ThermoFisher, Waltham, MA) as previously described (16).
Statistics
Saliva and serum antibody data were analyzed for participants in the per-protocol population who were study-compliant through the avoidance phase and had an evaluable blinded DBPCFC at the end of the maintenance and avoidance phases (per protocol population for the secondary endpoint). For comparisons between placebo and PnOIT groups, a linear mixed model was used with adjustment for baseline levels. For comparisons among PnOIT outcome groups, a linear mixed model was used without adjustment for baseline levels. The threshold for significance was p<0.05 (two-sided). All analyses were performed with SAS Version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Salivary IgG4 during PnOIT treatment and avoidance
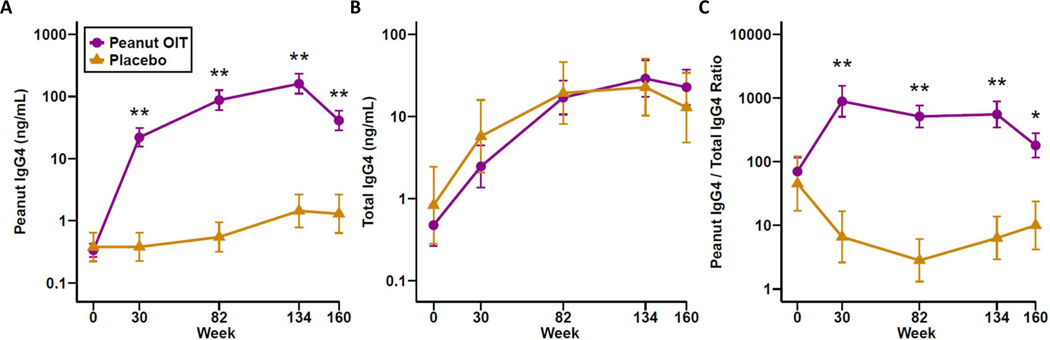
Saliva and serum samples were collected at baseline, 30, 82, 134, and 160 weeks for quantification of salivary peanut-specific and total IgG4, and serum peanut-specific IgG4 (PNsIgG4). Participants receiving PnOIT demonstrated significant increases in PNsIgG4 in saliva, whereas participants receiving placebo did not (placebo vs. PnOIT, p<0.01 at all time points; Figure 1A). After therapy was discontinued at week 134, PNsIgG4 sharply decreased in the PnOIT group. Total IgG4 was similar between placebo and PnOIT groups at baseline and increased in both treatment groups over time (Figure 1B). When looking at the ratio of PNsIgG4 to total IgG4 in the PnOIT group, there was a sharp increase at week 30, which plateaued through the remaining maintenance period and decreased during avoidance (weeks 134–160) (Figure 1C). PNsIgG4 to total IgG4 ratio did not increase in the placebo group during the trial.
Figure 1.
Peanut-specific and total IgG4 in saliva. Peanut-specific IgG4 (A), total IgG4 (B), and peanut IgG4/total IgG4 ratio (C) throughout the course of peanut (purple) or placebo (gold) OIT. Data are show as means ± standard error of the mean (SEM). *P<0.05, **P<0.01.
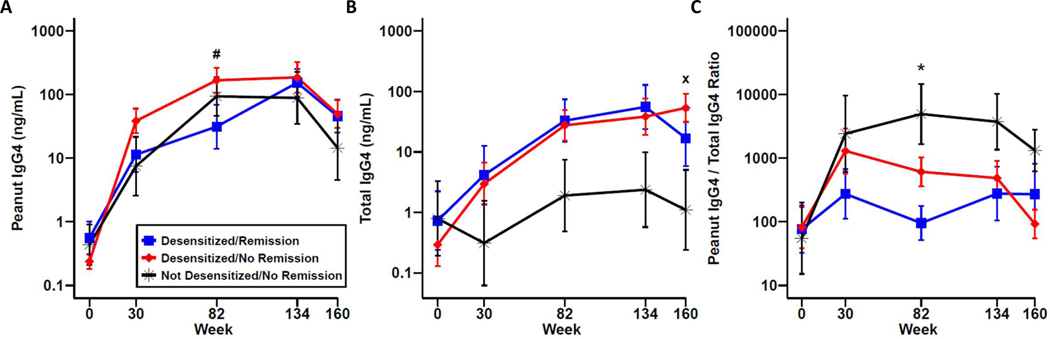
For the 69 participants that completed the protocol, when stratified by clinical outcome, a significant difference was noted in PNsIgG4 between the desensitized/remission and desensitized/no remission groups at week 82 (Figure 2A). For total IgG4, the not desensitized/no remission group trended towards lower levels throughout the course of treatment, and when therapy was stopped, there were statistically lower levels of total IgG4 between the not desensitized/no remission and desensitized/no remission groups at week 160 (Figure 2B). Taking into account the ratio of PNsIgG4 to total IgG4, the desensitized/remission group increased from baseline, and remained constant throughout the course of therapy, in comparison to the other groups where, on average, there was an increase at week 30 and remained elevated throughout treatment at week 134 (Figure 2C). Interestingly, the desensitized/remission group on average remained constant even off therapy, while the other groups had a trend towards a rapid decrease off treatment. Overall, PnOIT causes large increases in salivary PNsIgG4, but was not predictive of clinical outcome.
Figure 2.
Peanut-specific and total IgG4 in saliva by clinical outcome. Peanut-specific IgG4 (A), total IgG4 (B), and peanut IgG4/total IgG4 ratio (C) in desensitized/remission (blue), desensitized/no remission (red), and not desensitized/no remission (black) OIT participants. Data are show as mean ± standard error of the mean (SEM). # P<0.05 for desensitized/remission vs desensitized/no remission; x P<0.05 for desensitized/no remission vs not desensitized/no remission; * P<0.05 for desensitized/remission vs not desensitized/no remission.
Salivary IgA during PnOIT treatment and avoidance
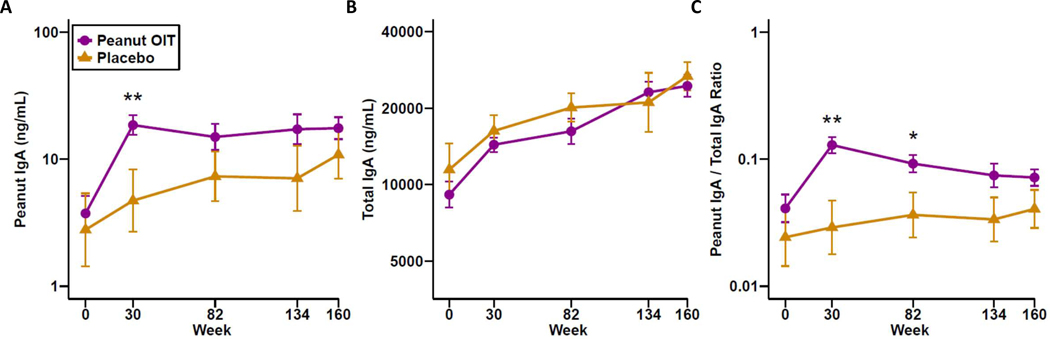
Peanut-specific IgA (PNsIgA) increased in participants treated with PnOIT and reached a plateau by week 30. PNsIgA was significantly higher in the PnOIT group compared to the placebo group at week 30 (Figure 3A). Total IgA was similar between placebo and PnOIT groups with both increasing over time (Figure 3B). When peanut-specific IgA was normalized to total IgA, the ratio of peanut-specific IgA to total IgA increased sharply in the PnOIT group by week 30 and gradually declined thereafter, whereas only small changes were observed in the placebo group (Figure 3C).
Figure 3.
Peanut-specific and total IgA in saliva. Peanut-specific IgA (A), total IgA (B), and peanut IgA/total IgA ratio (C) throughout the course of peanut (purple) or placebo (gold) OIT. Data are show as means ± standard error of the mean (SEM). *P<0.05, **P<0.01.
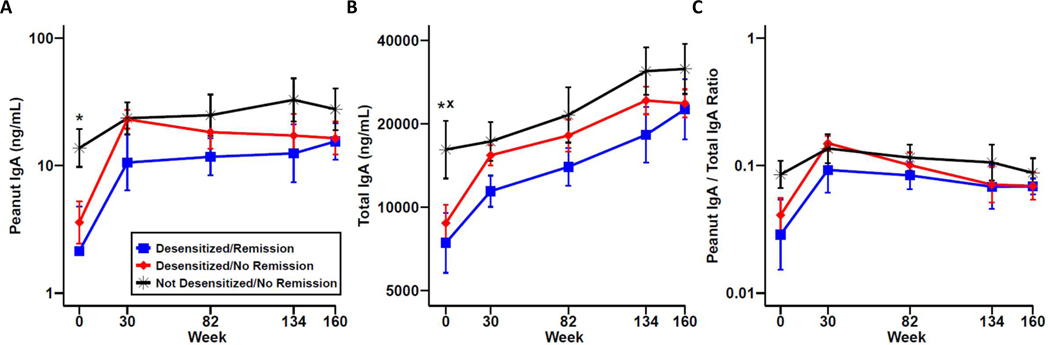
When stratified by clinical outcome within the PnOIT group, the not desensitized/no remission group had significantly higher salivary PNsIgA at baseline compared to the desensitized/remission group (Figure 4A). The same held true for total IgA (Figure 4B). When we normalized PNsIgA to total IgA, there was a similar trend as observed for PNsIgA (Figure 4C). There is also a trend for PNsIgA and total IgA in the not desensitized/no remission group at weeks 134 and 160 with higher levels noted when compared to both desensitized groups.
Figure 4.
Peanut-specific and total IgA in saliva by clinical outcome. Peanut-specific IgA (A), total IgA (B), and peanut IgA/total IgA ratio (C) in desensitized/remission (blue), desensitized/no remission (red), and not desensitized/no remission (black) OIT participants. Data are show as mean ± standard error of the mean (SEM). x P<0.05 for desensitized/no remission vs not desensitized/no remission; * P<0.05 for desensitized/remission vs not desensitized/no remission.
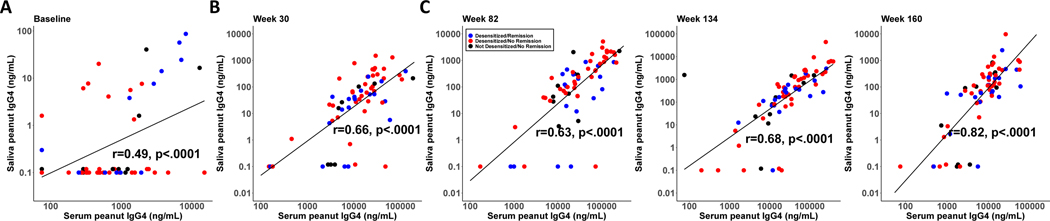
Correlations between salivary and serum immunoglobulin responses
To investigate local versus systemic responses, we compared salivary PNsIgG4 to serum PNsIgG4. Correlation plots were produced for each time point, and statistically significant correlations were identified (p<0.0001 for all time points, Figure 5). These results indicate a high correlation between local and systemic PNsIgG4 production. In contrast, serum PNsIgA was below the limit of detection for >80% of samples during treatment and avoidance, and therefore could not be correlated with salivary PNsIgA.
Figure 5.
Correlations between salivary and serum peanut-specific IgG4. Correlations in the PnOIT group at weeks 0 (A), 30 (B), 82 (C), 134 (D), and 160 (E). Lines represent linear regression lines and gray bands represent the 95% confidence intervals around regression lines.
Discussion
To investigate the effects of PnOIT on salivary immunoglobulins, we utilized saliva samples collected from the ITN IMPACT trial. Compared to the placebo group, the PnOIT group had significantly increased PNsIgG4 throughout the course of therapy, indicating a relationship between peanut exposure and production of PNsIgG4. There were similar levels of total IgG4 in the placebo and PnOIT groups, which was not surprising, since PnOIT only modulates the peanut-specific immune response. However, the increase in salivary IgG4 over time was unexpected and may be due to the developing immune system in these young children.
The PnOIT group had a sharp increase in PNsIgA by 30 weeks compared to the placebo group. These findings indicate a pronounced effect by PnOIT to enhance PNsIgA production at the oral mucosal surface. Daily administration of peanut antigen drives PNsIgA during build-up (weeks 0–30) and plateaus thereafter, indicating a maximum threshold for antigen-specific IgA production. Interestingly, serum PNsIgA was not detectable in the vast majority of subjects, indicating a more robust local response to orally administered peanut antigen. One interpretation of this finding is that salivary PNsIgA may play a role in the desensitization and remission mechanisms of PnOIT and may reflect the amount of PNsIgA present at the GI mucosal surface, as was shown in a mouse model with OVA. (18) Furthermore, mucosal IgA is responsible for immune exclusion (19) and in the context of PnOIT, may prevent peanut from being absorbed systemically. Further mechanistic studies are warranted to determine the role of mucosal IgA following OIT.
The IMPACT trial had well-defined endpoints for desensitization and remission based on DBPCFC outcomes at weeks 134 and 160. By using samples from this trial, we hypothesized that there would be distinct characteristics in the remission group. Contrary to what we expected, the desensitized/remission group did not have significantly higher salivary PNsIgG4 or PNsIgA than the other OIT outcome groups. In fact, the not desensitized/no remission group had significantly higher salivary PNsIgA at baseline. This surprising data suggests that the sharp increase from weeks 0 to 30 are associated with desensitization, and that the increase in the response rather than the quantity of the response is indicative of desensitization. Of note, a previous study examined salivary IgA after peanut SLIT and similarly found the increase in peanut IgA was correlated with increasing amounts of peanut protein tolerated during DBPCFC. (20) One possible interpretation of these results is that high levels of mucosal IgA may sequester the peanut antigen, preventing uptake by tolerogenic dendritic cells that are responsible for tolerance induction. Alternatively, the significantly higher PNsIgA in the not desensitized/no remission group at baseline may be an indicator of inflammation, which may limit successful outcomes on OIT. (21) Therefore, high levels of mucosal IgA at baseline may not be advantageous, and instead may indicate a decreased likelihood of tolerance induction. .
The clinical implications of this work are twofold: predicting which participants may respond positively to PnOIT, and monitoring outcomes throughout the course of therapy. Currently, there are no accepted assays available to predict participant-specific responses to OIT, although thresholds for baseline peanut-specific IgE in serum may be informative. (9, 22) Monitoring OIT is also challenging due to lack of predictive biomarkers. Direct basophil activation testing has emerged as a potential assay to monitor OIT, (22, 23) although these types of assays are technically difficult and require whole blood that needs to be processed quickly. Indirect basophil activation testing has recently been reported to have high diagnostic accuracy (24); however, it has not been tested in monitoring OIT outcomes. Alternatively, saliva is easily accessible, non-invasive, and can be stored frozen for later analysis. Here, although the sample size of groups stratified by clinical outcome was relatively small, we demonstrated that high levels of PNsIgA in saliva at baseline may indicate decreased likelihood of desensitization, which would be an impactful predictor if validated in other larger trials. Similarly, increases in PNsIgA within 30 weeks of starting OIT may be utilized to monitor successful outcomes.
In conclusion, we demonstrated that PnOIT has a substantial effect on peanut-specific IgA and IgG4 in saliva when compared to placebo treatment. These data give insight into mucosal responses induced by PnOIT and suggest a role for mucosal IgA and IgG4 in PnOIT-induced treatment outcomes. It is noteworthy that when therapy was discontinued, PNsIgG4 decreased sharply, while PNsIgA remained elevated. Importantly, both PNsIgG4 and PNsIgA remain higher than baseline levels even when therapy was discontinued. Since saliva is easily obtained, biomarkers within this sample type may provide further mechanistic insights as well as prove useful for monitoring clinical outcomes.
Highlights.
1. What is already known about this topic?
Serum peanut-specific IgG4 and IgA are significantly increased after peanut OIT, but do not correlate to an individual’s likelihood of success on therapy. Immunoglobulin responses have yet to be studied at the oral mucosal surface.
2. What does this article add to our knowledge?
PnOIT induces substantial increases in allergen-specific IgG4 and IgA in saliva. Salivary peanut IgA is higher at baseline in subjects that do not achieve desensitization, suggesting the increase during OIT is beneficial for desensitization.
3. How does this study impact current management guidelines?
These data provide insight into OIT-induced mucosal responses and suggest utility of these easily obtained samples for biomarker development.
Funding:
This research was performed as a project of the Immune Tolerance Network, an international clinical research consortium headquartered at the Benaroya Research Institute and supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JMS is funded by a T32 Allergy/Immunology Training Grant (AI007062) through Duke University and University of North Carolina at Chapel Hill.
Disclosure statement:
Johanna Smeekens has received funding from an NIH T32 training grant. Michael Kulis has received research support from NIH and DoD, and consulting fees from Ukko. Stacie Jones reports grants from NIH-NIAID, Food Allergy Research & Education (FARE), Aimmune Therapeutic, DBV Technologies, Astellas, Inc., Sanofi, Inc., Regeneron, Inc., and Genentech, Inc. and personal fees from Food Allergy Research and Education, EMMES Corporation, Aimmune Therapeutics.
Abbreviations:
- DBPCFC
Double-blind placebo-controlled food challenge
- OIT
Oral immunotherapy
- PnOIT
Peanut oral immunotherapy
- PNsIgA
Peanut-specific IgA
- PNsIgG4
Peanut-specific IgG4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lieberman JA, Gupta RS, Knibb RC, Haselkorn T, Tilles S, Mack DP, et al. The global burden of illness of peanut allergy: A comprehensive literature review. Allergy. 2021;76(5):1367–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300, e1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. 2010;105(6):444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Investigators PGoC, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med. 2018;379(21):1991–2001. [DOI] [PubMed] [Google Scholar]
- 5.Smeekens JM, Kulis MD. Evolution of Immune Responses in Food Immunotherapy. Immunol Allergy Clin North Am. 2020;40(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil SU, Ogunniyi AO, Calatroni A, Tadigotla VR, Ruiter B, Ma A, et al. Peanut oral immunotherapy transiently expands circulating Ara h 2-specific B cells with a homologous repertoire in unrelated subjects. J Allergy Clin Immunol. 2015;136(1):125–34 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134(6):1310–7 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood RA. Food allergen immunotherapy: Current status and prospects for the future. J Allergy Clin Immunol. 2016;137(4):973–82. [DOI] [PubMed] [Google Scholar]
- 11.Tordesillas L, Berin MC. Mechanisms of Oral Tolerance. Clin Rev Allergy Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinou GN, Nowak-Wegrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA. Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: is there a role in tolerance induction? Pediatr Allergy Immunol. 2014;25(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orgel K, Burk C, Smeekens J, Suber J, Hardy L, Guo R, et al. Blocking antibodies induced by peanut oral and sublingual immunotherapy suppress basophil activation and are associated with sustained unresponsiveness. Clin Exp Allergy. 2019;49(4):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135(5):1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. The Lancet. 2022;399(10322):359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bednar KJ, Hardy L, Smeekens J, Raghuwanshi D, Duan S, Kulis MD, et al. Antigenic Liposomes for Generation of Disease-specific Antibodies. Journal of visualized experiments : JoVE. 2018(140). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Externest D, Meckelein B, Schmidt MA, Frey A. Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage. Infect Immun. 2000;68(7):3830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Liu E, Gertie JA, Joseph J, Xu L, Pinker EY, et al. Divergent T follicular helper cell requirement for IgA and IgE production to peanut during allergic sensitization. Sci Immunol. 2020;5(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen IS, Baeten DLP, den Dunnen J. The inflammatory function of human IgA. Cell Mol Life Sci. 2019;76(6):1041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai M, Mukai K, Chinthrajah RS, Nadeau KC, Galli SJ. Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J Allergy Clin Immunol. 2020;145(3):885–96 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil SU, Steinbrecher J, Calatroni A, Smith N, Ma A, Ruiter B, et al. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. J Allergy Clin Immunol. 2019;144(5):1310–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruinemans-Koerts J, Brouwer ML, Schmidt-Hieltjes Y, Stevens P, Merkus P, Doggen CMJ, et al. The Indirect Basophil Activation Test Is a Safe, Reliable, and Accessible Tool to Diagnose a Peanut Allergy in Children. J Allergy Clin Immunol Pract. 2022;10(5):1305–11 e3. [DOI] [PubMed] [Google Scholar]